Abstract
During walking, the elevation angles of the thigh, shank, and foot (i.e., the angle between the segment and the vertical) covary along a characteristic loop constrained on a plane. Here, we investigate how the shape of the loop and the orientation of the plane, which reflect the intersegmental coordination, change with the slope of the terrain and the speed of progression. Ten subjects walked on an inclined treadmill at different slopes (between −9° and +9°) and speeds (from 0.56 to 2.22 m/s). A principal component analysis was performed on the covariance matrix of the thigh, shank, and foot elevation angles. At each slope and speed, the variance accounted for by the two principal components was >99%, indicating that the planar covariation is maintained. The two principal components can be associated to the limb orientation (PC1*) and the limb length (PC2*). At low walking speeds, changes in the intersegmental coordination across slopes are characterized mainly by a change in the orientation of the covariation plane and in PC2* and to a lesser extent, by a change in PC1*. As speed increases, changes in the intersegmental coordination across slopes are more related to a change in PC1*, with limited changes in the orientation of the plane and in PC2*. Our results show that the kinematic patterns highly depend on both slope and speed.
NEW & NOTEWORTHY In this paper, changes in the lower-limb intersegmental coordination during walking with slope and speed are linked to changes in the trajectory of the body center of mass. Modifications in the kinematic pattern with slope depend on speed: at slow speeds, the net vertical displacement of the body during each step is related to changes in limb length and orientation. When speed increases, the vertical displacement is mostly related to a change in limb orientation.
Keywords: intersegmental coordination, kinematics, planar covariation, slope, walking
INTRODUCTION
Walking is a rather complex task that involves the motion of all body segments at several degrees of freedom (45, 69). However, with an examination of the whole body dynamics, the description of walking appears remarkably simple and consistent: the out-of-phase fluctuations of the potential and kinetic energies of the center of mass (COM) of the body can be likened to an inverted pendulum (15). It has been suggested that these pendulum-like dynamics may serve as a target for motor control of walking (2, 26, 37, 48, 65). The displacement of the COM highly depends on the combined movements of the lower-limb segments (44, 45, 63). These segmental motions result from the interplay between passive dynamics and active neural control (37, 43), which in turn, contribute to whole body stability and economy.
In the mid-1990s, it was shown that the changes of the orientation of the lower-limb segments in the sagittal plane do not evolve independently during the stride (9, 12): when the orientation angles of the thigh, shank, and foot are plotted against each other in a three-dimensional (3D) space, they covary along a loop constrained close to a 2D plane. The orientation of this plane and the shape of the loop depend on the phase and amplitude relationships among the segmental angles. Changes in the planar covariation are related to how subjects perform in different walking conditions, e.g., with erect vs. bent posture (29), with different levels of body-weight unloading (38), along curved paths (19), or with slip-like perturbations (6). Furthermore, pathologies of the brain structures, such as the basal ganglia (28) or the cerebellum (51, 54), also affect the shape of the elevation angle loop and the orientation of the covariance plane.
With the analysis of the planar covariation of the lower-limb segments, Ivanenko et al. (35) has shown that the main axis of the covariance plane primarily represents the limb orientation, and the second axis primarily represents the limb length. In this way, the planar covariation of lower-limb segments describes the behavior of a “telescopic limb” (41) that shapes the COM trajectory as a function of body posture and gait (29, 35). However, open questions remain as to how the COM trajectories are achieved by the combined rotation and translation of the lower-limb segments when walking on various slopes and at different speeds.
The task of walking up or down slopes involves a modification of COM height with each step. At first approximation, the trajectory of the COM during level walking is often illustrated by a “compass gait” model (3, 56, 63, 66). In this model, the lower limbs are assimilated to rigid sticks, their mass is negligible, and the COM is located at the hip. In this way, the hip joint and the COM move along a series of arcs that are symmetric relative to the point of contact. In a previous study (21), we suggested that the changes in the transduction between kinetic and potential energies of the COM during walking on a slope can be illustrated by the tilting of the compass gait model backward in uphill walking and forward in downhill walking. However, these changes at the COM level could be accomplished in the following various ways: by changing 1) the limb length, 2) the limb orientation, or 3) both limb length and limb orientation in a coordinated fashion. Limb length and limb orientation are then a function of intersegmental coordination, particularly of the thigh, shank, and foot. The aim of the present study is to characterize how limb orientation and limb length, as well as intersegmental coordination patterns, change with walking slope and speed.
METHODS
Subjects and Experimental Procedure
Data were collected at the same time and on the same subjects as in the study of Dewolf et al. (21). Six men and four women (age: 22.2 ± 2.4 yr, mass: 69.2 ± 14.4 kg, height: 1.75 ± 0.10 m, means ± SD) participated in the study. All participants gave their written, informed consent. Experimental procedures were performed according the Declaration of Helsinki and were approved by the Ethics Committee of the Université Catholique de Louvain.
Subjects walked on an instrumented treadmill mounted on wedges. The instrumented treadmill consisted of a modified commercial treadmill (h/p/comos-Stellar, Nussdorf-Traunstein, Germany; belt surface: 1.6 × 0.65 m, mass: ~240 kg) combined with four force transducers (Arsalis, Louvain-la-Neuve, Belgium), measuring the components of the ground reaction forces (GRFs), parallel, normal, and lateral to the tread belt. Wedges of different size were attached under the four strain gages to incline the treadmill at different slopes.
The whole structure of the treadmill (i.e., the body, tread surface, belt, and motor) is mounted on force transducers. Since the fixed parts of the treadmill are rigid and firmly attached to each other and since the mobile parts are moving symmetrically, the acceleration and the velocity of the COM of the treadmill, relative to the reference frame of the laboratory, are nil. In this way, the forces exerted by the subject moving on the upper part of the treadmill are accurately transmitted to the transducers placed under it, and all internal forces, including friction, between the parts of the treadmill, are cancelling each other (68).
The trajectory of the COM was determined from the GRF using the procedure described in detail in Dewolf et al. (21, 22). The lateral (dy) and fore-aft (dx) position of the center of pressure was computed by the following
| (1) |
| (2) |
where Fx, Fy, and Fz are the lateral, fore-aft, and vertical GRFs; Mx and My are the moment components in the force transducer coordinate system; and h is the vertical distance between the force transducers and the tread surface (71). Note that the center of pressure is under one foot during the single-support phase and moves from one foot to the other during the double-support phase (59).
Reflective markers were glued on the skin of the subject at the following positions: chin-neck intersect, greater trochanter (GT), lateral femoral condyle, lateral malleolus, heel (HE), and fifth metatarsophalangeal joint (VM). An additional marker was also placed at a point midway between the GT and the lateral femoral condyle on the lateral side of the thigh in case the marker of the hip was hidden by the hand. The position of the markers in the sagittal plane was measured by means of a high-speed video camera (A501k; Basler, Ahrensburg, Germany; resolution 1,280 × 1,024 pixels, aperture time 3 ms), fixed on the ground, 3 m to the side of the treadmill, perpendicular to the plane of progression. Images were sampled at a rate of 100 frames/s. The horizontal and vertical coordinates of the reflectors in the sagittal plane were measured in each frame using Lynxzone software (Arsalis).
Seven different inclinations (0°, ±3°, ±6°, and ±9°) were explored: six subjects walked on all slopes. Due to the duration of the experiments, two subjects walked only at 0° and ±6° and two at 0° and ±9°. One-half of the subjects started with the belt turning forward to simulate uphill walking and the other half with the belt turning backward to simulate downhill walking. At each slope, subjects walked at seven different speeds, ranging between 0.56 m/s (slow walking speed) and 2.22 m/s (close to the walk-run transition), with at least a 3-min resting period between each speed. At 2.22 m/s, four subjects were spontaneously running, especially on steep slopes, and were thus not recorded. Each trial started with the belt still, and speed was ramped up until the desired speed. An average of 13.6 ± 5.6 strides (means ± SD) was recorded in each trial. The number of subjects recorded in each speed-slope class is presented in Table 1.
Table 1.
Number of subjects in each slope/speed class
| Speed, m/s | −9° | −6° | −3° | 0° | 3° | 6° | 9° |
|---|---|---|---|---|---|---|---|
| 0.56 | 8 | 8 | 6 | 10 | 6 | 8 | 8 |
| 0.83 | 8 | 8 | 6 | 10 | 6 | 8 | 8 |
| 1.11 | 8 | 8 | 6 | 10 | 6 | 8 | 8 |
| 1.39 | 8 | 8 | 6 | 10 | 6 | 8 | 8 |
| 1.67 | 8 | 8 | 6 | 10 | 6 | 8 | 8 |
| 1.94 | 8 | 8 | 6 | 10 | 6 | 8 | 8 |
| 2.22 | 5 | 6 | 5 | 8 | 5 | 6 | 5 |
Data Analysis
Stride period and stride length.
The stride was defined as the period between a right-foot contact (FC; 0%) and the next one (100%). FC and toe off (TO) were estimated from the displacement of the center of pressure on the belt (59). The stride duration T was calculated as the time between two successive right FCs.
Orientation of the body segments during the stride.
From the marker locations, the orientation of the thigh (GT–lateral femoral condyle), shank (lateral femoral condyle–lateral malleolus), foot (lateral malleolus–VM), and trunk (chin-neck intersect–GT), relative to the vertical axis (elevation angle), was computed, as described in Borghese et al. (12). The joint angles (hip, knee, and ankle) were computed from the elevation angle of adjacent segments. For each subject, the different strides of each trial were time interpolated to fit a normalized, 400-point time base and then averaged every 0.25% of the stride period.
To analyze the time course of the elevation angle during the stride, a Fourier series component was performed (9, 29). Amplitude (A), phase shift (P), and percentage of variance, accounted for by the first harmonic, were computed. The amplitude ratio and phase shift between two adjacent limb segments p and d (Gpd and ϕpd, respectively) were computed as Gpd = A(d)/A(p) and as ϕpd = P(d) − P(p).
Lower-limb length and orientation during the stride.
The length of the limb (L) was measured as the distance between the markers located on the GT and on the HE during standing. At each instant of stance, the actual limb axis should be defined as the connection of the proximal joint with the point of contact with the ground, i.e., with the instantaneous center-of-pressure position (35). However, during walking, the contact moves from the HE to the ball of the foot, as stance progresses from FC to TO. During double support, our system allows the measurement of only the global center of pressure under the two feet. Therefore, the angle, relative to vertical (θfc, θto), and the limb length (Lfc, Lto) were measured only at FC and TO. The angle θfc and the length Lfc were measured from the markers of the GT and the HE at FC, and θto and Lto were measured from the markers of the GT and the VM at TO.
Intersegmental coordination.
A principal component analysis was applied to the 3D covariance matrix of the segment elevation angles (thigh, shank, foot). Eigenvalues and eigenvectors ui were computed by the factoring of the covariance matrix from the set of original signals by using a singular value decomposition algorithm. The first two eigenvectors (u1 and u2) lay on the best-fitting plane of angular covariation, and the data projected onto these axes corresponded to the first (PC1) and second (PC2) principal components. The planarity was evaluated for each condition by finding the percentage of variance that was explained by u1 (PV1) and u2 (PV2). If the data were lying perfectly on a plane, then 100% of the variance would be explained by u1 and u2. The eigenvectors u1 and u2 defined the shape of the loop, whereas the third eigenvector u3, normal to the plane, provided a measure of its orientation. The parameters u3t, u3s, and u3f corresponded to the direction cosines with the positive semiaxis of the thigh, shank, and foot angular coordinates, respectively.
In a next step, the principal components PC1 and PC2 were reoriented to be associated to the limb orientation (PC1*) and the limb length (PC2*), as proposed by Ivanenko et al. (35). The reference axis PC1* corresponded to the projection of the vector t = s = f on the covariance plane, where t, s, and f referred to the thigh, shank, and foot elevation angles. The orientation of the second axis PC2* was determined from a previous study (35), in which subjects were walking in place; in this case, there was no change in the limb orientation, and the covariance loop was a line that could be associated to the limb length L. In the present study, the orientation of the in-place covariance line, computed by Ivanenko et al. (35), was used to determine the orientation of PC2*.
Since the trunk orientation can be regulated independently of the covariation of the lower-limb angles, we have also included the trunk into the coplanar analysis and redid the principal component analysis performed on the 4D covariance matrix [as in Borghese et al. (12)]. Due to the limited range of motion (ROM) of the trunk during walking, whatever the slope, the projection of the 4D space on the principal plane is very similar to the projection of the 3D space (see Table 2). Therefore, to obtain a better visualization of the angular covariations, the analysis was limited to the 3D space.
Table 2.
Percent of variance accounted for by PV1 and PV2 when the principal component analysis is performed on the 3D covariance matrix (first line) and on the 4D covariance matrix (second line)
| 0.56 m/s |
1.39 m/s |
2.22 m/s |
||||
|---|---|---|---|---|---|---|
| Slope, ° | PV1 | PV1 + PV2 | PV1 | PV1 + PV2 | PV1 | PV1 + PV2 |
| −9 | 89.4 ± 3.6 | 99.3 ± 3.4 | 90.7 ± 2.1 | 99.4 ± 2.1 | 90.9 ± 1.9 | 98.9 ± 2.2 |
| 89.2 ± 3.4 | 99.2 ± 0.3 | 90.6 ± 2.1 | 99.3 ± 0.2 | 90.7 ± 1.8 | 98.8 ± 0.7 | |
| −6 | 87.0 ± 2.9 | 99.3 ± 2.6 | 89.5 ± 1.6 | 99.5 ± 1.6 | 90.8 ± 1.0 | 99.1 ± 1.4 |
| 87.1 ± 2.9 | 99.1 ± 0.3 | 89.4 ± 1.6 | 99.4 ± 0.1 | 90.6 ± 0.9 | 99.0 ± 0.4 | |
| −3 | 85.1 ± 1.7 | 99.3 ± 1.6 | 88.5 ± 1.0 | 99.6 ± 1.1 | 89.4 ± 1.0 | 99.5 ± 1.1 |
| 84.9 ± 1.6 | 99.0 ± 0.3 | 88.3 ± 1.0 | 99.4 ± 0.1 | 89.2 ± 1.0 | 99.3 ± 0.1 | |
| 0 | 83.4 ± 3.0 | 99.4 ± 3.0 | 87.8 ± 1.4 | 99.5 ± 1.4 | 88.8 ± 1.0 | 99.4 ± 1.0 |
| 83.1 ± 2.9 | 99.2 ± 0.4 | 87.6 ± 1.4 | 99.4 ± 0.1 | 88.6 ± 1.1 | 99.3 ± 0.3 | |
| 3 | 80.6 ± 3.2 | 99.5 ± 3.3 | 86.0 ± 1.3 | 99.5 ± 1.3 | 86.9 ± 1.0 | 99.5 ± 1.1 |
| 80.4 ± 3.2 | 99.4 ± 0.2 | 85.8 ± 1.3 | 99.4 ± 0.1 | 86.7 ± 1.0 | 99.4 ± 0.1 | |
| 6 | 76.7 ± 3.6 | 99.5 ± 3.6 | 82.8 ± 2.6 | 99.5 ± 2.7 | 85.1 ± 1.9 | 99.5 ± 2.0 |
| 76.4 ± 3.5 | 99.4 ± 0.1 | 82.6 ± 2.5 | 99.4 ± 0.1 | 84.9 ± 1.9 | 99.3 ± 0.1 | |
| 9 | 70.6 ± 2.1 | 99.4 ± 2.1 | 79.2 ± 1.8 | 99.3 ± 2.0 | 83.1 ± 2.3 | 99.3 ± 2.4 |
| 70.4 ± 2.1 | 99.2 ± 0.2 | 79.0 ± 1.8 | 99.2 ± 0.4 | 82.8 ± 2.2 | 99.2 ± 0.2 | |
3D, 3-dimensional; 4D, 4-dimensional; PV1, variance explained by eigenvector 1; PV2, variance explained by eigenvector 2.
Estimation of the motion of the COM from the kinematic data.
One of the subgoals of our study was to investigate changes in kinematics of the lower-limb segments relative to changes in COM trajectory. Therefore, the trajectory of the COM, as estimated from the GRF, was compared with the trajectory of a point located at mid-distance between the two hips (on the assumption that the distance between this point and the COM remains constant during the stride). Due to the rotation of the pelvis in the frontal plane and in the transverse plane, the movements of one hip do not reflect the movements of the midpoint of the pelvis. To estimate the x–y position of this midpoint in the sagittal plane, we make the assumption that the x–y position of the hip on the nonrecorded side of the body during one-half of a stride is equal to the one on the side facing the cameras during the other one-half of the stride (9). From the coordinates of both hips, we estimate the projection on the sagittal plane of the point located at mid-distance between the two hips to assess if this point can be used as a surrogate for the COM.
Statistics
Data were grouped into speed-slope classes. To obtain one value per subject in each class, all strides of a subject in a given class were averaged. The mean and SD of the population were then computed in each class (grand mean). The variables (mean value per subject) were analyzed across all conditions using a repeated-measure ANOVA with post hoc Bonferroni correction (PASW Statistics 19; SPSS, IBM, Armonk, NY) to assess the individual and interaction effects of speed and slope on the calculated variables. Linear regression analysis, using Pearson’s correlation coefficient (r), was used to indicate the relationship between variables. An α threshold of 0.05 was used throughout to assess statistical significance.
RESULTS
General Gait Parameters and Limb Kinematics
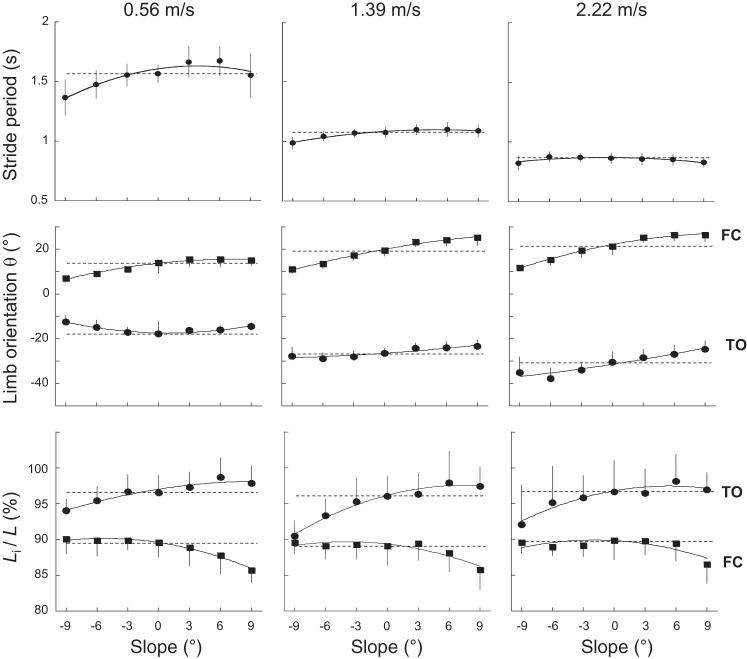
Stride period (T)—the angle between the limb axis and the vertical and the limb length at FC (θfc, Lfc) and at TO (θto, Lto)—is presented as a function of slope at three different speeds in Fig. 1. In accordance with Kawamura et al. (40) and Sun et al. (64), T decreases with speed (F6,363 = 580.8, P < 0.001). Bonferroni post hoc shows that T is not significantly affected by the slope of the terrain compared with the level (P > 0.063), except at −9° at 0.56 m/s (P < 0.001), where T is shorter.
Fig. 1.
Stride period, limb orientation, and limb length as a function of slope at slow, intermediate, and fast walking speed. Top: stride period; middle: orientation of the limb relative to vertical at foot contact [FC; θfc (■)] and toe off [TO; θto (●)]; bottom: length of the limb (Li), expressed as a percent of the length (L), measured during standing at FC [Lfc (■)] and at TO [Lto (●)]. Horizontal dotted lines correspond to the data obtained on the level. Symbols and bars represent the grand means of the subjects and the SDs (when the length of the bar exceeds the size of the symbol). The continuous lines were drawn through experimental data (using a weighted mean function, KaleidaGraph 4.5; Synergy Software, Reading, PA).
Compared with walking on the level at a given speed, θfc increases when walking uphill, whereas it decreases when walking downhill (F6,363 = 100.1, P < 0.001). The angle θto is also affected by slope (F6,363 = 24.6, P < 0.001): θto decreases from 0° to +9°. From 0° to −9°, θto decreases at low speeds, whereas θto increases at high speeds. At all slopes, both θfc (F6,363 = 46.3, P < 0.001) and θto (F6,363 = 163.2, P < 0.001) increase when speed becomes faster, because the stride length increases. Note that at 2.22 m/s, the change in orientation of the limb corresponds approximately to the change in inclination of the terrain.
The limb lengths at FC and at TO (respectively, Lfc and Lto; Fig. 1) are also affected by slope (F6,363 = 20.5, P < 0.001 and F6,363 = 25.9, P < 0.001, respectively) but not by speed (F6,363 = 0.27, P = 0.948 and F6,363 = 1.6, P = 0.150, respectively). When walking uphill, Lfc is shorter, and Lto is longer compared with the level. When walking downhill, Lto is longer, but Lfc remains unchanged compared with the level.
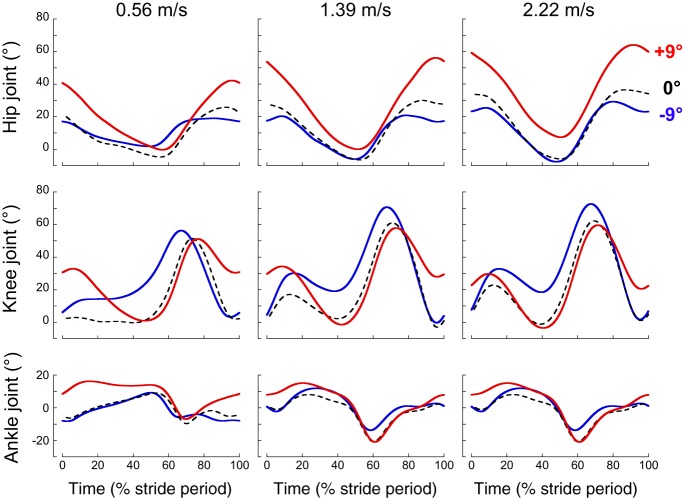
Figure 2 shows the average waveforms of lower-limb joint angles at low, intermediate, and high walking speeds on the level and on the steepest positive and negative slopes. The joint motion pattern observed on slope walking in this study shows good agreement with results obtained in other studies (24, 30, 49, 57). Compared with level walking, the major kinematic changes in uphill walking occur at FC, where the hip, knee, and ankle joints are more flexed. In downhill walking, the major kinematic modification is an increase of knee flexion during stance.
Fig. 2.
Lower-limb joint angles at 3 different slopes at slow-, intermediate-, and fast-walking speeds. Ensemble average of the hip (top), knee (middle), and ankle (bottom) joint angles over a stride during walking at +9° (red curves), −9° (blue curves), and 0° (black interrupted lines). All of the curves of each subject walking at a given speed and on a given slope were first averaged (mean curve). The curves presented here are the average of the mean curves of the 10 subjects (ensemble average). Zero percent and 100% correspond to the right-foot contact.
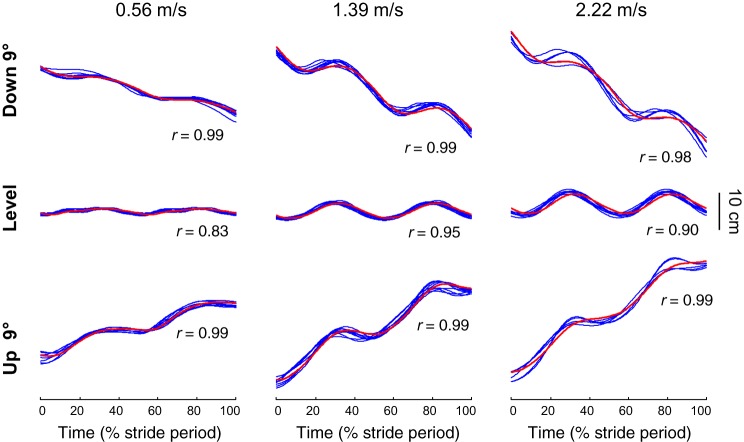
The vertical displacement of the midpoint between the two hips is presented in Fig. 3 at different slopes and speeds. The trajectory of this point is similar to the trajectory of the COM, measured from the GRF, both in uphill and downhill walking.
Fig. 3.
Center of mass (COM) and hips vertical displacement at slow-, intermediate-, and fast-walking speed. Ensemble-average vertical displacement of the COM of the 10 subjects (red curve) over a stride during walking at −9° (top), +9° (bottom), and on the level (middle). Each blue line represents the mean curve of the vertical displacement of the hips (see methods) of a subject. In each condition, r corresponds to the average Pearson’s correlation coefficient between the COM and the hips vertical displacement.
Lower-Limb Segment Angular Motion
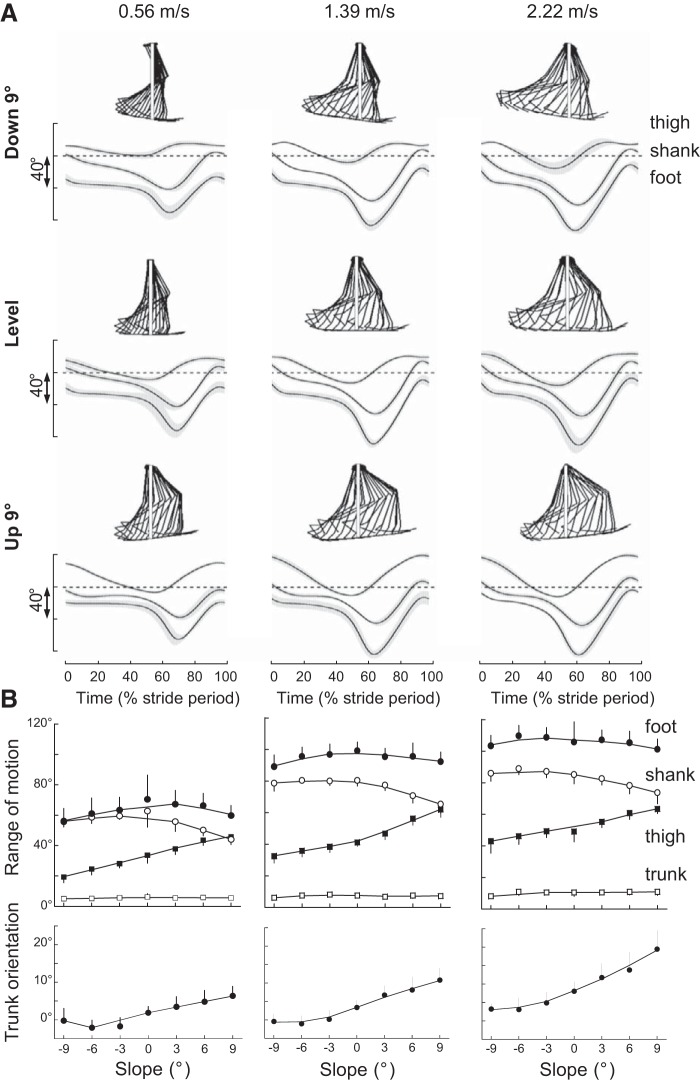
Figure 4A shows the time curve of the average elevation angles of lower-limb segments at low, intermediate, and high walking speeds on the level and on the steepest positive and negative slopes. All elevation angles display a typical biphasic shape (9, 12), and their ROM increases with increasing speed (Fig. 4B; F6,363> 89.1, P < 0.001). Compared with level walking, when walking uphill, the shank ROM decreases (Bonferroni post hoc, P < 0.001), whereas the thigh ROM increases (Bonferroni post hoc, P < 0.001). When walking downhill, the foot and the shank ROM remain fairly similar, whereas the thigh ROM decreases (Bonferroni post hoc, P < 0.003).
Fig. 4.
Elevation angles of lower-limb segments during walking at slow-, intermediate-, and fast-walking speeds. A: ensemble-average elevation angles of the thigh, shank, and foot over a stride at −9° (top), +9° (bottom), and on the level (middle) at 0.56 (left), 1.39 (middle), and 2.22 (right) m/s. The gray zone represents ±1 SD. The interrupted lines correspond to 0° for the thigh and shank segments and to 90° for the foot segment. In each condition, the stickman illustrates the position of the segments, every 5% of a typical stride of 1 subject. The white continuous lines correspond to vertical. B: range of motion of all body segments over 1 stride (top) and average orientation of the trunk (bottom) as a function of slope at 0.56 (left), 1.39 (middle), and 2.22 (right) m/s. Other indications are as in Fig. 1.
Even if the orientation of the trunk changes little during the gait cycle (Fig. 4B), its average orientation is affected by both slope (F6,363 = 134.8, P < 0.001) and speed (F6,363 = 43.5, P < 0.001). Compared with level walking, the trunk shows a forward tilt during uphill walking and a backward tilt during downhill walking. At each slope, the body bends more forward with increasing speed.
Planar Covariation of the Limb-Segment Elevation Angles
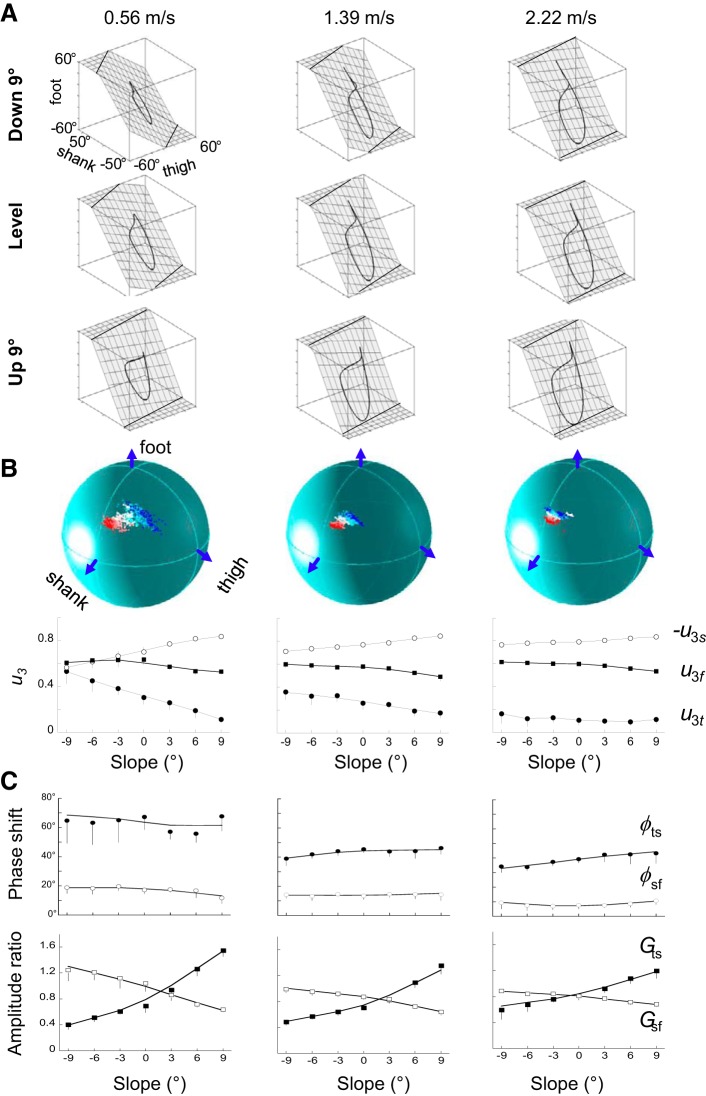
In each speed-slope class, the variance accounted for by the two first eigenvectors of the data covariance matrix (PV1 + PV2; Table 2) was, on average, 99.45 ± 0.24%. The variance accounted for by each principal axis changes with speed (F6,363 = 59.9, P < 0.001) and slope (F6,363 = 253.8, P < 0.001): PV1 decreases on positive slopes and increases on negative slopes and with increasing speed (Table 2).
The principal planes are illustrated in Fig. 5A. In agreement with previous studies (8, 9, 18, 23, 36, 38, 60), both slope and speed involve a rotation, mainly along the long axis of the gait loop (Fig. 5B): the covariation plane rotates counterclockwise when viewed from above. At low walking speed, with increasing slope from −9° to +9°, u3t and u3s decrease, whereas u3f remains fairly similar. As speed increases, both u3t and u3s are reduced. Note that the effect of speed is scaled with slope (F36,363 = 4.6, P < 0.001): the change due to speed is greater in downhill walking and almost nil in uphill walking. As a result, at high walking speed, the effect of slope on u3 tends to disappear. Because the effect of slope and speed is similar but greater on u3t than on u3s, we will only discuss the results for u3t.
Fig. 5.
Planar covariation of elevation angles. A: covariation of the limb-segment elevation angles during walking at −9° (top), +9° (bottom), and on the level (middle) at 0.56 (left), 1.39 (middle), and 2.22 (right) m/s. Each trace represents the ensemble average (see definition in Fig. 2). Grids show the best-fitting plane. B, bottom: third eigenvector direction cosines for thigh, shank, and foot (u3t, −u3s, u3f) of the normal to the covariation plane (u3 vector) as a function of slope at 0.56 (left), 1.39 (middle), and 2.22 (right) m/s. Other indications are as in Fig. 1. Top: spatial distribution of the normal to the principal plane (u3) in the 3-dimensional space, defined by the elevation angles in each slope at 0.56 (left), 1.39 (middle), and 2.22 (right) m/s. White symbols correspond to level walking. The red color gradients are for the positive slopes (the darker the color, the steeper the slope), whereas the blue color gradients are for the negative slopes. C: phase shift (ϕ; top) and amplitude ratio (G; bottom) between the first harmonics of adjacent lower-limb segments (ts, thigh/shank; sf, shank/foot). Other indications are as in Fig. 1.
The first harmonic of the elevation angles accounts for the major part of the variance for all segments in each speed-slope class (86.1 ± 4.8%, means ± SD). Thus the amplitude ratio (G) and the phase shift (ϕ) between pairs of adjacent lower-limb segments (thigh-shank and shank-foot) capture the amplitude and time relationship characteristics of the elevation angles (Fig. 5C). As slope changes from −9° to +9°, Gts increases (F6,363 = 281.7, P < 0.001), meaning that the amplitude of the thigh movements, relative to that of the shank movements tends to increase (Fig. 5B). To a lesser extent, Gsf shows the opposite tendency (F6,363 = 313.2, P < 0.001), i.e., the amplitude of the shank movements, relative to that of the foot, tends to decrease from −9 to +9°. However, the effect of slope on Gts and Gsf is lessened when walking speed increases. At each walking speed, the time changes of thigh-elevation-angle lead those of shank-elevation-angle, and the time changes of shank-elevation-angle lead those of foot-elevation-angle. However, both ϕts and ϕsf are reduced when speed increases (F6,363> 61.2, P < 0.001) but do not change with slope compared with the level (Bonferroni post hoc, P > 0.271), except at −9°, where ϕts is slightly reduced (Bonferroni post hoc, P = 0.003).
The rotation of the plane described above is related to the changes in amplitude ratio and phase shift between adjacent segments (9, 35). A multiple linear regression is calculated to predict u3t, based on Gts and ϕsf. A significant regression equation is found (P < 0.001), with an r2 = 0.92. Both Gts and ϕsf are significant predictors of u3t.
PC1* and PC2*: Length and Orientation of Limb Axis
Overall, PC1* and PC2* are in agreement with our lower-limb kinematics measurement. The time changes of PC1* are correlated with the time changes in lower-limb orientation. The lower-limb axis definition used here (i.e., GT-HE or GT-VM) did not affect appreciably the correlation between PC1* and limb orientation, likely because of a similar back-and-forth horizontal motion of any fixed point on the foot (35). The correlation is higher for the GT-VM (r2 = 0.99 ± 0.01) than the GT-HE limb axis (r2 = 0.96 ± 0.02). The behavior of PC2* is similar to that of knee-joint angle observed in Fig. 2. Indeed, PC2* is essentially correlated with knee angle (r2 = 0.91 ± 0.07). As a result, the differences (Δ), observed in ΔPC2*, are essentially dependent on the change in knee angle during stance, which affects limb length.
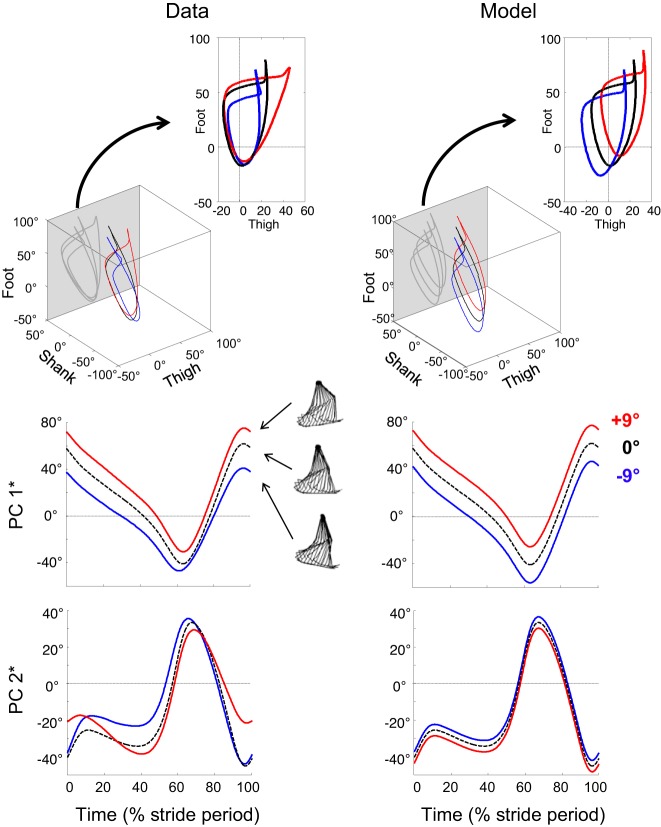
In a previous study (21), we suggested that the changes in the COM trajectory during walking on a slope can be illustrated by the tilting of the compass gait model backward in uphill walking and forward in downhill walking. In Fig. 6, the actual data of the covariation of limb-segment elevation angles are compared with data obtained by simulating a passive tilt of the compass-gait model relative to the slope: these last data are obtained by adding +9° (uphill) or −9° (downhill) to the elevation angles of the thigh, shank, and foot measured on the level. Changes in kinematic pattern across slope cannot be explained by a simple tilt of the compass gait model proportional to the inclination of the ground. The actual PC1* curves hardly differ from PC1* of the model, suggesting that there is a tilt of the limb-axis orientation. However, major differences in PC2* are observed with slope.
Fig. 6.
Decomposition of planar covariation in the reoriented principal component associated to limb orientation (PC1*) and to limb length (PC2*). Top: covariation of limb-segment elevation angles during walking at 1.39 m/s on a −9° (blue) and a +9° (red) slope and on the level (black interrupted line). Insets: projection of the gait loops on the thigh-foot plane (gray planes of the cubes). The loops were decomposed in PC1* and PC2* (see methods), corresponding to limb orientation and limb length, respectively. Middle and bottom: variation of PC1* and PC2* over a stride. All curves are ensemble average. Left: actual data of the covariation of limb-segment elevation angles. Right: data obtained by simulation of a passive tilt of the compass-gait model relative to the slope. Data on the level (black curve) are the same as the left. The red and blue loops and decomposition in PC1* and PC2* are obtained by addition of +9° (uphill) or −9° (downhill) to the elevation angles of the thigh, shank, and foot, measured on the level.
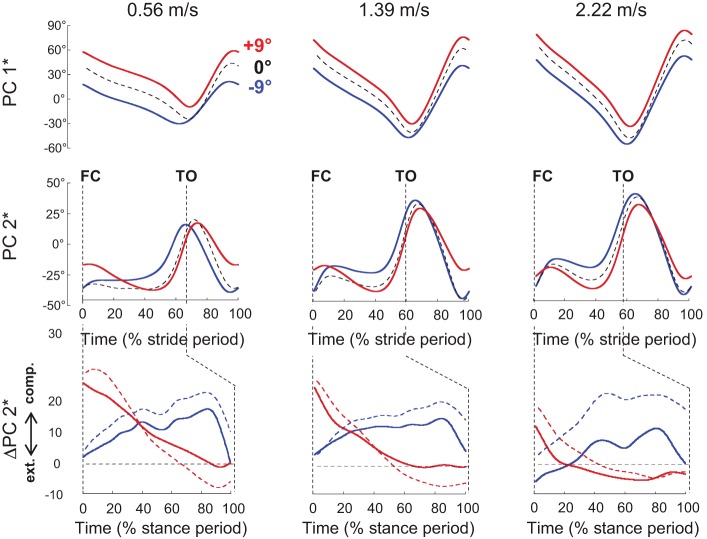
In uphill walking, the main change occurs at FC, where a higher PC2* is indicative of a more flexed limb (Fig. 7). In downhill walking, the major PC2* modification reflects an increase in limb flexion during stance. Compared with the level, ΔPC2* reflects that in uphill walking, the limb is extended throughout the stance, whereas in downhill walking, the limb is compressed during early stance and is extended before TO. However, both in uphill and downhill walking, the changes in limb length are reduced with increasing speed.
Fig. 7.
Limb length- and limb orientation-related angular covariance at slow-, intermediate-, and fast-walking speed. Top and middle: ensemble-average reoriented principal component associated to limb orientation (PC1*) and to limb length (PC2*) over a complete stride during walking at +9° (red), −9° (blue), and on the level (black interrupted line). Bottom: ΔPC2* is computed by subtracting PC2* on the level from PC2* at +9° (continuous red lines) and from PC2* at −9° (continuous blue lines) during the stance period. Likewise, the knee-angle time curves (presented in Fig. 2) on the level were subtracted from those at +9° (interrupted red lines) and at −9° (interrupted blue lines). comp., compressed; ext., extended; FC, foot contact; TO, toe off.
DISCUSSION
In this study, we investigated the combined effect of slope and speed on the lower-limb intersegmental coordination, limb orientation, and limb length. Whereas other studies have also reported the impact of speed and slope on joint kinematics and kinetics during walking (1, 4, 30–33, 39, 42, 46, 47, 49, 57, 61, 67, 74), here, we built on this work by the characterization of changes in intersegmental coordination to slopes.
Our findings extend previous observations that the limb-segment elevation angles covary along a plane, not only during level (9) or uphill walking (60) but also during downhill walking (Fig. 5), despite considerable differences in the joint kinetics and in the relative amplitude of proximal vs. distal segment motion across slopes (Fig. 4). It is worth stressing that the changes in the kinematic pattern across slopes cannot be accounted for by a simple tilt of the compass gait model proportional to the inclination of the ground, i.e., a simple tilt of the “dynamic template” of limb-segment motion (Fig. 6). Furthermore, we observe a cross-effect of speed and slope on the characteristics of the limb-segment covariation (orientation of the covariance plane and the width of the gait loop): at slow speeds, more changes were observed across slope, whereas at high walking speed, the effect of slope tends to disappear (Fig. 5). Below, we discuss the results in the context of the telescopic limb behavior (35, 41) and phase relationships among limb-segment motions during walking on a slope.
Effect of Speed and Slope on Limb-Segment Planar Covariation
At slow speeds, the effect of slope on covariance plane orientation, the shape of PC2*, and the shape of the gait loop is considerable (Fig. 5), whereas at higher speeds, this effect fades away. The fact that at fast walking speeds, the intersegmental coordination hardly changes with slope is not a simple result of high inertia, making it difficult for the planar covariation characteristics to adapt quickly to slope. Indeed, when running at ~3 m/s, for example, the characteristics of planar covariation change significantly: the compression of the limb at midstance corresponds to adjustments of the planar covariation (35).
At slow speed, when walking uphill or downhill, the difference in limb length between FC and TO is modified compared with the level and affects the net vertical displacement of the COM (Fig. 1). The change in covariation plane orientation across slopes is related to a change in the amplitude ratio between thigh- and shank-elevation angles (Gts). In turn, the modification of Gts affects the shape of the loop. In uphill walking, the greater Gts is associated with a larger PV2 (Table 2), indicating a wider loop most likely to facilitate the increase in toe clearance (51). On the contrary, in downhill walking, the smaller Gts is associated with a lower PV2 and thus a narrower loop.
The changes in limb orientation (and thus in PC1*) and in limb length (and thus in PC2*) confirm that there is a tilt in the orientation of the limbs but also an adjustment of limb length (Fig. 7). Because of the high correlation between PC2* (Fig. 7) and the knee joint angle (Fig. 2), the adjustment of limb length is thought to depend mainly on knee motion. ΔPC2* indicates that in uphill walking, the limb is compressed at FC and extends during stance, whereas in downhill walking, the limb is extended at FC and compressed during stance.
When walking downhill, the COM moves forward and downward, aided by gravity. Body balance must be ensured to avoid either foot slipping or a headlong rush, due to a greater forward “toppling moment.” Greater knee flexion throughout the stance (Fig. 2) has been related to shorter strides (Fig. 1) (31, 49), which may reduce the likelihood of slipping (31, 57). Greater knee flexion also tends to decrease the COM height, which may help reduce instability issues (10, 16, 34, 49, 55, 62). In addition, compared with the level, the trunk is tilted backward (Fig. 4) (49). When walking uphill, the COM moves forward and upward, against gravity. At the same time, body balance must be ensured to avoid a backward toppling moment. At low speeds, the increase in knee but also in hip and to a lesser extent, in ankle flexion at FC (Fig. 2) results in a shorter Lfc (49, 51, 57). Furthermore, the greater difference between Lfc and Lto (Fig. 1) leads to a higher range of limb-length change compared with the level. Compared with the level, the trunk is tilted forward in uphill walking (Fig. 4) (49). The more crouched posture brings the COM closer to the ground, which helps to counteract the issue of the toppling moment (10, 14).
When speed increases, the net vertical displacement of the COM during each stride increases (Fig. 3) in uphill and downhill walking. However, we did not observe any effect of speed on Lfc and Lto. With increasing speed, the orientation of the covariation plane tends to be independent of slope. Indeed, the gait loop is progressively stretched lengthwise with increasing speed (11, 38), as indicated by the increase in PV1 (Table 2). As a result, the changes in Gts and in PV2 with slope progressively fade away. Likewise, the time changes in ΔPC2* throughout the stance are reduced (Fig. 7). Thus at fast speeds, the change in COM height is more related to a tilt of the compass gait than at slow speeds (Figs. 6 and 7): the portion of the stance in which the foot is posterior to the hip joint decreases in uphill walking (backward tilt of the compass gait) and increases in downhill walking (forward tilt of the compass gait). Likewise, the fraction of stance dedicated to increase/decrease the gravitational potential energy of the COM also increases in uphill/downhill walking (21).
Changes in planar covariation are thought to be related to an ability to adapt to different walking conditions (9, 54). For instance, when toddlers walk on terrains with different inclinations, their ability to adapt is very limited, and they maintain a roughly constant planar orientation, suggesting a reduced flexibility of the kinematic pattern (23). The selection of the appropriate motor strategy from a large number of possibilities could indicate optimization of some physiological goals and properties (e.g., stability, energy expenditure, mechanical stress, etc.). The present results suggest that modifications of the kinematic coordination pattern highly depend on walking speed. At slow speeds, these changes might be necessary to maintain stability (17). Indeed, the COM remains within (or close to) the polygon of support formed by the foot/feet in contact with the ground. At fast speeds, the lack of modification observed may highlight a more active selection pressure on the walking gait. Indeed, it has been shown that the progressive reduction of u3t with increasing speed contains the increase in the mechanical energy expenditure (8). Furthermore, the trunk tilts forward with speed both in uphill and downhill walking (Fig. 4B). This forward leaning may be pursued to assist lower limbs in generating greater forward propulsion (49, 50).
Neural Underpinnings for the Differential Effect of Speed on Intersegmental Coordination
It has been hypothesized that the intersegmental coordination results from interplay between the activity of central pattern generators (CPGs; neural circuits that can generate rhythmic motor activity) and sensory signals originating in the limbs (13). The effect of speed on coordination patterns during walking has also been found in insects (20, 27, 70). It has been suggested that this change in coordination may be due to the contribution of central CPG coupling mechanisms for coordination and its dependence of walking speed (7). Indeed, the coordination in slow-walking insects is thought to be largely based on sensory input contributions, whereas in fast-walking insects, the central CPG coupling is thought to play a more important role (25, 53). In humans, Yokoyama et al. (73) showed that the neural control strategies for activating muscles depend on locomotor speed, which suggests that human locomotor networks may have speed dependency as in insects.
In sum, our results show that the kinematic patterns highly depend on the walking speed. The effect of speed on motor control of locomotion has been observed in animal studies (5, 58, 75), which have demonstrated distinct recruitment of spinal neuronal groups, depending on the speed of progression. In humans, different networks are recruited at various speeds as well (72, 73). Here, we report a speed-dependent modification in the intersegmental coordination to walking on slopes (Figs. 5 and 7). This observation might reflect the fact that the CPG neural networks are not overlapping for high and slow speeds (i.e., there is no simple scaling of motoneuron and interneuron activity with speed but the involvement of somewhat different neural circuits), since slow and fast speeds of progression exhibited different telescopic limb behavior on slopes.
Limitations of the Study
In this study, the movements were only analyzed in the sagittal plane, because those represent the major and most systematic component of walking gait (12, 52). Given the small ROM and low signal-to-noise ratio in the frontal plane, a larger sample size should be necessary to achieve reliable results. Indeed, at the highest speed and on the steepest slopes, due to their physical ability, only five of the 10 subjects were able to perform the walking task.
Conclusion
In conclusion, this study provides new quantitative details, consistent with prior literature (26, 72, 73), on how intersegmental coordination changes during uphill, downhill, and level walking. The results demonstrate that the kinematic patterns highly depend on both ground slope and walking speed.
GRANTS
Funding for this study was provided by the Université Catholique de Louvain (Belgium), Fonds de la Recherche Scientifique (Belgium), Italian Ministry of Health (IRCCS Ricerca Corrente), Italian Space Agency (Contract No. I/006/06/0), Italian Ministry of University and Research (PRIN Grant 2015HFWRYY_002), Horizon 2020 Robotics Program (ICT-23-2014 under Grant Agreement 644727-CogIMon), Lazio Region (INNOVA.1 FILAS-RU 2014_1033), Whitaker International Program, and in part by NIH Grant K12HD073945 (to K. E. Zelik).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.E.Z. and P.A.W. conceived and designed research; K.E.Z. and P.A.W. performed experiments; A.H.D., Y.I., F.L., and P.A.W. analyzed data; A.H.D., Y.I., K.E.Z., F.L., and P.A.W. interpreted results of experiments; A.H.D., Y.I., and P.A.W. prepared figures; A.H.D. and P.A.W. drafted manuscript; A.H.D., Y.I., K.E.Z., F.L., and P.A.W. edited and revised manuscript; A.H.D., Y.I., K.E.Z., F.L., and P.A.W. approved final version of manuscript.
REFERENCES
- 1.Alexander N, Schwameder H. Effect of sloped walking on lower limb muscle forces. Gait Posture 47: 62–67, 2016. doi: 10.1016/j.gaitpost.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Alexander RM. Human walking and running. J Biol Educ 18: 135–140, 1984. doi: 10.1080/00219266.1984.9654619. [DOI] [Google Scholar]
- 3.Alexander RM. Tendon elasticity and positional control. Behav Brain Sci 18: 745, 1995. doi: 10.1017/S0140525X00040711. [DOI] [Google Scholar]
- 4.Amirudin AN, Parasuraman S, Kadirvel A, Ahmed Khan MK, Elamvazuthi I. Biomechanics of hip, knee and ankle joint loading during ascent and descent walking. Proc Comp Sci 42: 336–344, 2014. doi: 10.1016/j.procs.2014.11.071. [DOI] [Google Scholar]
- 5.Ampatzis K, Song J, Ausborn J, El Manira A. Separate microcircuit modules of distinct v2a interneurons and motoneurons control the speed of locomotion. Neuron 83: 934–943, 2014. doi: 10.1016/j.neuron.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Aprigliano F, Martelli D, Tropea P, Pasquini G, Micera S, Monaco V. Aging does not affect the intralimb coordination elicited by slip-like perturbation of different intensities. J Neurophysiol 118: 1739–1748, 2017. doi: 10.1152/jn.00844.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berendes V, Zill SN, Büschges A, Bockemühl T. Speed-dependent interplay between local pattern-generating activity and sensory signals during walking in Drosophila. J Exp Biol 219: 3781–3793, 2016. doi: 10.1242/jeb.146720. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi L, Angelini D, Lacquaniti F. Individual characteristics of human walking mechanics. Pflugers Arch 436: 343–356, 1998. doi: 10.1007/s004240050642. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi L, Angelini D, Orani GP, Lacquaniti F. Kinematic coordination in human gait: relation to mechanical energy cost. J Neurophysiol 79: 2155–2170, 1998. doi: 10.1152/jn.1998.79.4.2155. [DOI] [PubMed] [Google Scholar]
- 10.Birn-Jeffery AV, Higham TE. The scaling of uphill and downhill locomotion in legged animals. Integr Comp Biol 54: 1159–1172, 2014. doi: 10.1093/icb/icu015. [DOI] [PubMed] [Google Scholar]
- 11.Bleyenheuft C, Detrembleur C. Kinematic covariation in pediatric, adult and elderly subjects: is gait control influenced by age? Clin Biomech (Bristol, Avon) 27: 568–572, 2012. doi: 10.1016/j.clinbiomech.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Borghese NA, Bianchi L, Lacquaniti F. Kinematic determinants of human locomotion. J Physiol 494: 863–879, 1996. doi: 10.1113/jphysiol.1996.sp021539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buschges A, Schmitz J, Bassler U. Rhythmic patterns in the thoracic nerve cord of the stick insect induced by pilocarpine. J Exp Biol 198: 435–456, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol 79: 1687–1701, 1998. doi: 10.1152/jn.1998.79.4.1687. [DOI] [PubMed] [Google Scholar]
- 15.Cavagna GA, Thys H, Zamboni A. The sources of external work in level walking and running. J Physiol 262: 639–657, 1976. doi: 10.1113/jphysiol.1976.sp011613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cham R, Redfern MS. Heel contact dynamics during slip events on level and inclined surfaces. Safety Sci 40: 559–576, 2002. doi: 10.1016/S0925-7535(01)00059-5. [DOI] [Google Scholar]
- 17.Cheron G, Bouillot E, Dan B, Bengoetxea A, Draye JP, Lacquaniti F. Development of a kinematic coordination pattern in toddler locomotion: planar covariation. Exp Brain Res 137: 455–466, 2001. doi: 10.1007/s002210000663. [DOI] [PubMed] [Google Scholar]
- 18.Chow JW, Stokic DS. Intersegmental coordination scales with gait speed similarly in men and women. Exp Brain Res 233: 3175–3185, 2015. [Erratum in Exp Brain Res 234: 2105–2106, 2016. doi: 10.1007/s00221-016-4652-2. 27113581]. doi:. [DOI] [PubMed] [Google Scholar]
- 19.Courtine G, Schieppati M. Tuning of a basic coordination pattern constructs straight-ahead and curved walking in humans. J Neurophysiol 91: 1524–1535, 2004. doi: 10.1152/jn.00817.2003. [DOI] [PubMed] [Google Scholar]
- 20.Cruse H. What mechanisms coordinate leg movement in walking arthropods? Trends Neurosci 13: 15–21, 1990. doi: 10.1016/0166-2236(90)90057-H. [DOI] [PubMed] [Google Scholar]
- 21.Dewolf AH, Ivanenko YP, Lacquaniti F, Willems PA. Pendular energy transduction within the step during human walking on slopes at different speeds. PLoS One 12: e0186963, 2017. doi: 10.1371/journal.pone.0186963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewolf AH, Peñailillo LE, Willems PA. The rebound of the body during uphill and downhill running at different speeds. J Exp Biol 219: 2276–2288, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Dominici N, Ivanenko YP, Cappellini G, Zampagni ML, Lacquaniti F. Kinematic strategies in newly walking toddlers stepping over different support surfaces. J Neurophysiol 103: 1673–1684, 2010. doi: 10.1152/jn.00945.2009. [DOI] [PubMed] [Google Scholar]
- 24.Franz JR, Kram R. Advanced age and the mechanics of uphill walking: a joint-level, inverse dynamic analysis. Gait Posture 39: 135–140, 2014. doi: 10.1016/j.gaitpost.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuchs E, Holmes P, Kiemel T, Ayali A. Intersegmental coordination of cockroach locomotion: adaptive control of centrally coupled pattern generator circuits. Front Neural Circuits 4: 125, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Full RJ, Koditschek DE. Templates and anchors: neuromechanical hypotheses of legged locomotion on land. J Exp Biol 202: 3325–3332, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Grabowska M, Godlewska E, Schmidt J, Daun-Gruhn S. Quadrupedal gaits in hexapod animals - inter-leg coordination in free-walking adult stick insects. J Exp Biol 215: 4255–4266, 2012. doi: 10.1242/jeb.073643. [DOI] [PubMed] [Google Scholar]
- 28.Grasso R, Peppe A, Stratta F, Angelini D, Zago M, Stanzione P, Lacquaniti F. Basal ganglia and gait control: apomorphine administration and internal pallidum stimulation in Parkinson’s disease. Exp Brain Res 126: 139–148, 1999. doi: 10.1007/s002210050724. [DOI] [PubMed] [Google Scholar]
- 29.Grasso R, Zago M, Lacquaniti F. Interactions between posture and locomotion: motor patterns in humans walking with bent posture versus erect posture. J Neurophysiol 83: 288–300, 2000. doi: 10.1152/jn.2000.83.1.288. [DOI] [PubMed] [Google Scholar]
- 30.Han S. The influence of walking speed on gait patterns during upslope walking. J Med Imag Health In 5: 89–92, 2015. doi: 10.1166/jmihi.2015.1354. [DOI] [Google Scholar]
- 31.Hansen AH, Childress DS, Miff SC. Roll-over characteristics of human walking on inclined surfaces. Hum Mov Sci 23: 807–821, 2004. doi: 10.1016/j.humov.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Hong SW, Leu TH, Li JD, Wang TM, Ho WP, Lu TW. Influence of inclination angles on intra- and inter-limb load-sharing during uphill walking. Gait Posture 39: 29–34, 2014. doi: 10.1016/j.gaitpost.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Hong SW, Wang TM, Lu TW, Li JD, Leu TH, Ho WP. Redistribution of intra- and inter-limb support moments during downhill walking on different slopes. J Biomech 47: 709–715, 2014. doi: 10.1016/j.jbiomech.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 34.Hunter LC, Hendrix EC, Dean JC. The cost of walking downhill: is the preferred gait energetically optimal? J Biomech 43: 1910–1915, 2010. doi: 10.1016/j.jbiomech.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 35.Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F. Modular control of limb movements during human locomotion. J Neurosci 27: 11149–11161, 2007. doi: 10.1523/JNEUROSCI.2644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivanenko YP, d’Avella A, Poppele RE, Lacquaniti F. On the origin of planar covariation of elevation angles during human locomotion. J Neurophysiol 99: 1890–1898, 2008. doi: 10.1152/jn.01308.2007. [DOI] [PubMed] [Google Scholar]
- 37.Ivanenko YP, Dominici N, Cappellini G, Dan B, Cheron G, Lacquaniti F. Development of pendulum mechanism and kinematic coordination from the first unsupported steps in toddlers. J Exp Biol 207: 3797–3810, 2004. doi: 10.1242/jeb.01214. [DOI] [PubMed] [Google Scholar]
- 38.Ivanenko YP, Grasso R, Macellari V, Lacquaniti F. Control of foot trajectory in human locomotion: role of ground contact forces in simulated reduced gravity. J Neurophysiol 87: 3070–3089, 2002. doi: 10.1152/jn.2002.87.6.3070. [DOI] [PubMed] [Google Scholar]
- 39.Jeong J, Oh YK, Shin CS. Measurement of lower extremity kinematics and kinetics during valley-shaped slope walking. Int J Precis Eng Man 16: 2725–2730, 2015. doi: 10.1007/s12541-015-0348-y. [DOI] [Google Scholar]
- 40.Kawamura K, Tokuhiro A, Takechi H. Gait analysis of slope walking: a study on step length, stride width, time factors and deviation in the center of pressure. Acta Med Okayama 45: 179–184, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Kuo AD, Donelan JM, Ruina A. Energetic consequences of walking like an inverted pendulum: step-to-step transitions. Exerc Sport Sci Rev 33: 88–97, 2005. doi: 10.1097/00003677-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Kuster M, Sakurai S, Wood GA. Kinematic and kinetic comparison of downhill and level walking. Clin Biomech (Bristol, Avon) 10: 79–84, 1995. doi: 10.1016/0268-0033(95)92043-L. [DOI] [PubMed] [Google Scholar]
- 43.Lacquaniti F, Grasso R, Zago M. Motor patterns in walking. News Physiol Sci 14: 168–174, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Lacquaniti F, Ivanenko YP, Zago M. Kinematic control of walking. Arch Ital Biol 140: 263–272, 2002. [PubMed] [Google Scholar]
- 45.Lacquaniti F, Ivanenko YP, Zago M. Patterned control of human locomotion. J Physiol 590: 2189–2199, 2012. doi: 10.1113/jphysiol.2011.215137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lange GW, Hintermeister RA, Schlegel T, Dillman CJ, Steadman JR. Electromyographic and kinematic analysis of graded treadmill walking and the implications for knee rehabilitation. J Orthop Sports Phys Ther 23: 294–301, 1996. doi: 10.2519/jospt.1996.23.5.294. [DOI] [PubMed] [Google Scholar]
- 47.Lay AN, Hass CJ, Gregor RJ. The effects of sloped surfaces on locomotion: a kinematic and kinetic analysis. J Biomech 39: 1621–1628, 2006. doi: 10.1016/j.jbiomech.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Lee CR, Farley CT. Determinants of the center of mass trajectory in human walking and running. J Exp Biol 201: 2935–2944, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Leroux A, Fung J, Barbeau H. Postural adaptation to walking on inclined surfaces: I. normal strategies. Gait Posture 15: 64–74, 2002. doi: 10.1016/S0966-6362(01)00181-3. [DOI] [PubMed] [Google Scholar]
- 50.Leteneur S, Gillet C, Sadeghi H, Allard P, Barbier F. Effect of trunk inclination on lower limb joint and lumbar moments in able men during the stance phase of gait. Clin Biomech (Bristol, Avon) 24: 190–195, 2009. doi: 10.1016/j.clinbiomech.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 51.MacLellan MJ, Dupré N, McFadyen BJ. Increased obstacle clearance in people with ARCA-1 results in part from voluntary coordination changes between the thigh and shank segments. Cerebellum 10: 732–744, 2011. doi: 10.1007/s12311-011-0283-0. [DOI] [PubMed] [Google Scholar]
- 52.Mah CD, Hulliger M, Lee RG, O’Callaghan IS. Quantitative analysis of human movement synergies: constructive pattern analysis for gait. J Mot Behav 26: 83–102, 1994. doi: 10.1080/00222895.1994.9941664. [DOI] [PubMed] [Google Scholar]
- 53.Mantziaris C, Bockemühl T, Holmes P, Borgmann A, Daun S, Büschges A. Intra- and intersegmental influences among central pattern generating networks in the walking system of the stick insect. J Neurophysiol 118: 2296–2310, 2017. doi: 10.1152/jn.00321.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martino G, Ivanenko YP, Serrao M, Ranavolo A, d’Avella A, Draicchio F, Conte C, Casali C, Lacquaniti F. Locomotor patterns in cerebellar ataxia. J Neurophysiol 112: 2810–2821, 2014. doi: 10.1152/jn.00275.2014. [DOI] [PubMed] [Google Scholar]
- 55.McAndrew Young PM, Dingwell JB. Voluntary changes in step width and step length during human walking affect dynamic margins of stability. Gait Posture 36: 219–224, 2012. doi: 10.1016/j.gaitpost.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGeer T. Passive dynamic walking. Int J Rob Res 9: 62–82, 1990. doi: 10.1177/027836499000900206. [DOI] [Google Scholar]
- 57.McIntosh AS, Beatty KT, Dwan LN, Vickers DR. Gait dynamics on an inclined walkway. J Biomech 39: 2491–2502, 2006. doi: 10.1016/j.jbiomech.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 58.McLean DL, Fetcho JR. Spinal interneurons differentiate sequentially from those driving the fastest swimming movements in larval zebrafish to those driving the slowest ones. J Neurosci 29: 13566–13577, 2009. doi: 10.1523/JNEUROSCI.3277-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meurisse GM, Dierick F, Schepens B, Bastien GJ. Determination of the vertical ground reaction forces acting upon individual limbs during healthy and clinical gait. Gait Posture 43: 245–250, 2016. doi: 10.1016/j.gaitpost.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Noble JW, Prentice SD. Intersegmental coordination while walking up inclined surfaces: age and ramp angle effects. Exp Brain Res 189: 249–255, 2008. doi: 10.1007/s00221-008-1464-z. [DOI] [PubMed] [Google Scholar]
- 61.Prentice SD, Hasler EN, Groves JJ, Frank JS. Locomotor adaptations for changes in the slope of the walking surface. Gait Posture 20: 255–265, 2004. doi: 10.1016/j.gaitpost.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Redfern MS, DiPasquale J. Biomechanics of descending ramps. Gait Posture 6: 119–125, 1997. doi: 10.1016/S0966-6362(97)01117-X. [DOI] [Google Scholar]
- 63.Saunders JB, Inman VT, Eberhart HD. The major determinants in normal and pathological gait. J Bone Joint Surg Am 35-A: 543–558, 1953. doi: 10.2106/00004623-195335030-00003. [DOI] [PubMed] [Google Scholar]
- 64.Sun J, Walters M, Svensson N, Lloyd D. The influence of surface slope on human gait characteristics: a study of urban pedestrians walking on an inclined surface. Ergonomics 39: 677–692, 1996. doi: 10.1080/00140139608964489. [DOI] [PubMed] [Google Scholar]
- 65.Ting LH, Chvatal SA, Safavynia SA, McKay JL. Review and perspective: neuromechanical considerations for predicting muscle activation patterns for movement. Int J Numer Methods Biomed Eng 28: 1003–1014, 2012. doi: 10.1002/cnm.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Usherwood JR, Szymanek KL, Daley MA. Compass gait mechanics account for top walking speeds in ducks and humans. J Exp Biol 211: 3744–3749, 2008. doi: 10.1242/jeb.023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogt L, Banzer W. Measurement of lumbar spine kinematics in incline treadmill walking. Gait Posture 9: 18–23, 1999. doi: 10.1016/S0966-6362(98)00038-1. [DOI] [PubMed] [Google Scholar]
- 68.Willems PA, Gosseye TP. Does an instrumented treadmill correctly measure the ground reaction forces? Biol Open 2: 1421–1424, 2013. doi: 10.1242/bio.20136379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winter DA. The Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological. Waterloo, Ontario: University of Waterloo Press, 1991. [Google Scholar]
- 70.Wosnitza A, Bockemühl T, Dübbert M, Scholz H, Büschges A. Inter-leg coordination in the control of walking speed in Drosophila. J Exp Biol 216: 480–491, 2013. doi: 10.1242/jeb.078139. [DOI] [PubMed] [Google Scholar]
- 71.Xu H, Wang Y, Greenland K, Bloswick D, Merryweather A. The influence of deformation height on estimating the center of pressure during level and cross-slope walking on sand. Gait Posture 42: 110–115, 2015. doi: 10.1016/j.gaitpost.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 72.Yokoyama H, Ogawa T, Kawashima N, Shinya M, Nakazawa K. Distinct sets of locomotor modules control the speed and modes of human locomotion. Sci Rep 6: 36275, 2016. doi: 10.1038/srep36275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yokoyama H, Ogawa T, Shinya M, Kawashima N, Nakazawa K. Speed dependency in α-motoneuron activity and locomotor modules in human locomotion: indirect evidence for phylogenetically conserved spinal circuits. Proc Biol Sci 284: 20170290, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zelik KE, Kuo AD. Human walking isn’t all hard work: evidence of soft tissue contributions to energy dissipation and return. J Exp Biol 213: 4257–4264, 2010. doi: 10.1242/jeb.044297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhong G, Sharma K, Harris-Warrick RM. Frequency-dependent recruitment of V2a interneurons during fictive locomotion in the mouse spinal cord. Nat Commun 2: 274, 2011. doi: 10.1038/ncomms1276. [DOI] [PMC free article] [PubMed] [Google Scholar]