Abstract
Oxidative stress and mitochondrial dysfunction exacerbate acute kidney injury (AKI), but their role in any associated progress to chronic kidney disease (CKD) remains unclear. Antioxidant therapies often benefit AKI, but their benefits in CKD are controversial since clinical and preclinical investigations often conflict. Here we examined the influence of the antioxidant N-acetyl-cysteine (NAC) on oxidative stress and mitochondrial function during AKI (20-min bilateral renal ischemia plus reperfusion/IR) and progression to chronic kidney pathologies in mice. NAC (5% in diet) was given to mice 7 days prior and up to 21 days post-IR (21d-IR). NAC treatment resulted in the following: prevented proximal tubular epithelial cell apoptosis at early IR (40-min postischemia), yet enhanced interstitial cell proliferation at 21d-IR; increased transforming growth factor-β1 expression independent of IR time; and significantly dampened nuclear factor-like 2-initiated cytoprotective signaling at early IR. In the long term, NAC enhanced cellular metabolic impairment demonstrated by increased peroxisome proliferator activator-γ serine-112 phosphorylation at 21d-IR. Intravital multiphoton microscopy revealed increased endogenous fluorescence of nicotinamide adenine dinucleotide (NADH) in cortical tubular epithelial cells during ischemia, and at 21d-IR that was not attenuated with NAC. Fluorescence lifetime imaging microscopy demonstrated persistent metabolic impairment by increased free/bound NADH in the cortex at 21d-IR that was enhanced by NAC. Increased mitochondrial dysfunction in remnant tubular cells was demonstrated at 21d-IR by tetramethylrhodamine methyl ester fluorimetry. In summary, NAC enhanced progression to CKD following AKI not only by dampening endogenous cellular antioxidant responses at time of injury but also by enhancing persistent kidney mitochondrial and metabolic dysfunction.
Keywords: acute kidney injury, chronic kidney disease, intravital multiphoton microscopyoxidative stress, metabolism, mitochondria
INTRODUCTION
Progression of chronic kidney disease (CKD) is driven by numerous deleterious and converging physiological processes, regardless of the cause. Oxidative stress contributes to progressive kidney disease and failure via its mechanistic role in fibrosis, apoptosis, senescence, autophagy, vascular insufficiency, and maladaptive repair. Metabolic alterations within the kidney are intimately linked to mitochondrial dysfunction and, consequently, oxidative stress and generation of reactive oxygen species (ROS). This presents oxidative stress and ROS as underlying common mediators of progression to CKD and as possible therapy targets to reduce CKD.
Antioxidant therapy has been successful in reducing acute kidney injury (AKI) in contrast-induced nephropathy (21, 29), renal ischemia-reperfusion (IR) injury (45, 53), and acute toxic nephropathies (24, 44). However, such antioxidant therapies in CKD patients have produced ambivalent outcomes. Use of small patient populations for the studies may contribute to the disparities, with outcomes that have insufficient statistical power to confirm any benefit. A recent meta-analysis of 10 studies assessing antioxidant therapy in transplant and non-dialysis CKD patients found that antioxidants significantly reduced development or progression of end-stage kidney disease (ESKD) but showed no clear benefit on cardiovascular and all-cause mortality in CKD patients (23). Recently, a well-powered trial investigating whether mixed tocopherols and α-lipoic acid altered oxidative stress and inflammation in maintenance hemodialysis patients was conducted (19). No detectable change was found compared with placebo controls. These data highlight the need for more basic research to design appropriate targeted therapies against oxidative stress.
Common therapeutic antioxidant compounds act via a variety of direct or indirect mechanisms (56). N-acetyl-cysteine (NAC) is an essential precursor to many endogenous antioxidants involved in decomposition of peroxides (69, 71), as well as replenishing intracellular glutathione stores. In addition, the sulfhydryl-thiol group of l-cysteine may exert direct antioxidant effects by scavenging free radicals. NAC has greatest antioxidant and anti-inflammatory properties when used against greatest injury, such as in ESKD patients receiving either hemodialysis or peritoneal dialysis (20, 41). In those cases, NAC reduced serum F2-isoprostane and multiple biomarkers of inflammation.
Oxidative stress is traditionally defined as an imbalance between production of ROS and endogenous antioxidants, but it is now also recognized that vital redox-sensitive intracellular signaling networks are disrupted by oxidative stress. For example, the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1) system alters during oxidative stress. The altered expression dynamics of this system gained attention from the BEACON trial that investigated bardoxolone methyl to slow progression of CKD. The trial was terminated because of increased mortality in the treatment arm (13). Bardoxolone methyl activates Nrf2, and thus the trial results may suggest that altering such upstream, highly conserved targets could have negative systemic consequences (62). We have previously demonstrated that oxidative stress-induced alterations to peroxisome proliferator-activated receptor-γ (PPARγ) can disrupt important PPARγ-dependent processes such as mitochondrial homeostasis and mitophagy in proximal tubular epithelial cells (57). It is not known whether these effects persist as maladaptive repair in progressive kidney pathologies following AKI (25, 33). Thus continuing antioxidant therapy, after AKI has resolved, but before onset of CKD, may be of benefit. The present study sought to investigate this hypothesis in a mouse model of IR-induced oxidative stress, in part by using intravital multiphoton microscopy (MPM) and fluorescence lifetime imaging (FLIM).
METHODS
Mice.
All experiments were approved by the University of Queensland Animal Ethics Committee 2013 (AEC No. MED/053/CKDR). Male C57BL/6 mice aged 4–6 wk old were housed in temperature-controlled boxes with a 12:12-h light-dark cycle. Mice (20–30 g) were fed normal powdered mouse chow or normal powdered chow plus 5% NAC since this dosage has previously been shown to be beneficial (36). Each mouse was allotted 4.5 g of food in a 24-h period. Mice were fed the diets 1 wk before bilateral ischemia-reperfusion (IR) kidney injury and for 21 days following kidney IR (21d-IR). Intravital MPM and kidney collection for histological and protein analysis occurred following initial kidney IR injury and at 21d-IR (n = 4–6 animals/group).
Kidney IR and intravital microscopy.
Mice were anesthetized with ketamine (10 mg/ml)/xylazine (1 mg/ml) via intraperitoneal injection at a dose of 10 μl/g body weight. They were placed on a temperature-controlled surgery table (38°C), and the left kidney was externalized via a flank incision. The renal vessels were microclamped, and the kidney was placed back into the body cavity. The same procedure was performed on the right side. Following bilateral 20-min ischemia, microclamps were removed, and reperfusion to kidneys was confirmed by a return to normal kidney color. For 21d-IR mice (recovery), muscle and skin layers were sutured using 4–0 silk and sterile techniques. For intravital MPM (nonrecovery) during ischemia (clamp on) and during initial 40-min reperfusion (clamp released), only the left kidney was externalized to prevent major animal trauma and temperature loss. After microclamp placement, the mouse was moved to the MPM stage, and the kidney was placed over the objective bathed in dH2O. A heated jacket (38°C) was placed over the mouse to maintain normal body temperature, and images were acquired over a 20-min period from when the microclamp was applied. Following 20-min ischemia, the microclamp was released, and the mouse was replaced onto the microscope stage to acquire images over a 40-min reperfusion period. Both left and right kidneys from healthy control mice and 21d-IR mice were imaged by the same procedure, but with no clamping at the time of MPM. Tetramethylrhodamine methyl ester (TMRM) (5 μg/ml) was infused via tail vein injection into healthy control mice and 21d-IR mice while the mouse was on the microscope stage and imaged immediately.
Intravital MPM image acquisition and analysis.
Images were captured using a ×20 objective and a Nikon Ti Eclipse-LaVision MPM (LaVision BioTec, Bielefeld, Germany) equipped with an ultrashort (85-fs pulse width) pulsed mode-locked 80-MHz titanium:sapphire MaiTai laser (Spectra-Physics, Mountain View, CA) and ImSpector Pro software. The excitation wavelength was set to 740 nm for NADH fluorescence and 800 nm for TMRM. Emitted light was collected using three photomultiplier tube filters: 447–460 nm (blue), 485–550 nm (green), and 575–630 nm (red). The objective was placed in a field of view that visualized detailed tubular structures, and serial penetrating slice images (1-µm step size) were acquired from the renal cortex to a depth of 50–70 μm. A minimum of three fields of view per kidney was obtained. Imaris x64 7.6.0 (Bitplane Scientific Software, Zurich, Switzerland) was used to adjust minimum and maximum exposure settings consistently throughout all acquired images for standardized quantification of fluorescence intensity. ImageJ (1.46r, National Institutes of Health, Bethesda, MD) was used to obtain mean fluorescent intensity values for NAD(P)H and TMRM-detected emission. Prior to TMRM infusion, images were recorded to exclude baseline autofluorescence within the 575- to 630-nm emission range. For each of the three fields captured per kidney, the epithelial layer of definitive continuous tubules was selected and measured for mean intensity. Eight tubules per z-slice image were measured from five selected z-slice images per z stack.
FLIM microscopy and analysis.
FLIM was performed on mouse kidney immediately posteuthanasia. Time from death to first image capture was no more than 30 min. Three areas each from cortex and medulla were captured for FLIM data per kidney. Images were captured using a DermaInspect system (JenLab, Jena, Germany) equipped with an ultrashort (85 fs pulse width) pulsed mode-locked 80-MHz titanium:sapphire MaiTai laser (Spectra-Physics). The excitation wavelength was set to 740 nm for kidney autofluorescence with an emission signal range of 350 to 650 nm established using a BG39 band-pass filter. Images were recorded with a ×40 objective and laser power set to 15 mW. FLIM data were collected with a time-correlated single-photon counting SPC-830 detector (Becker & Hickl, Berlin, Germany). Fluorescence emission was spectrally resolved between three linearly arranged photon counters through three dichroic filters in the beam path, spectrally dividing the emission light into three channels for each photon counter: 350–450, 450–515, and 515–620 nm. Each FLIM image was collected at an exposure of 13.4 s and an acquisition image size of 214 × 214 μm. SPCImage 4.8 (Becker and Hickl) was used to analyze FLIM data using a double-exponential decay model function. The fitted decay curve established the short (τ1) and long (τ2) fluorescence decay lifetimes (ps) with corresponding relative amplitude coefficients α1 and α2 (%), respectively. The lifetime of NAD(P)H was resolved as a two-component system with the short (~0.3–4 ns) and long (~2.3 ns) lifetime represented as the free and protein-bound conformations, respectively (2, 34, 42). The free-to-bound ratio of NAD(P)H, represented by the ratio of the amplitude coefficients for the short and long lifetimes (α1/α2), was related to the NADH/NAD+ ratio and was used as an indicator for redox changes within the cell (3) (2 animals/group, both kidneys analyzed, n = 4).
Kidney function.
Serum creatinine was measured using the DetectX Serum Creatinine Kit per the manufacturer’s protocol. Dipstick analysis was used to semiquantitate the presence of proteinuria.
Western immunoblotting.
Protein expression levels were determined for p-PPARγ, PPARγ, TGF-β1, Keap1, Nrf2, SOD2, and HO-1 from whole kidney protein extracts by using standard Western blotting methods previously described (57). GAPDH was used as a loading control, and densitometry using ImageJ software (National Institutes of Health) was used to semiquantitate protein expression.
Histology, immunohistochemistry, and ApopTag of mouse kidneys.
Paraffin-embedded formalin-fixed sections were used for staining protocols throughout. Kidneys were collected from control mice, those at early reperfusion, and at 21d-IR. Hematoxylin and eosin, Masson’s trichrome stain for collagen, in situ enzymatic ApopTag (Millipore) for apoptosis, and immunohistochemistry (IHC) for PCNA, 8-OHdG, p-PPARγ, and PPARγ were performed using standard techniques (46). Immunofluorescence was used to confirm the PCNA-expressing interstitial cells as positive for α-smooth muscle actin, a marker of myofibroblasts. Morphometry was performed using ImageScope following digital scanning of slides by using the Aperio ScanScope XT slide scanning system (Aperio Technologies, Vista, California). Entire kidney sections were analyzed and separated into cortex and medulla. The following algorithms were selected for quantification: IHC Nuclear v1, for ApopTag and PCNA staining, with strong positive (+3) and positive (+2) included into mean values; Positive Pixel Count v9, for 8-OHdG staining, with strong positive (+3) only included into mean values; and Masson’s trichrome stain using the Positive Pixel Count v9 algorithm, with total negative pixels included into mean values since blue is detected as negative staining.
Statistics.
Parameters were expressed as means ± SE for n = 4–6 mice per group. Independent samples t-test was used for comparison between two groups. A one-way analysis of variance (ANOVA) was used for comparison between more than two groups, and where relevant a Tukey’s post hoc comparison was carried out. A two-way ANOVA was used to test the interaction between two independent variables, and where relevant a Bonferroni’s post hoc comparison was used. Statistical significance was determined at P ≤ 0.05.
RESULTS
NAC attenuated cell damage in AKI but was associated with enhanced progression to chronic kidney pathologies.
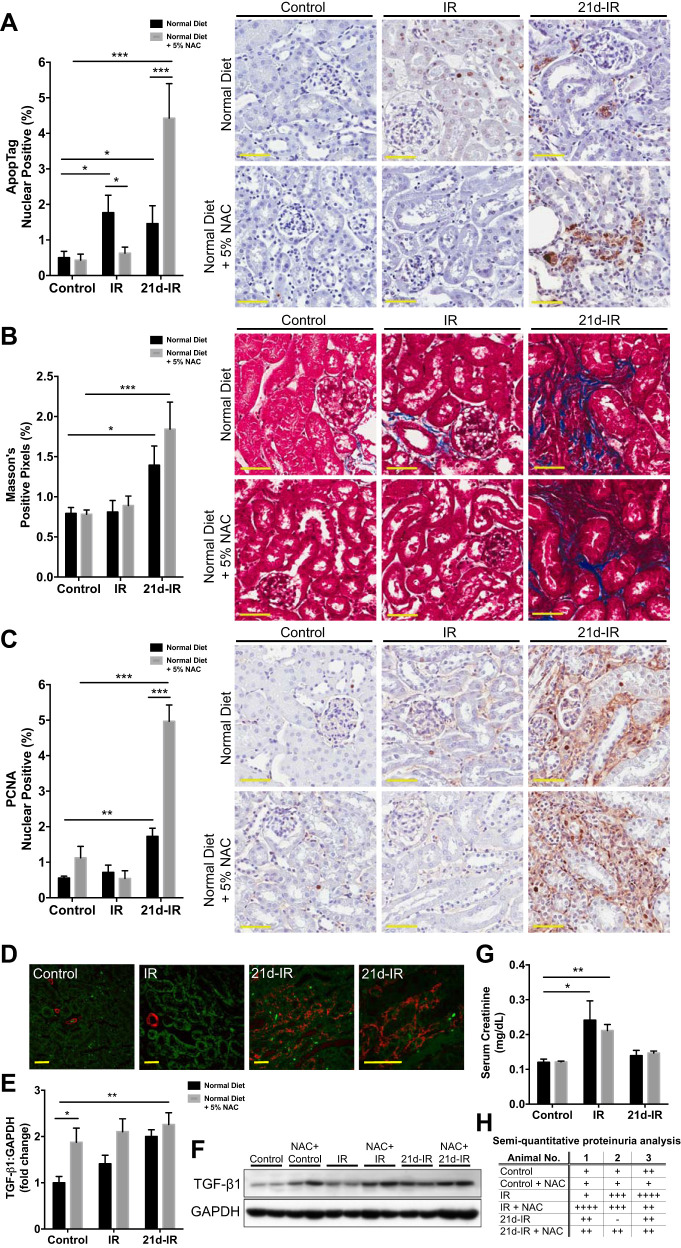
NAC was selected following in vitro experiments that demonstrated its superior antioxidant activity in oxidative stress-induced kidney epithelial cell injury. The following groups of mice were used for the in vivo study: untreated controls (control); 20-min bilateral renal ischemia with 40-min reperfusion (early IR); and IR injury with 21d-IR. Groups were given normal diet, or normal diet + 5% NAC. Figure 1 demonstrates outcomes on apoptosis and fibrosis and kidney function. NAC attenuated IR-induced tubular epithelial apoptosis at early IR yet significantly enhanced apoptosis at 21d-IR (Fig. 1A). Progressive fibrosis occurred at 21d-IR regardless of diet, demonstrated by increased collagen deposition (Fig. 1B), and increased interstitial cell proliferation that was significantly enhanced by NAC (Fig. 1C). α-SMA immunofluorescence confirmed tubular interstitial cell localization of PCNA-expressing cells (Fig. 1D). NAC significantly increased profibrotic transforming growth factor-β1 (TGF-β1) expression without injury and at 21d-IR in the control diet (Fig. 1, E and F). AKI was demonstrated by kidney functional changes (serum creatinine/SCr and proteinuria significantly increased at 40-min reperfusion). Function returned to control levels at 21d-IR regardless of diet (Fig. 1, F and H).
Fig. 1.
Maladaptive repair following acute kidney injury (AKI) is enhanced by the antioxidant N-acetyl-cysteine (NAC). Groups of mice were the following: untreated controls (control); 20-min bilateral renal ischemia with 40-min reperfusion [early ischemia-reperfusion (IR)]; and IR injury with 21-days reperfusion (21d-IR). Groups were given normal diet or normal diet + 5% NAC. Acute and long-term outcomes of apoptosis and fibrosis and kidney function are demonstrated. A: the enzymatic in situ stain ApopTag was used to identify and quantify apoptosis. Apoptosis significantly increased in tubular epithelial cells at IR and 21d-IR in mice on a normal diet. The 5% NAC diet prevented tubular cell apoptosis at IR but significantly enhanced apoptosis at 21d-IR compared with mice on a normal diet. B: Masson’s trichrome staining and morphometry demonstrated increased interstitial collagen deposition at 21d-IR compared with healthy controls and early IR for both normal diet and 5% NAC diet. C: progressive fibrosis was also evident at 21d-IR via increased interstitial cell proliferation (using proliferating cell nuclear antibody/PCNA labeling and quantification by morphometry) that was significantly enhanced by NAC. D: immunofluorescence was used to confirm interstitial cells expressing α-smooth muscle actin, a marker of myofibroblasts, in 21d-IR mice compared with control and IR animals on a normal diet (red, αSMA; green, autofluorescence). Regardless of IR duration, NAC in diet significantly increased profibrotic transforming growth factor-β1 (TGF-β1) expression, detected by Western immunoblotting (E) and quantified by densitometry (F). Kidney functional changes verified induction of AKI (serum creatinine/SCr) (G) and proteinuria by semiquantitative dipstick analysis (H) significantly increased at 40-min reperfusion, but function returned to control levels at 21d-IR regardless of diet. Results are expressed as means ± SE (n = 4–6 animals per group; *P < 0.05, **P < 0.01, ***P < 0.001 compared as indicated). Scale bar represents 50 µm.
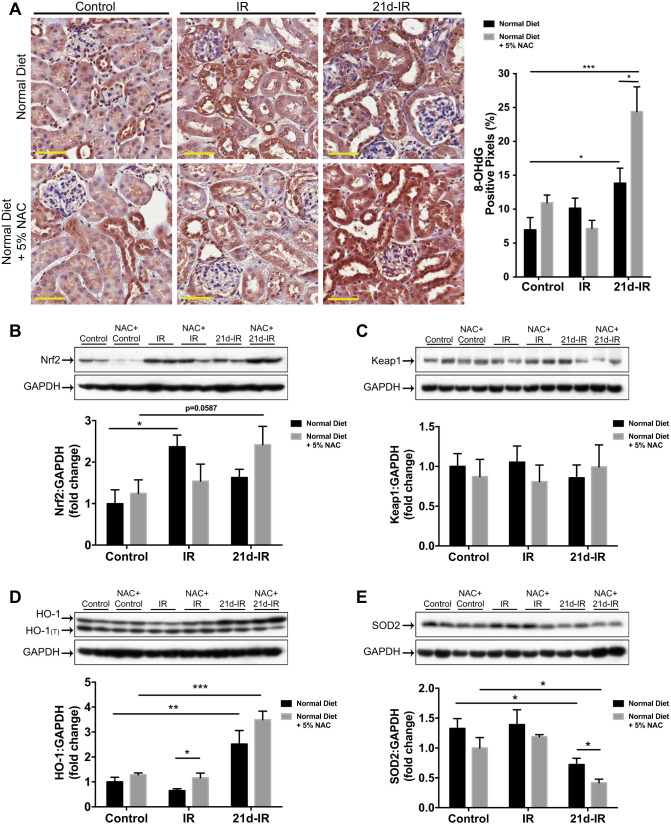
NAC enhanced oxidative damage in chronically damaged kidney following AKI.
The oxidative damage marker 8-hydroxy deoxy guanosine (8-OHdG) was unaltered from controls at early IR regardless of diet, yet at 21d-IR, 8-OHdG was significantly increased compared with controls regardless of diet and further enhanced by NAC (Fig. 2A). With normal diet, Nrf2 expression was significantly increased at early IR, but with NAC diet Nrf2 expression decreased. This trend may indicate dampened cytoprotective responses at early IR (Fig. 2B). Keap1 expression did not alter significantly (Fig. 2C). Heme oxygenase-1 (HO-1) expression at 32–34 kDa plus a lower molecular weight HO-1 protein (HO-1Truncated consistent with C-terminal truncation) were observed (Fig. 2D). Quantification of the nontruncated isoform revealed greater HO-1 expression with NAC at early IR compared with a normal diet, yet both diets resulted in significantly increased HO-1 at 21d-IR compared with controls. Superoxide dismutase-2 (SOD2), a superoxide scavenger in mitochondria, has significantly decreased expression with NAC at 21d-IR (Fig. 2E).
Fig. 2.
NAC enhanced oxidative damage in the chronically damaged kidney following AKI. A: 8-OHdG immunohistochemistry, and morphometry demonstrate increased oxidative damage 21 days following IR injury (21d-IR) for mice on a normal diet and with 5% NAC (NAC diet) compared with control kidneys. NAC diet significantly enhanced 8-OHdG positivity at 21d-IR compared with mice on a normal diet. B: nuclear factor-like-2 (Nrf2) protein expression increased with normal diet at early reperfusion (IR), and an increase trend was observed at 21d-IR with NAC diet. C: no changes were observed in Kelch-like ECH-associated protein 1 (Keap1) protein expression. D: heme oxygenase-1 (HO-1) protein expression increased at 21d-IR with normal diet and with NAC diet compared with controls. As well as the normal 32–34 kDa band, a band for truncated HO-1(T) was detected. E: superoxide dismutase-2 (SOD2) decreased at 21d-IR with normal diet compared with controls. SOD2 was further decreased with the NAC diet. Results are expressed as means ± SE (n = 4–6 animals per group; *P < 0.05, **P < 0.01, ***P < 0.001 compared as indicated). Scale bar represents 50 µm.
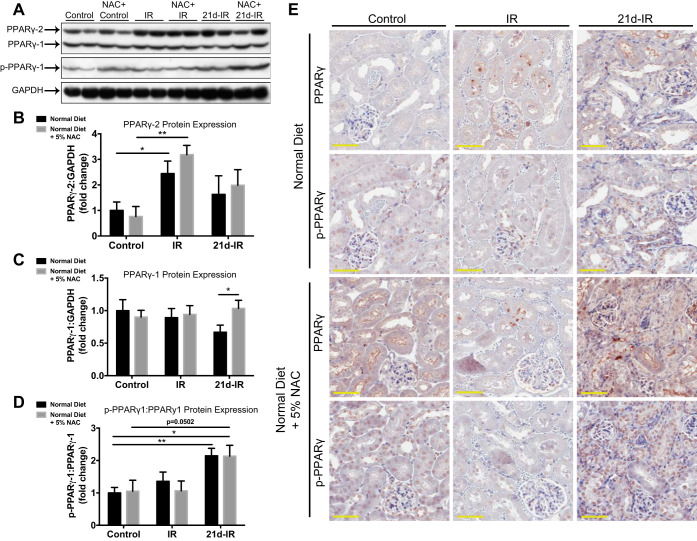
Serine-112 phosphorylation of PPARγ-1 was associated with chronic kidney pathology following AKI.
PPAR-γ1 and PPAR-γ2 isoforms were found, with only phosphorylation (ser112) of PPAR-γ1 (p-PPAR-γ1) detected (Fig. 3A). PPAR-γ2 expression was significantly increased at 40-min IR compared with controls regardless of diet (Fig. 3B), whereas significantly lower PPAR-γ1 was seen at 21d-IR in a normal vs. NAC diet (Fig. 3C). The p-PPAR-γ1:PPAR-γ1 ratio significantly increased at 21d-IR with both diets compared with controls (Fig. 3D). IHC of PPAR-γ and p-PPAR-γ was used to provide cellular localization (Fig. 3E). PPARγ expression was low in atrophic tubules at 21d-IR regardless of diet but localized to interstitial cells with NAC. Nuclear labeling of p-PPARγ within tubular epithelial cells was greater in atrophic areas at 21d-IR regardless of diet.
Fig. 3.
Ser112 phosphorylation of PPARγ is associated with chronic kidney pathology following AKI. PPARγ protein analysis was performed comparing normal with NAC diet in control kidneys, early reperfusion (IR), and 21 days following IR injury (21d-IR). A: Western blotting of whole kidney extracts for PPAR-γ protein expression and its phosphorylation at Ser112 detected PPAR-γ1 and PPAR-γ2 isoforms. Only phosphorylation of PPAR-γ1 was detected (p-PPAR-γ1). Densitometry demonstrated increased PPAR-γ2 expression at IR in mice regardless of diet (B) and decreased PPAR-γ1 expression at 21d-IR with normal diet compared with NAC diet (C). D: the p-PPAR-γ1:PPAR-γ1 ratio was significantly increased at 21d-IR with normal diet compared with controls. E: localization and intensity of protein expression for PPAR-γ and p-PPAR-γ were determined by immunohistochemistry. Control kidneys regardless of type of diet demonstrated cytoplasmic and brush-border localization of PPAR-γ within proximal tubular cells and nuclear localization of p-PPAR-γ. Punctate regions within tubular cells positive for PPAR-γ were apparent at IR regardless of the type of diet, and no change was observed in p-PPAR-γ localization. At 21d-IR, PPAR-γ expression was localized primarily to the interstitium, which matched nuclear localization of p-PPAR-γ. Results are expressed as means ± SE (n = 4–6 animals per group; *P < 0.05, **P < 0.01, ***P < 0.001 compared as indicated). Scale bar represents 50 µm.
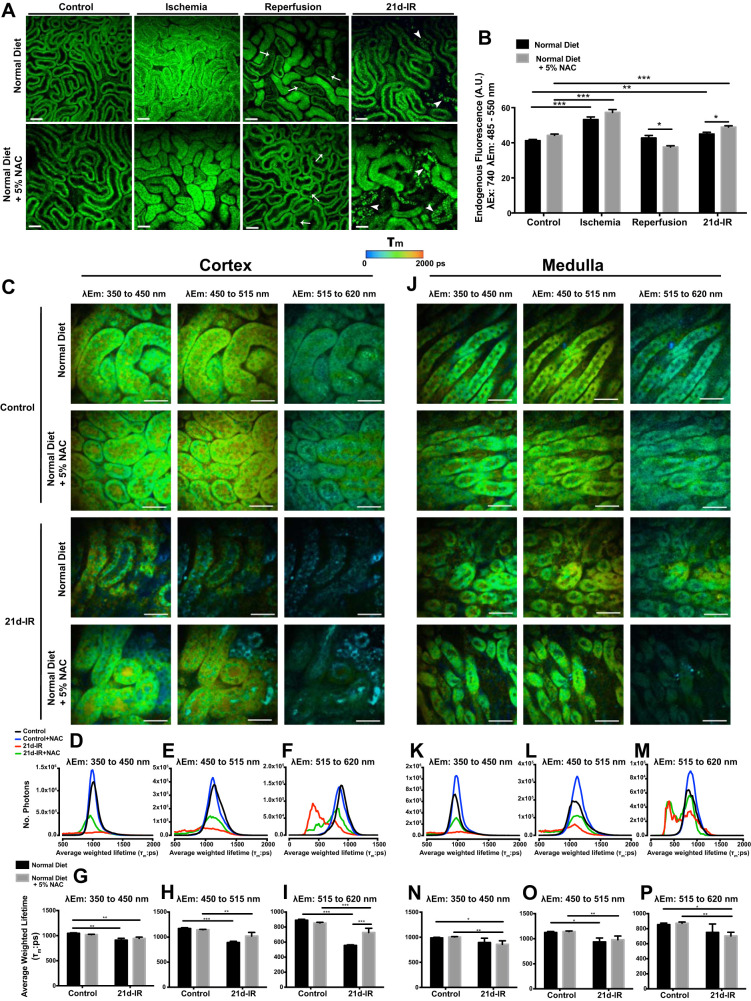
Metabolic dysfunction in kidney cortex during progressive chronic kidney pathology following AKI was not prevented by NAC.
Figure 4 demonstrates intravital MPM (740-nm excitation) as well as MPM-coupled FLIM. The molecular source of tissue autofluorescence are varied; however, it has been demonstrated that the main source, especially in kidney, arises from NADH and its phosphorylated partner, NADPH (17, 73). Flavin adenine dinucleotide (FAD) also contributes endogenous fluorescent species within the collected spectral range (73). The following is demonstrated in Fig. 4, A and B: for controls, diet alone induced no observable differences in the renal structure or mean endogenous fluorescence; during 20-min ischemia, tubular epithelial cell swelling and reduced interstitial space were associated with significantly increased mean endogenous fluorescence, regardless of diet; at 40-min reperfusion, tubular cast material evident with normal diet was reduced with NAC; at 21d-IR, only small areas of tubular atrophy were seen with a normal diet, whereas with NAC there was marked tubular atrophy with high-intensity fluorescence (Fig. 4B). To further study metabolic changes following IR, FLIM of autofluorescent species was performed with MPM (Fig. 4, C–P). Intravital MPM does not allow visualization of the medulla. Therefore FLIM of fresh kidney slices was performed to allow visualization of both cortex and medulla. FLIM constructs a spatial distribution map of fluorescence lifetimes. The fluorescence lifetime of a fluorophore is the mean time a fluorophore remains in an excited state before emitting a photon and returning to the initial ground state, and depends on the type of molecule, its conformation, and the way the molecule interacts with the surrounding environment. For kidney cortex, Fig. 4C demonstrates a FLIM spatial distribution map (pseudocolored according to average-weighted lifetime) across three emission spectral channels that are selected to capture dominant autofluorescent species: 350 to 450 nm [NAD(P)H}, 450 to 515 nm (NAD(P)H, and FAD], and 515 to 620 nm (FAD). In all spectral channels there was a decrease in average-weighted lifetime (τm) between controls vs. 21d-IR, regardless of diet (Fig. 4, G–I). This is indicated by a change from green to blue within representative images (Fig. 4C). This observation was also seen in τm lifetime histograms by a left shift in 21d-IR τm peaks compared with control τm peaks, regardless of diet (Fig. 4, D–F). FLIM imaging of the medulla (Fig. 4J) demonstrated alterations to the τm profile within all spectral channels at 21d-IR compared with controls, especially with NAC diet. These changes were not apparent in τm histograms (Fig. 4, K–M) yet were confirmed when τm was averaged (Fig. 4, N–P). Average τm significantly decreased at 21d-IR with NAC diet for all spectral channels compared with control NAC-fed mice (Fig. 4, N–P). Compared with control mice, 21d-IR mice on a normal diet had significantly decreased average τm within the 450- to 515-nm emission spectrum (Fig. 4O), typically associated with NAD(P)H and FAD autofluorescence (49, 59).
Fig. 4.
Intravital multiphoton microscopy (MPM) and fluorescence lifetime imaging (FLIM)-MPM demonstrate metabolic and structural alterations in chronic kidney pathology following AKI that is not attenuated by NAC. A: endogenous fluorescence, excited at 740 nm, in healthy control kidney cortex demonstrates the cortical tubular network with distal tubules, proximal tubules with associated brush border, and interstitial space that was unaltered in mice fed NAC diet. B: during 20-min ischemia, tubular swelling and a reduction in interstitial space was evident in both normal and NAC diet and was associated with a significant increase in tubular cell NAD(P)H endogenous fluorescence. Tubular and cellular damage was observed at the early stages of reperfusion (5- to 40-min IR), indicated by epithelial cell striations, cell effacement and the formation of cast material with either diet (A). At 21-days following IR injury (21d-IR), there was widespread tubular atrophy (arrowhead). Tubular structure was highly variable with structurally normal tubules appearing within areas of atrophy, perhaps allowing crosstalk between damaged tubules. There was a significantly greater endogenous fluorescence signal in the normal-looking tubules for normal and NAC diet (B). Results are expressed as means ± SE tubular epithelial cell fluorescence a 740 nm (3 random fields analyzed per kidney; n = 4–6 animals per group; *P < 0.05, **P < 0.01, ***P < 0.001 compared as indicated). Scale bar represents 50 µm. FLIM with chronically damaged mouse kidney are presented in C–P for cortex and medulla. Representative average-weighted fluorescence lifetime (τm) images for autofluorescent species in three spectral ranges are presented for cortex and medulla of control and 21d-IR mice on a normal diet or a normal diet + 5% NAC (NAC diet). Fluorescence lifetime data were measured within three spectral ranges (pseudocolor range from blue-red indicated in color bar): 350 to 450 (750 to 1,500 ps) to capture NAD(P)H, 450 to 515 (1,000 to 1,750 ps) to capture NAD(P)H/FAD, and 515 to 620 nm (500 to 1,750 ps) to capture FAD. The τm lifetime histograms, representing the average pixel intensity for each τm lifetime, are shown for cortex (G–I) and medulla (N–P). The corresponding average τm lifetimes from the three spectral channels are charted for control and 21d-IR cortical slices (D–F) and medullary slices (K–M). The τm lifetime histograms demonstrate decreased pixel intensity for 21d-IR kidneys in the cortex and medulla regardless of diet and within all three spectral channels. An observable left shift is apparent in 21d-IR kidney cortex compared with control kidneys, which is more pronounced in mice on a normal diet (D–F). The τm detected in all three spectral channels was significantly decreased in the cortex of 21d-IR kidneys compared with respective control kidneys, regardless of diet, and this was enhanced for 515–610 nm in mice on a normal diet. In the medulla, only mice on the NAC diet had a significantly reduced τm detected in the 350–450 and 515–620 nm channels at 21d-IR (J–M). The 450–515 nm channel detected a decreased τm in the medulla of the 21d-IR kidney regardless of diet. Results are expressed as means ± SE (*P < 0.05, **P < 0.01, ***P < 0.001 compared as indicated). Scale bar represents 30 µm.
Persistent oxidative stress and mitochondrial dysfunction occurred within remnant cortical tubules.
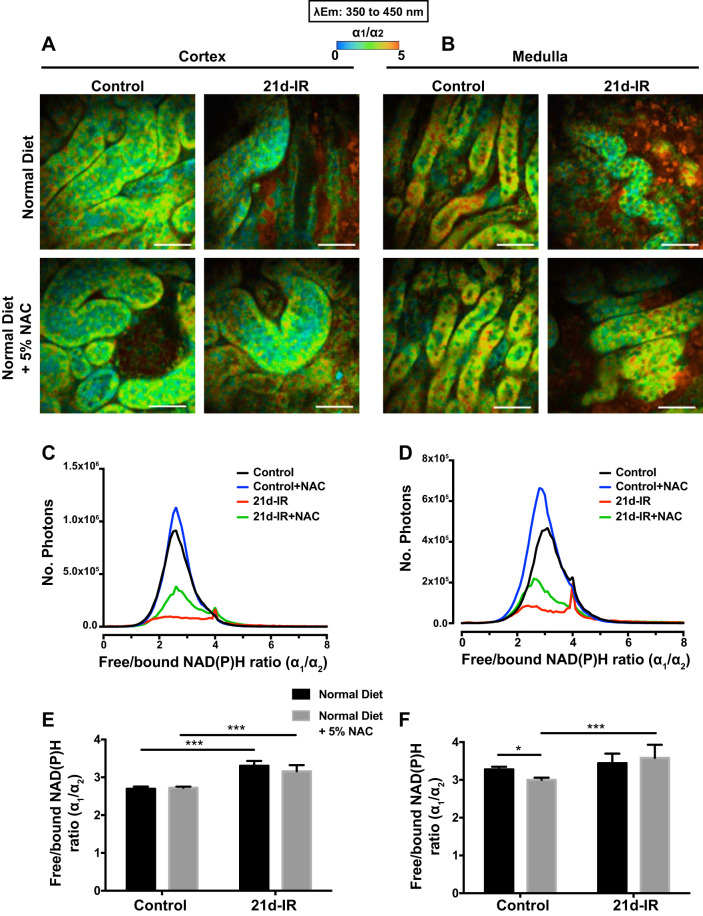
Figure 5, A and B, show representative redox FLIM images of kidney cortex and medulla, pseudocolored according to the ratio of the two components, short (α1) and long (α2) lifetimes, of the emission spectral range of 350 to 450 nm. This ratio indicates the redox state of cells that is commonly implicated with changes in free-to-bound NAD(P)H (5, 55, 65, 68). The α1/α2 ratio appeared higher at 21d-IR (red) compared with controls (green) regardless of diet, in both cortex and medulla. The α1/α2 histograms showed a similar change (right shift at 21d-IR), regardless of diet, primarily within a secondary peak in cortex and medulla (Fig. 5, C and D). The average cortical ratio significantly increased at 21d-IR regardless of diet (Fig. 5E), and NAC significantly increased the medullary ratio at 21d-IR while decreasing the medullary ratio in control mice (Fig. 5F).
Fig. 5.
Changes in the ratio of free/bound NAD(P)H (α1/α2) in the chronically damaged kidney following NAC. Representative redox images of cortex (A, C, E) and medulla (B, D, F) from controls and 21-days following IR injury (21d-IR) with normal diet or NAC diet. Changes in the free (α1) to bound (α2) NAD(P)H ratio are depicted. Fluorescence lifetime data were measured in the 350- to 450-nm spectral channel to capture NAD(P)H and pseudocolored according to the α1/α2 range 0 to 5 (blue to red). The ratio spectra, representing the average pixel intensity for each ratio value, are shown for the cortex (C) and for medulla (D). The corresponding average ratios are charted for the control and 21d-IR kidneys for cortex (E) and medulla (F). The pixel intensity for the average ratio was noticeably decreased in 21d-IR kidneys regardless of diet in both the cortex and medulla. The ratio was increased in the cortex of 21d-IR kidneys compared with controls regardless of diet (E). Mice on NAC diet had an increased ratio in the medulla of the kidney at 21d-IR compared with control mice (F). NAC diet decreased the ratio in the healthy control kidney (F). Results are expressed as means ± SE (*P < 0.05, ***P < 0.001 compared as indicated). Scale bar represents 30 µm.
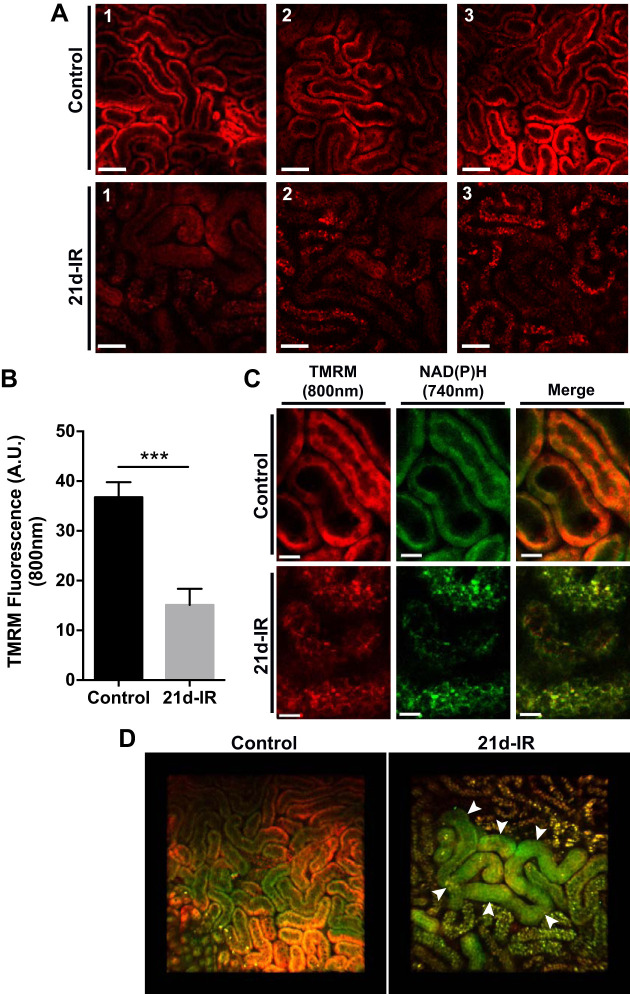
In Fig. 6 uptake of the mitochondrial potential-dependent dye TMRM into cortical tubules is demonstrated. Compared with healthy kidneys, TMRM uptake was impaired in tubules of 21d-IR kidneys, consistent with persistent mitochondrial dysfunction (Fig. 6A). TMRM uptake was significantly decreased at 21d-IR compared with controls (Fig. 6B). Impaired TMRM uptake could also reflect diminished perfusion due to vessel atrophy. TMRM accumulation colocalized with NAD(P)H autofluorescence within tubular epithelium in controls and was disrupted at 21d-IR (Fig. 6C). Z-stack 3D reconstructions of chronic cortical damage at 21d-IR demonstrated remnant, structurally normal tubules that retained NAD(P)H endogenous fluorescence yet displayed mitochondrial dysfunction through impaired TMRM uptake (Fig. 6D).
Fig. 6.
Multiphoton in vivo imaging reveals remnant tubules in the kidney cortex 21-days following ischemia-reperfusion that is a site of persistent mitochondrial dysfunction. A: the mitochondrial membrane potential-dependent fluorophore tetramethylrhodamine methyl ester (TMRM) was infused into mice and imaged with intravital MPM at excitation 800 nm (pseudocolored red) for controls and 21-days ischemia-reperfusion (21d-IR). Three representative images of the healthy control kidney revealed accumulation of TMRM into tubular epithelial cells. Accumulation of TMRM into tubular epithelial cells of the cortex of 21d-IR kidneys was noticeably reduced in three representative images. B: TMRM fluorescence quantification per tubule demonstrated a significant decrease in TMRM accumulation at 21d-IR compared with controls. C: the co-localization of TMRM and NAD(P)H (excitation at 740 nm) to the basolateral region of tubular epithelial cells confirmed mitochondrial accumulation. Basolateral localization of TMRM and NAD(P)H fluorescence was disrupted at 21d-IR with the formation of punctate vesicles, perhaps enlarged lysosomes, strongly detected at both excitations. D: representative z-stack 3-D reconstruction of a healthy control kidney cortex depict green NAD(P)H autofluorescence and red TMRM fluorescence accumulation within normal tubular epithelial cells, compared with kidney cortex at 21d-IR, demonstrating the appearance of remnant, structurally normal tubules within areas of tubular atrophy that have low TMRM accumulation yet retain NAD(P)H fluorescence (arrow heads). Results are expressed as means ± SE (n = 4–6 animals per group; ***P < 0.001 compared with control). Scale bar represents 50 µm (A) and 15 µm (C).
DISCUSSION
Despite numerous reported benefits of antioxidant therapy against AKI, targeting oxidative stress in progressive CKD has yielded limited and often disappointing results in patient cohorts (19, 23). This preclinical study confirmed a benefit for use of the antioxidant NAC to target systemic oxidative stress in IR-induced AKI. However, there may be a downside to this therapy. This study provides new evidence that progression to chronic kidney pathologies after AKI can be enhanced by NAC therapy, possibly by eliminating redox-sensitive endogenous cytoprotective Nrf2 signaling during AKI. Furthermore, evidence for metabolic and mitochondrial dysfunction is provided in chronically injured kidney that, to date, has previously been described only in models of acute injury.
NAC diet attenuated kidney cortical tubular epithelial cell apoptosis during early IR injury, demonstrating acute protection. These findings are consistent with human clinical trials that have reported benefits of NAC against AKI, including contrast-induced nephropathy (26) and cardiovascular bypass surgeries (52). IR injury of the kidney primarily occurs at reperfusion, when representation of oxygen occurs to components of the mitochondrial membrane electron transport chain (ETC) complexes, previously damaged from a failure of ATP-dependent actin polymerization during ischemia. Subsequent ROS production increases (1, 47). Visualizing NAD(P)H dynamics with intravital MPM during IR-induced injury also demonstrated these changes. Increased NAD(P)H fluorescence demonstrated a failure of ETC complex 1 to convert NAD+, in both normal and NAC-treated mice. The apparent decrease in NAD(P)H fluorescence with NAC diet at reperfusion, compared with normal diet, may represent efficient ETC complex I activity due to NAC detoxification of ROS and subsequent amelioration of mitochondrial membrane damage (9).
Recent studies demonstrate that Nrf2 dissociation from Keap1, a substrate adapter for the ubiquitin E3 ligase complex, occurs by ROS modification of Keap1 cysteine-288 residues (28). It is possible that NAC eliminates intracellular ROS and ROS signaling that are dependent on cysteine modification. This would result in maintained binding of Keap1 to Nrf2, subsequent degradation, and failure to upregulate cytoprotective responses. Furthermore, the cysteine residue of NAC likely forms a competitive antagonist-type mechanism for ROS-dependent Nrf2 activation. The antiapoptotic effect of NAC early after reperfusion can be attributed to immediate ROS detoxification and protection of the intrinsic mitochondrial apoptotic pathway (18). The underlying disruption to kidney cell redox control is apparent during the ensuing progression of disease following AKI. However, preventing highly conserved Nrf2-mediated cytoprotective responses by NAC, acutely in cells with disrupted redox control, may manifest as progression to chronic disease in the long term.
Disruption in the kidney redox environment due to prolonged NAC therapy promoted apoptosis and proliferation in the chronically damaged kidney. Increased PCNA and α-smooth muscle actin labeling was found in interstitial cells at 21d-IR, consistent with increased myofibroblast proliferation (15). This and increased secretion of TGF-β1 likely promote development of chronic kidney pathology (43). Interestingly, TGF-β1 increased in control mice given NAC therapy without the development of a profibrotic phenotype, indicating a requirement for TGF-β1 to be within a profibrotic environment, such as occurs following IR, to have an outcome of deposition of extracellular matrix in fibrogenesis. PCNA-positive cells were also found within the tubular epithelium, indicating an attempt to repopulate a functional tubule. This repair mechanism has gained recent attention and indicates the importance of remnant tubules within areas of focal fibrotic change (32). Our results demonstrate, for the first time, a lack of any antifibrotic benefit of NAC. The results are in contrast with previous studies reporting the antifibrotic actions of NAC within isolated profibrotic hepatic stellate cells (38) and fibroblasts (30). However, these studies were often confined to in vitro systems and highlight that NAC often demonstrates a superior antioxidant effect in the cell culture environment. Borkham-Kamphorst et al. (8) demonstrated that phosphorylation of platelet-derived growth factor (PDGF), extracellular signal-regulated protein kinase, or protein kinase B could not be inhibited despite high NAC administration to hepatic stellate cells in vitro. The studies by Ryoo et al. (48) support our conclusion that NAC dampens the Nrf2-dependent cytoprotective response following kidney IR injury and that TGF-β1 may suppress Nrf2-dependent activation.
Increased HO-1 expression is recognized as a protective response against virtually any insult, including AKI (6, 11, 61). Zager et al. (70) demonstrated that peak renal HO-1 expression occurred at 4 h following reperfusion. Increased HO-1 at 40-min reperfusion was, predictably, negligible. Despite genetic knock-in/out mouse models confirming a protective relationship, physiological HO-1 induction in response to IR injury does not always prevent damage (6, 27, 37). Thus HO-1 expression may also be a marker of oxidative stress rather than an indicator of a full cytoprotective response (67). The truncation of HO-1 (HO-1Truncated) at the C-terminal domain may aid nuclear-cytoplasmic HO-1 shuttling and alter binding of transcription factors involved in oxidative stress, thereby affording protection against oxidative stress (35). We observed a decrease in HO-1Truncated at 21d-IR compared with healthy controls, regardless of diet, suggesting that HO-1Truncated may be functionally relevant in a chronic oxidative stress environment in the kidney.
The results support the association between increased PPARγ ser112 phosphorylation and a failure to induce cytoprotective responses during oxidative stress in the kidney, previously shown by us in vitro (57). The PPAR-γ2 isoform was especially responsive during early IR compared with PPAR-γ1. Punctate regions within proximal tubular epithelial cells, seen via IHC, are likely attributable to PPAR-γ2 (39, 72). Differential expression profiles of PPAR-γ1 and PPAR-γ2 may indicate unique mechanisms of these isoforms in response to kidney injury, with PPAR-γ2 playing an early adaptive and protective role against injury, and PPAR-γ1 primarily influenced by posttranslational phosphorylation in the long term (57).
This study also presents the first demonstration of differences in the redox and metabolic state of healthy vs. IR-injured kidney tubular epithelial cells. Observations made of cortex and medulla were consistent with high cortical O2 tension (14) and the outer medulla as the main site of IR injury (7). Technically, in vivo MPM visualization is limited to the cortex. However, MPM-FLIM experiments also demonstrated that the cortex is a critical site of metabolic dysfunction in IR-injured kidney (51, 55, 65), with NAD(P)H protein recruitment in chronic injury contributing to persistent metabolic dysfunction. Metabolic differences may exist in fresh postmortem kidney slices compared with live kidney tissue. However, previous studies have demonstrated minimal changes in photon counts in excised skin after 4 days if kept at 4°C (51) and that cell viability remains 4 h postmortem at 4°C in the kidney (16, 63). The benefit of this method is that it allows visualization and analysis of the medulla that intravital MPM currently does not. We believe that the comparison of kidneys from control animals vs. treated animals, using standardized methods, guarantees the validity of our results. Increased cortical free-to-bound NAD(P)H (α1/α2) ratio demonstrated either an increase in free NAD(P)H or decrease in protein-bound NAD(P)H. Binding of NAD(P)H primarily occurs on mitochondrial membranes (complex I). Therefore, decreased free/bound ratio indicates impaired mitochondrial function and is consistent with intravital MPM data, describing complex I-deficient backing up of NAD+ at 21d-IR. Similar NAD(P)H binding dynamics were not observed in the medulla, except under NAC diet, indicating NAC contributes long term to metabolic dysfunction. It is also possible that metabolic effects on fluorescence lifetimes reflect the ratio of protein bound NADPH to protein-bound NADH (4). There is NAD(P)H emission within the collected FAD range, which presents a limitation; however, this is minimal compared with the FAD emission denoted by the observed τm differences between the three collected spectral channels. Previous studies have utilized the emission spectrum range of 515 to 620 nm to study metabolic changes of FAD (12, 22, 40). Decreased FAD τm likely demonstrates decreased protein-bound FAD to the succinate dehydrogenase enzyme complex of complex II of the ETC, and decreased free FAD due to decreased complex II utilization and representation of FAD to the citric acid cycle. That NAC significantly enhanced the decrease in FAD at 21d-IR is further evidence that dampening the kidney’s cellular redox responses through exogenous antioxidant therapy can worsen outcome after injury. Reduced TMRM uptake and mitochondrial function were also found in remnant tubules within focal atrophic areas. This has important implications for mitochondrial-targeted therapies (such as lipophilic triphenylphosphonium cations, for example, MitoQ) that rely on accumulation of mitochondrial membrane potential differences to exert beneficial effects (60). The tubules that remain in such damaged areas appear structurally normal yet have measurable metabolic dysfunction that could be manifested as persistent ROS generation, cell cycle arrest, and/or secretion of fibrogenic factors (10, 66).
In summary, this study demonstrates that AKI-induced metabolic dysfunction of tubular epithelial cells, at least in part, underlies progression to CKD. The antioxidant NAC did not reduce oxidative damage in the progressive kidney pathologies, but instead significantly altered the cellular redox environment to promote metabolic and mitochondrial dysfunction. The results of this study do not support the use of long-term NAC antioxidant therapy for progressive CKD. We believe that NAC saturates vital ROS-dependent intracellular signaling networks, thereby preventing conserved endogenous cytoprotective responses. The results do, however, present mitochondrial preservation and a reduction of excess mitochondrial-derived ROS as therapeutic targets. The contribution of oxidative stress to AKI and its associated progression to CKD is an important process within the cellular milieu of the kidney, which may require specific, targeted therapies rather than a general antioxidant approach.
GRANTS
D. Small was supported by an Australian Postgraduate Award and a National Health and Medical Research Council Top-up grant.
DISCLOSURES
D. Johnson has received consultancy fees, research funds, speaking honoraria and travel sponsorships from Jannsen-Cilag, Amgen, Pfizer, Baxter, and Roche. None of these funds have been used to support the research presented in this manuscript.
AUTHOR CONTRIBUTIONS
D.M.S., J.S.C., D.W.J., and G.C.G. conceived and designed research; D.M.S., W.Y.S., and S.F.R. performed experiments; D.M.S., W.Y.S., and H.L.B. analyzed data; D.M.S., C.M., H.L.B., and G.C.G. interpreted results of experiments; D.M.S. prepared figures; D.M.S. drafted manuscript; D.M.S., C.M., H.L.B., D.W.J., and G.C.G. edited and revised manuscript; D.M.S., W.Y.S., S.F.R., C.M., H.L.B., J.S.C., D.W.J., and G.C.G. approved final version of manuscript.
REFERENCES
- 1.Atkinson SJ, Hosford MA, Molitoris BA. Mechanism of actin polymerization in cellular ATP depletion. J Biol Chem 279: 5194–5199, 2004. doi: 10.1074/jbc.M306973200. [DOI] [PubMed] [Google Scholar]
- 2.Berezin MY, Achilefu S. Fluorescence lifetime measurements and biological imaging. Chem Rev 110: 2641–2684, 2010. doi: 10.1021/cr900343z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bird DK, Yan L, Vrotsos KM, Eliceiri KW, Vaughan EM, Keely PJ, White JG, Ramanujam N. Metabolic mapping of MCF10A human breast cells via multiphoton fluorescence lifetime imaging of the coenzyme NADH. Cancer Res 65: 8766–8773, 2005. doi: 10.1158/0008-5472.CAN-04-3922. [DOI] [PubMed] [Google Scholar]
- 4.Blacker TS, Duchen MR. Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radic Biol Med 100: 53–65, 2016. doi: 10.1016/j.freeradbiomed.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blacker TS, Mann ZF, Gale JE, Ziegler M, Bain AJ, Szabadkai G, Duchen MR. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat Commun 5: 3936, 2014. doi: 10.1038/ncomms4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolisetty S, Traylor AM, Kim J, Joseph R, Ricart K, Landar A, Agarwal A. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol 21: 1702–1712, 2010. doi: 10.1681/ASN.2010030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borkham-Kamphorst E, Meurer SK, Gressner AM, Weiskirchen R. Disruption of intermolecular disulfide bonds in PDGF-BB dimers by N-acetyl-l-cysteine does not prevent PDGF signaling in cultured hepatic stellate cells. Biochem Biophys Res Commun 338: 1711–1718, 2005. doi: 10.1016/j.bbrc.2005.10.139. [DOI] [PubMed] [Google Scholar]
- 9.Brunet J, Boily MJ, Cordeau S, Des Rosiers C. Effects of N-acetylcysteine in the rat heart reperfused after low-flow ischemia: evidence for a direct scavenging of hydroxyl radicals and a nitric oxide-dependent increase in coronary flow. Free Radic Biol Med 19: 627–638, 1995. doi: 10.1016/0891-5849(95)00077-B. [DOI] [PubMed] [Google Scholar]
- 10.Canaud G, Bonventre JV. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant 30: 575–583, 2015. doi: 10.1093/ndt/gfu230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Kapturczak MH, Wasserfall C, Glushakova OY, Campbell-Thompson M, Deshane JS, Joseph R, Cruz PE, Hauswirth WW, Madsen KM, Croker BP, Berns KI, Atkinson MA, Flotte TR, Tisher CC, Agarwal A. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc Natl Acad Sci USA 102: 7251–7256, 2005. doi: 10.1073/pnas.0502407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Combs CA, Balaban RS. Direct imaging of dehydrogenase activity within living cells using enzyme-dependent fluorescence recovery after photobleaching (ED-FRAP). Biophys J 80: 2018–2028, 2001. doi: 10.1016/S0006-3495(01)76172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM; BEACON Trial Investigators . Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369: 2492–2503, 2013. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein FH, Agmon Y, Brezis M. Physiology of renal hypoxia. Ann N Y Acad Sci 718: 72–82, 1994. doi: 10.1111/j.1749-6632.1994.tb55706.x. [DOI] [PubMed] [Google Scholar]
- 15.Forbes JM, Hewitson TD, Becker GJ, Jones CL. Ischemic acute renal failure: long-term histology of cell and matrix changes in the rat. Kidney Int 57: 2375–2385, 2000. doi: 10.1046/j.1523-1755.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghorbani R, Jalili C, Salahshoor MR, Shiasi M. The effect of time and temperature on viability and performance of Langerhans islets separated from Balb/c mouse after death. Adv Biomed Res 4: 93, 2015. doi: 10.4103/2277-9175.156657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall AM, Rhodes GJ, Sandoval RM, Corridon PR, Molitoris BA. In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int 83: 72–83, 2013. doi: 10.1038/ki.2012.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higuchi M, Honda T, Proske RJ, Yeh ET. Regulation of reactive oxygen species-induced apoptosis and necrosis by caspase 3-like proteases. Oncogene 17: 2753–2760, 1998. doi: 10.1038/sj.onc.1202211. [DOI] [PubMed] [Google Scholar]
- 19.Himmelfarb J, Ikizler TA, Ellis C, Wu P, Shintani A, Dalal S, Kaplan M, Chonchol M, Hakim RM. Provision of antioxidant therapy in hemodialysis (PATH): a randomized clinical trial. J Am Soc Nephrol 25: 623–633, 2014. doi: 10.1681/ASN.2013050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu SP, Chiang CK, Yang SY, Chien CT. N-acetylcysteine for the management of anemia and oxidative stress in hemodialysis patients. Nephron Clin Pract 116: c207–c216, 2010. doi: 10.1159/000317201. [DOI] [PubMed] [Google Scholar]
- 21.Hsu TF, Huang MK, Yu SH, Yen DH, Kao WF, Chen YC, Huang MS. N-acetylcysteine for the prevention of contrast-induced nephropathy in the emergency department. Intern Med 51: 2709–2714, 2012. doi: 10.2169/internalmedicine.51.7894. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Heikal AA, Webb WW. Two-photon fluorescence spectroscopy and microscopy of NAD(P)H and flavoprotein. Biophys J 82: 2811–2825, 2002. doi: 10.1016/S0006-3495(02)75621-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jun M, Venkataraman V, Razavian M, Cooper B, Zoungas S, Ninomiya T, Webster AC, Perkovic V. Antioxidants for chronic kidney disease. Cochrane Database Syst Rev 10: CD008176, 2012. doi: 10.1002/14651858.CD008176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadkhodaee M, Khastar H, Arab HA, Ghaznavi R, Zahmatkesh M, Mahdavi-Mazdeh M. Antioxidant vitamins preserve superoxide dismutase activities in gentamicin-induced nephrotoxicity. Transplant Proc 39: 864–865, 2007. doi: 10.1016/j.transproceed.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 25.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Kay J, Chow WH, Chan TM, Lo SK, Kwok OH, Yip A, Fan K, Lee CH, Lam WF. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: a randomized controlled trial. JAMA 289: 553–558, 2003. doi: 10.1001/jama.289.5.553. [DOI] [PubMed] [Google Scholar]
- 27.Kezic A, Thaiss F, Becker JU, Tsui TY, Bajcetic M. Effects of everolimus on oxidative stress in kidney model of ischemia/reperfusion injury. Am J Nephrol 37: 291–301, 2013. doi: 10.1159/000348496. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Lee HG, Park SA, Kundu JK, Keum YS, Cha YN, Na HK, Surh YJ. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS One 9: e85984, 2014. doi: 10.1371/journal.pone.0085984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kongkham S, Sriwong S, Tasanarong A. Protective effect of alpha tocopherol on contrast-induced nephropathy in rats. Nefrologia 33: 116–123, 2013. doi: 10.3265/Nefrologia.pre2012. [DOI] [PubMed] [Google Scholar]
- 30.Kopp J, Seyhan H, Müller B, Lanczak J, Pausch E, Gressner AM, Dooley S, Horch RE. N-acetyl-l-cysteine abrogates fibrogenic properties of fibroblasts isolated from Dupuytren’s disease by blunting TGF-β signalling. J Cell Mol Med 10: 157–165, 2006. doi: 10.1111/j.1582-4934.2006.tb00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA 111: 1527–1532, 2014. doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 21: 345–352, 2010. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakowicz JR, Szmacinski H, Nowaczyk K, Johnson ML. Fluorescence lifetime imaging of free and protein-bound NADH. Proc Natl Acad Sci USA 89: 1271–1275, 1992. doi: 10.1073/pnas.89.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem 282: 20621–20633, 2007. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 36.Malins DC, Hellstrom KE, Anderson KM, Johnson PM, Vinson MA. Antioxidant-induced changes in oxidized DNA. Proc Natl Acad Sci USA 99: 5937–5941, 2002. doi: 10.1073/pnas.082111199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer RD, Wang X, Maines MD. Nitric oxide inhibitor Nω-nitro-l-arginine methyl ester potentiates induction of heme oxygenase-1 in kidney ischemia/reperfusion model: a novel mechanism for regulation of the oxygenase. J Pharmacol Exp Ther 306: 43–50, 2003. doi: 10.1124/jpet.102.048686. [DOI] [PubMed] [Google Scholar]
- 38.Meurer SK, Lahme B, Tihaa L, Weiskirchen R, Gressner AM. N-acetyl-l-cysteine suppresses TGF-β signaling at distinct molecular steps: the biochemical and biological efficacy of a multifunctional, antifibrotic drug. Biochem Pharmacol 70: 1026–1034, 2005. doi: 10.1016/j.bcp.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Miglio G, Rosa AC, Rattazzi L, Grange C, Camussi G, Fantozzi R. Protective effects of peroxisome proliferator-activated receptor agonists on human podocytes: proposed mechanisms of action. Br J Pharmacol 167: 641–653, 2012. doi: 10.1111/j.1476-5381.2012.02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mironov SL, Richter DW. Oscillations and hypoxic changes of mitochondrial variables in neurons of the brainstem respiratory centre of mice. J Physiol 533: 227–236, 2001. doi: 10.1111/j.1469-7793.2001.0227b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nascimento MM, Suliman ME, Silva M, Chinaglia T, Marchioro J, Hayashi SY, Riella MC, Lindholm B, Anderstam B. Effect of oral N-acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo-controlled study. Perit Dial Int 30: 336–342, 2010. doi: 10.3747/pdi.2009.00073. [DOI] [PubMed] [Google Scholar]
- 42.Niesner R, Peker B, Schlüsche P, Gericke KH. Noniterative biexponential fluorescence lifetime imaging in the investigation of cellular metabolism by means of NAD(P)H autofluorescence. ChemPhysChem 5: 1141–1149, 2004. doi: 10.1002/cphc.200400066. [DOI] [PubMed] [Google Scholar]
- 43.Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol 16: 133–143, 2005. doi: 10.1681/ASN.2004040339. [DOI] [PubMed] [Google Scholar]
- 44.Parra Cid T, Conejo García JR, Carballo Alvarez F, de Arriba G. Antioxidant nutrients protect against cyclosporine A nephrotoxicity. Toxicology 189: 99–111, 2003. doi: 10.1016/S0300-483X(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 45.Peerapanyasut W, Thamprasert K, Wongmekiat O. Ubiquinol supplementation protects against renal ischemia and reperfusion injury in rats. Free Radic Res 48: 180–189, 2014. doi: 10.3109/10715762.2013.858148. [DOI] [PubMed] [Google Scholar]
- 46.Percy CJ, Brown L, Power DA, Johnson DW, Gobe GC. Obesity and hypertension have differing oxidant handling molecular pathways in age-related chronic kidney disease. Mech Ageing Dev 130: 129–138, 2009. doi: 10.1016/j.mad.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Plotnikov EY, Kazachenko AV, Vyssokikh MY, Vasileva AK, Tcvirkun DV, Isaev NK, Kirpatovsky VI, Zorov DB. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int 72: 1493–1502, 2007. doi: 10.1038/sj.ki.5002568. [DOI] [PubMed] [Google Scholar]
- 48.Ryoo IG, Ha H, Kwak MK. Inhibitory role of the KEAP1-NRF2 pathway in TGFβ1-stimulated renal epithelial transition to fibroblastic cells: a modulatory effect on SMAD signaling. PLoS One 9: e93265, 2014. doi: 10.1371/journal.pone.0093265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez WY, Obispo C, Ryan E, Grice JE, Roberts MS. Changes in the redox state and endogenous fluorescence of in vivo human skin due to intrinsic and photo-aging, measured by multiphoton tomography with fluorescence lifetime imaging. J Biomed Opt 18: 061217, 2013. doi: 10.1117/1.JBO.18.6.061217. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez WY, Prow TW, Sanchez WH, Grice JE, Roberts MS. Analysis of the metabolic deterioration of ex vivo skin from ischemic necrosis through the imaging of intracellular NAD(P)H by multiphoton tomography and fluorescence lifetime imaging microscopy. J Biomed Opt 15: 046008, 2010. doi: 10.1117/1.3466580. [DOI] [PubMed] [Google Scholar]
- 52.Santana-Santos E, Gowdak LH, Gaiotto FA, Puig LB, Hajjar LA, Zeferino SP, Drager LF, Shimizu MH, Bortolotto LA, De Lima JJ. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 97: 1617–1623, 2014. doi: 10.1016/j.athoracsur.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 53.Seguro AC, Poli de Figueiredo LF, Shimizu MH. N-acetylcysteine (NAC) protects against acute kidney injury (AKI) following prolonged pneumoperitoneum in the rat. J Surg Res 175: 312–315, 2012. doi: 10.1016/j.jss.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 55.Skala MC, Riching KM, Bird DK, Gendron-Fitzpatrick A, Eickhoff J, Eliceiri KW, Keely PJ, Ramanujam N. In vivo multiphoton fluorescence lifetime imaging of protein-bound and free nicotinamide adenine dinucleotide in normal and precancerous epithelia. J Biomed Opt 12: 024014, 2007. doi: 10.1117/1.2717503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton) 17: 311–321, 2012. doi: 10.1111/j.1440-1797.2012.01572.x. [DOI] [PubMed] [Google Scholar]
- 57.Small DM, Morais C, Coombes JS, Bennett NC, Johnson DW, Gobe GC. Oxidative stress-induced alterations in PPAR-γ and associated mitochondrial destabilization contribute to kidney cell apoptosis. Am J Physiol Renal Physiol 307: F814–F822, 2014. doi: 10.1152/ajprenal.00205.2014. [DOI] [PubMed] [Google Scholar]
- 59.Small DM, Sanchez WY, Roy S, Hickey MJ, Gobe GC. Multiphoton fluorescence microscopy of the live kidney in health and disease. J Biomed Opt 19: 020901, 2014. doi: 10.1117/1.JBO.19.2.020901. [DOI] [PubMed] [Google Scholar]
- 60.Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM; Protect Study Group . A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord 25: 1670–1674, 2010. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 61.Stec DE, Drummond HA, Gousette MU, Storm MV, Abraham NG, Csongradi E. Expression of heme oxygenase-1 in thick ascending loop of Henle attenuates angiotensin II-dependent hypertension. J Am Soc Nephrol 23: 834–841, 2012. doi: 10.1681/ASN.2011050455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tayek JA, Kalantar-Zadeh K. The extinguished BEACON of bardoxolone: not a Monday morning quarterback story. Am J Nephrol 37: 208–211, 2013. doi: 10.1159/000346950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tomita Y, Nihira M, Ohno Y, Sato S. Ultrastructural changes during in situ early postmortem autolysis in kidney, pancreas, liver, heart and skeletal muscle of rats. Leg Med (Tokyo) 6: 25–31, 2004. doi: 10.1016/j.legalmed.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Wang HW, Gukassyan V, Chen CT, Wei YH, Guo HW, Yu JS, Kao FJ. Differentiation of apoptosis from necrosis by dynamic changes of reduced nicotinamide adenine dinucleotide fluorescence lifetime in live cells. J Biomed Opt 13: 054011, 2008. doi: 10.1117/1.2975831. [DOI] [PubMed] [Google Scholar]
- 66.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshida T, Ishikawa K, Sato M. Degradation of heme by a soluble peptide of heme oxygenase obtained from rat liver microsomes by mild trypsinization. Eur J Biochem 199: 729–733, 1991. doi: 10.1111/j.1432-1033.1991.tb16177.x. [DOI] [PubMed] [Google Scholar]
- 68.Yu Q, Heikal AA. Two-photon autofluorescence dynamics imaging reveals sensitivity of intracellular NADH concentration and conformation to cell physiology at the single-cell level. J Photochem Photobiol B 95: 46–57, 2009. doi: 10.1016/j.jphotobiol.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci 60: 6–20, 2003. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zager RA, Johnson AC, Becker K. Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol 23: 1048–1057, 2012. doi: 10.1681/ASN.2011121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang F, Lau SS, Monks TJ. The cytoprotective effect of N-acetyl-l-cysteine against ROS-induced cytotoxicity is independent of its ability to enhance glutathione synthesis. Toxicol Sci 120: 87–97, 2011. doi: 10.1093/toxsci/kfq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu C, Huang S, Yuan Y, Ding G, Chen R, Liu B, Yang T, Zhang A. Mitochondrial dysfunction mediates aldosterone-induced podocyte damage: a therapeutic target of PPARγ. Am J Pathol 178: 2020–2031, 2011. doi: 10.1016/j.ajpath.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci USA 100: 7075–7080, 2003. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]