Abstract
High-molecular-weight kininogen (HK), together with factor XI, factor XII and prekallikrein, is part of the contact system that has proinflammatory, prothrombotic, and vasoactive properties. We hypothesized that HK plays a role in the host response during pneumonia-derived sepsis. To this end mice were depleted of kininogen (KNG) to plasma HK levels of 28% of normal by repeated treatment with a specific antisense oligonucleotide (KNG ASO) for 3 wk before infection with the common human sepsis pathogen Klebsiella pneumoniae via the airways. Whereas plasma HK levels increased during infection in mice treated with a scrambled control ASO (Ctrl ASO), HK level in the KNG ASO-treated group remained reduced to 25–30% of that in the corresponding Ctrl ASO group both before and after infection. KNG depletion did not influence bacterial growth in lungs or dissemination to distant body sites. KNG depletion was associated with lower lung CXC chemokine and myeloperoxidase levels but did not impact neutrophil influx, lung pathology, activation of the vascular endothelium, activation of the coagulation system, or the extent of distant organ injury. These results were corroborated by studies in mice with a genetic deficiency of KNG, which were indistinguishable from wild-type mice during Klebsiella-induced sepsis. Both KNG depletion and KNG deficiency were associated with strongly reduced plasma prekallikrein levels, indicating the carrier function of HK for this zymogen. This study suggests that KNG does not significantly contribute to the host defense during gram-negative pneumonia-derived sepsis.
Keywords: contact system, high-molecular weight kininogen, kallikrein-kinin system
INTRODUCTION
Sepsis, defined as a life-threatening syndrome caused by a dysregulated host response to infection (29), brings a great health burden associated with a high mortality worldwide despite substantial improvements in the knowledge of its pathogenesis and management (2, 14). Pneumonia represents the most common cause of sepsis, accounting for about one-half of all cases (2, 14). Klebsiella pneumoniae is one of the most common causative gram-negative pathogens in pneumonia and sepsis (2, 21). The emergence of carbapenemase-producing Klebsiella and other multidrug-resistant Enterobacteriaceae further accentuates the necessity to increase the understanding of pathophysiological mechanisms at play during these gram-negative infections (21).
High-molecular-weight kininogen (HK) and three additional zymogen factors in plasma [factor XII (FXII), factor XI (FXI), and prekallikrein (PKK)] compose the contact system (8, 16). In humans, HK is encoded by the kininogen (KNG) gene, which also produces low-molecular-weight KNG by alternative splicing. In mice, two KNG genes exist (Kng1 and Kng2), of which Kng2 does not contribute to the production of plasma kininogens (17). HK forms a complex with either FXI or PKK and facilitates their docking to negatively charged surfaces where FXII(a) is already present (8, 16). Autoactivation of FXII on these surfaces can further activate PKK and/or FXI (9). Activation of PKK generates the active form plasma kallikrein (PK), which can cleave HK and produce bradykinin in a cascade known as the kallikrein-kinin system. Activation of FXI initiates the intrinsic pathway of the coagulation system (8, 16). Our group recently showed that FXII deficiency enhances host defense and reduces mortality in a model of pneumonia-derived sepsis caused by K. pneumoniae (31). Studies using FXI-deficient mice did not reproduce this phenotype, suggesting that the detrimental role of FXII does not involve activation of the intrinsic pathway of the coagulation system (31). These results led us to hypothesize that the kallikrein-kinin system impairs host defense during Klebsiella-induced pneumonia and sepsis. To test this hypothesis we focused our experiments on the role of HK, considering that HK is the physiological substrate of PK and the main source of bradykinin (8, 16), and used the previously described mouse pneumonia model induced by infection with K. pneumoniae via the airways, producing an initially localized lung infection with subsequent sepsis and distant organ injury (1, 10, 31). To establish the function of KNG we used wild-type (WT) mice in which the expression of Kng1 was suppressed by a specific antisense oligonucleotide (KNG ASO) and mice deficient for Kng1.
MATERIALS AND METHODS
Animals.
Male C57BL/6J mice (7–8 wk old) were purchased from Charles River (Maastricht, The Netherlands). Homozygous Kng1 gene knockout mice (Kng1−/− mice) with a C57BL/6J genetic background were generated as described (17). All experiments were approved by the Animal Care and Use Committee of the Academic Medical Center.
Oligonucleotides.
KNG ASO and scrambled control ASO not targeting any specific gene (Ctrl ASO) were generated as described (3). The sequence of KNG ASO is GGCTATGAACTCAATAACAT, and its targeting sequence is shared by all three transcript variants of gene Kng1; the sequence of scrambled control ASO is CCTTCCCTGAAGGTTCCTCC.
Experimental design.
WT C57BL/6J mice were treated with KNG ASO or Ctrl ASO by subcutaneous injection two times weekly (40 mg/kg per injection) for 3 wk. This treatment regimen was based on previous studies (4, 24). After 3 wk of ASO treatment, mice were euthanized (to obtain baseline measurements) or inoculated with K. pneumoniae serotype 2 (ATCC 43816; American Type Culture Collection, Manassas, VA) by inhalation [104 colony-forming units (CFU) in 50 μl isotonic saline] as described (1, 10, 31). At predefined time points, citrated blood was collected and organs were harvested as described (1, 10, 31). Bacteria in blood and organ homogenates was quantified as described (1, 10, 31). All experimental groups consisted of eight mice per group at each time point.
Measurement of KNG mRNA.
Total RNA from liver samples was extracted with RNA isolation kits (Nucleospin RNA; MACHEREY-NAGEL, Düren, Germany) and reverse transcribed using oligo(dT) primer and Moloney murine leukemia virus reverse transcriptase (Promega Benelux, Leiden, The Netherlands). The following primers were used to quantify Kng1 gene expression level: forward primer ATCACAGCCACCTCTTTACTCTC, reverse primer TCCTCTACATTCACCATCATCAC; GAPDH was used as a reference gene (forward primer CTCATGACCACAGTCCATGC; reverse primer CACATTGGGGGTAGGAACAC). Real-time quantitative PCR (RT-qPCR) was performed with Sensifast SybrGreen mix (Bioline, London, UK) on a LightCycler system (LC480; Roche Applied Science, Mannheim, Germany). Amplification curves were analyzed with LinRegPCR software (version 2014.4).
Assays.
For Western blotting plasma proteins were separated by SDS-PAGE (10% separating gel). Plasma PKK and HK levels were analyzed by immunoblotting with goat anti-mouse PKK antibody (AF2498; R&D Systems) and rabbit anti-human HK domain 5 antibody, respectively. The latter antibody was raised in rabbit against a peptide derived from the human HK domain 5 sequence “DHGHKHKHGHGHGKHKNKGKKN” (a cysteine was added to the NH2-terminus for coupling to a column for affinity purification on the same peptide). Antisera was affinity purified on the same peptide used to raise the antibody. Protein levels were analyzed by densitometric quantification of the bands with Image J. Cytokines, chemokines, soluble E-selectin, and soluble vascular cell adhesion molecule-1 (VCAM-1) were measured by sandwich ELISAs (Duoset; R&D Systems) except the following: thrombin-antithrombin complexes (TATc) were measured by ELISA according to the manufacturer’s protocol (Affinity Biologicals, Ancaster, Canada); plasma tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-10, and interferon-γ were quantified by cytometric bead array multiplex assay (BD Biosciences).
Histopathology.
Four-micrometer lung tissue sections were stained with hematoxylin and eosin (H&E) and scored as described (1). For granulocyte staining, FITC-labeled anti-mouse Ly6G monoclonal antibody (Biolegend, San Diego, CA) was used as described (32). Ly6G expression level in lung tissue was quantified digitally as the percentage of Ly6G-positive area in the total lung area on the section calculated with Image J as described (1).
Statistical analysis.
Data are provided as medians with interquartile ranges or means with SE, as specified in the legends for Figs. 1–7. Mann-Whitney U-tests were performed for comparisons between groups. Kruskal-Wallis test, where appropriate followed by Mann-Whitney U-test, was used for groups of three or more. A P value <0.05 was considered statistically significant. All analyses was performed by GraphPad Prism 5.
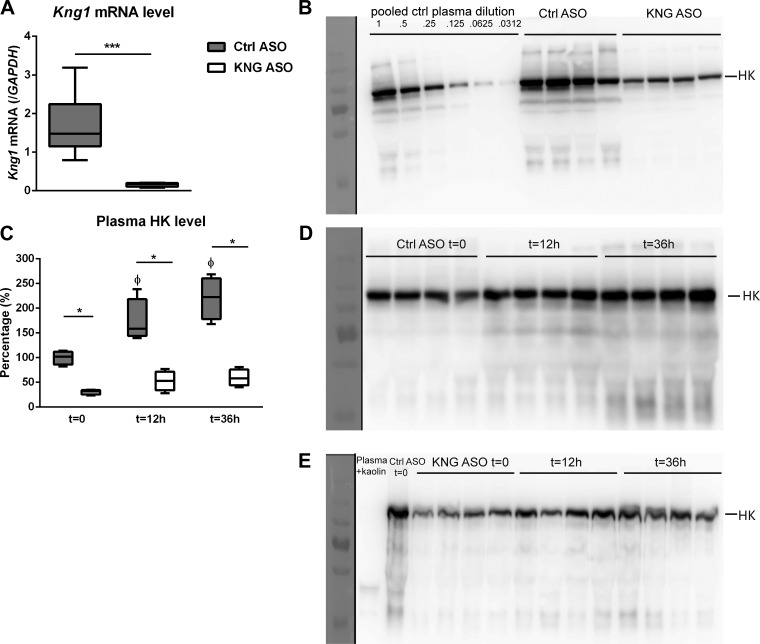
Fig. 1.
High-molecular-weight kininogen (HK) levels before and after induction of pneumonia-derived sepsis in mice pretreated with control (Ctrl) antisense oligonucleotide (ASO) or kininogen (KNG) ASO. Target gene (Kng1) expression level indicated as its mRNA by quantitative RT-PCR (A) and plasma HK level by Western blot after SDS-PAGE under reducing conditions (B); plasma HK levels before and during Klebsiella pneumonia in mice treated with Ctrl ASO (n = 8) or KNG ASO (n = 8) (D and E). Quantification of HK levels in naïve and infected mice (C) determined by densitometry of the HK bands at 120 kDa in B, D, and E and compared with a serial dilution of plasma as shown in B. Serial data were analyzed by Kruskal-Wallis test followed by Mann-Whitney U-tests. ΦP < 0.05 vs. corresponding time (t) = 0 group; *P < 0.05 and ***P < 0.001, Mann-Whitney U-tests between KNG ASO vs. Ctrl ASO.
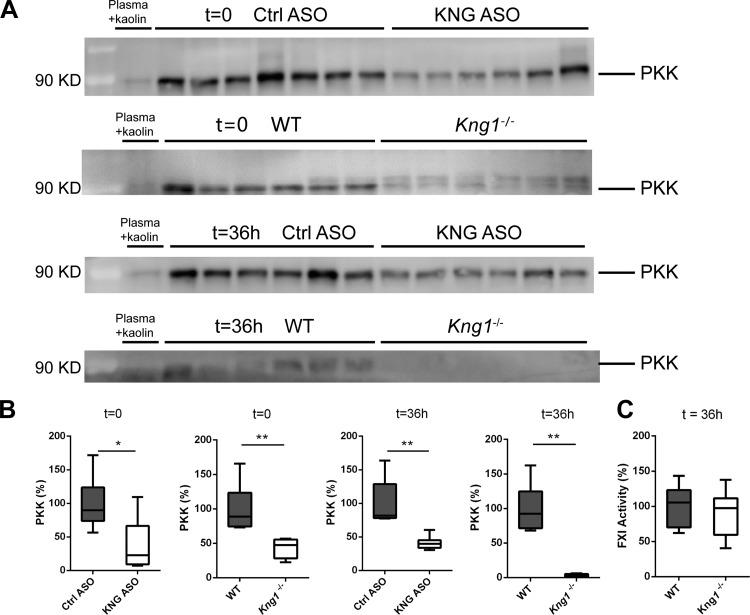
Fig. 7.
Effect of kininogen depletion or kininogen genetic deficiency on prekallikrein (PKK) plasma level and factor XI (FXI) activity level. Plasma PKK levels in noninfected and 36-h-infected mice were determined by Western blot (nonreduced, A); full-length PKK (85- and 88-kDa doublet) was quantified by densitometry of PKK band with ImageJ (B). The mean values from Ctrl ASO mice or WT mice are presented as 100%. Kaolin-activated mouse plasma was used as a negative control. C: plasma FXI activity of 36-h-infected WT and Kng1−/− mice. Data in B and C are presented as box-and-whisker plots showing interquartile ranges and medians. *P < 0.05 and **P < 0.01, Mann-Whitney U-test.
RESULTS
Inhibition of Kng1 gene expression by KNG ASO treatment.
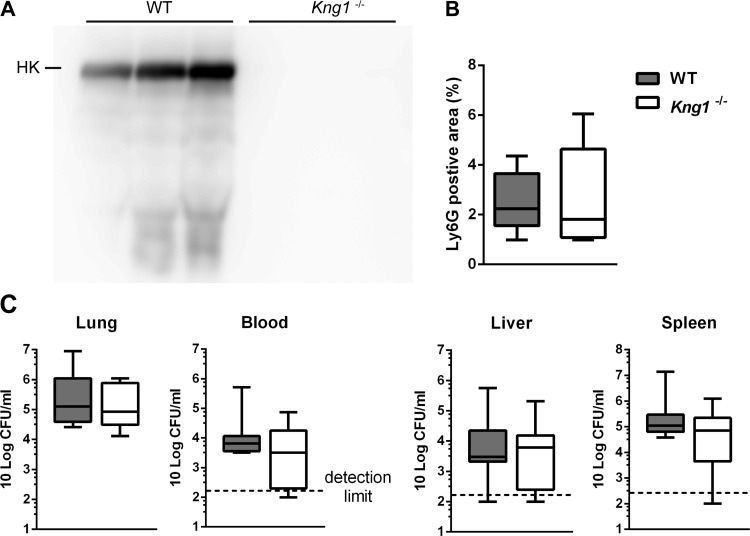
To study the role of HK in gram-negative pneumonia-derived sepsis, expression of Kng1, the gene encoding KNG, was suppressed by treating WT mice with KNG ASO for 3 wk; control mice received scrambled Ctrl ASO. After 3 wk of ASO treatment, before induction of pneumonia, KNG mRNA levels in the liver were reduced by ~90% compared with those in mice treated with Ctrl ASO (Fig. 1A), whereas plasma HK detected in KNG ASO mice was ~28% of that measured in Ctrl ASO mice (Fig. 1B). Induction of pneumosepsis resulted in an increase in plasma HK levels during infection in Ctrl ASO-treated mice [peaking at time (t) = 36 h, 220 ± 43% of t = 0 levels; P < 0.05, Fig. 1, C and D]. Plasma HK levels did not significantly increase after infection in KNG ASO-treated mice; HK levels remained much lower compared with Ctrl ASO treated-mice throughout (Fig. 1, C and E).
Kininogen depletion does not affect bacterial growth or dissemination during pneumonia-derived sepsis.
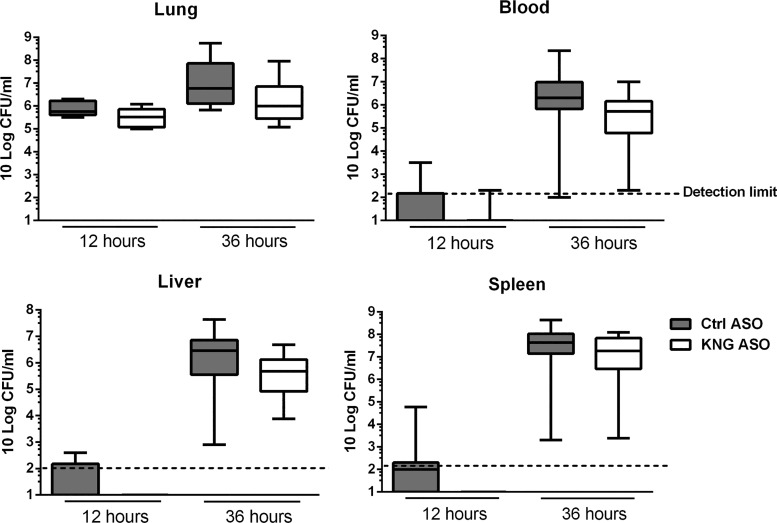
To investigate the role of KNG in the host response during pneumosepsis, we infected ASO-pretreated mice with K. pneumoniae via the airways. To obtain insight for a possible role of KNG in modulating bacterial growth and dissemination, we determined bacterial numbers at the primary site of infection (lungs) and distant body sites (blood, liver, and spleen) at 12 and 36 h after infection (Fig. 2). At the early time point (12 h), bacteria were mostly restricted to the lung with no differences between Ctrl and KNG ASO-treated mice. At 36 h, which represents late-stage sepsis, and a time point shortly before the first mice are expected to succumb (1, 32), bacteria had disseminated to blood, liver, and spleen, still with similar bacterial burdens in mice with or without KNG depletion. These data suggest that KNG does not influence bacterial growth or dissemination during gram-negative pneumosepsis.
Fig. 2.
Kininogen depletion does not influence bacterial growth or dissemination during pneumonia-derived sepsis. Bacterial loads [colony-forming units (CFUs)] in the lung, blood, liver, and spleen 12 and 36 h after infection with K. pneumoniae via the airways in mice pretreated with Ctrl ASO or KNG ASO. Data were expressed as box-and-whisker diagrams (10-Log) of 8 mice/group at each time point. At 12 h the vast majority of cultures from blood, liver, and spleen were sterile, reflecting the initially localized infection. Differences between groups were not significant with Mann-Whitney U-test.
Effect of kininogen depletion on lung pathology and inflammation during pneumosepsis.
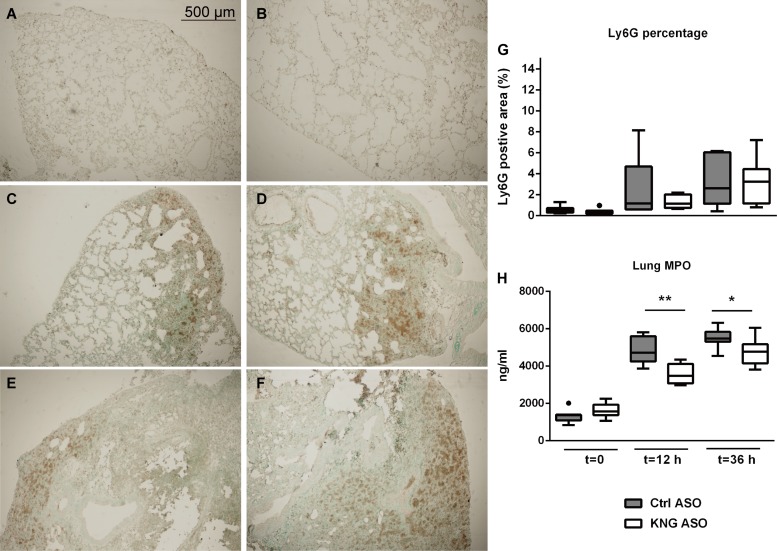
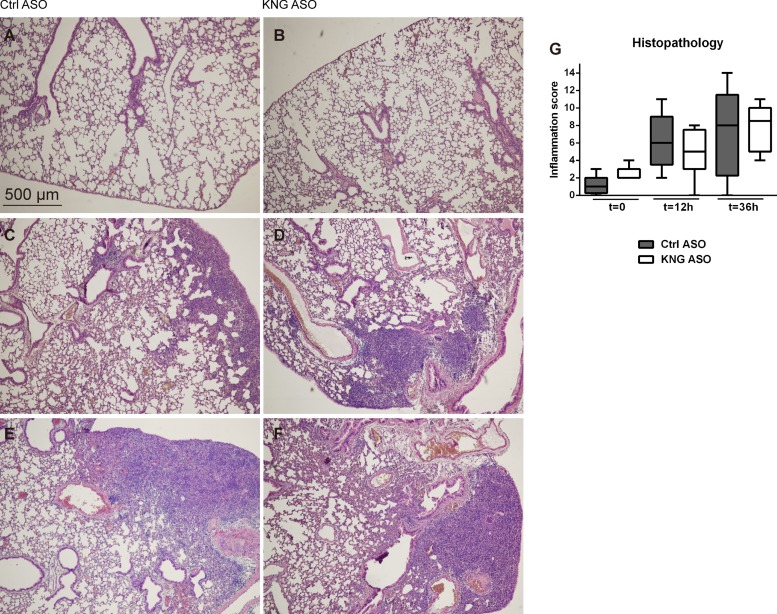
HK has been implicated as an important mediator of inflammation (28). Because neutrophil recruitment and activation are hallmark innate immune responses during infection, we measured neutrophil numbers in the lung tissue by quantifying Ly6G-positive cells in tissue slides (Fig. 3, A–G) and evaluated neutrophil influx and activation by determining myeloperoxidase (MPO) levels in lung homogenates (Fig. 3H). When compared with Ctrl ASO, KNG ASO treatment did not influence the increase in Ly6G-positive cell numbers in lungs, while lessening the rise in pulmonary MPO levels. Consistently, KNG ASO treatment was associated with reduced lung levels of the neutrophil-activating chemokines CXCL1 and CXCL2 at 12 h postinfection (Table 1). KNG ASO administration did not influence lung cytokine (TNF-α, IL-6, IL-1β) levels (Table 1). Pneumonia was associated with histological features characteristic of lower respiratory tract infection (Fig. 4). The extent of lung pathology, as determined by semiquantitative scores of H&E tissue slides, was not affected by KNG ASO treatment.
Fig. 3.
Kininogen depletion does not influence neutrophil influx but lowers myeloperoxidase (MPO) levels in lungs during pneumonia-derived sepsis. Ly6G (neutrophil) stainings of lung sections from mice before (A and B) and 12 (C and D) or 36 (E and F) h after infection with K. pneumoniae via the airways. A, C, and E are representative images from Ctrl ASO-treated mice; B, D, and F are from KNG ASO-treated mice. Original magnification ×4. G: percentage of Ly6G-positive area in lung analyzed with Image J. H: MPO levels in lung homogenates. Data are expressed as box-and-whisker diagrams showing interquartile ranges with medians of 8 mice/group at each time point. *P < 0.05 and **P < 0.01, Mann-Whitney U-test.
Table 1.
Lung cytokine and chemokine levels during pneumonia-derived sepsis in mice treated with KNG ASO or control ASO
|
Time 0 |
Time 12 h |
Time 36 h |
||||
|---|---|---|---|---|---|---|
| Lung levels | Ctrl ASO | KNG ASO | Ctrl ASO | KNG ASO | Ctrl ASO | KNG ASO |
| TNF-α, pg/ml | 1,770 (1,496–2,033) | 1,892 (1,785–2,135) | 2,827 (2,355–3,372) | 2,420 (2,208–3,274) | 3,098 (2,778–5,800) | 3,300 (2,666–5,842) |
| IL-1β, pg/ml | 1,706 (1,627–1,904) | 1,725 (1,565–1,755) | 2,486 (2,356–2,868) | 2,256 (2,055–2,541) | 3,154 (2,550–4,177) | 3,175 (2,660–4,138) |
| IL-6, pg/ml | 616 (560–921) | 835 (674–968) | 1,757 (1,400–2,200) | 1,326 (1,036–2,455) | 2,791 (1,462–6,848) | 2,432 (1,507–3,962) |
| CXCL1, pg/ml | 2,540 (2,041–3,095) | 2,549 (2,091–4,680) | 12,932 (7,783–17,236) | 6,607* (4,595–9,921) | 20,441 (15,328–36,056) | 19,190 (11,923–32,965) |
| CXCL2, pg/ml | 2,541 (2,384–2,757) | 2,541 (2,461–2,892) | 5,127 (4,030–7,195) | 3,216* (3,009–5,029) | 10,137 (8,755–19,167) | 9,588 (7,759–13,152) |
Values are medians (interquartile ranges). Ctrl, control; ASO, antisense oligonucleotide; KNG, kininogen; TNF-α, tumor necrosis factor-α; IL, interleukin; CXC, the CXC chemokines whose NH2-terminal cysteines are separated by one amino acid, represented in this name with an “X.” Mann-Whitney U-tests were performed for comparisons between groups (n = 8 mice/group for each time point).
P < 0.05 compared with the Ctrl ASO group.
Fig. 4.
Histopathological change in the lung during pneumonia-derived sepsis. Hematoxylin and eosin (H&E) staining of lung sections from mice before (A and B) and 12 (C and D) or 36 (E and F) h after infection with K. pneumoniae via the airways. A, C, and E are representative images from Ctrl ASO-treated mice; B, D, and F are from KNG ASO-treated mice. Original magnification ×4. G: severity of inflammation in the lung was evaluated and scored as described in materials and methods.
Effect of kininogen depletion on endothelium, coagulation, and organ injury.
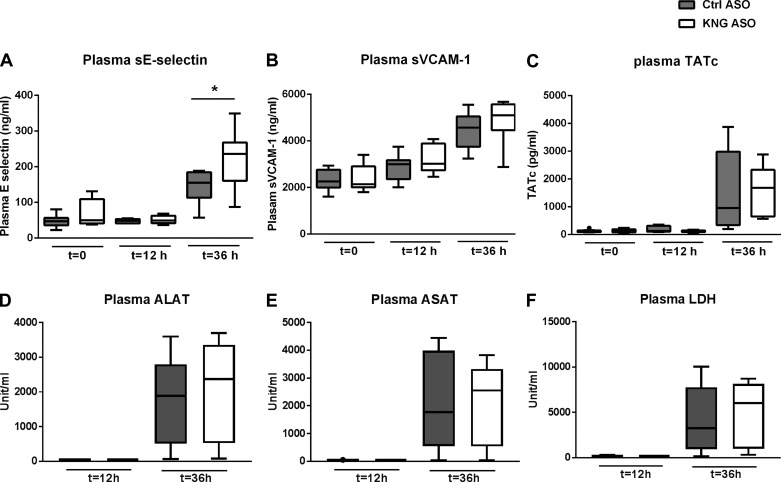
Akin to clinical sepsis, this model of pneumonia-derived sepsis is associated with activation of the vascular endothelium and the coagulation system and distant organ injury (10, 11). Considering the position of HK at the crossroad of inflammation and coagulation (28), we determined the impact of KNG ASO treatment on the plasma concentrations of soluble E-selectin, soluble VCAM-1 (reflecting endothelial cell activation), TATc (coagulation activation), alanine aminotransferase, aspartate aminotransferase (hepatocellular injury), and lactate dehydrogenase (cell injury in general). While all markers increased as a consequence of pneumosepsis, KNG ASO treatment did not influence these responses with the exception of soluble E-selectin levels, which were higher in KNG ASO-administered mice 36 h after infection (Fig. 5). Kininogen depletion also did not modify the systemic cytokine response (Table 2).
Fig. 5.
Effect of kininogen depletion on plasma biomarkers for endothelial activation, coagulation activation, and organ injury during pneumonia-derived sepsis. Endothelial (A and B) and coagulation activation (C) were investigated both before and 12 and 36 h after infection, whereas the biomarkers for liver (D and E) and general organ injury (F) were measured at 12 and 36 h after infection. Data are from mice pretreated with Ctrl or KNG ASO (8 mice/group at each time point) and presented as box-and-whisker plots showing interquartile ranges and medians. sE-selectin, soluble E-selectin; cVCAM-1, soluble vascular cell adhesion molecule-1; TATc, thrombin-antithrombin complexes; ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; LDH, lactate dehydrogenase. *P < 0.05, Mann-Whitney U-test.
Table 2.
Plasma cytokine and chemokine levels in noninfected and 12- and 36-h infected mice with Ctrl ASO or KNG ASO pretreatment
|
Time 0 |
Time 12 h |
Time 36 h |
||||
|---|---|---|---|---|---|---|
| Cytokines and Chemokines, pg/ml | Ctrl ASO | KNG ASO | Ctrl ASO | KNG ASO | Ctrl ASO | KNG ASO |
| IL-6 | ND | ND | 479 (384–908) | 368 (163–524) | 7,786 (3,294–15,780) | 5,492 (2,830–13,710) |
| TNF-α | 23 (ND–37.75) | 62 (7–75) | 68 (15–94) | 20 (1.6–44) | 921 (685–3,298) | 938 (622–2,163) |
| IL-10 | ND | ND | 4 (ND–19) | ND | 567 (313–1,038) | 300 (46–769) |
| MCP-1 | 1,058 (924–1,319) | 1,433 (1,317–2,118) | 1,307 (1,096–1,811) | 1,501 (1,201–1,612) | 9,804 (5,422–100,000) | 16,648 (8,209–100,000) |
| IFN-γ | 15 (14–20) | 15 (13–18) | 19 (17–22) | 14 (13–16) | 254 (65–2,109) | 343 (161–1,004) |
Values are medians (interquartile ranges). MCP-1, monocyte chemoattractant protein-1; IFN, interferon-γ; ND, not detectable, used for those groups if more than one-half was not detectable. The highest concentration for depicting the standard curve was 10,000 pg/ml. MCP-1 data were extrapolated until 100,000 pg/ml.
Impact of genetic kininogen deficiency on the host response during gram-negative pneumosepsis.
Considering that ASO treatment did not completely suppress production of HK (Fig. 1), we next sought to confirm essential data obtained in KNG ASO-treated mice in Kng1−/− mice. For this we infected WT and Kng1−/− mice with Klebsiella and determined bacterial loads and several host response readouts 36 h after infection. As expected, HK could not be detected in plasma of Kng1−/−mice (Fig. 6A). WT and Kng1−/− mice displayed similar neutrophil influx in the lung (Fig. 6B) and bacterial burdens in all body sites (Fig. 6C). In addition, genetic KNG deficiency did not affect lung cytokine and chemokine levels (Table 3), plasma soluable E-selectin, TATc, and parameters of hepatocellular and general cell injury (Table 4).
Fig. 6.
Kininogen deficiency does not influence neutrophil influx in lungs or bacterial growth or dissemination during pneumonia-derived sepsis. Data are from wild-type (WT) and Kng1−/− mice (8 mice/group) at 36 h after infection and presented as box-and-whisker plots showing interquartile ranges and medians. A: plasma HK level of WT and Kng1−/− mice indicated by Western blot after SDS-PAGE under reducing conditions. B: percentage of Ly6G-positive area in the lung. C: bacterial loads (CFUs) in the lung, blood, liver, and spleen at 36 h after infection with K. pneumoniae in WT and Kng1−/− mice. Differences between groups were not significant with Mann-Whitney U-test.
Table 3.
Lung cytokine, chemokine, and myeloperoxidase levels during pneumonia-derived sepsis in Kng1−/− and wild-type mice
|
Time 36 h |
||
|---|---|---|
| Lung Levels | Wild-type mice | Kng1−/− mice |
| TNF-α, pg/ml | 11,352 (9,255–18,981) | 11,744 (7,998–18,879) |
| IL-1β, pg/ml | 679 (363–1,569) | 552 (463–2,406) |
| IL-6, pg/ml | 11,072 (6,131–13,875) | 8,375 (5,385–14,420) |
| CXCL1, pg/ml | 24,000 (12,377–29,970) | 11,553 (10,487–19,457) |
| CXCL2, pg/ml | 16,612 (10,715–18,839) | 13,024 (11,575–19,696) |
| MPO, ng/ml | 5,926 (5,581–7,165) | 5,428 (5,031–5,735) |
Values are medians (interquartile ranges). MPO, myeloperoxidase. Mann-Whitney U-tests were performed for comparisons between groups (n = 8 mice/group). Differences between groups were not significant.
Table 4.
Plasma levels of markers of cellular injury and activation of coagulation and endothelial cells during pneumonia-derived sepsis in Kng1−/− and wild-type mice
|
Time 36 h |
||
|---|---|---|
| Plasma Levels | Wild-type mice (n = 8) | Kng1−/− mice (n = 8) |
| ASAT, U/ml | 616 (96–1,933) | 105 (66–2,120) |
| ALAT, U/ml | 468 (143–1,562) | 72 (44–790) |
| LDH, U/ml | 732 (272–2,395) | 358 (165–2,206) |
| TATc, pg/ml | 763 (176–3,104) | 1,247 (321–2,789) |
| sE-selectin, ng/ml | 47 (31–59) | 45 (28–67) |
Values are medians (interquartile ranges). ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase; LDH, lactate dehydrogenase; TATc, thrombin-antithrombin complexes; sE-selectin, soluble E-selectin. Mann-Whitney U-tests were performed for comparisons between groups. Differences between groups were not significant.
Kininogen is required to stabilize plasma PKK levels.
HK circulates as a carrier protein of FXI and PKK, and previous studies showed a correlation between HK (or KNG) deficiency and reduced PKK/FXI level in humans (7, 12) and animals. A previous in vitro study showed that binding with HK can protect plasma kallikrein from inactivation by complement 1inhibitor (26). To obtain an insight into the effect of KNG on PKK levels in vivo, we measured plasma PKK in KNG ASO-treated WT mice and Kng1−/− mice. Both KNG ASO-treated and Kng1−/− mice had much lower plasma PKK levels compared with their respective controls. In the naïve noninfected condition, plasma PKK levels of KNG ASO-treated mice and Kng1−/− were ~40% of the PKK levels in the Ctrl ASO group and WT group, respectively. Similarly, at 36 h after infection, PKK plasma levels were reduced to ~40% (KNG ASO relative Ctrl ASO) and ~5% (Kng1−/− vs. WT) (Fig. 7, A and B). Plasma FXI procoagulant activity was not different between infected Kng1−/− and WT mice (Fig. 7C).
DISCUSSION
HK occupies a central role in the kallikrein-kinin system that can exert strong proinflammatory and vascular effects at least in part through bradykinin, a peptide liberated from HK by PK. We hypothesized that HK is involved in the host response during sepsis originating from the lungs caused by the common human pathogen K. pneumoniae. In contrast to our expectations, we here show that KNG inhibition or deficiency did not importantly modify antibacterial, inflammatory, procoagulant, or vascular responses in a model that enables studies on both the early protective (local) immune response in the lungs as well as the late injurious (local and systemic) consequences of innate immune activation.
The role of the kallikrein-kinin system in bacterial infections likely is janus faced. Excessive activation of the contact system, such as during fulminant experimental sepsis, contributes to hypotension and organ injury (23). However, in the early phase of host-pathogen interaction, the kallikrein-kinin system can contribute to protective immunity. HK is upregulated during infection (30), a finding confirmed in our present study. Proteolytic products of HK can exert direct antimicrobial activity (13), and a peptide derived from HK had a protective effect in a mouse sepsis model (19). In addition, kinins are chemotactic and enhance the migration of neutrophils (22), and bradykinin triggers alveolar macrophages to release neutrophil chemotactic substances (25). Although these previously described antimicrobial and chemotactic effects mediated by HK may improve local host defense during bacterial pneumonia, bacterial growth in lungs was not altered by either KNG ASO treatment or genetic KNG deficiency. In KNG ASO-treated mice we detected lower lung levels of the neutrophil-attracting chemokines CXCL1 and CXCL2, and diminished neutrophil activation (as reflected by lower MPO concentrations), but this did not modify bacterial burdens. Genetic KNG deficiency was not associated with altered CXC chemokine or neutrophil responses. While the discrepancy with data obtained in KNG ASO-treated mice cannot be explained with certainty, compensatory mechanisms present in genetically modified mice and/or the difference between complete and partial HK elimination may have been involved. Whatever the reason, the combined KNG ASO and Kng1−/− results strongly argue against a role for KNG in antibacterial defense during K. pneumoniae infection.
Endothelial cell activation, disturbed barrier function of the vascular endothelium, and activation of the coagulation system are hallmark features of sepsis (2, 20). HK may enhance vascular permeability through generation of bradykinin (28). Although the role of FXII in thrombus formation has been documented in many studies (6), recent data suggest that HK also contributes to thrombin generation. Indeed, Kng1−/− mice demonstrated delayed thrombus formation in a model of carotid artery thrombosis (17), were protected from stroke induced by nylon filament injury of the middle cerebral artery (15), and showed reduced cancer-associated pulmonary embolism (18). In experimental stroke models, Kng1−/−mice also showed less severe blood-brain barrier damage and reduced local inflammation and edema (15). Together, these data suggested a role for KNG in the endothelial cell and hemostatic response during pneumonia-derived sepsis. However, such a role was not revealed in either KNG ASO-treated or Kng1−/− mice. KNG ASO-treated mice even had higher plasma E-selectin levels, indicating enhanced endothelial cell activation at 36 h after infection, although this was not confirmed in Kng1−/− mice. Similarly, neither inhibition nor elimination of KNG expression influenced the extent of distant organ injury. Thus, our data strongly suggest that KNG does not contribute to systemic injurious host responses in this experimental gram-negative pneumosepsis.
PKK and HK circulate in plasma as a bimolecular complex (5). Previous studies documented much lower PKK levels in patients with hereditary KNG deficiency (7, 12). Accordingly, we here report reduced PKK plasma levels in mice with inhibited or absent KNG expression. HK-PK complex formation has been implicated in FXII-independent activation of the kallikrein-kinin system (28). HK-PK complexes can assemble on the surface of the vascular endothelium, with HK serving as the binding site and cofactor of PKK activation, resulting in the formation of PK (27). This alternative (FXII-independent) route of contact system activation is considered to occur constitutively and to mediate basal bradykinin formation (28). Although at present the function of PKK in this model of gram-negative sepsis remains to be established, it is possible that the effects of KNG depletion are obscured by the consequentially reduced plasma PKK concentrations.
Our laboratory recently reported lower bacterial burdens and an improved survival in FXII KO mice in this same model of gram-negative pneumonia-derived sepsis by a mechanism that was independent of FXI (31). We therefore speculated that FXII exerts its detrimental effect via activating the HK-driven kallikrein-kinin system. The current results argue against this assumption. As such, further studies are warranted to determine how FXII negatively impacts host defense in this model.
The kallikrein-kinin system can mediate many inflammatory, vascular, and prothrombotic reactions. Because many of these are commonly activated during severe bacterial infection, we evaluated the role of KNG, a central mediator in the kallikrein-kinin system, in the host response to pneumonia-derived sepsis caused by a clinically relevant pathogen. By using two different approaches, inhibition of gene expression by treatment with a specific ASO and gene ablation, we show that KNG does not have a major part in either the early protective or the late detrimental host response.
GRANTS
C. Ding was supported by the State Scholarship Fund from the Chinese Scholarship Council.
DISCLOSURES
J. Crosby and A. S. Revenko are employees of Ionis Pharmaceuticals..
AUTHOR CONTRIBUTIONS
C.D., C.v.V., A.S.R., J.C., and T.v.d.P. conceived and designed research; C.D., C.v.V., J.J.T.H.R., and M.S. performed experiments; C.D., J.J.T.H.R., and M.S. analyzed data; C.D., C.v.V., M.S., K.R.M., A.S.R., J.C., and T.v.d.P. interpreted results of experiments; C.D. prepared figures; C.D. and T.v.d.P. drafted manuscript; C.D., C.v.V., K.R.M., A.S.R., J.C., and T.v.d.P. edited and revised manuscript; C.D., C.v.V., J.J.T.H.R., K.R.M., A.S.R., J.C., and T.v.d.P. approved final version of manuscript.
REFERENCES
- 1.Achouiti A, Vogl T, Urban CF, Röhm M, Hommes TJ, van Zoelen MA, Florquin S, Roth J, van ’t Veer C, de Vos AF, van der Poll T. Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS Pathog 8: e1002987, 2012. doi: 10.1371/journal.ppat.1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 369: 840–851, 2013. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 3.Baker BF, Lot SS, Condon TP, Cheng-Flournoy S, Lesnik EA, Sasmor HM, Bennett CF. 2′-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J Biol Chem 272: 11994–12000, 1997. doi: 10.1074/jbc.272.18.11994. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharjee G, Revenko AS, Crosby JR, May C, Gao D, Zhao C, Monia BP, MacLeod AR. Inhibition of vascular permeability by antisense-mediated inhibition of plasma kallikrein and coagulation factor 12. Nucleic Acid Ther 23: 175–187, 2013. doi: 10.1089/nat.2013.0417. [DOI] [PubMed] [Google Scholar]
- 5.Björkqvist J, Jämsä A, Renné T. Plasma kallikrein: the bradykinin-producing enzyme. Thromb Haemost 110: 399–407, 2013. doi: 10.1160/TH13-03-0258. [DOI] [PubMed] [Google Scholar]
- 6.Björkqvist J, Nickel KF, Stavrou E, Renné T. In vivo activation and functions of the protease factor XII. Thromb Haemost 112: 868–875, 2014. doi: 10.1160/TH14-04-0311. [DOI] [PubMed] [Google Scholar]
- 7.Colman RW, Bagdasarian A, Talamo RC, Scott CF, Seavey M, Guimaraes JA, Pierce JV, Kaplan AP. Williams trait. Human kininogen deficiency with diminished levels of plasminogen proactivator and prekallikrein associated with abnormalities of the Hageman factor-dependent pathways. J Clin Invest 56: 1650–1662, 1975. doi: 10.1172/JCI108247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Maat S, Tersteeg C, Herczenik E, Maas C. Tracking down contact activation—from coagulation in vitro to inflammation in vivo. Int J Lab Hematol 36: 374–381, 2014. doi: 10.1111/ijlh.12222. [DOI] [PubMed] [Google Scholar]
- 9.de Maat S, van Dooremalen S, de Groot PG, Maas C. A nanobody-based method for tracking factor XII activation in plasma. Thromb Haemost 110: 458–468, 2013. doi: 10.1160/TH12-11-0792. [DOI] [PubMed] [Google Scholar]
- 10.de Stoppelaar SF, van ’t Veer C, Claushuis TAM, Albersen BJA, Roelofs JJ, van der Poll T. Thrombocytopenia impairs host defense in gram-negative pneumonia-derived sepsis in mice. Blood 124: 3781–3790, 2014. doi: 10.1182/blood-2014-05-573915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Stoppelaar SF, Van’t Veer C, Roelofs JJ, Claushuis TAM, de Boer OJ, Tanck MWT, Hoogendijk AJ, van der Poll T. Platelet and endothelial cell P-selectin are required for host defense against Klebsiella pneumoniae-induced pneumosepsis. J Thromb Haemost 13: 1128–1138, 2015. doi: 10.1111/jth.12893. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson VH, Kleniewski J, Saito H, Sayed JK. Prekallikrein deficiency in a kindred with kininogen deficiency and Fitzgerald trait clotting defect. Evidence that high molecular weight kininogen and prekallikrein exist as a complex in normal human plasma. J Clin Invest 60: 571–583, 1977. doi: 10.1172/JCI108809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frick IM, Akesson P, Herwald H, Mörgelin M, Malmsten M, Nägler DK, Björck L. The contact system–a novel branch of innate immunity generating antibacterial peptides. EMBO J 25: 5569–5578, 2006. doi: 10.1038/sj.emboj.7601422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ 353: i1585, 2016. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 15.Langhauser F, Göb E, Kraft P, Geis C, Schmitt J, Brede M, Göbel K, Helluy X, Pham M, Bendszus M, Jakob P, Stoll G, Meuth SG, Nieswandt B, McCrae KR, Kleinschnitz C. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Blood 120: 4082–4092, 2012. doi: 10.1182/blood-2012-06-440057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long AT, Kenne E, Jung R, Fuchs TA, Renné T. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost 14: 427–437, 2016. doi: 10.1111/jth.13235. [DOI] [PubMed] [Google Scholar]
- 17.Merkulov S, Zhang WM, Komar AA, Schmaier AH, Barnes E, Zhou Y, Lu X, Iwaki T, Castellino FJ, Luo G, McCrae KR. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood 111: 1274–1281, 2008. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nickel KF, Ronquist G, Langer F, Labberton L, Fuchs TA, Bokemeyer C, Sauter G, Graefen M, Mackman N, Stavrou EX, Ronquist G, Renné T. The polyphosphate-factor XII pathway drives coagulation in prostate cancer-associated thrombosis. Blood 126: 1379–1389, 2015. doi: 10.1182/blood-2015-01-622811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oehmcke S, Shannon O, von Köckritz-Blickwede M, Mörgelin M, Linder A, Olin AI, Björck L, Herwald H. Treatment of invasive streptococcal infection with a peptide derived from human high-molecular weight kininogen. Blood 114: 444–451, 2009. doi: 10.1182/blood-2008-10-182527. [DOI] [PubMed] [Google Scholar]
- 20.Opal SM, van der Poll T. Endothelial barrier dysfunction in septic shock. J Intern Med 277: 277–293, 2015. doi: 10.1111/joim.12331. [DOI] [PubMed] [Google Scholar]
- 21.Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80: 629–661, 2016. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paegelow I, Trzeczak S, Böckmann S, Vietinghoff G. Migratory responses of polymorphonuclear leukocytes to kinin peptides. Pharmacology 66: 153–161, 2002. doi: 10.1159/000063797. [DOI] [PubMed] [Google Scholar]
- 23.Pixley RA, De La Cadena R, Page JD, Kaufman N, Wyshock EG, Chang A, Taylor FB Jr, Colman RW. The contact system contributes to hypotension but not disseminated intravascular coagulation in lethal bacteremia. In vivo use of a monoclonal anti-factor XII antibody to block contact activation in baboons. J Clin Invest 91: 61–68, 1993. doi: 10.1172/JCI116201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revenko AS, Gao D, Crosby JR, Bhattacharjee G, Zhao C, May C, Gailani D, Monia BP, MacLeod AR. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood 118: 5302–5311, 2011. doi: 10.1182/blood-2011-05-355248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato E, Koyama S, Nomura H, Kubo K, Sekiguchi M. Bradykinin stimulates alveolar macrophages to release neutrophil, monocyte, and eosinophil chemotactic activity. J Immunol 157: 3122–3129, 1996. [PubMed] [Google Scholar]
- 26.Schapira M, Scott CF, Colman RW. Protection of human plasma kallikrein from inactivation by C1 inhibitor and other protease inhibitors. The role of high molecular weight kininogen. Biochemistry 20: 2738–2743, 1981. doi: 10.1021/bi00513a006. [DOI] [PubMed] [Google Scholar]
- 27.Schmaier AH. Assembly, activation, and physiologic influence of the plasma kallikrein/kinin system. Int Immunopharmacol 8: 161–165, 2008. doi: 10.1016/j.intimp.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost 14: 28–39, 2016. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- 29.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801–810, 2016. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sriskandan S, Kemball-Cook G, Moyes D, Canvin J, Tuddenham E, Cohen J. Contact activation in shock caused by invasive group A Streptococcus pyogenes. Crit Care Med 28: 3684–3691, 2000. doi: 10.1097/00003246-200011000-00025. [DOI] [PubMed] [Google Scholar]
- 31.Stroo I, Zeerleder S, Ding C, Luken B, Roelofs JJTH, de Boer OJ, Meijers JCM, Castellino FJ, van ‘t Veer C, van der Poll T. Coagulation factor XI improves host defence during pneumonia-derived sepsis independent of factor XII activation. Thromb Haemostas 117: 1601–1614, 2017. doi: 10.1160/TH16-12-0920 . [DOI] [PubMed] [Google Scholar]
- 32.van Lieshout MHP, Anas AA, Florquin S, Hou B, van’t Veer C, de Vos AF, van der Poll T. Hematopoietic but not endothelial cell MyD88 contributes to host defense during gram-negative pneumonia derived sepsis. PLoS Pathog 10: e1004368, 2014. doi: 10.1371/journal.ppat.1004368. [DOI] [PMC free article] [PubMed] [Google Scholar]