Abstract
Previous studies have shown that muscle sympathetic nerve activity (MSNA) is reduced during low- and mild-intensity dynamic leg exercise. It has been suggested that such inhibition is mediated by loading of the cardiopulmonary baroreceptors and that this effect is overridden by muscle metaboreflex activation with higher-intensity exercise. However, limited data are available regarding the interaction between the cardiopulmonary baroreflex and the muscle metaboreflex. Therefore, we tested the hypothesis that cardiopulmonary baroreflex-mediated inhibition of MSNA is attenuated during high-intensity muscle metaboreflex activation. In nine young men, MSNA (right peroneal nerve), mean arterial pressure (MAP), and thoracic impedance were recorded. Graded isolation of muscle metaboreflex activation was achieved via postexercise ischemia (PEI) following low (PEI-L)-, moderate (PEI-M)-, and high (PEI-H)-intensity isometric handgrip performed at 20, 30, and 40% maximum voluntary contraction, respectively. Lower-body positive pressure (LBPP, +10 Torr) was applied at rest and during PEI, to load the cardiopulmonary baroreceptors. Handgrip exercise elicited intensity-dependent increases in MSNA and MAP that were maintained during PEI, indicating a graded muscle metaboreflex activation. LBPP at rest significantly decreased MSNA burst frequency (BF: −36.7 ± 4.7%, mean ± SE, P < 0.05), whereas MAP was unchanged. When LBPP was applied during PEI, MSNA BF decreased significantly at PEI-L (−40.0 ± 9.2%, P < 0.05) and PEI-M (−27.0 ± 6.3%, P < 0.05), but not at PEI-H (+1.9 ± 7.1%, P > 0.05). These results suggest that low- and moderate-intensity muscle metaboreflex activation does not modulate the inhibition of MSNA by cardiopulmonary baroreceptor loading, whereas high-intensity metaboreflex activation can override cardiopulmonary baroreflex-mediated inhibition of sympathetic vasomotor outflow.
NEW & NOTEWORTHY The interaction between the sympathoinhibitory influence of cardiopulmonary baroreflex and sympathoexcitatory effect of skeletal muscle metaboreflex is not completely understood. In the current study, light- to moderate-intensity muscle metaboreflex activation did not modulate the suppression of muscle sympathetic nerve activity by cardiopulmonary baroreceptor loading, whereas high-intensity muscle metaboreflex activation attenuated the cardiopulmonary baroreflex-mediated inhibition of muscle sympathetic nerve activity. These results provide important information concerning the neural reflex mechanisms regulating sympathetic vasomotor outflow during exercise.
Keywords: dynamic exercise, isometric handgrip, lower-body positive pressure, MSNA, postexercise ischemia
INTRODUCTION
An appropriate regulation of sympathetic vasomotor outflow is necessary during exercise to maintain arterial blood pressure (BP) and facilitate the delivery of blood flow to active muscles. Central command, a feedforward control mechanism, and the exercise pressor reflex, a feedback mechanism from metabolically (i.e., metaboreflex) and mechanically (i.e., mechanoreflex) sensitive skeletal muscle afferents, contribute to the exercise-induced increase in sympathetic vasomotor outflow (10, 12, 37) with further modulation provided by the arterial baroreflex. It has been demonstrated that muscle sympathetic nerve activity (MSNA) increases in proportion to exercise intensity when the exercise is static and performed using small muscle mass (i.e., isometric handgrip) (40, 44). However, if the exercise is dynamic (i.e., leg cycling) with light or mild intensity, MSNA decreases or does not change from that at rest (3, 19, 23, 24, 42). A recent study revealed that an enhanced muscle pump-induced increase in central blood volume (CBV) suppressed MSNA during leg cycling (23), indicating that loading of the cardiopulmonary baroreflex inhibits sympathetic vasomotor outflow during dynamic exercise. This finding is supported by previous studies that reported that increasing CBV, thereby loading of cardiopulmonary baroreceptors, suppressed the MSNA response (39) and reduced the magnitude of the exercise-induced increase in BP during dynamic exercise (32). These findings highlight the importance of the cardiopulmonary baroreflex during exercise.
Suppression of MSNA by cardiopulmonary baroreceptor loading does not occur in several circumstances, such as during prolonged or hypoxic exercise (21, 22, 38, 41). Additionally, MSNA increases during dynamic exercise above mild intensity (9, 19, 42). Likewise, increases in MSNA were observed during dynamic exercise when the circulation to the exercising muscle was occluded (12, 50). From these studies, it has been suggested that the sympathoinhibitory effect of the cardiopulmonary baroreflex could be modulated by a powerful sympathoexcitatory drive from the exercise pressor reflex (10, 12, 23, 44). However, limited studies have directly tested the modulatory influence of the skeletal muscle metaboreflex on the inhibition of MSNA via loading of cardiopulmonary baroreceptors.
Sanders and Ferguson (43) reported that the increased MSNA during sustained handgrip followed by postexercise ischemia (PEI) was not reduced after volume expansion with intravenous normal saline solution to load the cardiopulmonary baroreceptors. This result suggests that muscle metaboreflex control of sympathetic vasomotor outflow is not modulated by cardiopulmonary baroreflex loading. However, it should be noted that loading of the cardiopulmonary baroreceptors was constant and occurred before exercise and metaboreflex activation. Also, only moderate-intensity exercise and metaboreflex activation was achieved. Therefore, whether cardiopulmonary baroreflex control of sympathetic vasomotor outflow is affected by higher-intensity activation of the muscle metaboreflex remains unknown.
With this background in mind, we tested the hypothesis that cardiopulmonary baroreflex-mediated inhibition of MSNA is attenuated during high-intensity muscle metaboreflex activation. To test this hypothesis, we measured MSNA during PEI following three intensities of static handgrip to grade muscle metaboreflex activation from low to high. Lower-body positive pressure (LBPP) was applied at rest and during PEI to load the cardiopulmonary baroreceptors.
METHODS
Ethical Approval
All procedures conformed to the Declaration of Helsinki and were approved by the University of Texas at Arlington Institutional Review Board (no. 2016–0783). Each subject received a verbal and written explanation of the study objectives, measurement techniques, and the risks associated with the investigation and provided written informed consent before participation.
Subjects
Nine healthy men with a mean age of 23.7 ± 1.2 yr, height of 177.1 ± 1.9 cm, and weight of 76.8 ± 3.3 kg (means ± SE) were recruited for voluntary participation in the study. All subjects were studied ≥2 h postprandial, free of alcohol and exercise for 24 h and caffeine for 12 h. All subjects were free of any recognized pulmonary, cardiovascular, metabolic, or neurological disease, were nonsmokers, and not taking prescribed or over-the-counter medications. We chose to include men only since previous studies have shown that women exhibit differences in cardiopulmonary baroreflex control (4) and muscle metaboreflex activation (7) compared with men. Also, in women, muscle metaboreflex activation is influenced by variations in sex hormones (8).
Experimental Measurements and Instrumentation
Cardiovascular variables.
Heart rate (HR) was continuously monitored using a lead II surface electrocardiogram (ECG) (Q710; Quinton, Bothell, WA). Systolic (SBP) and diastolic (DBP) BP were measured on a beat-to-beat basis using servo-controlled finger photoplethysmography (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands) placed on the middle finger of the left hand and supported on a bedside table positioned at the level of the heart. Additionally, to validate absolute BP measurements from the Finometer, an automated sphygmomanometer (Welch Allyn, Skaneateles Falls, NY) recorded baseline BP every minute on the brachial artery of the right arm. Thoracic impedance was recorded using noninvasive impedance cardiography (model 304B; Minnesota Impedance Cardiograph; Instrumentation for Medicine, Greenwich, CT), and changes in thoracic admittance (1/thoracic impedance) were used as an index of changes in CBV (2, 32). Previous studies have indicated that thoracic admittance provides an accurate measure of changes in CBV compared with central venous pressure (2, 6). The impedance device that was used in the present study (model 304B; Minnesota Impedance Cardiograph) has been used extensively to measure changes in thoracic impedance and estimate central blood volume (4, 5, 32, 33, 45). Respiratory movements were monitored using a strain-gauge pneumobelt placed in a stable position around the abdomen (Pneumotrace; UFI, Morro Bay, CA).
MSNA.
Multiunit postganglionic MSNA was recorded using standard microneurographic techniques, as previously described (18, 48, 49). Briefly, a unipolar tungsten microelectrode was inserted in muscle fascicles of the peroneal nerve near the fibular head of the right leg. Neural signals were amplified, filtered (bandwidth, 700–2,000 Hz), rectified, and integrated (time constant, 0.1 s) to obtain mean voltage neurograms (University of Iowa Bioengineering, Iowa City, IA). MSNA recordings were identified by their pulse synchronous burst pattern and increased burst frequency to an end-expiratory breath hold without any responses to arousal or skin stroking.
Experimental Techniques
LBPP.
LBPP (+10 Torr) was applied to increase CBV and load the cardiopulmonary baroreceptors. The subject’s lower body distal to the iliac crest was sealed in a customized Plexiglas and composite wood pressure chamber (16, 36). Pressure was increased within the LBPP chamber by manually controlling variable autotransformers that regulated vacuum motors that blew into the LBPP chamber. To minimize the potential movement of the MSNA electrodes, chamber pressure was elevated gradually with the duration to achieve +10 Torr being ~1 min. The pressure inside the chamber was measured continuously by a pressure transducer (DP45 Pressure Transducer and CD15 Carrier Demodulator; Validyne Engineering, Northridge, CA) interfaced with PowerLab. LBPP has been used in a number of studies to load the cardiopulmonary baroreflex (16, 36, 46, 51), making it an ideal stimulus to mimic the loading of the cardiopulmonary baroreceptors as previously observed during cycling exercise (23, 32).
Maximal voluntary contraction and sustained handgrip exercise.
A handgrip dynamometer (model 56380; Stoelting, Wood Dale, IL) was used to establish the maximal voluntary contraction (MVC) and to perform sustained handgrip exercise in the right arm. MVC was determined as the highest force produced during three to five maximal efforts, each separated by ~1.5–2 min. During isometric handgrip exercise, visual feedback of the handgrip force exerted by the subject, expressed as a percentage of maximum (20, 30, or 40%), was displayed on a computer screen that was positioned at eye level of the subject.
Postexercise ischemia.
Postexercise ischemia (PEI) was used to isolate activation of the muscle metaboreflex control of sympathetic vasomotor outflow from central command and the muscle mechanoreflex (12, 18, 26). An occlusion cuff placed over the right upper arm was inflated to suprasystolic pressure (240 mmHg) 5 s before the cessation of handgrip exercise and maintained for 3.5 min (D. E. Hokanson, Bellevue, WA).
Experimental Protocols
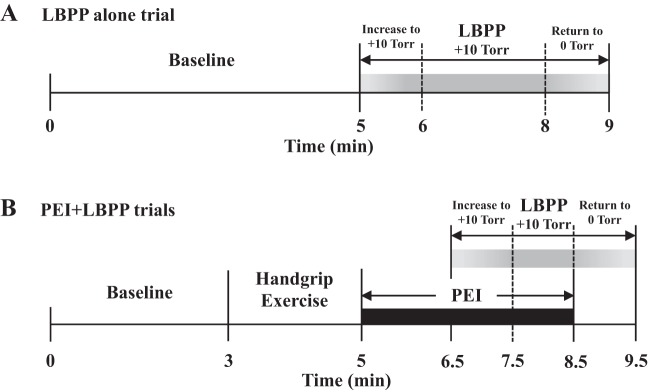
Experiments were performed in a climate-controlled (21–22°C) dimly lit quiet room. Subjects were placed in a semirecumbent position with the lower body sealed in the LBPP box at the level of the iliac crest. The arms were extended laterally and supported by bedside tables. Once a quality MSNA recording site was found, the LBPP box was closed and sealed. The initial LBPP alone trial was then performed (Fig. 1A). Following a minimum 5-min baseline period in which the subjects rested quietly, chamber pressure was elevated to +10 Torr and maintained for 2 min. The chamber pressure was then returned to 0 Torr over a duration of 1 min followed by recovery. For analysis (see below), only the 1st min of LBPP was used to compare with LBPP applied during PEI. Next, subjects performed three PEI + LBPP trials separated by ~15 min to ensure the reestablishment of baseline variables before commencing the subsequent trial (Fig. 1B). Baseline measurements were performed for 3 min after which the subjects carried out isometric handgrip exercise at 20% for 2 min. Ratings of perceived exertion (RPE) were obtained using the standard 6–20 Borg scale (1) immediately after exercise. Before the cessation of exercise (5 s), a cuff was inflated on the exercising arm to suprasystolic pressure (PEI: isolated muscle metaboreflex) for 3.5 min. After 1.5 min of PEI, chamber pressure was gradually increased to +10 Torr and maintained for 1 min. Next, the chamber pressure was slowly returned to 0 Torr followed by recovery (PEI-L). The same procedures were then followed for 30 and 40% MVC isometric exercise and PEI (PEI-M and PEI-H). The PEI + LBPP trials were not administered in random order because of the greater risk of losing the MSNA signal with higher-intensity handgrip. Therefore, the LBPP alone trial was repeated after the last PEI + LBPP trial to confirm similar responses to the first trial.
Fig. 1.
Schematic diagram of the experimental protocol. A: lower-body positive pressure (LBPP) alone trial. After a 5-min baseline, LBPP was gradually increased to +10 Torr (~1 min) and maintained. B: postexercise ischemia (PEI) + LBPP trials. After a 3-min baseline, handgrip exercise was performed for 2 min followed by PEI. After 1.5 min of PEI, LBPP was gradually increased to +10 Torr and maintained for 1 min. The same protocol was repeated for low-, medium-, and high-intensity handgrip exercise.
Data Acquisition and Analysis
MSNA, HR, mean arterial pressure (MAP), impedance, respiration rate, and chamber pressure were sampled at 1,000 Hz and stored for off-line analysis (PowerLab; AD Instruments, Bella Vista, NSW, Australia). MSNA bursts were identified from the mean voltage neurogram using a customized LabVIEW program employing fixed criteria, which accounted for the latency from the R wave of the ECG to the sympathetic burst (11) and incorporated a signal-to-noise ratio of at least 3:1 (17). Computer-identified bursts were subsequently evaluated and confirmed by an experienced microneurographer. For burst amplitude, the three largest bursts during baseline were assigned a mean value of 100 arbitrary units (AU), and all MSNA burst amplitudes were expressed as a percentage of this value (18). MSNA burst frequency (BF; in bursts/min), burst incidence (BI; in bursts/100 HR), and total activity (TA; i.e., burst frequency × mean burst amplitude, in AU/min) were calculated. All baseline data were averaged over 3-min periods. Variables during LBPP, exercise, PEI, and PEI + LBPP were averaged during 1 min. To compare the changes in variables among the four trials (LBPP alone, PEI-L, PEI-M, and PEI-H trials) during LBPP, the absolute and percent changes in variables were calculated as the difference from baseline to LBPP in the LBPP alone trial and from PEI to PEI + LBPP in the PEI + LBPP trials.
Statistical Analysis
Values are expressed as means ± SE. For all data, the assumption of normal distribution was examined using a Kolmogorov-Smirnov test. Changes in parameters during the LBPP alone trial (baseline vs. LBPP) were performed using paired t-test (parametric test) when the distribution was normal. When the distribution was not normal, a Wilcoxon test (nonparametric test) was used. A one-way repeated-measures ANOVA was used to evaluate the changes in variables during each PEI + LBPP trial. When a significant time effect was found, comparisons of parameters between the sessions (baseline, exercise, PEI, and PEI + LBPP) were performed using the Bonferroni test. The comparisons of parameters among the trials (e.g., LBPP alone, PEI-L, PEI-M, and PEI-H) were also examined by one-way repeated-measures ANOVA and Bonferroni test. Statistical significance was set at P < 0.05. Analyses were performed with StatView (5.0; SAS Institute, Tokyo, Japan) and SPSS (11.5; SPSS, Tokyo, Japan) statistical packages.
RESULTS
Cardiovascular and MSNA Responses to Graded Handgrip and PEI
Representative recordings of MSNA at rest and during handgrip and PEI are shown in Fig. 2, and mean values of HR, MAP, and MSNA at each session are provided in Table 1. HR, BP, and MSNA increased significantly during sustained handgrip exercise, with the magnitude of increase proportional to exercise intensity. RPE values during handgrip were also higher with increasing exercise intensity (PEI-L, 10.3 ± 0.4; PEI-M, 14.3 ± 0.4; PEI-H, 17.7 ± 0.4, F = 104.0, P < 0.05). During PEI trials, SBP, DBP, and MAP remained elevated, whereas HR returned to baseline values (Table 1). The increases in BP during PEI were graded from PEI-L to PEI-H. Likewise, MSNA BF and MSNA TA remained elevated during PEI in a graded fashion.
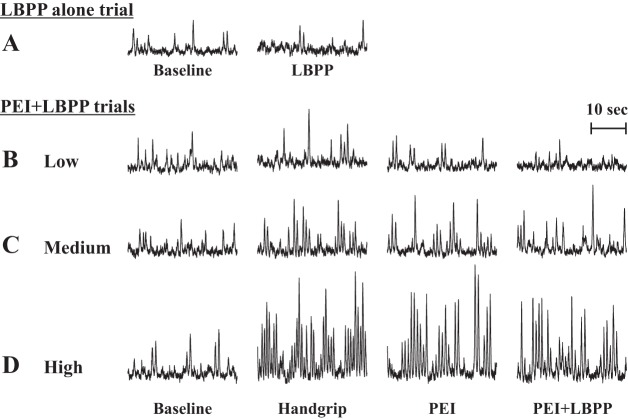
Fig. 2.
Original recordings of muscle sympathetic nerve activity (MSNA) in one individual at baseline followed by lower-body positive pressure (LBPP) (A) and at baseline, during low (B)-, medium (C)-, and high (D)-intensity isometric handgrip followed by postexercise ischemia (PEI), and PEI combined with LBPP (PEI + LBPP). The low, medium, and high intensities were 20, 30, and 40% maximal voluntary contraction, respectively.
Table 1.
Average hemodynamic parameters during baseline, isometric handgrip, PEI, and PEI combined with LBPP for low-, medium-, and high-intensity isometric handgrip
| Sessions |
||||
|---|---|---|---|---|
| Exercise Intensity | Baseline | Handgrip | PEI | PEI + LBPP |
| SBP, mmHg | ||||
| Low | 130 ± 3 | 140 ± 3* | 138 ± 3* | 139 ± 3* |
| Medium | 130 ± 3 | 148 ± 3*† | 150 ± 3*† | 152 ± 4*† |
| High | 131 ± 3 | 161 ± 2*‡ | 168 ± 4*‡ | 168 ± 5*‡ |
| DBP, mmHg | ||||
| Low | 74 ± 2 | 81 ± 2* | 80 ± 2* | 79 ± 2* |
| Medium | 73 ± 2 | 87 ± 2*† | 87 ± 2*† | 88 ± 2*† |
| High | 73 ± 1 | 96 ± 2*‡ | 96 ± 2*‡ | 95 ± 2*‡ |
| Thoracic admittance, S × 10−4 | ||||
| Low | 384.8 ± 10.0 | 383.9 ± 10.0 | 385.9 ± 10.1 | 391.7 ± 10.1*¶ |
| Medium | 384.9 ± 9.9 | 384.0 ± 9.8 | 386.6 ± 10.0 | 392.1 ± 9.8*¶ |
| High | 383.9 ± 9.8 | 381.9 ± 9.5 | 386.2 ± 9.8 | 391.9 ± 9.9*¶ |
| MSNA TA, AU/min | ||||
| Low | 861.1 ± 80.7 | 1,157.0 ± 145.7* | 1,077.2 ± 71.8* | 612.6 ± 95.9¶ |
| Medium | 954.4 ± 65.7 | 2,135.0 ± 279.7*† | 1,867.6 ± 362.1*† | 1,207.8 ± 240.2*¶† |
| High | 823.9 ± 112.4 | 3,289.3 ± 532.7*‡ | 2,896.7 ± 446.4*‡ | 2,509.3 ± 377.1*‡ |
| MSNA BI, bursts/100 HR | ||||
| Low | 25.3 ± 3.6 | 29.0 ± 3.8 | 31.3 ± 3.5 | 16.9 ± 3.1*¶ |
| Medium | 24.0 ± 1.7 | 41.4 ± 3.4*† | 41.1 ± 3.1*† | 28.1 ± 3.1¶† |
| High | 24.8 ± 1.9 | 50.8 ± 5.0*‡ | 51.7 ± 5.8*‡ | 49.5 ± 4.5*‡ |
Values are means ± SE. PEI, postexercise ischemia; LBPP, lower-body negative pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MSNA TA, muscle sympathetic nerve activity total activity; MSNA BI, muscle sympathetic nerve activity bursts incidence; AU, arbitrary units; HR, heart rate.
P < 0.05 vs. baseline.
P < 0.05 vs. PEI.
P < 0.05, low vs. medium.
P < 0.05, medium vs. high.
Cardiovascular and MSNA Responses to LBPP Alone and During PEI
During the application of LBPP, HR and MAP (baseline 92 ± 3 mmHg; LBPP 91 ± 2 mmHg, P > 0.05) did not change. This was consistent for when LBPP was applied at rest and during PEI (Table 1 and Fig. 3). In contrast, MSNA TA (baseline 980.1 ± 85.7 AU/min; LBPP 590.7 ± 63.6 AU/min, P < 0.05) and MSNA BF decreased significantly during the LBPP alone trials (Fig. 3). Likewise, in the PEI + LBPP trials, MSNA decreased significantly with LBPP during PEI-L and PEI-M. However, no significant reduction in MSNA was found with LBPP during PEI-H (Fig. 3). The magnitude of change in MSNA BF (Fig. 4B) and percent changes in MSNA BF and MSNA TA with LBPP during PEI-H were smaller (P < 0.05) than those in LBPP alone, PEI-L, and PEI-M (MSNA BF: LBPP alone, −36.7 ± 4.7%; PEI-L, −40.0 ± 9.2%; PEI-M, −27.0 ± 6.3%; PEI-H, +1.9 ± 7.1%, F = 10.9, P < 0.05; MSNA TA: LBPP alone, −38.6 ± 5.1%; PEI-L, −40.7 ± 9.5%, PEI-M, −35.1 ± 7.6%; PEI-H, −12.1 ± 7.6%, F = 5.9, P < 0.05). Thoracic admittance increased significantly during LBPP in all trials, and there were no significant differences in the magnitude of increase in thoracic admittance among the four trials (Fig. 4A).
Fig. 3.
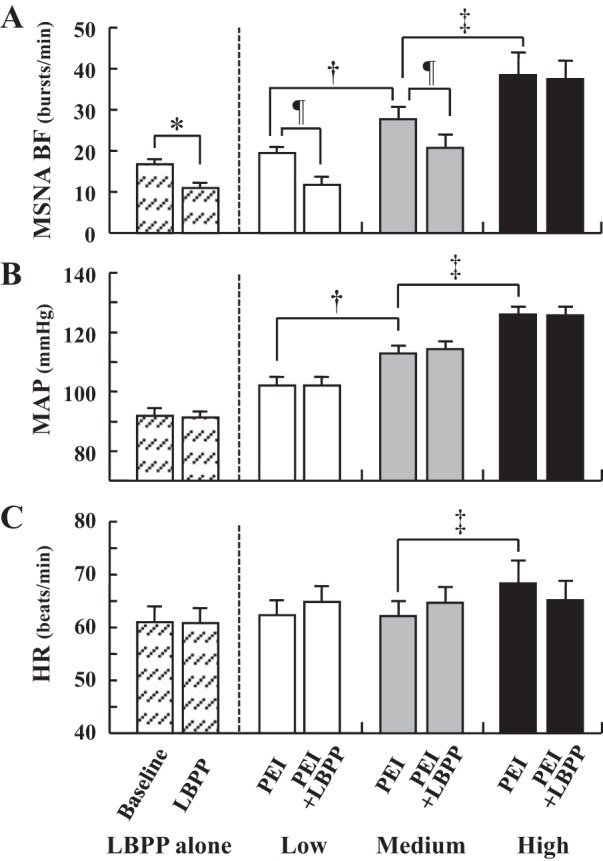
Mean summary data for muscle sympathetic nerve activity burst frequency (MSNA BF, A), mean arterial pressure (MAP, B), and heart rate (HR, C) during lower-body positive pressure (LBPP) at baseline (hatched bars) and during postexercise ischemia (PEI) following low (white bars)-, medium (gray bars)-, and high (black bars)-intensity isometric handgrip, and PEI combined with LBPP (PEI + LBPP). *P < 0.05, baseline vs. LBPP in LBPP alone trial. †P < 0.05, low-intensity PEI vs. medium-intensity PEI. ‡P < 0.05, medium-intensity PEI vs. high-intensity PEI. ¶P < 0.05 PEI vs. PEI + LBPP.
Fig. 4.
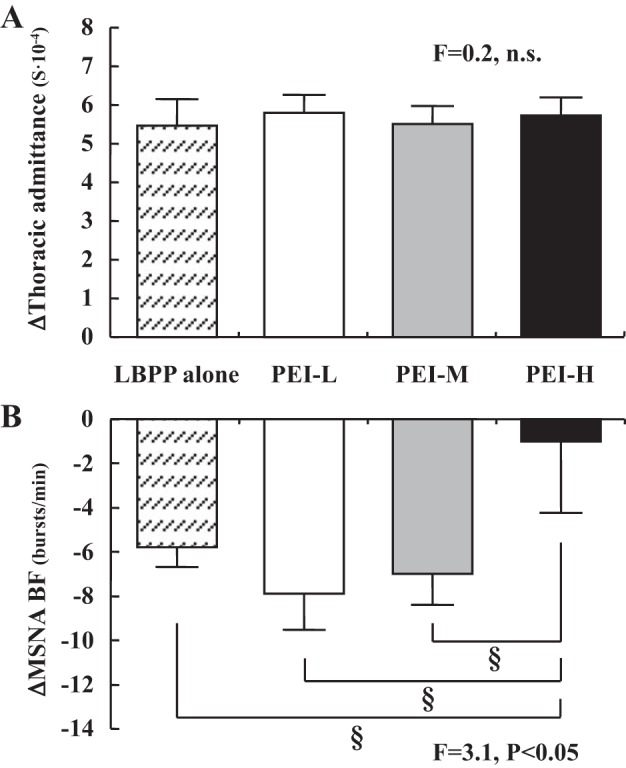
Absolute changes in thoracic admittance (A) and muscle sympathetic nerve activity burst frequency (MSNA BF, B) in response to lower-body positive pressure (LBPP) applied at rest (hatched bars), LBPP applied during postexercise ischemia (PEI) following low (PEI-L; white bars)-, medium (PEI-M; gray bars)-, and high (PEI-H; black bars)-intensity isometric handgrip. §P < 0.05.
Comparison of LBPP Alone Trials
There were no significant differences in the magnitude of decrease in MSNA BF or TA between the LBBP alone trial performed at the beginning of the protocol compared with the trial at the end of the protocol (BF: first trial, −5.8 ± 1.0 burst/min vs. second trial, −6.0 ± 0.9 burst/min, P = 0.95; MSNA TA: first trial, −38.6 ± 5.1% baseline vs. second trial, −40.6 ± 5.1% baseline, P = 0.68). Likewise, the increase in thoracic admittance was not different between LBPP alone trials (first trial, +5.5 ± 0.7 S × 10−4 vs. second trial, +5.1 ± 0.6 S × 10−4, P = 0.59).
DISCUSSION
The novel finding of the present study is that high-intensity muscle metaboreflex activation overrides the cardiopulmonary baroreflex-mediated inhibition of MSNA. These data support our hypothesis that the inhibitory effect of loading the cardiopulmonary baroreflex on sympathetic vasomotor outflow could be overridden when the magnitude of muscle metaboreflex control of MSNA is high. Our results provide important information concerning the neural reflex mechanisms controlling sympathetic vasomotor outflow during exercise.
There are ample data indicating that the cardiopulmonary baroreflex inhibits MSNA, particularly at low- and mild-intensity dynamic exercise (i.e., leg cycling) (3, 19, 23, 24, 39, 42). Because MSNA increases when dynamic exercise is performed above moderate intensity and exercise duration is prolonged, it has been suggested that the sympathoinhibitory influence of the cardiopulmonary baroreflex is overridden by the sympathoexcitatory effect of the exercise pressor reflex. Herein, we provide clear evidence of an interaction between the cardiopulmonary baroreflex and the metabolic component of the exercise pressor reflex (i.e., muscle metaboreflex). Indeed, cardiopulmonary baroreflex-mediated inhibition of MSNA present during PEI-L and PEI-M was overridden by high-intensity muscle metaboreflex activation (PEI-H) (Figs. 3 and 4B). To our knowledge, only one study by Sanders and Ferguson (43) attempted to examine an interaction between metaboreflex activation using PEI and loading of cardiopulmonary baroreceptors using volume expansion with intravenous saline solution. These investigators reported that the cardiopulmonary baroreceptors had no effect on MSNA responses during 30% MVC handgrip or during PEI. Notably, in their study, the cardiopulmonary baroreceptors were loaded before handgrip began, and only a single moderate exercise intensity was used. Thus, in the present study, to comprehensively examine the interaction between exercise-induced metaboreflex activation and loading of cardiopulmonary baroreceptors, we used PEI after three intensities of isometric handgrip (i.e., 20, 30, and 40% MVC) and then applied LBPP during isolated activation of the muscle metaboreflex with PEI. This approach identified clear cardiopulmonary baroreflex-mediated inhibition of MSNA during PEI following low- and moderate-intensity handgrip. However, with high-intensity muscle metaboreflex activation, LBPP had little effect on sympathetic vasomotor outflow, demonstrating that the muscle metaboreflex can override cardiopulmonary baroreflex-mediated inhibition of MSNA when the magnitude of the metabolic stimulus is high. These findings indicate that the effect of the cardiopulmonary baroreflex on efferent sympathetic responses to muscle metaboreflex activation is dependent on exercise intensity. This could explain the response of MSNA to graded-intensity dynamic exercise in which low- and mild-intensity dynamic exercise inhibits MSNA; however, as exercise intensity increases, this inhibition disappears, and graded sympathoexcitation occurs (20).
In the present study, we took advantage of previous work showing that +10 Torr LBPP increases central venous pressure and CBV, leading to a decrease in MSNA with no changes in MAP, indicating selective loading of the cardiopulmonary baroreceptors (15, 46). Indeed, consistent with these findings, we found that +10 Torr LBPP at rest induced an increase in thoracic admittance and a decrease in MSNA (Fig. 4) similar to that observed previously (15). Although our results and those of others (15, 46) indicate selective loading of the cardiopulmonary baroreflex with +10 LBPP, a potential influence of LBPP on the arterial baroreceptors cannot be completely ruled out (14). Nevertheless, important to our study design, +10 Torr LBPP-induced increases in thoracic admittance were consistent when applied at rest and during PEI, indicating a similar stimulus to the cardiopulmonary baroreflex among all trials (Fig. 4A).
Although the precise central mechanisms by which high-intensity muscle metaboreflex activation overrides the inhibition of MSNA by the cardiopulmonary baroreflex are not clear, some general discussion is warranted. Briefly, stimulation of mechanically sensitive stretch receptors located in the chambers of the heart, great veins, and blood vessels of the lungs, which comprise the cardiopulmonary baroreflex, activate vagal afferent fibers (10, 25). These vagal afferents innervate within the nucleus tractus solitarius (NTS) of the medulla oblongata, a major central integration site for cardiovascular regulation (12, 29). Indeed, both mechanically and metabolically sensitive skeletal muscle afferents also synapse in the NTS. Signals processed within the NTS are transmitted to both the caudal ventrolateral medulla and rostral ventrolateral medulla (RVLM), which cause sympathoinhibition and sympathoexcitation, respectively. Given the commonality of the central neural pathways for the cardiopulmonary baroreflex and the exercise pressor reflex, one possibility is that the expected increase in the excitability of cardiopulmonary sensitive NTS neurons, caused by the exercise-induced increase in CBV, is offset by the activation of an inhibitory neural circuit within the NTS by feedback from skeletal muscle afferents during high-intensity muscle metaboreflex activation. Thus, in the present study, we would speculate that the influence of cardiopulmonary loading on NTS neurons is counteracted by a metaboreceptor-activated inhibitory neural circuit when the activation of the muscle metaboreflex is high. A similar model has been proposed by Dr. Jeff Potts (35) for resetting of the arterial baroreflex during exercise. Given that the exercise pressor reflex also has direct projections to the RVLM (12, 29), it is also possible that, during high-intensity muscle metaboreflex activation, direct input into the RVLM simply overrides cardiopulmonary baroreflex inhibitory signals arising from the NTS. Future studies are needed to examine the central interactions between the exercise pressor reflex and the cardiopulmonary baroreflex.
Perspectives and Significance
The combined data in previous studies and the present one demonstrate that the cardiopulmonary baroreflex plays an important modulatory role in the neural cardiovascular responses to exercise at light and moderate intensities. Importantly, our current findings demonstrate, for the first time, that high-intensity muscle metaboreflex activation can override the inhibitory influence of the cardiopulmonary baroreflex on MSNA. In addition, input from the cardiopulmonary baroreceptors influences arterial baroreflex resetting and function during dynamic exercise (32). Therefore, an impaired cardiopulmonary baroreflex could lead to an exaggerated increase in sympathetic vasomotor outflow and consequent larger increase in blood pressure during exercise (10, 44). In this regard, previous studies have demonstrated that, in patients with heart failure, the cardiopulmonary baroreflex is impaired at rest (27, 28). Although not directly tested, the impaired cardiopulmonary baroreflex could be one of the potential mechanisms for abnormal hemodynamic and sympathoexcitatory responses to exercise in these patients (10, 13). Indeed, Notarius et al. (31) reported that, during mild to moderate dynamic one-legged cycling, MSNA decreased in healthy subjects, whereas MSNA increased in heart failure patients. Thus, an impaired cardiopulmonary baroreflex-mediated inhibition (30, 34, 47) may be a possible mechanism for exaggerated MSNA responses during exercise in heart failure patients. Additionally, recent findings have demonstrated that the muscle metaboreflex is augmented in type 2 diabetes patients (18). As such, heightened muscle metaboreflex activation in this patient population may override the sympathoinhibitory effect of cardiopulmonary baroreflex, particularly at light- to moderate-intensity exercise. Further research is needed to clarify the role of the cardiopulmonary baroreflex and its interaction with the muscle metaboreflex to better understand the exaggerated MSNA and pressor responses to exercise observed in various disease populations.
Conclusion
In summary, these results demonstrate that light- to moderate-intensity muscle metaboreflex activation does not modulate the inhibition of MSNA by cardiopulmonary baroreceptor loading, whereas high-intensity metaboreflex activation can override cardiopulmonary baroreflex-mediated inhibition of sympathetic vasomotor outflow. Thus, the effect of cardiopulmonary baroreceptor loading on efferent sympathetic responses to muscle metaboreflex activation is dependent on exercise intensity.
GRANTS
This study was supported by JSPS KAKENHI Grant No. JP16KK0201 and National Heart, Lung, and Blood Institute Grant R01-HL-127071.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.K. and P.J.F. conceived and designed research; K.K., J.K., B.E.Y., T.C.B., and P.J.F. performed experiments; K.K., B.E.Y., P.J.F., analyzed data; K.K., J.K., B.E.Y., T.C.B., S.O., and P.J.F. interpreted results of experiments; K.K., J.K., and P.J.F. drafted manuscript; K.K., J.K., B.E.Y., T.C.B., S.O., and P.J.F. edited and revised manuscript; K.K., J.K., B.E.Y., T.C.B., S.O., and P.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. D. M. Keller, R. M. Brothers, and J. R. Vranish (University of Texas at Arlington) for insight and assistance for these studies.
REFERENCES
- 1.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Cai Y, Holm S, Jenstrup M, Strømstad M, Eigtved A, Warberg J, Højgaard L, Friberg L, Secher NH. Electrical admittance for filling of the heart during lower body negative pressure in humans. J Appl Physiol (1985) 89: 1569–1576, 2000. doi: 10.1152/jappl.2000.89.4.1569. [DOI] [PubMed] [Google Scholar]
- 3.Callister R, Ng AV, Seals DR. Arm muscle sympathetic nerve activity during preparation for and initiation of leg-cycling exercise in humans. J Appl Physiol (1985) 77: 1403–1410, 1994. doi: 10.1152/jappl.1994.77.3.1403. [DOI] [PubMed] [Google Scholar]
- 4.Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 275: R1909–R1920, 1998. doi: 10.1152/ajpregu.1998.275.6.R1909. [DOI] [PubMed] [Google Scholar]
- 5.Convertino VA, Doerr DF, Ludwig DA, Vernikos J. Effect of simulated microgravity on cardiopulmonary baroreflex control of forearm vascular resistance. Am J Physiol Regul Integr Comp Physiol 266: R1962–R1969, 1994. doi: 10.1152/ajpregu.1994.266.6.R1962. [DOI] [PubMed] [Google Scholar]
- 6.Ebert TJ, Smith JJ, Barney JA, Merrill DC, Smith GK. The use of thoracic impedance for determining thoracic blood volume changes in man. Aviat Space Environ Med 57: 49–53, 1986. [PubMed] [Google Scholar]
- 7.Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol (1985) 80: 245–251, 1996. doi: 10.1152/jappl.1996.80.1.245. [DOI] [PubMed] [Google Scholar]
- 8.Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, Sinoway LI. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol (1985) 85: 2075–2081, 1998. doi: 10.1152/jappl.1998.85.6.2075. [DOI] [PubMed] [Google Scholar]
- 9.Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 280: H1383–H1390, 2001. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- 10.Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97: 39–50, 2012. doi: 10.1113/expphysiol.2011.057554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. J Neurol Sci 47: 433–448, 1980. doi: 10.1016/0022-510X(80)90098-2. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol 5: 475–512, 2015. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- 13.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54: 375–385, 2009. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 14.Fu Q, Shibata S, Hastings JL, Prasad A, Palmer MD, Levine BD. Evidence for unloading arterial baroreceptors during low levels of lower body negative pressure in humans. Am J Physiol Heart Circ Physiol 296: H480–H488, 2009. doi: 10.1152/ajpheart.00184.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Q, Sugiyama Y, Kamiya A, Shamsuzzaman AS, Mano T. Responses of muscle sympathetic nerve activity to lower body positive pressure. Am J Physiol Heart Circ Physiol 275: H1254–H1259, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher KM, Fadel PJ, Smith SA, Norton KH, Querry RG, Olivencia-Yurvati A, Raven PB. Increases in intramuscular pressure raise arterial blood pressure during dynamic exercise. J Appl Physiol (1985) 91: 2351–2358, 2001. doi: 10.1152/jappl.2001.91.5.2351. [DOI] [PubMed] [Google Scholar]
- 17.Hamner JW, Taylor JA. Automated quantification of sympathetic beat-by-beat activity, independent of signal quality. J Appl Physiol (1985) 91: 1199–1206, 2001. doi: 10.1152/jappl.2001.91.3.1199. [DOI] [PubMed] [Google Scholar]
- 18.Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am J Physiol Heart Circ Physiol 310: H300–H309, 2016. doi: 10.1152/ajpheart.00636.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichinose M, Saito M, Fujii N, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during incremental leg cycling. J Physiol 586: 2753–2766, 2008. doi: 10.1113/jphysiol.2007.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichinose M, Saito M, Kitano A, Hayashi K, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during mild orthostatic stress in humans. J Physiol 557: 321–330, 2004. doi: 10.1113/jphysiol.2003.057133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katayama K, Ishida K, Iwamoto E, Iemitsu M, Koike T, Saito M. Hypoxia augments muscle sympathetic neural response to leg cycling. Am J Physiol Regul Integr Comp Physiol 301: R456–R464, 2011. doi: 10.1152/ajpregu.00119.2011. [DOI] [PubMed] [Google Scholar]
- 22.Katayama K, Ishida K, Saito M, Koike T, Ogoh S. Hypoxia attenuates cardiopulmonary reflex control of sympathetic nerve activity during mild dynamic leg exercise. Exp Physiol 101: 377–386, 2016. doi: 10.1113/EP085632. [DOI] [PubMed] [Google Scholar]
- 23.Katayama K, Ishida K, Saito M, Koike T, Hirasawa A, Ogoh S. Enhanced muscle pump during mild dynamic leg exercise inhibits sympathetic vasomotor outflow. Physiol Rep 2: e12070, 2014. doi: 10.14814/phy2.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katayama K, Iwamoto E, Ishida K, Koike T, Saito M. Inspiratory muscle fatigue increases sympathetic vasomotor outflow and blood pressure during submaximal exercise. Am J Physiol Regul Integr Comp Physiol 302: R1167–R1175, 2012. doi: 10.1152/ajpregu.00006.2012. [DOI] [PubMed] [Google Scholar]
- 25.Mancia G, Mark A. Arterial baroreflexes in humans. In: Handbook of Physiology. The Cardiovascular System. Circulation. Bethesda, MD: Am Physiol Soc, 1983, p. 755–793. doi: 10.1002/cphy.cp020320. [DOI] [Google Scholar]
- 26.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. doi: 10.1161/01.RES.57.3.461. [DOI] [PubMed] [Google Scholar]
- 27.Modesti PA, Polidori G, Bertolozzi I, Vanni S, Cecioni I. Impairment of cardiopulmonary receptor sensitivity in the early phase of heart failure. Heart 90: 30–36, 2004. doi: 10.1136/heart.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohanty PK, Arrowood JA, Ellenbogen KA, Thames MD. Neurohumoral and hemodynamic effects of lower body negative pressure in patients with congestive heart failure. Am Heart J 118: 78–85, 1989. doi: 10.1016/0002-8703(89)90075-6. [DOI] [PubMed] [Google Scholar]
- 29.Mueller PJ, Clifford PS, Crandall CG, Smith SA, Fadel PJ. Integration of central and peripheral regulation of the circulation during exercise: acute and chronic adaptations. Compr Physiol 8: 103–151, 2017. doi: 10.1002/cphy.c160040. [DOI] [PubMed] [Google Scholar]
- 30.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol 280: H969–H976, 2001. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- 31.Notarius CF, Millar PJ, Murai H, Morris BL, Marzolini S, Oh P, Floras JS. Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age-matched healthy subjects. J Physiol 593: 715–722, 2015. doi: 10.1113/jphysiol.2014.281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogoh S, Fisher JP, Fadel PJ, Raven PB. Increases in central blood volume modulate carotid baroreflex resetting during dynamic exercise in humans. J Physiol 581: 405–418, 2007. doi: 10.1113/jphysiol.2006.125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parry MJ, McFetridge-Durdle J. Ambulatory impedance cardiography: a systematic review. Nurs Res 55: 283–291, 2006. doi: 10.1097/00006199-200607000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation 93: 940–952, 1996. doi: 10.1161/01.CIR.93.5.940. [DOI] [PubMed] [Google Scholar]
- 35.Potts JT. Inhibitory neurotransmission in the nucleus tractus solitarii: implications for baroreflex resetting during exercise. Exp Physiol 91: 59–72, 2006. doi: 10.1113/expphysiol.2005.032227. [DOI] [PubMed] [Google Scholar]
- 36.Potts JT, Shi X, Raven PB. Cardiopulmonary baroreceptors modulate carotid baroreflex control of heart rate during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 268: H1567–H1576, 1995. doi: 10.1152/ajpheart.1995.268.4.H1567. [DOI] [PubMed] [Google Scholar]
- 37.Raven PB, Chapleau MW. Blood pressure regulation XI: overview and future research directions. Eur J Appl Physiol 114: 579–586, 2014. doi: 10.1007/s00421-014-2823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray CA. Muscle sympathetic nerve responses to prolonged one-legged exercise. J Appl Physiol (1985) 74: 1719–1722, 1993. doi: 10.1152/jappl.1993.74.4.1719. [DOI] [PubMed] [Google Scholar]
- 39.Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol Heart Circ Physiol 264: H1–H7, 1993. doi: 10.1152/ajpheart.1993.264.1.H1. [DOI] [PubMed] [Google Scholar]
- 40.Saito M, Mano T, Abe H, Iwase S. Responses in muscle sympathetic nerve activity to sustained hand-grips of different tensions in humans. Eur J Appl Physiol Occup Physiol 55: 493–498, 1986. doi: 10.1007/BF00421643. [DOI] [PubMed] [Google Scholar]
- 41.Saito M, Sone R, Ikeda M, Mano T. Sympathetic outflow to the skeletal muscle in humans increases during prolonged light exercise. J Appl Physiol (1985) 82: 1237–1243, 1997. doi: 10.1152/jappl.1997.82.4.1237. [DOI] [PubMed] [Google Scholar]
- 42.Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol (1985) 75: 663–667, 1993. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- 43.Sanders JS, Ferguson DW. Cardiopulmonary baroreflexes fail to modulate sympathetic responses during isometric exercise in humans: direct evidence from microneurographic studies. J Am Coll Cardiol 12: 1241–1251, 1988. doi: 10.1016/0735-1097(88)92607-1. [DOI] [PubMed] [Google Scholar]
- 44.Seals DR. Cardiopulmonary baroreflexes do not modulate exercise-induced sympathoexcitation. J Appl Physiol (1985) 64: 2197–2203, 1988. doi: 10.1152/jappl.1988.64.5.2197. [DOI] [PubMed] [Google Scholar]
- 45.Sherwood A, McFetridge J, Hutcheson JS. Ambulatory impedance cardiography: a feasibility study. J Appl Physiol (1985) 85: 2365–2369, 1998. doi: 10.1152/jappl.1998.85.6.2365. [DOI] [PubMed] [Google Scholar]
- 46.Shi X, Crandall CG, Raven PB. Hemodynamic responses to graded lower body positive pressure. Am J Physiol Heart Circ Physiol 265: H69–H73, 1993. doi: 10.1152/ajpheart.1993.265.1.H69. [DOI] [PubMed] [Google Scholar]
- 47.Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol (1985) 84: 1551–1559, 1998. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- 48.Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 49.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012. doi: 10.1152/ajpheart.01105.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Victor RG, Seals DR. Reflex stimulation of sympathetic outflow during rhythmic exercise in humans. Am J Physiol Heart Circ Physiol 257: H2017–H2024, 1989. doi: 10.1152/ajpheart.1989.257.6.H2017. [DOI] [PubMed] [Google Scholar]
- 51.Williamson JW, Raven PB, Whipp BJ. Unaltered oxygen uptake kinetics at exercise onset with lower-body positive pressure in humans. Exp Physiol 81: 695–705, 1996. doi: 10.1113/expphysiol.1996.sp003970. [DOI] [PubMed] [Google Scholar]