Abstract
Sympathetically induced vasoconstrictor modulation of local vasodilation occurs in contracting skeletal muscle during exercise to ensure appropriate perfusion of a large active muscle mass and to maintain also arterial blood pressure. In this synthesis, we discuss the contribution of group III-IV muscle afferents to the sympathetic modulation of blood flow distribution to locomotor and respiratory muscles during exercise. This is followed by an examination of the conditions under which diaphragm and locomotor muscle fatigue occur. Emphasis is given to those studies in humans and animal models that experimentally changed respiratory muscle work to evaluate blood flow redistribution and its effects on locomotor muscle fatigue, and conversely, those that evaluated the influence of coincident limb muscle contraction on respiratory muscle blood flow and fatigue. We propose the concept of a “two-way street of sympathetic vasoconstrictor activity” emanating from both limb and respiratory muscle metaboreceptors during exercise, which constrains blood flow and O2 transport thereby promoting fatigue of both sets of muscles. We end with considerations of a hierarchy of blood flow distribution during exercise between respiratory versus locomotor musculatures and the clinical implications of muscle afferent feedback influences on muscle perfusion, fatigue, and exercise tolerance.
Keywords: blood flow, diaphragm, exercise, metaboreflex, sympathetic vasoconstriction, work of breathing
INTRODUCTION
In this synthesis, we focus on the competition for available blood flow during exercise between respiratory and locomotor muscles. We make the case that fatigue-producing deficits in blood flow to these two sets of muscles result to a significant extent from the selective influences of heightened sympathetic vasoconstriction arising principally from metaboreceptors in both the limb locomotor and respiratory muscles. We first review the established occurrence of sympathetically mediated vasoconstriction in locomotor muscles during exercise. This is followed by an examination of the conditions under which diaphragm and locomotor muscle fatigue occur. Emphasis is placed on the influence of experimentally altering respiratory muscle work on limb fatigue and blood flow distribution, and conversely, the influence of exercising locomotor muscles on respiratory muscle blood flow and fatigue. We conclude with consideration of whether respiratory muscles have a “priority” over locomotor muscles for blood flow distribution during exercise and discuss the implications of this “two-way street” of sympathetic activation for exercise limitation in chronic disease states.
EVIDENCE IN SUPPORT OF EXERCISE-INDUCED SYMPATHETIC VASOCONSTRICTION IN LIMB LOCOMOTOR MUSCLES
Regulation of blood flow to contracting skeletal muscle represents the net effect of local metabolic-induced vasodilation versus sympathetically induced vasoconstriction: each of these influences with a homeostatic purpose. The former serves O2 transport primarily, while the latter is required to preserve an adequate perfusion pressure because of the substantial vasodilator capacity of contracting skeletal muscle, if left unopposed (7, 67, 84). During low-intensity exercise, O2 delivery via local vasodilation is prioritized, while during higher intensity whole body exercise blood pressure regulation and therefore vasoconstriction increases in priority. The vasoconstriction is confined primarily to larger vessels while constriction in smaller vessels is blunted, thereby permitting total muscle blood flow to be limited but distributed more optimally (18).
There are several lines of evidence in support of significant sympathetic vasoconstrictor influences over local vasodilation in muscle during exercise in healthy humans, including the following examples: 1) increases in locomotor muscle vascular conductance and blood flow that occur via α-adrenergic blockade in rhythmically exercising humans and dogs (16, 44, 75); 2) the compromised leg vascular conductance and blood flow observed at near maximal and maximal exercise during two-legged cycling but not during one-legged knee extension exercise (55); 3) the reduced vascular conductance and flow in the vasculature of the arms and trunk relative to the legs during two-legged cycling at- and near-maximal work rates (17, 38); and 4) the specific preferential effects of training of the arms, which increased peak exercise work rate with the arms and increased the share of cardiac output to the arms, while maintaining the share of total flow to the trunk and reducing it to the legs: the latter achieved presumably via an enhanced sympathetic vasoconstriction (15).
Evidence documenting increased sympathetic activity to locomotor muscles during exercise includes an increase in norepinephrine spillover across the working muscle (68, 84). More direct evidence of exercise-induced muscle sympathetic tone is found in the significant increases above resting levels observed in directly recorded median nerve muscle sympathetic nerve activity (MSNA) in the resting arm during two-legged cycling exercise requiring >40% of maximal oxygen uptake (V̇o2max) (37, 66). Potential sources of exercise-induced sympathetic vasoconstrictor tone to contracting muscle include “central command” feed-forward mechanisms, which rise in parallel with central motor command to contracting limbs, as well as feedback control from muscle afferents, baroreceptors, and chemoreceptors (43, 75, 76).
EXERCISE-INDUCED FATIGUE OF RESPIRATORY AND LOCOMOTOR MUSCLES
Muscular fatigue is defined as a temporary condition in which there is a decrease in the capacity of muscle force generation that is reversible by rest (58). In health, ventilation is capable of being maintained during high-intensity endurance exercise at levels approximating 20–25 times resting levels. This requires increases in force output by the diaphragm and many accessary inspiratory muscles, which approximate 60–90% of their dynamic capacity for force generation (11, 41). The oxygen cost of the sustained ventilation during exercise approximates 7–10% of maximal O2 consumption (V̇o2max) in the untrained healthy subject (V̇o2max: 40–50 ml·kg−1·min−1) and 13–16% in the highly trained male subject (V̇o2max: >60 ml·kg−1·min−1) (2, 27) and upwards of 20% in some endurance-trained women (27). Diaphragm contractile strength [i.e., transdiaphragmatic twitch pressure (Pdi,tw) in response to stimulation of the phrenic nerves] progressively decreases during sustained high-intensity endurance exercise to exhaustion and remains depressed for an hour or more of recovery (8, 11, 40, 49, 85). In addition, there appears to be a strong relationship between diaphragm force output during exercise and the magnitude of fatigue suggesting that diaphragm fatigue is intrinsically linked to the amount of generated pressure and work performed (11). Accordingly, when diaphragm work was markedly reduced (via mechanical ventilation, see below), exercise-induced diaphragm fatigue was prevented (10). It is important to highlight that blood flow is a major determinant of diaphragmatic fatigue where it has been shown that diaphragm fatigue can be partially reversed by increasing phrenic artery blood flow (77).
Neither lower intensity sustained exercise (11, 40, 49) nor short duration incremental exercise to maximum elicits diaphragm fatigue (40, 47, 65). However, diaphragm fatigue does occur at lower exercise intensities of sustained exercise when the work of breathing is elevated and/or arterial hypoxemia is present as occurs in chronic obstructive pulmonary disease (COPD) (12, 48) or in healthy subjects exercising in hypoxic environments (4, 9, 81). Fatigue of the abdominal expiratory muscles has also been documented using supramaximal magnetic stimulation over the vertebral column between the 8th and the 11th thoracic vertebrae after sustained heavy-intensity exercise to exhaustion (79). Locomotor muscle fatigue has also been detected under conditions of sustained high-intensity exercise as determined via pre- versus postexercise comparisons of quadriceps force output achieved via supramaximal stimulation of the femoral nerve (3). As with the diaphragm, hypoxemia also exacerbates the rate of development of locomotor muscle fatigue in health and in COPD (5, 6). Limited evidence in elderly sedentary subjects showed that sustained submaximal cycling exercise to exhaustion resulted in significant leg locomotor muscle fatigue without coincident fatigue of the diaphragm (47).
MECHANICALLY UNLOADING THE WORK OF BREATHING INFLUENCES LOCOMOTOR MUSCLE BLOOD FLOW/FATIGUE
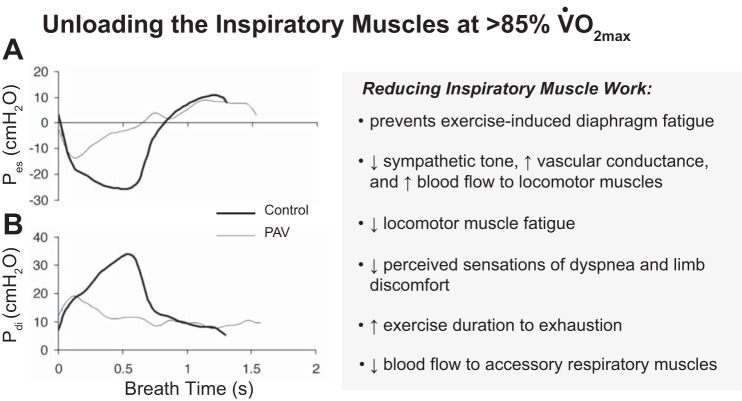
The effects of respiratory muscle work on blood flow distribution to and fatigue of the respiratory and locomotor muscles have been studied by artificially altering the work of the inspiratory muscles during exercise. Although studies involving both increases and decreases in respiratory muscle work have been conducted, it is the “unloading” studies that have yielded the most relevant information to the physiologic state, because this procedure quantifies the effects of preventing most of the normally observed exercise-induced increase in respiratory muscle work. Thus a series of experiments examined the effects of the normally occurring respiratory muscle work during high-intensity exercise by reducing the work of breathing by 50–70% with a proportional assist ventilator in healthy, fit subjects (see Fig. 1). Three major effects occurred: 1) diaphragm fatigue was prevented, thereby unmasking the work of the diaphragm as a critical determinant of its own exercise-induced fatigue (10); 2) locomotor muscle fatigue at end exercise was reduced by 25–30% below control (64), and in moderate hypoxic environments in health (4), or in COPD patients in normoxia (5), unloading the respiratory muscles elicited significant reductions in locomotor muscle fatigue even during exercise at only 50–70% of V̇o2max; and 3) exercise duration to exhaustion was prolonged 10–20% in healthy subjects in normoxia (34) or acute hypoxia (4) and in patients with congestive heart failure (CHF) (59). Subjective ratings of perceived dyspnea and leg discomfort, at equivalent time points and work rates, were also significantly reduced via respiratory muscle unloading (34, 64). These powerful effects of respiratory muscle unloading were primarily attributed to coincident changes in blood flow. Thus, with respiratory muscle unloading, limb vascular conductance and blood flow were increased significantly despite a coincident reduction in stroke volume and cardiac output (32, 33). Conversely, increasing the resistive load on the respiratory muscles reduced limb vascular conductance and blood flow and reduced endurance exercise time (32–34). These cardiovascular effects of respiratory muscle unloading and loading only occurred during heavy-intensity exercise in healthy individuals (60, 88). The fact that intense levels of respiratory muscle work are required to elicit limb vasoconstriction in health was also demonstrated in resting humans breathing with progressive, voluntary increases in respiratory work. Reductions in limb vascular conductance and blood flow occurred only when inspiratory muscle work achieved fatiguing levels of force output (70, 71). However, in heart failure patients with an elevated work of breathing during even submaximal exercise (secondary to reduced lung compliance and hyperventilation), respiratory muscle unloading caused 50 to 60% increases in limb vascular conductance and limb blood flow, which were out of proportion to the coincident increase in cardiac output (60).
Fig. 1.
Summary of the effects of unloading the inspiratory muscles during heavy-intensity exercise. Shown are representative within-breath esophageal (A: Pes) and transdiphragmatic pressure-time (B: Pdi) traces for 1 subject ensemble averaged over 1 min during spontaneous breathing (control) and proportional assist ventilation (PAV) at exercise iso-time. Note that with the use of PAV the esophageal and Pdi during inspiration were reduced by 50–70% vs control. Tracings from Romer et al. (64).
Further support for this concept of respiratory muscle work influencing limb locomotor muscle vascular conductance and blood flow was also found: 1) in a canine model of heart failure subjected to respiratory muscle loading and unloading during exercise (54), 2) in the increased locomotor muscle oxygenation with respiratory muscle unloading observed during heavy-intensity exercise in CHF patients (14), and 3) in the tendency for exercising limb V̇o2 to plateau rather than continue to increase at heavy exercise intensities in selected COPD patients who experienced the highest levels of respiratory muscle work (72).
A RESPIRATORY MUSCLE METABOREFLEX LIKELY EXPLAINS THE SYSTEMIC CARDIOVASCULAR EFFECTS OF CHANGING THE WORK OF BREATHING
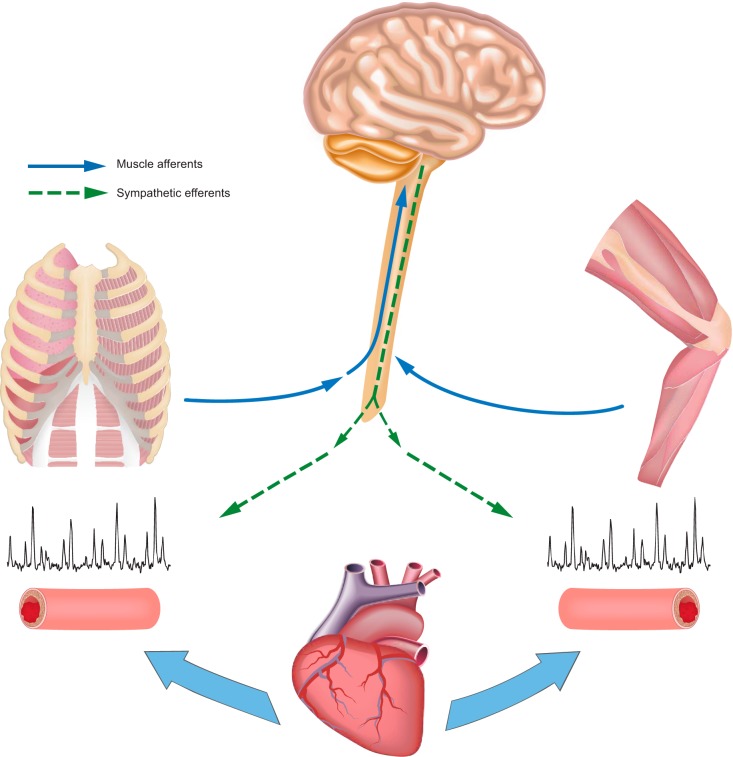
The consistent changes in norepinephrine spillover across the exercising limb when respiratory muscle work was altered during heavy exercise clearly implicates sympathetically mediated vasomotor effects (32, 88). This effect of a changing respiratory muscle work on sympathetically mediated vasoconstriction in the limb was recently confirmed and extended with the demonstration during two-legged cycling that directly measured MSNA in the median nerve of the resting forearm was reduced with respiratory muscle unloading and rose with respiratory muscle loading (26). Most evidence (as summarized below) suggests that a metaboreflex originating in the inspiratory and/or expiratory muscles is a major source of this sympathoexcitation. This feedback effect from the respiratory musculature is analogous to the way in which group III-IV afferents in limb muscles elicit increased sympathetic vasoconstriction to mediate the “pressor response” to rhythmic exercise (46).
It is well established that a majority of fibers in the phrenic nerve are sensory in function. Lightly myelinated group III mechanoreceptors and unmyelinated group IV metaboreceptors in the diaphragm have been shown to project to supraspinal centers in the brainstem involved in cardiorespiratory control and even higher to the cerebellum, hypothalamus, and somatosensory cortex (28, 57). Hussain and colleagues (36) used an anesthetized dog preparation to demonstrate that various types of stimulation of thin fiber phrenic afferents (e.g., lactic acid, capsaicin) elevated widespread systemic vasoconstriction that was absent when the phrenic nerve was sectioned. In anesthetized rodents, Hill (35) and Jammes and Balzamo (39) reported increased diaphragmatic metaboreceptor activity coincident with the onset of diaphragm fatigue in the anesthetized rodent. In resting and submaximally exercising dogs, Rodman et al. (63) caused limb muscle vasoconstriction and reduced blood flow and increased mean arterial pressure by infusing lactic acid solutions into the diaphragm via the phrenic artery. These systemic cardiovascular effects of diaphragmatic acidosis were prevented via adrenergic blockade meaning that the respiratory muscle metaboreflex contributed to an increased sympathetic tone and redistribution of blood flow during exercise. In humans, St. Croix et al. (74) and Derchak et al. (24) showed that steady-state MSNA in the resting limb was not responsive to voluntary increases in either central respiratory motor output or tidal volume but did show a time-dependent increase in MSNA frequency with increasing inspiratory or expiratory muscle work carried out to the point of task failure. Katayama et al. (45) also reported a significant increase in median nerve sympathetic vasomotor outflow elicited via voluntary increases in expiratory muscle work during leg cycling.
In summary, we interpret the available findings to mean that the sympathetically mediated vasoconstriction invoked via the respiratory muscle metaboreflex during the course of fatiguing exercise is sufficiently robust to overcome a significant portion of the powerful local vasodilator influence operative in near maximally contracting locomotor muscle. Thus adding or subtracting respiratory muscle work during high-intensity limb exercise has an influence on limb muscle vascular conductance and blood flow, which is analogous to that achieved via the addition of leg to arm work or to the difference between one vs two-legged exercise (see summary above).
COMPETITION FOR AVAILABLE BLOOD FLOW: A TWO-WAY STREET!
The above evidence on changes in limb vascular conductance and flow plus the coincident effects on limb fatigue with respiratory muscle loading and unloading might be interpreted to mean that during heavy exercise in humans distribution of cardiac output may preferentially prioritize the respiratory over the limb muscle vasculature (23, 60, 67), thereby negatively impacting fatigability of the locomotor muscles (64). On the other hand (as summarized below), there is also evidence supporting a significant influence of limb locomotor muscle activity on respiratory muscle blood flow and fatigue. Figure 2 shows a schematic of our view of this “two-way street” of metaboreceptor influences on blood flow distribution under conditions of heavy exercise.
Fig. 2.
Schematic of a “2-way street of sympathetic vasoconstrictor activity” emanating from both limb and respiratory muscle metaboreceptors during exercise, which serves to constrain blood flow and O2 transport. High-intensity contractions of both sets of muscles causes increased group III and IV afferent activity leading to a sympathetically mediated vasoconstriction of respiratory and limb locomotor muscle vasculatures, thereby contributing to their mutual fatigue during whole body exercise.
Sympathetic Vasoconstrictor Effects on Locomotor versus Respiratory Muscle Vasculature
First, in vivo studies in anesthetized canines reveal certain conditions in which substantial vasoconstrictor sympathetic tone exists in the diaphragm vasculature. When the force of diaphragm contractions was increased via resistive airway loading, diaphragm vascular conductance fell by 70–80% during superimposed high-frequency electrical stimulation of aortic baroreceptor depressor nerves (22). Diaphragm vasoconstriction also occurred with intravenously administered α-adrenoreceptor stimuli (36, 78). On the other hand, dose responses of isolated second order arterioles showed similar vasodilator response sensitivities (i.e., to isoproterenol) but markedly less vasoconstrictor response sensitivity to norepinephrine (or phenylephrine) in the predominantly high-oxidative, slow-twitch diaphragm versus either predominantly red or white gastrocnemius muscle arterioles (1). This blunted norepinephrine-induced vasoconstrictor response in the diaphragm versus limb muscle vasculature is similar to that observed in the coronary vasculature (42) and is not attributable to differences in either oxidative capacity or fiber type of the muscle (1). An as yet unexplored explanation may be found in fewer α1-adrenergic receptors in the arteriolar smooth muscle of the diaphragm vasculature.
Muscle Vasodilator Reserve in Maximal-Intensity Exercise?
In maximally exercising equines diaphragm and locomotor muscle vasculatures achieved similar, marked reductions in vascular resistance and increased blood flow rates (>300 ml·min−1·100 g−1). With adenosine infusion vascular resistance was unchanged in the diaphragm but fell an additional 20% in locomotor muscle (52). In humans, femoral arterial infusion of ATP during high-intensity cycling increased leg vascular conductance, but decreased O2 extraction, indicating the importance of vasoconstriction to ensure blood flow distribution to the most activated muscles (19). In dogs performing mild and moderate treadmill exercise, the greatest increase in perfusion occurred in the diaphragm relative to the heart or locomotor muscles (29). Resistance fell significantly in the muscle groups involved with exercise (i.e., heart, diaphragm, intercostal, and gastrocnemius) and increased in the renal and intestinal vasculatures. On the other hand, in the exercising rodent, blood flow and conductance through predominantly red muscle in the limbs exceeded that in the diaphragm by 25% (62). The blood flow to muscles traditionally labeled “inspiratory or expiratory” amounted to 15–16% of the total cardiac output in the equine at maximum exercise (51) (Fig. 3). Similar fractions of V̇o2max and cardiac output were reported for respiratory muscles at maximal exercise in fit humans (33). The latter was estimated by extrapolating the fall in cardiac output induced via respiratory muscle unloading during exercise, which agrees with the increase in cardiac output reported at rest while performing levels of hyperpnea achieved at maximal exercise (21, 83). These estimates of respiratory muscle blood flow and V̇o2max are similar to those based on measures of “trunk and head” flow using dye dilution cardiac output and thermodilution limb blood flow via catheterization of the subclavian and femoral veins (17). Figure 4 shows blood flow to the respiratory musculature using different experimental approaches and species. Of note is that despite the varied methodologies, a qualitative assessment indicates that in highly fit maximally exercising humans or ponies, the respiratory musculature commands ~14–20% of maximal or near-maximal cardiac output.
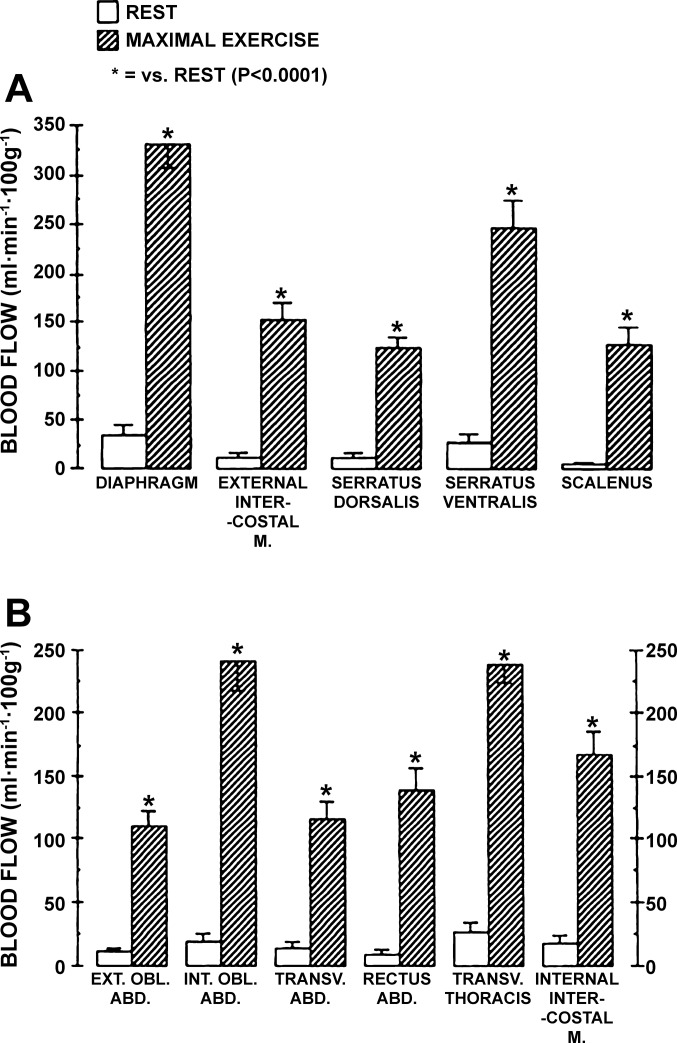
Fig. 3.
Blood flow to inspiratory (A) and expiratory (B) muscles of ponies at rest at rest and during maximal exercise. Data are from Manohar (51). EXT. OBL. ABD., external oblique abdominis; INT. OBL. ABD., internal oblique abdominis; TRANS. ABD., transverse abdominis; RECUTS ABD, rectus abdominis; TRANSV. THORACIS, transverse thoracis. Note, that without exception blood flow to the respiratory musculature during maximal exercise was significantly increased relative to resting conditions. Note that blood flow to the diaphragm exceeded 300 ml·min−1·100g−1; neither diaphragm flow or vascular conductance was increased further upon infusion of a vasodilator during maximal exercise.
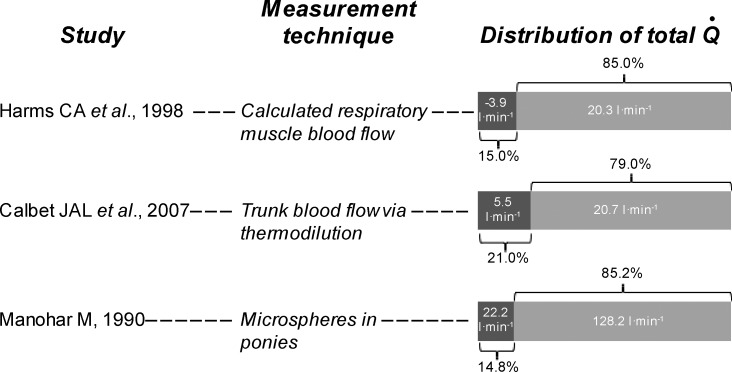
Fig. 4.
Respiratory muscle blood flow during maximal exercise. Shown are 3 distinct approaches to assessing respiratory muscle blood flow that yield comparable estimates of the fraction of cardiac output. Harms et al. (33) determined leg blood flow (thermodilution) during bouts of maximal exercise in trained cyclists (V̇o2max = 62 ml·kg−1·min−1). Respiratory muscle work was either unaltered (control condition), reduced (proportional assist ventilator), increased (resistive load). Blood flow to the respiratory muscles was assumed to be equal to a measured fall in cardiac output obtained with respiratory muscle unloading at V̇o2max and extrapolated to zero work of breathing. Calbet et al. (17) studied healthy active men (V̇o2max = 49 ml·kg−1·min−1; 3.7 l/min) who performed upright cycle exercise to exhaustion while blood flow to the arms and legs was determined (thermodilution). Blood flow to the “trunk” was calculated as the difference between cardiac output and arm and leg blood flow and included into a lumped parameter the blood flow directed to the head, neck, heart, abdominal viscera, kidneys, respiratory muscles, and gluteal muscles. Manohar (51) injected radionuclide-labeled microspheres into the left ventricle of maximally exercising ponies, and blood flow increased significantly in all respiratory muscles. Blood flow was measured in inspiratory and expiratory muscles (individual muscles are shown in Fig. 3).
Heart Failure Effects on Respiratory vs. Locomotor Muscle Blood Flow
The only other “direct” evidence, i.e., utilizing blood flow measurements in both diaphragm and locomotor muscles during exercise, that we are aware of that clearly supports a “preferential” distribution of blood flow to the diaphragm versus locomotor muscles occurred in the heart failure rodent model with microsphere measurements of blood flow distribution (56, 73). Very high levels of sympathetic vasoconstrictor activity, as well as an increased work of breathing (secondary to both a hyperventilatory response as well as reduced lung compliance), commonly accompany heart failure. During moderate-intensity exercise, this CHF rodent model showed, relative to healthy controls, an enhanced vascular conductance and blood flow in the diaphragm versus a reduced conductance and blood flow to the locomotor muscles. The degree of this preferential distribution of flow to the diaphragm correlated positively with an index of lung water accumulation and presumably of respiratory muscle work.
Respiratory Muscle Training Effects
Additional indirect evidence of “preferential” effects of blood flow distribution to respiratory versus locomotor muscles is found in human studies of respiratory muscle training (RMT) in both healthy subjects (53, 89) and in people with CHF (20). For example, Witt et al. (89) found that 5 wk of RMT attenuated the sympathetically mediated rise in mean arterial pressure that accompanies fatiguing inspiratory muscle work. In addition, Chiappa et al. (20) found that in heart failure patients RMT resulted in diaphragm hypertrophy and increased respiratory muscle strength and/or endurance. The RMT effects on systemic hemodynamics included enhanced limb vascular conductance and flow during moderate exercise and an increase in the resistive ventilatory load required to elicit a reduction in limb vascular conductance and flow. Finally, McConnell and Lomax (53) demonstrated that adding respiratory muscle work exacerbated, while chronic respiratory muscle training attenuated, exercise-induced fatigue of the plantar flexors.
LOCOMOTOR MUSCLE WORK INFLUENCES VASCULAR CONDUCTANCE, BLOOD FLOW, AND FATIGUE OF RESPIRATORY MUSCLES
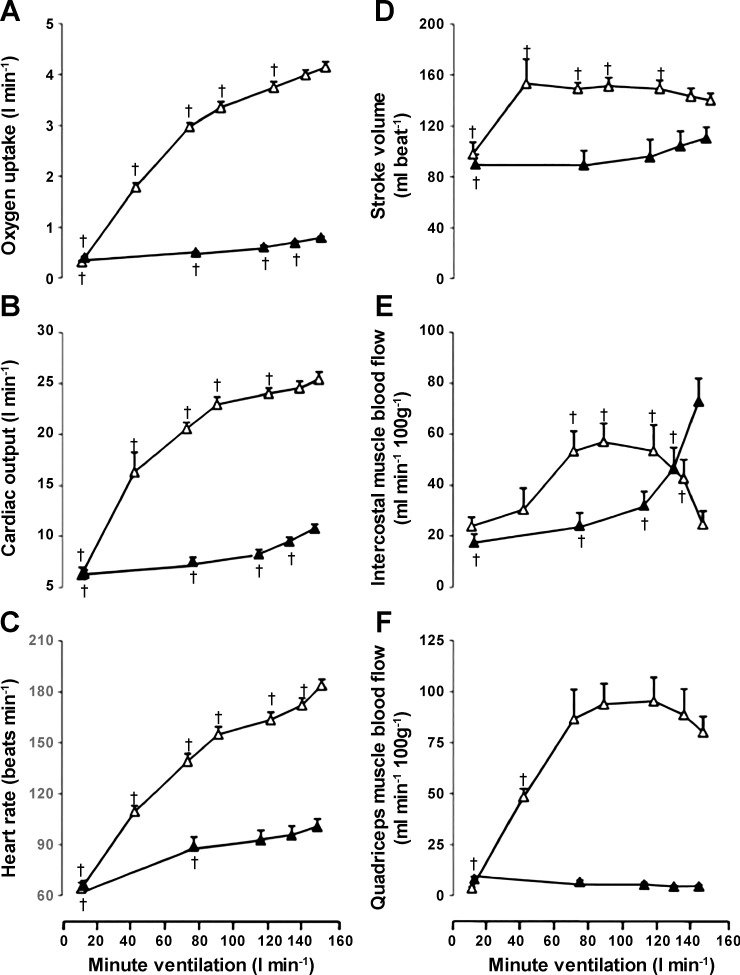
Vogiatzis et al. (81, 83) used near-infrared spectroscopy (NIRS) in combination with indocyanine green (ICG) dye infusion to estimate “respiratory” muscle blood flow in trained cyclists. Here optodes were placed over the seventh intercostal space with a penetration depth of ~2 cm, and blood flow values reflect both internal and external intercostal muscles (Q̇ic) and perhaps a minor contribution from the costal region of the diaphragm. They measured intercostal and quadriceps muscle blood flow during incremental exercise to maximum and compared the exercise-induced intercostal flow to intercostal flow at rest during voluntary increases in isocapnic hyperpnoea at minute ventilation levels up to those achieved at the maximal work rate. During resting isocapnic voluntary hyperpnoea, intercostal muscle flow increased linearly with work of breathing, whereas during exercise both intercostal and quadriceps flow increased only up to 60–80% of the maximal work rate (83). At higher workloads up to maximum, intercostal muscle blood flow fell by over 50% to the level observed at resting eupnea, whereas limb flow and conductance fell slightly but remained at very high levels (Fig. 5). These changes contrasted with their previous findings (81) and others (55, 88) where limb and trunk blood flow and conductance rose incrementally and then leveled off from high to maximum workloads. Furthermore, in COPD patients, they also compared Q̇ic during submaximal and maximal exercise with that observed at rest when the exercise ventilation and work of breathing were voluntarily mimicked (82). Here it was found that the Q̇ic increased severalfold above quiet eupneic breathing levels with voluntary hyperpnea while at rest, but during exercise Q̇ic fell below resting levels and was reduced to <10% of Q̇ic levels during resting hyperpnea, despite a fourfold increase in ventilation above resting levels (78). This reduced Q̇ic occurred presumably because the high level of sympathetically mediated vasoconstriction during heavy exercise, not present during resting hyperpnea, would override local vasodilatory mechanisms in the intercostal muscles (83). Vogiatzis et al. (83) interpreted their findings to mean that blood flow demands of both locomotor and respiratory muscles at maximal exercise could not be met “…requiring greater O2 extraction and likely contributing to respiratory muscle fatigue.” These authors and the accompanying editorial (69) also claim that these findings on Q̇ic contradicted the conclusions from respiratory muscle loading and unloading studies in humans (32, 33). As detailed above, these latter authors speculated that increases and decreases in the work of breathing resulting in measureable increases and decreases in limb vascular conductance and blood flow must have meant that reciprocal changes in flow occurred in the respiratory muscles. Given that the vasodilatory reactivity might differ among respiratory muscles, we need to ask if these findings in the intercostal muscles also apply to the diaphragm and other major inspiratory and expiratory muscles. In the rodent, blood flow to the diaphragm and other respiratory muscles, as measured by microsphere distribution, was substantially lower during heavy-intensity exercise than it was during CO2 or hypoxia-induced hyperpnea at rest (62). On the other hand, Poole et al. (62) further demonstrated that, unlike the diaphragm, blood flow to the intercostals, scalenus, and transverse abdominal muscles was substantially higher during the hyperpnea accompanying treadmill running when compared with similar levels of chemically induced hyperpnea at rest. Interpretation of this comparison of rest hyperpnea versus exercise hyperpnea effects on blood flow distribution in the rodent is confounded by the additional local vasodilatory effects of imposed hypercapnia and hypoxia in the resting animal.
Fig. 5.
Metabolic and hemodynamic responses during incremental exercise and isocapnic resting hyperpnoea. Data are from Vogiatzis et al. (83). Oxygen uptake (A), cardiac output (B), heart rate (C), stroke volume (D), intercostal muscle blood flow (E), and quadriceps muscle blood flow (F) at different levels of minute ventilation during exercise (△) and isocapnic, resting hyperpnoea (▲). Muscle blood flow was determined using near-infrared spectroscopy and indocyanine green (ICG) (see text). Values are means ± SE for 10 subjects. †Significant differences (P < 0.05) between exercise-induced hyperpnea and voluntary hyperpnea at rest at comparable levels of ventilation. Note that intercostal muscle blood flow increased progressively with mild- through moderate-intensity exercise, plateaued, and fell to resting levels as exercise intensity increased further; whereas intercostal blood flow increased progressively with increasing ventilation during voluntary hyperpnea at rest.
Babcock et al. (11) addressed this question of blood flow competition indirectly by conducting a similar comparison of the effects of high-intensity exercise versus similar levels and durations of the voluntarily produced high work of breathing at rest on diaphragm fatigue in humans. While some subjects with especially high levels of respiratory muscle work during exercise did show diaphragm fatigue at rest resulting from their resting hyperpnea trial, the majority did not, and even in those who did diaphragm twitch Pdi recovered much faster following the resting hyperpnea trial than following exercise. In most subjects, the work of the diaphragm had to be increased 60–80% greater than those levels achieved during exercise to elicit diaphragmatic fatigue in a resting state. These data likely reflect the critical influence of limited blood flow distribution to the diaphragm during coincident limb exercise, secondary to high levels of sympathetic vasoconstrictor activity emanating from locomotor muscle afferents, thereby contributing to diaphragmatic fatigue.
EVIDENCE THAT ALTERATIONS IN RESPIRATORY MUSCLE WORK INFLUENCE BOTH RESPIRATORY AND LOCOMOTOR MUSCLE BLOOD FLOW
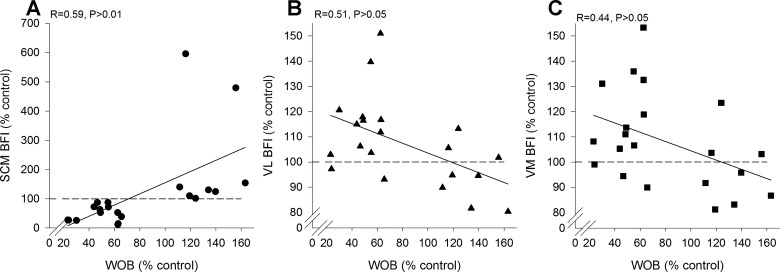
With respect to the distribution of blood flow during exercise with varying loads on the respiratory muscles, we recently asked if the now available “extra” blood flow with respiratory muscle unloading is, in fact, diverted away from the respiratory muscles (25). We utilized the NIRS + ICG method to assess blood flow to the sternocleidomastoid (SCM). We chose to investigate the SCM because it represents a superficial respiratory muscle with minimal movement-related artifacts and is highly active during intense exercise. We found that with respiratory muscle unloading, leg blood flow was increased and respiratory muscle blood flow was reduced (Fig. 6). On the other hand, when the work of breathing was increased with a resistive load, there was a commensurate reduction in limb blood flow and an increase in blood flow to the respiratory muscles. We take these observations as evidence that respiratory muscle work significantly influences the distribution of blood flow to both respiratory and locomotor muscles under conditions of intense exercise. Moreover, these findings demonstrate a bidirectional change in both respiratory and limb locomotor blood flow with changes in the work of breathing. Said differently, our findings support the concept that respiratory muscle work significantly influences the distribution of blood flow to both respiratory and locomotor muscles.
Fig. 6.
Individual values for sternocleidomastoid (A), vastus lateralis (B), and vastus medialis (C) blood flow index and work of breathing during cycling exercise at 85% of maximal work rate. Open shapes represent the respiratory muscle unloading condition with the proportional assist ventilator (PAV) and filled shapes represent the respiratory muscle loading condition with increased airway resistance. SCM, sternocleidomastoid; VL, vastus lateralis; VM, vastus medialis; WOB, work of breathing. Note that SCM flow increases and limb flow decreases with respiratory muscle loading and the SCM flow decreases and limb flow increases with respiratory muscle unloading. These findings demonstrate that respiratory muscle work significantly influences the distribution of blood flow to both respiratory and locomotor muscles under conditions of intense exercise. Data are from Dominelli et al. (25).
Our findings appear at odds with the observations of Vogiatzis et al. (82, 83) who have reported that blood flow to the intercostal region plateaus and then trends toward resting (or even below resting) values during heavy exercise (Fig. 5). These differences in findings merit comment. First, a restriction of blood flow of this size to an active muscle (i.e., high level of ventilation during exercise) implies a substantial vasoconstriction that is inconsistent with the lack of change in mean arterial pressure observed by others (32, 33). Second, in exercising ponies blood flow to all respiratory muscles measured increases proportionally as a function of ventilation (50–52) and this includes the intercostal musculature (Fig. 3). Third, there are also methodological differences that need to be considered when interpreting the observation that Q̇ic falls with high levels of ventilation during exercise. Specifically, surface probes utilized for the NIRS-ICG technique placed over the intercostal space have inherent limitations during exercise. Placing optodes over the seventh intercostal space will reflect blood flow values from both internal and external intercostal muscles and perhaps a minor contribution from the costal region of the diaphragm. However, during heavy exercise, there is an increase in end-inspiratory lung volume and alterations in end-expiratory lung volume. Furthermore, especially at near maximal V̇e, the chest wall can become distorted (31). In our view, it is possible that both of these factors could change the orientation of the muscle in the field of view of the optodes. At higher lung volumes, the depth of the NIRS penetration over the seventh intercostal space could potentially involve lung tissue and would not be indicative of muscle blood flow. The former points could explain why apparent respiratory muscle blood flow appears to fall below resting values in other studies (83). It is for these reasons we were led to use the SCM (Fig. 6). Nonetheless, it is possible that our findings differ from Vogiatzis et al. (83) because of the selection of respiratory muscle with which to assess blood flow and we cannot rule out this possibility.
SUMMARY/PRIORITIZATION OF FLOW DISTRIBUTION?
We propose that the evidence to date in humans and animals supports the postulate that the high level of sympathetic vasoconstrictor activity emanating from both limb and respiratory muscle metaboreceptors in heavy-intensity exercise constrains blood flow and O2 transport, thereby promoting both diaphragm and locomotor muscle fatigue during whole body exercise at high intensity. On one hand, the increased limb blood flow and relief of limb fatigue achieved via respiratory muscle unloading points to an effective tonic sympathetically mediated vasoconstrictor effect on the locomotor muscle vasculature emanating from respiratory muscles during high-intensity exercise. Conversely, that the threshold of respiratory muscle work required to elicit diaphragmatic fatigue was considerably higher under resting conditions versus heavy-intensity cycling in most healthy subjects implicates an effective vasoconstrictor effect on the diaphragm vasculature emanating from active locomotor muscles during heavy-intensity exercise.
Is sympatholysis in the face of sympathetically driven vasoconstriction during heavy-intensity exercise “greater” in the diaphragm than in limb locomotor muscles? In summary, there are four lines of indirect support for this postulate (see details in preceding sections). First, there is a preferential redistribution of blood flow to the diaphragm and away from the limb observed during submaximal exercise in response to the very high sympathetically driven vasoconstrictor activity plus high work of breathing accompanying heart failure in the rodent (56, 73). Second, there is an increase in vascular conductance and blood flow and reduced fatigue in exercising limbs resulting from specific training of the respiratory muscles (20, 53). Third, maximum vasodilation is observed in diaphragm, but not in limb vasculature, during maximal exercise in the equine (51, 52) (but not the rat: Ref. 62). Fourth, there is a relatively blunted vasoconstrictor response to norepinephrine in isolated phrenic versus locomotor muscle arterioles (1).
More definitive answers to this question of “prioritization” of blood flow distribution among locomotor and respiratory muscle in humans must await further experiments that incorporate blood flow and vascular conductance measures in both locomotor and respiratory muscles during exercise under conditions of altered sympathetic “tone.” Additional insight in to this complex problem would benefit greatly from a capability to measure blood flow to the diaphragm in the human as well as more fundamental study of the relative responsiveness and adrenergic receptor densities of diaphragm versus locomotor muscle vasculatures. We also need a means of effectively blocking phrenic afferents to permit quantification of their physiologic role in cardiorespiratory regulation during exercise.
CLINICAL RELEVANCE
There is growing evidence in animal models and humans to indicate that sensitized locomotor muscle afferents contribute significantly to excessive cardiorespiratory responses and sympathetic vasoconstrictor activity and to both symptom- and O2 transport-dependent limitations to exercise performance in CHF, COPD, and chronic hypertension. Thus, in CHF rodent models, direct recording from locomotor group III mechanoreceptor afferents showed increased sensitivity to muscle contraction to underlie their excessive exercise pressor response (86). Partial blockade of locomotor muscle afferents (via intrathecal administration of opiate agonists) reduced the ventilatory response, hyperinflation, and dyspnea and improved exercise performance in humans with COPD (30) and normalized the pressor response to exercise in hypertensive patients (13). With regard to respiratory muscle afferents, respiratory muscle unloading studies demonstrated substantial increases in locomotor muscle blood flow and vascular conductance in CHF patients (20, 54, 60) and reduced locomotor muscle fatigue and improved performance in COPD patients (5). Importantly, and unlike the reliance on heavy exercise intensities to demonstrate respiratory muscle unloading effects on vascular conductance in healthy young subjects (88), these effects were present in patients with COPD, hypertension, and CHF during relatively mild exercise intensities.
Chronic training of either locomotor or respiratory muscles would be expected to constrain exercise-induced muscle metabolite accumulation and its systemic consequences. Accordingly, in CHF patients, limb muscle training reduced their abnormal ventilatory and cardiovascular responses to exercise (61) and specific respiratory muscle training increased their limb muscle blood flow and oxygenation during exercise (20). In rodent CHF models, exercise training prevented excessive exercise-induced limb muscle mechanoreceptor response, sympathoexcitation, and the exercise pressor response (87). Furthermore, whole body exercise training in normal rodents enhanced nitric oxide production in both gastrocnemius and diaphragm muscles, an adaptive response important to both muscle contractile performance as well as to enhancing muscle perfusion by inducing both direct relaxation of vascular smooth muscle plus inhibition of adrenergic vasoconstriction of muscle resistance vessels (80). In summary, this limited evidence to date provides a clear rationale for pursuing in-depth studies in both humans and animal disease models into the role of respiratory and locomotor muscle afferents in both the pathogenesis and treatment of excessive exercise-induced sympathetic responses and their cardiovascular consequences.
GRANTS
The preparation of this work was supported, in part, by the Natural Sciences and Engineering Research Council of Canada (to A. W. Sheel and R. Boushel) and the National Heart, Lung, and Blood Institute (to J. A. Dempsey).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.W.S., R.C.B., and J.A.D. prepared figures, drafted manuscript, and edited and revised manuscript; A.W.S., R.C.B., and J.A.D. approved final version of manuscript.
REFERENCES
- 1.Aaker A, Laughlin MH. Diaphragm arterioles are less responsive to α1-adrenergic constriction than gastrocnemius arterioles. J Appl Physiol (1985) 92: 1808–1816, 2002. doi: 10.1152/japplphysiol.01152.2001. [DOI] [PubMed] [Google Scholar]
- 2.Aaron EA, Seow KC, Johnson BD, Dempsey JA. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol (1985) 72: 1818–1825, 1992. doi: 10.1152/jappl.1992.72.5.1818. [DOI] [PubMed] [Google Scholar]
- 3.Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol 586: 161–173, 2008. doi: 10.1113/jphysiol.2007.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Inspiratory muscle work in acute hypoxia influences locomotor muscle fatigue and exercise performance of healthy humans. Am J Physiol Regul Integr Comp Physiol 293: R2036–R2045, 2007. doi: 10.1152/ajpregu.00442.2007. [DOI] [PubMed] [Google Scholar]
- 5.Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, Dempsey JA. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol 299: R314–R324, 2010. doi: 10.1152/ajpregu.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amann M, Romer LM, Subudhi AW, Pegelow DF, Dempsey JA. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol 581: 389–403, 2007. doi: 10.1113/jphysiol.2007.129700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archiza B, Welch JF, Geary CM, Allen GP, Borghi-Silva A, Sheel AW. Temporal characteristics of exercise-induced diaphragmatic fatigue. J Appl Physiol (1985) 124: 906–914, 2018. doi: 10.1152/japplphysiol.00942.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babcock MA, Johnson BD, Pegelow DF, Suman OE, Griffin D, Dempsey JA. Hypoxic effects on exercise-induced diaphragmatic fatigue in normal healthy humans. J Appl Physiol (1985) 78: 82–92, 1995. doi: 10.1152/jappl.1995.78.1.82. [DOI] [PubMed] [Google Scholar]
- 10.Babcock MA, Pegelow DF, Harms CA, Dempsey JA. Effects of respiratory muscle unloading on exercise-induced diaphragm fatigue. J Appl Physiol (1985) 93: 201–206, 2002. doi: 10.1152/japplphysiol.00612.2001. [DOI] [PubMed] [Google Scholar]
- 11.Babcock MA, Pegelow DF, McClaran SR, Suman OE, Dempsey JA. Contribution of diaphragmatic power output to exercise-induced diaphragm fatigue. J Appl Physiol (1985) 78: 1710–1719, 1995. doi: 10.1152/jappl.1995.78.5.1710. [DOI] [PubMed] [Google Scholar]
- 12.Bachasson D, Wuyam B, Pepin JL, Tamisier R, Levy P, Verges S. Quadriceps and respiratory muscle fatigue following high-intensity cycling in COPD patients. PLoS One 8: e83432, 2013. doi: 10.1371/journal.pone.0083432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbosa TC, Vianna LC, Fernandes IA, Prodel E, Rocha HN, Garcia VP, Rocha NG, Secher NH, Nobrega AC. Intrathecal fentanyl abolishes the exaggerated blood pressure response to cycling in hypertensive men. J Physiol 594: 715–725, 2016. doi: 10.1113/JP271335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borghi-Silva A, Carrascosa C, Oliveira CC, Barroco AC, Berton DC, Vilaça D, Lira-Filho EB, Ribeiro D, Nery LE, Neder JA. Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high-intensity exercise in chronic heart failure. Am J Physiol Heart Circ Physiol 294: H2465–H2472, 2008. doi: 10.1152/ajpheart.91520.2007. [DOI] [PubMed] [Google Scholar]
- 15.Boushel R, Ara I, Gnaiger E, Helge JW, González-Alonso J, Munck-Andersen T, Sondergaard H, Damsgaard R, van Hall G, Saltin B, Calbet JA. Low-intensity training increases peak arm VO2 by enhancing both convective and diffusive O2 delivery. Acta Physiol (Oxf) 211: 122–134, 2014. doi: 10.1111/apha.12258. [DOI] [PubMed] [Google Scholar]
- 16.Buckwalter JB, Clifford PS. α-Adrenergic vasoconstriction in active skeletal muscles during dynamic exercise. Am J Physiol Heart Circ Physiol 277: H33–H39, 1999. doi: 10.1152/ajpheart.1999.277.1.H33. [DOI] [PubMed] [Google Scholar]
- 17.Calbet JA, Gonzalez-Alonso J, Helge JW, Søndergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol (1985) 103: 969–978, 2007. doi: 10.1152/japplphysiol.01281.2006. [DOI] [PubMed] [Google Scholar]
- 18.Calbet JA, Joyner MJ. Disparity in regional and systemic circulatory capacities: do they affect the regulation of the circulation? Acta Physiol (Oxf) 199: 393–406, 2010. doi: 10.1111/j.1748-1716.2010.02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calbet JA, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on V̇o2peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol 291: R447–R453, 2006. doi: 10.1152/ajpregu.00746.2005. [DOI] [PubMed] [Google Scholar]
- 20.Chiappa GR, Roseguini BT, Vieira PJ, Alves CN, Tavares A, Winkelmann ER, Ferlin EL, Stein R, Ribeiro JP. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol 51: 1663–1671, 2008. doi: 10.1016/j.jacc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 21.Coast JR, Krause KM. Relationship of oxygen consumption and cardiac output to work of breathing. Med Sci Sports Exerc 25: 335–340, 1993. doi: 10.1249/00005768-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 22.DeHaan SJ, Kendrick JE. Neural and metabolic control of blood flow to respiratory muscles of rabbits. Proc Soc Exp Biol Med 167: 485–492, 1981. doi: 10.3181/00379727-167-41202. [DOI] [PubMed] [Google Scholar]
- 23.Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol 151: 242–250, 2006. doi: 10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Derchak PA, Sheel AW, Morgan BJ, Dempsey JA. Effects of expiratory muscle work on muscle sympathetic nerve activity. J Appl Physiol (1985) 92: 1539–1552, 2002. doi: 10.1152/japplphysiol.00790.2001. [DOI] [PubMed] [Google Scholar]
- 25.Dominelli PB, Archiza B, Ramsook AH, Mitchell RA, Peters CM, Molgat-Seon Y, Henderson WR, Koehle MS, Boushel R, Sheel AW. Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp Physiol 102: 1535–1547, 2017. doi: 10.1113/EP086566. [DOI] [PubMed] [Google Scholar]
- 26.Dominelli PB, Katayama K, Vermuelen TD, Stuckless TJR, Brown CV, Foster GE, Sheel AW. Work of breathing influences muscle sympathetic nerve activity during whole-body exercise [Abstract]. Med Sci Sports Exerc 50: 122, 2018. doi: 10.1249/01.mss.0000535489.43958.a5. [DOI] [Google Scholar]
- 27.Dominelli PB, Render JN, Molgat-Seon Y, Foster GE, Romer LM, Sheel AW. Oxygen cost of exercise hyperpnoea is greater in women compared with men. J Physiol 593: 1965–1979, 2015. doi: 10.1113/jphysiol.2014.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duron B, Marlot D. The non-myelinated fibers of the phrenic and the intercostal nerves in the cat. Z Mikrosk Anat Forsch 94: 257–268, 1980. [PubMed] [Google Scholar]
- 29.Fixler DE, Atkins JM, Mitchell JH, Horwitz LD. Blood flow to respiratory, cardiac, and limb muscles in dogs during graded exercise. Am J Physiol 231: 1515–1519, 1976. doi: 10.1152/ajplegacy.1976.231.5.1515. [DOI] [PubMed] [Google Scholar]
- 30.Gagnon P, Bussières JS, Ribeiro F, Gagnon SL, Saey D, Gagné N, Provencher S, Maltais F. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186: 606–615, 2012. doi: 10.1164/rccm.201203-0404OC. [DOI] [PubMed] [Google Scholar]
- 31.Grimby G, Bunn J, Mead J. Relative contribution of rib cage and abdomen to ventilation during exercise. J Appl Physiol 24: 159–166, 1968. doi: 10.1152/jappl.1968.24.2.159. [DOI] [PubMed] [Google Scholar]
- 32.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol (1985) 82: 1573–1583, 1997. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 33.Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol (1985) 85: 609–618, 1998. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- 34.Harms CA, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol (1985) 89: 131–138, 2000. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- 35.Hill JM. Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res 856: 240–244, 2000. doi: 10.1016/S0006-8993(99)02366-5. [DOI] [PubMed] [Google Scholar]
- 36.Hussain SN, Chatillon A, Comtois A, Roussos C, Magder S. Chemical activation of thin-fiber phrenic afferents. 2. Cardiovascular responses. J Appl Physiol (1985) 70: 77–86, 1991. doi: 10.1152/jappl.1991.70.1.77. [DOI] [PubMed] [Google Scholar]
- 37.Ichinose M, Saito M, Fujii N, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during incremental leg cycling. J Physiol 586: 2753–2766, 2008. doi: 10.1113/jphysiol.2007.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob G, Costa F, Shannon J, Robertson D, Biaggioni I. Dissociation between neural and vascular responses to sympathetic stimulation: contribution of local adrenergic receptor function. Hypertension 35: 76–81, 2000. doi: 10.1161/01.HYP.35.1.76. [DOI] [PubMed] [Google Scholar]
- 39.Jammes Y, Balzamo E. Changes in afferent and efferent phrenic activities with electrically induced diaphragmatic fatigue. J Appl Physiol (1985) 73: 894–902, 1992. doi: 10.1152/jappl.1992.73.3.894. [DOI] [PubMed] [Google Scholar]
- 40.Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol 460: 385–405, 1993. doi: 10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol (1985) 73: 874–886, 1992. doi: 10.1152/jappl.1992.73.3.874. [DOI] [PubMed] [Google Scholar]
- 42.Jones CJ, Kuo L, Davis MJ, Chilian WM. α-Adrenergic responses of isolated canine coronary microvessels. Basic Res Cardiol 90: 61–69, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol Heart Circ Physiol 263: H1078–H1083, 1992. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- 45.Katayama K, Itoh Y, Saito M, Koike T, Ishida K. Sympathetic vasomotor outflow and blood pressure increase during exercise with expiratory resistance. Physiol Rep 3: e12421, 2015. doi: 10.14814/phy2.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufman M, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems, edited by Terjung R. Bethesda, MD: American Physiological Society, 1996, p. 381–447. doi: 10.1002/cphy.cp120110. [DOI] [Google Scholar]
- 47.Mador MJ, Kufel TJ, Pineda LA. Quadriceps and diaphragmatic function after exhaustive cycle exercise in the healthy elderly. Am J Respir Crit Care Med 162: 1760–1766, 2000. doi: 10.1164/ajrccm.162.5.2001005. [DOI] [PubMed] [Google Scholar]
- 48.Mador MJ, Kufel TJ, Pineda LA, Sharma GK. Diaphragmatic fatigue and high-intensity exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 161: 118–123, 2000. doi: 10.1164/ajrccm.161.1.9903010. [DOI] [PubMed] [Google Scholar]
- 49.Mador MJ, Magalang UJ, Rodis A, Kufel TJ. Diaphragmatic fatigue after exercise in healthy human subjects. Am Rev Respir Dis 148: 1571–1575, 1993. doi: 10.1164/ajrccm/148.6_Pt_1.1571. [DOI] [PubMed] [Google Scholar]
- 50.Manohar M. Costal vs. crural diaphragmatic blood flow during submaximal and near-maximal exercise in ponies. J Appl Physiol (1985) 65: 1514–1519, 1988. doi: 10.1152/jappl.1988.65.4.1514. [DOI] [PubMed] [Google Scholar]
- 51.Manohar M. Inspiratory and expiratory muscle perfusion in maximally exercised ponies. J Appl Physiol (1985) 68: 544–548, 1990. doi: 10.1152/jappl.1990.68.2.544. [DOI] [PubMed] [Google Scholar]
- 52.Manohar M. Vasodilator reserve in respiratory muscles during maximal exertion in ponies. J Appl Physiol (1985) 60: 1571–1577, 1986. doi: 10.1152/jappl.1986.60.5.1571. [DOI] [PubMed] [Google Scholar]
- 53.McConnell AK, Lomax M. The influence of inspiratory muscle work history and specific inspiratory muscle training upon human limb muscle fatigue. J Physiol 577: 445–457, 2006. doi: 10.1113/jphysiol.2006.117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller JD, Smith CA, Hemauer SJ, Dempsey JA. The effects of inspiratory intrathoracic pressure production on the cardiovascular response to submaximal exercise in health and chronic heart failure. Am J Physiol Heart Circ Physiol 292: H580–H592, 2007. doi: 10.1152/ajpheart.00211.2006. [DOI] [PubMed] [Google Scholar]
- 55.Mortensen SP, Damsgaard R, Dawson EA, Secher NH, González-Alonso J. Restrictions in systemic and locomotor skeletal muscle perfusion, oxygen supply and VO2 during high-intensity whole-body exercise in humans. J Physiol 586: 2621–2635, 2008. doi: 10.1113/jphysiol.2007.149401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Musch TI. Elevated diaphragmatic blood flow during submaximal exercise in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 265: H1721–H1726, 1993. doi: 10.1152/ajpheart.1993.265.5.H1721. [DOI] [PubMed] [Google Scholar]
- 57.Nair J, Streeter KA, Turner SM, Sunshine MD, Bolser DC, Fox EJ, Davenport PW, Fuller DD. Anatomy and physiology of phrenic afferent neurons. J Neurophysiol 118: 2975–2990, 2017. doi: 10.1152/jn.00484.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Heart, Lung, and Blood Institute NHLBI Workshop summary. Respiratory muscle fatigue. Report of the Respiratory Muscle Fatigue Workshop Group. Am Rev Respir Dis 142: 474–480, 1990. doi: 10.1164/ajrccm/142.2.474. [DOI] [PubMed] [Google Scholar]
- 59.O’Donnell DE, D’Arsigny C, Raj S, Abdollah H, Webb KA. Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am J Respir Crit Care Med 160: 1804–1811, 1999. doi: 10.1164/ajrccm.160.6.9808134. [DOI] [PubMed] [Google Scholar]
- 60.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol 588: 2487–2501, 2010. doi: 10.1113/jphysiol.2009.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation 93: 940–952, 1996. doi: 10.1161/01.CIR.93.5.940. [DOI] [PubMed] [Google Scholar]
- 62.Poole DC, Sexton WL, Behnke BJ, Ferguson CS, Hageman KS, Musch TI. Respiratory muscle blood flows during physiological and chemical hyperpnea in the rat. J Appl Physiol (1985) 88: 186–194, 2000. doi: 10.1152/jappl.2000.88.1.186. [DOI] [PubMed] [Google Scholar]
- 63.Rodman JR, Henderson KS, Smith CA, Dempsey JA. Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J Appl Physiol (1985) 95: 1159–1169, 2003. doi: 10.1152/japplphysiol.00258.2003. [DOI] [PubMed] [Google Scholar]
- 64.Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol 571: 425–439, 2006. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romer LM, Miller JD, Haverkamp HC, Pegelow DF, Dempsey JA. Inspiratory muscles do not limit maximal incremental exercise performance in healthy subjects. Respir Physiol Neurobiol 156: 353–361, 2007. doi: 10.1016/j.resp.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol (1985) 75: 663–667, 1993. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- 67.Saltin B, Rådegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- 68.Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise in humans: role of muscle mass. Am J Physiol 257: H1812–H1818, 1989. doi: 10.1152/ajpheart.1989.257.6.H1812. [DOI] [PubMed] [Google Scholar]
- 69.Secher NH, Richardson RS. A large slice of cardiac output or humble pie for the respiratory muscles? J Physiol 587: 3411, 2009. doi: 10.1113/jphysiol.2009.175679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol 537: 277–289, 2001. doi: 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheel AW, Derchak PA, Pegelow DF, Dempsey JA. Threshold effects of respiratory muscle work on limb vascular resistance. Am J Physiol Heart Circ Physiol 282: H1732–H1738, 2002. doi: 10.1152/ajpheart.00798.2001. [DOI] [PubMed] [Google Scholar]
- 72.Simon M, LeBlanc P, Jobin J, Desmeules M, Sullivan MJ, Maltais F. Limitation of lower limb V̇o2 during cycling exercise in COPD patients. J Appl Physiol (1985) 90: 1013–1019, 2001. doi: 10.1152/jappl.2001.90.3.1013. [DOI] [PubMed] [Google Scholar]
- 73.Smith JR, Hageman KS, Harms CA, Poole DC, Musch TI. Effect of chronic heart failure in older rats on respiratory muscle and hindlimb blood flow during submaximal exercise. Respir Physiol Neurobiol 243: 20–26, 2017. doi: 10.1016/j.resp.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol 529: 493–504, 2000. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stickland MK, Miller JD, Smith CA, Dempsey JA. Carotid chemoreceptor modulation of regional blood flow distribution during exercise in health and chronic heart failure. Circ Res 100: 1371–1378, 2007. doi: 10.1161/01.RES.0000266974.84590.d2. [DOI] [PubMed] [Google Scholar]
- 76.Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol 586: 1743–1754, 2008. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Supinski G, DiMarco A, Ketai L, Hussein F, Altose M. Reversibility of diaphragm fatigue by mechanical hyperperfusion. Am Rev Respir Dis 138: 604–609, 1988. doi: 10.1164/ajrccm/138.3.604. [DOI] [PubMed] [Google Scholar]
- 78.Supinski GS, DiMarco AF, Gonzalez J, Altose MD. Effect of norepinephrine on diaphragm contractility and blood flow. J Appl Physiol (1985) 69: 2019–2028, 1990. doi: 10.1152/jappl.1990.69.6.2019. [DOI] [PubMed] [Google Scholar]
- 79.Taylor BJ, How SC, Romer LM. Exercise-induced abdominal muscle fatigue in healthy humans. J Appl Physiol (1985) 100: 1554–1562, 2006. doi: 10.1152/japplphysiol.01389.2005. [DOI] [PubMed] [Google Scholar]
- 80.Vassilakopoulos T, Deckman G, Kebbewar M, Rallis G, Harfouche R, Hussain SN. Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol Lung Cell Mol Physiol 284: L452–L457, 2003. doi: 10.1152/ajplung.00270.2002. [DOI] [PubMed] [Google Scholar]
- 81.Vogiatzis I, Athanasopoulos D, Boushel R, Guenette JA, Koskolou M, Vasilopoulou M, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Contribution of respiratory muscle blood flow to exercise-induced diaphragmatic fatigue in trained cyclists. J Physiol 586: 5575–5587, 2008. doi: 10.1113/jphysiol.2008.162768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vogiatzis I, Athanasopoulos D, Habazettl H, Aliverti A, Louvaris Z, Cherouveim E, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Intercostal muscle blood flow limitation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 1105–1113, 2010. doi: 10.1164/rccm.201002-0172OC. [DOI] [PubMed] [Google Scholar]
- 83.Vogiatzis I, Athanasopoulos D, Habazettl H, Kuebler WM, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Intercostal muscle blood flow limitation in athletes during maximal exercise. J Physiol 587: 3665–3677, 2009. doi: 10.1113/jphysiol.2009.171694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Volianitis S, Secher NH. Arm blood flow and metabolism during arm and combined arm and leg exercise in humans. J Physiol 544: 977–984, 2002. doi: 10.1113/jphysiol.2002.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walker DJ, Walterspacher S, Schlager D, Ertl T, Roecker K, Windisch W, Kabitz HJ. Characteristics of diaphragmatic fatigue during exhaustive exercise until task failure. Respir Physiol Neurobiol 176: 14–20, 2011. doi: 10.1016/j.resp.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 86.Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol 588: 5033–5047, 2010. doi: 10.1113/jphysiol.2010.199562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang HJ, Li YL, Zucker IH, Wang W. Exercise training prevents skeletal muscle afferent sensitization in rats with chronic heart failure. Am J Physiol Regul Integr Comp Physiol 302: R1260–R1270, 2012. doi: 10.1152/ajpregu.00054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wetter TJ, Harms CA, Nelson WB, Pegelow DF, Dempsey JA. Influence of respiratory muscle work on V̇o2 and leg blood flow during submaximal exercise. J Appl Physiol (1985) 87: 643–651, 1999. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar]
- 89.Witt JD, Guenette JA, Rupert JL, McKenzie DC, Sheel AW. Inspiratory muscle training attenuates the human respiratory muscle metaboreflex. J Physiol 584: 1019–1028, 2007. doi: 10.1113/jphysiol.2007.140855. [DOI] [PMC free article] [PubMed] [Google Scholar]