Abstract
Epithelial-fibroblast interactions are thought to be very important in the adult lung in response to injury, but the specifics of these interactions are not well defined. We developed coculture systems to define the interactions of adult human alveolar epithelial cells with lung fibroblasts. Alveolar type II cells cultured on floating collagen gels reduced the expression of type 1 collagen (COL1A1) and α-smooth muscle actin (ACTA2) in fibroblasts. They also reduced fibroblast expression of hepatocyte growth factor (HGF), fibroblast growth factor 7 (FGF7, KGF), and FGF10. When type II cells were cultured at an air-liquid interface to maintain high levels of surfactant protein expression, this inhibitory activity was lost. When type II cells were cultured on collagen-coated tissue culture wells to reduce surfactant protein expression further and increase the expression of some type I cell markers, the epithelial cells suppressed transforming growth factor-β (TGF-β)-stimulated ACTA2 and connective tissue growth factor (CTGF) expression in lung fibroblasts. Our results suggest that transitional alveolar type II cells and likely type I cells but not fully differentiated type II cells inhibit matrix and growth factor expression in fibroblasts. These cells express markers of both type II cells and type I cells. This is probably a normal homeostatic mechanism to inhibit the fibrotic response in the resolution phase of wound healing. Defining how transitional type II cells convert activated fibroblasts into a quiescent state and inhibit the effects of TGF-β may provide another approach to limiting the development of fibrosis after alveolar injury.
Keywords: ARDS, fibroblast epithelial interactions, FGF7, FGF10, HGF, IPF, pulmonary fibrosis, type I cells, type II cells
INTRODUCTION
Epithelial cells are critical in the process of wound healing. Initially, epithelial cells at the edge of the wound spread and cells adjacent to the wound proliferate to close the wound, and later the epithelial cells reduce the fibrotic response of the underlying mesenchymal cells (53). Adamson and colleagues (3, 5), Haschek and Witschi (37), and Uhal and Nguyen (64) highlighted the importance of alveolar epithelial cells in regulating lung repair many years ago. Initial damage to the alveolar epithelium commonly occurs to type I cells, which are more susceptible to injury than type II cells, have limited ability to repair, and cover >95% of the alveolar surface (2, 3, 27, 62). If the epithelial injury is minor, the type II cells spread and proliferate to cover the alveolar surface, and the lung recovers without fibrosis (2, 12, 26). However, if the alveolar epithelium is injured more severely or if the ability of the type II cells to proliferate and restore the epithelium is impaired, then the injury results in fibrosis (5, 37).
Many investigators believe that the interactions between alveolar epithelial cells and fibroblasts are important in the development of pulmonary fibrosis, but the details of these interactions are not defined (11, 30, 55, 70). Both bleomycin- and radiation-induced fibrosis follow this pattern of initial injury to type I cells and subsequent fibrosis. Bleomycin injures type I cells in the first few days after instillation (1), and there is a loss of type I cells in the late phase of radiation lung injury as fibrosis develops (6). Importantly, there is an absence or severe reduction in the number of type I cells in the fibrotic portion of the idiopathic pulmonary fibrosis (IPF) lung, and several groups have suggested that the loss of type I cells might be important in the pathogenesis of IPF (23, 43, 44, 48). However, the manner in which the loss of type I cells contributes to pulmonary fibrosis is not known.
Epithelial-fibroblast interactions are well recognized to be critically important in fetal lung development (14, 57). However, the process is very complex and involves multiple interacting signaling cascades (67, 69). In the adult lung, epithelial-fibroblast interactions likely occur but are much less well defined and may be different from what occurs in the fetal lung. There is a very close physical association between type II cells and fibroblasts in the adult human and rodent lung (59, 60). In the human lung, type II cells reside near fibroblasts, which are situated in the valleys between alveolar capillaries (59). They are also both situated in the corners of the alveoli, where several alveolar septa meet (9). In vitro rat alveolar epithelial cells inhibit serum-induced fibroblast proliferation through production of PGE2 by both the fibroblasts and the epithelial cells (50, 51, 54). Recently, Epa et al. (24) reported that human bronchial epithelial cells, A459 cells, and primary alveolar epithelial cells inhibit TGF β-stimulated fibroblast α-smooth muscle and collagen expression through a PGE2-mediated mechanism.
Fibroblasts secrete hepatocyte growth factor (HGF), fibroblast growth factor 7 [FGF7; keratinocyte growth factor (KGF)], and fibroblast growth factor 10 (FGF10), which are important mitogens and differentiation factors for type II cells (52). These growth factors can reduce lung injury and subsequent fibrosis due to acid or bleomycin instillation in rodents (13, 21, 22, 36, 71). In addition, these growth factors are thought to be important in the therapeutic benefit of mesenchymal stem cell administration in the treatment of lung injury and the prevention of pulmonary fibrosis (13, 47). However, there is little information on the regulation of these growth factors by alveolar epithelial cells in the adult lung.
Although epithelial-fibroblast interactions have been implicated in a variety of human lung diseases, the precise interactions between these cell types and the multiple signaling pathways involved have not been delineated. We developed culture systems to study epithelial-fibroblast interactions and demonstrate that human alveolar epithelial cells inhibit fibroblast type 1 collagen (COL1A1) and smooth muscle actin (ACTA2) expression. Alterations in the in vitro culture conditions allow for the comparison of different alveolar epithelial cell phenotypes in these responses. The alveolar epithelial cells that inhibit these fibroblast genes express low levels of surfactant proteins and coexpress markers of type I cells. These culture systems provide the opportunity to define epithelial-fibroblast interactions more completely both for homeostatic regulation and for antifibrotic mechanisms.
METHODS
Type II cell isolation and culture.
Primary alveolar type II cells were isolated from human lungs from deidentified organ donors whose lungs were not suitable for transplantation. The Human Research Protection Program (HRPP) at National Jewish Health deemed this research as nonhuman subject research. The lung was perfused, lavaged, and digested with elastase as described previously (68). The lung was minced, and the cells were partially purified by centrifugation on a discontinuous density gradient made of Optiprep (Accurate Chemical Scientific, Westbury, NY) with densities of 1.085 and 1.040. The type II cells were then isolated either by nonadherence to IgG-coated petri dishes or positive selection with MACS MicroBeads human CD326 (EpCAM; Miltenyi Biotech, Bergisch Gladbach, Germany) (39). Most of the cells in this study were isolated by the IgG method, because this method produces cells with a higher plating efficiency. The isolated cells were suspended in Advanced DMEM/F-12 medium (Life Technologies, Grand Island, NY) or regular DMEM supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 2.5 µg/ml amphotericin B, 100 µg/ml streptomycin, 100 µg/ml penicillin G (Gibco, Life Technologies, Rockville, MD), and 10 µg/ml gentamicin (Sigma-Aldrich, St. Louis, MO) or frozen down in 90% FBS and 10% DMSO to be used for culture at a later date.
Fibroblast isolation and culture.
Primary normal fibroblasts were isolated from human lungs from deidentified organ donors whose lungs were not suitable for transplantation. The HRPP at National Jewish Health deemed this research as nonhuman subject research. Fibroblasts were also isolated from lungs of deidentified patients with idiopathic pulmonary fibrosis that had been resected at University of Colorado Hospital. These specimens have been deemed as nonhuman subject research by the Colorado Multiple Institutional Review Board. The demographics of the IPF patients from which the fibroblasts were derived have been published (17). The lung was minced and placed on scored tissue culture dishes and cultured with DMEM and 10% FBS. After 1 wk, fibroblasts migrated out from the minces, and the minces were removed. This culture was expanded up to two times and then frozen down. All subsequent experiments were done with cells passaged fewer than six additional times.
Epithelial and fibroblast cocultures in floating collagen gels.
Rat tail collagen (RTC), which we prepared (31), was mixed with 10× minimal essential medium (MEM) and NaOH to make a 1× rat tail collagen mixture at pH 7.4. This mixture was added to Matrigel at a ratio of 80:20 (vol/vol) RTC-Matrigel. The fibroblasts were counted to provide 600,000/cm2 in the gel and pelleted and then resuspended in the 80:20 RTC/Matrigel mixture. The cells were pipetted into the cell culture plate, and the gel was allowed to polymerize at 37°C for 10 min. At this point, alveolar epithelial cells were plated in regular or advanced DMEM + 10% FBS on top of these gels. The gels were incubated undisturbed for 2 days for epithelial cell adherence. The gels were washed twice with culture media. After washing, the gels were rimmed and released from the bottom of the well using a sterile Pasteur pipet. Then the final culture media [DMEM + 1% charcoal stripped FBS (CS-FBS) + 10 ng/ml KGF 10 + 10 nM dexamethasone (Dex) + antibiotics] was added. Media were changed every other day for 6 additional days, and the gels were harvested for analysis on the 8th day of culture.
Direct epithelial and fibroblast cocultures on collagen-coated dishes.
Alveolar type II cells (500,000 × 103/cm2) and fibroblasts (50 × 103/cm2) were plated together or individually on rat tail collagen-coated tissue culture wells in DMEM + 10% FBS. After 48 h, on day 2 of culture, the monolayers were washed and the media changed to DMEM with or without 5% FBS, 1 mg/ml bovine serum albumin (BSA), or 5 ng/ml transforming growth factor-β (TGF-β). The cells were harvested 3 days later.
Method for recovering the cell types.
In the cocultures and the individual cell types, the cells were reisolated at the end of the experiment by dissolving the gel with a mixture of 1 mg/ml collagenase (Worthington Biochemical Corporation, Lakewood, NJ) and 40 U/ml dispase (Corning, Corning, NY) and reisolating the epithelial cells by positive selection with EpCAM (CD326) magnetic beads (39).
Air-liquid interface conditions.
For air-liquid interface (ALI) cultures, the epithelial cells were plated on gels composed of 80% rat tail collagen and 20% Matrigel (Corning) at a density of 1.5 M cells/cm2 (17, 68). The fibroblasts were within the gel at a density of 0.4 M/cm2. The gels were formed on Corning Costar six-well 0.4 μM polycarbonate inserts. After 48 h the nonaherent cells were removed, the gel was rimmed so that it could contract, and culture medium was changed to DMEM with 1% charcoal-stripped FBS supplemented with 10 ng/ml KGF, and 10 nM dexamethasone with a small amount of fluid on the apical surface. Twenty-four hours later the apical fluid was removed, and the cells were cultured under ALI conditions. The media were changed on days 2, 4, and 6 of culture and harvested on day 8 of culture. The gels were dissolved with a mixture of collagenase and dispase as described above, and the epithelial cells and fibroblasts were separated with EpCAM (CD326) magnetic beads.
Cyclooxygenase inhibition.
Alveolar epithelial cells alone, fibroblasts alone, or cocultures were plated as described above. On day 2 (48 h after plating), the media were changed, and 10 μM indomethacin (Sigma-Aldrich, St. Louis, MO), 10 μM NS398 (Sigma Aldrich), or DMSO as a vehicle control was added. For the floating cocultures, the cells were plated in advance DMEM-F-12 with 10 FBS, and after day 2 the media were regular DMEM, 1% charcoal stripped FBS, KGF, and dexamethasone plus or minus the additives. The media were changed every 2 days, and the cells were harvested on day 8 (6 days with the additives).
Immunocytochemistry.
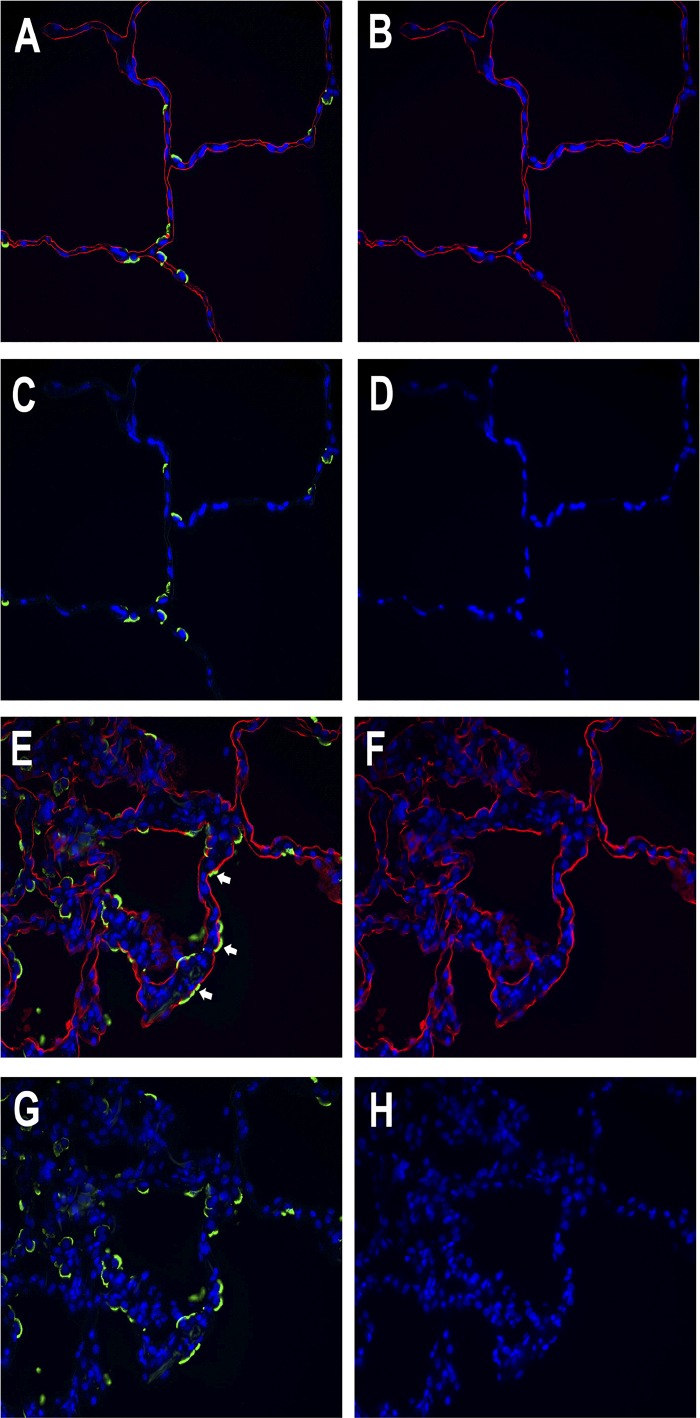
Cells in the collagen gels and pieces of lung were fixed with 4% parformaldehyde and paraffin embedded. The sections were deparaffinized, washed, and incubated with the primary antibody overnight. Collagen-coated coverslips were fixed with 4% paraformaldehyde. The primary antibodies were HTII-280 (a kind gift of Dr. Leland Dobbs and Robert Gonzalez, University of California San Francisco), MUC1 (05-652 clone 214D4; Millipore, Burlington, MA), E-cadherin (40772, clone EP700Y; Abcam, Cambridge, MA), β-catenin (610153, clone14; BD Biosystems, San Jose, CA), receptor for advanced glycation end products (RAGE) (AF1145; R&D Systems, Minneapolis, MN), epithelial membrane protein 2 (EMP2) (HPAA014711; Sigma-Aldrich, St. Louis, MO), SP-A (PE-10 mouse monoclonal antibody, a gift from Prof. Yoshio Kuroki, Sapporo, Japan), proSP-B (WRAB-55522; Seven Hills, Cincinnati, OH), and proSP-C (WRAB-9337; Seven Hills). We also used Dylight 594 (red) or fluorescein-labeled (green) lycopersicon esculentum (tomato) lectin (Vector Laboratories, Burlingame, CA) at a concentration of 0.5 ug/ml. The secondary antibodies were anti-mouse IgG Alexa Fluor 594 (A21-203; Molecular Probes), anti-rabbit IgG Alexa Fluor 488 (Molecular Probes, A21206), and anti-mouse IgM Heavy Chain Alexa Fluor 594 (A-21044; Molecular Probes). In Fig. 10, the lung with acute lung injury was from a 49-yr-old man who died of a cerebral vascular accident, was mechanically ventilated for 5 days, and had areas of consolidation on his chest radiographs presumably due to aspiration pneumonia. The normal-appearing lung is from a 48-yr-old man who died of head trauma.
Fig. 10.
Some alveolar epithelial cells express markers of both type II cells and type I cells in acute lung injury. Lungs from a normal organ donor and a donor with acute lung injury are shown. A through D are from a donor with normal appearing lung: A: combined image. B: tomato lectin, red. C: HTII-280, green. D: DAPI, blue. E through H are from a donor with acute lung injury: E: combined image. F: tomato lectin, red. G: HTII-280, green. H: DAPI, blue. White arrows point to cells that appear doubly labeled with the apical type II cell marker HTII-280 and the type I cell marker tomato lectin.
Real-Time RT-PCR.
RNA isolation was done using Qiagen RNeasy Kits according to the manufacturer’s instructions. For real-time RT-PCR, the expression levels of genes were expressed as a ratio to the expression of the constitutive probe GAPDH (46, 68). The specific verified primers and probes were purchased from Applied Biosystems (Foster City, CA).
Western blotting.
For the Western blotting analysis, polyacrylamide gradient gels (8–16%; Invitrogen) were run under reduced conditions in Tris glycine buffer to separate the proteins. Protein loading was normalized to β-actin. The primary antibodies were epithelial membrane protein 2 (EMP2) (HPAA014711; Sigma-Aldrich, St. Louis, MO), RAGE (AF1145; R&D Systems) smooth muscle actin (A2547; Sigma-Aldrich), and GAPDH (ab8245; Abcam, Cambridge, MA). The images were quantified using National Institutes of Health ImageJ software.
Statistics.
Because we were interested primarily in the direction of possible changes, we analyzed changes in mRNA levels using the Wilcoxon nonparametric signed rank test, when the number of samples was six or greater (Figs. 2, 3, and 6) (16). If the number of samples was five or fewer, the results were evaluated by t-tests, with the assumption that the resulting values were normally distributed (Figs. 3, 5, and 6). In all analyses, we focused on the magnitude of possible changes in outcomes, which is in keeping with current statistical practices (19, 20). Statistical analyses were done using R 3.3.2 (R statistical software, R Core Team, R Foundation), the SAS/STAT software package version 9.3 (SAS Institute, Cary, NC), or GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA).
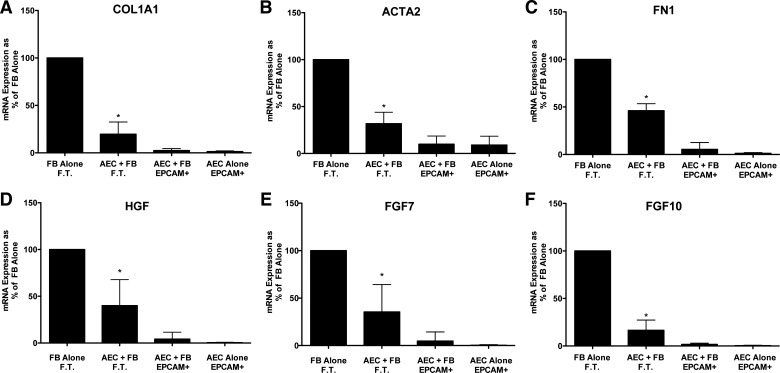
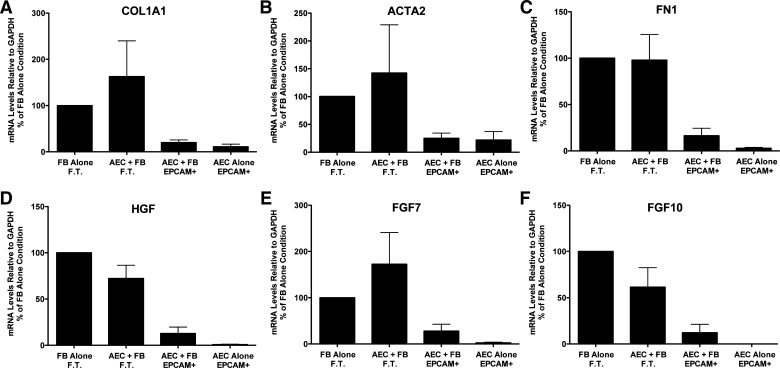
Fig. 2.
Alveolar epithelial cells (AEC) suppress fibroblast (FB) expression of type 1 collagen (COL1A1), fibronectin (FN1), α-smooth muscle actin (ACTA2), hepatocyte growth factor (HGF), fibroblast growth factor 7 (FGF7), and fibroblast growth factor 10 (FGF10) in floating submerged gels. The epithelial cells and the fibroblasts were cultured alone or together for 8 days. At the end of the experiment the gels were dissolved with dispase and collagenase and the epithelial cells were isolated with epithelial cellular adhesion molecule (EpCAM; CD326) magnetic beads. The fibroblasts were in the flow-through (FT) fraction. The mRNA was processed and quantitated by real-time qPCR as described above. Data were normalized to the level of gene expression in the fibroblast-alone cultures (100%). There were seven individual experiments with different epithelial cells for the comparisons. *Significant difference in the comparison of fibroblasts with or without alveolar epithelial cells, P = 0.02. The panels are as follows: A: COL1A1; B: ACTA2; C: FN1; D: HGF; E: FGF7; and F: FGF10. AEC, alveolar epithelial cell; EpCAM, epithelial cellular adhesion molecule; FN1, fibronectin.
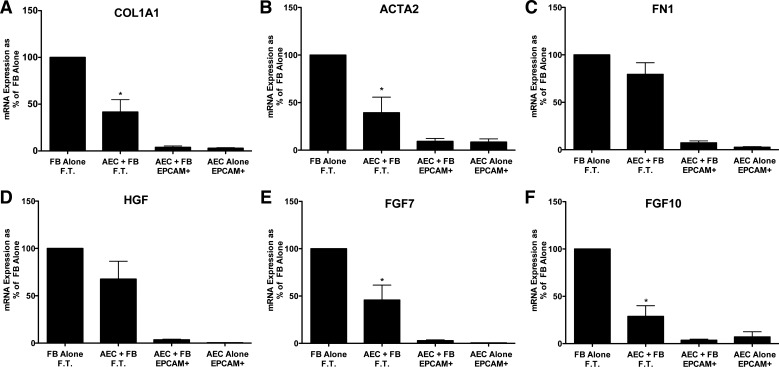
Fig. 3.
Alveolar epithelial cells (AEC) suppress expression of type 1 collagen (COL1A1), α-smooth muscle actin, fibroblast growth factor 7 (FGF7), and fibroblast growth factor 10 (FGF10) in idiopathic pulmonary fibrosis (IPF) fibroblasts in floating submerged gels. Epithelial cells and the fibroblasts were cultured alone or together for 8 days and then processed as described for Fig. 2. Data were normalized to the level of gene expression in the fibroblast-alone cultures (100%). There were six individual experiments with different epithelial cells and fibroblasts for the comparisons of COL1A1, ACTA2, and fibronectin (FN1) and five for hepatocyte growth factor (HGF), fibroblast growth factor 7 (FGF7), and fibroblast growth factor 10 (FGF10). *Significant difference in the comparison of the fibroblasts with and without the epithelial cells. P < 0.05. EpCAM, epithelial cellular adhesion molecule; FT, flow through. The panels are as follows: A: COL1A1; B: ACTA2; C: FN1; D: HGF; E: FGF7; and F: FGF10.
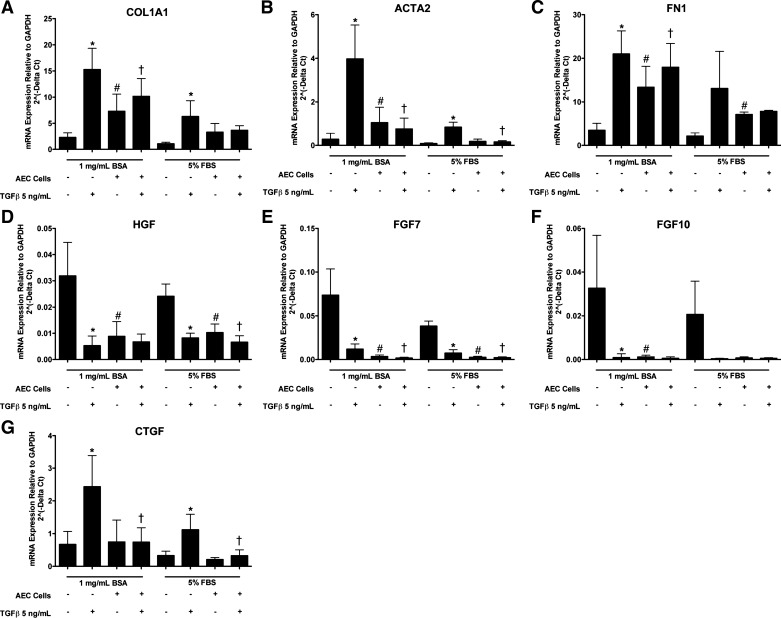
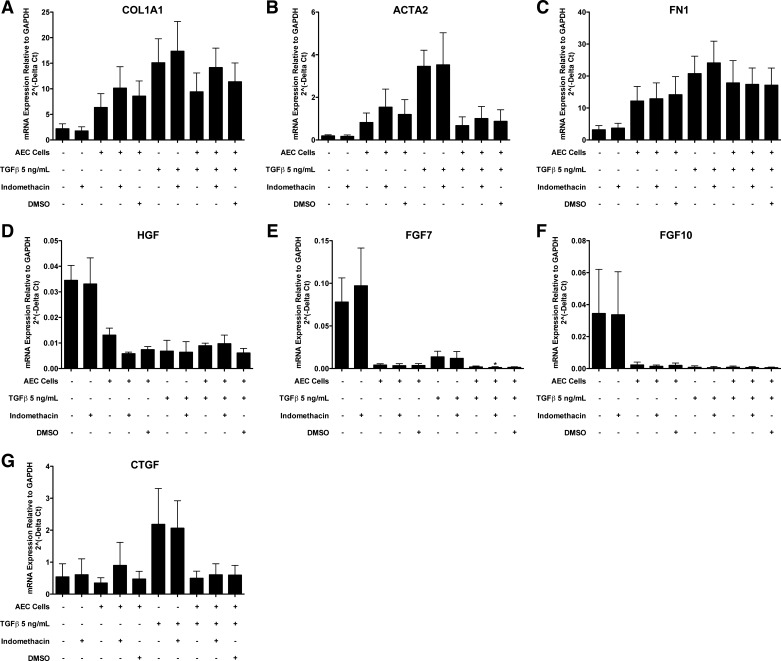
Fig. 6.
Alveolar epithelial cells (AEC) inhibit TGF-β-stimulated type 1 collagen (COL1A1) and smooth muscle actin (ACTA2) in fibroblasts in direct cocultures. Epithelial cells and fibroblasts were cultured alone or together on collagen-coated tissue culture wells. After 2 days for adherence, the cells were cultured with or without transforming growth factor-β (TGF-β) and with 1 mg/ml bovine serum albumin (BSA) or 5% FBS for 3 days until harvest. There were 7 independent experiments with 1 mg/ml BSA and 4 with 5% FBS. *Comparison of fibroblast expression with and without TGF-β under identical conditions, P < 0.05; #comparison of fibroblast expression with or without alveolar epithelial cells under identical conditions, P < 0.05; †comparison of fibroblast expression with or without alveolar epithelial cells in the presence of TGF-β, P < 0.05. The panels are as follows: A: COL1A1; B: ACTA2; C: FN1; D: HGF; E: FGF7; F: FGF10; and G: CTGF.
Fig. 5.
Alveolar epithelial cells (AEC) cultured under air-liquid interface conditions do not suppress fibroblast (FB) expression of type 1 collagen (COL1A1), α-smooth muscle actin (ACTA2), fibronectin (FN1), hepatocyte growth factor (HGF), fibroblast growth factor 7 (FGF7), or fibroblast growth factor 10 (FGF10). The epithelial cells and the fibroblasts were cultured alone or together for 8 days under air-liquid conditions. The type II cells were cultured on top of a gel on a culture insert under air-liquid interface conditions alone or with fibroblasts. The fibroblasts were cultured in the gel either alone or with the epithelial cells. The nonadherent cells were washed away on day 2 and then the cultures were kept under air-liquid conditions until harvest on day 8. The mRNA levels were normalized to the expression level of GAPDH and then compared with the levels to the fibroblasts alone on day 8. Results are from four independent experiments for most comparisons and three independent experiments for FGF10. EpCAM, epithelial cellular adhesion molecule; FT, flow through. The panels are as follows: A: COL1A1; B: ACTA2; C: FN1; D: HGF; E: FGF7; and F: FGF10.
RESULTS
Alveolar epithelial cells suppress matrix and growth factor expression in fibroblasts in floating gels.
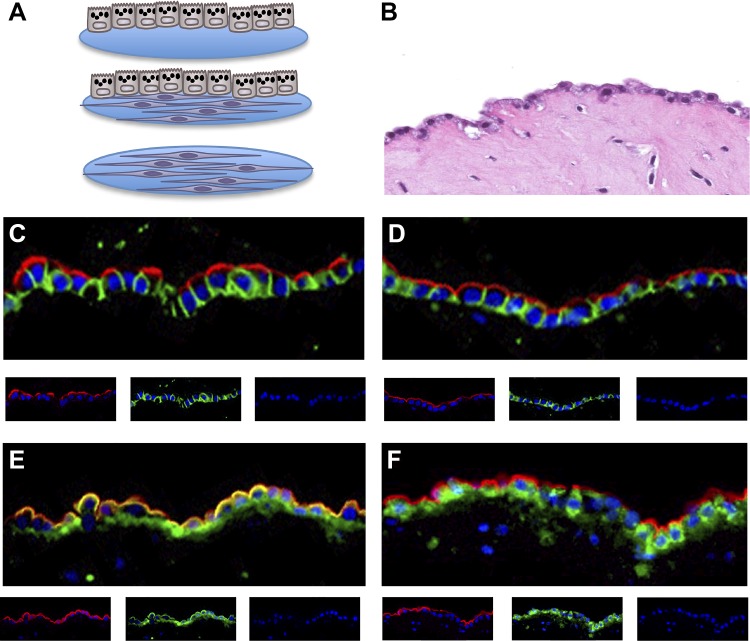
We designed a three-dimensional culture system to study the epithelial-fibroblast interactions as might occur in the fibrotic lung. We wanted the fibroblasts to be in an extracellular matrix and close to the epithelial cells, and we wanted the epithelial cells to be cuboidal in shape as in the IPF lung with well-maintained apical polarity and the basal surface facing the fibroblasts. We knew from previous studies with rat type II cells that the morphology of type II cells was well maintained on floating rat tail collagen gels and that fibroblasts improved the differentiation of type II cells in vitro (31, 58). Fibroblasts were embedded in a gel composed of rat tail collagen and Matrigel, and the type II cells were plated on top of the gel (Fig. 1). After 2 days to allow for adherence of the epithelial cells, gels were released and allowed to float. When the epithelial cells were cultured alone, the edges of the gel rolled up upon themselves. By contrast, with fibroblast embedded within the gel, the gel generally preserved its shape and contracted significantly. Hence, in this system, because the soft gel can contract, the fibroblasts should be under minimal tension. The individual cell types were reisolated at the end of the experiment by dissolving the gel with a mixture of collagenase and dispase and reisolating the epithelial cells by positive selection with EpCAM (CD326) magnetic beads (39). The purity of the re-isolated alveolar epithelial cells in these cocultures was 86.9 ± 2.1% (n = 7) based on Muc1 staining and the reisolated fibroblasts 88.5 ± 3.1% (n = 7) based on positive vimentin and negative Muc1 staining. As shown in Fig. 1, the epithelial monolayer is composed mostly of cuboidal type II cells. We believe that the lucencies seen in the apical cytoplasm of the type II cells represent lamellar bodies extracted in the process of paraffin embedding. The cells are polarized as shown by the expression of Muc1 and HTII-280 on the apical surface and E-cadherin along the basolateral surface (Fig. 1). RAGE appears at both the apical and the basolateral surfaces. The alveolar epithelial cells cultured on the floating collagen gels coexpressed markers of type II cells such as Muc1, HTII-280, and proSP-B and type I cell markers such as RAGE and EMP2 and binding the tomato lectin (Fig. 1, E and F, and data not shown) (29, 33). Under these submerged conditions, the cells express reduced levels of surfactant proteins (38).
Fig. 1.
Three-dimensional culture system with alveolar type II cells and fibroblasts. A: scheme of the culture system. The epithelial cells and the fibroblasts are cultured by themselves or together. The culture system is a floating gel composed of collagen and Matrigel, and the cultures can be under air-liquid or submerged conditions. The fibroblasts and the epithelial cells are reisolated at the end of the experiment for assays of gene expression under submerged conditions. B: hematoxylin and eosin-stained image of the floating coculture system. Cuboidal epithelial cells are on the top of the gel and fibroblasts within the gel. The alveolar type II cells in the cocultures are highly polarized. C: the red apical staining is HTII-280, the green basolateral marker is E-cadherin, and the nuclei are blue with DAPI. D: the red apical staining is Muc1, and the basolateral green staining is E-cadherin. E: the red apical staining is Muc1, and the green staining is receptor for advanced glycation end products. F: the red apical staining is Muc1, and the green staining is proSP-B.
To assess normal cell-cell interactions, we evaluated the effect of the epithelial cells on fibrogenic genes and growth factor expression by the fibroblasts isolated from normal lungs and the reciprocal effect of the fibroblasts on surfactant protein expression by the epithelial cells. As shown in Fig. 2, epithelial cells decreased the expression of ACTA2 and matrix genes (COL1A1 and FN1) and HGF, FGF7, and FGF10, growth and differentiation factors for alveolar type II cells compared with normal fibroblasts cultured alone. There was no consistent effect of the fibroblasts on surfactant protein gene expression, but surfactant protein mRNA was detected only in the EpCAM selected cells, indicating good separation of the epithelial cells from the fibroblasts (data not shown).
We next determined whether matrix and growth factor expression in fibroblasts isolated from IPF patients was inhibited in these cocultures. Alveolar epithelial cells suppressed expression of COL1A1, ACTA2, FGF7, and FGF10 in the IPF fibroblasts. The effects of the epithelial cells on IPF fibroblasts were similar but not identical to normal lung fibroblasts. HGF and FN1 expression was not inhibited by the epithelial cells in these experiments (Fig. 3).
Inhibition of cyclooxygenase 1 and cyclooxygenase 2 did not alter the suppression of matrix and growth factor mRNA levels in fibroblasts.
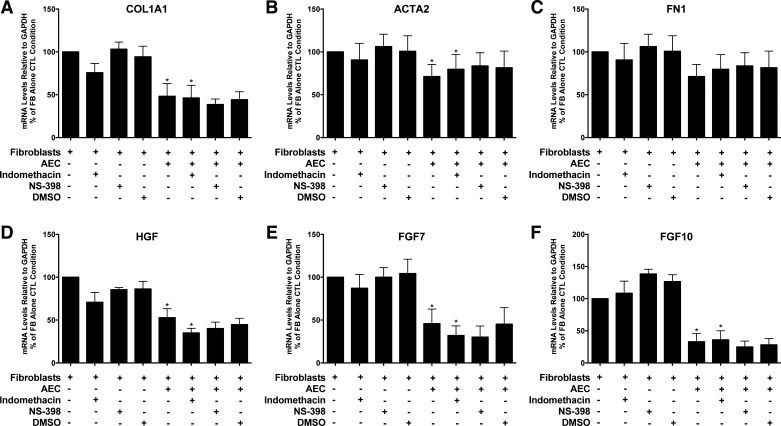
Inhibition of serum-induced fibroblast proliferation by rat alveolar epithelial cells has previously been shown to be PGE2 dependent (50, 51). Therefore, we tested whether human alveolar epithelial cells inhibit matrix and growth factor expression by fibroblasts in a PGE2-dependent manner. However, we didn’t find significant inhibition of the epithelial effect on fibroblasts for the expression of COL1A1, HGF, FGF7, or FGF10 in the presence of indomethacin, a cyclooxygenase 1 (COX1) and COX2 inhibitor, or NS298, a specific COX2 inhibitor. However, the levels of PGE2 in the media in these experiments were very low, below the level of detection in most samples (<8 pg/ml). In this series of experiments there was less epithelial inhibition of ACTA2 and FN1 than in the experiments described above (Fig. 4).
Fig. 4.
Inhibition of type 1 collagen (COL1A1), smooth muscle actin (ACTA2), hepatocyte growth factor (HGF), fibroblast growth factor 7 (FGF7), and fibroblast growth factor 10 (FGF10) by the epithelial cells was not blocked by cyclooxygenase 1 (COX1) and COX2 inhibitors. The epithelial cells and the fibroblasts were cultured alone or together for 8 days and then processed as described for Fig. 2. Indomethacin, DMSO, NS-398, and transforming growth factor-β were added 4 days before harvest. There were five individual experiments with different epithelial and fibroblasts cells for the comparisons of COL1A1, ACTA2, and FN1 and four for HGF, FGF7, and FGF10. *P < 0.05 by analysis of variance to assess if epithelial cells inhibited fibroblast gene expression and if they persisted in the presence of indomethacin (columns 1, 5, and 6). The panels are as follows: A: COL1A1; B: ACTA2; C: FN1; D: HGF; E: FGF7; and F: FGF10.
Type II cells cultured at an air-liquid interface did not inhibit fibroblast gene expression.
Although the epithelial cells were cuboidal and well polarized under submerged conditions on floating gels in the cocultures, the expression of surfactant proteins was low. To improve the level of surfactant gene expression, we cultured the type II cells and fibroblasts at an air-liquid interface (ALI), which improves the expression of the surfactant proteins (18, 68). Under ALI conditions, the alveolar epithelial cells express surfactant proteins at levels similar to the levels expressed by freshly isolated type II cells. Alveolar type II cells cultured under these conditions did not suppress the expression of ACTA2, COL1A1, FN1, or the growth factors HGF, FGF7, or FGF10 (Fig. 5).
Transitional type II cells cultured directly with fibroblasts suppressed TGF-β-stimulated ACTA2 and CTGF expression and inhibited basal HGF, KGF, and FGF10 expression in fibroblasts.
The results from the ALI cultures suggested that dedifferentiated type II cells were more capable of inhibiting selected fibroblast genes than fully differentiated type II cells. Type II cells cultured on a collagen-coated tissue culture plastic surface with 5% FBS lose their expression of surfactant proteins and acquire expression of markers associated with type I cells (68). Epithelial cells plated directly with the fibroblasts also allow for direct physical contact, and under these conditions, the fibroblasts would be under greater tension than fibroblasts in floating collagen gels. Because we were no longer interested in maintaining the type II cell phenotype, we cultured the cells with and without TGF-β to determine whether the epithelial cells could reduce TGF-β-stimulated expression of ACTA2 (18). Because there is a greater expression of ACTA2 in response to TGF-β under serum-starved conditions, we tested the effects of the epithelial cells in the presence and absence of serum (7). The critical comparison was to determine whether the epithelial cells would suppress fibroblast ACTA2 expression maximally stimulated by TGF-β. Under conditions with or without serum, alveolar epithelial cells inhibited TGF-β-stimulated expression of ACTA2 and CTGF in normal lung fibroblasts (Fig. 6). TGF-β increased the expression of COL1A1, ACTA2, FN1, and CTGF and decreased expression of HGF, FGF7, and FGF10 in fibroblasts, as reported previously (17). The basal level of expression of COL1A1 and ACTA2 was slightly increased by coculture with the alveolar epithelial cells under serum-free conditions but not in the presence of 5% FBS. However, type II cells cultured without serum appeared to be under considerable stress and tended to detach. Although inhibition of COX1 and COX 2 did not inhibit the effect of the alveolar epithelial cells on fibroblasts in the floating collagen gel system, we repeated these studies in the direct coculture system, because we were concerned that the potential PGE2 effect might require the fibroblasts to be cultured on a firm surface, such as tissue culture plastic. However, under the direct coculture conditions on tissue culture plastic, indomethacin, a COX1 and COX2 inhibitor, failed to inhibit the epithelial effect on TGF-β-induced expression of ACTA2 and CTGF (Fig. 7). Indomethacin also did not block the inhibition of HGF, FGF7, and FGF10 expression in fibroblasts by the alveolar epithelial cells (Fig. 7).
Fig. 7.
Alveolar epithelial inhibition of expression of smooth muscle actin (ACTA2), connective tissue growth factor (CTGF), hepatocyte growth factor (HGF), fibroblast growth factor 7 (FGF7), and fibroblast growth factor 10 (FGF10) is not altered by indomethacin. Cells were cultured as in Fig. 7. On day 2 of culture, 10 μM indomethacin was added and the media were changed to 1 mg/ml BSA. Cells were harvested on day 5. Results are from four independent experiments. There was no significant effect of indomethacin. Individual comparisons were not made since the data for the effects without indomethacin are included in the data in Fig. 6. The panels are as follows: A: COL1A1; B: ACTA2; C: FN1; D: HGF; E: FGF7; F: FGF10; and G: CTGF.
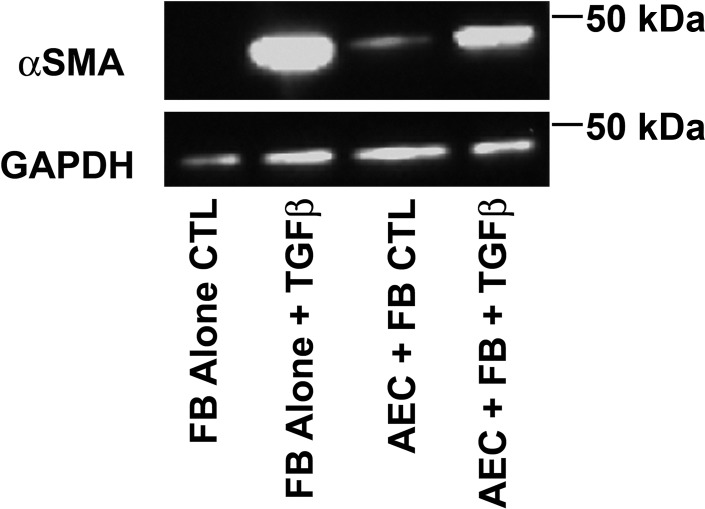
To document that the changes in mRNA expression translated into protein expression, we confirmed the epithelial suppression of fibroblast expression of α-smooth muscle actin stimulated by TGF-β (Fig. 8). However, the epithelial cells slightly increased the protein level of α-smooth muscle actin under serum-starved conditions. Although serum starvation is useful to augment the fibroblast response to TGF-β, this condition is not optimal for culturing epithelial cells.
Fig. 8.
Alveolar epithelial cells (AEC) inhibit transforming growth factor-β (TGF-β)-stimulated expression of α-smooth muscle actin (αSMA). Cells were cultured as in Fig. 6 with 1 mg/ml BSA and harvested on day 5 of culture. αSMA expression was measured by Western analyses. The image is from 1 of 3 independent experiments. In these experiments, alveolar epithelial cells decreased the expression of TGF-β-stimulated αSMA by 27 ± 6%.
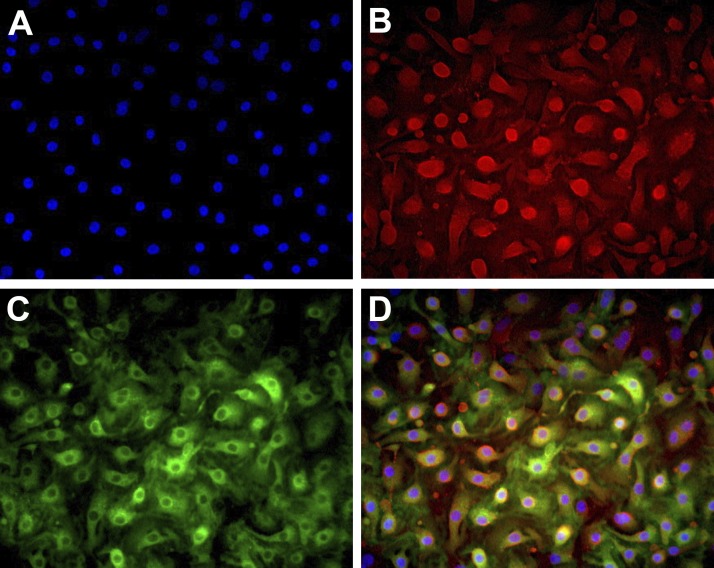
The phenotype of the type II cells cultured with 5% FBS on collagen-coated surfaces is transitional between type II cells and type I cells. The cells spread, flatten, and appear as type I-like cells but still retain lamellar body remnants (68). They lose expression of SP-A and HTII-280 by day 5 but retain expression of proSP-B and Muc1 for a longer period of time. In these cultures, there are numerous transitional type II cells that coexpress the type II cell marker Muc1 and the type I cell marker RAGE (Fig. 9).
Fig. 9.
Phenotype of the type II cells cultured on collagen-coated wells. Epithelial cells were cultured alone or with fibroblasts on collagen-coated coverslips or tissue culture wells. After 2 days for adherence the cells were cultured with 5% FBS, as in Fig. 6. By immunocytochemistry, the epithelial cells expressed both Muc1 and receptor for advanced glycation end-products (RAGE). A: blue DAPI. B: red Muc1. C: green RAGE. D: combined.
Transitonal type II cells are present in acute lung injury.
Although all of these studies were done in vitro, there is good evidence that transitional spreading type II cells exist in acute respiratory distress syndrome (ARDS) and animal models of acute lung injury (2, 27, 62). Transitional cells have been reported in ARDS by electron microscopy, and cells that express both type I and II cell markers appear in the rat lung after instillation of staphylococcus aureus (8, 15). In an organ donor with acute lung injury, we able to identify doubly labeled cells (Fig. 10). In the injured portion of the lung, the alveolar septa are significantly thickened, and there is an increased number of type II cells covering the alveolar surface. A small portion of them express markers of both type I cells and type II cells. The images appeared identical with either HTII-280 or Muc1 as apical markers for type II cells and tomato lectin staining for type I cells (29).
DISCUSSION
The coculture systems that we developed allow for functional assessment of human alveolar epithelial cells, fibroblasts, and their cell-cell interactions. We have shown that alveolar epithelial cells reduce basal ACTA2 and COL1A1 expression in the floating cocultures, when the fibroblasts should be under minimal tension, and TGF-β stimulated smooth muscle actin expression in fibroblasts cultured on collagen-coated dishes, when the fibroblasts should be under maximal physical tension. However, this inhibitory activity was not detected with fully differentiated type II cells cultured at an air-liquid interface. The type II cells that exhibited this activity were poorly differentiated as measured by the expression of surfactant proteins, and these cells expressed some markers of type I cells (38, 68). This is a gain of function by type II cells as they transition to become type I cells. Based on several experimental in vivo studies, we have termed these type II cells with markers both of both type I and type II cells as “reparative” type II cells and consider them to be the in vitro phenotype of the transitional type II cells seen after acute lung injury due to hyperoxia, nitrogen dioxide, or instillation of lipopolysaccharide or acid (2, 5, 8, 25, 26, 41). Bachofen and Weibel (8) described these transitional cells in ARDS many years ago. From our in vitro observations, these cells in vivo should be identified by the coexpression of type II cell markers such as Muc1, ATII-280, and the surfactant proteins and the type I cell markers such as RAGE, EMP2, or binding the tomato lectin. We have observed alveolar epithelial cells that express markers of both type II and type I cells in mild acute lung injury. However, there is always the possibility that in the intact lung visualized by fluorescent microscopy there may be overlapping of type I cell and type II cell plasma membranes. Flow cytometry or electron microscopy will be required to confirm these observations. Other researchers have used antibodies to MMC4 and RTI-40 to identify these transitional doubly labeled cells in a staphylococcus aureus model of acute lung injury in rats or surfactant protein D and the tomato lectin to identify the cells transitioning from type II cell hyperplasia induced by FGF7 to normal type I cells (15, 28). Recently, single-cell analyses have also shown that many alveolar epithelial cells coexpress type I and type II cell genes in the newborn mouse lung (35). During the newborn period in the mouse, there is marked thinning of the alveolar septal walls, expansion of the alveolar surface covered by type I cells, and reduction of the mesenchymal cell compartment.
Although this series of experiments was initially designed to examine type II cell fibroblast interactions as related to pulmonary fibrosis, analysis suggests that these observations are more applicable to the repair phase of the acute lung injury. We were not able to identify dual-positive cells in the fibrotic portion of lungs with advanced IPF (data not shown). IPF is characterized by progressive nonresolving fibrosis, and the type II cells are cuboidal and express high levels of the surfactant proteins and other type II cell markers (Muc 1 and HTII-280). There is a marked reduction in expression of RAGE, a type I cell marker, in the areas of fibrosis (23, 29). Others have also noted the absence of type I cells in the IPF lung and speculated that the absence of type I cells might contribute to the fibrotic response (43, 44, 48). The reason that type II cells do not differentiate into type I cells in the fibrotic portion of the IPF lung is not known.
There are two additional anatomic observations that support the hypothesis that type I cells inhibit fibroblast persistence, whereas highly differentiated type II cells do not. When fetal alveolar epithelial cells start to express type I cell markers, there is a marked reduction in the size of the mesenchymal compartment. The mechanisms that regulate the dissolution of the mesenchyme during the late stages of lung development have not been defined. The location of fibroblasts in the adult human and rodent lung also suggests that highly differentiated type II cells do not suppress fibroblasts. In the normal lung, fibroblasts tend to be absent from areas with type I cells and common adjacent to type II cells (9, 59, 60, 65).
The therapeutic implications of these observations for the treatment of lung injury or prevention of pulmonary fibrosis would be to limit the loss of type I cells or instill type II cells. Guillamat-Prats et al. (34) instilled isolated rat type II cells into rats 14 days after they were treated with bleomycin. The instilled type II cells reduced fibrosis, restored the level of surfactant proteins, and increased the weight of the injured rats (34). Hence, instilling type II cells to hasten the repair of alveolar epithelium has experimental support. Perhaps in the distant future, type II cells derived from pluripotent stem cells could be used therapeutically to restore the alveolar epithelium (40).
The mechanisms for this inhibition of fibroblast gene expression are not defined and will require a significant amount of additional study. Epa et al. (24) recently reported that small-airway epithelial cells, alveolar epithelial cells, and A549 cells inhibit TGF-β-stimulated α-smooth muscle actin and type I collagen expression in fibroblasts in a separated coculture system in a PGE2-dependent manner. We confirmed the inhibition of TGF-β-stimulated α-smooth muscle actin expression by alveolar epithelial cells. However, we were not able to confirm the PGE2 dependence in our studies with cells in the floating gels or cultured on collagen-coated tissue culture wells. In our experiments, COX1 and COX2 inhibition did not block these effects. The reason may be due to some technical detail on how the fibroblasts and epithelial cells were cultured. The PGE2 levels were very low in the floating collagen gel system. Epa et al. (24) studied TGF-β-stimulated fibroblasts cultured on plastic in the presence of 5% FBS. The phenotype of the type II cells in this study was not defined, but they would be expected to be poorly differentiated based on the culture conditions. In our study, transitional type II cells but not highly differentiated type II cells inhibited basal COL1A1 and ACTA2 expression in the absence of exogenous TGF-β when the fibroblasts were cultured in a soft gel. The alveolar epithelial cells inhibited TGF-β-stimulated COL1A1 and ACTA2 expression but not basal expression when the fibroblasts were cultured on collagen-coated tissue culture plates. We suspect that there are both PGE2-dependent and -independent pathways that result in inhibition of COL1A1 and ACTA2 expression.
Transitional type II cells but not highly differentiated type II cells suppress expression of HGF, KGF, and FGF10. These observations are compatible with the location of lipofibroblasts in the rodent lung. In the newborn rodent lung there are lipofibroblasts that reside under differentiated type II cells (65). These lipofibroblasts express FGF10 and provide a specialized niche for type II cells (56, 63, 66). In the human lung the role of lipofibroblasts is less well defined, in part because they are very difficult to identify in tissue sections (61). We speculate that transitional type II cells suppress the expression of a variety of genes in fibroblasts and that the growth factors just happen to be among the candidate genes that we selected. A more in-depth analysis of gene expression in these cultures is warranted.
There remain a number of unsettled questions related to these culture systems. A significant shortcoming in this field is the inability to isolate and culture human type I cells. Currently, one can form either a monolayer of mostly of cuboidal type II cells or a monolayer of squamous type II cells that lose the expression of some type II cell markers and acquire some markers expressed in type I cells. These squamous type II cells are not identical to type I cells (32). Hence, we can only speculate that the inhibition of matrix and growth factor genes is a likely property of type I cells. Single-cell RNA profiling of type I cells from human lung may provide additional insight on whether and how type I cells regulate fibroblast gene expression. Not all of the observations were consistent under all circumstances, and the results may be in part context dependent. For example, the epithelial inhibition of FN1 and HGF was not consistent in all conditions. In addition, when fibroblasts were cultured on collagen-coated tissue culture plastic, the epithelial cells inhibited TGF-β-stimulated COL1A1 and ACTA2 expression but not basal expression. In these conditions, the fibroblasts are cultured on a very firm surface which would tend to augment any profibrotic response. In addition, in some conditions there was a low level of expression of Col1A1 and growth factors in the epithelial EpCAM+ fractions. We consider this to be due most likely to fibroblast contamination, although human type II cells have been reported to express COL1A1 (10). More complete molecular phenotyping needs to be done with transitional type II cells in vitro. The reparative properties of type II cells are likely important in hastening the recovery of injured lung in ARDS. We also need to know more about the phenotypes of the alveolar epithelial cells in lungs from patients with acute lung injury. Finally, our results suggest a new function for type I cells that can be further defined once human type I cells are isolated and characterized more completely.
GRANTS
This work was supported by a grant from Gilead Sciences.
DISCLOSURES
A. Mikels-Vigdal is a research scientist for Gilead Sciences. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
R.L.Z., A.M.-V., and R.J.M. conceived and designed research; K.C., K.E., K.A.S., B.L.E., and R.J.M. performed experiments; E.F.R., D.C.-E., B.L.E., A.M.-V., and R.J.M. analyzed data; K.C., R.L.Z., E.F.R., D.C.-E., B.L.E., A.M.-V., and R.J.M. interpreted results of experiments; K.C., E.F.R., B.L.E., A.M.-V., and R.J.M. prepared figures; A.M.-V. and R.J.M. drafted manuscript; R.L.Z., E.F.R., K.A.S., D.C.-E., B.L.E., A.M.-V., and R.J.M. edited and revised manuscript; K.C., K.E., R.L.Z., E.F.R., K.A.S., D.C.-E., B.L.E., A.M.-V., and R.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the families of the de-identified organ donors and IIAM, NDRI, and the Donors Alliance, who made this research possible. We also thank Leland Dobbs and Robert Gonzalez, University of California San Francisco, for the HTII-280 antibody. Sarah Murrell helped prepare this manuscript for publication.
REFERENCES
- 1.Adamson IY, Bowden DH. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 77: 185–197, 1974. [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest 30: 35–42, 1974. [PubMed] [Google Scholar]
- 3.Adamson IY, Hedgecock C, Bowden DH. Epithelial cell-fibroblast interactions in lung injury and repair. Am J Pathol 137: 385–392, 1990. [PMC free article] [PubMed] [Google Scholar]
- 5.Adamson IY, Young L, Bowden DH. Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. Am J Pathol 130: 377–383, 1988. [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida C, Nagarajan D, Tian J, Leal SW, Wheeler K, Munley M, Blackstock W, Zhao W. The role of alveolar epithelium in radiation-induced lung injury. PLoS One 8: e53628, 2013. doi: 10.1371/journal.pone.0053628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arora PD, McCulloch CA. The deletion of transforming growth factor-beta-induced myofibroblasts depends on growth conditions and actin organization. Am J Pathol 155: 2087–2099, 1999. doi: 10.1016/S0002-9440(10)65527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachofen M, Weibel ER. Basic pattern of tissue repair in human lungs following unspecific injury. Chest 65, Suppl: 14S–19S, 1974. doi: 10.1378/chest.65.4_Supplement.14S. [DOI] [PubMed] [Google Scholar]
- 9.Badri L, Walker NM, Ohtsuka T, Wang Z, Delmar M, Flint A, Peters-Golden M, Toews GB, Pinsky DJ, Krebsbach PH, Lama VN. Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells. Am J Respir Cell Mol Biol 45: 809–816, 2011. doi: 10.1165/rcmb.2010-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballard PL, Lee JW, Fang X, Chapin C, Allen L, Segal MR, Fischer H, Illek B, Gonzales LW, Kolla V, Matthay MA. Regulated gene expression in cultured type II cells of adult human lung. Am J Physiol Lung Cell Mol Physiol 299: L36–L50, 2010. doi: 10.1152/ajplung.00427.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borensztajn K, Crestani B, Kolb M. Idiopathic pulmonary fibrosis: from epithelial injury to biomarkers—insights from the bench side. Respiration 86: 441–452, 2013. doi: 10.1159/000357598. [DOI] [PubMed] [Google Scholar]
- 12.Cabral-Anderson LJ, Evans MJ, Freeman G. Effects of NO2 on the lungs of aging rats. I. Morphology. Exp Mol Pathol 27: 353–365, 1977. doi: 10.1016/0014-4800(77)90006-5. [DOI] [PubMed] [Google Scholar]
- 13.Cahill EF, Kennelly H, Carty F, Mahon BP, English K. Hepatocyte growth factor is required for mesenchymal stromal cell protection against bleomycin-induced pulmonary fibrosis. Stem Cells Transl Med 5: 1307–1318, 2016. doi: 10.5966/sctm.2015-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao CM, Moiseenko A, Zimmer KP, Bellusci S. Alveologenesis: key cellular players and fibroblast growth factor 10 signaling. Mol Cell Pediatr 3: 17, 2016. doi: 10.1186/s40348-016-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clegg GR, Tyrrell C, McKechnie SR, Beers MF, Harrison D, McElroy MC. Coexpression of RTI40 with alveolar epithelial type II cell proteins in lungs following injury: identification of alveolar intermediate cell types. Am J Physiol Lung Cell Mol Physiol 289: L382–L390, 2005. doi: 10.1152/ajplung.00476.2004. [DOI] [PubMed] [Google Scholar]
- 16.Conover WJ. Practical Nonparametric Statistics (2nd ed.). New York: Wiley, 1980. [Google Scholar]
- 17.Correll KA, Edeen KE, Redente EF, Zemans RL, Edelman BL, Danhorn T, Curran-Everett D, Mikels-Vigdal A, Mason RJ. TGF beta inhibits HGF, FGF7, and FGF10 expression in normal and IPF lung fibroblasts. Physiol Rep 6: e13794, 2018. doi: 10.14814/phy2.13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correll KA, Edeen KE, Zemans RL, Redente EF, Mikels-Vigdal A, Mason RJ. TGF beta inhibits expression of SP-A, SP-B, SP-C, but not SP-D in human alveolar type II cells. Biochem Biophys Res Commun 499: 843–848, 2018. doi: 10.1016/j.bbrc.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curran-Everett D. CORP: Minimizing the chances of false positives and false negatives. J Appl Physiol (1985) 122: 91–95, 2017. doi: 10.1152/japplphysiol.00937.2016. [DOI] [PubMed] [Google Scholar]
- 20.Curran-Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society. Am J Physiol Regul Integr Comp Physiol 287: R247–R249, 2004. doi: 10.1152/ajpregu.00346.2004. [DOI] [PubMed] [Google Scholar]
- 21.Deterding RR, Havill AM, Yano T, Middleton SC, Jacoby CR, Shannon JM, Simonet WS, Mason RJ. Prevention of bleomycin-induced lung injury in rats by keratinocyte growth factor. Proc Assoc Am Physicians 109: 254–268, 1997. [PubMed] [Google Scholar]
- 22.Dohi M, Hasegawa T, Yamamoto K, Marshall BC. Hepatocyte growth factor attenuates collagen accumulation in a murine model of pulmonary fibrosis. Am J Respir Crit Care Med 162: 2302–2307, 2000. doi: 10.1164/ajrccm.162.6.9908097. [DOI] [PubMed] [Google Scholar]
- 23.Englert JM, Hanford LE, Kaminski N, Tobolewski JM, Tan RJ, Fattman CL, Ramsgaard L, Richards TJ, Loutaev I, Nawroth PP, Kasper M, Bierhaus A, Oury TD. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol 172: 583–591, 2008. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epa AP, Thatcher TH, Pollock SJ, Wahl LA, Lyda E, Kottmann RM, Phipps RP, Sime PJ. Normal human lung epithelial cells inhibit transforming growth factor-β induced myofibroblast differentiation via prostaglandin E2. PLoS One 10: e0135266, 2015. doi: 10.1371/journal.pone.0135266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 22: 142–150, 1975. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 26.Evans MJ, Cabral-Anderson LJ, Freeman G. Effects of NO2 on the lungs of aging rats. II. Cell proliferation. Exp Mol Pathol 27: 366–376, 1977. doi: 10.1016/0014-4800(77)90007-7. [DOI] [PubMed] [Google Scholar]
- 27.Evans MJ, Dekker NP, Cabral-Anderson LJ, Shami SG. Morphological basis of tolerance to ozone. Exp Mol Pathol 42: 366–376, 1985. doi: 10.1016/0014-4800(85)90086-3. [DOI] [PubMed] [Google Scholar]
- 28.Fehrenbach H, Kasper M, Tschernig T, Pan T, Schuh D, Shannon JM, Müller M, Mason RJ. Keratinocyte growth factor-induced hyperplasia of rat alveolar type II cells in vivo is resolved by differentiation into type I cells and by apoptosis. Eur Respir J 14: 534–544, 1999. doi: 10.1034/j.1399-3003.1999.14c10.x. [DOI] [PubMed] [Google Scholar]
- 29.Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Müller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand) 44: 1147–1157, 1998. [PubMed] [Google Scholar]
- 30.Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet 380: 680–688, 2012. doi: 10.1016/S0140-6736(12)61144-1. [DOI] [PubMed] [Google Scholar]
- 31.Geppert EF, Williams MC, Mason RJ. Primary culture of rat alveolar type II Cells on floating collagen membranes. Morphological and biochemical observations. Exp Cell Res 128: 363–374, 1980. doi: 10.1016/0014-4827(80)90072-5. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol 288: L179–L189, 2005. doi: 10.1152/ajplung.00272.2004. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez RF, Allen L, Gonzales L, Ballard PL, Dobbs LG. HTII-280, a biomarker specific to the apical plasma membrane of human lung alveolar type II cells. J Histochem Cytochem 58: 891–901, 2010. doi: 10.1369/jhc.2010.956433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillamat-Prats R, Gay-Jordi G, Xaubet A, Peinado VI, Serrano-Mollar A. Alveolar type II cell transplantation restores pulmonary surfactant protein levels in lung fibrosis. J Heart Lung Transplant 33: 758–765, 2014. doi: 10.1016/j.healun.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Guo M, Du Y, Gokey JJ, Ray S, Bell SM, Adam M, Sudha P, Perl AK, Deshmukh H, Potter SS, Whitsett JA, Xu Y. Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. Nat Commun 10: 37, 2019. doi: 10.1038/s41467-018-07770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupte VV, Ramasamy SK, Reddy R, Lee J, Weinreb PH, Violette SM, Guenther A, Warburton D, Driscoll B, Minoo P, Bellusci S. Overexpression of fibroblast growth factor-10 during both inflammatory and fibrotic phases attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 180: 424–436, 2009. doi: 10.1164/rccm.200811-1794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haschek WM, Witschi H. Pulmonary fibrosis–a possible mechanism. Toxicol Appl Pharmacol 51: 475–487, 1979. doi: 10.1016/0041-008X(79)90372-7. [DOI] [PubMed] [Google Scholar]
- 38.Ito Y, Ahmad A, Kewley E, Mason RJ. Hypoxia-inducible factor regulates expression of surfactant protein in alveolar type II cells in vitro. Am J Respir Cell Mol Biol 45: 938–945, 2011. doi: 10.1165/rcmb.2011-0052OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito Y, Correll K, Zemans RL, Leslie CC, Murphy RC, Mason RJ. Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-α/EGFR signaling. Am J Physiol Lung Cell Mol Physiol 308: L1178–L1188, 2015. doi: 10.1152/ajplung.00290.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacob A, Morley M, Hawkins F, McCauley KB, Jean JC, Heins H, Na CL, Weaver TE, Vedaie M, Hurley K, Hinds A, Russo SJ, Kook S, Zacharias W, Ochs M, Traber K, Quinton LJ, Crane A, Davis BR, White FV, Wambach J, Whitsett JA, Cole FS, Morrisey EE, Guttentag SH, Beers MF, Kotton DN. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 21: 472–488.e10, 2017. doi: 10.1016/j.stem.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jansing NL, McClendon J, Henson PM, Tuder RM, Hyde DM, Zemans RL. Unbiased quantitation of alveolar type II to alveolar type I cell transdifferentiation during repair after lung injury in mice. Am J Respir Cell Mol Biol 57: 519–526, 2017. doi: 10.1165/rcmb.2017-0037MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasper M, Barth K. Potential contribution of alveolar epithelial type I cells to pulmonary fibrosis. Biosci Rep 37: BSR20171301, 2017. doi: 10.1042/BSR20171301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949–1961, 2011. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 46.Kosmider B, Messier EM, Janssen WJ, Nahreini P, Wang J, Hartshorn KL, Mason RJ. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir Res 13: 43, 2012. doi: 10.1186/1465-9921-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McAuley DF, Curley GF, Hamid UI, Laffey JG, Abbott J, McKenna DH, Fang X, Matthay MA, Lee JW. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Physiol Lung Cell Mol Physiol 306: L809–L815, 2014. doi: 10.1152/ajplung.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McElroy MC, Kasper M. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur Respir J 24: 664–673, 2004. doi: 10.1183/09031936.04.00096003. [DOI] [PubMed] [Google Scholar]
- 50.Moore BB, Peters-Golden M, Christensen PJ, Lama V, Kuziel WA, Paine R III, Toews GB. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol 284: L342–L349, 2003. doi: 10.1152/ajplung.00168.2002. [DOI] [PubMed] [Google Scholar]
- 51.Pan T, Mason RJ, Westcott JY, Shannon JM. Rat alveolar type II cells inhibit lung fibroblast proliferation in vitro. Am J Respir Cell Mol Biol 25: 353–361, 2001. doi: 10.1165/ajrcmb.25.3.4004. [DOI] [PubMed] [Google Scholar]
- 52.Panos RJ, Rubin JS, Csaky KG, Aaronson SA, Mason RJ. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest 92: 969–977, 1993. doi: 10.1172/JCI116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M. Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle) 3: 445–464, 2014. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Portnoy J, Pan T, Dinarello CA, Shannon JM, Westcott JY, Zhang L, Mason RJ. Alveolar type II cells inhibit fibroblast proliferation: role of IL-1α. Am J Physiol Lung Cell Mol Physiol 290: L307–L316, 2006. doi: 10.1152/ajplung.00102.2005. [DOI] [PubMed] [Google Scholar]
- 55.Sakai N, Tager AM. Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim Biophys Acta 1832: 911–921, 2013. doi: 10.1016/j.bbadis.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schultz CJ, Torres E, Londos C, Torday JS. Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells. Am J Physiol Lung Cell Mol Physiol 283: L288–L296, 2002. doi: 10.1152/ajplung.00204.2001. [DOI] [PubMed] [Google Scholar]
- 57.Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol 66: 625–645, 2004. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- 58.Shannon JM, Pan T, Nielsen LD, Edeen KE, Mason RJ. Lung fibroblasts improve differentiation of rat type II cells in primary culture. Am J Respir Cell Mol Biol 24: 235–244, 2001. doi: 10.1165/ajrcmb.24.3.4302. [DOI] [PubMed] [Google Scholar]
- 59.Sirianni FE, Chu FS, Walker DC. Human alveolar wall fibroblasts directly link epithelial type 2 cells to capillary endothelium. Am J Respir Crit Care Med 168: 1532–1537, 2003. doi: 10.1164/rccm.200303-371OC. [DOI] [PubMed] [Google Scholar]
- 60.Sirianni FE, Milaninezhad A, Chu FS, Walker DC. Alteration of fibroblast architecture and loss of Basal lamina apertures in human emphysematous lung. Am J Respir Crit Care Med 173: 632–638, 2006. doi: 10.1164/rccm.200509-1434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tahedl D, Wirkes A, Tschanz SA, Ochs M, Mühlfeld C. How common is the lipid body-containing interstitial cell in the mammalian lung? Am J Physiol Lung Cell Mol Physiol 307: L386–L394, 2014. doi: 10.1152/ajplung.00131.2014. [DOI] [PubMed] [Google Scholar]
- 62.Tomashefski JF., Jr Pulmonary pathology of the adult respiratory distress syndrome. Clin Chest Med 11: 593–619, 1990. [PubMed] [Google Scholar]
- 63.Torday JS, Rehan VK. On the evolution of the pulmonary alveolar lipofibroblast. Exp Cell Res 340: 215–219, 2016. doi: 10.1016/j.yexcr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uhal BD, Nguyen H. The Witschi hypothesis revisited after 35 years: genetic proof from SP-C BRICHOS domain mutations. Am J Physiol Lung Cell Mol Physiol 305: L906–L911, 2013. doi: 10.1152/ajplung.00246.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaccaro C, Brody JS. Ultrastructure of developing alveoli. I. The role of the interstitial fibroblast. Anat Rec 192: 467–479, 1978. doi: 10.1002/ar.1091920402. [DOI] [PubMed] [Google Scholar]
- 66.Volckaert T, De Langhe S. Lung epithelial stem cells and their niches: Fgf10 takes center stage. Fibrogenesis Tissue Repair 7: 8, 2014. doi: 10.1186/1755-1536-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volckaert T, De Langhe SP. Wnt and FGF mediated epithelial-mesenchymal crosstalk during lung development. Dev Dyn 244: 342–366, 2015. doi: 10.1002/dvdy.24234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol 36: 661–668, 2007. doi: 10.1165/rcmb.2006-0410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Curr Top Dev Biol 90: 73–158, 2010. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu X, Dai H, Wang C. Epithelium-dependent profibrotic milieu in the pathogenesis of idiopathic pulmonary fibrosis: current status and future directions. Clin Respir J 10: 133–141, 2016. doi: 10.1111/crj.12190. [DOI] [PubMed] [Google Scholar]
- 71.Yano T, Deterding RR, Simonet WS, Shannon JM, Mason RJ. Keratinocyte growth factor reduces lung damage due to acid instillation in rats. Am J Respir Cell Mol Biol 15: 433–442, 1996. doi: 10.1165/ajrcmb.15.4.8879176. [DOI] [PubMed] [Google Scholar]