Abstract
Measures of aortic stiffness and pressure and flow pulsatility have emerged as correlates of and potential contributors to cardiovascular disease, dementia, and kidney disease. Higher aortic stiffness and greater pressure and flow pulsatility are associated with excessive pulsatile load on the heart, which increases mass and reduces global longitudinal strain of the left ventricle. Excessive stiffness and pulsatility are also associated with microvascular lesions in high-flow organs, such as the brain and kidney, suggesting that small vessels in these organs are damaged by pulsatility. This brief review will summarize evidence relating aortic stiffness to cardiovascular, brain, and kidney disease.
Keywords: aorta, cardiovascular disease, chronic kidney disease, dementia, hemodynamics, pulsatility index
INTRODUCTION
With advancing age, aortic wall stiffness increases monotonically, resulting in widening pulse pressure (PP; i.e., systolic minus diastolic pressure) and incident hypertension (71). Measures of aortic stiffness, such as carotid-femoral pulse-wave velocity (CFPWV) and pressure and flow pulsatility, have emerged as correlates of and potential contributors to cardiovascular disease (CVD), dementia, and kidney disease (12, 25–27, 64, 67, 100, 101). Higher aortic stiffness and greater pressure and flow pulsatility are associated with excessive pulsatile load on the heart, which contributes to an increase in mass and reduction in longitudinal strain of the left ventricle (LV) (8, 11, 49). Aortic stiffness and hemodynamic pulsatility are also associated with microvascular lesions in high-flow organs, such as the brain and kidney, suggesting that small vessels, which are exposed to arterial levels of pressure and flow pulsatility in these low-impedance organs, are damaged by excessive pulsatility (59). This brief review will summarize recent evidence showing strong relations of aortic stiffness with cardiovascular, brain, and kidney disease.
AORTIC STIFFNESS AND PULSATILE HEMODYNAMICS
Factors that contribute to aortic stiffness are complex and incompletely elucidated but include the remodeling and breakdown of long-lived elastic fibers and progressive deposition and engagement of much stiffer collagen fibers in the aortic wall. Elastic fibers in the aortic media are organized as a complex, lamellar mesh, consisting primarily of elastin, fibrillins, and fibulins (5). Elastic fibers are synthesized and crosslinked in a carefully orchestrated process that begins during embryonic organogenesis and ends at approximately the toddler stage of development for most mammals, including humans (82). After this early-life phase of development, the gene program for synthesis of elastic fibers is silenced, possibly through post-transcriptional mechanisms (48, 77), resulting in a relatively fixed complement of elastic fibers that must survive the full lifespan (29). Subsequent growth causes the early aorta to remodel to increase length and lumen diameter to accommodate increasing body height and blood flow, respectively. Aortic diameter continues to increase throughout the human life course, and the increase is exacerbated by obesity (52). Since the pool of elastin is relatively fixed from early childhood onward, aortic remodeling—to greater diameter and length—thins the pool of elastic fibers and by the law of Laplace, places the fibers under higher tension. The combination of thinning and increased tension increases elastic fiber stress, resulting in increased fractional engagement of collagen, which will stiffen the aortic wall (3). In addition, fragmentation and loss of elastic fibers amplify loading of remaining elastic fibers and are associated with increased deposition and crosslinking of collagen, all of which contribute to the stiffening of the aortic wall (82).
Prior work assumed that aortic stiffness was a consequence of accelerated degeneration of elastin in the presence of longstanding hypertension. However, work in humans (50) and animal models (98) has shown that stiffness antedates and contributes to the pathogenesis of hypertension. Aortic stiffness is already elevated in nonhypertensive children whose parents had hypertension, consistent with a genetic component and the concept that stiffness antedates development of hypertension (2). Aortic stiffness, like hypertension, is highly heritable. Prior work from the Framingham Heart Study (FHS) found that CFPWV has a heritability of 40% (61). The largest genome-wide association study to date, performed by the AortaGen Consortium, found a locus on chromosome 14 that is associated with CFPWV (69). The locus, which is in a gene desert that is nearly 1 million bp away from the nearest named gene, contains a distal 3′ enhancer for the gene BCL11B. The product of this gene is known to be involved in aortic and brain development and also serves as the master regulator of T cell fate (4), which may be relevant in light of known relations among T cell function, hypertension, and aortic stiffness (6). Furthermore, the locus was associated with excess risk for incident coronary heart disease and heart failure, consistent with the concept that aortic stiffness represents a true risk factor that contributes to the pathogenesis of CVD.
The effects of arterial remodeling on pressure and flow pulsatility compared with measures of wave propagation, such as pulse-wave velocity (PWV), are complex because of differing nonlinear relations of various “stiffness” measures with wall stiffness and lumen area. The principle determinant of pressure pulsatility in a single, uniform arterial segment is characteristic impedance (Zc), which quantifies the pulsatile pressure (dP) produced by a given pulsatile flow (dQ) in the absence of wave reflection: Zc = dP/dQ. Various stiffness measures (S), including Zc and PWV, have the general form: S ≈ √(Eh/rx), where the product of Young’s modulus (E) and wall thickness (h) is divided by lumen radius (r) raised to a power (x) that depends on the stiffness measure. The Moens-Korteweg equation shows that x = 1 for PWV, meaning that PWV is relatively insensitive to diameter. In contrast, the water hammer equation shows that x = 5 for characteristic impedance. Thus whereas Zc and PWV have similar relations with wall stiffness (Eh), Zc is five times more sensitive to lumen size than PWV (65). As a result, when the aorta remodels to a larger diameter, it is possible for PWV to increase, because of thinning and greater stress on the wall constituents, which increases E, even as Zc falls, because the increase in lumen radius, when raised to the 5th power, has a larger effect on Zc than the modest, early increase in E (58).
The aortic diameter increases continuously throughout the full life course, probably as a result of active remodeling and degenerative processes (52). However, people with higher PP have smaller aortic diameters overall and after adjusting for various confounders. The inverse relation between aortic diameter and PP suggests that excessive remodeling stress, due to an increase in cardiac output (somatic growth, obesity) or a limited elastin pool from birth, may lead to a mismatch among aortic wall stiffness, diameter, and flow, resulting in larger forward pressure-wave amplitude and higher PP. In a tracking study in FHS participants, the aortic root diameter adjusted for body size was smaller and increased less with age and obesity in women. The smaller initial size of the aorta (adjusted for height and weight) in women may limit the ability of the aorta to remodel in the face of hemodynamic stress, such as an increase in cardiac output that accompanies somatic growth or obesity (52, 60, 62, 65). In a study of secular change in PP and mean arterial pressure (MAP) in children during onset of the obesity epidemic, PP increased, whereas MAP fell (102). Differences in body mass index across the observation period explained 32% of the increase in PP. The presence of obesity amplified the relation between height and PP, especially in girls, suggesting that obesity imposes an additional remodeling stress on the aorta at a time of rapid growth, leading to mismatch between cardiac output and aortic diameter. At the opposite end of the spectrum, with the use of detailed MRI and hemodynamics in older adults, the Age, Gene/Environment Susceptibility (AGES)-Reykjavik investigators showed that despite the lifelong increase in aortic diameter with increasing age, higher PP was associated with a smaller aortic diameter (91). The wall of the aorta was stiffer, and LV volume was greater in those with higher PP, suggesting that mismatch in aortic and ventricular adaptation to aortic wall stiffness and hemodynamic demand contributed to higher PP (91).
CROSSTALK BETWEEN AORTIC STIFFNESS AND MICROVASCULAR STRUCTURE AND FUNCTION
In the normal arterial system, the highly compliant elastic aorta is coupled to much stiffer muscular conduit vessels, creating an impedance gradient that increases progressively from heart to periphery. The ascending impedance gradient in a normal arterial system creates wave reflection, which reduces distal flow pulsatility and limits the amount of pulsatile energy that penetrates into the microcirculation. Microvascular structure and myogenic tone may be sensitive to PP, as well as MAP, with pulsatility promoting hypertrophy and increasing tone of resistance vessels (59). The microvascular response may limit penetration of pulsatility into the capillaries of high-flow organs, such as the brain and kidney (55). However, based on Ohm’s law, mean flow to a vascular bed is equal to MAP divided by peripheral resistance; if resistance vessels constrict or remodel in response to an increase in PP with no change in MAP, then resistance will increase, and mean flow will fall (59). Resistance vessel remodeling in response to increased pressure pulsatility (7, 23, 47) also limits the vasodilatory reserve (70). Thus increased PP with unchanged or falling MAP, as commonly occurs beyond 60 yr of age (37), could interfere with autoregulation of blood flow in critical organs, such as the brain and kidney (59).
Accentuated blood pressure variability is common in older people and may further sensitize the brain to harmful effects of impaired autoregulation or limited vasoreactivity. With the change from a supine to standing position, MAP normally increases to compensate for a moderate reduction in hydrostatic pressure at the level of the brain, which is 20–30 cm above heart level. The increase in MAP generally is insufficient to offset the reduction in hydrostatic pressure, so cerebrovascular vasodilation is required to keep upright brain perfusion similar to supine values. Higher values for key measures of aortic stiffness (CFPWV, Zc, and forward pressure-wave amplitude) are associated with a blunted increase in MAP on standing (90). Higher aortic stiffness is associated with greater short-term blood pressure variability throughout the day (87). The basis for greater blood pressure variability is complex and may involve potentially bidirectional relations of stiffness with baroreceptor function, cardiac structure and function, and damage to brain regions involved in blood pressure regulation (87). Greater average real variability in ambulatory blood pressure was associated with prevalent cerebral small vessel disease in hypertensive adults (35). A blunted orthostatic increase in MAP was associated with smaller brain volumes and worse executive function, and effects were modulated by age and aortic stiffness in FHS (24). Orthostatic hypotension, greater orthostatic variability in systolic blood pressure (SBP), and blunted heart rate response to orthostasis were associated with increased risk for incident dementia during 15 yr of follow-up in the Rotterdam Study (99).
Excessive aortic stiffness also interferes with the conduit vessel response to changes in hemodynamic demand. Greater aortic stiffness is associated with diastolic flow reversal in the descending aorta and brachial and femoral arteries (15, 41, 66). Individuals with diastolic flow reversal have lower forearm microvascular-reactive hyperemia and lower flow-mediated dilation of the brachial artery (15). The relation between flow-mediated dilation and the hyperemic flow stimulus is blunted in the presence of diastolic flow reversal. Thus stiffening can interfere with flow-mediated dilation, both by the reduction of the hyperemic stimulus and by the production of abnormal flow patterns that adversely affect endothelial function. Conversely, abnormal endothelial function may modulate aortic stiffness by the alteration of the activation state of smooth muscle in the aortic wall (74), resulting in the potential for a vicious cycle of stiffening and endothelial dysfunction. As a result of the foregoing effects, blunted microvascular hyperemia is associated with CVD events and partially mediates the relation between aortic stiffness and CVD events (1, 25).
AORTIC STIFFNESS AND INCIDENT HYPERTENSION
Abnormal aortic stiffness is often considered a consequence of longstanding hypertension. The transition from predominant elevation of diastolic blood pressure (DBP) and MAP to predominant or isolated elevation of SBP and PP was thought to represent “burned out” essential hypertension, wherein prolonged elevation of MAP and DBP led to accelerated aging and stiffening of the aorta, widening of PP with a fall in DBP, and a transition to isolated systolic hypertension (18, 38). Because of nonlinearities in aortic wall properties, aortic stiffness increases with distending pressure, suggesting that hypertension could contribute to development of aortic stiffness (36). Indeed, there is evidence that aortic stiffening is accelerated in the presence of hypertension (13). However, recent evidence suggests that progression from isolated diastolic hypertension to systolic hypertension is relatively unusual. Franklin et al. (38) examined relations between antecedent blood pressure and the hypertension subtype in FHS participants and found that isolated systolic hypertension generally developed de novo in individuals with no history of hypertension. We subsequently measured blood pressures and aortic stiffness (CFPWV) at two points in time, separated by 6 yr in the FHS Offspring cohort, and found that baseline CFPWV was a strong predictor of blood pressure progression and incident hypertension (50). In contrast, after accounting for baseline CFPWV, no blood pressure measure (SBP, DBP, MAP, or PP) entered a model for follow-up CFPWV. As noted above, a parental history of hypertension is associated with higher aortic stiffness in their nonhypertensive children, consistent with the concept that stiffness antedates and contributes to the pathogenesis of hypertension (2). In a mouse model of dietary obesity, aortic stiffening (higher aortic PWV) developed at 1 mo and preceded the onset of hypertension by 5 mo (98). Importantly, blood pressure and aortic stiffness returned to normal within 2 mo after resuming a normal diet. The foregoing studies provide strong support for the hypothesis that aortic stiffness is a contributor to pathogenesis rather than a consequence of hypertension.
AORTIC STIFFNESS AND LV STRUCTURE AND FUNCTION: MECHANICAL COUPLING
The burden of heart failure-related disease has increased in the past two decades (85). The prevalence of heart failure with a preserved LV ejection fraction (HFpEF) is increasing out of proportion to heart failure with a reduced ejection fraction (78, 96). Hemodynamic mechanisms that contribute to development of heart failure are diverse, particularly in HFpEF, and rational treatment will require knowledge of the components of hemodynamic load in an individual patient (88). Despite a number of large-scale clinical trials, no treatment has been identified that modifies the course of HFpEF, possibly reflecting mechanistic diversity of the HFpEF syndrome (88). Although the pathophysiology of HFpEF is heterogeneous, age, sex, hypertension, and obesity are key risk factors, with LV diastolic dysfunction as a central pathophysiological substrate (39). Aortic stiffness is also associated with advancing age, hypertension, and obesity and has been proposed as a major contributor to HFpEF (30), particularly in women (28, 84). Aortic stiffening increases hemodynamic load on the heart and is associated with LV remodeling, hypertrophy, and impaired diastolic function (19, 28, 49). In the older FHS Offspring cohort, higher CFPWV was associated with increased risk for incident heart failure (HFpEF and heart failure with a reduced ejection fraction) after adjusting for standard risk factors, including MAP (94).
Whereas the foregoing adverse consequences of abnormal hemodynamic coupling between the LV and a stiff aorta are well known, the implications of impaired mechanical coupling between LV and proximal aorta have only recently been elucidated (9–11). The proximal aorta is stretched longitudinally by LV contraction, which pulls the base of the heart toward the stationary apex (Fig. 1). The force associated with the longitudinal stretch of the proximal “aortic spring” is considerable, compared with the force associated with pressure generation (11). This force places an asymmetric mechanical load on the LV long axis that had not been considered previously when assessing total LV load. Importantly, much of the work associated with aortic stretch may be recovered as diastolic elastic recoil, which lifts the base of the heart, elongates the LV, and facilitates transfer of blood from the left atrium into LV (Fig. 1). We have proposed that recoil of the aortic spring provides a major component of the LV recoil force that is required to generate ventricular suction and brisk filling during early diastole. Work done on the proximal aorta during systole, defined as the integral of longitudinal force and aortic displacement in systole, is recovered as enhanced early diastolic filling in older men but not older women in the AGES-Reykjavik cohort (11). Although reasons for these sex differences are unclear, the observation is consistent with the higher risk for HFpEF in older women. In FHS participants, as the proximal aorta stiffens, LV global longitudinal strain (GLS) is reduced, consistent with the hypothesis that the stiffening of the proximal aorta increases force required to stretch the aortic spring and specifically loads the LV long axis (8). We have also shown that higher aortic stiffness is associated with lower LV early diastolic filling, as assessed by mitral annulus tissue Doppler velocity (49). In addition, failure of the aortic spring mechanism would be expected to increase left atrial pressure and cause remodeling and fibrosis, which may provide the substrate for development of atrial fibrillation in individuals with a stiffened aorta (68).
Fig. 1.
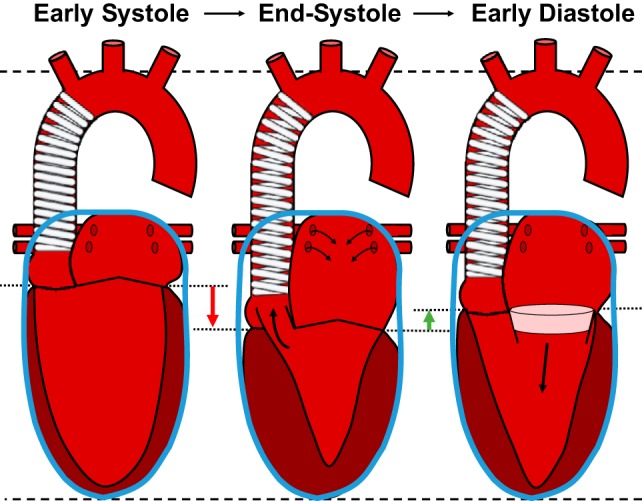
Proximal aortic “spring” plays a critical role in normal systolic and early diastolic function of the left ventricle (LV). The aortic arch and cardiac apex are relatively fixed during the cardiac cycle (dashed lines). In addition, total heart volume is nearly constant throughout the cardiac cycle (blue containers) because of pericardial constraint (only the left heart is shown for clarity). During systole, LV long axis shortening (global longitudinal strain) pulls the base of the heart (dotted lines) toward the apex (red arrow). This atrioventricular plane displacement ejects blood from the LV (middle; thick, black arrow) but also fills the left atrium (thin, black arrows) and stretches and stores energy in the walls of the proximal aortic spring. During diastole (right), recoil of the aortic spring pulls the base of the heart upward (green arrow), which translocates a volume of blood from the left atrium into the LV (pink disk), because of motion of the atrioventricular plane alone, but also stretches and thins the walls of the LV, creating suction that facilitates early diastolic filling (thick, black arrow). [Reprinted by permission from Springer Nature Current Hypertension Reports (c) 2015 Bell and Mitchell (9).]
Atrioventricular plane displacement (AVPD), because of LV GLS, is a strong determinant of global LV systolic and diastolic function. Approximately 60–80% of LV stroke volume and much of early diastolic filling are attributable to AVPD (20). Tight coupling between AVPD and global cardiac function is a consequence of fixed total heart volume during the cardiac cycle because of constraints imposed by the pericardium and helical myocardial fibers (Fig. 1). Fixed external cardiac volume increases pumping efficiency, because no energy is wasted moving surrounding tissues (14, 43). Because of fixed total volume, AVPD, in the apical direction during ventricular contraction, is analogous to a piston in a cylinder that pushes blood out of the ventricles, while simultaneously pulling blood into the atria (Fig. 1). The substantial volume of incompressible myocardium below the atrioventricular plane amplifies the LV ejection fraction by fivefold relative to GLS during AVPD, as inward thickening of the externally constrained myocardium further reduces LV chamber volume (21, 33). Thus modest LV GLS (12%) produces an amplified ejection fraction (60%); conversely, a modest reduction in GLS can have a marked effect on the LV ejection fraction.
Diastolic LV function underscores a limitation of our current understanding of the AVPD model of cardiac function. Various models (ventricular twist) and elastic elements (titin) have been proposed as the basis for restoring forces that create suction in and efficient filling of the LV during early diastole. We hypothesized that the considerable force generated during systolic loading of the aortic spring provides the elastic recoil required for early diastolic AVPD toward the atria. AVPD toward the atria will transfer a component of blood from atrium to ventricle by simply translocating the mitral annulus around a discoid volume of blood. In addition, the thinning of the myocardium, because of the long axis stretch associated with early diastolic AVPD and fixed total heart volume, will generate the suction that pulls bulk flow across the tricuspid and mitral valves and into the right ventricle and LV. Elastic recoil of compressed titin (40) and ventricular untwisting (83, 89) contributes to transfer of blood from atria to ventricles but is unlikely to provide adequate force for brisk and efficient transfer of a large volume of blood from atria to ventricles during early diastole. Similarly, although contractile elements can pull vigorously, they cannot push effectively and hence, cannot generate or add substantively to the restoring force required to generate ventricular suction and rapid early diastolic filling (16). However, active relaxation of contractile elements will modulate (i.e., may slow or limit) elastic recoil. For example, the calcium transient is prolonged, and active relaxation is impaired in the hypertrophied myocardium (17). Thus aortic stiffening can worsen diastolic function, both by the limitation of AVPD mechanically, as well as by the stimulation of hypertrophy, remodeling, and dysfunction at the level of the myocyte.
EXCESSIVE AORTIC STIFFNESS AND BRAIN STRUCTURE AND FUNCTION
Age is a powerful predictor of cognitive decline and incident dementia in the community. Brain blood flow and parenchymal volume decline and cortical and subcortical infarcts and white matter hyperintensities (WMHs) detectable by MR increase with age. Mild cognitive impairment is increasingly prevalent with advancing age and has emerged as an important preclinical stage of dementia, including Alzheimer’s disease. Although several important risk factors for abnormal brain structure and function, including vascular risk factors, have been identified, a considerable component of the age-related decline remains undefined.
Previous work suggests that increased aortic stiffness may contribute to the unexplained component of decline in brain function with age (54, 67, 79). A number of studies have demonstrated relations of vascular factors, including hypertension, with the presence or severity of subcortical infarcts, WMH, and brain atrophy (42, 51, 67). The AGES-Reykjavik investigators used comprehensive pulsatile hemodynamics and brain MR to examine relations among aortic stiffness, carotid stiffness, and brain small vessel disease. Greater aortic stiffness was associated with a reduction in local wave reflection and an increase in transmission of pulsatility into the cerebrovascular circulation (Fig. 2). Higher aortic stiffness and carotid flow pulsatility were associated with small vessel disease in the brain, including higher prevalence of subcortical infarcts, larger WMH volume, and lower brain volumes. The carotid pulsatility index was associated with lower scores in all cognitive domains. The foregoing observations are consistent with the hypothesis that disproportionate stiffening of the aorta leads to impedance matching, which reduces wave reflection and increases transmission of harmful pulsatility into the microcirculation, where it causes small vessel damage that is quantifiable by imaging and associated with reduced function, as was evident in cognitive testing.
Fig. 2.
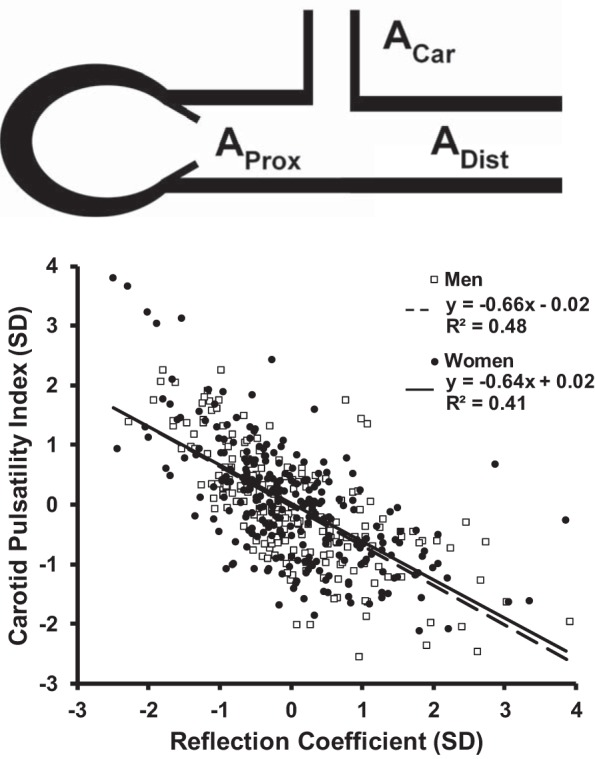
Impedance matching in the cerebrovasculature. Top: a simple model of the aorta and cerebrovascular circuit. To facilitate calculation of the reflection coefficient, local properties are expressed as the admittance, which is the reciprocal of local characteristic impedance. Aortic admittance (AProx) is coupled to a lumped bilateral carotid admittance (ACar) and distal aortic admittance (ADist). The reflection coefficient (RC) at this interface is RC = (AProx – ACar – ADist)/(AProx + ACar + ADist). Disproportionate stiffening of the proximal aorta reduces proximal admittance (AProx) and thereby reduces wave reflection. Bottom: a reduction in local wave reflection is associated with a reciprocal increase in the pulsatility of the flow waveform entering the carotid circulation. [Bottom is modified from Mitchell et al. (67) by permission of Oxford University Press.]
A limited number of studies have shown similar relations of aortic stiffness with cerebral microbleeds and dilated perivascular or Virchow-Robin spaces (32, 81, 86, 97). Carotid stiffness was associated with incident deep but not cortical cerebral microbleeds in the AGES-Reykjavik Study (31). Findings are consistent with greater exposure of deep structures, which are perfused by short, penetrating arteries that arise directly from the circle of Willis at the base of the brain, to higher pulsatility in patients with a stiff aorta. In contrast, the cortex is perfused by penetrating branches of the superficial, circuitous pial arterial network; these long, muscular arteries damp pulsations and protect the cortex. The Virchow-Robin spaces play a key role in the “glymphatic” system, which is responsible for clearing toxins, such as amyloid, from the brain (46). Higher aortic (brachial-ankle or carotid-femoral) PWV was associated with amyloid positivity and progression in cognitively intact older adults (44, 45). Higher PP was associated with higher levels of phosphorylated tau and lower levels of amyloid beta 1–42 in the cerebrospinal fluid and more rapid progression to dementia in 877 older adults without overt dementia at baseline (72, 73). Therefore, relations between arterial stiffness and amyloid deposition in the brain may involve pulsatile damage to the glymphatic system.
In the younger FHS Generation 3 cohort, higher CFPWV was associated with impaired processing speed and executive function, larger lateral ventricle volumes, and higher WMH volume (80). With the use of diffusion-weighted imaging, we demonstrated that CFPWV was associated with lower fractional anisotropy, indicative of early damage to white matter fiber tracts, and lower gray matter density in deep gray matter (57). The foregoing deep regions of the brain are particularly susceptible to excessive pulsatility, because they are perfused from below by penetrating branch vessels that are less able to damp pulsatility. We also found evidence that CFPWV and SBP were associated with higher free-water content, an early marker of microvascular brain injury, in a large volume (most) of cerebral white matter. Sequential mediation analyses demonstrated that higher free-water content was associated with lower anisotropy (greater disorganization) in a moderate volume of white matter and the presence of WMH in a modest but important volume of white matter, consistent with a pathophysiologic paradigm, wherein transmission of excessive pulsatility into the cerebrovasculature initially causes modest but diffuse damage in white matter that interferes with fiber organization and eventually progresses to fiber loss and scarring (56). In Generation 3, we also demonstrated that excessive aortic stiffness was associated with a blunted increase in MAP on standing, which can render the brain susceptible to ischemia, particularly if small vessel function is impaired as a result of excessive pulsatility (90).
We evaluated relations between vascular stiffness and change in measures of executive function, which are thought to be particularly sensitive to vascular insults. In a model that adjusted for age, sex, cognitive measure at baseline, and various potential confounders (depression scale, time to MR and neurocognitive testing, diabetes mellitus, atrial fibrillation, current smoking, hypertensive therapy, prevalent CVD, apolipoprotein E genotype, homocysteine, and the fourth quartile of waist/hip ratio), higher CFPWV and central PP were associated with deterioration in measures of executive performance (trails test and similarities test, respectively) (93).
In the AGES-Reykjavik cohort, mediation analysis demonstrated that 41% of the relation between higher CFPWV and lower memory scores was mediated by higher cerebrovascular resistance and greater WMH volume, which are markers of small vessel damage in the brain (27). Higher CFPWV was also associated with higher scores on the 15-item geriatric depression scale. A separate mediation analysis demonstrated that small vessel disease in deep white matter (subcortical infarcts and WMH volume) mediated the relation between CFPWV and depressive symptoms (95), possibly as a result of damage to deep white and gray matter structures that are perfused by penetrating arteries that arise directly from the circle of Willis and are less able to damp excessive pulsatility. Importantly, models that examined relations between cognitive function and arterial stiffness were adjusted for depressive symptoms. The foregoing studies suggest that aortic stiffness and microvascular damage play a fundamental role in the pathogenesis of depression, cognitive decline, and dementia in older people and may represent targets for interventions to prevent or delay development of cognitive impairment.
EXCESSIVE AORTIC STIFFNESS AND KIDNEY STRUCTURE AND FUNCTION
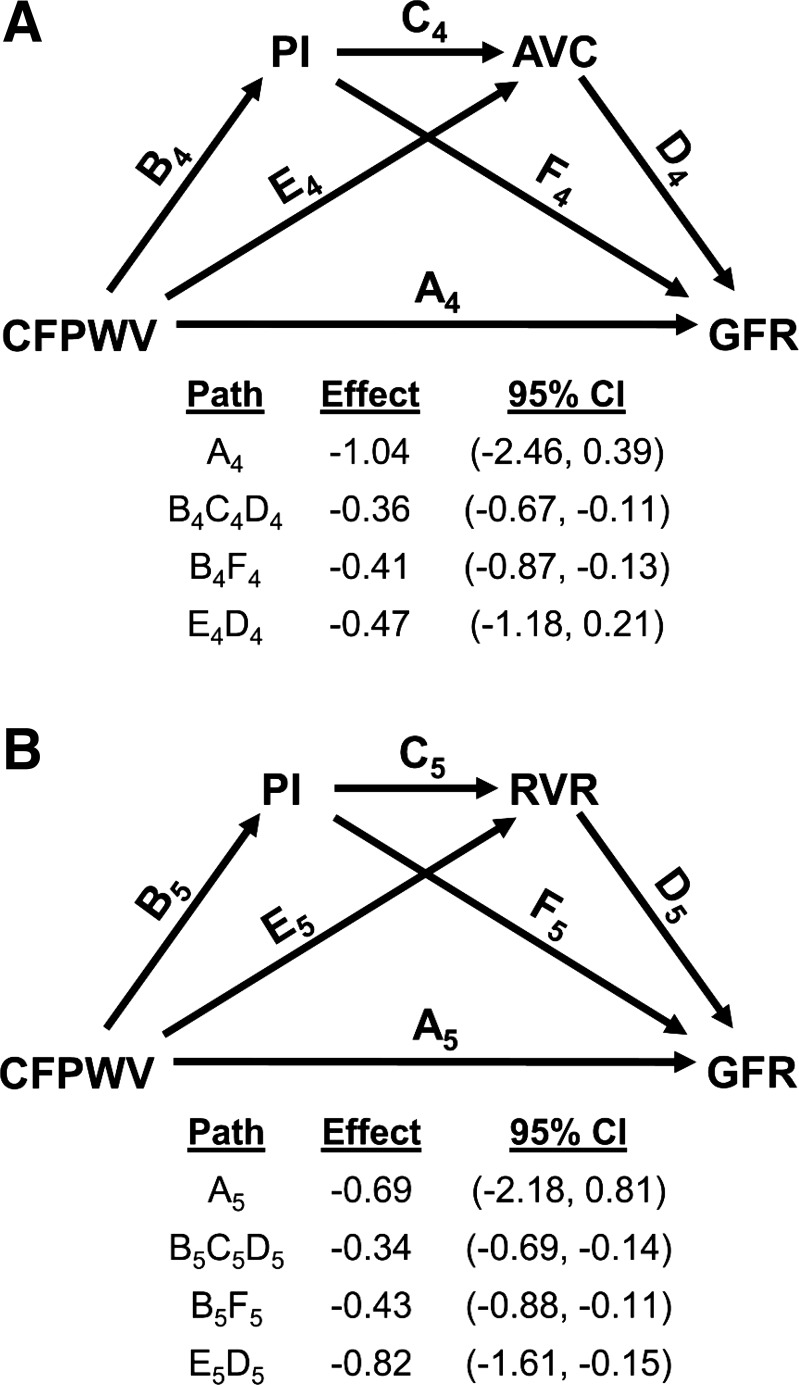
AGES-Reykjavik investigators demonstrated relations between higher aortic stiffness (CFPWV) and lower measured glomerular filtration rate (GFR) (34, 100). Higher CFPWV was associated with greater pulsatility of flow in the renal arteries, which was measured using phase-contrast MR. With the use of an MR kidney-segmentation approach, we were able to assess arterial microvascular volume of the renal cortex and demonstrated a lower volume of small arteries in the renal cortex in the presence of higher CFPWV (100, 101). Formal mediation analysis demonstrated that 34% of the relation between CFPWV and measured GFR was attributable to effects of aortic stiffness on the renal artery flow pulsatility index and that an additional 20% or 36% of the relation was mediated by lower arterial volume in the cortex or higher renal vascular resistance, respectively, when offered as mediators downstream from a higher pulsatility index (Fig. 3). The foregoing observations are consistent with the concept that the stiffening of the aorta reduces impedance mismatch between aorta and renal arteries, which reduces local wave reflection, increases flow pulsatility, damages small arteries and glomeruli in the renal cortex, and impairs function.
Fig. 3.
Relations among aortic stiffness, renal artery flow pulsatility, and kidney structure and function. In the older Age, Gene/Environment Susceptibility-Reykjavik Study cohort, there was a relation between carotid-femoral pulse-wave velocity (CFPWV) and measured glomerular filtration rate (GFR). The total effect for the relation between CFPWV and GFR was −2.28 ml/min per SD. Mediation analysis demonstrated that most of the effect was attributable to indirect effects mediated through excessive renal artery flow pulsatility index (PI), which damages small vessels, leading to loss of arterial volume in the cortex (AVC; A) or an increase in renal vascular resistance (RVR; B), accounting for 54% and 70% of the total effect, respectively. CI, confidence interval. [Modified from Woodard et al. (100) with permission.]
SEX DIFFERENCES IN PULSATILE HEMODYNAMICS
As noted above, substantive sex differences in age relations and adverse consequences of aortic stiffening have been observed. Beginning in childhood (102) and extending through midlife (65) and old age (52), aortic stiffening and impaired remodeling in response to age and various exposures, such as excess body weight, are amplified in women. Adverse effects of aortic stiffening on LV structure (22) and systolic (8) and diastolic (11) function are enhanced in women and may contribute to excess risk for HFpEF in women. These observations underscore the critical need for additional research that seeks to elucidate genetic, hormonal, and environmental contributors to sex differences in aortic structure and function and adverse implications of aortic stiffening.
EXCESSIVE AORTIC STIFFNESS AND MAJOR CVD EVENTS
Higher CFPWV, a measure of aortic wall stiffness and the kinetic energy carried in pressure and flow waves, is associated with higher CVD risk and meaningful reclassification of risk in models that include standard CVD risk factors (Fig. 4) (12, 64). Similarly, excessive pressure pulsatility, a measure of the potential energy of the pressure wave, is also associated with excess CVD risk. The risk associated with pressure pulsatility is attributable to the forward wave component, not reflected wave amplitude or the reflection coefficient, and persists when CFPWV is added to the risk model, indicating that excesses of either potential or kinetic energy or both in the pressure and flow waveforms are potentially harmful (26). As noted above, a component of global CVD risk associated with excessive aortic stiffness is attributable to abnormalities in microvascular reactivity (25). In an analysis of relations between CFPWV and hypertension, CFPWV was associated with events, regardless of hypertension status; participants with hypertension and elevated CFPWV were at the highest risk. The prevalence of high CFPWV was exceedingly high in participants with uncontrolled (90%) compared with controlled (60%) hypertension or normal blood pressure (34%), suggesting that high aortic stiffness may complicate control of blood pressure or that poorly controlled blood pressure may accelerate ongoing damage to the aorta (75).
Fig. 4.
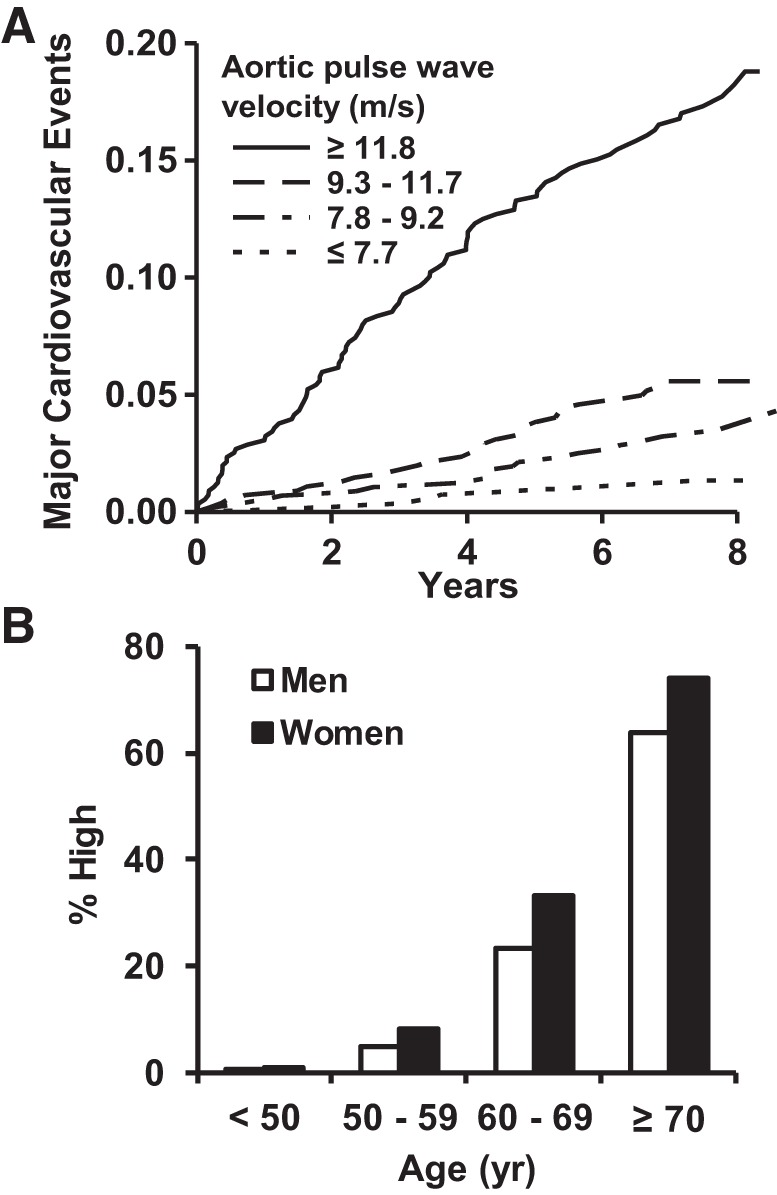
Relations between aortic stiffness and incident cardiovascular disease (CVD). A: relations between carotid femoral pulse-wave velocity (CFPWV) and a first major CVD event. B: the prevalence of CFPWV > 12 m/s, which corresponds to the highest quartile group in A. [Modified from Mitchell et al. (63, 64) with permission of Wolters Kluwer Health, Inc.]
Although research frequently focuses on abnormalities in function, a recent study examined correlates and implications of healthy vascular aging (HVA), defined as the absence of hypertension and CFPWV <7.6 m/s (76). HVA was uncommon by midlife (30% prevalence of HVA in the 50- to 59-yr age group) and vanishingly rare (<1%) after 70 yr of age. During a decade of follow-up, the presence of HVA was associated with a markedly reduced risk of CVD events (hazard ratio = 0.45, 0.26–0.77). Furthermore, a 1-unit increase in the American Heart Association’s Life’s Simple 7 was associated with 1.55-fold odds for the presence of HVA. The components of Life’s Simple 7 most closely associated with HVA were obesity and diabetes, which are also associated with longitudinal change in aortic stiffness (103), suggesting that relatively straightforward lifestyle interventions may prevent stiffening (53), promote HVA, and reduce CVD risk.
CONCLUSIONS
Over the past two decades, aortic stiffness has emerged as a major new CVD risk factor. In addition, strong relations among aortic stiffness, microvascular damage, and dysfunction in the brain and kidneys underscore the strong vascular contribution to dementia and chronic kidney disease. A recent statement from the American Heart Association underscored the importance of CFPWV as a measure of aortic stiffness and independent predictor of CVD risk (92). That review provides a comprehensive summary of important details of acquisition and interpretation of stiffness measures and highlights a number of additional challenges that require further work. There is presently strong evidence that aortic stiffening contributes to both risk for and pathogenesis of target organ damage and CVD. Several small studies have shown that aortic stiffening is not irreversible as once thought but rather, can be favorably modified by various lifestyle and pharmacologic interventions. However, no study, to date, has demonstrated that a treatment-related reduction in aortic stiffness translates into a reduction in events. Future studies will be required to close the loop and bring aortic stiffness into the mainstream of modifiable risk factors.
GRANTS
Support for this work was provided by the National Heart, Lung, and Blood Institute (Grants N01-HC-25195, HHSN268201500001I, HL-60040, HL-70100, HL-71039, HL-73551, HL-075795, HL-77234, HL-77447, HL-80124, and HL-126136) and by grants from the American Heart Association, Donald W. Reynolds Foundation, Bristol-Myers Squibb, AstraZeneca, and Novartis.
DISCLOSURES
G. F. Mitchell is owner of Cardiovascular Engineering, Inc., a company that designs and manufactures devices that measure vascular stiffness. He has received recent grants or consulting fees from Novartis, Servier, and Merck.
AUTHOR CONTRIBUTIONS
G.F.M. performed experiments; G.F.M. analyzed data; G.F.M. interpreted results of experiments; G.F.M. prepared figures; G.F.M. drafted manuscript; G.F.M. edited and revised manuscript; G.F.M. approved final version of manuscript.
REFERENCES
- 1.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) Study. Circulation 123: 163–169, 2011. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 2.Andersson C, Quiroz R, Enserro D, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Mitchell GF, Vasan RS. Association of parental hypertension with arterial stiffness in nonhypertensive offspring: the Framingham Heart Study. Hypertension 68: 584–589, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armentano RL, Barra JG, Levenson J, Simon A, Pichel RH. Arterial wall mechanics in conscious dogs. Assessment of viscous, inertial, and elastic moduli to characterize aortic wall behavior. Circ Res 76: 468–478, 1995. doi: 10.1161/01.RES.76.3.468. [DOI] [PubMed] [Google Scholar]
- 4.Avram D, Califano D. The multifaceted roles of Bcl11b in thymic and peripheral T cells: impact on immune diseases. J Immunol 193: 2059–2065, 2014. doi: 10.4049/jimmunol.1400930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin AK, Simpson A, Steer R, Cain SA, Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med 15: e8, 2013. doi: 10.1017/erm.2013.9. [DOI] [PubMed] [Google Scholar]
- 6.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T Regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 7.Baumbach GL. Effects of increased pulse pressure on cerebral arterioles. Hypertension 27: 159–167, 1996. doi: 10.1161/01.HYP.27.2.159. [DOI] [PubMed] [Google Scholar]
- 8.Bell V, McCabe EL, Larson MG, Rong J, Merz AA, Osypiuk E, Lehman BT, Stantchev P, Aragam J, Benjamin EJ, Hamburg NM, Vasan RS, Mitchell GF, Cheng S. Relations between aortic stiffness and left ventricular mechanical function in the community. J Am Heart Assoc 6: e004903, 2017. doi: 10.1161/JAHA.116.004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell V, Mitchell GF. Influence of vascular function and pulsatile hemodynamics on cardiac function. Curr Hypertens Rep 17: 68, 2015. doi: 10.1007/s11906-015-0580-y. [DOI] [PubMed] [Google Scholar]
- 10.Bell V, Mitchell WA, Sigurðsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, de Roos A, Gudnason V, Harris TB, Mitchell GF. Longitudinal and circumferential strain of the proximal aorta. J Am Heart Assoc 3: e001536, 2014. doi: 10.1161/JAHA.114.001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell V, Sigurdsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, Harris TB, Gudnason V, de Roos A, Mitchell GF. Relations between aortic stiffness and left ventricular structure and function in older participants in the Age, Gene/Environment Susceptibility–Reykjavik Study. Circ Cardiovasc Imaging 8: e003039, 2015. doi: 10.1161/CIRCIMAGING.114.003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646, 2014. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, Thomas F, Pannier B, Asmar R, Zureik M, Safar M, Guize L. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 105: 1202–1207, 2002. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 14.Bowman AW, Kovács SJ. Assessment and consequences of the constant-volume attribute of the four-chambered heart. Am J Physiol Heart Circ Physiol 285: H2027–H2033, 2003. doi: 10.1152/ajpheart.00249.2003. [DOI] [PubMed] [Google Scholar]
- 15.Bretón-Romero R, Wang N, Palmisano J, Larson MG, Vasan RS, Mitchell GF, Benjamin EJ, Vita JA, Hamburg NM. Cross-sectional associations of flow reversal, vascular function, and arterial stiffness in the Framingham Heart Study. Arterioscler Thromb Vasc Biol 36: 2452–2459, 2016. doi: 10.1161/ATVBAHA.116.307948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brutsaert DL, Housmans PR, Goethals MA. Dual control of relaxation. Its role in the ventricular function in the mammalian heart. Circ Res 47: 637–652, 1980. doi: 10.1161/01.RES.47.5.637. [DOI] [PubMed] [Google Scholar]
- 17.Brutsaert DL, Sys SU, Gillebert TC. Diastolic failure: pathophysiology and therapeutic implications. J Am Coll Cardiol 22: 318–325, 1993. doi: 10.1016/0735-1097(93)90850-Z. [DOI] [PubMed] [Google Scholar]
- 18.Bulpitt CJ, Palmer AJ, Fletcher AE, Bradley IC, Broxton JS, Davis AJ, Ganvir PL, Gostick NK, Mayhew SR, Mukerji D. Proportion of patients with isolated systolic hypertension who have burned-out diastolic hypertension. J Hum Hypertens 9: 675–678, 1995. [PubMed] [Google Scholar]
- 19.Canepa M, Alghatrif M, Strait JB, Cheng HM, Chuang SY, Chen CH, Brunelli C, Ferrucci L, Lakatta EG. Early contribution of arterial wave reflection to left ventricular relaxation abnormalities in a community-dwelling population of normotensive and untreated hypertensive men and women. J Hum Hypertens 28: 85–91, 2014. doi: 10.1038/jhh.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlsson M, Ugander M, Mosén H, Buhre T, Arheden H. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 292: H1452–H1459, 2007. doi: 10.1152/ajpheart.01148.2006. [DOI] [PubMed] [Google Scholar]
- 21.Çevik Y, Değertekin M, Başaran Y, Turan F, Pektaş O. A new echocardiographic formula to calculate ejection fraction by using systolic excursion of mitral annulus. Angiology 46: 157–163, 1995. doi: 10.1177/000331979504600210. [DOI] [PubMed] [Google Scholar]
- 22.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation 122: 570–578, 2010. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen KL. Reducing pulse pressure in hypertension may normalize small artery structure. Hypertension 18: 722–727, 1991. doi: 10.1161/01.HYP.18.6.722. [DOI] [PubMed] [Google Scholar]
- 24.Cooper LL, Himali JJ, Torjesen A, Tsao CW, Beiser A, Hamburg NM, DeCarli C, Vasan RS, Seshadri S, Pase MP, Mitchell GF. Inter-relations of orthostatic blood pressure change, aortic stiffness, and brain structure and function in young adults. J Am Heart Assoc 6: e006206, 2017. doi: 10.1161/JAHA.117.006206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper LL, Palmisano JN, Benjamin EJ, Larson MG, Vasan RS, Mitchell GF, Hamburg NM. Microvascular function contributes to the relation between aortic stiffness and cardiovascular events: the Framingham Heart Study. Circ Cardiovasc Imaging 9: e004979, 2016. doi: 10.1161/CIRCIMAGING.116.004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, Hamburg NM, Vasan RS, Mitchell GF. Components of hemodynamic load and cardiovascular events: the Framingham Heart Study. Circulation 131: 354–361, 2015. doi: 10.1161/CIRCULATIONAHA.114.011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper LL, Woodard T, Sigurdsson S, van Buchem MA, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Harris TB, Gudnason V, Launer LJ, Mitchell GF. Cerebrovascular damage mediates relations between aortic stiffness and memory. Hypertension 67: 176–182, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol 61: 96–103, 2013. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson JM, Hill KE, Alford JL. Developmental changes in collagen and elastin biosynthesis in the porcine aorta. Dev Biol 118: 103–111, 1986. doi: 10.1016/0012-1606(86)90077-1. [DOI] [PubMed] [Google Scholar]
- 30.Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail 15: 658–664, 2009. doi: 10.1016/j.cardfail.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Ding J, Mitchell GF, Bots ML, Sigurdsson S, Harris TB, Garcia M, Eiriksdottir G, van Buchem MA, Gudnason V, Launer LJ. Carotid arterial stiffness and risk of incident cerebral microbleeds in older people: the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study. Arterioscler Thromb Vasc Biol 35: 1889–1895, 2015. doi: 10.1161/ATVBAHA.115.305451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 41: 450–454, 2010. doi: 10.1161/STROKEAHA.109.564914. [DOI] [PubMed] [Google Scholar]
- 33.Emilsson K, Egerlid R, Nygren BM, Wandt B. Mitral annulus motion versus long-axis fractional shortening. Exp Clin Cardiol 11: 302–304, 2006. [PMC free article] [PubMed] [Google Scholar]
- 34.Fan L, Levey AS, Gudnason V, Eiriksdottir G, Andresdottir MB, Gudmundsdottir H, Indridason OS, Palsson R, Mitchell G, Inker LA. Comparing GFR estimating equations using cystatin c and creatinine in elderly individuals. J Am Soc Nephrol 26: 1982–1989, 2015. doi: 10.1681/ASN.2014060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filomena J, Riba-Llena I, Vinyoles E, Tovar JL, Mundet X, Castañé X, Vilar A, López-Rueda A, Jiménez-Baladó J, Cartanyà A, Montaner J, Delgado P; ISSYS Investigators . Short-term blood pressure variability relates to the presence of subclinical brain small vessel disease in primary hypertension. Hypertension 66: 634–640, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05440. [DOI] [PubMed] [Google Scholar]
- 36.Franklin SS. Arterial stiffness and hypertension: a two-way street? Hypertension 45: 349–351, 2005. doi: 10.1161/01.HYP.0000157819.31611.87. [DOI] [PubMed] [Google Scholar]
- 37.Franklin SS, Gustin W IV, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 96: 308–315, 1997. doi: 10.1161/01.CIR.96.1.308. [DOI] [PubMed] [Google Scholar]
- 38.Franklin SS, Pio JR, Wong ND, Larson MG, Leip EP, Vasan RS, Levy D. Predictors of new-onset diastolic and systolic hypertension: the Framingham Heart Study. Circulation 111: 1121–1127, 2005. doi: 10.1161/01.CIR.0000157159.39889.EC. [DOI] [PubMed] [Google Scholar]
- 39.Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, Rankin KM, Little WC. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 344: 17–22, 2001. doi: 10.1056/NEJM200101043440103. [DOI] [PubMed] [Google Scholar]
- 40.Hamdani N, Herwig M, Linke WA. Tampering with springs: phosphorylation of titin affecting the mechanical function of cardiomyocytes. Biophys Rev 9: 225–237, 2017. doi: 10.1007/s12551-017-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto J, Ito S. Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: potential implication for retrograde embolic stroke in hypertension. Hypertension 62: 542–549, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01318. [DOI] [PubMed] [Google Scholar]
- 42.Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, Lodder J, de Leeuw PW. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension 52: 1120–1126, 2008. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman EA, Ritman EL. Invariant total heart volume in the intact thorax. Am J Physiol 249: H883–H890, 1985. [DOI] [PubMed] [Google Scholar]
- 44.Hughes TM, Kuller LH, Barinas-Mitchell EJ, Mackey RH, McDade EM, Klunk WE, Aizenstein HJ, Cohen AD, Snitz BE, Mathis CA, Dekosky ST, Lopez OL. Pulse wave velocity is associated with β-amyloid deposition in the brains of very elderly adults. Neurology 81: 1711–1718, 2013. doi: 10.1212/01.wnl.0000435301.64776.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughes TM, Kuller LH, Barinas-Mitchell EJ, McDade EM, Klunk WE, Cohen AD, Mathis CA, Dekosky ST, Price JC, Lopez OL. Arterial stiffness and β-amyloid progression in nondemented elderly adults. JAMA Neurol 71: 562–568, 2014. doi: 10.1001/jamaneurol.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123: 1299–1309, 2013. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.James MA, Watt PA, Potter JF, Thurston H, Swales JD. Pulse pressure and resistance artery structure in the elderly. Hypertension 26: 301–306, 1995. doi: 10.1161/01.HYP.26.2.301. [DOI] [PubMed] [Google Scholar]
- 48.Johnson DJ, Robson P, Hew Y, Keeley FW. Decreased elastin synthesis in normal development and in long-term aortic organ and cell cultures is related to rapid and selective destabilization of mRNA for elastin. Circ Res 77: 1107–1113, 1995. doi: 10.1161/01.RES.77.6.1107. [DOI] [PubMed] [Google Scholar]
- 49.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Cheng S, Aragam J, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Relations of central hemodynamics and aortic stiffness with left ventricular structure and function: the Framingham Heart Study. J Am Heart Assoc 5: e002693, 2016. [Erratum in J Am Heart Assoc 5: e002100, 2016.] doi: 10.1161/JAHA.115.002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 308: 875–881, 2012. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, Lecompte T, Lacolley P, Benetos A, Zannad F. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke 40: 1229–1236, 2009. doi: 10.1161/STROKEAHA.108.532853. [DOI] [PubMed] [Google Scholar]
- 52.Lam CS, Xanthakis V, Sullivan LM, Lieb W, Aragam J, Redfield MM, Mitchell GF, Benjamin EJ, Vasan RS. Aortic root remodeling over the adult life course: longitudinal data from the Framingham Heart Study. Circulation 122: 884–890, 2010. doi: 10.1161/CIRCULATIONAHA.110.937839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LaRocca TJ, Martens CR, Seals DR. Nutrition and other lifestyle influences on arterial aging. Ageing Res Rev 39: 106–119, 2017. doi: 10.1016/j.arr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao D, Cooper L, Cai J, Toole J, Bryan N, Burke G, Shahar E, Nieto J, Mosley T, Heiss G. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology 16: 149–162, 1997. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- 55.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maillard P, Mitchell GF, Himali JJ, Beiser A, Fletcher E, Tsao CW, Pase MP, Satizabal CL, Vasan RS, Seshadri S, DeCarli C. Aortic stiffness, increased white matter free water, and altered microstructural integrity: a continuum of injury. Stroke 48: 1567–1573, 2017. doi: 10.1161/STROKEAHA.116.016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maillard P, Mitchell GF, Himali JJ, Beiser A, Tsao CW, Pase MP, Satizabal CL, Vasan RS, Seshadri S, DeCarli C. Effects of arterial stiffness on brain integrity in young adults from the Framingham Heart Study. Stroke 47: 1030–1036, 2016. doi: 10.1161/STROKEAHA.116.012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension 64: 210–214, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 105: 1652–1660, 2008. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell GF, Conlin PR, Dunlap ME, Lacourcière Y, Arnold JM, Ogilvie RI, Neutel J, Izzo JL Jr, Pfeffer MA. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension 51: 105–111, 2008. doi: 10.1161/HYPERTENSIONAHA.107.099721. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell GF, DeStefano AL, Larson MG, Benjamin EJ, Chen MH, Vasan RS, Vita JA, Levy D. Heritability and a genome-wide linkage scan for arterial stiffness, wave reflection, and mean arterial pressure: the Framingham Heart Study. Circulation 112: 194–199, 2005. doi: 10.1161/CIRCULATIONAHA.104.530675. [DOI] [PubMed] [Google Scholar]
- 62.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility–Reykjavik Study. Hypertension 51: 1123–1128, 2008. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation 115: 2628–2636, 2007. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- 64.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitchell GF, Lacourcière Y, Ouellet JP, Izzo JL Jr, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation 108: 1592–1598, 2003. doi: 10.1161/01.CIR.0000093435.04334.1F. [DOI] [PubMed] [Google Scholar]
- 66.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension 44: 134–139, 2004. [Erratum in Hypertension 45: e9, 2005.] doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 67.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik Study. Brain 134: 3398–3407, 2011. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, D’Agostino RB Sr, Kannel WB, Levy D, Benjamin EJ. Pulse pressure and risk of new-onset atrial fibrillation. JAMA 297: 709–715, 2007. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- 69.Mitchell GF, Verwoert GC, Tarasov KV, Isaacs A, Smith AV. Yasmin, Rietzschel ER, Tanaka T, Liu Y, Parsa A, Najjar SS, O’Shaughnessy KM, Sigurdsson S, De Buyzere ML, Larson MG, Sie MPS, Andrews JS, Post WS, Mattace-Raso FUS, McEniery CM, Eiriksdottir G, Segers P, Vasan RS, van Rijn MJE, Howard TD, McArdle PF, Dehghan A, Jewell ES, Newhouse SJ, Bekaert S, Hamburg NM, Newman AB, Hofman A, Scuteri A, De Bacquer D, Arfan Ikram M, Psaty BM, Fuchsberger C, Olden M, Wain LV, Elliott P, Smith NL, Felix JF, Erdmann J, Vita JA, Sutton-Tyrrell K, Sijbrands EJ, Sanna S, Launer LJ, De Meyer T, Johnson AD, Schut AF, Herrington DM, Rivadeneira F, Uda M, Wilkinson IB, Aspelund T, Gillebert TC, Van Bortel L, Benjamin EJ, Oostra BA, Ding J, Gibson Q, Uitterlinden AG, Abecasis GR, Cockcroft JR, Gudnason V, De Backer GG, Ferrucci L, Harris TB, Shuldiner AR, van Duijn CM, Levy D, Lakatta EG, Witteman JC. Common genetic variation in the 3′-BCL11B gene desert is associated with carotid-femoral pulse wave velocity and excess cardiovascular disease risk. Circ Cardiovasc Genet 5: 81–90, 2012. doi: 10.1161/CIRCGENETICS.111.959817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation 112: 3722–3728, 2005. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 71.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation 122: 1379–1386, 2010. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nation DA, Edland SD, Bondi MW, Salmon DP, Delano-Wood L, Peskind ER, Quinn JF, Galasko DR. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology 81: 2024–2027, 2013. doi: 10.1212/01.wnl.0000436935.47657.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nation DA, Edmonds EC, Bangen KJ, Delano-Wood L, Scanlon BK, Han SD, Edland SD, Salmon DP, Galasko DR, Bondi MW; Alzheimer’s Disease Neuroimaging Initiative Investigators . Pulse pressure in relation to tau-mediated neurodegeneration, cerebral amyloidosis, and progression to dementia in very old adults. JAMA Neurol 72: 546–553, 2015. doi: 10.1001/jamaneurol.2014.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicholson CJ, Singh K, Saphirstein RJ, Gao YZ, Li Q, Chiu JG, Leavis P, Verwoert GC, Mitchell GF, Porter T, Morgan KG, Tarasov KV, Isaacs A, Smith AV, Yasmin, Rietzschel ER, Tanaka T, Liu Y, Parsa A, Najjar SS, O’Shaughnessy KM, Sigurdsson S, De Buyzere ML, Larson MG, Sie MPS, Andrews JS, Post WS, Mattace-Raso FUS, McEniery CM, Eiriksdottir G, Segers P, Vasan RS, van Rijn MJE, Howard TD, McArdle PF, et al.; AortaGen Consortium. Reversal of aging-induced increases in aortic stiffness by targeting cytoskeletal protein-protein interfaces. J Am Heart Assoc 7: e008926, 2018. doi: 10.1161/JAHA.118.008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niiranen TJ, Kalesan B, Hamburg NM, Benjamin EJ, Mitchell GF, Vasan RS. Relative contributions of arterial stiffness and hypertension to cardiovascular disease: the Framingham Heart Study. J Am Heart Assoc 5: e004271, 2016. doi: 10.1161/JAHA.116.004271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niiranen TJ, Lyass A, Larson MG, Hamburg NM, Benjamin EJ, Mitchell GF, Vasan RS. Prevalence, correlates, and prognosis of healthy vascular aging in a Western community-dwelling cohort: the Framingham Heart Study. Hypertension 70: 267–274, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ott CE, Grünhagen J, Jäger M, Horbelt D, Schwill S, Kallenbach K, Guo G, Manke T, Knaus P, Mundlos S, Robinson PN. MicroRNAs differentially expressed in postnatal aortic development downregulate elastin via 3′ UTR and coding-sequence binding sites. PLoS One 6: e16250, 2011. doi: 10.1371/journal.pone.0016250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251–259, 2006. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 79.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke 47: 2256–2261, 2016. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S. Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: the Framingham Third Generation Cohort Study. Hypertension 67: 513–519, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol 26: 1512–1520, 2005. [PMC free article] [PubMed] [Google Scholar]
- 82.Powell JT, Vine N, Crossman M. On the accumulation of D-aspartate in elastin and other proteins of the ageing aorta. Atherosclerosis 97: 201–208, 1992. doi: 10.1016/0021-9150(92)90132-Z. [DOI] [PubMed] [Google Scholar]
- 83.Rademakers FE, Buchalter MB, Rogers WJ, Zerhouni EA, Weisfeldt ML, Weiss JL, Shapiro EP. Dissociation between left ventricular untwisting and filling. Accentuation by catecholamines. Circulation 85: 1572–1581, 1992. doi: 10.1161/01.CIR.85.4.1572. [DOI] [PubMed] [Google Scholar]
- 84.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112: 2254–2262, 2005. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 85.Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289: 194–202, 2003. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 86.Rouhl RP, van Oostenbrugge RJ, Knottnerus IL, Staals JE, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol 255: 692–696, 2008. doi: 10.1007/s00415-008-0777-y. [DOI] [PubMed] [Google Scholar]
- 87.Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P, Battista F, Settimi L, Desamericq G, Dolbeau G, Faini A, Salvi P, Mannarino E, Parati G. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension 60: 369–377, 2012. doi: 10.1161/HYPERTENSIONAHA.112.197491. [DOI] [PubMed] [Google Scholar]
- 88.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 134: 73–90, 2016. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol 54: 36–46, 2009. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 90.Torjesen A, Cooper LL, Rong J, Larson MG, Hamburg NM, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Relations of arterial stiffness with postural change in mean arterial pressure in middle-aged adults: the Framingham Heart Study. Hypertension 69: 685–690, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Torjesen AA, Sigurðsson S, Westenberg JJ, Gotal JD, Bell V, Aspelund T, Launer LJ, de Roos A, Gudnason V, Harris TB, Mitchell GF. Pulse pressure relation to aortic and left ventricular structure in the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study. Hypertension 64: 756–761, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsao CW, Himali JJ, Beiser AS, Larson MG, DeCarli C, Vasan RS, Mitchell GF, Seshadri S. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology 86: 619–626, 2016. doi: 10.1212/WNL.0000000000002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, Benjamin EJ, Mitchell GF, Vasan RS. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc 4: e002189, 2015. doi: 10.1161/JAHA.115.002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Sloten TT, Mitchell GF, Sigurdsson S, van Buchem MA, Jonsson PV, Garcia ME, Harris TB, Henry RM, Levey AS, Stehouwer CD, Gudnason V, Launer LJ. Associations between arterial stiffness, depressive symptoms and cerebral small vessel disease: cross-sectional findings from the AGES–Reykjavik Study. J Psychiatry Neurosci 41: 162–168, 2016. doi: 10.1503/jpn.140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vasan RS, Xanthakis V, Lyass A, Andersson C, Tsao C, Cheng S, Aragam J, Benjamin EJ, Larson MG. Epidemiology of left ventricular systolic dysfunction and heart failure in the Framingham Study: an echocardiographic study over 3 decades. JACC Cardiovasc Imaging 11: 1–11, 2018. doi: 10.1016/j.jcmg.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 70: 1208–1214, 2008. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 98.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 62: 1105–1110, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolters FJ, Mattace-Raso FU, Koudstaal PJ, Hofman A, Ikram MA; Heart Brain Connection Collaborative Research Group . Orthostatic hypotension and the long-term risk of dementia: a population-based study. PLoS Med 13: e1002143, 2016. doi: 10.1371/journal.pmed.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woodard T, Sigurdsson S, Gotal JD, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Gudnason V, Harris TB, Launer LJ, Levey AS, Mitchell GF. Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol 26: 1181–1187, 2015. doi: 10.1681/ASN.2014050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Woodard T, Sigurdsson S, Gotal JD, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Gudnason V, Harris TB, Launer LJ, Levey AS, Mitchell GF. Segmental kidney volumes measured by dynamic contrast-enhanced magnetic resonance imaging and their association with CKD in older people. Am J Kidney Dis 65: 41–48, 2015. [Erratum in Am J Kidney Dis 65: 639, 2015.] doi: 10.1053/j.ajkd.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zachariah JP, Graham DA, de Ferranti SD, Vasan RS, Newburger JW, Mitchell GF. Temporal trends in pulse pressure and mean arterial pressure during the rise of pediatric obesity in US children. J Am Heart Assoc 3: e000725, 2014. doi: 10.1161/JAHA.113.000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zachariah JP, Rong J, Larson MG, Hamburg NM, Benjamin EJ, Vasan RS, Mitchell GF. Metabolic predictors of change in vascular function: prospective associations from a community-based cohort. Hypertension 71: 237–242, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]