Abstract
This review summarizes a presentation given at the 2016 Gerontological Society of America Annual Meeting as part of the Vascular Aging Workshop. The development of age-related vascular dysfunction increases the risk of cardiovascular disease as well as other chronic age-associated disorders, including chronic kidney disease and Alzheimer’s disease. Healthy lifestyle behaviors, most notably regular aerobic exercise and certain dietary patterns, are considered “first-line” strategies for the prevention and/or treatment of vascular dysfunction with aging. Despite the well-established benefits of these strategies, however, many older adults do not meet the recommended guidelines for exercise or consume a healthy diet. Therefore, it is important to establish alternative and/or complementary evidence-based approaches to prevent or reverse age-related vascular dysfunction. Time-efficient forms of exercise training, hormetic exposure to mild environmental stress, fasting “mimicking” dietary paradigms, and nutraceutical/pharmaceutical approaches to favorably modulate cellular and molecular pathways activated by exercise and healthy dietary patterns may hold promise as such alternative approaches. Determining the efficacy of these novel strategies is important to provide alternatives for adults with low adherence to conventional healthy lifestyle practices for healthy vascular aging.
Keywords: caloric restriction, energy sensing, inflammation, nitric oxide, oxidative stress
CARDIOVASCULAR DISEASES AND VASCULAR AGING
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in the US and most modern societies (5, 34a). Advancing age is the primary risk factor for CVD, and as such, >90% of CVDs occur in middle-aged and older adults (5). Importantly, a new epidemic of CVD is projected in the near future as a consequence of a demographic shift toward older populations in developed nations (132a, 43). In the US alone, the number of older adults is expected to double by 2050 (132a); without effective intervention, it is predicted that 40% of adults in the US will have one or more forms of CVD by 2030 (43).
The key intermediate event linking aging with increased risk of CVD is the development vascular dysfunction (56, 88, 117). Although numerous adverse changes in vascular function occur with advancing age, two primary expressions of vascular aging that increase CVD risk are endothelial dysfunction and stiffening of the large elastic arteries (i.e., the aorta and carotid arteries) (56, 117). Importantly, healthy lifestyle behaviors such as regular aerobic exercise and certain dietary approaches favorably modulate these processes and reduce CVD risk with aging (62, 70, 86, 114, 116, 117).
Despite the well-documented benefits of these behaviors, however, many older adults do not meet the recommended guidelines for exercise and diet (104, 143). Thus, as we move toward the age of precision (personalized) medicine, alternative and complementary strategies must be established to provide options to those individuals for whom adherence to current guidelines may be unrealistic. Circumventing established barriers to healthy lifestyle behaviors with novel, time-efficient forms of aerobic exercise and/or via mild, controlled exposure to environmental stress to promote physiological adaptation, and more adherable dietary practices may hold promise in this context. Targeting specific cellular and molecular mechanisms of action by which healthy lifestyle behaviors exert their favorable effects with nutraceutical and/or pharmacological approaches represents another promising strategy for improving vascular health with aging.
In this summary of our presentation, which focused on strategies for enhancing vascular function with aging for the Vascular Aging Workshop at the 2016 Gerontological Society of America’s annual meeting, we will provide an overview of the physiology underlying the development of age-related vascular dysfunction, discuss how healthy lifestyle behaviors favorably modulate this process, and provide examples of novel lifestyle and nutraceutical-based interventions to recapitulate, at least in part, the benefits of these healthy lifestyle behaviors.
VASCULAR AGING
Vascular Endothelial Dysfunction
The vascular endothelium plays a critical role in the regulation of vascular tone and systemic blood flow, immune function, metabolism, thrombosis, and numerous other processes (115), in part through the release of the vasodilatory and vasoprotective molecule nitric oxide (NO). Mechanical (i.e., blood flow) and chemical (e.g., acetylcholine) stimuli elicit NO production in endothelial cells by activation of endothelial NO synthase (eNOS), which catalyzes the conversion of l-arginine and oxygen to NO. Endothelium-derived NO subsequently diffuses to vascular smooth muscle cells, where it activates an intracellular signaling cascade, leading to vasodilation [endothelium-dependent dilation (EDD)]. The degree of NO-mediated EDD can be assessed experimentally in humans by the vasodilatory response of the brachial artery to an increase in blood flow produced by temporary forearm ischemia (i.e., brachial artery flow-mediated dilation), which is considered the gold standard noninvasive assessment of macrovascular (conduit artery) function (115). In addition, microvascular (resistance vessel) EDD can be assessed by the forearm blood flow response to brachial artery-infused acetylcholine (ACh) (115). In rodents, flow-mediated dilation and the change in diameter of isolated arterial segments in response to pharmacological stimuli such as ACh can also be used to assess EDD (110, 112). Both macrovascular and microvascular EDD are key indices of endothelial health and independent predictors of CVD risk in older adults (22, 66, 115, 126, 127, 150, 151).
Mechanisms of Endothelial Dysfunction
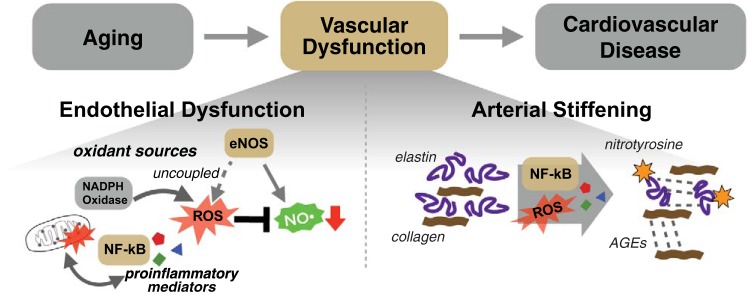
Multiple molecular and cellular mechanisms underlie the adverse effects of aging on endothelial function. Of these, superoxide-related oxidative stress, characterized by an excess of reactive oxygen species (ROS) production relative to endogenous antioxidant defenses, is a primary mechanism contributing to reduced NO bioavailability and vascular dysfunction with age (Fig. 1) (21, 22, 28, 82). Excessive ROS within endothelial cells react directly with NO, leading to its deactivation and formation of another ROS, peroxynitrite. Moreover, ROS can oxidize tetrahydrobiopterin, an essential cofactor for NO synthesis by eNOS, which further compromises NO bioavailability and results in eNOS uncoupling, whereby eNOS itself becomes a ROS generator, producing superoxide instead of NO (13, 58). Additional important sources of vascular ROS include increased NADPH oxidase activity (21, 25) and excess ROS produced as a consequence of age-related declines in mitochondrial health/function (7, 38, 144). Unchanged or decreased endogenous antioxidant enzyme defenses with aging, including superoxide dismutases (115), contribute to this state of vascular oxidative stress.
Fig. 1.
Primary mechanisms of age-related vascular dysfunction. Vascular dysfunction is a key intermediary event linking aging to increased cardiovascular disease risk. Endothelial dysfunction (bottom left) is characterized by reduced bioavailability of nitric oxide (NO) as a result of excess reactive oxygen (ROS) production by dysfunctional mitochondria, increased NADPH oxidase activity, and uncoupling of endothelial NO synthase (eNOS). Increased proinflammatory cytokine production and an upregulation of the proinflammatory mediator NF-κB also contribute. Arterial stiffening (bottom right) occurs with age as a consequence of increased collagen deposition, loss/fragmentation of elastin, and formation of advanced glycation end products (AGEs). These processes are driven, at least in part, by age-associated increases in oxidative stress and inflammation.
Increases in chronic, low-grade inflammation with age also play a prominent role in promoting endothelial dysfunction (Fig. 1) (55, 115). Central inflammatory mediators such as the master transcription factor nuclear factor-κB (NF-κB) increase with aging in endothelial cells and contribute to impaired endothelial function, at least in part, by promoting increased oxidative stress (20, 100, 141).
Large Elastic Artery Stiffness
The large elastic arteries (i.e., the aorta and carotid arteries) expand and recoil with each bolus of blood that is ejected by the left ventricle during systole. This action dampens the oscillatory pulse of blood ejected into the arterial system, aids in the propulsion of blood to the periphery, and helps to maintain perfusion of the heart during diastole (110). The pulsatility-dampening effect of large elastic arteries is critical for reducing the transmission of high pulsatile pressures to low-impedance, high-flow-sensitive organs such as the kidneys and the brain (78). However, the large elastic arteries stiffen with age, and blood must be ejected into a stiffer aorta, which increases central and peripheral systolic blood pressure as well as the work of the heart to overcome the consequent increase in afterload (114). Moreover, the forward-moving pressure wave generated by the ejection of blood into the aorta travels at a higher velocity along the stiffer arteries, which alters the timing of the pressure wave reflected by points of impedance in the arterial tree, such that the returning pressure wave reaches the heart during systole; the early return of the reflected pressure wave further augments central systolic blood pressure and afterload and negates the ability of the reflected wave to support perfusion of the heart during diastole (77, 114). The change in timing of the reflected wave also results in a greater transmission of the forward-moving pressure wave to the microcirculation, which may damage small arterioles and capillaries leading to reduced blood flow and oxygen delivery to distal organs, such as the kidneys and brain (78). The pathophysiology and broad clinical implications of arterial stiffening for age-associated CVD, brain aging, and chronic kidney disease are the focus of another review from this series.
Arterial stiffness can be assessed regionally by measuring the velocity of the arterial pressure pulse wave [pulse wave velocity (PWV)] traveling through a defined arterial segment. The gold standard measure of PWV in humans is carotid-femoral (aortic) PWV (132), and aortic PWV can also be assessed in mice (110). Similar to EDD, carotid-femoral PWV independently predicts CVD risk with aging (79). Growing evidence also implicates aortic stiffening in the pathogenesis of Alzheimer’s disease (46) and declines in cognition (113, 139), as well as decreases in renal function (71, 142), consistent with the notion of greater pulsatility transmission-related damage to these high-flow organs (78). Arterial stiffness can also be evaluated locally by measuring arterial distensibility, which is most commonly assessed in the carotid artery as carotid artery compliance (inverse of stiffness). Carotid artery compliance decreases with advancing age (81, 130) and is associated with an increased risk of cardiovascular events, particularly incident stroke (134).
Mechanisms of Arterial Stiffening
Age-related stiffening of the large elastic arteries is mediated by structural changes in the arterial wall as well as functional changes, leading to increases in vascular smooth muscle tone (Fig. 1) (90). Structural changes include extracellular matrix remodeling (increased collagen deposition and elastin degradation) and formation of advanced glycation end products, which increase stiffening by cross-linking structural proteins (29, 56, 57). These stiffness-promoting processes are driven by mechanical events (i.e., repeated mechanical loads associated with cyclical changes in arterial pressure). In addition, preclinical findings indicate that oxidative stress may also contribute, as age-related collagen deposition is reversed with short-term treatment with the superoxide dismutase mimetic TEMPOL (29, 31). Oxidative stress and proinflammatory signaling also likely play a role in arterial stiffening with aging by increasing vascular smooth muscle tone, at least in part, by reducing NO bioavailability (132, 145). Greater sympathetic nervous system activity, renin-angiotensin-aldosterone system signaling, and endothelin-1 system activation also contribute to increased vascular smooth muscle tone and arterial stiffening with aging (67, 93, 123), and augmented intrinsic vascular smooth muscle cell stiffness also plays a role (102).
HEALTHY LIFESTYLE STRATEGIES FOR VASCULAR AGING
Aerobic Exercise
Regular aerobic exercise is considered a first-line strategy for preventing or reversing age-related arterial endothelial dysfunction and large elastic artery stiffening and reducing CVD risk with aging (Fig. 2) (26, 114). Regular aerobic exercise is advanced as the most effective overall approach, at least in part, because it acts to preserve vascular function with aging and improves function in previously sedentary late middle-aged and older adults. For example, macro- and microvascular EDD assessed in regularly exercising older men is higher than in their sedentary peers and, in some cases, similar to young, healthy control subjects (17, 80, 99). Moreover, exercise training in previously sedentary older men nearly restores EDD back to levels observed in young adults (17, 99).
Fig. 2.
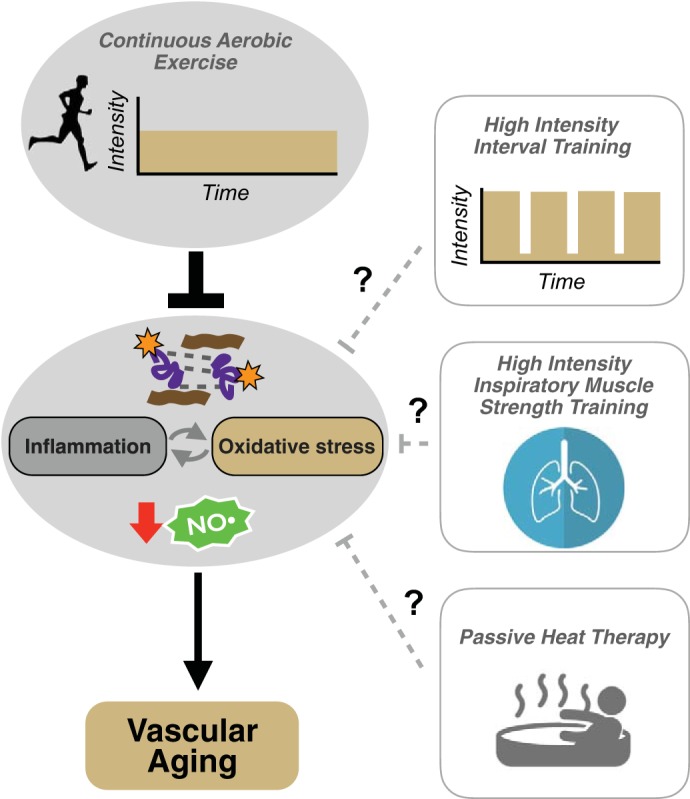
Aerobic exercise and “exercise-inspired” approaches for preventing and reversing vascular aging. Continuous moderate-intensity aerobic exercise is one of the most evidence-based strategies for ameliorating vascular aging. The beneficial effects of aerobic exercise are mediated by reduced inflammation and oxidative stress and increased nitric oxide (NO) bioavailability. Aerobic exercise also exerts favorable effects on the composition of the arterial wall (i.e., reduces collagen deposition and advanced glycation end product formation). The efficacy of novel, potentially more adherable forms of exercise and/or “exercise-inspired” approaches such as high-intensity interval training, high-intensity inspiratory muscle strength training, and passive heat therapy for healthy vascular aging remains to be established.
Evidence for a beneficial effect of regular aerobic exercise on endothelial function in postmenopausal women, however, is less clear and consistent. In estrogen-deficient postmenopausal women, a large cross-sectional comparison of aerobic exercise-trained and untrained subjects (99) as well as selective exercise intervention studies (11, 99) fail to show exercise-related differences. In contrast, some studies in estrogen-deficient postmenopausal women suggest improved brachial artery macrovascular EDD (6, 125) or leg microvascular EDD (91) following aerobic exercise interventions, although the latter is in recently postmenopausal women. Results of a clinical trial assessing the interactive effects of training and sex hormones suggest that “premenopausal” circulating concentrations of estrogen may be necessary for transducing the beneficial effects of aerobic exercise on endothelial function in postmenopausal women, as a 12-wk aerobic exercise intervention improves brachial artery FMD in estrogen-supplemented but not estrogen-deficient individuals (84).
Lifelong aerobic exercise also favorably modulates arterial stiffness with aging in both men and postmenopausal women. Carotid-femoral PWV is lower and arterial compliance higher in older habitually exercising adults versus their sedentary peers (63, 81, 118, 129, 130, 133), and greater physical activity levels are associated with lower carotid-femoral PWV (35). Even lifelong causal exercisers (i.e., older adults exercising 2–3 days/wk for ≥20 yr) exhibit greater arterial compliance than sedentary older adults; however, a higher lifelong exercise dose of ≥4–5 days/wk appears to be necessary for preventing age-related aortic stiffening (118). Moreover, aerobic exercise interventions in previously sedentary adults have favorable effects on large elastic artery stiffness, although these effects are more clearly established for carotid artery compliance (81, 96, 130). Importantly, in contrast with endothelial function, aerobic exercise interventions appear to improve carotid artery compliance in both estrogen-deficient postmenopausal women (3, 128) and postmenopausal women on hormone replacement therapy (81). In terms of aortic stiffness, although some studies suggest beneficial effects of aerobic exercise interventions on carotid-femoral PWV in middle-aged and older men and women (42, 137, 152), this is not a universal observation (94, 96).
Mechanisms of Aerobic Exercise
The mechanisms by which exercise prevents or reverses vascular dysfunction with aging are multifactorial. In humans and animal models, the contribution of specific pathways to vascular dysfunction can be quantified by acutely inhibiting the pathway and evaluating the improvement in function; the greater the improvement, the greater the contribution of the pathway to the dysfunction observed without pathway inhibition (i.e., under basal conditions). This experimental paradigm has been employed to elucidate the mechanisms by which aerobic exercise preserves/restores vascular function with aging. For example, a primary mechanism of age-related vascular dysfunction affected by exercise is oxidative stress (114). Incubation with the general, superoxide-scavenging antioxidant TEMPOL to inhibit oxidative stress restores age-related declines in EDD in arteries from old sedentary mice, with no effect in old mice that underwent voluntary wheel running (25). Similar effects are observed following incubation with the NADPH oxidase inhibitor apocynin in arteries from sedentary but not voluntary wheel-running old mice (25). In sedentary but not endurance-trained older adult humans, infusion of supraphysiological doses of the superoxide scavenging antioxidant vitamin C acutely improves macro- and microvascular EDD (28, 126), and endothelial cells biopsied from the brachial artery exhibit increased abundance of nitrotyrosine, a marker of oxidant stress (98). Moreover, in estrogen-supplemented postmenopausal women, the aerobic exercise training-associated improvement in macrovascular endothelial function is mediated by reduced oxidative stress (84). Collectively, these findings suggest that oxidative stress contributes to the tonic suppression of endothelial function with sedentary and preserved endothelial function in exercising older adults is due to an absence of oxidative stress.
Aerobic exercise training also reverses age-related increases in inflammation. In old mice, voluntary wheel running normalizes NF-κB and proinflammatory cytokine expression in arteries, which is associated with improved EDD (64). In humans, NF-κB expression is lower in biopsied endothelial cells from older aerobic exercise-trained men versus their sedentary peers (98). Moreover, inhibition of NF-κB signaling with 4 days of administration of the anti-inflammatory drug salsalate restores macrovascular EDD in older sedentary men to levels observed in older endurance-trained men; this effect is mediated in part by a suppression of oxidative stress (141). Collectively, these findings implicate an overall suppression of ROS and reduced inflammation as primary mechanisms by which aerobic exercise improves endothelial function (Fig. 2).
Increased vascular stress resistance, i.e., the ability to maintain function in the presence of external stressors, is another mechanism by which regular aerobic exercise preserves/restores vascular function with aging. In support of this concept, macrovascular EDD in older adults who perform regular aerobic exercise is similar to EDD in healthy young subjects even in the presence of traditional CVD risk factors, such as elevated low-density lipoprotein cholesterol and impaired fasting glucose (18, 140). Moreover, voluntary wheel running mitigates the adverse effects of a high-fat/high-sugar “Western”-style diet on EDD in old mice (65). Similarly, acute exposure to a simulated Western-style diet stress (i.e., high-fat, high-sugar media) reduces EDD in arteries isolated from old sedentary mice, but not in those from young mice or old mice engaged in voluntary wheel running (37). This enhanced stress resistance appears to be related to improved mitochondrial health/reduced mitochondrial reactive oxygens species (ROS) in old arteries (37).
Reduced oxidative stress and inflammation are also mechanisms by which aerobic exercise reduces age-associated increases in arterial stiffness. Exercise-induced improvements in arterial stiffness in old mice are associated with reduced abundance of the oxidant stress marker nitrotyrosine in the aorta (30). Moreover, in sedentary, but not endurance-trained, postmenopausal women, an acute vitamin C infusion increases carotid artery compliance (83); similar effects, however, have not been observed in older men (27), suggesting that other factors apart from reductions in oxidative stress-related increases in vascular smooth muscle tone may contribute. Observations in old mice suggest that wheel running-associated reductions in oxidative stress may decrease arterial stiffness by modifying the composition of the arterial wall, namely by reducing collagen and advanced glycation end products (30, 89). With regard to inflammation, 4 days of treatment with the anti-inflammatory agent salsalate to inhibit NF-κB signaling reduces (improves) carotid-femoral PWV in older sedentary men, with no effect in older endurance exercise-trained men (47).
Alternative “Exercise-Inspired” Approaches
Despite the large evidence base supporting conventional aerobic exercise training (i.e., 150 min/wk of moderate-intensity exercise; see Ref. 105) for improving vascular function with aging, adherence remains low; it is estimated that only 20–50% of older adults meet current recommendations for physical activity (1). The reasons for low adherence are not completely understood and vary by sex, race, geography, and socioeconomic status; however, commonly cited barriers include limited time, motivation, access to facilities, and safety (85, 108). To circumvent these barriers, increasing attention is being paid to novel, time-efficient and/or easier-to-adopt strategies involving physical training or controlled exposure to environmental stress. In this section, we will highlight three such strategies that may be relevant for older adults and hold promise for healthy vascular aging (Fig. 2).
Perhaps the most well characterized of these interventions to date in younger adults and patients with clinical disorders is high-intensity interval training (HIIT), which consists of short exercise intervals performed at higher intensities (e.g., 85–95% of maximal heart rate) interspersed with periods of rest. HIIT has been reported to induce physiological adaptations similar to or even greater than conventional (continuous) moderate-intensity exercise (44, 68, 146). A growing body of evidence supports benefits of HIIT on vascular function in clinical populations, including patients with type 2 diabetes, hypertension, and established CVD (103). The efficacy of HIIT to improve endothelial function and/or arterial stiffness in older adults, however, remains to be fully elucidated and is currently under investigation (clinicaltrials.gov no. NCT01883271).
Another novel, potentially time-efficient form of exercise is high-intensity inspiratory muscle strength training (IMST). IMST involves inhaling against a resistance while exhaling unimpeded (15, 138). Using a modified version of IMST involving only 30 breaths against higher resistance (∼5 min/day) performed on most days of the week, clinically significant reductions in blood pressure (e.g., 10–12 mmHg) have been observed in normotensive young adults and patients with obstructive sleep apnea following 6 wk of training (15, 138). Interestingly, these reductions in blood pressure with IMST appear to be greater than those achieved with traditional aerobic exercise (typically ≤5 mmHg). Whether the benefits of IMST on blood pressure extend to older adults and include improvements in vascular function is unknown and currently being evaluated (Clinicaltrials.gov no. NCT03266510).
Lastly, passive heat therapy, characterized by repeated hot water immersion to raise core body temperature ∼1.0–1.5°C, improves macro- and microvascular (cutaneous) EDD and reduces arterial stiffness in young healthy subjects (8, 9). The cardiovascular benefits of passive heat therapy are thought to be mediated by some of the same physiological mechanisms that induce adaptations to aerobic exercise, including increases in core temperature, heart rate, and cardiac output, blood flow-related shear stress in the peripheral circulation, and activation of protective stress response mediators, such as heat shock proteins (9). The efficacy of heat therapy for improving vascular function in older adults is not known and is currently being investigated (Clinicaltrials.gov no. NCT03264508).
Additional investigation is needed to establish the safety, short- and long-term adherence, and efficacy of these novel exercise and/or exercise-like interventions for improving vascular function with aging.
Energy Intake
Caloric restriction, or energy intake restriction without malnutrition, is the most powerful lifestyle-based strategy for extending maximal lifespan and healthspan (period of healthy living) in rodents (7, 24). In regard to vascular aging, long-term (i.e., life-long) caloric restriction in mice prevents age-related declines in endothelial function and increases in large elastic artery stiffness (23); these effects are related to reduced oxidative stress (23). Short-term (i.e., 3–8 wk) caloric restriction also reverses age-related vascular dysfunction in old mice (106, 153). In humans, 3 mo of caloric restriction-based weight loss in overweight and obese middle-aged and older adults improves macrovascular and microvascular endothelial function (97) and large elastic artery stiffness (16) and is associated with reduced oxidative stress (97).
Mechanisms of Caloric Restriction
The beneficial effects of caloric restriction on vascular function most likely result from activation of multiple energy-sensing cellular signaling networks, including sirtuin-1 (SIRT-1) and AMP-activated protein kinase (AMPK), and inhibition of pro-growth mediators such as mammalian target of rapamycin (mTOR) (Fig. 3) (70, 73). These networks respond to the energy state of the cell; under low-energy conditions (e.g., high NAD/NADH ratio, low ATP levels, low glucose and amino acid availability), they activate cell stress resistance pathways such as autophagy and mitochondrial homeostasis networks, which ultimately increase NO bioavailability and reduce oxidative stress and inflammation in arteries (22, 70). SIRT-1 and AMPK may also directly increase NO bioavailability by posttranslationally modifying eNOS (70). As such, these intracellular mediators of the beneficial effects of caloric restriction can be considered as therapeutic targets for diet-based and/or pharmacological strategies for improving vascular function with aging (62, 70).
Fig. 3.
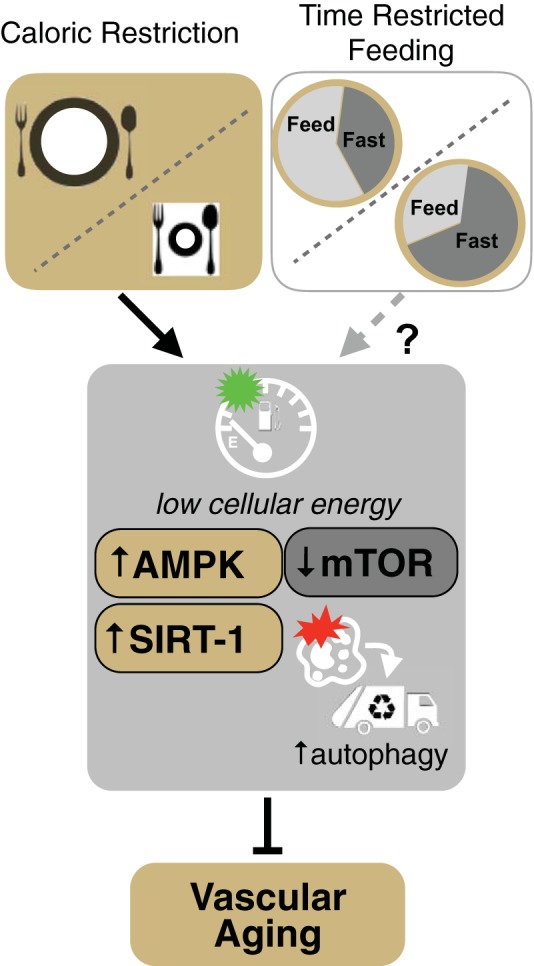
Calorie restriction and “calorie restriction-inspired” approaches for preventing and reversing vascular aging. Caloric restriction (i.e., energy intake restriction without malnutrition) prevents or reverses vascular aging, primarily by inducing a low-cellular energy state and modulating key energy-sensing pathways such as AMP-activated protein kinase (AMPK), the deacetylase sirtuin-1 (SIRT-1), mammalian target of rapamycin (mTOR), and autophagy. However, chronic caloric restriction is not practical in older adults. As a result, there is considerable interest in determining the safety, efficacy, and adherence of alternative, caloric restriction-inspired approaches for healthy vascular aging. One such caloric restriction-inspired approach presently under investigation is time-restricted feeding, which entails consuming all daily calories within a shorter time period (e.g., 8 h) than normal and fasting for the remainder of the day.
Alternative Caloric Restriction-Inspired Approaches
Despite the strong evidence for efficacy of caloric restriction in both preclinical models and humans, adherence to chronic caloric restriction is poor due to several factors, including readily available calorie-rich foods in developed societies and the social and cultural importance of food (72). Moreover, caloric restriction reduces skeletal muscle and bone mass, which are significant concerns for normal-weight older adults (76, 136). An alternative, potentially safer and more adherable strategy is “intermittent fasting,” characterized by alternating periods of unrestricted feeding with periods of caloric restriction to activate energy-sensing networks (70, 72, 73). One novel form of intermittent fasting that may recapitulate the benefits of caloric restriction while minimizing its risks is time-restricted feeding (Fig. 3). Time-restricted feeding involves consuming all daily calories within a shorter time period (e.g., 8 h) than normal and fasting for the remainder of the day (74). Recent findings in young, prediabetic men support beneficial effects of time-restricted feeding on cardiovascular function (reduced blood pressure) and oxidative stress without any changes in body weight, although no effects on arterial stiffness were observed (124). The efficacy of time-restricted feeding for improving vascular function in older adults remains to be determined and is currently being investigated (Clinicaltrials.gov no. NCT02970188); however, the safety, feasibility, and effects on muscle and bone mass must first be established in this population (70).
Dietary Influences
Broad dietary patterns prioritizing fruit and vegetables, whole grains, low-fat dairy, and moderate consumption of lean meats (e.g., the Mediterranean and DASH diets), as well as dietary patterns associated with increased fiber and fish consumption, are supported by both observational data and clinical trials for improving endothelial function and reducing arterial stiffness, particularly in older adults with CVD risk factors (62). Conversely, suboptimal dietary patterns appear to accelerate vascular aging; preclinical evidence demonstrates that consumption of a high-fat, high-sugar, low-fiber Western-style diet reduces endothelial function and increases arterial stiffness with age (45, 65). The micronutrient composition of the diet appears to also modulate vascular function with aging; specific electrolytes, such as magnesium, potassium, and calcium improve vascular function, whereas excess dietary sodium consumption exerts adverse effects on vascular function (62). Accordingly, low sodium intake is associated with better endothelial function and lower arterial stiffness, and dietary sodium restriction lowers blood pressure and reduces large elastic artery stiffness and improves both macro- and microvascular function in middle-aged and older adults (34, 48, 49). Future research is needed to distinguish between the efficacy of broad, healthy dietary patterns versus specific foods or bioactive ingredients within those patterns for promoting healthy vascular aging.
HEALTHY LIFESTYLE-MIMICKING STRATEGIES
Despite the well-documented benefits and robust effects of conventional aerobic exercise and certain dietary approaches, issues with adherence may preclude their utility in some individuals. As such, there is significant interest in repurposed pharmaceutical drugs (e.g., statins, antihypertensive or anti-inflammatory agents) or nutraceuticals, natural food ingredients or components with bioactive properties that may benefit human health. One approach for identifying potentially efficacious pharmaceutical or nutraceutical compounds is to select those that target the same mechanisms of action as healthy lifestyle behaviors, such as aerobic exercise and select dietary approaches (Fig. 4). In the sections below, we will illustrate this concept with examples of nutraceutical-based, healthy lifestyle-mimicking strategies focusing on those supported by preclinical and clinical evidence and highlighting work performed in our laboratory.
Fig. 4.
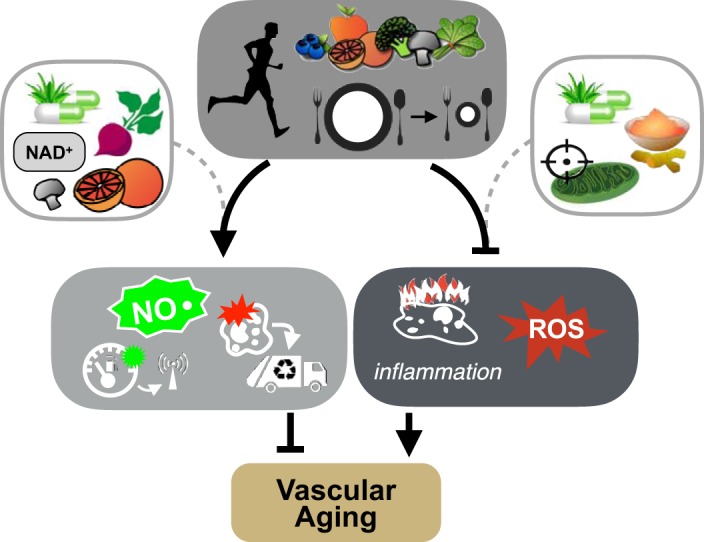
Nutraceutical compounds selected to activate signaling pathways of aerobic exercise, caloric restriction, and a healthy diet. Certain nutraceuticals targeting the signaling pathways modulated by exercise, caloric restriction, and/or a healthy diet hold promise for preventing or treating vascular aging. Examples include nitric oxide (NO)-boosting strategies through increased intake of nitrates or nitrites, which are naturally found in high concentrations in beets, compounds to modulate energy-sensing pathways by increasing levels of the sirtuin-1 substrate NAD+ or activation of autophagy via trehalose (found in mushrooms) or spermidine (found in grapefruit) supplementation, anti-inflammatory therapies such as curcumin, and mitochondria-targeted antioxidants to reduce reactive oxygen species (ROS).
NO Signaling
Because reductions in NO bioavailability play a central role in vascular aging, and restoration of NO signaling is a primary mechanism by which aerobic exercise and a healthy diet improve vascular function, NO-boosting strategies are ideal candidates for improving vascular function in older adults. There is now considerable preclinical and clinical evidence for the potential benefits of supplementation with nitrates and/or nitrites, which are found in high concentrations in beets and green leafy vegetables. In the blood and tissues, nitrate, nitrites, and related molecules serve as precursors for NO. Increasing nitrate primarily through diet-based approaches such as consumption of concentrated beetroot juice has been well studied for its blood pressure lowering effects, largely in groups with hypertension and/or other CVD risk factors (2). In these populations, nitrate also improves macrovascular endothelial function and reduces arterial stiffness (52, 135). Direct supplementation with inorganic nitrite is another, more direct approach for increasing nitrite levels (119). Nitrite supplementation improves endothelial function and reduces arterial stiffness in old mice, which is associated with reduced oxidative stress and inflammation in arteries (120). Nitrite also has favorable effects on macrovascular endothelial function in healthy middle-aged and older adults and may reduce large elastic artery stiffness (i.e., reduce carotid artery stiffness) in this population (19).
Inflammation and Oxidative Stress
Targeting inflammation and oxidative stress is another logical approach given the centrality of these processes in mediating age-related vascular dysfunction. Curcumin, considered to be the major bioactive component of the Indian spice turmeric, is a naturally occurring phenolic compound with antioxidant and anti-inflammatory properties. Curcumin supplementation in old mice completely restores age-related endothelial dysfunction and large elastic artery stiffening back to young adult levels, which is mediated by increased NO bioavailability and reduced oxidative stress and inflammation (32). Three months of curcumin supplementation also improves both macro- and microvascular endothelial function, the latter by increasing NO bioavailability and reducing oxidative stress, in middle-aged and older adults (111). Evidence suggests that curcumin may also reduce arterial stiffness (increase carotid artery compliance) in healthy postmenopausal women (3), although a lack of such effects also has been reported (111).
Given the prominent role of age-related increases in oxidative stress as a mechanism of vascular dysfunction, substantial research has focused on various antioxidant strategies for improving vascular function. However, the general consensus is that oral antioxidants such as vitamin C and E are ineffective for vascular aging and reducing CVD risk and may actually be harmful (28, 41, 50, 54). The short half-lives and inability of these traditional exogenous antioxidants to accumulate at key cellular sources of ROS are likely key contributors to their lack of efficacy. To circumvent these issues, considerable effort has been directed toward the development of more targeted antioxidant strategies (24, 87). Among these, mitochondria-targeted therapies are emerging as a promising option for age-related vascular dysfunction (95, 121). The mitochondria-specific antioxidant MitoQ is a compound consisting of the natural antioxidant coenzyme Q10 conjugated to a lipophilic compound; these properties enable MitoQ to accumulate in the mitochondrial matrix, where it is optimally positioned to reduce ROS produced by mitochondria (122). In old mice, MitoQ supplementation completely ameliorates age-related endothelial dysfunction by increasing NO bioavailability and reducing mitochondrial ROS and reverses age-associated arterial stiffening (36, 38). Recent evidence indicates that MitoQ also improves macrovascular endothelial function in late middle-aged and older adults and may reduce aortic stiffness in individuals exhibiting age-related arterial stiffening (107).
Energy Sensing Pathways
As mentioned above, interventions targeting the energy sensing networks activated by caloric restriction are another promising approach for preventing/reversing vascular dysfunction in older adults. This general approach is the subject of a recent review from our laboratory (70) and a focus of another review in this series (40).
Work from our laboratory has demonstrated favorable effects on cardiovascular function with aging through pharmacological activation of the SIRT-1 network using both pharmaceutical-based sirtuin-activating compounds (33) and by increasing levels of the SIRT-1 activation-dependent substrate NAD+ (14, 69). Treatment with the sirtuin-activating compound SRT1720 restores age-related decreases in aortic SIRT-1 expression and activity in mice and reverses the age-associated impairment in EDD by enhancing cyclooxygenase-2 dilation and normalizing arterial superoxide production, oxidative stress, and inflammation in old mice (33). Supplementation with nicotinamide mononucleotide, a natural (nutraceutical) precursor molecule for NAD+ biosynthesis, increases arterial NAD+ and SIRT-1 activity and restores endothelial function in old mice by reversing excessive aortic superoxide production, oxidative stress, and inflammation while also ameliorating age-associated increases arterial stiffness (aortic PWV and intrinsic stiffness) and aortic fibrosis (14). More recently, supplementation with nicotinamide riboside, another NAD+ precursor, was shown to increase NAD+ levels in healthy middle-aged and older adults and reduce arterial stiffness and systolic blood pressure in subjects with elevated blood pressure without influencing endothelial function (69). However, resveratrol, a polyphenol and strong SIRT-1 activator, improves macrovascular endothelial function in older obese adults and older adults with impaired glucose tolerance (101, 148).
Finally, activation of autophagy, the intracellular degradation and recycling of damaged macromolecules and organelles, holds promise as a therapeutic target, given its central role as an effector of the beneficial impact of caloric restriction (4). Nutraceutical approaches for enhancing autophagy include spermidine (a polyamine found in grapefruit and fermented soy products) and trehalose (a disaccharide found in mushrooms). Both of these compounds reverse age-related endothelial dysfunction and arterial stiffening in mice (59–61), and trehalose improves microvascular endothelial function by increasing NO bioavailability in late middle-aged and older healthy men and women (53).
Considerations
An important consideration for the strategies described above is the possibility of their interaction with other healthy lifestyle practices, such as aerobic exercise. For example, although not well-studied in the context of vascular function per se, evidence exists for adverse interactions between aerobic exercise training and concurrent administration of some supplements, such as nonspecific antioxidants (75) and resveratrol (39, 109). In contrast to adverse interactions, consumption of nitrate-rich beetroot juice before each exercise bout of a supervised exercise training program enhances the training-associated benefits of the program (e.g., augments the increase in 6-min walk distance after training) in individuals with peripheral artery disease (147); a similar enhancement of exercise training-associated improvements in physiological function is observed in young subjects with concurrent nitrate-rich beetroot juice supplementation during a training program (131). Clearly, more research is needed to determine the nature of the interaction (if any) between nutraceutical strategies and other healthy lifestyle practices in the context of vascular aging.
Another important consideration is whether adherence to the alternative strategies described in this review will actually be better than more established strategies. Although it is tempting to speculate that alternatives to exercise and eating a healthy diet may have broader appeal and be associated with greater adherence, recent data indicate that many older adults may not even adhere to such practices. For example, only 60% of older adults are classified as “highly adherent” to antihypertensive medication, defined as meeting their recommended dose >80% of the time (149). Similar low rates (<50%) are observed for long-term adherence to statins prescribed for primary prevention of CVD in older adults (92). Considerably less is known about the use of nutraceuticals or dietary supplements and adherence to recommended dosage for specific indications (51), and it is important to consider that adherence to the nutraceuticals described above may be less than assumed.
Regardless, it is likely that certain individuals are more or less motivated to adhere to specific types of strategies, and therefore, it is necessary to establish diverse, evidence-based options that may be utilized by these individuals to preserve vascular health with aging. Indeed, personalized medicine is based on the concept of tailoring medical decisions, practices, and interventions to the individual based on their predicted response or risk of disease. Thus, it is biomedically compelling to continue to identify and assess novel interventions that may be the best fit for a given person. In contrast to advancing pharmaceutical or nutraceutical strategies as panaceas for the adherence issues commonly associated with following healthy lifestyle guidelines, the goal of this research should be to establish evidence for alternative interventions to ultimately provide options to individuals for whom current practices are ineffective.
SUMMARY AND CONCLUSIONS
The development of age-related vascular dysfunction is a key intermediary event linking aging with increased CVD risk and other common age-associated disorders. Healthy lifestyle behaviors, including regular aerobic exercise and select dietary practices, are the most well-established strategies for enhancing vascular function with aging. Determining the safety, feasibility, and efficacy of novel interventions intended to recapitulate the effects of established healthy lifestyle behaviors on healthy vascular aging, to ultimately provide preventive and therapeutic options to individuals, should be viewed as a biomedical research priority.
GRANTS
Work from the authors’ research was supported by National Institutes of Health Awards AG-013038 (MERIT), HL-134887, AG0-49451, AG-053009, AG-000279, HL-107120, HL-107105, AG-042795, AG-006537, and RR-000051/TR001082.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.R., T.J.L., and C.R.M. prepared figures; M.J.R. and D.R.S. drafted manuscript; M.J.R., T.J.L., C.R.M., and D.R.S. edited and revised manuscript; M.J.R., T.J.L., C.R.M., and D.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Erzsebet Nagy for contributions to the figures.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) Prevalence of self-reported physically active adults—United States, 2007. MMWR Morb Mortal Wkly Rep 57: 1297–1300, 2008. [PubMed] [Google Scholar]
- 2.Ahluwalia A, Gladwin M, Coleman GD, Hord N, Howard G, Kim-Shapiro DB, Lajous M, Larsen FJ, Lefer DJ, McClure LA, Nolan BT, Pluta R, Schechter A, Wang CY, Ward MH, Harman JL. Dietary nitrate and the epidemiology of cardiovascular disease: report from a National Heart, Lung, and Blood Institute workshop. J Am Heart Assoc 5: e003402, 2016. doi: 10.1161/JAHA.116.003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R, Maeda S. Effects of curcumin intake and aerobic exercise training on arterial compliance in postmenopausal women. Artery Res 7: 67–72, 2013. doi: 10.1016/j.artres.2012.09.003. [DOI] [Google Scholar]
- 4.Alfaras I, Di Germanio C, Bernier M, Csiszar A, Ungvari Z, Lakatta EG, de Cabo R. Pharmacological Strategies to Retard Cardiovascular Aging. Circ Res 118: 1626–1642, 2016. doi: 10.1161/CIRCRESAHA.116.307475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 135: e146–e603, 2017. [Erratum in: Circulation 135: e646, 2017. doi: 10.1161/CIR.0000000000000491. . Comment in: J Card Fail 23: 271, 2017. doi:. ] doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol 27: 1941–1946, 2007. doi: 10.1161/ATVBAHA.107.146852. [DOI] [PubMed] [Google Scholar]
- 8.Brunt VE, Eymann TM, Francisco MA, Howard MJ, Minson CT. Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J Appl Physiol (1985) 121: 716–723, 2016. doi: 10.1152/japplphysiol.00424.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594: 5329–5342, 2016. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey DP, Pierce GL, Howe KS, Mering MC, Braith RW. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol 100: 403–408, 2007. doi: 10.1007/s00421-007-0447-2. [DOI] [PubMed] [Google Scholar]
- 13.Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, West N, Channon KM, Volpe M, Lüscher TF. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol 21: 496–502, 2001. doi: 10.1161/01.ATV.21.4.496. [DOI] [PubMed] [Google Scholar]
- 14.de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15: 522–530, 2016. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLucia CM, De Asis RM, Bailey EF. Daily inspiratory muscle training lowers blood pressure and vascular resistance in healthy men and women. Exp Physiol 103: 201–211, 2018. doi: 10.1113/EP086641. [DOI] [PubMed] [Google Scholar]
- 16.Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM, Davy KP. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension 55: 855–861, 2010. doi: 10.1161/HYPERTENSIONAHA.109.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. doi: 10.1161/01.CIR.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 18.DeVan AE, Eskurza I, Pierce GL, Walker AE, Jablonski KL, Kaplon RE, Seals DR. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clin Sci (Lond) 124: 325–331, 2013. doi: 10.1042/CS20120291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeVan AE, Johnson LC, Brooks FA, Evans TD, Justice JN, Cruickshank-Quinn C, Reisdorph N, Bryan NS, McQueen MB, Santos-Parker JR, Chonchol MB, Bassett CJ, Sindler AL, Giordano T, Seals DR. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J Appl Physiol (1985) 120: 416–425, 2016. doi: 10.1152/japplphysiol.00879.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805–812, 2008. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 22.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol 89: 122–135, 2015. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 12: 772–783, 2013. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471, 2011. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63: 2960–2984, 2014. [Erratum in: J Am Coll Cardiol 63: 3027–3028, 2014. doi: 10.1016/j.jacc.2013.11.003. ] doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol 286: H1528–H1534, 2004. doi: 10.1152/ajpheart.00879.2003. [DOI] [PubMed] [Google Scholar]
- 28.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleenor BS. Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis 4: 76–83, 2012. [PMC free article] [PubMed] [Google Scholar]
- 30.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588: 3971–3982, 2010. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11: 269–276, 2012. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, Seals DR. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol 48: 269–276, 2013. doi: 10.1016/j.exger.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gano LB, Donato AJ, Pasha HM, Hearon CM Jr, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol 307: H1754–H1763, 2014. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension 44: 35–41, 2004. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- 34a.GBD 2013 Risk Factors Collaborators; Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, Burnett R, Casey D, Coates MM, Cohen A, Delwiche K, Estep K, Frostad JJ, Astha KC, Kyu HH, Moradi-Lakeh M, Ng M, Slepak EL, Thomas BA, Wagner J, Aasvang GM, Abbafati C, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, Aboyans V, Abraham B, Abraham JP, Abubakar I, Abu-Rmeileh NM, Aburto TC, Achoki T, Adelekan A, . et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386: 2287–2323, 2015. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Germano-Soares AH, Andrade-Lima A, Menêses AL, Correia MA, Parmenter BJ, Tassitano RM, Cucato GG, Ritti-Dias RM. Association of time spent in physical activities and sedentary behaviors with carotid-femoral pulse wave velocity: A systematic review and meta-analysis. Atherosclerosis 269: 211–218, 2018. doi: 10.1016/j.atherosclerosis.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985) 124: 1194–1202, 2018. doi: 10.1152/japplphysiol.00670.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gioscia-Ryan RA, Battson ML, Cuevas LM, Zigler MC, Sindler AL, Seals DR. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging (Albany NY) 8: 2897–2914, 2016. doi: 10.18632/aging.101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592: 2549–2561, 2014. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gliemann L, Schmidt JF, Olesen J, Biensø RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol 591: 5047–5059, 2013. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gogulamudi VR, Cai J, Lesniewski LA. Reversing age-associated arterial dysfunction: insight from preclinical models. J Appl Physiol (1985). In press. doi: 10.1152/japplphysiol.00086.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guallar E, Stranges S, Mulrow C, Appel LJ, Miller ER III. Enough is enough: Stop wasting money on vitamin and mineral supplements. Ann Intern Med 159: 850–851, 2013. doi: 10.7326/0003-4819-159-12-201312170-00011. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T. Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. Jpn J Physiol 55: 235–239, 2005. doi: 10.2170/jjphysiol.S2116. [DOI] [PubMed] [Google Scholar]
- 43.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research . Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 44.Helgerud J, Høydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J. Aerobic high-intensity intervals improve V̇o2max more than moderate training. Med Sci Sports Exerc 39: 665–671, 2007. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 45.Henson GD, Walker AE, Reihl KD, Donato AJ, Lesniewski LA. Dichotomous mechanisms of aortic stiffening in high-fat diet fed young and old B6D2F1 mice. Physiol Rep 2: e00268, 2014. doi: 10.1002/phy2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes TM, Craft S, Lopez OL. Review of ‘the potential role of arterial stiffness in the pathogenesis of Alzheimer’s disease’. Neurodegener Dis Manag 5: 121–135, 2015. doi: 10.2217/nmt.14.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jablonski KL, Donato AJ, Fleenor BS, Nowlan MJ, Walker AE, Kaplon RE, Ballak DB, Seals DR. Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: potential role of suppressed nuclear factor κ B signalling. J Hypertens 33: 2477–2482, 2015. doi: 10.1097/HJH.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jablonski KL, Fedorova OV, Racine ML, Geolfos CJ, Gates PE, Chonchol M, Fleenor BS, Lakatta EG, Bagrov AY, Seals DR. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clin J Am Soc Nephrol 8: 1952–1959, 2013. doi: 10.2215/CJN.00900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol 61: 335–343, 2013. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse R, Vieth R, Blanco Mejia S, Viguiliouk E, Nishi S, Sahye-Pudaruth S, Paquette M, Patel D, Mitchell S, Kavanagh M, Tsirakis T, Bachiri L, Maran A, Umatheva N, McKay T, Trinidad G, Bernstein D, Chowdhury A, Correa-Betanzo J, Del Principe G, Hajizadeh A, Jayaraman R, Jenkins A, Jenkins W, Kalaichandran R, Kirupaharan G, Manisekaran P, Qutta T, Shahid R, Silver A, Villegas C, White J, Kendall CWC, Pichika SC, Sievenpiper JL. Supplemental vitamins and minerals for CVD prevention and treatment. J Am Coll Cardiol 71: 2570–2584, 2018. doi: 10.1016/j.jacc.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 51.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999-2012. JAMA 316: 1464–1474, 2016. doi: 10.1001/jama.2016.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65: 320–327, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaplon RE, Hill SD, Bispham NZ, Santos-Parker JR, Nowlan MJ, Snyder LL, Chonchol M, LaRocca TJ, McQueen MB, Seals DR. Oral trehalose supplementation improves resistance artery endothelial function in healthy middle-aged and older adults. Aging (Albany NY) 8: 1167–1183, 2016. doi: 10.18632/aging.100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL; Nutrition Committee of the American Heart Association Council on Nutrition, Physical Activity, and Metabolism . Antioxidant vitamin supplements and cardiovascular disease. Circulation 110: 637–641, 2004. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 55.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003. doi: 10.1161/01.CIR.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 56.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 57.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107: 346–354, 2003. doi: 10.1161/01.CIR.0000048893.62841.F7. [DOI] [PubMed] [Google Scholar]
- 58.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. doi: 10.1172/JCI200314172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LaRocca TJ, Gioscia-Ryan RA, Hearon CM Jr, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev 134: 314–320, 2013. doi: 10.1016/j.mad.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LaRocca TJ, Hearon CM Jr, Henson GD, Seals DR. Mitochondrial quality control and age-associated arterial stiffening. Exp Gerontol 58: 78–82, 2014. doi: 10.1016/j.exger.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 590: 3305–3316, 2012. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaRocca TJ, Martens CR, Seals DR. Nutrition and other lifestyle influences on arterial aging. Ageing Res Rev 39: 106–119, 2017. doi: 10.1016/j.arr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laurent P, Marenco P, Castagna O, Smulyan H, Blacher J, Safar ME. Differences in central systolic blood pressure and aortic stiffness between aerobically trained and sedentary individuals. J Am Soc Hypertens 5: 85–93, 2011. doi: 10.1016/j.jash.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 64.Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301: H1025–H1032, 2011. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lesniewski LA, Zigler ML, Durrant JR, Nowlan MJ, Folian BJ, Donato AJ, Seals DR. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol 48: 1218–1225, 2013. doi: 10.1016/j.exger.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lind L, Berglund L, Larsson A, Sundström J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation 123: 1545–1551, 2011. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- 67.Lyle AN, Raaz U. Killing me unsoftly: causes and mechanisms of arterial stiffness. Arterioscler Thromb Vasc Biol 37: e1–e11, 2017. doi: 10.1161/ATVBAHA.116.308563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol 595: 2915–2930, 2017. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun 9: 1286, 2018. doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martens CR, Seals DR. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J Physiol 594: 7177–7195, 2016. doi: 10.1113/JP272348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsuda N, Takei T, Fujiu A, Ogawa T, Nitta K. Arterial stiffness in patients with non-diabetic chronic kidney disease (CKD). J Atheroscler Thromb 16: 57–62, 2009. doi: 10.5551/jat.E602. [DOI] [PubMed] [Google Scholar]
- 72.Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, Seyfried TN, Varady KA, Panda S. Meal frequency and timing in health and disease. Proc Natl Acad Sci USA 111: 16647–16653, 2014. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem 16: 129–137, 2005. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Melkani GC, Panda S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J Physiol 595: 3691–3700, 2017. doi: 10.1113/JP273094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merry TL, Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol 594: 5135–5147, 2016. doi: 10.1113/JP270654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging 12: 487–491, 2008. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- 77.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension 64: 210–214, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 105: 1652–1660, 2008. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Montero D, Padilla J, Diaz-Cañestro C, Muris DM, Pyke KE, Obert P, Walther G. Flow-mediated dilation in athletes: influence of aging. Med Sci Sports Exerc 46: 2148–2158, 2014. doi: 10.1249/MSS.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 81.Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res 57: 861–868, 2003. doi: 10.1016/S0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- 82.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension 45: 1107–1112, 2005. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 83.Moreau KL, Gavin KM, Plum AE, Seals DR. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause 13: 951–958, 2006. doi: 10.1097/01.gme.0000243575.09065.48. [DOI] [PubMed] [Google Scholar]
- 84.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morland K, Wing S, Diez Roux A. The contextual effect of the local food environment on residents’ diets: the atherosclerosis risk in communities study. Am J Public Health 92: 1761–1768, 2002. doi: 10.2105/AJPH.92.11.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation 123: 2870–2891, 2011. doi: 10.1161/CIRCULATIONAHA.110.968735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murphy MP. Antioxidants as therapies: can we improve on nature? Free Radic Biol Med 66: 20–23, 2014. doi: 10.1016/j.freeradbiomed.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 88.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46: 454–462, 2005. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 89.Nosaka T, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol 28: 204–212, 2003. doi: 10.1139/h03-016. [DOI] [PubMed] [Google Scholar]
- 90.Nowak KL, Rossman MJ, Chonchol M, Seals DR. Strategies for Achieving Healthy Vascular Aging. Hypertension 71: 389–402, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nyberg M, Egelund J, Mandrup CM, Nielsen MB, Mogensen AS, Stallknecht B, Bangsbo J, Hellsten Y. Early postmenopausal phase is associated with reduced prostacyclin-induced vasodilation that is reversed by exercise training: The Copenhagen Women Study. Hypertension 68: 1011–1020, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07866. [DOI] [PubMed] [Google Scholar]
- 92.Ofori-Asenso R, Jakhu A, Zomer E, Curtis AJ, Korhonen MJ, Nelson M, Gambhir M, Tonkin A, Liew D, Zoungas S. Adherence and Persistence Among Statin Users Aged 65 Years and Over: A Systematic Review and Meta-analysis. J Gerontol A Biol Sci Med Sci 73: 813–819, 2018. doi: 10.1093/gerona/glx169. [DOI] [PubMed] [Google Scholar]
- 93.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension 59: 98–104, 2012. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oudegeest-Sander MH, Olde Rikkert MG, Smits P, Thijssen DH, van Dijk AP, Levine BD, Hopman MT. The effect of an advanced glycation end-product crosslink breaker and exercise training on vascular function in older individuals: a randomized factorial design trial. Exp Gerontol 48: 1509–1517, 2013. doi: 10.1016/j.exger.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oyewole AO, Birch-Machin MA. Mitochondria-targeted antioxidants. FASEB J 29: 4766–4771, 2015. doi: 10.1096/fj.15-275404. [DOI] [PubMed] [Google Scholar]
- 96.Pierce GL. Aortic stiffness in aging and hypertension: prevention and treatment with habitual aerobic exercise. Curr Hypertens Rep 19: 90, 2017. doi: 10.1007/s11906-017-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension 52: 72–79, 2008. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 10: 1032–1037, 2011. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 120: 13–23, 2011. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-kappaB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 119: 1284–1292, 2009. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pollack RM, Barzilai N, Anghel V, Kulkarni AS, Golden A, O’Broin P, Sinclair DA, Bonkowski MS, Coleville AJ, Powell D, Kim S, Moaddel R, Stein D, Zhang K, Hawkins M, Crandall JP. Resveratrol improves vascular function and mitochondrial number but not glucose metabolism in older adults. J Gerontol A Biol Sci Med Sci 72: 1703–1709, 2017. doi: 10.1093/gerona/glx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res 107: 615–619, 2010. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med 45: 679–692, 2015. doi: 10.1007/s40279-015-0321-z. [DOI] [PubMed] [Google Scholar]
- 104.Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary Intake Among US Adults, 1999-2012. JAMA 315: 2542–2553, 2016. doi: 10.1001/jama.2016.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riebe D, Ehrman J, Liguori G. ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Wolters Kluwer, 2016. [Google Scholar]
- 106.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell 9: 304–312, 2010. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 71: 1056–1063, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sallis JF, Hovell MF. Determinants of exercise behavior. Exerc Sport Sci Rev 18: 307–330, 1990. doi: 10.1249/00003677-199001000-00014. [DOI] [PubMed] [Google Scholar]
- 109.Santos-Parker JR, Kaplon RE. Supplementing exercise: translational considerations for nutraceutical and lifestyle interventions. J Physiol 592: 427–428, 2014. doi: 10.1113/jphysiol.2013.268029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santos-Parker JR, LaRocca TJ, Seals DR. Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv Physiol Educ 38: 296–307, 2014. doi: 10.1152/advan.00088.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Santos-Parker JR, Strahler TR, Bassett CJ, Bispham NZ, Chonchol MB, Seals DR. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY) 9: 187–208, 2017. doi: 10.18632/aging.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schuler D, Sansone R, Freudenberger T, Rodriguez-Mateos A, Weber G, Momma TY, Goy C, Altschmied J, Haendeler J, Fischer JW, Kelm M, Heiss C. Measurement of endothelium-dependent vasodilation in mice–brief report. Arterioscler Thromb Vasc Biol 34: 2651–2657, 2014. doi: 10.1161/ATVBAHA.114.304699. [DOI] [PubMed] [Google Scholar]
- 113.Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens 25: 1035–1040, 2007. doi: 10.1097/HJH.0b013e3280895b55. [DOI] [PubMed] [Google Scholar]
- 114.Seals DR. Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol (1985) 117: 425–439, 2014. doi: 10.1152/japplphysiol.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol 594: 2001–2024, 2016. doi: 10.1113/jphysiol.2014.282665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 29: 250–264, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shibata S, Fujimoto N, Hastings JL, Carrick-Ranson G, Bhella PS, Hearon CM Jr, Levine BD. The effect of lifelong exercise frequency on arterial stiffness. J Physiol 596: 2783–2795, 2018. doi: 10.1113/JP275301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sindler AL, Devan AE, Fleenor BS, Seals DR. Inorganic nitrite supplementation for healthy arterial aging. J Appl Physiol (1985) 116: 463–477, 2014. doi: 10.1152/japplphysiol.01100.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith RA, Hartley RC, Murphy MP. Mitochondria-targeted small molecule therapeutics and probes. Antioxid Redox Signal 15: 3021–3038, 2011. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 122.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci 1201: 96–103, 2010. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 123.Stauffer BL, Westby CM, DeSouza CA. Endothelin-1, aging and hypertension. Curr Opin Cardiol 23: 350–355, 2008. doi: 10.1097/HCO.0b013e328302f3c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 27: 1212–1221.e3, 2018. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Swift DL, Earnest CP, Blair SN, Church TS. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: results from the DREW study. Br J Sports Med 46: 753–758, 2012. doi: 10.1136/bjsports-2011-090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. doi: 10.1161/01.CIR.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 127.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. doi: 10.1161/01.HYP.38.2.274. [DOI] [PubMed] [Google Scholar]
- 128.Tanahashi K, Akazawa N, Miyaki A, Choi Y, Ra SG, Matsubara T, Kumagai H, Oikawa S, Maeda S. Aerobic exercise training decreases plasma asymmetric dimethylarginine concentrations with increase in arterial compliance in postmenopausal women. Am J Hypertens 27: 415–421, 2014. doi: 10.1093/ajh/hpt217. [DOI] [PubMed] [Google Scholar]
- 129.Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 18: 127–132, 1998. doi: 10.1161/01.ATV.18.1.127. [DOI] [PubMed] [Google Scholar]
- 130.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 131.Thompson C, Vanhatalo A, Kadach S, Wylie LJ, Fulford J, Ferguson SK, Blackwell JR, Bailey SJ, Jones AM. Discrete physiological effects of beetroot juice and potassium nitrate supplementation following 4-wk sprint interval training. J Appl Physiol (1985) 124: 1519–1528, 2018. doi: 10.1152/japplphysiol.00047.2018. [DOI] [PubMed] [Google Scholar]
- 132.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the american heart association. Hypertension 66: 698–722, 2015. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132a.US Census Bureau Table 12. Projections of the populations by age and sex for the United States: 2010 to 2050 (NP2008-T12) (Online) https://www.census.gov/data/tables/2008/demo/popproj/2008-summary-tables.html, 2008.
- 133.Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88: 1456–1462, 1993. doi: 10.1161/01.CIR.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 134.van Sloten TT, Sedaghat S, Laurent S, London GM, Pannier B, Ikram MA, Kavousi M, Mattace-Raso F, Franco OH, Boutouyrie P, Stehouwer CDA. Carotid stiffness is associated with incident stroke: a systematic review and individual participant data meta-analysis. J Am Coll Cardiol 66: 2116–2125, 2015. doi: 10.1016/j.jacc.2015.08.888. [DOI] [PubMed] [Google Scholar]
- 135.Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, Van Eijl S, Sagi-Kiss V, Chowdhury TA, Curtis M, Kuhnle GG, Wade WG, Ahluwalia A. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr 103: 25–38, 2016. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M, Lewis M, Schwartz AV; CALERIE Study Group . Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J Bone Miner Res 31: 40–51, 2016. doi: 10.1002/jbmr.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vogel T, Leprêtre PM, Brechat PH, Lonsdorfer-Wolf E, Kaltenbach G, Lonsdorfer J, Benetos A. Effect of a short-term intermittent exercise-training programme on the pulse wave velocity and arterial pressure: a prospective study among 71 healthy older subjects. Int J Clin Pract 67: 420–426, 2013. doi: 10.1111/ijcp.12021. [DOI] [PubMed] [Google Scholar]
- 138.Vranish JR, Bailey EF. Inspiratory muscle training improves sleep and mitigates cardiovascular dysfunction in obstructive sleep apnea. Sleep 39: 1179–1185, 2016. doi: 10.5665/sleep.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 51: 99–104, 2008. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 140.Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. Am J Hypertens 22: 250–256, 2009. doi: 10.1038/ajh.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Walker AE, Kaplon RE, Pierce GL, Nowlan MJ, Seals DR. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor κB. Clin Sci (Lond) 127: 645–654, 2014. doi: 10.1042/CS20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis 45: 494–501, 2005. doi: 10.1053/j.ajkd.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 143.Ward BW, Clarke TC, Nugent NC, Schiller JS. Early release of selected estimates based on data from the 2015 National Health Interview Survey (Online) Hyattsville, MD: National Center for Health Statistics, 2015. http://www.cdc.gov/nchs/nhis.htm. [Google Scholar]
- 144.Wenzel P, Schuhmacher S, Kienhöfer J, Müller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K, Münzel T, Bürkle A, Bachschmid MM, Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res 80: 280–289, 2008. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension 44: 112–116, 2004. doi: 10.1161/01.HYP.0000138068.03893.40. [DOI] [PubMed] [Google Scholar]
- 146.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115: 3086–3094, 2007. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 147.Woessner MN, VanBruggen MD, Pieper CF, Sloane R, Kraus WE, Gow AJ, Allen JD. Beet the best? dietary inorganic nitrate to augment exercise training in lower extremity peripheral artery disease with intermittent claudication. Circ Res. In press.29976553 [Google Scholar]
- 148.Wong RH, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I, Howe PR. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens 31: 1819–1827, 2013. doi: 10.1097/HJH.0b013e328362b9d6. [DOI] [PubMed] [Google Scholar]
- 149.Yang Q, Chang A, Ritchey MD, Loustalot F. Antihypertensive Medication Adherence and Risk of Cardiovascular Disease Among Older Adults: A Population-Based Cohort Study. J Am Heart Assoc 6: e006056, 2017. doi: 10.1161/JAHA.117.006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 151.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yoshizawa M, Maeda S, Miyaki A, Misono M, Saito Y, Tanabe K, Kuno S, Ajisaka R. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: a randomised controlled trial in women aged 32-59 years. Br J Sports Med 43: 615–618, 2009. doi: 10.1136/bjsm.2008.052126. [DOI] [PubMed] [Google Scholar]
- 153.Zanetti M, Gortan Cappellari G, Burekovic I, Barazzoni R, Stebel M, Guarnieri G. Caloric restriction improves endothelial dysfunction during vascular aging: Effects on nitric oxide synthase isoforms and oxidative stress in rat aorta. Exp Gerontol 45: 848–855, 2010. doi: 10.1016/j.exger.2010.07.002. [DOI] [PubMed] [Google Scholar]