Abstract
Hospitals are required to report all-cause 30-day resadmissions for patients discharged with heart failure. Same-cause readmissions have received less attention but may differ for heart failure with reduced ejection fraction (HFrEF) versus heart failure with preserved ejection fraction (HFpEF). The ARIC study began abstracting medical records for cohort members hospitalized with acute decompensated heart failure (ADHF) in 2005. ADHF was validated by physician review, with HFrEF defined by ejection fraction <50%. Recurrent admissions for ADHF were analyzed within 30 days, 90 days, 6 months, and 1 year of the index hospitalization using repeat-measures Cox regression models. All recurrent ADHF admissions per patient were counted rather than the more typical analysis of only the first occurring readmission. From 2005 to 2014, 1,133 cohort members survived at least 1 hospitalization for ADHF and had ejection fraction recorded. Half were classified as HFpEF. Patients with HFpEF were more often women and had more comorbidities. The overall ADHF readmission rate was greatest within 30 days of discharge but was higher for patients with HFrEF (115 vs 88 readmissions per 100 person-years). After adjustments for demographics, year of admission, and co-morbidities, there was a trend for higher ADHF readmissions with HFrEF, relative to HFpEF, at 30 days (hazard ratio [HR] 1.41, 95% confidence interval [CI] 0.92 to 2.18), 90 days (HR 1.39, 95% CI 1.05 to 1.85), 6 months (HR 1.47, 95% CI, 1.18 to 1.84), and 1 year (HR 1.42, 95% CI 1.18 to 1.70) of follow-up. In conclusion, patients with HFrEF have a greater burden of short- and long-term readmissions for recurrent ADHF.
Each year, ~6 million Americans with heart failure spend a collective total of ~6.5 million days in the hospital.1 Heart failure is not only a leading cause of hospitalization for Medicare beneficiaries but also the most common discharge diagnosis for patients subsequently readmitted within 30 days.2 The Affordable Care Act requires hospitals to publicly report 30-day all-cause readmissions for patients discharged with heart failure, with excessive readmissions penalized by the Centers for Medicare and Medicaid Services.3 However, the majority of readmissions are for causes other than recurrent acute decompensated heart failure (ADHF).2,4 The presentation of heart failure is heterogenous,5 and the clinical course and frequency of acute decompensation may differ by heart failure type. We investigated recurrent ADHF readmissions in patients with heart failure with reduced ejection fraction (HFrEF) versus patients with heart failure with preserved ejection fraction (HFpEF) in the Atherosclerosis Risk in Communities (ARIC) Study. Unique to our analysis, short- and long-term readmissions were analyzed by counting all hospitalizations per patient as the outcome rather than only the first readmission.
Methods
The ARIC study is an observational, population-based cohort of 15,792 mostly white or black adults in 4 US communities: Forsyth County, North Carolina; Washington County, Maryland; Jackson, Mississippi; and Minneapolis, Minnesota. Study participants were recruited with informed consent and have been prospectively followed since enrollment (1987 to 1989). To date, participation in the ARIC cohort study has involved 6 completed in-person study visits, with annual telephone contact during interim years and surveillance of hospitalized events. All research activities are approved by local institutional review boards from the 4 ARIC communities.
In 2005, the ARIC study began abstracting medical records for cohort members hospitalized with heart failure, as previously described.6 At the time of this writing, heart failure events were adjudicated through December 31, 2015. To allow at least 1 year of follow-up for recurrent ADHF readmissions, we considered hospitalizations with discharge dates from January 1, 2005 to December 31, 2014 eligible for study inclusion. Demographics, medical histories, and laboratory values were collected from the hospital record by trained abstractors. Laboratory values were abstracted by recording the worst and last values over the course of the hospitalization. For the purposes of our analysis, the last laboratory values were analyzed because these are temporally closer to hospital discharge and likely more pertinent to subsequent outcomes.
When available, left ventricular ejection fraction (EF) was abstracted from inpatient transthoracic echocardiography reports. In the event that patients were not evaluated by echocardiography during the hospital visit, an available EF documented within 2 years before hospital admission was abstracted from the medical record. In these instances, transthoracic echocardiography was prioritized first for EF abstraction, followed by cardiac magnetic resonance imaging, computed tomography, radionucleotide ventriculograms, coronary angiography, stress testing, and transesophageal echocardiograms.
Using standardized criteria, hospitalizations were classified by physician reviewers as definite ADHF, probable ADHF, stable chronic heart failure, not heart failure, or unclassifiable, based on diagnostic reports from the hospital record, physician notes, and discharge summaries.6 ADHF was differentiated from stable, chronic heart failure by evidence of new onset or worsening signs or symptoms. Consistent with previous analyses of hospital readmissions in patients discharged with heart failure,7–9 we defined HFrEF by an EF <50% and considered an EF ≥50% to be evidence of HFpEF. However, other cut points have been defined in the literature.10 Recently, the European Society of Cardiology proposed sub-dividing an EF <50% into midrange (40% to 49%) and reduced (<40%) EF classifications.11
We considered the first occurring hospitalization for definite or probable ADHF with an in-hospital assessment of EF to be the index hospitalization. In the event that no ADHF hospitalizations per patient included inpatient echocardiography, the first occurring ADHF hospitalization from 2005 to 2014 with a historical EF recorded within 2 years was considered the index hospitalization. Subsequent hospitalizations for definite or probable ADHF within 30 days, 90 days, 6 months, and 1 year of the index hospitalization discharge date were considered ADHF readmissions. Transfers were defined by relocation to another acute care hospital or relocation to a rehabilitation unit of the same hospital generating a separate admission. To avoid misclassification with readmission, transfers were excluded from the analysis.
All statistical analyses were carried out using SAS 9.4 (SAS Institute, Cary, North Carolina). Continuous variables were assessed for normality and compared using t tests or Wilcoxon rank-sum tests, as appropriate. Categorical variables were compared using chi-square test. Each recurrent ADHF readmission per patient was counted within 30 days, 90 days, 6 months, and 1 year of the index hospitalization discharge date, with censoring only at death or at the end of the observation period. Point estimates for the readmission, death, or composite incidence rates were calculated by summing all occurrences of the outcomes of interest, divided by the total amount of person-time at risk. Hazard ratios (HRs) of ADHF readmissions, death, or composite were analyzed using repeat measures Cox regression, with robust estimators accounting for within-subject correlation between recurrent readmissions.
Previous research suggests that hospital readmissions for patients with heart failure are strongly influenced by race, socioeconomic status, insurance type, age, and medical literacy.12–15 To account for these factors, regression models were adjusted for demographics (age, sex, race), insurance status, geographic location (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; Washington County, Maryland), and education (<12 years, 12 to 16 years, >16 years). To account for temporal differences in medical management and readmission policies, we also adjusted for year of hospital admission. Because HFrEF and HFpEF are associated with multiple co-morbidities that may influence precipitation and onset of ADHF, our final models additionally adjusted for baseline characteristics that were routinely measured and statistically differed between the 2 types of heart failure. Model adjustment decisions were made a priori.
Several sensitivity analyses were also carried out. In the first sensitivity analysis, we excluded 143 patients with heart failure type classified by a historical EF. In the second sensitivity analysis, we constructed competing risk repeat-measures Cox regression models using the subdistribution hazard as described by Fine and Gray.16 In the third sensitivity analysis, we examined the impact of various EF partition values on readmissions HRs. HFrEF was compared with HFpEF using EF cut points of <55% versus ≥55%, <45% versus ≥45%, 40% to 49% versus ≥50%, and <40% versus ≥50%.
Results
From January 1, 2005 to December 31, 2014, a total of 1,282 cohort members survived at least 1 hospitalization for definite or probable ADHF. Of these, 1,133 (88%) had available EF data, with 990 (87%) based on inpatient echocardiograms from the index hospitalization. Half were classified as HFrEF. Patients with HFrEF more often had a history of coronary heart disease; however, patients with HFpEF carried a greater number of co-morbidities (Table 1).
Table 1.
Demographics and clinical characteristics of Atherosclerosis Risk in Communities Study participants who were hospitalized with acute decompensated heart failure (ADHF) and discharged alive January 1, 2005 – December 31, 2014
| Characteristic | Overall (N = 1133) | HFrEF (EF<50%) (N = 560) | HFpEF (EF ≥ 50%) (N = 573) | |
|---|---|---|---|---|
| Mean ± S.D., Median (IQR) or No. (%) | P-value* | |||
| Age (years) | 77 ± 6 | 76 ± 6 | 77 ± 6 | 0.003 |
| Women | 608 (54%) | 246 (44%) | 362 (63%) | <0.0001 |
| White | 756 (67%) | 355 (64%) | 401 (70%) | 0.02 |
| Medical insurance (% yes) | 1082 (96%) | 542 (97%) | 540 (94%) | 0.1 |
| Education (years) | 0.6 | |||
| <12 | 390 (35%) | 199 (36%) | 191 (33%) | |
| 12–16 | 426 (38%) | 203 (36%) | 223 (39%) | |
| >16 | 312 (28%) | 155 (28%) | 157 (28%) | |
| Geographic location | 0.002 | |||
| Forsyth County, NC | 280 (25%) | 151 (27%) | 129 (23%) | |
| Jackson, MS | 326 (29%) | 177 (32%) | 149 (26%) | |
| Minneapolis, MN | 233 (21%) | 113 (20%) | 120 (21%) | |
| Washington County, MD | 294 (26%) | 119 (21%) | 175 (31%) | |
| Index hospitalization Year | 2009 ± 3 | 2009 ± 3 | 2010 ± 3 | 0.0006 |
| Smoker | 117 (10%) | 62 (11%) | 55 (10%) | 0.4 |
| Body mass index ≥30 kg/m2 | 400 (35%) | 152 (27%) | 248 (43%) | <0.0001 |
| Hypertension | 989 (87%) | 481 (86%) | 508 (89%) | 0.1 |
| Diabetes mellitus | 550 (49%) | 270 (48%) | 280 (50%) | 0.8 |
| Coronary heart disease† | 654 (58%) | 350 (63%) | 304 (53%) | 0.001 |
| Myocardial infarction | 261 (23%) | 161 (29%) | 100 (17%) | <0.0001 |
| Atrial fibrillation / flutter | 368 (33%) | 197 (34%) | 171 (31%) | 0.2 |
| COPD / chronic bronchitis | 346 (31%) | 153 (27%) | 193 (34%) | 0.02 |
| Chronic kidney disease‡ | 404 (43%) | 199 (41%) | 205 (44%) | 0.5 |
| Dialysis | 52 (5%) | 23 (4%) | 29 (5%) | 0.4 |
| Stroke or TIA | 213 (19%) | 92 (16%) | 121 (22%) | 0.04 |
| Thyroid disease | 203 (18%) | 76 (14%) | 127 (22%) | 0.0002 |
| Depression | 169 (15%) | 58 (10%) | 111 (19%) | <0.0001 |
| At Index Hospitalization | ||||
| Ejection fraction (%) | 45 ± 16 | 31 ± 10 | 58 ± 7 | |
| B-type natriuretic peptide (pg/ml)§ | 589 (304–1134) | 745 (374 – 1525) | 484 (246 – 921) | <0.0001 |
| Hemoglobin (g/dL) | 11.4 ± 1.8 | 11.6 ± 1.9 | 11.1 ± 1.7 | <0.0001 |
| Creatinine (mg/dL) | 1.7 ± 1.4 | 1.6 ± 1.3 | 1.7 ± 1.5 | 0.3 |
| Sodium (mEq/L) | 138 ± 4 | 138 ± 4 | 139 ± 4 | 0.4 |
| Length of stay (days) | 8 ± 13 | 8 ± 11 | 8 ± 15 | 0.8 |
P-value contrasting patients with acute decompensated heart failure with preserved vs. reduced ejection fraction.
Coronary heart disease includes history of angina, coronary atherosclerosis, acute coronary syndrome, myocardial infarction, and coronary revascularization.
Chronic kidney disease defined by glomerular filtration rate < 45 ml/min/1.73 m2, from CKD-Epi formula in patients with available serum creatinine (n = 949).
Based on patients with available abstractions (n = 747).
HFpEF = heart failure with preserved ejection fraction. HFrEF = heart failure with reduced ejection fraction. COPD = chronic obstructive pulmonary disease. TIA = transient ischemic attack.
As shown in Table 2, the majority of readmitted patients had a single ADHF rehospitalization irrespective of observation period. The maximum number of recurrent ADHF readmissions per patient was 2, 4, 5, and 8, within 30 days, 90 days, 6 months, and 1 year of the index hospitalization discharge date, respectively. Compared with HFpEF, a greater percentage of patients with HFrEF was readmitted at least once for recurrent ADHF by 30 days (8% vs 6%), 90 days (16% vs 13%), 6 months (25% vs 19%), and 1 year (32% vs 26%) of follow-up. The unadjusted mortality was comparable for the 2 types of heart failure but slightly higher with HFrEF at 30 days and 1 year of follow-up.
Table 2.
Maximum numbers of recurrent acute decompensated heart failure readmissions per patient and mortality, among participants of the Atherosclerosis Risk in Communities Study who survived at least one hospitalization for acute decompensated heart failure during 2005–2014
| Observation | Maximum Numbers of Recurrent Acute Decompensated Heart Failure Readmissions | Deaths | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Period | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| 30 Days | ||||||||||
| HFrEF | 512 (92%) | 45 (8%) | 3 (0.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 38 (7%) |
| HFpEF | 536 (94%) | 34 (6%) | 3 (0.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 30 (5%) |
| 90 Days | ||||||||||
| HFrEF | 469 (84%) | 70 (13%) | 16 (3%) | 4 (0.7%) | 1 (0.2%) | 0 | 0 | 0 | 0 | 63 (11%) |
| HFpEF | 501 (87%) | 58 (10%) | 12 (2%) | 1 (0.2%) | 1 (0.2%) | 0 | 0 | 0 | 0 | 67 (12%) |
| 6 Months | ||||||||||
| HFrEF | 421 (75%) | 96 (17%) | 28 (5%) | 8 (1.4%) | 4 (0.7%) | 3 (0.5%) | 0 | 0 | 0 | 107 (19%) |
| HFpEF | 465 (81%) | 85 (15%) | 16 (3%) | 4 (0.7%) | 2 (0.4%) | 1 (0.2%) | 0 | 0 | 0 | 111 (19%) |
| 1 Year | ||||||||||
| HFrEF | 378 (68%) | 110 (20%) | 43 (8%) | 17 (3%) | 7 (1.2%) | 3 (0.5%) | 1 (0.2%) | 0 | 1 (0.2%) | 172 (31%) |
| HFpEF | 425 (74%) | 102 (18%) | 29 (5%) | 10 (2%) | 5 (0.9%) | 0 | 1 (0.2%) | 0 | 1 (0.2%) | 161 (28%) |
HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction.
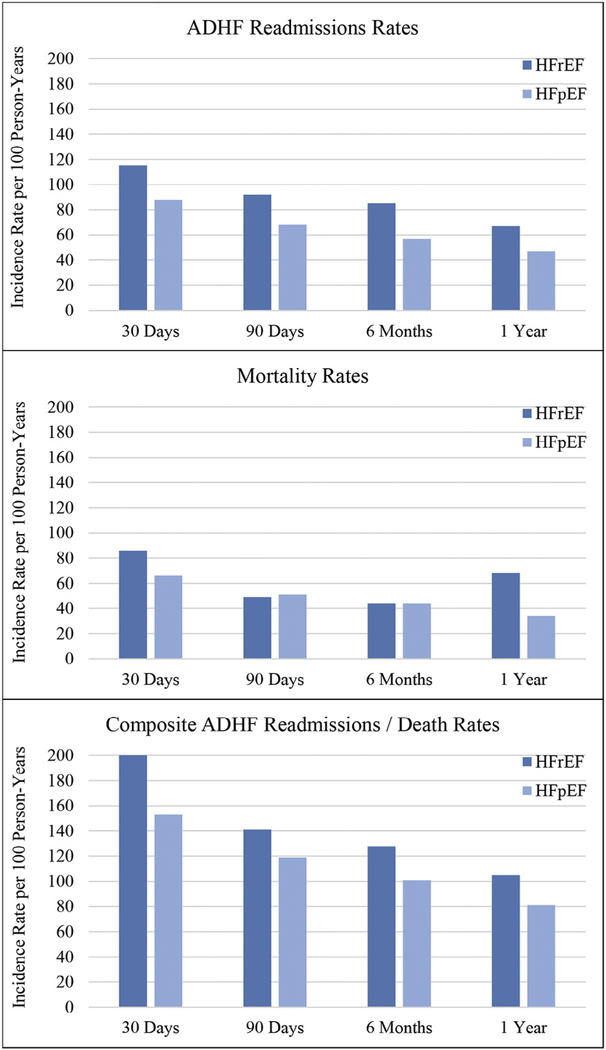
When analyzed as an incidence rate, the overall ADHF readmissions peaked during the first 30 days (101 readmissions per 100 person-years [PYs]) and then decreased dramatically until reaching 57 readmissions per 100 PYs by 1 year of follow-up. When stratified by heart failure type (Figure 1), ADHF readmissions rates were consistently higher for HFrEF than HFpEF. Death rates followed a similar pattern, peaking at 30 days (76 deaths per 100 PYs) then falling to 36 deaths per 100 PYs by 1 year of follow-up. As shown in Figure 1, the 30-day and 1-year death rates were higher with HFrEF but comparable with HFpEF at 90 days and 6 months. As with ADHF readmissions rates and death rates, composite event rates were highest within the first 30 days of the index hospital discharge (177 events per 100 PYs) and steadily decreased until reaching 93 events per 100 PYs by 1 year of follow-up. When stratified by heart failure type, composite event rates were consistently higher with HFrEF at 30 days (200 vs 153 events per 100 PYs), 90 days (141 vs 119 events per 100 PYs), 6 months (128 vs 101 events per 100 PYs), and 1 year (105 vs 81 events per 100 PYs).
Figure 1.
Crude incidence rates* of ADHF readmissions for participants of the Atherosclerosis Risk in Communities Study who survived at least 1 hospitalization for ADHF during 2005 to 2014. *Incidence rates are per 100 PYs and count all recurrent ADHF admissions per patient within 30 days, 90 days, 6 months, and 1 year of the index hospitalization.
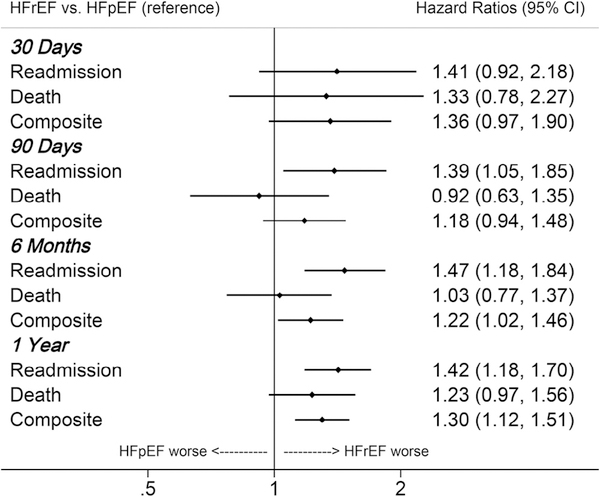
After adjustment for demographics and co-morbidities, a 40% higher hazard of ADHF readmissions was consistently observed for HFrEF relative to HFpEF across all observation periods (Figure 2). Although this trend did not achieve significance until 90 days of follow-up, readmission HRs were similar at 30 days (HR 1.41), 90 days (HR 1.39), 6 months (HR 1.47), and 1 year (HR 1.42). In contrast, the relative hazard of death was not significantly greater with HFrEF at any observation period but was marginally higher by 1 year of follow-up. When composite outcomes were considered, HFrEF was associated with a 20% to 30% higher hazard of ADHF readmissions and/or death, which attained significance by 6 months and 1 year of follow-up (Figure 2).
Figure 2.
Adjusted* HRs of death, ADHF readmission, or composite in participants of the ARIC study who survived at least 1 hospitalization for ADHF during 2005 to 2014. HRs contrast HFrEF to HFpEF. *Adjusted for age, race, sex, year of index hospitalization, insurance status, geographic location (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; Washington County, Maryland), education (<12 years, 12 to 16 years, >16 years), obesity, hypertension, coronary heart disease, chronic obstructive pulmonary disease, stroke, thyroid disease, depression, and hemoglobin.
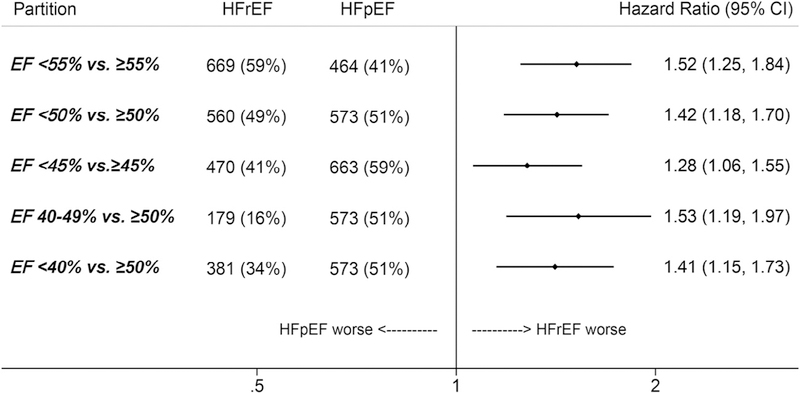
HRs from unadjusted and minimally adjusted models are shown in Appendix Table S1 and are comparable with the fully adjusted models. HRs were also similar when excluding the 143 patients with heart failure type classified by a historical EF (Appendix Table S2). Likewise, analyzing ADHF readmissions by repeat-measures competing risk models had minimal impact on the estimates (Appendix Table S3). When defining heart failure type by various EF partition values, the 1-year hazard of ADHF readmissions remained higher with HFrEF irrespective of the cut point (Figure 3). Maximum numbers of ADHF readmissions per patient and mortality by 1 year of follow-up are shown for various classifications of HFrEF in Appendix Table S4.
Figure 3.
Adjusted* HRs of recurrent ADHF readmission within 1 year of discharge from the index hospitalization in participants of the ARIC study surviving at least 1 hospitalization for ADHF during 2005 to 2014. HRs contrast HFrEF with HFpEF using various ejection fraction partition values to define HFrEF and HFpEF. *Adjusted for age, race, sex, year of index hospitalization, insurance status, geographic location (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; Washington County, Maryland), education (<12 years, 12 to 16 years, >16 years), obesity, hypertension, coronary heart disease, chronic obstructive pulmonary disease, stroke, thyroid disease, depression, and hemoglobin.
Discussion
In this analysis from the ARIC study, we investigate the burden of recurrent ADHF readmissions for patients discharged with HFrEF and HFpEF. Unique to our investigation, we examined total repeated admissions in short- and long-term follow-up intervals, which were validated by physician review. Despite evidence-based treatment options,17 patients with HFrEF were consistently observed to have a 40% higher hazard of ADHF readmissions than those with HFpEF, both in short- and long-term follow-up. Composite event rates were also higher with HFrEF but largely driven by the greater number of ADHF readmissions rather than differences in mortality.
In the global literature, most7–9,18 but not all19 registries report marginally higher unadjusted long-term ADHF readmissions for patients with HFrEF compared with HFpEF. These registries considered ADHF readmission to be a single event, defined by the first recurrent hospitalization for worsening heart failure. Others reported a composite cumulative incidence of ADHF readmission and/or death. However, none considered multiple ADHF readmissions per patient. Unlike previous investigations, we counted all recurrent ADHF readmissions per patient, allowing a more comprehensive evaluation of the ADHF readmission burden. The Enhanced Feedback for Effective Cardiac Treatment trial is one of the few other studies counting all recurrent heart failure readmissions as an outcome.20 Heart failure readmissions were significantly higher for Canadian patients with HFrEF compared with HFpEF (43 vs 35 per 100 PYs); however, follow-up time for incidence rates was divided into varying patient-specific intervals, limiting generalizability to 30-day, 90-day, 6-month, and 1-year benchmarks. As we observed in the ARIC study, readmission rates for ADHF are not constant over time but peak within the first 30 days of hospital discharge and then steadily decrease.
The Centers for Medicare and Medicaid Services currently penalize excessive 30-day all-cause readmissions for patients discharged with heart failure.3 Recent analyses suggest these policies may have had unintended consequences by increasing mortality for patients with heart failure.21 Critics have proposed quality metrics based on longer duration of follow-up, with a focus on same-cause rather than all-cause readmissions.22,23 If such changes were enacted, hospitals with a greater case mix of HFrEF may receive more penalties due to the greater burden of recurrent ADHF readmissions. However, impending modification of the existing readmissions policy is far from certain.
One potential application of our analysis may be the planning and design of clinical trials. Recent investigations have incorporated multiple recurrent readmissions into study end points. This captures a more comprehensive assessment of the patient experience and may also increase the statistical power compared with traditional end points based on only the first readmission. Notable examples of heart failure trials analyzing multiple recurrent readmissions include the Prospective Comparison of ARNI with ARB Global Outcomes in HF With Preserved Ejection Fraction trial24 and the Torsemide Comparison With Furosemide for Management of Heart Failure trial.
Our study has some limitations. This was an observational analysis and was based on data available in the hospital record. B-type natriuretic peptide was not uniformly measured across the hospitals, with 33% of index hospitalizations recording pro-B-type natriuretic peptide. Although the ARIC study was initiated in 1987, ADHF hospitalizations before 2005 were not included. Approximately 10% of patients discharged alive with a classification of ADHF were missing EF data. Additionally, echocardiograms were clinically indicated and unstandardized, with inevitable variations in imaging protocols, quality, and interpretations. We classified heart failure type by an EF cut point of 50%, but other partition values have been used in the literature. Importantly, the results were comparable in our sensitivity analysis defining HFrEF by various other EF cut points. It is possible our analysis of short-term readmissions was underpowered, given the low number of ADHF readmissions within 30 days of discharge. Nonetheless, relative hazards of ADHF readmissions remained higher for HFrEF irrespective of the observation period.
Our analysis also has several noteworthy strengths. The ARIC study is a population-based biracial cohort representing 4 economically diverse regions of the United States. Rather than relying solely on ICD-9 codes as in previous studies, ADHF hospitalizations in the ARIC study were verified (classified and adjudicated) by physician review. Hospitalizations for cohort members were closely monitored, providing information not only on the first readmission following hospital discharge but also on all readmissions within an observation period. Altogether, these strengths allow an interpretation of “real-world” heart failure readmissions outcomes, which are socially, financially, and clinically relevant.
Supplementary Material
Acknowledgment:
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts HHSN268201100005C, HHSN268201100006C, HHSN 268201100007C, HHSN268201100008C, HHSN2682011 00009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Disclosures
The authors have no relevant disclosures to report.
Supplementary data
Supplementary data associated with this article can be found, in the online version, https://doi.org/10.1016/j.amjcard.2018.03.011.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 3.McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation 2015;131:1796–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015;175:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang PP, Chambless LE, Shahar E, Bertoni AG, Russell SD, Ni H, He M, Mosley TH, Wagenknecht LE, Samdarshi TE, Wruck LM, Rosamond WD. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2014;113:504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, Yokota T, Goto D, Yokoshiki H, Kato N, Takeshita A, Tsutsui H, JCARE-CARD Investigators. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 2009;73:1893–1900. [DOI] [PubMed] [Google Scholar]
- 8.Senni M, Gavazzi A, Oliva F, Mortara A, Urso R, Pozzoli M, Metra M, Lucci D, Gonzini L, Cirrincione V, Montagna L, Di Lenarda A, Maggioni AP, Tavazzi L, IN HF Outcome Investigators. In-hospital and 1-year outcomes of acute heart failure patients according to presentation (de novo vs. worsening) and ejection fraction. Results from IN-HF Outcome Registry. Int J Cardiol 2014;173:163–169. [DOI] [PubMed] [Google Scholar]
- 9.Kaplon-Cieslicka A, Tyminska A, Peller M, Balsam P, Ozieranski K, Galas M, Marchel M, Crespo-Leiro MG, Maggioni AP, Drozdz J, Filipiak KJ, Opolski G. Diagnosis, clinical course, and 1-year outcome in patients hospitalized for heart failure with preserved ejection fraction (from the Polish Cohort of the European Society of Cardiology Heart Failure Long-Term Registry). Am J Cardiol 2016;118:535–542. [DOI] [PubMed] [Google Scholar]
- 10.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B,Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 11.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members, Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 12.Joynt KE, Jha AK. Who has higher readmission rates for heart failure, and why? Implications for efforts to improve care using financial incentives. Circ Cardiovasc Qual Outcomes 2011;4:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen LA, Smoyer Tomic KE, Smith DM, Wilson KL, Agodoa I. Rates and predictors of 30-day readmission among commercially insured and Medicaid-enrolled patients hospitalized with systolic heart failure. Circ Heart Fail 2012;5:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu JR, Moser DK, DeWalt DA, Rayens MK, Dracup K. Health literacy mediates the relationship between age and health outcomes in patients with heart failure. Circ Heart Fail 2016;9:e002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durstenfeld MS, Ogedegbe O, Katz SD, Park H, Blecker S. Racial and ethnic differences in heart failure readmissions and mortality in a large municipal healthcare system. JACC Heart Fail 2016;4:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. Aproportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 17.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society ofAmerica. Circulation 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 18.Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J 2014;168:721–730. [DOI] [PubMed] [Google Scholar]
- 19.Kajimoto K, Minami Y, Sato N, Kasanuki H, Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) Registry. Etiology of heart failure and outcomes in patients hospitalized for acute decompensated heart failure with preserved or reduced ejection fraction. Am J Cardiol 2016;118:1881–1887. [DOI] [PubMed] [Google Scholar]
- 20.Chun S, Tu JV, Wijeysundera HC, Austin PC, Wang X, Levy D, Lee DS. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail 2012;5:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Allen LA, Bhatt DL, Cox M, DeVore AD, Heidenreich PA, Hernandez AF, Peterson ED, Matsouaka RA, Yancy CW, Fonarow GC. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol 2018;3:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konstam MA. Heart failure in the lifetime of Musca domestica (the common housefly). JACC Heart Fail 2013;1:178–180. [DOI] [PubMed] [Google Scholar]
- 23.Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day réadmissions: the clock is ticking. JAMA 2013;309:345–346. [DOI] [PubMed] [Google Scholar]
- 24.Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Shi VC, Lefkowitz MP, McMurray JJV. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF trial. JACC Heart Fail 2017;5:471–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.