Abstract
Background
This study aims to investigate the descending aortic morphological alterations caused by pathological changes in acute and chronic Type B aortic dissection (TBAD) and morphological remodeling after thoracic endovascular aortic repair (TEVAR).
Patients and methods
From February 2012 to January 2016, 86 TBAD patients undergoing TEVAR were divided into an acute group (n=63) and a chronic group (n=23). The areas of the true, false and maximal lumen and descending aorta morphological parameters, including the taper ratio (TR), oversizing ratio (OR), mismatch ratio (MR), radius of curvature (RoC) and tortuosity index (TI), were evaluated. Follow-ups were performed postoperatively before discharge, at 1 and 6 months, and yearly thereafter.
Results
Before TEVAR, the TR (0.57 (0.49) vs 0.74 (0.60); P < 0.05), OR (2.75 ± 1.89 vs 3.96 ± 2.27; P < 0.05) and TI (1.22 (1.19) vs 1.41 ± 0.17; P < 0.05) were significantly higher in the chronic group than in the acute group. The TEVAR technical success rate was 100%. No differences were found in 30-day and >30-day mortality between the two groups. Pathology-specific devices, such as tapered stent grafts and distal bare stents, were used more frequently in the chronic group than in the acute group (47.8% vs 23.8%, P = 0.03; 43.5% vs 12.7%, P = 0.002, respectively). The OR and TI changes that occurred after TEVAR were significantly higher in the chronic group than in the acute group (1.80 ± 0.86 vs 2.98 ± 1.85, P = 0.028; 0.00 ± 0.09 vs 0.09 ± 0.10, P < 0.001, respectively).
Conclusion
TEVAR is a safe and effective therapy for acute and chronic TBAD. Compared to acute TBAD, chronic TBAD resulted in an adverse preoperative descending aorta morphology. Pathology-specific devices may be a feasible treatment option for endovascular repair of chronic TBAD. A larger series of cases with longer follow-up are needed to draw definitive conclusions.
Keywords: thoracic endovascular aortic repair, aortic remodeling, type B aortic dissection, morphology, stent graft
Introduction
With advances in endovascular technology, thoracic endovascular aortic repair (TEVAR) has been recommended as the mainstream treatment for type B aortic dissection (TBAD).1,2 However, despite advances in endovascular devices and techniques for complex aortic dissections involving the distal arch and descending thoracic aorta, previous studies have demonstrated increasing stent-related complications, such as distal stent graft-induced new entry (SINE), endoleak, aneurysm degeneration and aortic rupture.3–6
These complications are mainly caused by unsatisfactory compliance by stent grafts (SG) in a dissected aorta. On the one hand, TBAD is different from descending thoracic aorta aneurysm (DTAA). The intima of the distal landing zone (DLZ) is dissected, and the stent graft is usually tubular or has a small tapered design with a rigid length. On the other hand, the dissection pathology causes different changes to occur, such as aorta dilatation, continued false lumen (FL) patency, and thickening of the intimal flap, between acute and chronic dissection.7 Therefore, different dissection stages may be associated with different outcomes after TEVAR. Increasing evidence supports the value of using TEVAR to treat complicated and uncomplicated aortic dissection.1,2,8–10 However, the role of TEVAR in chronic TBAD remains controversial. Because pathological changes in the aortic lumen are difficult to evaluate, aortic morphological alterations caused by pathological changes can be feasibly assessed by computed tomography. Therefore, assessing the morphology of the descending aorta in the acute and chronic phases may improve evaluations of the outcomes of TEVAR.
Unlike DTAA, for which there are protocols to characterize aortic morphology,11 to our knowledge, no well-defined protocol is available for thoracic dissection. The distal diameters of the true lumen (TL) and FL have commonly been used to describe the descending aorta morphology,12 but they fail to balance stent-graft selection and the distal aortic diameter and the global descending aorta morphology was not considered. Therefore, we attempted to use the distal taper ratio (TR), oversizing ratio (OR), mismatch rate (MR), radius of curvature (RoC) and tortuosity index (TI) to evaluate descending aorta morphology, which is related to distal stent-graft complications. The purpose of this study was to compare the influence of TEVAR on descending aortic morphology between acute and chronic TBAD and investigate whether changes are associated with the outcomes of TEVAR.
Patients And Methods
The study design was a retrospective analysis of prospectively collected registry data. The study was conducted according to the principles of the Declaration of Helsinki and approved by the ethics review committee of the First Affiliated Hospital of Anhui Medical University, P.R. China. The need for written patient consent was waived because of the observational nature of the study, and the CT image data were anonymized and maintained with confidentiality.
Selection Of Patients And Descending Aorta Morphological Parameters
An analysis of consecutive patients diagnosed with TBAD at our institution between February 2012 and January 2016 was performed. The indications for TEVAR for TBAD were impending rupture, organ malperfusion, resistant hypertension unresponsive to medical therapy, refractory pain (ongoing symptoms in the back and/or chest pain requiring narcotic medications), aortic growth (aortic diameter increase >5 mm within 3 months) and the patient’s will or surgeon’s decision. Patients who underwent TEVAR for intramural hematomas, penetrating atherosclerotic ulcers, ulcer-like projections or traumatic dissection were excluded from the analysis, and patients with infectious or connective tissue diseases were also excluded.
Acute aortic dissection was defined as dissection occurring ≤14 days from the onset of symptoms, whereas chronic aortic dissection was defined as dissection with an elapsed time >14 days from symptom onset.13 A total of 86 patients were included in the present analysis. Of these patients, 63 had acute aortic dissection, and 23 had chronic aortic dissection.
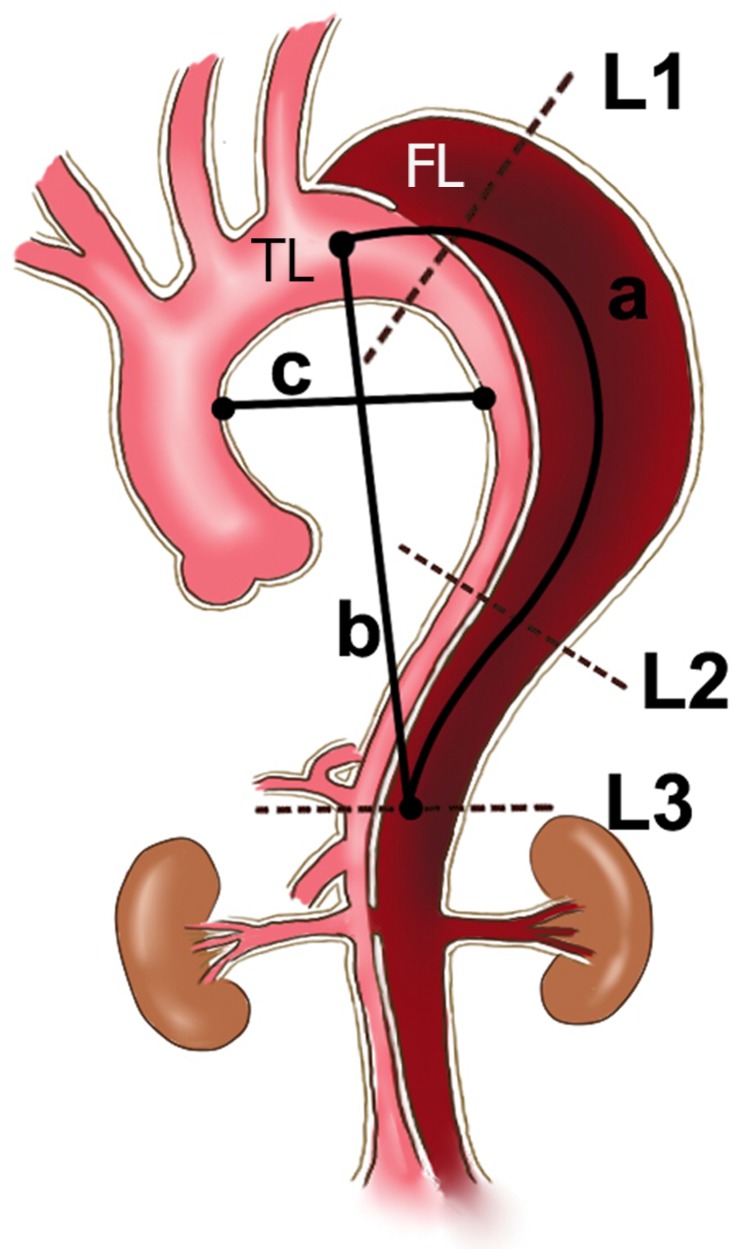
The following clinical data were collected: demographic characteristics, preoperative and postoperative morphological characteristics and follow-up data. All patients underwent pre- and postoperative computed tomography angiography (CTA) of the entire aorta. Digital imaging and communications in medicine (DICOM) standard data obtained in eligible patients were transferred for further analysis in an anonymized fashion. Data from CT images were transferred to 3D Recon software (version 6.0.9037.9003; Vital Images Inc., Minnetonka, Minnesota) for measurement. The maximal lumen (ML) area, TL area, and FL area were each measured directly at the following levels: the bronchial bifurcation (L1), the lower edge of the left atrium (L2), and the celiac trunk (L3) (Figure 1, dotted lines). The status of the FL was qualitatively defined as patent, partial thrombosis or complete thrombosis at the L1, L2 and L3 levels.
Figure 1.
Schematic diagram showing the TL, FL and ML area measured directly at the L1, L2 and L3 (dotted lines) levels and the measurements of TI (a/b) and RoC (c/2).
Notes: Level 1: the bronchial bifurcation level; Level 2: the lower edge of the left atrium level; and Level 3: the level of the celiac trunk; a, intra-aorta centerline length between the left subclavian artery and celiac trunk; b, linear distance between the left subclavian artery and celiac trunk; c, distance from the inner wall of the ascending aorta to the inner wall of the descending aorta at the level of the pulmonary artery.
Abbreviations: TL, true lumen; FL, false lumen; ML, maximal lumen; TI, tortuosity index; RoC, radius of curvature.
The definitions and mathematical measurements used to obtain the morphological parameters were as follows:
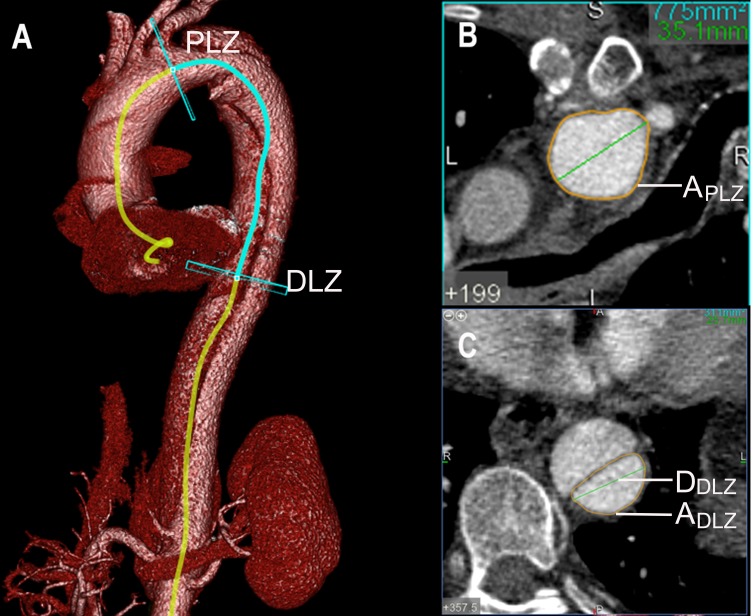
1. TR: pre-TR = 1 - (ADLZ/APLZ); post-TR = 1 - (ADLZ’/APLZ’),14 where ADLZ is the TL area of the presumed DLZ before TEVAR, and APLZ is the area of the presumed proximal landing zone (PLZ) before TEVAR. ADLZ’ is the TL area of the DLZ after TEVAR, and APLZ’ is the area of the PLZ after TEVAR (Figures 2 and 3);
Figure 2.
The definitions and mathematical measurements of the taper ratio (TR), the oversizing ratio (OR) and the mismatch rate (MR) before TEVAR. Pre-TR = 1−(ADLZ/APLZ); Pre-OR = (ASG/ADLZ)−1; Pre-MR = DSG/DDLZ.
Notes: (A) VR reconstruction images from a patient affected by TBAD. Measurement of the aorta and TL on two planes: DLZ and PLZ. (B) Axial computed tomography scans obtained in the PLZ plane showing the area (APLZ) of the TL. (C) Axial computed tomography scans obtained in the DLZ plane showing the area (ADLZ) and maximal diameter (DDLZ) of the TL.
Abbreviations: TEVAR, thoracic endovascular aortic repair; DLZ, distal landing zone; PLZ, proximal landing zone; TL, true lumen; ASG, distal area of the fully expanded stent graft; DSG, distal diameter of the selected stent graft.
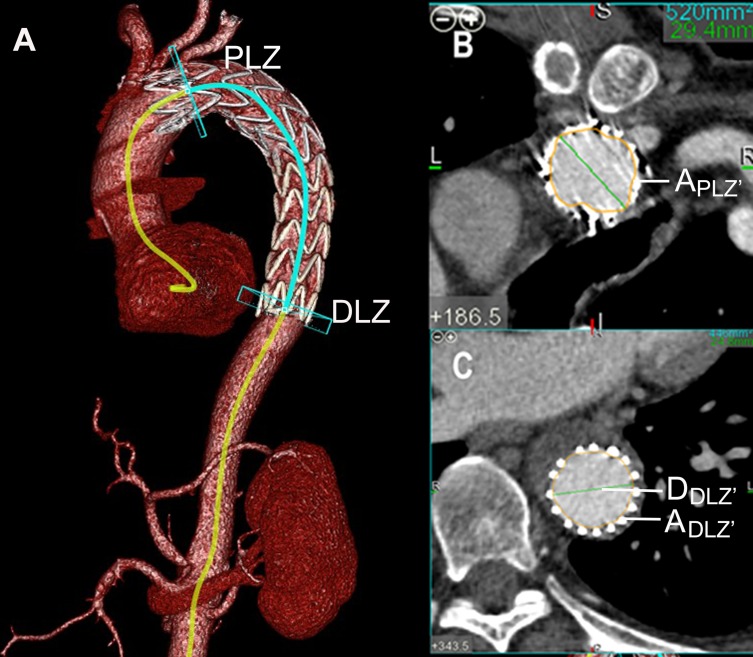
Figure 3.
The definitions and mathematical measurements used to calculate the taper ratio (TR), oversizing ratio (OR) and mismatch rate (MR) after TEVAR. Post-TR = 1−(ADLZ’/APLZ’); Post-OR = (ASG’/ADLZ)−1; Post-MR = DSG/DDLZ’.
Notes: (A) VR reconstruction images from the same patient affected by TBAD. (B) Axial computed tomography scans obtained in the PLZ plane showing the area (APLZ’) of the TL. (C) Axial computed tomography scans obtained in the DLZ plane showing the distal TL area (ADLZ’) and maximal diameter (DDLZ’) of the TL of the partially expanded stent.
Abbreviations: TEVAR, thoracic endovascular aortic repair; DLZ, distal landing zone; PLZ, Proximal landing zone; TL, true lumen; ASG’, the distal area of the partially expanded stent graft; DSG, the distal diameter of the selected stent graft; A DLZ, is the TL area of the presumed DLZ before TEVAR
2. OR: pre-OR = (ASG/ADLZ) - 1; post-OR = (ASG’/ADLZ) – 1,14 where ASG is the distal area of the fully expanded stent graft before TEVAR, ASG’ is the distal area of the partially expanded stent after TEVAR, and ADLZ is the TL area of the presumed DLZ before TEVAR (Figures 2 and 3);
3. MR: Pre-MR = DSG/DDLZ; Post-MR = DSG/DDLZ’,15 where DSG is the distal diameter of the selected stent graft, DDLZ is the maximal diameter of the presumed DLZ graft before TEVAR, and DDLZ’ is the maximal diameter of the DLZ after TEVAR (Figures 2 and 3);
4. TI was defined as the intra-aorta centerline length divided by the linear distance between the left subclavian artery and celiac trunk (Figure 1, TI = a/b);16 and
5. RoC was defined as the distance from the inner wall of the ascending aorta to the inner wall of the descending aorta at the level of the pulmonary artery divided by two (Figure 1, RoC = c/2) and was also measured to describe the degree of distortion of the aortic arch.17
Surgical Procedure
All procedures were performed under general anesthesia. Intraoperative thoracic and abdominal aortic angiography were routinely performed to assess the patency of the visceral vessels and their origins from the TL or FL. The size of the proximal stent graft was selected according to the centerline diameter of the proximal nondissected aorta. Some patients required partial or total exclusion of the left subclavian artery and implantation of a chimney graft to obtain an adequate proximal landing zone if the right vertebral artery was patent and the left artery was not dominant. In cases with TL compression or a tortuous descending aorta at the fixation site, pathology-specific devices, such as a taper stent or distal bare stent, were used before stent-graft implantation.15,18–20 In cases of persistent malperfusion syndrome, an adjunctive procedure (distal bare stent extension or open surgery) was performed. Technical success was defined as stent graft deployment without type I endoleaks, open surgical conversion or death within 24 hrs of the operation. CTA was performed before discharge and at 1, 6, and 12 months postoperatively, followed by annual examinations during the extended follow-up period.
Statistical Analyses
All data were analyzed using SPSS statistical software version 17.0 (SPSS Inc., Chicago, IL). Continuous variables are expressed as the mean ± standard deviation (SD) or median (interquartile range) according to whether they exhibited a normal distribution and were compared by a t-test or the Wilcoxon test. Categorical variables are expressed as percentages and were analyzed by chi-square and Fisher’s exact tests. A value of P < 0.05 was considered to indicate statistical significance.
Results
Patient Demographics
Eighty-six patients were included in the present analysis: 63 patients (acute group, 73.3%) underwent TEVAR during the acute phase, and 23 patients (chronic group, 26.7%) underwent TEVAR during the chronic phase of TBAD. The demographics of the patient cohort are illustrated in Table 1. The two groups had similar age distributions, and no significant difference was found between the acute and chronic groups in terms of baseline demographics.
Table 1.
Baseline Characteristics
| Variables | Acute Group No. (%) Or Mean±SD | Chronic Group No. (%) Or Mean±SD | P-value |
|---|---|---|---|
| Age (years) | 59.13 ± 9.91 | 55.92 ± 12.06 | 0.451 |
| Male | 52 (82.5) | 18 (78.3) | 0.890 |
| Characteristics | |||
| Hypertension | 58 (92.1) | 20 (87.0) | 0.762 |
| Smoking | 41 (65.1) | 16 (69.6) | 0.697 |
| Uremia | 5 (7.9) | 3 (13.0) | 0.762 |
| Diabetes | 11 (17.5) | 4 (17.4) | 1 |
| COPD | 2 (3.2) | 2 (8.7) | 0.619 |
| Cardiac disease | 5 (7.9) | 1 (4.3) | 0.920 |
| Carotid disease | 2 (3.2) | 0 | 1 |
| Indications for TEVAR | |||
| Impending rupture | 17 (27.0) | 4 (17.4) | 0.977 |
| Organ malperfusion | 19 (30.2) | 2 (8.7) | 0.040 |
| Resistant hypertension | 15 (23.8) | 5 (21.7) | 0.841 |
| Refractory pain | 24 (38.1) | 8 (34.8) | 0.778 |
| Aortic growth | 4 (6.3) | 8 (34.8) | 0.003 |
Abbreviation: TEVAR, thoracic endovascular aortic repair.
The indications reported in all patients who underwent TEVAR are summarized in Table 1. More organ malperfusion patients were included in the acute group than in the chronic group (30.2% vs 8.7% for the acute vs chronic groups, respectively, P = 0.040). Among the 23 patients with chronic TBAD, aortic growth was the indication for TEVAR in 8 cases, and there was therefore a significantly higher proportion of such patients in this group than in the acute TBAD group (34.8% vs 6.3% for the chronic vs acute groups, respectively, P = 0.003).
TEVAR Procedure
The median time to interventions for acute dissection events was 11 days (range, 1–15 days) and the median time to interventions for chronic dissection events was 58 days (range, 23 days −37 months). There was no significant difference in the types of grafts used between the acute and chronic groups (P = 0.23). The mean length of the aorta that was covered was significantly longer in the chronic group than in the acute group (182.96 ± 22.32 mm vs 193.35 ± 13.60 mm in the chronic vs acute groups, respectively, P = 0.02). Tapered SGs were more frequently used in the chronic group (47.8% vs 23.8%, P = 0.03). The distal bare stent technique was used in 43.5% of the chronic group (12.7% in the acute group, P = 0.002). Notably, the technical success rate of TEVAR was 100% in both groups. Detailed procedural information is listed in Table 2.
Table 2.
Procedural Details
| Variables | Acute Group No. (%) Or Mean±SD | Chronic Group No. (%) Or Mean±SD | P-value |
|---|---|---|---|
| Stent graft type | 0.23 | ||
| Captivia | 43 (68.3) | 12 (52.2) | |
| Zenith | 7 (11.1) | 2 (8.7) | |
| Ankura | 12 (19.0) | 7 (30.4) | |
| Grink | 1 (1.6) | 2 (8.7) | |
| Tapered SG | 15 (23.8) | 11(47.8) | 0.03 |
| Median length, mm | 182.96±22.32 | 193.35±13.60 | 0.02 |
| Coverage LSA (n, %) | 0.44 | ||
| Total coverage | 26 (41.3) | 13 (56.5) | |
| Partial coverage | 20 (31.7) | 5 (21.7) | |
| Adjunctive procedures | |||
| Subclavian chimney | 3 (4.8) | 0 | 0.29 |
| Restrictive bare stent | 8 (12.7) | 10 (43.5) | 0.002 |
| Distal stent graft extensions | 0.91 | ||
| Renal artery bare stent | 2 (3.2) | 2 (8.7) | |
| Mesenteric artery bare stent | 3 (4.8) | 0 | |
| Ilio-femoral artery bare stent | 1 (1.6) | 0 |
Abbreviation: LSA, left subclavian artery.
Follow-Up Outcomes
The 30-day mortality rate was 4.3% (1/63) in the chronic group and was not different between the two groups; one patient died of aortic rupture 7 days after TEVAR. The incidence of endoleak was 6.3% (4/63) in the acute group and 8.7% (2/23) in the chronic group, resulting in no difference between the two groups. One patient with chronic TBAD had a slight type I endoleak, and another patient in the chronic group had a type III endoleak. Four patients had endoleaks in the acute group; two were type II, and two were type III. All six patients with endoleaks were asymptomatic and did not require any intervention.
A 30-day follow-up was completed by all patients. The median follow-up period was 34 months (range, 24–66 mo) for acute TBAD patients and 36 months (range, 1–75 mo) for chronic TBAD patients. No difference was found in >30-day aortic-related mortality between the two groups (P = 0.453). Two deaths occurred in the chronic group due to aortic aneurysm rupture. Moreover, the incidence of distal SINE was 3.2% (2/63) in the acute group and 4.3% (1/23) in the chronic group, resulting in no difference between the two groups (P = 1). One of the two patients with acute TBAD who suffered distal SINE was asymptomatic and experienced spontaneous resolution. The other patient was treated with a secondary endovascular intervention because of resistant chest pain at 10 months after TEVAR. Distal SINE also occurred in a chronic-phase patient who presented with chest pain, and this patient was treated with a secondary endovascular intervention. Only one retrograde type A dissection (RTAD) occurred in the acute group. This patient, who was found to have an asymptomatic RTAD by CTA on the seventh postoperative day, was treated with aortic arch replacement. No stent collapse occurred in the acute group, but in the chronic group, three distal bare stents collapsed, resulting in a significant difference between the two groups (P = 0.017). No significant differences were observed in other postoperative complications, including stroke (P = 1), spinal ischemia or paraplegia (P = 1) and renal failure (P = 0.576). The complications and results are summarized in Table 3.
Table 3.
Complications And Results
| Variables | Acute Group No. (%) Or Median | Chronic Group No. (%) Or Median | P-value |
|---|---|---|---|
| Technical success | 63 (100) | 23 (100) | |
| Follow-up, months | 34(26) | 36(30) | |
| 30-day mortality | 0 | 1 (4.3) | 0.267 |
| 30-day aortic-related mortality | 0 | 1 (4.3) | 0.267 |
| >30-day mortality | 2 (3.2) | 2 (9.1) | 0.587 |
| >30-day aortic-related mortality | 1 (1.6) | 1 (4.5) | 0.453 |
| Aortic rupture | 0 | 2 (8.7) | 0.069 |
| Endoleak | 4 (6.3) | 2 (8.7) | 0.576 |
| Distal SINE | 2 (3.2) | 1 (4.3) | 1 |
| RTAD | 1 (1.6) | 0 | 1 |
| Distal stent collapse | 0 | 3 (13.04) | 0.017 |
| Stroke | 3 (4.8) | 1 (4.3) | 1 |
| Spinal ischemia or paraplegia | 2 (3.2) | 1 (4.3) | 1 |
| Renal failure | 4 (6.3) | 3 (13.0) | 0.576 |
| Secondary intervention | 2 (3.2) | 1 (4.3) | 1 |
Abbreviations: SINE, stent graft-induced new entry; RTAD, retrograde type A dissection.
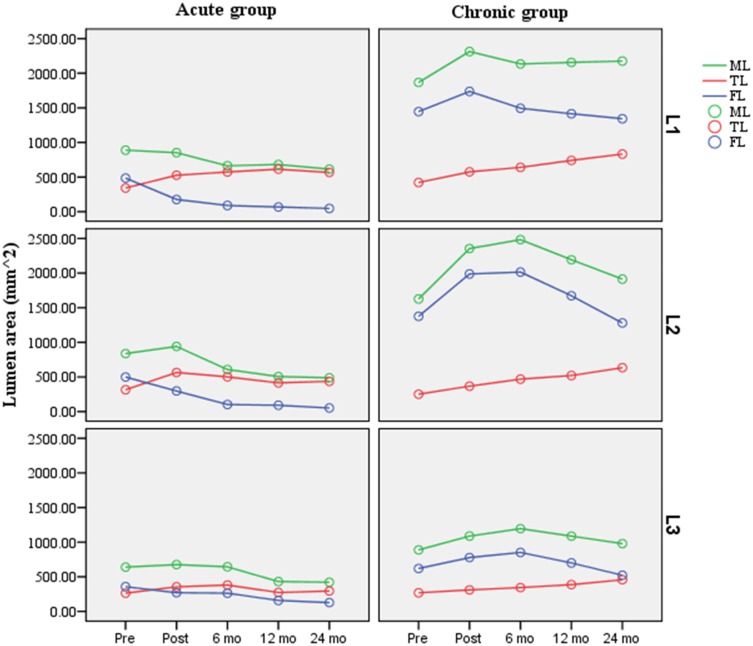
Aortic Remodeling
The TL area at the L1 level and the FL area and ML area at the L1, L2, and L3 levels were all significantly higher in the chronic TBAD patients than in the acute TBAD patients before TEVAR (P < 0.005) (Table 4). Remodeling of the aorta at the three different levels is plotted in Figure 4. In the acute group, the TL area significant increased at the L1 and L2 levels and remained stable at the L3 level. The FL area was significantly smaller at all three levels. The ML area decreased at the L1 and L3 level but increased before decreasing at the L2 level. In the chronic group, TEVAR significantly increased the TL area at all three levels. The FL area and ML area remained stable at the L1 level, although both increased before decreasing at the L2 and L3 levels.
Table 4.
Preoperative Descending Aorta Morphology Between The Acute And Chronic Groups
| Variables | Acute Group Mean±SD Or Median | Chronic Group Mean±SD Or Median | P-value |
|---|---|---|---|
| True lumen area, mm2 | |||
| L1 | 342.3 ± 107.1 | 421.5 ± 121.7 | 0.004 |
| L2 | 314.4 ± 97.3 | 250.1 ± 84.2 | 0.006 |
| L3 | 263.4 ± 95.3 | 268.2 ± 78.1 | 0.829 |
| False lumen area, mm2 | |||
| L1 | 485.4 ± 292.6 | 1445.0 ± 643.3 | 0.000 |
| L2 | 498.5 ± 199.4 | 1375.2 ± 581.5 | 0.000 |
| L3 | 356.9 ± 114.6 | 619.5 ± 273.6 | 0.000 |
| Maximal lumen area, mm2 | |||
| L1 | 827.7 ± 384.4 | 1866.5 ± 712.4 | 0.000 |
| L2 | 812.9 ± 516.9 | 1625.3 ± 535.7 | 0.000 |
| L3 | 620.3 ± 324.4 | 887.7 ± 422.6 | 0.003 |
| TR | 0.57 (0.49) | 0.74 (0.60) | 0.000 |
| OR | 2.75 ± 1.89 | 3.96 ± 2.27 | 0.022 |
| MR | 1.23 ± 0.15 | 1.20 ± 0.19 | 0.467 |
| TI | 1.22 (1.19) | 1.41 ± 0.17 | 0.000 |
| RoC, mm | 26.05 ± 4.64 | 28.26 ± 4.56 | 0.052 |
Abbreviations: TEVAR, thoracic endovascular aortic repair; TR, taper ratio; OR, oversizing ratio; MR, mismatch ratio; TI, tortuosity index; RoC, radius of curvature.
Figure 4.
Area of the TL, FL and ML regression trends at different measured levels, along with the time before and after thoracic endovascular aortic repair (TEVAR).
Notes: Level 1: the bronchial bifurcation level; Level 2: the lower edge of the left atrium level; and Level 3: the level of the celiac trunk.
Abbreviations: TL, true lumen; FL, false lumen; ML, maximal lumen; Pre, pre-TEVAR; Post, Post-TEVAR; mo, months.
Complete thrombosis occurred in the FL at L1 in 88.89% (56/63) of the acute TBAD patients and 85.71% (18/21) of the chronic TBAD patients, and there was no difference between the two groups (P = 0.70). Complete FL thrombosis at L2 occurred less frequently and was observed in 50.79% (32/63) of the acute TBAD patients and 33.33% (7/21) of the chronic TBAD patients, resulting in no difference between the two groups (P = 0.66). Complete FL thrombosis at L3 occurred in 7.94% (5/63) of the acute TBAD patients and 4.76% (1/21) of the chronic TBAD patients, resulting in no difference between the two groups (P = 0.61).
A comparison of preoperative descending aorta morphology data between the two groups is presented in Table 4. The TR (P < 0.001), OR (P = 0.022) and TI (P < 0.001) were all significantly higher in the chronic TBAD patients than in the acute TBAD patients; there was no difference in the MR (P = 0.467) and RoC (P = 0.052). A comparison of morphological remodeling between the acute and chronic groups is presented in Table 5. The changes in OR (P = 0.028) and the TI (P < 0.001) observed after TEVAR were significantly higher in the chronic group than in the acute group. There were no differences in the changes observed in the TR (P = 0.685), MR (P = 0.323) and RoC (P = 0.116) between the two groups.
Table 5.
Changes In Preoperative To Postoperative Descending Aorta Morphology Between The Acute And Chronic Groups
| Variables | Acute Group Mean±SD | Chronic Group Mean±SD | P-value |
|---|---|---|---|
| Change in TR | 0.29 ± 0.17 | 0.28 ± 0.16 | 0.685 |
| Change in OR | 1.80 ± 0.86 | 2.98 ± 1.85 | 0.028 |
| Change in MR | 0.11 ± 0.22 | 0.03 ± 0.21 | 0.323 |
| Change in TI | 0.00 ± 0.09 | 0.09 ± 0.10 | 0.000 |
| Change in RoC, mm | 1.53 ± 2.65 | −0.030 ± 2.63 | 0.116 |
Abbreviations: TR, taper ratio; OR, oversizing ratio; MR, mismatch ratio; TI, tortuosity index; RoC, radius of curvature.
Discussion
The disease course, pathological changes and progression levels of dissection observed in chronic TBAD were considerably different from those observed during the acute phase, and these differences in the natural process of TBAD may influence the outcomes of its treatment.7,21–25 Several studies have described results related to changes in the diameter of the aorta in acute and chronic TBAD before and after TEVAR treatment,21,22 but no study has focused on the effects of different stages on overall morphological parameters, including the TR, OR, MR, RoC and TI, which are related to several distal stent-graft complications.26–28 Our analysis of 63 patients with acute TBAD and 23 patients with chronic TBAD demonstrates that having chronic TBAD is associated with an adverse morphology before TEVAR. Furthermore, we found that the preoperative to postoperative changes observed in the OR and TI were different between the acute and chronic groups.
In our report, compared to acute dissection, chronic dissection resulted in a larger aortic size and was associated with an adverse morphology before the intervention, consistent with what is known about the changes in pathology of the thoracic aorta that occur from acute to chronic dissection.7,25 It is noted that there was a higher proportion (34.8%, n=8) of patients with aortic growth in the chronic group. Thus, aortic remodeling secondary to surgical indication is a reasonable assumption; however, aortic remodeling, which included FL thrombosis and FL shrinkage cannot reflect the entire aorta remodeling process. It should also include TL changes and SG-aorta interactions, and the acute TBAD patients with aortic growth also exhibited satisfactory aortic remodeling. Therefore, aortic remodeling is governed by the pathological differences and SG-aorta interactions. In addition, significantly greater changes occurred in the OR after TEVAR in the chronic group than in the acute group, indicating that chronic TBAD was associated with a lower stent expansion rate (i.e., a larger change in the OR results in a lower stent expansion rate). Similarly, the TR in the acute and chronic phases may be attributed to similarities in tapered remodeling between the two groups.
Previous studies have revealed that high TR and OR values may be associated with some distal stent graft-related complications, such as distal SINE, RTAD and endoleaks. However, our data indicate that the incidence rates of these complications were similar in the acute and chronic groups. The possible explanations for this finding are as follows. First, in our report, tapered SGs were more frequently used in the chronic group (47.8% vs 23.8%, P = 0.03). The distal bare stent technique, which has been proposed to prevent distal SINE in TEVAR,15,28 was used in 43.5% of the chronic group (12.7% of the acute group, P = 0.002). Second, Ma et al proposed that a stent length <165 mm may increase the risks of RTAD and distal SINE.26 The stent grafts used in this study were relatively long, and they were longer in the chronic group than in the acute group (182.96 ± 22.32 mm vs 193.35 ± 13.60 mm in the chronic vs acute groups, P = 0.02), which may buffer the distal radical force. Third, the sample in our study was relatively small. In addition, compared to the OR, the MR, another parameter used to assess the response between the stent graft and the TL, may not be as effective because diameter measurements do not accurately reflect the changes that occur in the size of a three-dimensional blood vessel with a complex curvature and a spiraling dissection flap. Hence, area may be a more appropriate parameter when measuring the size of a dissected aorta.3
The TI and RoC, which reflect the global anatomy, are other important factors associated with adverse outcomes after TEVAR.26,29,30 Aortic dissections with higher TI values are associated with a stronger spring-back force at the distal end of the stent graft, and this type of force across the tortuous descending aorta fixation site may cause the dissected intima to be potentially vulnerable. Our data show that preoperative TI values were higher in the chronic phase of TBAD than in its acute phase and that larger changes occurred after TEVAR. The collapse of the three distal bare stents in our study were all related to high TI values. The higher TI value and stiffer intima observed in chronic TBAD were associated with inadequate radial force of the distal stent, and this may explain the high incidence of distal stent collapse observed during the chronic phase. The RoC can reflect the tortuosity of the proximal deployment zone, which is correlated with the risk of developing type I endoleaks.29,31 Although we did note a difference in the TI in our results, there was no difference in the RoC between the acute phase and the chronic phase. Thus, the dissection phase exerted little influence on the tortuosity of the proximal deployment zone.
The results of our study may provide support for clinical decision-making and the following recommendations for further research. First, in our study, chronic dissection with an adverse morphology was not correlated with a higher incidence of distal stent-graft complications. This effect appeared to be partly attributable to the pathology-specific devices used in chronic TBAD, such as tapered stents and distal bare stents, which may compensate for the limitations of current stent grafts and reduce distal stent oversizing and intimal damage.16,20,28 Second, in chronic dissection, which is related to a high TI, our experience suggests that the distal landing zone should be located in a relatively straight portion of the descending aorta and that in some cases, distal bare stents can be used to reduce the angle between the distal end of the stent and the aorta.18,32 Third, although there was no significant difference in mortality between the two groups (P= 0.069), all deaths caused by aneurysm rupture occurred in the chronic group. Furthermore, our data also show that FL thrombosis in the abdominal segment was less successfully treated in terms of aortic coverage, a finding that may indicate that it is necessary to close all the entry and reentry sites in chronic aneurysmal aortic dissection cases.33 However, we need more evidence to verify these assertions.
Limitations
There are some limitations to this study. First, the results were derived from a retrospective analysis of data collected from a single institution. The number of patients was limited, and there may have been selection biases. Data derived from a prospective long-term larger patient cohort study are needed to resolve this issue. Second, a comparison of patients treated with pathology-specific devices with patients anatomically suitable for such treatment who did not receive pathology-specific devices would be helpful for establishing the true value of pathology-specific devices, but this comparison was not possible in this retrospective review. Finally, several types of stent grafts were used, and the differences among them may have influenced the final clinical results.
Conclusions
TEVAR is a safe and effective therapy for acute and chronic TBAD. Compared to acute TBAD, chronic TBAD results in an adverse preoperative morphology. The changes in OR and TI observed after TEVAR were different between the acute and chronic groups. Pathology-specific devices, such as tapered stents and distal bare stents, may be a viable treatment option for endovascular repair of chronic TBAD. However, further evaluation of more patients with longer follow-up is needed to substantiate these results.
Acknowledgements
The schematic was created by Xinyuan Li. We also thank Dr. Xinwu Lu, Professor in the Vascular Surgery Division of Shanghai Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, and the Vascular Center of Shanghai Jiaotong University, for his valuable suggestions in preparing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Riambau V, Bockler D, Brunkwall J, et al. Editor’s choice - management of descending thoracic aorta diseases: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53(1):4–52. doi: 10.1016/j.ejvs.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 2.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281 [DOI] [PubMed] [Google Scholar]
- 3.Pantaleo A, Jafrancesco G, Buia F, et al. Distal stent graft-induced new entry: an emerging complication of endovascular treatment in aortic dissection. Ann Thorac Surg. 2016;102(2):527–532. doi: 10.1016/j.athoracsur.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 4.Chen CK, Liang IP, Chang HT, et al. Impact on outcomes by measuring tortuosity with reporting standards for thoracic endovascular aortic repair. J Vasc Surg. 2014;60(4):937–944. doi: 10.1016/j.jvs.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 5.Famularo M, Meyermann K, Lombardi JV. Aneurysmal degeneration of type B aortic dissections after thoracic endovascular aortic repair: a systematic review. J Vasc Surg. 2017;66(3):924–930. doi: 10.1016/j.jvs.2017.06.067 [DOI] [PubMed] [Google Scholar]
- 6.Fattori R, Nienaber CA, Rousseau H, et al. Results of endovascular repair of the thoracic aorta with the talent thoracic stent graft: the talent thoracic retrospective registry. J Thorac Cardiovasc Surg. 2006;132(2):332–339. doi: 10.1016/j.jtcvs.2006.03.055 [DOI] [PubMed] [Google Scholar]
- 7.Peterss S, Mansour AM, Ross JA, et al. Changing pathology of the thoracic aorta from acute to chronic dissection: literature review and insights. J Am Coll Cardiol. 2016;68(10):1054–1065. doi: 10.1016/j.jacc.2016.05.091 [DOI] [PubMed] [Google Scholar]
- 8.Qin YL, Wang F, Li TX, et al. Endovascular repair compared with medical management of patients with uncomplicated type B acute aortic dissection. J Am Coll Cardiol. 2016;67(24):2835–2842. doi: 10.1016/j.jacc.2016.03.578 [DOI] [PubMed] [Google Scholar]
- 9.Iannuzzi JC, Stapleton SM, Bababekov YJ, et al. Favorable impact of thoracic endovascular aortic repair on survival of patients with acute uncomplicated type B aortic dissection. J Vasc Surg. 2018;68(6):1649–1655. doi: 10.1016/j.jvs.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 10.Brunkwall J, Kasprzak P, Verhoeven E, et al. Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodelling: 1 year results of the ADSORB trial. Eur J Vasc Endovasc Surg. 2014;48(3):285–291. doi: 10.1016/j.ejvs.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 11.Ammar CP, Larion S, Ahanchi SS, Lavingia KS, Dexter DJ, Panneton JM. Anatomic severity grading score for primary descending thoracic aneurysms predicts procedural difficulty and aortic-related reinterventions after thoracic endovascular aortic repair. J Vasc Surg. 2016;64(4):912–920 e911. doi: 10.1016/j.jvs.2016.03.451 [DOI] [PubMed] [Google Scholar]
- 12.Patterson BO, Cobb RJ, Karthikesalingam A, et al. A systematic review of aortic remodeling after endovascular repair of type B aortic dissection: methods and outcomes. Ann Thorac Surg. 2014;97(2):588–595. doi: 10.1016/j.athoracsur.2013.07.128 [DOI] [PubMed] [Google Scholar]
- 13.Grabenwoger M, Alfonso F, Bachet J, et al. Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2012;42(1):17–24. doi: 10.1093/ejcts/ezs107 [DOI] [PubMed] [Google Scholar]
- 14.Chen IM, Huang CY, Weng SH, et al. Implantation sequence modification averts distal stent graft-induced new entry after endovascular repair of Stanford type B aortic dissection. J Vasc Surg. 2016;64(2):281–288. doi: 10.1016/j.jvs.2016.02.032 [DOI] [PubMed] [Google Scholar]
- 15.Feng J, Lu Q, Zhao Z, et al. Restrictive bare stent for prevention of stent graft-induced distal redissection after thoracic endovascular aortic repair for type B aortic dissection. J Vasc Surg. 2013;57(2Suppl):44S–52S. doi: 10.1016/j.jvs.2012.06.117 [DOI] [PubMed] [Google Scholar]
- 16.Shirali AS, Bischoff MS, Lin HM, et al. Predicting the risk for acute type B aortic dissection in hypertensive patients using anatomic variables. JACC Cardiovasc Imaging. 2013;6(3):349–357. doi: 10.1016/j.jcmg.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 17.Alberta HB, Secor JL, Smits TC, Farber MA, Jordan WD, Matsumura JS. Differences in aortic arch radius of curvature, neck size, and taper in patients with traumatic and aortic disease. J Surg Res. 2013;184(1):613–618. doi: 10.1016/j.jss.2013.05.098 [DOI] [PubMed] [Google Scholar]
- 18.Zha B, Xu G, Zhu H, et al. Endovascular repair of type B aortic dissection with the restrictive bare stent technique: morphologic changes, technique details, and outcomes. Ther Clin Risk Manag. 2018;14:1993–2002. doi: 10.2147/TCRM.S177757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He H, Yao K, Nie WP, et al. Modified petticoat technique with pre-placement of a distal bare stent improves early aortic remodeling after complicated acute stanford type B aortic dissection. Eur J Vasc Endovasc Surg. 2015;50(4):450–459. doi: 10.1016/j.ejvs.2015.04.035 [DOI] [PubMed] [Google Scholar]
- 20.Xu SD, Huang FJ, Du JH, et al. A study of aortic dimension in type B aortic dissection. Interact Cardiovasc Thorac Surg. 2008;7(2):244–248. doi: 10.1510/icvts.2007.163154 [DOI] [PubMed] [Google Scholar]
- 21.Zhang MH, Du X, Guo W, Liu XP, Jia X, Ge YY. Early and midterm outcomes of thoracic endovascular aortic repair (TEVAR) for acute and chronic complicated type B aortic dissection. Medicine (Baltimore). 2017;96(28):e7183. doi: 10.1097/MD.0000000000007183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou HW, Chan CY, Chang CH, et al. Comparisons of aortic remodelling and outcomes after endovascular repair of acute and chronic complicated type B aortic dissections. Interact Cardiovasc Thorac Surg. 2018;27(5):733–741. doi: 10.1093/icvts/ivy167 [DOI] [PubMed] [Google Scholar]
- 23.Conway AM, Qato K, Mondry LR, Stoffels GJ, Giangola G, Carroccio A. Outcomes of thoracic endovascular aortic repair for chronic aortic dissections. J Vasc Surg. 2018;67(5):1345–1352. doi: 10.1016/j.jvs.2017.08.098 [DOI] [PubMed] [Google Scholar]
- 24.Tolenaar JL, van Keulen JW, Jonker FH, et al. Morphologic predictors of aortic dilatation in type B aortic dissection. J Vasc Surg. 2013;58(5):1220–1225. doi: 10.1016/j.jvs.2013.05.031 [DOI] [PubMed] [Google Scholar]
- 25.Fanelli F, Cannavale A, O’Sullivan GJ, et al. Endovascular repair of acute and chronic aortic type B dissections: main factors affecting aortic remodeling and clinical outcome. JACC Cardiovasc Interv. 2016;9(2):183–191. doi: 10.1016/j.jcin.2015.10.027 [DOI] [PubMed] [Google Scholar]
- 26.Ma T, Dong ZH, Fu WG, et al. Incidence and risk factors for retrograde type A dissection and stent graft-induced new entry after thoracic endovascular aortic repair. J Vasc Surg. 2018;67(4):1026–1033.e1022. doi: 10.1016/j.jvs.2017.08.070 [DOI] [PubMed] [Google Scholar]
- 27.Sze DY, van Den Bosch MA, Dake MD, et al. Factors portending endoleak formation after thoracic aortic stent-graft repair of complicated aortic dissection. Circ Cardiovasc Interv. 2009;2(2):105–112. doi: 10.1161/CIRCINTERVENTIONS.108.819722 [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Yin H, Chen Y, et al. Restrictive bare stent prevents distal stent graft-induced new entry in endovascular repair of type B aortic dissection. J Vasc Surg. 2018;67(1):93–103. doi: 10.1016/j.jvs.2017.04.066 [DOI] [PubMed] [Google Scholar]
- 29.Boufi M, Aouini F, Guivier-Curien C, et al. Examination of factors in type I endoleak development after thoracic endovascular repair. J Vasc Surg. 2015;61(2):317–323. doi: 10.1016/j.jvs.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 30.Boufi M, Guivier-Curien C, Dona B, et al. Risk factor analysis for the mal-positioning of thoracic aortic stent grafts. Eur J Vasc Endovasc Surg. 2016;52(1):56–63. doi: 10.1016/j.ejvs.2016.03.025 [DOI] [PubMed] [Google Scholar]
- 31.Ueda T, Fleischmann D, Dake MD, Rubin GD, Sze DY. Incomplete endograft apposition to the aortic arch: bird-beak configuration increases risk of endoleak formation after thoracic endovascular aortic repair. Radiology. 2010;255(2):645–652. doi: 10.1148/radiol.10091468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X, Huang L, Sun L, et al. Endovascular repair of stanford B aortic dissection using two stent grafts with different sizes. J Vasc Surg. 2015;62(1):43–48. doi: 10.1016/j.jvs.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 33.Sharafuddin MJ, Bhama JK, Bashir M, Aboul-Hosn MS, Man JH, Sharp AJ. Distal landing zone optimization before endovascular repair of aortic dissection. J Thorac Cardiovasc Surg. 2019;157(1):88–98. doi: 10.1016/j.jtcvs.2018.06.095 [DOI] [PubMed] [Google Scholar]