Abstract
Adult survivors of very preterm (≤32 wk gestational age) birth without (PRE) and with bronchopulmonary dysplasia (BPD) have variable degrees of airflow obstruction at rest. Assessment of the shape of the maximal expiratory flow-volume (MEFV) curve in PRE and BPD may provide information concerning their unique pattern of airflow obstruction. The purposes of the present study were to 1) quantitatively assess the shape of the MEFV curve in PRE, BPD, and healthy adults born at full-term (CON), 2) identify where along the MEFV curve differences in shape existed between groups, and 3) determine the association between an index of MEFV curve shape and characteristics of preterm birth (i.e., gestational age, mass at birth, duration of oxygen therapy) in PRE and BPD. To do so, we calculated the average slope ratio (SR) throughout the effort-independent portion of the MEFV curve and at increments of 5% of forced vital capacity (FVC) between 20 and 80% of FVC in PRE (n = 19), BPD (n = 25), and CON (n = 20). We found that average SR was significantly higher in PRE (1.34 ± 0.35) and BPD (1.33 ± 0.45) compared with CON (1.03 ± 0.22; both P < 0.05) but similar between PRE and BPD (P = 0.99). Differences in SR between groups occurred early in expiration (i.e., 20–30% of FVC). There was no association between SR and characteristics of preterm birth in PRE and BPD groups (all P > 0.05). The mechanism(s) of increased SR during early expiration in PRE/BPD relative to CON is unknown but may be due to differences in the structural and mechanical properties of the airways.
Keywords: bronchopulmonary dysplasia, expiratory flow limitation, pulmonary function, slope ratio, ventilatory constraints
INTRODUCTION
Very preterm neonates (i.e., those born at a gestational age of ≤32 wk) have arrested development of the lungs and airways, which results in airflow obstruction at birth (40). Some individuals born very preterm require prolonged supplemental oxygen therapy (i.e., FIO2 > 0.21 for ≥28 days) to maintain blood gas homeostasis, resulting in the development of bronchopulmonary dysplasia (18). Supplemental oxygen delivery can cause oxidative stress, leading to damage of the airways (3) and worsening pulmonary function in individuals born preterm (9, 22–24). In the absence of compensatory lung development in those born preterm (44), adult survivors of preterm birth who did not (PRE) and those who did develop bronchopulmonary dysplasia (BPD) have worse pulmonary function compared with their counterparts who were born at full term (≥37 wk of gestation) (7–10, 23, 24, 36, 38, 43). Although the magnitude of impairment is variable, PRE and BPD generally have chronic mild airflow obstruction, as evidenced by the size and shape of their maximum expiratory flow-volume (MEFV) curves.
Analysis of the MEFV curve provides physiologically relevant information that is used to assess pulmonary function, which can subsequently be employed to diagnose and determine the severity of obstructive lung diseases (33). In addition to standard pulmonary function measurements, the shape of the MEFV curve can also be evaluated at specific lung volumes to describe an individual’s expiratory airflow pattern using the slope ratio (SR) method (31). We recently used the SR method to dissociate the effect of pathological airway obstruction in individuals with mild chronic obstructive pulmonary disease (COPD) from that of the age-related increase in airflow obstruction in healthy, age-matched controls (5). Assessing SR in individuals with conditions that involve mild to moderate degrees of airflow obstruction may provide important information that complements that obtained from standard spirometry. Specifically, the SR provides index of the degree of homogeneity (or heterogeneity) of lung emptying as well as the overall pattern of expiratory airflow across the effort-independent zone and/or within a discrete volume increment. This additional information derived from the SR can be used to further characterize the pattern of airflow obstruction resulting from preterm birth (i.e., PRE) as well as the effect of prolonged supplemental oxygen therapy during the neonatal period (i.e., BPD). Additionally, SR can be used to identify the lung volume(s) where the shape of the MEFV curve differs between groups, particularly since the decrement in pulmonary function in PRE/BPD individuals has been likened to that of patients with mild COPD (26, 28) and healthy older individuals (29) despite presumed differences in the mechanisms of airflow obstruction.
Accordingly, the primary purpose of the present study was threefold: 1) to assess whether the shape of the MEFV curve is altered by preterm birth, 2) to determine whether BPD individuals have a higher SR (indicative of greater convexity) than PRE individuals, and 3) to determine the association between SR and subject characteristics in the PRE and BPD groups, such as gestational age, birth weight, and duration of oxygen therapy. An additional purpose of the study was to examine whether acute changes in airway resistance had an effect on the SR in a subset of PRE and BPD individuals. We hypothesized that SR would be higher in PRE and BPD individuals than in healthy controls and that the higher SR would be most evident during early expiration, as in the case in patients with obstructive lung disease. We further hypothesized that BPD would have a higher SR than PRE and that gestational age and duration of oxygen therapy would be significantly associated with SR. Finally, we hypothesized that breathing a helium-oxygen gas mixture (heliox), which reduces airway resistance, would decrease the SR in PRE and BPD individuals.
METHODS
Ethical approval and subjects.
The present study is a retrospective analysis of previously collected data on PRE and BPD individuals (8–10, 22–24) and CON individuals (34). In total, data from 64 subjects were analyzed, with 19 (n = 7 female) having been born preterm without bronchopulmonary dysplasia (PRE; see below), n = 25 (n = 12 female) having been born preterm with bronchopulmonary dysplasia (BPD; see below), and n = 20 (n = 11 female) healthy, young control subjects (CON). To be included in the preterm groups, individuals were required to have been born ≤32 wk gestational age, which was verified by a board-certified neonatologist using medical records. Subjects included in the PRE or BPD group were born between 1979 and 1997. Diagnosis and classification of preterm birth with bronchopulmonary dysplasia was determined by a board-certified neonatologist, using medical records described in detail elsewhere (18), and validated (12). Briefly, bronchopulmonary dysplasia was considered present if the infant received oxygen therapy of >21% oxygen for ≥28 days after birth. Severity of bronchopulmonary dysplasia was determined based on the percent of oxygen breathed at 36 wk postmenstrual age (if born <32 wk gestational age) or at 56 days postnatal age (if born ≥32 wk gestational age) or at discharge, whichever occurred first. Mild BPD was the diagnosis if the infant was breathing room air (21% oxygen) at the gestational age at the birth-specific time point. Moderate BPD was the diagnosis if the infant was breathing <30% oxygen at the gestational age-specific time point. Finally, severe BPD was the diagnosis if the infant was breathing ≥30% oxygen and/or receiving mechanical ventilation at the gestational age-specific time point. Based on these criteria, BPD subjects were classified as having mild (n = 10), moderate (n = 13), or severe bronchopulmonary dysplasia (n = 2). Individuals included in the CON group were self-reported to be born at full term (i.e., born at >37 wk of gestation) and had normal pulmonary function based on prediction equations (37), a body mass index between 18 and 30 kg/m−2, and peak aerobic power of ≥80% predicted based on population-specific normative values (19). All subjects volunteered to participate in the associated initial studies after being advised both verbally and in writing as to the nature of the experiments. Informed consent was obtained both orally and in writing. The study protocols of the initial investigations were approved by the institutional ethics boards at the University of Oregon, Ohio University, and the University of British Columbia. All studies were performed in accordance with the standards set forth by the Declaration of Helsinki, except for registration in a database.
Pulmonary function testing.
Spirometry and whole body plethysmography were performed using a commercially available system (CON = Vmax Encore 229, V62J Autobox; CareFusion, Yorba Linda, CA; PRE and BPD = Elite Series Plethysmograph; MedGraphics, St. Paul, MN) according to standard recommendations (26, 33, 46). Pulmonary function measurements were expressed as absolute values and as percentages of predicted values (37).
MEFV curves.
To generate MEFV curves, subjects performed forced expiratory maneuvers from total lung capacity to residual volume at maximum effort according to societal standards (33). All subjects performed multiple maneuvers (i.e., ≥3) to ensure repeatability, and the maneuver with the largest forced vital capacity (FVC) was selected for analysis.
Slope ratio.
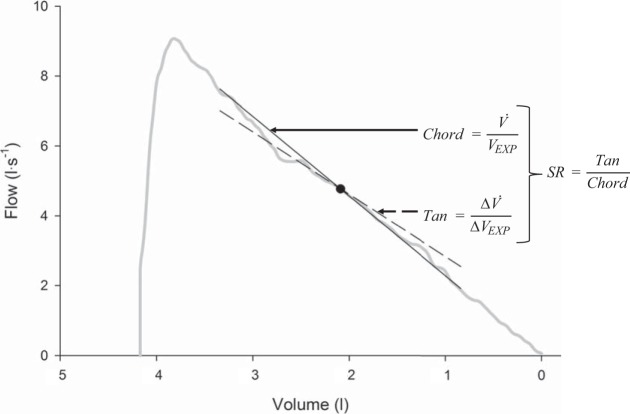
To quantify the shape of the MEFV curves, we used the SR method, as previously described (5, 6, 31). Briefly, raw flow and volume data for each subject’s largest FVC were analyzed. A line of tangency and a chord line were mathematically determined at each point of interest (i.e., in 1% increments of FVC) along the volume axis of each individual’s MEFV curve, as shown in Fig. 1. The Δflow and volume used to determine the tangent line was 0.1 liter above and below a given point of interest. At each 1% increment of FVC, SR was calculated as the ratio of the tangent and chord lines. The SR provides additional information regarding airflow obstruction that is complimentary to standard spirometry. The SR is a measure of the degree of curvature of the MEFV curve within a given portion of its volume, whereby a SR of 1 indicates a linear decrease in flow with decreasing volume (i.e., no curvature), whereas a SR <1 designates a concave (i.e., “bump”) shape and a SR >1 defines a convex (i.e., “scoop”) shape. The SR is a unitless measure that can be used to describe the average curvature of the entire effort-independent zone of the MEFV curve (i.e., 20–80% of FVC). To do this, the SR was computed in 1% volume increments from 20 to 80% of FVC and then averaged to represent the SR for each individual. Additionally, the SR was reported at specific fractions of FVC. SR data for a given individual were then averaged in 5% increments of FVC, whereby SR data in 1% increments from 20 to 25 and from 25 to 30% of FVC were averaged and considered the SR at 20 and 25% of FVC, respectively. During preliminary analyses we used different fractions of expired volume (i.e., 1, 2, 10%, etc.) but found that 5% increments provided the best resolution without compromising the signal-to-noise ratio. The SR was not calculated for lung volumes <20% or >80% of expired vital capacity due to the effect of effort dependence and passive opposition of the chest wall overriding the flow-limiting mechanism (30, 31).
Fig. 1.
Schematic of the method of determining the slope ratio (SR) of a single point along the maximal expiratory flow-volume (MEFV) curve. Example represents an MEFV curve from a healthy female subject in the control (CON) group. The same procedure was repeated at each point of interest along each subject’s MEFV curve. Tan, tangent; V̇, flow; Vexp, volume remaining to be expired; ΔV̇, change in flow; ΔVexp, change in volume.
We have previously noted that PRE and BPD individuals have a smaller dysanapsis ratio (i.e., an index of airway size relative to lung size) compared with CON (9). Smaller airways in PRE/BPD relative to CON would increase airflow resistance, which may result in a higher SR. To determine the effect of airflow resistance on the SR, a subset of our PRE and BPD subjects (n = 10) performed forced expiratory maneuvers while breathing heliox (in addition to forced expiratory maneuvers while breathing ambient air). The heliox MEFV curves were generated in an identical manner described above. Helium has a lower density than nitrogen, thereby promoting laminar flow and decreasing airflow resistance (2, 6, 10, 13, 35). If high airflow resistance contributed to SR in PRE/BPD individuals, then breathing heliox during a forced expiratory maneuver would alter SR.
Statistical analysis.
All statistical analyses were performed using Prism statistical software (version 7.0a; GraphPad Software, San Diego, CA), and α was set at 0.05. Anthropometric and pulmonary function data were compared across groups using one-way analysis of variance (ANOVA) with Tukey’s HSD post hoc test where appropriate. To compare the average SR across groups, a one-way ANOVA with Tukey’s HSD post hoc test was computed. To compare SR across groups at 5% FVC increments, a two-way ANOVA was computed with a priori planned comparisons at each increment of FVC (i.e., t tests with a Bonferroni-adjusted α). To compare the average SR while breathing air and heliox in a subset of PRE and BPD subjects, we computed a dependent-samples t test. To determine the association between SR and gestational age, mass at birth, Z-score of mass at birth (14), and duration of supplemental oxygen therapy, multiple (i.e., 1 per parameter) Pearson correlation coefficients (r) were computed.
RESULTS
Anthropometric and pulmonary function data.
Subject characteristics and anthropometric data are shown in Table 1. At birth, PRE subjects weighed 1.24 ± 0.45 kg and were born at 28.2 ± 2.0 wk gestation, whereas BPD subjects weighed 0.99 ± 0.27 kg and were born at 27.2 ± 1.8 wk gestation. Additionally, BPD subjects received 74 ± 45 days of oxygen therapy at birth (range: 29–175 days) and PRE subjects received 17 ± 6 days of oxygen therapy (range: 0–900 days). The CON group was significantly older than both PRE and BPD groups (P < 0.05), but there were no significant differences in height (P = 0.27), body mass (P = 0.37), or body mass index (P = 0.15).
Table 1.
Subject characteristics and anthropometric
| CON (n = 20) | PRE (n = 19) | BPD (n = 25) | |
|---|---|---|---|
| Sex (men/women) | 11/9 | 12/9 | 13/12 |
| Age, yr | 24.1 ± 0.5 | 21.2 ± 0.6* | 21.8 ± 0.7* |
| Height, cm | 171.7 ± 1.9 | 172.0 ± 1.8 | 167.6 ± 2.4 |
| Weight, kg | 64.9 ± 2.4 | 68.1 ± 3.1 | 67.9 ± 3.1 |
| BMI, kg/m2 | 21.9 ± 0.4 | 22.9 ± 0.8 | 24.2 ± 1.1 |
| Gestational age, wk | 28.8 ± 0.5 | 27.2 ± 0.4† | |
| Mass at birth, kg | 1.24 ± 0.10 | 0.99 ± 0.05† | |
| Mass at birth, Z-score | 0.033 ± 0.323 | −0.048 ± 0.156 | |
| Duration of supplemental oxygen therapy, days | 17 ± 6 | 74 ± 9† |
All values are expressed as means ± SD; n, number of subjects. BMI, body mass index; BPD, bronchopulmonary dysplasia; CON, control; PRE, preterm without bronchopulmonary dysplasia. Z-score for mass at birth determined via Fenton and Kim (14).
Statistically significant difference from CON, P < 0.05;
statistically significant difference between PRE and BPD, P < 0.05.
Pulmonary function data at the time of study are displayed in Table 2. When expressed in absolute terms (i.e., liters or L/s) or as a percentage of predicted normal values (i.e., %predicted), measures of spirometry were consistently lower in the BPD group than the in CON group (all P < 0.05), with the exception of absolute FVC (P = 0.07). Absolute and %predicted peak expiratory flow, forced expired flow at 25% of FVC, forced expired flow between 25 and 75% of FVC, and %predicted forced expired volume in 1 s were lower in the PRE group than the CON group (all P < 0.05). Absolute and %predicted FVC, forced expired flow at 50 and 75% of FVC, and absolute forced expired volume in 1 s were similar between the PRE and CON groups (all P > 0.05). Additionally, when compared with the PRE group, the BPD group had lower absolute forced expired volume in 1 s, forced expired flow at 25% of FVC, and absolute and %predicted forced expired flow between 25 and 75% of FVC. Absolute and %predicted total lung capacity and functional residual capacity as well as absolute residual volume were similar between groups (all P > 0.05), whereas %predicted residual volume was lower in the BPD group than in the CON group (P = 0.04). Z-score values were computed using appropriate prediction equations (37) for FVC, forced expiratory volume in 1 s, ratio of forced expiratory volume in 1 s to FVC, forced expired flow between 25 and 75% of FVC, and forced expired flow at 75% of FVC. These data are displayed in brackets in Table 2. Not unlike the %predicted data, BPD had a significantly lower Z-score than CON for all parameters, but only forced expired flow between 25 and 75% of FVC Z-score differed between PRE and CON. No differences in Z-scores were present between PRE and BPD groups (all P > 0.05).
Table 2.
Pulmonary function data
| CON (n = 20) |
PRE (n = 19) |
BPD (n = 25) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute values | %Predicted | Z-score | Absolute values | %Predicted | Z-score | Absolute values | %Predicted | Z-score | |
| Spirometry | |||||||||
| FVC, liters | 4.9 ± 0.2 | 103 ± 2 | 0.34 ± 0.16 | 4.7 ± 0.2 | 95 ± 3 | −0.17 ± 0.21 | 4.3 ± 0.3 | 90 ± 3* | −0.54 ± 0.24* |
| FEV1, liters | 3.9 ± 0.2 | 103 ± 2 | −0.13 ± 0.15 | 3.7 ± 0.2 | 92 ± 2* | −0.90 ± 0.26 | 3.1 ± 0.2*† | 82 ± 4* | −1.61 ± 0.29* |
| FEV1/FVC | 81 ± 1 | 95 ± 1 | −0.76 ± 0.16 | 78 ± 2 | 92 ± 2 | −1.11 ± 0.23 | 74 ± 2* | 85 ± 3* | −1.62 ± 0.26* |
| PEF, l × s−1 | 9.1 ± 0.4 | 112 ± 2 | 7.6 ± 0.4* | 88 ± 3* | 6.5 ± 0.4* | 78 ± 4* | |||
| FEF25, l × s−1 | 8.0 ± 0.3 | 106 ± 3 | 6.3 ± 0.4* | 78 ± 4* | 5.0 ± 0.4*† | 65 ± 4*† | |||
| FEF50, l × s−1 | 4.7 ± 0.2 | 85 ± 4 | 3.9 ± 0.3 | 70 ± 5 | 3.1 ± 0.3* | 58 ± 5* | |||
| FEF75, l × s−1 | 2.0 ± 0.1 | 88 ± 6 | −0.16 ± 0.19 | 1.7 ± 0.2 | 71 ± 6 | −0.95 ± 0.24 | 1.4 ± 0.1* | 60 ± 6* | −1.41 ± 0.28* |
| FEF25–75, l × s−1 | 3.9 ± 0.2 | 94 ± 3 | −0.46 ± 0.15 | 3.3 ± 0.3 | 75 ± 5* | −1.33 ± 0.24* | 2.7 ± 0.2*† | 63 ± 5* | −1.96 ± 0.28* |
| Lung volumes | |||||||||
| TLC, liters | 6.2 ± 0.2 | 103 ± 2 | 6.3 ± 0.3 | 102 ± 3 | 5.9 ± 0.3 | 102 ± 2 | |||
| IC, liters | 3.1 ± 0.1 | 108 ± 4 | 3.1 ± 0.1 | 100 ± 4 | 2.7 ± 2 | 95 ± 4* | |||
| FRC, liters | 3.1 ± 0.1 | 99 ± 4 | 3.3 ± 0.2 | 107 ± 5 | 3.2 ± 0.2 | 109 ± 4 | |||
| ERV, liters | 1.8 ± 0.1 | 109 ± 4 | 1.7 ± 0.1 | 100 ± 6 | 1.6 ± 0.1 | 96 ± 6 | |||
| RV, liters | 1.2 ± 0.1 | 87 ± 6 | 1.5 ± 0.1 | 111 ± 9 | 1.6 ± 0.1 | 126 ± 9* | |||
All values are expressed as means ± SD; n, number of subjects. BPD, bronchopulmonary dysplasia; CON, control; ERV, expiratory reserve volume; FEV1, forced expiratory volume in 1 s; FEF25, forced expiratory flow at 25% of FVC; FEF50, forced expiratory flow at 50% of FVC; FEF75, forced expiratory flow at 75% of FVC; FEF25–75, average forced expiratory flow from 25 to 75% of FVC; FRC, functional residual capacity; FVC, forced vital capacity; IC, inspiratory capacity; PEF, peak expiratory flow; PRE, preterm without bronchopulmonary dysplasia; RV, residual volume; TLC, total lung capacity.
Statistically significant difference from CON, P < 0.05;
statistically significant difference between PRE and BPD, P < 0.05.
Slope ratio.
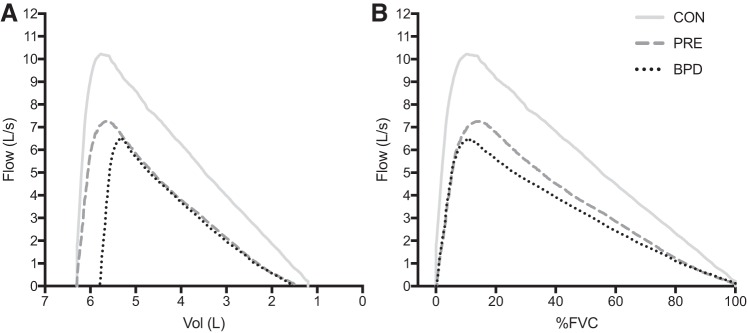
Composite average MEFV curves for each group are displayed in Fig. 2, whereby expiratory flow is expressed as a function of absolute volume (i.e., scaled to total lung capacity, in liters; Fig. 2A), and as a function of relative FVC (i.e., %FVC; Fig. 2B). Figure 3 displays box and whisker plots of the average SR from 20 to 80% of FVC for the CON (1.03 ± 0.22), PRE (1.34 ± 0.35), and BPD (1.33 ± 0.45) groups. When compared with CON, SR was significantly greater in PRE (P = 0.03) and BPD (P = 0.02) but not significantly different from one another (P = 0.99). Figure 4 displays the SR from 20 to 80% of FVC in 5% increments. The omnibus two-way ANOVA revealed that the interaction between %FVC and group was not statistically significant (P = 0.36), but there was a significant effect for group (P = 0.01) and a trend toward a significant effect of %FVC (P = 0.06). Our preplanned comparisons revealed that there were no significant differences between PRE and BPD at any fraction of FVC between 20 and 80% (all P > 0.05). However, CON had a significantly lower SR than both PRE (P = 0.006) and BPD (P = 0.007) at 20, 25 (P = 0.008, 0.04), and 35% (P = 0.02, 0.04) of FVC. CON also had a significantly lower SR than BPD at 65% of FVC (P = 0.04). No other statistically significant differences were present at any other relative lung volumes.
Fig. 2.
Maximal expiratory flow-volume (MEFV) curves for each group. A: MEFV curves for each group as a function of absolute volume, scaled to total lung capacity. B: MEFV curves for each group as a function of %forced vital capacity (%FVC). BPD, bronchopulmonary dysplasia; CON, control; PRE, preterm without bronchopulmonary dysplasia.
Fig. 3.
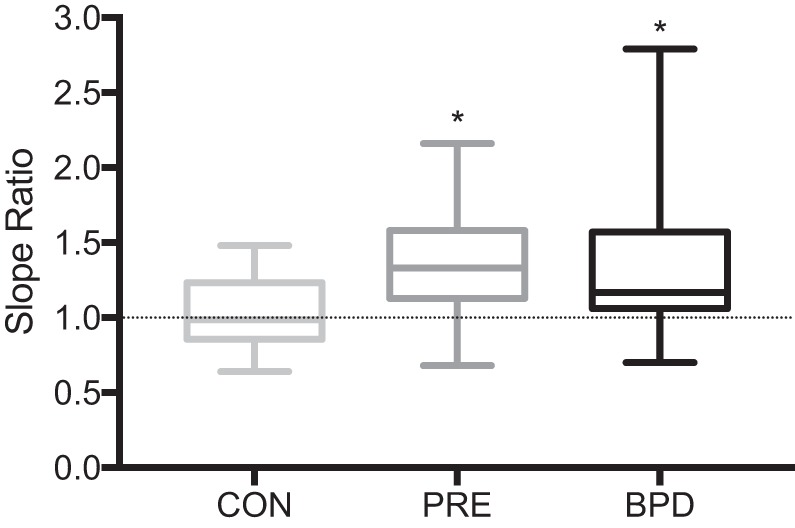
Box and whisker plot of mean slope ratio (SR) data for each group. Line in the middle of the box denotes the median SR value for each group. Box extends from the 25th (bottom) to the 75th (top) percentiles. Whiskers extend to the minimum and maximum values within each group. *Significant difference from the control (CON) group, P < 0.05. BPD, bronchopulmonary dysplasia; PRE, preterm without bronchopulmonary dysplasia.
Fig. 4.
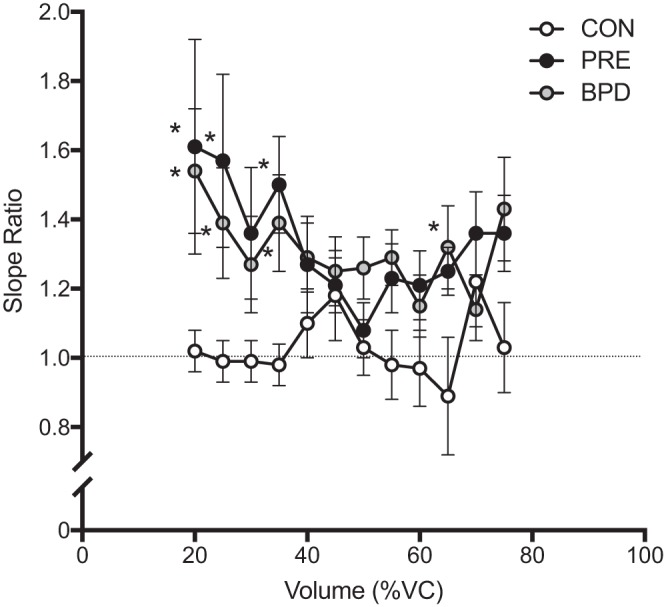
Mean ± SE of slope ratio data at intervals of 5% of forced vital capacity (FVC) from 20 to 80% of FVC. ○, Control (CON) data; gray circles, preterm without bronchopulmonary dysplasia (PRE) data; ●, bronchopulmonary dysplasia (BPD) data. *Significant difference from CON within a given interval, P < 0.05. %VC, %vital capacity.
Figure 5 displays box and whisker plots of the average SR for the subset of PRE and BPD subjects (n = 10) that performed FVC maneuvers while breathing ambient air and heliox. Despite a significant increase in forced expired flow between 25 and 75% of FVC (2.96 ± 1.32 vs. 4.31 ± 2.15 L/s, P = 0.0009) there was no change in the average SR when heliox was breathed (P = 0.76).
Fig. 5.
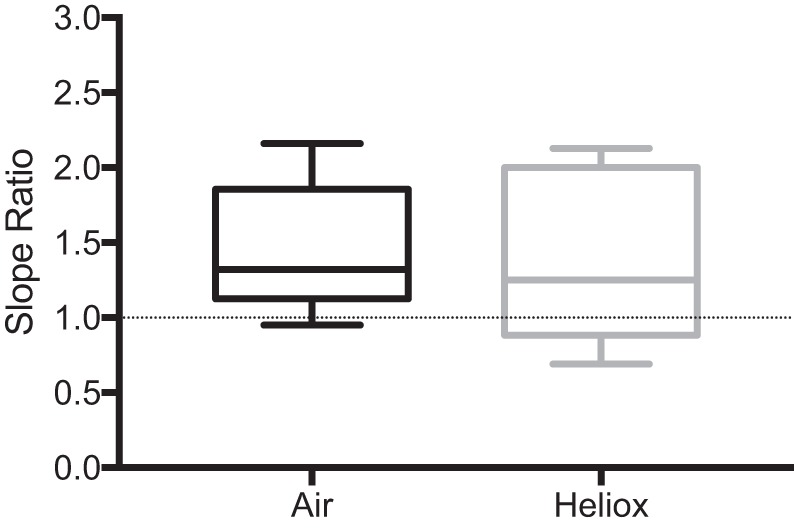
Box and whisker plot of mean slope ratio (SR) data for a subset (n = 10) of preterm without bronchopulmonary dysplasia (PRE) and bronchopulmonary dysplasia (BPD) subjects while breathing air and heliox. Line in the middle of the box denotes the median SR value for each group. Box extends from the 25th (bottom) to the 75th (top) percentiles. Whiskers extend to the minimum and maximum values within each group.
Relationships between SR and subject characteristics of PRE and BPD.
There was no relationship between average SR and gestational age in the PRE (P = 0.61) or BPD (P = 0.92) groups or with PRE and BPD subjects pooled together (P = 0.86). Likewise, there was no relationship between SR and mass at birth in kilograms in PRE (P = 0.54) or BPD (P = 0.46) or with PRE and BPD subjects pooled together (P = 0.99), nor was there a relationship between SR and mass at birth in Z-score in PRE (P = 0.65) or BPD (P = 0.24) or with PRE and BPD subjects pooled together (P = 0.76). Finally, there was no relationship between SR and the duration of supplemental oxygen therapy in PRE (n = 12, P = 0.39) or BPD individuals (P = 0.51) or with PRE and BPD subjects pooled together (P = 0.81).
DISCUSSION
Main findings.
The main findings of this study are threefold. First, the average SR, a measure of the shape of the MEFV curve, was significantly higher in PRE and BPD compared with CON but was not different between PRE and BPD groups despite differences in spirometry (FEV1, forced expired flow at 25% of FVC, and forced expired flow between 25 and 75% of FVC). Accordingly, the SR is in slight contrast with the spirometry data and suggests that the severity of airflow obstruction may not differ significantly between PRE and BPD, at least in our cohort of subjects. Second, the difference in SR between the PRE and BPD groups relative to CON occurred early during forced expiration. Third, there was no apparent relationship between SR and characteristics of preterm birth (i.e., birthweight, gestational age, and days on supplemental oxygen). Finally, we observed no change in the SR with heliox breathing in a subset of PRE and BPD individuals. Collectively, we interpret our findings to mean that the arrested development of the lungs in individuals that are born preterm alters the shape of their MEFV curve in adulthood; however, in individuals that are born preterm, the shape of the MEFV curve is not affected by prolonged supplemental oxygen therapy during the neonatal period.
Effect of very preterm birth on SR.
We noted that average SR was lower in the CON group relative to the PRE and BPD groups (Fig. 3), in whom the MEFV curve demonstrated an overall convexity or “scoop” (Fig. 2). As expected, our results indicate that the mild degree of airflow obstruction in PRE and BPD individuals appears to increase their average SR. Despite being significantly higher than CON individuals, an average SR of 1.34 ± 0.41 implies that PRE and BPD individuals are still within the limits that are thought to reflect homogeneous lung emptying (i.e., SR < 2.5) (31). Nevertheless, it is noteworthy that the PRE and BPD individuals in our study had a similar average SR compared with healthy individuals ∼40 yr older (i.e., 63 ± 9 yr) (5). Based on this observation, it is reasonable to speculate that the arrested lung development in PRE and BPD individuals may have a similar effect on SR than the age-related changes to the structural and mechanical properties of the lungs. If this were true, then we would likely have noted that the higher SR in PRE and BPD individuals would be evident at similar lung volumes. We previously found that healthy older individuals have a SR that is similar to healthy young individuals early in expiration (i.e., a SR of ∼1), followed by a progressive increase in SR later in expiration (5), likely due to premature airway closure (4). We noted that the average SR throughout expiration in PRE and BPD individuals had an opposite pattern, whereby SR was relatively high during early expiration and progressively declined to a point that is similar to CON individuals during late expiration (Figs. 3 and 4). The pattern of SR during expiration in PRE and BPD individuals more closely resembles that of patients with COPD, but with SR values being consistently lower in the PRE and BPD individuals (5, 29). Our findings raise important questions regarding the trajectory of change in pulmonary function over time in PRE and BPD individuals. It has previously been suggested that PRE and BPD individuals may be at increased risk of developing obstructive lung disease later in life than healthy individuals born full term (11, 20, 25), and the similarity of the pattern of SR during exercise between PRE/BPD and patients with mild COPD (5) may support this idea. Thus, the age-related decline in pulmonary function, which increases SR and is thought to be accelerated in PRE and BPD individuals (39), may lead to the early development of COPD (18). At present, these ideas remain speculative, and work examining the SR longitudinally in PRE/BPD over the course of the aging process is needed.
The mechanistic basis of the curvilinearity in the MEFV curve of those in the PRE and BPD groups is difficult to elucidate but could be the result of differences in airway size, static recoil pressure, or a combination thereof. Under conditions where expiratory effort is maximal, a lower static recoil pressure at high lung volumes could explain the higher SR in those in the PRE and BPD groups compared with those in the CON group. Static recoil pressure is determined by the mechanical tissue and surface forces (41), both of which may be affected by stunted lung/airway growth in adults born preterm. The convexity of the MEFV curve at higher lung volumes in PRE and BPD subjects may also be caused by differences in airway resistance, which is known to be increased in preterm infants with and without BPD (21) as well as adolescents (42, 45). The high airway resistance may be related to differences in airway cross-sectional area. Using the dysanapsis ratio as an index for airway size, we previously showed that PRE/BPD individuals appear to have smaller airways than age-, sex-, and height-matched healthy controls (9). It should be noted, however, that dysanapsis ratio is an indirect measure that is partially determined by static recoil pressure (32), which precludes our ability to differentiate the influence of airway cross-sectional area from that of tissue and surfaces forces upon SR in PRE and BPD individuals. To determine whether altering airflow resistance had an effect on SR in PRE/BPD, we computed average SR in a subset of PRE and BPD individuals (n = 10) while they breathed heliox. There was no difference on average SR between ambient air or heliox (Fig. 5), in contrast to our hypothesis, which suggests that changes in resistance do not affect SR and/or that the differences in SR between PRE/BPD and CON are due to differences in lung tissue elasticity. We speculate that the high SR in PRE and BPD individuals is likely due to a combination of factors that includes altered tissue and/or surface forces as well as relatively smaller airways in PRE and BPD compared with CON. Along with altered tissue as a result of preterm birth, work using computed tomography suggests that alveolar simplification may be present in those born preterm (1, 27, 28, 47). The presence of alveolar simplification would reduce lung elastic recoil pressure and perhaps results in a decrease in ability to generate expiratory airflow (i.e., greater obstruction), which would manifest as a SR >1 signifying a “scoop.” Likewise, recent work has noted the presence of oxidative stress/inflammation in adolescents born preterm (15). Both of these factors could also contribute to the SR pattern we have observed. However, we emphasize that studies involving detailed pulmonary function measures in combination with assessments of respiratory mechanics, measures of airway morphology, and/or the effect of oxidative stress/inflammation on airway function are required to confirm these hypotheses. Additionally, studies investigating changes in the MEFV curve, and thus the SR and pre- and post-bronchodilator therapy in PRE and BPD, would be valuable to further understand the underlying causes of an increased SR.
In an attempt to determine whether specific attributes of preterm birth had an effect on SR, we computed correlation coefficients between the SR and characteristics of preterm birth (e.g., gestational age, mass at birth, and duration of supplemental oxygen therapy). We found no statistically significant relationships, which may be due to having a relatively small and homogenous (i.e., narrow range of gestational age/mass) sample of individuals who were born preterm but had a wide range in SR values in PRE and BPD. These data suggest that gestational age and mass at birth as well as the duration of supplemental oxygen therapy are insufficient to describe patterns of expiratory airflow in our sample. In other words, the variability in SR is likely explained by other aspects associated with lung development and growth, which are influenced by underlying genetics as well as pre- and antenatal factors. However, it is noteworthy that the relationship between pulmonary function and neonatal factors such as birth weight and/or duration of supplemental oxygen therapy is equivocal, with several conflicting reports (11). Overall, our findings relating to the associations between SR and specific attributes of preterm birth should be interpreted with caution. It is possible that factors such as preterm birth and/or duration of supplemental oxygen therapy may be associated with SR in a larger sample of PRE/BPD individuals.
Lack of difference in SR between the PRE and BPD groups.
We found no difference in SR between PRE and BPD groups, which was unexpected given the between-group differences in pulmonary function (Table 2). There are several possible explanations for this observation. First, our BPD group predominantly included those with mild or moderate, rather than severe, bronchopulmonary dysplasia. Individuals with severe bronchopulmonary dysplasia likely have correspondingly severe airflow obstruction (16, 17, 44), but little is known about this population, and more work is needed. Nevertheless, it is reasonable to speculate that the degree of pulmonary function impairment within the BPD group relative to the PRE group in our study may not have been of a sufficient magnitude to result in between-group differences in SR. Second, the duration of supplemental oxygen therapy is a significant component of the pathophysiology of bronchopulmonary dysplasia, with the cutoff being a duration of ≥28 days (18). However, some of the individuals in the PRE group received supplemental oxygen therapy for <28 days (Table 1), an insufficient length of time to result in a diagnosis of bronchopulmonary dysplasia (18). Thus, there is likely some overlap between individuals in the PRE and BPD groups in terms of deleterious effect of supplemental oxygen therapy on lung and airway growth. Finally, it is well known that there is substantial variability in the pulmonary consequences of preterm birth. The aforementioned variability may be related to host of factors, including but not limited to genetic factors, socioeconomic factors, perinatal exposures (e.g., cigarette smoking), and differences in the timing and type of treatment as well as the interaction of various treatments. Overall, we speculate that differences in SR between PRE and BPD individuals may only be evident at the extremes of each group; however, we emphasize that a larger sample of PRE and BPD individuals is required to test this hypothesis.
Limitations.
There are limitations to our study that should be considered when interpreting our findings. First, our assessment of MEFV curves did not account for the effect of thoracic gas compression, which would likely have decreased our absolute SR values. However, we chose to perform our SR analyses on the same FVC maneuvers from which spirometry measures were derived and would be derived clinically. Second, the BPD group was composed primarily of individuals with mild to moderate bronchopulmonary dysplasia, and only two individuals had severe bronchopulmonary dysplasia. It follows that our findings may not be generalizable to adult survivors of preterm birth with severe bronchopulmonary dysplasia. Future work should focus on physiological differences across severities of bronchopulmonary dysplasia. Third, the current study was a retrospective analysis of previously published data (8–10, 23, 24, 34) collected in different laboratories. Therefore, we acknowledge that small differences in experimental setup and measurement equipment could have influenced our findings; however, this possibility was mitigated by a rigorous calibration protocol, the fact that measures of pulmonary function are highly reproducible and that the SR analyses were performed by a single investigator using a standardized method that accounts for slight differences in sampling frequency. Fourth, we obtained information relating to the duration of supplemental oxygen therapy from clinical records of individuals in the BPD group but could not obtain this information for all individuals in the PRE group. Accordingly, some individuals in the PRE group received supplemental oxygen therapy that was not of a sufficient duration to cause bronchopulmonary dysplasia but could have still resulted in overlap between the PRE and BPD groups in terms of their decrement in pulmonary function. Finally, a larger sample size may have allowed us to establish meaningful relationships between neonatal parameters and the SR.
Perspectives and Significance
Overall, our findings have several implications that merit brief comment. First, we have demonstrated that despite having lower pulmonary function than CON, PRE and BPD individuals still have relatively homogeneous lung emptying according to the SR. Thus, our data lend support to the hypothesis that PRE/BPD have relatively normal rather than diseased lungs that may be anatomically altered by premature birth. Second, the fact that we did not observe a difference in SR between the PRE and BPD groups suggests that arrested lung development resulting from preterm birth, rather than lung injury due to chronic supplemental oxygen therapy, is likely the primary factor that explains the difference in the shape of the MEFV curve in adults born preterm either with or without BPD relative to adults born at full term. Third, our study provides insight into the mechanism(s) of airflow obstruction in PRE/BPD. That SR in the PRE/BPD individuals was unaffected by an acute reduction in airway resistance suggests that airway resistance may not be the major cause of the differences between PRE/BPD and CON we have observed. We surmise that differences in SR are likely due to differences in the mechanical properties of the airways; however, this hypothesis awaits experimental testing. Finally, our findings suggest that the PRE/BPD individuals in our study have a pattern of airflow obstruction similar to patients with mild COPD, albeit with a lesser magnitude of obstruction. However, the PRE/BPD individuals in the present study are almost 50 years younger than the patients with mild COPD (5). Perhaps the most important unanswered question in this area concerns what will happen to PRE/BPD individuals over the course of the aging process. Will their age-associated changes in lung function follow those of healthy individuals that were born at full term, or will the decline be “steeper,” resulting in debilitating lung function at an earlier age? Unfortunately, the answers to these questions are largely unknown, but it remains timely and important to understand the mechanisms responsible for the obstructive airflow pattern we have observed in the present study.
In conclusion, we found that the average SR was significantly greater in PRE and BPD individuals compared with CON, which was evident early in expiration. These findings suggest that the pattern of airflow obstruction in PRE and BPD is similar to that in patients with mild COPD (5), but with an SR that is closer to 1 (i.e., less “scooping” and closer to normal). These findings lend support to the notion that PRE and BPD individual are at increased risk of developing COPD later in life (11, 25). Unfortunately, the mechanism(s) responsible for the greater average SR and “early scooping” in PRE/BPD compared with CON remain unknown. However, we demonstrated that SR in PRE and BPD individuals was unaffected by acute changes in airway resistance resulting from breathing heliox, which may suggest that their airflow obstruction is the result of more than just having smaller airways. Indeed, further research in this population is required to clearly define the physiological basis for a high SR during early expiration. Because this population continues to grow with advances in neonatal medicine, gaining a more thorough understanding of the unique cardiopulmonary physiology of PRE and BPD individuals is timely and of significant importance.
GRANTS
This research was supported by American Heart Association Scientist Development Grant no. 2280238 (A. T. Lovering), the American Physiological Society’s Giles F. Filley Memorial Award for Excellence in Respiratory Physiology and Medicine (A. T. Lovering), a Medical Research Foundation of Oregon Early Clinical Investigator Award (J. W. Duke), and an Ohio University Research Committee Award (J. W. Duke). Additional funding was provided by the Natural Science and Engineering Research Council of Canada (NSERC) and the British Columbia Lung Association (BCLA). Y. Molgat-Seon and C. M. Peters were supported by graduate scholarships from NSERC. J. A. Guenette was supported by a Scholar Award from the Michael Smith Foundation for Health Research and a Canadian Institutes of Health Research Clinical Rehabilitation New Investigator Award. The funders had no role in the study design, data collection and analysis, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.M.-S., P.B.D., C.M.P., J.A.G., A.W.S., I.M.G., A.T.L., and J.W.D. conceived and designed research; Y.M.-S., P.B.D., C.M.P., J.A.G., and J.W.D. performed experiments; Y.M.-S., P.B.D., C.M.P., J.A.G., A.W.S., I.M.G., A.T.L., and J.W.D. analyzed data; Y.M.-S., P.B.D., C.M.P., J.A.G., A.W.S., I.M.G., A.T.L., and J.W.D. interpreted results of experiments; Y.M.-S., P.B.D., J.A.G., and J.W.D. prepared figures; Y.M.-S., P.B.D., J.A.G., and J.W.D. drafted manuscript; Y.M.-S., P.B.D., C.M.P., J.A.G., A.W.S., I.M.G., A.T.L., and J.W.D. edited and revised manuscript; Y.M.-S., P.B.D., C.M.P., J.A.G., A.W.S., I.M.G., A.T.L., and J.W.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the subjects for participation. We also thank Drs. J. E. Elliott, S. S. Laurie, C. Gust, S. Milind Nigam, X. Yang, as well as K. M. Beasley, J. Kern, T. Mangum, T. Olson, T. Straley, H. Straub, K. Ortega-Schwartz, and C. Bryce for assistance with data collection and analysis.
REFERENCES
- 1.Aukland SM, Rosendahl K, Owens CM, Fosse KR, Eide GE, Halvorsen T. Neonatal bronchopulmonary dysplasia predicts abnormal pulmonary HRCT scans in long-term survivors of extreme preterm birth. Thorax 64: 405–410, 2009. doi: 10.1136/thx.2008.103739. [DOI] [PubMed] [Google Scholar]
- 2.Babb TG. Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol (1985) 82: 746–754, 1997. doi: 10.1152/jappl.1997.82.3.746. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari V. Hyperoxia-derived lung damage in preterm infants. Semin Fetal Neonatal Med 15: 223–229, 2010. doi: 10.1016/j.siny.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotes J. The lung: scientific foundations. In: Barnes, edited by Crystal RG, West JB, Weibel ER, and Barnes PJ. Philadelpha, PA: Lippincott-Raven, 1997, p. 2193–2203. [Google Scholar]
- 5.Dominelli PB, Foster GE, Guenette JA, Haverkamp HC, Eves ND, Dominelli GS, Henderson WR, O’Donnell DE, Sheel AW. Quantifying the shape of the maximal expiratory flow-volume curve in mild COPD. Respir Physiol Neurobiol 219: 30–35, 2015. doi: 10.1016/j.resp.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Dominelli PB, Molgat-Seon Y, Foster GE, Dominelli GS, Haverkamp HC, Henderson WR, Sheel AW. Quantifying the shape of maximal expiratory flow-volume curves in healthy humans and asthmatic patients. Respir Physiol Neurobiol 220: 46–53, 2016. doi: 10.1016/j.resp.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle LW, Adams A-M, Robertson C, Ranganathan S, Davis NM, Lee KJ, Cheong JL; Victorian Infant Collaborative Study Group . Increasing airway obstruction from 8 to 18 to years in extremely preterm/low-birthweight survivors born in the surfactant era. Thorax 72: 712–719, 2017. doi: 10.1136/thoraxjnl-2016-208524. [DOI] [PubMed] [Google Scholar]
- 8.Duke JW, Elliott JE, Laurie SS, Beasley KM, Mangum TS, Hawn JA, Gladstone IM, Lovering AT. Pulmonary gas exchange efficiency during exercise breathing normoxic and hypoxic gas in adults born very preterm with low diffusion capacity. J Appl Physiol (1985) 117: 473–481, 2014. doi: 10.1152/japplphysiol.00307.2014. [DOI] [PubMed] [Google Scholar]
- 9.Duke JW, Gladstone IM, Sheel AW, Lovering AT. Premature birth affects the degree of airway dysanapsis and mechanical ventilatory constraints. Exp Physiol 103: 261–275, 2018. doi: 10.1113/EP086588. [DOI] [PubMed] [Google Scholar]
- 10.Duke JW, Zidron AM, Gladstone IM, Lovering AT. Alleviating mechanical constraints to ventilation with heliox improves exercise endurance in adult survivors of very preterm birth. Thorax 74: 302–304, 2019. doi: 10.1136/thoraxjnl-2018-212346. [DOI] [PubMed] [Google Scholar]
- 11.Eber E, Zach MS. Long term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy). Thorax 56: 317–323, 2001. doi: 10.1136/thorax.56.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K; National Institutes of Child Health and Human Development Neonatal Research Network . Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 116: 1353–1360, 2005. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 13.Eves ND, Petersen SR, Haykowsky MJ, Wong EY, Jones RL. Helium-hyperoxia, exercise, and respiratory mechanics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174: 763–771, 2006. doi: 10.1164/rccm.200509-1533OC. [DOI] [PubMed] [Google Scholar]
- 14.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13: 59, 2013. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filippone M, Bonetto G, Corradi M, Frigo AC, Baraldi E. Evidence of unexpected oxidative stress in airways of adolescents born very pre-term. Eur Respir J 40: 1253–1259, 2012. doi: 10.1183/09031936.00185511. [DOI] [PubMed] [Google Scholar]
- 16.Jacob SV, Coates AL, Lands LC, MacNeish CF, Riley SP, Hornby L, Outerbridge EW, Davis GM, Williams RL. Long-term pulmonary sequelae of severe bronchopulmonary dysplasia. J Pediatr 133: 193–200, 1998. doi: 10.1016/S0022-3476(98)70220-3. [DOI] [PubMed] [Google Scholar]
- 17.Jacob SV, Lands LC, Coates AL, Davis GM, MacNeish CF, Hornby L, Riley SP, Outerbridge EW. Exercise ability in survivors of severe bronchopulmonary dysplasia. Am J Respir Crit Care Med 155: 1925–1929, 1997. doi: 10.1164/ajrccm.155.6.9196097. [DOI] [PubMed] [Google Scholar]
- 18.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729, 2001. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 19.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 131: 700–708, 1985. doi: 10.1164/arrd.1985.131.5.700. [DOI] [PubMed] [Google Scholar]
- 20.Kalhan R, Dransfield MT, Colangelo LA, Cuttica MJ, Jacobs DR Jr, Thyagarajan B, Estepar RSJ, Harmouche R, Onieva JO, Ash SY, Okajima Y, Iribarren C, Sidney S, Lewis CE, Mannino DM, Liu K, Smith LJ, Washko GR. Respiratory symptoms in young adults and future lung disease. The CARDIA Lung Study. Am J Respir Crit Care Med 197: 1616–1624, 2018. doi: 10.1164/rccm.201710-2108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kao LC, Warburton D, Platzker AC, Keens TG. Effect of isoproterenol inhalation on airway resistance in chronic bronchopulmonary dysplasia. Pediatrics 73: 509–514, 1984. [PubMed] [Google Scholar]
- 22.Laurie SS, Elliott JE, Beasley KM, Mangum TS, Goodman RD, Duke JW, Gladstone IM, Lovering AT. Exaggerated increase in pulmonary artery pressure during exercise in adults born preterm. Am J Respir Crit Care Med 197: 821–823, 2018. doi: 10.1164/rccm.201704-0740LE. [DOI] [PubMed] [Google Scholar]
- 23.Lovering AT, Elliott JE, Laurie SS, Beasley KM, Gust CE, Mangum TS, Gladstone IM, Duke JW. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reduced exercise capacity. Ann Am Thorac Soc 11: 1528–1537, 2014. doi: 10.1513/AnnalsATS.201312-466OC. [DOI] [PubMed] [Google Scholar]
- 24.Lovering AT, Laurie SS, Elliott JE, Beasley KM, Yang X, Gust CE, Mangum TS, Goodman RD, Hawn JA, Gladstone IM. Normal pulmonary gas exchange efficiency and absence of exercise-induced arterial hypoxemia in adults with bronchopulmonary dysplasia. J Appl Physiol (1985) 115: 1050–1056, 2013. doi: 10.1152/japplphysiol.00592.2013. [DOI] [PubMed] [Google Scholar]
- 25.Luu TM, Katz SL, Leeson P, Thébaud B, Nuyt A-M. Preterm birth: risk factor for early-onset chronic diseases. CMAJ 188: 736–746, 2016. doi: 10.1503/cmaj.150450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacIntyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CPM, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 26: 720–735, 2005. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 27.Mahut B, De Blic J, Emond S, Benoist M-R, Jarreau P-H, Lacaze-Masmonteil T, Magny J-F, Delacourt C. Chest computed tomography findings in bronchopulmonary dysplasia and correlation with lung function. Arch Dis Child Fetal Neonatal Ed 92: F459–F464, 2007. doi: 10.1136/adc.2006.111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margraf LR, Tomashefski JF Jr, Bruce MC, Dahms BB. Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis 143: 391–400, 1991. doi: 10.1164/ajrccm/143.2.391. [DOI] [PubMed] [Google Scholar]
- 29.Mead J, Goldman M, Grimby G. Action of respiratory muscles during exercise as inferred from rib-cage and abdominal pressure-volume partitioning. Scand J Respir Dis Suppl 77: 8–13, 1971. [PubMed] [Google Scholar]
- 30.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol 22: 95–108, 1967. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- 31.Mead J. Analysis of the configuration of maximum expiratory flow-volume curves. J Appl Physiol 44: 156–165, 1978. doi: 10.1152/jappl.1978.44.2.156. [DOI] [PubMed] [Google Scholar]
- 32.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121: 339–342, 1980. doi: 10.1164/arrd.1980.121.2.339. [DOI] [PubMed] [Google Scholar]
- 33.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force . Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 34.Molgat-Seon Y, Dominelli PB, Ramsook AH, Schaeffer MR, Molgat Sereacki S, Foster GE, Romer LM, Road JD, Guenette JA, Sheel AW. The effects of age and sex on mechanical ventilatory constraint and dyspnea during exercise in healthy humans. J Appl Physiol (1985) 124: 1092–1106, 2018. doi: 10.1152/japplphysiol.00608.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molgat-Seon Y, Ramsook AH, Peters CM, Schaeffer MR, Dominelli PB, Romer LM, Road JD, Guenette JA, Sheel AW. Manipulation of mechanical ventilatory constraint during moderate intensity exercise does not influence dyspnoea in healthy older men and women. J Physiol 597: 1383–1399, 2019. doi: 10.1113/JP277476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Näsänen-Gilmore P, Sipola-Leppänen M, Tikanmäki M, Matinolli H-M, Eriksson JG, Järvelin M-R, Vääräsmäki M, Hovi P, Kajantie E. Lung function in adults born preterm. PLoS One 13: e0205979, 2018. doi: 10.1371/journal.pone.0205979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MSM, Zheng J, Stocks J; ERS Global Lung Function Initiative . Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 40: 1324–1343, 2012. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson SJ, Logie KM, O’Dea CA, Banton GL, Murray C, Wilson AC, Pillow JJ, Hall GL. Altered lung structure and function in mid-childhood survivors of very preterm birth. Thorax 72: 702–711, 2017. doi: 10.1136/thoraxjnl-2016-208985. [DOI] [PubMed] [Google Scholar]
- 39.Simpson SJ, Turkovic L, Wilson AC, Verheggen M, Logie KM, Pillow JJ, Hall GL. Lung function trajectories throughout childhood in survivors of very preterm birth: a longitudinal cohort study. Lancet Child Adolesc Health 2: 350–359, 2018. doi: 10.1016/S2352-4642(18)30064-6. [DOI] [PubMed] [Google Scholar]
- 40.Smith LJ, McKay KO, van Asperen PP, Selvadurai H, Fitzgerald DA. Normal development of the lung and premature birth. Paediatr Respir Rev 11: 135–142, 2010. doi: 10.1016/j.prrv.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Turner JM, Mead J, Wohl ME. Elasticity of human lungs in relation to age. J Appl Physiol 25: 664–671, 1968. doi: 10.1152/jappl.1968.25.6.664. [DOI] [PubMed] [Google Scholar]
- 42.Udomittipong K, Sly PD, Patterson HJ, Gangell CL, Stick SM, Hall GL. Forced oscillations in the clinical setting in young children with neonatal lung disease. Eur Respir J 31: 1292–1299, 2008. doi: 10.1183/09031936.00058107. [DOI] [PubMed] [Google Scholar]
- 43.Vollsæter M, Clemm HH, Satrell E, Eide GE, Røksund OD, Markestad T, Halvorsen T. Adult respiratory outcomes of extreme preterm birth. A regional cohort study. Ann Am Thorac Soc 12: 313–322, 2015. doi: 10.1513/AnnalsATS.201406-285OC. [DOI] [PubMed] [Google Scholar]
- 44.Vollsæter M, Røksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax 68: 767–776, 2013. doi: 10.1136/thoraxjnl-2012-202980. [DOI] [PubMed] [Google Scholar]
- 45.Vrijlandt EJ, Boezen HM, Gerritsen J, Stremmelaar EF, Duiverman EJ. Respiratory health in prematurely born preschool children with and without bronchopulmonary dysplasia. J Pediatr 150: 256–261, 2007. doi: 10.1016/j.jpeds.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J 26: 511–522, 2005. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 47.Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J 32: 321–328, 2008. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]