Abstract
Muscle sympathetic nerve activity (MSNA) decreases during low-intensity dynamic one-leg exercise in healthy subjects but increases in patients with heart failure with reduced ejection fraction (HFrEF). We hypothesized that increased peak oxygen uptake (V̇o2peak) after aerobic training would be accompanied by less sympathoexcitation during both mild and moderate one-leg dynamic cycling, an attenuated muscle metaboreflex, and greater skin vasodilation. We studied 27 stable, treated HFrEF patients (6 women; mean age: 65 ± 2 SE yr; mean left ventricular ejection fraction: 30 ± 1%) and 18 healthy age-matched volunteers (6 women; mean age: 57 ± 2 yr). We assessed V̇o2peak (open-circuit spirometry) and the skin microcirculatory response to reactive hyperemia (laser flowmetry). Fibular MSNA (microneurography) was recorded before and during one-leg cycling (2 min unloaded and 2 min at 50% of V̇o2peak) and, to assess the muscle metaboreflex, during posthandgrip ischemia (PHGI). HFrEF patients were evaluated before and after 6 mo of exercise-based cardiac rehabilitation. Pretraining V̇o2peak and skin vasodilatation were lower (P < 0.001) and resting MSNA higher (P = 0.01) in HFrEF than control subjects. Training improved V̇o2peak (+3.0 ± 1.0 mL·kg−1·min−1; P < 0.001) and cutaneous vasodilation and diminished resting MSNA (−6.0 ± 2.0, P = 0.01) plus exercise MSNA during unloaded (−4.0 ± 2.5, P = 0.04) but not loaded cycling (−1.0 ± 4.0 bursts/min, P = 0.34) and MSNA during PHGI (P < 0.05). In HFrEF patients, exercise training lowers MSNA at rest, desensitizes the sympathoexcitatory metaboreflex, and diminishes MSNA elicited by mild but not moderate cycling. Training-induced downregulation of resting MSNA and attenuated reflex sympathetic excitation may improve exercise capacity and survival.
Keywords: exercise training, handgrip, microneurography, muscle metaboreflex, one-leg cycling
INTRODUCTION
Two characteristics of patients with heart failure and reduced left ventricular ejection fraction (HFrEF) that predict independently foreshortened survival are diminished exercise capacity and augmented resting state sympathetic nervous system activity (8, 16, 33, 38, 53). Consequently, interventions such as exercise training that moderate these abnormalities and have, as well, the potential to alleviate symptom burden and prolong life expectancy are of great interest to the heart failure community (16, 54).
Clinical trials of training patients with HFrEF have demonstrated, consistently, an increase in mean values for V̇o2peak (1). In experimental models of HFrEF, exercise has been shown to lower the central set point for sympathetic outflow (19) and improve cardiopulmonary reflex responsiveness (44). Thus one potential mechanism for the increase in V̇o2peak achieved with training may be a reduction in sympathetically mediated vasoconstriction (2).
In healthy subjects in whom sympathetic firing rates are highly reproducible over time (10), exercise training has little or no impact on muscle sympathetic nerve activity (MSNA), measured at rest. In contrast, several trials from a single center involving cohorts with HFrEF reported training-induced reductions in resting MSNA (42, 48).
Importantly, resting data provide little or no predictive insight into such patients’ autonomic responses to exercise. Further sympathetic recruitment from a higher initial baseline could expose patients with HFrEF to greater risk of adrenergically mediated arrhythmias or sudden death and by increasing vascular resistance and left ventricular afterload trigger dyspnea and reduce exercise capacity (16, 54).
Seminal kinetic studies have revealed, in patients with HFrEF relative to controls, substantially higher cardiac and renal norepinephrine spillover into plasma in response to supine steady-state bicycle exercise at 50% V̇o2peak, thus refuting the prevailing concept of impaired sympathetic reserve in this condition (21). However, for pragmatic considerations, initial investigations of MSNA during exercise focused on neural responses to static or dynamic handgrip (2, 30, 34, 35, 43, 51).
Both modes of handgrip exercise elicit reflex increases in MSNA at lower workloads and of greater magnitude in patients with HFrEF than in healthy age and sex-matched subjects (34). Such elevations are sustained during posthandgrip ischemia (PHGI), a maneuver that prolongs type III and IV muscle afferent stimulation by metabolites released during muscle contraction, triggering the muscle metaboreflex (34, 35).
Recognizing that handgrip exercise engages a smaller muscle mass and is less representative of routine daily locomotor activities than is leg exercise, we proceeded to record MSNA during dynamic contralateral one-leg cycling at mild (0 load) and moderate (50% V̇o2peak) loads (39). In healthy, middle-aged subjects, we documented a fall in MSNA burst frequency and incidence during exercise, similar to the reduction found previously in healthy young subjects and presumed it to be a consequence of muscle pump-induced activation of the sympathoinhibitory cardiopulmonary baroreflex by increased venous return (22, 25, 47). In distinct contrast, in patients with HFrEF, both mild and moderate one-leg cycling elicited an increase in MSNA burst frequency during both mild- and moderate-cycling intensities (39). Moreover, and in support of the concept that sympathetically mediated vasoconstriction may constrain exercise performance, there was a significant inverse relationship between MSNA, recorded at 50% V̇o2peak and participants’ V̇o2peak (39).
The impact of training individuals with HFrEF on these sympathoexcitatory responses to dynamic one-leg exercise at mild to moderate intensities, which represents more generally than handgrip day-to-day activities of such patients, is unknown. We hypothesized that in patients with HFrEF a 6-mo cardiac rehabilitation program based on aerobic exercise training, shown to increase V̇o2peak, would reduce muscle sympathetic nerve firing rates both at rest and during dynamic one-leg exercise.
Such training, which involves upper and lower body resistance as well as aerobic training, would be anticipated to exert a range of systemic effects. For example, 4 mo of aerobic training has been reported to normalize both MSNA and vasoconstrictor responses to static handgrip (52). Positing that one mechanism responsible for the divergent MSNA responses to one-leg cycling in HFrEF and control subjects may be an exaggerated metaboreflex in the former cohort (20, 34, 35, 43, 51), we hypothesized that cardiac rehabilitation would also attenuate both arm- and leg- muscle metaboreflex-elicited sympathetic excitation. We recently reported that in patients with HFrEF, but not similarly aged healthy subjects, aortic wave reflection characteristics such as the augmentation index correlate positively with MSNA (32). Since the current literature lacks consensus as to whether exercise training improves arterial compliance (11, 41), we quantified, as well, aortic wave characteristics before and after training. Attenuated cutaneous vascular reactivity in patients with HFrEF has emerged as an additional disturbance associated with increased risk of adverse outcome (14, 40). In healthy subjects, training has been shown to augment skin vasodilation (18) and improve cutaneous vascular reactivity (28). Whether similar changes occur with training in HFrEF patients is unknown. Consequently, we tested the hypothesis that 6 mo of exercise training would improve skin blood flow responsiveness to reactive hyperemia.
A cohort of healthy untrained individuals was also studied but at a single session. The purpose of their recruitment as control subjects was to determine how closely such posttraining variables approximated responses of middle-aged men and women without heart failure participating in the identical protocol.
METHODS
Subjects
HFrEF patients.
We studied 27 stable, training-naïve HFrEF patients [6 women; mean age: 65 ± 2 SE (range 42 to 80) yr] without diabetes mellitus (to avoid any confounding by autonomic neuropathy), referred to our Cardiovascular Prevention and Rehabilitation Program. Of these, 25 were in sinus rhythm and 2 in atrial fibrillation. For 18, the diagnosis was ischemic and in 9 dilated nonischemic cardiomyopathy. Mean left ventricular ejection fraction was 30 ± 1% (range 12–40). For clinical relevance, and to avoid adverse consequences of drug withdrawal, participants continued with all prescribed therapy: angiotensin-converting enzyme inhibitors (n = 16; 59%), β-adrenoceptor antagonists (n = 27; 100%), diuretics (n = 15; 55%), aspirin (n = 20; 74%), anticoagulants (n = 19; 70%), and statins (n = 17; 63%). Of those administered diuretics, five were prescribed a loop diuretic, two a mineralocorticoid receptor antagonist, and eight their combination.
Healthy Controls
Eighteen healthy medication-free volunteers (6 women) of similar age were recruited through local advertisement and screened by medical history. Their mean age was 57 ± 2 (range 48–72) yr.
This study complied with the Declaration of Helsinki and was approved by the Research Ethics Boards of University Health Network, the Toronto Rehabilitation Institute (now part of this Network), and the Mount Sinai Hospital. Informed written consent was obtained from all participants. Pretraining baseline MSNA data from 16 patients and 13 controls have been reported (38, 39).
Procedures and Protocol
Before the laboratory study days, peak oxygen uptake (V̇o2peak) was assessed on a cycle ergometer (17 W/min) by open-circuit spirometry (HFrEF patients: Sensormedics Vmax Encore Metabolic Cart, Yorba Linda, CA; control subjects: Quark CPET system, Cosmed, Chicago, IL). Briefly, subjects exercised until they could no longer maintain pedal speed and the respiratory exchange ratio (V̇co2/V̇o2) exceeded 1.1. V̇o2peak was expressed both as milliliters per kilogram per minute and as percentage of the age, sex, body weight, and height-predicted V̇o2peak (23).
The experimental protocol was conducted in a quiet temperature-controlled laboratory following 12 h of caffeine abstinence and 2 h after any food intake. With participants supine, pulse-wave reflection was measured by applanation tonometry and forearm skin blood flow by laser-Doppler flowmetry. Subjects then sat upright, with the left leg supported (see below), while the right foot was secured to the pedal of a cycle ergometer (Monark Rehab Ergometer Trainer 881, Stockholm, Sweden). Right upper arm blood pressure was acquired automatically every minute (Dinamap Pro 100; Critikon, Tampa, FL). Heart rate was derived from lead II of an electrocardiogram. A respiratory belt encircled the abdomen. Maximum voluntary contraction (MVC) was determined using a handgrip dynamometer (model 78010; Lafayette Instruments, Lafayette, IN).
After 10 min of quiet rest, baseline signals were acquired during spontaneous breathing. We recorded heart rate, blood pressure, and MSNA (microneurography; left fibular nerve) at rest and during right leg cycling for 4 min (2 min at zero load and 2 min at 50% of the work rate at V̇o2peak, but halved, since only 1 leg exercised). After a 10-min recovery and recontrol period, we continued these recordings during 2 min of isometric handgrip at 30% of MVC, followed immediately by 2 min of PHGI, achieved by inflating an upper arm cuff to 200 mmHg then 2 min of recovery. By removing the mechanics of the muscle contraction (mechanoreflex) plus any volitional engagement (i.e., central command), PHGI permits specific quantitation of the reflex response to stimulation of metabolically sensitive muscle afferents (metaboreflex). Heart rate, blood pressure, and rating of perceived exertion (modified Borg scale 0–10) also were assessed. Cycling and handgrip exercise were performed in random order.
Patients were then enrolled in the Toronto Rehabilitation Institute’s standard 6-mo cardiac rehabilitation program. This prescribed walking, five times per week, at a heart rate corresponding to 60–70% of peak V̇o2. To maintain the same relative training intensity as fitness improves, walking duration (from an initial 15 min as tolerated to a maximum of 1 h) and subsequently its intensity were adjusted every week at a supervised session. After 8 wk, mild-to-moderate upper and lower body resistance training was added. The other four sessions each week were performed at home; patients documented their exercise heart rates on written logs for weekly review at the supervised sessions. The program also included an extensive education program covering topics such as living with heart disease, nutrition, medications, exercise safety, and stress management. The pretraining experimental protocol was repeated at this program's conclusion.
Applanation Tonometry
Central blood pressure and aortic wave reflection characteristics were derived from the radial pulse waveform during supine rest by automated radial artery applanation tonometry (SphygmoCor; AtCor Medical, Itasca, IL). The difference in the pressure wave between peak flow and peak pressure, termed the augmentation index (AI), increases as arteries stiffen. AI was calculated as the difference between the first and second systolic peaks of the ascending aortic wave form, expressed as a percentage of the central pulse pressure and calibrated to a heart rate of 75 beats/min (AIx at 75) (41).
Laser Doppler Flowmetry
Skin blood flow (SBF) was calculated from changes in red blood cell flux assessed by laser Doppler flowmetry (Periflux 5001; Perimed, Ardmore, PA), a noninvasive measure of microcirculatory function made by placing a probe on the forearm 10 cm distal to the antecubital fossa. The output signal relates linearly to red cell flow. After stabilization with skin temperature constant, a 5-min recording of skin blood flow was acquired at rest, then to assess forearm skin vasodilatory capacity, during 5 min of reactive hyperemia induced by 3 min of brachial artery occlusion. Skin blood flow was expressed in perfusion units and as percent change from baseline. Cutaneous vascular conductance (CVC) was calculated as: (SBF/mean arterial pressure) ×100.
Microneurography
Multiunit recordings of postganglionic MSNA were obtained with a unipolar tungsten electrode inserted selectively into a muscle-nerve fascicle of the left peroneal (fibular) nerve, posterior to the fibular head, and quantified using a customized analytic program based on a LabVIEW (National Instruments, Austin, TX) platform, as previously described (33). Analysis was restricted to recordings in which the burst to background noise ratio exceeded 3:1. This was to assure confidence in burst detection in anticipation of muscle tension at the recording site on transition from rest to unloaded one-leg cycling or from mild-to-moderate exercise load. MSNA was expressed both as burst frequency (bursts/min) and, to allow for differences in heart rate between groups and with training, burst incidence (bursts/100 heartbeats), but not as total MSNA, which, as emphasized by other experienced investigators (26, 56), is unsuitable as an end point variable in conditions such as one-leg exercise in which in some or many subjects muscle action in the contralateral leg could cause baseline-altering tension in muscles adjacent to the recording site. Because burst detection is the most robust and reliable means of quantifying nerve firing during dynamic one-leg exercise, and because neural norepinephrine release rate, the consequent vasoconstriction, and V̇o2peak are coupled most closely with burst frequency (55), burst/min was the principal variable of interest in the present analysis.
Statistical Analysis
Data are presented as means ± SE. One-way ANOVA (SigmaStat for Windows, Ver. 3.5; Systat Software, Chicago, IL) was used to test for differences between descriptive and resting dependent variables among groups (HFrEF pre- and postexercise training and control subjects). A post hoc Student-Newman-Keuls test was applied to assess individual differences between means. Two-way ANOVA assessed between- group changes in skin blood flow and conductance during postocclusion reactive hyperemia. During cycling and handgrip exercise, preselected time point differences were determined by paired t test or Mann-Whitney Rank sum tests (if the data did not follow a Normal Gaussian distribution) to assess the effect of training in the HFrEF group.
RESULTS
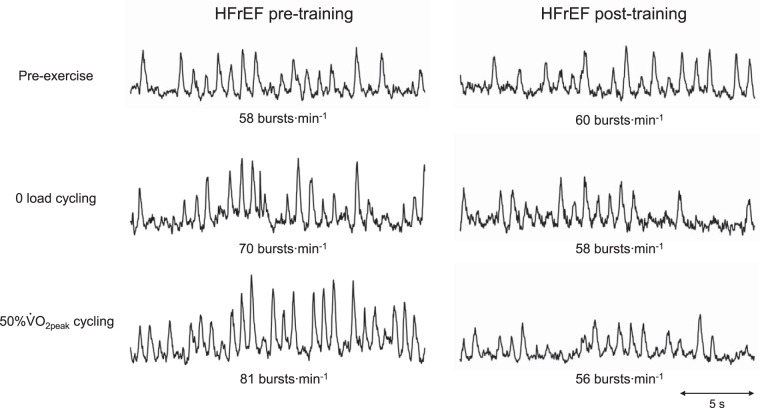
Physical characteristics and baseline measures in HFrEF pre- and posttraining and control groups are summarized in Table 1. HFrEF patients were maintained on the same medical therapy pre- and posttraining. Figure 1 shows representative MSNA recordings at rest and during exercise pre- and posttraining.
Table 1.
Resting and descriptive data
| Variable | HFrEF Pretraining (n = 27) | HFrEF Posttraining (n = 27) | Healthy Subjects (n = 18) |
|---|---|---|---|
| Age, yr | 65 ± 2 | 66 ± 2 | 57 ± 2† |
| Height, cm | 171 ± 2 | 171 ± 2 | 172 ± 3 |
| Body weight, kg | 78.5 ± 3 | 80.7 ± 3 | 74 ± 4 |
| BMI, kg/m2 | 26 ± 1 | 27 ± 1 | 25 ± 1 |
| Heart rate, beats/min | 63 ± 2 | 63 ± 2 | 64 ± 2 |
| Systolic blood pressure, mmHg | 111 ± 3 | 113 ± 3 | 115 ± 4 |
| Diastolic blood pressure, mmHg | 62 ± 1 | 63 ± 2 | 68 ± 2† |
| Augmentation index, % | 27.4 ± 1.7 | 28.6 ± 2.0 | 16.7 ± 3.2† |
| Augmentation index at HR75, % | 20.7 ± 1.8 | 23.6 ± 2.0 | 12.9 ± 2.3† |
| Skin blood flow, PU | 7.4 ± 0.6 | 7.9 ± 0.5 | 8.4 ± 1.0 |
| Cutaneous vascular conductance, PU/mmHg | 9.6 ± 0.8 | 9.9 ± 0.5 | 10.3 ± 1.5 |
| MSNA, bursts/min | 52.6 ± 2.5 | 47.0 ± 2.6* | 44.0 ± 2.0** |
| MSNA, bursts/100 heartbeats | 83.7 ± 3.5 | 76.4 ± 4.5* | 70.0 ± 3.6** |
| Peak work rate, W | 96 ± 14 | 119 ± 15* | 152 ± 18** |
| V̇o2peak, L/min | 1.47 ± 0.1 | 1.69 ± 0.1* | 2.3 ± 0.2† |
| V̇o2peak, mL·kg−1·min−1 | 18.3 ± 1.5 | 21.4 ± 1.7* | 30.6 ± 2.2† |
| V̇o2peak, %predicted | 76.8 ± 5.2 | 87.6 ± 5.3* | 110.3 ± 6.5† |
Values are means ± SE; n as shown except for muscle sympathetic nerve activity (MSNA; n = 18). BMI, body mass index; V̇o2peak, peak oxygen uptake; HFrEF heart failure with reduced ejection fraction; HR75, heart rate of 75 beats/min; PU, perfusion units.
P < 0.05 pre- vs. posttraining HFrEF subjects;
P < 0.05 healthy controls vs. pretraining only;
P < 0.05 healthy controls vs. posttraining.
Fig. 1.
Representative muscle sympathetic nerve activity (MSNA) recordings at rest and during mild and moderate 1-leg cycling, pre- and posttraining. HFrEF, heart failure with reduced ejection fraction; V̇o2peak, peak oxygen uptake.
Effects of Training HFrEF Patients on Functional Capacity
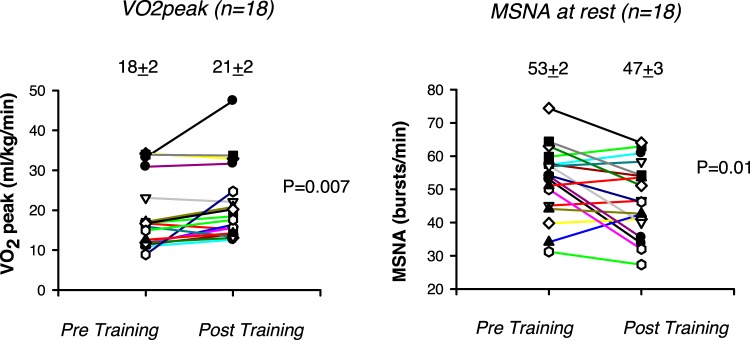
Before training, mean peak V̇o2 in HFrEF patients was less than that of control subjects (P < 0.001), and at rest, MSNA burst frequency and incidence were significantly greater (P < 0.05) Individual data are shown in Fig. 2. Peak oxygen uptake increased after training (+0.2 ± 0.05 L/min; +3.0 ± 1.0 mL·kg−1·min−1; +11.2 ± 2.6% of predicted based on age and body size; all P < 0.001, n = 27), but the group mean values achieved did not attain that of control subjects (P < 0.001; Table 1).
Fig. 2.
Individual heart failure with reduced ejection fraction data pre- and posttraining (n = 18) with group means ± SE indicated for peak oxygen uptake (V̇o2peak; left) and muscle sympathetic nerve activity (MSNA; right) burst frequency at rest. P values as indicated.
Effects of Training HFrEF Patients on Resting Variables
MSNA.
Stable high-quality recordings were obtained at rest both before and after training in 18 of the 27 patients with HFrEF. Mean resting MSNA burst frequency (−6.0 ± 2.0 burst/min, P = 0.01) and incidence (−7.3 ± 3.4 burst/100 heartbeats, P = 0.04) were significantly lower after training; posttraining group mean values did not differ from those of control participants (P = 0.35 and 0.27, respectively, Table 1).
Augmentation index.
Whether expressed as a percentage or normalized to a heart rate of 75 beats/min, the augmentation index at rest was greater in HFrEF patients than in control subjects (both P < 0.05). These values were unaffected by exercise training (P = 0.68 and P = 0.28, respectively) (Table 1).
Laser flowmetry.
There was no difference in resting skin blood flow or vascular conductance between pre- and posttraining HFrEF groups and age-matched controls (Table 1). Before training, SBF and CVC responses to reactive hyperemia after 5 min of circulatory occlusion were blunted in the HFrEF group compared with controls (P < 0.001; Fig. 3). However, after training, the vasodilatory response of patients with HFrEF, whether expressed in absolute perfusion units or percent change from baseline, was not significantly different from that of control subjects (P = 0.78) (Fig. 3). There was no correlation between the increases in SBF or CVC and the decreases in resting MSNA observed after training (r = 0.02, P = 0.95; r = 0.05, P = 0.84, respectively).
Fig. 3.
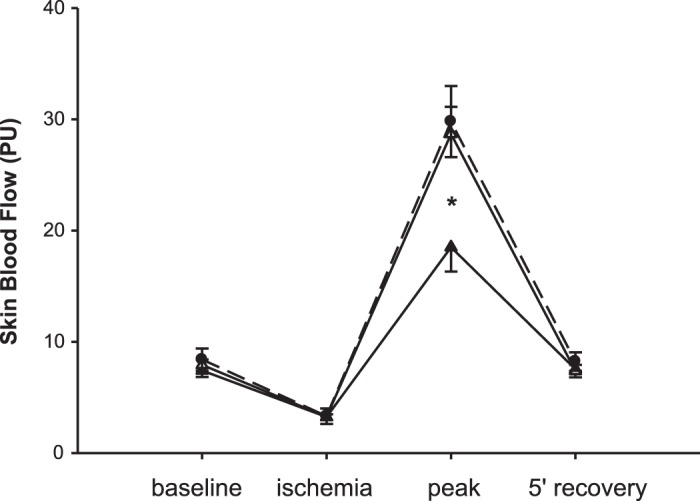
Skin blood flow response to reactive hyperemia is normalized after exercise training in heart failure with reduced ejection fraction (HFrEF) patients (pretraining, closed triangles; posttraining, open triangles). Data are means ± SE. PU, perfusion units. Effect of group, P = 0.03; effect of time and interaction, P < 0.001; *P < 0.001 HFrEF pre- vs. both posttraining (n = 27) and healthy control subjects (closed circles; n = 18).
Effect of Training HFrEF Patients on MSNA During Exercise and PHGI
Cycling.
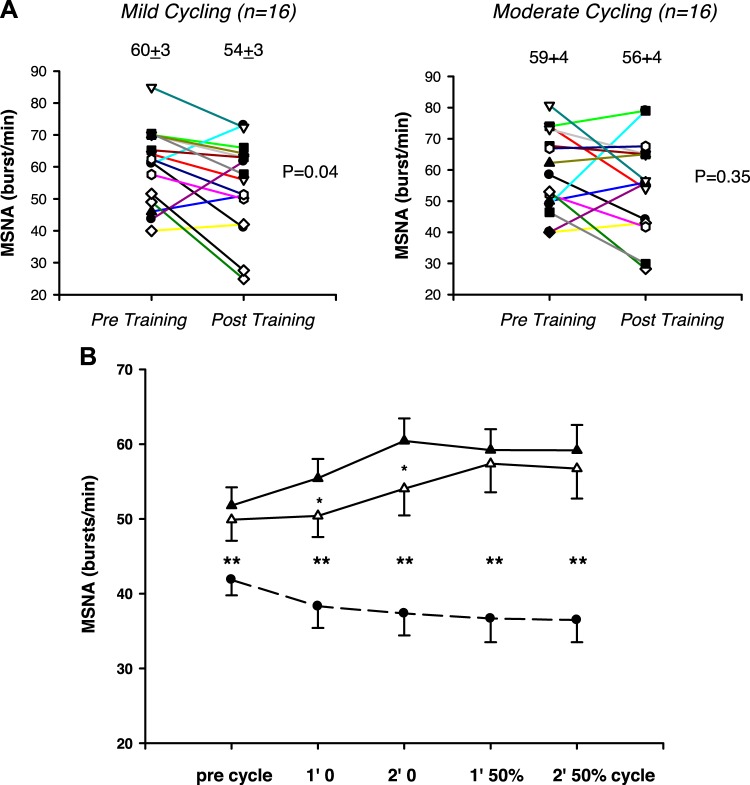
Stable high-quality recordings were obtained during exercise both before and after training in 16 of 27 patients. Individual and mean data appear in Fig. 4. Before training, mean MSNA burst frequency and incidence fell at both exercise intensities in control participants (P = 0.01) but increased in patients with HFrEF (P < 0.001).
Fig. 4.
Individual heart failure with reduced ejection fraction (HFrEF) data pre- and posttraining during the 2nd minute of mild (0 load) cycling (A; left) and moderate load [50% of peak oxygen uptake (V̇o2peak) adjusted for 1-leg] cycling (A, right). Muscle sympathetic nerve activity (MSNA; B) burst frequency (means ± SE) is reduced after exercise training during 1st and 2nd minute of mild- but not moderate-intensity 1-leg cycling exercise in HFrEF patients (pretraining, closed triangles; posttraining, open triangles; n = 16) but is still higher than in healthy control subjects (closed circles; n = 18). Effect of group, P < 0.001; *P = 0.04; HFrEF pre- vs. posttraining; **P < 0.001 HFrEF vs. controls.
Patients’ rating of perceived exertion was greater after than before training during mild (P = 0.002) but not moderate cycling (P = 0.37). Their rise in heart rate during unloaded one-leg cycling was similar pre- and posttraining, as would be anticipated in a cohort receiving guideline-directed β-blockade, although it was slightly higher during moderate exercise posttraining (P = 0.04) (Table 1). Systolic and diastolic blood pressures during one-leg cycling were similar before and after training (all P > 0.05).
Exercise training attenuated the increases in both MSNA burst frequency (Fig. 4B) and incidence (Table 2) elicited by mild-intensity cycling (P = 0.04). However, in some participants the attenuation observed at mild-intensity dissipated when workload intensified (Fig. 4A). Thus, during moderate-intensity cycling, sympathoinhibition was evident in some individuals but not in the cohort as a whole (P = 0.34 and 0.13; Table 2 and Fig. 4).
Table 2.
Cycling data in HFrEF patients
| Variable | Precycle | Mild Cycling | Moderate Cycling |
|---|---|---|---|
| HR, beats/min | |||
| Pre | 64 ± 2 | 73 ± 2 | 75 ± 2 |
| Post | 64 ± 2 | 75 ± 2 | 79 ± 3* |
| SBP, mmHg | |||
| Pre | 113 ± 4 | 127 ± 6 | 128 ± 6 |
| Post | 118 ± 3 | 129 ± 6 | 136 ± 5 |
| DBP, mmHg | |||
| Pre | 65 ± 2 | 69 ± 3 | 71 ± 3 |
| Post | 66 ± 2 | 71 ± 3 | 73 ± 3 |
| MAP, mmHg | |||
| Pre | 81 ± 2 | 88 ± 4 | 90 ± 3 |
| Post | 83 ± 2 | 88 ± 3 | 93 ± 3 |
| Work rate, W | |||
| Pre | – | – | 21 ± 3 |
| Post | – | – | 26 ± 3* |
| MSNA/100 heartbeats | |||
| Pre | 82 ± 4 | 82 ± 4 | 78 ± 5 |
| Post | 79 ± 5 | 73 ± 5* | 71 ± 5 |
Values are means ± SE; n = 16 except for systolic blood pressure (SBP) and diastolic blood pressure (DBP) (n = 12 mild and n = 13 moderate exercise). HR, heart rate; MAP, mean arterial pressure; MSNA/100, muscle sympathetic nerve activity (burst incidence); HFrEF, heart failure with reduced ejection fraction.
P < 0.05 pre- vs. posttraining
Static handgrip.
Isometric handgrip exercise at 30% MVC and PHGI elicited similar increases in heart rate (P = 0.87) and blood pressure before and after training (P > 0.49). During PHGI, systolic and diastolic blood pressure remained above resting values both pre- and posttraining (P > 0.48) (Table 3).
Table 3.
Handgrip and posthandgrip ischemia data in HFrEF patients
| Variable | Pre-HG | 30% Static HG | PHGI |
|---|---|---|---|
| HR, beats/min | |||
| Pre | 63 ± 2 | 72 ± 2 | 64 ± 2 |
| Post | 64 ± 2 | 71 ± 3 | 65 ± 2 |
| SBP, mmHg | |||
| Pre | 108 ± 4 | 130 ± 5 | 126 ± 5 |
| Post | 111 ± 4 | 133 ± 5 | 128 ± 5 |
| DBP, mmHg | |||
| Pre | 62 ± 2 | 75 ± 3 | 68 ± 2 |
| Post | 62 ± 2 | 74 ± 3 | 70 ± 2 |
| MAP, mmHg | |||
| Pre | 77 ± 2 | 93 ± 4 | 87 ± 3 |
| Post | 78 ± 2 | 94 ± 3 | 89 ± 3 |
| Work load, W | |||
| Pre | – | 28 ± 3 | – |
| Post | – | 28 ± 3 | – |
| MSNA/100 heartbeats | |||
| Pre | 85 ± 3 | 86 ± 3 | 88 ± 3 |
| Post | 78 ± 5 | 83 ± 5 | 78 ± 4* |
Values are means ± SE; n = 16. Data are from minute 2 of handgrip (HG) and posthandgrip ischemia (PHGI). HR, heart rate; SBP, systolic blood pressure in mmHg; DBP, diastolic blood pressure; MAP, mean arterial pressure; MSNA/100, muscle sympathetic nerve activity (burst incidence); HFrEF, heart failure with reduced ejection fraction.
P < 0.05 pre- vs. posttraining.
Increases in MSNA burst frequency and incidence elicited by handgrip exercise (effect of time, P < 0.001) were unaffected by exercise training in HFrEF (effect of intervention, P = 0.13). Compared with control subjects, MSNA remained higher in HFrEF patients posttraining at rest and throughout handgrip exercise and PHGI (P < 0.001). However, the elevation during PHGI present before training, indicative of muscle metaboreflex activation, was no longer present during the second minute of PHGI after training (Student-Newman-Keuls, P = 0.04; Fig. 5).
Fig. 5.
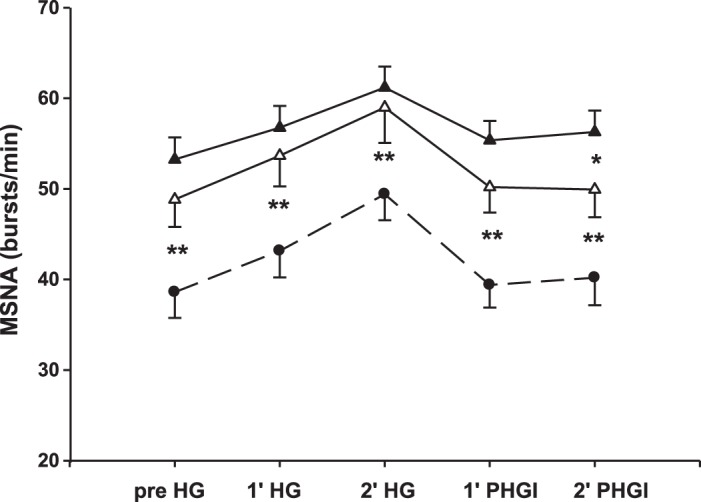
Muscle metaboreflex activation is reduced after exercise training in heart failure with reduced ejection fraction (HFrEF) patients (pretraining, closed triangles; posttraining, open triangles). Muscle sympathetic nerve activity (MSNA) burst frequency in HFrEF during 30% static handgrip (HG) is similar pre- and posttraining but when the metaboreflex is isolated during posthandgrip ischemia (PHGI) is reduced in the 2nd minute of PHGI posttraining. Healthy control subjects (closed circles) have a lower MSNA burst frequency throughout HG and PHGI compared with HFrEF. Data are means ± SE. *P < 0.05; HFrEF (n = 16) pre- vs. posttraining; **P < 0.001 HFrEF vs. controls (n = 18).
DISCUSSION
With sustained sympathetic activation placing patients with HFrEF at increased risk of morbid and mortal events, its modulation has become a key target of contemporary drug and device therapy (16, 54). Further sympathetic stimulation, atop this upwardly reset baseline, such as that elicited by mental and physical activity, may have greater pathophysiological consequences for patients with HFrEF than resting measures alone (16). In this context, exercise training becomes an attractive potential nonpharmacological sympathomodulatory intervention, as it could be offered to the majority of patients with this condition.
In experimental models of heart failure, exercise training has been shown to increase survival; diminish tonic and reflexively stimulated efferent renal sympathetic nerve firing by downregulating central excitatory angiotensin II, redox, and nitric oxide signaling pathways; and attenuate exaggerated peripheral chemoreceptor responsiveness to hypoxia (16, 19, 29). In patients with HFrEF, one center has found reductions in resting MSNA after exercise training, but, as noted in a recent meta-analysis (42), in ~30% of trial participants heart failure was caused by a region-specific parasite. The authors of this meta-analysis urged replication of this finding by other investigators in cohorts with etiologies more representative of heart failure populations globally.
The impact of training protocols on muscle sympathetic firing rates during dynamic leg exercise, a stimulus that, paradoxically, further increases MSNA in patients with HFrEF (39), has yet to be reported. In the present study, we tested the hypothesis in optimally treated patients with HFrEF that 6 mo of exercise training within the context of a clinical cardiac rehabilitation program would attenuate the reflex increase in MSNA elicited by dynamic one-leg cycling. We also sought to determine whether exercise training attenuated the muscle metaboreflex or improved cutaneous vascular responsiveness to hyperemia.
Two-thirds of the present cohort was classified clinically as having an ischemic cardiomyopathy and one-third as having a nonischemic dilated cardiomyopathy. As anticipated from prior training literature (6, 7), V̇o2peak increased, on average, by 3 mL·kg−1⋅min−1, or 17%. This mean value lies within the 15–17% range achieved in randomized clinical trials (1). The principal sympathoneural adaptations observed in conjunction with this increase in exercise capacity were 1) lower resting MSNA burst frequency and incidence; 2) a reduction in MSNA during one-leg cycling exercise at mild but not moderate intensity; 3) attenuation of the MSNA response elicited by muscle metaboreflex stimulation; and, 4) normalization of skin vasodilator responsiveness. As anticipated from prior literature involving patients also treated with β-adrenoceptor antagonists, resting heart rate, blood pressure, and aortic augmentation index were unaffected (6, 41).
Effect of Training on Variables at Rest
As highlighted by Carter and Ray (10), the impact of aerobic exercise training on resting MSNA differs according to the population studied. In healthy young subjects, exercise training has no impact on resting MSNA (10, 33, 46), whereas in patients with HFrEF, as calculated in a recent meta-analysis, 4 mo of dynamic exercise training reduced resting MSNA burst frequency and incidence in HFrEF, on average, by −11 bursts/min and −15 burst/100 heartbeats, respectively (range 12–37%) (42). This effect dissipated when patients were re-evaluated after a further 4 mo of home-based training (13). Importantly, all studies selected originated from a single laboratory in Sao Paulo, Brazil (4–7, 17, 48, 52) and differ from the present series in two important respects: subjects were, on average, 10 yr younger, and in approximately one-third of their, patients HFrEF was attributed to Chagas’ disease, which because of its singular etiology may respond differently than ischemic or idiopathic dilated cardiomyopathies to training. Nonetheless, in the present HFrEF cohort responses to training were similar in magnitude to those reported in the meta-analysis (42): resting burst frequency fell 6.0 ± 2.0 bursts/min, or 11.3% (P = 0.01, n = 18) and resting burst incidence by 7.3 ± 3.4 burst/100 heartbeats or 17.8% (P = 0.04).
Neither blood pressure nor arterial stiffness was affected by training, despite sympathoinhibition and normalization of the microvascular dilator response to reactive hyperemia. Lack of improvement in the augmentation index is in agreement with previous work (41) but in contrast to an increase of 29% reported in a randomized clinical trial of HFrEF patients in which 33 completed 12 wk of high-intensity interval training (11). This suggests that a higher training intensity and different modality may be required to achieve alterations in arterial stiffness in HFrEF, in contrast to the aerobic exercise-based cardiac rehabilitation in the present study.
Effect of Training on MSNA During Exercise
In healthy young subjects, the mode of exercise determines the reflex sympathetic response elicited. For example, Saito and Mano (49) reported a rise in MSNA during mild and moderate isometric leg exercise but a fall in MSNA during dynamic leg exercise at the same intensities. This drop in MSNA has also been observed in the median nerve of the arm in young healthy subjects during two-leg cycling but only at work rates <40% of V̇o2peak (22, 24). This response has been attributed to stimulation of the sympathoinhibitory cardiopulmonary baroreflex as a consequence of a rostral shift in blood volume due to activation of the leg-muscle pump during exercise. The results of subsequent studies supported this interpretation (25). However, at higher intensities this reflex response was overridden by the muscle metaboreflex, resulting in graded increases in MSNA (26).
Two of the Brazilian studies examined the impact of training on the neural response to isometric exercise in patients with HFrEF. Both resting MSNA and the increase in MSNA elicited by moderate-intensity ischemic handgrip were lower following exercise training (52), and yet after the identical exercise training protocol, the increase in MSNA induced by moderate ischemic leg contractions was, paradoxically, higher (6). In the present study, we observed, in conjunction with greater peak V̇o2, a training-induced reduction in MSNA burst frequency and incidence during mild-intensity, but not moderate-intensity, one-leg cycling exercise suggesting that the higher workload in both protocols stimulated similar sympathoexcitatory mechanisms.
Effect of Training on MSNA Response to Stimulation of Muscle Afferents
In healthy young subjects, a 6-wk high-intensity endurance training protocol attenuated the MSNA response to one-leg cycling at 40 W (46). Ray (46) attributed this result to diminished muscle metaboreflex activation. Previously, we documented an augmented sympathoexcitatory muscle metaboreflex response to both dynamic handgrip exercise and PHGI in patients with HFrEF relative to healthy age-matched control subjects (34). Thus one potential mechanism for the decrease in MSNA during mild cycling observed after training in the present cohort that is consistent with prior findings in both experimental (20) and human heart failure (43, 51) is desensitization through conditioning of either metabo- or mechanoreflexes activated by muscle exercise.
In a rabbit model, the sympathoinhibitory cardiopulmonary baroreflex regulation of renal sympathetic nerve traffic was depressed in HFrEF, but partially restored by exercise training (44). Thus a second possible mechanism for the present finding is training-induced augmentation of the sympathoinhibitory cardiopulmonary reflex by leg-muscle pump-stimulated venous return. A third possibility is less atrial distension, during exercise, or attenuation following training of a recently described excitatory reflex response to increased atrial pressure emergent in HFrEF (31). However, at present we can offer no data in support of such speculation.
Group mean values for MSNA during moderate exercise were not affected by training, but, as is evident from Figs. 1 and 4, sympathetic inhibition at this workload was evident in some subjects. This may relate to qualitative or quantitative between-patient differences in the impact of training on reflex arterial chemoreceptor reflex elicited increases in sympathetic activity (26, 27). This supposition represents a topic for future research. Moderate-intensity cycling may also engage additional sympathoexcitatory circuits that are not attenuated by contemporary training protocols, thus offsetting any exercise-induced desensitization of muscle metabolic or other reflexes (26).
Although in the present series training did not significantly affect the increase in MSNA elicited by static handgrip (which engages both metabo- and mechanoreflexes), it abolished its sustained elevation during PHGI, an intervention designed to assess sympathetic responses specific to muscle metaboreflex stimulation by eliminating any confounding autonomic responses to volitional contraction. Thus our data suggest that activation of the muscle metaboreflex in HFrEF patients may be reduced by exercise training, although the effect was small. This may be because we quantified a forearm, rather than a leg metaboreflex response. However, similar attenuation as a consequence of training involving walking is a plausible mechanism for the observation, after 6 mo, of lower MSNA during mild-intensity cycling. Importantly, our findings do not support the concept of an augmented muscle mechanoreflex in this population (30) that reverses with exercise training (6). This discordance with others' work may be due to differences in HFrEF characteristics, exercise protocols, and subject posture during microneurography.
Functional Implications
One functional consequence of reflex sympathetic excitation is peripheral vasoconstriction (12). In patients with HFrEF, neurogenically mediated reductions in leg muscle blood flow (2) might constrain exercise performance. In an important affirmation of this concept, involving a cohort with HFrEF, Amann et al. (3) documented a fall in norepinephrine spillover and an increase in oxygen delivery during mild but not higher intensity single leg extension exercise when neural input from type III and IV muscle afferents arising from skeletal muscle was blocked by intrathecal fentanyl. Thus the exercise training-induced reduction in MSNA that we observed during mild exercise has the potential to attenuate muscle vasoconstriction (2) and to improve peak V̇o2 (16, 37). It could also have a positive impact on activities of daily living, as most such are performed at mild intensities (45).
Limitations
Adherence to the exercise training program was tracked subjectively by patient reports.
Because of the technical challenge of securing high-quality and stable MSNA recordings from an exercising leg, we measured this in the contralateral stationary leg and assumed that MSNA measured in the stationary leg was congruent.
The forearm, rather than the leg muscle metaboreflex was assessed for two reasons. Conceptually, our 6 mo cardiac rehabilitation program, which involves upper body as well as lower body training, would be anticipated to reset tonic and reflexively stimulated central sympathetic outflow (19) and thereby modify the forearm as well as the leg muscle metabo-reflex. Consistent with this concept, Soares-Miranda et al. (52) demonstrated a training effect on the sympathetic response to handgrip, as noted earlier. Pragmatically, we tested the metaboreflex in the forearm to ensure a stable leg MSNA signal. Had we instead stimulated the metaboreflex by encircling the contralateral thigh with an inflatable cuff, there would have been great risk of nerve site loss. MSNA during isolation of the metaboreflex during PHGI could have been under- or overestimated by measuring this in the arm, as has been observed when pressor and ventilatory responses of patients with HFrEF and control subjects have been compared (9, 50). Although the absolute moderate (loaded)-cycling work rate was slightly but significantly higher posttraining, it comprised the same relative load and as such would not independently impact sympathetic outflow.
The healthy controls did not train. The purpose of their recruitment was to determine how closely sympathetic responses of trained HFrEF patients approximated those of middle-aged healthy subjects. Although the mean age of the control group was slightly lower than that of participants with HFrEF, in healthy subjects age does not affect the MSNA burst frequency response to exercise at these intensities (36).
Perspectives and Significance
Predictors of adverse outcome in patients with HFrEF include diminished exercise capacity, excessive resting sympathetic drive (8, 16, 33, 38, 53), and attenuated cutaneous vascular reactivity (14, 40). Our prior work involving patients with HFrEF documented a further increase, rather than the anticipated decrease in MSNA observed in middle-aged controls during one-leg cycling and an inverse relationship between MSNA during moderate exercise and subjects’ peak V̇o2 (39). Elucidating the neurogenic mechanisms of exercise intolerance in HFrEF and how these may be favorably impacted by exercise training have been identified as research priorities by the National Heart Lung and Blood Institute Working Group on exercise training in heart failure (15). The present work confirms that a contemporary clinical cardiac rehabilitation program that increases exercise capacity of patients with HFrEF also lowers muscle sympathetic burst frequency at rest. Importantly, we now provide the first evidence in patients with HFrEF of training-induced attenuation of the additional reflex sympathoexcitation elicited by mild dynamic leg exercise. Training also blunted the MSNA response to PHGI and normalized skin microvascular reactivity. The present data thus provide novel insight into a neurogenic mechanism by which downregulation of resting sympathetic discharge, alleviation of vasoconstriction, and normalization of cutaneous microvascular reactivity by aerobic training could improve heart failure symptoms, exercise capacity, and prognosis.
GRANTS
This work was supported by operating grants from the Heart and Stroke Foundation of Ontario Grants T4938 and NA6298 (to J. S. Floras), Canadian Institutes of Health Research Grant PJT148836 (to J. S. Floras), and Natural Science and Engineering Council of Canada Grant 06019 (to P. J. Millar). P. J. Millar was the recipient of Post-Doctoral Fellowships from the Heart and Stroke/Richard Lewar Centre of Excellence, Heart and Stroke Foundation of Canada, and Canadian Institutes of Health Research. J. S. Floras held the Tier 1 Canada Research Chair in Integrative Cardiovascular Biology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.F.N., P.O., and J.S.F. conceived and designed research; S.M. recruited patients; C.F.N., P.J.M., D.A.K., H.M., N.H., and E.O. performed experiments; C.F.N. analyzed data; C.F.N., P.J.M., D.A.K., H.M., N.H., E.O., and J.S.F. interpreted results of experiments; C.F.N. prepared figures; C.F.N. drafted manuscript; C.F.N., P.J.M., D.A.K., E.O., S.M., and J.S.F. edited and revised manuscript; C.F.N., P.J.M., D.A.K., H.M., N.H., E.O., S.M., P.O., and J.S.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Beverley Morris for the technical and administrative support.
REFERENCES
- 1.Ades PA, Keteyian SJ, Balady GJ, Houston-Miller N, Kitzman DW, Mancini DM, Rich MW. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 1: 540–547, 2013. doi: 10.1016/j.jchf.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alves MJ, Rondon MU, Santos AC, Dias RG, Barretto AC, Krieger EM, Middlekauff HR, Negrão CE. Sympathetic nerve activity restrains reflex vasodilatation in heart failure. Clin Auton Res 17: 364–369, 2007. doi: 10.1007/s10286-007-0448-6. [DOI] [PubMed] [Google Scholar]
- 3.Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Jonathan Groot H, Walter Wray D, Stehlik J, Richardson RS. Group III/IV muscle afferents impair limb blood flowin patients with chronic heart failure. Int J Cardiol 174: 368–375, 2014. doi: 10.1016/j.ijcard.2014.04.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antunes-Correa LM, Kanamura BY, Melo RC, Nobre TS, Ueno LM, Franco FG, Roveda F, Braga AM, Rondon MU, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Exercise training improves neurovascular control and functional capacity in heart failure patients regardless of age. Eur J Prev Cardiol 19: 822–829, 2012. doi: 10.1177/1741826711414626. [DOI] [PubMed] [Google Scholar]
- 5.Antunes-Correa LM, Melo RC, Nobre TS, Ueno LM, Franco FG, Braga AM, Rondon MU, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Impact of gender on benefits of exercise training on sympathetic nerve activity and muscle blood flow in heart failure. Eur J Heart Fail 12: 58–65, 2010. doi: 10.1093/eurjhf/hfp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antunes-Correa LM, Nobre TS, Groehs RV, Alves MJ, Fernandes T, Couto GK, Rondon MU, Oliveira P, Lima M, Mathias W, Brum PC, Mady C, Almeida DR, Rossoni LV, Oliveira EM, Middlekauff HR, Negrao CE. Molecular basis for the improvement in muscle metaboreflex and mechanoreflex control in exercise-trained humans with chronic heart failure. Am J Physiol Heart Circ Physiol 307: H1655–H1666, 2014. doi: 10.1152/ajpheart.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antunes-Correa LM, Ueno-Pardi LM, Trevizan PF, Santos MR, da Silva CH, Franco FG, Alves MJ, Rondon MU, Negrao CE. The influence of aetiology on the benefits of exercise training in patients with heart failure. Eur J Prev Cardiol 24: 365–372, 2017. doi: 10.1177/2047487316683530. [DOI] [PubMed] [Google Scholar]
- 8.Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrão CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135: 302–307, 2009. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 9.Carrington CA, Fisher JP, Davies MK, White MJ. Muscle afferent inputs to cardiovascular control during isometric exercise vary with muscle group in patients with chronic heart failure. Clin Sci (Lond) 107: 197–204, 2004. doi: 10.1042/CS20040038. [DOI] [PubMed] [Google Scholar]
- 10.Carter JR, Ray CA. Sympathetic neural adaptations to exercise training in humans. Auton Neurosci 188: 36–43, 2015. doi: 10.1016/j.autneu.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Chrysohoou C, Angelis A, Tsitsinakis G, Spetsioti S, Nasis I, Tsiachris D, Rapakoulias P, Pitsavos C, Koulouris NG, Vogiatzis I, Tousoulis D.. Cardiovascular effects of high-intensity interval aerobic training combined with strength exercise in patients with chronic heart failure. A randomized phase III clinical trial. Int J Cardiol 179: 269–274, 2015. doi: 10.1016/j.ijcard.2014.11.067. [DOI] [PubMed] [Google Scholar]
- 12.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- 13.de Mello Franco FG, Santos AC, Rondon MU, Trombetta IC, Strunz C, Braga AM, Middlekauff H, Negrão CE, Pereira Barretto AC. Effects of home-based exercise training on neurovascular control in patients with heart failure. Eur J Heart Fail 8: 851–855, 2006. doi: 10.1016/j.ejheart.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Edvinsson ML, Uddman E, Andersson SE. Deteriorated function of cutaneous microcirculation in chronic congestive heart failure. J Geriatr Cardiol 8: 82–87, 2011. doi: 10.3724/SP.J.1263.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleg JL, Cooper LS, Borlaug BA, Haykowsky MJ, Kraus WE, Levine BD, Pfeffer MA, Piña IL, Poole DC, Reeves GR, Whellan DJ, Kitzman DW; National Heart, Lung, and Blood Institute Working Group . Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail 8: 209–220, 2015. doi: 10.1161/CIRCHEARTFAILURE.113.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J 36: 1974–1982, 2015. doi: 10.1093/eurheartj/ehv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR, Negrão CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail 9: 630–636, 2007. doi: 10.1016/j.ejheart.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Franzoni F, Galetta F, Morizzo C, Lubrano V, Palombo C, Santoro G, Ferrannini E, Quiñones-Galvan A. Effects of age and physical fitness on microcirculatory function. Clin Sci (Lond) 106: 329–335, 2004. doi: 10.1042/CS20030229. [DOI] [PubMed] [Google Scholar]
- 19.Haack KK, Zucker IH. Central mechanisms for exercise training-induced reduction in sympatho-excitation in chronic heart failure. Auton Neurosci 188: 44–50, 2015. doi: 10.1016/j.autneu.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O’Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. doi: 10.1152/ajpheart.2000.278.3.H818. [DOI] [PubMed] [Google Scholar]
- 21.Hasking GJ, Esler MD, Jennings GL, Dewar E, Lambert G. Norepinephrine spillover to plasma during steady-state supine bicycle exercise. Comparison of patients with congestive heart failure and normal subjects. Circulation 78: 516–521, 1988. doi: 10.1161/01.CIR.78.3.516. [DOI] [PubMed] [Google Scholar]
- 22.Ichinose M, Saito M, Fujii N, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during incremental leg cycling. J Physiol 586: 2753–2766, 2008. doi: 10.1113/jphysiol.2007.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 131: 700–708, 1985. [DOI] [PubMed] [Google Scholar]
- 24.Katayama K, Ishida K, Iwamoto E, Iemitsu M, Koike T, Saito M. Hypoxia augments muscle sympathetic neural response to leg cycling. Am J Physiol Regul Integr Comp Physiol 301: R456–R464, 2011. doi: 10.1152/ajpregu.00119.2011. [DOI] [PubMed] [Google Scholar]
- 25.Katayama K, Ishida K, Saito M, Koike T, Hirasawa A, Ogoh S. Enhanced muscle pump during mild dynamic leg exercise inhibits sympathetic vasomotor outflow. Physiol Rep 2: e12070, 2014. doi: 10.14814/phy2.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katayama K, Saito M. Muscle sympathetic nerve activity during exercise. J Physiol Sci 69: 589–598, 2019. doi: 10.1007/s12576-019-00669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keir DA, Duffin J, Millar PJ, Floras JS. Simultaneous assessment of central and peripheral chemoreflex regulation of muscle sympathetic nerve activity and ventilation in healthy young men. J Physiol 597: 3281–3296, 2019. doi: 10.1113/JP277691. [DOI] [PubMed] [Google Scholar]
- 28.Lanting SM, Johnson NA, Baker MK, Caterson ID, Chuter VH. The effect of exercise training on cutaneous microvascular reactivity: A systematic review and meta-analysis. J Sci Med Sport 20: 170–177, 2017. doi: 10.1016/j.jsams.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Li YL, Ding Y, Agnew C, Schultz HD. Exercise training improves peripheral chemoreflex function in heart failure rabbits. J Appl Physiol (1985) 105: 782–790, 2008. doi: 10.1152/japplphysiol.90533.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol 287: H1937–H1943, 2004. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- 31.Millar PJ, Murai H, Floras JS. Paradoxical muscle sympathetic reflex activation in human heart failure. Circulation 131: 459–468, 2015. doi: 10.1161/CIRCULATIONAHA.114.010765. [DOI] [PubMed] [Google Scholar]
- 32.Millar PJ, Notarius CF, Haruki N, Floras JS. Heart failure-specific relationship between muscle sympathetic nerve activity and aortic wave reflection. J Card Fail 25: 404–408, 2019. doi: 10.1016/j.cardfail.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Notarius CF, Ando S, Rongen GA, Floras JS. Resting muscle sympathetic nerve activity and peak oxygen uptake in heart failure and normal subjects. Eur Heart J 20: 880–887, 1999. doi: 10.1053/euhj.1998.1447. [DOI] [PubMed] [Google Scholar]
- 34.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol 280: H969–H976, 2001. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- 35.Notarius CF, Atchison DJ, Rongen GA, Floras JS. Effect of adenosine receptor blockade with caffeine on sympathetic response to handgrip exercise in heart failure. Am J Physiol Heart Circ Physiol 281: H1312–H1318, 2001. doi: 10.1152/ajpheart.2001.281.3.H1312. [DOI] [PubMed] [Google Scholar]
- 36.Notarius CF, Millar PJ, Doherty CJ, Incognito AV, Haruki N, O’Donnell E, Floras JS. Microneurographic characterization of sympathetic responses during 1-leg exercise in young and middle-aged humans. Appl Physiol Nutr Metab 44: 194–199, 2019. doi: 10.1139/apnm-2018-0101. [DOI] [PubMed] [Google Scholar]
- 37.Notarius CF, Millar PJ, Floras JS. Muscle sympathetic activity in resting and exercising humans with and without heart failure. Appl Physiol Nutr Metab 40: 1107–1115, 2015. doi: 10.1139/apnm-2015-0289. [DOI] [PubMed] [Google Scholar]
- 38.Notarius CF, Millar PJ, Murai H, Morris BL, Floras JS. Inverse relationship between muscle sympathetic activity during exercise and peak oxygen uptake in subjects with and without heart failure. J Am Coll Cardiol 63: 605–606, 2014. doi: 10.1016/j.jacc.2013.08.693. [DOI] [PubMed] [Google Scholar]
- 39.Notarius CF, Millar PJ, Murai H, Morris BL, Marzolini S, Oh P, Floras JS. Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age-matched healthy subjects. J Physiol 593: 715–722, 2015. doi: 10.1113/jphysiol.2014.281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paine NJ, Hinderliter AL, Blumenthal JA, Adams KF Jr, Sueta CA, Chang PP, O’Connor CM, Sherwood A. Reactive hyperemia is associated with adverse clinical outcomes in heart failure. Am Heart J 178: 108–114, 2016. doi: 10.1016/j.ahj.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parnell MM, Holst DP, Kaye DM. Exercise training increases arterial compliance in patients with congestive heart failure. Clin Sci (Lond) 102: 1–7, 2002. doi: 10.1042/cs1020001. [DOI] [PubMed] [Google Scholar]
- 42.Pearson MJ, Smart NA. Exercise therapy and autonomic function in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 23: 91–108, 2018. doi: 10.1007/s10741-017-9662-z. [DOI] [PubMed] [Google Scholar]
- 43.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation 93: 940–952, 1996. doi: 10.1161/01.CIR.93.5.940. [DOI] [PubMed] [Google Scholar]
- 44.Pliquett RU, Cornish KG, Patel KP, Schultz HD, Peuler JD, Zucker IH. Amelioration of depressed cardiopulmonary reflex control of sympathetic nerve activity by short-term exercise training in male rabbits with heart failure. J Appl Physiol (1985) 95: 1883–1888, 2003. doi: 10.1152/japplphysiol.00486.2003. [DOI] [PubMed] [Google Scholar]
- 45.Pozehl BJ, Mcguire R, Duncan K, Hertzog M, Deka P, Norman J, Artinian NT, Saval MA, Keteyian SJ. Accelerometer-measured daily activity levels and related factors in patients with heart failure. J Cardiovasc Nurs 33: 329–335, 2018. doi: 10.1097/JCN.0000000000000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ray CA. Sympathetic adaptations to one-legged training. J Appl Physiol (1985) 86: 1583–1587, 1999. doi: 10.1152/jappl.1999.86.5.1583. [DOI] [PubMed] [Google Scholar]
- 47.Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol 264: H1–H7, 1993. doi: 10.1152/ajpheart.1993.264.1.H1. [DOI] [PubMed] [Google Scholar]
- 48.Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrão CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 42: 854–860, 2003. doi: 10.1016/S0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- 49.Saito M, Mano T. Exercise mode affects muscle sympathetic nerve responsiveness. Jpn J Physiol 41: 143–151, 1991. doi: 10.2170/jjphysiol.41.143. [DOI] [PubMed] [Google Scholar]
- 50.Scott AC, Davies LC, Coats AJ, Piepoli M. Relationship of skeletal muscle metaboreceptors in the upper and lower limbs with the respiratory control in patients with heart failure. Clin Sci (Lond) 102: 23–30, 2002. doi: 10.1042/cs1020023. [DOI] [PubMed] [Google Scholar]
- 51.Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol (1985) 84: 1551–1559, 1998. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- 52.Soares-Miranda L, Franco FG, Roveda F, Martinez DG, Rondon MU, Mota J, Brum PC, Antunes-Correa LM, Nobre TS, Barretto AC, Middlekauff HR, Negrao CE. Effects of exercise training on neurovascular responses during handgrip exercise in heart failure patients. Int J Cardiol 146: 122–125, 2011. doi: 10.1016/j.ijcard.2010.09.091. [DOI] [PubMed] [Google Scholar]
- 53.Stelken AM, Younis LT, Jennison SH, Miller DD, Miller LW, Shaw LJ, Kargl D, Chaitman BR. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J Am Coll Cardiol 27: 345–352, 1996. doi: 10.1016/0735-1097(95)00464-5. [DOI] [PubMed] [Google Scholar]
- 54.van Bilsen M, Patel HC, Bauersachs J, Böhm M, Borggrefe M, Brutsaert D, Coats AJ, de Boer RA, de Keulenaer GW, Filippatos GS, Floras J, Grassi G, Jankowska EA, Kornet L, Lunde IG, Maack C, Mahfoud F, Pollesello P, Ponikowski P, Ruschitzka F, Sabbah HN, Schultz HD, Seferovic P, Slart RH, Taggart P, Tocchetti CG, Van Laake LW, Zannad F, Heymans S, Lyon AR. The autonomic nervous system as a therapeutic target in heart failure: a scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 19: 1361–1378, 2017. doi: 10.1002/ejhf.921. [DOI] [PubMed] [Google Scholar]
- 55.Wallin BG, Esler MD, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings GL. Simultaneous measurements of cardiac norepinephrine spillover and sympathetic outflow to skeletal muscle in humans. J Physiol 453: 45–58, 1992. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White DW, Shoemaker JK, Raven PB. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Auton Neurosci 193: 12–21, 2015. doi: 10.1016/j.autneu.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]