Abstract
Controversy exists regarding the role played by transient receptor potential vanilloid-1 (TRPV1) in evoking the exercise pressor reflex. Here, we determine the role played by TRPV1 in evoking this reflex while assessing possible confounding factors arising from TRPV1 antagonists or from the vehicle in which they were dissolved. The exercise pressor reflex was evoked in decerebrated, anesthetized Sprague-Dawley rats by electrical stimulation of the tibial nerve to contract the triceps surae muscles statically. This procedure was repeated before and after injection of the TRPV1 blockers: capsazepine (100 μg/100 μL), ruthenium red (100 μg/100 μL), or iodoresiniferatoxin (IRTX; 1 μg/100 μL). We found that capsazepine decreased the exercise pressor reflex when the drug was dissolved in DMSO (−10 ± 9 mmHg; P = 0.015; n = 7). However, similar reduction was found when DMSO alone was injected (−8 ± 5 mmHg; P = 0.023; n = 5). Capsazepine, dissolved in ethanol (2 ± 6 mmHg; P = 0.49; n = 7), ruthenium red (−4 ± 12 mmHg; P = 0.41; n = 7), or IRTX (4 ± 18 mmHg; P = 0.56; n = 7), did not significantly decrease the exercise pressor reflex. In addition, we found that capsazepine and ruthenium red had “off-target” effects. Capsazepine decreased the pressor response evoked by intra-arterial injection of bradykinin (500 ng/kg; −12 ± 13 mmHg; P = 0.028; n = 9) and α-β-methylene ATP (10 μg/kg; −7 ± 8 mmHg; P = 0.019; n = 10), whereas ruthenium red decreased the ability of the muscle to produce and sustain force (−99 ± 83 g; P = 0.020; n = 7). Our data therefore suggest that TRPV1 does not play a role in evoking the exercise pressor reflex. Additionally, given their strong off-target effects, capsazepine and ruthenium red should not be used for studying the role played by TRPV1 in evoking the exercise pressor reflex.
Keywords: blood pressure, exercise, rats, transient receptor potential vanilloid-1
INTRODUCTION
The exercise pressor reflex, in combination with sympatholysis, functions to increase arterial blood flow to contracting muscles (1, 32, 36). The afferent arm of the reflex is composed of group III and IV fibers (25) whose endings are found in the interstitial space of skeletal muscle, small vessels, or connective tissue (40, 46a). For the most part, the endings of group III afferents respond to mechanical distortion of their receptive fields (20, 28, 33), whereas the endings of group IV afferents respond to byproducts of muscle contraction (20, 21, 27, 28). Group III and IV muscle afferents synapse in laminae I, II, and V of the dorsal horn of the spinal cord (5, 26) and then project to brainstem to exert their sympathoexcitatory effects (17).
Recently, studies have identified the receptors that activate the group III–IV afferent fibers, whose contraction-induced stimulation evokes the exercise pressor reflex. So far, there is good agreement that purinergic 2X receptors (15, 42), acid-sensing ion channels (42), and transient receptor potential (TRP) ankyrin-1 (23) play important roles in evoking the reflex. In contrast, conflicting results exist regarding the role played by TRP vanilloid-1 (TRPV1) in evoking the exercise pressor reflex (22, 29, 38, 45). TRPV1 is expressed in rat dorsal root ganglion cells innervating the hindlimb muscles (29); it is activated by low pH (<5.5) and heat (>43°C) and by vanilloid compounds, such as capsaicin (4, 31). The hypothesis that TRPV1 plays a role in evoking the exercise pressor reflex may have arisen from findings that the injection of capsaicin into the arterial supply of hindlimb skeletal muscle evoked a strong reflex pressor response (19, 47), an effect that, in turn, was blunted by injection of TRPV1 antagonists (22, 38, 45). Two findings, however, cast doubt on this hypothesis, namely, that muscle interstitial pH does not decrease below 6.9 (43) and that during exhaustive exercise muscle temperature, does not increase above 39°C (12). Furthermore, skeletal muscle does not produce vanilloid substances (8).
The lack of consensus regarding the role played by TRPV1 in evoking the exercise pressor reflex might have been caused by differences in animal species (i.e., cats vs. rats), animal health, TRPV1 antagonists, and/or the solvents used to dissolve the antagonists. For example, the findings showing that capsazepine or iodoresiniferatoxin (IRTX) decreased the pressor response to static contraction have not been accompanied by the proper vehicle controls (29, 38), raising the possibility that the reduced pressor reflex found following capsazepine or IRTX injections was caused by the vehicle alone. In addition, the effect of TRPV1 antagonists might have been misinterpreted because of the drugs acting on other receptors. For example, capsazepine, in addition to inhibiting TRPV1 effectively, has been found to inhibit voltage-gated calcium channels in vitro (10). Likewise, ruthenium red, acting as a nonselective TRP antagonist (2), also inhibits TRP ankyrin-1 (18), receptors that, in turn, have been shown to play a role in evoking the exercise pressor reflex (23). In addition, ruthenium red blocks ryanodine receptors (48), an effect that might have reduced the ability of the muscle to produce force by reducing the sarcoplasmic release of Ca2+. It is therefore possible that the injection of TRPV1 antagonists might have reduced the exercise pressor reflex by mechanisms other than just TRPV1 inhibition.
Therefore, the main purpose of the present study was to determine the role played by TRPV1 in evoking the exercise pressor reflex in healthy rats. To block TRPV1, we injected capsazepine, ruthenium red, or IRTX into the arterial supply of the triceps surae muscles. To determine if the vehicles in which these TRPV1 antagonists were dissolved affected the exercise pressor reflex, we tested the effect of dissolving capsazepine in two different solvents, namely, DMSO and ethanol. Finally, to determine possible confounding factors of TRPV1 antagonism, we measured the effect of capsazepine on the pressor response evoked by bradykinin, α-β-methylene ATP, and diprotonated phosphate, each of which is a metabolic byproduct of contraction but does not directly activate TRPV1. We also determined the effect of ruthenium red on the ability of the muscle to produce and sustain force. We tested the following hypotheses that: 1) capsazepine dissolved in DMSO decreased the exercise pressor reflex, but this decrease was due to the DMSO alone; 2) capsazepine dissolved in ethanol decreased the exercise pressor reflex, but this decrease was confounded by the fact that capsazepine decreased the pressor responses to bradykinin, α-β-methylene ATP, and diprotonated phosphate; 3) ruthenium red decreased the exercise pressor reflex, but this decrease was confounded by the fact that the ability of the muscle to produce force was reduced; and 4) IRTX had no effect on the exercise pressor reflex.
METHODS
Ethical Approval
The Institutional Care and Use Committee of the Pennsylvania State University College of Medicine approved all of the procedures. The authors understand and conformed to the ethical guidelines of the journal for animal use in research.
Animal Characteristics, Wellness, and Sample Size
Experiments were conducted at constant room air temperature (21°C) on 91 male Sprague-Dawley rats (Charles River), weighing 300–500 g.
Rats were housed within the central animal facility of the Pennsylvania State University College of Medicine, with access to food and water ad libitum, and were exposed to a 50:50 light/dark cycle. All attempts were made to minimize animal discomfort and pain.
Surgical Procedures
At the beginning of every surgery, the animal was anesthetized by inhalation of 4% of isoflurane with oxygen. We started the surgical procedure only when the corneal reflex stopped and when pinching the hindpaw did not produce a withdrawal reflex.
With the rat in supine position, the neck area was opened to expose the trachea, which was cannulated with a 2-cm long 14G Teflon tube. Lungs were mechanically ventilated (model 683; Harvard Apparatus, Holliston, MA), and the amount of isoflurane was reduced to 2%. The left and right common carotids were isolated from the vagus nerve and cannulated using RenaPulse High Fidelity Pressure Tubing (RPT040; Braintree Scientific, Braintree, MA) to record arterial blood pressure (P23XL; Gould-Statham Instruments, Los Angeles, CA) and to draw arterial blood samples, respectively. Similarly, the right jugular vein was cannulated (RPT040) to inject drugs into the systemic circulation.
An incision was made on the ventral part of the left thigh to expose the inguinal fat pad. Once the inguinal fat pad has been reflected upward to expose the junction of the femoral, superficial epigastric, muscular branch, and saphenous arteries, the superficial epigastric artery was cannulated with the tip of the tubing (SUBL-140; Braintree Scientific) positioned just before the anastomosis with the femoral artery. This cannulation permits injection of solutions directly into the arterial circulation of the hindlimb. A snare (2.0 silk suture) was placed around the femoral artery and vein, which when tightened, trapped the solution into the hindlimb circulation. Finally, the muscular branch and saphenous arteries were ligated (4.0 or 5.0 silk suture) to increase the probability that any injected solution entered the circulation of the triceps surae muscles.
For the contracting or stretching experiments, an incision was made on the skin located at mid-distance between the two great trochanters with the rat in prone position. Two other incisions were made along each side of the pelvis to hold the ilium bones with a metal clamp. For the contracting experiments, the popliteal fossa was opened to expose and isolate the tibial nerve, which was hooked with bipolar stainless-steel electrodes. The femur was attached to a small metal clamp to prevent knee movements during the contracting or stretch procedure. Each paw was secured to the experimental table, and the head of the rat was secured using a customized stereotaxic unit. To record the force produced by the triceps surae muscles, the calcaneus bone was severed, and its attached Achilles tendon was connected to a force transducer (FT03; Grass Instrument, Quincy, MA) and a rack and pinion.
With the use of a blunted spatula, we decerebrated the rat by sectioning <1 mm rostral to the superior colliculus (9). The isoflurane was then discontinued, and the lungs were ventilated with room air. Blood arterial Po2 (100–150 mmHg), Pco2 (35–40 mmHg), pH (7.35–7.45), and [] (22–26 mM) was regularly monitored and kept within physiological range. Body temperature was maintained around 37°C using a heating lamp. At the end of the experiment, the decerebrated rats were killed by intravenous injection of 3 mL of a supersaturated KCl solution into the systemic circulation.
Experimental Procedures
Contraction of the triceps surae muscles.
Baseline tension of the triceps surae muscles was set at 100 g. The motor threshold was then determined by progressively increasing the current of a single pulse (0.01 ms) applied to the tibial nerve until a muscle twitch was observed. The stimulator output was then set at a current intensity that evoked a twitch tension equal to 90% of the maximal twitch tension (~1.60 times motor threshold). Given that ruthenium red inhibits ryanodine channels in the muscle fibers (48), we anticipated that peak tension of the static contraction would be reduced after injecting the drug. Therefore, the stimulation current intensity was set to evoke a twitch equal to 50% of the maximal twitch tension (~1.25 times motor threshold). This procedure allowed us to increase the current intensity once the drug had been injected to match the peak twitch tension. During static contraction, the tibial nerve was stimulated for 30 s at 40 Hz (0.01 ms pulse duration) to increase arterial blood pressure reflexively (39). After a recovery period of at least 10 min, the contraction procedure was repeated to verify that the pressor response was reproducible. After 10 min of recovery, the snare around the femoral artery and vein was tightened, and capsazepine (100 μg/100 μL; 0.1 mL), ruthenium red (100 μg/100 μL; 0.1 mL), or IRTX (1 μg/100 μL; 0.1 mL) was injected into the femoral artery using the superficial epigastric artery catheter. The snare was released 2 min after injection of capsazepine or 10 min after injection of ruthenium red or IRTX (38). Following a washout period of 5 min, the stimulation current intensity was adjusted, if necessary, to evoke the same twitch tension as that evoked before injecting the drug.
To control for the effect of ruthenium red on the ability of the muscle to produce force, a supramaximal twitch was evoked just before injecting the drug and just before contracting the triceps surae muscles after the TRPV1 blocker injection. Given that the supramaximal twitch elicits a complete spatial recruitment of the muscle fibers, a decrease of the peak tension demonstrated that the ability of the muscle fiber pool to produce force has been reduced.
To control that the tibial nerve stimulation did not electrically activate the axons of the group III and IV afferents, which evoke the exercise pressor reflex, the rat was paralyzed by intravenous injection of pancuronium bromide (1 mg/mL; 0.2 mL), and the tibial nerve was stimulated for 30 s at 40 Hz, with the highest stimulation parameters used to evoke contraction. If an increase in blood pressure was observed, then the data were excluded from the data set.
Stretch of the triceps surae muscles.
The pressor response evoked by stretching the triceps surae muscles was compared before and after injecting the TRPV1 antagonists, capsazepine, ruthenium red, and IRTX. To stretch the triceps surae muscles, the tension was passively raised using a rack and pinion until reaching a fixed tension corresponding to ~750–850 g from a baseline tension of 100 g (Δ650–750 g). The tension was released 30 s later. After 10 min of recovery, the snare around the femoral artery and vein was tightened, and capsazepine (100 μg/100 μL; 0.1 mL), ruthenium red (100 μg/100 μL; 0.1 mL), or IRTX (1 μg/100 μL; 0.1 mL) was injected into the femoral artery using the superficial epigastric artery catheter. The snare was released 2 min after injecting capsazepine or 10 min after injecting ruthenium red or IRTX. After a washout period of 5 min, the stretching procedure was repeated.
Chemical injection.
We first determined the effect of capsazepine, ruthenium red, and IRTX on the pressor responses to the TRPV1 agonist capsaicin, injected into the superficial epigastric artery. We also determined the effect of capsazepine on the pressor responses to “non-TRPV1” agonists (i.e., bradykinin, α-β-methylene ATP, and diprotonated phosphate) injected into the femoral artery using the superficial epigastric artery catheter. A non-TRPV1 agonist was defined as a drug that does not activate TRPV1 (i.e., pH < 5.5, heat > 43°C, or vanilloids). Bradykinin, ATP, and diprotonated phosphate were chosen because they are produced by the contracting muscles and are known to evoke a reflex pressor response when injected into the arterial supply of skeletal muscle (13, 14, 24, 41). During these experiments, the rat was paralyzed with intravenous injection of pancuronium bromide (1 mg/mL; 0.2 mL). Before the injection, the snare placed around the femoral artery and vein was tightened to trap the solution into the hindlimb circulation. Then, capsaicin (0.5 μg/mL; 0.1 mL), bradykinin (500 ng/kg; 0.2 mL) (35), α-β-methylene ATP (10 μg/kg; 0.2 mL) (14), and diprotonated phosphate (86 mM; pH 6.0; 0.2 mL) (13, 23) were injected into the femoral artery using the superficial epigastric artery catheter to increase blood pressure reflexively. The snare was released 2 min after injecting the drug. After 15 min of recovery (30 min for the bradykinin trials), capsazepine (100 μg/100 μL; 0.1 mL), ruthenium red (100 μg/100 μL; 0.1 mL), or IRTX (1 μg/100 μL; 0.1 mL) was injected into the femoral artery using the superficial epigastric artery catheter. The snare was released 2 min after injecting capsazepine or 10 min after injecting ruthenium red or IRTX. After a washout period of 5 min, capsaicin, bradykinin, α-β-methylene ATP, or diprotonated phosphate was reinjected. Because bradykinin can be considered as a secondary TRPV1 agonist (37, 44), the bradykinin experiment was reproduced using IRTX to verify that if capsazepine blocks the response to bradykinin, then the effect was mediated through TRPV1 inhibition.
Control for an effect of the drug vehicle.
If one of the TRPV1 antagonists decreased the pressor response to one of the stimuli studied, then we attempted to replicate the decrease in another group of rats for which we injected the vehicle in which the drug was dissolved. This experiment was conducted to test the possibility that the decrease in any pressor response was caused by the drug and not caused by the vehicle in which it was dissolved.
Blue dye control.
To determine that injections into the femoral artery accessed the arterial circulation of the triceps surae muscles, we injected 0.2–0.3 mL Evans Blue dye into the femoral artery using the superficial epigastric artery catheter after tightening the snare placed around the femoral artery and vein. We considered that the solution spread into the muscle when the belly of the triceps surae muscles was stained blue. If the color of the muscles did not change, then we excluded the data from the study.
Drug preparation.
The stock solution for each of the drugs used in the present experiments was stored in 1 mL aliquots at −20°C. On the day of the experiment, we diluted the stock solution to the needed concentration with 0.9% saline solution. Bradykinin (Sigma-Aldrich), α-β-methylene ATP (Tocris), and ruthenium red (Tocris) were dissolved in saline. Diprotonated phosphate was made by dissolving 153 mg (43 mM) Na2HPO4 and 129 mg (43 mM) NaH2PO4 into 25 mL of 10 mM HEPES. The pH of the solution was decreased to 6.0 by adding HCl and if necessary, adjusted on a weekly basis.
Capsaicin (10 mg; Sigma-Aldrich) was dissolved in 0.1 mL ethanol with a drop of Tween 80. Then, 9.9 mL saline was added. The solution was stirred and gently heated to avoid visible flakes. Saline (40 mL) was finally added until obtaining a clear solution.
Capsazepine (MedChemExpress) was dissolved in ethanol or DMSO. For the ethanol solution, 10 mg capsazepine was diluted into 1 mL ethanol. Tween 80 (1 mL) was added, and the solution was shaken several times to avoid visible flakes. The final volume was obtained by the addition of 8 mL saline. For the DMSO solution, 10 mg capsazepine was diluted in 50 μL DMSO, which was the smallest volume of solvent that could be used without producing visible flakes or precipitate. Five microliters of that solution was withdrawn and mixed with a drop of Tween 80 and 0.995 mL saline to obtain a 0.5% DMSO solution.
IRTX (1 mg; Tocris) was diluted in 0.1 mL ethanol with a drop of Tween 80. Saline (0.9 mL) was added, and the solution was properly shaken to avoid the appearance of visible flakes. That solution (0.1 mL) was mixed with 9.9 mL saline to bring the concentration of the stock solution down to 100 μg/mL.
Data Analysis
Tension and arterial blood pressure signals were amplified (Gould Universal and Pressure Processors; Gould-Statham Instruments, Los Angeles, CA), displayed, and recorded at 1 kHz using an analog-to-digital converter (Micro1401 MKII; Cambridge Electronic Design, Cambridge, UK) and its associated commercially available software (Spike2; 7.20; RRID:SCR_000903; Cambridge Electronic Design). Heart rate was determined beat by beat from the pulsatile wave of the blood pressure signal and expressed as beats per minute. To determine the effect of the TRPV1 antagonists on the pressor response to chemical injections, we calculated the difference between the peak mean arterial pressure and its corresponding baseline value, measured before and after injection of the antagonist. For α-β-methylene ATP injections, specifically, the pressor response depicted a double-peak response. As the second peak was most likely caused by recirculation of the drug, we analyzed the peak pressor response recorded during the first peak only. To determine the effect of TRPV1 antagonists on the pressor response to 30 s of static contraction or a passive stretch, we calculated the peak pressor response (as described above) and the change in the blood pressure index. The blood pressure index was calculated by first integrating the area under the curve during the 30-s contraction or stretch period and then subtracting from this value the area under the curve measured 1 s before the contraction or stretch and multiplied by 30. With the use of a similar method, we calculated the change in peak tension produced by the contraction or stretch, as well as the tension-time index (i.e., the equivalent of the blood pressure index for tension).
Statistical Analysis
Data in results are presented as the means ± SD difference between baseline and post-TRPV1 antagonist. With the use of a Kolmogorov-Smirnoff test, we verified that our samples respected a normal distribution. Then, paired Student’s t tests were used to determine the following: 1) the pre- to poststimulus (i.e., contraction, stretch, capsaicin, etc.) change in peak pressor response and peak heart rate or 2) the pre- to postinjection effect of TRPV1 blockers on the peak pressor response and peak heart rate evoked by the different stimuli. For contraction or stretch experiments, these analyses were also conducted on the blood pressure index, peak tension, and tension-time index. The level of significance was set at P < 0.05. The effect size was calculated using Cohen’s d (3). A Cohen’s d index for effect size was considered small, medium, or large when d was close to 0.2, 0.5, or 0.8, respectively (3). When individual data are not presented, effect size was also calculated using 95% confidence intervals (∆CI) (6). ∆CI is presented as the lower and upper limit of the interval that should, if this experiment is repeated, contain 95% of the time of the true value of the treated effect (6). Statistical analyses were conducted using Statistica 8.0 (RRID:SCR_014213; StatSoft, Tulsa, OK).
RESULTS
Pressor Response Evoked by Chemical Injections
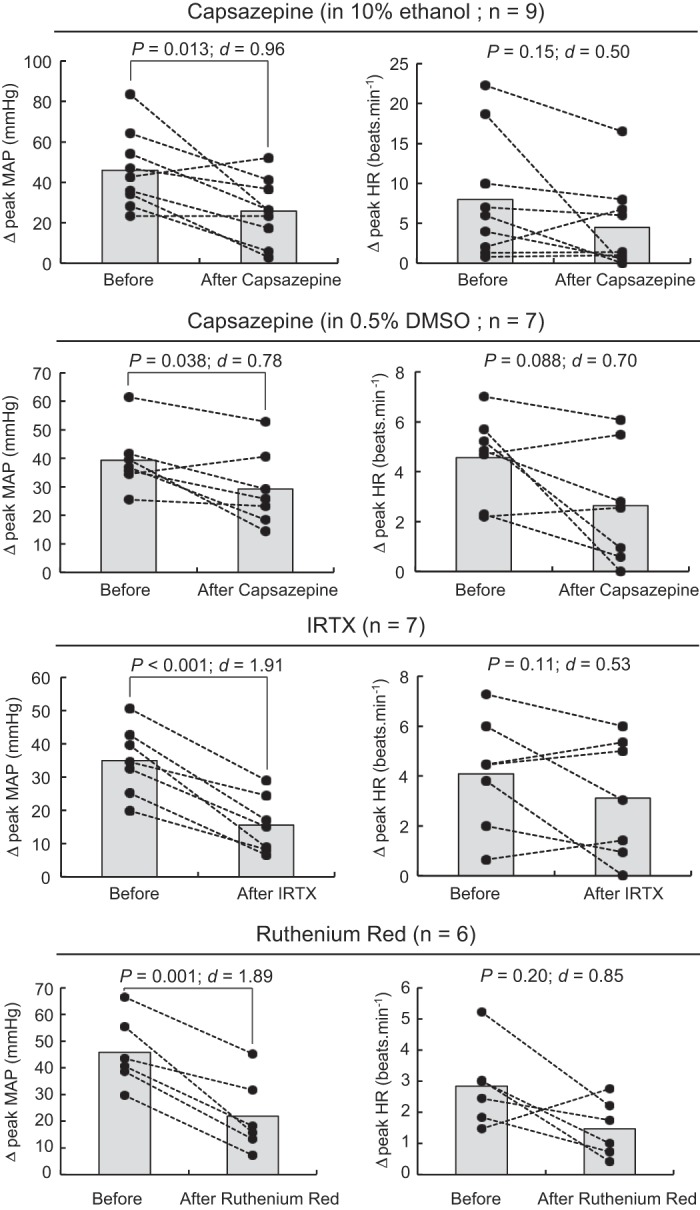
Injections of capsaicin, bradykinin, α-β-methylene ATP, and diprotonated phosphate into the superficial epigastric artery significantly increased arterial blood pressure and heart rate (Table 1). Capsazepine, ruthenium red, and IRTX significantly attenuated the pressor response to capsaicin injection, showing that these antagonists effectively blocked TRPV1 (Fig. 1). However, capsazepine also decreased the pressor responses evoked by injections of bradykinin and α-β-methylene ATP, showing that the effect of capsazepine was not limited to TRPV1 antagonism (Fig. 2). The effect of capsazepine on bradykinin was not replicated using IRTX (12 ± 20 mmHg; ∆CI = [−9 34]; P = 0.20; d = 0.15; n = 6), suggesting that the inhibition of the bradykinin pressor response by capsazepine was not secondary to TRPV1 antagonism. Capsazepine had no effect on the peak pressor response evoked by injection of diprotonated phosphate. The effect of IRTX on the blood pressure response to α-β-methylene ATP or diprotonated phosphate was not tested. The vehicle in which capsazepine (10% ethanol, 10% Tween 80, 80% saline), ruthenium red (saline), or IRTX (0.01% ethanol) was dissolved had no effect on the blood pressure or the cardioaccelerator responses to the different drugs tested (P > 0.12; Table 2).
Table 1.
Peak pressor and cardioaccelerator responses to intra-arterial injection of chemicals, static contraction, or stretch of triceps surae muscles
| Stimulus (Index) | Baseline | Peak | 95% CI | P Value | Cohen’s d |
|---|---|---|---|---|---|
| Capsaicin (n = 35) | |||||
| MAP | 132 ± 24 | 176 ± 29 | [29 59] | P < 0.001 | d = 1.67 |
| HR | 455 ± 64 | 461 ± 65 | [3 6] | P < 0.001 | d = 0.92 |
| Bradykinin (n = 15) | |||||
| MAP | 116 ± 31 | 138 ± 32 | [6 38] | P < 0.001 | d = 1.37 |
| HR | 419 ± 54 | 421 ± 54 | [1 4] | P < 0.001 | d = 0.59 |
| α-β-Methylene ATP (n = 10) | |||||
| MAP | 127 ± 39 | 150 ± 38 | [7 40] | P < 0.001 | d = 2.16 |
| HR | 461 ± 57 | 464 ± 57 | [1 6] | P < 0.001 | d = 0.58 |
| Diprotonated phosphate (n = 10) | |||||
| MAP | 127 ± 25 | 166 ± 37 | [11 66] | P < 0.001 | d = 1.68 |
| HR | 473 ± 55 | 479 ± 52 | [2 10] | P < 0.001 | d = 1.32 |
| Static contraction (n = 34) | |||||
| MAP | 105 ± 25 | 130 ± 31 | [17 35] | P < 0.001 | d = 1.53 |
| HR | 386 ± 42 | 396 ± 40 | [7 14] | P < 0.001 | d = 0.94 |
| BPI | 2,999 ± 757 | 3,328 ± 856 | [216 442] | P < 0.001 | d = 1.11 |
| Passive stretch (n = 33) | |||||
| MAP | 120 ± 23 | 158 ± 30 | [37 51] | P < 0.001 | d = 2.04 |
| HR | 394 ± 41 | 401 ± 39 | [4 9] | P < 0.001 | d = 0.76 |
| BPI | 3,474 ± 699 | 3,989 ± 738 | [425 605] | P < 0.001 | d = 1.46 |
Results are presented as means ± SD; n = sample size. Ninety-five percent confidence interval (CI) is presented as the lower and upper boundary of the interval containing the true value of the effect of stimulus on the corresponding index. BPI, blood pressure index (in millimeters of mercury times s); HR, heart rate (in beats/minute); MAP, mean arterial blood pressure (in millimeters of mercury).
Fig. 1.
Capsazepine, iodoresiniferatoxin (IRTX), and ruthenium red reduced the pressor response to intra-arterial injection of capsaicin. Individual (closed circles) or group (shaded bars) data for mean arterial blood pressure (MAP) or heart rate (HR) are presented as the peak difference (Δ) measured before vs. after injecting capsaicin (0.5 μg/mL) into the superficial epigastric artery. Transient receptor potential vanilloid-1 was inhibited by injecting 0.1 mL capsazepine (100 μg/100 μL), ruthenium red (100 μg/100 μL), or IRTX (1 μg/100 μL) into the superficial epigastric artery.
Fig. 2.
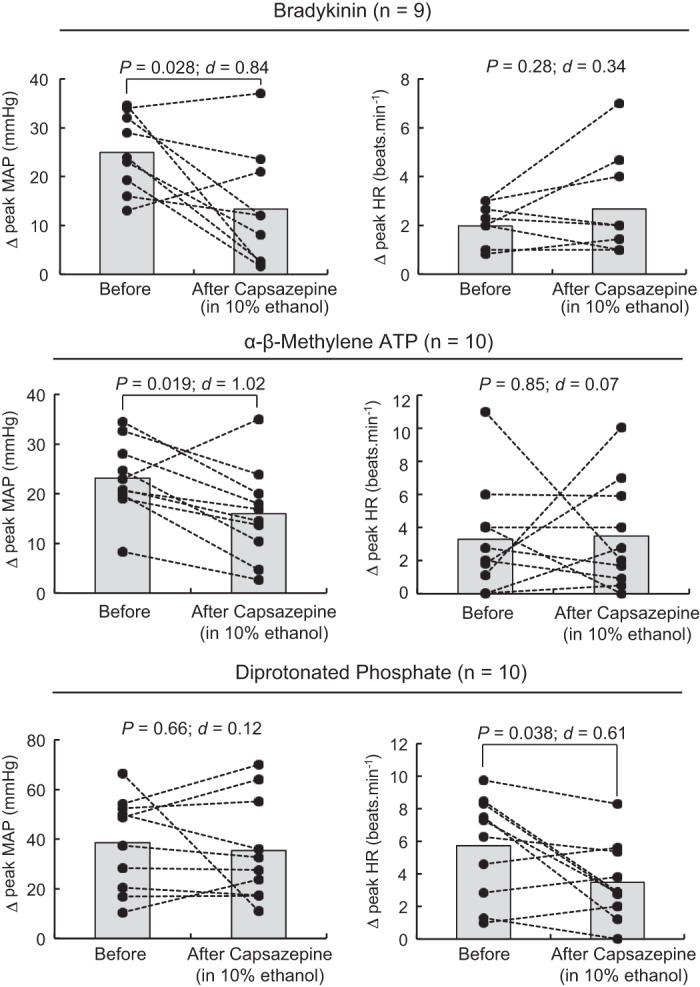
Capsazepine decreased the pressor responses to intra-arterial injection of bradykinin and α-β-methylene ATP. Individual (closed circles) or group (shaded bars) data for mean arterial blood pressure (MAP) and heart rate (HR) are presented as the peak difference (Δ) measured before and after injecting bradykinin (500 ng/kg), α-β-methylene ATP (10 μg/kg), or diprotonated phosphate (86 mM; pH = 6.0) into the superficial epigastric artery. Transient receptor potential vanilloid-1 was inhibited by injecting 0.1 mL capsazepine (100 μg/100 μL), dissolved in 10% ethanol and 10% Tween 80, into the superficial epigastric artery.
Table 2.
Effect of vehicle controls for TRPV1 antagonists on the peak pressor and cardioaccelerator responses to injecting chemicals into superficial epigastric artery
| 10% Ethanol/10% Tween 80 |
0.5% DMSO |
100% Saline |
0.1% Ethanol |
|||||
|---|---|---|---|---|---|---|---|---|
| Chemicals | Before | After | Before | After | Before | After | Before | After |
| Capsaicin | ||||||||
| MAP | 36 ± 11 | 42 ± 25 | 63 ± 29 | 70 ± 26 | 35 ± 8 | 38 ± 10 | 34 ± 20 | 43 ± 25 |
| HR | 10 ± 9 | 13 ± 11 | 4 ± 2 | 4 ± 2 | 2 ± 3 | 3 ± 2 | 3 ± 1 | 2 ± 1 |
| n | 6 | 4 | 4 | 4 | ||||
| Bradykinin | ||||||||
| MAP | 23 ± 8 | 29 ± 14 | Not tested | Not tested | 21 ± 12 | 19 ± 8 | ||
| HR | 2 ± 1 | 3 ± 2 | 1 ± 1 | 2 ± 2 | ||||
| n | 5 | 3 | ||||||
| α-β-Methylene ATP | ||||||||
| MAP | 22 ± 12 | 21 ± 9 | Not tested | Not tested | Not tested | |||
| HR | 3 ± 1 | 2 ± 1 | ||||||
| n | 9 | |||||||
| Diprotonated phosphate | ||||||||
| MAP | 33 ± 24 | 37 ± 26 | Not tested | Not tested | Not tested | |||
| HR | 4 ± 4 | 4 ± 2 | ||||||
| n | 5 | |||||||
Results are presented as means ± SD increase in mean arterial blood pressure (MAP, in mmHg) or heart rate (HR, in beats/min), evoked by each chemical; n = sample size. Note that no significant difference in the pressor or cardioaccelerator response was found for any of the vehicles that were injected into the superficial epigastric artery. TRPV1, transient receptor potential vanilloid-1.
Pressor Responses Evoked by Contraction
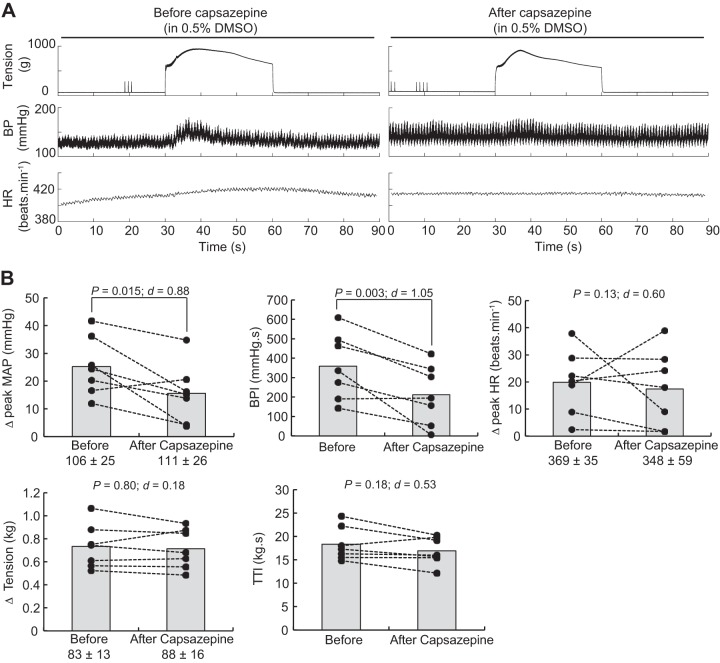
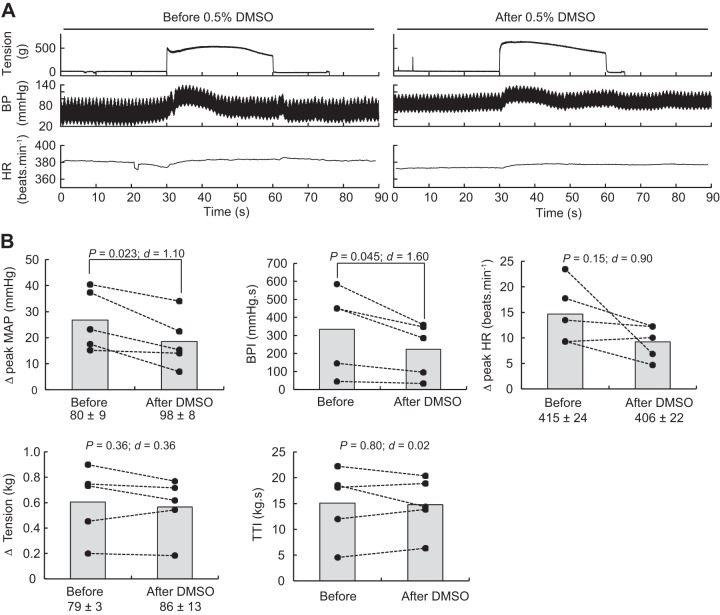
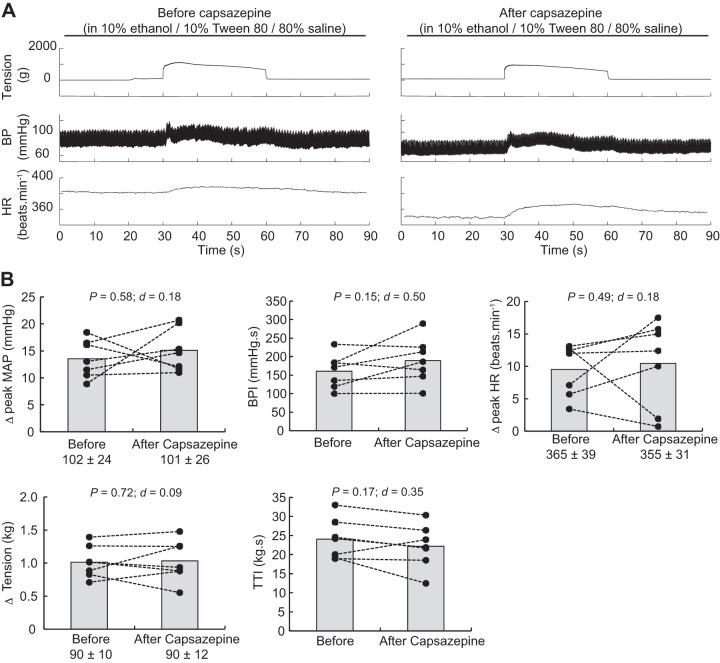
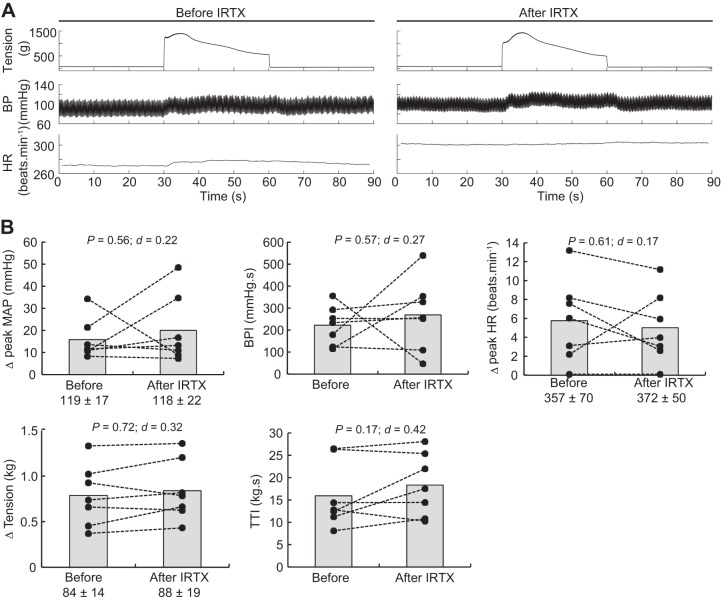
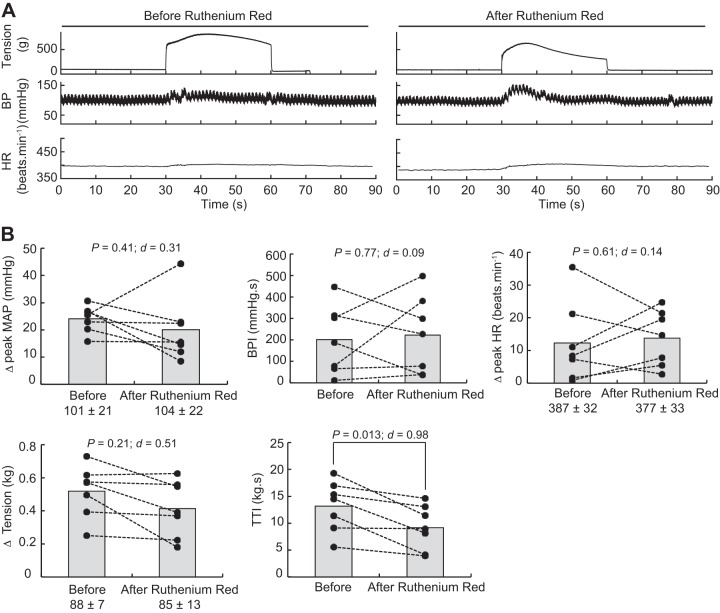
Static contractions of the triceps surae muscles significantly elevated mean arterial blood pressure, blood pressure index, and heart rate (Table 1). The blocking of TRPV1 with capsazepine dissolved in 0.5% DMSO significantly reduced the peak pressor response and the blood pressure index evoked by static contraction of the triceps surae muscles (Fig. 3). However, the injection of a capsazepine-free solution, containing only 0.5% DMSO to control for an effect of the vehicle, produced a similar reduction of the peak pressor response and blood pressure index evoked by static contraction, suggesting that the effect of capsazepine was, at least partly, due to the vehicle in which capsazepine was dissolved (Fig. 4). In contrast, capsazepine dissolved into 10% ethanol and 10% Tween 80, IRTX, or ruthenium red did not have a significant effect on the peak pressor response or the blood pressure index evoked by static contraction (Figs. 5, 6, and 7). Except for ruthenium red, injection of TRPV1 antagonists or 0.5% DMSO solution did not significantly change peak tension or the tension-time index. Ruthenium red significantly decreased the tension-time index evoked by a 30-s static contraction and decreased the peak force produced by a supramaximal twitch (−99 ± 83 g; ∆CI = [−176 − 22]; P = 0.020; d = 0.99; n = 7), showing that the drug impaired the ability of the muscle to produce and sustain force.
Fig. 3.
Effect of capsazepine dissolved in DMSO on the exercise pressor reflex. A: representative sample. B: individual (closed circles) and group (gray bars; n = 7) data for mean arterial pressure (MAP), blood pressure (BP) index (BPI), heart rate (HR), peak tension, and tension-time index (TTI) evoked by static contraction of the triceps surae muscles before and after injecting 0.1 mL capsazepine (100 μg/100 μL) dissolved in 0.5% DMSO into the superficial epigastric artery. The numbers (±) below the MAP, HR, and tension graphs represent the baseline values (in grams for tension). Δ, difference.
Fig. 4.
Effect of DMSO on the exercise pressor reflex. A: representative sample. B: individual (closed circles) and group (shaded bars; n = 5) data for mean arterial pressure (MAP), blood pressure (BP) index (BPI), heart rate (HR), peak tension, and tension-time index (TTI) evoked by static contraction of the triceps surae muscles before and after injecting 0.5% DMSO (0.1 mL) into the superficial epigastric artery. The numbers (±) below the MAP, HR, and tension graphs represent the baseline values. Δ, difference.
Fig. 5.
Effect of capsazepine dissolved in ethanol on the exercise pressor reflex. A: representative sample. B: individual (closed circles) and group (shaded bars; n = 7) data for mean arterial pressure (MAP), blood pressure (BP) index (BPI), heat rate (HR), peak tension, and tension-time index (TTI) evoked by static contraction of the triceps surae muscles before and after injecting 0.1 mL capsazepine (100 μg/100 μL) dissolved in 10% ethanol and 10% Tween 80 into the superficial epigastric artery. The numbers (±) below the MAP, HR, and tension graphs represent the baseline values. Δ, difference.
Fig. 6.
Effect of iodoresiniferatoxin (IRTX) on the exercise pressor reflex. A: representative sample. B: individual (closed circles) and group (gray bars; n = 7) data for mean arterial pressure (MAP), blood pressure (BP) index (BPI), heat rate (HR), peak tension, and tension-time index (TTI) evoked by static contraction of the triceps surae muscles before and after injecting 0.1 mL IRTX (1 μg/100 μL) into the superficial epigastric artery. The numbers (±) below the MAP, HR, and tension graphs represent the baseline values. Δ, difference.
Fig. 7.
Effect of ruthenium red on the exercise pressor reflex. A: representative sample. B: individual (closed circles) and group (shaded bars; n = 7) data for mean arterial pressure (MAP), blood pressure (BP) index (BPI), heat rate (HR), peak tension, and tension-time index (TTI) evoked by static contraction of the triceps surae muscles before and after injecting 0.1 mL ruthenium red (100 μg/100 μL) into the superficial epigastric artery. The numbers (±) below the MAP, HR, and tension graphs represent the baseline values. Δ, difference.
Pressor Response Evoked by Passive Stretch
Passive stretch of the triceps surae muscles significantly elevated blood pressure, blood pressure index, and heart rate (Table 1). Except for capsazepine dissolved in 0.5% DMSO, TRPV1 inhibition did not significantly change the peak pressor response, blood pressure index, or peak heart rate evoked by passive stretch (Table 3). Capsazepine dissolved in 0.5% DMSO significantly decreased the blood pressure index evoked by passive stretch, whereas the peak pressor response remained unchanged (Table 3). Similar to the static contraction data, vehicle (i.e., 0.5% DMSO) injection decreased the blood pressure index evoked by passive stretch without significantly changing the peak pressor response (Table 3).
Table 3.
Effect of TRPV1 antagonists on the peak pressor and cardioaccelerator responses to stretch of triceps surae muscles
| TRPV1 Antagonist (Index) | Before Injection | After Injection | 95% CI | P Value | Cohen’s d |
|---|---|---|---|---|---|
| Capsazepine (10% ethanol/10% Tween 80; n = 8) | |||||
| MAP | 27 ± 13 | 25 ± 16 | [−9 5] | P = 0.29 | d = 0.18 |
| HR | 9 ± 9 | 7 ± 6 | [−6 2] | P = 0.25 | d = 0.40 |
| BPI | 417 ± 247 | 328 ± 208 | [−367 189] | P = 0.47 | d = 0.28 |
| Capsazepine (0.5% DMSO; n = 9) | |||||
| MAP | 38 ± 12 | 41 ± 15 | [−4 9] | P = 0.40 | d = 0.30 |
| HR | 9 ± 8 | 8 ± 8 | [−2 2] | P = 0.93 | d = 0.24 |
| BPI | 589 ± 378 | 397 ± 350 | [−905 −273] | P = 0.003 | d = 0.95 |
| Ruthenium red (n = 4) | |||||
| MAP | 47 ± 8 | 44 ± 19 | [−36 29] | P = 0.74 | d = 0.17 |
| HR | 4 ± 1 | 4 ± 2 | [−5 5] | P = 0.22 | d = 0.19 |
| BPI | 540 ± 174 | 468 ± 176 | [−274 130] | P = 0.22 | d = 0.42 |
| IRTX (n = 8) | |||||
| MAP | 39 ± 15 | 37 ± 17 | [−9 7] | P = 0.48 | d = 0.18 |
| HR | 5 ± 4 | 4 ± 3 | [−3 1] | P = 0.23 | d = 0.39 |
| BPI | 555 ± 220 | 549 ± 233 | [−113 103] | P = 0.47 | d = 0.50 |
Results are presented as means ± SD increase in mean arterial blood pressure (MAP, in mmHg), heart rate (HR, beats/min), or blood pressure index (BPI; measured as mmHg·s), evoked by passive stretch; n = sample size. Ninety-five percent confidence interval (CI) is presented as the lower and upper boundary of the interval containing the true value of the effect of the transient receptor potential vanilloid-1 (TRPV1) antagonist. IRTX, iodoresiniferatoxin.
DISCUSSION
The primary goal of our experiments was to shed light on the controversy over the role played by TRPV1 in evoking the exercise pressor reflex arising from static contraction of the triceps surae muscles. We found that three structurally different TRPV1 antagonists, namely, capsazepine, IRTX, and ruthenium red, when dissolved in either ethanol or saline, had no effect on the exercise pressor reflex, despite the fact that each of these antagonists significantly attenuated the reflex pressor responses to capsaicin injection into the arterial supply of the hindlimb muscles. Although we found that capsazepine, dissolved in DMSO, did attenuate the exercise pressor reflex, we replicated this finding when we injected DMSO in the same concentration and volume as that used to place capsazepine into solution. Our results thus showed that TRPV1 played no role in the pressor response evoked by static contraction in healthy rats.
Our finding that blockade of TRPV1 played no role in evoking the exercise pressor reflex agreed with studies in cats or rats with chronic femoral artery ligation, showing that IRTX had no effect on this reflex (22, 45). This finding, however, contrasts with that of Smith et al. (38) and Mizuno et al. (29), who found that injection of capsazepine, IRTX, and ruthenium red into the arterial supply of the hindlimb muscles of decerebrated rats with freely perfused hindlimb muscles attenuated the exercise pressor reflex. In part, this contrast might be explained by the solvents used to place capsazepine and IRTX into solution. This possibility, however, is difficult to assess because the solvent used to dissolve these antagonists was not described by Smith et al. (38) and Mizuno et al. (29).
Ruthenium red was the third TRPV1 antagonist used in our experiments. As ruthenium red was dissolved in saline, our result, showing that the exercise pressor reflex was not attenuated following ruthenium red injection, cannot be explained by the solvent in which the drug was dissolved. Alternatively, we found that ruthenium red markedly decreased the ability of the triceps surae muscles to produce force, an effect that was most likely caused by the drug acting as a ryanodine channel inhibitor (48) that, in turn, decreased the ability of the muscle fibers to release Ca2+. With the knowledge beforehand that ruthenium red would decrease the peak force of contractions, we evoked a submaximal contraction before injecting the drug. Most of the time, this procedure allowed us, after injecting ruthenium red, to increase the current intensity to match the peak tension of the initial contraction. Despite this precaution, we were still not able to match the tension-time index, suggesting that in addition to ruthenium red decreasing the ability of the muscle to produce force, it decreased the ability of the muscle to sustain force. Smith et al. (38) did not report the tension-time index. Consequently, our data reveal the possibility that the decrease in blood pressure, resulting from the injection of ruthenium red in their study, was the result of a decrease in the tension-time index.
Studies using analgesic balms containing capsaicin, topically applied to the skin overlying limb muscles, have been reported to attenuate the exercise pressor reflex in both animals (16, 30) and humans (7, 46). At first glance, these studies might be viewed as complementary to the findings of Smith et al. (38) and Mizuno et al. (29). Likewise, some investigators may interpret this finding as evidence that TRPV1 plays a role in evoking the exercise pressor reflex. However, it is important to realize that capsaicin is an explosive stimulus to group IV muscle afferents (19) and that its prolonged application may prevent these thin fiber afferents from evoking the reflex because they have been depleted of a neurotransmitter. In addition, this interpretation does not address the issue of a vehicle-induced effect, which is just as applicable in these experiments as it was in the experiments described above in which TRPV1 antagonists were reported to attenuate the exercise pressor reflex.
The secondary goal of our experiments was to determine the selectivity of capsazepine for TRPV1. We found that capsazepine, dissolved in ethanol and Tween 80, attenuated the pressor reflex evoked by injection of bradykinin and α-β-methylene ATP into the arterial supply of the hindlimb muscles. This finding was surprising because there is no evidence that either bradykinin or α-β-methylene ATP directly stimulates TRPV1. Instead, one can argue that bradykinin and α-β-methylene ATP stimulate TRPV1 by an indirect action (34, 37, 44). For example, activation of bradykinin 2 receptors has been found to release a lipoxygenase byproduct that, in turn, activated TRPV1 (37). If this were the case in our experiments, then IRTX should have also decreased the pressor response to bradykinin injection. It did not, which suggests that the effect of capsazepine on the pressor response to bradykinin injection was not the result of TRPV1 antagonism. We speculate that the decrease in the pressor responses to bradykinin and α-β-methylene ATP injections was the result of an off-target action of capsazepine, of which the blocking of voltage-gated calcium channels is one (10). This off-target action might nevertheless also be expected to decrease the pressor response to diprotonated phosphate injection, but it did not, and we can offer no explanation as to why this is the case.
Study Limitation
Although our findings strongly suggest that TRPV1 does not play a role in evoking the exercise pressor reflex, the possibility exists that a compensatory mechanism could have hidden the antagonism of TRPV1 in our experiments. For example, Stone et al. (42) showed in healthy rats that the exercise pressor reflex was attenuated only if a combination of three antagonists was administered; in contrast, the injection of each antagonist separately had no effect. Stone et al. (42) concluded that the exercise pressor reflex was determined by redundant mechanisms that compensated one for the other. Nevertheless, given the unphysiological thresholds required for the activation of the TRPV1 (i.e., pH < 5.5, heat > 43°C) and given the absence of an attenuation of the exercise pressor reflex following injection of capsazepine, IRTX, or ruthenium red, we think it is unlikely that static contraction activated TRPV1 in decerebrated rats with freely perfused hindlimb muscles.
Perspectives and Significance
Our results suggest the following: 1) TRPV1 does not play a role in evoking the exercise pressor reflex in healthy rats; 2) capsazepine and ruthenium red should not be used to study the role played by TRPV1 in evoking the exercise pressor because of their “off-target” effects; and 3) DMSO, in a concentration of 0.5% or greater, should be avoided as a vehicle to dissolve TRPV1 antagonists to study the exercise pressor reflex.
GRANTS
Funding for this study was provided by the National Institute of Arthritis and Muskuloskeletal and Skin Diseases (Grant R01-AR-059397) and National Heart, Lung, and Blood Institute (Grant P01-HL-134609).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.P.D. and M.P.K. conceived and designed research; G.P.D. performed experiments; G.P.D. analyzed data; G.P.D., J.A.E., J.S.K., and M.P.K. interpreted results of experiments; G.P.D. prepared figures; G.P.D. drafted manuscript; G.P.D., J.A.E., J.S.K., and M.P.K. edited and revised manuscript; G.P.D., J.A.E., J.S.K., and M.P.K. approved final version of manuscript.
REFERENCES
- 1.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev 57: 427–450, 2005. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic, 1977. [Google Scholar]
- 4.Cortright DN, Crandall M, Sanchez JF, Zou T, Krause JE, White G. The tissue distribution and functional characterization of human VR1. Biochem Biophys Res Commun 281: 1183–1189, 2001. doi: 10.1006/bbrc.2001.4482. [DOI] [PubMed] [Google Scholar]
- 5.Craig ADB, Mense S. The distribution of afferent fibers from the gastrocnemius-soleus muscle in the dorsal horn of the cat, as revealed by the transport of horseradish peroxidase. Neurosci Lett 41: 233–238, 1983. doi: 10.1016/0304-3940(83)90456-1. [DOI] [PubMed] [Google Scholar]
- 6.Curran-Everett D. Explorations in statistics: confidence intervals. Adv Physiol Educ 33: 87–90, 2009. doi: 10.1152/advan.00006.2009. [DOI] [PubMed] [Google Scholar]
- 7.Dawson AN, Walser B, Jafarzadeh M, Stebbins CL. Topical analgesics and blood pressure during static contraction in humans. Med Sci Sports Exerc 36: 632–638, 2004. doi: 10.1249/01.MSS.0000121949.43010.F4. [DOI] [PubMed] [Google Scholar]
- 8.Di Marzo V, Blumberg PM, Szallasi A. Endovanilloid signaling in pain. Curr Opin Neurobiol 12: 372–379, 2002. doi: 10.1016/S0959-4388(02)00340-9. [DOI] [PubMed] [Google Scholar]
- 9.Dobson KL, Harris J. A detailed surgical method for mechanical decerebration of the rat. Exp Physiol 97: 693–698, 2012. doi: 10.1113/expphysiol.2012.064840. [DOI] [PubMed] [Google Scholar]
- 10.Docherty RJ, Yeats JC, Piper AS. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br J Pharmacol 121: 1461–1467, 1997. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Febbraio MA, Snow RJ, Stathis CG, Hargreaves M, Carey MF. Effect of heat stress on muscle energy metabolism during exercise. J Appl Physiol (1985) 77: 2827–2831, 1994. doi: 10.1152/jappl.1994.77.6.2827. [DOI] [PubMed] [Google Scholar]
- 13.Gao Z, Henig O, Kehoe V, Sinoway LI, Li J. Vanilloid type 1 receptor and the acid-sensing ion channel mediate acid phosphate activation of muscle afferent nerves in rats. J Appl Physiol (1985) 100: 421–426, 2006. doi: 10.1152/japplphysiol.00659.2005. [DOI] [PubMed] [Google Scholar]
- 14.Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol (1985) 96: 1166–1169, 2004. doi: 10.1152/japplphysiol.01020.2003. [DOI] [PubMed] [Google Scholar]
- 15.Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol (1985) 94: 1437–1445, 2003. doi: 10.1152/japplphysiol.01011.2002. [DOI] [PubMed] [Google Scholar]
- 16.Ichiyama RM, Ragan BG, Bell GW, Iwamoto GA. Effects of topical analgesics on the pressor response evoked by muscle afferents. Med Sci Sports Exerc 34: 1440–1445, 2002. doi: 10.1097/00005768-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto GA, Waldrop TG, Kaufman MP, Botterman BR, Rybicki KJ, Mitchell JH. Pressor reflex evoked by muscular contraction: contributions by neuraxis levels. J Appl Physiol (1985) 59: 459–467, 1985. doi: 10.1152/jappl.1985.59.2.459. [DOI] [PubMed] [Google Scholar]
- 18.Jordt S-E, Bautista DM, Chuang H-H, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res 50: 133–139, 1982. doi: 10.1161/01.RES.50.1.133. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- 22.Kindig AE, Heller TB, Kaufman MP. VR-1 receptor blockade attenuates the pressor response to capsaicin but has no effect on the pressor response to contraction in cats. Am J Physiol Heart Circ Physiol 288: H1867–H1873, 2005. doi: 10.1152/ajpheart.00735.2004. [DOI] [PubMed] [Google Scholar]
- 23.Koba S, Hayes SG, Sinoway LI. Transient receptor potential A1 channel contributes to activation of the muscle reflex. Am J Physiol Heart Circ Physiol 300: H201–H213, 2011. doi: 10.1152/ajpheart.00547.2009. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol 283: H2636–H2643, 2002. doi: 10.1152/ajpheart.00395.2002. [DOI] [PubMed] [Google Scholar]
- 25.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mense S, Craig ADB Jr. Spinal and supraspinal terminations of primary afferent fibers from the gastrocnemius-soleus muscle in the cat. Neuroscience 26: 1023–1035, 1988. doi: 10.1016/0306-4522(88)90117-0. [DOI] [PubMed] [Google Scholar]
- 27.Mense S, Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol 363: 403–417, 1985. doi: 10.1113/jphysiol.1985.sp015718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol 342: 383–397, 1983. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589: 6191–6204, 2011. doi: 10.1113/jphysiol.2011.214429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson AJ, Ragan BG, Bell GW, Ichiyama RM, Iwamoto GA. Capsaicin-based analgesic balm decreases pressor responses evoked by muscle afferents. Med Sci Sports Exerc 36: 444–450, 2004. doi: 10.1249/01.MSS.0000117163.67344.7B. [DOI] [PubMed] [Google Scholar]
- 31.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 32.O’Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol 276: H1399–H1403, 1999. doi: 10.1152/ajpheart.1999.276.4.H1399. [DOI] [PubMed] [Google Scholar]
- 33.Paintal AS. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol 152: 250–270, 1960. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan H-L, Chen S-R. Sensing tissue ischemia: another new function for capsaicin receptors? Circulation 110: 1826–1831, 2004. doi: 10.1161/01.CIR.0000142618.20278.7A. [DOI] [PubMed] [Google Scholar]
- 35.Pan HL, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol (1985) 75: 2061–2068, 1993. doi: 10.1152/jappl.1993.75.5.2061. [DOI] [PubMed] [Google Scholar]
- 36.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962. doi: 10.1161/01.RES.11.3.370. [DOI] [PubMed] [Google Scholar]
- 37.Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA 99: 10150–10155, 2002. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH, Garry MG. The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol 588: 1179–1189, 2010. doi: 10.1113/jphysiol.2009.184952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. doi: 10.1113/jphysiol.2001.012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stacey MJ. Free nerve endings in skeletal muscle of the cat. J Anat 105: 231–254, 1969. [PMC free article] [PubMed] [Google Scholar]
- 41.Stebbins CL, Longhurst JC. Bradykinin-induced chemoreflexes from skeletal muscle: implications for the exercise reflex. J Appl Physiol (1985) 59: 56–63, 1985. doi: 10.1152/jappl.1985.59.1.56. [DOI] [PubMed] [Google Scholar]
- 42.Stone AJ, Copp SW, Kim JS, Kaufman MP. Combined, but not individual, blockade of ASIC3, P2X, and EP4 receptors attenuates the exercise pressor reflex in rats with freely perfused hindlimb muscles. J Appl Physiol (1985) 119: 1330–1336, 2015. doi: 10.1152/japplphysiol.00630.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Street D, Bangsbo J, Juel C. Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J Physiol 537: 993–998, 2001. doi: 10.1113/jphysiol.2001.012954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol 88: 544–548, 2002. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- 45.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010. doi: 10.1152/ajpheart.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vianna LC, Fernandes IA, Barbosa TC, Teixeira AL, Nóbrega ACL. Capsaicin-based analgesic balm attenuates the skeletal muscle metaboreflex in healthy humans. J Appl Physiol (1985) 125: 362–368, 2018. doi: 10.1152/japplphysiol.00038.2018. [DOI] [PubMed] [Google Scholar]
- 46a.von Düring M, Andres KH. Topography and ultrastructure of group III and IV nerve terminals of the cat’s gastrocnemius-soleus muscle. In: The Primary Afferent Neuron, edited by Zenker W and Neuhuber WL. Boston, MA: Springer, 1990, p. 35–41. [Google Scholar]
- 47.Webb-Peploe MM, Brender D, Shepherd JT. Vascular responses to stimulation of receptors in muscle by capsaicin. Am J Physiol 222: 189–195, 1972. doi: 10.1152/ajplegacy.1972.222.1.189. [DOI] [PubMed] [Google Scholar]
- 48.Xu L, Tripathy A, Pasek DA, Meissner G. Ruthenium red modifies the cardiac and skeletal muscle Ca(2+) release channels (ryanodine receptors) by multiple mechanisms. J Biol Chem 274: 32680–32691, 1999. doi: 10.1074/jbc.274.46.32680. [DOI] [PubMed] [Google Scholar]