Abstract
Idiopathic pulmonary fibrosis (IPF) is the most common and devastating of the interstitial lung diseases. Epithelial dysfunction is thought to play a prominent role in disease pathology, and we sought to characterize secreted signals that may contribute to disease pathology. Transcriptional profiling of senescent type II alveolar epithelial cells from mice with epithelial-specific telomere dysfunction identified the transforming growth factor-β family member, growth and differentiation factor 15 (Gdf15), as the most significantly upregulated secreted protein. Gdf15 expression is induced in response to telomere dysfunction and bleomycin challenge in mice. Gdf15 mRNA is expressed by lung epithelial cells, and protein can be detected in peripheral blood and bronchoalveolar lavage following bleomycin challenge in mice. In patients with IPF, GDF15 mRNA expression in lung tissue is significantly increased and correlates with pulmonary function. Single-cell RNA sequencing of human lungs identifies epithelial cells as the primary source of GDF15, and circulating concentrations of GDF15 are markedly elevated and correlate with disease severity and survival in multiple independent cohorts. Our findings suggest that GDF15 is an epithelial-derived secreted protein that may be a useful biomarker of epithelial stress and identifies IPF patients with poor outcomes.
Keywords: aging, MIC-1, NAG-1, SASP
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is the most common and devastating of the idiopathic interstitial pneumonias (47). As its name suggests, the full etiology of IPF is not fully understood; however, strides have been made in recent years, largely driven by genetic association and familial studies that have focused interest on epithelial dysfunction as a primary driver of disease (12, 41). Mutations in surfactant genes, including SFTPC, SFTPA1, and SFTPA2, cause type II alveolar epithelial cell (AEC2)-specific injury and are responsible for 1–3% of familial pulmonary fibrosis cases (41). Mutations in genes related to telomere biology, responsible for ~30% of familial (7, 8, 13, 18, 50) and ~10% of sporadic cases (23, 46), likely limit the proliferative capacity of the epithelium and increase epithelial senescence (3, 12). The downstream signals from epithelial dysfunction that lead to organ remodeling and failure remain poorly understood.
Telomeres are DNA protein caps on the ends of chromosomes that function to maintain genome stability. Telomeres shorten each time a cell divides and limit the proliferative capacity of most somatic cells (28). Critically short telomeres trigger apoptosis or senescence, depending on the specific cellular context (20). Numerous transcriptional and phenotypic changes occur in senescent cells, including changes to the repertoire of proteins secreted, which has been termed the senescence-associated secretory phenotype (SASP) (17). While the function of all SASP-associated proteins is not known, several components are thought to promote inflammation and wound healing (19, 21).
Growth differentiation factor 15 [GDF15; also known as NAG-1 (NSAID activated gene-1) and MIC-1 (macrophage inhibitory cytokine-1)] is a divergent member of the transforming growth factor (TGF)-β family of secreted proteins (15, 54). GDF15 has been previously reported to be a stress-induced gene that is upregulated in the context of several disease states, including heart, kidney, and liver disease (26, 33, 36, 38, 39, 42), and in response to exogenous injury (29, 58, 62). In the context of lung disease, GDF15 levels have been associated with an increased frequency of exacerbations, subclinical cardiovascular disease, declining lung function, and poor outcomes in chronic obstructive pulmonary disease (25, 31, 35, 40). GDF15 levels are elevated in systemic scleroderma patients with lung involvement and upregulated in response to bleomycin exposure in mice (37). However, Gdf15 is dispensable for bleomycin-induced pulmonary fibrosis in mice (37). Exogenous GDF15 is sufficient to cause weight loss in mice, and GDF15-neutralizing antibodies prevent tumor-associated weight loss (32). While GDF15 has been reported to signal through the canonical TGF-β receptors, TGF-β receptors I and II (14, 32), recent reports have identified a novel high-affinity receptor glial cell-derived neurotrophic factor family receptor-α life (GFRAL) (24, 30, 44, 61). The full tissue-specific distribution of GFRAL is not known, but evidence to date suggests that its expression is limited to the brain stem (24, 30, 61).
While searching for signaling molecules that mediate inflammation in response to telomere dysfunction, we identified Gdf15 as an epithelial-derived secreted factor. Gdf15 is expressed in response to prosenescence and profibrotic challenges in mice. In humans, we detected markedly high levels of GDF15 expression in blood and lung tissue from IPF patients compared with controls, with the highest levels identifying individuals with severe disease and poor outcomes. Our data suggest that GDF15 is a novel epithelial “stress signal” and biomarker of IPF that identifies patients with severe, progressing disease.
METHODS
Human subjects.
All studies were approved by the relevant Institutional Review Board and the Committee for Oversight of Research and Clinical Training Involving Decedents at the University of Pittsburgh and Yale University. All subjects provided written, informed consent before enrollment in the research study. IPF subjects were recruited from the Simmons Center for Interstitial Lung Diseases at the University of Pittsburgh Medical Center. Clinical, physiologic, and high-resolution computed tomography studies of these patients supported the diagnosis of IPF. Patients fulfilled the criteria of the American Thoracic Society and European Respiratory Society for the diagnosis of IPF (9, 47). Patients with known causes of interstitial lung disease were excluded. Control patients consisted of unrelated healthy subjects, randomly recruited from the University of Pittsburgh Medical Center, and had no self-reported advanced lung diseases. Yale participants were recruited from the Yale ILD Center of Excellence and the criteria for IPF that were current at the time of enrollment (10, 47). Healthy, age-matched controls without known inflammatory or fibrotic disease were recruited from the greater New Haven community, as previously described (49). Explanted lungs were obtained from subjects undergoing lung transplantation at the University of Pittsburgh Medical Center or from The Center for Organ Recovery & Education (CORE).
Animal studies.
All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Mice were housed at the University of Pittsburgh and given ad libitum access to food and water. Adult (8–12 wk of age) mice were treated with bleomycin (1 U/kg) via intratracheal instillation. Tracheal intubation for each mouse was confirmed by observing the oscillation of a water bubble attached to the tracheal cannula due to tidal breathing. Bleomycin was diluted in sterile saline to 50 μL and pipetted into the tracheal cannula until it was completely aspirated. Plasma, bronchoalveolar lavage (BAL), and lungs were collected on days 3, 7, 14, and 21 following bleomycin or saline administration, as described previously (5). Lung injury, fibrosis (at days 14 and 21), or lack thereof was confirmed by histologic analysis of the left lung from each mouse. GDF15 instillation experiments were carried out similar to bleomycin experiments. Recombinant murine GDF15 (R&D Systems) was resuspended in sterile saline and instilled directly into the trachea via intubation with an 18-gauge angiocatheter. Animals were euthanized, and BAL was collected by flushing the lung three times with 0.75 mL of saline 24, 48, and 96 h after GDF15 administration.
Gene expression and immunoblotting.
Gene expression data and clinical attributes were obtained as part of the Lung Genomics Research Consortium (www.lung-genomics.org). We performed a pairwise comparison of control and IPF samples for GDF15 expression from microarray data. Secretome analysis of senescent murine AEC2s was carried out on previously published microarray profiling (GEO GSE56892) (3). Briefly, lineage labeled AEC2s isolated from Trf2Fl/+RosamTmG/mTmGSftpc-CreER and Trf2Fl/FlRosamTmG/mTmGSftpc-CreER mice following tamoxifen treatment by fluorescent-activated cell sorting. Purity of sorted cells was shown to be >90% by immunostaining for prosurfactant protein C in sorted cells that had been spun onto slides. Significantly upregulated genes were compared with a curated list of secreted murine proteins from the MetazSecKB database (bioinformatics.ysu.edu/secretomes). For validation expression studies on murine AEC2s, RNA was purified from cells isolated from mice with and without telomere dysfunction, as described above. For tissue studies, RNA was purified from fat, small intestine, heart, kidney, liver, lung, muscle, spleen, and thymus (n = 3, for all tissues). All tissues were homogenized using a Bullet Blender (Next Advance), and RNA was extracted using Trizol (Invitrogen). Medulla RNA was purchased from Zyagen. cDNA was generated using iScript cDNA synthesis kit (Bio-Rad), according to the manufacturer’s protocol. Quantitative PCR was performed on a CFX96 real-time instrument (BioRad) using Sybergreen (Bio-Rad) and primers listed in Supplemental Table S2 (All supplemental material is available at https://doi.org/10.6084/m9.figshare.8320709.). Expression changes were normalized to Hprt and B2m transcripts. Lung tissues were obtained from excess pathologic tissue after lung transplantation, as described (22). Control lungs (donor) were donated organs not suitable for transplantation from the Center for Organ Recovery & Education (CORE). Tissues were prepared for Western blotting by homogenizing in RIPA buffer containing Halt protease inhibitors (Thermo Fisher Scientific) in a bullet blender. Lysates were separated on 4–15% SDS-PAGE gels and transferred to PVDF membranes (Bio-Rad). Membranes were blocked and incubated with primary antibodies at 4°C overnight from the following sources: GDF15 (G-5, Santa Cruz Biotechnology; recognizes proGDF15), and GAPDH (FL-335, Santa Cruz Biotechnology).
Immunohistochemistry.
Biopsies were obtained from explanted lungs, fixed in formalin, and embedded in paraffin. Sections were deparaffinized and stained following standard procedures. Slides were stained with GDF15 antibodies (G-5, Santa Cruz Biotechnology), developed with 3, 3′-diaminobenzidine, and counterstained with hematoxylin QS, according to the manufacturer’s protocol (Vector Laboratories). Slides were scanned at HistoWiz. Differential counts were performed on BAL cells that had been spun onto slides and stained with Kwik-Diff kit, according to the manufacturer’s protocol (Thermo Fisher Scientific).
Luminex and ELISA assays.
Plasma samples from participating subjects were used for the Bioplex and ELISA analysis. Plasma samples were prepared from blood samples immediately after sample collection and stored at −80°C. For the first cohort, GDF15 levels were analyzed using a custom multiplex Luminex assay (R&D Systems). For the second and third cohorts, GDF15 levels were analyzed using a human GDF15 Quantikine ELISA Kit (R&D Systems), according to the manufacturer’s protocol.
Single-cell RNA sequencing analysis.
Single-cell RNA sequencing data were downloaded from GEO (GSE128033) (43). Three donor and three IPF samples were used in our analysis [see Morse et al. (43) for a full description of the explant samples]. Data cleaning, normalization, clustering, and cluster identification were carried out exactly as described. Violin plots were generated using the Seurat package in R.
Telomere length measurement.
Peripheral blood mononuclear cells were isolated from patients using Ficoll-Paque density centrifugation. Telomere length was measured using flow cytometry, combined with fluorescence in situ hybridization (flowFISH), at Johns Hopkins University, as described previously (6).
RNA in situ hybridization.
Probes specific for the indicated genes were purchased from Advanced Cell Diagnostics (ACD; RNAscope). Tissues were processed according to the manufacturer’s protocol. Multiplex fluorescents images were acquired on an Olympus Fluorview 1000 confocal microscope in the Center for Biologic Imaging at the University of Pittsburgh. Negative control probes were purchased from ACD. Bright-field images were acquired on a Nikon Eclipse 55i upright microscope.
Statistical analysis.
Natural log transformation of GDF15 was compared between groups using Welch’s t test. We used Pearson correlation to assess the correlation between baseline percent forced vital capacity (FVC%) and percent diffusing capacity for carbon monoxide (DlCO%) scores with GDF15 concentration. Kaplan-Meier and log-rank tests were used to compare the survival function (of time to mortality or first lung transplant) between two groups of patients based on the GDF15 level and were age adjusted. We used mixed-effect models with random coefficients to assess the interaction between baseline GDF15 and follow-up duration (in years). This interaction represents the effect of baseline GDF15 in annualized rate of FVC% and DlCO% change. All analyses were performed in Stata 15.0 (StataCorp, College Station, TX).
RESULTS
Gdf15 is upregulated in AEC2s in response to telomere dysfunction.
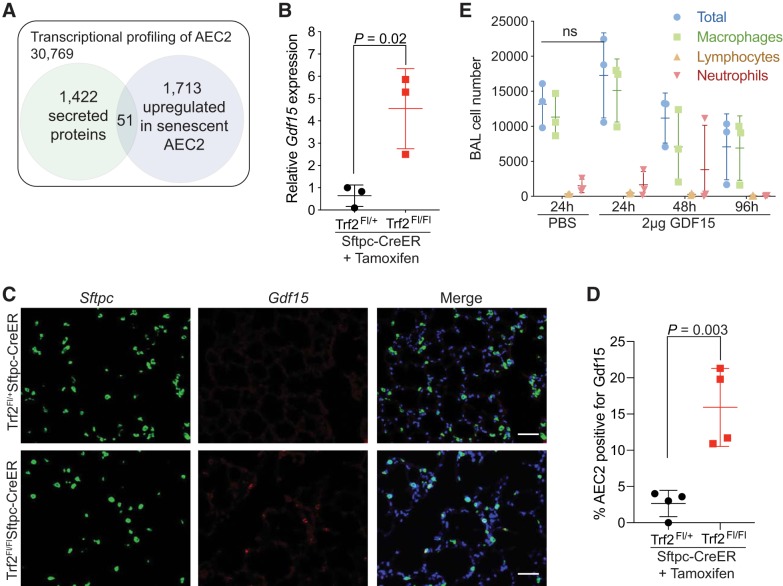
We previously developed an animal model that permitted the induction of telomere dysfunction and cellular senescence specifically in AEC2s (3). Animals with AEC2-specific telomere dysfunction developed pulmonary inflammation 14–21 days following induction of telomere dysfunction. To identify AEC2-derived signals that may be responsible for recruiting inflammatory cells, we analyzed transcriptional profiling data from purified AEC2s, focusing on 1,422 curated secreted proteins from the MetazSecKB database (http://bioinformatics.ysu.edu) that could be mapped to our data. We identified 51 transcripts that encoded secreted proteins that were upregulated in response to telomere dysfunction-mediated senescence (Table 1 and Supplemental Table S1). The top transcript from this analysis mapped to Gdf15, and we confirmed its differential expression by quantitative PCR in purified AEC2s (Fig. 1, A and B). GDF15 was strongly expressed by airway cells by RNA in situ hybridization (RNA-ISH) and could be identified in AEC2s following induction of telomere dysfunction in Trf2Fl/FlSftpc-CreER mice (Fig. 1, C and D, and Supplemental Fig. S1A). GDF15 has been identified in several disease contexts and has been reported to both support and inhibit inflammatory cell recruitment (14, 34, 55). We tested if GDF15 was sufficient to induce inflammation by instilling 2 µg of recombinant GDF15 via intratracheal instillation into the lungs and examined bronchoalveolar lavage (BAL) fluid 24, 48, and 96 h later. We did not observe any significant changes in the total number of specific inflammatory cell types in BAL from GDF15-instilled mice, despite the high levels of GDF15 (Fig. 1E). Together, these data support that Gdf15 is upregulated in response to telomere dysfunction, but alone it is not sufficient to cause inflammation in the lung.
Table 1.
Genes encoding secreted proteins that are transcriptionally upregulated in senescent murine type II alveolar epithelial cells
| Gene | Gene Name | Fold Change | P Value |
|---|---|---|---|
| Gdf15 | Growth differentiation factor 15 | 2.31 | 0.001 |
| Tgfbi | Transforming growth factor, β induced | 1.59 | 0.011 |
| Ssc5d | RIKEN cDNA A430110N23 gene | 1.47 | 0.041 |
| Tff2 | Trefoil factor 2 (spasmolytic protein 1) | 1.46 | 0.041 |
| Cma1 | Chymase 1, mast cell | 1.40 | 0.012 |
| Spon1 | Spondin 1, (f-spondin) extracellular matrix protein | 1.40 | 0.023 |
| Ncan | Neurocan | 1.39 | 0.016 |
| Fetub | Fetuin-β | 1.39 | 0.014 |
| Mif | Macrophage migration inhibitory factor | 1.37 | 0.008 |
| Fibin | Fin bud initiation factor homolog (zebrafish) | 1.35 | 0.040 |
| Ang2 | Angiogenin, ribonuclease A family, member 2 | 1.33 | 0.048 |
| Ephx3 | Epoxide hydrolase 3 | 1.31 | 0.032 |
| Il17b | Interleukin 17B | 1.28 | 0.046 |
| SlpI | Secretory leukocyte peptidase inhibitor | 1.26 | 0.012 |
| Il17c | Interleukin 17C | 1.26 | 0.050 |
Fold change is relative change in expression calculated by dividing the expression in senescent type II alveolar epithelial cells (AEC2) compared with control AEC2.
Fig. 1.
Growth and differentiation factor 15 (Gdf15) is upregulated in response to telomere dysfunction. A: schematic of our analysis strategy for identifying secreted proteins from transcriptional profiling data. Transcriptional data were obtained as described previously (3). Differentially upregulated genes that were also annotated as secreted proteins are identified. B: quantitative real-time PCR for Gdf15 from sorted type II alveolar epithelial cells (AEC2s) from Trf2Fl/+Sftpc-CreER (control) and Trf2Fl/FlSftpc-CreER (senescent) AEC2s. Cells were sorted 10 days after treatment with tamoxifen based on green fluorescent protein expression from mTmG reporter allele (3). Gene expression was normalized to Hprt and B2m. C: representative images of RNA in situ hybridization staining for Gdf15 from mouse lungs 6 wk after treatment with tamoxifen showing AEC2-specific expression of Gdf15. AEC2s were identified by expression of the Sftpc transcript. Scale bar is 50 µM. D: quantitation of the colocalization of Gdf15 and Sftpc transcripts (n = 4 mice per group). E: bronchoalveolar lavage cell counts from mice treated with 2 µg of GDF15. GDF15 or sterile saline was instilled directly into the lungs and the bronchoalveolar lavage was collected thereafter at the indicated times. Total viable cells were quantitated by trypan blue staining, and a differential count was performed on >100 cells. Values are means and standard deviation (SD). Student’s t test (two-tailed) was used to compare groups.
GDF15 is expressed in response to bleomycin.
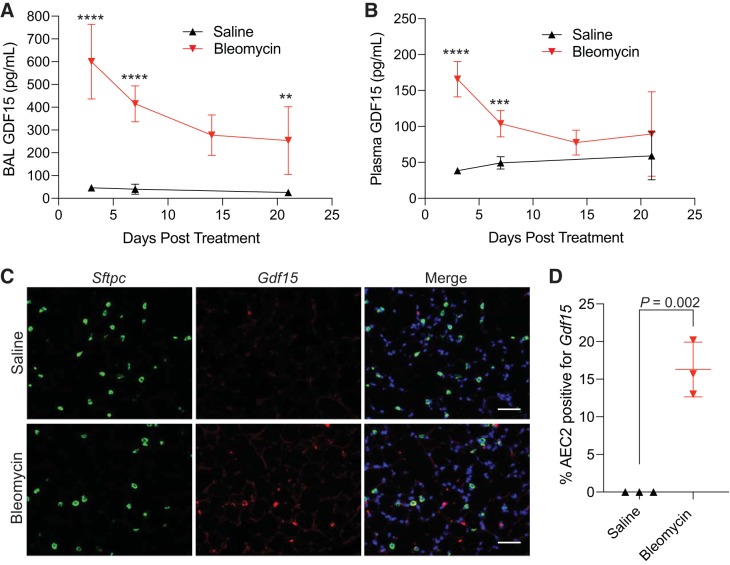
We next explored if Gdf15 upregulation was specific to telomere dysfunction or if additional tissue stressors would induce its expression, as has been reported (53, 58, 59, 62). We administered bleomycin, a widely used pulmonary toxin, via intratracheal instillation and examined the lungs, BAL, and plasma at several time points. BAL GDF15 protein levels were highest 3 days after bleomycin administration and remained significantly higher than in saline-treated animals (Fig. 2A) at all time points examined. Similarly, plasma levels of GDF15 were elevated in response to bleomycin, but returned to baseline levels after 21 days (Fig. 2B). Gdf15-expressing AEC2s could be identified in mouse lungs 3 days after treatment with bleomycin, in addition to a large number of non-AEC2s, suggesting that additional cell types express Gdf15 (Fig. 2, C and D). These results are consistent with previous results, demonstrating that Gdf15 expression is increased in whole lung lysates following treatment with bleomycin (37). These findings suggest that Gdf15 expression is induced following bleomycin treatment, in addition to telomere dysfunction.
Fig. 2.
Bleomycin induces expression of growth and differentiation factor 15 (GDF15). A and B: quantitative ELISA of GDF15 levels in bronchoalveolar lavage (BAL; A) and plasma (B) from mice treated with intratracheal bleomycin or saline. Mice were treated on day 0, and groups of 6–7 mice (at least 3 male and 3 female at each time point) were harvested at the indicated time points. C: representative RNA in situ hybridization of day 3 lungs showing alveolar expression of Gdf15. D: quantitation of colocalization of Sftpc and Gdf15 transcripts in RNA in situ hybridization staining. Values are means and SD. Student’s t test (two-tailed) was used to compare groups. ****P < 0.0001, ***P < 0.001, and **P < 0.01.
GDF15 is upregulated in IPF and is expressed by epithelial cells.
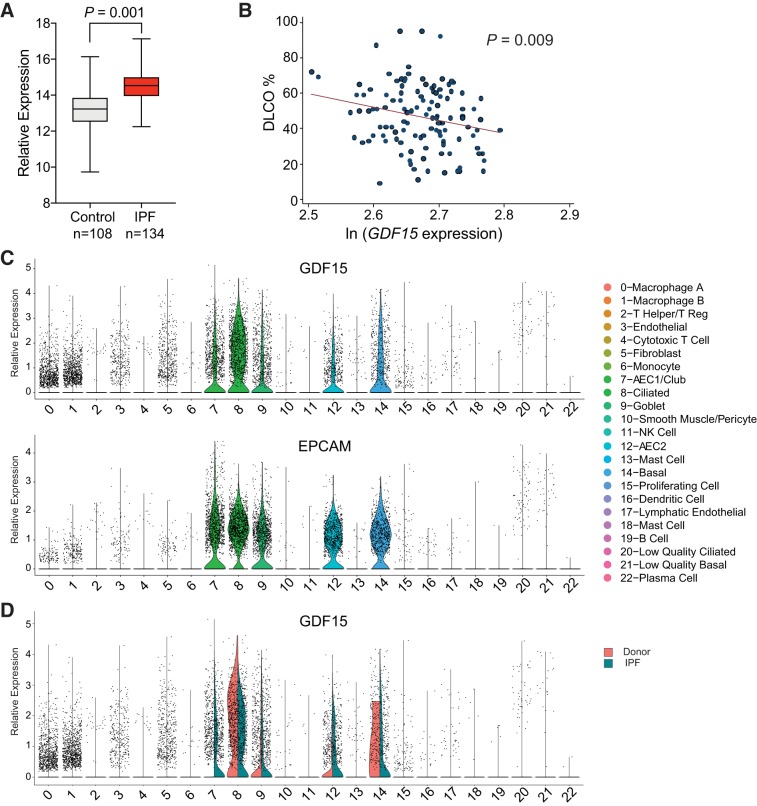
Telomere dysfunction and bleomycin are both known to induce pulmonary fibrosis in humans (1, 11, 13). We sought to determine whether GDF15 was upregulated in the context of IPF in clinical samples. To accomplish this, we examined gene expression data from the Lung Genomics Research Consortium (LGRC; www.lung-genomics.org), consisting of 134 IPF samples and 108 age-matched controls (51). GDF15 expression was significantly higher in IPF lung homogenates compared with controls (P = 0.001; Fig. 3A) in a pairwise comparison. Furthermore, GDF15 gene expression was inversely related to lung function, as measured by the DlCO in IPF patients (Fig. 3B). Because the LGRC data are derived from whole lung homogenates, the precise cell-type responsible for GDF15 expression is unclear from this analysis due to the heterogeneity of the biopsies. To address this question, we reanalyzed single-cell RNA sequencing (scRNA-Seq) data from three marginal donor lungs that were declined for transplantation (donor hereafter) and three explanted lungs from patients with IPF (43). GDF15 was expressed primarily by epithelial cells, marked by EPCAM (epithelial cell adhesion molecule), in addition to a subset of macrophages (Fig. 3C). In the context of IPF, a greater proportion of AEC1, AEC2, and club cells expressed GDF15. However, given the decreased abundance of cells expressing markers of AEC1 and AEC2 in IPF, club, ciliated, and basal cells are likely the most significant source of GDF15 (Fig. 3D) (43). Similar results were found in scRNA-seq data sets from Reyfman et al. (48) and Xu et al. (60). Together, our gene expression analysis demonstrates that GDF15 is expressed primarily by epithelial cells, its expression is increased in IPF, and that its expression is associated with impaired gas exchange.
Fig. 3.
Growth and differentiation factor 15 (GDF15) is upregulated in idiopathic pulmonary fibrosis (IPF) and expressed by epithelial cells. A: box-and-whisker plot of GDF15 expression data from the Lung Genomics Research Consortium (LGRC). Horizontal line marks the median value, box boundaries show the upper and lower quartiles, and whiskers show high and low values. Relative expression was calculated from normalized hybridization signal from microarray data. Welch’s t test, two tailed, was used to compare groups. B: correlation between natural log of GDF15 expression from IPF patients and carbon monoxide diffusion capacity (DlCO) in LGRC samples (Pearson correlation = −0.24). C: violin plots of GDF15 and EPCAM (epithelial cell adhesion molecule) expression in scRNA-seq data demonstrating epithelial specific expression of GDF15 (43). Data were processed and clustered exactly as described (43). The identity of each cluster is listed in the legend on the right. D: violin plot comparing GDF15 expression in donor and IPF lungs. AEC1 and AEC2, type I and II alveolar epithelial cell, respectively; NK, natural killer.
GDF15 receptor expression.
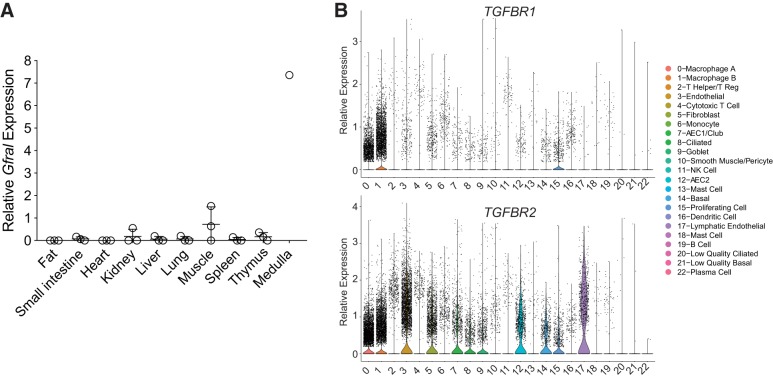
We next investigated the expression of GDF15 receptors to identify the potential target of this ligand. The high-affinity receptor for GDF15, GFRAL, was recently identified and shown to be expressed in the brain stem (24, 30, 44, 61). We queried our scRNA-seq data to determine whether GFRAL or its coreceptor RET was expressed in any cell types within the lung and found no evidence of expression in donor or IPF lungs (not shown). We also stained mouse lungs with antibodies to GFRAL, but found no evidence expression by immunohistochemistry (not shown). We further surveyed several mouse tissues for Gfral expression and, similar to previous reports (24, 30, 44, 61), only found evidence of expression in the medulla of the mouse brain (Fig. 4A). Previous investigations have identified the canonical TGF-β receptors, TGFBR1 and TGFBR2, as potential receptors for GDF15 (14, 32). TGFBR1 and TGFBR2 were broadly expressed in the human lung, with TGFBR2 being far more abundant (Fig. 4B). Thus the precise cellular target of the GDF15 ligand in the lung is unclear.
Fig. 4.
Expression of putative growth and differentiation factor 15 (GDF15) receptors. A: Gfral expression was measured in the mouse tissues shown (n = 3 for all tissues, except medulla for which only a single sample was measured). No signal was detected in the majority of samples, except skeletal muscle and medulla. Values are means and SD. B: violin plots of TGFBR1 and TGFBR2 (transforming growth factor-β receptors I and II, respectively) in scRNA-seq data from human lungs showing macrophage predominant expression of TGFBR1 and broad expression of TGFBR2 (43). AEC1 and AEC2, type I and II alveolar epithelial cell, respectively; NK, natural killer.
GDF15 is expressed by epithelial cells in fibrotic regions of the lung.
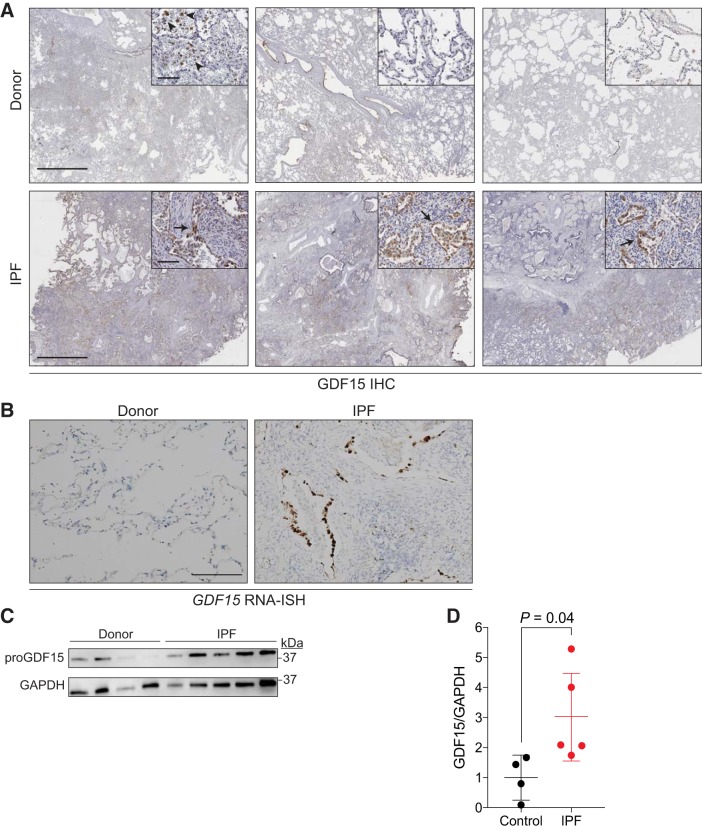
We next investigated the expression of GDF15 in situ. We obtained lung tissue from donor and IPF explanted lungs and examined GDF15 expression by immunohistochemistry. GDF15 expression was localized primarily to epithelial cells in fibrotic regions of the lung and within macrophages in those areas (Fig. 5A). We also observed GDF15 expression in macrophages from donor lungs (Fig. 5A). As GDF15 is a secreted protein, we reasoned that macrophages could potentially take up GDF15 that has been secreted by other cells. To address this possibility, we performed RNA-ISH for GDF15 in human lungs. RNA-ISH staining was limited to the epithelial cells of the lung and sparsely stained cells in the rest of the lung, suggesting that epithelial cells are the primary source of GDF15 (Fig. 5B). Western blotting of lung tissue samples from the lower lobe demonstrated higher expression of proGDF15 in IPF lungs (Fig. 5, C and D). These data are consistent with the mRNA expression data and demonstrate that GDF15 is expressed primarily in epithelial cells from IPF lungs.
Fig. 5.
Growth and differentiation factor 15 (GDF15) is expressed by honeycomb cyst epithelial cells. A: representative photomicrographs from three independent donor and idiopathic pulmonary fibrosis (IPF) lungs. Slides were stained for GDF15 (brown) and counterstained with hematoxylin. GDF15 expression is present in macrophages from healthy lungs (arrowheads) but rarely in epithelial cells. In contrast, GDF15 expression was abundant in epithelial cells (arrows) and macrophages in fibrotic lungs. Scale bar in micrographs is 2 mm and 100 μm in insets. B: GDF15 RNA in situ hybridization (RNA-ISH) in donor and IPF lungs showing epithelial-specific expression of GDF15. Scale bar is 100 μm. C: Western blot of whole lung lysate from donor and IPF lungs for proGDF15 and GAPDH as a load control. D: quantitation of proGDF15 in Western blot in C. Values are means and SD. Student’s t test, two tailed was used for comparison in D. IHC, immunohistochemistry.
Plasma GDF15 is elevated in IPF patients and correlates with disease progression.
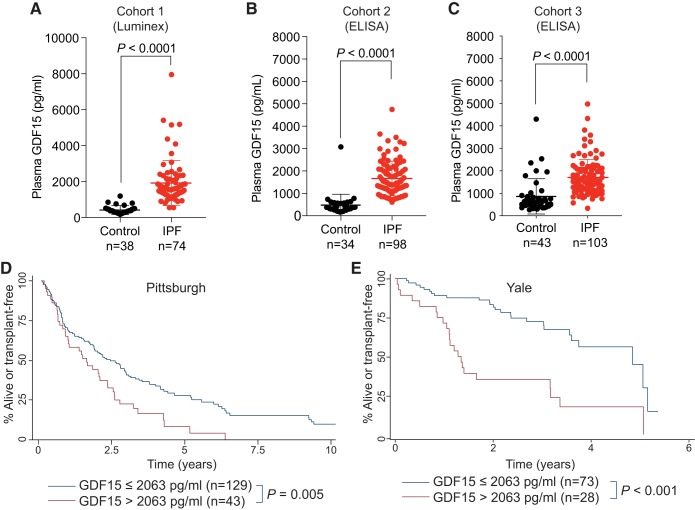
Because of our finding of increased GDF15 expression in IPF lungs and the association with lung function, we explored if GDF15 could be detected in plasma samples from IPF patients. Using a discovery cohort of 38 control and 74 IPF patients that were approximately age and sex matched, we found significantly higher GDF15 in IPF patients compared with controls (Fig. 6A; 1,918 pg/mL versus 420 pg/mL, P < 0.0001, Welch’s t test). We validated these findings by examining a larger, independent cohort (34 controls and 98 IPF) utilizing an independent technique (quantitative ELISA) and found similar results with significantly higher levels of GDF15 in IPF patients (Fig. 6B; 1,666 pg/mL versus 475 pg/mL, P < 0.0001, Welch’s t test). We extended these findings in a third cohort from an independent medical center and found similarly elevated levels of GDF15 (Fig. 6C; 1,712 pg/mL versus 866 pg/mL, P < 0.0001, Welch’s t test). Pulmonary function data were available for a subset of these patients, and we explored if GDF15 correlated with lung function of IPF patients in these cohorts (Table 2). The DlCO of IPF patients was strongly and inversely correlated with GDF15 levels in all three cohorts. There was also a trend toward decreasing forced vital capacity in two of the three cohorts (Table 2). Patients with the highest plasma levels (>2,063 pg/mL; top quartile) of GDF15 had significantly shorter transplant-free survival (P = 0.005, University of Pittsburgh Medical Center, P < 0.001, Yale; Fig. 6, D and E), suggesting that high GDF15 identifies high-risk individuals. Because we identified GDF15 in the context of mice with telomere dysfunction, we examined the relationship between telomere length and GDF15 levels in a limited number of patients (n = 22). We did not observe any relationship between telomere length, measured by flowFISH, and plasma GDF15 levels (not shown).
Fig. 6.
Growth and differentiation factor 15 (GDF15) is a biomarker of idiopathic pulmonary fibrosis (IPF) and identifies high-risk patients. A–C: plasma levels of GDF15 in three cohorts of controls and IPF patients. GDF15 was measured using Luminex assay in cohort 1 (A) and ELISA in cohorts 2 and 3 (B and C, respectively). Values are means and SD. D and E: Kaplan-Meier graph showing the proportion of patients who were alive or transplant free as a function of time. IPF patients were split into two groups based on GDF15 level. Patients from the upper quartile were compared with the lower three quartiles. Age-adjusted P values are from Cox regression analysis. Comparisons in A, B, and C used Welch’s t test, two tailed.
Table 2.
Clinical characteristics and correlation between plasma GDF15 and pulmonary function in patients with IPF
| Cohort 1 | Cohort 2 | Cohort 3 | |
|---|---|---|---|
| No. of samples with spirometry | 62 | 90 | 103 |
| Age (SD), yr | 66.3 (8.9) | 67.7 (7.7) | 70.9 (6.6) |
| Men, n (%) | 38 (61%) | 61 (68) | 79 (77) |
| Smoking status, n (%) | |||
| No | 20 (33%) | 27 (30%) | 27 (26%) |
| Former | 38 (62%) | 60 (68%) | 73 (71%) |
| Current | 3 (5%) | 2 (2%) | 3 (3%) |
| FVC, mean (SD), % | 71 (18.7) | 62 (19.5) | 74 (17.0) |
| DlCO, mean (SD), % | 48 (14.3) | 43 (16.1) | 47 (16.2) |
| GAP, median (IQR) | 3 (2–5) | 4 (3–5) | 4 (3–5) |
| Method for measuring GDF15 | Luminex | ELISA | ELISA |
| Plasma GDF15 (SD), pg/ml | 1,918 (1,228) | 1,666 (779) | 1,712 (794) |
| Correlation with clinical characteristics, regression β (P value) | |||
| FVC% | −0.07 (0.57) | 0.03 (0.78) | −0.17 (0.089) |
| DlCO% | −0.30 (0.022) | −0.25 (0.03) | −0.23 (0.023) |
| GAP | 0.23 (0.07) | 0.09 (0.41) | 0.37 (<0.001) |
| Change in FVC%* | −5.9 (0.083) | −3.7 (0.23) | −5.9 (0.005) |
| Change in DlCO%* | 1.2 (0.71) | 3.3 (0.29) | NA |
Values are means (SD). DlCO, diffusing capacity for carbon monoxide; FVC, forced vital capacity; GAP, gender, age, and physiology score; GDF15, growth and differentiation factor 15; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range; NA, not applicable; SD, standard deviation.
Annualized rate of change.
DISCUSSION
Here we identified GDF15 in an animal model of the most common genetic risk factor for IPF, telomere dysfunction, as a lung-derived secreted factor that is expressed in response to genetic and environmental stress. GDF15 is expressed primarily by epithelial cells, and its expression is elevated in IPF lungs, by whole lung gene expression analysis and protein analysis. In three independent cohorts of IPF patients, we found plasma GDF15 levels were inversely associated with diffusion capacity and declining FVC, and we identified patients with shorter transplant-free survival. Together, these findings identify GDF15 as a novel cell-specific marker of epithelial injury, as well as a novel biomarker of IPF severity.
Cellular senescence induces a constellation of changes within cells, including the activation of the senescence-associated secretory phenotype (SASP) (19). Unexpectedly, very few transcripts from previously reported SASP proteins were found upregulated in senescent AEC2 from mice. Indeed, only GDF15 and MIF (macrophage migration inhibitory factor) have been previously reported as SASP components (2, 19). This suggests that the response to senescence and the SASP may be cell-type specific. GDF15 levels have also been associated with aging (52, 57). Senescent cells accumulate with age and likely contribute to many age-associated pathologies (16). This connection would point to a novel use for GDF15 in quantifying the burden of senescent cells in the lung epithelium. In a small cohort of patients, we did not observe any relationship between telomere length and GDF15. Our data support that GDF15 is upregulated by cells undergoing a stress response, and it is possible that peripheral blood mononuclear cell telomere length may not reflect the stress status of all epithelial cells. Furthermore, telomeres are thought to function until they reach a specific threshold when their length is too short to carry out their function in suppressing the DNA damage response. Therefore, GDF15 is likely to be elevated only when telomeres reach their functional threshold length. Additional studies with larger numbers of samples are warranted to fully evaluate the relationship between telomere length and GDF15 expression. It remains unclear what the contribution of GDF15 may be to age-associated phenotypes and how to distinguish cellular senescence from cellular stress.
We identified Gdf15 in the context of searching for secreted factors that could be responsible for pulmonary inflammation seen in mice with telomere dysfunction (3). However, our findings suggest that GDF15 alone is not sufficient to cause inflammation in the lung. This is in contrast to several reports that have described GDF15 as a regulator of the inflammatory response (14, 59) through the canonical TGF-β-family receptors TGFBR1 and TGFBR2. While we did not observe increased inflammation in response to exogenous GDF15, we cannot draw conclusions about a potential inhibitory role for GDF15 due to the low baseline inflammation in the mouse lung. To begin to explore the downstream consequences of GDF15 signaling in the lung, we searched for cells that expressed the putative GDF15 receptors. We were unable to detect expression of the high-affinity receptor for GDF15, GFRAL, in mouse or human lungs. It is possible that GFRAL is expressed in some exceptionally rare cell types or under specific conditions (i.e., injury or additional cytokines) and that our experiments did not include these conditions. The other putative receptors for GDF15, TGFBR1 and TGFBR2, were expressed widely in the lung, including abundant expression on macrophages. However, given the recent genome-wide screens of all transmembrane proteins and failure to identify any additional receptors for GDF15 besides GFRAL (30, 44, 61), structural data supporting the unique interaction between GDF15 and GFRAL (30), and failure to identify any interaction between GDF15 and TGFβ-family receptors other than GFRAL (24, 30, 44), it is unclear what role GDF15 signaling plays locally within the lung. The downstream consequences of GDF15 signaling remain uncertain.
Numerous blood-derived biomarkers have been identified in IPF, including MMP-7, MUC-1 (KL-6), ICAM-1, IL-8, VCAM-1, SP-A, SP-D, CXCL13, CCL18, COMP, and markers of extracellular matrix turnover, among others (27, 56). Together with previous reports, our data suggest that GDF15 may be useful as a marker of epithelial injury or stress in the lung. How GDF15 could be used alone or in combination with other biomarkers to diagnose, identify distinct disease endotypes, or measure the effectiveness of clinical interventions is unknown, but merits prospective study, perhaps as a secondary end point of an IPF clinical trial.
Our study further connects telomere dysfunction with pulmonary disease, as we identified GDF15 while studying an animal model of AEC2-specific telomere dysfunction. Despite the differences between telomere dysfunction induced by deletion of TRF2 and telomere shortening in humans, modeling telomere dysfunction in mice using this model was sufficient to identify GDF15 and translate these finding into patients with IPF. While mutations in genes responsible for telomere maintenance are identifiable in a subset of cases, short telomeres are present in the majority of all IPF patients (4), supporting a strong link between telomere dysfunction and lung disease. How telomere dysfunction leads to GDF15 expression is not known; however, it likely depends on p53 signaling (45). We showed previously that telomere dysfunction can limit the capacity of the lung epithelium to proliferate and repair after injury (3, 5) and our present findings suggest that GDF15 expression represents an additional consequence of telomere dysfunction in the lung epithelium that may have systemic repercussions.
To our knowledge, this is the first study to combine an animal model, whole tissue expression data, and scRNA-Seq data to identify the source of a plasma biomarker potentially linking epithelial stress or senescence to a quantifiable and accessible surrogate. Our study is limited by its nonprospective nature; however, our findings were validated in a large, independent replication cohort and further supported by expression and in situ studies. Our findings connect one of the best know biomarkers of chronologic aging (GDF15) (52) with one of the best characterized mechanisms of aging (telomeres) in the context of an age-associated disease (IPF). We expect that these finding will spur novel investigations exploring the usefulness of GDF15 as a biomarker of sever disease and its potential role in disease pathology.
GRANTS
This project was supported by National Heart, Lung, and Blood Institute Grants HL-113105, HL-135062 (both to J. K. Alder), HL-1226990 (D. J. Kass), HL-123766 (M. Rojas), and HL-109233 and HL-125850 (both to E. L. Herzog), funding from the Samuel and Emma Winters Foundation (J. K. Alder), funding from the Pulmonary Fibrosis Foundation (Y. Zhang), and the Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease.
DISCLOSURES
D. J. Kass reports collaborative research funding from Regeneron Pharmaceuticals in pulmonary hypertension, which is unrelated to this article. R. Lafyatis has received consulting fees from PRISM Biolab, Merck, Bristol Myers Squibb, Biocon, Formation, Genentech/Roche, UCB, and Sanofi and grant support from Elpidera and Regeneron, not related to the submitted work. K. F. Gibson reports membership on the advisory board of Bayer Pharmaceuticals, outside the scope of the submitted work. E. L. Herzog has received grant funding from Sanofi, Bristol Myers, and Biogen, and consulting fees from Boehringer Ingelheim, Genentech, and Merck, all unrelated to the submitted work. M. Rojas reports funding from Regeneron and MedImmune, unrelated to this work. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
Y.Z., J.F.M., D.J.K., and J.K.A. conceived and designed research; M.J., M.G.R., T.T., S.W., X.C., J.S., Y.C., N.C., C.R., and J.K.A. performed experiments; Y.Z., M.J., M.N., M.G.R., T.T., S.W., R.M.T., E.L.H., C.R., J.F.M., D.J.K., and J.K.A. analyzed data; Y.Z., M.N., M.G.R., S.W., E.L.H., M.R., R.L., K.F.G., J.F.M., D.J.K., and J.K.A. interpreted results of experiments; M.J., M.N., and J.K.A. prepared figures; J.K.A. drafted manuscript; Y.Z., M.N., M.G.R., R.M.T., E.L.H., M.R., R.L., D.J.K., and J.K.A. edited and revised manuscript; Y.Z., M.J., M.N., M.G.R., T.T., S.W., X.C., J.S., Y.C., N.C., R.M.T., E.L.H., C.R., M.R., R.L., K.F.G., J.F.M., D.J.K., and J.K.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to all of the patients who agreed to participate in this study and acknowledge The Center for Organ Recovery & Education (CORE) as well as organ donors and their families for the generous donation of tissues used in this study. In addition, we acknowledge the help and expertise of Amber Luketich and Carlos Castro at the McGee-Womens Research Institute & Foundation histology core and Daniel Sullivan for constructive feedback on this manuscript.
REFERENCES
- 1.Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol 13: 242–248, 2001. doi: 10.1097/00001622-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15: 978–990, 2013. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, Hogan BL, Mitzner W, Armanios M. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci USA 112: 5099–5104, 2015. doi: 10.1073/pnas.1504780112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA III, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 105: 13051–13056, 2008. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, Gorgy AI, Walsh MF, Sussan T, Biswal S, Mitzner W, Tuder RM, Armanios M. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med 184: 904–912, 2011. doi: 10.1164/rccm.201103-0520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alder JK, Hanumanthu VS, Strong MA, DeZern AE, Stanley SE, Takemoto CM, Danilova L, Applegate CD, Bolton SG, Mohr DW, Brodsky RA, Casella JF, Greider CW, Jackson JB, Armanios M. Diagnostic utility of telomere length testing in a hospital-based setting. Proc Natl Acad Sci USA 115: E2358–E2365, 2018. [Erratum in Proc Natl Acad Sci USA 115: E4312, 2018.] doi: 10.1073/pnas.1720427115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alder JK, Parry EM, Yegnasubramanian S, Wagner CL, Lieblich LM, Auerbach R, Auerbach AD, Wheelan SJ, Armanios M. Telomere phenotypes in females with heterozygous mutations in the dyskeratosis congenita 1 (DKC1) gene. Hum Mutat 34: 1481–1485, 2013. doi: 10.1002/humu.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alder JK, Stanley SE, Wagner CL, Hamilton M, Hanumanthu VS, Armanios M. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest 147: 1361–1368, 2015. doi: 10.1378/chest.14-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med 161: 646–664, 2000. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 10.American Thoracic Society/European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 165: 277–304, 2002. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 11.Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet 10: 45–61, 2009. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet 13: 693–704, 2012. [Erratum in Nat Rev Genet 14: 235, 2013.] doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA III, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356: 1317–1326, 2007. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 14.Artz A, Butz S, Vestweber D. GDF-15 inhibits integrin activation and mouse neutrophil recruitment through the ALK-5/TGF-βRII heterodimer. Blood 128: 529–541, 2016. doi: 10.1182/blood-2016-01-696617. [DOI] [PubMed] [Google Scholar]
- 15.Böttner M, Laaff M, Schechinger B, Rappold G, Unsicker K, Suter-Crazzolara C. Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1). Gene 237: 105–111, 1999. doi: 10.1016/S0378-1119(99)00309-1. [DOI] [PubMed] [Google Scholar]
- 16.Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM, Bardeesy N, Castrillon DH, Beach DH, Sharpless NE. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 152: 340–351, 2013. doi: 10.1016/j.cell.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8: 729–740, 2007. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 18.Cogan JD, Kropski JA, Zhao M, Mitchell DB, Rives L, Markin C, Garnett ET, Montgomery KH, Mason WR, McKean DF, Powers J, Murphy E, Olson LM, Choi L, Cheng DS, Blue EM, Young LR, Lancaster LH, Steele MP, Brown KK, Schwarz MI, Fingerlin TE, Schwartz DA, Lawson WE, Loyd JE, Zhao Z, Phillips JA III, Blackwell TS. Rare variants in RTEL1 are associated with familial interstitial pneumonia. Am J Respir Crit Care Med 191: 646–655, 2015. doi: 10.1164/rccm.201408-1510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99–118, 2010. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198, 2003. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 21.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31: 722–733, 2014. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol 279: C461–C479, 2000. doi: 10.1152/ajpcell.2000.279.2.C461. [DOI] [PubMed] [Google Scholar]
- 23.Dressen A, Abbas AR, Cabanski C, Reeder J, Ramalingam TR, Neighbors M, Bhangale TR, Brauer MJ, Hunkapiller J, Reeder J, Mukhyala K, Cuenco K, Tom J, Cowgill A, Vogel J, Forrest WF, Collard HR, Wolters PJ, Kropski JA, Lancaster LH, Blackwell TS, Arron JR, Yaspan BL. Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: a candidate gene sequencing study. Lancet Respir Med 6: 603–614, 2018. doi: 10.1016/S2213-2600(18)30135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, Coskun T, Hamang MJ, Sindelar DK, Ballman KK, Foltz LA, Muppidi A, Alsina-Fernandez J, Barnard GC, Tang JX, Liu X, Mao X, Siegel R, Sloan JH, Mitchell PJ, Zhang BB, Gimeno RE, Shan B, Wu X. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med 23: 1215–1219, 2017. doi: 10.1038/nm.4393. [DOI] [PubMed] [Google Scholar]
- 25.Freeman CM, Martinez CH, Todt JC, Martinez FJ, Han MK, Thompson DL, McCloskey L, Curtis JL. Acute exacerbations of chronic obstructive pulmonary disease are associated with decreased CD4+ & CD8+ T cells and increased growth & differentiation factor-15 (GDF-15) in peripheral blood. Respir Res 16: 94, 2015. doi: 10.1186/s12931-015-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamal SM, Elgengehy FT, Kamal A, El Bakry SA, Shabaan E, Elgendy A, Bassyouni IH. Growth differentiation factor-15 (GDF-15) level and relation to clinical manifestations in Egyptian systemic sclerosis patients: preliminary data. Immunol Invest 46: 703–713, 2017. doi: 10.1080/08820139.2017.1360340. [DOI] [PubMed] [Google Scholar]
- 27.Guiot J, Moermans C, Henket M, Corhay JL, Louis R. Blood biomarkers in idiopathic pulmonary fibrosis. Lung 195: 273–280, 2017. doi: 10.1007/s00408-017-9993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460, 1990. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 29.Hsiao EC, Koniaris LG, Zimmers-Koniaris T, Sebald SM, Huynh TV, Lee SJ. Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol Cell Biol 20: 3742–3751, 2000. doi: 10.1128/MCB.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu JY, Crawley S, Chen M, Ayupova DA, Lindhout DA, Higbee J, Kutach A, Joo W, Gao Z, Fu D, To C, Mondal K, Li B, Kekatpure A, Wang M, Laird T, Horner G, Chan J, McEntee M, Lopez M, Lakshminarasimhan D, White A, Wang SP, Yao J, Yie J, Matern H, Solloway M, Haldankar R, Parsons T, Tang J, Shen WD, Alice Chen Y, Tian H, Allan BB. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 550: 255–259, 2017. [Erratum in Nature 551: 398, 2017.] doi: 10.1038/nature24042. [DOI] [PubMed] [Google Scholar]
- 31.Husebø GR, Grønseth R, Lerner L, Gyuris J, Hardie JA, Bakke PS, Eagan TM. Growth differentiation factor-15 is a predictor of important disease outcomes in patients with COPD. Eur Respir J 49: 1601298, 2017. doi: 10.1183/13993003.01298-2016. [DOI] [PubMed] [Google Scholar]
- 32.Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, Hunter M, Fairlie WD, Lee NJ, Enriquez RF, Baldock PA, Corey E, Apple FS, Murakami MM, Lin EJ, Wang C, During MJ, Sainsbury A, Herzog H, Breit SN. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med 13: 1333–1340, 2007. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 33.Kastritis E, Papassotiriou I, Merlini G, Milani P, Terpos E, Basset M, Akalestos A, Russo F, Psimenou E, Apostolakou F, Roussou M, Gavriatopoulou M, Eleutherakis-Papaiakovou E, Fotiou D, Ziogas DC, Papadopoulou E, Pamboucas C, Dimopoulos MA, Palladini G. Growth differentiation factor-15 is a new biomarker for survival and renal outcomes in light chain amyloidosis. Blood 131: 1568–1575, 2018. doi: 10.1182/blood-2017-12-819904. [DOI] [PubMed] [Google Scholar]
- 34.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, Bavendiek U, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D, Wollert KC. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med 17: 581–588, 2011. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 35.Kim M, Cha SI, Choi KJ, Shin KM, Lim JK, Yoo SS, Lee J, Lee SY, Kim CH, Park JY, Yang DH. Prognostic value of serum growth differentiation factor-15 in patients with chronic obstructive pulmonary disease exacerbation. Tuberc Respir Dis (Seoul) 77: 243–250, 2014. doi: 10.4046/trd.2014.77.6.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koo BK, Um SH, Seo DS, Joo SK, Bae JM, Park JH, Chang MS, Kim JH, Lee J, Jeong WI, Kim W. Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease. Liver Int 38: 695–705, 2018. doi: 10.1111/liv.13587. [DOI] [PubMed] [Google Scholar]
- 37.Lambrecht S, Smith V, De Wilde K, Coudenys J, Decuman S, Deforce D, De Keyser F, Elewaut D. Growth differentiation factor 15, a marker of lung involvement in systemic sclerosis, is involved in fibrosis development but is not indispensable for fibrosis development. Arthritis Rheumatol 66: 418–427, 2014. doi: 10.1002/art.38241. [DOI] [PubMed] [Google Scholar]
- 38.Lee ES, Kim SH, Kim HJ, Kim KH, Lee BS, Ku BJ. Growth differentiation factor 15 predicts chronic liver disease severity. Gut Liver 11: 276–282, 2017. doi: 10.5009/gnl16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magnussen C, Blankenberg S. Biomarkers for heart failure: small molecules with high clinical relevance. J Intern Med 283: 530–543, 2018. doi: 10.1111/joim.12756. [DOI] [PubMed] [Google Scholar]
- 40.Martinez CH, Freeman CM, Nelson JD, Murray S, Wang X, Budoff MJ, Dransfield MT, Hokanson JE, Kazerooni EA, Kinney GL, Regan EA, Wells JM, Martinez FJ, Han MK, Curtis JL; COPDGene Investigators . GDF-15 plasma levels in chronic obstructive pulmonary disease are associated with subclinical coronary artery disease. Respir Res 18: 42, 2017. doi: 10.1186/s12931-017-0521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathai SK, Newton CA, Schwartz DA, Garcia CK. Pulmonary fibrosis in the era of stratified medicine. Thorax 71: 1154–1160, 2016. doi: 10.1136/thoraxjnl-2016-209172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meadows CA, Risbano MG, Zhang L, Geraci MW, Tuder RM, Collier DH, Bull TM. Increased expression of growth differentiation factor-15 in systemic sclerosis-associated pulmonary arterial hypertension. Chest 139: 994–1002, 2011. doi: 10.1378/chest.10-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morse C, Tabib T, Sembrat J, Buschur KL, Bittar HT, Valenzi E, Jiang Y, Kass DJ, Gibson K, Chen W, Mora A, Benos PV, Rojas M, Lafyatis R. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur Respir J 54: 1802441, 2019. doi: 10.1183/13993003.02441-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, Beck SC, South VJ, Dinh TQ, Cash-Mason TD, Cavanaugh CR, Nelson S, Huang C, Hunter MJ, Rangwala SM. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med 23: 1150–1157, 2017. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- 45.Osada M, Park HL, Park MJ, Liu JW, Wu G, Trink B, Sidransky D. A p53-type response element in the GDF15 promoter confers high specificity for p53 activation. Biochem Biophys Res Commun 354: 913–918, 2007. doi: 10.1016/j.bbrc.2007.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrovski S, Todd JL, Durheim MT, Wang Q, Chien JW, Kelly FL, Frankel C, Mebane CM, Ren Z, Bridgers J, Urban TJ, Malone CD, Finlen Copeland A, Brinkley C, Allen AS, O’Riordan T, McHutchison JG, Palmer SM, Goldstein DB. An exome sequencing study to assess the role of rare genetic variation in pulmonary fibrosis. Am J Respir Crit Care Med 196: 82–93, 2017. doi: 10.1164/rccm.201610-2088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, Fernandez R, Akbarpour M, Chen CI, Ren Z, Verma R, Abdala-Valencia H, Nam K, Chi M, Han S, Gonzalez-Gonzalez FJ, Soberanes S, Watanabe S, Williams KJN, Flozak AS, Nicholson TT, Morgan VK, Winter DR, Hinchcliff M, Hrusch CL, Guzy RD, Bonham CA, Sperling AI, Bag R, Hamanaka RB, Mutlu GM, Yeldandi AV, Marshall SA, Shilatifard A, Amaral LAN, Perlman H, Sznajder JI, Argento AC, Gillespie CT, Dematte J, Jain M, Singer BD, Ridge KM, Lam AP, Bharat A, Bhorade SM, Gottardi CJ, Budinger GRS, Misharin AV. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 199: 1517–1536, 2019. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryu C, Sun H, Gulati M, Herazo-Maya JD, Chen Y, Osafo-Addo A, Brandsdorfer C, Winkler J, Blaul C, Faunce J, Pan H, Woolard T, Tzouvelekis A, Antin-Ozerkis DE, Puchalski JT, Slade M, Gonzalez AL, Bogenhagen DF, Kirillov V, Feghali-Bostwick C, Gibson K, Lindell K, Herzog RI, Dela Cruz CS, Mehal W, Kaminski N, Herzog EL, Trujillo G. Extracellular mitochondrial DNA is generated by fibroblasts and predicts death in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 196: 1571–1581, 2017. doi: 10.1164/rccm.201612-2480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuart BD, Choi J, Zaidi S, Xing C, Holohan B, Chen R, Choi M, Dharwadkar P, Torres F, Girod CE, Weissler J, Fitzgerald J, Kershaw C, Klesney-Tait J, Mageto Y, Shay JW, Ji W, Bilguvar K, Mane S, Lifton RP, Garcia CK. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat Genet 47: 512–517, 2015. doi: 10.1038/ng.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan J, Tedrow JR, Dutta JA, Juan-Guardela B, Nouraie M, Chu Y, Trejo Bittar H, Ramani K, Biswas PS, Veraldi KL, Kaminski N, Zhang Y, Kass DJ. Expression of RXFP1 is decreased in idiopathic pulmonary fibrosis. Implications for relaxin-based therapies. Am J Respir Crit Care Med 194: 1392–1402, 2016. doi: 10.1164/rccm.201509-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka T, Biancotto A, Moaddel R, Moore AZ, Gonzalez-Freire M, Aon MA, Candia J, Zhang P, Cheung F, Fantoni G, CHI consortium, Semba RD, Ferrucci L. Plasma proteomic signature of age in healthy humans. Aging Cell 17: e12799, 2018. doi: 10.1111/acel.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tiwari KK, Moorthy B, Lingappan K. Role of GDF15 (growth and differentiation factor 15) in pulmonary oxygen toxicity. Toxicol In Vitro 29: 1369–1376, 2015. doi: 10.1016/j.tiv.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verhamme FM, Freeman CM, Brusselle GG, Bracke KR, Curtis JL. GDF-15 in pulmonary and critical care medicine. Am J Respir Cell Mol Biol 60: 621–628, 2019. doi: 10.1165/rcmb.2018-0379TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verhamme FM, Seys LJM, De Smet EG, Provoost S, Janssens W, Elewaut D, Joos GF, Brusselle GG, Bracke KR. Elevated GDF-15 contributes to pulmonary inflammation upon cigarette smoke exposure. Mucosal Immunol 10: 1400–1411, 2017. doi: 10.1038/mi.2017.3. [DOI] [PubMed] [Google Scholar]
- 56.Vuga LJ, Milosevic J, Pandit K, Ben-Yehudah A, Chu Y, Richards T, Sciurba J, Myerburg M, Zhang Y, Parwani AV, Gibson KF, Kaminski N. Cartilage oligomeric matrix protein in idiopathic pulmonary fibrosis. PLoS One 8: e83120, 2013. doi: 10.1371/journal.pone.0083120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiklund FE, Bennet AM, Magnusson PK, Eriksson UK, Lindmark F, Wu L, Yaghoutyfam N, Marquis CP, Stattin P, Pedersen NL, Adami HO, Grönberg H, Breit SN, Brown DA. Macrophage inhibitory cytokine-1 (MIC-1/GDF15): a new marker of all-cause mortality. Aging Cell 9: 1057–1064, 2010. doi: 10.1111/j.1474-9726.2010.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Q, Jiang D, Chu HW. Cigarette smoke induces growth differentiation factor 15 production in human lung epithelial cells: implication in mucin over-expression. Innate Immun 18: 617–626, 2012. doi: 10.1177/1753425911429837. [DOI] [PubMed] [Google Scholar]
- 59.Wu Q, Jiang D, Schaefer NR, Harmacek L, O’Connor BP, Eling T, Eickelberg O, Chu HW. Overproduction of growth differentiation factor 15 promotes human rhinovirus infection and virus-induced inflammation in the lung. Am J Physiol Lung Cell Mol Physiol 314: L514–L527, 2018. doi: 10.1152/ajplung.00324.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, Wikenheiser-Brokamp KA, Perl AT, Funari VA, Gokey JJ, Stripp BR, Whitsett JA. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1: e90558, 2016. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjær SB, Wu X, Huang T, Hultman K, Paulsen SJ, Wang J, Bugge A, Frantzen JB, Nørgaard P, Jeppesen JF, Yang Z, Secher A, Chen H, Li X, John LM, Shan B, He Z, Gao X, Su J, Hansen KT, Yang W, Jørgensen SB. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med 23: 1158–1166, 2017. doi: 10.1038/nm.4394. [DOI] [PubMed] [Google Scholar]
- 62.Zimmers TA, Jin X, Hsiao EC, McGrath SA, Esquela AF, Koniaris LG. Growth differentiation factor-15/macrophage inhibitory cytokine-1 induction after kidney and lung injury. Shock 23: 543–548, 2005. doi: 10.1097/01.shk.0000163393.55350.70. [DOI] [PubMed] [Google Scholar]