Abstract
The alveolus participates in gas exchange, which can be impaired by environmental factors and toxins. There is an increase in using electronic cigarettes (e-cigarettes); however, their effect on human primary alveolar epithelial cells is unknown. Human lungs were obtained from nonsmoker organ donors to isolate alveolar type II (ATII) cells. ATII cells produce and secrete pulmonary surfactant and restore the epithelium after damage, and mitochondrial function is important for their metabolism. Our data indicate that human ATII cell exposure to e-cigarette aerosol increased IL-8 levels and induced DNA damage and apoptosis. We also studied the cytoprotective effect of DJ-1 against ATII cell injury. DJ-1 knockdown in human primary ATII cells sensitized cells to mitochondrial dysfunction as detected by high mitochondrial superoxide production, decreased mitochondrial membrane potential, and calcium elevation. DJ-1 knockout (KO) mice were more susceptible to ATII cell apoptosis and lung injury induced by e-cigarette aerosol compared with wild-type mice. Regulation of the oxidative phosphorylation (OXPHOS) is important for mitochondrial function and protection against oxidative stress. Major subunits of the OXPHOS system are encoded by both nuclear and mitochondrial DNA. We found dysregulation of OXPHOS complexes in DJ-1 KO mice after exposure to e-cigarette aerosol, which could disrupt the nuclear/mitochondrial stoichiometry, resulting in mitochondrial dysfunction. Together, our results indicate that DJ-1 deficiency sensitizes ATII cells to damage induced by e-cigarette aerosol leading to lung injury.
Keywords: alveolar type II cells, DJ-1, e-cigarette aerosol, lung, mitochondria
INTRODUCTION
Electronic cigarettes (e-cigarettes) belong to electronic nicotine delivery systems (ENDS) (8). These devices are used to heat e-liquid and generate aerosol for inhalation. There is an increase in using e-cigarettes especially by adolescents (10, 11, 37). It was reported that 24.2% high school students were using e-cigarettes in 2011 and the number of users increased to 27.1% in 2018. The primary addictive chemical in e-liquid is nicotine, which is commonly present in concentrations between 8 mg/mL and 24 mg/mL. It was shown that the high temperature used in e-cigarette devices has a direct effect on nicotine yield and is associated with greater nicotine aerosolization (43). Data indicate that, among 3,000 reported exposures to nicotine-containing e-liquids, over 1,000 required medical attention in 2015 (34). It was reported that e-cigarettes generate reactive oxygen species (ROS), which contribute to small airway epithelial cell injury, inflammatory and pro-senescence responses, fibroblast, and murine lung damage (25, 26, 45, 50). Moreover, e-cigarette aerosols suppress the cellular total antioxidant capacity (14). In addition, recent studies showed that e-cigarette components can be metabolized leading to changes in DNA methylation and oxidation and induction of DNA damage in the lung (7, 23). Severe DNA damage or impairment of DNA damage repair pathways may lead to cell death (47).
The alveolus participates in gas exchange, which can be impaired by inhaled environmental factors and toxins (17, 31). The interface with the environment consists of the surfactant surface film and alveolar epithelial cells. The alveolar epithelium is composed of the alveolar type I (ATI) cells and alveolar type II (ATII) cells. ATII cells cover ~5% of the alveolar surface area and restore the epithelium after damage to the very sensitive ATI cells. ATII cells secrete a variety of factors, which limit inflammation and have numerous mitochondria to provide the energetics for metabolism and active sodium transport. Therefore, ATII cells are critical to maintain homeostasis in the distal lung and their damage can cause atelectasis.
We have previously reported on human and murine primary ATII cell death induced by exposure to cigarette smoke (3, 27, 32, 33, 46). However, the impact of e-cigarette aerosol on ATII cell injury is unknown. Manigrasso et al. (30) used a computational model to predict particle deposition generated from this exposure. Obtained results indicated that most particle deposition would occur in the alveolar region. In addition, the thickness of only 10 puffs of the e-cigarette liquid deposited on the alveolar epithelium would be comparable to the thickness of the surfactant layer. The presence of nicotine in e-cigarettes will increase deposited doses of ~40%.
Antioxidant defense systems protect ATII cells against injury induced by environmental factors (3, 6, 21). DJ-1 is a multifunctional protein localized in the cytoplasm, nucleus, and mitochondria (2, 44, 48). It has a cytoprotective role and participates in the regulation of mitochondrial function. DJ-1 possesses quenching activity against ROS by self-oxidation of its cysteine residue. We have previously shown that DJ-1 is expressed in human primary ATII cells (3). Our data indicate decreased DJ-1 levels in freshly isolated ATII cells from heavy smokers, which suggests that cigarette smoke-induced oxidative stress affects DJ-1-mediated cell response. Furthermore, we reported that DJ-1 knockout (KO) mice are more susceptible to lung injury induced by cigarette smoke compared with wild-type mice (46). However, the role of DJ-1 after in vitro or in vivo exposure to e-cigarette aerosol is unknown.
To our knowledge, all published studies on the effect of e-cigarettes in the alveolar epithelium in vitro have used the A549 epithelial cell line derived from human adenocarcinoma (8). Unlike primary human ATII cells, A549 cells do not form functional tight junctions in culture. We report for the first time the effect of e-cigarette aerosol on cultured human primary ATII cells. In addition, we determined DJ-1 cytoprotective function against oxidative stress generated during this exposure using loss-of-function in vitro approach and DJ-1 KO mice.
MATERIALS AND METHODS
Human lungs and ATII cell isolation.
Human lungs were obtained from organ donors whose lungs were not suitable for transplantation and donated for medical research through the International Institute for the Advancement of Medicine (IIAM), National Disease Research Interchange (NDRI), and Gift of Life Foundation. We selected donors without a history of chronic lung disease with reasonable lung function with a / ratio of > 225, X-ray, and a clinical history that did not indicate infection and limited time on a ventilator. Lungs were obtained from nonsmoker organ donors who never smoked (n = 3–8 lungs, women and men, 25–37 yr old). We isolated ATII cells as we previously reported (22). Briefly, the right middle lobe was perfused, lavaged, and then instilled with elastase (6.45 U/mL; Roche Diagnostics, Indianapolis, IN). The lung was minced and subsequently, the cells were filtrated and purified by centrifugation on a density gradient made of Optiprep (Accurate Chemical Scientific, Westbury, NY). We used a positive selection by Ep-CAM microbeads (Miltenyi Biotech, Germany) and obtained >90% purity of ATII cells (22). The Committee for the Protection of Human Subjects at National Jewish Health and Temple University approved this research using human lungs.
ATII cell culture.
The isolated ATII cells were cultured as we previously described (3). Briefly, cells were resuspended in DMEM supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Franklin, MA), 2 mM glutamine (Thermo Fisher Scientific, Franklin, MA), 2.5 mg/mL amphotericin B (Mediatech, Manassas, VA), 100 mg/mL streptomycin (Thermo Fisher Scientific, Franklin, MA), 100 U/mL penicillin (Thermo Scientific, Franklin, MA), and 10 mg/mL gentamicin (Sigma Chemicals, St. Louis, MO). To maintain their differentiated state, ATII cells were plated for 2 days on a mixture of 20% Engelbreth-Holm-Swarm tumor matrix (BD Biosciences, San Jose, CA) and 80% rat-tail collagen in DMEM with 10% FBS and then cultured for two days with 1% charcoal-stripped FBS along with 10 ng/mL keratinocyte growth factor (R&D Systems, Minneapolis, MN), and for additional 2 days with 10 ng/mL keratinocyte growth factor, 0.1 mM isobutylmethylxanthine, 0.1 mM 8-Br-cAMP, and 10 nM dexamethasone (all of them from Sigma, St. Louis, MO) in addition to glutamine, amphotericin B, streptomycin, penicillin, and gentamicin as mentioned above. ATII cells were treated with 15 ng/mL or 50 ng/mL nicotine (Wizard Labs, Altamonte Springs, FL).
ATII cells were exposed one time for 1 h to an aerosol generated from either nicotine-free e-liquid (vehicle) or e-liquid containing 24 mg/mL nicotine (Liberty Stix, Cleveland, OH) in TE-2 machine (Teague Enterprises, Woodland, CA) as previously reported (38, 39). Briefly, two ports were used to generate an aerosol from e-liquid. A control box activated one of the two puff ports for 2 s and each port every 30 s. The aerosol was generated by heating 1 mL e-liquid per 1 h to 204°C, which indicates that the coil temperature was in the normal range used by vapers (200°C–250°C) (39). ATII cell analysis was performed 24 h postexposure.
ATII cell transfection with DJ-1 siRNA.
For gene knockdown in ATII cells, DJ-1 siRNA (sense: 5′-GGAGCAGGAAAACCGGAAGtt-3′; antisense: 5′-CUUCCGGUUUUCCUGCUCCtt-3′) (5) was purchased from Thermo Fisher Scientific (Waltham, MA). To confirm the specificity of the inhibition, nontargeting (NT) siRNA was used as a control as we reported (20). ATII cells were transfected with 300 nM of siRNA duplexes using GenomONE HVJ Envelope Vector Kit (Cosmo Bio, Carlsbad, CA) for 48 h according to the manufacturer’s instructions. Knockdown of DJ-1 was confirmed by Western blotting.
ROS generation.
ATII cells were stained with 10 μM cell-permeant 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA, Thermo Fisher Scientific, Waltham, MA), which is a chemically reduced form of fluorescein and was used as an indicator of ROS in cells. Upon cleavage of the acetate groups by intracellular esterases and oxidation, the nonfluorescent H2DCF-DA is converted to the highly fluorescent 2′,7′-dichlorofluorescein (DCF). Live cell confocal microscope (Zeiss) was used for ROS detection in ATII cells.
Calcium levels measurement.
Measurement of calcium changes was performed using the calcium-sensitive nonfluorescent acetoxymethyl ester Fluo-4 (Thermo Fisher Scientific, Waltham, MA), which is cleaved inside the cell to give the free, fluorescent reagent. ATII cells were stained with 5 μM Fluo-4 for 30 min, followed by an additional 10-min incubation in a dye-free medium. Confocal live cell imaging was performed using a live cell confocal microscope with excitation at 488 nm.
Mitochondrial membrane potential.
The cationic potentiometric fluorescent dye, tetramethylrhodamine, methyl ester (TMRM; Thermo Fisher Scientific, Waltham, MA) was used to measure mitochondrial membrane potential (ΔΨm). TMRM (100 nM) was added to ATII cell medium and allowed to equilibrate for at least 15 min. This red-orange fluorescent dye is readily sequestered by active mitochondria. Confocal live cell imaging was performed with excitation at 568 nm.
Mitochondrial superoxide generation.
ATII cells were loaded with the mitochondrial superoxide-sensitive fluorophore MitoSOX Red (10 μM; Thermo Fisher Scientific, Waltham, MA) at 37°C for 10 min. ATII cells were then washed and imaged using a live cell confocal microscope at 580 nm emission. Oxidation of MitoSOX Red reagent by superoxide produces red fluorescence.
Mice exposure to e-cigarette aerosol.
Wild-type C57BL/6 mice and DJ-1 KO mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were age- and sex-matched and randomly assigned to control or treatment groups (n = 5 - 7). We used 4-wk-old mice for all experiments. Mice were exposed to e-cigarette aerosol in Teague TE-2 machine (Teague Enterprises; Woodland, CA) for 2 h/day for 3 days. We used the same settings as described for in vitro exposure and exposed mice to 48 mg aerosolized nicotine per day by using 2 mL e-liquid containing 24 mg/mL nicotine. Comparable levels of exposure to nicotine (48–50 mg/m3) are achieved using 3R4F cigarettes (University of Kentucky, Lexington, KY) in vivo (39). Paraffin sections were stained with hematoxylin and eosin (H&E) for morphological examination of the lung and alveolar macrophage quantification as previously reported (4). Mice were euthanized immediately after the last exposure. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Temple University.
Western blotting.
Protein expression was measured by Western blotting as we described previously (32). Briefly, polyacrylamide gradient gels (8–16%; Thermo Fisher Scientific, Waltham, MA) were run in tris glycine buffer to separate the proteins. Protein loading was normalized to β-actin (Sigma, St. Louis, MO). DJ-1, COXIV, NDUFS1, ATP5A, SDHB, UQCRC2, and ND1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Dallas, TX). Active caspase-3 antibody was obtained from Cell Signaling Technology (Danvers, MA) and 53BP1 antibody was purchased from Abcam (Cambridge, MA). Horseradish peroxidase (HRP)-conjugated AffiniPure donkey anti-mouse IgG was purchased from Jackson ImmunoResearch (West Grove, PA). The blots were then developed using an enhanced chemiluminescence Western blotting kit (Millipore, Bedford, MA) according to the manufacturer’s instructions. Images were quantitated using NIH Image 1.62 software (Bethesda, MD).
Enzyme-linked immunosorbent assay.
Interleukin (IL)-8 levels were measured in ATII cell supernatant by ELISA (ELISA Tech, Aurora, CO) according to manufacturer’s recommendations. We used a MicroQuant microplate spectrophotometer (BioTek Instruments, Winooski, VT). Data were analyzed using KCJunior Data Analysis Software (BioTek Instruments, Winooski, VT).
TUNEL assay.
Cultured human ATII cells were exposed to e-cigarette aerosol as described above. Apoptosis was determined using TUNEL (TdT-mediated dUTP nick-end labeling) assay (Promega, Madison, WI) as we previously described (3). Briefly, ATII cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS and permeabilized with 0.2% Triton X-100 (Sigma Chemicals, St. Louis, MO). Then, slides with cells were incubated for 1 h at 37°C in a humid chamber in the presence of terminal deoxynucleotidyl transferase (TdT). Cells were mounted with Vectashield medium containing DAPI (Vector Laboratories, Burlingame, CA) to identify nuclei. Images were obtained using a confocal laser-scanning microscope (Zeiss). We also analyzed apoptotic ATII cells in murine lung after exposure to e-cigarette aerosol. Lung tissue sections were embedded in paraffin and TUNEL assay was performed as described above followed by incubation with prosurfactant protein C (proSP-C; Millipore, Bedford, MA) overnight to identify ATII cells. Secondary antibody Alexa Fluor 594 (Invitrogen, Carlsbad, CA) was applied for 1 h and nuclei were stained with Vectashield medium.
Nuclear DNA levels.
The amount of double-strand (ds) DNA and single-strand (ss) DNA levels were determined using QuantiFluor dsDNA and QuantiFluor ssDNA Systems, respectively (Promega, Madison, WI) according to the manufacturer’s instructions. Nuclear DNA amount was evaluated in murine bronchoalveolar lavage (BAL) fluid as previously described with slight modifications (13, 41). Briefly, we amplified 85 bp of nuclear DNA by qPCR using the following primers purchased from Thermo Fisher Scientific (Waltham, MA): forward 5′-TGCTGTCTCCATGTTTGATGTATCT-3′ and reverse 5′-TCTCTGCTCCCCACCTCTAAGT-3′. All results were normalized to 1 µL of BAL fluid and Ct method was used for analysis.
Statistical analysis.
To evaluate statistical differences among experimental groups, one-way ANOVA was used. A value of P < 0.05 was considered significant. Data are shown as means ± SE from at least three independent experiments.
RESULTS
Mitochondrial dysfunction in ATII cells exposed to nicotine.
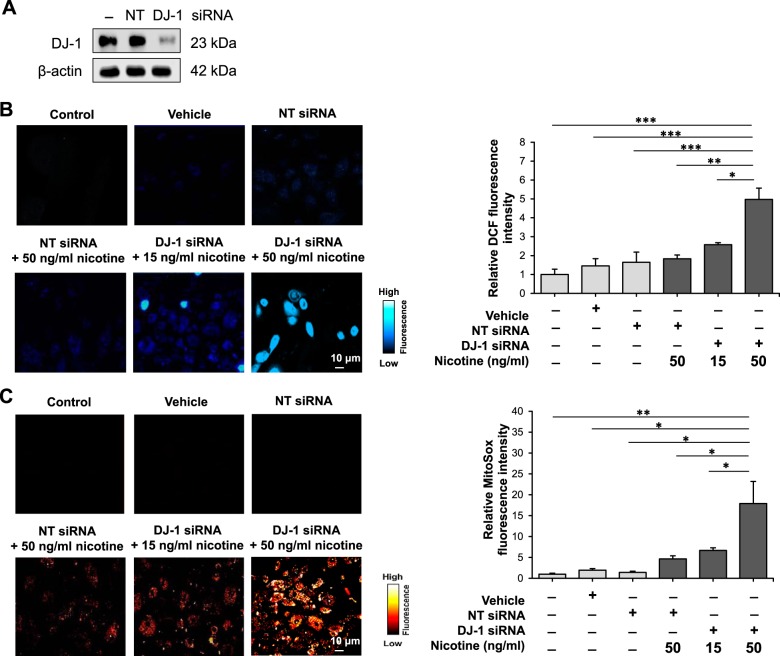
We previously showed that cigarette smoke extract decreased mitochondrial potential in human primary ATI cells (20). In addition, our data indicated decreased DJ-1 expression in ATII cells isolated from heavy smokers, which correlated with high oxidative stress and a proinflammatory response as detected by increased 4-HNE and IL-8 levels, respectively (3). Here we wanted to determine mitochondrial function in human primary ATII cells exposed to nicotine, which is a key ingredient in e-cigarettes (43). We knocked down DJ-1 in cultured human primary ATII cells using Sendai and siRNA strategy (Fig. 1A). Cultured ATII cells with DJ-1 knocked down were treated with 15 ng/mL or 50 ng/mL nicotine. We analyzed cellular ROS generation using DCF staining by live cell confocal microscope (Fig. 1B). Our results indicate significantly higher ROS levels in ATII cells with DJ-1 knockdown compared with controls. Moreover, high nicotine concentration (50 ng/mL) led to greater ROS generation compared with treatment with 15 ng/mL nicotine. We also checked superoxide production in mitochondria using MitoSOX (Fig. 1C). We found a similar correlation showing that DJ-1 knockdown increased ATII cell susceptibility to superoxide generation induced by nicotine compared with controls. In addition, 50 ng/mL nicotine had a greater effect than 15 ng/mL nicotine.
Fig. 1.
Nicotine increases reactive oxygen species levels in human primary alveolar type II (ATII) cells with DJ-1 knockdown. A: DJ-1 knockdown in ATII cells. B and C: ATII cells were treated with 15 ng/mL or 50 ng/mL nicotine as described in materials and methods to determine 2′,7′-dichlorofluorescein (DCF; B) and MitoSOX (C) levels using live cell confocal microscope. Representative images and quantification of fluorescence intensity are shown (NT, nontargeting siRNA; *P < 0.05, **P < 0.001, *** P < 0.0001, n = 3 lungs).
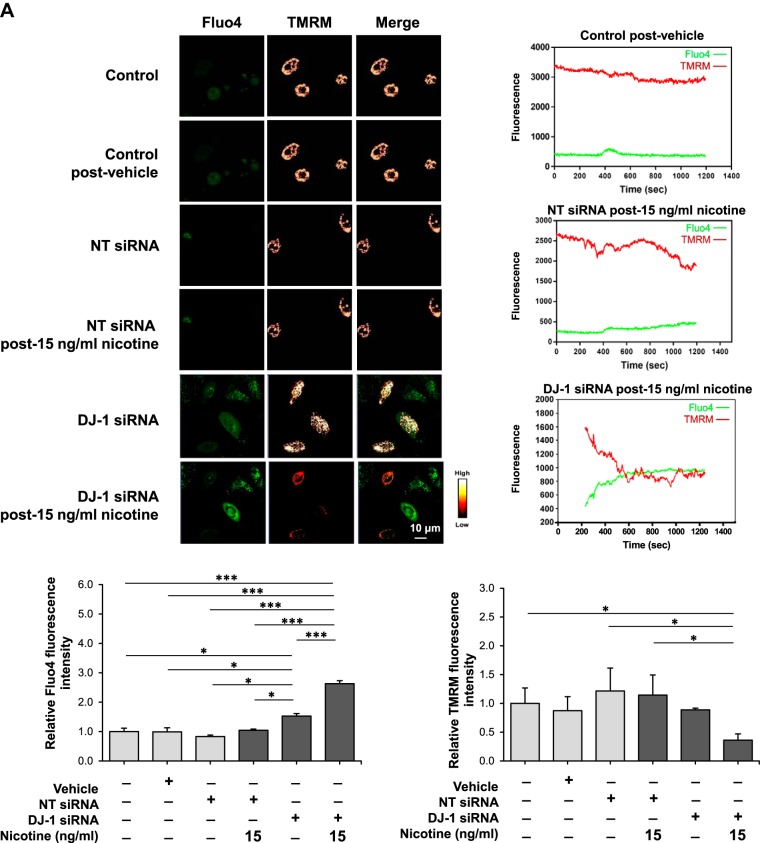
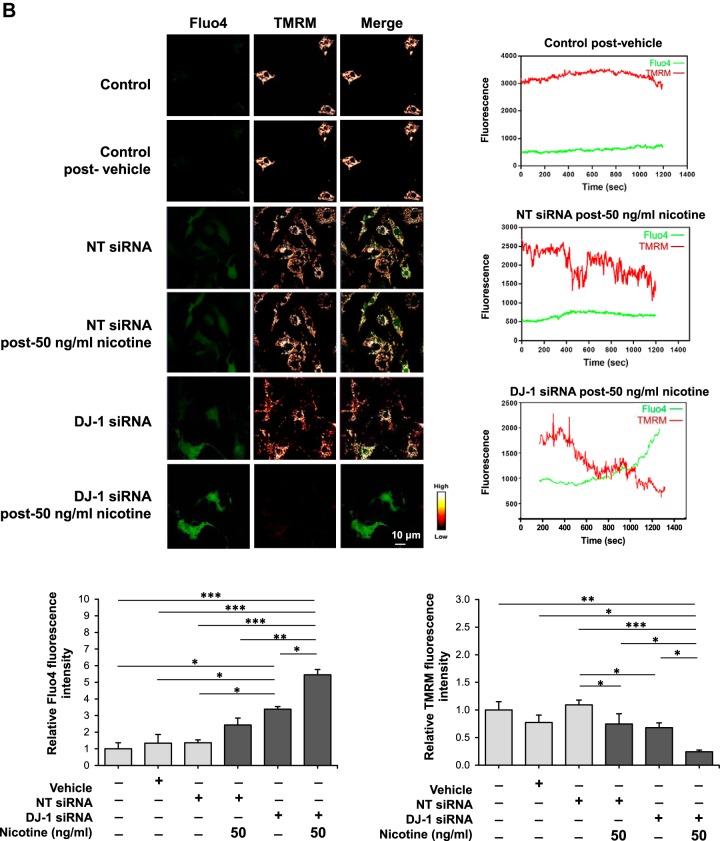
Mitochondrial membrane potential (ΔΨm) is a critical factor in maintaining the physiological function of mitochondria and is a major determinant of cell fate (28). Mitochondria regulate intracellular calcium concentration by acting as local buffers. We analyzed the effect of 15 ng/mL and 50 ng/mL nicotine on ΔΨm and calcium in control ATII cells and in ATII cells with DJ-1 knocked down using TMRM and Fluo-4 staining, respectively. Exposure to 15 ng/mL nicotine led to significantly elevated calcium only in ATII cells with DJ-1 knocked down as evidenced by an increase in Fluo-4 fluorescence (Fig. 2A). We also found decreased ΔΨm in these cells, as detected by live cell analysis using confocal microscopy. We detected a similar correlation for ATII cells treated with 50 ng/mL nicotine showing significantly induced calcium levels and lowered ΔΨm in ATII cells with DJ-1 knockdown (Fig. 2B). Our results indicate that DJ-1 knockdown sensitizes ATII cells to nicotine leading to mitochondrial dysfunction.
Fig. 2.
Nicotine alters calcium levels and mitochondrial membrane potential in human primary alveolar type II (ATII) cells with DJ-1 knockdown. A and B: DJ-1 was knocked down in ATII cells followed by treatment with 15 ng/mL (A) or 50 ng/mL (B) nicotine. Calcium levels and mitochondrial membrane potential were analyzed by Fluo-4 (green) and tetramethylrhodamine, methyl ester (TMRM, red) staining, respectively, in single cells using live cell confocal microscope. Representative images and quantification of fluorescence intensity are shown (NT, nontargeting siRNA; *P < 0.05, *** P < 0.0001, n = 3 lungs).
E-cigarette aerosol induces apoptosis and pro-inflammatory response in ATII cells.
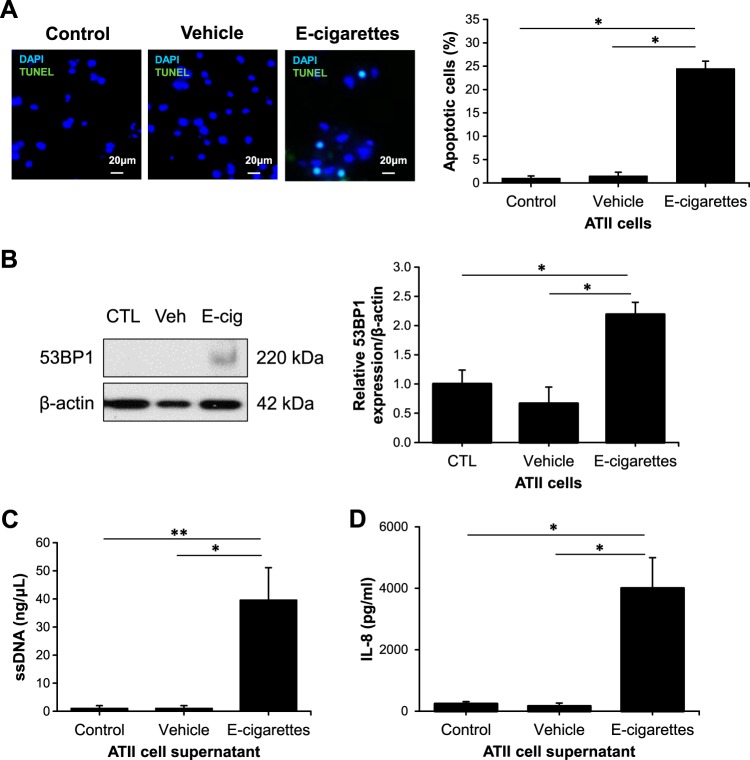
It has been reported that ROS drives proinflammatory response and cell death (35, 42). Human primary ATII cells were cultured under air/liquid conditions as we previously described (3, 32) followed by exposure to e-cigarette aerosol for 1 h. We detected significantly higher ATII cell apoptosis after 24 h by TUNEL assay (Fig. 3A). We further analyzed DNA damage repair signaling and detected increased 53BP1 levels after this treatment (Fig. 3B). Apoptotic cells secrete cell-free DNA (cfDNA) originated from nuclear and/or mitochondrial genome fragments (19). Here we found a significant increase in single-strand DNA (ssDNA) levels in ATII cell supernatant after exposure to e-cigarette aerosol compared with controls (Fig. 3C). Human cells bearing DNA damage had an increase in cytokines secretion (36, 40). Therefore, we analyzed the effect of e-cigarette aerosol on IL-8 expression. Interestingly, we found higher IL-8 levels after this treatment (Fig. 3D). Together, our data indicate that e-cigarette aerosol-induces DNA damage and proinflammatory response, which may contribute to ATII cell death.
Fig. 3.
E-cigarette aerosol induces human primary alveolar type II (ATII) cell injury. ATII cells were exposed to e-cigarette aerosol for 1 h and analyzed after 24 h. A: apoptotic ATII cells were detected by TUNEL assay (green) and DAPI (blue). Cell quantification is also shown. B: 53BP1 expression was determined by Western blotting. Densitometric quantification is also shown. C: single-strand (ss) DNA levels in ATII cell supernatant determined by QuantiFluor ssDNA System. D: IL-8 levels in ATII cell supernatant as measured by ELISA (CTL, control; Veh, vehicle; E-cig, e-cigarette aerosol; *P < 0.05, **P < 0.001, n = 8 lungs).
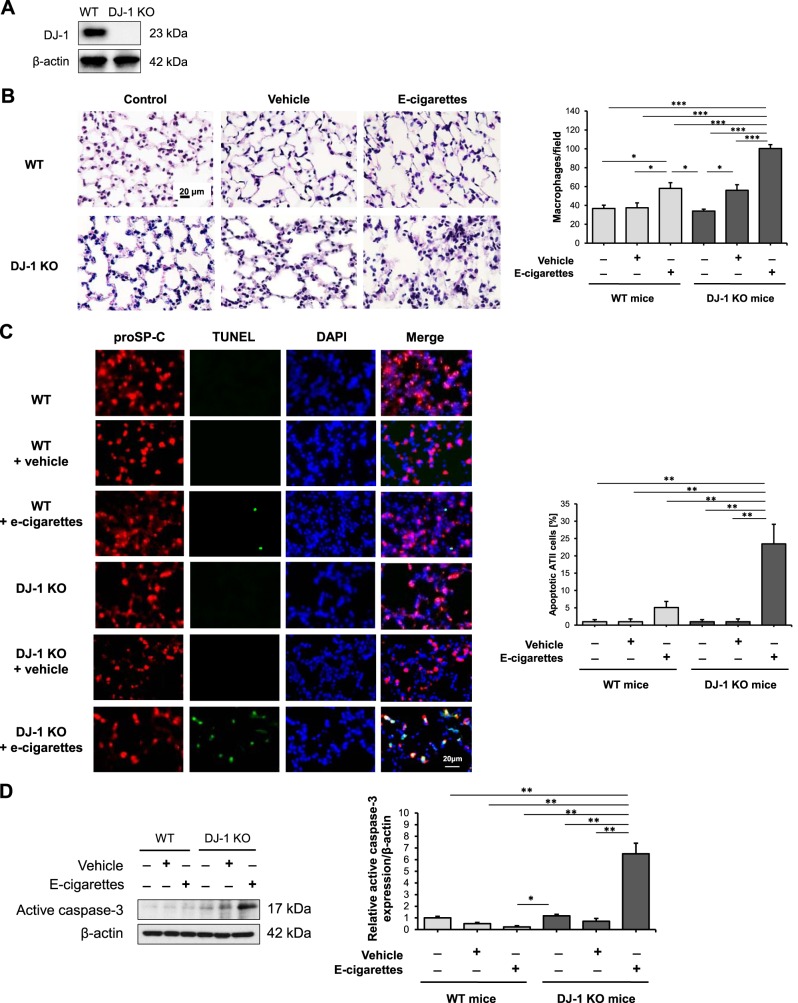
E-cigarette aerosol induces ATII cell damage, lung injury, and mitochondrial dysfunction in DJ-1 KO mice.
We have recently reported a proinflammatory response in DJ-1 KO mice exposed to cigarette smoke (46). Here, we exposed wild-type and DJ-1 KO mice (Fig. 4A) to e-cigarette aerosol to determine its effect on lung injury. We found a significant increase in alveolar macrophages in both wild-type and DJ-1 KO mice after this exposure compared with controls (Fig. 4B). In addition, the number of macrophages was higher in DJ-1 KO compared with wild-type mice. This exposure also significantly increased ATII cell apoptosis in DJ-1 KO mice as detected by proSP-C staining and TUNEL assay (Fig. 4C). We observed a similar correlation by analysis of active caspase-3 expression in murine lung tissue by Western blotting (Fig. 4D).
Fig. 4.
Murine alveolar type II (ATII) cell and lung injury in DJ-1 knockout (KO) mice exposed to e-cigarette aerosol. A: DJ-1 expression in murine lung tissue by Western blotting. B: H&E staining in murine lung tissue. Mice were exposed to e-cigarette aerosol as described in materials and methods. Number of alveolar macrophages per field is also shown. C: apoptotic ATII cells were identified in murine lung tissue using proSP-C (red), TUNEL assay (green), and DAPI (blue) by immunohistofluorescence. Cell quantification is also shown. D: active caspase-3 expression in murine lung tissue by Western blotting. Densitometric quantification is also shown (WT, wild-type mice; *P < 0.05, **P < 0.001, *** P < 0.0001, n = 5 mice per group).
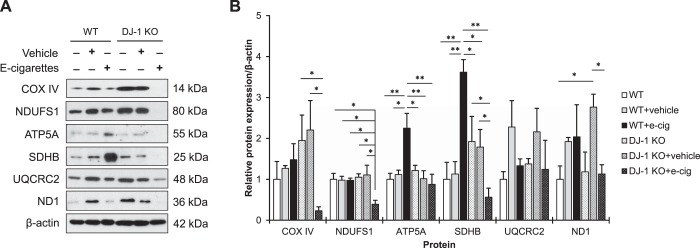
To further determine the effect of e-cigarette aerosol on mitochondrial function in vivo, we checked the expression of nuclear and mitochondrial-encoded components of the OXPHOS complexes in wild-type and DJ-1 KO mice (Fig. 5A). We found statistically significant decrease in COX IV (complex IV), NDUFS1 (complex I), ATP5A (complex V), SDHB (complex II), and ND1 (complex I) levels in DJ-1 KO mice after this exposure (Fig. 5B). We did not find any difference in UQCRC2 (complex III) expression in both wild-type and DJ-1 KO mice.
Fig. 5.
Oxidative phosphorylation (OXPHOS) complexes expression in wild-type (WT) and DJ-1 knockout (KO) mice exposed to e-cigarette aerosol. A: mice were exposed to e-cigarette aerosol as described in materials and methods. COX IV, NDUFS1, ATP5A, SDHB, UQCRC2 and ND1 expression in murine lung tissue by Western blotting. B: protein expression was normalized to β-actin and densitometric quantification is also shown (e-cig, e-cigarette aerosol; *P < 0.05, **P < 0.001, n = 7 mice per group).
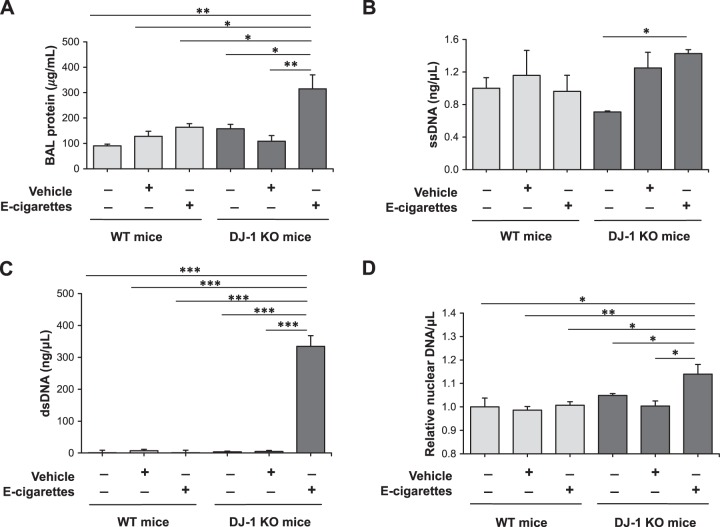
We analyzed lung injury and DNA damage in wild-type and DJ-1 KO mice exposed to e-cigarette aerosol. We detected increased total protein levels in bronchoalveolar lavage (BAL) fluid only in DJ-1 KO mice (Fig. 6A). Moreover, we found significantly higher ssDNA levels in BAL fluid in DJ-1 KO mice compared with wild-type mice (Fig. 6B). In addition, we observed a more pronounced effect for dsDNA levels, which were increased in DJ-1 KO mice exposed to e-cigarette aerosol (Fig. 6C). Increased nuclear DNA levels were detected only in DJ-KO mice exposed to e-cigarette aerosol and were significantly higher in comparison with wild-type mice (Fig. 6D). Our data indicate that e-cigarette aerosol dysregulates OXPHOS complexes and induces ATII cell death leading to lung injury especially in DJ-1 KO mice.
Fig. 6.
DNA release in bronchoalveolar lavage (BAL) fluid in DJ-1 knockout (KO) mice exposed to e-cigarette aerosol. Wild-type (WT) and DJ-1 KO mice were exposed to e-cigarette aerosol as described in materials and methods. Analysis was performed in BAL fluid. A: total protein concentration. B: single-strand (ss) DNA levels determined by QuantiFluor ssDNA System. C: double-strand (ds) DNA levels evaluated by QuantiFluor dsDNA System. D: nuclear DNA levels by qPCR (*P < 0.05, **P < 0.001, *** P < 0.0001, n = 7 mice per group).
DISCUSSION
E-cigarettes have been promoted as a safer substitute for tobacco; however, this does not equate to safer forms of nicotine (23, 29). In addition, e-cigarette vaping is gaining popularity in young individuals. This exposure may induce distal lung injury since the particle number distribution measured in e-cigarette aerosols was detected in the range of 100–600 nm and a computational model predicted their deposition between 7% and 18% in the alveoli (49). ATII cells are the progenitor cells for the alveolar epithelium (31). Here, we found that e-cigarette aerosol induces a proinflammatory response in human primary ATII cells. It has been reported that epithelial H292 cells exposed to e-cigarette aerosols secreted IL-8 into culture media (26). Flavored e-liquid also increased IL-8 levels in neutrophils (9). Furthermore, we detected DNA damage and ATII cell apoptosis after exposure to e-cigarette aerosols. Our results are in agreement with observations showing that this exposure induced DNA fragmentation in cultured lung human fibroblasts as determined by comet assay (25). Mitochondrial function is important for ATII cell metabolism. Mitochondrial potential (ΔΨm) provides the driving force for calcium uptake into mitochondria and stimulates ATP production in response to an increased energy demand by the cell. However, under high oxidative stress, excessive uptake of calcium by mitochondria can trigger the mitochondrial permeability transition, which can cause a collapse of ΔΨm, so ATP cannot be resynthesized (15). Our data indicate decreased ΔΨm and increased calcium after ATII cell treatment with nicotine, which suggests mitochondrial dysfunction and the deleterious effect of this exposure.
DJ-1 has a cytoprotective activity and is localized in mitochondria, cytoplasm, and nucleus (2, 48). We have previously shown that it protects human primary ATII cells against injury induced by cigarette smoke (3, 46). DJ-1 overexpression decreased ATII cell apoptosis induced by cigarette smoke extract. Here, we found that DJ-1 knockdown in human primary ATII cells sensitized cells to mitochondrial superoxide generation, increased calcium, and decreased mitochondrial membrane potential after exposure to nicotine. It has been shown that MDA-MB-435 cells with DJ-1 knockdown had decreased mitochondrial membrane potential after treatment with 2′-benzoyloxycinnamaldehyde, which is used as a flavoring compound (18). Notably, DJ-1 overexpression inhibited mitochondrial damage, which indicates its crucial role in maintaining mitochondrial function under oxidative stress conditions.
Oxidative stress contributes to high mutation rates and DNA can accumulate mutations (16). Several ROS, particularly the highly reactive hydroxyl radical, promote 8-hydroxy-2′-deoxyguanosine formation. Moreover, exposure to e-cigarette aerosol and nicotine induced DNA adducts in the murine lung (23, 24). In addition, DNA adducts were two- to threefold higher in the lung compared with bladder and heart. They were the result of DNA damaging agents contained in e-cigarette aerosol and indicate that the ability for nicotine absorption and metabolism of different organs determine their susceptibility to e-cigarette aerosol-induced DNA adduct formation. In addition, it was suggested that the accumulation of DNA damage may be a result of inhibited DNA damage repair (23). We reported decreased DJ-1 levels in ATII cells obtained from heavy compared with moderate smokers, which indicates the impairment of this pathway (3). Here, our data suggest that nicotine contributes to ATII cell dysfunction in a DJ-1-mediated manner. Our previous study indicated the susceptibility of DJ-1 KO mice to lung injury induced by cigarette smoke (46). Here, we observed increased nuclear DNA, ssDNA and dsDNA levels in BAL fluid in DJ-1 KO mice exposed to e-cigarette aerosol containing nicotine compared with wild-type mice. We also found higher ATII cell apoptosis in the former genotype after this exposure, which indicates lung injury. Aoshiba et al. (1) observed apoptosis in rats exposed to cigarette smoke generated from 10 cigarettes for 5 h.
Regulation of OXPHOS is important for mitochondrial function and protection against oxidative stress (12). OXPHOS complexes I–IV are located in the tubular membranes of mitochondrial cristae and complex V localizes to cristae bends. Subunits of the electron transport chain of the OXPHOS are encoded by both nuclear and mitochondrial DNA except of the complex II subunit SDHB, which is encoded only by nuclear DNA. Nuclear-encoded subunits are translated in the cytoplasm and imported inside the mitochondria. Assembling these complexes requires synchronizing systems for the proper expression of nuclear and mitochondrial genes. Coordinated expression of nuclear and mitochondrial encoded components is necessary to avoid the accumulation of unassembled OXPHOS subunits or partial assembly intermediates, which may lead to mitochondrial dysfunction. Our results indicate downregulation of OXPHOS complexes in DJ-1 knockout mice exposed to e-cigarette aerosol, which can potentially disrupt the nuclear/mitochondrial stoichiometry, resulting in mitochondrial dysfunction in the murine lung.
Our data suggest that DJ-1 deficiency in human primary ATII cells and mice contributes to mitochondrial dysfunction and DNA damage induced by exposure to e-cigarette aerosol, leading to cell death and lung injury.
GRANTS
This work was partially supported by National Institutes of Health/National Heart, Lung, and Blood Institute Grant R01 HL118171 (B. Kosmider), the Catalyst Award from the American Lung Association (K. Bahmed), and National Institute on Drug Abuse Grant P30 DA013429 (E. M. Unterwald and T. K. Eisenstein).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.B. and B.K. conceived and designed research; K.B., C.-R.L., H.S., L.K., M.A., D.T., E.M., E.M.U., T.K.E., and B.K. performed experiments; K.B., S.K., D.T., M.M., J.W.E., R.J.M., G.J.C., and B.K. interpreted results of experiments; C.-R.L., H.S., L.K., M.A., D.T., E.M., E.M.U., and T.K.E. analyzed data; C.-R.L., H.S., L.K., and M.A. prepared figures; K.B. and B.K. drafted manuscript; K.B. and B.K. edited and revised manuscript; K.B., C.-R.L., H.S., L.K., M.A., S.K., D.T., M.M., J.W.E., E.M., R.J.M., E.M.U., T.K.E., G.J.C., and B.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the Lung Center Tissue Bank at Temple University for providing the human lung specimens.
REFERENCES
- 1.Aoshiba K, Tamaoki J, Nagai A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 281: L1392–L1401, 2001. doi: 10.1152/ajplung.2001.281.6.L1392. [DOI] [PubMed] [Google Scholar]
- 2.Ariga H, Takahashi-Niki K, Kato I, Maita H, Niki T, Iguchi-Ariga SM. Neuroprotective function of DJ-1 in Parkinson’s disease. Oxid Med Cell Longev 2013: 683920, 2013. doi: 10.1155/2013/683920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahmed K, Messier EM, Zhou W, Tuder RM, Freed CR, Chu HW, Kelsen SG, Bowler RP, Mason RJ, Kosmider B. DJ-1 modulates nuclear erythroid 2-related factor-2-mediated protection in human primary alveolar type II cells in smokers. Am J Respir Cell Mol Biol 55: 439–449, 2016. doi: 10.1165/rcmb.2015-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balli D, Ren X, Chou FS, Cross E, Zhang Y, Kalinichenko VV, Kalin TV. Foxm1 transcription factor is required for macrophage migration during lung inflammation and tumor formation. Oncogene 31: 3875–3888, 2012. doi: 10.1038/onc.2011.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batelli S, Albani D, Rametta R, Polito L, Prato F, Pesaresi M, Negro A, Forloni G. DJ-1 modulates alpha-synuclein aggregation state in a cellular model of oxidative stress: relevance for Parkinson’s disease and involvement of HSP70. PLoS One 3: e1884, 2008. doi: 10.1371/journal.pone.0001884. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Boukhenouna S, Wilson MA, Bahmed K, Kosmider B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid Med Cell Longev 2018: 5730395, 2018. doi: 10.1155/2018/5730395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Li G, Chan YL, Chapman DG, Sukjamnong S, Nguyen T, Annissa T, McGrath KC, Sharma P, Oliver BG. Maternal e-cigarette exposure in mice alters DNA methylation and lung cytokine expression in offspring. Am J Respir Cell Mol Biol 58: 366–377, 2018. doi: 10.1165/rcmb.2017-0206RC. [DOI] [PubMed] [Google Scholar]
- 8.Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol 313: L193–L206, 2017. doi: 10.1152/ajplung.00071.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clapp PW, Pawlak EA, Lackey JT, Keating JE, Reeber SL, Glish GL, Jaspers I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am J Physiol Lung Cell Mol Physiol 313: L278–L292, 2017. doi: 10.1152/ajplung.00452.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the Field: Use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 67: 1276–1277, 2018. doi: 10.15585/mmwr.mm6745a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diez SL, Cristello JV, Dillon FR, De La Rosa M, Trucco EM. Validation of the electronic cigarette attitudes survey (ECAS) for youth. Addict Behav 91: 216–221, 2019. doi: 10.1016/j.addbeh.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Formosa LE, Ryan MT. Mitochondrial OXPHOS complex assembly lines. Nat Cell Biol 20: 511–513, 2018. doi: 10.1038/s41556-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 13.Furda A, Santos JH, Meyer JN, Van Houten B. Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol Biol 1105: 419–437, 2014. doi: 10.1007/978-1-62703-739-6_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganapathy V, Manyanga J, Brame L, McGuire D, Sadhasivam B, Floyd E, Rubenstein DA, Ramachandran I, Wagener T, Queimado L. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS One 12: e0177780, 2017. doi: 10.1371/journal.pone.0177780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths EJ. Mitochondria–potential role in cell life and death. Cardiovasc Res 46: 24–27, 2000. doi: 10.1016/S0008-6363(00)00020-1. [DOI] [PubMed] [Google Scholar]
- 16.Hahn A, Zuryn S. The cellular mitochondrial genome landscape in disease. Trends Cell Biol 29: 227–240, 2019. doi: 10.1016/j.tcb.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Herzog EL, Brody AR, Colby TV, Mason R, Williams MC. Knowns and unknowns of the alveolus. Proc Am Thorac Soc 5: 778–782, 2008. doi: 10.1513/pats.200803-028HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail IA, Kang HS, Lee HJ, Kwon BM, Hong SH. 2′-Benzoyloxycinnamaldehyde-mediated DJ-1 upregulation protects MCF-7 cells from mitochondrial damage. Biol Pharm Bull 35: 895–902, 2012. doi: 10.1248/bpb.35.895. [DOI] [PubMed] [Google Scholar]
- 19.Jiang P, Chan CW, Chan KC, Cheng SH, Wong J, Wong VW, Wong GL, Chan SL, Mok TS, Chan HL, Lai PB, Chiu RW, Lo YM. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc Natl Acad Sci USA 112: E1317–E1325, 2015. doi: 10.1073/pnas.1500076112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosmider B, Messier EM, Chu HW, Mason RJ. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS One 6: e26059, 2011. doi: 10.1371/journal.pone.0026059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosmider B, Messier EM, Janssen WJ, Nahreini P, Wang J, Hartshorn KL, Mason RJ. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir Res 13: 43, 2012. doi: 10.1186/1465-9921-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosmider BM, Mason, Bahmed RJ. Isolation and characterization of human alveolar type II cells. Methods Mol Biol 1809: 83–90, 2018. doi: 10.1007/978-1-4939-8570-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HW, Park SH, Weng MW, Wang HT, Huang WC, Lepor H, Wu XR, Chen LC, Tang MS. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc Natl Acad Sci USA 115: E1560–E1569, 2018. doi: 10.1073/pnas.1718185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HW, Wang HT, Weng MW, Chin C, Huang W, Lepor H, Wu XR, Rom WN, Chen LC, Tang MS. Cigarette side-stream smoke lung and bladder carcinogenesis: inducing mutagenic acrolein-DNA adducts, inhibiting DNA repair and enhancing anchorage-independent-growth cell transformation. Oncotarget 6: 33226–33236, 2015. doi: 10.18632/oncotarget.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lerner CA, Rutagarama P, Ahmad T, Sundar IK, Elder A, Rahman I. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem Biophys Res Commun 477: 620–625, 2016. doi: 10.1016/j.bbrc.2016.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10: e0116732, 2015. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CR, Bahmed K, Criner GJ, Marchetti N, Tuder RM, Kelsen S, Bolla S, Mandapati C, Kosmider B. S100A8 protects human primary alveolar type II cells against injury and emphysema. Am J Respir Cell Mol Biol 60: 299–307, 2019. doi: 10.1165/rcmb.2018-0144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logan A, Pell VR, Shaffer KJ, Evans C, Stanley NJ, Robb EL, Prime TA, Chouchani ET, Cochemé HM, Fearnley IM, Vidoni S, James AM, Porteous CM, Partridge L, Krieg T, Smith RA, Murphy MP. Assessing the mitochondrial membrane potential in cells and in vivo using targeted click chemistry and mass spectrometry. Cell Metab 23: 379–385, 2016. doi: 10.1016/j.cmet.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowry JA. Electronic cigarettes: another pediatric toxic hazard in the home? Clin Toxicol (Phila) 52: 449–450, 2014. doi: 10.3109/15563650.2014.918998. [DOI] [PubMed] [Google Scholar]
- 30.Manigrasso M, Buonanno G, Fuoco FC, Stabile L, Avino P. Aerosol deposition doses in the human respiratory tree of electronic cigarette smokers. Environ Pollut 196: 257–267, 2015. doi: 10.1016/j.envpol.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Mason RJ. Biology of alveolar type II cells. Respirology 11, Suppl: S12–S15, 2006. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 32.Messier EM, Bahmed K, Tuder RM, Chu HW, Bowler RP, Kosmider B. Trolox contributes to Nrf2-mediated protection of human and murine primary alveolar type II cells from injury by cigarette smoke. Cell Death Dis 4: e573, 2013. doi: 10.1038/cddis.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messier EM, Day BJ, Bahmed K, Kleeberger SR, Tuder RM, Bowler RP, Chu HW, Mason RJ, Kosmider B. N-acetylcysteine protects murine alveolar type II cells from cigarette smoke injury in a nuclear erythroid 2-related factor-2-independent manner. Am J Respir Cell Mol Biol 48: 559–567, 2013. [Erratum in Am J Respir Cell Mol Biol 49: 165, 2013.] doi: 10.1165/rcmb.2012-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila) 54: 924–1109, 2016. doi: 10.1080/15563650.2016.1245421. [DOI] [PubMed] [Google Scholar]
- 35.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med 208: 417–420, 2011. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakad R, Schumacher B. DNA Damage response and immune defense: links and mechanisms. Front Genet 7: 147, 2016. doi: 10.3389/fgene.2016.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Loughlin J, Wellman RJ, Potvin L. Whither the e-cigarette? Int J Public Health 61: 147–148, 2016. doi: 10.1007/s00038-016-0800-5. [DOI] [PubMed] [Google Scholar]
- 38.Rau AS, Reinikovaite V, Schmidt EP, Taraseviciene-Stewart L, Deleyiannis FW. Electronic Cigarettes Are as Toxic to Skin Flap Survival as Tobacco Cigarettes. Ann Plast Surg 79: 86–91, 2017. doi: 10.1097/SAP.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 39.Reinikovaite V, Rodriguez IE, Karoor V, Rau A, Trinh BB, Deleyiannis FW, Taraseviciene-Stewart L. The effects of electronic cigarette vapour on the lung: direct comparison to tobacco smoke. Eur Respir J 51: 51, 2018. doi: 10.1183/13993003.01661-2017. [DOI] [PubMed] [Google Scholar]
- 40.Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11: 973–979, 2009. [Erratum in Nat Cell Biol 11: 1272, 2009.] doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos JH, Meyer JN, Mandavilli BS, Van Houten B. Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol Biol 314: 183–199, 2006. doi: 10.1385/1-59259-973-7:183. [DOI] [PubMed] [Google Scholar]
- 42.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 24: R453–R462, 2014. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeder MJ, Hoffman AC. Electronic cigarettes and nicotine clinical pharmacology. Tob Control 23, Suppl 2: ii30–ii35, 2014. doi: 10.1136/tobaccocontrol-2013-051469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith N, Wilson MA. Structural biology of the DJ-1 superfamily. Adv Exp Med Biol 1037: 5–24, 2017. doi: 10.1007/978-981-10-6583-5_2. [DOI] [PubMed] [Google Scholar]
- 45.Sundar IK, Javed F, Romanos GE, Rahman I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 7: 77196–77204, 2016. doi: 10.18632/oncotarget.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan LH, Bahmed K, Lin CR, Marchetti N, Bolla S, Criner GJ, Kelsen S, Madesh M, Kosmider B. The cytoprotective role of DJ-1 and p45 NFE2 against human primary alveolar type II cell injury and emphysema. Sci Rep 8: 3555, 2018. doi: 10.1038/s41598-018-21790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torgovnick A, Schumacher B. DNA repair mechanisms in cancer development and therapy. Front Genet 6: 157, 2015. doi: 10.3389/fgene.2015.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waak J, Weber SS, Görner K, Schall C, Ichijo H, Stehle T, Kahle PJ. Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J Biol Chem 284: 14245–14257, 2009. doi: 10.1074/jbc.M806902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Sumner W, Chen DR. In vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicotine Tob Res 15: 501–508, 2013. doi: 10.1093/ntr/nts165. [DOI] [PubMed] [Google Scholar]
- 50.Zhao J, Zhang Y, Sisler JD, Shaffer J, Leonard SS, Morris AM, Qian Y, Bello D, Demokritou P. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J Hazard Mater 344: 549–557, 2018. doi: 10.1016/j.jhazmat.2017.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]