Abstract
Dysregulated mitochondrial quality control leads to mitochondrial functional impairments that are central to the development and progression of hepatic steatosis to nonalcoholic steatohepatitis (NASH). Here, we identify hepatocellular localized endothelial nitric oxide synthase (eNOS) as a novel master regulator of mitochondrial quality control. Mice lacking eNOS were more susceptible to Western diet-induced hepatic inflammation and fibrosis in conjunction with decreased markers of mitochondrial biogenesis and turnover. The hepatocyte-specific influence was verified via magnetic activated cell sorting purified primary hepatocytes and in vitro siRNA-induced knockdown of eNOS. Hepatic mitochondria from eNOS knockout mice revealed decreased markers of mitochondrial biogenesis (PPARγ coactivator-1α, mitochondrial transcription factor A) and autophagy/mitophagy [BCL-2-interacting protein-3 (BNIP3), 1A/1B light chain 3B (LC3)], suggesting decreased mitochondrial turnover rate. eNOS knockout in primary hepatocytes exhibited reduced fatty acid oxidation capacity and were unable to mount a normal BNIP3 response to a mitophagic challenge compared with wild-type mice. Finally, we demonstrate that eNOS is required in primary hepatocytes to induce activation of the stress-responsive transcription factor nuclear factor erythroid 2-related factor 2 (NRF2). Thus, our data demonstrate that eNOS is an important regulator of hepatic mitochondrial content and function and NASH susceptibility.
Keywords: endothelial nitric oxide synthase, mitophagy, NAFLD, steatohepatitis
INTRODUCTION
The progressive form of nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), is independently associated with mortality risk (1, 3) and has emerged as the most rapidly growing cause for liver transplantation in the United States (54). Unfortunately, a lack of understanding of the molecular events leading to the progression of hepatic steatosis to NASH has limited effective therapeutic development. Detriments in hepatic mitochondrial function, including decreased oxidative capacity and increased reactive oxygen species (ROS) generation, play a central role in the etiology of NASH (20, 38). However, underlying causes of these mitochondrial impairments remain poorly understood.
Mounting evidence supports an association between endothelial nitric oxide synthase (eNOS) dysregulation and NASH susceptibility. We have previously reported in obese rats that attenuated eNOS activation is associated with the transition from hepatic steatosis to NASH and that chronic pharmacological NOS inhibition exacerbates NAFLD in conjunction with diminished mitochondrial respiratory capacity and biogenesis (44, 45). Additionally, others have shown that lack of eNOS exacerbates hepatic steatosis (26, 33), activation of hepatic stellate cells (55) and Kupffer cells (21, 49), and the dysregulation of liver hemodynamics (34, 35, 43). However, much less is known about the role of eNOS in susceptibility to Western diet-induced NASH or in relation to mitochondrial turnover.
eNOS is well known to mediate vascular tone and provide anti-inflammatory signals in the cardiovascular system. It is generally thought to be expressed solely in the endothelial cells, but there is some discrepancy regarding the eNOS expression profile in the liver. Some reports demonstrate that eNOS is restricted to the sinusoidal endothelium (43); whereas, others identify expression in hepatocytes (2, 24, 25). This is intriguing given the high mitochondrial content of hepatocytes and the clear influence of eNOS on mitochondrial biogenesis (29, 31, 32, 50, 52) and electron transport chain activity (5, 6, 9). Furthermore, mitophagy is another essential component of mitochondrial quality control, and loss of the essential mitophagy effector BNIP3 can lead to an accumulation of poorly functioning mitochondria (12a). Whether eNOS also coordinates mitophagy to preserve hepatic mitochondrial content is unknown.
Here, we tested the hypothesis that loss of eNOS drives NAFLD progression to NASH through dysregulation of mitochondrial quality control. With a model of Western diet-induced NASH in eNOS KO mice, our results demonstrate that eNOS is necessary to preserve mitochondrial function. In complementary primary hepatocyte experiments, we show that eNOS is expressed in these cells and is necessary for stimulation of BCL-2/adenovirus E1B 19-kDa protein-interacting protein-3 (BNIP3) production in response to a mitophagic challenge. Collectively, our results encourage further study of the hepatocellular autonomous role of eNOS in the etiology of NASH.
MATERIALS AND METHODS
Animal protocol.
For the knockout study, male congenic wild-type C57/Bl6J (WT) and B6.129P2-Nos3tm1Unc/J mice (eNOS KO) were obtained from Jackson Laboratories (no. 002684; Bar Harbor, ME) at 8 wk of age and maintained in a temperature-controlled environment (22°C) on a 12:12-h light-dark cycle with ad libitum access to drinking water. Animals were randomly selected to receive either a semipurified control diet (CON; no. D12110704; Research Diets, New Brunswick, NJ) containing 10% kcal fat, 70% kcal carbohydrate (3.5% kcal sucrose), and 20% kcal protein, or a Western style high-fat, high-sucrose diet with cholesterol (WD; D09071604, Research Diets) containing 44.9% kcal fat, 35.1% kcal carbohydrate (17% sucrose), 20% kcal protein, and 1% wt/wt cholesterol for 18 wk. Food consumption and body weight were measured weekly, and body composition (4in1-1100 Analyzer; EchoMRI, Houston, TX) was collected biweekly throughout the duration of the study. Food consumption was measured by taking the difference in grams of food given and grams of food remaining 7 days later and multiplying total grams consumed by energy content per gram of the diet and dividing by 7 to give kilocalories per day. On the day of euthanasia, animals were fasted for 5 h by pulling food at 0300 and then anesthetized with pentobarbital sodium (100 mg/kg) at 0800. Blood was collected via cardiac puncture, and the animals were euthanized via removal of the heart. The liver was rapidly removed and prepared for mitochondrial isolation, nuclear extraction, homogenization for palmitate oxidation, and fixed in formalin or snap-frozen in liquid nitrogen for later processing as described in detail in the following sections. All animal protocols were approved by the University of Missouri and the Harry S Truman Animal Care and Use Committees.
Primary hepatocyte isolation and culture.
A separate cohort of chow-fed WT and eNOS KO mice was used for primary hepatocyte studies. Primary hepatocytes were isolated from 9- to 13-wk-old mice by using the two-step collagenase method as described previously (13, 27, 36). Hepatocyte purification was achieved by preplating on plastic bottom dishes for 1 h. Nonparenchymal cells adhere to the plastic dish while hepatocytes remain suspended in the medium. The medium was collected from each preplate and centrifuged for 3 min at 50 g to pellet hepatocytes. Cellular preparations were assessed for viability using trypan blue, and preparations with <90% viability were discarded. Hepatocytes were plated on type 1 collagen-coated plates for 2 days for other outcomes (40, 46). Growth medium [Williams Medium E (no. 12551-032, ThermoFisher), 10% FBS, 4 mM l-glutamine, 1% penicillin-streptomycin (no. 15140122, ThermoFisher), 20 ng/mL mouse epidermal growth factor (EGF), insulin, transferrin, and selenium (ITS), 100 nM dexamethasone, 10 mM sodium pyruvate, 0.1% BSA] was exchanged daily throughout culture.
Small interfering RNA transfection of primary hepatocytes.
Silencer Select small interfering (si)RNAs (ThermoFisher) were introduced via reverse transfection according to the manufacturer’s instructions. Briefly, siRNAs for NOS3 (nos. s70696 and s70697) or a scrambled negative control (Scr; no. 4390843) were complexed to lipofectamine RNAi Max (no. 13778, ThermoFisher) in the appropriate volume of Opti-MEM (no. 31985070, ThermoFisher) for 15 min. Following preplating, cells were pelleted (50 g for 3 min), resuspended in growth medium without antibiotics, and added to wells containing the appropriate siRNA-lipofectamine complex in Opti-MEM. Following a 6-h incubation to allow cell seeding and transfection, medium was changed to fresh growth medium. All siRNA experiments were conducted 44–48 h posttransfection.
Measurement of BNIP3 accumulation in primary hepatocytes.
Twenty-four hours after plating, primary hepatocytes were switched into starvation medium (William Medium E, 20 mM glutamine, 0.1% FBS) containing 1 µM chloroquine (CQ) and/or 250 µM CCCP with or without 500 µM fatty acids (250 µM palmitate complexed to BSA + 250 µM oleate). In preliminary experiments, cells were incubated for 1, 2, 4, 8, 16, and 24 h and then processed for Western blot and/or qPCR (see next section). Sixteen hours of treatment was determined to maximize BNIP3 accumulation and was used for subsequent studies.
Hepatocyte processing for Western blot and qPCR.
Cells prepared for Western blot analysis were washed with ice-cold PBS and lysed with cell lysis buffer [1% Triton X-100, 100 mM NaCl, 20 mM Tris, 2 mM EDTA, 10 mM MgCl2, 10 mM NaF, 40 mM β-glycerol phosphate, protease inhibitor (Roche), and phosphatase inhibitors (Sigma)]. Samples were sonicated and centrifuged at 15,000 g for 15 min, and the supernatant was evaluated for total protein content using BCA. Cells prepared for RNA extraction were washed with ice-cold PBS and lysed in buffer RLT (Qiagen) with 1% (vol/vol) β-mercaptoethanol and sonicated. RNA was isolated using the RNeasy mini kit (Qiagen) per the manufacturer’s instructions.
Magnetic activated cell sorting of primary hepatocytes.
The crude isolated hepatocyte pellet described above was resuspended in a 43.5% Percoll solution [11 mL Percoll, 11 mL growth medium (Williams E, Invitrogen), 10% FBS, 4 mM l-glutamine, 100 U penicillin-100 mg streptomycin, 2 ng/mL rat EGF, 100 nM insulin, 100 nM dexamethasone, 0.1% BSA, and 10 mM sodium pyruvate, and 3.3 mL PBS] and centrifuged at 40 g for 15 min at 4°C without braking. Cell viability and number were determined with trypan blue and a manual hemocytometer. Modified from Azimifar et al. (2) and according to manufacturer’s directions, 108 cells were pelleted at 300 g for 10 min at 4°C. The cell pellet was resuspended in 80 μL of degassed magnetic-activated cell sorting (MACS) working buffer (PBS, 0.5% BSA, 2 mM EDTA, pH 7.2), 10 μL of CD146 (endothelial marker) microbeads, and 10 μL of CD11b (macrophage marker) microbeads (Miltenyi Biotec, Auburn, CA) and incubated on ice for 15 min. The cells were then washed with 2 mL of MACS working buffer and centrifuged at 300 g for 10 min at 4°C. The supernatant was aspirated, and the pellet was resuspended in 500 μL of MACS working buffer. The large cell column was washed with 500 vols of MACS working buffer, the cells were then applied to the column, and the flow-through was collected containing the unlabeled cells, the column was then washed three times with 500 μL of MACS working buffer and combined with the unlabeled cell flow-through. The cells were then pelleted and either plated with growth medium or lysed for RNA and protein analysis.
Immunofluorescence of MACS-purified primary hepatocytes.
MACS-purified primary hepatocytes were plated onto collagen coated glass chamber slides for 24 h in growth medium. Modified from Werner et al. (53), cells were fixed with prechilled methanol-acetone (1:2 vol/vol) for 10 min on ice, washed with PBS, permeabilized with 0.3% Triton X-100 for 30 min, and then washed with PBS. The slides were then blocked with 2% BSA for 1 h. The following primary antibodies were diluted 1:1,000 and incubated overnight at 4°C: albumin (Antibodies-Online, Atlanta, GA), eNOS (BD Bioscience, San Jose, CA), CD146, and CD11b (Novus Biologicals, Littleton, CO). After a washing with PBS, the slides were incubated with the appropriate secondary antibody diluted 1:1,000 for 3 h at 4°C in the dark. The slides were washed with PBS, stained with DAPI for 1 min, washed with PBS, and mounted with Mowiol mounting medium (3.6 M glycerol, 4.3 mM Mowiol 4-88, 130 mM Tris). Omission of the primary antibody was used as negative control. Back-end immunofluorescence for CD11b and CD146 in purified hepatocytes was used to confirm the removal of Cd11b+ and CD146+ cells.
Mitochondrial isolation and respiration.
Hepatic mitochondria were isolated as previously described (37, 39). Briefly, roughly 100 mg of fresh liver tissue was minced and homogenized using a Teflon pestle in mitochondrial isolation buffer (220 mM mannitol, 70 mM sucrose, 10 mM Tris base, 1 mM EDTA, pH 7.4). The sample was centrifuged at 1,500 g for 10 min. The supernatant was retained and underwent a series of three centrifugations (6,000–8,000 g) and pellet resuspensions using gentle glass-on-glass homogenizations. Sample was maintained at 4°C throughout the isolation protocol. The final pellet was suspended in mitochondrial incubation buffer (110 mM sucrose, 60 mM KMES, 20 mM glucose, 20 mM HEPES, 10 mM KH2PO4, 3 mM MgCl2, 0.5 mM EGTA, pH 7.4) and used for mitochondrial respiration, [1-14C]palmitate oxidation, or stored at −80°C for Western blot analysis.
In the eNOS KO study, hepatic mitochondrial respiration was assessed in isolated hepatic mitochondria by using Clark electrodes (Strathkelvin Instruments), and all values were corrected to total mitochondrial protein loaded. Respiration was assessed by first loading mitochondria and then substrates (as indicated below) to observe state II respiration, followed by the addition of excess 200 µM ADP to stimulate state III respiration. In separate replicates, FCCP was added in place of ADP in the presence of both complex I and II substrates in serial 1 µM additions until maximal uncoupled respiration was reached. Complex I respiration was stimulated with 1 mM malate and either 10 mM glutamate or 10 µM palmitoylcarnitine, as indicated in the figures. Complex I+II respiration was stimulated by the same complex I substrates plus 10 mM succinate. State III (200 µM ADP), and maximal uncoupled respiration were achieved by serial additions of 1 µM FCCP. Increases in maximal uncoupled respiration >20% following the addition of reduced cytochrome c (2 µM) was used for mitochondrial preparation quality control. Each substrate condition was tested in a technical replicate from a single mitochondrial preparation per mouse.
Plasma analysis.
Quantification of plasma glucose, total cholesterol, triglyceride, alanine aminotransferase (ALT), and free fatty acid (FFA) assays were performed by a commercial laboratory (Comparative Clinical Pathology Services, Columbia, MO) using an Olympus AU680 automated chemistry analyzer (Beckman Coulter, Brea, CA) according to the manufacturer’s instructions. Plasma insulin concentrations were determined using a commercially available, mouse-specific ELISA (Alpco Diagnostics, Salem, NH).
Nuclear/cytosolic extractions.
Roughly 50 mg of fresh liver tissue was placed in ice-cold PBS, minced, and homogenized with a Teflon pestle. Nuclear and cytosolic fractions were obtained using a commercially available kit per the manufacturer’s instructions (no. 78833; ThermoFisher Scientific, Grand Island, NY).
Liver histology and biochemical TAG.
Fresh liver tissue was immediately fixed in formalin. Samples were later imbedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) or picrosirius red (to assess fibrosis) by IDEXX RADIL (Columbia, MO). NAFLD activity score (19) and fibrosis staging of liver sections were conducted by a trained and blinded observer. Intrahepatic triacylglycerol (TAG) content was measured using commercially available reagents (no. F6428, Sigma) using methanol-chloroform (2:1 ratio) lipid extraction as described previously (37). Triglyceride content is reported as nanomoles per gram wet weight of tissue.
Western blots.
Whole liver homogenate, isolated hepatic mitochondria, and hepatocyte lysates were prepared for Western blot analysis. Sample protein concentration was determined by Pierce BCA protein assay (no. 23225, ThermoFisher Scientific). Primary antibodies used are as follows: eNOS (no. 610297; BD Biosciences, San Jose, CA), oxidative phosphorylation (OXPHOS) mitochondrial profile (ab110413; Abcam, Cambridge, MA), mitochondrial transcription factor A (TFAM; Santa Cruz Biotechnology, Dallas, TX), Bnip3 (no. 3769; Cell Signaling Technology, Danvers, MA), 1A/1B light chain 3B (LC3; no. 4108S, Cell Signaling), voltage-dependent anion-selective channel protein (VDAC; ab15895, Abcam), β-actin (no. sc81178, Santa Cruz Biotechnology), p62 (no. 5114, Cell Signaling Technology), Kelch-like ECH-associated protein-1 (Keap1; no. 8047, Cell Signaling Technology), and nuclear factor erythroid 2-related factor 2 (Nrf2; no. 12721, Cell Signaling). Primary antibodies were used at 1:1,000 dilution, and secondary antibody at 1:5,000 dilution. Blots were analyzed via densometric analysis (Image Laboratory Beta 3, Bio-Rad Laboratories, Hercules, CA). Total protein was assessed with Amido black (0.1%, Sigma) to control for differences in protein loading and transfer as previously described (37). Blots in primary hepatocytes were normalized to β-actin.
Quantitative real-time PCR.
RNA was extracted from liver tissue or hepatocyte cultures with a commercially available kit (no. 74104, Qiagen), and a cDNA library was synthesized (Promega). Purity and quality of RNA and cDNA were assessed with a Nanodrop spectrometer. Quantitative real-time PCR (qPCR) was conducted using Sybr Green reagents (172–5121, BioRad) and primer pairs listed in Table 1 (Sigma). PCR product melt curves were used to assess primer specificity. Data are represented relative to cyclophillin B (Ppib) using the 2−ΔΔCT method.
Table 1.
Mouse qPCR primer pairs
| Primers (5′–3′) |
||
|---|---|---|
| Gene | Forward | Reverse |
| Acta2 | AAACAGGAATACGACGAAG | CAGGAATGATTTGGAAAGGA |
| Bnip3 | ACCACAAGATACCAACAGAG | AATCTTCCTCAGACAGAGTG |
| Ccr2 | ACCACATGTGCTAAGAATTG | CTGGTTTTATGACAAGGCTC |
| Cd11c | TCACACCTGCAGAGATTT | TACTCAGACGGCCATGGT |
| Cd163 | AGTCTGCTCACGATACATAG | TCCTTCTGGAATAGATTGGG |
| Cd68 | GTGTCTGATCTTGCTAGGACC | GTGCTTTCTGTGGCTGTAG |
| Col1al | ACGCCATCAAGGTCTACTGC | ACTCGAACGGGAATCCATCG |
| Emr1 | TTTCAAATGGATCCAGAAGG | CAGAAGGAAGCATAACCAAG |
| Il1b | TCACAGCAGCACATCAACAACAA | TGTCCTCATCCTGGAAGGCT |
| Keap1 | GTGGAGAGATATGAGCCAG | CTCTCTGGATAGTAACATTCTG |
| Mrc1 | AAATGATGAGCTGTGGATTG | CCATCCTTGCCTTTCATAAC |
| Nos2 | TCAACTGCAAGAGAACGGAGAA | ACATTCTGTGCTGTCCCAGTG |
| Nos3 | GCTTCAGGAAGTGGAGGCTG | CTGCAGTCCCGAGCATCAA |
| Nqo1 | CCTTTCCAGAATAAGAAGACC | AATGCTGTAAACCAGTTGAG |
| Nfe2l2 | CATTCCCGAATTACAGTGTC | GGAGATCGATGAGTAAAAATGG |
| Pgc1a | AGTGGTGTAGCGACCAATCG | TCTTCATCCACGGGGAGACT |
| Ppib | TGGAGATGAATCTGTAGGAC | CAAATCCTTTCTCTCCTGTAG |
| Tgfb1 | AAGTTGGCATGGTAGCCCTT | GCCCTGGATACCAACTATTGC |
| Tnfa | AGGCACTCCCCCAAAAGATG | CTTGGTGGTTTGCTACGACG |
Statistical analysis.
Statistical analysis was conducted in R (v. 3.2.2). In vivo studies were analyzed via two-way (2×2) ANOVA. When a significant interaction term was observed by two-way ANOVA (P < 0.05), a Fisher’s least significant difference post hoc test was performed. In vitro data were analyzed with either two-way ANOVA or paired t-test as appropriate and are indicated in the corresponding figure legends. For primary hepatocyte experiments, a biological replicate was defined as cells isolated from a single mouse, and each condition was tested in technical triplicate and averaged. Data from such experiments, and n size listed in the figure legends are representative of independent biological replicates. Data were confirmed as normally distributed using GraphPad Prism 8.1. All data are presented as means ± SE.
RESULTS
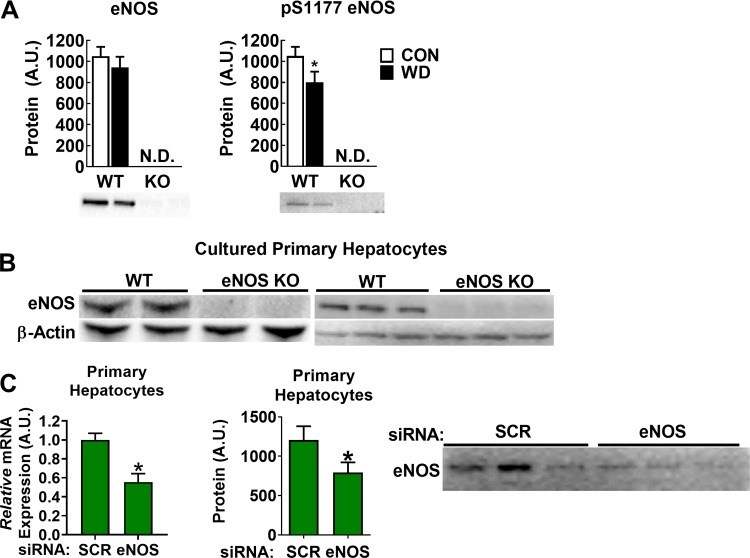
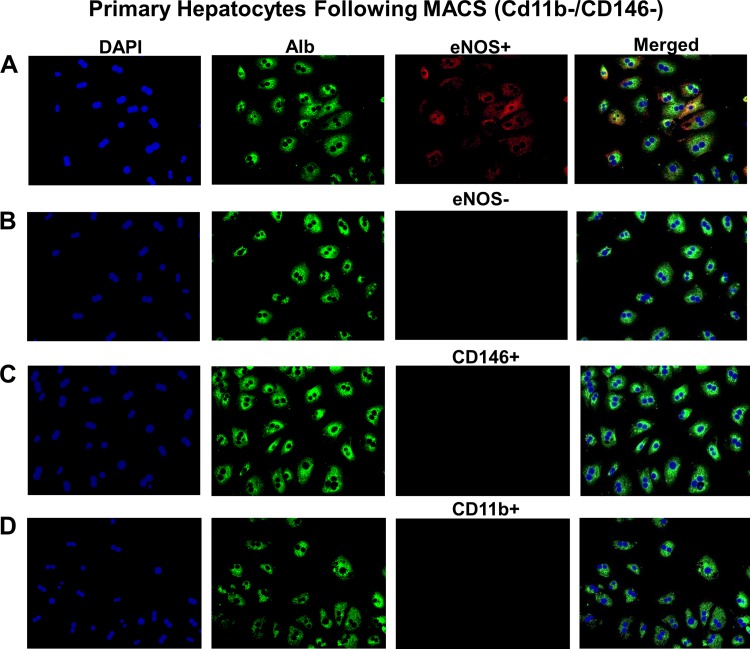
Hepatic eNOS protein content was unaffected by diet in WT mice, though its activation status via Ser1177 phosphorylation was decreased with WD (Fig. 1A). This is consistent with our previous report (44). Neither eNOS nor p-Ser1177 eNOS was detectable in eNOS KO mice. We next asked whether eNOS was detectable in isolated and cultured primary hepatocytes. Indeed, eNOS was present in WT primary hepatocyte cultures but not in hepatocytes isolated from eNOS KO mice (Fig. 1B). Next, we used siRNA to knockdown eNOS in WT primary hepatocytes and observed an ~50% decrease in eNOS mRNA and protein content (Fig. 1C). To address the possibility of nonhepatocyte contamination giving a false positive in our primary hepatocyte experiments, we used MACS to separate liver cell types following two-step collagenase digestion. Antibodies against CD11b and CD146 were used to separate endothelial cells and Kupffer cells, respectively, from hepatocytes. CD11b−/CD146− cells were cultured and stained for albumin (hepatocyte-specific marker) and eNOS (Fig. 2). These cells showed a clear hepatocyte morphology and were positive for both albumin and eNOS and negative for CB11b and CD146. Thus, our results strongly support the presence of eNOS in purified hepatocytes.
Fig. 1.
Endothelial NO synthase (eNOS) in liver tissue and primary hepatocytes. A: representative Western blot for eNOS and phosphorylated (p-)S1177 eNOS in liver homogenates from wild-type (WT) and eNOS knockout (KO) mice fed either control (CON) or Western diet (WD), n = 7–8/group. B: Western blot in cultured primary hepatocyte lysates from WT and eNOS KO mice. Blots on the left and right are from independent experiments (n = 5/genotype). C: small interfering (si)RNA-mediated knockdown of eNOS mRNA (left) and protein content (right) in WT hepatocytes; n = 7/group of independent replicates. A.U., arbitrary units; N.D., not detected; SCR, scramble. *P < 0.05 by paired two-tailed t-test.
Fig. 2.
Endothelial NO synthase (eNOS) expression in purified hepatocytes. A: immunofluorescence for albumin (Alb) and eNOS in cultured hepatocytes following magnetic-activated cell sorting (MACS) purification from WT mice. B–D: same experiment as in A except primary antibody was omitted (B) or primary antibody for CD146 (C) or CD11b (D) was used to confirm removal of CD146+ and CD11b-positive cell types from MACS-purified hepatocyte cultures.
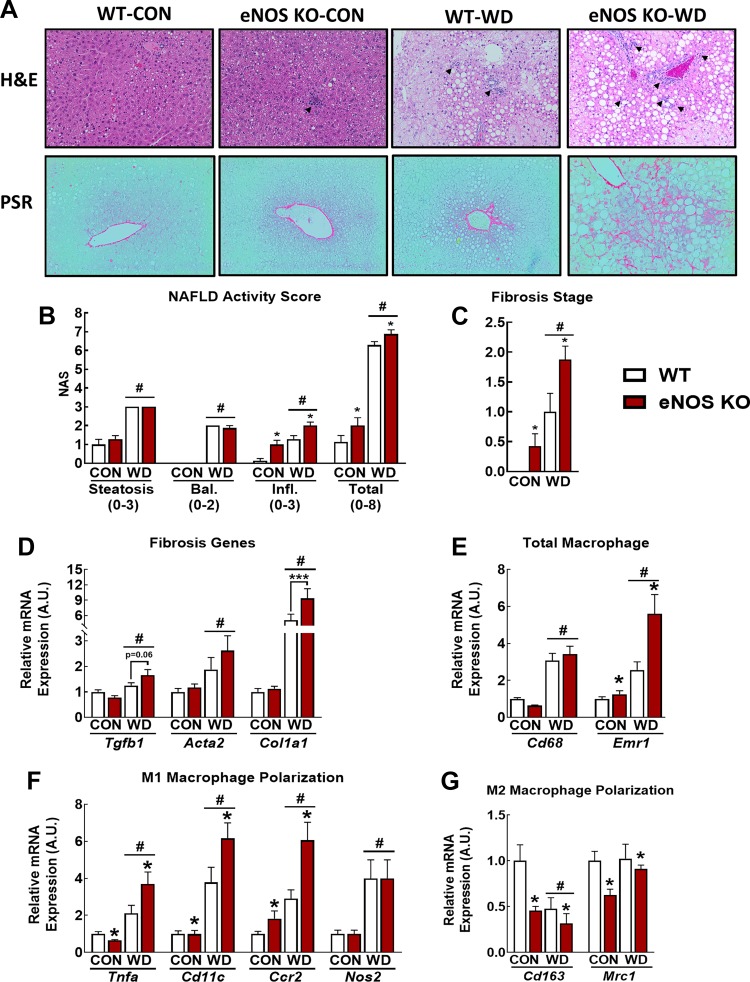
eNOS and NO modulate multiple disease processes associated with NASH, yet this role remains to be clearly defined (44, 45). To address this, we examined the liver phenotype in WT and eNOS KO mice fed either CON or WD (45% fat, 17% sucrose, 1% wt/wt cholesterol) for 18 wk to induce NASH. Portions of the animal characteristics from these mice have been previously published (16) and are summarized in Table 2. WD feeding induced widespread hepatic steatosis (Fig. 3A) and biochemical TAG accumulation (Table 2) in WT and eNOS KO mice. Gross histological examination revealed that eNOS KO mice were more susceptible to WD-induced hepatic inflammatory cell infiltration and perivenular and perisinusoidal fibrosis compared with WT mice (Fig. 3A). NAFLD activity score (NAS) revealed that eNOS KO mice on both diets had significantly increased total NAS relative to WT counterparts. This was driven by an increase in hepatocellular inflammation (Fig. 3B). Furthermore, fibrosis scores were significantly elevated in eNOS KO mice compared with WT mice (Fig. 3C). We confirmed these histological findings with qPCR analysis of mRNA markers of inflammation and fibrosis. WD feeding increased the expression of key genes involved in fibrogenesis [tranforming growth factor-β1 (Tgfb1), collagen I-α1 (Col1α1)] in eNOS KO vs. WT mice (Fig. 3D). We also observed significant increased expression of markers of total macrophage content [EGF module-containing mucin-like receptor (Emr1)], M1 macrophage markers [tumor necrosis factor-α (Tnfa), Cd11c], and decreased expression of M2 macrophage polarization markers CD163 and CD206 (Mrc1) in eNOS KO vs. WT mice (Fig. 3, E–G). These findings substantiate a role for eNOS in the exacerbation of Western diet-induced NASH.
Table 2.
Animal and plasma characteristics from eNOS KO study
| WT |
eNOS KO |
|||
|---|---|---|---|---|
| CON | WD | CON | WD | |
| Final BW, g | 33.4 ± 0.6 | 45.8 ± 1.5* | 29.6 ± 0.7† | 37.8 ± 1.3*† |
| Body fat, % | 26.0 ± 0.9 | 41.3 ± 1.4* | 23.1 ± 1.7† | 35.6 ± 1.3*† |
| Energy intake, kcal/wk | 70.1 ± 1.4 | 83.4 ± 2.5* | 63.8 ± 1.6† | 72.7 ± 1.7*† |
| Liver TG, nmol/g wet wt | 23.0 ± 2.9 | 70.6 ± 4.9* | 16.8 ± 1.5 | 59.5 ± 3.3* |
| LW/BW, mg/g | 42.1 ± 1.0 | 62.5 ± 3.9* | 41.7 ± 1.1 | 60.7 ± 5.3* |
| Fat pad weight, g | 1.9 ± 0.1 | 3.7 ± 0.1* | 1.7 ± 0.2† | 2.5 ± 0.1*† |
| ALT, U/liter | 38.3 ± 4.8 | 210.6 ± 50.3* | 42.3 ± 5.3 | 250.8 ± 72.3* |
| Plasma FFA, mmol/L | 0.73 ± 0.05 | 0.69 ± 0.07 | 0.80 ± 0.05† | 0.92 ± 0.10† |
| Plasma TG, mg/dL | 51.1 ± 4.8 | 42.0 ± 3.3 | 59.9 ± 4.1 | 52.1 ± 9.7 |
| Total cholesterol, mg/dL | 145.4 ± 6.9 | 215.6 ± 25.7* | 134.6 ± 4.9 | 247.1 ± 25.6* |
| Insulin, ng/mL | 0.8 ± 0.1 | 2.4 ± 0.5* | 1.2 ± 0.2 | 3.2 ± 1.2* |
| Glucose, mg/dL | 290.5 ± 25.5 | 309.0 ± 30.4 | 316.1 ± 14.6 | 315.5 ± 46.4 |
| HOMA-IR | 17.5 ± 2.5 | 57.3 ± 12.4* | 27.9 ± 5.8 | 74.9 ± 30.6* |
Values are means ± SE; n = 7 or 8/group).eNOS, endothelial NO synthase; KO, knockout; CON, control; WD, Western diet; LW, liver weight. BW, body weight. TG, triglycerides. ALT, alanine aminotransferase. FFA, free fatty acids. HOMA-IR, homeostasis model assessment of insulin resistance.
Diet main effect;
genotype main effect.
Fig. 3.
Endothelial NO synthase knockout (eNOS KO) increases hepatic fibrosis and inflammation. A: representative liver hematoxylin-eosin (H&E; top) and Picrosirius red (PSR; bottom) stains in control (CON)- and Western diet (WD)-fed wild-type (WT) and eNOS KO mice. Arrowheads indicate immune cell infiltrate. B: nonalcoholic fatty liver disease (NAFLD) activity scores. C: fibrosis staging. D: qPCR markers of fibrosis. E: qPCR markers of total macrophage content. F: qPCR markers of proinflammatory M1 macrophage markers. G: qPCR markers of anti-inflammatory M2 macrophage polarization; n = 7–8 mice/group. #P < 0.05 diet main effect; *P < 0.05 genotype main effect; ***P < 0.05 significant post hoc pairwise comparison indicated by bracket.
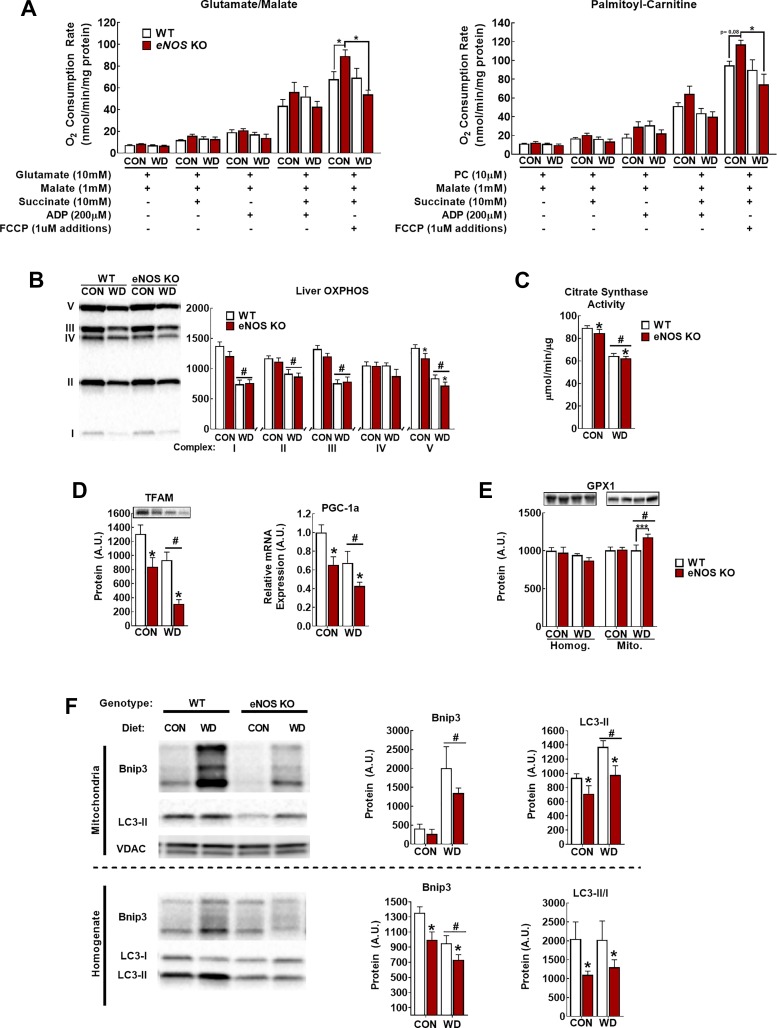
Diminished mitochondrial respiratory capacity, increased ROS production, and excess substrate pressure are important driving mechanisms of NASH (20, 38, 42, 48). Ex vivo mitochondrial respiration did not differ between genotypes except that maximal uncoupled respiration (+FCCP) was elevated in CON diet-fed eNOS KO mice compared with WT (Fig. 3). However, this elevated uncoupled respiration was lost with WD feeding in eNOS KO mice. This is interesting and in agreement with our previous report with systemic NOS inhibition in lean vs. obese rats (45). NO can compete with O2 binding in complex IV and throttle respiration (7, 8). In eNOS-deficient mice, there may be reduced throttling of the electron transport chain (ETC), which may lead to aberrant ETC flux and damage from the high reducing pressure introduced by WD feeding and perhaps have increased susceptibility to liver mitochondria oxidative damage. As expected, markers of hepatic mitochondrial content was decreased with WD feeding (Fig. 4, B and C); however, with the exception of small but significant decreases in complex V content and citrate synthase activity (P < 0.05, main effect of genotype), markers of hepatic mitochondrial content were not dramatically different in eNOS KO vs. WT mice (Fig. 4, B and C).
Fig. 4.
Endothelial NO synthase knockout (eNOS KO) alters mitochondrial function and turnover. A: glutamate/malate- and palmitoylcarnitine (PC)-supported respiration. B–C: assessment of mitochondrial content by oxidative phosphorylation (OXPHOS; B) and citrate synthase activity (C). D: markers of mitochondrial biogenesis peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) mRNA and mitochondrial transcription factor A (TFAM) protein. E: total liver (left) and mitochondrial (right) glutathione peroxidase-1 (GPX-1) content. F: representative Western blot images and densitometric quantification of BCL-2-interacting protein-3 (BNIP3) and light-chain 3B (LC3-II) in the mitochondrial fraction (top) and whole liver homogenate (bottom). VDAC, voltage-dependent anion-selective channel protein. Note that the arrangement of samples in representative Western blot image is not in the same order as the presentation of quantified data; n = 7–8 mice/group. #P < 0.05 diet main effect; *P < 0.05 genotype main effect; ***P < 0.05 significant post hoc pairwise comparison indicated by bracket.
eNOS also plays an important role in maintaining mitochondrial integrity through the regulation of mitochondrial biogenesis. Indeed, despite minimal effects of eNOS deficiency on markers of hepatic mitochondrial content, we observed substantial decreases in markers of mitochondrial biogenesis [peroxisome proliferator-activated receptor-γ coactivtor 1α (Pgc1a) mRNA and TFAM protein content; Fig. 4D]. This was associated with an increase in mitochondrial glutathione peroxidase-1 (GPX-1) content, suggestive of greater mitochondrial oxidative stress in the eNOS KO vs. WT mice. This led us to hypothesize that eNOS might clamp mitochondrial pool size under these conditions through concomitant regulation of mitophagy as well as biogenesis.
To address the potential role of eNOS in the regulation of mitophagy, we measured mitochondrial localization of the activated autophagosome coat protein LC3-II and mitochondrial BNIP3 content, a pro-mitophagy protein that can serve as a mitochondrial receptor for LC3-II (15, 57), as indicators of mitophagy. WD increased the protein content of BNIP3 (Fig. 4E) in the mitochondrial fraction, whereas activated mitochondrial LC3-II content was decreased in eNOS KO mice (Fig. 4E; LC3-I was not detected in isolated mitochondria). This indicates that WD drives activation of the LC3-II-BNIP3 mitophagy pathway and that this response is attenuated in the mitochondria from eNOS KO mice. Furthermore, in whole liver homogenate, both LC3-II/I ratio and BNIP3 protein content were decreased in eNOS KO vs. WT mice (Fig. 4E). We also assessed PTEN-induced kinase-1 (PINK1) and Parkin to gain insight into other major autophagic/mitophagic pathways, but these were either not different between groups or not detected, respectively (data not shown), suggesting a potential BNIP3-specific mechanism. Collectively, these data suggest that eNOS KO mice are unable to mount a normal mitophagic response to WD feeding.
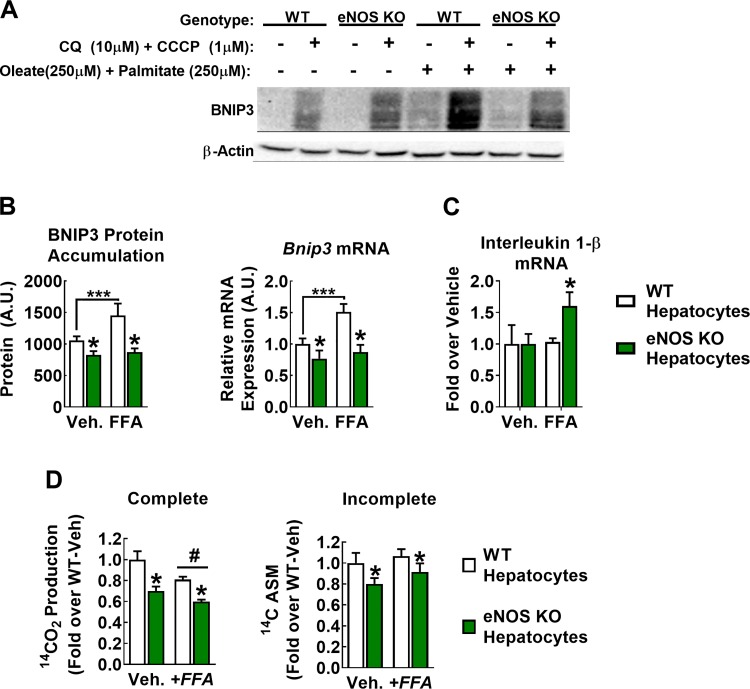
We next sought to identify whether the effects of eNOS KO on mitophagy occurred via a hepatocyte autonomous mechanism. Cultured WT and eNOS KO primary hepatocytes were treated with 10 µM CQ [an inhibitor of autophagosomal degradation (23)] and 1 µM CCCP (a mitochondrial uncoupler to induce depolarization, a stimulatory signal for mitophagy) (4, 12, 28). Since BNIP3 is typically cleared by the autophagosome, blocking this with CQ allows for assessment of BNIP3 accumulation as a proxy for the mitophagic drive of the cell. Preliminary experiments indicated that 16-h treatment at the concentrations indicated produced a significant accumulation of both LC3 and BNIP3 and that CCCP was necessary for BNIP3 induction. Primary hepatocytes from eNOS KO mice accumulated significantly less BNIP3 protein than WT cells when treated with CQ and CCCP (Fig. 5, A and B; P < 0.05). In addition, BNIP3 accumulation was further increased following 24 h of FFA challenge (250 µM oleate + 250 µM palmitate; +40%) in WT but not eNOS KO hepatocytes (P < 0.05; genotype × FFA interaction). This effect appeared to occur transcriptionally, as WT hepatocytes responded to this challenge with a ~50% increase (P < 0.05) in Bnip3 mRNA expression, whereas eNOS KO hepatocytes failed to respond (Fig. 5B). Importantly, FFA treatment increased expression on the proinflammatory cytokine interleukin-1β in eNOS KO but not WT hepatocytes (Fig. 5C). Finally, eNOS KO hepatocytes demonstrated diminished palmitate oxidation capacity compared with WT hepatocytes (Fig. 5D), indicative of reduced hepatocyte mitochondrial function in eNOS-deficient conditions. Collectively, these data support a hepatocellular autonomous role for eNOS in maintaining mitochondrial function.
Fig. 5.
Hepatocellular endothelial NO synthase (eNOS) regulates BCL-2-interacting protein-3 (BNIP3) flux in vitro. A–C: wild-type (WT) and eNOS knockout (KO) hepatocytes were exposed for 24 h with control starvation medium [−free fatty acid (FFA)] or starvation medium conditioned with 500 µM FFA (+FFA; 250 µM palmitate + 250 µM oleate) and treated with 10 µM chloroquine (CQ) and 1 µM CCCP for the final 16 h. A: representative blot of BNIP3 indicating that CQ + CCCP is necessary to induce BNIP3 protein accumulation. B, left: quantification of BNIP3 protein content only in samples that received CQ + CCCP. Right: in a separate experiment, 24-h fatty acid treatment increased BNIP3 mRNA expression in WT but not eNOS KO cells. A.U., arbitrary units; Veh., vehicle. Data are representative of 5–7 biological replicates. #P < 0.05 diet main effect; *P < 0.05 genotype main effect; ***P < 0.05 significant post hoc pairwise comparison indicated by bracket. C: 24-h fatty acid treatment induced mRNA expression of the proinflammatory cytokine interleukin-1β in eNOS KO but not WT hepatocytes. Data are representative of 4 biological replicates. *P < 0.05 paired t-test. D: complete (left) and incomplete (right) [1-14C]palmitate oxidation in WT and eNOS KO hepatocytes. Data are representative of 5–7 biological replicates. #P < 0.05 diet main effect; *P < 0.05 genotype main effect.
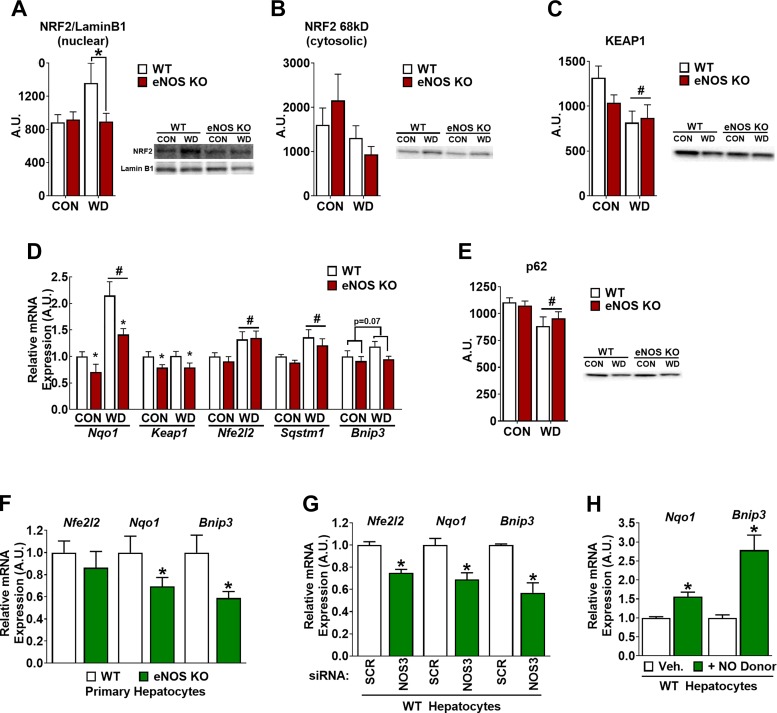
Here, we identified a novel eNOS-BNIP3 association. To gain mechanistic insight, we focused on the antioxidant and detoxifying transcription factor nuclear factor erythroid 2-related factor (2NFE2L2)/NRF2, as a potential link between eNOS and BNIP3. NRF2 can be activated by NO (22, 51) and has pro-autophagy/mitophagic effects through its transcriptional target p62 (Sqstm1). Consistent with its cell stress-responsive role, hepatic activation (nuclear localization) of NRF2 was elevated with WD feeding (P < 0.05, diet main effect) in WT mice. This effect was attenuated in eNOS KO mice (Fig. 6A; P < 0.05, genotype main effect). No genotype differences were observed in cytosolic NRF2 content (Fig. 6B) or mRNA expression (Fig. 6D), suggesting that decreased nuclear NRF2 in eNOS KO mice resulted from specific alteration in subcellular distribution rather than broad changes to the expression profile. Along these lines, protein content of KEAP1 (Fig. 6C), which binds and sequesters NRF2 in the cytosol content did not differ between genotypes.
Fig. 6.
Evidence for endothelial NO synthase (eNOS) regulation of nuclear factor erythroid 2-related factor 2 (NRF2) and BCL-2-interacting protein-3 (BNIP3). A: NRF2 protein content in hepatic nuclear extracts normalized to nuclear marker LaminB1. A.U., arbitrary units. Cytosolic NRF2 (B) and Kelch-like ECH-associated protein-1 (KEAP1; C) in whole liver lysates. Note that the arrangement of samples in representative Western blot image is not in the same order as the presentation of quantified data. D: mRNA expression of NRF2-associated genes in liver of wild-type (WT) and eNOS knockout (KO) mice fed control (CON) or Western diet (WD) for 18 wk. E: protein expression of the known mitophagy effector of NRF2 activation p62; n = 7–8/group. #Diet main effect P < 0.05; *genotype main effect P < 0.05. F: primary hepatocyte experiments: Nrf2, quinone oxidoreductase-1 (Nqo1), and Bnip3 mRNA expression in WT and eNOS KO hepatocytes. Data are representative of 6 biological replicates. *P < 0.05 vs. WT. G: effects of small interfering (si)RNA-mediated knockdown of eNOS in WT hepatocytes on Nrf2, Nqo1, and Bnip3 mRNA. *P < 0.05 vs. scramble (SCR) control; n = 4 biological replicates. H: effects of exogenous NO donor diethylenetriamine NONOate on Nqo1 and Bnip3 mRNA in primary hepatocytes. Data are representative of 6 independent experiments. *P < 0.05 vs. Vehicle.
Consistent with transcriptional activation by nuclear NRF2, a biomarker of NRF2 activation, NAD(P)H:quinone oxidoreductase-1 (Nqo1) mRNA expression, was induced in the liver with WD feeding (P < 0.05, diet main effect) and attenuated in eNOS KO mice (Fig. 6D; P < 0.05, genotype main effect). Interestingly, mRNA expression of Keap1 was also decreased in eNOS KO compared with WT mice (Fig. 6D; P < 0.05, genotype main effect), perhaps serving as a compensatory response to restore NRF2 activation. The hepatic mRNA expression of NRF2 and the autophagy adapter gene p62 (Sqstm1) were elevated with WD feeding (P < 0.05, diet main effect) but not affected by genotype (Fig. 6D). Similarly, p62 protein was decreased with WD but not affected by genotype despite decrease NRF2 activation with eNOS KO (Fig. 6E). In contrast, Bnip3 mRNA expression trended (P = 0.07) toward being decreased in (Fig. 6D), and protein content was significantly decreased in eNOS KO vs. WT liver (Fig. 4D). These data demonstrate a novel association between lack of eNOS and lower NRF2 and BNIP3 in the liver, which we sought to further clarify in primary hepatocytes.
Primary hepatocytes isolated from eNOS KO mice exhibited significant reductions in Nqo1 and Bnip3 mRNA expression compared with WT (Fig. 6F). In addition, siRNA-mediated knockdown of eNOS (NOS3) in WT primary hepatocytes (Fig. 1) resulted in attenuated expression of Nfe2l2 (NRF2), Nqo1, and Bnip3 (Fig. 6G). On the other hand, administration of the NO donor diethylenetriamine NONOate (50 µM) on WT hepatocytes significantly increased Nqo1 and Bnip3 mRNA expression (P < 0.05; Fig. 6H). These studies provide direct evidence in primary hepatocytes of the functional role of eNOS in maintaining antioxidant defense and regulation of BNIP3.
DISCUSSION
Functional alterations of hepatic mitochondria contribute to the etiology of NASH, yet underlying mechanisms remain poorly understood. Using a combined approach of systemic genetic eNOS knockout and primary hepatocyte culture, the present study demonstrates the importance of eNOS in NASH susceptibility and a potential novel role for hepatocellular eNOS in the maintenance of mitochondrial quality. We provide evidence that eNOS promotes mitochondrial quality through promotion of markers of mitophagy and antioxidant responsiveness via potential regulation of NRF2 and BNIP3. This work extends conventional knowledge of eNOS as a stimulator of mitochondrial biogenesis. Thus, loss of eNOS content/activation in NASH may contribute to mitochondrial dysfunction by causing reduced mitochondrial degradation and attenuated NRF2 activation, thus allowing for the accumulation of oxidative damage. Importantly, primary hepatocyte studies strongly support the notion that the observed hepatic mitochondrial effects of eNOS may be acting directly in the hepatocyte. This underscores the need to further delineate eNOS biology in hepatocytes and perhaps other hepatic cell types. Collectively, the current findings provide biological insight into eNOS as a regulator of mitochondrial quality control, which may have broad implications in other tissues and pathologies.
A discrepancy exists in the literature regarding the distribution of eNOS within cells of the liver. Some immunohistochemical evidence places eNOS exclusively in the sinusoidal lumen (43). However, other immunohistochemical and in situ hybridization data depict a more ubiquitous eNOS distribution in the liver, including within hepatocytes (24, 25). More importantly and more precisely, recent cell type-resolved proteomics have clearly identified that eNOS protein is positively expressed in hepatocytes (2), which we confirm here by utilizing the exact same MACS methodology followed by immunofluorescence in cultured primary hepatocytes. Our results extend these findings by delineating a physiological role for eNOS specifically in hepatocytes. Importantly, it should also be noted that eNOS is expressed in other cell types beyond the endothelium in other organ systems (reviewed in Ref. 11), including cardiomyocytes (10), skeletal myocytes (17, 18), developing glomeruli (14), and proximal tubule epithelial cells (41). Despite this, little evidence exists that ascribes a functional role to hepatocyte eNOS expression. Whether the role for hepatocellular eNOS that we describe in the current report extends to these other cell types remains to be determined.
The biological effects of NO are concentration dependent, and NO concentrations decay rapidly with distance from the originating source. Thus, hepatocellular eNOS could allow for cell-autonomous control over the magnitude and location of NO production. We propose that this maintains mitochondrial homeostasis on multiple levels by 1) attenuating unnecessary respiratory flux due to increased reducing equivalents by the known competitive inhibition of NO on cytochrome c oxidase (5, 6, 9), thus throttling respiration and potentially limiting ROS production 2), stimulating the NRF2 antioxidant response (Fig. 6 and in Ref. 51), and 3) coordinating mitochondrial turnover to limit the persistence of oxidative damage (Figs. 3, 4, and 6 and in Refs. 29–32, 52).
Our present data indicate that WD feeding induced markers of hepatic autophagy and mitophagy in the current study (Fig. 4). This is in contrast to previously published work that demonstrated that high-fat diet-fed mice exhibit decreased autophagic and mitophagic fluxes (47, 56). Whether the differences in the diet used or the severity of the NAFLD phenotype induced between these studies underlying this discrepancy is unclear. In addition, these processes are highly dynamic and are influenced by many factors including circadian rhythms and fasting length. Care should be taken to control for and report these variables in future investigations. It is clear that autophagy/mitophagy are involved in NAFLD and future efforts should work to characterize their role across the disease spectrum.
In conclusion, here, we demonstrate that the absence of eNOS leads to increased susceptibility to Western diet-induced NASH and fibrosis. In addition, primary hepatocyte culture studies reveal a hepatocyte-specific function for eNOS, which maintains mitochondrial homeostasis and hepatic inflammatory health, perhaps through either NRF2 and/or BNIP3. These findings unveil a potentially new mechanism though which hepatocytes can sense and respond to mitochondrial stress. Future studies should more thoroughly interrogate the role of hepatocyte-specific manipulation of eNOS in vivo, as well as explore the utility of NRF2/BNIP3 as potential rescue targets, in the context of mitochondrial health and NASH development.
GRANTS
Funding for this work was provided by Veterans Affaris Merit Grant I01BX003271 (R. S. Rector) and Veterans Affairs Merit Grant I01 RX000123 (J. P. Thyfault), National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-088940 (J. P. Thyfault), partially supported by American Heart Association Grant 14POST20110034 (E. M. Morris), University of Missouri Department of Medicine Research Council Grant (R. S. Rector), the Missouri Foundation for Medical Research (R. S. Rector), and Richard Wallace Foundation (R. S. Rector). This work was supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.D.S., G.M.M., E.M.M., M.A.L., J.P.T., M.H.L., and R.S.R. conceived and designed research; R.D.S., G.M.M., E.M.M., M.A.L., and R.P.C. performed experiments; R.D.S., G.M.M., M.A.L., R.P.C., and R.S.R. analyzed data; R.D.S., G.M.M., E.M.M., M.A.L., R.P.C., J.I., J.P.T., M.H.L., and R.S.R. interpreted results of experiments; R.D.S., G.M.M., and R.S.R. prepared figures; R.D.S. and R.S.R. drafted manuscript; R.D.S., R.P.C., J.I., J.P.T., M.H.L., and R.S.R. edited and revised manuscript; R.D.S., G.M.M., E.M.M., M.A.L., R.P.C., J.I., J.P.T., M.H.L., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Tasnim Haq, Radheya Naik, and Kathryn Phillips for excellent assistance with data collection.
REFERENCES
- 1.Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 59: 1174–1197, 2014. doi: 10.1002/hep.26717. [DOI] [PubMed] [Google Scholar]
- 2.Azimifar SB, Nagaraj N, Cox J, Mann M. Cell-type-resolved quantitative proteomics of murine liver. Cell Metab 20: 1076–1087, 2014. doi: 10.1016/j.cmet.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol 20: 1724–1745, 2014. doi: 10.3748/wjg.v20.i7.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berezhnov AV, Soutar MP, Fedotova EI, Frolova MS, Plun-Favreau H, Zinchenko VP, Abramov AY. Intracellular pH Modulates Autophagy and Mitophagy. J Biol Chem 291: 8701–8708, 2016. doi: 10.1074/jbc.M115.691774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta 1504: 46–57, 2001. doi: 10.1016/S0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 6.Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta 1658: 44–49, 2004. doi: 10.1016/j.bbabio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 356: 295–298, 1994. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 8.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett 345: 50–54, 1994. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 9.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci USA 95: 7631–7636, 1998. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felaco M, Grilli A, De Lutiis MA, Patruno A, Libertini N, Taccardi AA, Di Napoli P, Di Giulio C, Barbacane R, Conti P. Endothelial nitric oxide synthase (eNOS) expression and localization in healthy and diabetic rat hearts. Ann Clin Lab Sci 31: 179–186, 2001. [PubMed] [Google Scholar]
- 11.Förstermann U, Boissel JP, Kleinert H. Expressional control of the ‘constitutive’ isoforms of nitric oxide synthase (NOS I and NOS III). FASEB J 12: 773–790, 1998. doi: 10.1096/fasebj.12.10.773. [DOI] [PubMed] [Google Scholar]
- 12.Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12: 119–131, 2010. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 12a.Glick D, Zhang W, Beaton M, Marsboom G, Gruber M, Simon MC, Hart J, Dorn GW, 2nd, Brady MJ, Macleod KF. BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol 32: 2570–2584, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guguen-Guillouzo C. Isolation and culture of animal and human hepatocytes. In Culture of Epithelial Cells (2nd Ed.). Edited by Freshney R. Ian and Freshney Mary G Hoboken, NJ: Wiley-Liss, 2002, p. 337–379, 2002. [Google Scholar]
- 14.Han KH, Lim JM, Kim WY, Kim H, Madsen KM, Kim J. Expression of endothelial nitric oxide synthase in developing rat kidney. Am J Physiol Renal Physiol 288: F694–F702, 2005. doi: 10.1152/ajprenal.00085.2004. [DOI] [PubMed] [Google Scholar]
- 15.Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson AB. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem 287: 19094–19104, 2012. doi: 10.1074/jbc.M111.322933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurrissen TJ, Sheldon RD, Gastecki ML, Woodford ML, Zidon TM, Rector RS, Vieira-Potter VJ, Padilla J. Ablation of eNOS does not promote adipose tissue inflammation. Am J Physiol Regul Integr Comp Physiol 310: R744–R751, 2016. doi: 10.1152/ajpregu.00473.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapur S, Bédard S, Marcotte B, Côté CH, Marette A. Expression of nitric oxide synthase in skeletal muscle: a novel role for nitric oxide as a modulator of insulin action. Diabetes 46: 1691–1700, 1997. doi: 10.2337/diab.46.11.1691. [DOI] [PubMed] [Google Scholar]
- 18.Kawagishi K, Terasawa F, Nakamura A, Moriizumi T, Ueda H. Endothelial nitric oxide synthase expression in the sarcoplasmic reticulum of mouse skeletal muscle. Acta Histochem Cytochem 37: 307–311, 2004. doi: 10.1267/ahc.37.307. [DOI] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 20.Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, Schlensak M, Roden M. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 21: 739–746, 2015. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Lee WJ, Tateya S, Cheng AM, Rizzo-DeLeon N, Wang NF, Handa P, Wilson CL, Clowes AW, Sweet IR, Bomsztyk K, Schwartz MW, Kim F. M2 macrophage polarization mediates anti-inflammatory effects of endothelial nitric oxide signaling. Diabetes 64: 2836–2846, 2015. doi: 10.2337/db14-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CQ, Kim MY, Godoy LC, Thiantanawat A, Trudel LJ, Wogan GN. Nitric oxide activation of Keap1/Nrf2 signaling in human colon carcinoma cells. Proc Natl Acad Sci USA 106: 14547–14551, 2009. doi: 10.1073/pnas.0907539106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, Coppes RP, Engedal N, Mari M, Reggiori F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 14: 1435–1455, 2018. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNaughton L, Puttagunta L, Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, Hamid Q, Radomski MW. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc Natl Acad Sci USA 99: 17161–17166, 2002. doi: 10.1073/pnas.0134112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mei Y, Thevananther S. Endothelial nitric oxide synthase is a key mediator of hepatocyte proliferation in response to partial hepatectomy in mice. Hepatology 54: 1777–1789, 2011. doi: 10.1002/hep.24560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan S, Reddick RL, Musi N, Horn DA, Yan B, Prihoda TJ, Natarajan M, Abboud-Werner SL. Diabetic eNOS knockout mice develop distinct macro- and microvascular complications. Lab Invest 88: 515–528, 2008. doi: 10.1038/labinvest.2008.23. [DOI] [PubMed] [Google Scholar]
- 27.Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol 303: G979–G992, 2012. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183: 795–803, 2008. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci 119: 2855–2862, 2006. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- 30.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299: 896–899, 2003. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 31.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba MO, Moncada S, Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA 101: 16507–16512, 2004. [Erratum in Proc Natl Acad Sci USA 102: 5635, 2005]. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314–317, 2005. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 33.Nozaki Y, Fujita K, Wada K, Yoneda M, Shinohara Y, Imajo K, Ogawa Y, Kessoku T, Nakamuta M, Saito S, Masaki N, Nagashima Y, Terauchi Y, Nakajima A. Deficiency of eNOS exacerbates early-stage NAFLD pathogenesis by changing the fat distribution. BMC Gastroenterol 15: 177, 2015. doi: 10.1186/s12876-015-0409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasarín M, Abraldes JG, Rodríguez-Vilarrupla A, La Mura V, García-Pagán JC, Bosch J. Insulin resistance and liver microcirculation in a rat model of early NAFLD. J Hepatol 55: 1095–1102, 2011. doi: 10.1016/j.jhep.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 35.Pasarín M, La Mura V, Gracia-Sancho J, García-Calderó H, Rodríguez-Vilarrupla A, García-Pagán JC, Bosch J, Abraldes JG. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS One 7: e32785, 2012. doi: 10.1371/journal.pone.0032785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rector RS, Morris EM, Ridenhour S, Meers GM, Hsu FF, Turk J, Ibdah JA. Selective hepatic insulin resistance in a murine model heterozygous for a mitochondrial trifunctional protein defect. Hepatology 57: 2213–2223, 2013. doi: 10.1002/hep.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 38.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol 300: G874–G883, 2011. doi: 10.1152/ajpgi.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Enriquez S, Kai Y, Maldonado E, Currin RT, Lemasters JJ. Roles of mitophagy and the mitochondrial permeability transition in remodeling of cultured rat hepatocytes. Autophagy 5: 1099–1106, 2009. doi: 10.4161/auto.5.8.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudnicki M, Eder S, Perco P, Enrich J, Scheiber K, Koppelstätter C, Schratzberger G, Mayer B, Oberbauer R, Meyer TW, Mayer G. Gene expression profiles of human proximal tubular epithelial cells in proteinuric nephropathies. Kidney Int 71: 325–335, 2007. doi: 10.1038/sj.ki.5002043. [DOI] [PubMed] [Google Scholar]
- 42.Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston KA, Fu X, Merritt ME, Sherry AD, Malloy CR, Shelton JM, Lambert J, Parks EJ, Corbin I, Magnuson MA, Browning JD, Burgess SC. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest 125: 4447–4462, 2015. doi: 10.1172/JCI82204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah V, Haddad FG, Garcia-Cardena G, Frangos JA, Mennone A, Groszmann RJ, Sessa WC. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J Clin Invest 100: 2923–2930, 1997. doi: 10.1172/JCI119842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheldon RD, Laughlin MH, Rector RS. Reduced hepatic eNOS phosphorylation is associated with NAFLD and type 2 diabetes progression and is prevented by daily exercise in hyperphagic OLETF rats. J Appl Physiol (1985) 116: 1156–1164, 2014. doi: 10.1152/japplphysiol.01275.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheldon RD, Padilla J, Jenkins NT, Laughlin MH, Rector RS. Chronic NOS inhibition accelerates NAFLD progression in an obese rat model. Am J Physiol Gastrointest Liver Physiol 308: G540–G549, 2015. doi: 10.1152/ajpgi.00247.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M, Pandak WM, Dent P, Spiegel S, Shi R, Xu W, Liu X, Bohdan P, Zhang L, Zhou H, Hylemon PB. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology 55: 267–276, 2012. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun N, Yun J, Liu J, Malide D, Liu C, Rovira II, Holmström KM, Fergusson MM, Yoo YH, Combs CA, Finkel T. Measuring in vivo mitophagy. Mol Cell 60: 685–696, 2015. doi: 10.1016/j.molcel.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 14: 804–810, 2011. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tateya S, Rizzo NO, Handa P, Cheng AM, Morgan-Stevenson V, Daum G, Clowes AW, Morton GJ, Schwartz MW, Kim F. Endothelial NO/cGMP/VASP signaling attenuates Kupffer cell activation and hepatic insulin resistance induced by high-fat feeding. Diabetes 60: 2792–2801, 2011. doi: 10.2337/db11-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L, Fabris R, Serra R, Quarta M, Reggiani C, Nisoli E, Vettor R. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 63: 2800–2811, 2014. doi: 10.2337/db13-1234. [DOI] [PubMed] [Google Scholar]
- 51.Um HC, Jang JH, Kim DH, Lee C, Surh YJ. Nitric oxide activates Nrf2 through S-nitrosylation of Keap1 in PC12 cells. Nitric Oxide 25: 161–168, 2011. doi: 10.1016/j.niox.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Vettor R, Valerio A, Ragni M, Trevellin E, Granzotto M, Olivieri M, Tedesco L, Ruocco C, Fossati A, Fabris R, Serra R, Carruba MO, Nisoli E. Exercise training boosts eNOS-dependent mitochondrial biogenesis in mouse heart: role in adaptation of glucose metabolism. Am J Physiol Endocrinol Metab 306: E519–E528, 2014. doi: 10.1152/ajpendo.00617.2013. [DOI] [PubMed] [Google Scholar]
- 53.Werner M, Driftmann S, Kleinehr K, Kaiser GM, Mathé Z, Treckmann JW, Paul A, Skibbe K, Timm J, Canbay A, Gerken G, Schlaak JF, Broering R. All-in-one: advanced preparation of human parenchymal and non-parenchymal liver cells. PLoS One 10: e0138655, 2015. doi: 10.1371/journal.pone.0138655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 59: 2188–2195, 2014. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 55.Xie G, Wang X, Wang L, Wang L, Atkinson RD, Kanel GC, Gaarde WA, Deleve LD. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 142: 918–927.e6, 2012. doi: 10.1053/j.gastro.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 11: 467–478, 2010. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Y, Massen S, Terenzio M, Lang V, Chen-Lindner S, Eils R, Novak I, Dikic I, Hamacher-Brady A, Brady NR. Modulation of serines 17 and 24 in the LC3-interacting region of Bnip3 determines pro-survival mitophagy versus apoptosis. J Biol Chem 288: 1099–1113, 2013. doi: 10.1074/jbc.M112.399345. [DOI] [PMC free article] [PubMed] [Google Scholar]