Abstract
Leptin administration into the hindbrain, and specifically the nucleus of the solitary tract, increases phosphorylated signal transducer and activator of transcription 3 (pSTAT3), a marker of leptin receptor activation, in hypothalamic nuclei known to express leptin receptors. The ventromedial nucleus of the hypothalamus (VMH) shows the greatest response, with a threefold increase in pSTAT3. This experiment tested the importance of VMH leptin receptor-expressing neurons in mediating weight loss caused by fourth ventricle (4V) leptin infusion. Male Sprague-Dawley rats received bilateral VMH 75-nL injections of 260 ng/μL of leptin-conjugated saporin (Lep-Sap) or blank-saporin (Blk-Sap). After 23 days they were fitted with 4V infusion cannulas and 1 wk later adapted to housing in a calorimeter before they were infused with 0.9 μg leptin/day for 14 days. There was no effect of VMH Lep-Sap on weight gain or glucose clearance before leptin infusion. Leptin inhibited food intake and respiratory exchange ratio in Blk-Sap but not Lep-Sap rats. Leptin had no effect on energy expenditure or brown adipose tissue temperature of either group. Inguinal and epididymal fat were significantly reduced in leptin-treated Blk-Sap rats, but the response was greatly attenuated in Lep-Sap rats. VMH pSTAT3 was increased in leptin-treated Blk-Sap but not Lep-Sap rats. These results support the concept that leptin-induced weight loss results from an integrated response across different brain areas. They also support previous reports that VMH leptin receptors do not play a significant role in maintaining energy balance in basal conditions but limit weight gain during positive energy balance.
Keywords: body fat, food intake, integration, leptin-saporin, rats
INTRODUCTION
Since the discovery of leptin in 1994 (44), a majority of the work investigating the central mechanisms by which leptin inhibits food intake has focused on the arcuate nucleus of the hypothalamus (Arc) (13) and, to a lesser extent, the nucleus of the solitary tract (NTS) in the hindbrain (17). Recently, we have investigated the integration of leptin activity in the forebrain and hindbrain. Infusion of subthreshold doses of leptin into either the third or the fourth ventricle (3V, 4V) that do not inhibit food intake when given independently causes hypophagia and significant weight loss when applied simultaneously (8, 18), suggesting that under normal physiological conditions the energetic response to leptin is achieved through a coordinated response from multiple brain areas. Leptin responsiveness of hypothalamic nuclei was identified by measurement of phosphorylated signal transducer and activator of transcription 3 (pSTAT3), which is considered a reliable marker of leptin receptor (ObR) activation (39). Expression of ΔFosB, an early-response gene that accumulates in cells subjected to chronic activation (5), was used to confirm that forebrain activation in rats receiving simultaneous 3V and 4V infusions of leptin was limited to the hypothalamic areas that showed increased levels of pSTAT3 (9). Further support for an integrated leptin response by the forebrain and hindbrain is provided by evidence that 4V infusions (20) or injections (38) of leptin that are high enough to cause weight loss are associated with increased pSTAT3 expression in the same hypothalamic areas as were identified in the dual-infusion experiments.
The hypothalamic areas that respond to simultaneous 3V and 4V infusions of leptin and to 4V infusions of leptin are limited to the Arc, the ventromedial nucleus of the hypothalamus (VMH), the dorsomedial hypothalamus (DMH), and the posterior hypothalamus, all of which are known to express the long-form leptin receptor (ObRb). Of these, the dorsomedial portion of the ventromedial hypothalamus (VMHdm) stands out, in that there is a threefold increase in pSTAT3 under conditions of leptin-induced weight loss (9, 20). This is a much larger response than the other nuclei identified in the dual-infusion experiments. A role for the VMH as a mediator in leptin control of energy balance is supported by previous observations that twice-daily bilateral injections of 50 ng of leptin into the VMH of rats caused a 50% reduction in food intake and significant weight loss (25). The weight loss was fully reversed once the injections stopped. In mice, the use of Cre recombinase to selectively delete ObRb in steroidogenic factor 1 (SF1) neurons of the VMH resulted in an 80% reduction in VMH pSTAT3 after a peripheral leptin injection and an absence of depolarization and activation of SF1 neurons by leptin (10). The mice in which ObRb had been ablated showed a 15% increase in weight gain over 20 wk compared with control mice, which was accounted for by an increase in body fat with no change in growth of lean tissue, bone density, reproductive function, or function of the hypothalamic-pituitary-adrenal axis. The loss of VMH ObRb increased sensitivity to high-fat diets, which was attributed to a failure to promote diet-induced thermogenesis (10). These results were confirmed in a second publication (3) in which high-fat-fed knockout mice were fatter, hypertriglyceridemic, and glucose intolerant compared with their wild-type controls, although there was no obvious phenotype on a low-fat diet.
The objective of the experiment described here was to determine the importance of the VMH in the central response to leptin and specifically in weight loss caused by 4V infusions. This was achieved by deleting ObR-expressing neurons in the VMH with site-specific injections of leptin-conjugated saporin (Lep-Sap). Saporin is a ribosome-inactivating protein. Lep-Sap binds to leptin receptors, is internalized, and causes cell death (43). Controls in this experiment were animals that received VMH injections of saporin conjugated with a nonsense sequence peptide [blank-saporin (Blk-Sap)]. Wiater et al. (42) and Li et al. (28) have previously used Lep-Sap to delete ObR-expressing neurons in the Arc of rats as part of an investigation of feeding rhythms. These animals became obese, stabilizing at a new increased weight ~8 wk after the Lep-Sap injections. Lep-Sap produced a >80% reduction in cells that were known to express ObRb. In addition to making injections into the Arc, Li et al. (28) injected Lep-Sap into the VMH as a control for the specificity of their Arc injections. The VMH injections appeared to be very effective in deleting ObR-expressing cells, but there was only a small increase in body weight compared with the extreme obesity induced by Lep-Sap injections into the Arc. Others have used adeno-associated virally mediated RNA interference to knock down ObRb in the hindbrain NTS (24, 26) but found only a 40% reduction in receptor expression. Therefore, we chose to use Lep-Sap for receptor ablation, acknowledging the limitation that Lep-Sap resulted in loss of all activity of ObR-expressing neurons and not just those activities initiated by leptin.
METHODS
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Augusta University and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (35). Male Sprague-Dawley rats weighing 275–300 g (Envigo, Indianapolis, IN) were initially housed in individual hanging wire cages in a room maintained at 21–23°C with lights on from 7 AM to 7 PM. They had free access to chow (Harlan Teklad Rodent Diet 8604) and water unless indicated otherwise. A Nylabone (Nylabone Products, Neptune, NJ) was placed in each cage for enrichment. Body weights were recorded daily.
Dye injections were used to confirm injection coordinates for the VMHdm. Rats were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg). Injections of 100 nL of 0.9% India ink diluted 1:4 with 0.9% saline were made with a 28-gauge needle from a Hamilton syringe with a manual stereotaxic injector (Stoelting, Wood Dale, IL). All injections were delivered over 2 min, and the needle was held in place for an additional 2 min before being withdrawn. Thirty minutes after injection, rats were perfused with 300 mL of 0.9% heparinized saline followed by 500 mL of 4% paraformaldehyde. Brains were collected and fixed in 4% paraformaldehyde at 4°C overnight and then transferred to 25% sucrose azide solution and stored at 4°C. Serial 30-μm sections were made through the hypothalamus to view the dye injection site. Appropriate coordinates were determined to be 2.9 mm posterior to bregma, ±0.4 mm lateral to the midline, and 9.3 mm dorsal to the surface of the skull.
To determine an appropriate injection volume of conjugated saporin that would be limited to dispersion through the VMHdm, rats were anesthetized with ketamine and xylazine and treated with analgesic (2 mg/kg ketofen injected subcutaneously: Fort Dodge Animal Health, Fort Dodge, IA). Lep-Sap at a concentration of 260 ng/μL (Lep-SAP kit; Advanced Targeting Systems, San Diego, CA) was injected in volumes of 25, 50, or 100 nL. After 4 h, rats were anesthetized a second time and perfused. Brains were sectioned, and Lep-Sap diffusion was visualized by immunohistochemistry using a primary anti-saporin antibody (1:5,000 dilution, AB-15; Advanced Targeting Systems) with the secondary antibody Alexa Fluor 488 Donkey Anti-Goat IgG (AffiniPure, Jackson ImmunoResearch Laboratories, West Grove, PA).
Immunohistochemistry for pSTAT3 was used to test for effective deletion of ObR-expressing cells by Lep-Sap. Rats were anesthetized with ketamine-xylazine and treated with analgesic immediately before surgery and again 24 h after surgery. Bilateral 75-nL injections of 260 ng/μL Lep-Sap or Blk-Sap were made into the VMH. Twenty days after surgery the rats were food deprived for 4 h and then were given intraperitoneal injections of 1 mg leptin/kg (recombinant rat leptin; R&D Systems, Minneapolis, MN). Exactly 45 min later each rat was anesthetized and perfused with paraformaldehyde and the brains collected. Immunohistochemistry for pSTAT3 was performed on free-floating 30-μm coronal sections with the protocol described by Ellacott et al. (12). The primary antibody was rabbit anti-pSTAT3 (Tyr705) (catalog no. 9145, Cell Signaling, Danvers, MA), and pSTAT3-positive nuclei were detected with ABC and DAB Kits (Vector Laboratories, Burlingame, CA). Images were obtained with an Olympus BX51 system microscope.
A separate set of rats were used to confirm the loss of ObR-expressing cells by RNAscope in situ hybridization (Advanced Cell Diagnostics, Middleton, WI). Rats were treated with Lep-Sap or Blk-Sap, and brains were collected after perfusion with paraformaldehyde 22 days after saporin injections. The fixed brains were frozen in optimal cutting temperature compound, and 14-µm sections were collected and mounted onto Superfrost Plus slides. RNAscope was performed according to manufacturer’s directions for the 2.5 HD Detection Reagents-RED assay with the RNAscope Probe-Hs-LEPR probe. Sections were counterstained with 25% hematoxylin and coverslipped with Ecomount Mounting medium (Advanced Cell Diagnostics).
To test the importance of VMHdm ObR-expressing neurons in the energetic response to 4V leptin infusions, a total of 44 rats weighing 300−330 g received bilateral VMH injections of either Lep-Sap or Blk-Sap, as described above. Because we have only 12 calorimeter cages the experiment was carried out with four cohorts of rats, with the different treatment groups represented in each cohort. Daily body weights were recorded throughout the experiment. Twenty-two days after the saporin injections, rats were fitted with 26-gauge 4V infusion cannulas (Plastics One, Roanoke, VA) placed on the midline, 2.5 mm anterior to the occipital suture and 6.5 mm ventral to the skull surface (36). After 1 wk of recovery, a glucose tolerance test (GTT) was performed. Food was removed from the cages at 8:00 AM. Starting at 12:00 PM tail blood was collected for glucose measurements with Easy Gluco blood glucose test trips (US Diagnostics, Huntsville, AL), and a 200-μL blood sample was taken for insulin measurement (rat insulin radioimmunoassay kit; Millipore Sigma, Burlington, MA). The rats were injected intraperitoneally with 1 g glucose/kg. Blood glucose was measured 15, 30, 45, 60, 90, and 120 min after the injection. Additional blood samples were collected 15, 30, 60, and 120 min after injection for measurement of insulin. Food was returned to the cages at the end of the GTT.
The following day animals were placed in individual indirect calorimeter cages (TSE LabMaster Metabolic Research Platform; TSE Systems International, Chesterfield, MO). Air was sampled from each cage for 3 min every 39 min, and oxygen consumption and carbon dioxide production were calculated from values recorded during the last minute of sampling. Food intake, energy expenditure, and respiratory exchange ratio as an index of macronutrient oxidation were recorded every 39 min. Each morning the calorimeter was stopped at 8:30 AM for 30 min while rats were weighed, bedding was changed as necessary, and food hoppers and water bottles were refilled. The rats habituated to the calorimeter cages for 5 days, and then an Alzet miniosmotic pump (model 1002; Durect, Cupertino, CA) with an infusion rate of 0.25 µL/h was attached to each cannula and this was considered day 0 of the experiment. The pumps delivered either isotonic saline or 0.9 μg leptin/day. At the same time as the pumps were attached, iButtons (Embedded Data Systems, Lawrenceburg, KY) were placed over interscapular brown adipose tissue (IBAT). IBAT temperature was measured every 30 min starting 24 h after placement until the end of the study. Four rats were removed from the experiment because they stopped eating once the pumps were attached to the cannulas. It was assumed that this was due to a misplaced cannula.
On day 14 of infusion, food was removed from the cages at 8:30 AM. At 10:00 AM a blood sample was collected from the tail of each rat for measurement of serum leptin (Millipore Multi-Species Leptin RIA Kit; Millipore Sigma). Starting at 12:30 PM rats were anesthetized with ketamine and xylazine. One epididymal and one inguinal fat pad were dissected and weighed, and then the rats were perfused for collection of the brain. pSTAT3-positive nuclei in the hypothalamus were detected by immunohistochemistry as described above and quantified by manual counting. pSTAT3-positive nuclei were quantified in the dorsomedial nucleus, VMHdm, ventrolateral nucleus of the VMH (VMHvl), and medial and lateral Arc. Bilateral expression was determined on one section at each of four different levels between −2.56 and –3.30 mm from bregma [plates 30−34 of the Paxinos and Watson rat brain atlas (36)], and the expression from the four sections was summed for each rat.
If the level of pSTAT3 in the VMHdm of a Lep-Sap rat was equal to or higher than the average amount of pSTAT3 measured in PBS-infused Blk-Sap rats, then it was assumed that the Lep-Sap injection had not been effective; two leptin-infused Lep-Sap rats were removed from the experiment for this reason. Thus 38 rats completed the experiment: 10 Blk-Sap PBS, 8 Blk-Sap Lep, 11 Lep-Sap PBS, and 9 Lep-Sap Lep.
STATISTICA software version 9.0 (StatSoft, Tulsa, OK) was used for all statistical analysis. Differences were considered significant at P < 0.05. Repeated-measures ANOVA was used to compare daily measurements. If the repeated measures indicated a significant interaction, then two-way ANOVA was used to compare values on individual days. Tukey’s honestly significant difference (HSD) post hoc test was used to identify differences between individual groups when the ANOVA indicated a significant overall effect or interaction. Single end point measures were compared by two-way ANOVA and Tukey’s HSD post hoc test.
RESULTS
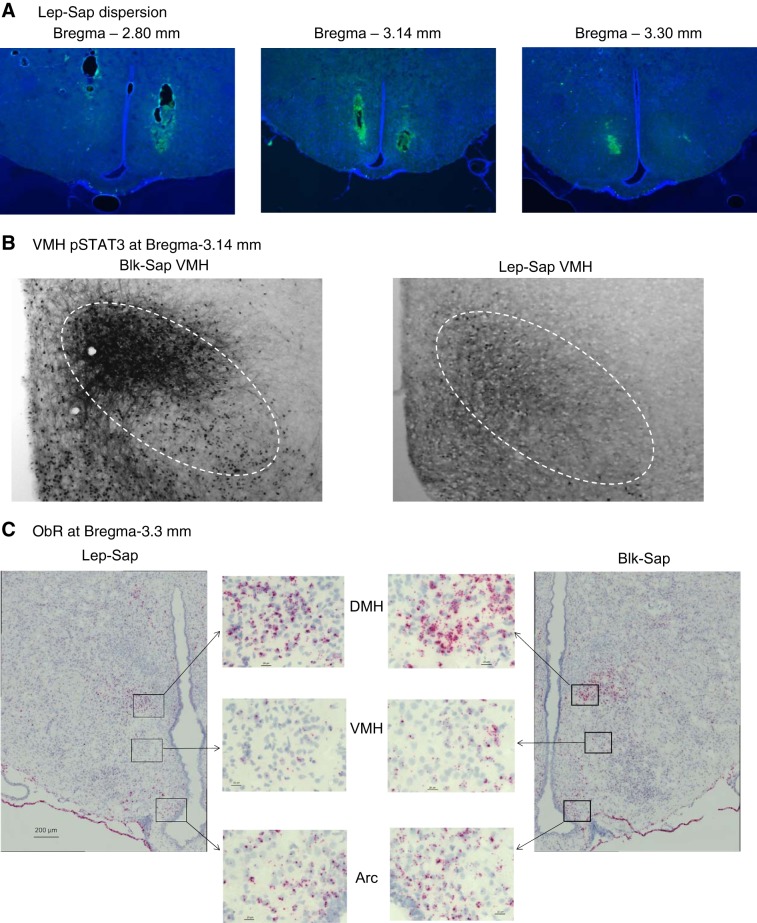
Preliminary studies detecting the dispersion of Lep-Sap injections determined that 75 nL of a solution containing 260 μg/μL was the appropriate volume needed to selectively delete ObR-expressing neurons in the VMH (Fig. 1A). These injections effectively prevented phosphorylation of STAT3 in the VMHdm following an intraperitoneal injection of leptin (Fig. 1B), and the absence of ObR was confirmed with RNAscope in situ hybridization. The in situ hybridization also confirmed that Lep-Sap injections did not delete ObR in either the Arc or the DMH (Fig. 1C).
Fig. 1.
A: the dispersion of leptin-conjugated saporin (Lep-Sap) 4 h after a 75-nL injection of a 260 mg/μL solution. B: ventromedial nucleus of the hypothalamus (VMH) phosphorylated signal transducer and activator of transcription 3 (pSTAT3) detected 45 min after a 1 mg leptin/kg intraperitoneal injection 20 days after VMH injections of blank-saporin (Blk-Sap) or Lep-Sap. C: leptin receptor (ObR) mRNA detected by RNAscope in situ hybridization conducted 21 days after bilateral VMH injections of Blk-Sap or Lep-Sap. High-magnification images show presence of ObR in the dorsomedial hypothalamus (DMH) and arcuate nucleus of the hypothalamus (Arc) of a Lep-Sap rat.
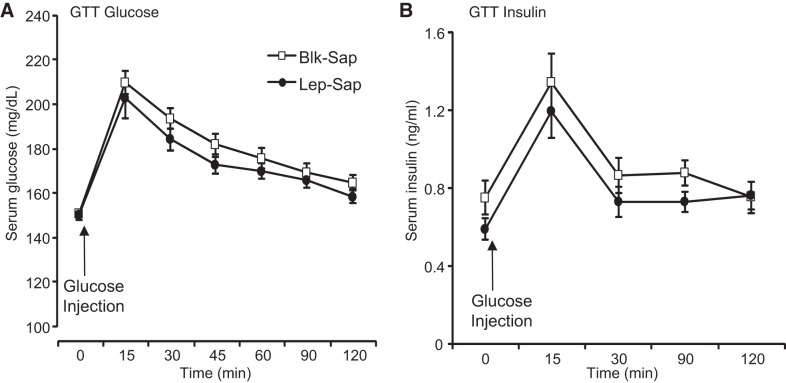
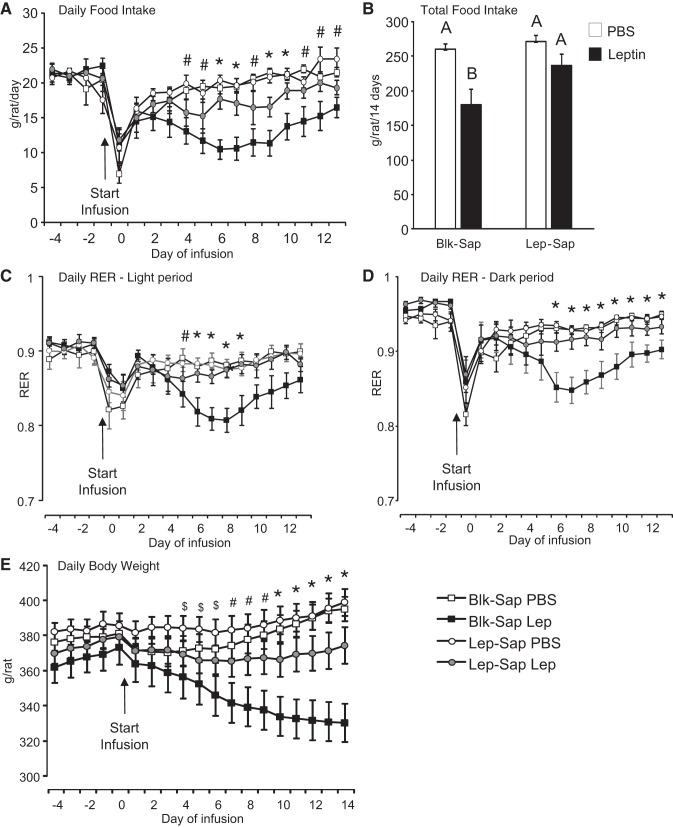
Lep-Sap injections into the VMH of rats used in the 4V leptin infusions had no effect on weight gain of the rats during the 22 days between Lep-Sap injections and placement of the 4V infusion cannula (Blk-Sap = 41 ± 4 g/22 days, Lep-Sap = 44 ± 3 g/22 days). In addition, there was no effect of VMH Lep-Sap on glucose clearance during the GTT performed before the leptin infusions started (Fig. 2). All of the rats ate less than normal on the first day of 4V infusion, but this was reversed by the second day of infusion for all groups except the Blk-Sap rats infused with leptin (Fig. 3A; saporin: P < 0.01, leptin: P < 0.0001, day: P < 0.0001, leptin × day: P < 0.0001, saporin × leptin × day: P < 0.04). The intake of these rats was lower than that of the PBS-infused rats by day 4 of infusion and remained inhibited throughout the period of leptin infusion. The intake of Lep-Sap leptin-infused rats tended to be lower than that of the PBS-infused rats, but this did not reach significance on any day of infusion. Total food intake during the 14 days of infusion was not different between Blk-Sap and Lep-Sap PBS-infused groups (Fig. 3B). Intake of the Blk-Sap leptin-infused rats was significantly inhibited compared with their controls, whereas that of Lep-Sap leptin-infused rats was inhibited by 16% compared with Lep-Sap PBS-infused control rats, but this did not represent a significant difference (Fig. 3B). Respiratory exchange ratio of the rats followed the same pattern of response as food intake, being significantly reduced in Blk-Sap leptin-infused rats compared with all other groups, and this was apparent during both the light and dark periods [Fig. 3, C and D: saporin: not significant (NS), leptin: P < 0.04, day: P < 0.0001, leptin × day: P < 0.0001, saporin × leptin × day: P < 0.006]. Despite this apparent difference in substrate utilization there were no differences in energy expenditure of the four groups of rats (data not shown).
Fig. 2.
Serum glucose (A) and insulin (B) during a glucose tolerance test (GTT) performed 29 days after rats received bilateral ventromedial nucleus of the hypothalamus (VMH) injections of blank-saporin (Blk-Sap) or leptin-conjugated saporin (Lep-Sap). There was no effect of saporin treatment on glucose clearance or insulin release. Data are means ± SE for 16 Blk-Sap and 18 Lep-Sap rats. Significant differences were tested for by a repeated-measures ANOVA.
Fig. 3.
A and B: daily (A) and total (B) food intake of rats during the experiment. Leptin infusion into the 4th ventricle significantly inhibited food intake in blank-saporin (Blk-Sap) but not leptin-conjugated saporin (Lep-Sap) rats. C and D: daily respiratory exchange ratio (RER) averaged either during the light period (C) or during the dark period (D). Infusions of 0.9 μg leptin/day or PBS started on day 0. E: daily body weight of the rats. Data are means ± SE for groups of 8–10 rats. A, C, D and E: *Blk-Sap leptin-treated rats (Blk-Sap Lep) are significantly different from all other groups; #Blk-Sap Lep rats are different from both groups of PBS-infused rats; $Blk-Sap Lep rats are different from Lep-Sap leptin-treated rats (Lep-Sap Lep). In B, values that do not share a common superscript are significantly different. Differences were considered significant at P < 0.05. For the daily data, significant differences were initially tested for by a repeated-measures 2-way ANOVA. If there was a significant interaction, then data on each day were tested by 2-way ANOVA followed by a post hoc Tukey’s honestly significant difference (HSD) test. Significant differences in total food intake were determined by 2-way ANOVA followed by a post hoc Tukey’s HSD test.
Leptin-infused Blk-Sap rats lost weight throughout the experimental period and weighed significantly less than any other group during the last 5 days of the experiment (Fig. 3E; saporin: P < 0.04, leptin: P < 0.002, day: P < 0.0001, saporin × day: P < 0.0008, leptin × day: P < 0.0001, saporin × leptin × day: P < 0.0001). In contrast, PBS-infused Blk-Sap rats stopped gaining weight during the first 5 days of infusion but then started to gain weight at a steady rate. Lep-Sap rats receiving PBS infusions gained weight during the second week of infusion and weighed the same as Blk-Sap PBS-infused rats at the end of the experiment. Leptin infusions in Lep-Sap rats inhibited weight gain but did not cause the weight loss experienced by the Blk-Sap leptin rats. At the end of infusion Lep-Sap leptin-infused rats weighed less than either group of PBS-infused rats, but the difference did not reach significance. Inguinal and epididymal fat pad weights were significantly smaller in Blk-Sap leptin-infused rats compared with either group of PBS-infused rats. The amount of inguinal fat in Lep-Sap leptin-infused rats was not different from that in either group of PBS-infused rats (Fig. 4); epididymal fat was reduced compared with that in Lep-Sap PBS-infused rats but was not different from that in either PBS- or leptin-infused Blk-Sap rats (Fig. 4).
Fig. 4.
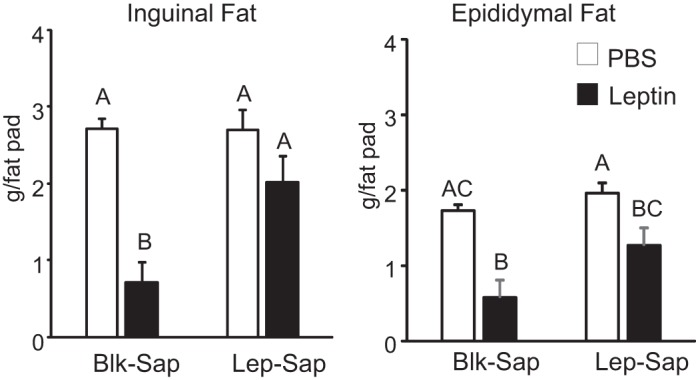
Weight of 1 inguinal or 1 epididymal fat pad dissected and weighed 14 days after the start of 4th ventricle infusions of PBS or leptin in rats that had received bilateral ventromedial nucleus of the hypothalamus injections of blank-saporin (Blk-Sap) or leptin-conjugated saporin (Lep-Sap) 35 days before the start of infusion. Values for either inguinal or epididymal fat that do not share a common superscript are significantly different at P < 0.05. Data are means + SE for groups of 8–11 rats. Significant differences were determined by 2-way ANOVA followed by a post hoc Tukey’s honestly significant difference test.
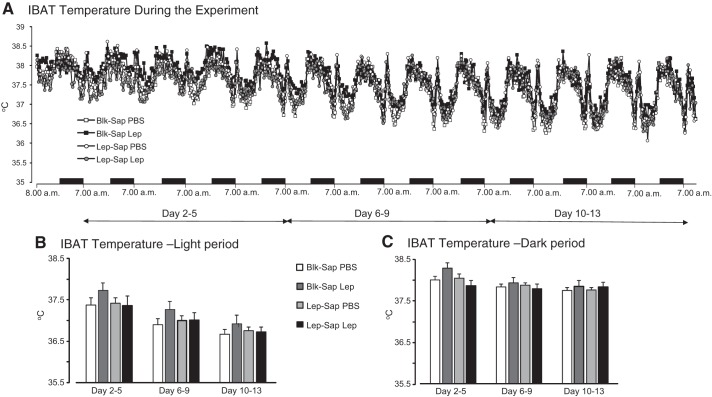
IBAT temperature was measured every 30 min starting from day 1 of the infusion period (Fig. 5A). Diurnal variation in temperature appeared to be attenuated during the first 4 days of infusion, but the rhythm appeared stable after that. To test for an effect of leptin or of Lep-Sap treatment, the lowest and highest daily IBAT temperatures for each rat were calculated in three phases of the infusion period. Temperature was averaged for the 5 h between 10:00 AM and 3:00 PM and between 10:00 PM and 3:00 AM on days 2–5, when food intake of the Blk-Sap leptin rats was declining, days 6–9, when intake of the Blk-Sap leptin rats was at a stable but reduced level, and days 10–13, when the food intake of Blk-Sap leptin rats was starting to increase. There was no effect of treatment or leptin on the daytime temperature (Fig. 5B; saporin: NS, leptin: NS, time: P < 0.0001). Repeated-measures analysis suggested an effect of treatment on nighttime temperature (Fig. 5C; saporin: NS, leptin: NS, time: P < 0.0001, saporin × time: P < 0.06, saporin × leptin × time: P < 0.03), but post hoc analysis did not identify any differences between groups during any phase of infusion.
Fig. 5.
A: interscapular brown adipose tissue (IBAT) temperature measured every 30 min throughout the infusion period starting at 8:00 AM on day 1 of infusion. The brief daily spike in temperature at 8:30 AM coincided with the daily weighing of the animals. B: average IBAT temperature during the 5-h period of lowest temperature between 10:00 AM and 3:00 PM in the light period. C: average IBAT temperature during the 5-h period of highest temperature between 10:00 PM and 3:00 AM in the dark period. Averages were calculated for 3 phases during the infusion: days 2–5, when intake of blank-saporin (Blk-Sap) leptin-treated (Blk-Sap Lep) rats was declining, days 6–9, when intake of Blk-Sap Lep rats was stable, and days 10–13, when intake of Blk-Sap Lep rats was starting to return to control levels. There were no differences between any of the groups at any of the time points tested. Data are means + SE for groups of 8–11 rats. Blk-Sap PBS, Blk-Sap PBS-treated rats; Lep-Sap Lep, leptin-conjugated saporin (Lep-Sap) leptin-treated rats; Lep-Sap PBS, Lep-Sap PBS-treated rats.
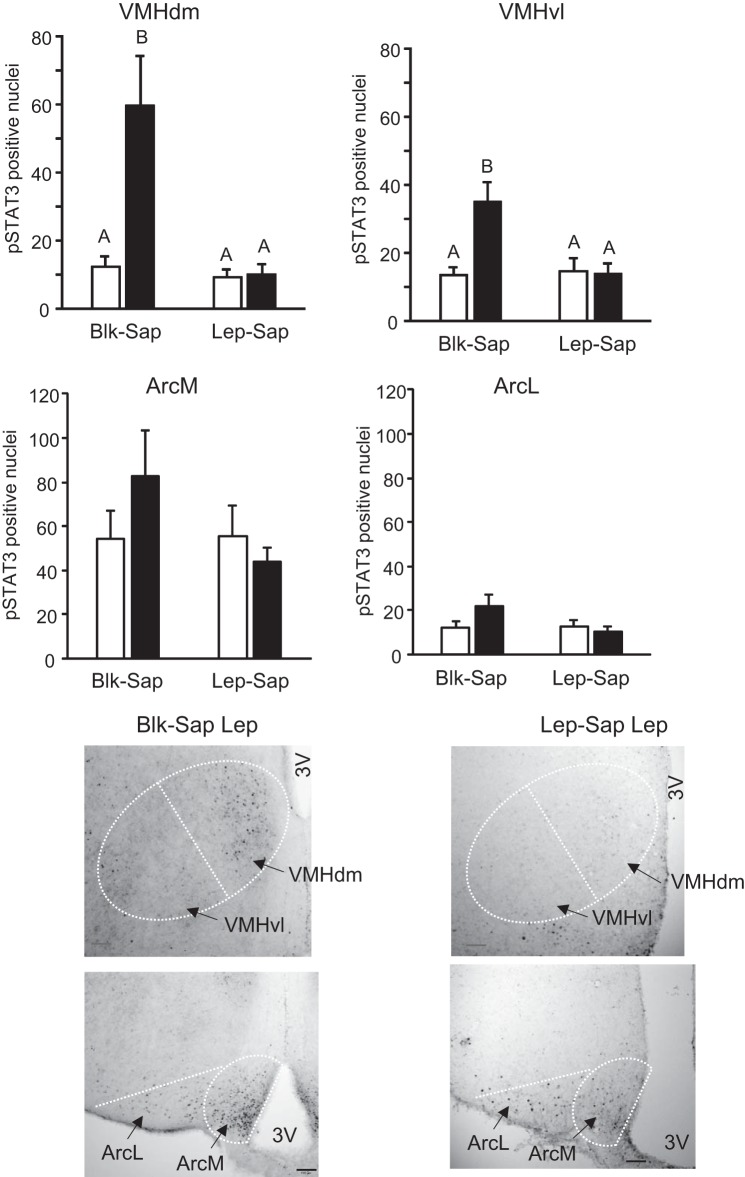
Hypothalamic pSTAT3 measured at the end of the experiment showed negligible levels of pSTAT3 in the DMH, and there was no difference between treatment groups (data not shown). Leptin infusion increased pSTAT3 in both the VMHdm and the VMHvl of Blk-Sap-treated rats, but there was no effect of leptin in the VMH of Lep-Sap treated animals (Fig. 6; VMHvl: saporin: P < 0.01, leptin: P < 0.001, interaction: P < 0.007; VMHdm: saporin: P < 0.001, leptin: P < 0.002, interaction: P < 0.003). There was a trend for leptin infusion to increase pSTAT3 in both the lateral Arc (P < 0.08) and the medial Arc (P < 0.16) of Blk-Sap-treated rats, but there were no significant differences between any of the groups at either site (Fig. 6). Representative images of pSTAT3 expression for leptin-treated Blk-Sap and Lep-Sap rats are shown in Fig. 6.
Fig. 6.
Quantification of phosphorylated signal transducer and activator of transcription 3 (pSTAT3)-positive nuclei in the ventromedial nucleus of the hypothalamus (VMH; top) and the arcuate nucleus of the hypothalamus (Arc; middle) of rats that had received bilateral VMH injections of blank-saporin (Blk-Sap) or leptin-conjugated saporin (Lep-Sap) and then received 14 day 4th ventricle infusions of either PBS or 0.9 μg leptin/day. Data are means + SE for groups of 8–11 rats. Statistical differences were detected by 2-way ANOVA followed by Tukey’s honestly significant difference test. Values within a panel that do not share a common superscript are significantly different at P < 0.05. Bottom: representative images of VMH and Arc of leptin-infused Blk-Sap or Lep-Sap rats. Images have been adjusted for brightness and contrast. ArcL, lateral Arc; ArcM, medial Arc; Blk-Sap Lep, Blk-Sap leptin-treated rats; Lep-Sap Lep, Lep-Sap leptin-treated rats; VMHdm, dorsomedial nucleus of VMH; VMHvl, ventrolateral nucleus of VMH; 3V, 3rd ventricle. Black bars in lower images represent 100 μm.
DISCUSSION
In this experiment we used leptin-conjugated saporin (Lep-Sap) to delete VMH neurons that expressed ObR. Others have used adeno-associated virally mediated RNA interference to knock down ObRb in the hindbrain of rats (24, 26), but they reported only a 40% reduction in receptor expression. By contrast, Li et al. (28) and Wiater et al. (42) reported that Lep-Sap destroyed 80% of ObRb-expressing cells in the Arc. For this reason we selected Lep-Sap as the more efficient treatment, even though it was less selective, killing the entire neuron rather than just downregulating receptor expression. In this experiment the Lep-Sap treatment prevented 85% of the VMH pSTAT3 response to leptin infusion. The measurement of Lep-Sap dispersion 4 h after injection indicated that it was confined to the VMH and had a smaller distribution pattern than injections delivered by picospritzer (28). Nonetheless, measurement of leptin receptor distribution with RNAscope and the pSTAT3 response to peripheral leptin injections confirmed that the Lep-Sap injections were effective in reaching a majority of ObR-expressing neurons in the VMH without destroying those located in the DMH or Arc of the rats. This information allows us to be confident that the effects of Lep-Sap treatment on the response to 4V leptin infusions was due to loss of ObR-expressing cells in the VMH and not due to a more general disruption of leptin signaling.
Even though Lep-Sap resulted in the loss of the neuronal population in the VMH that expressed ObR, there was no overt phenotype of the rats before leptin infusion. The Lep-Sap and Blk-Sap rats gained the same amount of weight between the saporin injections and the start of the infusion experiment, and food intake measured during the days in which they adapted to the calorimeter before 4V infusions started was not different between groups. These observations are consistent with those reported by Li et al. (28), who used VMH Lep-Sap injections as a negative control for Arc Lep-Sap injections, and provide indirect evidence that our Lep-Sap injections into the VMH did not affect Arc ObR-expressing cells. Injection of Lep-Sap into the Arc causes overeating and rapid weight gain for ~8 wk after the injection (28), neither of which were apparent in our animals. Loss of Arc ObR-expressing cells also disrupts the circadian rhythms of feeding (28), activity, and core body temperature (42). Although detailed analysis of circadian rhythmicity was not performed in this study, there was no obvious change in the diurnal pattern of IBAT temperature of rats in this experiment. Finally, ObRb in proopiomelanocortin-expressing cells of the Arc have been reported to be essential for normal glucostasis in mice (2), and we found no evidence of either hyperglycemia or an abnormal glucose clearance rate during a GTT in the rats in the study described here. Taken together, these data suggest that leptin response in the Arc was intact in the rats that received VMH injections of Lep-Sap.
The objective of this experiment was to test the importance of VMH ObR-expressing cells in mediating the response to leptin infused into the 4V. The justification was based on previous observations that when subthreshold doses of leptin are infused simultaneously into the 3V and 4V there is substantial weight loss (9) and that 4V infusions of leptin stimulate pSTAT3 in hypothalamic nuclei known to express ObRb (20). These data suggested that weight loss in response to central administration of leptin results from an integrated response from multiple brain areas, and it appeared that the VMHdm may have a critical role in this integrated response because it showed the greatest stimulation of pSTAT3 activity in response to 4V leptin infusions (20). Leptin receptor expression in the hindbrain is limited to the NTS and area postrema (AP). Injection of leptin directly into the NTS inhibits food intake and also stimulates hypothalamic pSTAT3 (38), and infusions of leptin into the NTS lower the threshold for hypothalamic response to leptin, whereas similar doses of leptin into the 4V do not increase sensitivity to leptin in the hypothalamus (23). These data exclude the possibility that the increase in hypothalamic pSTAT3 results from diffusion of leptin from the 4V through the subarachnoid space to the hypothalamus, consistent with a report by Ruiter et al. (38) that the pattern of hypothalamic pSTAT3 caused by 4V injections of 3 μg of leptin did not overlap with the distribution of Fluoro-Gold injected at the same site.
In the AP ObR are coexpressed on neurons that express amylin receptors (29). Amylin appears to act synergistically with leptin to inhibit food intake, with an associated increase in VMH, but not Arc, pSTAT3 expression (37). It has been reported that the increase in VMH pSTAT3 is due to promotion of release of IL-6 from hypothalamic microglia (27). In the study reported here we cannot exclude a potential contribution of AP neurons that respond to leptin and amylin in mediating the weight loss that requires involvement of VMH leptin receptor-expressing neurons. However, the results of this experiment imply that such an effect in the VMH would be specific to neurons that express ObR and that either IL-6-dependent stimulation of pSTAT3 is limited to neurons that express ObR or IL-6 cross-reacts with ObR. Evidence that parenchymal injections or infusions of leptin into the NTS increase VMH pSTAT3, whereas similar doses applied to the 4V do not (27, 38), indicates that increased hypothalamic pSTAT3 can occur independent of ObR activation in the AP but does not exclude the possibility that there may be both direct and indirect mechanisms in place by which hindbrain leptin enhances hypothalamic STAT3 activation. This experiment also does not exclude the possibility that, under normal conditions, projections from leptin-responsive neurons in the VMH increase leptin sensitivity of neurons in the hindbrain. Although there is evidence for direct afferent projections from the VMHdm to the NTS in both rats (4) and mice (30), this scenario is unlikely. We have previously reported that leptin infusion into the 4V increases sensitivity to 3V leptin injections but that leptin infusions into the 3V do not change the response to 4V leptin injections (21). If VMH afferents were sensitizing NTS neurons to leptin, then we would have expected the reverse outcome, with 3V leptin enhancing the response to 4V leptin injections.
In the study described here deletion of VMH neurons that express ObR with Lep-Sap significantly attenuated the effect of 4V leptin infusions on food intake and body weight, supporting our hypothesis that leptin-induced weight loss only occurs when there is simultaneous activation of ObRb in the hindbrain and forebrain. This is not the first evidence that ObRb in the VMH influence energy balance. Although leptin injection into the VMH inhibits food intake in rats (25), loss of ObRb from VMH neurons in mice does not produce a significant phenotype until energy balance is disrupted by offering the animals a high-fat diet (10). Absence of VMH ObRb appears to accelerate the onset of obesity and insulin insensitivity in mice (3, 10), suggesting that under normal conditions ObRb in the VMH play only a small role in maintenance of energy expenditure but are more important when the system is stressed and they function to restrain inappropriate overeating or weight gain.
Leptin receptors in the Arc clearly play an essential role in the maintenance of energy balance under normal, baseline conditions. Loss of leptin signaling in the Arc results in hyperphagia and obesity that is reversed if receptor activation is corrected (34). The importance of ObRb in other hypothalamic nuclei has received less attention. However, a study examining the activation of ObRb in different nuclei in response to increasing doses of leptin given by intraperitoneal injection suggested different thresholds for response to leptin in the Arc versus the VMHdm (31). ObRb in the Arc were fully activated by an injection of only 50 μg/kg, whereas pSTAT3 in the VMHdm did not show an increase in pSTAT3 until the injected dose reached 200 μg/kg, and then there was a stepwise increase in response as the injected dose increased to 800 μg/kg. For perspective, in our studies we use peripheral injections of 2 mg leptin/kg to produce a reliable inhibition of food intake in leptin-responsive rats (19, 22). Thus these observations support those from the SF1-ObRb knockout mice in suggesting that ObRb in the Arc are fully engaged at normal physiological concentrations of leptin but VMH ObRb respond when leptin levels are stimulated by an intervention that puts an animal into a state of positive energy balance and potentially increased body fat mass (3, 10).
Others have reported that leptin in the hindbrain stimulates thermogenesis (41). In this study we did not find an effect of 4V leptin infusion or loss of VMH ObR-expressing cells on energy expenditure of the rats. There also was no effect of leptin on IBAT temperature in the leptin-infused Blk-Sap or Lep-Sap rats. One of the biggest differences between our studies and those in which IBAT is seen to be activated is the dose and method of leptin administration. We were infusing 0.9 μg leptin/24 h, whereas thermogenesis has been reported to be stimulated by acute 4V injections of 3 μg of leptin (41). The response is dependent upon activation of melanocortin receptors and is largely reversed within 6 h of injection (41). Thus the thermogenic response to hindbrain leptin may be a pharmacological response that is not initiated with the lower doses of leptin used in our infusion experiments. In addition, Dhillon et al. (10) reported that a failure to increase oxygen consumption contributed to weight gain in high-fat-fed mice in which ObRb had been deleted from SF1 neurons. However, the difference in oxygen consumption was not apparent in chow-fed mice. In this study we did not find any change in energy expenditure of the different groups of rats, all of which were fed chow, and it is possible that we would have found a difference if the rats had been put in a situation that promoted weight gain rather than weight loss.
The absence of a change in IBAT temperature or energy expenditure in leptin-infused Blk-Sap rats implies that weight loss is primarily determined by a reduction in energy intake. Inhibition of food intake in rats by leptin is achieved by a reduction in meal size (11, 16), and it has been shown repeatedly that peripheral (1, 33) and central (14, 15, 32) leptin amplify the response to peripheral signals of satiation such as cholecystokinin and gastric distension. There are ObRb on vagal afferent neurons that feed into the NTS, and it is clear that activation of these receptors enhances the efficacy of gut-derived signals of satiation (6, 7). However, it has also been reported that central leptin amplifies the response to cholecystokinin through a mechanism that is independent of increased vagal afferent activity, and it has been hypothesized that leptin increases signaling from the NTS to an integrative site in the forebrain, possibly the paraventricular nucleus of the hypothalamus (40). Further studies are required to test whether activation of ObRb in the NTS lowers the threshold for leptin response in the VMHdm as part of a pathway that amplifies satiety responses through a network that also includes the paraventricular nucleus of the hypothalamus.
In summary, this experiment demonstrated that loss of ObRb-expressing neurons from the VMH had no effect on food intake or weight gain of ad libitum-fed rats offered chow. The loss of the leptin receptors did, however, prevent hypophagia and weight loss when the rats received 4V infusions of leptin. These results support our hypothesis that weight loss in leptin-treated animals only occurs when there is simultaneous activation of receptors in the hindbrain and forebrain. Previous experiments have shown that hindbrain leptin lowers the threshold for a hypothalamic response to leptin and that this is likely mediated by a neural network. The results of this study demonstrate a critical role for the VMH in this network, consistent with previous observations that stimulation of pSTAT3 in the hypothalamus of rats treated with leptin in the hindbrain is greater in the VMH than in any other nucleus (9, 20).
GRANTS
This work was supported by NIH Grant R01 DK-053903 awarded to R. B. S, Harris, NIH Grant DK-051496 awarded to S. Ritter, and American Diabetes Association Grant 18-IBS-156 to S. Ritter.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S., A.-J.L., S.R., and R.B.S.H. conceived and designed research; M.S. and W.A. performed experiments; W.A. and R.B.S,H. analyzed data; R.B.S,H. interpreted results of experiments; R.B.S.H. prepared figures; R.B.S.H. drafted manuscript; A.-J.L., S.R., and R.B.S.H. edited and revised manuscript; M.S., W.A., A.-J.L., S.R., and R.B.S.H. approved final version of manuscript.
REFERENCES
- 1.Akieda-Asai S, Poleni PE, Date Y. Coinjection of CCK and leptin reduces food intake via increased CART/TRH and reduced AMPK phosphorylation in the hypothalamus. Am J Physiol Endocrinol Metab 306: E1284–E1291, 2014. doi: 10.1152/ajpendo.00664.2013. [DOI] [PubMed] [Google Scholar]
- 2.Berglund ED, Vianna CR, Donato J Jr, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, Coppari R, Elmquist JK. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest 122: 1000–1009, 2012. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149: 2138–2148, 2008. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348: 41–79, 1994. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci 17: 4933–4941, 1997. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS One 7: e32967, 2012. doi: 10.1371/journal.pone.0032967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lartigue G, Lur G, Dimaline R, Varro A, Raybould H, Dockray GJ. EGR1 is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology 151: 3589–3599, 2010. doi: 10.1210/en.2010-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai BN, Harris RB. Integrated effects of leptin in the forebrain and hindbrain of male rats. Endocrinology 154: 2663–2675, 2013. doi: 10.1210/en.2013-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai BN, Harris RB. Leptin in the hindbrain facilitates phosphorylation of STAT3 in the hypothalamus. Am J Physiol Endocrinol Metab 308: E351–E361, 2015. doi: 10.1152/ajpendo.00501.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49: 191–203, 2006. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Eckel LA, Langhans W, Kahler A, Campfield LA, Smith FJ, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol Regul Integr Comp Physiol 275: R186–R193, 1998. doi: 10.1152/ajpregu.1998.275.1.R186. [DOI] [PubMed] [Google Scholar]
- 12.Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology 147: 3190–3195, 2006. doi: 10.1210/en.2005-0877. [DOI] [PubMed] [Google Scholar]
- 13.Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Int J Obes Relat Metab Disord 25, Suppl 5: S78–S82, 2001. doi: 10.1038/sj.ijo.0801918. [DOI] [PubMed] [Google Scholar]
- 14.Emond M, Ladenheim EE, Schwartz GJ, Moran TH. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol Behav 72: 123–128, 2001. doi: 10.1016/S0031-9384(00)00393-0. [DOI] [PubMed] [Google Scholar]
- 15.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol Regul Integr Comp Physiol 276: R1545–R1549, 1999. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- 16.Flynn MC, Scott TR, Pritchard TC, Plata-Salamán CR. Mode of action of OB protein (leptin) on feeding. Am J Physiol Regul Integr Comp Physiol 275: R174–R179, 1998. doi: 10.1152/ajpregu.1998.275.1.R174. [DOI] [PubMed] [Google Scholar]
- 17.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143: 239–246, 2002. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 18.Harris RB. Evidence that leptin-induced weight loss requires activation of both forebrain and hindbrain receptors. Physiol Behav 120: 83–92, 2013. doi: 10.1016/j.physbeh.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris RB, Apolzan JW. Changes in glucose tolerance and leptin responsiveness of rats offered a choice of lard, sucrose, and chow. Am J Physiol Regul Integr Comp Physiol 302: R1327–R1339, 2012. doi: 10.1152/ajpregu.00477.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris RB, Desai BN. Fourth-ventricle leptin infusions dose-dependently activate hypothalamic signal transducer and activator of transcription 3. Am J Physiol Endocrinol Metab 311: E939–E948, 2016. doi: 10.1152/ajpendo.00343.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RB. Low-dose leptin infusion in the fourth ventricle of rats enhances the response to third-ventricle leptin injection. Am J Physiol Endocrinol Metab 313: E134–E147, 2017. doi: 10.1152/ajpendo.00052.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris RB. Development of leptin resistance in sucrose drinking rats is associated with consuming carbohydrate-containing solutions and not calorie-free sweet solution. Appetite 132: 114–121, 2019. doi: 10.1016/j.appet.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris RB. Low-dose infusions of leptin into the nucleus of the solitary tract increase sensitivity to third ventricle leptin. Am J Physiol Endocrinol Metab 316: E719–E728, 2019. doi: 10.1152/ajpendo.00562.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83, 2010. [Erratum in Cell Metab 23: 744, 2016.] doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob RJ, Dziura J, Medwick MB, Leone P, Caprio S, During M, Shulman GI, Sherwin RS. The effect of leptin is enhanced by microinjection into the ventromedial hypothalamus. Diabetes 46: 150–152, 1997. doi: 10.2337/diab.46.1.150. [DOI] [PubMed] [Google Scholar]
- 26.Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, De Jonghe BC, Bence KK, Hayes MR, Grill HJ. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab 303: E496–E503, 2012. doi: 10.1152/ajpendo.00205.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Foll C, Johnson MD, Dunn-Meynell AA, Boyle CN, Lutz TA, Levin BE. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes 64: 1621–1631, 2015. doi: 10.2337/db14-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li AJ, Wiater MF, Oostrom MT, Smith BR, Wang Q, Dinh TT, Roberts BL, Jansen HT, Ritter S. Leptin-sensitive neurons in the arcuate nuclei contribute to endogenous feeding rhythms. Am J Physiol Regul Integr Comp Physiol 302: R1313–R1326, 2012. doi: 10.1152/ajpregu.00086.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberini CG, Boyle CN, Cifani C, Venniro M, Hope BT, Lutz TA. Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. Eur J Neurosci 43: 653–661, 2016. doi: 10.1111/ejn.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindberg D, Chen P, Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. J Comp Neurol 521: 3167–3190, 2013. doi: 10.1002/cne.23338. [DOI] [PubMed] [Google Scholar]
- 31.Maniscalco JW, Rinaman L. Systemic leptin dose-dependently increases STAT3 phosphorylation within hypothalamic and hindbrain nuclei. Am J Physiol Regul Integr Comp Physiol 306: R576–R585, 2014. doi: 10.1152/ajpregu.00017.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matson CA, Ritter RC. Long-term CCK-leptin synergy suggests a role for CCK in the regulation of body weight. Am J Physiol Regul Integr Comp Physiol 276: R1038–R1045, 1999. doi: 10.1152/ajpregu.1999.276.4.R1038. [DOI] [PubMed] [Google Scholar]
- 33.Matson CA, Wiater MF, Kuijper JL, Weigle DS. Synergy between leptin and cholecystokinin (CCK) to control daily caloric intake. Peptides 18: 1275–1278, 1997. doi: 10.1016/S0196-9781(97)00138-1. [DOI] [PubMed] [Google Scholar]
- 34.Morton GJ, Niswender KD, Rhodes CJ, Myers MG Jr, Blevins JE, Baskin DG, Schwartz MW. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fak/fak) rats. Endocrinology 144: 2016–2024, 2003. doi: 10.1210/en.2002-0115. [DOI] [PubMed] [Google Scholar]
- 35.National Research Council Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press, 2011. [Google Scholar]
- 36.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA 105: 7257–7262, 2008. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiter M, Duffy P, Simasko S, Ritter RC. Increased hypothalamic signal transducer and activator of transcription 3 phosphorylation after hindbrain leptin injection. Endocrinology 151: 1509–1519, 2010. doi: 10.1210/en.2009-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scarpace PJ, Matheny M, Shek EW. Impaired leptin signal transduction with age-related obesity. Neuropharmacology 39: 1872–1879, 2000. doi: 10.1016/S0028-3908(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz GJ, Moran TH. Leptin and neuropeptide Y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology 143: 3779–3784, 2002. doi: 10.1210/en.2002-220352. [DOI] [PubMed] [Google Scholar]
- 41.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology 150: 1705–1711, 2009. doi: 10.1210/en.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiater MF, Li AJ, Dinh TT, Jansen HT, Ritter S. Leptin-sensitive neurons in the arcuate nucleus integrate activity and temperature circadian rhythms and anticipatory responses to food restriction. Am J Physiol Regul Integr Comp Physiol 305: R949–R960, 2013. doi: 10.1152/ajpregu.00032.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiley RG, Kline RH 4th. Neuronal lesioning with axonally transported toxins. J Neurosci Methods 103: 73–82, 2000. doi: 10.1016/S0165-0270(00)00297-1. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]