Abstract
Androgen depletion in humans leads to significant atrophy of the limb muscles. However, the pathways by which androgens regulate limb muscle mass are unclear. Our laboratory previously showed that mitochondrial degradation was related to the induction of autophagy and the degree of muscle atrophy following androgen depletion, implying that decreased mitochondrial quality contributes to muscle atrophy. To increase our understanding of androgen-sensitive pathways regulating decreased mitochondrial quality, total RNA from the tibialis anterior of sham and castrated mice was subjected to microarray analysis. Using this unbiased approach, we identified significant changes in the expression of genes that compose the core molecular clock. To assess the extent to which androgen depletion altered the limb muscle clock, the tibialis anterior muscles from sham and castrated mice were harvested every 4 h throughout a diurnal cycle. The circadian expression patterns of various core clock genes and known clock-controlled genes were disrupted by castration, with most genes exhibiting an overall reduction in phase amplitude. Given that the core clock regulates mitochondrial quality, disruption of the clock coincided with changes in the expression of genes involved with mitochondrial quality control, suggesting a novel mechanism by which androgens may regulate mitochondrial quality. These events coincided with an overall increase in mitochondrial degradation in the muscle of castrated mice and an increase in markers of global autophagy-mediated protein breakdown. In all, these data are consistent with a novel conceptual model linking androgen depletion-induced limb muscle atrophy to reduced mitochondrial quality control via disruption of the molecular clock.
Keywords: autophagy, muscle atrophy, testosterone
INTRODUCTION
Maintaining skeletal muscle mass is directly linked to a reduced risk of morbidity and mortality (34, 62, 63). In males, a decrease in the production and/or bioavailability of testicular androgens (termed “hypogonadism”) contributes to the loss of muscle mass during various pathological conditions (6, 9, 21, 22, 66, 72). Though multiple muscle groups are affected by hypogonadism, atrophy of the limb muscles is particularly important as they comprise the large majority of total muscle mass (37) and they are the primary muscles involved with physical function. Whereas limb muscle atrophy is a consequence of hypogonadism, the pathways by which androgens regulate limb muscle mass remain equivocal. This is likely attributed in large part to a lack of information defining the androgen-sensitive pathways in limb muscles. For instance, the levator ani muscle is commonly used as a model system to study androgen signaling because levator ani muscle mass is highly sensitive to androgen depletion (46, 59), and use of this model has shown that the canonical androgen receptor (AR) signaling pathway is a predominant pathway regulating muscle mass (51, 59). Pharmacological inhibition of AR in primary myocytes and C2C12 cell culture systems yielded results similar to those observed in vivo (5, 28). Despite the necessity of AR signaling in those models, deletion of AR did not affect mass of the limb muscles [e.g., tibialis anterior (TA); 2, 15, 51, 52, 67]. Rather, it was the presence of androgens themselves that dictated limb muscle mass (67). This dichotomy between model systems is further illustrated by work from our laboratory that showed that signaling through mechanistic target of rapamycin complex 1 (mTORC1) was dispensable for androgen-mediated growth of the limb skeletal muscles (54), even though mTORC1 was absolutely required for androgen-mediated growth in an AR-dependent model system (1, 5), implying that androgens regulate limb muscle mass through distinct pathways that have yet to be fully defined.
In pursuit of novel androgen-sensitive pathways in limb skeletal muscle, our laboratory recently found that mitochondrial degradation was increased in the atrophied TA of mice subjected to androgen depletion via castration surgery (56). Importantly, the measures of degradation were related to measures of autophagy activation and the degree of muscle atrophy, implying that changes in mitochondrial quality control may contribute to autophagy activation and subsequent limb muscle atrophy under androgen-deprived conditions (56). With this idea in mind, the present study subjected total RNA from the TA of those sham and castrated mice to microarray analysis to define novel androgen-sensitive pathways in the limb skeletal muscle known to regulate mitochondrial quality control. Using this unbiased approach, we identified significant castration-induced changes in the expression of genes that compose the core molecular clock. The core molecular clock is a transcription-translation feedback system that regulates various metabolic processes, including mitochondrial quality, by mediating diurnal changes in gene expression (3, 8, 29). The positive portion of the core clock is regulated by the dimer between brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like 1 (Bmal1) and circadian locomotor output cycles protein kaput (Clock), which induce transcription of various clock-controlled genes (8). The period genes [period circadian regulator 1–3 (Per1–3, respectively)] and cryptochrome genes [cryptochrome circadian regulator 1 and 2 (Cry1 and Cry2, respectively)] are among those transcribed by the Bmal1-Clock dimer (8, 58), which when translated into proteins, feed back to inhibit the transcriptional activity of Bmal1-Clock, thus creating the negative portion of the core clock (8). Herein we demonstrate that cycling and function of the core molecular clock are disrupted within the limb skeletal muscle by depletion of testicular androgens and that this disruption coincides with altered expression of genes that regulate mitochondrial quality control. Alterations in mitochondrial quality control overlapped with increased mitochondrial degradation (i.e., mitophagy) and global autophagy activation, supporting a novel conceptual model linking androgen depletion-induced limb muscle atrophy to reduced mitochondrial quality control via disruption of the molecular clock.
MATERIALS AND METHODS
Animals, Castration Surgery, and Experimental Design
Microarray sample generation.
The muscle samples analyzed by microarray were generated from a previous study conducted by our laboratory where the objective was to determine whether androgen depletion altered the molecular response following anabolic stimuli (64). In brief, mice from that study were subjected to a sham or castration surgery (n = 7–8 per group). Seven weeks following surgery, all mice were subjected to an overnight fast beginning at 1700. The next morning, sham and castrated mice were given access to food for 30 min. After the refeeding, mice were fasted for an additional 4.5 h with unrestricted access to water until euthanasia occurring between 1200 and 1500. The TA muscles were used for analysis as androgens regulate mass of the TA in an AR-independent manner (67). The Institutional Animal Care and Use Committee at the University of Central Florida approved these procedures and the animal facility.
Circadian study.
Male, C57BL/6NHsd mice (12 wk of age) were purchased from Envigo (Indianapolis, IN). Upon arrival, all mice were housed individually for 2 wk with ad libitum access to food (5001 rodent chow; LabDiet, St. Louis, MO) and water before being randomized into two groups of equal body weight. One group was subjected to a castration surgery to effectively stop testicular androgen production, whereas the other group was subjected to a sham surgery in which testicular androgen production was left intact. All mice were given buprenorphine (0.05 mg/kg) immediately following surgery and again 5 h later to alleviate postoperative pain. Mice recovered for 8 wk before testing. Testing included euthanizing a subset of mice from each group (n = 3 per group per time point) in alternating fashion (i.e., sham/castrated) every 4 h beginning at the onset of the dark cycle (1900). Food and water were consumed ad libitum throughout the data collection. For euthanasia during the dark cycle, the mouse cage was placed into a black Tupperware container under dim red light for transport to a separate surgical suite. In the surgical suite, mice were lightly anesthetized with isoflurane under dim red light, and then mice were euthanized via cervical dislocation. The lights were then turned on, and the limb muscles were rapidly extracted and flash-frozen in liquid nitrogen. Preliminary tests using a HOBO light detector (Onset Computer, Bourne, MA) performed before the experiment showed that the Tupperware container prevented light exposure inside the container during transport, and therefore, light exposure was assumed to be negligible during the actual experiment. The Animal Care and Use Committee at Florida State University approved these procedures and the animal facility.
RNA Extraction, Microarray, and Microarray Data Analysis
TA muscles (~20 mg) were homogenized in 600 µL of Zymo TRI Reagent (Zymo Research, Irvine, CA), and RNA was isolated using a Zymo RNA MiniPrep extraction kit (cat. no. R-2071) with on-column DNase treatment (Irvine, CA). RNA quantity was determined spectrophotometrically by the 260 nm-to-280 nm ratio. The quality of total RNA was assessed by the Agilent Bioanalyzer Nano Chip (Agilent Technologies, Santa Clara, CA), and RNA integrity scores >6 were required for subsequent microarray analysis. Microarray analysis was conducted as previously described (25). The microarray and microarray analysis were performed at the Sanford Burnham Prebys Medical Discovery Institutes (Orlando, FL, and La Jolla, CA, respectively; n = 3 per group). Lists of differentially expressed genes (DEGs) were generated using a flexible P value (fold change ≥1.5 and P value without false discovery rate <0.05) as this resulted in >100 DEGs (Supplemental Table S1; all Supplemental Material for this article is available online at https://doi.org/10.6084/m9.figshare.8939567.v1). A rigorous P value was not used for microarray analysis as it resulted in only a handful of DEGs. The list of DEGs was uploaded into the publicly available Database for Annotation, Visualization and Integrated Discovery (DAVID) software (https://david.ncifcrf.gov/) and analyzed using two separate algorithms: 1) functional category analysis and 2) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis (Tables S2 and S3, respectively). Gene expression data for this study have been made available at Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo; GSE126965).
cDNA Synthesis and RT-PCR
cDNA was synthesized from 1.5 µg of total RNA using High Capacity cDNA Reverse Transcription Kit (cat. no. 4368814; Thermo Fisher Scientific, Waltham, MA). RT-PCR was conducted on either a QuantStudio3 (Thermo Fisher Scientific) or a CFX Connect (Bio-Rad, Hercules, CA) RT-PCR thermal cycler using PowerUp SYBR Green Master Mix (cat. no. A-2742; Thermo Fisher Scientific) or TaqMan Fast Advanced Master Mix (cat. no. 4444557; Thermo Fisher Scientific). The conditions for RT-PCR with SYBR Green included an initial 2 min at 50°C and 2 min at 95°C, followed by 40 cycles that included a 15-s denature step at 95°C, a 15-s annealing step at 55°C, and a 1-min extension step at 72°C within each cycle. A melt curve analysis was performed for each primer pair to ensure that a single product was efficiently amplified, and the product sizes for each primer pair were verified via agarose gel electrophoresis before experimentation. Measurement of BCL2/adenovirus E1B 19-kDa protein-interacting protein-3 (Bnip3, assay ID Mm01275600_g1), transcription factor A, mitochondrial (Tfam, assay ID Mm00447485_m1), nuclear respiratory factor 1 (Nrf1, assay ID Mm01135606_m1), Parkin RBR E3 ubiquitin-protein ligase (Parkin, assay ID Mm00450187_m1), phosphatase and tensin homolog (PTEN)-induced putative kinase protein-1 (Pink1, assay ID Mm00550827_m1), Clock (assay ID Mm00455950_m1), microtubule-associated proteins 1A/1B light chain 3B (LC3B, assay ID Mm00782868_sH), and muscle atrophy F-box (MAFbx, assay ID Mm00499523_m1) was quantified using TaqMan predesigned primer probes according to the manufacturer-recommended conditions for the QuantStudio3. Relative expression levels of all genes were normalized using the ΔΔCt method (where Ct is threshold cycle). Gapdh was used as the internal control for validation of the microarray, whereas ribosomal protein lateral stalk subunit P0 (Rplp0) was used as the internal control for the circadian study as Rplp0 expression was not affected by either time or castration (Table S4). Primer sequences for all SYBR Green RT-PCR reactions are listed in Table 1. The mean reaction efficiency for all experimental genes was 86.6 ± 6.3% (standard deviation). The reaction efficiencies of Rplp0 and Gapdh were 87.3 and 81.3%, respectively.
Table 1.
Primer sequences for RT-PCR using SYBR Green
| Gene Symbol | Forward (5′–3′) | Reverse (5′–3′) | Amplicon Size, bp |
|---|---|---|---|
| Bmal1 | TGGAGGGACTCCAGACATTC | TTGCTGCCTCATCGTTACTG | 173 |
| Per1 | GTCCCCTGGTCCTCTACACA | GCCCGAGATTCAATGAAGAG | 159 |
| Per2 | AGCCACCCTGAAAAGGAAGT | GGTGAGGGACACCACACTCT | 184 |
| Per3 | GTCGAGAGGAGGTGCTGAAG | TCTGTCTTCACAGGCGACAC | 173 |
| Rev-Erbα | GGCACCTGCCAACAGTCTA | GCTGAGAAAGGTCACGGAAG | 197 |
| Rora | GGAAGAGTTTGTGTTCTATGCACC | TTCCATCTTCTCGGTGGTTC | 177 |
| Cry1 | TTCACTGCTACTGCCCTGTG | CACTTGGCAACCTTCTGGAT | 151 |
| Myod | TACCCAAGGTGGAGATCCTG | CATCATGCCATCAGAGCAGT | 200 |
| Dbp | TCTAGGGACACACCCAGTCC | TGGTTGAGGCTTCAGTTCCT | 159 |
| Rplp0 | CAACCCAGCTCTGGAGAAAC | GTTCTGAGCTGGCACAGTGA | 169 |
| Drp1 | AGGAACCAACAACAGGCAAC | TCACAATCTCGCTGTTCTCG | 190 |
| Fis1 | AAGTATGTGCGAGGGCTGTT | ACAGCCAGTCCAATGAGTCC | 167 |
| Opa1 | GATGACACGCTCTCCAGTGA | TCGGGGCTAACAGTACAACC | 177 |
| Mfn1 | GCTGTCAGAGCCCATCTTTC | CAGCCCACTGTTTTCCAAAT | 195 |
| Mfn2 | AGCGCCAGTTTGTGGAATAC | CTTTCTTGTTCATGGCAGCA | 177 |
| Ppargc1a | AAGACGGATTGCCCTCATTT | AGTGCTAAGACCGCTGCATT | 191 |
| Sirt1 | AGTTCCAGCCGTCTCTGTGT | CTCCACGAACAGCTTCACAA | 198 |
| Gapdh | GTTGTCTCCTGCGACTTCA | TGCTGTAGCCGTATTCATTG | 124 |
| Id1 | TACGACATGAACGGCTGCTA | GTGGTCCCGACTTCAGACTC | 155 |
Bmal1, brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like 1; bp, base pair; Cry1, cryptochrome circadian regulator 1; Dbp, D-box-binding proline- and acid-rich basic region leucine zipper (PAR bZIP) transcription factor; Drp1, dynamin-1-like protein; Fis1, mitochondrial fission 1 protein; Id1, inhibitor of DNA binding 1, helix-loop-helix (HLH) protein; Mfn1 and Mfn2, mitofusin-1 and -2, respectively; Myod, myogenic differentiation; Opa1, dynamin-like 120-kDa protein, mitochondrial; Per1–3, period circadian regulator 1–3, respectively; Ppargc1a, peroxisome proliferator-activated receptor-γ coactivator 1α; Rev-Erbα, nuclear receptor subfamily 1 group D member 1α; Rora, retinoic acid receptor (RAR)-related orphan receptor-α; Rplp0, ribosomal protein lateral stalk subunit P0; Sirt1, sirtuin 1.
Micro-RNA Analysis
Micro-RNA expression was determined using TaqMan assay primer probes (Thermo Fisher Scientific) against micro-RNA-181a (miR-181a, cat. no. 4427975; assay ID 000480). As per the manufacturer’s recommendations, 15 ng of total RNA (isolated using Zymo MiniPrep extraction kit as described above) were reverse transcribed using a micro-RNA reverse transcription kit (cat. no. 4427975; Thermo Fisher Scientific) with micro-RNA-specific primers provided with each TaqMan assay. Relative micro-RNA expression levels were normalized using the ΔΔCt method. snRNA U6 (cat. no. 4427975; assay ID 001973) was used as the internal control as previously recommended (43). Expression of U6 was not affected by either time or castration (Table S4). Reaction efficiencies for U6 and miR-181a were 95.3 and 96.8%, respectively.
Western Blot Analysis
Western blot analysis was conducted as previously described with slight modifications (64). Whole muscle protein from the TA was extracted by glass-on-glass homogenization in 10 volumes of buffer (10 µL/mg muscle) consisting of 50 mM HEPES (pH 7.4), 0.1% Triton X-100, 4 mM EGTA, 10 mM EDTA, 50 mM Na4P2O7, 100 mM β-glycerophosphate, 25 mM NaF, 5 mM Na3VO4, and 10 µL/mL protease inhibitor cocktail (cat. no. P-8340; Sigma-Aldrich). Muscle extract was centrifuged for 10 min at 10,000 g at 4°C, and the supernatant fraction was quantified via the Bradford method. After quantification, all samples were diluted to the same concentration in 2X Laemmli buffer. At each circadian time point, equal amounts of protein from the three samples within a group (sham or castrated) were pooled together before Western blot analysis as a way to identify potential differences between groups in diurnal protein expression patterns (29). Though this method has clear limitations (i.e., inability to estimate variability at each time point), this method was chosen because of limited statistical power at each time point (i.e., n = 3 per group) and the lack of feasibility for determining relative protein expression by Western blot analysis from a single cohort consisting of 36 samples. Though pooling is not the preferred method for detecting differences as it can mask individual variation, others have shown that pooling samples can be a viable approach to identify potential differences when experimental constraints limit traditional analysis (17, 36). Thus, future work that is sufficiently powered will be needed to verify differences in protein expression within each time point. Once the samples were pooled, 20–60 µg of protein from each group and time point were fractionated on 4–20% Bio-Rad Tris-Glycine Criterion precast gels (Hercules, CA) and transferred to polyvinylidene difluoride membranes. Ponceau-S staining was used to assess effective transfer and equal protein loading. Membranes were blocked with 5% nonfat dried milk in Tris-buffered saline + 0.1% Tween 20 (Tris-buffered saline-Tween 20). Membranes were then incubated overnight at 4°C with antibodies against BNIP3 (cat. no. 3769), Parkin (cat. no. 2132), LC3B (cat. no. 2775), ubiquitin-binding protein p62 (p62, cat. no. 5114), cytochrome-c oxidase subunit IV (COX IV, cat. no. 4844), voltage-dependent anion-selective channel (VDAC, cat. no. 4866), Bcl-2-like protein-1 isoform L (Bcl-xL, cat. no. 2764), glycogen synthase kinase 3β (Ser9) [GSK3β (Ser9), cat. no. 5558], total GSK3β (cat. no. 12456), Akt (Ser473) (cat. no. 4060), total Akt (cat. no. 9272), and sirtuin 1 (Sirt1, cat. no. 2028), which were all obtained from Cell Signaling Technology (Danvers, MA). Antibodies against Per2 were produced in-house (14). Antibodies against Bmal1 were obtained from Sigma-Aldrich (cat. no. SAB-4300614). Antibodies against 4-hydroxynonenal (4-HNE) were obtained from Alpha Diagnostics (cat. no. HNE 13-M; San Antonio, TX). After incubation with secondary antibodies (cat. no. A-120-101P or A-90-116P, Bethyl Laboratories; cat. no. A-7289, Sigma-Aldrich), the antigen-antibody complex was visualized by enhanced chemiluminescence using Clarity reagent (Bio-Rad) on a Bio-Rad ChemiDoc Touch imaging system. The exposure time for all blots occurred within 10 min. The pixel density for total protein was quantified as the ratio of total protein to the 45-kDa band on the Ponceau-S stain using ImageJ software (National Institutes of Health, Bethesda, MD) or Image Lab Software (Bio-Rad), while the pixel density for all other blots was quantified as the phosphorylated-to-total protein ratio using ImageJ software. We have previously shown that our anti-mouse secondary antibody reacts nonspecifically with the presumed endogenous heavy-chain (~50 kDa) and light-chain (~25 kDa) IgG within our mouse muscle extracts (24). As such, those bands were excluded from the quantification of 4-HNE. The antibodies used in this study have been previously validated by our laboratory (23, 24, 55, 56) or by others (7, 12, 16, 26, 60, 71, 74, 77).
Cytosolic-Nuclear Fraction Separation
Whole gastrocnemius muscle samples were homogenized using glass on glass in 10 volumes (10 µL/mg tissue) of buffer (hereinafter referred to as buffer A) consisting of 10 mM NaCl, 1.5 mM MgCl2, 20 mM HEPES, 20% glycerol, 0.1% Triton X-100, 1 mM DTT, and 10 µL/mL protease inhibitor cocktail (cat. no. P-8340; Sigma-Aldrich, St. Louis, MO). Samples were centrifuged for 5 min at 2,400 g and 4°C. The supernatant was collected and saved as the cytosolic-enriched fraction. This fraction was further centrifuged three times, each for 5 min at 3,500 g and 4°C, to pellet and remove any remaining noncytosolic material. The pellet containing the nuclear-enriched fraction was then gently washed three times in buffer A. Between each wash, the nuclear pellet was centrifuged for 5 min at 2,400 g and 4°C. Proteins in the final nuclear pellet were then extracted using glass-on-glass homogenization in 400 µL of the protein extraction buffer described in materials and methods, Western Blot Analysis. The sample was then centrifuged for 15 min at 21,000 g and 4°C. The supernatant was collected and saved as the nuclear-enriched fraction. The protein content of each fraction was quantified by the Bradford method, and equal quantities of protein were diluted into 2X Laemmli buffer. At each circadian time point, equal amounts of protein from each fraction generated from the three samples within a group (sham or castrated) were pooled together before Western blot analysis.
Statistical Analysis
Circadian protein expression data from pooled samples are presented as a single value at each time point. All other data are presented as means ± SE. Analysis of the microarray was described above. Student’s t test was used to compare body and tissue masses between groups and to validate the microarray. Two-way ANOVA was used to assess changes in mRNA across the sampling period using castration and time as the two factors. If an interaction was observed, Fisher’s least significant difference test was used post hoc to define specific differences. Otherwise, main effects are shown. Differences in protein expression patterns from ≥3 consecutive time points were initially detected visually. If an expression pattern was visually observed, differences in the mean pixel intensity values obtained from the ≥3 time points were assessed by Student’s t test. Given that this study is underpowered to detect differences in protein expression at each time point, future work will need to confirm the specific changes in protein expression at each time point. All analysis was performed using GraphPad Prism software (La Jolla, CA). Significance for all analysis was set at P ≤ 0.05.
RESULTS
Androgen Depletion Disrupts Core Clock Phase and Function in the Limb Skeletal Muscle
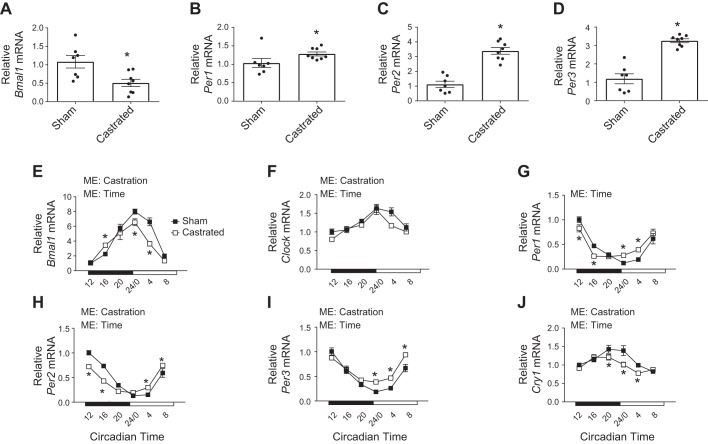
Total RNA from the TA of sham and castrated mice was analyzed by microarray. DAVID bioinformatics software identified significant changes in genes that compose “biological rhythms” and “circadian rhythm” using functional category and KEGG pathway analyses, respectively (Tables 2 and 3). Bmal1, Per2, and Per3 were common genes included in both analyses (Table 4), and RT-PCR validation confirmed that expression of Bmal1 was decreased and expression of Per1–3 was increased in the atrophied TA muscle of castrated mice relative to values in sham mice (Fig. 1, A–D). To assess the magnitude by which androgen depletion disrupted the core clock, TA muscles were harvested at 4-h intervals across a single circadian cycle from mice that were previously subjected to a sham or castration surgery. The muscle and tissue characteristics of those mice are presented in Tables 5 and 6, respectively. As expected (64, 71), mass of various limb muscles was lower in the castrated mice compared with the sham group without a corresponding change in tibia length or fat pad mass (Tables 5 and 6). The efficacy of the castration surgery was also confirmed by changes in the mass of various androgen-sensitive tissues, including the seminal vesicle (55, 64; Table 6). Gene expression of Bmal1 and Per1–3 in the TA of sham mice exhibited the expected circadian patterns (58), and these patterns were altered in the TA of castrated mice (Fig. 1, E and G–I). The diurnal expression pattern of Clock and Cry1 was also disrupted (Fig. 1, F and J), whereas circadian expression of Cry2 was not different between groups (data not shown).
Table 2.
Top 5 functional gene categories altered by castration
| Functional Category | No. of Genes | P Value |
|---|---|---|
| Polyamine biosynthesis | 3 | 0.0011 |
| Biological rhythms | 5 | 0.0019 |
| Receptor | 22 | 0.0021 |
| Olfaction | 13 | 0.0030 |
| Decarboxylase | 3 | 0.0034 |
Table 3.
Top 5 KEGG pathways altered by castration
| KEGG Pathway | No. of Genes | P Value |
|---|---|---|
| Glutathione metabolism | 4 | 0.0037 |
| Systemic lupus erythematosus | 5 | 0.0095 |
| Circadian rhythm | 3 | 0.013 |
| Herpes simplex infection | 5 | 0.030 |
| Arginine and proline metabolism | 3 | 0.032 |
KEGG, Kyoto Encyclopedia of Genes and Genomes.
Table 4.
RT-PCR confirmation of the molecular clock/circadian genes identified via microarray
| Gene Symbol | Fold Change (Castrated vs. Sham) |
P Value | 95% Confidence Interval |
|---|---|---|---|
| Bmal1 | −0.57351 | 0.0102 | −0.985 to −0.1609 |
| Id1 | 1.8576 | 0.0327 | 1.08249 to 2.633 |
| Per2 | 3.27 | <0.0001 | 2.598 to 3.937 |
| Per3 | 3.062 | <0.0001 | 2.467 to 3.658 |
| Ppargc1a | 1.8999 | 0.0269 | 1.1203 to 2.68 |
n = 7–8 mice per group. Bmal1, brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like 1; Id1, inhibitor of DNA binding 1, helix-loop-helix (HLH) protein; Per2 and Per3, period circadian regulator 2 and 3, respectively; Ppargc1a, peroxisome proliferator-activated receptor-γ coactivator 1α. Ppargc1a gene expression from this data set has been reported previously (56).
Fig. 1.
Characterization of changes to the core clock gene expression in the limb skeletal muscle following androgen depletion. A–D: microarray analysis was confirmed by measuring the relative mRNA content of brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like 1 (Bmal1) and period circadian regulator 1–3 (Per1–3) in the tibialis anterior (TA) of sham and castrated mice by RT-PCR (n = 7/8 per group for microarray validation). These samples were collected between 1300 and 1500. E–J: circadian expression patterns of Bmal1, circadian locomotor output cycles protein kaput (Clock), Per1–3, and cryptochrome circadian regulator 1 (Cry1) mRNA were determined in the TA of sham and castrated mice by RT-PCR (n = 3 per group per time point for circadian measurements). Student’s t test was used to confirm microarray analysis. Two-way ANOVA was used to assess changes in circadian expression patterns. ME, main effect. *Significantly different from the mean value in the Sham group, or significantly different from the Sham value at the given circadian time. P ≤ 0.05 for all other analyses.
Table 5.
Muscle mass and tibia length
| Sham | Castrated | |
|---|---|---|
| Tibialis anterior, mg | 50.98 ± 0.82 | 42.60 ± 1.18* |
| Gastrocnemius, mg | 140.53 ± 1.57 | 130.66 ± 1.60* |
| Plantaris, mg | 22.73 ± 0.52 | 20.06 ± 0.48* |
| Soleus, mg | 9.79 ± 0.20 | 8.58 ± 0.21* |
| Tibia length, mm | 17.39 ± 0.10 | 17.17 ± 0.09 |
Values are means ± SE; n = 18 mice for both sham and castrated groups.
Significance at P < 0.05.
Table 6.
Body and tissue mass
| Sham | Castrated | |
|---|---|---|
| Initial body weight, g | 26.7 ± 0.40 | 26.7 ± 0.39 |
| Final body weight, g | 32.6 ± 0.61 | 29.4 ± 0.62* |
| Heart, mg | 139.29 ± 3.44 | 121.87 ± 2.54* |
| Spleen, mg | 64.56 ± 2.39 | 85.32 ± 1.39* |
| Epididymal fat pad, mg | 1,035.64 ± 65.72 | 1,129.28 ± 112.61 |
| Seminal vesicle, mg | 364.42 ± 14.16 | 11.03 ± 1.23* |
Values are means ± SE; n = 18 mice for both sham and castrated groups.
Significance at P < 0.05.
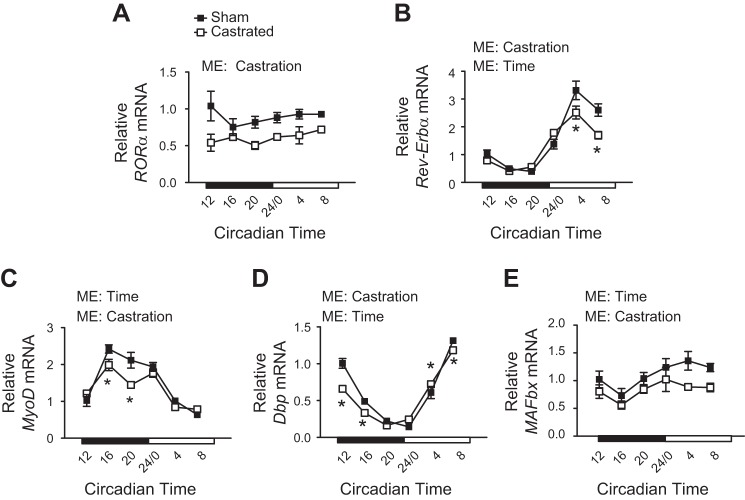
Previous work has identified various genes in skeletal muscle whose expression is under the control of the core clock (45, 58), including the nuclear receptors retinoic acid receptor (RAR)-related orphan receptor-α (Rora) and nuclear receptor subfamily 1 group D member 1α (Rev-Erbα), myogenic differentiation (Myod), D-box-binding proline- and acid-rich basic region leucine zipper (PAR bZIP) transcription factor (Dbp), and MAFbx. Rev-Erbα, Myod, Dbp, and MAFbx each exhibited a circadian expression pattern in the TA of sham mice, whereas Rora did not (Fig. 2, A–E). Androgen depletion significantly altered the rhythmicity or overall expression of each gene (Fig. 2, A–E), suggesting that androgens are required for proper core clock function in addition to the normal cycling of core clock genes.
Fig. 2.
Assessment of core clock function in the limb muscle following androgen depletion. A–E: circadian expression patterns of various clock-controlled genes including retinoic acid receptor (RAR)-related orphan receptor-α (Rora), nuclear receptor subfamily 1 group D member 1α (Rev-Erbα), myogenic differentiation (Myod), D-box-binding proline- and acid-rich basic region leucine zipper (PAR bZIP) transcription factor (Dbp), and muscle atrophy F-box (MAFbx) were determined in the tibialis anterior of sham and castrated mice by RT-PCR (n = 3 per group per time point for circadian measurements). Student’s t test was used to confirm microarray analysis. Two-way ANOVA was used to assess changes in circadian expression patterns. ME, main effect. *Significantly different from the Sham value at the given circadian time. P ≤ 0.05 for all other analyses.
Expression of Various Mitochondrial Quality Control Genes is Disrupted by Androgen Depletion
The core molecular clock has been implicated in regulating the expression of genes involved with mitochondrial quality control (29). Indeed, disruption of the core clock decreased measures of mitochondrial function in skeletal muscle (3). This is pertinent to androgen depletion as disruption of mitochondrial quality control can induce muscle atrophy (53, 61), and our group previously observed a strong relationship between indexes of impaired mitochondrial quality and the degree of muscle atrophy following androgen depletion (56). Moreover, the magnitude of change in expression of core clock genes identified by the microarray was strongly related to both the indexes of impaired mitochondrial quality (e.g., r = 0.94) and the degree of muscle atrophy (e.g., r = 0.58) previously reported in those same samples (56).
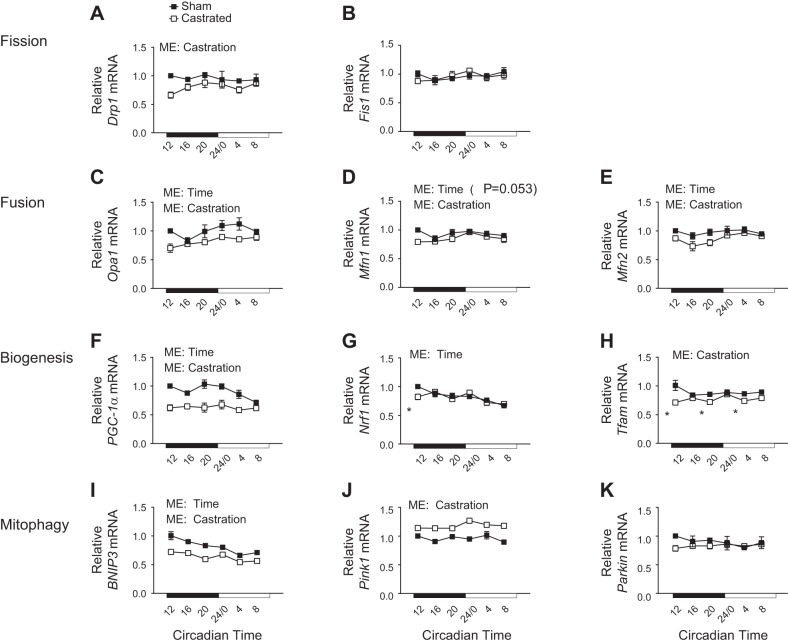
Mitochondria are dynamic organelles that change in number, size, and network complexity to handle metabolic demand by the coordinated balance of mitochondrial fission-fusion and mitochondrial biogenesis-degradation (50, 65). Mitofusin-1 (Mfn1), Mfn2, and the mitochondrial dynamin-like GTPase [dynamin-like 120-kDa protein, mitochondrial (Opa1)] promote fusion of mitochondria into larger networks (73), whereas mitochondrial fission 1 protein (Fis1) and dynamin-1-like protein (Drp1) promote network fragmentation (73). Though genes involved with mitochondrial fission (Drp1 and Fis1) changed in expression over time in other tissues (29), expression of these genes did not differ by time in the present study (Fig. 3, A and B). However, castration led to an overall reduction in the mRNA expression of Drp1. Conversely, expression of genes involved with mitochondrial fusion (Opa1 and Mfn2) changed over time, and castration led to an overall decrease in expression of these genes (Fig. 3, C and E). There was a strong trend for expression of Mfn1 to change over time (P = 0.053) with castration causing a significant overall reduction in expression (Fig. 3D).
Fig. 3.
Assessment of circadian expression patterns for genes involved with mitochondrial quality control in the limb skeletal muscle following androgen depletion. The circadian expression patterns of genes involved with mitochondrial fission (A and B), mitochondrial fusion (C–E), mitochondrial biogenesis (F–H), and mitophagy (I–K) were determined in the tibialis anterior of sham and castrated mice by RT-PCR. Two-way ANOVA was used to assess circadian expression patterns; n = 3 per group per time point for circadian measurements. ME, main effect. Bnip3, BCL2/adenovirus E1B 19-kDa protein-interacting protein-3; Drp1, dynamin-1-like protein; Fis1, mitochondrial fission 1 protein; Mfn1 and Mfn2, mitofusin-1 and -2, respectively; Nrf1, nuclear respiratory factor 1; Opa1, dynamin-like 120-kDa protein, mitochondrial; Parkin, Parkin RBR E3 ubiquitin-protein ligase; Pgc-1α, peroxisome proliferator-activated receptor-γ coactivator 1α (Ppargc1a); Pink1, phosphatase and tensin homolog (PTEN)-induced putative kinase protein-1; Tfam, transcription factor A, mitochondrial. *Significantly different from the Sham value at the given circadian time. P ≤ 0.05 for all other analyses.
Mitochondrial biogenesis is mediated in large part by the peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α; gene, Ppargc1a) signaling nexus, which regulates the expression of various mitochondrial-associated genes from both nuclear and mitochondrial genomes (44). Ppargc1a is a known clock-controlled gene in skeletal muscle (58), and as such, Ppargc1a mRNA exhibited a change in expression over time (Fig. 3F). Consistent with disruption of the core clock, the overall expression of Ppargc1a was significantly reduced in the TA of castrated mice (Fig. 3F). Expression of Nrf1, a coactivator of PGC-1α within the biogenesis nexus (20), was largely unaffected by castration (Fig. 3G). However, expression of Tfam, a target gene of the Nrf1-PGC-1α transcriptional complex (33), exhibited an overall reduction (Fig. 3H), suggesting impaired signaling downstream of the Nrf1-PGC-1α complex.
Mitochondrial degradation is an important quality control mechanism for the removal of old or dysfunctional mitochondria via the lysosomal-mediated process termed “mitophagy” (40), which occurs through at least two distinct pathways. BNIP3 shuttles mitochondria to the phagophore for disposal into the lysosome, whereas degradation via Pink1 and Parkin involves ubiquitylation of mitochondrial proteins and subsequent shuttling to the phagophore by the p62 adaptor protein (32, 49). Bnip3 was the only mitophagy-related gene to exhibit a change in expression over time, and this occurred in both groups (Fig. 3I). However, castration led to an overall reduction in the expression of Bnip3 as well as an overall increase in Pink1 (Fig. 3, I and J). Parkin mRNA was not affected by castration (Fig. 3K). In all, these data suggest that genes involved with mitochondrial quality control, including those that exhibit changes in expression over time, were disrupted by androgen depletion.
Indexes of Mitophagy and Autophagy Activation Are Enhanced Following Androgen Deprivation, and this Coincides with Changes in Mitochondrial Protein Expression Patterns
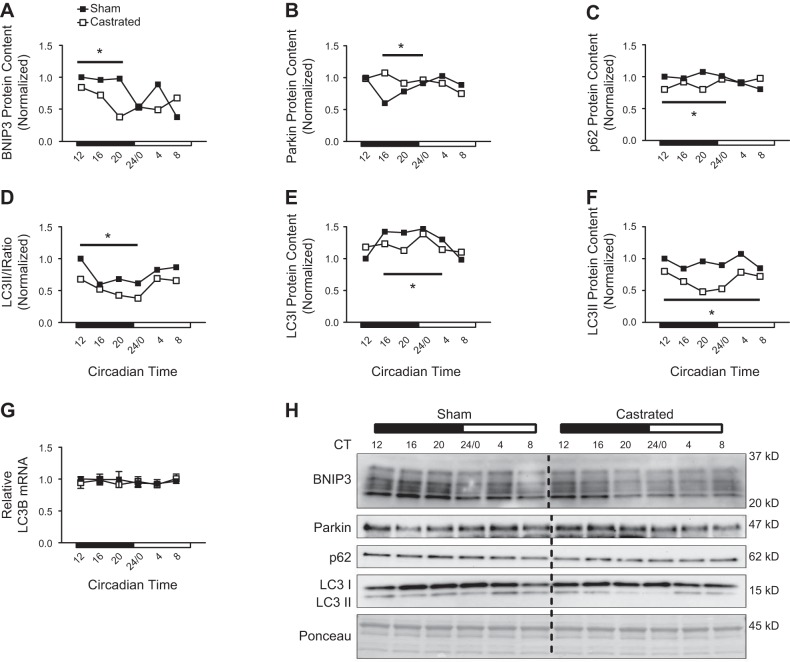
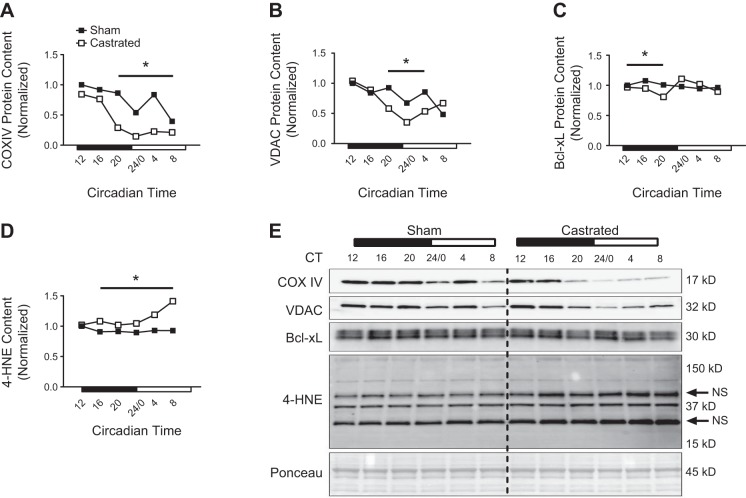
Mitochondrial degradation and global autophagy are enhanced when mitochondrial quality is impaired to protect the cell against adverse events such as apoptosis (10, 11, 47). Our previous work found that BNIP3 protein content was reduced in the TA muscle of castrated mice 4 h following consumption of a meal, but the change in BNIP3 protein did not coincide with a change in the corresponding BNIP3 transcript (56). Because BNIP3 protein is turned over during the mitophagy process (75), it was concluded that activation of that degradative process was increased. Consistent with those previous data, the BNIP3 protein expression pattern in the muscle of castrated mice exhibited an accelerated decrease from circadian time 12 to 20 corresponding to the period of time when food consumption in mice is elevated (Fig. 4, A and H). This pattern was not matched by a change in the corresponding transcript (i.e., Figure 3I), suggesting enhanced activation of this degradative pathway. In addition to BNIP3, the pattern of Parkin protein was elevated from circadian time 16 to 24 (Fig. 4, B and H), implying activation of this degradative pathway as well. Since Parkin-induced mitophagy occurs via p62 (32), and p62 protein is degraded during this process (38), the pattern of p62 protein was decreased in the muscle of castrated mice at those same time points, further supporting activation of this degradative pathway (Fig. 4, C and H).
Fig. 4.
Assessment of mitophagy and autophagy activation patterns in the limb skeletal muscle following androgen depletion. A–C: circadian protein expression patterns for mitophagy-related proteins were determined in the tibialis anterior (TA) of sham and castrated mice by Western blot analysis. D–F: circadian protein expression patterns of the microtubule-associated proteins 1A/1B light chain 3B-II (LC3 II)-to-LC3 I ratio, LC3 I, and LC3 II were determined in the TA of sham and castrated mice by Western blot analysis. G: circadian expression pattern for LC3B mRNA was determined in the TA of sham and castrated mice by RT-PCR. For Western blot analysis, equal amounts of protein from each sample within a group (n = 3 sham or castrated) at each time point were pooled together for analysis. If a visual difference in the expression patterns across ≥3 consecutive time points was observed, differences in the mean pixel intensity values obtained from the ≥3 time points for each group (sham or castrated) were assessed statistically. H: Western blot. Dashed black line on blot is used to visually separate the sham and castrated groups. Two-way ANOVA was used to assess the circadian expression pattern of LC3B mRNA. Student’s t test was used to assess difference in the pixel intensity of ≥3 consecutive time points of protein in the TA; n = 3 per group per time point for circadian measurement of LC3B mRNA. CT, circadian time; ME, main effect. BNIP3, BCL2/adenovirus E1B 19-kDa protein-interacting protein-3; p62, ubiquitin-binding protein p62; Parkin, Parkin RBR E3 ubiquitin-protein ligase. *Significant difference from ≥3 consecutive time points between groups under the solid black line. P ≤ 0.05 for analysis.
The LC3 II-to-LC3 I ratio (LC3 II/I ratio) is used to represent global autophagy activation since lipidation of the LC3 protein (conversion of LC3 I to LC3 II) is required for closure of the autophagosome and subsequent disposal of components at the lysosome (35). Typically, a decrease in the ratio of LC3 II to LC3 I suggests autophagy inhibition, and vice versa (35, 38). In sham mice, the pattern of the LC3 II/I ratio was lowest from circadian time 16 to 24/0 (Fig. 4, D and H), consistent with nutrient-mediated inhibition of this degradative process when mice consume the majority of food [i.e., the dark cycle (19, 35)], and this pattern was higher from circadian time 24/0 to 8, when mice are typically fasting. Interestingly, the LC3 II/I ratio pattern was lower in the muscle of castrated mice from circadian time 12 to 24/0 (Fig. 4, D and H), which would initially suggest greater autophagy inhibition. However, comparison of the individual changes in the LC3 I and LC3 II patterns suggests that LC3 I lipidation (i.e., conversion to LC3 II) and LC3 II clearance were higher in the muscle of castrated mice during this time, implying enhanced autophagy activation. For instance, the LC3 I pattern increased in the TA of sham mice, but not in the castrated group (Fig. 4, E and H), even though LC3B mRNA content did not differ between groups (Fig. 4G). This suggests that the conversion of LC3 I to LC3 II was blunted in the muscle of sham mice but this conversion was maintained in the muscle of castrated mice. Furthermore, LC3 II is degraded when autophagy is activated (35), and thus, the decrease in the LC3 II pattern that occurred only in the muscle of castrated mice likely resulted from enhanced LC3 II turnover (Fig. 4, F and H). The notion that autophagy was increased in the muscle of castrated mice is further supported by the decrease in the p62 expression pattern at these same time points (i.e., Fig. 4C), which serves as a complimentary marker of autophagy activation (38). In all, these data suggest that disruption of mitochondrial quality control gene expression in the limb muscle following androgen depletion coincides with increased activation of BNIP3 and Pink/Parkin-mediated mitochondrial degradation and global autophagy activation.
Markers of mitochondrial content were assessed to determine whether changes in these markers coincided with the upregulation of mitochondrial degradation. Though COX IV and VDAC expression appeared similar between groups at circadian times 12–16, the pattern of COX IV was significantly lower in the muscle of castrated mice from circadian time 20 to 8, and the pattern of VDAC expression was lower at circadian times 20–4 (Fig. 5, A, B, and E), coinciding with the observed increase in markers of mitophagy pathway activation (i.e., Fig. 4). The Bcl-xL expression pattern was also lower in the muscle of castrated mice from circadian time 12 to 20 before returning to sham values for the remainder of the time course (Fig. 5, C and E), suggesting a compensatory effect by the muscle to maintain levels of this antiapoptotic protein (30). The pattern of 4-HNE, a free-radical by-product, was higher in the muscle of castrated mice before the change in the mitochondrial protein expression pattern, and this elevation persisted throughout the remaining sampling time course (Fig. 5, D and E), suggesting that production of reactive oxygen species coincided with enhanced turnover of mitochondrial proteins following androgen depletion.
Fig. 5.
Assessment of mitochondrial protein expression patterns in the limb skeletal muscle following androgen depletion. A–D: circadian expression patterns for cytochrome-c oxidase subunit IV (COX IV, A), voltage-dependent anion-selective channel (VDAC, B), Bcl-2-like protein-1 isoform L (Bcl-xL, C), and 4-hydroxynonenal (4-HNE, D) were determined in the tibialis anterior (TA) of sham and castrated mice by Western blot analysis. E: Western blot. Dashed black line on blot is used to visually separate the sham and castrated groups. For Western blot analysis, equal amounts of protein from each sample within a group (n = 3 sham or castrated) at each time point were pooled together for analysis. If a visual difference in the expression patterns across ≥3 consecutive time points was observed, differences in the mean pixel intensity values obtained from the ≥3 time points for each group (sham or castrated) were assessed statistically. Student’s t test was used to assess difference in the pixel intensity of ≥3 consecutive time points of protein in the TA. CT, circadian time. The black arrows indicate nonspecific (NS) reactivity by the anti-mouse secondary antibody. For illustrative purposes only, the lanes of the 4-HNE blot were made vertical in Adobe Photoshop after quantification. *Significant difference from ≥3 consecutive time points between groups under the solid black line. P ≤ 0.05 for analysis.
Various Regulators of the Core Clock Are Altered in the Limb Muscle Following Androgen Depletion
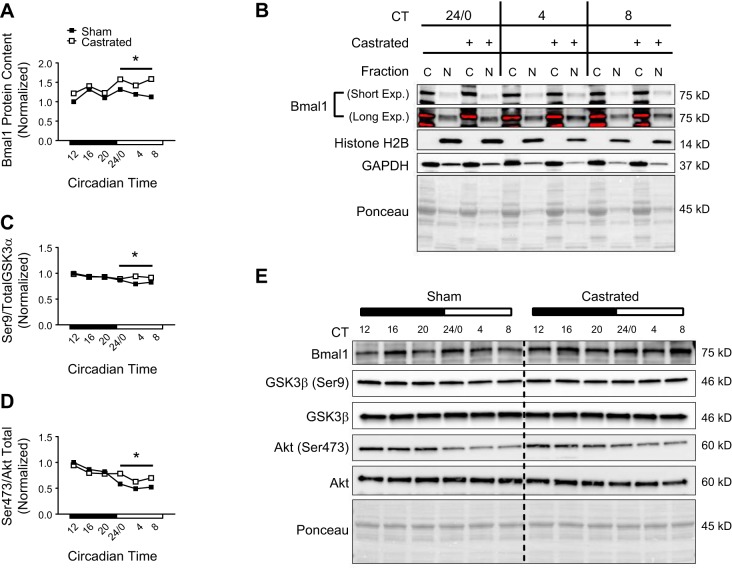
The most evident change to the core clock following androgen depletion was an overall reduction in the phase amplitude of clock-controlled genes. In addition to regulating Bmal1 gene transcription, MyoD also feeds back to enhance the transcriptional activity of the Bmal1-Clock dimer in skeletal muscle (27). As such, the reduction in Myod (i.e., Fig. 2) may have contributed to impaired transcription of Bmal1-Clock target genes (i.e., Dbp). Despite reduced amplitude of Bmal1-Clock target genes, we observed a contradictory increase in the protein expression pattern of Bmal1 in the muscle of castrated mice from circadian time 24/0 to 8 (Fig. 6, A and E). Further analysis indicates that impairment of Bmal1-Clock transcription was not due to Bmal1 protein export from the nucleus as Bmal1 protein content appeared to be higher in the nuclear-enriched fraction from gastrocnemius muscles of castrated mice from circadian time 24/0 to 8 (long exposure in Fig. 6B).
Fig. 6.
Assessment of brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like 1 (Bmal1) regulation in the limb skeletal muscle following androgen depletion. A: circadian protein expression pattern of Bmal1 was determined in the tibialis anterior (TA) of sham and castrated mice by Western blot analysis. B: enrichment of Bmal1 protein in the cytosolic (C)- and nuclear (N)-enriched fractions was determined in the gastrocnemius of sham and castrated mice by Western blot analysis. C and D: circadian patterns for the ratio of phosphorylated to total protein for GSK3β (Ser9) (C) and Akt (Ser473) (D) were determined in the TA of sham and castrated mice by Western blot analysis. E: Western blot. Dashed black line on blot is used to visually separate the sham and castrated groups. For Western blot analysis, equal amounts of protein from each sample within a group (n = 3 sham or castrated) at each time point were pooled together for analysis. If a visual difference in the expression patterns across ≥3 consecutive time points was observed, differences in the mean pixel intensity values obtained from the ≥3 time points for each group (sham or castrated) were assessed statistically. Student’s t test was used to assess difference in the pixel intensity of ≥3 consecutive time points of protein in the TA. CT, circadian time; Exp., exposure. *Significant difference from ≥3 consecutive time points between groups under the solid black line. P ≤ 0.05 for all analyses.
Previous work found that loss of GSK3β function not only impaired Bmal1-Clock transcriptional activity but also resulted in accumulation of Bmal1 protein (57). Accordingly, the pattern of GSK3β phosphorylation on the inhibitory Ser9 site was higher in the muscle of castrated mice at the circadian times when the Bmal1 protein pattern was also elevated (Fig. 6, C and E), suggesting that inhibition of GSK3β function may have contributed in part to disruption of the muscle clock and the observed increase in Bmal1 protein expression pattern. Akt phosphorylates GSK3β on Ser9 to inhibit function (13), and we previously found Akt phosphorylation to be increased in the muscle following androgen deprivation (55, 56). Though the phosphorylation pattern of Akt (Thr308) did not appear different between groups (data not shown), the phosphorylation pattern of Akt (Ser473) was higher in the muscle of castrated mice from circadian time 24/0 to 8 (Fig. 6, D and E), implying that activation of Akt may have also contributed to disruption of the clock via GSK3β.
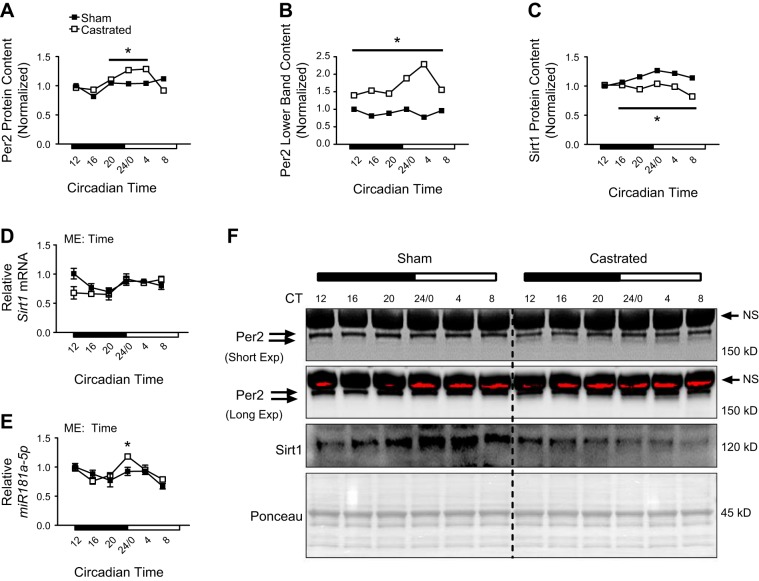
The Per2-Cry dimer also represses Bmal1-Clock transcriptional activity (8), with changes in expression of Per2 being the primary factor mediating this repression (12). Consistent with reduced amplitude of Bmal1-Clock target genes, the pattern of total Per2 protein was higher in the TA of castrated mice from circadian time 20 to 4 (Fig. 7, A and F). Per2 undergoes extensive phosphorylation as a signal to induce degradation (41, 69), and changes in Per2 phosphorylation can be observed by altered migration by SDS-PAGE (41). Consequently, the pattern of Per2 in the faster-migrating immunoreactive band was higher in the muscle of castrated mice (lower band in Fig. 7, B and F), indicating that accumulation of Per2 was due at least in part to reduced phosphorylation.
Fig. 7.
Assessment of period circadian regulator 2 (Per2) regulation in the limb skeletal muscle following androgen depletion. A–C: circadian protein expression patterns of Per2 (A), Per2 lower band (B), and sirtuin 1 (Sirt1, C) were assessed by Western blot analysis. For Western blot analysis, equal amounts of protein from each sample within a group (n = 3 sham or castrated) at each time point were pooled together for analysis. If a visual difference in the expression patterns across ≥3 consecutive time points was observed, differences in the mean pixel intensity values obtained from the ≥3 time points for each group (sham or castrated) were assessed statistically. Student’s t test was used to assess difference in the pixel intensity of ≥3 consecutive time points of protein in the tibialis anterior (TA). D and E: circadian expression patterns for Sirt1 mRNA (D) and micro-RNA-181a (miR-181a, E) were determined in the TA of sham and castrated mice by RT-PCR analysis; n = 3 per group per time point. Two-way ANOVA was used to assess circadian expression patterns of each RNA. F: Western blot. Dashed black line on blot is used to visually separate the sham and castrated groups. CT, circadian time; Exp, exposure; ME, main effect. The black arrows indicate nonspecific (NS) immunoreactive band. *Significant difference from ≥3 consecutive time points between groups under the solid black line (Western blots) or significantly different from the Sham values at the given circadian time (RT-PCR). P ≤ 0.05 for all analyses.
Sirt1 is an NAD+-dependent deacetylase whose protein not only exhibits a circadian expression pattern but also regulates function of the core clock by promoting degradation of the Per2 protein (4). Sirt1 protein exhibited the anticipated circadian expression pattern in the TA of sham mice (Fig. 7, C and F), but this pattern was completely lost in the muscle of castrated mice (Fig. 7, C and F) as the overall pattern was lower throughout the sampling period. Interestingly, this overall reduction in the Sirt1 protein pattern was independent of changes in the corresponding Sirt1 transcript (Fig. 7D), implying posttranscriptional regulation of the Sirt1 protein.
miR-181a not only influenced core clock function in other tissues (39) but also repressed Sirt1 expression at the level of translation (76), implying a potential role for change in this micro-RNA in the regulation of Sirt1. miR-181a expression oscillated in the muscle of both groups across the sampling period (main effect of time), but miR-181a levels were significantly higher in the TA of castrated mice at circadian time 24/0 (Fig. 7E), corresponding to the circadian time when Sirt1 protein expression pattern peaked in sham mice (i.e., Fig. 7C). Although this implies a potential repressive role for miR-181a at that time point, other factors likely contributed to the overall reduction in the Sirt1 protein pattern at other time points. In all, these data suggest that disruption of the clock following androgen deprivation is likely due to changes to various known clock regulatory components.
DISCUSSION
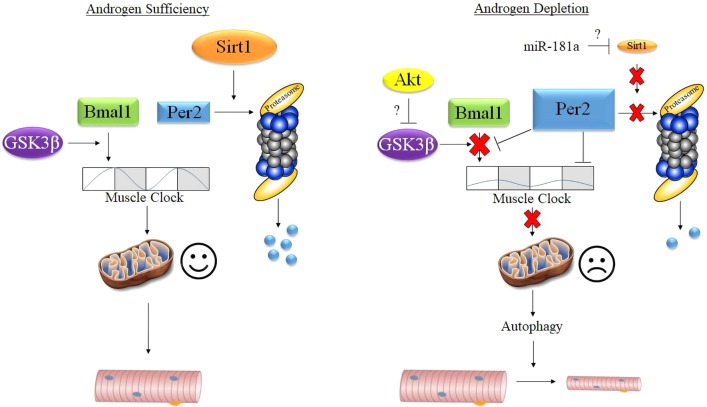
The absence of circulating androgens contributes to atrophy of the limb skeletal muscles (21, 22, 68); however, the underlying mechanisms remain ill defined. For instance, the canonical AR signaling pathway is not required for maintenance of limb skeletal muscle mass (2, 15, 51, 67). Previous work by our laboratory provided initial evidence that altered mitochondrial quality may contribute to limb muscle atrophy as mitochondrial degradation was related to the induction of autophagy markers and the degree of muscle atrophy (56). During our search for regulatory mechanisms that mediate mitochondrial quality, we identified changes in genes associated with the core clock (e.g., Bmal1). Since the core clock regulates mitochondrial quality in skeletal muscle (3) and other tissues [i.e., liver (29)], we hypothesized that disruption to the core clock may coincide with impaired mitochondrial quality control following androgen depletion. Consequently, our present findings show that disruption of the core clock following androgen depletion coincides with altered expression of various genes involved with mitochondrial quality control, including those that exhibit a change in expression over time. This disruption overlapped with indexes of enhanced mitochondrial degradation (mitophagy), global autophagy activation, and subsequent muscle atrophy. Therefore, we posit a novel conceptual model linking androgen depletion-induced limb muscle atrophy to reduced mitochondrial quality control via disruption of the molecular clock (Fig. 8).
Fig. 8.
Theoretical model for the androgen-mediated regulation of limb muscle mass. Bmal1, brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like 1; miR-181a, micro-RNA-181a; Per2, period circadian regulator 2; Sirt1, sirtuin 1.
The results of the present study provide evidence that atrophy of the limb muscle following androgen depletion may be due in part to changes in core clock-mediated regulation of mitochondrial quality control. Despite this notion, in previous work, disrupting the core clock in adult skeletal muscle via inducible deletion of Bmal1 was not sufficient to induce atrophy (18, 58), questioning this as a mechanism in the regulation of limb muscle mass. One possible explanation is that only some mitochondrial quality control genes exhibited a change in expression over time, implying that disruption of the core clock contributes to, but is not the only cause of, decreased mitochondrial quality control following androgen depletion. Indeed, analysis of publicly available gene arrays from the gastrocnemius of adult mice in which the muscle clock was disrupted via inducible Bmal1 deletion (58) showed that Pink1, which did not exhibit changes in expression over time in our study, was unaffected by deletion of Bmal1. Conversely, Ppargc1a and Bnip3, which did exhibit changes in expression over time in our study, were altered by deleting Bmal1. This suggests that the Bmal1-Clock transcriptional dimer is responsible for expression of some, but not all, mitochondrial quality control genes. An alternative explanation is that disruption of Bmal1-Clock in combination with changes in other core clock regulatory factors may contribute to the regulation of mitochondrial quality control and subsequent muscle mass. For instance, maintaining Sirt1 protein expression following nutrient deprivation preserved muscle mass by inhibiting the transcription of genes involved with muscle protein breakdown including MAFbx and Bnip3 (42). That mechanism of Sirt1 action with fasting would not be consistent with findings in the present study as MAFbx and Bnip3 mRNA were suppressed in the muscle under androgen-deprived conditions (i.e., Figs. 2 and 3; 31, 56), suggesting an alternative mechanism. A more likely explanation is that the decrease in muscle mass was due to the failure of Sirt1 to mediate degradation of Per2, which would disrupt not only the Bmal1-Clock transcriptional activity but also possibly expression of other genes that regulate muscle mass as well. For instance, in addition to mediating Bmal1-Clock function, Per2 has also been shown to regulate expression of mitochondrial genes (48) and to regulate growth in ovarian tumors (70). Therefore, the way in which the clock is disrupted under androgen-deprived conditions (i.e., decreased Sirt1 and increased Per2) may have a different effect on muscle mass compared with inhibition of just the Bmal1-Clock transcriptional complex.
It is known that testosterone is released in a circadian manner, but the sensitivity of the limb muscle clock to changes in testosterone concentration remains unknown. Our group showed that castration decreased plasma testosterone in mice by ~70% (56, 64), which is sufficient to disrupt the clock (Figs. 1 and 2). A previous study from our laboratory showed that administering nandrolone decanoate (ND) to previously castrated mice restored muscle mass (54). In that study, the TA muscles were harvested 7 days following the final ND injection. Even though muscle mass was completely restored by ND administration (54), testosterone levels at the time of euthanasia were reduced by ~50% compared with sham values (data not shown). Although that study was not properly designed to analyze changes to the core clock, the expression of some core clock genes (e.g., Per1 and Per3) was restored to sham levels by ND administration, whereas others (e.g., Clock and Rora) were not (data not shown). This suggests that the sensitivity of the core clock to changes in circulating testosterone may be gene dependent, and it will require additional work to assess the sensitivity of the core clock to changes in circulating testosterone.
In humans, androgens have been deemed to regulate muscle mass in large part by blunting muscle protein breakdown during the fasted metabolic state (21, 22). In contrast, our data show that markers of autophagy, oxidative stress, and mitochondrial degradation (e.g., increased Parkin and BNIP3 protein content and decreased p62 protein content) were increased in the muscle of castrated mice during a time when these nocturnal animals consume much of their food (i.e., circadian times 12–24). Given that we previously showed a strong relationship between mitochondrial degradation and autophagy activation (56), it is possible that nutrient consumption initiates mitochondrial stress, leading to the subsequent increase in autophagy-mediated protein breakdown that persists into the postabsorptive state (10, 11). This idea is supported by our recent finding that markers of mitophagy were increased in the muscle of castrated mice that were refed following an overnight fast (56) but those same markers of mitophagy were not altered if castrated mice remained fasted despite elevated markers of autophagy (64; unpublished observations). Future work is needed to understand whether increased availability of ATP-producing substrates (i.e., fatty acids and/or NADH+) might impose a detrimental stress on the mitochondria in the limb muscle under androgen-deprived conditions and whether this effect is augmented by disruption of the core clock.
In conclusion, we provide evidence that androgen depletion disrupts the core molecular clock in the limb skeletal muscle, and this disruption coincides with changes in mitochondrial quality control and subsequent muscle atrophy. The change in expression of mitochondrial quality control genes coincides with an increase in mitochondrial degradation pathway activation and subsequent change to the circadian expression pattern of mitochondrial proteins. As mitochondrial health is an emerging and important regulator of skeletal muscle mass, these data support a novel conceptual model linking androgen depletion-induced limb muscle atrophy to reduced mitochondrial quality control via disruption of the molecular clock. Such knowledge will be useful for developing therapies that treat limb muscle atrophy in hypogonadal males who are unable to receive androgen replacement therapy.
GRANTS
This project was supported through funds provided by the University of Central Florida and the Institute for Successful Longevity (to B. S. Gordon) and National Institutes of Health Grants R01-AR-066082 (to K. A. Esser) and NS-099813 (to C. Lee).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A.E. and B.S.G. conceived and designed research; M.L.R. and B.S.G. performed experiments; M.L.R., A.M.E., and B.S.G. analyzed data; M.L.R., K.A.E., C.L., R.J.T., and B.S.G. interpreted results of experiments; M.L.R. and B.S.G. prepared figures; M.L.R. and B.S.G. drafted manuscript; M.L.R., K.A.E., C.L., R.J.T., and B.S.G. edited and revised manuscript; M.L.R., K.A.E., C.L., R.J.T., A.M.E., and B.S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jennifer Steiner for critical discussion and review of the manuscript.
REFERENCES
- 1.Altamirano F, Oyarce C, Silva P, Toyos M, Wilson C, Lavandero S, Uhlén P, Estrada M. Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J Endocrinol 202: 299–307, 2009. doi: 10.1677/JOE-09-0044. [DOI] [PubMed] [Google Scholar]
- 2.Altuwaijri S, Lee DK, Chuang KH, Ting HJ, Yang Z, Xu Q, Tsai MY, Yeh S, Hanchett LA, Chang HC, Chang C. Androgen receptor regulates expression of skeletal muscle-specific proteins and muscle cell types. Endocrine 25: 27–32, 2004. doi: 10.1385/ENDO:25:1:27. [DOI] [PubMed] [Google Scholar]
- 3.Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci USA 107: 19090–19095, 2010. doi: 10.1073/pnas.1014523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134: 317–328, 2008. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 5.Basualto-Alarcón C, Jorquera G, Altamirano F, Jaimovich E, Estrada M. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med Sci Sports Exerc 45: 1712–1720, 2013. doi: 10.1249/MSS.0b013e31828cf5f3. [DOI] [PubMed] [Google Scholar]
- 6.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab 82: 407–413, 1997. doi: 10.1210/jc.82.2.407. [DOI] [PubMed] [Google Scholar]
- 7.Brown JL, Rosa-Caldwell ME, Lee DE, Blackwell TA, Brown LA, Perry RA, Haynie WS, Hardee JP, Carson JA, Wiggs MP, Washington TA, Greene NP. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle 8: 926–938, 2017. doi: 10.1002/jcsm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhr ED, Takahashi JS. Molecular components of the Mammalian circadian clock. Handb Exp Pharmacol 217: 3–27, 2013. doi: 10.1007/978-3-642-25950-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burney BO, Garcia JM. Hypogonadism in male cancer patients. J Cachexia Sarcopenia Muscle 3: 149–155, 2012. doi: 10.1007/s13539-012-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cárdenas C, Miller RA, Smith I, Bui T, Molgó J, Müller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142: 270–283, 2010. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cárdenas C, Müller M, McNeal A, Lovy A, Jaňa F, Bustos G, Urra F, Smith N, Molgó J, Diehl JA, Ridky TW, Foskett JK. Selective vulnerability of cancer cells by inhibition of Ca2+ transfer from endoplasmic reticulum to mitochondria. Cell Reports 15: 219–220, 2016. doi: 10.1016/j.celrep.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Schirmer A, Lee Y, Lee H, Kumar V, Yoo SH, Takahashi JS, Lee C. Rhythmic PER abundance defines a critical nodal point for negative feedback within the circadian clock mechanism. Mol Cell 36: 417–430, 2009. doi: 10.1016/j.molcel.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785–789, 1995. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 14.D’Alessandro M, Beesley S, Kim JK, Jones Z, Chen R, Wi J, Kyle K, Vera D, Pagano M, Nowakowski R, Lee C. Stability of wake-sleep cycles requires robust degradation of the PERIOD protein. Curr Biol 27: 3454–3467.e8, 2017. doi: 10.1016/j.cub.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Gendt K, Verhoeven G. Tissue- and cell-specific functions of the androgen receptor revealed through conditional knockout models in mice. Mol Cell Endocrinol 352: 13–25, 2012. doi: 10.1016/j.mce.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Dennis MD, Coleman CS, Berg A, Jefferson LS, Kimball SR. REDD1 enhances protein phosphatase 2A-mediated dephosphorylation of Akt to repress mTORC1 signaling. Sci Signal 7: ra68, 2014. doi: 10.1126/scisignal.2005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diz AP, Truebano M, Skibinski DO. The consequences of sample pooling in proteomics: an empirical study. Electrophoresis 30: 2967–2975, 2009. doi: 10.1002/elps.200900210. [DOI] [PubMed] [Google Scholar]
- 18.Dyar KA, Hubert MJ, Mir AA, Ciciliot S, Lutter D, Greulich F, Quagliarini F, Kleinert M, Fischer K, Eichmann TO, Wright LE, Peña Paz MI, Casarin A, Pertegato V, Romanello V, Albiero M, Mazzucco S, Rizzuto R, Salviati L, Biolo G, Blaauw B, Schiaffino S, Uhlenhaut NH. Transcriptional programming of lipid and amino acid metabolism by the skeletal muscle circadian clock. PLoS Biol 16: e2005886, 2018. [Erratum in PLoS Biol 16: e3000035, 2018.] doi: 10.1371/journal.pbio.2005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellacott KL, Morton GJ, Woods SC, Tso P, Schwartz MW. Assessment of feeding behavior in laboratory mice. Cell Metab 12: 10–17, 2010. doi: 10.1016/j.cmet.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93: 884S–890S, 2011. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab 88: 358–362, 2003. doi: 10.1210/jc.2002-021041. [DOI] [PubMed] [Google Scholar]
- 22.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282: E601–E607, 2002. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 23.Gordon BS, Delgado-Diaz DC, Carson J, Fayad R, Wilson LB, Kostek MC. Resveratrol improves muscle function but not oxidative capacity in young mdx mice. Can J Physiol Pharmacol 92: 243–251, 2014. doi: 10.1139/cjpp-2013-0350. [DOI] [PubMed] [Google Scholar]
- 24.Gordon BS, Liu C, Steiner JL, Nader GA, Jefferson LS, Kimball SR. Loss of REDD1 augments the rate of the overload-induced increase in muscle mass. Am J Physiol Regul Integr Comp Physiol 311: R545–R557, 2016. doi: 10.1152/ajpregu.00159.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon BS, Steiner JL, Rossetti ML, Qiao S, Ellisen LW, Govindarajan SS, Eroshkin AM, Williamson DL, Coen PM. REDD1 induction regulates the skeletal muscle gene expression signature following acute aerobic exercise. Am J Physiol Endocrinol Metab 313: E737–E747, 2017. doi: 10.1152/ajpendo.00120.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodge BA, Wen Y, Riley LA, Zhang X, England JH, Harfmann BD, Schroder EA, Esser KA. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle 5: 17, 2015. doi: 10.1186/s13395-015-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodge BA, Zhang X, Gutierrez-Monreal MA, Cao Y, Hammers DW, Yao Z, Wolff CA, Du P, Kemler D, Judge AR, Esser KA. MYOD1 functions as a clock amplifier as well as a critical co-factor for downstream circadian gene expression in muscle. eLife 8: e43017, 2019. doi: 10.7554/eLife.43017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes DC, Stewart CE, Sculthorpe N, Dugdale HF, Yousefian F, Lewis MP, Sharples AP. Testosterone enables growth and hypertrophy in fusion impaired myoblasts that display myotube atrophy: deciphering the role of androgen and IGF-I receptors. Biogerontology 17: 619–639, 2016. doi: 10.1007/s10522-015-9621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, Unluturk U, Li X, Kong X, Hyde AL, Gangl MR, Mair WB, Lee CH. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab 22: 709–720, 2015. doi: 10.1016/j.cmet.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janumyan YM, Sansam CG, Chattopadhyay A, Cheng N, Soucie EL, Penn LZ, Andrews D, Knudson CM, Yang E. Bcl-xL/Bcl-2 coordinately regulates apoptosis, cell cycle arrest and cell cycle entry. EMBO J 22: 5459–5470, 2003. doi: 10.1093/emboj/cdg533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao Q, Pruznak AM, Huber D, Vary TC, Lang CH. Castration differentially alters basal and leucine-stimulated tissue protein synthesis in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 297: E1222–E1232, 2009. doi: 10.1152/ajpendo.00473.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci 125: 795–799, 2012. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem 47: 69–84, 2010. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joskova V, Patkova A, Havel E, Najpaverova S, Uramova D, Kovarik M, Zadak Z, Hronek M. Critical evaluation of muscle mass loss as a prognostic marker of morbidity in critically ill patients and methods for its determination. J Rehabil Med 50: 696–704, 2018. doi: 10.2340/16501977-2368. [DOI] [PubMed] [Google Scholar]
- 35.Ju JS, Varadhachary AS, Miller SE, Weihl CC. Quantitation of “autophagic flux” in mature skeletal muscle. Autophagy 6: 929–935, 2010. doi: 10.4161/auto.6.7.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kendziorski C, Irizarry RA, Chen KS, Haag JD, Gould MN. On the utility of pooling biological samples in microarray experiments. Proc Natl Acad Sci USA 102: 4252–4257, 2005. doi: 10.1073/pnas.0500607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr 76: 378–383, 2002. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 38.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre-Ghiso J, Airoldi EM, Ait-Si-Ali S, Akematsu T, Akporiaye ET, Al-Rubeai M, Albaiceta GM, Albanese C, Albani D, Albert ML, Aldudo J, Algül H, Alirezaei M, Alloza I, Almasan A, Almonte-Beceril M, Alnemri ES, , et al. . Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12: 1–222, 2016. [Erratum in Autophagy 12: 443, 2016.] doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knarr M, Nagaraj AB, Kwiatkowski LJ, DiFeo A. miR-181a modulates circadian rhythm in immortalized bone marrow and adipose derived stromal cells and promotes differentiation through the regulation of PER3. Sci Rep 9: 307, 2019. doi: 10.1038/s41598-018-36425-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubli DA, Gustafsson ÅB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res 111: 1208–1221, 2012. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107: 855–867, 2001. doi: 10.1016/S0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 42.Lee D, Goldberg AL. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J Biol Chem 288: 30515–30526, 2013. doi: 10.1074/jbc.M113.489716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee DE, Brown JL, Rosa ME, Brown LA, Perry RA Jr, Wiggs MP, Nilsson MI, Crouse SF, Fluckey JD, Washington TA, Greene NP. microRNA-16 is downregulated during insulin resistance and controls skeletal muscle protein accretion. J Cell Biochem 117: 1775–1787, 2016. doi: 10.1002/jcb.25476. [DOI] [PubMed] [Google Scholar]
- 44.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 31: 86–95, 2007. doi: 10.1152/physiolgenomics.00066.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mobley CB, Mumford PW, Kephart WC, Conover CF, Beggs LA, Balaez A, Yarrow JF, Borst SE, Beck DT, Roberts MD. Effects of testosterone treatment on markers of skeletal muscle ribosome biogenesis. Andrologia 48: 1055–1065, 2016. doi: 10.1111/and.12539. [DOI] [PubMed] [Google Scholar]
- 47.Nahapetyan H, Moulis M, Grousset E, Faccini J, Grazide MH, Mucher E, Elbaz M, Martinet W, Vindis C. Altered mitochondrial quality control in Atg7-deficient VSMCs promotes enhanced apoptosis and is linked to unstable atherosclerotic plaque phenotype. Cell Death Dis 10: 119, 2019. doi: 10.1038/s41419-019-1400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, Nir D, Rousso-Noori L, Kuperman Y, Golik M, Mann M, Asher G. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci USA 113: E1673–E1682, 2016. doi: 10.1073/pnas.1519650113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen TN, Padman BS, Lazarou M. Deciphering the molecular signals of PINK1/Parkin mitophagy. Trends Cell Biol 26: 733–744, 2016. doi: 10.1016/j.tcb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol 4: 6–13, 2015. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ophoff J, Van Proeyen K, Callewaert F, De Gendt K, De Bock K, Vanden Bosch A, Verhoeven G, Hespel P, Vanderschueren D. Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology 150: 3558–3566, 2009. doi: 10.1210/en.2008-1509. [DOI] [PubMed] [Google Scholar]
- 52.Rana K, Chiu MW, Russell PK, Skinner JP, Lee NK, Fam BC, Zajac JD, MacLean HE. Muscle-specific androgen receptor deletion shows limited actions in myoblasts but not in myofibers in different muscles in vivo. J Mol Endocrinol 57: 125–138, 2016. doi: 10.1530/JME-15-0320. [DOI] [PubMed] [Google Scholar]
- 53.Romanello V, Sandri M. Mitochondrial quality control and muscle mass maintenance. Front Physiol 6: 422, 2016. doi: 10.3389/fphys.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossetti ML, Fukuda DH, Gordon BS. Androgens induce growth of the limb skeletal muscles in a rapamycin-insensitive manner. Am J Physiol Regul Integr Comp Physiol 315: R721–R729, 2018. doi: 10.1152/ajpregu.00029.2018. [DOI] [PubMed] [Google Scholar]
- 55.Rossetti ML, Gordon BS. The role of androgens in the regulation of muscle oxidative capacity following aerobic exercise training. Appl Physiol Nutr Metab 42: 1001–1007, 2017. doi: 10.1139/apnm-2017-0230. [DOI] [PubMed] [Google Scholar]
- 56.Rossetti ML, Steiner JL, Gordon BS. Increased mitochondrial turnover in the skeletal muscle of fasted, castrated mice is related to the magnitude of autophagy activation and muscle atrophy. Mol Cell Endocrinol 473: 178–185, 2018. doi: 10.1016/j.mce.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 57.Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3β-mediated phosphorylation. PLoS One 5: e8561, 2010. doi: 10.1371/journal.pone.0008561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schroder EA, Harfmann BD, Zhang X, Srikuea R, England JH, Hodge BA, Wen Y, Riley LA, Yu Q, Christie A, Smith JD, Seward T, Wolf Horrell EM, Mula J, Peterson CA, Butterfield TA, Esser KA. Intrinsic muscle clock is necessary for musculoskeletal health. J Physiol 593: 5387–5404, 2015. doi: 10.1113/JP271436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serra C, Sandor NL, Jang H, Lee D, Toraldo G, Guarneri T, Wong S, Zhang A, Guo W, Jasuja R, Bhasin S. The effects of testosterone deprivation and supplementation on proteasomal and autophagy activity in the skeletal muscle of the male mouse: differential effects on high-androgen responder and low-androgen responder muscle groups. Endocrinology 154: 4594–4606, 2013. doi: 10.1210/en.2013-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen Q, Li J, Mai J, Zhang Z, Fisher A, Wu X, Li Z, Ramirez MR, Chen S, Shen H. Sensitizing non-small cell lung cancer to BCL-xL-targeted apoptosis. Cell Death Dis 9: 986, 2018. doi: 10.1038/s41419-018-1040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smuder AJ, Sollanek KJ, Nelson WB, Min K, Talbert EE, Kavazis AN, Hudson MB, Sandri M, Szeto HH, Powers SK. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic Biol Med 115: 179–190, 2018. doi: 10.1016/j.freeradbiomed.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srikanthan P, Horwich TB, Tseng CH. Relation of muscle mass and fat mass to cardiovascular disease mortality. Am J Cardiol 117: 1355–1360, 2016. doi: 10.1016/j.amjcard.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 63.Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med 127: 547–553, 2014. doi: 10.1016/j.amjmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steiner JL, Fukuda DH, Rossetti ML, Hoffman JR, Gordon BS. Castration alters protein balance after high-frequency muscle contraction. J Appl Physiol (1985) 122: 264–272, 2017. doi: 10.1152/japplphysiol.00740.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suliman HB, Piantadosi CA. Mitochondrial quality control as a therapeutic target. Pharmacol Rev 68: 20–48, 2016. doi: 10.1124/pr.115.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szeszycki EE. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. JPEN J Parenter Enteral Nutr 21: 241–242, 1997. doi: 10.1177/0148607197021004241. [DOI] [PubMed] [Google Scholar]
- 67.Ueberschlag-Pitiot V, Stantzou A, Messéant J, Lemaitre M, Owens DJ, Noirez P, Roy P, Agbulut O, Metzger D, Ferry A. Gonad-related factors promote muscle performance gain during postnatal development in male and female mice. Am J Physiol Endocrinol Metab 313: E12–E25, 2017. doi: 10.1152/ajpendo.00446.2016. [DOI] [PubMed] [Google Scholar]
- 68.Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol Endocrinol Metab 269: E820–E826, 1995. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 69.Vanselow K, Vanselow JT, Westermark PO, Reischl S, Maier B, Korte T, Herrmann A, Herzel H, Schlosser A, Kramer A. Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS). Genes Dev 20: 2660–2672, 2006. doi: 10.1101/gad.397006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Z, Li L, Wang Y. Effects of Per2 overexpression on growth inhibition and metastasis, and on MTA1, nm23-H1 and the autophagy-associated PI3K/PKB signaling pathway in nude mice xenograft models of ovarian cancer. Mol Med Rep 13: 4561–4568, 2016. doi: 10.3892/mmr.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol 365: 174–186, 2013. doi: 10.1016/j.mce.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White JP, Puppa MJ, Narsale A, Carson JA. Characterization of the male ApcMin/+ mouse as a hypogonadism model related to cancer cachexia. Biol Open 2: 1346–1353, 2013. doi: 10.1242/bio.20136544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science 337: 1062–1065, 2012. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang S, Eitan E, Mattson MP. Early involvement of lysosome dysfunction in the degeneration of cerebral cortical neurons caused by the lipid peroxidation product 4-hydroxynonenal. J Neurochem 140: 941–954, 2017. doi: 10.1111/jnc.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang T, Xue L, Li L, Tang C, Wan Z, Wang R, Tan J, Tan Y, Han H, Tian R, Billiar TR, Tao WA, Zhang Z. BNIP3 protein suppresses PINK1 kinase proteolytic cleavage to promote mitophagy. J Biol Chem 291: 21616–21629, 2016. doi: 10.1074/jbc.M116.733410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou B, Li C, Qi W, Zhang Y, Zhang F, Wu JX, Hu YN, Wu DM, Liu Y, Yan TT, Jing Q, Liu MF, Zhai QW. Downregulation of miR-181a upregulates sirtuin-1 (SIRT1) and improves hepatic insulin sensitivity. Diabetologia 55: 2032–2043, 2012. doi: 10.1007/s00125-012-2539-8. [DOI] [PubMed] [Google Scholar]
- 77.Zou T, Chen D, Yang Q, Wang B, Zhu MJ, Nathanielsz PW, Du M. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J Physiol 595: 1547–1562, 2017. doi: 10.1113/JP273478. [DOI] [PMC free article] [PubMed] [Google Scholar]