Keywords: brainstem, electrophysiology, gastric motility, vagus
Abstract
Functional gastrointestinal disorders, including delayed gastric emptying and decreased gastric motility, are more prevalent in women, suggesting a potential role for circulating gonadal hormones, including estrogen. Gastric motility is tuned by the vagal inputs arising from the dorsal motor nucleus of the vagus (DMV), which is itself controlled by tonic GABAergic inputs. Estrogen increases GABA functions in various central nervous system areas; however, the effect of the estrus cycle in modulating GABAergic inputs onto DMV neurons, hence vagal control of gastric motility, has not been investigated. The aim of the present study was to test the hypothesis that GABAergic tone to DMV neurons, hence the vagal output to the stomach, varies according to sex and the estrus cycle. Experiments were performed on age-matched Sprague-Dawley male and virgin female rats; females were subdivided according to the high-estrogen (HE) or low-estrogen (LE) period of their cycle. Whole-cell patch-clamp recordings were made from gastric-projecting DMV neurons, and the response to perfusion with the GABAA receptor antagonist bicuculline was examined. The response of corpus and antrum tone and motility to bicuculline microinjected in the dorsal vagal complex, recorded via strain gauges sewn to the anterior gastric surface, was also assessed. Bicuculline increased the firing rate of DMV neurons, as well as gastric tone and motility, to a larger extent in HE compared with LE or male rats, suggesting a higher GABAergic tone in HE female rats. Taken together, the data support the hypothesis that GABAergic tone to DMV neurons varies according to sex and estrus cycle.
NEW & NOTEWORTHY GABAergic neurotransmission to the dorsal motor nucleus of the vagus (DMV) plays a pivotal role in the modulation of gastric tone and motility. Gastric motility is reduced in women and may contribute to the higher incidence of functional gastrointestinal disorders. In the present study, we report that GABAergic tone to rat DMV neurons, hence vagal output to the stomach, varies according to sex and estrus cycle, and the GABAergic tone is increased during the high-estrogen period of the estrus cycle.
INTRODUCTION
The pathophysiology of functional gastrointestinal disorders (FGID), including functional dyspepsia and irritable bowel syndrome, is multifactorial and complex and remains to be elucidated fully, although several lines of evidence suggest impairment or dysregulation of extrinsic vagal efferent inputs (9, 11, 18, 19). FGID are more prevalent in women than in men, implying that circulating gonadal hormones, such as estrogen, play an important role (11, 12, 39). Gastric motility patterns appear to be modulated by circulating estrogen, with gastric emptying and motility being slower in women during high-estrogen phases of the estrus cycle (1, 5, 25, 27). Indeed, premenopausal women have a slower gastric emptying rate compared with age-matched men (23, 25), and this difference is attenuated following menopause (23), again suggesting that circulating gonadal hormones modulate and affect gastric functions.
Vagal pathways originating in the dorsal motor nucleus of the vagus (DMV) provide the fine modulation of the upper gastrointestinal (GI) tract, from the lower third of the esophagus to the splenic flexure in the transverse colon (33, 36). The spontaneous activity of DMV neurons is subject to modulation by local tonic GABAergic synapses originating in the nucleus tractus solitarius (NTS) (2, 16, 33, 34). Indeed, microinjections of the GABAA receptor antagonist bicuculline in the dorsal vagal complex (DVC; i.e., DMV, NTS, and area postrema) increase both gastric tone and motility (32). Similarly, perfusion of brainstem slices containing the DVC with bicuculline increases the firing rate of DMV neurons significantly (2). The GABAergic synapse between NTS and DMV neurons, therefore, plays a major role in the control of efferent vagal activity and, hence, gastric motor functions.
Both genomic and membrane-bound estrogen receptors are present in many brain areas including the DVC (20, 31, 37). The expression of estrogen receptors in the DVC varies in concert with the estrus cycle (20), i.e., increasing levels of circulating estrogen induce an increase of estrogen receptor expression. The physiological role of activation of estrogen receptors in neurons of the DVC has been studied in detail in the neurocircuits devoted to the control of cardiovascular functions. Indeed, variations in the levels of circulating estrogen alter the responses of both vagal sensory and motoneurons related to baroreflex neurocircuits (6–8, 10, 28, 30), suggesting that estrogen may also ultimately modulate vagal output to the adjacent GI-related neurocircuits. Furthermore, estrogen also potentiates GABA currents through modulation of the synthesis, transport, and release of GABA, and GABA receptor expression in brain areas such as the preoptic area and the bed nucleus of the stria terminalis (21, 22).
Taken together, this evidence points toward a possible role of circulating estrogen in the reduction of gastric motility via the modulation of the GABAergic synapse between NTS and DMV neurons.
The aim of the present study was to test the hypothesis that GABAergic tone to DMV neurons, hence the vagal output to the stomach, varies according to sex and the estrus cycle.
MATERIALS AND METHODS
Animals.
Age-matched male and virgin female Sprague-Dawley rats (7–10 wk) were housed in an American Association for the Accreditation of Laboratory Animal Care accredited Animal Care Facility maintained at 24°C on a 12-h:12-h light/dark cycle with food and water provided ad libitum. All procedures were conducted in accordance with the National Institutes of Health guidelines and with the approval of the Penn State University College of Medicine Institutional Animal Care and Use Committee.
Estrus cycle determination.
Estrus cycling was characterized using daily vaginal smears for 8 consecutive days before, and on the day of, experimentation. A cotton-tipped applicator soaked with saline was inserted 1 cm into the vagina; the cells collected were smeared onto glass slides and observed microscopically. The estrus cycle phase was defined as diestrus and metestrus, i.e., low estrogen (LE), or proestrus and estrus, i.e., high estrogen (HE), following identification of the predominant cell type. All male animals were swabbed across the external perineum to control for handling.
In vitro electrophysiological recordings.
Rats were anesthetized deeply with isoflurane (5% with air), and the brainstem was removed rapidly and immersed in ice-cold Krebs solution. Coronal brain slices containing the DVC were cut at 300-μm thickness using a vibratome and incubated in oxygenated Krebs solution at 30°C for at least 90 min before use. A single brainstem slice was then transferred in a perfusion chamber, held in place with a nylon mesh on the stage of a microscope (Nikon E600FN), and maintained at 32 ± 1°C by continuous perfusion with Krebs solution.
Whole cell patch-clamp recordings were made in 34 DMV neurons from 10 male rats, 24 neurons from 7 LE female rats, and 17 neurons from 7 HE female rats. Recordings were conducted in medial, i.e., gastric-projecting (24), DMV neurons using glass pipette with 2–5 MΩ tip resistance when filled with potassium gluconate-based internal solution. Recordings were done using a single-electrode voltage clamp amplifier (Axopatch 200A; Molecular Devices, Union City, CA). Data were filtered at 2 kHz, digitized via a Digidata 1440A interface, and analyzed using pClamp10 software (Molecular Devices). Only recordings with a series resistance less than 20 MΩ were used.
Basic electrophysiological properties measured included the membrane input resistance (measured from the current deflection obtained by stepping the membrane from −50 to −60 mV), the duration of the action potential measured at the threshold, the amplitude of the afterhyperpolarization following the firing of a single action potential, the frequency of action potential firing in response to depolarizing direct current (DC) pulses of 400-ms duration, and intensities ranging from 10 pA to 90 pA in step increments; calcium-dependent potassium current [IK(Ca)] was evoked with a 16-ms-long step depolarization from −50 to 0 mV.
To assess the effect of bicuculline, the firing rate of DMV neurons was set to ~1 pulse/s via injection of DC, and the perfusing Krebs was supplemented with the nonselective ionotropic glutamate antagonist kynurenic acid (1 mM; Sigma Aldrich). After a 1-min baseline period, different concentrations of bicuculline (0.5–5 μM; Sigma Aldrich, St. Louis, MO) were applied in random order to the bath until a plateau response was obtained, but no longer than 5 min. Drugs were washed out for a sufficient period of time to allow the firing rate to recover toward baseline values. Firing rates were expressed as percent change relative to baseline values.
Spontaneous and miniature inhibitory postsynaptic currents (sIPSCs and mIPSCs, respectively) were recorded from DMV neurons using a potassium chloride intracellular solution at a holding potential of −50 mV in slices perfused with 1 mM kynurenic acid. To block action potential-dependent synaptic transmission, tetrodotoxin (0.3 μM) was included in the perfusing Krebs solution when mIPSCs were recorded. The effects of GABAergic transmission on holding current were assessed in DMV neurons voltage clamped at −50 mV using potassium gluconate intracellular solution. After a 1-min baseline period, different concentrations of bicuculline (0.5–10 μM) were applied in random order for a period of time sufficient for the response plateau, but no longer than 5 min. Drugs were then washed out for a sufficient period of time to allow the firing rate to recover toward baseline values
In vivo gastric recordings.
Gastric tone and motility recordings were made from 23 male rats, 18 female HE rats, and 13 female LE rats. Rats were fasted overnight (water ad libitum) and anesthetized with thiobutabarbital sodium (Inactin; 100–150 mg/kg ip). The anesthesia level was monitored continuously throughout the experiment, and core temperature was kept at 37°C with a heating pad. Once a deep plan of anesthesia was achieved (absence of palpebral reflex), rats were intubated with a tracheal catheter, and a midline laparotomy was performed to expose the anterior gastric wall. An encapsulated miniature strain gauge (6 × 8 mm; AT Engineering, Hershey, PA) was aligned with the gastric circular smooth muscle and sutured to the anterior corpus and antrum; the laparotomy was closed with a 5–0 suture, and the strain gauge leads were exteriorized. Strain gauge signals were amplified (EXP CLSG-2; Quanta Metrics, Newton, PA), filtered (low-pass cutoff = 0.1 Hz, AT Engineering), digitized via a Digidata 1320 interface, and recorded using AxoScope 10.3 software (Molecular Devices). Rats were then placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA), and the lower medulla was exposed via blunt dissection. The meningeal membranes above the vagal trigone were dissected, and the exposed lower medulla was covered with a gauze soaked in prewarmed saline for at least 1 h to stabilize.
A glass micropipette (20–30-μm tip diameter) was directed into the DVC (in mm: 0.4–0.6 rostrocaudal from calamus scriptorius, 0.1–0.3 mediolateral from midline, 0.6–0.65 dorsoventral from the brainstem dorsal surface). Drugs were dissolved in isotonic phosphate-buffered saline (PBS) and microinjected in 60-nL volumes over a period of 2 min via a picospritzer (Parker Hannifin, Hollis, NH). Fluorescent microspheres were included in the injectate for post hoc verification of the injection site. Gastric tone and motility were monitored for 5 min before drug application and for at least 15 min after the microinjection. Different doses of bicuculline were injected into the DVC in random order. Gastric tone and motility were allowed to recover for a minimum of 1 h between injections.
Gastric tone was measured as absolute tone variation (in mg) from baseline. Gastric motility was calculated using the following formula, as described previously (24): motility index = (N1X1 + N2X2 + N3X4 + N4X8)/t × 100% where N equals the number of peaks in a particular force range (N1 = 25–50 mg, N2 = 51–100 mg, N3 = 101–200 mg, N4 > 201 mg) and t equals the time interval over which the gastric motility was measured. The effect of drugs on gastric motility was measured relative to the averaged value of gastric motility before microinjection (baseline = 100%).
At the conclusion of the experiment, rats were perfused transcardially with 0.1 M PBS followed by paraformaldehyde (4%) in 0.1 M PBS. Brainstems were removed and postfixed in 4% paraformaldehyde overnight and then transferred to 0.1 M PBS with 20% sucrose for 48 h. The brainstems were then frozen, and coronal sections (50-μm thickness) throughout the rostrocaudal extent of the DVC were cut using a microtome. Every fourth slice was mounted to identify the injection site using a Nikon E400 microscope.
Drugs and solutions.
Krebs solution was composed of the following (in mM): 126 NaCl, 25 NaHCO3, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, and 11 dextrose, maintained at pH = 7.4 by bubbling with 95% O2-5% CO2. Potassium gluconate intracellular solution was composed of the following (in mM): 128 K-gluconate, 10 KCl, 0.3 CaCl2, 1 MgCl2, 10 HEPES, 1 EGTA, 2 Na2ATP, and 0.25 NaGTP, adjusted to pH = 7.36 with KOH. Potassium chloride intracellular solution was composed of the following (in mM): 140 KCl, 1 CaCl2, 10 HEPES, 10 EGTA, 2 Na2ATP, and 0.25 NaGTP, adjusted to pH = 7.36 with KOH. PBS was composed of the following (in mM): 124 NaCl, 26 NaHCO3, and 2 KH2PO4, pH = 7.4.
Data analysis.
Data were tested, and normal distributions and equal variances in the sampled distributions were found. Student’s t tests were used for parametric data; one-way ANOVA followed by a post hoc Tukey’s comparison were used for intergroup comparisons using GraphPad Prism 5 (GraphPad Software, San Diego, CA). Data are reported as means ± SE with a significance defined as P < 0.05.
RESULTS
Basic electrophysiological properties.
Whole cell patch-clamp recordings were made from 34 neurons from 10 male rats, 17 neurons from 7 female rats during HE cycle (female-HE), and 24 neurons from 7 female rats during LE cycle (female-LE).
The input resistance was similar among the groups (male: 360 ± 32.0 MΩ; female-HE: 334 ± 26.7 MΩ; and female-LE: 394 ± 33.5 MΩ; one-way ANOVA F2,71 = 0.8508, Tukey’s multiple comparison test P > 0.05 for all). The action potential duration was also similar among the groups (male: 2.2 ± 0.1 ms; female-HE: 2.1 ± 0.1 ms; female-LE: 2.1 ± 0.1 ms; one-way ANOVA F2,71 = 0.3017, Tukey’s multiple comparison test P > 0.05 for all).
The amplitude of the afterhyperpolarization, however, was significantly larger in neurons from female-HE (20 ± 0.6 mV, N = 17) compared with neurons from male (17 ± 1.1 mV, N = 34, t49 = 1.835, P < 0.05) or female-LE (18 ± 1.2 mV, N = 24, t39 = 1.731, P < 0.05; Fig. 1, where t is the t value and the subscripted number is the degree of freedom.). Since the afterhyperpolarization of DMV neurons is determined, in part, by the apamin-sensitive IK(Ca) (4, 29, 35), in a different group of rats we tested whether there were differences in the amplitude of the IK(Ca) between males and females at different estrogen cycle phases. The amplitude of the IK(Ca) was significantly higher in female-HE (390 ± 27.0 pA, N = 13) or female-LE (425 ± 34.8 pA, N = 9) versus males (300 ± 30.8 pA, N = 13; t24 = 2.208 vs. female-HE, P < 0.05; t20 = 2.656 vs. female-LE, P < 0.05). In all these groups, perfusion of the slice with the SK channel blocker apamin (100 nM) reduced the amplitude of the afterhyperpolarization to a similar but significant extent, from 17 ± 1.37 to 13 ± 1.19 mV (N = 14, t13 = 6.422, P < 0.05) and of the IK(Ca) from 398 ± 33.2 to 269 ± 32.4 pA (N = 14, t13 = 7.617, P < 0.05).
Fig. 1.
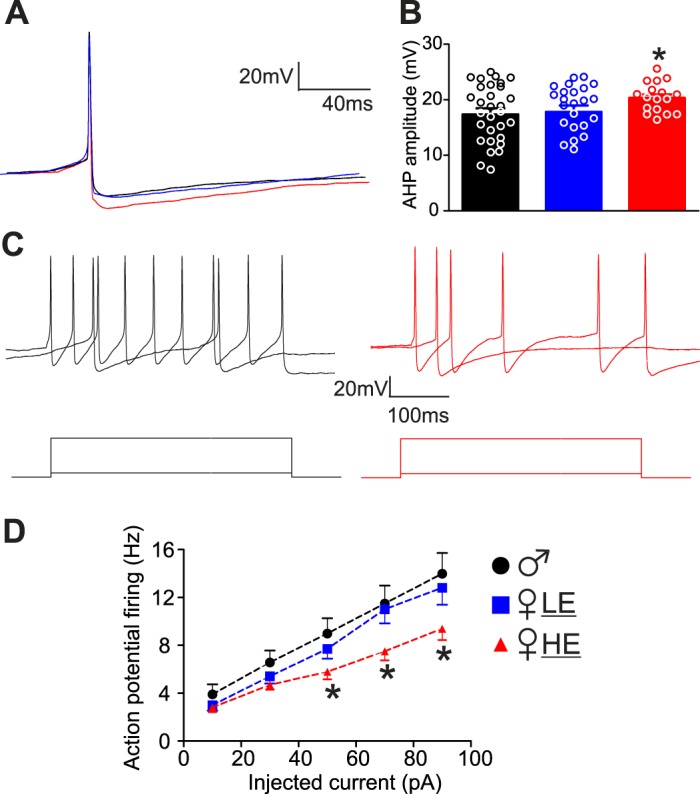
Dorsal motor nucleus of the vagus (DMV) neurons from female high estrogen (HE) rats are less excitable. A: representative traces of single action potentials evoked in DMV neurons from male (black), female low estrogen (LE) (blue), and female-HE (red) groups. Neurons were clamped at −60 mV before injection of a short (16 ms) depolarizing current pulse of intensity sufficient to evoke a single action potential at its offset. Note that the amplitude of the action potential from female-HE rats displayed a larger and slower hyperpolarization (AHP). B: graphic representation of the AHP amplitude in DMV neurons from male (34 neurons from 10 rats), female-LE (17 neurons from 7 rats), and female-HE (24 neurons from 7 rats). *P < 0.05 vs. male and female-LE. C: representative traces showing the response of DMV neurons from male (black, left) and female-HE (red, right) rats following injection of 10 pA and 90 pA DC (400 ms long). D: frequency-response curves for DMV neurons from male, female-LE (blue), and female-HE (red) rats. Note the neurons from female-HE rats are less excitable and fire fewer action potentials than neurons from other groups when injected with 50–90 pA current. Holding potential = −60 mV. *P < 0.05 vs. male and female-LE. DC, direct current.
Neurons from female-HE also had a lower frequency of action potentials in response to injected current (10–90 pA, P < 0.05 vs. male and female-LE, Fig. 1).
These data suggest that gastric-projecting DMV neurons from female-HE have less excitability compared with male or female-LE.
The GABAergic inputs onto vagal motoneurons increase with estrogen levels.
In the presence of kynurenic acid (1 mM), perfusion of brainstem slices with the GABAA receptor antagonist bicuculline increased action potential firing rate in a concentration-dependent manner in neurons from both male and female rats. Perfusion of brainstem slices with a low concentration (0.5 μM) of bicuculline increased action potential firing rate from 0.95 ± 0.07 to 1.11 ± 0.14 events/s, i.e., 115 ± 10.4% of baseline in 11 neurons from male rats (Fig. 2). In 12 neurons from female-LE rats, perfusion with 0.5 μM bicuculline increased action potential firing rate from 0.97 ± 0.07 to 1.15 ± 0.12 events/s (118 ± 12.4% of baseline, P > 0.05 vs. males). Conversely, in 10 neurons from female-HE rats, perfusion with 0.5 μM of bicuculline induced a significantly larger increase in action potential firing rate (from 0.9 ± 0.08 to 1.26 ± 0.11 events/s, P < 0.05 vs. male and female-LE, Fig. 2 and Table 1).
Fig. 2.
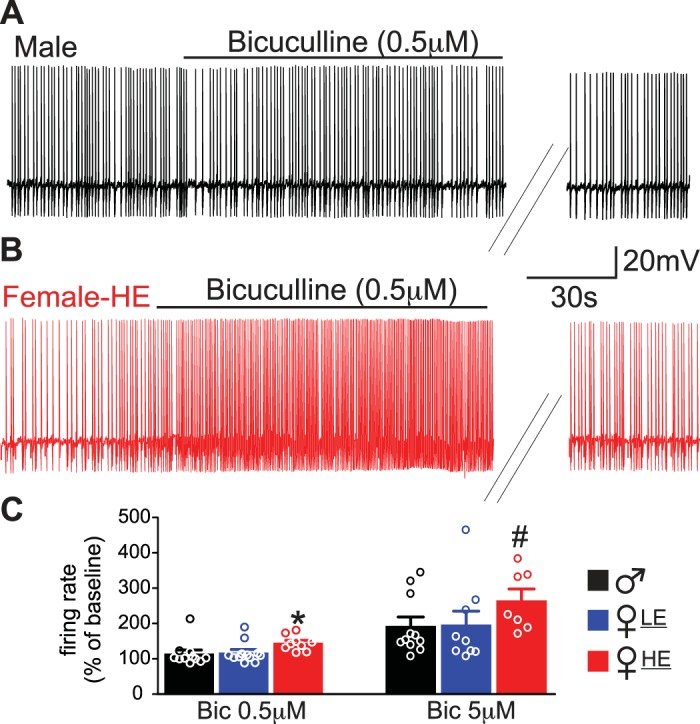
Dorsal motor nucleus of the vagus (DMV) neurons from female high estrogen (HE) rats have a larger response to bicuculline application. A: representative trace of action potentials recorded in a DMV neuron from male rats. Note that application of 0.5 μM bicuculline did not increase action potential firing rate. B: representative trace of action potentials recorded in a DMV neuron from female-HE rats. Note that application of 0.5 μM bicuculline increased action potential firing rate to a larger extent compared with male. C: summary graph representing the responsiveness in firing rate to the application of 0.5 μM and 5 μM bicuculline. Note neurons from female-HE have a larger response in firing rate. *P < 0.05 vs. male and female low estrogen (LE), #P < 0.05 vs. male.
Table 1.
Effects of bicuculline on action potential firing rate
| Bicuculline |
||||
|---|---|---|---|---|
| 0.5 μM |
5 μM |
|||
| % of baseline (N) | t (df) vs. male | % of baseline (N) | t (df) vs. male | |
| Male | 115 ± 10.4 (11) | 193 ± 25.2 (11) | ||
| Female-HE | 142 ± 7.4 (10) | 2.995 (18)* | 266 ± 31.9 (7) | 1.79 (16)* |
| Female-LE | 118 ± 12.4 (12) | 0.4824 (21) | 196 ± 38.5 (9) | 0.072 (18) |
Values are means ± SE; N, number of rats in parentheses. HE, high estrogen; LE, low estrogen; t, t value; df, degrees of freedom.
P < 0.05.
In male rats (n = 11 neurons), perfusion of brainstem slices with 5 μM of bicuculline induced a significantly larger increase in action potential firing rate, i.e., 193 ± 25.2% of baseline compared with a lower concentration of bicuculline (t10 = 3.244, P < 0.05 vs. baseline, t20 = 3.096, P < 0.05 vs. 0.5 μM bicuculline). In female-LE (n = 9 neurons), perfusion with 5 μM of bicuculline significantly increased the firing rate over baseline (196 ± 38.5%; t8 = 3.054, P < 0.05 vs. baseline). This increase was significantly greater than that induced by a lower dose of bicuculline (t19 = 2.293, P < 0.05 vs. 0.5 μM bicuculline) but was not different from the increase observed in neurons from male rats (P > 0.05). In female-HE rats (n = 7 neurons), perfusion with 5 μM of bicuculline induced a significant increase in the firing rate over baseline (266 ± 31.9%; t6 = 4.013, P < 0.05). This increase was significantly greater than that induced by a lower concentration of bicuculline (t14 = 4.134, P < 0.05 vs. 0.5 μM bicuculline). Notably, the increase in the firing rate of DMV neurons from female-HE rats induced by 5 μM was also significantly greater than the increase observed in male rats (P < 0.05).
To assess whether basal inhibitory synaptic transmission contributes to the higher GABAergic input to DMV neurons from female-HE rats, the frequency and amplitude of spontaneous and miniature IPSCs were analyzed at baseline. The frequency of sIPSCs was similar among the groups, i.e., 2.25 ± 0.66, 2.71 ± 1.07, and 1.48 ± 0.80 events/s in males, female-HE, or female-LE, respectively (one-way ANOVA F2,22 = 0.5055, Tukey’s multiple comparison test P > 0.05 for all). Likewise, the amplitude of sIPSCs was similar among the groups, i.e., 77 ± 7.2 pA, 75 ± 8.2 pA, and 69 ± 11.3 pA, in males, female-HE, or female-LE, respectively (one-way ANOVA F2,22 = 0.2065, Tukey’s multiple comparison test P > 0.05 for all). The frequency and amplitude of mIPSCs were also similar among the groups (data not shown).
In 5 neurons from 4 male, 7 neurons from 4 female-HE, and 5 neurons from 4 female-LE rats, we assessed the shift in the holding current induced by perfusion with bicuculline (0.5–10 μM). In DMV neurons, the shift in the holding current induced by 0.5 μM bicuculline in female-HE rats was significantly greater than in neurons from males (14.6 ± 4.6 vs. 3.8 ± 1.7 pA, t12 = 1.95, P < 0.05) or in female-LE (14.6 ± 4.6 pA vs. 3.2 ± 2.6 pA, t11 = 1.84, P < 0.05). The response of neurons from female-HE rats to a larger concentration of bicuculline (10 μM) was also significantly greater compared with males (27.5 ± 4.2 pA vs. 14.0 ± 4.3 pA, t10 = 2.18, P < 0.05) but not to female-LE (27.5 ± 4.2 pA vs. 20.6 ± 7.7 pA, t10 = 0.8445, P > 0.05). Perfusion of DMV neurons with gabazine (25 μM) did not induce any significant variation in the holding current (N = 7; P > 0.05).
These data suggest that the GABAergic inhibitory inputs to DMV neurons from female-HE rats are higher compared with neurons from male or female-LE rats and that the inhibitory GABAergic effects are likely mediated by activation of extrasynaptic receptors.
GABAergic inputs onto vagal output to the stomach increase with estrogen levels.
To investigate the effect of GABAergic inhibitory tone on gastric tone and motility, the GABAA receptor antagonist bicuculline (0.1–5 pmol/60 nL) was microinjected into the DVC of male rats and female rats at various phases of the estrus cycle.
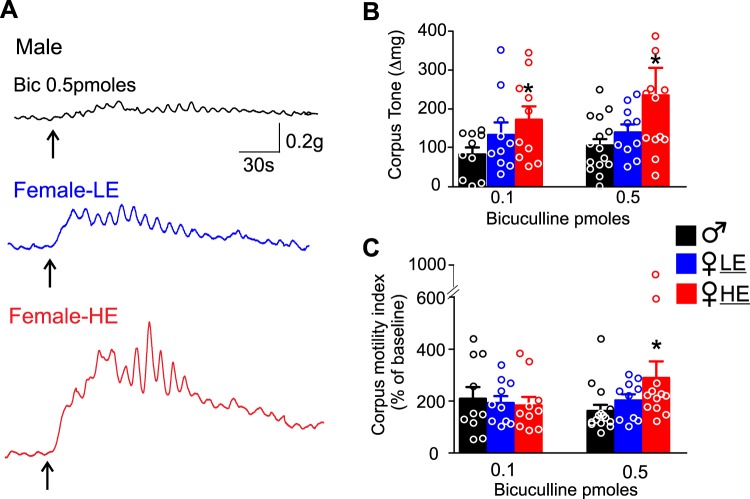
In male rats (n = 10), DVC microinjection of 0.1 pmol bicuculline induced an 83 ± 18.1 mg increase in corpus tone, with a 109 ± 45.2% increase in corpus motility. In female-LE rats (n = 10), microinjection of 0.1 pmol of bicuculline in the DVC induced a 133 ± 31.9 mg increase in corpus tone and a 92 ± 26.2% increase in corpus motility over baseline. The increase in corpus tone and motility was not significantly different between female-LE and male rats (P > 0.05). In female-HE rats (n = 10), microinjection of 0.1 pmole of bicuculline in the DVC induced a 173 ± 34.4 mg increase in corpus tone, which was significantly greater than the increase induced in males (P < 0.05). The increase in corpus motility in female-HE rats (83 ± 37.7%) was not significantly different from that in female-LE or male rats (P > 0.05 vs. male and female-LE).
Microinjection of 0.5 pmole of bicuculline in the DVC of 15 male rats induced a 106 ± 18.4 mg increase in corpus tone and a 62 ± 23.4% increase in corpus motility. Similarly, microinjection of 0.5 pmole of bicuculline in the DVC of 10 female-LE rats increased corpus tone and motility by 140 ± 20.3 mg and 92 ± 26.2% versus baseline, respectively (P > 0.05). Conversely, microinjection of the same dose of bicuculline into the DVC of 12 female-HE rats induced a significantly larger increase in corpus tone (235 ± 70.7 mg, P < 0.05 vs. male), as well as a larger increase in corpus motility (222 ± 84.0%, P < 0.05 vs. male; Fig. 3 and Table 2).
Fig. 3.
Microinjection of bicuculline in the dorsal vagal complex (DVC) induced a larger increase in corpus tone and motility in female high estrogen (HE) rats. A: representative recordings from the corpus of a male (top, black), female low estrogen (LE) (middle, blue), and female-HE (bottom, red) showing the increase in tone and motility upon microinjection (upward arrow) of bicuculline (0.5 pmol/60 nL). Note the increase in tone and motility in female-HE rats is larger compared with male and female-LE rats. B: summary graph representing the effects of bicuculline (0.1 pmol and 0.5 pmol) microinjection in the DVC on corpus tone in male (black), female-LE (blue), and female-HE (red) rats. Note that both 0.1 and 0.5 pmol bicuculline-induced increase in corpus tone is significantly larger in female-HE rats. *P < 0.05 vs. male. C: summary graph representing the effects of bicuculline (0.1 pmol and 0.5 pmol) microinjection in the DVC on corpus motility in male (black), female-LE (blue), and female-HE (red) rats. Note that 0.5 pmol bicuculline-induced increase in corpus motility is significantly larger in female-HE rats. *P < 0.05 vs. male.
Table 2.
Effect of bicuculline microinjection in the DVC on corpus tone and motility
| Bicuculline |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0.1 pmol |
0.5 pmol |
|||||||
| Corpus tone, mg | t (df) vs. male | Corpus motility, % | t (df) vs. male | Corpus tone, mg | t (df) vs. male | Corpus motility, % | t (df) vs. male | |
| Male | 83 ± 18.1 (N = 10) | 109 ± 45.2 (N = 10) | 106 ± 18.4 (N = 15) | 62 ± 23.4 (N = 15) | ||||
| Female-HE | 173 ± 34.4 (N = 10) | 2.298 (18)* | 83 ± 37.7 (N = 10) | 0.461 (18) | 235 ± 70.7 (N = 12) | 1.95 (25)* | 222 ± 84.0 (N = 12)* | 2.02 (25) |
| Female-LE | 133 ± 31.9 (N = 10) | 1.372 (18) | 92 ± 26.2 (N = 10) | 0.312 (18) | 140 ± 20.3 (N = 10) | 0.240 (23) | 92 ± 26.2 (N = 10) | 1.170 (10) |
Values are means ± SE; N, number of rats. DVC, dorsal vagal complex; HE, high estrogen; LE, low estrogen; t, t value; df, degrees of freedom.
P < 0.05.
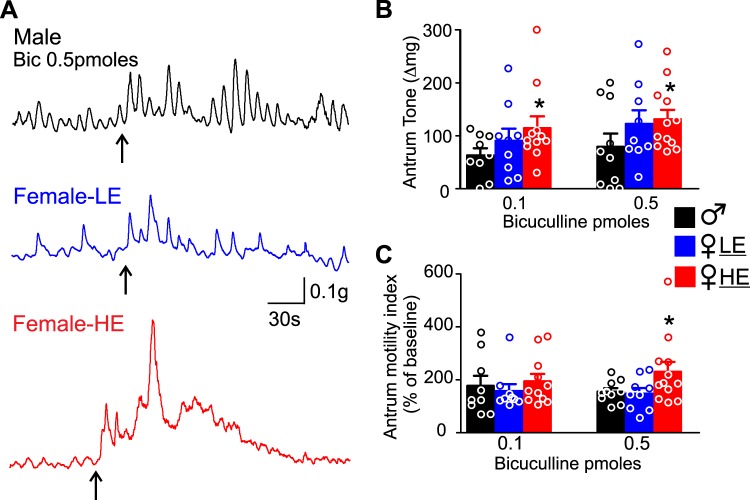
Regarding the antrum tone and motility, microinjection of 0.1 pmole of bicuculline in the DVC of male rats induced a 63 ± 13.5 mg increase in antrum tone and a 77 ± 37% increase in antrum motility (N = 9). In 9 female-LE rats, microinjection of 0.1 pmole of bicuculline in the DVC increased antrum tone and motility by 90 ± 22.8 mg and 28 ± 6.7% versus baseline, respectively (P > 0.05 vs. male). Conversely, microinjection of 0.1 pmole of bicuculline in the DVC of 11 female-HE rats induced a significantly higher increase in antrum tone (115 ± 22.2 mg, P < 0.05) and a similar increase in antrum motility (94 ± 27.6%, P > 0.05) compared with male rats.
A higher dose of bicuculline (0.5 pmole) microinjection into the DVC of male rats induced a 79 ± 25 mg increase in antrum tone and a 58 ± 14% increase in antrum motility (N = 10). Microinjection of 0.5 pmole of bicuculline in the DVC of 9 female-LE rats increased antrum tone and motility by 122 ± 25 mg and 48 ± 20.9% versus baseline, respectively (P > 0.05). Conversely, microinjection of 0.5 pmole of bicuculline in the DVC of 12 female-HE rats induced a significantly higher increase in both antrum tone (131 ± 17.7 mg, P < 0.05 vs. male) and antrum motility (230 ± 37.3%, P < 0.05 vs. male, Fig. 4 and Table 3).
Fig. 4.
Microinjection of bicuculline in the dorsal vagal complex (DVC) induced a larger increase in antrum tone and motility in female high estrogen (HE) rats. A: representative recordings from the antrum of a male (top, black), female low estrogen (LE) (middle, blue), and female-HE (bottom, red) showing the increase in antrum tone and motility upon microinjection (upward arrow) of bicuculline (0.5 pmol/60 nL). Note the increase in tone and motility in female-HE rats is larger compared with male and female-LE rats. B: summary graph representing the effects of bicuculline (0.1 pmol and 0.5 pmol) microinjection in the DVC on antrum tone in male (black), female-LE (blue), and female-HE (red) rats. Note that both 0.1 and 0.5 pmol bicuculline-induced increase in antrum tone is significantly larger in female-HE rats. *P < 0.05 vs. male. C: summary graph representing the effects of bicuculline (0.1 pmol and 0.5 pmol) microinjection in the DVC on corpus motility in male (black), female-LE (blue), and female-HE (red) rats. Note that 0.5 pmol bicuculline-induced increase in antrum motility is significantly larger in female-HE rats. *P < 0.05 vs. male.
Table 3.
Effect of bicuculline microinjection in the DVC on antrum tone and motility
| Bicuculline |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0.1 pmol |
0.5 pmol |
|||||||
| Antrum tone, mg | t (df) vs. male | Antrum motility, % | t (df) vs. male | Antrum tone, mg | t (df) vs. male | Antrum motility, % | t (df) vs. male | |
| Male | 63 ± 13.5 (N = 9) | 77 ± 37.0 (N = 9) | 79 ± 25.0 (N = 10) | 58 ± 14.0 (N = 10) | ||||
| Female-HE | 115 ± 22.2 (N = 11) | 1.871 (18)* | 94 ± 27.6 (N = 11) | 0.372 (18) | 131 ± 17.7 (N = 12) | 1.732 (20)* | 230 ± 37.3 (N = 12) | 1.761 (20)* |
| Female-LE | 90 ± 22.8 (N = 9) | 1.041 (16) | 28 ± 6.7 (N = 9) | 0.431 (16) | 122 ± 25 (N = 9) | 1.2 (17) | 48 ± 20.9 (N = 9) | 0.308 (17) |
Values are means ± SE; N, number of rats. DVC, dorsal vagal complex; HE, high estrogen; LE, low estrogen; t, t value; df, degrees of freedom.
P < 0.05.
These data indicated that in female-HE rats, the gastric tone and motility are decreased because of increased GABAergic inhibitory inputs to DMV neurons.
DISCUSSION
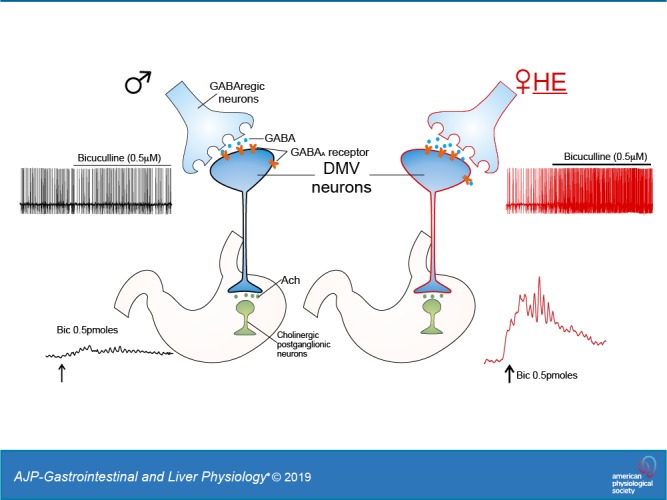
In the present study, we have shown that GABAergic tone to DMV neurons, hence the vagal output to the stomach, varies according to sex and the phase of the estrus cycle. In particular, we have shown the following: 1) gastric-projecting DMV neurons from female-HE rats have a larger action potential afterhyperpolarization and a lower membrane excitability; 2) the magnitude of the increase in action potential firing rate following perfusion of a low concentration of bicuculline is larger in DMV neurons from female-HE rats compared with male rats; and 3) microinjection of bicuculline into the DVC induced a larger increase in gastric tone and motility in female-HE rats. Taken together, these data suggest that the GABAergic inhibitory inputs onto gastric-projecting DMV neurons are higher in female-HE rats.
The functions of upper GI tract, including gastric tone and motility, are regulated by the efferent vagus nerve, the output of which is largely dependent on the activity of vagal preganglionic DMV neurons (16, 33). The excitability of pacemaking DMV motoneurons is influenced heavily by robust GABAergic synaptic inputs originating mainly from NTS neurons (2, 32). Removal of GABAergic inputs by application of GABA receptor antagonists has been shown to increase firing rates of DMV neurons and to increase gastric tone and motility (2, 26, 32). We have shown here that the influence of GABAergic inputs to the DMV neurons, hence to gastric motility, is further regulated by sex and the phase of the estrus cycle. Application of low concentrations of the GABAA receptor antagonist bicuculline induced a significantly larger increase in firing rate in female-HE rats, suggesting that during this cycle stage, the inhibitory inputs to gastric-projecting DMV neurons are more robust. These stronger inhibitory inputs to DMV neurons contribute, at least in part, to the lower excitability of DMV neurons observed in female-HE rats, and by consequence, to the decreased vagal output to the stomach.
It is well recognized that the GABAergic inhibitory current in gastric-projecting DMV neurons can be divided into phasic and tonic currents, which are associated with activation of synaptic or extrasynaptic GABAA receptors, respectively (13). Both phasic and tonic GABA currents seem to play a role in the modulation of gastric tone and motility in rats (26). Here, we used a low concentration of bicuculline and observed a larger inhibition in DMV neurons from female-HE rats. This GABAergic inhibition appears to be determined by tonic, extrasynaptic GABAergic receptors since we observed a larger shift in holding current in DMV neurons in female-HE rats in response to superfusion with bicuculline, but not gabazine, and we did not observe any differences in frequency or amplitude in spontaneous or miniature IPSCs among the groups.
In this study, application of bicuculline to DMV neurons induced a larger increase in the firing rate of DMV neurons in female-HE rats than in male and female-LE rats. Considering that the vagal output that modulates gastric motility (i.e., smooth muscle contractility) is largely dependent on the activity of DMV neurons, female-HE rats were expected to have a lesser vagal output and thus a lower gastric motility than male or female-LE rats. Indeed, consistent with the in vitro data, we have shown here that the gastric tone and motility response to microinjection of a low dose of bicuculline in the DVC is larger in female-HE rats, indicating a decreased tonic vagal output to the stomach due to a higher inhibitory input to DMV neurons.
Although the cellular mechanisms of such sex/estrus cycle dependent inhibitory modulation of DMV neurons need further investigation, we suggest that circulating estrogen levels may influence the strength of the inhibitory GABA inputs onto DMV neurons, hence the vagal output to the stomach. Both genomic and membrane-bound estrogen receptors are abundant in the DVC (20, 31, 37), and the number of NTS neurons that express estrogen receptors fluctuates throughout the estrus cycle (20). It is thus possible that circulating estrogen acts through its receptors in the DVC to modulate the critical GABAergic neurotransmission between NTS and DMV. In fact, estrogen treatment increases vagal afferent projections to the NTS (6) and enhances baroreflex function through an increase in cardiac vagal tone that involves the nucleus ambiguus (30). Since the cardiac vagal tone is driven by a glutamatergic excitatory projection from the NTS to the nucleus ambiguus (15), it is plausible that estrogen augments the excitability of subsets of NTS neurons while inhibiting others (38). The increased excitability of NTS neurons by estrogen may explain the increased release of GABA onto DMV neurons, resulting in the higher GABAergic inputs to gastric-projecting DMV neurons from female-HE rats observed in our study.
Despite the potential action of estrogen on GABAergic presynaptic terminals, several studies also suggest estrogen or other neurosteroids may modulate directly GABA currents through multiple pathways. In other central nervous systems areas, estrogen has been shown to increase the activity and mRNA expression of the GABA transporter, increase the expression of α-2 and γ-1 subunit of the GABAA receptor, and increase the sensitivity to the GABAA receptor agonist THIP (14, 21, 22). Whether estrogen modulates GABA receptors in DMV neurons remains unclear, but other neurosteroids have been shown to potentiate GABA currents in the DMV through increasing both duration and contribution of the slower decay component of the inhibitory current (34). Thus, it is possible that the higher GABAergic inhibitory inputs to DMV neurons from female-HE rats modulate postsynaptic GABA currents by estrogen.
FGID, including functional dyspepsia and irritable bowel syndrome, are more prevalent in women. Many symptoms observed in FGID, including delayed gastric emptying and impaired gastric motility, are associated with suppressed vagal output to the stomach. Several studies suggest that estradiol administration delays gastric emptying and inhibits gastric motility in rodents (3, 5, 17). Our data indicate that a higher circulating estrogen level increased inhibitory inputs to the DMV, and by consequence, decreased the vagal output to the stomach. This may provide a mechanistic explanation of the reduced gastric motility observed in women.
GRANTS
This work was supported by National Institutes of Health Grant DK 120170 to R. A. Travagli, and an American Neurogastroenterology and Motility Society research grant to T. Babic.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.J., T.B., and R.A.T. conceived and designed research; Y.J. and T.B. performed experiments; Y.J., T.B., and R.A.T. analyzed data; Y.J., T.B., and R.A.T. interpreted results of experiments; Y.J. and R.A.T. prepared figures; Y.J. and R.A.T. drafted manuscript; Y.J., T.B., and R.A.T. edited and revised manuscript; Y.J., T.B., and R.A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Cesare M. and Zoraide Travagli for support and encouragement, and Dr. Kirsteen N. Browning for comments on previous versions of the manuscript.
REFERENCES
- 1.Aytuğ N, Giral A, Imeryüz N, Enç FY, Bekiroğlu N, Aktaş G, Ulusoy NB. Gender influence on jejunal migrating motor complex. Am J Physiol Gastrointest Liver Physiol 280: G255–G263, 2001. doi: 10.1152/ajpgi.2001.280.2.G255. [DOI] [PubMed] [Google Scholar]
- 2.Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol 300: G21–G32, 2011. doi: 10.1152/ajpgi.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond EF, Heitkemper MM, Perigo R. Gastric emptying and gastric-intestinal transit in rats with varying ovarian hormone status. Nurs Res 45: 218–224, 1996. doi: 10.1097/00006199-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Browning KN, Babic T, Holmes GM, Swartz E, Travagli RA. A critical re-evaluation of the specificity of action of perivagal capsaicin. J Physiol 591: 1563–1580, 2013. doi: 10.1113/jphysiol.2012.246827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol Gastrointest Liver Physiol 268: G171–G176, 1995. doi: 10.1152/ajpgi.1995.268.1.G171. [DOI] [PubMed] [Google Scholar]
- 6.Ciriello J, Caverson MM. Effect of estrogen on vagal afferent projections to the brainstem in the female. Brain Res 1636: 21–42, 2016. doi: 10.1016/j.brainres.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira CV, Rosas-Arellano MP, Solano-Flores LP, Babic T, Li Z, Ciriello J. Estrogen alters the bradycardia response to hypocretin-1 in the nucleus tractus solitarius of the ovariectomized female. Brain Res 978: 14–23, 2003. doi: 10.1016/S0006-8993(03)02724-0. [DOI] [PubMed] [Google Scholar]
- 8.Dias DP, Oliveira M, Salgado HC, Fazan R Jr. Ovariectomy does not affect the cardiac sympathovagal balance of female SHR but estradiol does. Braz J Med Biol Res 43: 969–975, 2010. doi: 10.1590/S0100-879X2010007500105. [DOI] [PubMed] [Google Scholar]
- 9.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology 150: 1262–1279.e2, 2016. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 10.El-Mas MM, Abdel-Rahman AA. Estrogen enhances baroreflex control of heart rate in conscious ovariectomized rats. Can J Physiol Pharmacol 76: 381–386, 1998. doi: 10.1139/y98-031. [DOI] [PubMed] [Google Scholar]
- 11.Enck P, Azpiroz F, Boeckxstaens G, Elsenbruch S, Feinle-Bisset C, Holtmann G, Lackner JM, Ronkainen J, Schemann M, Stengel A, Tack J, Zipfel S, Talley NJ. Functional dyspepsia. Nat Rev Dis Primers 3: 17081, 2017. doi: 10.1038/nrdp.2017.81. [DOI] [PubMed] [Google Scholar]
- 12.Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut 64: 1049–1057, 2015. doi: 10.1136/gutjnl-2014-307843. [DOI] [PubMed] [Google Scholar]
- 13.Gao H, Smith BN. Tonic GABAA receptor-mediated inhibition in the rat dorsal motor nucleus of the vagus. J Neurophysiol 103: 904–914, 2010. doi: 10.1152/jn.00511.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Flores O, Sánchez N, García-Juárez M, Lima-Hernández FJ, González-Mariscal G, Beyer C. Estradiol and testosterone modulate the anesthetic action of the GABA-A agonist THIP, but not of the neurosteroid 3alpha,5beta-pregnanolone in the rat. Psychopharmacology (Berl) 172: 283–290, 2004. doi: 10.1007/s00213-003-1649-x. [DOI] [PubMed] [Google Scholar]
- 15.Gourine AV, Machhada A, Trapp S, Spyer KM. Cardiac vagal preganglionic neurones: An update. Auton Neurosci 199: 24–28, 2016. doi: 10.1016/j.autneu.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Goyal RK, Guo Y, Mashimo H. Advances in the physiology of gastric emptying. Neurogastroenterol Motil 31: e13546, 2019. doi: 10.1111/nmo.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Günal O, Bozkurt A, Deniz M, Sungur M, Yeğen BC. Effect of sex steroids on colonic distension-induced delay of gastric emptying in rats. J Gastroenterol Hepatol 19: 975–981, 2004. doi: 10.1111/j.1440-1746.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 18.Guo WJ, Yao SK, Zhang YL, Du SY, Wang HF, Yin LJ, Li HL. Impaired vagal activity to meal in patients with functional dyspepsia and delayed gastric emptying. J Int Med Res 46: 792–801, 2018. doi: 10.1177/0300060517726442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hausken T, Svebak S, Wilhelmsen I, Haug TT, Olafsen K, Pettersson E, Hveem K, Berstad A. Low vagal tone and antral dysmotility in patients with functional dyspepsia. Psychosom Med 55: 12–22, 1993. doi: 10.1097/00006842-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Haywood SA, Simonian SX, van der Beek EM, Bicknell RJ, Herbison AE. Fluctuating estrogen and progesterone receptor expression in brainstem norepinephrine neurons through the rat estrous cycle. Endocrinology 140: 3255–3263, 1999. doi: 10.1210/endo.140.7.6869. [DOI] [PubMed] [Google Scholar]
- 21.Herbison AE, Augood SJ, Simonian SX, Chapman C. Regulation of GABA transporter activity and mRNA expression by estrogen in rat preoptic area. J Neurosci 15: 8302–8309, 1995. doi: 10.1523/JNEUROSCI.15-12-08302.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbison AE, Fénelon VS. Estrogen regulation of GABAA receptor subunit mRNA expression in preoptic area and bed nucleus of the stria terminalis of female rat brain. J Neurosci 15: 2328–2337, 1995. doi: 10.1523/JNEUROSCI.15-03-02328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology 96: 11–17, 1989. doi: 10.1016/0016-5085(89)90758-0. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y, Browning KN, Toti L, Travagli RA. Vagally mediated gastric effects of brain stem α2-adrenoceptor activation in stressed rats. Am J Physiol Gastrointest Liver Physiol 314: G504–G516, 2018. doi: 10.1152/ajpgi.00382.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight LC, Parkman HP, Brown KL, Miller MA, Trate DM, Maurer AH, Fisher RS. Delayed gastric emptying and decreased antral contractility in normal premenopausal women compared with men. Am J Gastroenterol 92: 968–975, 1997. [PubMed] [Google Scholar]
- 26.McMenamin CA, Travagli RA, Browning KN. Perinatal high fat diet increases inhibition of dorsal motor nucleus of the vagus neurons regulating gastric functions. Neurogastroenterol Motil 30: e13150, 2018. doi: 10.1111/nmo.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mearadji B, Penning C, Vu MK, van der Schaar PJ, van Petersen AS, Kamerling IM, Masclee AA. Influence of gender on proximal gastric motor and sensory function. Am J Gastroenterol 96: 2066–2073, 2001. doi: 10.1111/j.1572-0241.2001.03940.x. [DOI] [PubMed] [Google Scholar]
- 28.Pamidimukkala J, Taylor JA, Welshons WV, Lubahn DB, Hay M. Estrogen modulation of baroreflex function in conscious mice. Am J Physiol Regul Integr Comp Physiol 284: R983–R989, 2003. doi: 10.1152/ajpregu.00761.2001. [DOI] [PubMed] [Google Scholar]
- 29.Sah P, McLachlan EM. Potassium currents contributing to action potential repolarization and the afterhyperpolarization in rat vagal motoneurons. J Neurophysiol 68: 1834–1841, 1992. doi: 10.1152/jn.1992.68.5.1834. [DOI] [PubMed] [Google Scholar]
- 30.Saleh TM, Connell BJ. 17beta-estradiol modulates baroreflex sensitivity and autonomic tone of female rats. J Auton Nerv Syst 80: 148–161, 2000. doi: 10.1016/S0165-1838(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 31.Schlenker EH, Hansen SN. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res 1123: 89–100, 2006. doi: 10.1016/j.brainres.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 32.Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil 10: 305–313, 1998. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 33.Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol 13: 389–401, 2016. doi: 10.1038/nrgastro.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 260: G531–G536, 1991. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- 35.Travagli RA, Gillis RA, Vicini S. Effects of thyrotropin-releasing hormone on neurons in rat dorsal motor nucleus of the vagus, in vitro. Am J Physiol Gastrointest Liver Physiol 263: G508–G517, 1992. doi: 10.1152/ajpgi.1992.263.4.G508. [DOI] [PubMed] [Google Scholar]
- 36.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanderHorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-α and -β immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol 488: 152–179, 2005. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- 38.Xue B, Hay M. 17β-estradiol inhibits excitatory amino acid-induced activity of neurons of the nucleus tractus solitarius. Brain Res 976: 41–52, 2003. doi: 10.1016/S0006-8993(03)02629-5. [DOI] [PubMed] [Google Scholar]
- 39.Zia JK, Heitkemper MM. Upper gastrointestinal tract motility disorders in women, gastroparesis, and gastroesophageal reflux disease. Gastroenterol Clin North Am 45: 239–251, 2016. doi: 10.1016/j.gtc.2016.02.003. [DOI] [PubMed] [Google Scholar]