Keywords: biotin, inflammasome, intestinal inflammation, microbiota, NF-κB activation, Slc5a6, SMVT, tamoxifen-inducible knockout
Abstract
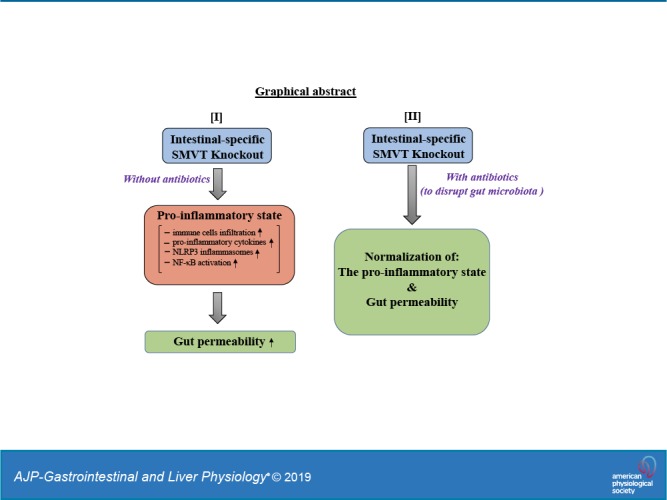
The sodium-dependent multivitamin transporter (SMVT; SLC5A6) is involved in intestinal absorption of vitamin B7 (biotin). We have previously shown that mice with an embryonic intestinal-specific SMVT knockout (KO) develop biotin deficiency and severe spontaneous intestinal inflammation in addition to growth retardation, developmental delays, and death within the first 6–7 wk of life. The profound morbidity and mortality associated with the SMVT-KO has limited our ability to further characterize the intestinal inflammation and other sequelae of this deletion in adult mice with a mature gut microbiota. To overcome this limitation, we generated an intestine-specific, tamoxifen-inducible, conditional SMVT-KO (SMVT-icKO). Our results showed that adult SMVT-icKO mice have reduced body weight, biotin deficiency, shorter colonic length, and bloody diarrhea compared with age- and sex-matched control littermates. All SMVT-icKO mice also developed spontaneous intestinal inflammation associated with induction of calprotectin (S100a8/S100a9), proinflammatory cytokines (IL-1β, TNF-α, IFN-γ, and IL-6), and an increase in intestinal permeability. Additionally, the intestines of SMVT-icKO showed activation of the NF-κB pathway and the nucleotide-binding domain and leucine-rich repeat pyrin 3 domain (NLRP3) inflammasome. Notably, administration of broad-spectrum antibiotics reduced lethality and led to normalization of intestinal inflammation, proinflammatory cytokines, altered mucosal integrity, and reduced expression of the NLRP3 inflammasome. Overall, these findings support our conclusion that the biotin transport pathway plays an important role in the maintenance of intestinal homeostasis, and that NF-κB and the NLRP3 inflammasome, as well as gut microbiota, drive the development of intestinal inflammation when SMVT is absent.
NEW & NOTEWORTHY This study demonstrates that deletion of the intestinal biotin uptake system in adult mice leads to the development of spontaneous gut inflammation and that luminal microbiota plays a role in its development.
INTRODUCTION
The sodium-dependent multivitamin transporter (SMVT; product of the SLC5A6 gene) is exclusively responsible for intestinal absorption of biotin (vitamin B7), a member of the water-soluble family of vitamins. The vitamin is essential for normal human health and well-being because of its involvement as a cofactor in a variety of critical metabolic reactions (e.g., fatty acid, glucose, and amino acid metabolism), as well as in gene expression (expression of over 2,000 human genes appears to be regulated by biotin) and in cell proliferation and survival (35, 36, 43, 45, 54, 57). Emerging evidence also demonstrates an important role for biotin in normal cellular energy metabolism (ATP production), the regulation of intracellular level of reactive oxygen species, and innate and adaptive immunity (2, 5, 11, 19–22, 32, 39). With regard to the role of biotin in immune function, studies have shown an important role for the vitamin in the activity, generation, maturation, and responsiveness of a variety of immune cells, and that its deficiency leads to induction of proinflammatory cytokines (2, 5, 11, 20–22, 39, 44). Biotin also appears to affect the colonization and invasiveness of certain enteropathogenic bacteria (59) and mediates the effect of probiotic bacteria on intestinal microbial population (51, 60). Hence, it is not surprising that deficiency of this vitamin negatively impacts the normal homeostasis of different tissues/systems in the body and ultimately the overall health and well-being of humans (reviewed in 36, 45, 58). Such a state of deficiency occurs in conditions like inflammatory bowel diseases (IBD) and inborn error of biotin metabolism (1, 14, 49).
Humans (and other mammals) cannot synthesize biotin endogenously, but rather they obtain the micronutrient from exogenous sources (i.e., diet and gut microbiota) via intestinal absorption (9, 38). Dietary biotin is processed and absorbed in the small intestine, whereas gut- microbiota-generated biotin is absorbed in the large intestine (46). Studies from our laboratory and others have established the involvement of SMVT in a carrier-mediated process for the uptake of biotin in both the small and large intestine (6, 47, 48). We have characterized different aspects of the SMVT system, including its physiology, cell biology, and regulation at both transcriptional and posttranscriptional levels (10, 16, 23, 42, 50). Using a conditional, intestine-specific SMVT knockout (KO) mouse model, we have also demonstrated an exclusive role for SMVT in intestinal absorption of biotin (15). Interestingly, in the latter study, we made the unexpected observation that all of the SMVT-KO animals developed spontaneous enterocolitis associated with changes in gut permeability/integrity, and they all died prematurely during the first 6-7 wk of life (15, 44). The lethality of this mutation has impeded our ability to further characterize the effects of biotin deficiency in the developed adult intestine with a mature microbiome. In the current investigation, we overcame this limitation by generating an intestinal-specific, Villin-CreERT2–driven, tamoxifen-inducible, conditional SMVT-KO (SMVT-icKO) mouse model. This unique mouse allowed us to fully characterize the phenotype that develops in a mature intestine following SMVT deletion. The results showed that the SMVT-icKO mice develop similar characteristics to those observed with the embryonic SMVT-KO mouse model, including development of severe, spontaneous enterocolitis. The improvements to this disease model allowed us to uncover a role for nucleotide-binding domain and leucine-rich repeat pyrin 3 domain (NLRP3) inflammasomes, NF-kB, and gut microbiota in the inflammation that develops following SMVT deletion.
MATERIALS AND METHODS
Materials
All chemicals and reagents used in this study were purchased from commercial vendors and were of analytical/molecular biology grade. Mouse anti-claudin-2 (catalog no. 325600), mouse anti-β-actin (catalog no. MA5–15739), and mouse anti-zonula occludens (ZO)-1 (catalog no. sc-8146) antibodies were obtained from Life Technologies and Santa Cruz Biotechnology, respectively. Mouse anti-NLRP3 (catalog no. 15101S), mouse anti-IL1β (catalog no. 12242S), and mouse anti-pNF-κB (catalog no. 3033S) were obtained from Cell Signaling and mouse anti-NF-κB (catalog no. ab16502) from abcam.
Methods
Generation of SMVT-icKO in adult mice.
SMVT-icKO mice were generated by crossing our previously generated homozygous SMVT-LoxP+/+ mice (15) with heterozygous tamoxifen-driven Villin-Cre-ERT2 mice (12). The SMVT-LoxP mice have LoxP sites flanking exons 4 to 6 of the slc5a6 gene; the Cre-ERT2 mice have a Cre-tamoxifen receptor fusion protein driven by a Villin promoter for selective expression in intestinal epithelial cells. Both strains had previously been backcrossed to a C57BL/6J background. We identified CreERT2+/SMVT-LoxP+/− pups in the F1 generation and backcrossed them to the SMVT-LoxP+/+ parental line. This pairing led to the generation of SMVT-icKO (i.e., CreERT2+/SMVT-LoxP+/+) mice and their littermates (i.e., CreERT2+/SMVT-LoxP+/−), which was confirmed with PCR genotyping (12, 15). For experiments, we used 16-wk-old SMVT-icKO mice and their sex-matched (CreERT2+/SMVT-LoxP+/−) littermates as controls (i.e., control littermates). To induce deletion of SMVT, mice received tamoxifen (2 mg/mouse, dissolved in corn oil) intraperitoneally on days 1, 2, and 3 according to methods previously described (28). Control littermates also received similar amounts of tamoxifen. All breeding and animal studies were approved by the University of California, Irvine Institutional Animal Care and Use Committee.
Oral antibiotics treatment of mice.
SMVT-icKO mice were treated with an antibiotic cocktail (1 g/L ampicillin, 0.35 g/L vancomycin, 1 g/L metronidazole, 1 g/L neomycin; Sigma) in the drinking water starting 2 wk before tamoxifen (2 mg/mouse) was administered according to methods previously described (28). Antibiotics were continued for 7 more days following tamoxifen injection, which was the experimental end point. Bacterial depletion in the fecal material of the antibiotic-treated mice was verified by fecal plating on Luria-Bertani agar followed by colony counts after an overnight culture at 37°C as described before (29).
Real-time PCR and Western blotting.
Total RNA was extracted from the tissue of interest using QIAzol reagent (Qiagen) and RNeasy Kit (Qiagen) following the manufacturer’s protocol. The cDNA was prepared from RNA samples using the verso-cDNA synthesis kit (Invitrogen). Quantitative real-time PCR (RT-qPCR) analysis was performed using the CFX96 real-time PCR system (Bio-Rad) according to manufacturer’s instructions using the gene-specific primers for mouse TNF-α, IFN-γ, IL-1β, IL-6, S100a8, S100a9, ZO-1, claudin-2, NLRP3, NLRP4, nucleotide-binding domain–like receptor family pyrin domain containing 6 (NLRP6), absent in melanoma (AIM)-2, apoptosis-associated speck-like protein containing a COOH-terminal caspase-recruitment domain (ASC), caspase-1, and GAPDH (Table 1). Relative gene expression was quantified by normalizing threshold cycle values to the corresponding GAPDH.
Table 1.
List of mouse-specific primer sequences used for RT-qPCR
| Gene Name | Forward and Reverse Primer Sequences (5′-3′) |
|---|---|
| S100a8 | F: TGTCCTCAGTTTGTGCAGAATATAAA R: TCACCATCGCAAGGAACTCC |
| S100a9 | F: GGTGGAAGCACAGTTGGCA R: GTGTCCAGGTCCTCCATGATG |
| Claudin-2 | F: TTAGCCCTGACCGAGAAAGA R: AAAGGACCTCTCTGGTGCTG |
| ZO-1 | F: TTCAAAGTCTGCAGAGACAATAGC R: TCACATTGCTTAGTCCAGTTCC |
| IL-6 | F: GAGGATACCACTCCCAACAGACC R: AAGTGCATCATCGTTGTTCATACA |
| IFN-γ | F: TCAAGTGGCATAGATGTGGAAGAA R: TGGCTCTGCAGGATTTTCATG |
| TNF-α | F: CATCTTCTCAAAATTCGAGTGACAA R: TCGGAGTAGACAAGGTACAACCC |
| IL-1β | F: CTCTCCAGCCAAGCTTCCTTGTGC; R: GCTCTCATCAGGACAGCCCAGGT |
| GAPDH | F: CTACAGCAACAGGGTGGTGG R: TATGGGGGTCTGGGATGG |
| SMVT | F: CGTAGGAACTTTGGTAGCCCTGG R: CTTAGGTGTGATGGGTCTCTCC |
| NLRP3 | F: ATTACCCGCCCGAGAAAGG R: TCGCAGCAAAGATCCACACAG |
| NLRP6 | F: CTCGCTTGCTAGTGACTACAC R: AGTGCAAACAGCGTCTCGTT |
| NLRC4 | F: ATCGTCATCACCGTGTGGAG R: GCCAGACTCGCCTTCAATCA |
| AIM-2 | F: GTCACCAGTTCCTCAGTTGTG R: CACCGTGACAACAAGTGGAT |
| ASC | F: CTTGTCAGGGGATGAACTCAAAA R: GCCATACGACTCCAGATAGTAGC |
| Caspase-1 | F: ACAAGGCACGGGACCTATG R: TCCCAGTCAGTCCTGGAAATG |
AIM, absent in melanoma; ASC, apoptosis-associated speck-like protein containing a C-terminal caspase-recruitment domain; F, forward; NLRC4, nucleotide-binding domain and leucine-rich repeat family Caspase activation and recruitment domain containing 4; NLRP3, nucleotide-binding domain and leucine-rich repeat pyrin 3 domain; NLRP6, nucleotide-binding domain–like receptor family pyrin domain containing 6; R, reverse; RT-qPCR, quantitative real-time PCR; SMVT, sodium-dependent multivitamin transporter; ZO, zonula occludens.
For Western blot analysis, tissues were homogenized in radioimmunoprecipitation assay buffer (Sigma) containing complete protease inhibitor cocktail (Roche) by using Fisherbrand Bead Mill 24 Homogenizer (1.4-mm beads). Total protein homogenates were then separated by centrifugation at 12,000 g for 25 min, and an equal amount (60 μg) of the total proteins was loaded on a 4%–12% mini gel (Invitrogen). The proteins were then transferred to a PVDF membrane and probed simultaneously with mouse claudin-2, ZO-1, NLRP3, IL-1β antibodies (raised in mouse or rabbit) and monoclonal β-actin antibody (raised in mouse). The blots were then incubated with anti-rabbit/anti-mouse IR 800 dye and anti-mouse IR 680 dye (LI-COR) secondary antibodies (1:25,000) for 1 h at room temperature. Relative expression was quantified by comparing the fluorescence intensities in an Odyssey Infrared imaging system (LI-COR) using Odyssey application software (version 3.0) with respect to corresponding β-actin.
Measurement of intestinal permeability.
Gut permeability was assessed in vivo by oral gavage (40 mg/100 g body wt) of 4 kDa FITC-dextran (Sigma-Aldrich) followed by determination of its appearance in the blood, as previously described (8, 44).
Biotin uptake investigations.
We used a previously described in vivo jejunal loop approach to study biotin absorption (15, 41). Briefly, a 1-cm jejunal loop from either SMVT-icKO mice or the littermate control was filled with 0.1 mL of Krebs-Ringer buffer containing [3H]biotin, and uptake was determined 5 min later (presented as fmol/mg tissue wet wt per 5 min).
Histopathology.
Tissue samples from the intestine of experimental and control mice were sectioned immediately following euthanization, fixed in 10% formalin, and embedded in paraffin. Hematoxylin and eosin-stained slides were prepared using standard histological techniques (Long Beach Veterans Medical Center Clinical Histology Laboratory). Gross and microscopic evaluations and reporting were performed by a board-certified anatomic pathologist.
Statistical Analysis
Data presented in this study are means ± SE and plotted with “box and dot-whisker” plots. Statistical comparison of the presented data sets is calculated using nonparametric and Mann-Whitney tests (by GraphPad Prism software) when n < 8. Statistical significance between two groups was set as P < 0.05. Statistical comparison for the loss of body weight between mice groups is determined by using Student’s t-tests from eight pairs of animals. Uptake of [3H]biotin by the carrier-mediated process was determined by subtracting uptake in the presence of unlabeled biotin from that in its absence. For the Kaplan-Meier survival curve, eight pairs of animals were used.
RESULTS
Characterization of the SMVT-icKO Adult Mouse Model
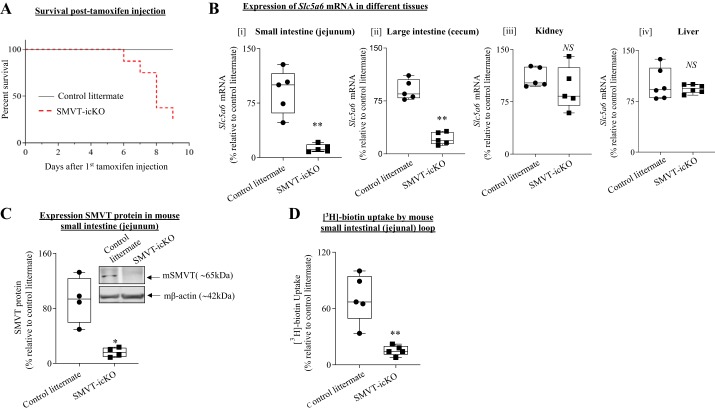
To characterize the newly developed SMVT-icKO mouse, we injected tamoxifen on 3 consecutive days and observed that 50% of the SMVT-icKO mice die spontaneously within 8–10 days; however, no death was observed in the control littermates during this period (Fig. 1A). For this reason, we limited our experimental end point in all future experiments to 7 days. To confirm the intestinal specificity of the SMVT (Slc5a6) deletion in the SMVT-icKO mouse, we determined the level of expression of SMVT mRNA in the large and small intestine as well as in unrelated tissue, including the liver and kidney. The data demonstrate a substantial reduction in the expression of the SMVT mRNA in the intestine of SMVT-icKO mice compare with their age- and sex-matched littermate controls following tamoxifen treatment. Notably, no such reduction in level of SMVT mRNA expression was observed in the liver or the kidney (Fig. 1B). The dramatic reduction in SMVT expression from the intestine of the SMVT-icKO mouse following tamoxifen treatment was also confirmed at the protein level (Fig. 1C). In a final functional assay, we quantified biotin uptake from the intestine of tamoxifen-treated SMVT-icKO mice and controls in vivo using jejunal loops (15). The results indicate substantial inhibition in carrier-mediated biotin uptake in the intestine of SMVT-icKO mice compared with their control littermates (Fig. 1D). Collectively, these findings confirm that SMVT expression is specifically and substantially inhibited in the intestine of adult SMVT-icKO mice treated with tamoxifen.
Fig. 1.
Characterization and validation of inducible conditional (intestine-specific) sodium-dependent multivitamin transporter (SMVT)-inducible, conditional knockout (icKO) mice. A: Kaplan-Meier survival curve for intestine-specific SMVT-icKO mice (n = 8). SMVT-icKO (labeled as red dotted line) mice die between days 5 and 9 posttamoxifen treatment. B: expression of SMVT mRNA in small intestine (jejunum) (i), large intestine (cecum) (ii), kidney (iii), and liver (iv) of SMVT-icKO mice and their sex-matched control littermates [n = 5 in each group; **P < 0.01 and not significant (NS)]. C: Western blot analysis of SMVT protein in small intestine (jejunum) of SMVT-icKO mice and their control littermates (n = 4 in each group; *P < 0.05). D: carrier-mediated [3H]biotin uptake in vivo using intact jejunal loops of SMVT-icKO mice and their sex-matched control littermates (n = 5 in each group; **P < 0.01). Data are presented as box and dot-whisker plots, and statistical significance is analyzed by Mann-Whitney test.
Phenotype of the SMVT-icKO Mice
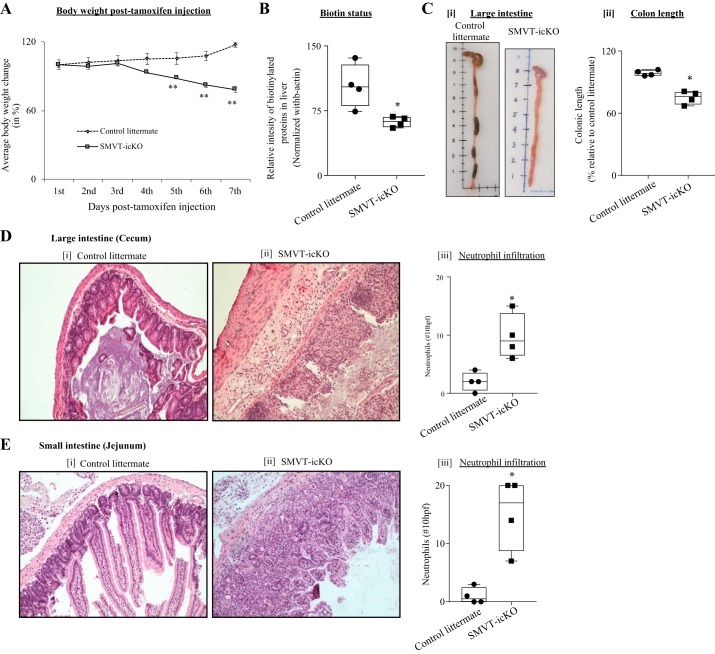
We observed a significant and progressive decline in body weight of the SMVT-icKO mice during the experimental period of 7 days (Fig. 2A). Also, biotin level in the SMVT-icKO mice was significantly (P < 0.05) reduced compared with control littermates (Fig. 2B). In addition, we observed a significant (P < 0.05) shortening of the colon of the SMVT-icKO mice compared with their control littermates (Fig. 2C). Furthermore, severe bloody diarrhea was observed in the SMVT-icKO mice but not in the control group. Finally, histological evaluation of the large intestine (cecum) of the SMVT-icKO mice showed mucosal erosions and severe chronic active inflammation with cryptitis, crypt abscesses, and loss of crypts. Notably, there was no inflammation or altered crypt architecture in the littermate controls (Fig. 2D). Similarly, histological evaluation of the small intestine showed acute inflammation with significant increase in neutrophils infiltration (Fig. 2E).
Fig. 2.
Phenotype of the sodium-dependent multivitamin transporter (SMVT)-inducible, conditional knockout (icKO) mice. A: growth chart showing a significant difference in weight loss of SMVT-icKO mice compared with their sex-matched control littermates after tamoxifen injection (n = 8; **P < 0.01; statistical significance is calculated using unpaired Student’s t test). B: level of total biotinylated proteins (a measure of biotin status) in liver of SMVT-icKO mice and their sex-matched control littermates. C: representative photographs showing length of large intestine (i) and colon length (ii) at day 7 posttamoxifen treatment. D: histology section of the large intestine (cecum) of control littermates (i) and SMVT-icKO mice (ii). A semiquantitative analysis of the number of neutrophils in 10 high-power fields (×400, left y-axis) presented from the large intestine (cecum; iii). E: histology section of the small intestine (jejunum) of control littermates (i) and SMVT-icKO mice (ii). A semiquantitative analysis of the number of neutrophils in 10 high-power fields (×400, left y-axis) presented from the small intestine (jejunum; iii). Both representative sections were stained using hematoxylin and eosin (×40). Data in B–E are presented as box and dot-whisker plots from four separate sets of mice, and statistical significance (*P < 0.05) is calculated with the Mann-Whitney test.
Intestine-Specific Deletion of SMVT in Adult Mice Leads to the Development of Spontaneous Inflammation and Decreased Intestinal Permeability and Tight-Junction Integrity
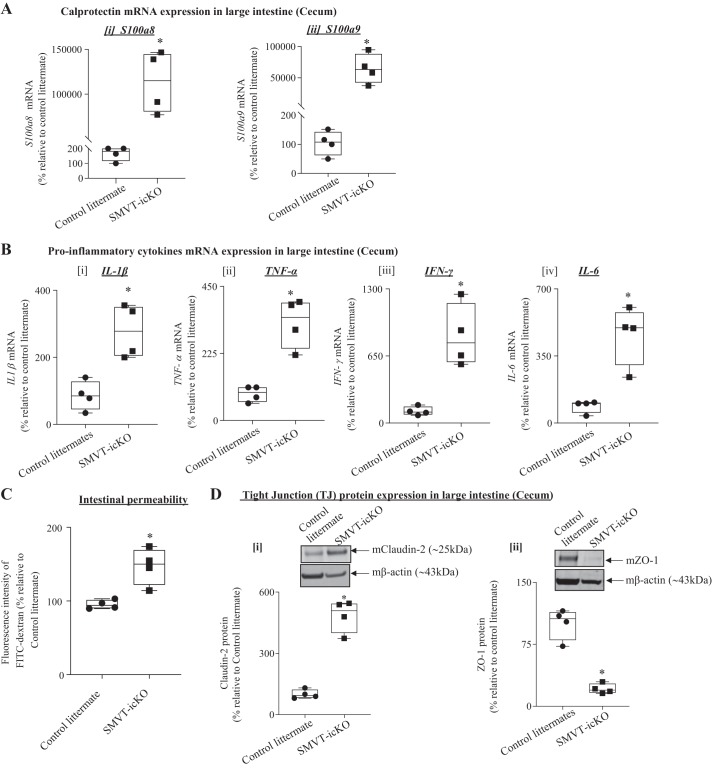
In the next series of experiments, we investigated changes in the intestinal permeability and tight-junction (TJ) integrity of SMVT-icKO mice treated with tamoxifen. First, we determined the level of mRNA expression of calprotectin (S100a8 and S100a9), a marker of intestinal inflammation and neutrophil infiltration (56), in the cecum of the SMVT-icKO mice and observed a significant (P < 0.01 for both) induction in its level compared with control littermates (Fig. 3A); similar changes were also observed in the small intestine (jejunum; data not shown). We then determined the level of expression of proinflammatory cytokines in the cecum of the SMVT-icKO mice and observed a significant (P < 0.05 for all) induction in level of expression of IL-1β, TNF-α, IFN-γ, and IL-6 compared with their levels in control littermates (Fig. 3B); similar results were observed in the small intestine (jejunum; data not shown).
Fig. 3.
Level of mRNA expression of calprotectin, proinflammatory cytokines, and gut permeability as well as level of expression of tight-junction (TJ) proteins in sodium-dependent multivitamin transporter (SMVT)-inducible, conditional knockout (icKO) mice and their control littermates. A: level of mRNA expression of calprotectin [s100a8 (i) and s100a9 (ii)] in the cecum of SMVT-icKO mice and their control littermates. B: level of mRNA expression of proinflammatory cytokines IL-1β (i), TNF-α (ii), IFN-γ (iii), and IL-6 (iv) in the cecum of SMVT-icKO mice and their control littermates. C: reduced gut permeability in the intestine of SMVT-icKO compared with control littermates determined with FITC-dextran assay. D: effect of intestinal SMVT-icKO on the level of TJ protein expression in the large intestine. The bar diagram shows relative protein expression of claudin-2 (i) and zonula occludens (ZO)-1 (ii) in the SMVT-icKO mice and their sex-matched control littermates. Data are presented as box and dot-whisker plots from four separate sets of mice, and statistical significance (*P < 0.05) is calculated with the Mann-Whitney test.
Finally, we examined possible changes in intestinal permeability and TJ integrity of the SMVT-icKO mice. Intestinal permeability was assessed using the FITC-dextran method (8, 44). The results showed a significant increase (P < 0.05) in gut permeability in the SMVT-icKO mice compared with control littermates (Fig. 3C). We also determined possible changes in level of expression of important TJ proteins in the cecum of the SMVT-icKO mice, with the results showing a significant (P < 0.05) increase in the level of “leaky” TJ protein claudin-2, and significant (P < 0.05) decrease in the level of expression of the “tight” TJ protein ZO-1 (Fig. 3D). Similar changes in level of expression of TJ proteins were observed in the small intestine (jejunum) of the SMVT-icKO mice (data not shown).
Role of the NF-κB Signaling Pathway and Inflammasomes in Intestinal Inflammation in the SMVT-icKO Mice
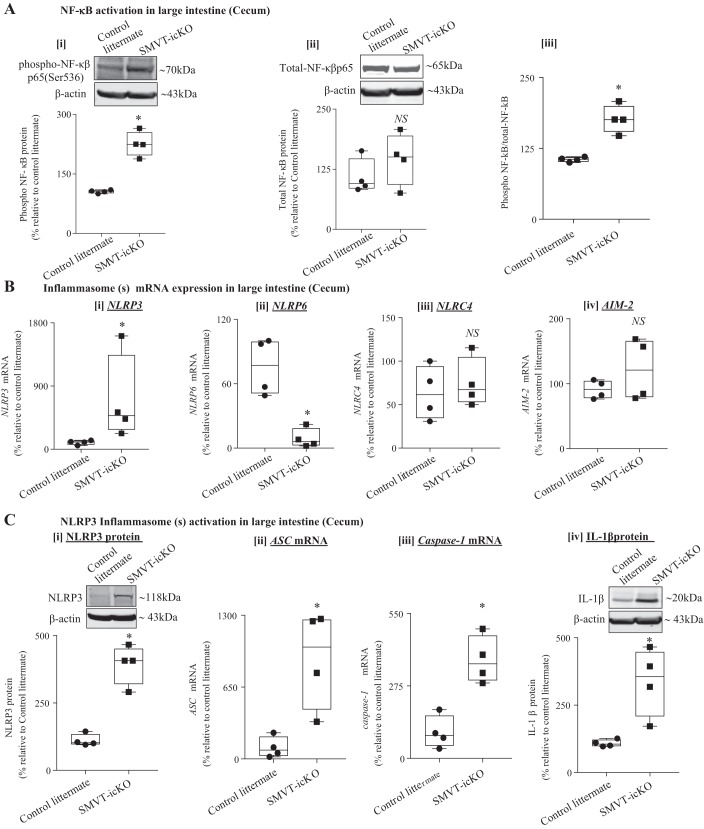
The nuclear factor NF-κB represents a major proinflammatory signaling pathway and controls the transcription of many proinflammatory cytokines (including TNF-α, IL-6, IFN-γ, etc.) as well as inflammasomes (including the NLRP3 inflammasomes) (7, 17, 31). This pathway is activated in patients with IBD, and this activation strongly influences the development of mucosal inflammation (4). NF-κB is generally retained in the cytoplasm in its inactive form via binding to its inhibitor, Iκβ. Phosphorylation activates NF-κB, leading to its dissociation from Iκβ and translocation into the nucleus, where it exerts its transcriptional effects. Considering the crucial role of the NF-κB signaling pathway, we investigated (by mean of Western blotting; see materials and methods) the phosphorylation status of NF-κB in the cecum of SMVT-icKO mice and observed a marked increase in the level of phosphorylated, but not in total, NF-κB in the cecum of the SMVT-icKO mice compared with their control littermates (Fig. 4A). The ratio of phosphorylated to nonphosphorylated NF-κB was significantly (P < 0.05) higher in the SMVT-icKO mice compared with control littermates, suggesting activation of the NF-κB pathway in the SMVT-deleted inflamed intestinal tissue.
Fig. 4.
Involvement of NF-κβ signaling pathway and inflammasome(s) in the intestine of sodium-dependent multivitamin transporter (SMVT)-inducible, conditional knockout (icKO) mice. A: representative Western blotting bands and the bar diagrams show the relative phospho-NF-κB (i), total-NF-κB (ii), and ratio of phospho- and total-NF-κB (iii) protein expression in cecum of SMVT-icKO mice and their control littermates. B: level of mRNA expression of different inflammasomes [nucleotide-binding domain and leucine-rich repeat pyrin 3 domain (NLRP3) (i), nucleotide-binding domain–like receptor family pyrin domain containing 6 (NLRP6) (ii), nucleotide-binding domain and leucine-rich repeat family caspase activation and recruitment domain containing 4 (NLRC4) (iii), and absent in melanoma (AIM)-2 (iv)] in the cecum of SMVT-icKO mice and their control littermates. C: activation of NLRP3 inflammasome in the cecum of SMVT-icKO mice. Level of expression of NLRP3 protein (i), level of mRNA expression of apoptosis-associated speck-like protein containing a COOH-terminal caspase-recruitment domain (ASC) (ii), level of mRNA expression of caspase-1 (iii), and level of expression of mature IL-1β protein in cecum of SMVT-icKO mice and their control littermates (iv). mRNA levels were determined by RT-qPCR, and data were normalized relative to GAPDH. Protein expression levels were determined by Western blot, and data were normalized to β-actin. All RT-qPCR and Western blot data are presented as box and dot-whisker plots from four separate sets of mice and statistical significance (*P < 0.05) are calculated with Mann-Whitney test. NS, not significant.
In other studies, we investigate the role of inflammasome(s) in the observed intestinal inflammation in the SMVT-icKO mice. Inflammasomes are key intracellular signaling mediators that sense pathogens and intestinal epithelial cell damage to promote inflammation (27). Their assembly triggers proteolytic cleavage of pro-caspase-1 into its active form, which then converts important proinflammatory cytokines, like pro-IL-1β, into their active/mature form (34, 55). Formation of an inflammasome requires a pattern recognition receptor (as a sensor) and, among other things, the adaptor apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC) (24, 33). Thus, we first examined the level of mRNA expression of several inflammasomes [NLRP3, NLRP6, nucleotide-binding domain and leucine-rich repeat family caspase activation and recruitment domain containing 4 (NLRC4), and AIM-2] in the cecum of SMVT-icKO mice and compared the results with their levels in control littermates. The results showed a significant (P < 0.05) increase in level of mRNA expression of NLRP3, and a significant (P < 0.05) decrease in the expression of NLRP6 in the cecum of the SMVT-icKO mice and compared their control littermates (Fig. 4B); no significant change in the level of expression of NLRC4 and AIM-2 was observed between the two groups (Fig. 4B). Similar results were observed in the small intestine (jejunum) of SMVT-icKO mice (data not shown). Focusing on NLRP3 (because of its apparent involvement in IBD and other human diseases (18, 26, 40, 61), we determined whether the changes in its level of expression in the cecum of the SMVT-icKO mice is also reflected at the protein level. This was done by means of Western blot analysis (see methods), which again showed a significant (P < 0.05) increase in its level in the SMVT-icKO mice compared with control littermates (Fig. 4C). Since the NLRP3 inflammasome requires the adaptor protein ASC to activate caspase-1, we also investigated possible changes in the level of mRNA expression of both ASC and caspase-1 in the cecum of the SMVT-icKO. The results showed a significant (P < 0.05) increase in the level of mRNA expression of both ASC and caspase-1 in the cecum of SMVT-icKO mice compared with control littermates (Fig. 4C). In the same tissue samples, we also observed a significant (P < 0.05) induction in the protein level of mature IL-1β in the SMVT-icKO mice compared with their control littermates (Fig. 4C). Similar changes in the level of expression of the different inflammasomes described above were also observed in the small intestine (jejunum) of the SMVT-icKO mice (data not shown).
Role of the Gut Microbiota in the Development of Intestinal Inflammation in the SMVT-icKO Mice: Effect of Treatment with Broad-Spectrum Antibiotics
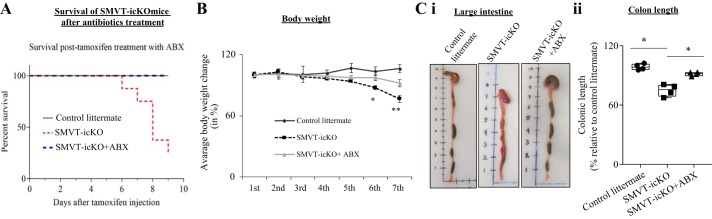
In this study, we examined the contribution of gut microbiota in the development of intestinal inflammation in the SMVT-icKO mice. For that, we administrated (in the drinking water) a cocktail of broad-spectrum antibiotics [ampicillin, vancomycin, neomycin sulfate, and metronidazole (37, 53)] to the SMVT-icKO mice. The results showed that such a treatment prevents death in the SMVT-icKO mice during the experimental period (Fig. 5A) and leads to a significant increase in body weight (Fig. 5B) and colonic length (Fig. 5C) compared with SMVT-icKO mice that were not treated with the antibiotics. Furthermore, no bloody diarrhea and a well-formed fecal pellet were observed in the SMVT-icKO treated with antibiotics.
Fig. 5.
Phenotype of sodium-dependent multivitamin transporter (SMVT)-inducible, conditional knockout (icKO) mice following administration of antibiotics (ABX). A: Kaplan-Meier survival curve for intestine-specific SMVT-icKO mice after rescue with antibiotics treatment (n = 8). Blue dotted line indicates SMVT-icKO mice treated with antibiotic and red dotted line is for nontreated SMVT-icKO mice. Black solid line is for control littermates. B: plot showing clear recovery from weight loss of antibiotic-treated SMVT-icKO mice compared with nontreated SMVT-icKO and their sex-matched control littermates starting from the 6th day after tamoxifen injection [n = 8; statistical significance (**P < 0.01) is calculated using unpaired Student’s t test]. C: representative photographs and bar diagram showing recovery in length of large intestine (i) and colon length (ii) of SMVT-icKO mice after administration of antibiotic cocktail. Colonic length of each group of mice is presented as box and dot-whisker plots from four separate sets of mice, and statistical significance (*P < 0.05) is calculated with the Mann-Whitney test.
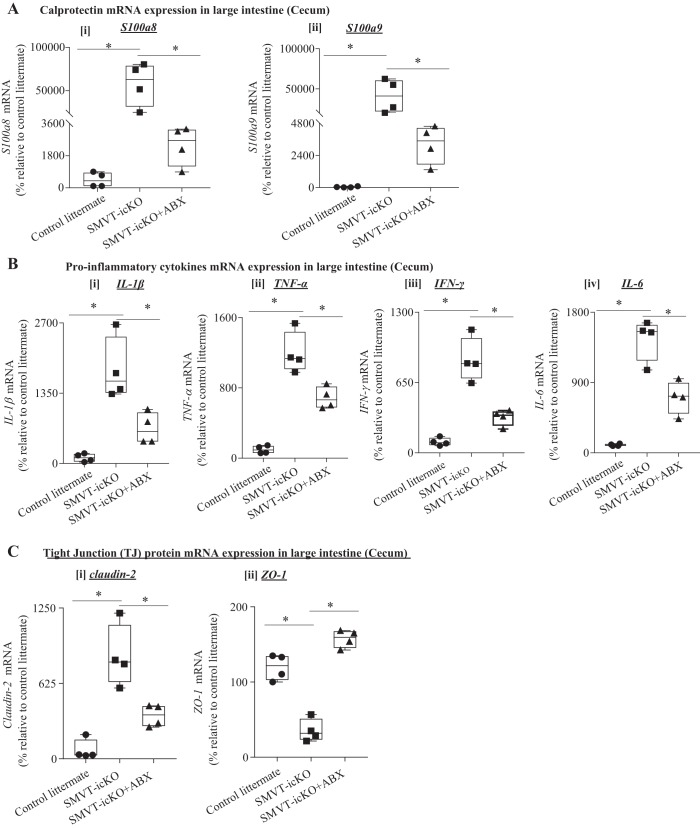
In other studies, we examined the effect of treating the SMVT-icKO mice with antibiotics on level of expression of calprotectin (S100a8 and S100a9) in the cecum and observed a significant (P < 0.05 for both) reduction in its level compared with the untreated SMVT-icKO mice (Fig. 6A; similar changes were observed in the small intestine; data not shown). In addition, the antibiotic-treated SMVT-icKO mice showed a significant (P < 0.05 for all) reduction in the level of expression of the proinflammatory cytokines IL-1β, TNF-α, IFN-γ, and IL-6 in the cecum compared with the untreated SMVT-icKO mice (Fig. 6B); again, similar changes were seen in the small intestine (jejunum; data not shown).
Fig. 6.
Effect of antibiotic (ABX) administration to sodium-dependent multivitamin transporter (SMVT)-inducible, conditional knockout (icKO) on level of expression of calprotectin, proinflammatory cytokines, and tight-junction proteins in the cecum. A: level of mRNA expression of calprotectin genes [S100a8 (i) and S100a9 (ii)] in cecum of antibiotic-treated and nontreated SMVT-icKO mice as well as their control littermates. B: level of mRNA expression of proinflammatory cytokines [IL-1β (i), TNF-α (ii), IFN-γ (iii), and IL-6 (iv)] in the cecum of antibiotic-treated and nontreated SMVT-icKO mice and their control littermates. C: level of mRNA expression of tight-junction (TJ) proteins [claudin-2 (i), and ZO-1 (ii)] in the cecum of antibiotic-treated and nontreated SMVT-icKO mice as well as their control littermates. mRNA levels were determined by RT-qPCR, and data were normalized relative to GAPDH, as described in methods. All RT-qPCR data are presented as box and dot-whisker plots from four separate sets of mice, and statistical significance (*P < 0.05) is calculated with the Mann-Whitney test. ZO, zonula occludens.
We also examined the effect of treatment of the SMVT-icKO mice with antibiotics on the level of mRNA expression of the TJ proteins claudin-2 and ZO-1 in the cecum. The results showed a clear normalization in level of mRNA expression of both proteins compared with untreated SMVT-icKO mice (Fig. 6C); similar findings were observed in the small intestine (jejunum; data not shown).
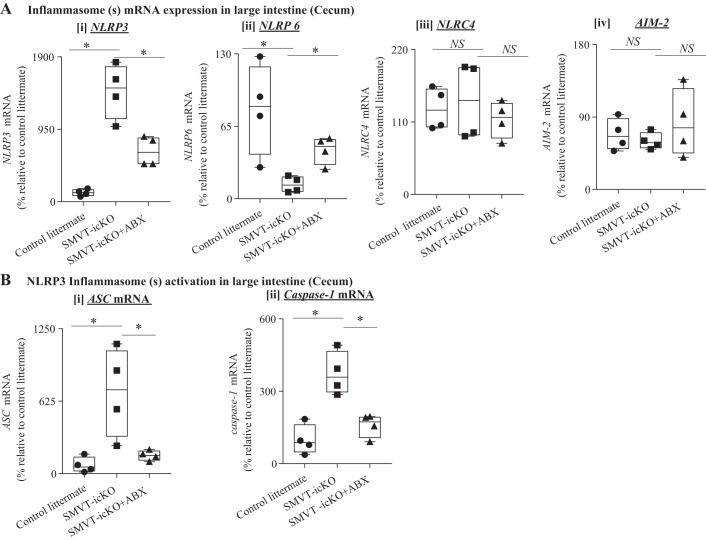
Finally, the effect of treating the SMVT-icKO mice with antibiotics on the level of expression of inflammasomes was also examined, with the results showing a significant normalization in levels of NLRP3 and NLRP6 in the cecum of the antibiotic-treated compared with the untreated SMVT-icKO (Fig. 7A). We also observed a significant normalization in the level of expression of the NLRP3 adaptor protein ASC and that of caspase-1 following treatment of the SMVT-icKO mice with antibiotics (Fig. 7B); similar results were seen in the small intestine (jejunum; data not shown).
Fig. 7.
Effect of antibiotics administration to sodium-dependent multivitamin transporter (SMVT)-inducible, conditional knockout (icKO) mice on level of expression of inflammasome(s). A: level of mRNA expression of nucleotide-binding domain and leucine-rich repeat pyrin 3 domain (NLRP3) (i), nucleotide-binding domain–like receptor family pyrin domain containing 6 (NLRP6) (ii), nucleotide-binding domain and leucine-rich repeat family Caspase activation and recruitment domain containing 4 (NLRC4) (iii), and absent in melanoma (AIM)-2 (iv) in the cecum of antibiotic-treated and nontreated SMVT-icKO mice as well as their control littermates. B: effect of antibiotics administration on the activation of NLRP3 inflammasome in cecum of SMVT-icKO mice. Level of mRNA expression of apoptosis-associated speck-like protein containing a COOH-terminal caspase-recruitment domain (ASC) (i) and caspase-1 (ii) in cecum of antibiotic-treated and nontreated SMVT-icKO mice as well as their control littermates. All data are presented as box and dot-whisker plots from four separate sets of mice, and statistical significance (*P < 0.05) is calculated with Mann-Whitney test. NS, not significant.
DISCUSSION
As mentioned earlier, intestinal absorption of biotin occurs via a carrier-mediated process that involves the SMVT system. This transporter is the only uptake system involved in the absorption of this vitamin in the gut, as shown by studies utilizing a gene-specific siRNA knockdown approach (6) as well as embryonic KO of the intestinal SMVT (SMVT-KO) in mice (15). In working with the latter model, we unexpectedly observed that all the SMVT-KO animals also developed spontaneous gut inflammation and died within 6–7 wk after birth (15, 44). The latter has limited our ability to further characterize the observed inflammation and the role of gut microbiota in its development, especially in fully developed adult intestine. To overcome this limitation, we generated and characterized an intestinal-specific SMVT-KO (SMVT-icKO) model in adult mice using the tamoxifen induction method (12), then used these animals to address these issues. First, we validated the specificity of the intestinal SMVT-KO in this model by demonstrating drastic reduction in expression (and function) of SMVT in the intestine of the SMVT-icKO mice but not in other tissues. The phenotype of these animals showed similarity to what we have observed before with the embryonic SMVT-KO model (15, 44), in that a significant reduction body weight and in biotin status was observed in the SMVT-icKO mice compared with their control littermates. Additionally, there was shortening of the colon and a marked increase in neutrophil infiltration of the gut mucosa developing histological changes reminiscent of that seen in ulcerative colitis. Similarly, levels of proinflammatory cytokines (IL-1β, TNF-α, IFN-γ and IL-6) were markedly increased in the intestine of the SMVT-icKO mice. In addition, an increase in intestinal permeability together with changes in level of expression of important TJ proteins (reduction in level of expression of the tight TJ protein ZO-1 and induction in the level of expression of the leaky TJ protein claudin-2) were observed in the SMVT-icKO mouse compared with their control littermates. These findings are similar to those observed previously with the embryonic intestinal-specific SMVT-KO model.
With the new SMVT-icKO model, we also observed a significant induction in level of expression of calprotectin, a marker of intestinal inflammation and neutrophil infiltration (15, 44). Using the SMVT-icKO model, we then examined possible involvement of the NF-κB pathway in mediating the inflammation observed in the SMVT-icKO mouse intestine. Clear evidence for involvement of this pathway was obtained as indicated by the increase in the ratio of the phosphorylated (active) form of NF-κB compared with its total level (which was not changed) in the intestine of the SMVT-icKO mice compared with their control littermates. The role of inflammasomes was then examined, with the results showing a significant induction in the level of expression of the NLRP3 inflammasome (as well as its adaptor protein ASC and its downstream target caspase-1) in the intestine of the SMVT-icKO mice compared with their control littermates. The level of the NLRP6 inflammasomes, on the other hand, was significantly reduced in the SMVT-icKO mice compared with controls, and no change in level of expression of the NLRC4 and AIM-2 inflammasomes was observed between the two groups. The increase in level of expression of the NLRP-3 inflammasome (18, 30) and the reduction in level of expression of the NLRP-6 inflammasome is similar to what has been reported in the intestine of patients with IBD (3, 13, 52).
A role for gut microbiota in the development of intestinal inflammation has been well documented (37, 53). Thus, we investigated whether gut microbiota also play a role in the development of inflammation in the intestine of the SMVT-icKO mouse model. The use of adult SMVT-icKO mice to address this issue is appropriate since gut microbiota in mice reaches a stable homeostatic state at 8 wk onward (25). Thus, a cocktail of antibiotics (in the drinking water) was administered to 16-wk-old SMVT-icKO mice followed by determination of different phenotypic and intestinal parameters. The results showed that antibiotic administration to the SMVT-icKO mice prevents early death, improves body weight as well as colonic length, and reduces the level of intestinal calprotectin compared with untreated SMVT-icKO animals. Similarly, a marked normalization in the level of proinflammatory cytokines, the NLRP-3 and NLRP6 inflammasomes, as well as in the level of expression of TJ proteins was observed in the antibiotics treated compared with untreated SMVT-icKO mice. These findings suggest that gut microbiota plays a role in the development of inflammation in the intestine of SMVT-icKO mice.
In summary, our findings in the current investigation establish the suitability of the SMVT-icKO mouse as a model for further characterization of intestinal inflammation associated with SMVT intestinal deletion in adulthood and demonstrate the involvement of the NF-κB mediated pathway and inflammasomes as well as gut microbiota in the development of this inflammation.
GRANTS
This study was supported by grants from the National Institutes of Health (DK-58057, DK-56061, and AA-018071 to H. M. Said; TR-001415 to J. Skupsky; AG-045216 to A. Agrawal) and the Department of Veterans Affairs (Merit I01BX001142 to H. M. Said and CDA 5IK2BX003518 to J. Skupsky).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S., A.A., and H.M.S. conceived and designed research; S.S., J.S., R.K., K.C., and N.W.L. performed experiments; S.S., J.S., R.K., K.C., N.W.L., A.A., and H.M.S. analyzed data; S.S., J.S., N.W.L., A.A., and H.M.S. interpreted results of experiments; S.S., J.S., R.K., K.C., N.W.L., and A.A. prepared figures; S.S., J.S., N.W.L., and H.M.S. drafted manuscript; S.S., J.S., R.K., K.C., N.W.L., A.A., and H.M.S. edited and revised manuscript; S.S., J.S., R.K., K.C., N.W.L., A.A., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Abad-Lacruz A, Fernandez-Bañares F, Cabre E, Gil A, Esteve M, Gonzalez-Huix F, Xiol X, Gassull MA. The effect of total enteral tube feeding on the vitamin status of malnourished patients with inflammatory bowel disease. Int J Vitam Nutr Res 58: 428–435, 1988. [PubMed] [Google Scholar]
- 2.Agrawal S, Agrawal A, Said HM. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am J Physiol Cell Physiol 311: C386–C391, 2016. doi: 10.1152/ajpcell.00141.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alipour M, Zaidi D, Valcheva R, Jovel J, Martínez I, Sergi C, Walter J, Mason AL, Wong GK, Dieleman LA, Carroll MW, Huynh HQ, Wine E. Mucosal barrier depletion and loss of bacterial diversity are primary abnormalities in paediatric ulcerative colitis. J Crohn’s Colitis 10: 462–471, 2016. doi: 10.1093/ecco-jcc/jjv223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med 263: 591–596, 2008. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 5.Báez-Saldaña A, Díaz G, Espinoza B, Ortega E. Biotin deficiency induces changes in subpopulations of spleen lymphocytes in mice. Am J Clin Nutr 67: 431–437, 1998. doi: 10.1093/ajcn/67.3.431. [DOI] [PubMed] [Google Scholar]
- 6.Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: role of the SMVT system. Am J Physiol Gastrointest Liver Physiol 285: G73–G77, 2003. doi: 10.1152/ajpgi.00059.2003. [DOI] [PubMed] [Google Scholar]
- 7.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183: 787–791, 2009. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandl K, Rutschmann S, Li X, Du X, Xiao N, Schnabl B, Brenner DA, Beutler B. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc Natl Acad Sci USA 106: 3300–3305, 2009. doi: 10.1073/pnas.0813036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burkholder PR, McVeigh I. Synthesis of vitamins by intestinal bacteria. Proc Natl Acad Sci USA 28: 285–289, 1942. doi: 10.1073/pnas.28.7.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee NS, Kumar CK, Ortiz A, Rubin SA, Said HM. Molecular mechanism of the intestinal biotin transport process. Am J Physiol 277: C605–C613, 1999. doi: 10.1152/ajpcell.1999.277.4.C605. [DOI] [PubMed] [Google Scholar]
- 11.Cowan MJ, Wara DW, Packman S, Ammann AJ, Yoshino M, Sweetman L, Nyhan W. Multiple biotin-dependent carboxylase deficiencies associated with defects in T-cell and B-cell immunity. Lancet 314: P115–P118, 1979. doi: 10.1016/S0140-6736(79)90002-3. [DOI] [PubMed] [Google Scholar]
- 12.el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39: 186–193, 2004. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 13.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145: 745–757, 2011. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989. [PubMed] [Google Scholar]
- 15.Ghosal A, Lambrecht N, Subramanya SB, Kapadia R, Said HM. Conditional knockout of the Slc5a6 gene in mouse intestine impairs biotin absorption. Am J Physiol Gastrointest Liver Physiol 304: G64–G71, 2013. doi: 10.1152/ajpgi.00379.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosal A, Subramanian VS, Said HM. Role of the putative N-glycosylation and PKC-phosphorylation sites of the human sodium-dependent multivitamin transporter (hSMVT) in function and regulation. Biochim Biophys Acta 1808: 2073–2080, 2011. doi: 10.1016/j.bbamem.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41: 1012–1021, 2016. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim EH, Park MJ, Park S, Lee ES. Increased expression of the NLRP3 inflammasome components in patients with Behçet’s disease. J Inflamm (Lond) 12: 41, 2015. doi: 10.1186/s12950-015-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kung JT, Mackenzie CG, Talmage DW. The requirement for biotin and fatty acids in the cytotoxic T-cell response. Cell Immunol 48: 100–110, 1979. doi: 10.1016/0008-8749(79)90103-5. [DOI] [PubMed] [Google Scholar]
- 20.Kuroishi T. Regulation of immunological and inflammatory functions by biotin. Can J Physiol Pharmacol 93: 1091–1096, 2015. doi: 10.1139/cjpp-2014-0460. [DOI] [PubMed] [Google Scholar]
- 21.Kuroishi T, Endo Y, Muramoto K, Sugawara S. Biotin deficiency up-regulates TNF-α production in murine macrophages. J Leukoc Biol 83: 912–920, 2008. doi: 10.1189/jlb.0607428. [DOI] [PubMed] [Google Scholar]
- 22.Kuroishi T, Kinbara M, Sato N, Tanaka Y, Nagai Y, Iwakura Y, Endo Y, Sugawara S. Biotin status affects nickel allergy via regulation of interleukin-1beta production in mice. J Nutr 139: 1031–1036, 2009. doi: 10.3945/jn.108.097543. [DOI] [PubMed] [Google Scholar]
- 23.Lakhan R, Subramanian VS, Said HM. Role of microRNA-423-5p in posttranscriptional regulation of the intestinal riboflavin transporter-3. Am J Physiol Gastrointest Liver Physiol 313: G589–G598, 2017. doi: 10.1152/ajpgi.00238.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell 157: 1013–1022, 2014. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev 40: 117–132, 2016. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev 243: 152–162, 2011. doi: 10.1111/j.1600-065X.2011.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei-Leston AC, Murphy AG, Maloy KJ. Epithelial cell inflammasomes in intestinal immunity and inflammation. Front Immunol 8: 1168, 2017. doi: 10.3389/fimmu.2017.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B, Staron M, Hong F, Wu BX, Sun S, Morales C, Crosson CE, Tomlinson S, Kim I, Wu D, Li Z. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc Natl Acad Sci USA 110: 6877–6882, 2013. doi: 10.1073/pnas.1302933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu B, Yang Y, Dai J, Medzhitov R, Freudenberg MA, Zhang PL, Li Z. TLR4 up-regulation at protein or gene level is pathogenic for lupus-like autoimmune disease. J Immunol 177: 6880–6888, 2006. doi: 10.4049/jimmunol.177.10.6880. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Dong Y, Ye M, Jin S, Yang J, Joosse ME, Sun Y, Zhang J, Lazarev M, Brant SR, Safar B, Marohn M, Mezey E, Li X. The pathogenic pole of NLRP3 inflammasome activation in inflammatory bowel diseases of both mice and humans. J Crohns Colitis 11: 737–750, 2017. doi: 10.1093/ecco-jcc/jjw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther 2: 17023, 2017. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madsen CT, Sylvestersen KB, Young C, Larsen SC, Poulsen JW, Andersen MA, Palmqvist EA, Hey-Mogensen M, Jensen PB, Treebak JT, Lisby M, Nielsen ML. Biotin starvation causes mitochondrial protein hyperacetylation and partial rescue by the SIRT3-like deacetylase Hst4p. Nat Commun 6: 7726, 2015. doi: 10.1038/ncomms8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev 265: 6–21, 2015. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10: 417–426, 2002. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 35.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr 22: 221–239, 2002. doi: 10.1146/annurev.nutr.22.121101.112819. [DOI] [PubMed] [Google Scholar]
- 36.Mock D. Biotin. In: Handbook of Vitamins, edited by Rucker RB, Zempleni J, Suttie JW, McCormick DB. Boca Raton, FL: CRC, 2007, p. 361–377. [Google Scholar]
- 37.Nitzan O, Elias M, Peretz A, Saliba W. Role of antibiotics for treatment of inflammatory bowel disease. World J Gastroenterol 22: 1078–1087, 2016. doi: 10.3748/wjg.v22.i3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Keefe SJ, Ou J, Aufreiter S, O’Connor D, Sharma S, Sepulveda J, Fukuwatari T, Shibata K, Mawhinney T. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr 139: 2044–2048, 2009. doi: 10.3945/jn.109.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okabe N, Urabe K, Fujita K, Yamamoto T, Yao T, Doi S. Biotin effects in Crohn’s disease. Dig Dis Sci 33: 1495–1496, 1988. doi: 10.1007/BF01537009. [DOI] [PubMed] [Google Scholar]
- 40.Paramel Varghese G, Folkersen L, Strawbridge RJ, Halvorsen B, Yndestad A, Ranheim T, Krohg-Sørensen K, Skjelland M, Espevik T, Aukrust P, Lengquist M, Hedin U, Jansson JH, Fransén K, Hansson GK, Eriksson P, Sirsjö A. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc 5: e003031, 2016. doi: 10.1161/JAHA.115.003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reidling JC, Lambrecht N, Kassir M, Said HM. Impaired intestinal vitamin B1 (thiamin) uptake in thiamin transporter-2-deficient mice. Gastroenterology 138: 1802–1809, 2010. doi: 10.1053/j.gastro.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reidling JC, Said HM. Regulation of the human biotin transporter hSMVT promoter by KLF-4 and AP-2: confirmation of promoter activity in vivo. Am J Physiol Cell Physiol 292: C1305–C1312, 2007. doi: 10.1152/ajpcell.00360.2006. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Melendez R, Zempleni J. Regulation of gene expression by biotin (review) J Nutr Biochem 14: 680–690, 2003. doi: 10.1016/j.jnutbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Sabui S, Bohl JA, Kapadia R, Cogburn K, Ghosal A, Lambrecht NW, Said HM. Role of the sodium-dependent multivitamin transporter (SMVT) in the maintenance of intestinal mucosal integrity. Am J Physiol Gastrointest Liver Physiol 311: G561–G570, 2016. doi: 10.1152/ajpgi.00240.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Said HM. Biotin: biochemical, physiological and clinical aspects. Subcell Biochem 56: 1–19, 2012. doi: 10.1007/978-94-007-2199-9_1. [DOI] [PubMed] [Google Scholar]
- 46.Said HM. Cell and molecular aspects of human intestinal biotin absorption. J Nutr 139: 158–162, 2009. doi: 10.3945/jn.108.092023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin S. Biotin uptake by human colonic epithelial NCM460 cells: a carrier-mediated process shared with pantothenic acid. Am J Physiol Cell Physiol 275: C1365–C1371, 1998. doi: 10.1152/ajpcell.1998.275.5.C1365. [DOI] [PubMed] [Google Scholar]
- 48.Said HM, Redha R. Biotin transport in rat intestinal brush-border membrane vesicles. Biochim Biophys Acta 945: 195–201, 1988. doi: 10.1016/0005-2736(88)90482-8. [DOI] [PubMed] [Google Scholar]
- 49.Schwantje M, de Sain-van der Velden M, Jans J, van Gassen K, Dorrepaal C, Koop K, Visser G. Genetic defect of the sodium-dependent multivitamin transporter: a treatable disease, mimicking biotinidase deficiency. JIMD Rep 48: 11–14, 2019. doi: 10.1002/jmd2.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am J Physiol Cell Physiol 296: C663–C671, 2009. doi: 10.1152/ajpcell.00396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugahara H, Odamaki T, Fukuda S, Kato T, Xiao JZ, Abe F, Kikuchi J, Ohno H. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci Rep 5: 13548, 2015. doi: 10.1038/srep13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y, Zhang M, Chen CC, Gillilland M III, Sun X, El-Zaatari M, Huffnagle GB, Young VB, Zhang J, Hong SC, Chang YM, Gumucio DL, Owyang C, Kao JY. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology 144: 1478–1487.e8, 2013. doi: 10.1053/j.gastro.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ubeda C, Pamer EG. Antibiotics, microbiota, and immune defense. Trends Immunol 33: 459–466, 2012. doi: 10.1016/j.it.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Velázquez-Arellano A. From an inborn error patient to a search for regulatory meaning: a biotin conducted voyage. Mol Genet Metab 87: 194–197, 2006. doi: 10.1016/j.ymgme.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, Geddes BJ, Briskin M, DiStefano PS, Bertin J. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem 277: 29874–29880, 2002. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front Immunol 9: 1298, 2018. doi: 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiedmann S, Rodriguez-Melendez R, Ortega-Cuellar D, Zempleni J. Clusters of biotin-responsive genes in human peripheral blood mononuclear cells. J Nutr Biochem 15: 433–439, 2004. doi: 10.1016/j.jnutbio.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Wolf B. Disorders of biotin metabolism. In: The Metabolic and Molecular Bases of Inherited Disease, edited by Scriver CR, Beaudet AL, Aly WS, Valle D, Childs B, Kinzler KW, and Vogelstein B. New York: McGraw-Hill Medical Publishing Division, 2001, p. 3935–3396. [Google Scholar]
- 59.Yang B, Feng L, Wang F, Wang L. Enterohemorrhagic Escherichia coli senses low biotin status in the large intestine for colonization and infection. Nat Commun 6: 6592, 2015. doi: 10.1038/ncomms7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao C, Chou J, Wang T, Zhao H, Zhang B. Pantothenic acid, vitamin C, and biotin play important roles in the growth of Lactobacillus helveticus. Front Microbiol 9: 1194, 2018. doi: 10.3389/fmicb.2018.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhen Y, Zhang H. NLRP3 inflammasome and inflammatory bowel disease. Front Immunol 10: 276, 2019. doi: 10.3389/fimmu.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]