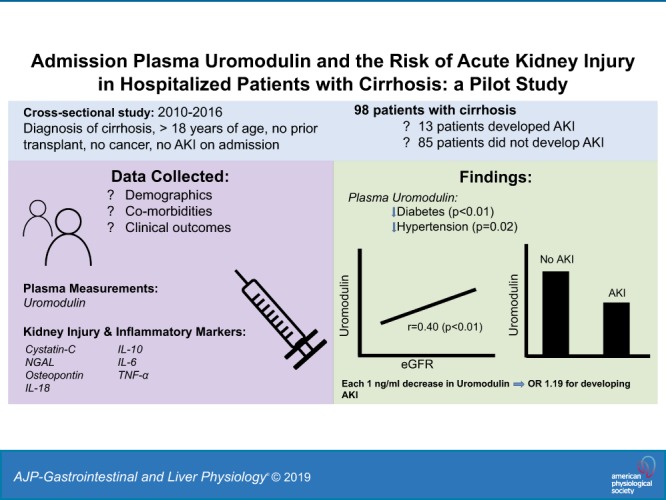
Keywords: AKI, cirrhosis, incident AKI, kidney biomarker, uromodulin
Abstract
Acute kidney injury (AKI) is a common complication in hospitalized patients with cirrhosis. Uromodulin, a protein uniquely produced by the kidney and released both in the urine and circulation, has been shown to regulate AKI and is linked to tubular reserve. Although low levels of urine uromodulin are associated with AKI after cardiac surgery, it is unclear whether circulating uromodulin can stratify the risk of AKI, particularly in a susceptible population such as hospitalized patients with cirrhosis. Thus, we investigated whether plasma uromodulin measured at the time of admission is associated with subsequent hospital-acquired AKI (defined by a rise in serum creatinine >0.3mg/dL within 48 h or ≥ 1.5 times baseline) in patients with cirrhosis. A total of 98 patients [mean age 54 yr, Model for Endstage Liver Disease Sodium (MELD-Na) score 19, and baseline creatinine of 0.95 mg/dL] were included, of which 13% (n = 13) developed AKI. Median uromodulin levels were significantly lower in patients who developed AKI compared with patients who did not (9.30 vs. 13.35 ng/mL, P = 0.02). After adjusting for age, sex, diabetes, hypertension, albumin, and MELD-Na score as covariates on multivariable logistic regression, uromodulin was independently associated with AKI [odd ratios of 1.19 (95% confidence interval 1.02, 1.37; P = 0.02)]. Lower uromodulin levels on admission are associated with increased odds of subsequent AKI in hospitalized patients with cirrhosis. Further studies are needed to better understand the role of uromodulin in the pathogenesis and as a predictive biomarker of AKI in this population.
NEW & NOTEWORTHY In this study, we found that admission plasma uromodulin levels are significantly lower in patients who developed subsequent acute kidney injury (AKI) during their hospital stay compared with patients who did not. Additionally, uromodulin is independently associated with AKI development after adjusting for clinically relevant parameters such as age, sex, diabetes, hypertension, severity of cirrhosis, and kidney function. To our knowledge, this is the first study linking plasma uromodulin with AKI development in patients with cirrhosis.
INTRODUCTION
Acute kidney injury (AKI) is a common complication that occurs in patients with decompensated cirrhosis (13) and is included in the definition of acute on chronic liver failure, a syndrome characterized by acute decompensation and extra-hepatic organ failures driven by excessive systemic inflammation (30, 32). In patients admitted with decompensated cirrhosis, AKI is present in 20% at the time of admission to the hospital (19) and develops in up to 19% after admission (19). Development of in-hospital AKI is independently associated with high short-term mortality (44) and poor outcomes after liver transplantation (31). AKI is also associated with progressive loss of kidney function, which can lead to future episodes of AKI (i.e., AKI on top of chronic kidney disease); thus, it contributes to significant morbidity (21). To improve outcomes related to hospital-acquired AKI in the setting of cirrhosis, identifying patients at high risk is essential. A reliable approach for AKI prediction and risk stratification could be valuable in this context, as it may allow for timely preventative and possible therapeutic care (2) and appropriate allocation of hospital resources.
Uromodulin, also known as Tamm-Horsfall protein, is a glycoprotein exclusively expressed in the thick ascending limb and distal convoluted tubule of the kidneys. This protein is excreted as the most abundant urinary protein in healthy humans (10) but is also released by the kidney into the circulation through the peritubular capillary network (8, 28). Because of its unique production in thick ascending limb cells, uromodulin, unlike other conventional markers for kidney function, is linked to tubular health and reserve (23, 29, 33, 34, 39). In fact, measurement of uromodulin in the urine and the serum has been positively correlated with kidney function (6, 23, 33, 37, 41). Furthermore, a protective role of uromodulin in experimental models of AKI has been demonstrated (11, 28). This positive effect against injury occurs through an immunomodulatory function of uromodulin that downregulates inflammation both in the kidney and systemically. Therefore, it is not surprising to observe emerging interest in the value of measuring uromodulin to prognosticate kidney disease and/or cardiovascular events (5, 14, 15, 23, 29).
Indeed, many recent studies linked low levels of urinary or circulating uromodulin with the risk of chronic kidney disease progression and mortality (5, 6, 14, 16, 23, 39). In AKI, available data are limited to cardiac surgery, showing that low urine uromodulin is associated with higher odds of AKI and higher peak serum creatinine after cardiac surgery (4, 15). However, it may be even more impactful to determine whether the prognostic value of uromodulin could be extended to other clinical settings. Furthermore, there is no available data on the value of measuring circulating uromodulin in predicting the risk of AKI. This could be quite relevant because serum or plasma measurements may be easier to handle and store and could be less prone to enzymatic degradation by proteases compared with the urine (46). Additionally, serum or plasma levels may be easier to interpret, as compared with urinary levels whereby a correction for urinary concentration may be required (27). Thus, the primary objective of this study was to evaluate whether admission plasma uromodulin is associated with AKI development within 7 days of hospitalization in cirrhotic patients. Other relevant biomarkers were also assessed and compared with the performance of uromodulin. Additionally, given the link of uromodulin and inflammation in patients without cirrhosis (22, 23, 39), we evaluated the association of uromodulin with inflammatory cytokines relevant to the pathophysiology of AKI and cirrhosis (1), such as tumor necrosis α (TNF-α), interleukin (IL)-6, and IL-10.
MATERIALS AND METHODS
Study design.
The study population comprised hospitalized cirrhotic patients who were nonconsecutively enrolled in a study evaluating urea metabolism (17). The enrollment period was from September 2010 to April 2016 and included patients who were nonelectively admitted to Indiana University Hospital. The diagnosis of cirrhosis was made based on clinical parameters involving laboratory tests, endoscopic or radiologic evidence of cirrhosis, evidence of decompensation (hepatic encephalopathy, ascites, variceal bleeding, jaundice), and liver biopsy if available. Inclusion criteria included a known diagnosis of cirrhosis, age ≥ 18 yr, and plasma samples obtained within 24 h of admission. Exclusion criteria included prior kidney or liver transplant, history of active cancer, AKI on admission, hemodialysis at the time of admission, and confirmed pregnancy. Patients received written information about the study and a signed consent form. All study protocols were submitted to and approved by the institutional review board at our center.
Data collection.
For patients who met inclusion criteria, data on etiology of cirrhosis (hepatitis C, alcohol, nonalcoholic steatohepatitis, hepatitis C with current alcohol use, and other), demographics, presence of infection on admission, history of complications related to cirrhosis (ascites, hepatic encephalopathy, history of portal hypertension related bleed), history of diabetes, history of hypertension, baseline creatinine, baseline estimated glomerular filtration rate (eGFR) calculated by Chronic Kidney Disease Epidemiology Collaboration creatinine equation (24), admission laboratory data (creatinine, eGFR, albumin, sodium, total bilirubin, internal normalized ratio, and white blood cell count), and mean arterial pressure were collected. Additionally, the severity of cirrhosis was recorded on admission through calculation of the Model for End-stage Liver Disease Sodium (MELD-Na) (12) and Child Turcotte Pugh (CTP) (35) scores. The MELD-Na score includes total bilirubin, international normalized ratio, creatinine, and sodium. The CTP score includes total bilirubin, albumin, international normalized ratio, and severity of ascites and hepatic encephalopathy.
Definition of AKI.
AKI was defined as a rise in creatinine of 0.3 mg/dL or 50% increase from baseline, as recommended by Kidney Disease Improving Global Outcomes (18). Baseline creatinine was defined per the Kidney Disease Improving Global Outcomes (18) as the availability of outpatient serum creatinine within the past year. When more than one creatinine value was available, the closest to admission time to the hospital was used. The median [interquartile range (IQR)] time between baseline creatinine and admission was 15 days (3, 42). In patients who did not have a baseline creatinine (n = 10), the admission creatinine was used. Furthermore, to ensure that these 10 patients had stable kidney function at the time of admission (i.e., did not already have AKI), we excluded any of them whose serum creatinine changed by > 0.30 mg/dL within 48 h from the time of admission, similar to what Belcher et al. (3) used previously .
Sample collection and biomarker measurement.
Blood samples (30 mL) were obtained via venipuncture or drawn from an established peripheral or central line. Samples were immediately refrigerated and then centrifuged for 10 min at −4°C for plasma. Aliquots of 0.5 mL of supernatant were subsequently stored within 6 h of collection in cryovials at −80°C. For the purpose of this investigation, in addition to uromodulin, the following kidney biomarkers and inflammatory cytokines were measured at the time of admission: cystatin-C (CysC), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), osteopontin, IL-18, IL-10, IL-6, and TNF-α. One aliquot was needed for uromodulin, CysC, NGAL, KIM-1, osteopontin, IL-18, IL-10, IL-6, and TNF-α. Samples were stored for a median (IQR) of 1,883 (1,669–2,164) days until biomarker measurements were made. All biomarkers were measured from frozen aliquots that did not undergo any freeze-thaw cycles. Laboratory measurements were performed by personnel blinded to patient information at the Multiplex Analysis Core at the Indiana University Melvin and Bren Simon Cancer Center.
Cytokine and kidney biomarker concentrations in the plasma were assayed using MilliporeSigma multiplex kits (Human kidney Injury Panel 4-2-Plex, Human Kidney Injury Panel 5-2-Plex, Human Cytokine/Chemokine Panel 6-Plex, and Human Cytokine 5-Plex). The kits contain spectrally distinct antibody-immobilized beads (2 or 5 bead sets specifically for the above biomarkers), cytokine standard cocktail, cytokine quality control I and II, detection antibody cocktail, streptavidin-phycoerythrin, assay buffer, wash buffer, serum matrix, and a microtiter plate. The assay was performed according to the manufacturer’s protocol. After preparation, samples were processed (50 beads per bead set in 25 microliter sample size) on a Bio-Plex 200 System with High Throughput Fluidics (HTF) Multiplex Array System (Bio-Rad Laboratories, Hercules, CA). All samples were run in duplicate. The detection ranges for the biomarkers were as follows: osteopontin 1.172–1,284.08 ng/mL; KIM-1 0.18–223.37 ng/mL; NGAL 0.05–48.36 ng/mL; uromodulin 0.05–50.00 ng/mL; IL-18 8.04–24,703.45 pg/mL, and CysC 0.06–61.03 ng/mL. The detection ranges for inflammatory biomarkers were as follows: IL-10 3.17–10,021.54 pg/mL; IL-6 3.21–9,872.59 pg/mL; and TNF-α 3.18–10,031.73 pg/mL.
Statistical analysis.
Patients were stratified based on the development of AKI or not, and their characteristics on admission were compared. Continuous variables were presented as means ± SD and median ± IQR where deemed appropriate. Categorical variables were presented as frequencies and percentages. Bivariate differences across groups with respect to categorical variables were analyzed using chi-square tests, using Fisher’s Exact tests when cell counts were small, whereas continuous variables were analyzed using Wilcoxon rank-sum tests when data were nonlinear and Student’s t tests when data were linear. Correlations between uromodulin with clinical variables and inflammatory cytokines were analyzed through Spearman nonparametric correlations because of data skewness. A two-sided nominal P value ≤ 0.05 was considered significant. Multivariable logistic regression models were used to determine whether uromodulin was independently associated with AKI development. Variables of clinical significance were chosen for the multivariable model. The final list of covariates was also determined by removing variables that caused high collinearity, as accessed by Variance Inflation Factors. We reported odds ratios and their corresponding 95% confidence intervals. All analytic assumptions were verified, and all analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 160 patients with cirrhosis were enrolled during the study period. Of these, 62 patients were excluded (21 with hepatocellular cancer, 5 on hemodialysis, and 36 with AKI on admission), leaving 98 patients for inclusion in this study analysis.
Patient population.
The mean age, MELD-Na score, and CTP score were 54.3 ± 8.4 yr, 19.4 ± 6.2, and 9.4 ± 2.1, respectively. The mean baseline and admission creatinine were 0.95 ± 0.34 and 0.99 ± 0.39 mg/dL, respectively. Among those patients who did not have a baseline creatinine (n = 10), the mean admission creatinine was 0.83 mg/dL and the mean change of creatinine within 48 h was 0.13 mg/dL. Of these 10 patients without prior baseline creatinine, only 1 patient developed AKI on day 4 of hospitalization. The median (IQR) of uromodulin was 12.71 ng/mL (8.31–18.31 ng/mL). Nonalcoholic steatohepatitis (35%), alcohol (22%), and hepatitis C with concurrent alcohol (17%) were the most common etiologies of cirrhosis. The most common reasons for admission were hepatic encephalopathy (36%), infection (26%), ascites and anasarca management (16%), and portal hypertension-related bleeding (11%).
Development of AKI.
Thirteen patients (13%) developed AKI and 85 patients did not develop AKI. There were no statistical differences in age, gender, race, etiology of cirrhosis, history of complications related to cirrhosis, and history of diabetes or hypertension between the two groups at the time of admission (Table 1). Additionally, there were no significant differences in baseline creatinine and eGFR, admission creatinine and eGFR, admission mean arterial pressure, presence of infection on admission, admission white blood cell count, and admission MELD-Na and CTP scores between the two groups. However, patients who developed AKI had a significantly lower albumin compared with those who did not develop AKI (2.5 vs. 2.9 g/dL, P = 0.02). Median uromodulin levels were found to be significantly lower in patients who developed AKI compared with patients who did not develop AKI [9.30 (6.31–13.22) vs. 13.35 (8.55–18.96) ng/mL, P = 0.02] (Fig. 1).
Table 1.
Baseline patient demographics stratified by AKI status
| No AKI Development | AKI Development | P | |
|---|---|---|---|
| n | 85 | 13 | |
| Age | 54.02 (8.38) | 55.77 (8.32) | 0.49 |
| Gender, n (%) male | 46 (54) | 6 (46) | 0.78 |
| Race, n (%) white | 77 (91) | 12 (92) | 0.52 |
| Etiology of cirrhosis, n (%) | |||
| HCV | 10 (12) | 3 (23) | |
| HCV + alcohol | 16 (19) | 1 (8) | |
| NASH | 29 (34) | 5 (38) | 0.75 |
| Alcohol | 19 (22) | 3 (23) | |
| Other | 11 (13) | 1 (8) | |
| Infection on admission, n (%) | 18 (21) | 5 (38) | 0.18 |
| History of ascites, n (%) | 61 (72) | 12 (92) | 0.17 |
| History of HE, n (%) | 58 (68) | 11 (85) | 0.19 |
| History of PHTN bleed, n (%) | 13 (15) | 1 (8) | 0.69 |
| DM, n (%) | 35 (41) | 6 (46) | 0.77 |
| HTN, n (%) | 29 (34) | 5 (38) | 0.76 |
| MAP | 84.08 (12.90) | 80.38 (11.78) | 0.33 |
| Baseline creatinine, mg/dL | 0.94 (0.35) | 0.99 (0.30) | 0.60 |
| Baseline eGFR, mL·min−1·1.73 m−2* | 86.06 (26.86) | 76.77 (21.34) | 0.23 |
| Admit creatinine, mg/dL | 0.98 (0.38) | 1.08 (0.41) | 0.39 |
| Admit eGFR, mL·min−1·1.73 m−2* | 82.82 (27.01) | 73.62 (26.82) | 0.26 |
| Admit sodium, mmol/L | 133.56 (12.00) | 132.54 (6.62) | 0.65 |
| Admit albumin, g/dL | 2.94 (0.62) | 2.51 (0.57) | 0.02 |
| Admit total bilirubin, mg/dL | 4.19 (4.95) | 2.84 (2.60) | 0.14 |
| Admit WBC, 109/L | 6.98 (4.50) | 7.92 (3.84) | 0.48 |
| Admit INR | 1.65 (0.58) | 1.61 (0.57) | 0.84 |
| CTP score | 9.31 (2.10) | 9.77 (1.88) | 0.45 |
| CTP class, n (%) | |||
| A | 10 (12) | 0 (0) | |
| B | 33 (39) | 6 (46) | 0.57 |
| C | 42 (49) | 7 (54) | |
| MELD-sodium score | 19.27 (6.22) | 20.23 (6.37) | 0.61 |
AKI, acute kidney injury; CTP, Child Turcotte Pugh; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HCV, hepatitis C; HE, hepatic encephalopathy; HTN, hypertension; INR, international normalized ratio; MAP, mean arterial pressure; MELD, Model for Endstage Liver Disease; NASH, nonalcoholic steatohepatitis; PTHN, portal hypertension; WBC, white blood cell.
Based on Chronic Kidney Disease Epidemiology Collaboration creatinine equation (24).
Fig. 1.
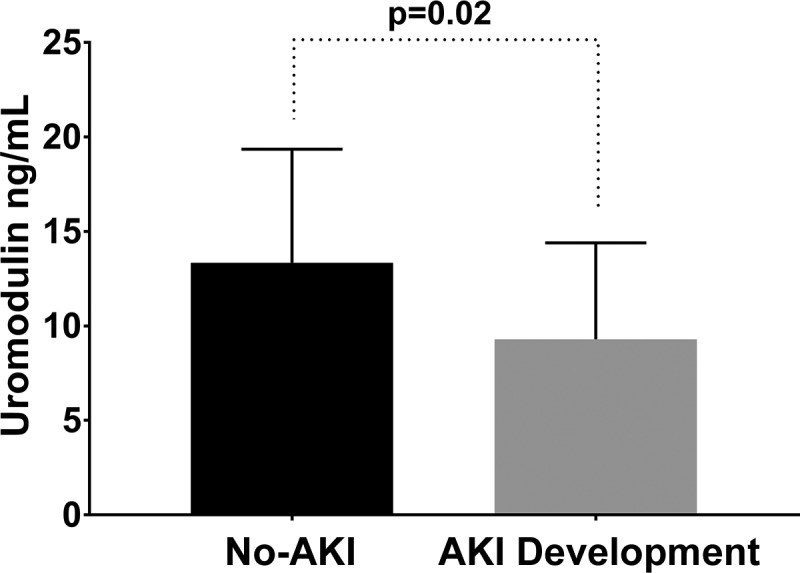
Comparison of uromodulin stratified by acute kidney injury (AKI) status. Bar graph showing the median interquartile ranges of uromodulin for patients who developed AKI compared with patients who did not.
The median time (IQR) for AKI development was 4 (2, 5) hospital days, and all 13 patients were categorized as stage 1 at the time of diagnoses. Of these, 4 and 5 patients progressed to stage 2 and 3, respectively, and 4 patients subsequently required hemodialysis. The most common precipitants identified for AKI development were infection (31%), aggressive diuresis (27%), and large volume paracentesis (27%).
Clinical factors associated with uromodulin levels at admission.
There were no significant differences in uromodulin levels between race, sex, etiology of cirrhosis, and CTP classes. Patients with diabetes (n = 41) had significantly lower median uromodulin levels compared with those without diabetes [9.30 (6.89–14.91) vs. 15.45 (10.94–21.43) ng/mL, P < 0.01]. Similarly, patients with hypertension (n = 34) had a significantly lower median uromodulin level compared with patients without hypertension [9.76 (7.04–18.31) vs. 14.48 (10.20–19.31) ng/mL, P = 0.02]. Furthermore, uromodulin was significantly and positively correlated with admission eGFR (r = 0.40, P < 0.01). There were no significant correlations between uromodulin and MELD-Na score, CTP score, MAP, albumin, or white blood cell count.
Correlations between uromodulin and kidney biomarkers and inflammatory cytokines.
Uromodulin was significantly correlated inversely with CysC (r = −0.46, P < 0.01) and directly with osteopontin (r = 0.23, P = 0.03) and IL-18 (r = 0.22, P = 0.03). There were no significant correlations between uromodulin and NGAL and KIM-1 (Table 2). There were no significant correlations between uromodulin and the inflammatory cytokines, IL-10, IL-6, and TNF-α (Table 2).
Table 2.
Correlations between uromodulin and kidney biomarkers and inflammatory cytokines
| Uromodulin | P | |
|---|---|---|
| Kidney biomarkers | ||
| NGAL | −0.17 | 0.10 |
| Cystatin-C | −0.46 | <0.01 |
| KIM-1 | 0.03 | 0.76 |
| Osteopontin | 0.23 | 0.03 |
| IL-18 | 0.22 | 0.03 |
| Inflammatory cytokines | ||
| TNF-α | −0.08 | 0.41 |
| IL-10 | −0.01 | 0.90 |
| IL-6 | 0.03 | 0.77 |
IL, interleukin; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; TNF, tumor necrosis factor.
Differences in kidney biomarkers and inflammatory molecules stratified by AKI status.
Median NGAL (203.69 vs. 126.67 ng/mL, P = 0.01) and osteopontin (83.89 vs. 55.05 ng/mL P < 0.01) levels were found to be significantly higher in patients who developed AKI compared with patients who did not. There were no differences between the two groups for KIM-1, CysC, and IL-18 (Table 3). Of the inflammatory cytokines, median levels for IL-6 and TNF-α were found to be statistically higher in the AKI development group versus no AKI development group [23.76 vs. 16.24 pg/mL (P = 0.02) and 46.33 vs. 26.6 pg/mL (P < 0.01), respectively] (Table 3).
Table 3.
Comparison of kidney biomarkers and inflammatory cytokines stratified by AKI status
| No AKI Development | AKI Development | P | |
|---|---|---|---|
| n | 85 | 13 | |
| Kidney biomarkers, median (IQR) | |||
| NGAL, ng/mL | 126.67 (99.49–173.45) | 203.69 (155.53–284.98) | 0.01 |
| Cystatin C, ng/mL | 843.59 (652.42–1,144.28) | 997.08 (897.15–1,322.00) | 0.14 |
| KIM-1, ng/mL | 1.19 (0.47–2.05) | 0.97 (0.66–2.68) | 0.10 |
| Osteopontin, ng/mL | 55.05 (35.57–76.14) | 83.89 (57.25–94.85) | <0.01 |
| IL-18, pg/mL | 77.03 (46.67–171.52) | 99.94 (84.99–110.68) | 0.59 |
| Inflammatory cytokines, median (IQR) | |||
| IL-10, pg/mL | 11.27 (7.10–19.33) | 15.39 (12.21–25.45) | 0.12 |
| IL-6, pg/mL | 16.24 (8.54–24.23) | 23.76 (20.72–63.65) | 0.02 |
| TNF-α, pg/mL | 26.60 (17.79–38.08) | 46.33 (39.02–55.11) | <0.01 |
n, number of patients. AKI, acute kidney injury; IL, interleukin; IQR, interquartile range; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; TNF, tumor necrosis factor.
Association of uromodulin with AKI.
Each 1 ng/mL lower, uromodulin was associated with 19% increased odds for AKI development [1.19 (95% confidence interval 1.02, 1.37); P = 0.02] after adjusting for age, sex, diabetes, hypertension, serum albumin, and MELD-Na score. We found that none of the other kidney biomarkers were associated with AKI after similar adjustment for confounders.
DISCUSSION
In this study, we found that admission plasma uromodulin levels are significantly lower in patients who developed subsequent AKI during their hospital stay compared with patients who did not. Although other commonly used biomarkers such as NGAL and osteopontin also showed differences between the two groups, only uromodulin remained independently associated with AKI development after adjusting for clinically relevant parameters such as age, sex, diabetes, hypertension, severity of cirrhosis, and kidney function. To our knowledge, this is the first study linking plasma uromodulin with AKI development, albeit in a unique population of patients with cirrhosis.
The function of uromodulin is not fully understood; however, recent discoveries have underscored its importance as a regulatory protein for renal function and its association with functional renal reserve (5, 33, 34, 39). In the setting of AKI, the expression of uromodulin has been found to be significantly decreased at the onset of injury in experimental models, suggesting that early AKI leads to uromodulin deficiency in the kidney (10, 11). These data have been supported by findings in kidney transplant patients showing that serum uromodulin levels correlate with graft function after transplant (39). As far as uromodulin and the risk of AKI, the available data are on urine uromodulin levels in clinical studies, whereby low urine uromodulin levels have been linked to increased AKI risk after cardiac surgery (4, 15) and liver transplantation (38). Our study is the first linking circulating uromodulin measured on admission to hospital-acquired AKI. Therefore, measurement of circulating uromodulin on admission may allow for early risk assessment for timely prevention and intervention (2). Nevertheless, further prospective validation studies in larger populations are needed to validate its potential use as a theranostic tool to guide prevention and management of patients with cirrhosis.
Interestingly, we found no correlation between uromodulin and TNF-α. This is in contrast to a previous study that found associations of low urine uromodulin levels with TNF-α(22). The cause of this discrepancy is likely due to the small sample size of our population and the bias introduced by cirrhosis, itself a state of systemic inflammation (1). However, albeit weak direct associations, we found IL-18 and osteopontin to be correlated to uromodulin. IL-18 is a proinflammatory cytokine that is released by the proximal tubules in response to inflammation and kidney injury and has been linked with AKI in patients with cirrhosis (36). Osteopontin is an extracellular matrix protein involved in the inflammatory response (20) that has been found to be significantly increased in all tubular segments in AKI (45). Given its possible protective, anti-inflammatory functions and role in maintaining homeostasis (29), uromodulin’s correlation with IL-18 and osteopontin is perhaps due to a compensatory increase in response to a systemic illness (9), such as a state of cirrhosis. However, larger studies in patients with cirrhosis are required to substantiate this correlation. It is interesting to note the diverging association of NGAL, TNF-α, and IL-6 to the risk of AKI but not to uromodulin. This could be related to the strength of the association of uromodulin to the risk of AKI, whereby this link remains uniquely significant even after adjustment for potential confounders. Of course, we cannot rule out the presence of biological heterogeneity in the clinical context of cirrhosis (3, 7, 40), which could weaken the correlation of inflammatory and injury markers on admission to the risk of AKI in this small cohort.
Higher serum concentrations of CysC have been associated with AKI in patients with cirrhosis (25, 26). However, in comparison to these studies, we did not find CysC (or any of the other biomarkers except for uromodulin) to be associated with AKI. Possible reasons for these differences may be attributed to differences in study population, impact and prevalence of bacterial infections, differences in baseline values of CysC, and adjusting for known influences of CysC such as serum albumin (42). It is important to note that in regard to the latter two, our patients had a much lower baseline CysC compared with aforementioned studies and our multivariable model was adjusted for serum albumin. Furthermore, these differences may be attributable to the different assay platform for CysC. It is also not surprising to find an association between uromodulin and CysC, which reflects the known association between uromodulin levels in the urine and circulation with kidney function (41).
There are several limitations to our study. This is an exploratory study performed at a single center. The small sample size may have reduced our ability to link more variables on admission to the odds of developing subsequent AKI. Therefore, our findings will need to be validated in a larger study before generalization. It is also unclear if the findings could be extended to an outpatient setting of stable decompensated cirrhotic patients, which is also very clinically relevant given their risk for AKI (43). Furthermore, serial measurements of uromodulin may add significant insights to its usefulness in predicting the risk of AKI and will likely need to be incorporated in future studies. Lastly, although no harmful effects have been reported on the storage time for uromodulin, we cannot rule out possible degradation of the protein over time, which may lead to dilution and therefore potential misclassification.
Despite these limitations, our study also had a number of strengths. First, all patients included in the study had detailed laboratory information, which allowed for serial creatinine monitoring and discernment for AKI development during the course of the hospitalization. Furthermore, we were able to obtain baseline creatinine in 90% of our cohort, which was not available in prior studies linking low uromodulin to AKI. Lastly, we have included hard outcomes such as AKI stage 3 and hemodialysis use, which were unavailable in the aforementioned prior studies due to mild AKI.
In conclusion, in this study, we report that low plasma uromodulin levels at the time of admission are independently associated with higher odds of AKI in hospitalized patients with cirrhosis. If validated, the measurement of uromodulin could enhance our clinical decision making for risk assessment of AKI in patients with cirrhosis.
GRANTS
This study was supported by the National Institute of Diabetes Digestive and Kidney Disease Grants 1R01DK111651 (to T. M. El-Achkar), K23 DK114556 (to P. S. Garimella), and K23 DK109202 (to E. S. Orman) and Veterans Affairs Merit Award (T. M. El-Achkar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.R.P. and T.M.E.-A. conceived and designed research; K.R.P., J.E.S., and T.M.E.-A. analyzed data; K.R.P., P.S.G., E.M., and T.M.E.-A. interpreted results of experiments; K.R.P. and T.M.E.-A. prepared figures; K.R.P., J.E.S., and T.M.E.-A. drafted manuscript; K.R.P. and T.M.E.-A. edited and revised manuscript; K.R.P., P.S.G., E.M., J.E.S., M.S.G., R.E.W., M.D.A., E.S.O., L.D.N., A.P.D., N.C., and T.M.E.-A. approved final version of manuscript.
REFERENCES
- 1.Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 61: 1385–1396, 2014. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramanian G, Al-Aly Z, Moiz A, Rauchman M, Zhang Z, Gopalakrishnan R, Balasubramanian S, El-Achkar TM. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot study. Am J Kidney Dis 57: 228–234, 2011. doi: 10.1053/j.ajkd.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, Coca SG, Garcia-Tsao G, Parikh CR; TRIBE-AKI Consortium . Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 60: 622–632, 2014. doi: 10.1002/hep.26980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett MR, Pyles O, Ma Q, Devarajan P. Preoperative levels of urinary uromodulin predict acute kidney injury after pediatric cardiopulmonary bypass surgery. Pediatr Nephrol 33: 521–526, 2018. doi: 10.1007/s00467-017-3823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostom A, Steubl D, Garimella PS, Franceschini N, Roberts MB, Pasch A, Ix JH, Tuttle KR, Ivanova A, Shireman T, Kim SJ, Gohh R, Weiner DE, Levey AS, Hsu CY, Kusek JW, Eaton CB. Serum uromodulin: a biomarker of long-term kidney allograft failure. Am J Nephrol 47: 275–282, 2018. doi: 10.1159/000489095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado GE, Kleber ME, Scharnagl H, Krämer BK, März W, Scherberich JE. Serum uromodulin and mortality risk in patients undergoing coronary angiography. J Am Soc Nephrol 28: 2201–2210, 2017. doi: 10.1681/ASN.2016111162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande P, Rausa K, Turner J, Johnson M, Golestaneh L. Acute kidney injury as a causal factor in mortality associated with hepatorenal syndrome. Hepatol Int 5: 751–758, 2011. doi: 10.1007/s12072-011-9269-8. [DOI] [PubMed] [Google Scholar]
- 8.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol 304: F1066–F1075, 2013. doi: 10.1152/ajprenal.00543.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Achkar TM, Plotkin Z, Marcic B, Dagher PC. Sepsis induces an increase in thick ascending limb Cox-2 that is TLR4 dependent. Am J Physiol Renal Physiol 293: F1187–F1196, 2007. doi: 10.1152/ajprenal.00217.2007. [DOI] [PubMed] [Google Scholar]
- 10.El-Achkar TM, Wu XR. Uromodulin in kidney injury: an instigator, bystander, or protector? Am J Kidney Dis 59: 452–461, 2012. doi: 10.1053/j.ajkd.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol 295: F534–F544, 2008. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elwir S, Lake J. Current status of liver allocation in the United States. Gastroenterol Hepatol (NY) 12: 166–170, 2016. [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 48: 2064–2077, 2008. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 14.Garimella PS, Biggs ML, Katz R, Ix JH, Bennett MR, Devarajan P, Kestenbaum BR, Siscovick DS, Jensen MK, Shlipak MG, Chaves PH, Sarnak MJ. Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int 88: 1126–1134, 2015. doi: 10.1038/ki.2015.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garimella PS, Jaber BL, Tighiouart H, Liangos O, Bennett MR, Devarajan P, El-Achkar TM, Sarnak MJ. Association of preoperative urinary uromodulin with AKI after cardiac surgery. Clin J Am Soc Nephrol 12: 10–18, 2017. doi: 10.2215/CJN.02520316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garimella PS, Katz R, Ix JH, Fried LF, Kritchevsky SB, Devarajan P, Bennett MR, Parikh CR, Shlipak MG, Harris TB, Gutiérrez OM, Sarnak MJ. Association of urinary uromodulin with kidney function decline and mortality: the health ABC study. Clin Nephrol 87: 278–286, 2017. doi: 10.5414/CN109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotur R, Hainline BE, Sun Q, Orman ES, Chalasani NP, Ghabril M. Hepatic encephalopathy may be associated with enzymatic impairment of ammonia metabolism: Is there a case for studying urea cycle function in cirrhosis? Hepatology 60: 394a–395a, 2014. [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012. doi: 10.1038/kisup.2012.1 [DOI] [Google Scholar]
- 19.Huelin P, Piano S, Solà E, Stanco M, Solé C, Moreira R, Pose E, Fasolato S, Fabrellas N, de Prada G, Pilutti C, Graupera I, Ariza X, Romano A, Elia C, Cárdenas A, Fernández J, Angeli P, Ginès P. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute-on-chronic liver failure. Clin Gastroenterol Hepatol 15: 438–445.e5, 2017. doi: 10.1016/j.cgh.2016.09.156. [DOI] [PubMed] [Google Scholar]
- 20.Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem 59: 17–24, 2018. doi: 10.1016/j.clinbiochem.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735, 2014. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 22.Jian L, Fa X, Zhou Z, Liu S. Functional analysis of UMOD gene and its effect on inflammatory cytokines in serum of essential hypertension patients. Int J Clin Exp Pathol 8: 11356–11363, 2015. [PMC free article] [PubMed] [Google Scholar]
- 23.Leiherer A, Muendlein A, Saely CH, Brandtner EM, Geiger K, Fraunberger P, Drexel H. The value of uromodulin as a new serum marker to predict decline in renal function. J Hypertens 36: 110–118, 2018. doi: 10.1097/HJH.0000000000001527. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiwall R, Kumar A, Bhardwaj A, Kumar G, Bhadoria AS, Sarin SK. Cystatin C predicts acute kidney injury and mortality in cirrhotics: a prospective cohort study. Liver Int 38: 654–664, 2018. doi: 10.1111/liv.13600. [DOI] [PubMed] [Google Scholar]
- 26.Markwardt D, Holdt L, Steib C, Benesic A, Bendtsen F, Bernardi M, Moreau R, Teupser D, Wendon J, Nevens F, Trebicka J, Garcia E, Pavesi M, Arroyo V, Gerbes AL. Plasma cystatin C is a predictor of renal dysfunction, acute-on-chronic liver failure, and mortality in patients with acutely decompensated liver cirrhosis. Hepatology 66: 1232–1241, 2017. doi: 10.1002/hep.29290. [DOI] [PubMed] [Google Scholar]
- 27.McMahon GM, Waikar SS. Biomarkers in nephrology: core curriculum 2013. Am J Kidney Dis 62: 165–178, 2013. doi: 10.1053/j.ajkd.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micanovic R, Khan S, Janosevic D, Lee ME, Hato T, Srour EF, Winfree S, Ghosh J, Tong Y, Rice SE, Dagher PC, Wu XR, El-Achkar TM. Tamm-Horsfall protein regulates mononuclear phagocytes in the kidney. J Am Soc Nephrol 29: 841–856, 2018. doi: 10.1681/ASN.2017040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micanovic R, LaFavers K, Garimella PS, Wu XR, El-Achkar TM. Uromodulin (Tamm-Horsfall protein): guardian of urinary and systemic homeostasis. Nephrol Dial Transplant, In press. doi: 10.1093/ndt/gfy394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium . Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144: 1426–1437.e9, 2013. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 31.O’Leary JG, Levitsky J, Wong F, Nadim MK, Charlton M, Kim WR. Protecting the kidney in liver transplant candidates: practice-based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant 16: 2516–2531, 2016. doi: 10.1111/ajt.13790. [DOI] [PubMed] [Google Scholar]
- 32.Piano S, Brocca A, Angeli P. Renal function in cirrhosis: a critical review of available tools. Semin Liver Dis 38: 230–241, 2018. doi: 10.1055/s-0038-1661372. [DOI] [PubMed] [Google Scholar]
- 33.Pivin E, Ponte B, de Seigneux S, Ackermann D, Guessous I, Ehret G, Pechère-Bertschi A, Olinger E, Mohaupt M, Vogt B, Martin PY, Burnier M, Bochud M, Devuyst O, Pruijm M. Uromodulin and nephron mass. Clin J Am Soc Nephrol 13: 1556–1557, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruijm M, Ponte B, Ackermann D, Paccaud F, Guessous I, Ehret G, Pechère-Bertschi A, Vogt B, Mohaupt MG, Martin PY, Youhanna SC, Nägele N, Vollenweider P, Waeber G, Burnier M, Devuyst O, Bochud M. Associations of urinary uromodulin with clinical characteristics and markers of tubular function in the general population. Clin J Am Soc Nephrol 11: 70–80, 2016. doi: 10.2215/CJN.04230415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60: 646–649, 1973. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 36.Puthumana J, Ariza X, Belcher JM, Graupera I, Ginès P, Parikh CR. Urine interleukin 18 and lipocalin 2 are biomarkers of acute tubular necrosis in patients with cirrhosis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 15: 1003–1013.e3, 2017. doi: 10.1016/j.cgh.2016.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Risch L, Lhotta K, Meier D, Medina-Escobar P, Nydegger UE, Risch M. The serum uromodulin level is associated with kidney function. Clin Chem Lab Med 52: 1755–1761, 2014. doi: 10.1515/cclm-2014-0505. [DOI] [PubMed] [Google Scholar]
- 38.Romero MC, Zanaro N, González L, Trigo P, Imventarza O, Nesse A. Tamm-Horsfall protein excretion to predict the onset of renal insufficiency. Clin Biochem 35: 65–68, 2002. doi: 10.1016/S0009-9120(02)00274-6. [DOI] [PubMed] [Google Scholar]
- 39.Scherberich JE, Gruber R, Nockher WA, Christensen EI, Schmitt H, Herbst V, Block M, Kaden J, Schlumberger W. Serum uromodulin-a marker of kidney function and renal parenchymal integrity. Nephrol Dial Transplant 33: 284–295, 2018. doi: 10.1093/ndt/gfw422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solé C, Solà E, Huelin P, Carol M, Moreira R, Cereijo U, Mas JM, Graupera I, Pose E, Napoleone L, dePrada G, Juanola A, Fabrellas N, Torres F, Morales-Ruiz M, Farrés J, Jiménez W, Ginès P. Characterization of inflammatory response in hepatorenal syndrome: Relationship with kidney outcome and survival. Liver Int 39: 1246–1255, 2019. doi: 10.1111/liv.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Satanovskij R, Angermann S, Hasenau AL, Stecher L, Heemann U, Renders L, Scherberich J. Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Medicine (Baltimore) 95: e3011, 2016. doi: 10.1097/MD.0000000000003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 75: 652–660, 2009. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut 62: 131–137, 2013. doi: 10.1136/gutjnl-2011-301255. [DOI] [PubMed] [Google Scholar]
- 44.Wong F, O’Leary JG, Reddy KR, Patton H, Kamath PS, Fallon MB, Garcia-Tsao G, Subramanian RM, Malik R, Maliakkal B, Thacker LR, Bajaj JS; North American Consortium for Study of End-Stage Liver Disease . New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology 145: 1280–8.e1, 2013. doi: 10.1053/j.gastro.2013.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Y, Sakatsume M, Nishi S, Narita I, Arakawa M, Gejyo F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int 60: 1645–1657, 2001. doi: 10.1046/j.1523-1755.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 46.Youhanna S, Weber J, Beaujean V, Glaudemans B, Sobek J, Devuyst O. Determination of uromodulin in human urine: influence of storage and processing. Nephrol Dial Transplant 29: 136–145, 2014. doi: 10.1093/ndt/gft345. [DOI] [PubMed] [Google Scholar]


