Abstract
Gravin, an A-kinase anchoring protein, is known to play a role in regulating key processes that lead to inflammation and atherosclerosis development, namely, cell migration, proliferation, and apoptosis. We investigated the role of gravin in the development of high-fat diet (HFD)-induced atherosclerosis and hyperlipidemia. Five-week-old male wild-type (WT) and gravin-t/t mice were fed a normal diet or an HFD for 16 wk. Gravin-t/t mice showed significantly lower liver-to-body-weight ratio, cholesterol, triglyceride, and very low-density lipoprotein levels in serum as compared with WT mice on HFD. Furthermore, there was less aortic plaque formation coupled with decreased lipid accumulation and liver damage, as the gravin-t/t mice had lower levels of serum alanine aminotransferase and aspartate aminotransferase. Additionally, gravin-t/t HFD-fed mice had decreased expression of liver 3-hydroxy-3-methyl-glutaryl-CoA reductase, an essential enzyme for cholesterol synthesis and lower fatty acid synthase expression. Gravin-t/t HFD-fed mice also exhibited inhibition of sterol regulatory element binding protein-2 (SREBP-2) expression, a liver transcription factor associated with the regulation of lipid transportation. In response to platelet-derived growth factor receptor treatment, gravin-t/t vascular smooth muscle cells exhibited lower intracellular calcium transients and decreased protein kinase A- and protein kinase C-dependent substrate phosphorylation, notably involving the Erk1/2 signaling pathway. Collectively, these results suggest the involvement of gravin-dependent regulation of lipid metabolism via the reduction of SREBP-2 expression. The absence of gravin-mediated signaling lowers blood pressure, reduces plaque formation in the aorta, and decreases lipid accumulation and damage in the liver of HFD mice. Through these processes, the absence of gravin-mediated signaling complex delays the HFD-induced hyperlipidemia and atherosclerosis.
NEW & NOTEWORTHY The gravin scaffolding protein plays a key role in the multiple enzymatic pathways of lipid metabolism. We have shown for the first time the novel role of gravin in regulating the pathways related to the initiation and progression of atherosclerosis. Specifically, an absence of gravin-mediated signaling decreases the lipid levels (cholesterol, triglyceride, and VLDL) that are associated with sterol regulatory element binding protein-2 downregulation.
Keywords: A-kinase anchoring protein 12, atherosclerosis, gravin, high-fat diet, hyperlipidemia, lipoproteins
INTRODUCTION
Atherosclerosis contributes to the development of cardiovascular diseases and is a leading cause of death in the United States (15, 39, 49). Atherosclerosis is a chronic inflammatory disease involving plaque formation in the blood vessel wall characterized by vascular inflammation, lipid accumulation, and smooth muscle cell proliferation/migration (39, 57). The development of atherosclerosis results from atherogenic factors, such as hyperlipidemia, hypertension, diabetes, smoking, and diets rich in fats (39). Hyperlipidemia leads to the retention of lipoproteins in the vessel walls, which further initiates the inflammatory response in the cells (15). Specifically, changes in the metabolism of lipoproteins, which include very-low-density lipoproteins (VLDL), low-density lipoproteins (LDL), high-density lipoproteins (HDL), triglycerides, and total cholesterol, are well recognized as important mediators in the pathogenesis of atherosclerosis (39). These atherogenic factors subsequently cause endothelial injury/dysfunction, triggering the release of cytokines and growth factors by inflammatory cells (macrophages/monocytes), endothelial cells, and vascular smooth muscle cells (VSMCs) (50). VSMC proliferation, migration, apoptosis, and extracellular matrix (ECM) synthesis induce the formation of the atherosclerotic lesion, which is regulated by matrix metalloproteinases (MMPs) (44). Once stimulated and activated, MMPs facilitate VSMC migration by degrading ECM components and downregulating their biosynthesis (11, 21). Diets high in fats are directly associated with aortic atherosclerosis, as the migrated and proliferated VSMCs and excessive amounts of lipids in the ECM can form plaques on the aortic luminal wall. This aortic plaque is then followed by calcification and hardening, which eventually restricts the flow of blood (39). Together, these events lead to hypertension and eventually cause myocardial infarction or ischemic stroke because of plaque rupture (54). The elevation of circulating cholesterol and hypertension are two of the most important factors that induce and promote the internal damage of arterial walls (57). Although many insightful studies have identified key signaling components involved in atherosclerosis, the full understanding of signaling pathways involved in the development and progression of atherosclerosis are not fully understood (3, 30).
The initiation and progression of atherosclerosis involves cytokines and growth factors, such as platelet-derived growth factor (PDGF) receptors released by inflammatory cells, endothelial cells, and VSMCs, to mediate downstream signaling through protein kinase-dependent substrate phosphorylation (7, 19). Activated growth factors are associated with VSMC proliferation, migration, and ECM synthesis (7, 19). The released growth factors then converge on VSMCs to stimulate a number of common pathways, triggering pathological vascular remodeling. The intracellular signaling pathways of activated VSMCs commonly involve the substrate-dependent phosphorylation mediated by protein kinase A (PKA) and protein kinase C (PKC), along with the elevation of intracellular calcium ([Ca2+]i) (38). In particular, during atherosclerosis, the over-release of PDGF is essential in the proliferation of VSMC activation in response to several atherogenic factors and other growth factors (51, 52), and this PDGF can then induce a rapid increase of [Ca2+]i (16, 58).
Gravin, also known as A-kinase anchoring protein (AKAP)12, AKAP250, or Src-suppressed C kinase substrate, is a member of the AKAP family of signalosome proteins that scaffold PKA, PKC, protein phosphatase 2B, β-adrenergic receptors (β2-ARs), as well as other protein kinases, protein phosphatases, and phosphodiesterases to specific intracellular locations, resulting in localization of the binding complex (22, 24, 64). Thus, gravin assembles intracellular signaling proteins into a specific complex within the cell, thereby enhancing the spatial and temporal signaling specificity as well as efficiency (18, 66). Particularly, gravin acts as a signalosome that coordinates the localization of PKA with its substrate complex, including [Ca2+]i and PKC (62). AKAPs regulate cAMP-dependent PKA signaling events that control lipolysis (55), including the regulation of lipoprotein lipase (LPL) expression via the inhibition of its translation (53). Additionally, it has been previously reported that gravin mediates cellular lipid metabolism via inducing the activation of sterol regulatory element binding proteins (SREBPs) in hepatocytes, which are transcription factors that bind to the sterol regulatory element DNA sequence of cholesterol-associated genes (13).
There are two main conceptual approaches to treat atherosclerosis: targeting plasma lipoprotein metabolism and targeting the inflammatory process. The objective of this study was to determine whether the absence of gravin-mediated signaling would prevent the initiation and progression of atherosclerosis induced by a high-fat diet (HFD; 42% kcal from fat). To study gravin’s role in the accelerated and enhanced development of HFD-induced hyperlipidemia and atherosclerosis, gravin mutant (gravin-t/t) mice that lack the scaffolding sites for PKA, PKC, protein phosphatase 2B, β2-AR, protein kinases, phosphatases, and phosphodiesterases were used. We found that the absence of gravin-mediated signaling complex delays the HFD-induced hyperlipidemia and atherosclerosis.
MATERIALS AND METHODS
Animals and diet.
Gravin mutant mice were produced using gene trap technology to ablate the AKAP12 (gravin) gene (NM_031185) (24). Homozygous mice lacking functional gravin protein (designated gravin-t/t, in which t refers to truncation) do not express the critical region exon 3, which encodes the binding sites for β2-AR, PKA, or PKC binding. Wild-type (WT) mice and gravin-t/t were bred on the C57BL/6J background. Gene expression was characterized by polymerase chain reaction (PCR) and reverse transcriptase quantitative PCR (RT-qPCR). Protein estimation was performed using Western blot analysis. Both gene and protein expression experiments were used to confirm the absence or near absence of expression. Male WT and gravin-t/t mice were maintained at room temperature (22°C) with a 12-h:12-h light-dark cycle and on a standard rodent chow until 5 wk of age. Mice were then randomized to receive either normal diet (ND) or HFD, resulting in four groups: WT ND, gravin-t/t ND, WT HFD, and gravin-t/t HFD. In this study, only male mice were used. The mice were then fed a standard chow ND (total fat: 4.7% by weight; 14% kcal from fat; Rodent Diet 20, PicoLab) or an HFD (total fat: 21% by weight; 42% kcal from fat; TD.88137, Teklad, Harlan) for 16 wk (63). Specifically, the fatty acid profile of the TD.88137 HFD contained 12.8% saturated fat, 5.6% monounsaturated fat, 1.0% polyunsaturated fat (including oleic, linoleic, and linolenic fatty acids), and 1.3% unidentified fatty acids and/or those contributing <0.5% of total fatty acids, for a total of 20.7% of the diet, as it is known that each type of fatty acid has a different impact on cardiac health (28). At the end of the 16 wk of the specialized diet, the animals were euthanized and tissue was collected for further analysis.
Blood pressure measurements.
A mouse pressure-volume conductance catheter (PVR-1045; Millar Instruments) was inserted into the left carotid artery to measure systolic and diastolic blood pressure, mean arterial pressure, and heart rate, as previously described (47). Data collection and analysis were performed using LabChart Pro (ADInstruments).
RT-qPCR.
Gravin gene expression was quantified using RT-qPCR. Total RNA was isolated from mouse heart tissue using the RNeasy mini kit (Qiagen) according to the manufacturer’s instructions and then assayed using a spectrophotometer. First, strand cDNA was synthesized using the Invitrogen Superscript III Kit (Invitrogen) according to the manufacturer’s instructions. The mRNA levels were quantified with SYBR Green (Applied Biosystems) using an ABI 7900 sequence detection system (StepOnePlus, Applied Biosystems). Each gene was measured in triplicate reactions to obtain the mean cycle threshold value. The mean cycle threshold value was normalized against the mean expression of the housekeeping gene encoding 18s RNA. A complete list of the RT-qPCR primers is described in Table 1.
Table 1.
List of the reverse transcriptase quantitative PCR primers
| Gene (Primer) | Sequence |
|---|---|
| 3′ end of exon 3 of gravin | |
| Forward | 5′-GCCAGTGAAGAACATGAGCA-3′ |
| Reverse | 5′-TGCAATCTGCTTTGTCTTGG-3′ |
| 5′ end of exon 3 of gravin | |
| Forward | 5′-GAGCAGGAGACCACCAAGAG-3′ |
| Reverse | 5′-TTCTCCATCTTTGGCTGCTT-3′ |
| FAS | |
| Forward | 5′-GGAGGTGGTGATAGCCGGTAT-3′ |
| Reverse | 5′-TGGGTAATCCATAGAGCCCAG-3′ |
| LPL | |
| Forward | 5′-TTGCCCTAAGGACCCCTGAA-3′ |
| Reverse | 5′-ACAGAGTCTGCTAATCCAGGAAT-3′ |
| SREBP-1c | |
| Forward | 5′-GATGTGCGAACTGGACA-3′ |
| Reverse | 5′-CATAGGGGGCGTCAAACAG-3′ |
| HMGCR | |
| Forward | 5′-GGCATTTGACAGCACTAGCA-3′ |
| Reverse | 5′-CTTTGCATGCTCCTTGAACA-3′ |
| LDLR | |
| Forward | 5′-CTCGCTGGTGACTGAAAACA-3′ |
| Reverse | 5′-CAAAGGAAGACGAGGAGCAC-3′ |
| SREBP-2 | |
| Forward | 5′-AAGTCTGGCGTTCTGAGGAA-3′ |
| Reverse | 5′-CACAAAGACGCTCAGGACAA-3′ |
| Exon 1 of gravin | |
| Forward | 5′-ATGGGTGCAGGCAGTTCC-3′ |
| Reverse | 5′-CGGGATCTCCAGCTGCTC-3′ |
| Exon 2 of gravin | |
| Forward | 5′-CTCCCACAGAAGAATGGTCAG-3′ |
| Reverse | 5′-GACTTCTTCCTCTTGCCCATC-3′ |
| Exon 3A of gravin | |
| Forward | 5′-GAGCAGGAGACCACCAAGAG-3′ |
| Reverse | 5′-TTCTCCATCTTTGGCTGCTT-3′ |
| Exon 3B of gravin | |
| Forward | 5′-TGGGCATCCTTCAAAAAGATG-3′ |
| Reverse | 5′-CCTTAAGCTCTTCTTCCTTGT-3′ |
| Exon 3C of gravin | |
| Forward | 5′-GCCAGTGAAGAACATGAGCA-3′ |
| Reverse | 5′-TGCAATCTGCTTTGTCTTGG-3′ |
| 18s RNA | |
| Forward | 5′-TCAAGAACGAAAGTCGGAGG-3′ |
| Reverse | 5′-GGACATCTAAGGGCATCA C-3′ |
FAS, fatty acid synthase; HMGCR, 3-hydroxy-3-methyl-glutaryl-CoA reductase; LDLR, low-density lipoprotein receptor; LPL, lipoprotein lipase; SREBP, sterol regulatory element binding protein.
Western blot analysis.
Tissues were homogenized using a radioimmunoprecipitation assay buffer containing 50 mM Tris base pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 10% glycerol, and 0.25% Na-deoxycholate, as previously described (42). To prepare nuclear and cytosolic extracts of liver, ~0.1 g of the frozen liver was homogenized. Briefly, 0.1 g frozen liver was homogenized by a homogenizer in a cold hypotonic buffer containing 10 mM HEPES (pH 7.5), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, and protease inhibitors (VWR). Cytosolic fractions were isolated by centrifugation for 5 min at 5,000 revolutions/min at 4°C. Nuclear fractions were then incubated with a lysis buffer containing 140 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, 50 mM of Tris·HCl, and protease inhibitor cocktail (VWR). After incubation on ice, nuclear extracts were centrifuged at 13,000 revolutions/min for 5 min at 4°C followed by a collection of the supernatant. In VSMC studies, isolated VSMCs were cultured and then treated by PDGF (10 ng/mL) for 15 min, after which the cell lysates were collected and then assayed. Protein homogenates were resolved by SDS-PAGE (4–12%) gradient gels and then transferred to polyvinylidene difluoride membranes for immunoblot analysis using primary antibodies to gravin [AKAP12; Santa Cruz; cat. no. sc-33578; molecular mass (MM, 250 kDa], MMP-9 (Cell Signaling; cat. no. 3852; MM, 84 and 92 kDa), F4/80 (AbDSerotec; cat. no. MCA497BT; MM, 160 kDa), SREBP-2n (Thermo Scientific; cat. no. PA1-338; MM, 68 kDa), phospho(Thr197)-PKA catalytic (Cell Signaling; cat. no. 5661; 42kDa), phospho(Ser/Thr)-PKA substrates (Cell Signaling; cat. no. 9621), phospho(Thr638/641)-PKCα/βII (Cell Signaling; cat. no. 9375; 80 kDa), phospho(Ser)-PKC substrates (Cell Signaling; cat. no. 2261), phospho(Thr202/Tyr204)-Erk1/2 (Cell Signaling; cat. no. 9106; MM, 42 and 44 kDa), phospho(Ser473)-Akt (Cell Signaling; cat. no. 9271; MM, 60 kDa), and GAPDH (Cell Signaling; cat. no. 2118; MM, 37 kDa). Blots were then incubated overnight at 4°C with primary antibodies. The blots were washed with Tris-buffered saline containing 0.1% Tween 20 and then probed with the appropriate horseradish peroxidase-conjugated secondary antibodies (anti-mouse or anti-rabbit; Cell Signaling) for 1 h at room temperature. The signal was detected by using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). Densitometric analyses of the immunoblots were performed via ImageJ Data Acquisition Software [National Institutes of Health (NIH), Bethesda, MD]. The antiprotein antibody signal was normalized to the GAPDH protein antibody signal.
Oil Red O staining.
Whole aortas and livers were isolated and stained with en face Oil Red O solution (39, 40). The freshly dissected livers were covered in optimum cutting temperature compound for 10–20 min in a labeled cryomold surrounded with dry ice. The liver cryostat sections (10 μm) were used for Oil Red O staining. The entire length of the aorta, including the aortic arch, was isolated and en face stained for atherosclerosis lesions by Oil Red O staining. The aortic tree from the root to the abdominal aorta was dissected from mice. After being mechanically cleaned of adventitia, the aortas were immersed in an Oil Red O solution (0.5% solubilized in 60% isopropanol) and differentiated in 85% propylene glycol. The stained aortas were then photographed and the images digitized by blind observation. The brightfield images of the aortas were taken using the Nikon Eclipse Ti microscope. Quantification of the atherosclerosis lesion (red) was measured via Image-Pro Plus (Media Cybernetics). The positive percentage of the areas of the atherosclerotic lesion was calculated as the sum of a red-stained area divided by the sum of the whole aorta area (31).
Histological and immunohistochemical analysis.
Tissues (whole aorta and liver) were harvested from WT and gravin-t/t mice and then fixed in 10% formalin. In separate animal experiments, regions of the thoracic and abdominal aorta were paraffin embedded. The cross sections (5 μm) were immunoblotted using antibodies for MMP-9 (Cell Signaling) and F4/80 (AbD Serotec). After deparaffinization and hydration, sections were blocked for endogenous peroxidase for 30 min using 3% H2O2 in deionized water and then washed with phosphate-buffered saline (PBS). The sections were incubated in a staining vessel containing preheated sodium citrate buffer (10 mM citric acid, pH 6.0) at 95–100°C for 10 min. After cooling for 20 min, the sections were rinsed with PBS. Tissue sections were then blocked with diluted with normal blocking serum for 20 min. Aortic sections were then incubated overnight at 4°C with primary antibodies. The sections were washed with PBS containing 0.1% Tween 20 and then probed with the appropriate biotinylated-conjugated secondary antibodies (anti-mouse or anti-rabbit; Vector). The VECTASTAIN Elite ABC Reagent (Vector) and ImmPACT DAB Peroxidase Substrates (Vector) were used to detect the protein signal, as according to manufacturers’ instructions. For morphological analysis, liver cryostat sections (10 μm) were stained with hematoxylin and eosin (H&E) solution. The images were then visualized using the BX41 Olympus microscope with a digital camera (Spot Insight 2, Diagnostic Instruments).
Blood collection.
A total volume of 250 μL of blood was collected from the mandibulofacial artery. The blood sample was collected in an Eppendorf tube and allow to clot for 30–45 min at room temperature. The sample was then centrifuged at 3,000–5,000 revolutions/min for 10 min. The serum was then separated by aliquoting into another tube and then stored at −20°C until ready for analysis.
Blood lipid panel measurements.
Plasma lipid levels from serum samples were collected for analysis for lipoprotein subtypes. Total cholesterol, triglyceride, VLDL, LDL, HDL, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were measured with the Cobas Integra 400 Plus (Roche; Baylor College of Medicine Core Facility) and Piccolo Express Chemistry Analyzer (Roche) using the Piccolo Lipid Panel Plus reagent (Abbott), according to the manufacturers’ instructions.
VSMC isolation and culture.
Primary VSMCs were isolated from the mice. Briefly, thoracic arteries were removed and placed in Petri dishes containing ice-cold Hank’s Balanced Salt Solution (HBSS; Ca2+, Mg2+ free; Sigma) with 0.2 mM added Ca2+. The thoracic arteries were cleaned to remove fat, adventitia, and venous tissues under a stereomicroscope, cut into small pieces, and transferred into 3.5-cm Petri dishes containing 4.0 mL of enzyme dissociation mixture: HBSS (Ca2+, Mg2+ free) with 0.2 mM added Ca2+, 15 mM HEPES buffer (pH 7.2–7.3), 0.125 mg/mL elastase (Sigma, Type III, 8 U/mg), and 1.0 mg/mL collagenase (Sigma, CLS Type I, 184 U/mg). After incubation at 37°C for 15 min in a gyrating shaker bath, the suspension was gently triturated several times through a 12-gage stainless steel cannula every 15 min to separate dispersed cells from undigested vessel wall fragments and debris. Enzymatic digestion was terminated by the addition of 5 mL of fresh Dulbecco’s modified Eagle’s medium (DMEM free of enzymes) supplemented with 10% fetal bovine serum (FBS). The cell suspension was centrifuged at 1,400 revolutions/min for 5 min, and the cell pellet resuspended in 1 mL of DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin (M. A. Bioproducts). Explants were incubated at 37°C in 5% CO2 for 7–10 days, and outgrown VSMCs were passaged by treatment with 0.25× trypsin. VSMCs that formed a confluent monolayer between passages 5–8 were used for subsequent experiments. The cells were starved for 72 h by incubation in DMEM supplemented with 0.1% FBS for quiescence and then treated with growth factor.
In vitro VSMC migration from scratch wound assays.
Migration of mouse aortic VSMCs was determined using a scratch wound assay. Briefly, VSMCs were placed in 6-well cell culture plates at 2.5 × 104 cells per well in DMEM supplemented with 10% FBS in a humidified atmosphere of 5% CO2 at 37°C. Once confluent, the cell monolayer was incubated in DMEM supplemented with 0.1% FBS. Twenty-four hours later, the confluent cells were scratched with a sterile rubber policeman to create cell-free zones. The cells were gently washed with PBS to remove unattached cells and incubated in DMEM supplemented with 0.1% FBS with or without PDGF (10 ng/mL)/BAPTA-AM (10 µM) at 37°C. The migration of cells into the cell-free zone was then monitored by photographing 3 random fields of view at ×40 magnification using a phase contrast microscope (Nikon Instruments, Melville, NY) at baseline (immediately after creating the zones) and 24 h after wounding. The distance between the leading edge of migrating cells and the wound edge was measured and averaged on 10 random photos from each experimental sample by using NIH ImageJ software.
VSMC proliferation measured using MTS assays.
VSMC proliferation was characterized using MTS colorimetric cell proliferation assays (Promega, Madison, WI) as previously described (34). Briefly, the VSMCs were seeded in 96-well culture plates at 2.5 × 103 cells/well and incubated at 37°C in 5% CO2. After 24 h, the confluent cell monolayers were incubated in DMEM with 0.1% FBS. After another 24 h, the cells were incubated in DMEM supplemented with 0.1% FBS with or without PDGF (10 ng/mL)/BAPTA-AM (10 µM) at 37°C and allowed to proliferate for 1, 3, or 5 days, followed by incubation with MTS reagent for 2 additional hours. The absorbance of each well was measured at 490 nm using a microplate reader (Model 680, Bio-Rad, Hercules, CA).
Measurements of intracellular calcium using Fluo-8.
VSMCs were grown in a DMEM supplemented with 10% FBS, harvested with trypsin, and then plated into 12-well plates (BD-Biocoat polylysine, black/clear) at 3 × 105 cells per well. The VSMCs were starved with serum-free DMEM for 24 h. The cells were then incubated with 4 µM of the calcium fluorescence indicator fluo-8 AM (AAT Bioquest), dissolved in DMEM, and supplemented with 2.5 mM probenecid acid (AAT Bioquest) and Pluronic F-127 (Molecular Probes cat. no. P-3000) at 37°C for 15 min (12). The cells on the plate were washed three times with a calcium indicator-free buffer (DMEM supplemented with 2.5 mM probenecid acid plus 1% Pluronic F-68 to remove excess probes. The experiment was run at 490 nm/514 nm (excitation/emission) using a Nikon Ti-S eclipse microscope (×40 objective). After 10 s of baseline recording, calcium signaling for cells within each well was individually recorded in the presence as well as the absence of PDGF.
Statistical analysis.
Data were processed using Microsoft Excel and GraphPad Prism 7.0. All values were presented as mean ± SE. Comparisons between the two groups were determined using an unpaired two-tailed Student’s t test. Comparisons between multiple groups were analyzed by one-way ANOVA followed by a post hoc Tukey test for multiple comparisons. P values of ≤0.05 were considered significant.
Ethical considerations.
All procedures involving experimental animals have been approved by the Institutional Animal Care and Use Committee and ethics committee at the University of Houston (UH; no. UH-ACP-14–023), which are in accordance with NIH guidelines. Animal care was provided for in American Association for the Accreditation of Laboratory Animal Care-accredited animal barrier facilities at UH and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
RESULTS
Characterization of gravin-t/t mice in response to HFD.
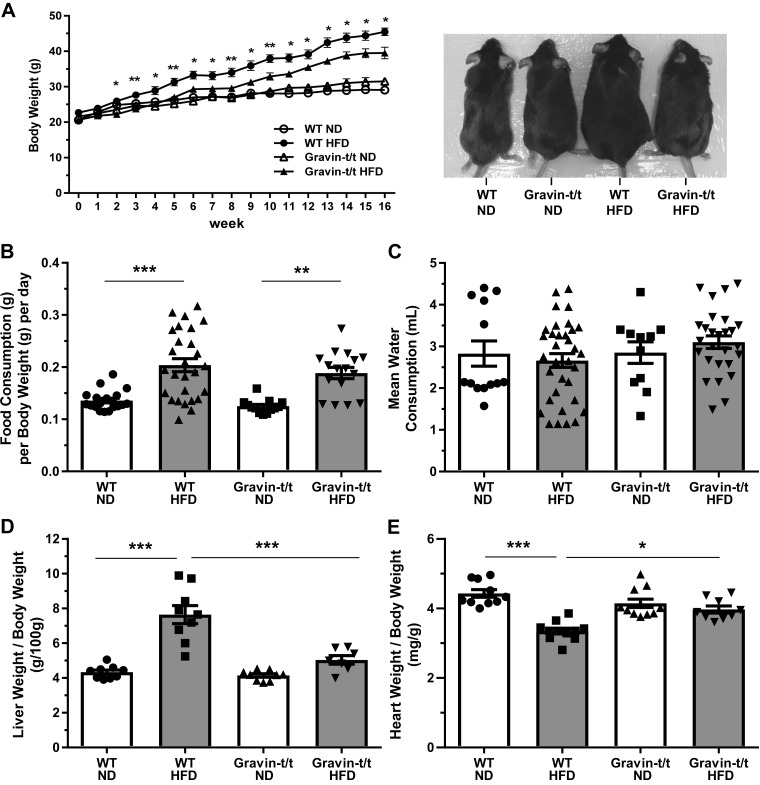
To characterize the effects of gravin on vascular inflammation during atherosclerosis, 5-wk-old male WT and gravin-t/t mice were fed either a standard ND or HFD for 16 wk. As expected, the HFD (42% kcal from fat) resulted in increased body weights of both WT and gravin-t/t mice (Fig. 1A; left). However, starting at 2 wk through the duration of the 16 wk of HFD treatment, the increase in body weight was significantly less in gravin-t/t HFD versus WT HFD-fed mice (116% increase in body weight in WT mice versus 93% increase in body weight in gravin-t/t mice). There was no significant difference between WT and gravin-t/t ND-fed mice. Figure 1A (right) shows a representative image of a mouse from each cohort at the end of the 16 wk treatment of either ND or HFD. Although food consumption for both WT and gravin-t/t mice was higher in the HFD-fed versus ND-fed control groups, there was no significant difference in the amount of food consumed between WT and gravin-t/t mice on either diet (Fig. 1B). There was also no significant difference in water consumption by the mice between groups at the end of the 16 wk treatment of either ND or HFD (Fig. 1C).
Fig. 1.
Gravin-t/t and wild-type (WT) whole animal response to normal diet (ND) and high-fat diet (HFD). Left shows quantification analysis of body weight, and right shows representative mouse images of each study group (A). Body weights of the mice were collected from 0 to 16 wk. Gravin-t/t HFD mice showed lower body weights compared with WT HFD starting from 2 wk of age. There was no significant body weight difference for ND groups for the 16-wk treatment. *Comparison between HFD-fed WT and Gravin-t/t mice. Results are presented as mean ± SE. n = 6; *P < 0.05 and **P < 0.01. There were no significant differences in food consumption (B) and water consumption (C) between WT and gravin-t/t on the HFD during the 16 wk of treatment. Results are presented as mean ± SE. n = 13; *P < 0.05 and **P < 0.01. Liver weight/body weight ratio (D) and heart weight/body weight ratio (E) of WT and gravin-t/t mice were measured at 16 wk of age. Results are presented as mean ± SE; n = 10 (WT ND), n = 11 (WT HFD), n = 11 (Gravin-t/t ND), n = 9 (Gravin-t/t HFD; D); n = 9 (WT ND), n = 9 (WT HFD), n = 9 (Gravin-t/t ND), n = 7 (Gravin-t/t HFD; E); *P < 0.05; **P < 0.01; ***P < 0.001. Comparisons between two groups were determined by an unpaired two-tailed Student’s t test, and comparisons between multiple groups were determined by one-way ANOVA followed by post hoc Tukey test.
To determine the effect of the HFD treatment on key organs involved in lipid metabolism and cardiac function, liver-weight-to-body-weight (LW/BW) ratio and heart-weight-to-body-weight (HW/BW) ratio were measured. The LW/BW ratio was significantly greater in WT HFD versus WT ND-fed mice, whereas no significant difference was observed between the gravin-t/t HFD versus gravin-t/t ND-fed mice (Fig. 1D). Furthermore, the LW/BW ratio was significantly reduced in gravin-t/t HFD versus WT HFD-fed mice. Although there was no difference in the HW/BW ratio in WT ND and gravin-t/t ND-fed mice, we did observe a significant increase in the HW/BW ratio in the gravin-t/t HFD versus WT HFD-fed mice (Fig. 1E). These results indicate that gravin-t/t mice lacking functional gravin have significantly lower liver weight but increased heart weight in response to HFD as compared with the WT mice with functional gravin.
Gravin-t/t mice protect against hypertension.
Diets rich in fat have been shown to lead to increased blood pressure and the development of hypertension, which is a known risk factor for atherosclerosis (65). Therefore, at the end of the 16-wk treatment of either ND or HFD, we measured the blood pressure using a pressure-volume conductance catheter and heart rate from electrocardiogram recordings. We observed a significant increase in systolic blood pressure, diastolic blood pressure, and mean arterial pressure in WT HFD-fed mice as compared with WT ND-fed mice (Fig. 2), whereas no significant differences were observed between gravin-t/t ND, gravin-t/t HFD, and WT ND-fed mice. In contrast, there was a significant decrease in systolic blood pressure (Fig. 2A), diastolic blood pressure (Fig. 2B), and mean arterial pressure (Fig. 2C) in gravin-t/t HFD versus WT HFD-fed mice. No differences in the heart rate were observed (Fig. 2D). These results indicate that gravin-t/t mice lacking functional gravin were protected against increased blood pressure in response to HFD.
Fig. 2.
Gravin-t/t mice show decreased blood pressure in response to high-fat diet (HFD). HFD-fed Gravin-t/t mice showed reduced systolic blood pressure (A), reduced diastolic blood pressure (B), and reduced mean blood pressure (C) as compared with wild-type (WT) mice measured at 16 wk of age. No difference in heart rate (D) was observed. Results are presented as mean ± SE. n = 8 [(WT normal diet (ND)], n = 7 (WT HFD), n = 8 (Gravin-t/t ND), and n = 7 (Gravin-t/t HFD) (A); n = 8 (WT ND), n = 8 (WT HFD), n = 8 (Gravin-t/t ND), and n = 7 (Gravin-t/t HFD) (B); n = 8 (WT ND), n = 8 (WT HFD), n = 7 (Gravin-t/t ND), and n = 7 (Gravin-t/t HFD) (C); and n = 9 (WT ND), n = 7 (WT HFD), n = 7 (Gravin-t/t ND), and n = 7 (Gravin-t/t HFD) (D). **P < 0.01, and ***P < 0.001. Comparisons between two groups were determined by an unpaired two-tailed Student’s t test, and comparisons between multiple groups were determined by one-way ANOVA followed by post hoc Tukey test.
Gravin-t/t mice protects against atherosclerosis.
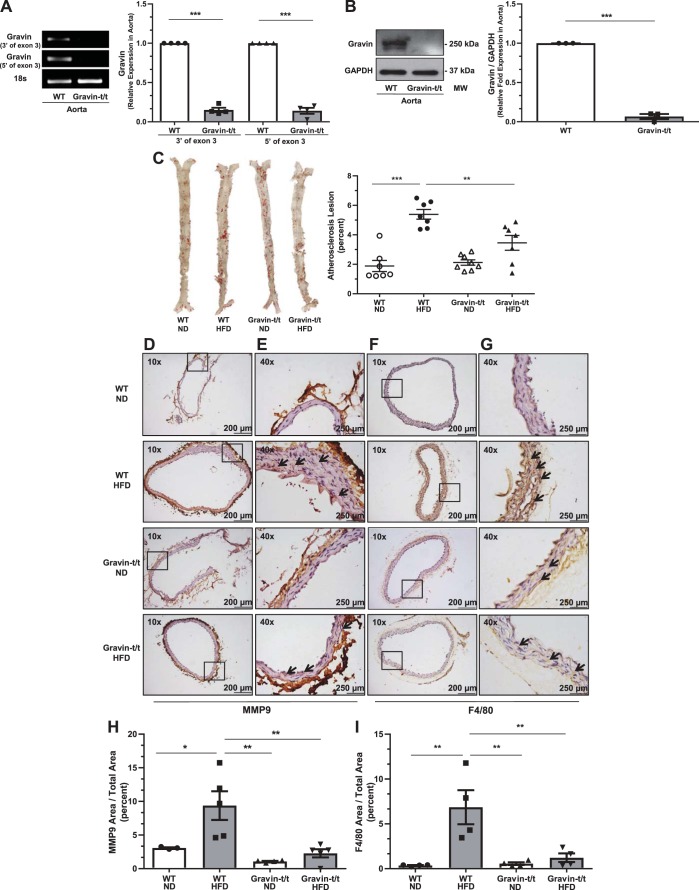
We previously reported (24) that gravin gene (AKAP12) transcription was significantly reduced (approximately 90%), with <10% of the gravin protein being expressed in gravin-t/t versus WT hearts of mice. These mice were generated using gene trap technology, permitting generation of global truncated gravin protein (designate gravin-t/t, in which t refers to truncated alleles) (24). In the current study, we confirmed that aortic tissue from gravin-t/t mice also did not express the gravin gene and protein (Fig. 3, A and B). As shown in Fig. 3A, the PCR products of gravin’s exon 3 were absent in gravin-t/t aortic tissue, where aortic gravin gene expression was significantly reduced by 85% (3′-end of exon 3) and 86% (5′-end of exon 3). Western blot analysis using a gravin antibody specific to the carboxyl-terminal amino acids (encoded by exon 3) confirmed that aortic gravin protein expression was also absent in gravin-t/t mice (Fig. 3B).
Fig. 3.
Gravin-t/t mice show resistance to atherosclerosis induced by high-fat diet (HFD). Gravin mRNA expression in the aorta was quantified by reverse transcriptase quantitative PCR from wild-type (WT) and gravin-t/t mice (A). Gravin-specific primers amplified regions of exon 3 that were either 3′ or 5′ of the exon. The aorta from gravin-t/t mice showed significantly decreased gravin mRNA expression compared with WT mice. Results are presented as mean ± SE. n = 4; ***P = 0.0006. Gravin protein expression was measured in the aorta by Western blot from WT and gravin-t/t mice, where the gravin antibody recognized the monomeric (250 kDa) forms of the gravin protein (B). The aorta from gravin-t/t mice showed significantly decreased gravin protein expression compared with WT mice. Results are presented as mean ± SE. n = 3; ***P < 0.001. Dissected whole aortas were isolated from gravin-t/t and WT mice and stained with Oil Red O solution following 16 wk treatment with either normal diet (ND) or HFD, where representative whole aorta images show atherosclerotic plaques (C). Aortas were then quantified for atherosclerotic plaque formation using Image-Pro Plus. Results are presented as mean ± SE. n = 7 (WT ND), n = 7 (WT HFD), n = 8 (Gravin-t/t ND), and n = 8 (Gravin-t/t HFD); **P < 0.01 and ***P < 0.001. Cross sections of paraffin-embedded thoracic and abdominal aortas (D–G) were stained with matrix metalloproteinase (MMP)-9 (D and E) and F4/80 antibodies (F and G). Images were measured using brightfield Nikon microscopy at ×10 (D and F) and ×40 (E and G). Gravin-t/t HFD-fed mice showed lower aortic MMP-9 and aortic F4/80 expression compared with WT HFD-fed mice. Arrows indicate the expression of the protein of interest. Boxed areas shown in D and F are shown in higher magnification in E and G, respectively. MMP-9 area to total area (H) and F4/80 area to total area (I) were both decreased in gravin-t/t HFD-fed mice compared with WT HFD-fed mice. Results are presented as mean ± SE. n = 3 (WT ND), n = 5 (WT HFD), n = 4 (Gravin-t/t ND), and n = 5 (Gravin-t/t HFD) (H); n = 4 for each group (I). *P < 0.05, **P < 0.01, and ***P < 0.001. Comparisons between two groups were determined by an unpaired two-tailed Student’s t test, and comparisons between multiple groups were determined by one-way ANOVA followed by post hoc Tukey test. MM, molecular mass.
To characterize the development of atherosclerosis in gravin-t/t mice, whole aortas were isolated and stained with Oil Red O of the enfaced aortas at the end of the 16-wk treatment of either ND or HFD. In addition, regions of abdominal aortas were sectioned and stained for MMP-9 or F4/80 expression. A diet enriched in dietary fats (42% kcal from fat) induces plaque formation. As expected, we observed increased aortic lesions in WT HFD-fed mice as compared with WT ND-fed mice (Fig. 3C), whereas no significant differences were observed between gravin-t/t ND, gravin-t/t HFD, and WT ND-fed mice. In contrast, there were significantly reduced (P = 0.0073) aortic lesions in gravin-t/t HFD versus WT HFD-fed mice (Fig. 3C). To confirm the reduction in aortic lesions in gravin-t/t HFD versus WT HFD-fed mice, immunohistochemical analysis of the abdominal aorta was performed using the known atherosclerotic marker (MMP-9; Fig. 3, D and E) and monocyte/macrophage-specific surface marker (F4/80) (Fig. 3, F and G). We observed reduced abdominal aorta atherosclerosis formation, as shown by decreased MMP-9 aortic expression (Fig. 3, D, E, and H) and decreased F4/80 aortic expression (Fig. 3, F, G, and I), in gravin-t/t HFD versus WT HFD-fed mice. These results indicate that gravin-t/t mice lacking functional gravin developed significantly less plaque formation and may exhibit decreased inflammatory response after HFD treatment.
Reduced serum lipids in gravin-t/t mice.
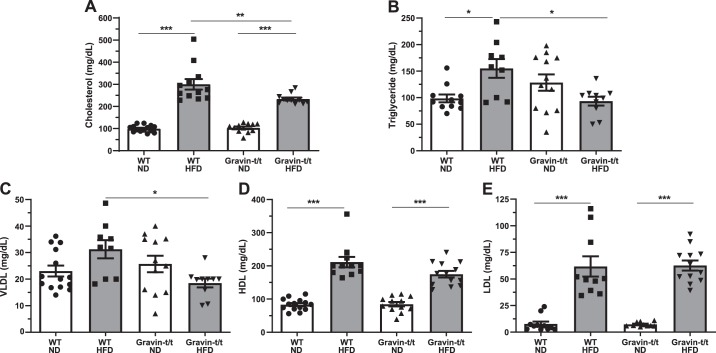
Dysregulation of plasma lipids is known to be associated with the development of aortic plaques and atherosclerosis (2). Therefore, we measured the cholesterol, triglycerides, HDL, LDL, and VLDL at the end of the 16-wk treatment with ND and HFD. We observed the expected increase in serum cholesterol, triglycerides, HDL, and LDL in WT HFD-fed mice as compared with WT ND-fed mice (Fig. 4). Interestingly, we observed decreases in cholesterol (Fig. 4A), triglycerides (Fig. 4B), and VLDL (Fig. 4C) in gravin-t/t HFD versus WT HFD-fed mice, whereas no significant differences were found between HDL (Fig. 4D) and LDL (Fig. 4E) in gravin-t/t HFD and WT HFD-fed mice. In addition, no significant differences were observed between gravin-t/t ND and WT ND-fed mice (Fig. 4, A–E). These results suggest that absence of gravin influences the lipid levels post-HFD treatment and may have a potential role in lipid metabolism in response to hyperlipidemia, likely because of differences in the lipid clearance and/or the reverse cholesterol transport process.
Fig. 4.
Gravin-t/t mice show plasma lipid differences in response to high-fat diet (HFD). Plasma lipid levels were measured from wild-type (WT) and gravin-t/t mice following 16 wk of treatment with either normal diet (ND) or HFD. Plasma level of cholesterol (A), triglyceride (B), and VLDL (C) were significantly lower in gravin-t/t HFD-fed mice compared with WT HFD-fed mice. Plasma levels of HDL (D) and LDL (E) were not significantly different between WT HFD-fed and gravin-t/t HFD-fed mice. Results are presented as mean ± SE. n = 14 (WT ND), n = 12 (WT HFD), n = 12 (Gravin-t/t ND), and n = 11 (Gravin-t/t HFD) (A); n = 11 (WT ND), n = 9 (WT HFD), n = 12 (Gravin-t/t ND), and n = 10 (Gravin-t/t HFD) (B); n = 14 (WT ND), n = 9 (WT HFD), n = 12 (Gravin-t/t ND), and n = 10 (Gravin-t/t HFD) (C); n = 14 (WT ND), n = 11 (WT HFD), n = 12 (Gravin-t/t ND), and n = 13 (Gravin-t/t HFD) (D); and n = 11 (WT ND), n = 10 (WT HFD), n = 10 (Gravin-t/t ND), and n = 12 (Gravin-t/t HFD) (E). *P < 0.05, **P < 0.01, and ***P < 0.001. Comparisons between two groups were determined by an unpaired two-tailed Student’s t test, and comparisons between multiple groups were determined by one-way ANOVA followed by post hoc Tukey test.
Decreased hepatic gene expression involved in lipid and cholesterol metabolism in gravin-t/t mice.
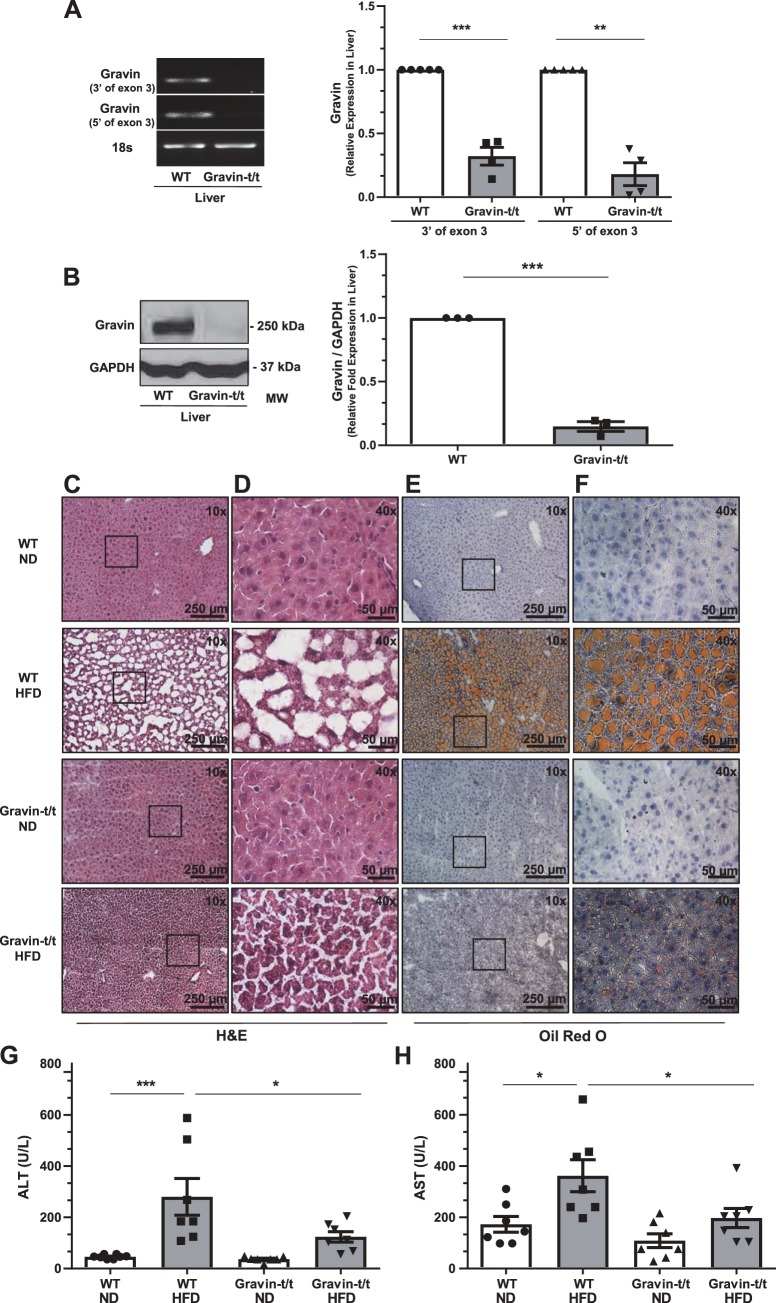
Given that gravin-t/t HFD-fed mice had an altered serum lipid profile and liver is a known regulator of lipid metabolism, we sought to elucidate gravin’s role in the liver-mediated lipid metabolism. We confirmed that liver tissue from gravin-t/t mice also did not express the gravin gene and protein (Fig. 5, A and B). As shown in Fig. 5A, the PCR products of gravin’s exon 3 were decreased in gravin-t/t liver tissue, where liver gravin gene expression was significantly reduced by 64% (3′-end of exon 3) and 78% (5′-end of exon 3). Western blot analysis using the carboxyl-terminal specific gravin antibody confirmed that liver gravin protein expression was also absent in gravin-t/t mice (Fig. 5B).
Fig. 5.
Gravin-t/t mice show reduced hepatic fat accumulation in response to high-fat diet (HFD). Gravin mRNA expression in the liver was quantified by reverse transcriptase quantitative PCR from wild-type (WT) and gravin-t/t mice (A). The liver from gravin-t/t mice showed significantly decreased gravin mRNA expression compared with WT mice. Results are presented as mean ± SE. n = 4; ***P = 0.0006 and **P = 0.0063. Gravin protein expression was measured in the liver by Western blot from WT and gravin-t/t mice, where the gravin antibody recognized the monomeric (250 kDa) forms of the gravin protein (B). The liver from gravin-t/t mice showed significantly decreased gravin protein expression compared with WT mice. Results are presented as mean ± SEM; n = 3; ***P < 0.001). Dissected livers (C–F) were isolated from gravin-t/t and WT mice, sectioned, frozen, and stained with hematoxylin and eosin (H&E; C and D) and Oil Red O (counterstained with hematoxylin; E and F) following 16 wk of treatment with either normal diet (ND) or HFD. Images were measured using brightfield Nikon microscopy at ×10 (C and E) and ×40 (D and F). Boxed areas shown in C and E are shown in higher magnification in D and F, respectively. Serum alanine aminotransferase (ALT; G) and serum aspartate aminotransferase (AST; H) were both decreased in gravin-t/t HFD-fed mice compared with WT HFD-fed mice, showing greater liver damage and more lipid accumulation in gravin-t/t HFD-fed mice compared with WT HFD-fed mice. Results are presented as mean ± SE; n = 7 (G and H); *P < 0.05, **P < 0.01, and ***P < 0.001. Comparisons between two groups were determined by an unpaired two-tailed Student’s t test, and comparisons between multiple groups were determined by one-way ANOVA followed by post hoc Tukey test.
As the liver is a major organ involved in lipogenesis and lipid clearance, we therefore investigated whether fat accumulation in the liver was altered between WT and gravin-t/t mice at the end of the 16-wk treatment with ND or HFD. In Fig. 1D, we showed that the LW/BW ratio was significantly increased in WT HFD-fed mice, but no increase was observed in gravin-t/t HFD-fed mice. Consistent with this observation, we also observed an increase in liver fat accumulation in WT HFD-fed mice as compared with WT ND-fed mice, as indicated by both H&E (Fig. 5, C and D) and Oil Red O (counterstained with hematoxylin; Fig. 5, E and F) stained liver sections. Interestingly, this liver fat accumulation was significantly reduced in gravin-t/t HFD-fed mice compared with WT HFD-fed mice, and no differences in liver fat accumulation were observed between WT ND and gravin-t/t ND-fed mice (Fig. 5C–F). Furthermore, H&E and Oil Red O-stained liver sections from gravin-t/t HFD-fed mice showed less liver damage as compared with WT HFD-fed mice (Fig. 5, C–F). To confirm that the decreased liver fat accumulation in gravin-t/t HFD versus WT HFD-fed mice was associated with decreased liver injury as well, we quantified the most important liver enzyme biomarkers indicated of any kind of liver injury (serum ALT and AST). Consistent with liver damage, we observed an increase in both ALT (Fig. 5G) and AST (Fig. 5H) in WT HFD-fed mice, whereas no significant increase in either ALT or AST levels was observed in gravin-t/t HFD-fed mice. These results indicate that gravin-t/t mice lacking functional gravin in the liver had significantly decreased lipid accumulation and liver damage.
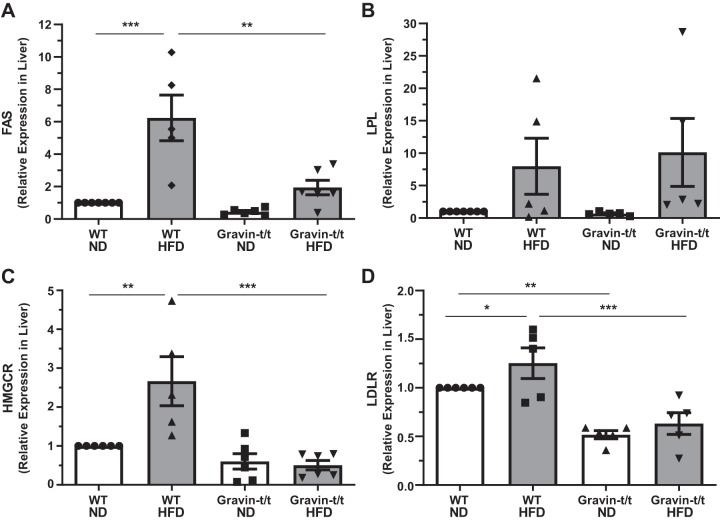
To determine whether altered serum lipid metabolism, as well as liver fat accumulation and damage, were associated with altered lipogenesis in gravin-t/t mice, we next determined whether key hepatic enzymes and receptors involved in lipid and cholesterol metabolism were altered between WT and gravin-t/t mice at the end of the 16-wk treatment of either ND or HFD. We observed a significant increase in liver fatty acid synthase (FAS) gene expression, the enzyme that catalyzes fatty acid synthesis, in WT HFD-fed mice as compared with WT ND-fed mice (Fig. 6A). However, liver FAS gene expression was significantly decreased in gravin-t/t HFD versus WT HFD-fed mice, and no differences were observed between gravin-t/t ND, gravin-t/t HFD, and WT ND-fed mice. In contrast, we observed no significant differences in liver LPL gene expression, the enzyme that hydrolyzes triglycerides into lipoproteins, between the groups of mice (Fig. 6B). Similar to FAS expression, we also observed a significant increase in liver 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR) gene expression, the rate-limiting enzyme within the mevalonate pathway involved in cholesterol biosynthesis, in WT HFD-fed mice as compared with WT ND-fed mice (Fig. 6C), whereas no significant differences were observed between gravin-t/t ND, gravin-t/t HFD, and WT ND-fed mice. Additionally, liver gene expression of low-density lipoprotein receptor (LDLR), the receptor that mediates endocytosis of cholesterol-rich LDL, was decreased in gravin-t/t HFD versus WT HFD-fed mice (Fig. 6D). Interestingly, LDLR liver gene expression was also significantly decreased in gravin-t/t versus WT mice in the absence of increased dietary fat for 16 wk. These results indicate that the lack of functional gravin in the liver is associated with reduced hepatic gene expression involved in lipid and cholesterol metabolism.
Fig. 6.
Gravin-t/t mice show reduced lipid and cholesterol metabolism-related gene expression in response to high-fat diet (HFD). mRNA expression of genes involved in lipid and cholesterol metabolism was quantified in the liver using reverse transcriptase quantitative PCR (RT-qPCR). Genes expressed in the liver related to lipid metabolism, fatty acid synthase (FAS; A) and lipoprotein lipase (LPL; B), and genes expressed in the liver related to cholesterol metabolism, 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR; C) and low-density lipoprotein receptor (LDLR; D), were quantified. Gravin-t/t HFD-fed mice showed significantly decreased liver expression of FAS (A), HMGCR (C), and LDLR (D) as compared with wild-type (WT) HFD-fed mice. Results are presented as the mean ± SE. n = 7 [WT normal diet (ND)], n = 5 (WT HFD), n = 6 (Gravin-t/t ND), and n = 6 (Gravin-t/t HFD) (A); n = 7 (WT ND), n = 5 (WT HFD), n = 5 (Gravin-t/t ND), and n = 5 (Gravin-t/t HFD) (B); and n = 6 (WT ND), n = 5 (WT HFD), n = 6 (Gravin-t/t ND), and n = 6 (Gravin-t/t HFD) (C and D). *P < 0.05, **P < 0.01, and ***P < 0.001. Comparisons between two groups were determined by an unpaired two-tailed Student’s t test, and comparisons between multiple groups were determined by one-way ANOVA followed by post hoc Tukey test.
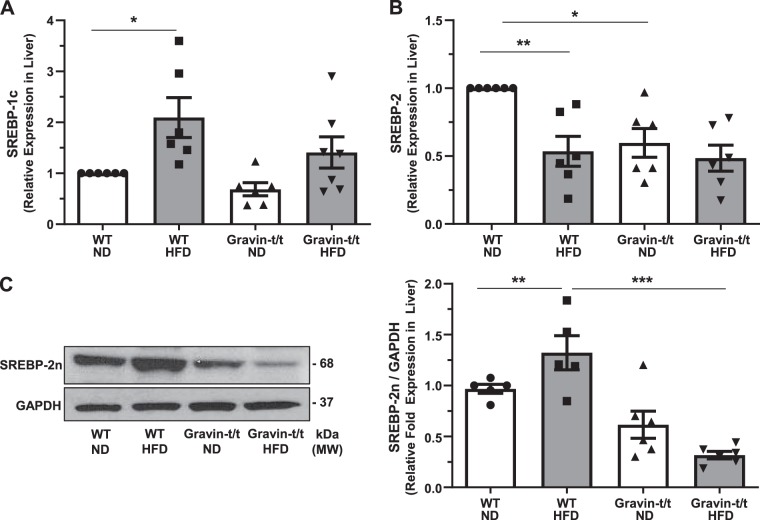
Gravin-dependent regulation of lipid and cholesterol metabolism is associated with SREBP-2.
SREBPs are transcription factors that regulate cholesterol and fatty acid biosynthesis. A decrease in liver SREBP expression may account for the decreased expression of the liver genes, FAS, HMGCR, and LDLR that we observed in Fig. 6. Therefore, we evaluated whether the expression of SREPB genes was altered between WT and gravin-t/t mice at the end of the 16-wk treatment of either ND or HFD. The expression of SREBP-1c, an isoform that regulates de novo lipogenesis, was significantly increased in WT HFD-fed as compared with WT ND-fed mice (Fig. 7A), whereas no significant differences were observed between gravin-t/t ND, gravin-t/t HFD, and WT ND-fed mice. In contrast, gene expression of SREBP-2, the SREBP isoform that regulates genes involved in cholesterol metabolism, was significantly decreased in gravin-t/t ND versus WT ND-fed mice (Fig. 7B). We then determined whether the decreased liver SREBP-2 gene expression correlated with decreased liver protein expression. Interestingly, we uniquely identified that less SREBP-2 precursor was cleaved into the biologically activated nuclear SREBP-2n protein in the gravin-t/t HFD versus WT HFD-fed mice (Fig. 7C). Thus, gravin-dependent regulation of lipid metabolism is likely to be mediated by SREBP-2.
Fig. 7.
Gravin-t/t mice show decreased activation of sterol regulatory element binding protein (SREBP)-2 in response to high-fat diet (HFD). mRNA expression of the cholesterol metabolism genes SREBP-1c (A), SREBP-2 (B), and SREBP-2n (C) were quantified in the liver using reverse transcriptase quantitative PCR. Expression of SREBP-2 (B) was significantly decreased in gravin-t/t normal diet (ND)-fed versus wild-type (WT) ND-fed mice, whereas expression of SREBP-2n, the active form of SREBP-2 found in liver homogenized lysates, was significantly decreased in both gravin-t/t ND-fed and gravin-t/t HFD-fed mice. Results are presented as the mean ± SE. n = 6 (A–C); *P < 0.05, **P < 0.01, and ***P < 0.0001. Comparisons between two groups were determined by an unpaired two-tailed Student’s t test, and comparisons between multiple groups were determined by one-way ANOVA followed by post hoc Tukey test. MM, molecular mass.
Absence of gravin-mediated signaling inhibits PDGF-induced VSMC migration via PKA and PKC signaling.
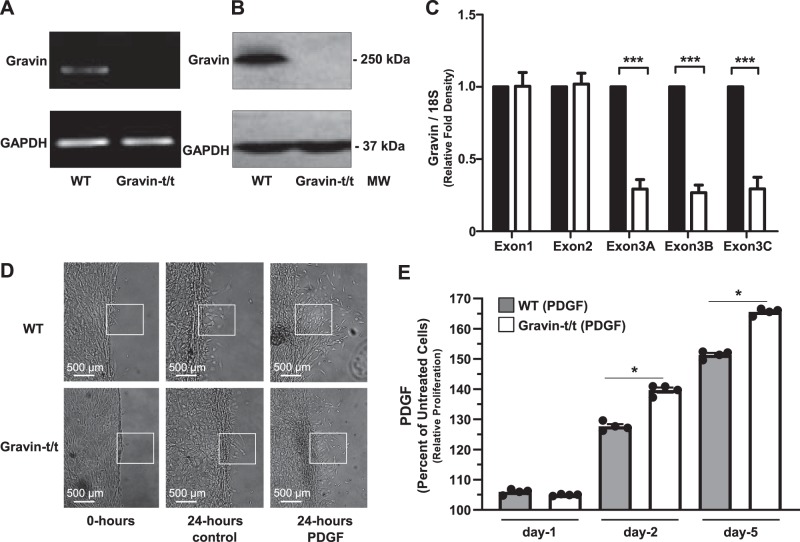
VSMC migration and proliferation are regulated by various growth factors, such as PDGF (51). During atherosclerosis and various other pathological conditions, the expression of PDGF is induced in both vascular and inflammatory cells (51). Therefore, to determine the potential function of gravin in VSMCs, we determined the effects of PDGF as an inducer of VSMC proliferation and migration in VSMCs isolated from the thoracic arteries of nonfed WT and gravin-t/t mice. We confirmed that the expression of the gravin gene was absent in VSMCs isolated from the thoracic arteries of gravin-t/t mice (Fig. 8, A and C). Specifically, three different regions of gravin’s exon 3 were quantified by RT-qPCR, and each region was found to be significantly reduced by ~70% in VSMCs isolated from gravin-t/t mice, whereas gravin’s exon 1 and exon 2 were similarly expressed in the isolated VSMCS. Western blot analysis using the carboxyl-terminal specific gravin antibody confirmed that gravin protein expression was also absent in isolated VSMCS from gravin-t/t mice (Fig. 8B).
Fig. 8.
Migration and proliferation of aortic vascular smooth muscle cells (VSMCs) isolated from wild-type (WT) and gravin-t/t mice in response to PDGF stimulation. Gravin mRNA expression in aortic VSMCs isolated from WT and gravin-t/t mice was quantified using reverse transcriptase quantitative PCR (A). Gravin protein expression was measured in VSMCs by Western blot from WT and gravin-t/t mice, in which the gravin antibody recognized the monomeric (250 kDa) forms of the gravin protein (B). VSMCs isolated from gravin-t/t mice showed significantly decreased gravin protein expression compared with WT mice. Quantification of the RT-PCR data in A shows that exon 3 of gravin (over three different regions) was significantly decreased in gravin-t/t VSMCs compared with WT VSMCs, as expected (C). Gravin’s exon 1 and exon 2 showed no differences in expression, as expected in this gene-trapped model. Results are presented as mean ± SE. n = 3; ***P < 0.001. Scratch wound assay was used to determine VSMC migration in WT and gravin-t/t mice following 24 h with or without PDGF (10 ng/mL) stimulation (D). Boxed areas emphasize areas of cell migration. Quantification of VSMC proliferation in the presence or absence of PDGF was determined using the MTS colorimetric cell proliferation assay on day 1, 3, and 5 after PDGF stimulation (E). Results are presented as the mean ± SE. n = 4; *P < 0.005. Comparisons between two groups were determined by an unpaired two-tailed Student’s t test, and comparisons between multiple groups were determined by one-way ANOVA followed by post hoc Tukey test. MM, molecular mass.
First, we tested whether gravin plays a role in the migration and proliferation of VSMCs in response to PDGF stimulation. WT and gravin-t/t VSMCs were isolated, cultured, and then fed with PDGF (10 ng/mL) to induce cell migration (Fig. 8D). We observed a significant decrease in VSMC migration upon PDGF treatment following 24 h in cells isolated from gravin-t/t mice as compared with WT mice, as determined by the scratch wound test assay (Fig. 8D). Furthermore, in the aortas of gravin-t/t mice, we expect to also observe a corresponding decrease in smooth muscle cell migration from the tunica media into the intima. Furthermore, VSMCs isolated from gravin-t/t mice showed greater cell proliferation versus WT after 2 and 3 days of PDGF treatment, whereas no significant differences were observed between VSMCs isolated from WT and gravin-t/t mice after 1 day of PDGF treatment, as determined by the MTS colorimetric assay (Fig. 8E). These results demonstrate that gravin inhibits PDGF-induced VSMC migration but does not inhibit promoting cell proliferation.
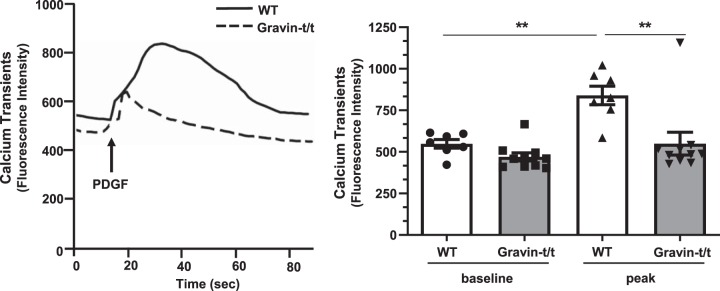
We then tested whether intracellular Ca2+ transients in VSMCs isolated from gravin-t/t mice were different from those of VSMCs isolated from WT mice in response to 10 ng/mL PDGF stimulation. As shown in Fig. 9, PDGF-mediated peak [Ca2+]i transient amplitude was inhibited by ablation of gravin in VSMCs. Furthermore, there was no significant difference between baseline [Ca2+]i between VSMCs isolated from WT and gravin-t/t mice. Next, we tested whether the absence of gravin-mediated signaling in VSMCs affected PKA and PKC signaling in response to 10 ng/mL PDGF stimulation. In contrast to an increase in PKA activity in VSMCs isolated from WT mice in response to PDGF stimulation, we observed no significant difference in PKA activity in VSMCs isolated from gravin-t/t mice, as determined by phosphorylation (Thr197) of the PKA catalytic subunit (Fig. 10A). Furthermore, treatment with the cell permeant calcium chelator BAPTA-AM to remove intracellular Ca2+ did not affect this PKA catalytic subunit phosphorylation in VSMCs isolated from gravin-t/t mice. As expected, PKA-dependent substrate phosphorylation (Ser/Thr) was increased in VSMCs isolated from WT mice in response to PDGF stimulation, and this increased phosphorylation was independent of the presence of BAPTA-AM (Fig. 10B). However, no significant differences were observed in PKA-dependent substrate phosphorylation from VSMCs isolated from gravin-t/t mice in response to PDGF treatment, with or without BAPTA-AM, and where this phosphorylation was significantly less than that observed in VSMCs isolated from WT mice. Thus, PKA activity and substrate-dependent phosphorylation were reduced in VSMCs isolated from gravin-t/t mice. Similar to the PKA catalytic subunit phosphorylation, we also observed an increase in PKC activity in VSMCs isolated from WT mice in response to PDGF stimulation, as determined by the phosphorylation (Thr638/641) of PKCα/βII (Fig. 10C). However, pretreatment with the calcium chelator BAPTA-AM prevented this increase in phosphorylation. Also, we observed no significant difference in PKC phosphorylation in VSMCs isolated from gravin-t/t mice in response to PDGF, and the pretreatment with BAPTA-AM did not affect this PKC phosphorylation. In addition, PKC-dependent substrate phosphorylation (Ser) was increased in VSMCs isolated from WT mice in response to PDGF stimulation; however, this increased phosphorylation was dependent on the presence of intracellular Ca2+ (Fig. 10D). No significant differences were observed in PKC-dependent substrate phosphorylation from VSMCs isolated from gravin-t/t mice in response to PDGF treatment, with or without BAPTA-AM, and this phosphorylation was significantly less than that observed in VSMCs isolated from WT mice. Thus, PKC activity and substrate-dependent phosphorylation were reduced in VSMCs isolated from gravin-t/t mice. Furthermore, our results suggest that [Ca2+]i may be involved in mediating both PKA and PKC signaling pathways in response to treatment with PDGF.
Fig. 9.
Intracellular Ca2+ transient in vascular smooth muscle cells (VSMCs) isolated from wild-type (WT) and gravin-t/t mice in response to PDGF stimulation. Intracellular Ca2+ transients tracing (left) recorded from aortic VSMCs isolated from WT and gravin-t/t mice before and after PDGF (10 ng/mL) stimulation. Quantitative analysis of fluorescence intensity of Ca2+ transients (right) demonstrated that absence of gravin-mediated signaling inhibited PDGF-induced Ca2+ release. Results are presented as the mean ± SE. n = 7 (WT ND), n = 7 [WT high-fat diet (HFD)], n = 10 (Gravin-t/t ND), and n = 10 (Gravin-t/t HFD). **P < 0.01. Comparisons between two groups were determined by an unpaired two-tailed Student’s t test, and comparisons between multiple groups were determined by one-way ANOVA followed by post hoc Tukey test.
Fig. 10.
PKA and PKC activity in vascular smooth muscle cells (VSMCs) isolated from wild-type (WT) and gravin-t/t mice in response to PDGF stimulation. Western blot analysis of homogenates isolated from aortic VSMCs from WT and gravin-t/t mice using antibodies to the phosphorylated (Thr197) PKA catalytic subunit (p-PKA; A), phosphorylated (Ser/Thr) PKA-dependent substrate (p-PKA substrates; B), phosphorylated (Thr638/641) PKCα/βII (p-PKC; C), phosphorylated (Ser) PKC-dependent substrate (p-PKC substrates; D), phosphorylated (Thr202/Tyr204) Erk1/2 (p-Erk1/2; E), and phosphorylated (Ser473) Akt (p-Akt; F), in response to 10 ng/mL PDGF stimulation as well as in the absence or presence of membrane-permeable selective calcium chelator, BAPTA-AM. In each of the images shown in A–F, the top shows a representative Western bot and the bottom shows the corresponding Western blot quantification of the phospho-protein expression. Results are presented as the mean ± SE. n = 5 (WT, Gravin-t/t), n = 4 (WT PDGF, Gravin-t/t PDGF, WT PDGF BAPTA-AM, Gravin-t/t PDGF BAPTA-AM) (A); n = 5 (WT, Gravin-t/t), n = 7 (WT PDGF), n = 6 (Gravin-t/t PDGF), n = 5 (WT PDGF BAPTA-AM, Gravin-t/t PDGF BAPTA-AM) (B); n = 4 (WT, Gravin-t/t, WT PDGF, Gravin-t/t PDGF), n = 3 (WT PDGF BAPTA-AM, Gravin-t/t PDGF BAPTA-AM) (C); n = 5 (WT), n = 4 (Gravin-t/t), n = 4 (WT PDGF), n = 6 (Gravin-t/t PDGF), n = 5 (WT PDGF BAPTA-AM), n = 4 (Gravin-t/t PDGF BAPTA-AM) (D); n = 4 (WT), n = 3 (Gravin-t/t), n = 5 (WT PDGF), n = 4 (Gravin-t/t PDGF, WT PDGF BAPTA-AM, Gravin-t/t PDGF BAPTA-AM) (E); n = 4 (WT, Gravin-t/t) n = 5 WT PDGF, Gravin-t/t PDGF), n = 3 (WT PDGF BAPTA-AM, Gravin-t/t PDGF BAPTA-AM) (F). *P < 0.05, **P < 0.01, and ***P < 0.0001. Comparisons between two groups were determined by an unpaired two-tailed Student’s t test, and comparisons between multiple groups were determined by one-way ANOVA followed by post hoc Tukey test. MM, molecular mass.
Finally, to specifically determine whether PKC-dependent Erk1/2 phosphorylation was depending on gravin-mediated signaling, we measured PDGF (10 ng/mL) induced Erk1/2 phosphorylation in VSMCs isolated from gravin-t/t and WT mice. We observed an increase in Erk1/2 phosphorylation (Thr202/Tyr204) in VSMCs isolated from both WT and gravin-t/t mice in response to PDGF stimulation (Fig. 10E). However, this PDGF-induced increase in Erk1/2 phosphorylation was lower in VSMCs isolated from gravin-t/t versus WT mice. Although pretreatment with BAPTA-AM decreased the PDGF-induced Erk1/2 phosphorylation in VSMCs isolated from WT mice, BAPTA-AM pretreatment had no effect in VSMCs isolated from gravin-t/t mice. Lastly, to determine whether gravin-mediated signaling may prevent the initiation and progression of atherosclerosis, we determined whether disruption of PKC/gravin interaction affected Akt activity. As shown in Fig. 10F, no significant differences were observed in PDGF-stimulated Akt phosphorylation (Ser473) in VSMCs isolated from WT and gravin-t/t mice. These results indicate that gravin modulates PDGF receptor-mediated signaling via the PKC/Erk1/2 signaling pathway and that this PDGF-mediated/Ca2+-dependent cell migration is suppressed in VSMCs isolated from gravin-t/t mice.
DISCUSSION
Cardiovascular diseases (including hypertension, stroke, and coronary artery diseases) remain the number one cause of mortality in the United State (5). In particular, atherosclerosis is a chronic lipid-driven inflammatory disease that is marked with arterial lumen narrowing (26). Atherosclerosis is linked with a systemic inflammatory state characterized by endothelial dysfunction, extensive lipid deposition in the intima, exacerbated innate and adaptive immune responses, proliferation of VSMCs, and remodeling of the ECM, which together lead to the formation of the atherosclerotic plaque (4, 6, 26, 33).
Cell signaling pathways cause numerous cellular responses and the diversity of complex cellular responses is mediated, in part, by scaffolding proteins. Scaffolding proteins assemble intracellular signaling components of a network cascade into a specific assembly within the cell, thereby enhancing signaling specificity as well as enhancing signaling efficiency. In particular, AKAPs are scaffolding protein “signalosomes” that fine-tune cellular responses by forming multicomponent protein complexes. In regard to cardiac muscle, the vigor of the contraction of the muscle is regulated by multiple processes that direct the movement of actin and myosin filaments in the cardiac sarcomere. Cardiac contractility is mainly dependent on the level of Ca2+, in which it is controlled mainly through β-AR signaling (14). It has been shown previously that, because of β-AR activation, cAMP/PKA-dependent substrates phosphorylation is highly dependent on the colocalization of PKA in close proximity to its substrates (10, 41, 48). Hence, AKAPs play an important role in the regulation of cardiac signaling (1, 9, 24, 32, 37, 61, 68, 69). More specifically, gravin facilitates the process of phosphorylation of the β2-AR by both PKA and PKC as well as regulating the desensitization/resensitization progression to terminate the signaling cycle of the receptor (20). Hence, impediment of the termination of β2-AR signaling would be expected to increase cardiac performance.
In the current study, we present data supporting a new role of gravin as a regulator of vascular inflammation and lipid metabolism. We report, for the first time, the function of gravin in HFD-induced atherosclerosis. Our findings, using our gravin-t/t mouse model, provide insights between gravin and proatherosclerotic signaling involved in hepatic lipogenesis and VSMC proliferation and migration. We revealed that the absence of gravin-mediated signaling prevents the development of atherosclerosis induced by HFD in mice.
Our results demonstrate that the absence of gravin-mediated signaling protects against hyperlipidemia and the development and progression of atherosclerosis induced by HFD. Gravin-t/t HFD-fed mice showed decreased body weight gain as well as decreased cholesterol, triglyceride, and VLDL, which together resulted in fewer aorta lipid lesions, whereas no difference was observed in LDL, presumably because of the LDL cholesterol protein content as well as the reduction in the HMGCR enzyme observed in gravin-t/t HFD-fed mice. As expected in WT mice, HFD treatment caused atherosclerosis-related phenotypes, including an excessive gain in body weight, aortic plaque formation, altered lipid profiles, and a fatty liver. This aortic plaque formation is associated with narrowing of the arteries, limiting blood flow to the heart and other parts of the body, and thus increasing the risk for atherosclerosis and cardiovascular diseases.
Others have shown that MMP, with its endopeptidase activity, is able to cleave various ECM proteins, including those involved in atherosclerosis and heart failure, and thus regulate both physiological as well as pathological signaling (27). Furthermore, it has been shown that MMP-9 can be used as a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation (27). We found in the current report that gravin-t/t mice showed decreased MMP-9 expression in aortic sections, which could potentially explain the reduced atherosclerotic vascular remodeling and plaque formation. Activation of macrophage and increased expression of MMPs have been shown to contribute to the development of human atherosclerotic lesions (21, 35). It has also been shown that overexpression of gravin downregulates the expression of MMP-9 in a carcinogenic cell line, where MMP-9 is involved in endothelial cell migration via downregulation of activator protein-1 and its transcriptional activity (35). A possible explanation for gravin’s distinct roles in regulating MMPs may instead be related to the different substrates’ specificities between the endothelial cell and VSMCs. It has been shown that gravin binds to PKC and plays a central role in regulating several cell signaling pathways that are related to cell remodeling and apoptosis (25). In addition, PKC regulates MMP-9 expression and VSMC migration (17). Thus, gravin may regulate expression of MMP-9 via the PKC/Ca2+/ERK signaling pathway, which in turn mediates macrophage-induced atherosclerotic plaque formation. Hence, blockade of functional gravin protein would reduce the expression of aortic MMPs, specifically MMP-9, and thus cause a reduction in the risk to develop atherosclerosis. Furthermore, a decrease in MMP-9 expression is consistent with the reduction we observed in aortic F4/80 expression in gravin-t/t HFD-fed mice. Additionally, these results are further confirmed by the decreased PKC-dependent substrate phosphorylation induced by PDGF observed in VSMCs isolated from gravin-t/t versus WT mice.
PDGF is a key survival growth factor that promotes cell growth and proliferation (43) while inhibiting apoptosis (56). It is one of the most important mitogens released at vasculature injury, resulting in proliferation of the VSMCs (59). It provides the necessary stimulation that prevents apoptosis, the default of proliferating cells. We have shown a decrease in PKA and PKC-dependent substrate phosphorylation, as well as a decreased Ca2+ transient in PDGF-stimulated gravin-t/t VSMCs. This observation indicates that gravin is involved in PDGF signaling via Ca2+-dependent PKA and PKC signaling pathways. In addition, it is known that through protein kinase B/Akt signaling, NF-κB can be activated via PDGF stimulation. This NF-κB activation can then induce cell proliferation and thus inhibit apoptosis (56). Furthermore, PKC can regulate the activation of Akt to modify cell apoptosis (36). However, we observed that disrupting the PKC/gravin interactions did not affect Akt activity in our study. The observed differences in PDGF-stimulated cell proliferation in WT versus gravin-t/t VSMCs is not mediated through the protein kinase B/Akt signaling pathway, and other pathways may be involved.
Lipid metabolism disorders induced by HFD cause excessive accumulation of lipids in various tissues, especially in the liver. A complex network of molecular mediators regulates lipid metabolism, including several proteins related to lipid and cholesterol biosynthesis. Our study showed that the pathological effects of HFD were decreased in the absence of gravin-mediated signaling. Specifically, we observed decreased liver damage and lipid accumulation in the gravin-t/t HFD-fed mice.
In the liver, SREBPs are the protein sensors that monitor sterol concentrations and are the primary transcription factors that regulate cholesterol, fatty acid, and triglyceride biosynthesis and uptake (8, 23). When cholesterol concentrations are low, the SREBPs form a complex with the SREBP cleavage-activating protein (SCAP), which then moves from the endoplasmic reticulum into the Golgi apparatus. Following the cleavage of SREBP, the active form of the protein stimulates the transcription of the genes essential for cholesterol biosynthesis and transport (23). In mammalian cells, SREBP-1c and SREBP-2 are two related isoforms of the SREBP family of transcription factors. SREBP-1c mainly stimulates the expression of genes related to fatty acid synthesis, such as FAS. In contrast, cholesterol synthesis is regulated by SREBP-2, in which SREBP-2 targets the transcription of key cholesterogenic genes, including HMGCR and LDL receptors (8). Although there was no effect of gravin on SREBP-1c expression in the presence or absence of HFD treatment, SREBP-2 showed an effect. We observed in gravin-t/t mice that gene expression of both LDLR and SREBP-2 were downregulated and that this gene expression was decreased irrespective of the ND or HFD diet. Relevant to our findings, Choi and colleagues (13) reported that gravin participates in cholesterol metabolism via SREBP-2 activation and that overexpression of gravin activates SREBP-2 in a SCAP-dependent manner. This is consistent with our results, in which we showed that gravin-t/t mice had decreased SREBP-2 gene expression, resulting in downregulation of HMGCR and LDLR and thus lower circulatory cholesterol levels. Thus, we believe that gravin is involved in the escort process of SREBP-2 to the Golgi apparatus in a SCAP-dependent manner and that the decreased gravin-dependent SREBP-2 activation consequently decreased the circulatory plasma lipids. Alternatively, the absence of gravin-mediated signaling in gravin-t/t mice resulted in decreased SREBP-2 gene expression and specifically decreased activation of the mature nuclear SREBP-2 (SREBP-2n) protein isoform that is transported into the nucleus, resulting in downregulation of the cholesterogenic gene targets. Together, by inhibiting gravin-mediated signaling, SREBP-2 is downregulated, leading to decreased levels of SREBP-2-dependent cholesterol synthesis. This, in turn, reduces serum lipid levels and ultimately lowers the risk of vascular plaque formation.
We acknowledge limitations in our study. Our gravin-t/t mouse model was bred into the C57BL/6J strain and treated to an HFD to induce atherosclerosis. However, it has been shown that the C57BL/6J strain is very sensitive and susceptible to the development of atherosclerosis (45, 46, 60) and thus potentially masks the ability to observe differences between ND and HFD-fed mice. Although there is no single animal model that perfectly mimics the pathology of atherosclerosis (67), we believe the observation showing the lack of atherosclerosis development in our gravin-t/t mice provides strong evidence that gravin plays an important role in HFD-induced hyperlipidemia/atherosclerosis in this mouse strain. An additional limitation of this study is that only adult male mice were studied. Thus, our future studies will characterize both females and males in aged mice, since cardiovascular disease is known to be prevalent in the aging population (29). Overall, our study is the first to demonstrate that the absence of gravin-mediated signaling exhibits resistance to the development of hyperlipidemia and atherosclerosis. Future studies utilizing other models of atherosclerosis, including the carotid artery ligation model to induce arterial injury, as well as breeding our gravin-t/t onto transgenic mouse models of atherosclerosis, LDLR−/− and ApoE−/− mouse models, will be useful to further characterize the absence of gravin-mediated signaling complex and its potential role in delaying hyperlipidemia and atherosclerosis.
In conclusion, our findings showed the involvement of gravin in regulating lipid metabolism and plaque formation during atherosclerosis initiation and progression. We have shown that gravin-t/t mice fed with HFD exhibit resistance to the development of atherosclerosis, as evidenced by lower levels of extracellular lipids, which are associated with downregulation of SREBP-2 and its downstream targets. These data provide insights into the novel role of gravin in regulating extracellular lipids. Further studies to understand the exact mechanism underlying this association are warranted and can further aid in the development of gravin as a potential target for atherosclerosis therapeutics.
GRANTS
Research reported in this paper was supported by the National Heart, Lung, and Blood Institute Grants R01-HL-085487 (to B. K. McConnell), R15-HL-124458 (to B. K. McConnell), R15-HL-141963 (to B. K. McConnell), and R01-HL-122769 (to Y. Zhang)]; American Heart Association Grant 18AIREA33960175 (to B. K. McConnell)]; and a grant from the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation (to B. K. McConnell).
DISCLAIMERS
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.F., X.Y., and B.K.M. conceived and designed research; Q.F., X.Y., A.D.D., C.S.W., S.V.S., H.H.V., and M.S. performed experiments; Q.F., X.Y., A.D.D., C.S.W., S.V.S., H.H.V., M.S., and B.K.M. analyzed data; Q.F., X.Y., A.R., A.D.D., C.S.W., S.S., S.V.S., H.H.V., M.S., Y.Z., and B.K.M. interpreted results of experiments; Q.F. and X.Y. prepared figures; Q.F. and B.K.M. drafted manuscript; Q.F., A.R., A.D.D., S.S., M.S., Y.Z., and B.K.M. edited and revised manuscript; Q.F., X.Y., A.R., A.D.D., C.S.W., S.S., S.V.S., H.H.V., M.S., Y.Z., and B.K.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The data in this article are based on the dissertation thesis submitted in partial fulfillment of the requirements for a PhD (Pharmacology) in the Department of Pharmacological and Pharmaceutical Sciences in the College of Pharmacy at the University of Houston (Q. Fan).
REFERENCES
- 1.Appert-Collin A, Cotecchia S, Nenniger-Tosato M, Pedrazzini T, Diviani D. The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates α1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci USA 104: 10140–10145, 2007. doi: 10.1073/pnas.0701099104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assini JM, Mulvihill EE, Sutherland BG, Telford DE, Sawyez CG, Felder SL, Chhoker S, Edwards JY, Gros R, Huff MW. Naringenin prevents cholesterol-induced systemic inflammation, metabolic dysregulation, and atherosclerosis in Ldlr−/− mice. J Lipid Res 54: 711–724, 2013. doi: 10.1194/jlr.M032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäck M, Hansson GK. Anti-inflammatory therapies for atherosclerosis. Nat Rev Cardiol 12: 199–211, 2015. doi: 10.1038/nrcardio.2015.5. [DOI] [PubMed] [Google Scholar]
- 4.Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med 276: 618–632, 2014. doi: 10.1111/joim.12296. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137: e67–e492, 2018. [Erratum in: Circulation 137: e493, 2018.] doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 6.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res 114: 1852–1866, 2014. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 7.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev 81: 999–1030, 2001. doi: 10.1152/physrev.2001.81.3.999. [DOI] [PubMed] [Google Scholar]
- 8.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89: 331–340, 1997. doi: 10.1016/S0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 9.Cariolato L, Cavin S, Diviani D. A-kinase anchoring protein (AKAP)-Lbc anchors a PKN-based signaling complex involved in α1-adrenergic receptor-induced p38 activation. J Biol Chem 286: 7925–7937, 2011. doi: 10.1074/jbc.M110.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnegie GK, Means CK, Scott JD. A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life 61: 394–406, 2009. doi: 10.1002/iub.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm 2013: 1–14, 2013. doi: 10.1155/2013/928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chillar AJ, Karimi P, Tang K, Ruan KH. An agonist sensitive, quick and simple cell-based signaling assay for determination of ligands mimicking prostaglandin E2 or E1 activity through subtype EP1 receptor: Suitable for high throughput screening. BMC Complement Altern Med 11: 11, 2011. doi: 10.1186/1472-6882-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi MC, Lee YU, Kim SH, Lee JH, Park JH, Streb JW, Oh DY, Im SA, Kim TY, Jong HS, Bang YJ. Overexpression of A-kinase anchoring protein 12A activates sterol regulatory element binding protein-2 and enhances cholesterol efflux in hepatic cells. Int J Biochem Cell Biol 40: 2534–2543, 2008. doi: 10.1016/j.biocel.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Chung JH, Biesiadecki BJ, Ziolo MT, Davis JP, Janssen PM. Myofilament calcium sensitivity: role in regulation of in vivo cardiac contraction and relaxation. Front Physiol 7: 562, 2016. doi: 10.3389/fphys.2016.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen Tervaert JW. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Pract Res Clin Rheumatol 27: 33–44, 2013. doi: 10.1016/j.berh.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Diliberto PA, Gordon GW, Yu CL, Earp HS, Herman B. Platelet-derived growth factor (PDGF) alpha receptor activation modulates the calcium mobilizing activity of the PDGF beta receptor in Balb/c3T3 fibroblasts. J Biol Chem 267: 11888–11897, 1992. [PubMed] [Google Scholar]
- 17.Ding Q, Chai H, Mahmood N, Tsao J, Mochly-Rosen D, Zhou W. Matrix metalloproteinases modulated by protein kinase Cε mediate resistin-induced migration of human coronary artery smooth muscle cells. J Vasc Surg 53: 1044–1051, 2011. doi: 10.1016/j.jvs.2010.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diviani D, Maric D, Pérez López I, Cavin S, Del Vescovo CD. A-kinase anchoring proteins: molecular regulators of the cardiac stress response. Biochim Biophys Acta 1833: 901–908, 2013. doi: 10.1016/j.bbamcr.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med 8: 1249–1256, 2002. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 20.Fan G, Shumay E, Wang H, Malbon CC. The scaffold protein gravin (cAMP-dependent protein kinase-anchoring protein 250) binds the beta 2-adrenergic receptor via the receptor cytoplasmic Arg-329 to Leu-413 domain and provides a mobile scaffold during desensitization. J Biol Chem 276: 24005–24014, 2001. doi: 10.1074/jbc.M011199200. [DOI] [PubMed] [Google Scholar]
- 21.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 94: 2493–2503, 1994. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelman IH. The role of SSeCKS/gravin/AKAP12 scaffolding proteins in the spaciotemporal control of signaling pathways in oncogenesis and development. Front Biosci 7: d1782–d1797, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell 124: 35–46, 2006. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Guillory AN, Yin X, Wijaya CS, Diaz Diaz AC, Rababa’h A, Singh S, Atrooz F, Sadayappan S, McConnell BK. Enhanced cardiac function in Gravin mutant mice involves alterations in the β-adrenergic receptor signaling cascade. PLoS One 8: e74784, 2013. doi: 10.1371/journal.pone.0074784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo LW, Gao L, Rothschild J, Su B, Gelman IH. Control of protein kinase C activity, phorbol ester-induced cytoskeletal remodeling, and cell survival signals by the scaffolding protein SSeCKS/GRAVIN/AKAP12. J Biol Chem 286: 38356–38366, 2011. doi: 10.1074/jbc.M111.258830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta KK, Ali S, Sanghera RS. Pharmacological options in atherosclerosis: a review of the existing evidence. Cardiol Ther 8: 5–20, 2019. doi: 10.1007/s40119-018-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 139: 32–40, 2013. doi: 10.1016/j.pharmthera.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halade GV, Kain V. Obesity and cardiometabolic defects in heart failure pathology. Compr Physiol 7: 1463–1477, 2017. doi: 10.1002/cphy.c170011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging (Albany NY) 8: 2611–2634, 2016. doi: 10.18632/aging.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ketelhuth DF, Hansson GK. Modulation of autoimmunity and atherosclerosis - common targets and promising translational approaches against disease. Circ J 79: 924–933, 2015. doi: 10.1253/circj.CJ-15-0167. [DOI] [PubMed] [Google Scholar]
- 31.Koupenova M, Johnston-Cox H, Vezeridis A, Gavras H, Yang D, Zannis V, Ravid K. A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation 125: 354–363, 2012. doi: 10.1161/CIRCULATIONAHA.111.057596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kritzer MD, Li J, Passariello CL, Gayanilo M, Thakur H, Dayan J, Dodge-Kafka K, Kapiloff MS. The scaffold protein muscle A-kinase anchoring protein β orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circ Heart Fail 7: 663–672, 2014. doi: 10.1161/CIRCHEARTFAILURE.114.001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kume N. [Molecular mechanisms of coronary atherosclerotic plaque formation and rupture]. Nihon Rinsho 68: 637–641, 2010. [PubMed] [Google Scholar]
- 34.Langenickel TH, Olive M, Boehm M, San H, Crook MF, Nabel EG. KIS protects against adverse vascular remodeling by opposing stathmin-mediated VSMC migration in mice. J Clin Invest 118: 3848–3859, 2008. doi: 10.1172/JCI33206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SW, Jung KH, Jeong CH, Seo JH, Yoon DK, Suh JK, Kim KW, Kim WJ. Inhibition of endothelial cell migration through the down-regulation of MMP-9 by A-kinase anchoring protein 12. Mol Med Rep 4: 145–149, 2011. doi: 10.3892/mmr.2010.389. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Sampat K, Hu N, Zakari J, Yuspa SH. Protein kinase C negatively regulates Akt activity and modifies UVC-induced apoptosis in mouse keratinocytes. J Biol Chem 281: 3237–3243, 2006. doi: 10.1074/jbc.M512167200. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Singh S, Suryavanshi SV, Ding W, Shen X, Wijaya CS, Gao WD, McConnell BK. Force development and intracellular Ca2+ in intact cardiac muscles from gravin mutant mice. Eur J Pharmacol 807: 117–126, 2017. doi: 10.1016/j.ejphar.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Palmer D, Jimmo SL, Tilley DG, Dunkerley HA, Pang SC, Maurice DH. Expression of phosphodiesterase 4D (PDE4D) is regulated by both the cyclic AMP-dependent protein kinase and mitogen-activated protein kinase signaling pathways. A potential mechanism allowing for the coordinated regulation of PDE4D activity and expression in cells. J Biol Chem 275: 26615–26624, 2000. doi: 10.1074/jbc.M001634200. [DOI] [PubMed] [Google Scholar]
- 39.Lusis AJ. Atherosclerosis. Nature 407: 233–241, 2000. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maganto-Garcia E, Tarrio M, Lichtman AH. Mouse models of atherosclerosis. Curr Protoc Immunol 96: 15.24.1–15.24.23, 2012. 10.1002/0471142735.im1524s96. [DOI] [PubMed] [Google Scholar]
- 41.Manni S, Mauban JH, Ward CW, Bond M. Phosphorylation of the cAMP-dependent protein kinase (PKA) regulatory subunit modulates PKA-AKAP interaction, substrate phosphorylation, and calcium signaling in cardiac cells. J Biol Chem 283: 24145–24154, 2008. doi: 10.1074/jbc.M802278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McConnell BK, Popovic Z, Mal N, Lee K, Bautista J, Forudi F, Schwartzman R, Jin JP, Penn M, Bond M. Disruption of protein kinase A interaction with A-kinase-anchoring proteins in the heart in vivo: effects on cardiac contractility, protein kinase A phosphorylation, and troponin I proteolysis. J Biol Chem 284: 1583–1592, 2009. doi: 10.1074/jbc.M806321200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monje P, Marinissen MJ, Gutkind JS. Phosphorylation of the carboxyl-terminal transactivation domain of c-Fos by extracellular signal-regulated kinase mediates the transcriptional activation of AP-1 and cellular transformation induced by platelet-derived growth factor. Mol Cell Biol 23: 7030–7043, 2003. doi: 10.1128/MCB.23.19.7030-7043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol 28: 2108–2114, 2008. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 45.Nishina PM, Lowe S, Verstuyft J, Naggert JK, Kuypers FA, Paigen B. Effects of dietary fats from animal and plant sources on diet-induced fatty streak lesions in C57BL/6J mice. J Lipid Res 34: 1413–1422, 1993. [PubMed] [Google Scholar]
- 46.Nishina PM, Wang J, Toyofuku W, Kuypers FA, Ishida BY, Paigen B. Atherosclerosis and plasma and liver lipids in nine inbred strains of mice. Lipids 28: 599–605, 1993. doi: 10.1007/BF02536053. [DOI] [PubMed] [Google Scholar]
- 47.Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rababa’h A, Singh S, Suryavanshi SV, Altarabsheh SE, Deo SV, McConnell BK. Compartmentalization role of A-kinase anchoring proteins (AKAPs) in mediating protein kinase A (PKA) signaling and cardiomyocyte hypertrophy. Int J Mol Sci 16: 218–229, 2014. doi: 10.3390/ijms16010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature 451: 904–913, 2008. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 50.Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med 5: 927–946, 2014. [PMC free article] [PubMed] [Google Scholar]
- 51.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev 15: 237–254, 2004. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Raines EW, Koyama H, Carragher NO. The extracellular matrix dynamically regulates smooth muscle cell responsiveness to PDGF. Ann NY Acad Sci 902: 39–52, 2000. doi: 10.1111/j.1749-6632.2000.tb06299.x. [DOI] [PubMed] [Google Scholar]
- 53.Ranganathan G, Pokrovskaya I, Ranganathan S, Kern PA. Role of A kinase anchor proteins in the tissue-specific regulation of lipoprotein lipase. Mol Endocrinol 19: 2527–2534, 2005. doi: 10.1210/me.2005-0144. [DOI] [PubMed] [Google Scholar]
- 54.Robinson JG, Fox KM, Bullano MF, Grandy S; SHIELD Study Group . Atherosclerosis profile and incidence of cardiovascular events: a population-based survey. BMC Cardiovasc Disord 9: 46, 2009. doi: 10.1186/1471-2261-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogne M, Taskén K. Compartmentalization of cAMP signaling in adipogenesis, lipogenesis, and lipolysis. Horm Metab Res 46: 833–840, 2014. doi: 10.1055/s-0034-1389955. [DOI] [PubMed] [Google Scholar]
- 56.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401: 86–90, 1999. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 57.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 340: 115–126, 1999. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 58.Saqr HE, Guan Z, Yates AJ, Stokes BT. Mechanisms through which PDGF alters intracellular calcium levels in U-1242 MG human glioma cells. Neurochem Int 35: 411–422, 1999. doi: 10.1016/S0197-0186(99)00092-3. [DOI] [PubMed] [Google Scholar]
- 59.Sarjeant JM, Lawrie A, Kinnear C, Yablonsky S, Leung W, Massaeli H, Prichett W, Veinot JP, Rassart E, Rabinovitch M. Apolipoprotein D inhibits platelet-derived growth factor-BB-induced vascular smooth muscle cell proliferated by preventing translocation of phosphorylated extracellular signal regulated kinase 1/2 to the nucleus. Arterioscler Thromb Vasc Biol 23: 2172–2177, 2003. doi: 10.1161/01.ATV.0000100404.05459.39. [DOI] [PubMed] [Google Scholar]
- 60.Schreyer SA, Wilson DL, LeBoeuf RC. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis 136: 17–24, 1998. doi: 10.1016/S0021-9150(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 61.Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature 364: 240–243, 1993. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- 62.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 78: 539–552, 2009. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang CY, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol 821: 421–433, 2012. doi: 10.1007/978-1-61779-430-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang HY, Tao J, Shumay E, Malbon CC. G-protein-coupled receptor-associated A-kinase anchoring proteins: AKAP79 and AKAP250 (gravin). Eur J Cell Biol 85: 643–650, 2006. doi: 10.1016/j.ejcb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Wilde DW, Massey KD, Walker GK, Vollmer A, Grekin RJ. High-fat diet elevates blood pressure and cerebrovascular muscle Ca(2+) current. Hypertension 35: 832–837, 2000. doi: 10.1161/01.HYP.35.3.832. [DOI] [PubMed] [Google Scholar]
- 66.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol 5: 959–970, 2004. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 67.Ye P, Cheah IK, Halliwell B. High fat diets and pathology in the guinea pig. Atherosclerosis or liver damage? Biochim Biophys Acta 1832: 355–364, 2013. doi: 10.1016/j.bbadis.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 68.Yu H, Yuan C, Westenbroek RE, Catterall WA. The AKAP Cypher/Zasp contributes to β-adrenergic/PKA stimulation of cardiac CaV1.2 calcium channels. J Gen Physiol 150: 883–889, 2018. doi: 10.1085/jgp.201711818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang M, Patriarchi T, Stein IS, Qian H, Matt L, Nguyen M, Xiang YK, Hell JW. Adenylyl cyclase anchoring by a kinase anchor protein AKAP5 (AKAP79/150) is important for postsynaptic β-adrenergic signaling. J Biol Chem 288: 17918–17931, 2013. doi: 10.1074/jbc.M112.449462. [DOI] [PMC free article] [PubMed] [Google Scholar]