Abstract
Atherosclerosis is the most common underlying cause of cardiovascular morbidity and mortality worldwide. c-Kit (CD117) is a member of the receptor tyrosine kinase family, which regulates differentiation, proliferation, and survival of multiple cell types. Recent studies have shown that c-Kit and its ligand stem cell factor (SCF) are present in arterial endothelial cells and smooth muscle cells (SMCs). The role of c-Kit in cardiovascular disease remains unclear. The aim of the current study is to determine the role of c-Kit in atherogenesis. For this purpose, atherosclerotic plaques were quantified in c-Kit-deficient mice (KitMut) after they were fed a high-fat diet (HFD) for 16 wk. KitMut mice demonstrated substantially greater atherosclerosis compared with control (KitWT) littermates (P < 0.01). Transplantation of c-Kit-positive bone marrow cells into KitMut mice failed to rescue the atherogenic phenotype, an indication that increased atherosclerosis was associated with reduced arterial c-Kit. To investigate the mechanism, SMC organization and morphology were analyzed in the aorta by histopathology and electron microscopy. SMCs were more abundant, disorganized, and vacuolated in aortas of c-Kit mutant mice compared with controls (P < 0.05). Markers of the “contractile” SMC phenotype (calponin, SM22α) were downregulated with pharmacological and genetic c-Kit inhibition (P < 0.05). The absence of c-Kit increased lipid accumulation and significantly reduced the expression of the ATP-binding cassette transporter G1 (ABCG1) necessary for lipid efflux in SMCs. Reconstitution of c-Kit in cultured KitMut SMCs resulted in increased spindle-shaped morphology, reduced proliferation, and elevated levels of contractile markers, all indicators of their restored contractile phenotype (P < 0.05).
NEW & NOTEWORTHY This study describes the novel vasculoprotective role of c-Kit against atherosclerosis and its function in the preservation of the SMC contractile phenotype.
Keywords: atheroma, atherosclerosis, c-Kit, mouse, phenotypic switch, smooth muscle cell
INTRODUCTION
Atherosclerosis is the most common underlying cause of cardiovascular morbidity and mortality worldwide. Three major factors contribute to the progression of atherosclerosis: pathological accumulation of cholesterol in the arterial wall, inflammation, and invasion of synthetic pro-inflammatory smooth muscle cells (SMCs) into the vascular subendothelium to further promote plaque growth and encapsulate lipid-rich cells beneath a fibrous cap (18). Multiple studies indicate that phenotypic switching of SMCs is influenced by the activity of receptor tyrosine kinases (RTKs), such as the platelet-derived growth factor receptor (PDGFR) and vascular endothelium growth factor receptor (VEGFR) (2, 20, 47). Increased activity of PDGFRβ or VEGFR promotes plaque formation in hyperlipidemic mice compared with littermate controls (20, 21). However, in a phase III clinical trial, RTK inhibition failed to benefit patients in their fight against SMC-proliferative diseases such as restenosis (50).
c-Kit (CD117) is a member of the RTK family that regulates differentiation, proliferation, and survival of hematopoietic stem cells (16). This receptor is also commonly used to identify stem cells in the heart, and gain or loss of c-Kit function has been implicated in carcinogenesis (28). Recent studies have shown that c-Kit and its ligand stem cell factor (SCF) are present in arterial endothelial cells and SMCs (22, 34). Evidence of c-Kit’s role in vascular disease is presently limited to descriptive data for progression of neointimal hyperplasia after balloon injury in rats (22, 35). Knockdown of c-Kit decreased expression of myocardin-regulated contractile genes in primary SMC cultures (15), suggesting a key role for this RTK in promoting full SMC differentiation. We have recently revealed the importance of c-Kit in the preservation of SMC’s anti-inflammatory signaling when challenged with oxidized phospholipids (43). In light of this in vitro evidence, the influence of c-Kit signaling on vascular disease deserves further attention.
In the present work, we studied the role of c-Kit signaling in atherogenesis to answer questions such as the following: What is the function of c-Kit during atherosclerosis? Does it behave like other RTKs? Does c-Kit control the SMC phenotype in vivo? We investigated the role of c-Kit in vivo by inducing atherosclerosis in mice with global loss of c-Kit function (KitMut).
METHODS
Animals
Kit promoter activity was monitored in c-KitBac-eGFP mice obtained from Dr. Michael I. Kotlikoff’s laboratory (Cornell University, Ithaca, NY) (44). This transgenic mouse allows eGFP expression under the full Kit promoter. Kit mutant mice KitW/+ and KitW-v/+ mice (stock no. 100410; The Jackson Laboratories, Bar Harbor, ME; https://www.jax.org/strain/100410) were individually cross-bred with the atherosclerosis prone apolipoprotein E (apoE)−/− mouse (stock no. 002052; The Jackson Laboratories; https://www.jax.org/strain/002052) (48). Crossing of KitW/+ apoE−/− and KitW-v/+ apoE−/− mice generated KitMut (KitW-v/W apoE−/−) and control littermates KitWT (Kit+/+ apoE−/−). Both KitMut mice and control littermates were fed a high-fat diet (HFD; TD 88137; Harlan, Indianapolis, IN) for 16 wk, followed by quantification of atherosclerotic burden in the aorta.
Kit Mutant Chimeric Mice
To examine the effect of bone marrow-derived c-Kit on atherosclerosis, lethally irradiated KitMut (KitW-v/W apoE−/−) and control littermates KitWT (Kit+/+ apoE−/−) were recipients of bone marrow transplants from KitWT (Kit+/+ apoE−/−) donors. The bone marrow was flushed from donor femurs and tibia with cold 2% FBS DMEM. Red blood cells (RBC) were removed from the single-cell suspension using a RBC lysis solution (155 mM NH4Cl, 12 mM NaHCO3, and 0.1 mM EDTA). Lethally irradiated recipient mice received 2 × 106 bone marrow (BM) mononuclear cells intravenously from age-matched KitWT donors (27). Chimeric mice recovered for 4 wk on normal chow and antibiotic-supplemented water before an additional 16-wk period of HFD feeding. RBC counts were used as surrogate marker of BM engraftment and chimerism. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami Miller School of Medicine.
Human Sample Collection
Human coronary arteries were procured from the Life Alliance Organ Recovery Agency (Miami, FL). Specimen collection was approved by the University of Miami Institutional Review Board (IRB No. 20110443). Experiments conformed to the principles set out in the Declaration of Helsinki and the Department of Health and Human Services Belmont Report.
Atherosclerosis Burden
Murine aortas in neutral-buffered 10% formalin (Sigma, St. Louis, MO) were submitted to Eehscience LLC (Pickerington, OH) for Oil Red O staining and independent quantification of atherosclerotic burden (13). Digital images of stained aortas were individually overlapped to a reference template, and the extent of disease was quantified by counting Oil Red O pixels vs. total aortic area. Eehscience remained blinded to experimental groups during atherosclerosis assessment.
Blood Chemistry and Lipid Profile
Blood chemistry, lipid profile, and complete blood counts (CBC) were determined in heparinized venous blood collected from each mouse before euthanasia. Blood samples were submitted to the University of Miami Comparative Pathology laboratory for automated analysis.
Confocal Immunofluorescence Microscopy
Green fluorescent protein and smooth muscle actin co-detection in the aorta of c-KitBac-eGFP mice.
Fixative (0.5% PFA-0.1% glutaraldehyde-20% sucrose) was washed out using 20% sucrose-PBS before embedding and cryosectioning of mouse aortas (8). The mouse anti-human smooth muscle actin (SMA) antibody (1:1,000, cat. no. M0851; Dako) was added to Triton X-100-permeabilized sections in blocking solution (cat. no. FP1020; Perkin-Elmer, Waltham, MA) for 1 h at 4°C. Bound anti-SMA antibodies were further detected with Alexa 546 goat anti-mouse polyclonal antibody (1:1,000; cat. no. A21202; Thermo Fisher Scientific).
Green fluorescent protein and SMA co-detection in primary smooth muscle cell cultures of c-KitBac-eGFP mice.
c-KitBac-eGFP primary SMCs cells were plated in chamber slides (cat. no. 154526; Nalge Nunc International) for 24 h. Cells were fixed in 4% PFA in PBS for 15 min, blocked with 0.1% Triton X-100 10% donkey serum, and incubated with mouse anti-human SMA (1:100, cat. no. M0851; Dako) and rabbit anti-GFP (1:100, cat. no. 2956S; Cell Signaling Technology) antibodies overnight. After cells were washed with PBS, secondary antibodies donkey anti-mouse Alexa 546 (1:1,000, cat. no. A10036; Life Technologies) and goat anti-rabbit Alexa 488 (1:1,000, cat. no. A27034; Life Technologies) were added, followed by 300 μM DAPI solution for 3 min before being mounted using DABCO anti-fading mounting media (cat. no. 10981; Sigma-Aldrich).
c-Kit and SMA co-detection in paraffin-embedded human and mouse arterial tissues.
Antigens were retrieved from rehydrated sections using a Tris 1-mM pH 9 1% EDTA solution for 10 min in a pressure cooker before treatment with 3% hydrogen peroxide and TNB blocking solution (cat. no. FP1020; Perkin-Elmer, Waltham, MA). Slides were then incubated with rabbit polyclonal antibodies against c-Kit (1:250; cat. no. 4502, Dako, Carpinteria, CA) overnight at 4οC. The next day, slides were incubated with biotinylated swine anti-rabbit polyclonal antibodies for 1 h (1:1,000, cat. no. E0353; Dako), streptavidin horseradish peroxidase (HRP; 1:1,000; cat. no. P0397; Dako, Carpinteria, CA) for 30 min, and finally amplified with a Tyramide Signal Amplification kit (1:50, cat. no. NEL700A001KT; Perkin Elmer). Amplification of the biotinylated antibody was detected through incubation with streptavidin-conjugated Alexa 546 secondary antibody (1:1,000, cat. no. S11029; Thermo Fisher Scientific, Waltham, MA) for 1 h. To co-detect SMA, samples were further incubated with mouse anti-human SMA (1:200, cat. no. M0851; Dako) and Alexa Fluor 488 goat anti-mouse secondary antibodies (1:1,000, cat. no. A11225; Thermo Fisher Scientific, Waltham, MA).
Mounting, image acquisition, and quantification.
Sections were counterstained using a 300 μM DAPI solution (cat. no. D1306; Invitrogen) in PBS for 2 min and mounted in polyvinyl alcohol mounting media with DABCO anti-fading mounting media (cat. no. 10981; Sigma-Aldrich). Sections were examined with a confocal scanning laser microscope Zeiss LSM 510 META (Carl Zeiss, Thornwood, NY) in an inverted configuration, and data were captured and analyzed with Zeiss LSM 510 Meta and Image Browser software (Carl Zeiss, Oberkochen, Germany). Images were acquired with the use of sequential capture mode to avoid potential fluorescence bleed-through between channels. Settings were fixed at the beginning using unstained controls and kept during the acquisition step. Brightness and contrast were adjusted equally.
Electron Microscopy
Murine aortas fixed in 4% PFA in PBS (wt/vol) were transferred to the University of Miami Transmission Electronic Microscope core facility for further fixation with 1% osmium tetroxide diluted in 100 mM phosphate buffer, followed by dehydration, plastic embedding, and sectioning. The ultrastructure of SMCs was examined in a Phillips CM10 transmission electron microscope (Eindhoven, The Netherlands). At least three fields were chosen from each section, and at least four images were captured per field.
Cell Culture
Murine primary aortic SMCs were isolated with the explant technique and cultured in DMEM/F-12-FBS (5:3:2; Thermo Fisher Scientific) supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 0.1 mM glutamine, 10 mM sodium pyruvate, and 0.75% sodium bicarbonate (32). Primary cells were used for three passages to avoid extensive loss of contractile features. The primary cultures stained positively for SMA and expressed calponin and SM22α. For proliferation assays, cells were seeded overnight in 24-well plates (5 × 104 cells/well) and starvation was induced with serum-deprived medium to synchronize the cell growth cycle. Seventy-two hours later, regular medium was added, and cells were counted daily. Alternatively, cells were grown in 0.1 mM of imatinib mesylate (Gleevec; Santa Cruz Biotechnology, CA) in complete medium for 24 h.
The foam-like cell phenotype was induced in subconfluent SMCs cultured with 0.2% BSA medium supplemented with 20 μg/ml water-soluble cholesterol (cat. no. C4951, Chol:MβCD; Sigma) for 72 h (38). Intracellular lipids were detected in formalin-fixed cells with Oil Red O staining (IHC World, Woodstock, MD) in a viewing area of 80,000 cells. Cellular cholesterol was quantified after hexane-isopropyl (3:2) (9) extraction with an Amplex Red Cholesterol Assay kit (Thermo Fisher Scientific). The remaining proteins were solubilized with protein lysis buffer and quantified using a Bradford protein assay (Bio-Rad, Hercules, CA). The results were presented as quantity of cellular lipids per milligram of total proteins.
Gene Rescue
Expression of c-Kit in KitMut SMCs (KitW/W-v) was rescued using a lentiviral vector (PRVPG24). This rescue vector was constructed by inserting a blunted BsrBI-NotI DNA fragment (3.6 Kb) containing the coding region of the mouse Kit cDNA under the murine phosphoglycerate kinase (PGK) promoter into the blunted pLenti CMV PuroDest vector (Addgene Inc., Cambridge, MA) cut with EcoRV and ClaI. Third-generation lentiviral stocks were produced in HEK-293 cells co-transfected with the lentiviral vector and the packaging and envelope plasmids psPAX2 and pMD2G (Addgene, Inc.). Transfections were done with a jetPRIME transfection kit (Polyplus, New York, NY). Infected cells (100 MOI) were selected in complete medium containing 10 μg/ml puromycin (Sigma). Gene rescue was confirmed by Western blot (see below) and fluorescence-activated cell sorting (FACS) analysis using an anti-c-Kit antibody (CD117-PE, cat. no. 130-091730; Miltenyi Biotec, San Diego, CA). Analytical flow cytometry was performed on BD FACS Canto II (BD Biosciences, San Jose, CA) using BD FACSDiva software (Becton Dickinson, Franklin Lakes, NJ). Data were analyzed using the FlowJo software (Ashland, OR).
Quantitative Real-Time PCR
Cell and tissue RNAs were isolated using the Quick-RNA MiniPrep kit (Zymo Research, Irvine, CA). cDNAs were generated with the High Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific). The relative abundance of c-Kit (Kit), calponin (Cnn1), SM22α (Tagln), ATP-binding cassette transporter G1 (ABCG1), and SM-MHC (Myh11) was assessed using TaqMan Gene Expression Assays (Thermo Fisher Scientific) and normalized with respect to β-actin. Real-time PCR was performed on an ABI Prism 7500 Fast Real-Time PCR System (96-well plate; Applied Biosystems). Relative gene expression was determined using the ΔΔCT method (29).
Western Blot and Immunoprecipitation
Protein lysates were prepared in RIPA buffer supplemented with 200 mM phenylmethylsulfonyl fluoride, 100 mM sodium orthovanadate (Santa Cruz Biotechnology, Dallas, TX), and a complete protease inhibitor cocktail (Roche Life Science). The protein concentration was determined using a commercial Bradford protein assay kit (Bio-Rad). Proteins were resolved in 4–12% SDS-polyacrylamide gels (NuPAGE; Thermo Fisher Scientific) before being transferred onto PVDF membranes (GE Healthcare, Marlborough, MA). Specific proteins were detected using antibodies against c-Kit (1:1,000, cat. no. sc-1494; Santa Cruz Biotechnology), SM22α (1:1,000, cat. no. ab10135; Abcam, Cambridge, MA), calponin (1:1,000, cat. no. ab46794; Abcam), and β-actin (1:5,000, cat. no. A5316; Sigma). Bound antibodies were detected after sequentially incubating the membranes with HRP-conjugated secondary antibodies and Amersham ECL Western Blotting Detection Reagent (GE Healthcare) or Supersignal West Femto Maximum Sensitivity Substrate (cat. no. 34095; Thermo Fisher). c-Kit was pulled down from 200 μg of arterial or cellular lysate using 1 μg of an anti-c-Kit antibody (cat. no. A4502; Dako) and 20 μl of Protein A/G PLUS-Agarose microbeads (cat. no. sc-2003; Santa Cruz Biotechnology). Microbeads were washed with pre-cooled RIPA buffer before analysis by Western blot.
Statistics
Results were presented as means ± SE. A two-tailed Student’s t-test was used to compare the difference between two groups, and a one-way ANOVA followed by a Newman-Keuls test was applied to compare the difference between multiple groups. Nonparametric values were compared using the Mann-Whitney test. A P value of <0.05 was considered significant.
RESULTS
c-Kit is Expressed in the Murine Aorta and Human Coronary Arteries
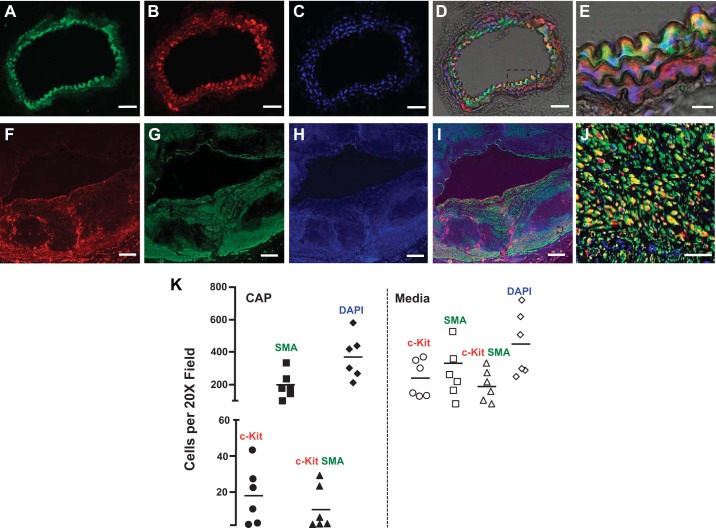
The activity of the c-Kit promoter was traced in SMCs using the c-KitBac-eGFP reporter mouse (44). In this model, the enhanced green fluorescent protein (eGFP) is downstream of the full c-Kit promoter. c-Kit+ (eGFP) cells surrounded the endothelial boundary of the aorta, but not the vena cava (Fig. 1, A–E). These cells stained positively for SMA (red), which confirmed their SMC lineage (Fig. 1, A–E). Moreover, more than 50% of outgrown primary SMCs from a c-KitBac-eGFP explant retained the eGFP expression during initial passages (Supplemental Fig. S1, A and B). These cells were also SMA positive (Supplemental Fig. S1; Supplemental Figures for this article can be found online at https://figshare.com/s/33c7b9eb380de387028b). We further confirmed that medial and cap SMCs in human coronary arteries with atherosclerosis expressed c-Kit. In humans, c-Kit was more abundant in cells in the media than in the cap (Fig. 1, F–K), suggesting a downregulation of this receptor during cell migration. The above data prompted further research on the role of this RTK in atherosclerosis.
Fig. 1.
Smooth muscle cells (SMCs) of murine and human aortas express the receptor tyrosine kinase c-Kit. A–E: green fluorescent protein (GFP) expression indicates the presence of c-Kit promoter activity in arterial SMCs in the c-KitBac-enhanced GFP reporter mouse. c-Kit+ cells (green; A) in the murine aorta also stained positively for smooth muscle actin (SMA) (red; B) using immunofluorescence confocal microscopy. Nuclei were counterstained with DAPI (blue; C). The section within the box in the overlaid image in D is magnified in E. Double-positive SMCs appear in yellow. F–J: c-Kit expression in an atherosclerotic human coronary artery. c-Kit (red; F), SMA (green; G), and double-positive cells (yellow; I) are abundantly found in the cap and tunica media of the human coronary artery. Nuclei were counterstained with DAPI (blue; H). The section within the box in the overlaid image in I is magnified in J. Scale bars: A–D, 50 µm; E, 20 µm; F–H, 500 µm; J, 50 µm. K: number of c-Kit+, SMA+ and double-positive (SMA+ c-Kit+) cells in the tunica media and cap of atherosclerotic lesions in human coronary arteries, n = 6.
c-Kit Deficiency Increases Atherosclerotic Burden in Hyperlipidemic Mice
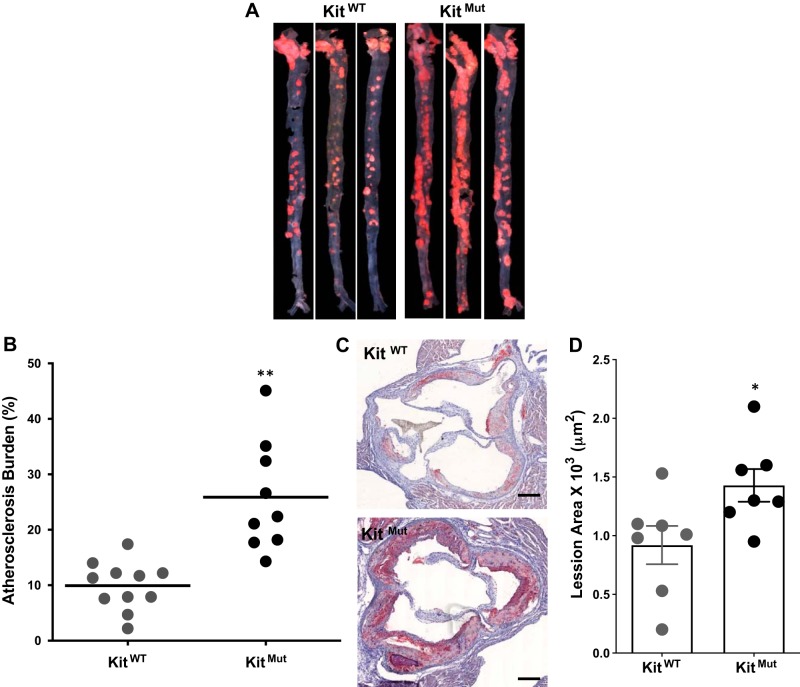
To assess the role of c-Kit in atherosclerosis, we measured atherosclerotic burden in hyperlipidemic c-Kit mutant mice (KitMut) fed a HFD for 16 wk. These mice carry a c-Kit null mutation (W) and a hypomorphic allele (W-v). The resulting mouse exhibits reduced kinase activity, and represents the severest c-Kit mutant that survives gestation (6). c-Kit deficient mice have marked erythropenia due to the lack of c-Kit function that compromises normal hematopoiesis (Supplemental Table S1; Supplemental Tables for this article can be found online at https://figshare.com/s/a9281a10d2f963356062). Mutant mice had lower body weight than littermate controls after 16 wk of HFD feeding (23.9 ± 4.7 vs. 32.2 ± 8.1 g, P < 0.01). However, both c-Kit mutant and wild-type littermates had similar numbers of monocytes in circulation (Table S1). Atherosclerosis burden was 2.5 times greater in mutant versus control mice (P < 0.01; Fig. 2, A and B). c-Kit deficiency was associated with significant atherosclerosis in all three main sections of the aorta (arch, thoracic, and abdominal) (Supplemental Fig. S2, compared with KitWT), and in the aortic sinus (P < 0.05 compared with KitWT; Fig. 2, C and D). The extent of disease at all aortic positions was greater in compound heterozygous mice (KitMut) than in those carrying a single c-Kit mutant allele (KitW/+ apoE−/− or KitW-v/+ apoE−/−), suggesting a direct link between this RTK and vasculoprotection. Although all animals were hyperlipidemic with plasma cholesterol levels above threshold, mutant mice (KitMut mice) had significantly lower plasma cholesterol than control littermates (Table S1) despite more atherosclerotic burden. These results support our hypothesis that c-Kit signaling is anti-atherogenic and vasculoprotective in mice.
Fig. 2.
Defective c-Kit exacerbates atherosclerosis in hyperlipidemic mice. A: representative Oil Red O-stained aortas showing increased aortic atherosclerotic burden in hyperlipidemic c-Kit mutant (KitMut) mice compared with control littermates (KitWT). Mice were fed high-fat diet (HFD) for 16 wk and euthanized. The percentage of the aorta affected by atherosclerosis in each experimental animal is plotted in the dot blot graphic in B. The horizontal line indicates the group’s mean. P values were assessed using a 2-tailed t-test assuming unequal variance. **P < 0.01. C: representative Oil Red O-stained sections from the aortic sinus of KitWT (control) and KitMut mice. Scale bars, 200 µm. Bars in D represent the means ± SE. P values were assessed using a 2-tailed t-test assuming unequal variance. *P < 0.05.
Bone Marrow Transplantation Does Not Alleviate Atherosclerosis in c-Kit-Deficient Mice
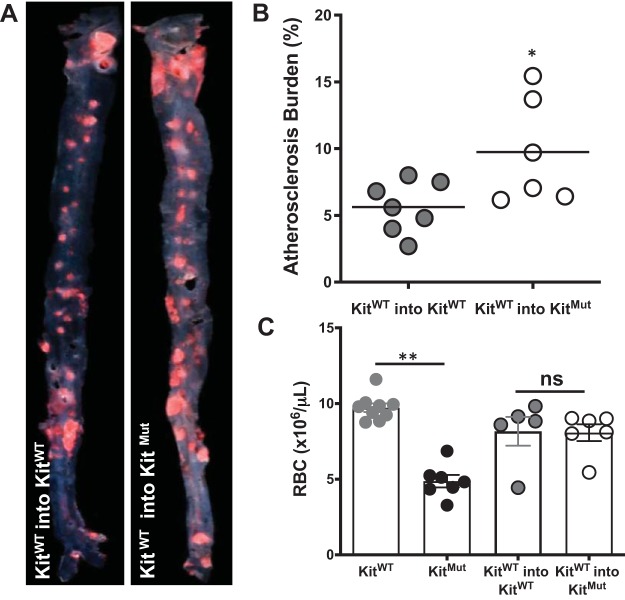
We further sought to delineate the role of c-Kit expressing BM cells in atherosclerosis in mice. Lethally irradiated KitMut mice and KitWT were rescued with KitWT mononuclear BM cells from littermate donors. The low number of hematopoietic stem cells in KitMut mice prevents their use as donor animals. Four weeks later, all chimeric mice were fed with HFD for an additional 16 wk. Bone marrow transplantation failed to prevent the exacerbation of atherosclerosis in mutant chimeric mice (Fig. 3, A and B) despite the alleviated erythropenia after successful engraftment of KitWT BM cells (Fig. 3C and Supplemental Table S2). The analysis of atherosclerosis in each examined area is further presented in Supplemental Fig. S3. Chimeric mice developed less disease than nonirradiated animals (Fig. 2). It is known that ionic irradiation before BM transplantation negatively impacts plaque buildup in hypercholesterolemic mice (39). Interestingly, chimeric KitMut mice had lower plasma cholesterol levels than chimeric control animals and nonirradiated mutant mice. These results imply a key role for vascular c-Kit signaling in the prevention of lesion formation and indicate that reconstitution of BM-derived c-Kit activity does not ameliorate the effects of its local deficiency.
Fig. 3.
Bone marrow (BM) transplant does not protect c-Kit-deficient mice from excessive atherosclerosis. A: representative Oil Red O-stained aortas from lethally irradiated c-KitMut and KitWT mice rescued with KitWT BM cells. Mice recovered for 4 wk on normal chow before they were fed high-fat diet (HFD) for 16 wk to induce atherosclerosis. B: atherosclerosis area in the aorta of each chimeric mouse. The horizontal line indicates the group’s mean. P values were assessed using a 2-tailed t-test assuming unequal variance. *P < 0.05. C: number of red blood cells (RBC) in nonirradiated and chimeric mice. Bars represent the means ± SE. P values were assessed using a 1-way ANOVA, followed by a Newman-Keuls test. **P < 0.01. NS, not significant with respect to the control chimeric mouse (KitWT into KitWT).
c-Kit Inhibits the Phenotypic Switch of SMCs and Prevents the Foam Cell Phenotype
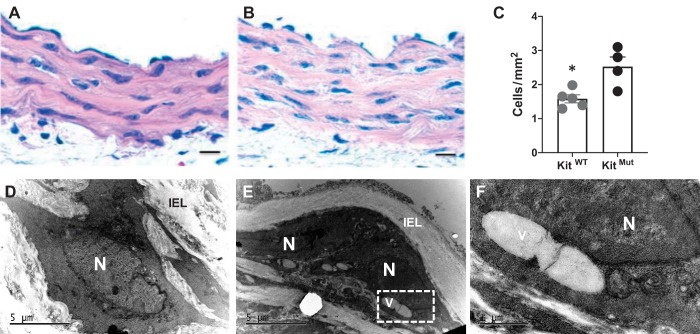
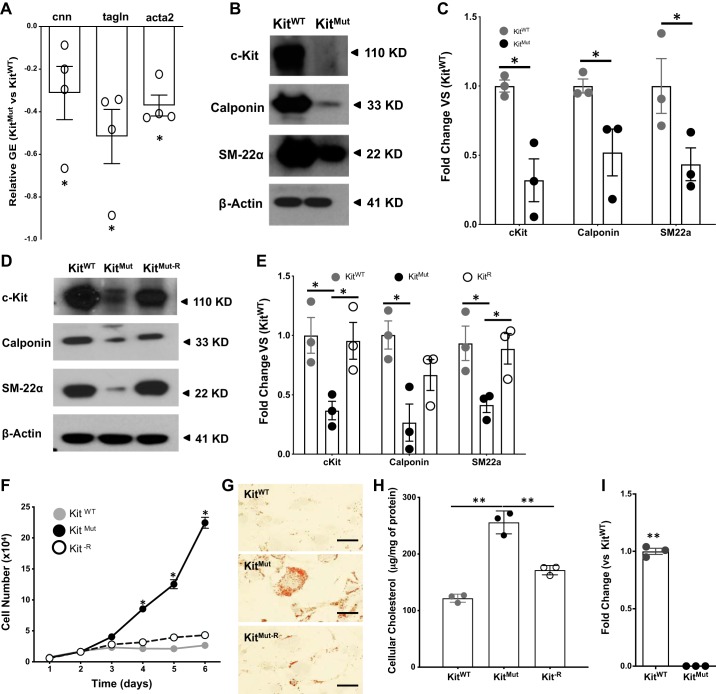
Because the vasculoprotective role of c-Kit resides in the vascular cells, we wanted to investigate how loss of function of c-Kit affects SMCs. Bright-field and electron microscopy of murine aortas revealed that aortic SMCs were more abundant (P < 0.05; Fig. 4, A–C), disorganized, and vacuolated (giant vacuoles) in KitMut compared with KitWT mice (Fig. 4, D–F). As expected, markers for the contractile phenotype in SMCs (calponin and SM22α) were significantly downregulated in the aorta of c-KitMut mice with respect to controls (P < 0.05; Fig. 5, A and B).
Fig. 4.
c-Kit deficiency leads to hypercellularity and hypertrophy of medial smooth muscle cells (SMCs) in c-Kit mutant mice. A–C: representative hematoxylin and eosin (H & E)-stained sections from control littermates (KitWT; A) and c-Kit-deficient mice (KitMut; B) depicting the increased number of cells in the aorta of c-Kit-deficient mice. The dot plot in C represents the number of cells/area in H & E-stained sections. Scale bar, 20 µm. The P value was assessed using a 2-tailed t-test assuming unequal variance. *P < 0.05. D–F: in contrast with SMCs in the aorta of KitWT mice (D), the cytoplasm of c-Kit-deficient SMCs (E and F) contained giant vacuoles like the one shown in microphotographs taken under the transmission electron microscope. The vacuole within the box in E is further amplified in F. IEL, internal elastic lamina; N, nucleus; V, vacuole.
Fig. 5.
c-Kit deficiency facilitates the synthetic phenotype in smooth muscle cells (SMCs) of c-Kit mutant mice. A–C: downregulation of contractile gene expression (GE) in aortas of KitMut versus KitWT, as determined by quantitative RT-PCR (A) and Western blot (B and C). Protein expression in C was normalized using β-actin levels and then standardized against KitWT. The P value was assessed using a 2-tailed t-test assuming unequal variance. D and E: reconstitution of c-Kit restored the expression of contractile markers in cultured SMCs, as determined by Western blot. Proteins were extracted from primary KitWT SMCs and primary KitMut SMCs transduced with either a lentiviral vector containing the murine c-Kit cDNA under a PGK promoter (KitMut-R) or the empty lentivirus (KitMut). Data are presented as means ± SE. Protein expression was normalized using β-actin levels and then standardized against KitWT. Groups were compared using a 1-way ANOVA, followed by a Newman-Keuls test. F–H: reconstitution of c-Kit (KitMut-R) slowed down proliferation of c-Kit mutant SMCs (F) and prevented intracellular accumulation of cholesterol as detected with Oil Red O staining (G). Cellular cholesterol levels were determined after 72 h culture in Chol:MβCD-loaded (20 μg/ml) 0.2% BSA medium and standardized against whole lysate protein concentration (H). Scale bars, 40 µm. Data in the bar graph are presented as means ± SE. The P values were assessed using a 1-way ANOVA, followed by a Newman-Keuls test. I: deficient c-Kit activity led to significant downregulation of the ATP-binding cassette subfamily G member 1 gene (ABCG1). Data in the bar graph are presented as means ± SE. The P value was assessed using a 2-tailed t-test assuming equal variance. *P < 0.05; **P < 0.01.
To validate that loss of c-Kit determines the SMC synthetic phenotype, we cultured primary SMCs from both KitMut and KitWT aortas. Lack of c-Kit activity compromised the expression of contractile markers (calponin and SM22α) in SMCs (Fig. 5, D and E), as previously observed in vivo (Fig. 5, A–C), whereas rescue of c-Kit expression in mutant cells by lentiviral transduction (Supplemental Fig. S4) restored the synthesis of contractile proteins (Fig. 5, D and E). In agreement with mutant cells, pharmacological inhibition of c-Kit with Gleevec caused a profound downregulation of contractile genes in KitWT SMCs (Supplemental Fig. S5). c-Kit-deficient SMCs had a higher proliferation rate than those from control littermates and c-Kit rescued cells (KitMut-R) (Fig. 5F).
Furthermore, we assessed whether the phenotypic switch due to lack of c-Kit favors the formation of lipid-loaded cells (foam cells) under hypercholesterolemic conditions. c-Kit-deficient SMCs showed higher accumulation of cytoplasmic lipids (Fig. 5, G and H) and total cholesterol levels compared with c-Kit rescued cells and those from controls (P < 0.01). These results prompted us to search our transcriptomic data on KitMut and KitWT SMCs for differentially expressed genes that code for scavenger receptors and lipid hemostasis proteins (43). We discovered that the ATP-binding cassette transporter G1 (ABCG1) gene was significantly downregulated in KitMut versus KitWT cells (Fig. 5I). These results identify the c-Kit receptor as a critical regulator of SMC phenotypic switching toward an atherogenic foam-like phenotype.
DISCUSSION
The present work demonstrates that impaired c-Kit signaling in the vascular wall increases atherosclerosis in hyperlipidemic mice. We have shown that c-Kit deficiency increases aortic atherosclerosis burden and favors the phenotypic switching of SMCs toward a synthetic phenotype, which is prone to higher accumulation of intracellular lipids and transformation into foam-like cells. To our knowledge, we are the first to provide evidence that a RTK in SMCs plays a pivotal and protective role in the pathophysiology of atherosclerosis.
Atherosclerosis is the most common underlying condition behind cardiovascular complications and deaths worldwide. Atheroma growth depends upon the phenotypic switching of SMCs from contractile to synthetic early in atherogenesis (3, 4). Synthetic SMCs migrate and proliferate in the intima, where they contribute to the inflammatory milieu and to the retention and oxidation of cholesterol. It is estimated that more than 50% of foam cells in human coronary lesions are derived from SMCs (1), hence the importance in regulating their pathological dedifferentiation. Our results identify c-Kit as a determinant and/or modulator of the SMC phenotype in the setting of atherosclerosis. We demonstrated that c-Kit expression preserves the contractile SMC phenotype, decreases intracellular cholesterol accumulation, and hinders cell proliferation. In agreement with our results, it has been shown that targeted inhibition of c-Kit in SMCs in vitro decreased the expression of SMC-specific contractile markers by reducing the activity of the transcriptional coactivator myocardin (15). Active myocardin is a potent co-activator of serum response factor (SRF), one of the master MADS-box transcription factors that control expression of SMC contractile genes (46).
Interestingly, we discovered that c-Kit deficiency leads to downregulation of the ATP-binding cassette transporter G1 (ABCG1) gene in SMCs. The roles of ABCG1 in lipid metabolism, intracellular lipid trafficking, and lipid efflux are widely recognized. Targeted disruption of ABCG1 in mice results in massive accumulation of lipids in multiple tissues (23). Moreover, overexpression of ABCG1 protects murine tissues from dietary fat-induced lipid accumulation (23). We believe that impaired cholesterol efflux in c-KitMut SMCs is one of several mechanisms exacerbating atherosclerosis in mutant mice. SMCs with impaired lipid transport contribute up to one-half of foam cells in human and mouse atherosclerotic lesions (17, 41). These cells uptake lipids through the LDL receptor (42), CD36 (31), or micropinocytosis (36). However, SMC-derived foam cells exhibit much lower expression of cholesterol exporters compared with macrophage-derived foam cells (36), indicating a predisposition to lipid retention. Our work indicates that c-Kit mutant cells have a significant downregulation of the ABCG1 exporter compared with wild-type SMCs. The mechanism by which c-Kit signaling is linked to higher ABCG1 expression is not clear at the moment. The retinoic acid receptors, crucial transcriptional activators of ABCG1 (40, 49), were recently found to activate both transcription and translation of c-Kit in other cell types (10, 24). Taking into consideration that retinoic acid inhibits foam cell formation and increases cholesterol efflux in atherosclerosis models (49) and promotes the contractile phenotype in SMCs (33), this potential mechanism deserves further attention.
The role of c-Kit in promoting the contractile SMC phenotype contrasts previous reports on other RTKs such as PDGFR and VEGFR (2, 20, 47). Stimulation of these receptors by ligands released from endothelial cells, platelets, and macrophages during vascular disease promotes synthetic SMC dedifferentiation and migration (25). Elevated PDGFRβ or VEGFR activity increased plaque formation in hyperlipidemic mice compared with littermate controls (20, 21). However, the evidence on PDGFRβ and VEGFR comes from either overexpression or knock-in activation models, which likely do not represent physiological levels of RTK activity (20, 21). Our experiments suggest that in the presence of physiological PDGFRβ and VEGFR signaling, the protective role of c-Kit predominates in WT animals. Not surprisingly, RTK inhibition failed to benefit patients against SMC proliferative conditions in a phase III clinical trial (50).
We demonstrated that c-Kit deficiency exacerbates atherosclerosis in global mutant mice with respect to the littermate control. Of note, our littermate controls had less atherosclerosis than inbred apoE-knockout mice in the C57BL/6 background, which was likely due to the influence of the genetic background (14). Both the KitMut and KitWT animals were outbred mice derived from mating the C57BL/6J mouse carrying the W-v and the WB/ReJ mouse carrying the W allele (6). It is also important to notice that Kit mutant mice possess multiple pleiotropic defects on melanocytes, germ cells, red blood cells, and self-renewal hematopoietic stem cells that produce the myeloid and lymphoid cells involved in atherogenesis (6). c-Kit expression vanishes during differentiation of most blood cells, with the exception of mast cells, that require this RTK for survival (37). There was no difference in the number of peripheral monocytes between hyperlipidemic KitMut and KitWT mice, which are the first inflammatory cells to enter the intima to initiate plaque formation. Our results do not rule out changes in the monocyte/macrophage inflammatory phenotype in mutant mice with respect to control littermates. However, the importance of inflammatory cells in the exacerbated atherosclerosis in c-KitMut mice appears to be not significant, as increased disease prevailed under normal hematopoiesis after BM transplantation. This further supports that vascular c-Kit signaling is responsible for the atheroprotective role of this receptor.
Interestingly, KitMut mice displayed ∼30% lower plasma cholesterol and LDL relative to their wild type littermates, despite similar levels of TG, HDL, and VLDL in both mouse strains. Hyperlipidemia was far above the clinical threshold values in both experimental groups, and KitMut mice developed more atherosclerosis despite having lower total cholesterol and LDL levels. In addition, the reconstitution of lethally irradiated c-Kit mutant mice with KitWT BM cells alleviated erythropenia without modifying plasma cholesterol in recipient mice, which ruled out the importance of systemic c-Kit signaling on cholesterol homeostasis. Our results contrast with a prior study that found spontaneous hyperlipidemia among KitMut mice (19). Mice in the latter study did not carry the apoE mutation. Cholesterol hemostasis relies on an intricate network of multi-organ cellular processes that control biosynthesis, uptake through LDL receptors, lipoprotein transport, storage by esterification, and degradation and secretion as bile acids (45). The decreased plasma cholesterol in mutant mice may be due to decreased food intake and body weight secondary to gastrointestinal dysfunction due to the notable loss of gastrointestinal Cajal cells (12). Alternatively, the lack of c-Kit signaling could also interfere with normal cholesterol hemostasis in the liver (30). c-Kit acts through several signaling pathways known to control lipid metabolism in the liver, such as the phosphatidylinositol 3-kinase/Akt/mTOR and JAK/STAT pathways (26). Similar to other RTKs, c-Kit signaling could directly regulate the signaling pathways of lipoprotein receptors (7). This potential new role of c-Kit in cholesterol hemostasis prompts further investigations in tissues responsible for lipid absorption and lipoprotein metabolism.
Finally, the localization of c-Kit in SMCs of mouse and human atherosclerotic plaques contrasts a vast literature where c-Kit was considered a functional marker for vascular progenitor cells (3, 44). c-Kit expressing progenitors generate small resistance arterioles and capillary structures during heart development and myocardium healing after infarction (3, 44). However, the contribution of progenitors to the atherosclerotic plaque has been controversial, and subsequent studies showed that the majority of plaque cells originate from pre-existing SMCs of the vessel wall (5). In the setting of other vascular diseases, prior studies have shown reduced neointimal hyperplasia secondary to the loss of c-Kit function in progenitors and SMCs (11). This discrepancy reflects the differences between neointimal hyperplasia (restenosis) and atherosclerosis in terms of etiology, natural history, culprit lesions, and progenitor cell contribution to lesion progression.
In summary, our data demonstrate that c-Kit is an important regulator of SMC differentiation in atherogenesis and that its presence is necessary to prevent the formation of SMC-derived macrophage like cells that contribute to disease burden. To our knowledge, this study is the first one to evaluate the role of c-Kit in atherosclerosis in vivo and furnishes new therapeutic targets to prevent and eventually mitigate the devastating effects of this condition. Our new findings may also shed light on the pathophysiology of other vascular occlusive diseases such as restenosis, transplant arteriosclerosis, vein graft failure, and pulmonary vascular occlusive disease.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grant R01-HL-109582 (to R. I. Vazquez-Padron, O. C. Velazquez, and K. A. Webster).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.S. and R.I.V.-P. conceived and designed research; L.S., Z.M.Z., A.S., and R.I.V.-P. performed experiments; L.S., Z.M.Z., L.M., R.M.L.-S., O.C.V., Z.-J.L., A.S., K.A.W., and R.I.V.-P. analyzed data; L.S., Z.M.Z., L.M., R.M.L.-S., O.C.V., Z.-J.L., and R.I.V.-P. interpreted results of experiments; L.S. and R.I.V.-P. prepared figures; L.S., Z.M.Z., L.M., and R.I.V.-P. drafted manuscript; L.S., Z.M.Z., L.M., A.E.M., A.S., K.A.W., and R.I.V.-P. edited and revised manuscript; L.S. and R.I.V.-P. approved final version of manuscript.
REFERENCES
- 1.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 129: 1551–1559, 2014. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 2.Arnott C, Punnia-Moorthy G, Tan J, Sadeghipour S, Bursill C, Patel S. The vascular endothelial growth factor inhibitors ranibizumab and aflibercept markedly increase expression of atherosclerosis-associated inflammatory mediators on vascular endothelial cells. PLoS One 11: e0150688, 2016. doi: 10.1371/journal.pone.0150688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bearzi C, Leri A, Lo Monaco F, Rota M, Gonzalez A, Hosoda T, Pepe M, Qanud K, Ojaimi C, Bardelli S, D’Amario D, D’Alessandro DA, Michler RE, Dimmeler S, Zeiher AM, Urbanek K, Hintze TH, Kajstura J, Anversa P. Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci USA 106: 15885–15890, 2009. doi: 10.1073/pnas.0907622106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res 118: 692–702, 2016. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol 26: 2696–2702, 2006. doi: 10.1161/01.ATV.0000247243.48542.9d. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein A, Chabot B, Dubreuil P, Reith A, Nocka K, Majumder S, Ray P, Besmer P. The mouse W/c-kit locus. Ciba Found Symp 148: 158–166, 1990. [PubMed] [Google Scholar]
- 7.Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, Herz J. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low Density lipoprotein receptor-related protein in caveolae. J Biol Chem 277: 15507–15513, 2002. doi: 10.1074/jbc.M200428200. [DOI] [PubMed] [Google Scholar]
- 8.Brazelton TR, Blau HM. Optimizing techniques for tracking transplanted stem cells in vivo. Stem Cells 23: 1251–1265, 2005. doi: 10.1634/stemcells.2005-0149. [DOI] [PubMed] [Google Scholar]
- 9.Brown MS, Ho YK, Goldstein JL. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem 255: 9344–9352, 1980. [PubMed] [Google Scholar]
- 10.Busada JT, Chappell VA, Niedenberger BA, Kaye EP, Keiper BD, Hogarth CA, Geyer CB. Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse. Dev Biol 397: 140–149, 2015. doi: 10.1016/j.ydbio.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caplice NM, Wang S, Tracz M, Croatt AJ, Grande JP, Katusic ZS, Nath KA. Neoangiogenesis and the presence of progenitor cells in the venous limb of an arteriovenous fistula in the rat. Am J Physiol Renal Physiol 293: F470–F475, 2007. doi: 10.1152/ajprenal.00067.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi MM, Powley TL. c-Kit mutant mouse behavioral phenotype: altered meal patterns and CCK sensitivity but normal daily food intake and body weight. Am J Physiol Regul Integr Comp Physiol 285: R1170–R1183, 2003. doi: 10.1152/ajpregu.00015.2003. [DOI] [PubMed] [Google Scholar]
- 13.Cornhill JF, Barrett WA, Herderick EE, Mahley RW, Fry DL. Topographic study of sudanophilic lesions in cholesterol-fed minipigs by image analysis. Arteriosclerosis 5: 415–426, 1985. doi: 10.1161/01.ATV.5.5.415. [DOI] [PubMed] [Google Scholar]
- 14.Dansky HM, Charlton SA, Sikes JL, Heath SC, Simantov R, Levin LF, Shu P, Moore KJ, Breslow JL, Smith JD. Genetic background determines the extent of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 19: 1960–1968, 1999. doi: 10.1161/01.ATV.19.8.1960. [DOI] [PubMed] [Google Scholar]
- 15.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem 284: 3728–3738, 2009. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edling CE, Hallberg B. c-Kit–a hematopoietic cell essential receptor tyrosine kinase. Int J Biochem Cell Biol 39: 1995–1998, 2007. doi: 10.1016/j.biocel.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res 115: 662–667, 2014. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 18.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res 95: 156–164, 2012. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatanaka K, Tanishita H, Ishibashi-Ueda H, Yamamoto A. Hyperlipidemia in mast cell-deficient W/WV mice. Biochim Biophys Acta 878: 440–445, 1986. doi: 10.1016/0005-2760(86)90254-7. [DOI] [PubMed] [Google Scholar]
- 20.He C, Medley SC, Hu T, Hinsdale ME, Lupu F, Virmani R, Olson LE. PDGFRβ signalling regulates local inflammation and synergizes with hypercholesterolaemia to promote atherosclerosis. Nat Commun 6: 7770, 2015. doi: 10.1038/ncomms8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinonen SE, Kivelä AM, Huusko J, Dijkstra MH, Gurzeler E, Mäkinen PI, Leppänen P, Olkkonen VM, Eriksson U, Jauhiainen M, Ylä-Herttuala S. The effects of VEGF-A on atherosclerosis, lipoprotein profile, and lipoprotein lipase in hyperlipidaemic mouse models. Cardiovasc Res 99: 716–723, 2013. doi: 10.1093/cvr/cvt148. [DOI] [PubMed] [Google Scholar]
- 22.Hollenbeck ST, Sakakibara K, Faries PL, Workhu B, Liu B, Kent KC. Stem cell factor and c-kit are expressed by and may affect vascular SMCs through an autocrine pathway. J Surg Res 120: 288–294, 2004. doi: 10.1016/j.jss.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy MA, Barrera GC, Nakamura K, Baldán A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab 1: 121–131, 2005. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Koli S, Mukherjee A, Reddy KV. Retinoic acid triggers c-kit gene expression in spermatogonial stem cells through an enhanceosome constituted between transcription factor binding sites for retinoic acid response element (RARE), spleen focus forming virus proviral integration oncogene (SPFI1) (PU.1) and E26 transformation-specific (ETS). Reprod Fertil Dev 29: 521–543, 2017. doi: 10.1071/RD15145. [DOI] [PubMed] [Google Scholar]
- 25.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134, 2010. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev 92: 1619–1649, 2012. doi: 10.1152/physrev.00046.2011. [DOI] [PubMed] [Google Scholar]
- 27.Liang A, Wang Y, Han G, Truong L, Cheng J. Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am J Physiol Renal Physiol 304: F1413–F1420, 2013. doi: 10.1152/ajprenal.00585.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang J, Wu YL, Chen BJ, Zhang W, Tanaka Y, Sugiyama H. The C-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci 9: 435–443, 2013. doi: 10.7150/ijbs.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Magnol L, Chevallier MC, Nalesso V, Retif S, Fuchs H, Klempt M, Pereira P, Riottot M, Andrzejewski S, Doan BT, Panthier JJ, Puech A, Beloeil JC, de Angelis MH, Hérault Y. KIT is required for hepatic function during mouse post-natal development. BMC Dev Biol 7: 81, 2007. doi: 10.1186/1471-213X-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto K, Hirano K, Nozaki S, Takamoto A, Nishida M, Nakagawa-Toyama Y, Janabi MY, Ohya T, Yamashita S, Matsuzawa Y. Expression of macrophage (Mphi) scavenger receptor, CD36, in cultured human aortic smooth muscle cells in association with expression of peroxisome proliferator activated receptor-gamma, which regulates gain of Mphi-like phenotype in vitro, and its implication in atherogenesis. Arterioscler Thromb Vasc Biol 20: 1027–1032, 2000. doi: 10.1161/01.ATV.20.4.1027. [DOI] [PubMed] [Google Scholar]
- 32.Metz RP, Patterson JL, Wilson E. Vascular smooth muscle cells: isolation, culture, and characterization. Methods Mol Biol 843: 169–176, 2012. doi: 10.1007/978-1-61779-523-7_16. [DOI] [PubMed] [Google Scholar]
- 33.Miano JM, Berk BC. Retinoids: new insight into smooth muscle cell growth inhibition. Arterioscler Thromb Vasc Biol 21: 724–726, 2001. doi: 10.1161/01.ATV.21.5.724. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto T, Sasaguri Y, Sasaguri T, Azakami S, Yasukawa H, Kato S, Arima N, Sugama K, Morimatsu M. Expression of stem cell factor in human aortic endothelial and smooth muscle cells. Atherosclerosis 129: 207–213, 1997. doi: 10.1016/S0021-9150(96)06043-1. [DOI] [PubMed] [Google Scholar]
- 35.Orlandi A, Di Lascio A, Francesconi A, Scioli MG, Arcuri G, Ferlosio A, Spagnoli LG. Stem cell marker expression and proliferation and apoptosis of vascular smooth muscle cells. Cell Cycle 7: 3889–3897, 2008. doi: 10.4161/cc.7.24.7323. [DOI] [PubMed] [Google Scholar]
- 36.Rivera J, Walduck AK, Thomas SR, Glaros EN, Hooker EU, Guida E, Sobey CG, Drummond GR. Accumulation of serum lipids by vascular smooth muscle cells involves a macropinocytosis-like uptake pathway and is associated with the downregulation of the ATP-binding cassette transporter A1. Naunyn Schmiedebergs Arch Pharmacol 386: 1081–1093, 2013. doi: 10.1007/s00210-013-0909-5. [DOI] [PubMed] [Google Scholar]
- 37.Roberts R, Govender D. Gene of the month: KIT. J Clin Pathol 68: 671–674, 2015. doi: 10.1136/jclinpath-2015-203207. [DOI] [PubMed] [Google Scholar]
- 38.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci USA 100: 13531–13536, 2003. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiller NK, Kubo N, Boisvert WA, Curtiss LK. Effect of gamma-irradiation and bone marrow transplantation on atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 21: 1674–1680, 2001. doi: 10.1161/hq1001.096724. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz G, Langmann T, Heimerl S. Role of ABCG1 and other ABCG family members in lipid metabolism. J Lipid Res 42: 1513–1520, 2001. [PubMed] [Google Scholar]
- 41.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21: 628–637, 2015. [Erratum in: Nat Med 22: 217, 2016.] 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sima AV, Botez GM, Stancu CS, Manea A, Raicu M, Simionescu M. Effect of irreversibly glycated LDL in human vascular smooth muscle cells: lipid loading, oxidative and inflammatory stress. J Cell Mol Med 14: 2790–2802, 2010. doi: 10.1111/j.1582-4934.2009.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song L, Martinez L, Zigmond ZM, Hernandez DR, Lassance-Soares RM, Selman G, Vazquez-Padron RI. c-Kit modifies the inflammatory status of smooth muscle cells. PeerJ 5: e3418, 2017. doi: 10.7717/peerj.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, Fisher PJ, Steffey M, Hesse M, Doran RM, Woods A, Singh B, Yen A, Fleischmann BK, Kotlikoff MI. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA 106: 1808–1813, 2009. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trapani L, Segatto M, Pallottini V. Regulation and deregulation of cholesterol homeostasis: The liver as a metabolic “power station”. World J Hepatol 4: 184–190, 2012. doi: 10.4254/wjh.v4.i6.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105: 851–862, 2001. doi: 10.1016/S0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 47.Yu K, Zheng B, Han M, Wen JK. ATRA activates and PDGF-BB represses the SM22α promoter through KLF4 binding to, or dissociating from, its cis-DNA elements. Cardiovasc Res 90: 464–474, 2011. doi: 10.1093/cvr/cvr017. [DOI] [PubMed] [Google Scholar]
- 48.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258: 468–471, 1992. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 49.Zhou W, Lin J, Chen H, Wang J, Liu Y, Xia M. Retinoic acid induces macrophage cholesterol efflux and inhibits atherosclerotic plaque formation in apoE-deficient mice. Br J Nutr 114: 509–518, 2015. doi: 10.1017/S0007114515002159. [DOI] [PubMed] [Google Scholar]
- 50.Zohlnhöfer D, Hausleiter J, Kastrati A, Mehilli J, Goos C, Schühlen H, Pache J, Pogatsa-Murray G, Heemann U, Dirschinger J, Schömig A. A randomized, double-blind, placebo-controlled trial on restenosis prevention by the receptor tyrosine kinase inhibitor imatinib. J Am Coll Cardiol 46: 1999–2003, 2005. doi: 10.1016/j.jacc.2005.07.060. [DOI] [PubMed] [Google Scholar]