OBESOGENIC DIET AND ATHEROGENESIS
In the United States, atherosclerosis is an underlying cause of endothelial dysfunction, chronic peripheral vascular disease, and subsequent heart attack or stroke, thereby increasing morbidity and mortality (10). Obesity aggravates the progress of atherosclerosis by providing a consistent low-grade chronic inflammation that worsens cardiovascular health. Fundamentally, calorie intake (primarily from fat)-dependent inflammation is a key transition from initial atherogenesis to further advancement, leading to thrombosis as a final complication of atherosclerosis (4). Atheromas contain a yellow fatty substance that was first discovered by Virchow more than 100 years ago. Later, Windaus identified the yellow substance as cholesterol and proposed the role of lipids in the pathogenesis of atherosclerosis (8). Lipoproteins transport cholesterol and triglycerides in plasma to maintain homeostasis. Lipoproteins can efficiently cross the protective endothelial layer and penetrate into the intima of the artery wall and, depending on particle size, plasma concentration, blood pressure, and arterial injury, defines their affinity and ability to bind proteoglycans and to enter or leave subendothelial space. The aggregation of triglyceride-rich lipoproteins, including intermediate-density lipoprotein, chylomicron remnants, and very-low-density lipoprotein (VLDL), drive the activation of leukocytes (foam cell macrophages) by enzymatic modification leading to an aggravated inflammatory response. Foam cell macrophages provoke maladaptive responses, such as activation of Toll-like receptors, the NLRP3 inflammasome and interleukin-1β, apoptosis, and other prothrombotic pathways (9). In this issue of the American Journal of Physiology-Heart and Circulatory Physiology, Fan et al. (2) recently showed that gravin is critical for lipid metabolism via the reduction of sterol regulatory element-binding proteins-2 (SREBP-2) expression, and the absence of gravin-mediated signaling delays the high-fat diet (HFD)-induced hyperlipidemia and limits atherogenesis progression to atherosclerosis.
GRAVIN GRAVITATES INFLAMMATION
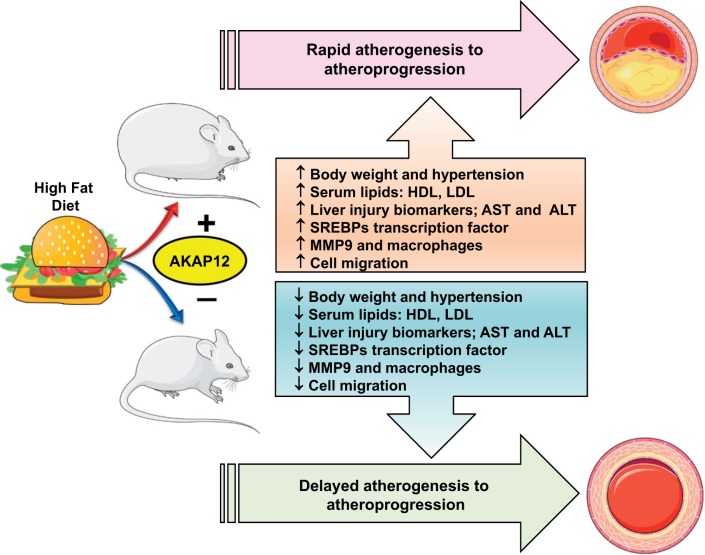
Gravin, also known as A-kinase anchor proteins (AKAPs) and Src-suppressed C-kinase substrate (SSeCKS), is a tumor suppressor protein belonging to a group of structurally diverse proteins, having the common function such as binding to the regulatory subunit of protein kinase A (PKA) (1, 7). Gravin is associated with protein PKA and PKC, as well as their associated phosphatase, thereby serving as a scaffold protein in signal transduction (5). Gravin has a pivotal role in controlling cell migration by managing cellular events through scaffolding key signaling molecules such as cyclin D1, calmodulin, PKA, and PKC. Gravin (AKAP12) has two major transcripts, α and β, ubiquitously expressed in the embryo and the adult as 305- or 290-kDa products (rodents, 290 or 280 kDa), respectively. One of the key mechanisms of AKAP is to control lipolysis via cAMP-dependent PKA signaling, including the regulation of lipoprotein lipase (LPL) expression through inhibition of its translation. The most recent role reported for gravin is mediating cellular lipid metabolism by the activation of SREBPs, which are transcription factors that bind to the sterol regulatory element DNA sequence of cholesterol-associated genes. Gravin, therefore, piled up in vascular inflammation and impairs lipid metabolism to localize it to the region of atheroma. Diversified composition and density of lipoproteins are well known in atherosclerosis. Atherosclerosis is nonresolving coronary artery disease that adversely increases the risk of heart attack. Lipid metabolism disorders induced by HFD cause excessive accumulation of lipids in various tissues, especially in the liver. A complex network of metabolic enzymes, molecular mediators regulate lipid metabolism, including several proteins related to lipid and cholesterol biosynthesis. Fan et al. (2) used a simple but twofold approach to develop a therapeutic target to treat atherosclerosis through gravin-mediated signaling. The first approach targeted plasma lipoprotein metabolism, and the second approach determined the inflammation connection. As expected, the HFD model (42% kCal from fat) aggravated hyperlipidemia and atherosclerosis in gravin mutant mouse model, thus defining the functional role of gravin in facilitating atherogenesis to atherosclerosis with a marked increase of hyperlipidemia. Gravin serves the novel role as a regulator of vascular inflammation and lipid metabolism. In contrast to wild-type C57BL/6 mice, the gravin mutant (gravin-t/t) mice lacked the scaffolding sites for PKA, PKC, PP2B, β2-adrenoreceptor, protein kinases, phosphatases, and phosphodiesterases. Gravin-t/t mouse model provided insights between gravin and proatherosclerotic signaling involved in hepatic lipogenesis and vascular smooth muscle cells proliferation and migration. The study emphasizes multiple aspects of gravin abrogation in HFD-induced atherosclerosis. HFD-fed gravin-t/t mice showed decreased body weight gain with decrease in cholesterol, triglyceride, and VLDL, which together resulted in fewer aorta lipid lesions and reduced 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) enzyme concentration (Fig. 1). Macrophage accumulation was lower in gravin-t/t mice with reduced matrix metallopeptidase 9 (MMP-9) expression in aortic lesions as a sign of dampened inflammation. The study highlighted the novel role of gravin in platelet-derived growth factor (PDGF) signaling via Ca2+-dependent PKA and PKC pathways involved in cell proliferation and migration. The presented report (2) provided a distinct role of gravin in low-grade chronic inflammation and lipid metabolism disorder in obesogenic stress using HFD. Despite nutrient-mediated stress of HFD, gravin deficiency reduced liver damage and lipid accumulation in mice, thereby lower atherosclerosis. Gravin transcriptionally controls of SREBPs, which regulate lipid and cholesterol metabolism. Gravin-t/t mice displayed decrease in liver gene expression of low-density lipoprotein receptor (LDLR and SREBP-2) irrespective of diet factor. The current model showed that gravin is involved in the escort process of SREBP-2 to the Golgi apparatus in an SREBP cleavage-activating protein (SCAP)-dependent manner and leads to decrease in gravin-dependent SREBP-2 activation. As a consequence, the decrease in circulating plasma lipids was noticed. Thus, absence of gravin resulted in downregulation of SREBP-2 gene expression in particular decreased mature nuclear SREBP-2 (SREBP-2n) protein isoform, resulting in downregulation of the cholesterogenic gene targets, thereby lowering levels of SREBP-2-dependent cholesterol synthesis (Fig. 1).
Fig. 1.
Influence of high-fat diet (HFD) and gravin signaling in progress of HFD-induced atherogenesis and atherosclerosis. HDL, high-density lipoprotein; LDL, low-density lipoprotein; AST, aspartate transaminase; ALT, alanine transaminase; SREBPs, sterol regulatory element-binding proteins; MMP9, matrix metallopeptidase 9; AKAP12, A-kinase anchor protein 12.
CONCLUSIONS AND FUTURE PERSPECTIVE
Fan and colleagues (2) describe a novel aspect that targeting gravin reduces the risk of atherosclerosis by targeting multiple signaling pathways. There are a few caveats to mention. The study was performed in nonhyperlipidemic young mice using a HFD. However, systemic hyperlipidemia, as well as aging, are profound and universal risk factors that aggravate inflammation by altering immune response. Thus, nutrient stress in aging is an obvious future goal to define immune response and risk of coronary artery disease. Future studies using hyperlipidemic mice [apolipoprotein E (ApoE)−/− or LDLR−/− or an adeno-associated virus (AAV)-based proprotein convertase subtilisin-like/kexin type 9 (PCSK9) overexpression approach] with a major focus on detailed quantitative targeting of specific leukocyte populations, impact of aging, and mechanism of immune response will advance new knowledge of gravin in cardiovascular medicine. At present, it is unclear whether a decrease in macrophages or MMP-9 expression really dampened inflammation through gravin signaling is beneficial for long-term targeting in cardiomyopathy of obesity, which is a model for heart failure with preserved ejection fraction. The protective role of MMP-9 deletion is well reported in inflammation and cardiovascular disease (3). Successive studies should identify quantitative and specific macrophages phenotypes to validate the role of gravin in atherosclerosis. It would be of interesting to know whether gravin has a similar role in obesogenic patients for pursuing therapeutic targets in atherosclerosis and hyperlipidemia. These results are in line with the previous report wherein a tight relationship is seen between age-dependent diet interaction with host intestinal microbiota being the frontline in the defense training of the immune system (6). Future studies delineating the role of gravin in microbiome interaction with immune cells should provide more information for translational outcome. When these encouraging findings are taken into consideration, further studies shedding more light on gravin signaling with an integrative multiorgan approach for leukocyte biology in the setting of obesogenic aging are warranted.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant HL-132989 and HL-144788.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.V.H. conceived and designed research; G.V.H. and V.K. prepared figures; V.K. and G.V.H. drafted manuscript; V.K. and G.V.H. edited and revised manuscript; G.V.H. approved final version of manuscript.
REFERENCES
- 1.Burgers PP, Ma Y, Margarucci L, Mackey M, van der Heyden MA, Ellisman M, Scholten A, Taylor SS, Heck AJ. A small novel A-kinase anchoring protein (AKAP) that localizes specifically protein kinase A-regulatory subunit I (PKA-RI) to the plasma membrane. J Biol Chem 287: 43789–43797, 2012. doi: 10.1074/jbc.M112.395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan Q, Yin X, Rababa'h A, Diaz AD, Wijaya CS, Singh S, Suryavanshi SV, Vo HH, Saeed M, Zhang Y, McConnell BK. Absence of gravin-mediated signaling inhibits development of high-fat diet-induced hyperlipidemia and atherosclerosis. Am J Physiol Heart Circ Physiol 317: TBA, 2019. doi: 10.1152/ajpheart.00215.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 139: 32–40, 2013. doi: 10.1016/j.pharmthera.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halade GV, Kain V. Obesity and cardiometabolic defects in heart failure pathology. Compr Physiol 7: 1463–1477, 2017. doi: 10.1002/cphy.c170011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han B, Poppinga WJ, Schmidt M. Scaffolding during the cell cycle by A-kinase anchoring proteins. Pflugers Arch 467: 2401–2411, 2015. doi: 10.1007/s00424-015-1718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kain V, Van Der Pol W, Mariappan N, Ahmad A, Eipers P, Gibson DL, Gladine C, Vigor C, Durand T, Morrow C, Halade GV. Obesogenic diet in aging mice disrupts gut microbe composition and alters neutrophil:lymphocyte ratio, leading to inflamed milieu in acute heart failure. FASEB J 33: 6456–6469, 2019. doi: 10.1096/fj.201802477R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohmann SM, DeCamilli P, Einig I, Walter U. High-affinity binding of the regulatory subunit (RII) of cAMP-dependent protein kinase to microtubule-associated and other cellular proteins. Proc Natl Acad Sci USA 81: 6723–6727, 1984. doi: 10.1073/pnas.81.21.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayerl C, Lukasser M, Sedivy R, Niederegger H, Seiler R, Wick G. Atherosclerosis research from past to present--on the track of two pathologists with opposing views, Carl von Rokitansky and Rudolf Virchow. Virchows Arch 449: 96–103, 2006. doi: 10.1007/s00428-006-0176-7. [DOI] [PubMed] [Google Scholar]
- 9.Morita SY. Metabolism and Modification of Apolipoprotein B-Containing Lipoproteins Involved in Dyslipidemia and Atherosclerosis. Biol Pharm Bull 39: 1–24, 2016. doi: 10.1248/bpb.b15-00716. [DOI] [PubMed] [Google Scholar]
- 10.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 6: 399–409, 2009. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]