Abstract
Induced vascular progenitor cells (iVPCs) were created as an ideal cell type for regenerative medicine and have been reported to positively promote collateral blood flow and improve cardiac function in a rat model of myocardial ischemia. Exosomes have emerged as a novel biomedicine that mimics the function of the donor cells. We investigated the angiogenic activity of exosomes from iPVCs (iVPC-Exo) as a cell-free therapeutic approach for ischemia. Exosomes from iVPCs and rat aortic endothelial cells (RAECs) were isolated using a combination of ultrafiltration and size-exclusion chromatography. Nanoparticle tracking analysis revealed that exosome isolates fell within the exosomal diameter (<150 nm). These exosomes contained known markers Alix and TSG101, and their morphology was validated using transmission electron microscopy. When compared with RAECs, iVPCs significantly increased the secretion of exosomes. Cardiac microvascular endothelial cells and aortic ring explants were pretreated with RAEC-Exo or iVPC-Exo, and basal medium was used as a control. iVPC-Exo exerted an in vitro angiogenic effect on the proliferation, tube formation, and migration of endothelial cells and stimulated microvessel sprouting in an ex vivo aortic ring assay. Additionally, iVPC-Exo increased blood perfusion in a hindlimb ischemia model. Proangiogenic proteins (pentraxin-3 and insulin-like growth factor-binding protein-3) and microRNAs (-143-3p, -291b, and -20b-5p) were found to be enriched in iVPC-Exo, which may mediate iVPC-Exo induced vascular growth. Our findings demonstrate that treatment with iVPC-Exo promotes angiogenesis in vitro, ex vivo, and in vivo. Collectively, these findings indicate a novel cell-free approach for therapeutic angiogenesis.
NEW & NOTEWORTHY The results of this work demonstrate exosomes as a novel physiological mechanism by which induced vascular progenitor cells exert their angiogenic effect. Moreover, angiogenic cargo of proteins and microRNAs may define the biological contributors in activating endothelial cells to form a new capillary plexus for ischemic vascular diseases.
Listen to this article's corresponding podcast at https://ajpheart.podbean.com/e/angiogenic-exosomes-from-vascular-progenitor-cells/.
Keywords: angiogenesis, endothelial cell, exosomes, microRNA, progenitor cell
INTRODUCTION
Studies suggest that individuals burdened with ischemic vascular diseases such as coronary artery disease (53), cerebral infarction (19), and critical limb ischemia (1) would benefit from angiogenic therapy. Angiogenesis is a dynamic biological process occurring between vascular cells and the extracellular environment that promotes blood vessel growth. This natural recovery mechanism is enticing, and the science of therapeutic angiogenesis has been intensively investigated by applying well-established angiogenic initiators as a method for inducing, augmenting, and controlling the host angiogenic response to supply a sufficient blood flow to ischemic tissue (11, 14, 43). Several approaches have been adopted to accomplish therapeutic angiogenesis, in which cell-based therapies have been utilized in preclinical and clinical trials (24, 29, 52), but an ideal cell source has yet to be identified. Recently, induced pluripotent stem cells (iPSCs) have been proposed as an attractive cellular candidate for cell-based therapy applications. iPSCs are fully reprogrammed somatic cells that can be differentiated into various terminal cell types and present valuable characteristics for replenishing functional cells in target tissues. It has been reported that implantation of iPSCs can increase endothelial cell growth and enhance new blood vessel formation (4). However, tumorigenesis remains as a limitation to their full clinical application. Considering this caveat, induced vascular progenitor cells (iVPCs) have been created as a novel cell candidate for angiogenic therapy. iVPCs are rat aortic endothelial cells that are partially reprogrammed using the same transcription cocktail employed for iPSCs to induce the development of stem cell-like characteristics. When compared with iPSCs, iVPCs have been shown to engraft into vessel wall cellular components and stimulate coronary collateral flow, thereby improving heart function in an ischemic heart model, without any recorded risk of tumor formation (23, 50). Indeed, a well-established collateral circulation can improve the physical outcome of the ischemic tissue, and Yin et al. have provided compelling evidence in both in vitro and in vivo models that iVPCs are an ideal cell type for proangiogenic therapy. Yet the mechanisms of how iVPC cell-based therapies elucidate their proangiogenic response are not fully understood.
It is well established that cellular secretomes comprise an essential component of the beneficial properties of transplanted stem cells, in addition to the differentiation of cells, and the focus of cell-based therapies has therefore shifted (3, 10, 31, 35). Exosomes are 30–150 nm cell-derived vesicles that exist in almost all biological fluids, including blood, urine, saliva, cerebrospinal fluid, and cell-preconditioned medium (27). Exosomes shuttle proteins, lipids, RNAs, microRNAs (miRs), and other molecular constituents to achieve cell-to-cell communication and modulate the function of recipient cells (25, 26). Furthermore, exosomes have been identified as a novel drug delivery system and therapeutic agent (9). Exosomes deliver a wide array of proteins and miRs that play critical roles in stimulating cellular processes. Here, we demonstrate that, like iVPCs, iVPC-secreted exosomes (iVPC-Exo) stimulate angiogenesis in in vitro, ex vivo, and in vivo hindlimb ischemia models compared with parent rat aortic endothelial cell-secreted exosomes (RAEC-Exo). Additionally, the biological content of the exosomes was examined, and proangiogenic proteins and miRs were identified as potential regulators of the angiogenic response in recipient endothelial cells. Overall, our data indicate that iVPC-Exo can serve as a novel cell-free angiogenic therapy.
MATERIALS AND METHODS
Cell culture.
Rat iVPCs were generated via lentiviral transduction expressing Oct-4, Klf4, Sox2, and c-Myc transcription factors in the laboratory of Dr. William Chillian at Northeast Ohio Medical University. iVPCs were maintained in knockout DMEM (Thermo Fisher Scientific; Waltham, MA) supplemented with 10% embryonic stem cell-qualified fetal bovine serum (FBS) (American Type Culture Collection; Manassas, VA), 1% l-glutamine (Thermo Fisher Scientific), 1% nonessential amino acids (Thermo Fisher Scientific), 0.1 mmol/L β-mercaptoethanol (Sigma-Aldrich; St. Louis, MO), 0.01% leukemia inhibitory factor protein (Millipore Sigma; Burlington, MA), and 1% antibiotic-antimycotic (Thermo Fisher Scientific). RAECs (Cell Applications; San Diego, CA) and rat cardiac microvascular endothelial cells (CMVECs; Cell Biologics; Chicago, IL) were maintained in RAEC growth medium and CMVEC growth medium, respectively, according to the manufacturers’ instructions. All cell types were grown in a humidified atmosphere of 5% CO2-95% air at 37°C. The medium was replaced every 2 to 3 days. Primary cells for all experiments were used in passages 4–6.
Exosome isolation.
iVPCs or RAECs were incubated in basal culture medium supplemented with 1% exosome-depleted FBS for 24 h. In all cases, the FBS used in this study was exosome-depleted FBS, which was obtained by ultracentrifugation of FBS at 100,000 g for 18 h at 4°C. Exosomes were isolated from the medium by using combinational techniques of ultrafiltration and size-exclusion chromatography. Briefly, the medium was collected from the culture vessel and subjected to centrifugation at 3,000 g for 15 min to remove detached cells and debris. The supernatant was then run through a membrane with a 100-kDa molecular mass cutoff in a centrifugal filter unit (Millipore Sigma). The concentrated medium was eluted in a size-exclusion chromatography qEV unit (iZON Science; Medford, MA) according to the manufacturer’s manual. The eluate containing exosomes was collected and reconcentrated with a 10-kDa molecular mass cutoff centrifugal filter unit (Millipore Sigma).
The protein concentration of the isolated exosomes was detected by using a total exosome RNA and Protein Isolation Kit (Thermo Fisher Scientific) and a BCA Protein Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Nanoparticle tracking analysis.
An LM10 nanoparticle tracking analysis (NTA) device (Malvern; Amesbury, UK) was used according to the manufacturer’s recommendations. Each exosome sample was analyzed by detecting the rate of the Brownian motion of particles in liquid suspension. The analysis settings were optimized, and each video was analyzed to obtain the mean, mode, median, and estimated concentration of each particle size. A total of 500 µL of a 1:5 diluted exosome sample in PBS was injected into a NanoSight sample cubicle, which yielded a particle concentration of 1 × 108 particles/mL. All samples were analyzed in triplicate.
Transmission electron microscopy.
Each exosome sample was fixed with 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer for 2 h at 4°C and postfixed with 1% osmium tetroxide in 0.1 mol/L sodium cacodylate buffer for 1 h at 4°C. The sample was then incubated in 0.5% aqueous uranyl acetate for 2 h at room temperature for en bloc staining, followed by a graded ethanol series for dehydration. Thereafter, the sample was embedded in Embed 812 resin, cut into ultrathin sections, and poststained with uranyl acetate and lead citrate. These sections were examined using a JEOL 1200EX transmission electron microscope (Tokyo, Japan) (33).
Western blot analysis.
Protein samples were resolved by electrophoresis in a 4–12% precast Bis-Tris gel (Thermo Fisher Scientific). Using an iBlot Dry Blotting System, the proteins were then transferred from the gel to a nitrocellulose membrane. The membrane was blocked using 5% dry milk in Tris-buffered saline-Tween 20. Western blot analysis for exosome markers was subsequently performed. Proteins were detected using primary anti-Alix (ab-186429, Abcam; Cambridge, MA), anti-TSG101 (T-5701, Sigma-Aldrich), anti-IGFBP3 (GTX-100454, GeneTex; Irvine, CA), and anti-Pentraxin (125007, Abcam) antibodies. Exposure of the resultant protein bands was performed with an ImageQuant LAS 4000 Luminescent Image Analyzer (GE Healthcare; Chicago, IL).
Exosome uptake.
Exosomes were labeled using the ExoGlow-Protein EV Labeling Kit (System Biosciences; Palo Alto, CA) according to the manufacturer’s instructions. Briefly, the exosomes were incubated in labeling dye at 37°C for 20 min and then in precipitation solution at 4°C overnight. The labeled iVPC-Exo were precipitated by centrifugation at 10,000 revolutions/min for 10 min and resuspended in CMVEC growth medium. CMVECs at 70% confluence were incubated in medium containing various concentrations of labeled exosomes (0, 25, 50, 100, or 200 µg/mL) for different times (0, 6, 12, or 24 h). Exosome uptake efficiency was determined using flow cytometry or fluorescence microscopy.
For flow cytometry, the cells were collected and analyzed by using a Guava easyCyte Flow Cytometer (Millipore Sigma). The gain controls were kept at their default settings. Endothelial cell autofluorescence was accounted for by gating the cell population above the 5% threshold. Each cell was measured for forward scatter (FSC-HLog) versus green fluorescence (GRN-HLog). The results were analyzed using FlowJo software.
Fluorescent microscopy was used to visualize the uptake of iVPC-Exo into CMVECs. CMVECs were washed with PBS 3 times and then incubated in a 1:2,000 dilution of Hoechst 33342 (Thermo Fisher Scientific) in culture medium for 30 min at 37°C. Images were obtained after costaining was completed.
Tube formation assay.
CMVECs were incubated in basal medium containing 1% FBS with or without 200 μg/mL of RAEC-Exo or iVPC-Exo for 24 h. The cells were then seeded in a 96-well plate at 1 × 104 cells/well in 75 μL of basal medium. The wells were preloaded with growth factor-reduced Matrigel (BD Biosciences; San Jose, CA) at 50 μL/well. The cells were subsequently incubated at 37°C under 5% CO2 for 4 h and stained with 10 µmol/L of calcein AM (Thermo Fisher Scientific) at 37°C under 5% CO2 for 30 min. Tube formation was visualized using fluorescence microscopy. Total vessel length, vessel area, the number of junctions, and network complexity were calculated using AngioTool v.2 software.
Scratch wound healing assay.
CMVECs were incubated in basal medium containing 1% FBS with or without 200 μg/mL of RAEC-Exo or iVPC-Exo for 24 h. The cells were then seeded in a 24-well plate to create a confluent monolayer. The cell monolayer was scratched with a sterile pipette tip. The migration of CMVECs was captured using a bright-field microscope at 0-, 6-, and 12-h postscratch. The percentage of wound coverage, taking the value at 0 h as 0%, was calculated using ImageJ software.
Cell proliferation assay.
CMVECs were seeded in a 96-well plate at 3.0 × 103 cells/well and serum starved for 24 h. The cells were then incubated in basal medium containing 1% FBS with or without 200 μg/mL of RAEC-Exo or iVPC-Exo for 4 days. The same fresh medium with or without exosomes was replaced at day 2. Cell growth was measured using a Cell Counting Kit-8 (Dojindo Molecular Technologies; Rockville, MD). Briefly, 10 μL of CCK-8 solution was added to the medium in each well, followed by incubation for 4 h at 37°C. The absorbance spectrum at 450 nm was measured using a SpectraMAX 190 spectrophotometer. To remove background, CMVEC basal medium containing 1% FBS without cells was incubated in parallel, and the acquired absorbance value was deducted.
Aortic ring assay.
All animal experiments conducted in this study were approved by the Institutional Animal Care and Use Committee of the Atlanta University Center and complied with National Institutes of Health guidelines for the care and use of laboratory animals. Male Sprague-Dawley rats aged 8–10 wk were obtained from Taconic Biosciences (Rensselaer, NY). The aortas were collected, cleaned of fibrofatty tissue, and cut into rings of ~1 mm in width under sterile conditions. The aortic rings were transferred to a 96-well plate and serum starved for 24 h in Opti-MEM supplemented with 1% FBS. The following day, the rings were embedded in 100 µL of Matrigel in an 8-well glass chamber slide with 1 ring/well. The embedded rings were incubated in Opti-MEM containing 1% FBS with or without 200 μg/mL of RAEC-Exo or iVPC-Exo for 5 days. The same fresh medium with or without exosomes was replaced at day 3. The rings were stained with 10 μmol/L calcein AM for 30 min, and images of the rings were captured using an Olympus microscope. Quantification of sprouting was performed via AngioTool v.2 software.
Hindlimb ischemia model.
Male Sprague-Dawley rats aged 5 wk were anesthetized via inhalation of 3% isoflurane. The limbs of the rats were fixed on a heated pad with adhesive tape. The hair on their left lower abdominal quadrant and left leg was removed. A skin incision was made on the medial aspect of the left thigh to expose the neurovascular bundle of the femoral nerve, artery, and vein. An opening of 1 to 2 mm was created between the femoral artery and vein. The femoral artery was ligated ~5 to 6 mm distal to the inguinal ligament with 3-0 surgical silk using triple surgical knots. The rats were randomized into three groups by the type of intramuscular injection into the adductor muscle of the left limb with either 100 µL of PBS (control), 30 µg of RAEC-Exo, or 30 µg of iVPC-Exo in 100 µL of PBS immediately after the ligation. The same intramuscular injection was performed at the surgery site 4 and 8 days postsurgery. Blood perfusion on both hindlimbs was recorded under general anesthesia with 3% isoflurane by using laser speckle contrast imaging the day before (baseline) or 0, 3, 7, 14, or 21 days after the surgery. Carprofen (5 mg/kg) was subcutaneously administered to manage pain the day of surgery and 3 days postsurgery. The rats were euthanized after the last blood perfusion assessment by inhalation of excess carbon dioxide, followed by cervical dislocation. The gastrocnemius muscle of the left hindlimb was harvested for histological evaluation of vascular growth by using immunohistochemistry.
Immunohistochemistry.
The gastrocnemius muscle was collected for frozen sectioning. The frozen blocks were cryosectioned at a thickness of 8 µm using a microtome cryostat (Leica Microsystems; Buffalo Grove, IL). The sections were mounted onto glass slides. The slides were dried at room temperature for 2 h and fixed in cold methanol for 15 min at −20°C. The slides were dried for 30 min at room temperature and washed with PBS twice. The sections were blocked with 3% bovine serum albumin in PBS at room temperature for 1 h. Each section was incubated with the primary anti-CD31 antibody (ab-64543, Abcam) at a dilution of 1:2,000 in blocking buffer at 4°C overnight. The slides were washed with PBS and incubated with the secondary antibody Alexa Fluor 488 donkey anti-mouse IgG (Thermo Fisher Scientific) at a dilution of 1:500 in blocking buffer at room temperature for 1 h. The slides were washed with PBS and counterstained with Hoechst 33342 nuclear dye at a dilution of 1:1,000 for 20 min at room temperature. Aqueous mounting medium (Electron Microscopy Science; Hatfield, PA) was added to mount the coverslip. Images were acquired using fluorescence microscopy. Regions with high-intensity CD31+ areas (hotspots) were chosen for quantification. Six hotspots per section and 3 sections per limb were analyzed at ×40 magnification. ImagePro Plus software (Media Cybernetics) was used to measure CD31+ areas in each hotspot (26).
Angiogenesis antibody array.
An Angiogenesis Antibody Array Kit was used to probe for proangiogenic and antiangiogenic proteins (R&D Systems; Minneapolis, MN) according to the manufacturer’s manual. Fifty-three capture antibodies were spotted in duplicate on a nitrocellulose membrane. A 100-µg sample of lysates of RAEC-Exo or iVPC-Exo was incubated with a cocktail of biotinylated detection antibodies for 1 h at room temperature. The angiogenesis antibody membrane was incubated in 2 mL of array blocking buffer for 1 h at room temperature on a rocking platform. The membrane was washed twice with 1× array wash buffer and incubated with the prepared lysate and antibody cocktail mixture in array blocking buffer at 4°C overnight on a rocking platform. The membrane was then washed with 1× array wash buffer for 5 min and incubated in 2 mL of streptavidin-horseradish peroxidase solution for 30 min at room temperature. The membrane was washed with 1× array wash buffer three times. Exposure of proteins on the membrane was achieved by using a Chemi Reagent and an ImageQuant LAS 4000 Luminescent Image Analyzer (GE Healthcare). ImageJ software was used to quantify the exposed protein dots. The array was performed twice for each independent exosomal sample.
Bioanalyzer RNA analysis.
Total RNA in cells or exosomes was extracted using a miRNeasy Isolation Kit (Qiagen; Venlo, Netherlands) according to the manufacturer’s instructions. The concentration of RNA was determined with a NanoDrop 1000 spectrophotometer. The isolated RNA from iVPCs and iVPC-Exo was analyzed using an Agilent 2100 Bioanalyzer, an RNA 6000 Nano Kit, and an RNA 6000 ladder (Agilent Technologies; Santa Clara, CA) following the manufacturer’s protocol.
MiR profiling.
The miR profiling of isolated total RNA from cells or exosomes was performed by LC Sciences (Houston, TX). Briefly, microarray assays were performed using a microfluidic chip in which the coding sequence of the detection probes was complementary to a specific miR sequence. The miR detection signal threshold was defined as twice the maximum background signal. Adjustments including data filtering, log2 transformation, gene centering, and normalization were performed.
TaqMan miR assay.
Reverse transcription of total RNA from exosomes, cDNA preamplification, and quantitative RT-PCR assays were performed using the specific TaqMan MiRNA Assay, TaqMan MiRNA Reverse Transcription Kit, TaqMan PreAmp Master Mix, and TaqMan Universal PCR Master Mix (Thermo Fisher Scientific) according to the manufacturer’s manual and a previous report by our group. Relative miR levels were normalized to those of the endogenous U6 small nuclear RNA, a highly conserved small nuclear RNA in vertebrate genomes, for each sample.
Statistical analysis.
All values are reported as the means ± SE based on experiments performed in at least triplicates unless otherwise noted. Protein concentrations were analyzed using Student’s t test. Exosome uptake, tube formation, and aortic ring assays were analyzed using one-way ANOVA. Analyses of proliferation, migration, and hindlimb perfusion recovery assays were conducted using two-way ANOVA with a post hoc Fisher’s least significant difference test to adjust for multiple group comparisons. For miR profiling, Student’s t test analysis was performed. All statistical analyses were carried out using the statistical package of SigmaPlot 7.0. All experiments were independently repeated at least three times. A difference was considered significant when P < 0.05.
RESULTS
Characterization of iVPC-Exo and RAEC-Exo.
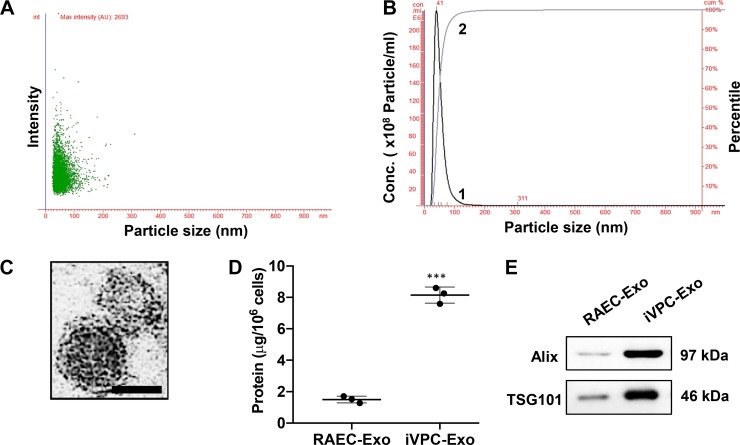
NTA was used to determine the size distribution profile and relative concentration of exosomes. NTA recorded the movement of even-sized exosomes under Brownian motion. When this method was used, both RAEC-Exo and iVPC-Exo fell within the 30–150 nm exosomal range (40). RAEC-Exo had an average peak diameter of 44 ± 5.4 nm [Supplemental Fig. S1A and S1B (https://doi.org/10.5281/zenodo.3261792)]. However, there was no significant difference in the size distribution between RAEC-Exo and iVPC-Exo (peak diameter: 50 ± 6.5 nm (Fig. 1, A and B). iVPC-Exo clearly exhibited typical morphologic characteristics of exosomes, appearing as submicron vesicles lacking a nucleus with a condensed core protected by a lipid bilayer, in line with the reported characteristics of exosomes (Fig. 1C). Protein content was used as a relative measure of the number of exosomes. The protein content of exosomes secreted from iVPCs was 4.5-fold higher than that of exosomes from the same number of RAECs (Fig. 1D). Similarly, when the same volume of exosome protein lysate was loaded from the same number of cells, the contents of Alix and TSG101 in iVPC-Exo were higher than those from the same number of RAECs. (Fig. 1E). Alix and TSG101 are well-known exosomal markers (39) and are categorized under a family of proteins known as the endosomal sorting complexes required for transport (ESCRT), which are directly involved in the biogenesis of exosomes (15). These results suggest that the reprogramming of RAECs (terminally differentiated adult cells) to iVPCs (stem/progenitor-like cells) promotes the secretion of exosomes.
Fig. 1.
Characterization of iVPC-Exo. iVPCs were incubated in culture medium containing 1% exosome-free fetal bovine serum for 24 h. Medium was collected and subjected to exosome isolation. Isolated exosomes were examined using NTA to determine exosomal size and concentration. Scatter plot graphs of exosomes demonstrating the particle size vs. light intensity of iVPC-Exo (A) and the distribution of particle size vs. concentration of iVPC-Exo (B) are shown. Curve 1 illustrates the relationship between particle number and distribution of particle size (concentration/mL; left, y-axis). Curve 2 describes the correlation between the cumulative percentage distribution of particles (percentile; right, y-axis) and particle size (x-axis). Morphology of iVPC-Exo was visualized under transmission electron microscopy (scale bar = 50 nm; C). Protein amount in exosomes from the same number of RAECs or iVPCs was compared (D) (***P < 0.001 vs. RAEC-Exo). Exosomal markers, Alix and TSG101, in RAEC-Exo and iVPC-Exo were determined via Western blot analysis (E). Each lane represented an exosomal lysate collected from 2.5 × 106 cells. For A, B, D, and E: n = 3. iVPC, induced vascular progenitor cell; iVPC-Exo, iVPC-secreted exosomes; NTA, nanoparticle tracking analysis; RAEC-Exo, rat aortic endothelial cell-secreted exosomes.
Internalization of iVPC-Exo into CMVECs.
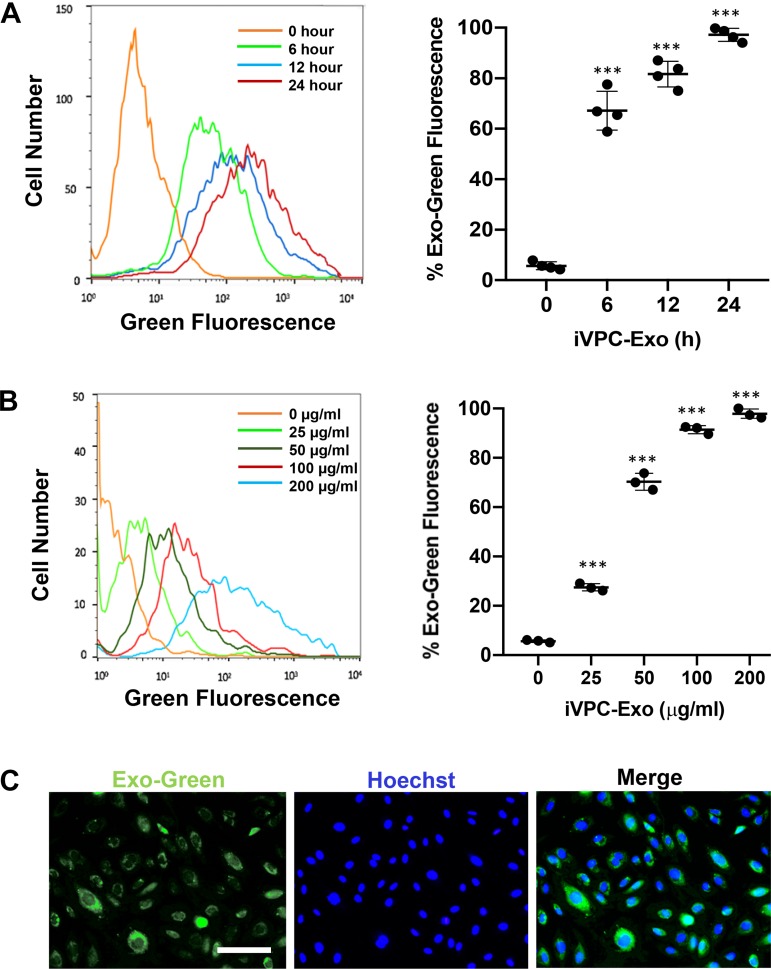
Exosome uptake kinetics were investigated in CMVECs. Purified exosomes were prelabeled using a green fluorescent dye and cocultured with CMVECs at different times and doses. The internalization of iVPC-Exo into CMVECs significantly increased with longer incubation times compared with that of nontreated CMVEC controls. Notably, more than 66.8% of CMVECs contained iVPC-Exo by 6 h, and by 12 h this percentage had increased to 83.6%, with 96.3% saturation occurring at 24 h (Fig. 2A). When using increasing concentrations of labeled iVPC-Exo, it was found that uptake operated in a dose-dependent manner (Fig. 2B). After coculturing CMVECs with varying concentrations of labeled exosomes, it was shown that increasing the dose increased the internalization of iVPC-Exo by recipient cells, where saturation occurred at 200 µg/mL (97.9%). The maximum fluorescence intensity could be observed at the 24-h time point under treatment with 200 µg/mL iVPC-Exo, and these conditions were used for all downstream experiments.
Fig. 2.
Uptake of iVPC-Exo into CMVECs. CMVECs were incubated with Exo-Green labeled iVPC-Exo to measure the efficiency of uptake. A: flow cytometry histogram of CMVECs after incubation with 200 µg/mL of labeled iVPC-Exo for 0 h, 6 h, 12 h, or 24 h (left). Quantification of time-course assay result from flow cytometry analysis (right) (***P < 0.001 vs. 0 h; n = 4). B: flow cytometry histogram of CMVECs after incubation with 0, 25, 50, 100, or 200 µg/mL of labeled iVPC-Exo for 24 h (left). Quantification of dose-response assay result from flow cytometry analysis (right) (***P < 0.001 vs. 0 µg/mL). C: representative images demonstrated iVPC-Exo uptake into CMVECs after incubation with 200 µg/mL of iVPC-Exo for 24 h using fluorescent microscopy. Images displayed Exo-Green (green) for labeled exosomes in iVPCs, Hoechst 33342 (blue) staining of nuclei, and merged image, respectively (scale bar = 100 µm). For B and C: n = 3. CMVEC, cardiac microvascular endothelial cells; iVPC, induced vascular progenitor cell; iVPC-Exo, iVPC-secreted exosomes.
The internalization of exosomes was confirmed visually using fluorescence microscopy in the presence of intracellular green fluorescence. The visualization of labeled exosomes revealed that they tended to disperse within the cytoplasm (Fig. 2C). Overall, these data indicated that iVPC-Exo could be taken up into the target CMVECs used in the angiogenic functional analyses after treatment with RAEC-Exo and iVPC-Exo.
iVPC-Exo promote angiogenesis in vitro.
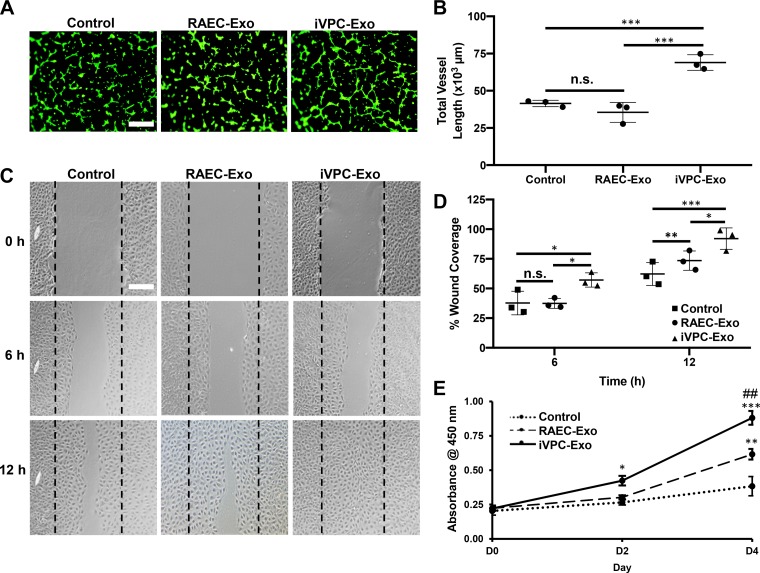
Exosomes from mesenchymal stem cells (34, 46), embryonic stem cells (28), and human pericardial fluid (5) are involved in the regulation of angiogenesis; however, the role of iVPC-Exo has not been investigated. To examine the angiogenic potential of iVPC-Exo, the tube formation, migration, and proliferation of CMVECs were examined in vitro. Our results demonstrated that the vessel length and vessel area of endothelial cells were significantly increased upon treatment with iVPC-Exo compared with RAEC-Exo. There was no significant difference in the vessel length or area of cells treated with RAEC-Exo or the control [Fig. 3, A and B and Supplemental Fig. S2A (https://doi.org/10.5281/zenodo.3261792)]. Additionally, RAEC-Exo and iVPC-Exo significantly increased the total number of junctions and reduced the lacunarity of these cells [Supplemental Fig. S2B and S2C (https://doi.org/10.5281/zenodo.3261792)]. These data provide compelling evidence that treatment with iVPC-Exo is more effective than treatment with RAEC-Exo in enhancing endothelial cell tubule formation, which is an essential step in the angiogenic process.
Fig. 3.
iVPC-Exo enhance tube formation, migration, and proliferation of CMVECs. CMVECs were left untreated (control) or pretreated with 200 µg/mL of RAEC-Exo or iVPC-Exo for 24 h and subjected to the following angiogenic assay in vitro. A and B: cells were seeded on top of Matrigel and used for tube formation assay and stained with calcein AM (green) for visualization, and representative images were acquired using fluorescent microscopy. Tube formation was quantified by vessel length using NIH AngioTool software (***P < 0.001). C and D: representative images of the scratch wound healing assay acquired at time points 0 h, 6 h, and 12 h using a bright-field microscope. Percentage of wound coverage was quantified by calculating area of gap using ImageJ software (*P < 0.05, **P < 0.01, and ***P < 0.001). E: CMVEC proliferation was measured by using CCK-8 analysis (*P < 0.05 iVPC-Exo vs. control on day 2, **P < 0.01 RAEC-Exo vs. control on day 4, ***P < 0.001 iVPC-Exo vs. control on day 4, and ##P < 0.01 iVPC-Exo vs. RAEC-Exo on day 4). For A and C: scale bar = 100 µm. For A–E: n = 3. CMVEC, cardiac microvascular endothelial cells; D, day; iVPC-Exo, induced vascular progenitor cell-secreted exosomes; n.s., not significant; RAEC-Exo, rat aortic endothelial cell-secreted exosomes.
The effect of iVPC-Exo on CMVEC migration was investigated by using a scratch wound healing assay. The wound coverage of CMVECs treated with iVPC-Exo was 1.5-fold greater than that of CMVECs treated with RAEC-Exo or left untreated at 6-h postscratch and 2.2-fold or 1.3-fold greater than that of CMVECs treated with RAEC-Exo or left untreated at 12-h postscratch, respectively (Fig. 3, C and D). These observations showed that iVPC-Exo increased the migration rate of CMVECs. In addition, iVPC-Exo enhanced CMVEC proliferation after 2 days of incubation in comparison with RAEC-Exo and the untreated control (Fig. 3E). Consistent with the tube formation assay results, iVPC-Exo had a stronger in vitro proangiogenic effect than RAEC-Exo.
iVPC-Exo enhance microvessel sprouting and angiogenesis in a hindlimb ischemia model.
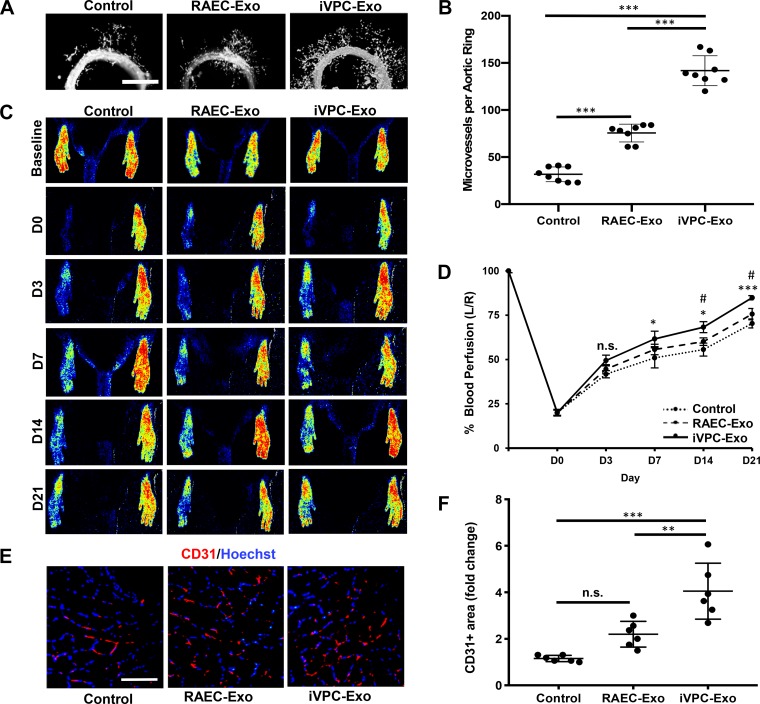
The aortic ring model of angiogenesis is an ex vivo model that has been frequently used to study factors that affect angiogenesis on the basis of observing microvessels sprouting from sections of the aorta (2). This assay recapitulates all the main steps in the angiogenic process, including matrix degradation, cell proliferation, and migration. An aortic ring assay was used to investigate the effect of iVPC-Exo on microvessel sprouting. Treatment of aortic rings with iVPC-Exo increased microvessel sprouting 4.5-fold and 1.9-fold compared with the untreated control and RAEC-Exo, respectively, whereas RAEC-Exo increased sprouting by 2.4-fold compared with the controls (Fig. 4, A and B).
Fig. 4.
iVPC-Exo promotes microvessel sprouting from aortic ring and recovery of blood perfusion in rat ischemic hindlimb. A and B: aortic ring explants were left untreated (control) or treated with 200 µg/mL RAEC-Exo or iVPC-Exo (n = 8). A: images were taken using fluorescent microscopy to visualize microvessel sprouts emerging from aortic rings after 5 days of treatment. B: number of microvessels per ring was then quantified (***P < 0.001). C–F: ligated limbs were left untreated (control) or treated with 30 µg RAEC-Exo or iVPC-Exo (n = 10). C: blood perfusion in both hind limbs was assessed before ligation (baseline) and on day 0, 3, 7, 14, and 21 postligation by using laser speckle imaging. D: perfusion was measured, and the percentage of perfusion was calculated measuring left limb/right limb (L/R) (*P < 0.05 iVPC-Exo vs. control on the same day, ***P < 0.001 iVPC-Exo vs. control on day 21, #P < 0.05 iVPC-Exo vs. RAEC-Exo on the same day, n.s. = not significant). E and F: sections of the gastrocnemius muscle on the ligated side were subjected to immunohistochemistry analysis for CD31, an endothelial cell marker, and counterstained with Hoechst 33342 (scale bar = 200 µm). E: representative images after staining of CD31-positive cells in the gastrocnemius were taken at day 21 postligation. F: quantification of the CD31-positive area was shown (**P < 0.05, ***P < 0.001, n.s. = not significant). D, day; iVPC-Exo, induced vascular progenitor cell-secreted exosomes; RAEC-Exo, rat aortic endothelial cell-secreted exosomes.
The angiogenic effect of iVPC-Exo was further examined in a hindlimb ischemia model. The PBS was used as a control to monitor the natural healing of the ischemic limb. Our results demonstrated that the percentage of blood perfusion in the ischemic hindlimb was enhanced from 20% to 84.5% by the intramuscular and intravenous administration of iVPC-Exo compared with PBS (from 20% to 70.4%) at day 21, or RAEC-Exo, starting at 7 and 14 days after femoral artery ligation, respectively (Fig. 4, C and D). Gastrocnemius muscle recovery after ischemia is predominantly contingent on angiogenesis because neovascularization is required for necessary nutrient and oxygen transfer between tissues. Therefore, immunohistochemical staining for CD31, an endothelial cell marker, was performed in the gastrocnemius muscle. The presence of CD31-positive cells in the ischemic muscles demonstrated that iVPC-Exo could increase the capillary area compared with the control and RAEC-Exo (Fig. 4, E and F), consistent with increased perfusion. Taken together, these results suggest that iVPC-Exo mediate proangiogenesis both ex vivo and in vivo.
iVPC-Exo contain angiogenesis-related growth factors and cytokines.
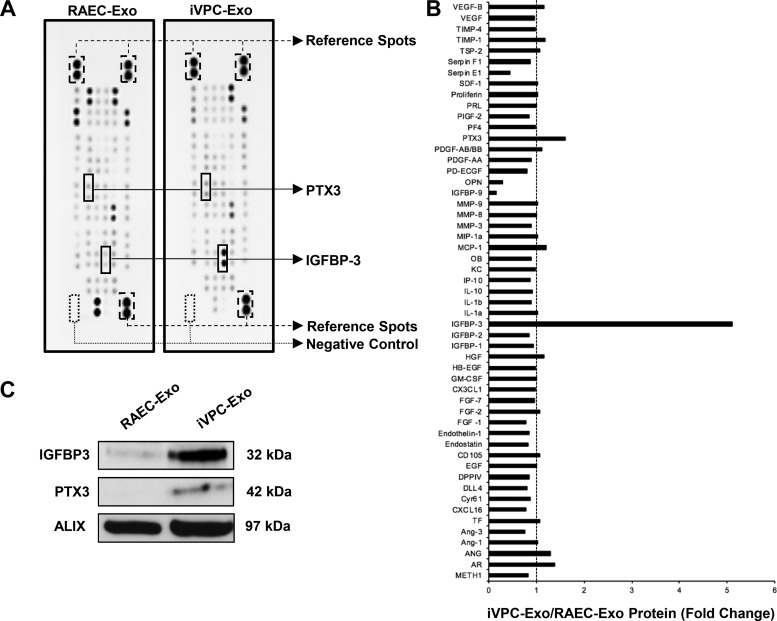
The release of exosome cargo into recipient cells has been regarded as a prominent mechanism for the modulation of function. To screen the contents of the exosomes for angiogenic regulators, an angiogenesis antibody array was employed. On the array, 53 immobilized antibodies against known angiogenesis-related peptides were probed. Two angiogenic factors, insulin-like growth factor-binding protein-3 (IGFBP3) and pentaxtrin-3 (PTX3), were found to exhibit higher levels in iVPC-Exo compared with RAEC-Exo (Fig. 5, A and B). IGFBP3 presented a 5.1-fold change, and PTX3 presented a 1.6-fold change when treated with the iVPC-Exo lysate. The angiopeptides were then validated using immunoblot analysis (Fig. 5C), in which the fold change of IGFBP-3 was 3.9-fold. The fold change of PTX3 was 1.3-fold. It is widely accepted that exosomes aid in the transmission of proteins, and our findings suggest that IGFBP3 and PTX3 may mediate overall angiogenesis induced by iVPC-Exo.
Fig. 5.
Identification of angiopeptides in RAEC-Exo and iVPC-Exo. A and B: a total of 53 angiogenesis-related proteins in RAEC-Exo and iVPC-Exo were examined using an angiogenesis antibody array (n = 2). A: when compared with RAEC-Exo, insulin-like growth factor-binding protein-3 (IGFBP3) and pentraxin-3 (PTX3) in iVPC-Exo are most increased and labeled (two dots represent one angiopeptide in duplicate). Positive (reference spots) and negative membrane controls are also labeled. B: signal intensities were quantified using ImageJ densitometry analysis. Level of protein from RAEC-Exo was set to 1 (dashed line). C: IGFBP3 and PTX3 were then validated using Western blot analysis. Exosomal marker Alix was used as a loading control. n = 3. iVPC-Exo, induced vascular progenitor cell-secreted exosomes; RAEC-Exo, rat aortic endothelial cell-secreted exosomes.
iVPC-Exo contain differential levels of proangiogenic miRs.
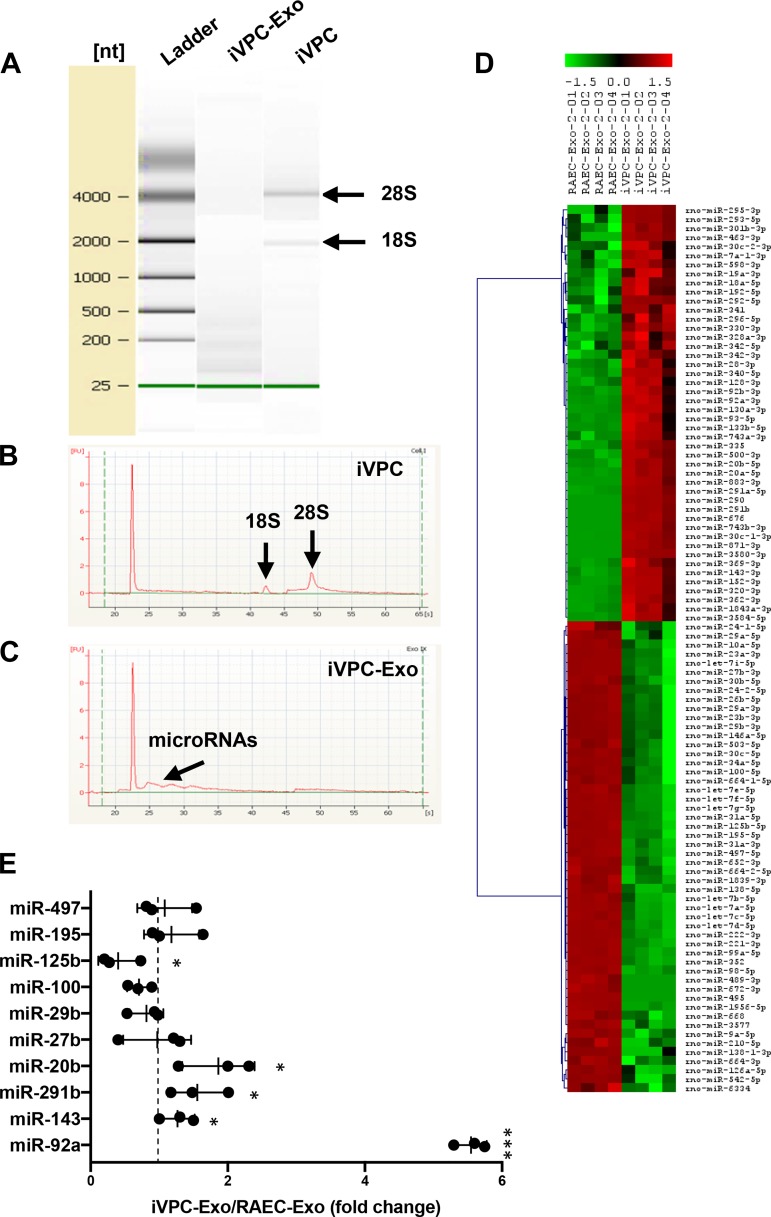
Exosomes have been shown to carry diverse biologically active molecules (45). Of these molecules, miRs have been indicated as key modulators of the initiation/inhibition of cellular functions in angiogenesis. To identify miRs that contribute to therapeutic angiogenesis, exosomal RNA contents were examined. Bioanalyzer RNA analysis demonstrated that iVPCs contained 28S and 18S ribosomal RNA, whereas iVPC-Exo lacked these units, indicating that cellular contamination was minimal (Fig. 6A). Further analysis of electrographs from iVPCs indicated two peaks, which are a known feature of cells (Fig. 6B). Ribosomal units are not expected to occur in iVPC-Exo, but there was a small distribution of peaks occurring at ~25 s, suggesting that miR may be enriched in iVPC-Exo, along with angiogenic proteins (Fig. 6C).
Fig. 6.
RNA analysis and microRNA profiling of RAEC-Exo and iVPC-Exo. A–C: an equal amount of RNA from RAEC-Exo and iVPC-Exo was analyzed using a Bioanalyzer. A: electrophoretic separation of RNA from iVPC and iVPC-Exo. RNA 6000 ladder standard marked six RNA fragments ranging in size from 0.25 to 4 kb. Bands of 18S and 28S ribosomal RNA units are indicated. B: iVPC electropherogram indicates peaks that correspond to ribosomal RNA. C: iVPC-Exo electropherogram shows possible microRNAs. D: exosomal microRNA profiling was performed (n = 2). The microRNAs with different levels between RAEC-Exo and iVPC-Exo (P < 0.01) was shown in a heat map. Hierarchical clustering displayed on the axis to the left demonstrates microRNA cluster relationships. E: angiogenic-related microRNAs (angiomiR) in D were validated using RT-PCR. Level of microRNA in RAEC-Exo was set to 1 (dashed line). U6 was used as an internal control (*P < 0.05, ***P < 0.001 iVPC-Exo vs. RAEC-Exo). n = 3. iVPC, induced vascular progenitor cell; iVPC-Exo, iVPC-secreted exosomes; miR, microRNA; RAEC-Exo, rat aortic endothelial cell-secreted exosomes.
To confirm the presence of miR and examine the pattern of miR in RAEC-Exo and iVPC-Exo, miR array analysis was performed. A total of 46 miRs were found to show statistically significant differences, and a unique miR profile was observed in iVPC-Exo compared with RAEC-Exo (Fig. 6D). Among these 46 miRs, miR-20b, -291b, -143, -42a, -497, -195, -125b, -100, -29b, and -27b were found to be differentially expressed in iVPC-Exo, all of which have reported roles in angiogenesis. When the levels of the angiomiRs in RAEC-Exo and iVPC-Exo were examined using RT-PCR (Fig. 6E), five miRs were shown to exhibit statistically significant differences. The results demonstrated that miR-92a, which has proangiogenic and antiangiogenic functions, was the most enriched miR in iVPC-Exo, whereas miR-125b, an antiangiogenic miR, was carried by iVPC-Exo at low concentrations. The data suggest a mechanism of action whereby iVPC-Exo induce angiogenesis.
DISCUSSION
Stem cell transplantation to stimulate angiogenesis has been used to treat ischemic diseases, including myocardial infarction (8), stroke (16), and peripheral arterial disease (20). Moreover, iVPCs are proposed to be an ideal cell candidate for proangiogenic therapy because these cells have been shown to successfully increase angiogenesis and coronary collateral growth in vitro and in vivo. It is now known that the benefits of stem/progenitor cells are mainly due to the secretion of paracrine factors rather than the differentiation of stem cells. This is referred to as the “paracrine hypothesis” and has shifted the paradigm of proangiogenic therapy. Among these factors, the role of exosomes in intercellular communication has become increasingly well established, and the data support the promotion of ischemia recovery by exosomes (5, 21). This method introduces a cell-free approach to therapeutic angiogenesis. In the present study, iVPC-Exo were shown to increase CMVEC tube formation, migration, proliferation, and ex vivo microvessel sprouting. Additionally, the administration of iVPC-Exo increased blood perfusion after in vivo femoral artery occlusion. Taken together, these results indicate that iVPC-Exo have the same putative effects as iVPCs, which may be mediated by the transmission of angiogenic proteins and miRs.
It was observed that iVPCs exhibited increased secretion of exosomes compared with parent RAECs. Stem cells have a unique capability to secrete abundant exosomes, and the partial reprogramming of RAECs via viral expression of embryonic stem cell transcription factors may increase exosome secretion in iVPCs. For example, MSCs are multipotent stem cells found in bone marrow that readily produce exosomes in large quantities, which has been attributed to their stem cell nature. Similarly, cancer cells secrete a greater number of exosomes than normal proliferating cells (48). Moreover, iVPC-Exo have been shown to contain increased endogenous levels of ESCRT proteins Alix and TSG101. In dendritic cells, Alix depletion was shown to decrease exosome secretion in half of the donor population. Additionally, this same study demonstrated that knockdown of TSG101 reduced the secretion of exosomes (17). Our data suggest that iVPCs may also be an excellent therapeutic donor of exosomes compared with RAECs.
Therapeutic angiogenesis is a tightly regulated process during ischemia, and three main steps must work in a cooperative manner to achieve vessel formation: 1) proliferation, 2) tube formation, and 3) migration of endothelial cells. It is still unclear whether exosomes must be internalized to elicit cellular responses. However, several studies have suggested mechanisms of uptake, including fusion, phagocytosis, micropinocytosis, and receptor-mediated endocytosis (36). Time-course and dose-response assays of exosome uptake have been used to evaluate the delivery and release of exosome cargo. Our uptake studies suggested that internalization may be necessary to elicit the angiogenic response. Thus, we investigated the angiogenic effects of CMVECs and aortic ring tissues after pretreatment with iVPC-Exo, and the results indicated that exosomes could mimic the properties and function of donor cells. Interestingly, RAEC-Exo promoted some proangiogenic effects, but iVPC-Exo were shown to be more effective. This finding could indicate that the method of the partial reprogramming of adult endothelial cells could be used as an autologous therapeutic approach for angiogenesis. Furthermore, iVPC-Exo significantly improved microcirculation in a rat model of hindlimb ischemia compared with the natural healing of the ischemic limb. The underlying mechanisms could be arteriogenesis induced by locally administered exosomes to bypass the ligated site of the femoral artery and angiogenesis induced by ischemia on the hindlimb (32). It has been proposed that exosomes shuttle proteins and genetic information that can be transferred to target cells and alter their bioactivity (6, 7). Because the exosomal content is linked to the cell of origin, it is not surprising that proangiogenic factors were found within iVPC-Exo. Our results demonstrate that proteins and miRs are enriched in iVPC-Exo and that functions related to the expression of proangiogenic and antiangiogenic cargo could be further investigated as a potential mechanism of action.
Our angiogenesis antibody array analysis showed that iVPC-Exo harbor the signaling molecules PTX3 and IGFBP3, which have been proven to contribute to proangiogenic activity in ischemic conditions. In a study performed by Rodrigues-Grande et al. (42), PTX3 knockout mice exhibit significantly reduced angiogenesis and decreased expression of VEGFR2 after in vivo middle cerebral artery occlusion. Moreover, recombinant expression of PTX3 induces endothelial cell proliferation and tube formation. PTX3 has also been shown to promote long-term cerebral blood flow recovery, angiogenesis, and neuronal survival in a preclinical mouse stroke-induced model (38). Coupled with the effects of PTX3, IGFBP3 is critical for neovascular formation and positively regulates angiogenesis through IGF-1R signaling (13). These findings reveal an underlying molecular mechanism at the protein level for angiogenic therapy based on iVPC-Exo.
In addition to evaluating the protein content of iVPC-Exo, miRs were also examined for their contribution to the observed angiogenic effect. MiRs are small, evolutionarily conserved, noncoding RNAs that are involved in the regulation of gene expression. Certain miRs have been shown to regulate the proliferation, migration, and tubule formation capacity of endothelial cells (30). We have previously demonstrated that microvesicles from adipose-derived stem cells promote angiogenesis via the transmission of miR-31 (26). Our miR profiling analysis revealed that iVPC-Exo exhibited unique enrichment of angiogenesis-promoting miRs (miR-92a-3p, -143-3p, -291b, and -20b-5p) and low levels of antiangiogenic miRs (miR-27b-3p, -29b, -100-5p, -125b-5p, -195-5p, and -497-5p). MiR-92a, a member of the miR-17-92 cluster, was shown to be highly expressed in endothelial cells and to present the greatest fold change in our study (41). The role of miR-92a in regulating angiogenesis is largely unknown and controversial. In a study conducted by Zhang et al. (51), pre-miR-92a treatment was found to enhance capillary tube formation of human umbilical vein endothelial cells under oxidative stress. However, the authors noted that inhibition of miR-92a also enhanced angiogenesis. These authors concluded that miR-92a in its normal “homeostatic” range induces endothelial cell angiogenesis because both overexpression and downregulation of miR-92a exerted proangiogenic effects. Interestingly, based on miR target prediction analysis, we found that miR-143 targets Serpin E1 with high affinity. Serpin E1, also known as plasminogen activator inhibitor-1 (PAI-1), is an antiangiogenic protein that inhibits VEGF/VEGFR2 signaling (18, 49). It was observed that Serpin E1 was reduced in iVPC-Exo compared with RAEC-Exo, and our study could benefit from validating the presence of Serpin E1 as a proposed molecular mechanism. Next, miR-20b was observed because of its role in an angiogenic-promoting mechanism. Similar to miR-92a, miR-20b has been reported to play proangiogenic and antiangiogenic roles that depend on the cell type and the physiological state of the body. For example, suppression of miR-20b decreased VEGF mRNA expression in both human umbilical vein endothelial cells and BeWo trophoblast cells, which may indicate cell-specific effects of miR-20b (47). Furthermore, miR-291b is a known stem cell-specific cell cycle-regulating miR that may also contribute to promoting the proliferation of target cells (22). Moreover, our analysis indicated downregulation of the antiangiogenic miR-125b. Studies have shown that overexpression of miR-125b suppresses tumor angiogenesis via the HIF-1α/VEGF pathway. VEGF is a key proangiogenic activator that is activated by the transcription level of HIF-1α (12). Hence, miR-92a, -143, -291b, -20b, and -125b may all play a role in regulating angiogenesis in CMVECs after exosome cargo release. Further genetic knockdown or overexpression studies in CMVECs will also be worthwhile for the future evaluation, application, and therapeutic understanding of iVPC-Exo.
Exosome therapy is advantageous in that the lipid bilayer of exosomes provides protection from degradation, to allow the transfer of biological contents locally and distally, similar to “physiological lipofection” for the modulation of gene expression in target cells (37, 44). Exosomes are naturally produced, and as our study indicates, they carry both angiogenesis-stimulating proteins and miRs that can be clinically applied for the treatment of ischemic disease. In conclusion, the present study has demonstrated that transplantation of iVPC-Exo can stimulate angiogenesis, suggesting that exosomes are a key mediator of the paracrine effects exerted by iVPCs. This work may provide a foundation for additional investigations using exosomes as an alternative approach for proangiogenic therapy.
GRANTS
This work was supported, in whole or in part, by National Institutes of Health Grants SC2GM099629, P50HL117929, and SC1HL134212 (to D. Liu) and R25-GM058268-18 (to T. K. Johnson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.L. conceived and designed research; T.K.J., L.Z., D.Z., Y.W., Y.X., B.O., and X.Z. performed experiments; T.K.J., L.Z., D.Z., Y.W., Y.X., B.O., X.Z., and D.L. analyzed data; T.K.J., D.Z., Y.W., Y.X., B.O., X.Z., and D.L. interpreted results of experiments; T.K.J., L.Z., D.Z., Y.W., Y.X., and D.L. prepared figures; T.K.J. and D.L. drafted manuscript; T.K.J., L.Y., W.M.C., and D.L. edited and revised manuscript; X.Z., W.G.K., L.Y., W.M.C., and D.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ming Bo Huang for technical assistance with nanoparticle tracking analysis.
REFERENCES
- 1.Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol 10: 387–396, 2013. doi: 10.1038/nrcardio.2013.70. [DOI] [PubMed] [Google Scholar]
- 2.Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D’Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K. Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc 7: 89–104, 2011. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- 3.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 103: 530–541, 2014. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 4.Belair DG, Whisler JA, Valdez J, Velazquez J, Molenda JA, Vickerman V, Lewis R, Daigh C, Hansen TD, Mann DA, Thomson JA, Griffith LG, Kamm RD, Schwartz MP, Murphy WL. Human vascular tissue models formed from human induced pluripotent stem cell derived endothelial cells. Stem Cell Rev Rep 11: 511–525, 2015. doi: 10.1007/s12015-014-9549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltrami C, Besnier M, Shantikumar S, Shearn AIU, Rajakaruna C, Laftah A, Sessa F, Spinetti G, Petretto E, Angelini GD, Emanueli C. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and promotes therapeutic angiogenesis. Mol Ther 25: 679–693, 2017. doi: 10.1016/j.ymthe.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78: 838–848, 2010. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 7.Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans 41: 283–287, 2013. doi: 10.1042/BST20120192. [DOI] [PubMed] [Google Scholar]
- 8.Choi SH, Jung SY, Kwon SM, Baek SH. Perspectives on stem cell therapy for cardiac regeneration. Advances and challenges. Circ J 76: 1307–1312, 2012. doi: 10.1253/circj.CJ-11-1479. [DOI] [PubMed] [Google Scholar]
- 9.Conlan RS, Pisano S, Oliveira MI, Ferrari M, Mendes Pinto I. Exosomes as Reconfigurable Therapeutic Systems. Trends Mol Med 23: 636–650, 2017. doi: 10.1016/j.molmed.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol 24: 199–206, 2015. doi: 10.1016/j.carpath.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Deveza L, Choi J, Yang F. Therapeutic angiogenesis for treating cardiovascular diseases. Theranostics 2: 801–814, 2012. doi: 10.7150/thno.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4613, 1996. doi: 10.1128/MCB.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granata R, Trovato L, Lupia E, Sala G, Settanni F, Camussi G, Ghidoni R, Ghigo E. Insulin-like growth factor binding protein-3 induces angiogenesis through IGF-I- and SphK1-dependent mechanisms. J Thromb Haemost 5: 835–845, 2007. doi: 10.1111/j.1538-7836.2007.02431.x. [DOI] [PubMed] [Google Scholar]
- 14.Grochot-Przeczek A, Dulak J, Jozkowicz A. Therapeutic angiogenesis for revascularization in peripheral artery disease. Gene 525: 220–228, 2013. doi: 10.1016/j.gene.2013.03.097. [DOI] [PubMed] [Google Scholar]
- 15.Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab 19, Suppl 1: 137–146, 2017. doi: 10.1111/dom.13027. [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J, Otero-Ortega L, Fuentes B, Díez-Tejedor E. Stem cells for brain repair and recovery after stroke. Expert Opin Biol Ther 13: 1479–1483, 2013. doi: 10.1517/14712598.2013.824420. [DOI] [PubMed] [Google Scholar]
- 17.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75: 193–208, 2018. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirahata M, Osaki M, Kanda Y, Sugimoto Y, Yoshioka Y, Kosaka N, Takeshita F, Fujiwara T, Kawai A, Ito H, Ochiya T, Okada F. PAI-1, a target gene of miR-143, regulates invasion and metastasis by upregulating MMP-13 expression of human osteosarcoma. Cancer Med 5: 892–902, 2016. doi: 10.1002/cam4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann CJ, Harms U, Rex A, Szulzewsky F, Wolf SA, Grittner U, Lättig-Tünnemann G, Sendtner M, Kettenmann H, Dirnagl U, Endres M, Harms C. Vascular signal transducer and activator of transcription-3 promotes angiogenesis and neuroplasticity long-term after stroke. Circulation 131: 1772–1782, 2015. doi: 10.1161/CIRCULATIONAHA.114.013003. [DOI] [PubMed] [Google Scholar]
- 20.Hou L, Kim JJ, Woo YJ, Huang NF. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease. Am J Physiol Heart Circ Physiol 310: H455–H465, 2016. doi: 10.1152/ajpheart.00726.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther 6: 10, 2015. doi: 10.1186/scrt546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakob P, Landmesser U. Role of microRNAs in stem/progenitor cells and cardiovascular repair. Cardiovasc Res 93: 614–622, 2012. doi: 10.1093/cvr/cvr311. [DOI] [PubMed] [Google Scholar]
- 23.Jamaiyar A, Wan W, Ohanyan V, Enrick M, Janota D, Cumpston D, Song H, Stevanov K, Kolz CL, Hakobyan T, Dong F, Newby BZ, Chilian WM, Yin L. Alignment of inducible vascular progenitor cells on a micro-bundle scaffold improves cardiac repair following myocardial infarction. Basic Res Cardiol 112: 41, 2017. doi: 10.1007/s00395-017-0631-4. [DOI] [PubMed] [Google Scholar]
- 24.Johnson T, Zhao L, Manuel G, Taylor H, Liu D. Approaches to therapeutic angiogenesis for ischemic heart disease. J Mol Med (Berl) 97: 141–151, 2019. doi: 10.1007/s00109-018-1729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang K, Ma R, Cai W, Huang W, Paul C, Liang J, Wang Y, Zhao T, Kim HW, Xu M, Millard RW, Wen Z, Wang Y. Exosomes secreted from CXCR4 overexpressing mesenchymal stem cells promote cardioprotection via Akt signaling pathway following myocardial infarction. Stem Cells Int 2015: 659890, 2015. doi: 10.1155/2015/659890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE, Bond VC, Chen YE, Liu D. Adipose-derived stem cells induce angiogenesis via microvesicle transport of miRNA-31. Stem Cells Transl Med 5: 440–450, 2016. doi: 10.5966/sctm.2015-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett 107: 102–108, 2006. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Kervadec A, Bellamy V, El Harane N, Arakélian L, Vanneaux V, Cacciapuoti I, Nemetalla H, Périer MC, Toeg HD, Richart A, Lemitre M, Yin M, Loyer X, Larghero J, Hagège A, Ruel M, Boulanger CM, Silvestre JS, Menasché P, Renault NK. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant 35: 795–807, 2016. doi: 10.1016/j.healun.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 117: 52–64, 2015. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosaka N, Yoshioka Y, Hagiwara K, Tominaga N, Ochiya T. Functional analysis of exosomal microRNA in cell-cell communication research. Methods Mol Biol 1024: 1–10, 2013. doi: 10.1007/978-1-62703-453-1_1. [DOI] [PubMed] [Google Scholar]
- 31.Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery - a novel application for the mesenchymal stem cell. Biotechnol Adv 31: 543–551, 2013. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Lieder HR, Kleinbongard P, Skyschally A, Hagelschuer H, Chilian WM, Heusch G. Vago-splenic axis in signal transduction of remote ischemic preconditioning in pigs and rats. Circ Res 123: 1152–1163, 2018. doi: 10.1161/CIRCRESAHA.118.313859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu D, Lin Y, Kang T, Huang B, Xu W, Garcia-Barrio M, Olatinwo M, Matthews R, Chen YE, Thompson WE. Mitochondrial dysfunction and adipogenic reduction by prohibitin silencing in 3T3-L1 cells. PLoS One 7: e34315–e34315, 2012. doi: 10.1371/journal.pone.0034315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, Qian H, Xu W, Zhu W. Exosomes derived from Act-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med 6: 51–59, 2017. doi: 10.5966/sctm.2016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez MC, Andriantsitohaina R. Microparticles in angiogenesis: therapeutic potential. Circ Res 109: 110–119, 2011. doi: 10.1161/CIRCRESAHA.110.233049. [DOI] [PubMed] [Google Scholar]
- 36.McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: mechanisms of uptake. J Circ Biomark 4: 7, 2015. doi: 10.5772/61186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radenkovic D, Arjun S, Poma A, Nyberg S, Battaglia B, Yellon DM, Davidson S. 162 Polymersomes functionalized with HSP70 – novel, synthetic cardioprotective nanovesicles. Heart 102, Suppl 6: A115–A115, 2016. doi: 10.1136/heartjnl-2016-309890.162. [DOI] [Google Scholar]
- 38.Rajkovic I, Wong R, Lemarchand E, Rivers-Auty J, Rajkovic O, Garlanda C, Allan SM, Pinteaux E. Pentraxin 3 promotes long-term cerebral blood flow recovery, angiogenesis, and neuronal survival after stroke. J Mol Med (Berl) 96: 1319–1332, 2018. doi: 10.1007/s00109-018-1698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rashed HM, Bayraktar E, Helal KG, Abd-Ellah MF, Amero P, Chavez-Reyes A, Rodriguez-Aguayo C. Exosomes: from garbage bins to promising therapeutic targets. Int J Mol Sci 18: E538, 2017. doi: 10.3390/ijms18030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribeiro MF, Zhu H, Millard RW, Fan GC. Exosomes function in pro- and anti-angiogenesis. Curr Angiogenes 2: 54–59, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rippe C, Blimline M, Magerko KA, Lawson BR, LaRocca TJ, Donato AJ, Seals DR. MicroRNA changes in human arterial endothelial cells with senescence: relation to apoptosis, eNOS and inflammation. Exp Gerontol 47: 45–51, 2012. doi: 10.1016/j.exger.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Grande B, Varghese L, Molina-Holgado F, Rajkovic O, Garlanda C, Denes A, Pinteaux E. Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia. J Neuroinflammation 12: 15, 2015. doi: 10.1186/s12974-014-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimamura M, Nakagami H, Koriyama H, Morishita R. Gene therapy and cell-based therapies for therapeutic angiogenesis in peripheral artery disease. Biomed Res Int 2013: 186215–186215, 2013. doi: 10.1155/2013/186215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics 6: 267–283, 2009. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 45.Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3: 15, 2011. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang N, Chen C, Yang D, Liao Q, Luo H, Wang X, Zhou F, Yang X, Yang J, Zeng C, Wang WE. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta Mol Basis Dis 1863: 2085–2092, 2017. doi: 10.1016/j.bbadis.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 47.Wang W, Feng L, Zhang H, Hachy S, Satohisa S, Laurent LC, Parast M, Zheng J, Chen DB. Preeclampsia up-regulates angiogenesis-associated microRNA (i.e., miR-17, -20a, and -20b) that target ephrin-B2 and EPHB4 in human placenta. J Clin Endocrinol Metab 97: E1051–E1059, 2012. doi: 10.1210/jc.2011-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem 74: 103–141, 2016. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Strawn TL, Luo M, Wang L, Li R, Ren M, Xia J, Zhang Z, Ma W, Luo T, Lawrence DA, Fay WP. Plasminogen activator inhibitor-1 inhibits angiogenic signaling by uncoupling vascular endothelial growth factor receptor-2-αVβ3 integrin cross talk. Arterioscler Thromb Vasc Biol 35: 111–120, 2015. doi: 10.1161/ATVBAHA.114.304554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin L, Ohanyan V, Pung YF, Delucia A, Bailey E, Enrick M, Stevanov K, Kolz CL, Guarini G, Chilian WM. Induction of vascular progenitor cells from endothelial cells stimulates coronary collateral growth. Circ Res 110: 241–252, 2012. doi: 10.1161/CIRCRESAHA.111.250126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Zhou M, Qin G, Weintraub NL, Tang Y. MiR-92a regulates viability and angiogenesis of endothelial cells under oxidative stress. Biochem Biophys Res Commun 446: 952–958, 2014. doi: 10.1016/j.bbrc.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther 8: 125, 2017. doi: 10.1186/s13287-017-0578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao X, Balaji P, Pachon R, Beniamen DM, Vatner DE, Graham RM, Vatner SF. Overexpression of cardiomyocyte α1A-adrenergic receptors attenuates postinfarct remodeling by inducing angiogenesis through heterocellular signaling. Arterioscler Thromb Vasc Biol 35: 2451–2459, 2015. doi: 10.1161/ATVBAHA.115.305919. [DOI] [PMC free article] [PubMed] [Google Scholar]