Abstract
Kidney fibrosis is associated with an increased lymphangiogenesis, characterized by the formation and expansion of new lymphatic vessels. However, the trigger and underlying mechanism responsible for the growth of lymphatic vessels in diseased kidney remain poorly defined. Here, we report that tubule-derived sonic hedgehog (Shh) ligand is a novel lymphangiogenic factor that plays a crucial role in mediating lymphatic endothelial cell proliferation and expansion. Shh was induced in renal tubular epithelium in various models of fibrotic chronic kidney disease, and this was accompanied by an expansion of lymphatic vessels in adjacent areas. In vitro, Shh selectively promoted the proliferation of human dermal lymphatic endothelial cells (HDLECs) but not human umbilical vein endothelial cells, as assessed by cell counting, MTT assay, and bromodeoxyuridine incorporation. Shh also induced the expression of vascular endothelial growth factor receptor-3, cyclin D1, and proliferating cell nuclear antigen in HDLECs. Shh did not affect the expression of Gli1, the downstream target and readout of canonical hedgehog signaling, but activated ERK-1/2 in HDLECs. Inhibition of Smoothened with small-molecule inhibitor or blockade of ERK-1/2 activation abolished the lymphatic endothelial cell proliferation induced by Shh. In vivo, inhibition of Smoothened also repressed lymphangiogenesis and attenuated renal fibrosis. This study identifies Shh as a novel mitogen that selectively promotes lymphatic, but not vascular, endothelial cell proliferation and suggests that tubule-derived Shh plays an essential role in mediating lymphangiogenesis after kidney injury.
Keywords: chronic kidney disease, inflammation, kidney fibrosis, lymphangiogenesis, lymphatic endothelial cells, sonic hedgehog
INTRODUCTION
Chronic kidney disease (CKD) is becoming an enormous public health problem, and it is considered as one of the fastest rising causes of death worldwide (34). Extensive studies have shown that the pathomechanism of CKD is very complex, which often involves many pathological processes such as tubular injury and dysfunction, renal inflammation, myofibroblast activation, and tissue scar formation (11, 26, 39). There is growing evidence that dysregulation of the lymphatic system is also a common pathological feature in a variety of fibrotic CKD (20, 25, 30, 38).
The lymphatic system plays an essential role in modulating tissue fluid balance and transportation of immune cells and nutrients (3). Recent studies have identified several specific proteins that are either exclusively or predominantly expressed in the capillary endothelial cells of lymphatic origin. Such markers for lymphatic endothelium include lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), VEGF receptor-3 (VEGFR-3), transmembrane protein podoplanin, and transcription factor Prox-1 (1, 6, 17). Using these lymphatic endothelial markers, one can readily identify the lymphatic network in solid organs under a variety of normal or pathological conditions.
In the normal physiological state, the lymphatic vasculature is relatively quiescent. However, under pathological conditions, such as inflammation, fibrosis, and cancer, the lymphatic system is characterized by lymphangiogenesis, the formation and expansion of new lymphatic vessels. Dysregulation of lymphatic vessel growth or function is involved in the pathogenesis of many human disorders such as lymphedema, transplant rejection, rheumatoid arthritis, and tumor metastasis (2, 15, 16, 19). Lymphangiogenesis is presumably regulated by various growth factors and their corresponding membrane receptors. Much attention in the past years, however, has focused on the VEGF-C, VEGF-D, and VEGFR-3 (their receptor) signaling axis (8, 20, 25, 30, 40). It has also been reported that transforming growth factor (TGF)-β or connective tissue growth factor (CTGF) regulates lymphangiogenesis by either inducing VEGF-C expression or promoting CTGF/VEGF-C interaction (20, 32). Besides the VEGF-C/VEGF-D andVEGFR-3 axis, whether there are new mediator(s) regulating lymphangiogenesis in vivo remains to be investigated.
Sonic hedgehog (Shh) is a secreted, lipid-modified glycoprotein that belongs to the family of hedgehog ligands (23, 44). Shh transduces its signal across the plasma membrane in responding cells via either the Gli-dependent, canonical pathway or Gli-independent, noncanonical pathway. Upon binding to the cell surface receptor Patched-1 (Ptch1), Shh triggers the derepression of Smoothened (Smo), the seven-pass transmembrane G protein-coupled receptor-like protein. This leads to the activation of the Gli family of transcription factors via the so-called canonical pathway. In addition, Shh also triggers cellular responses via two separate noncanonical pathways: one pathway is through Ptch1 but is unrelated to its derepression of Smo and another pathway is through Smo but is irrelevant to Gli regulation (28, 44). In the normal adult kidney, Shh protein is hardly detectable (9). However, it is induced specifically in the renal tubular epithelium and promotes interstitial fibroblast proliferation and activation (9, 18, 29, 43). Whether Shh also regulates lymphangiogenesis in fibrotic CKD, in which both Shh induction and lymphatic vessel expansion are evident, is completely unknown.
In the present study, we investigated the role of Shh in mediating lymphangiogenesis in mouse models of CKD. We show that Shh selectively promotes lymphatic endothelial cell proliferation through a Smo-dependent but Gli-independent, noncanonical pathway. Furthermore, blockade of Shh/Smo signaling with small-molecule inhibitor suppresses lymphatic endothelial cell growth in vitro and reduces lymphangiogenesis in vivo. Our results illustrate that Shh is a new and novel lymphangiogenic factor that selectively promotes lymphatic endothelial cell proliferation in fibrotic CKD.
MATERIALS AND METHODS
Animal models.
All animal experiments were performed by procedures approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Male BALB/c mice weighing ~20–25 g were obtained from Harlan Sprague Dawley (Indianapolis, IN). Mice were administered with adriamycin (ADR; doxorubicin hydrochloride, Sigma-Aldrich, St. Louis, MO) by a single intravenous injection at 10 mg/kg body wt, as previously reported (13). Mice were euthanized at 5 wk after ADR injection.
Male C57/BL6 mice weighing ~20–23 g were obtained from Harlan Sprague Dawley. Unilateral ureteral obstruction (UUO) was induced as previously described (45). Briefly, mice were anesthetized, and the left ureter was isolated. With the use of 4-0 silk suture, the ureter was permanently ligated and the abdomen was closed. Mice were euthanized at different time points (days 1, 3, and 7), respectively. For sham control, mice were manipulated and the ureter was exposed but unobstructed.
Bilateral ischemia-reperfusion injury (IRI) was performed in male BALB/c mice using an established protocol as previously described elsewhere (7, 12, 24). Briefly, bilateral renal pedicles were clamped for 30 min using microaneurysm clamps. During the ischemic period, body temperature was maintained between 35 and 37.5°C using a temperature-controlled heating system. At 3 days after IRI, mice were subjected to daily intraperitoneal injections of cyclopamine (CPN; Sigma-Aldrich) at 5 mg/kg body wt for 7 days as previously reported (43). Mice were euthanized 10 days after IRI, and kidney tissues were collected for various analyses. Another set of normal mice was injected with CPN at 5 mg/kg body wt for 10 days.
Cell culture.
Human dermal lymphatic endothelial cells (HDLECs) and human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Walkersville, MD). HUVECs were cultured in EGM-2 Bullet Kit Medium (CC-3162, Lonza), while HDLECs were cultured in EGM-2MV Bullet Kit Medium (CC-3202, Lonza). Cells were treated with recombinant human Shh protein (StemRD, Burlingame, CA) at different concentrations for various periods of time as indicated. For some experiments, cells were pretreated with CPN (5 μM) or PD98059 (5 μM) for 30 min, as previously described (9, 37), followed by an incubation with Shh. Cells were then collected and subjected to various analyses.
Cell proliferation assay.
Cell proliferation was assessed by two approaches: cell counting and a quantitative colorimetric MTT assay. Cell numbers were counted using a hemocytometer. HDLECs and HUVECs were detached after trypsinization, stained by trypan blue to exclude dead cells, and counted with hemocytometer in a blinded fashion. Cell proliferation was also determined quantitatively by a MTT assay (11). Briefly, HDLECs and HUVECs were seeded into 96-well plates at a density of 2 × 103 cells/well. After adherence of cells, cultures were changed to the serum-free medium and incubated for 24 h followed by treatment with or without Shh at different concentrations for various periods of time as indicated. MTT (5 mg/ml) was added to the medium at 10 µl/well followed by incubation at 37°C for 4 h. After the medium was removed, cells were lysed with 100 µl DMSO. Absorbance of each well was measured by a microplate reader at 490-nm wavelength.
Bromodeoxyuridine incorporation assay.
The effect of Shh on HDLEC and HUVEC DNA synthesis was evaluated by bromodeoxyuridine (BrdU) incorporation (43). Briefly, cells were seeded onto 24-well plates and treated with various concentrations of Shh for 48 h followed by pulsing with BrdU (10 mM) for 24 h. Cells were then fixed with ice-cold 70% ethanol for 20 min, and DNA was denatured by incubation with 2.5 N HCl for 20 min followed by neutralization with 0.1 M boric acid. Endogenous peroxidase activity was quenched by incubating the cells with 3% H2O2 in PBS for 20 min, and nonspecific binding was blocked by incubating the cells with 10% donkey serum for 10 min at room temperature as previously described (11). Incorporated BrdU was detected with mouse monoclonal anti-BrdU antibody (B2531, Sigma-Aldrich) followed by incubation with cyanine Cy3-conjugated, affinity-purified secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Stained cells were mounted with Vectashield anti-fade mounting media using SYTO-Green to visualize nuclei. Stained samples were viewed under an Eclipse E600 epifluorescence microscope equipped with a digital camera (Nikon, Melville, NY).
Real-time quantitative PCR.
The piece of entire kidney containing both the cortex and medulla was used to prepare RNA and protein samples. Total RNA was extracted using the TRIzol RNA isolation system (Invitrogen, Carlsbad, CA). First-strand cDNA synthesis was carried out using a reverse transcription system kit according to the instructions provided by the manufacturer (Promega, Madison, WI). Quantitative real-time PCR was performed on an ABI PRISM7000 sequence detection system (Applied Biosystems, Foster City, CA) as previously described (9). The sequences of primer pairs for different genes are shown in Supplemental Table S1 (Supplemental Data are available online at https://doi.org/10.6084/m9.figshare.8985932.v1). PCR was run using standard conditions. mRNA levels of various genes were calculated after normalization with β-actin.
Western blot analysis.
The piece of the entire kidney containing both the cortex and medulla was lysed with RIPA buffer containing 1% Tergitol-type Nonidet P-40, 0.1% SDS, 100 μg/ml PMSF, 1% protease inhibitor cocktail, and 1% phosphatase I and II inhibitor cocktail (Sigma-Aldrich) in PBS on ice. Supernatants were collected after centrifugation at 13,000 g at 4°C for 15 min. Protein expression was analyzed by Western blot analysis as previously described (41). The primary antibodies used were anti-Shh (sc-9024), anti-proliferating cell nuclear antigen (PCNA; sc-56, Santa Cruz Biotechnology, Santa Cruz, CA), anti-VEGFR-3 (ab27278, Abcam, Cambridge, MA), anti-cyclin D1 (RB-9041-PO, ThermoFisher, Fremont, CA), anti-phosphorylated (p)ERK-1/2 (phospho-p44/42 MAPK, no. 9101), anti-ERK-1/2 (no. 4695, Cell Signaling Technology, Danvers, MA), and anti-α-tubulin (T9026, Sigma-Aldrich).
Histology and immunohistochemical staining.
Paraffin-embedded mouse kidney sections (3 µm thickness) were prepared by a routine procedure. Sections were stained with Sirius red staining reagents by standard protocol, as previously described (42). Immunohistochemical staining was performed according to the established protocol as previously described (43). Antibodies against Shh (sc-9024, Santa Cruz Biotechnology) and VEGFR-3 (no. 552857, Santa Cruz Biotechnology) were used. To visualize the primary antibodies, slides were stained with biotin anti-rabbit (Jackson ImmunoResearch Laboratories) and anti-mouse (Millipore, Burlington, MA) secondary antibodies.
Immunofluorescence staining.
Kidney cryosections were fixed with 3.7% paraformaldehyde for 15 min at room temperature and immersed in 0.2% Triton X-100 for 10 min. After being blocked with 10% donkey serum in PBS for 1 h, slides were immunostained with the following antibodies: anti-CD31 (no. 550274, BD PharMingen), anti-LYVE-1 (no. 11-034, AngioBio), and anti-p-ERK-1/2 (p-p44/42 MAPK, Cell Signaling Technology). To visualize the primary antibodies, slides were stained with cyanine Cy2- or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Stained slides were viewed under an Eclipse E600 epifluorescence microscope equipped with a digital camera (Nikon). The assessment of lymphangiogenesis was carried out by an independent pathologist (H. Mo) who was blinded to treatment groups on accounting of lymphatic vessels per high-power field (36). The percentage of LYVE-1+ cells in each field was calculated by ImageJ software. The average value of 10 high-power fields from each animal was counted, and 3−5 animals/group were used.
Statistical analysis.
All data are expressed as means ± SE. Statistical analyses of the data were performed using IBM SPSS19.0 statistical software. Comparison between groups was made using a one-way ANOVA test. Comparison between two groups was made by a t-test. P values of <0.05 were considered significant.
RESULTS
Kidney fibrosis after injury is associated with lymphangiogenesis in vivo.
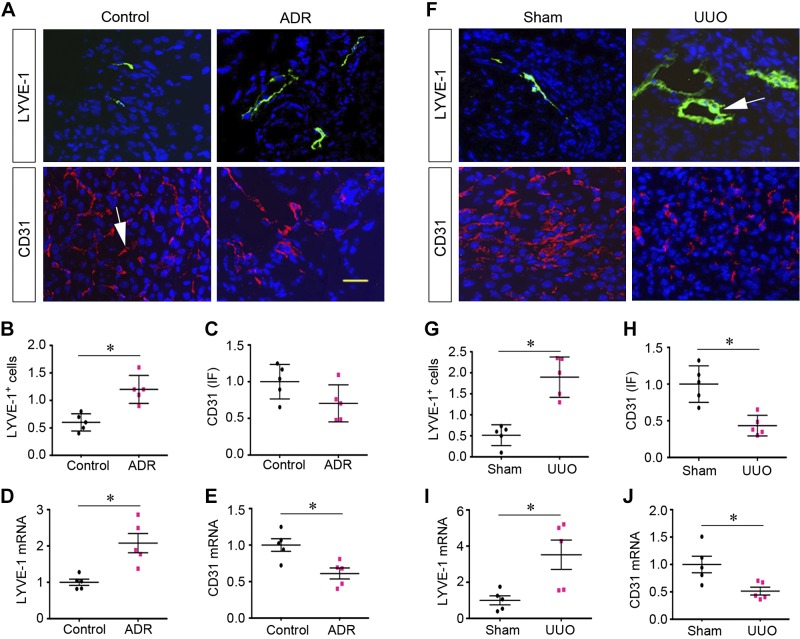
We first investigated the regulation of the lymphatic and vascular endothelial cell systems in two well-established models of CKD induced by ADR and UUO (13, 45). As shown in Fig. 1, A and B, expression of LYVE-1, a marker of lymphatic endothelial cells, was significantly induced after ADR administration. It appeared that the density and sizes of LYVE-1+ lymphatic vessels were increased in the injured kidneys at 5 wk after ADR compared with normal controls. Consistently, LYVE-1 mRNA was also induced in the diseased kidneys of mice injected with ADR (Fig. 1D). In contrast, renal expression of the vascular endothelial cell marker CD31 exhibited a tendency toward downregulation at 5 wk after ADR (Fig. 1, A and C). Consistently, CD31 mRNA was significantly repressed in the injured kidneys after ADR injection (Fig. 1E).
Fig. 1.
Chronic kidney disease is associated with an increased lymphangiogenesis and vascular rarefaction. A–C: immunofluorescent staining showing the expression of lymphatic endothelial cell marker lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) and vascular endothelial cell marker CD31 in the fibrotic kidneys at 5 wk after adriamycin (ADR) administration. Representative micrographs (A) and quantitative data (B and C) of CD31+ and LYVE-1+ cells are shown. *P < 0.05 vs. controls (n = 5). D and E: quantitative RT-PCR analyses showing the mRNA expression of CD31 and LYVE-1 in the ADR nephropathy model at 5 wk. *P < 0.05 vs. controls (n = 5). F–H: immunofluorescent (IF) staining showing the protein expression of LYVE-1 and CD31 in the fibrotic kidneys at 7 days after unilateral ureteral obstruction (UUO). Representative micrographs (F) and quantitative data (G and H) are shown. *P < 0.05 vs. sham controls (n = 5). I and J: quantitative RT-PCR analyses showing the mRNA expression of CD31 and LYVE-1 in the obstructed kidneys at 7 days after UUO. *P < 0.05 vs. sham controls (n = 5). Arrows indicate positive staining. Scale bar = 50 μm.
We further examined the regulation of the lymphatic and vascular systems in mouse model of UUO. As shown in Fig. 1, F and G, the numbers and sizes of LYVE-1+ lymphatic vessels were also increased at 7 days after UUO compared with sham controls. Similarly, renal expression of LYVE-1 mRNA was induced in obstructive nephropathy (Fig. 1I). However, both CD31 protein and mRNA levels were clearly decreased after UUO (Fig. 1, H and J). These results suggest that, in contrast to vascular rarefaction, lymphatic vessels are clearly expanded through lymphangiogenesis in the injured kidney in both models of CKD induced by ADR or UUO.
Tubular induction of Shh is associated with lymphangiogenesis in vivo.
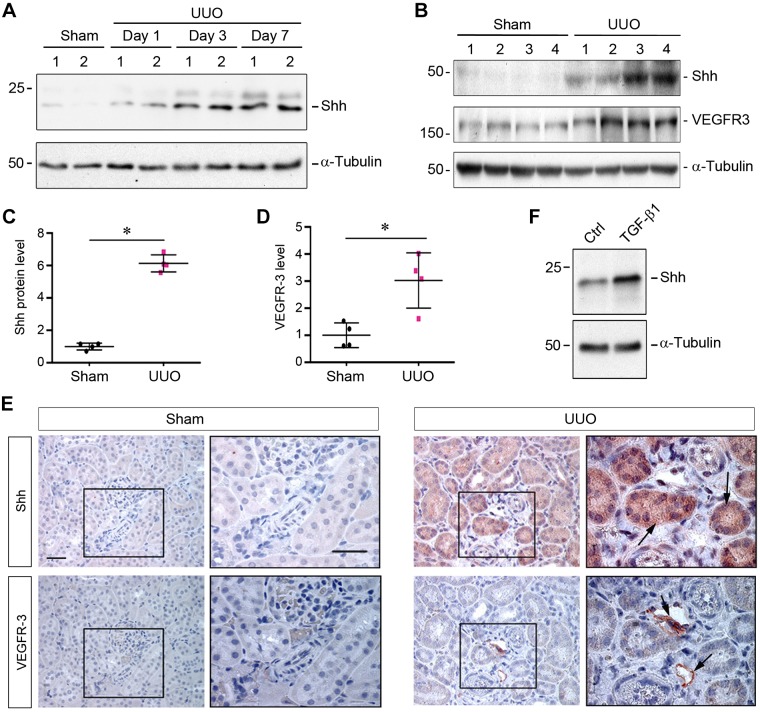
To search for the potential factor(s) mediating lymphangiogenesis in CKD, we studied the role of Shh in this process, as earlier studies have suggested its involvement in various models of CKD (9, 43). As shown in Fig. 2A, active Shh protein (N-Shh, 19 kDa) was upregulated in a time-dependent fashion in the obstructed kidney after UUO. Notably, this was accompanied by an increase in the expression of full-length Shh (45 kDa) and VEGFR-3 (Fig. 2, B–D), a tyrosine kinase receptor that is involved in lymphangiogenesis and maintenance of the lymphatic endothelium (21). We further examined the localization of Shh and VEGFR-3 proteins in the fibrotic kidneys after UUO. As shown in Fig. 2E, immunohistochemical staining revealed that little Shh or VEGFR-3 protein was detectable in the normal kidney after sham operation. However, a marked induction of both Shh and VEGFR-3 was observed in the fibrotic kidneys after UUO. As expected, VEGFR-3 protein was exclusively localized in the lymphatic vessels, which were surrounded by adjacent tubules with a high level of Shh protein (Fig. 2E). These results suggest a possible connection between tubule-derived Shh and lymphatic growth in diseased kidneys.
Fig. 2.
Induction of sonic hedgehog (Shh) is associated with lymphangiogenesis in fibrotic kidney. A: Western blot analyses showing a time-dependent induction of Shh protein in the obstructed kidneys after unilateral ureteral obstruction (UUO). Kidney tissue lysates from sham or UUO mice were immunoblotted with antibodies against Shh and α-tubulin. N-Shh (19 kDa) is shown. B–D: Western blot analyses showing the concomitant induction of both Shh and VEGF receptor-3 (VEGFR-3) proteins in the obstructed kidneys at 7 days after UUO. Full-length Shh (45 kDa) is shown. Representative (B) and quantitative data (C and D) are shown. *P < 0.05 vs. sham controls (Ctrl; n = 4). E: representative micrographs showing an increased expression of Shh and VEGFR-3 in the obstructed kidneys at 7 days after UUO. The series of sections was stained immunohistochemically with anti-Shh and anti-VEGFR-3, respectively. Boxed areas are enlarged at the right. Arrows indicate positive staining. Scale bar = 50 μm. F: transforming growth factor (TGF)-β1 induced Shh expression in human proximal tubular epithelial cells (HKC-8) in vitro. N-Shh (19 kDa) is shown.
To further validate tubular induction of Shh, we investigated Shh expression in human proximal tubular epithelial cells (HKC-8) in vitro. As shown in Fig. 2F, Shh was detectable in HKC-8 cells under basal condition, and it was induced after TGF-β1 stimulation. Therefore, tubular cells are clearly able to express and secrete Shh protein in response to injury.
Shh selectively promotes lymphatic endothelial cell proliferation in vitro.
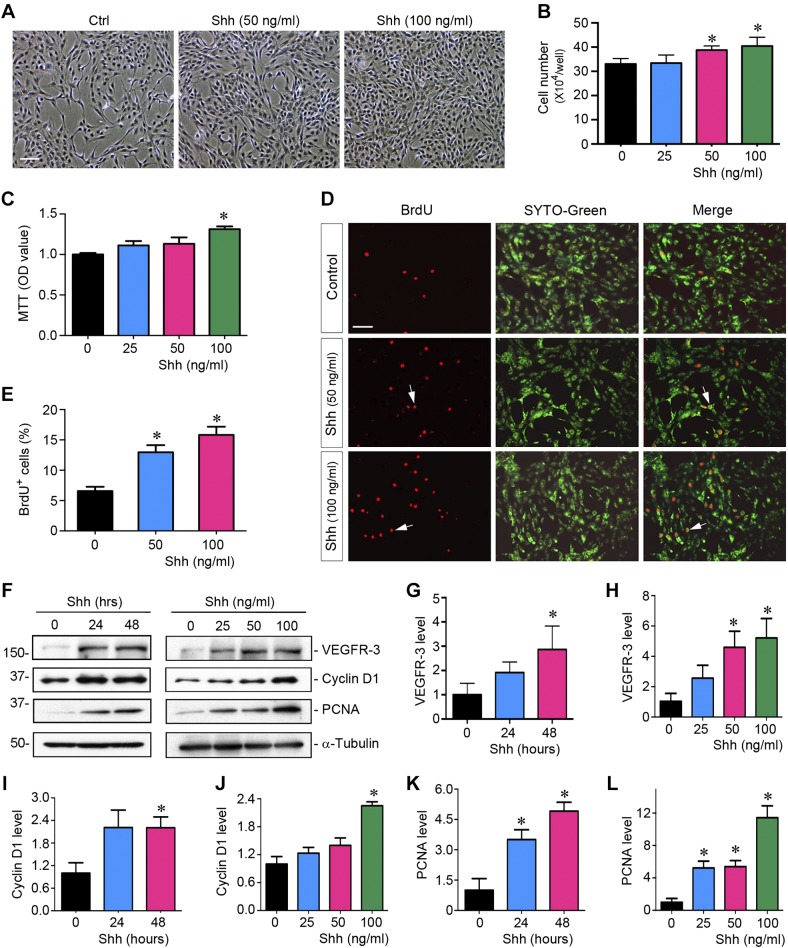
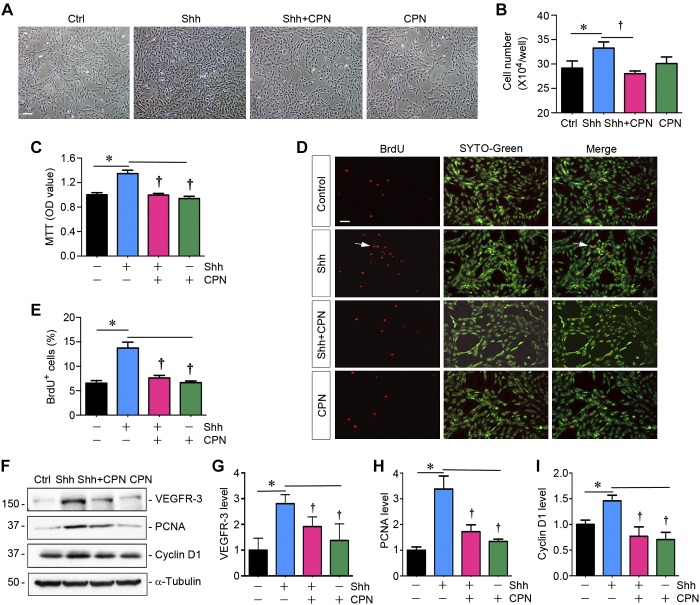
To test whether Shh has a role in lymphangiogenesis after kidney injury, we investigated its ability to promote lymphatic endothelial cell proliferation in vitro. To this end, we obtained primary HDLECs and incubated them with different concentrations of recombinant human Shh protein for various periods of time. As shown in Fig. 3A, an increase in HDLEC density was observed after Shh treatment, as illustrated in the phase-contrast images. Cell counting confirmed that Shh increased the number of HDLECs in a dose-dependent manner (Fig. 3B). Similar results were obtained using a quantitative colorimetric MTT assay (Fig. 3C). We further assessed the ability of Shh to promote HDLECs entering the cell cycle and undergoing DNA synthesis by BrdU incorporation. As shown in Fig. 3, D and E, increased BrdU incorporation was observed in HDLECs after incubation with Shh compared with controls.
Fig. 3.
Sonic hedgehog (Shh) promotes lymphatic endothelial cell proliferation in vitro. A: representative micrographs showing phase-contrast images of human dermal lymphatic endothelial cells (HDLECs) after incubation with recombinant Shh protein. HDLECs were incubated with Shh for 48 h at different concentrations as indicated. Scale bar = 30 µm. B: Shh promotes HDLEC proliferation in a dose-dependent manner. Cell numbers were counted at 48 h after incubation with Shh and are shown. Data were obtained from three independent experiments. *P < 0.05 vs. controls (Ctrl; n = 3). C: graphic presentation showing that Shh promoted HDLEC density assessed by a colorimetric MTT assay. *P < 0.05 vs. controls (n = 3). OD, optical density. D: representative micrographs showing that Shh promoted HDLEC DNA synthesis and entering the S phase as shown by bromodeoxyuridine (BrdU) incorporation. Cells were immunostained with anti-BrdU antibody (red) at 48 h after incubation with different concentrations of Shh. SYTO-Green (green) was used to visualize nuclei. Scale bar = 30 µm. E: quantitative determination of the percentage of BrdU+ cells after Shh treatment. *P < 0.05 vs. controls (n = 3). F: Western blots showing that Shh promoted the expression of VEGFR-3 and proliferation-related genes. HDLECs were incubated with different concentration of Shh for 48 h or Shh (50 ng/ml) with various durations. G–L: cell lysates were subjected to Western blot analyses for VEGFR-3 (G and H), cyclin D1 (I and J), and proliferating cell nuclear antigen (PCNA; K and L), respectively. *P < 0.05 vs. controls (n = 3).
To delineate the mechanism of Shh-mediated HDLEC proliferation, we next investigated the expression of proliferation-related genes. As shown in Fig. 3, F–L, both cyclin D1 and PCNA were induced by Shh in HDLECs in a time- and dose-dependent manner. Moreover, an increase in VEGFR-3 protein was also confirmed in HDLECs after Shh stimulation (Fig. 3, F–H). These results suggest that Shh acts as a potent mitogen for lymphatic endothelial cells and activates multiple proliferation-related genes, thereby leading to their proliferation and expansion.
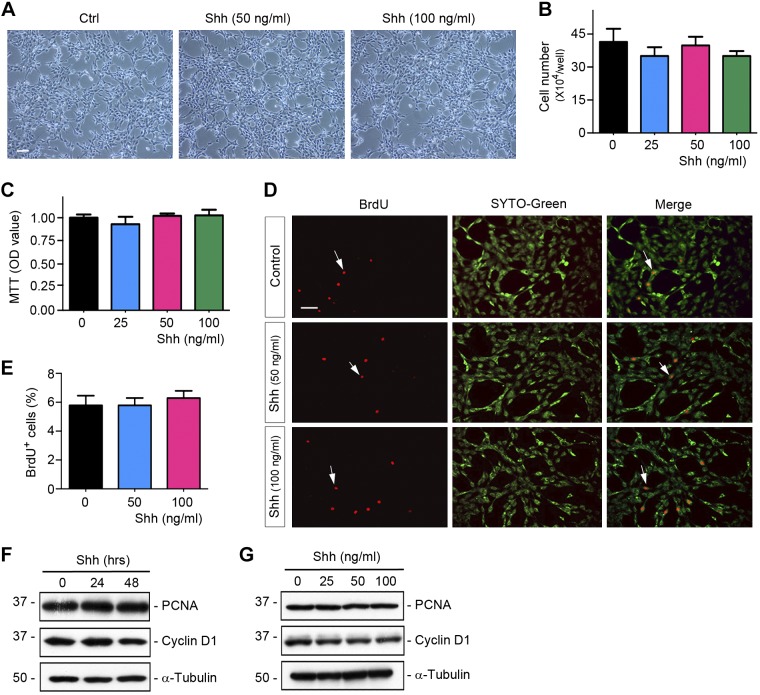
We also examined the effect of Shh on vascular endothelial cells. To this end, HUVECs were stimulated with different doses of Shh for various periods of time. As shown in Fig. 4, A–E, there was virtually no effect of Shh on HUVEC proliferation, as assessed by cell density, cell counting, MTT assay, and BrdU incorporation. Similarly, no induction of the proliferation-related proteins was observed in HUVECs after Shh incubation (Fig. 4, F and G). Therefore, it is clear that Shh selectively promotes lymphatic, but not vascular, endothelial cell proliferation.
Fig. 4.
Sonic hedgehog (Shh) has no influence on vascular endothelial cell proliferation. A: representative micrographs showing phase-contrast images of human umbilical vein endothelial cells (HUVECs) after incubation with recombinant Shh. HUVECs were incubated with different concentrations of Shh for 48 h. Scale bar = 30 µm. B: cell numbers of HUVECs after incubation with different concentrations of Shh for 48 h (n = 3). C: colorimetric MTT assay showing that Shh did not affect HUVEC growth (n = 3). OD, optical density. D: representative micrographs showing that Shh did not have an influence on HUVEC DNA synthesis as shown by bromodeoxyuridine (BrdU) incorporation. HUVECs were incubated with 50 and 100 ng/ml Shh for 48 h. Cells were immunostained with mouse anti-BrdU antibody (red). SYTO-Green (green) was used to visualize nuclei. Scale bar = 30 µm. E: quantitative determination of the percentage of BrdU+ cells after Shh treatment (n = 3). F and G: Western blots showing that Shh did not affect protein expression of proliferation-related genes in HUVECs after incubation with different concentrations of Shh for 48 h or Shh (50 ng/ml) for various durations as indicated. Cell lysates were subjected to Western blot analyses for cyclin D1, proliferating cell nuclear antigen (PCNA), and α-tubulin. Ctrl, control.
Blockade of Smo signaling inhibits lymphatic endothelial cell proliferation in vitro.
To investigate the signal pathway mediating lymphatic endothelial cell proliferation, we next explored whether pharmacological inhibition of Smo signaling affects Shh action. To this end, we used CPN, a small-molecule Smo inhibitor, to treat HDLECs, as previously reported (9). As shown in Fig. 5A, CPN effectively blocked the increase in HDLEC density induced by Shh, as illustrated in phase-contrast images, whereas it had no influence on HDLEC density in the absence of Shh. Cell counting, MTT assay, and BrdU incorporation also gave rise to similar results (Fig. 5, B–E). Consistently, Western blot analyses also revealed that CPN inhibited protein expression of cyclin D1, PCNA, and VEGFR-3 (Fig. 5, F–I). It is therefore concluded that Shh-induced lymphatic endothelial cell proliferation is dependent on Smo signaling.
Fig. 5.
Blockade of Smoothened (Smo) inhibits sonic hedgehog (Shh)-induced lymphatic endothelial cell proliferation. A: representative micrographs showing phase-contrast images of human dermal lymphatic endothelial cells (HDLECs) after incubation with recombinant Shh (50 ng/ml) in the absence or presence of cyclopamine (CPN; 5 μM) for 48 h. Scale bar = 30 µm. B: CPN blocks Shh-induced HDLEC proliferation. HDLECs were incubated with Shh (50 ng/ml) in the absence or presence of CPN (5 μM) for 48 h. Cell numbers (×104 cells/ml) were counted and are shown. *P < 0.05 vs. controls (Ctrl); †P < 0.05 vs. the Shh group (n = 3). C: graphic presentation showing that CPN inhibited Shh-induced HDLEC proliferation as assessed by a colorimetric MTT assay. OD, optical density. *P < 0.05 vs. controls; †P < 0.05 vs. the Shh group (n = 3). D: representative micrograph showing that CPN inhibited Shh-induced HDLEC DNA synthesis as assessed by bromodeoxyuridine (BrdU) incorporation. HDLECs were incubated with 50 ng/ml Shh in the absence or presence of CPN (5 μM) for 48 h. Scale bar = 30 µm. E: quantitative determination of the percentage of BrdU+ cells after Shh treatment with or without CPN. *P < 0.05 vs. controls; †P < 0.05 vs. the Shh group (n = 3). F–I: Western blots showing that CPN inhibited Shh-induced expression of VEGF receptor-3 (VEGFR-3) and proliferation-related genes in HDLECs. HDLECs were incubated with Shh (50 ng/ml) with or without CPN (5 μM) for 48 h. Cell lysates were subjected to Western blot analyses for VEGFR-3, cyclin D1, proliferating cell nuclear antigen (PCNA), and α-tubulin (n = 3).
Shh promotes lymphangiogenesis through noncanonical pathway.
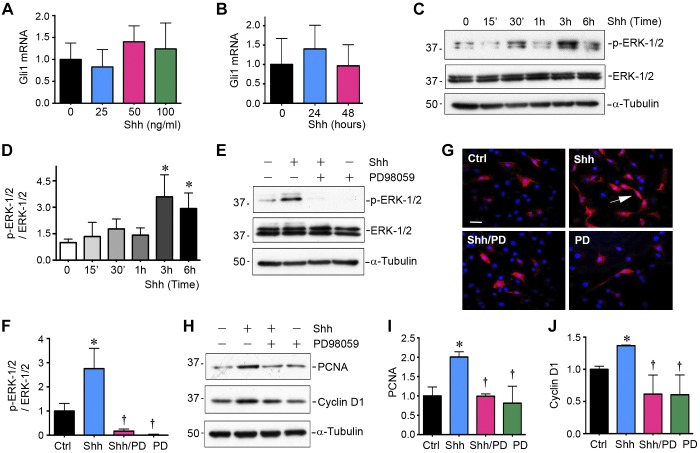
To further dissect the signaling pathway by which Shh induces HDLEC proliferation, we assessed the expression of Gli1, the downstream target and readout of hedgehog canonical signaling (10, 29, 44). As shown in Fig. 6, A and B, there was no significant change in Gli1 mRNA expression in HDLECs after incubation with different concentrations of Shh for 48 h or 50 ng/ml Shh for various durations. These results suggest that Shh induces lymphatic endothelial cell proliferation by a mechanism independent of the canonical pathway.
Fig. 6.
Sonic hedgehog (Shh) induces lymphatic endothelial cell proliferation through the noncanonical pathway. A and B: quantitative RT-PCR showing that Shh did not affect Gli1 mRNA expression in lymphatic endothelial cells. Human dermal lymphatic endothelial cells (HDLECs) were incubated with different concentrations of Shh for 48 h or 50 ng/ml Shh for various durations as indicated. C and D: Western blot analyses showing that Shh activated ERK-1/2 in lymphatic endothelial cells. HDLECs were stimulated with Shh (50 ng/ml) for various durations as indicated. Representative (C) and quantitative data (D) are shown. The relative abundance of phosphorylated (p-)ERK-1/2 after normalization with total ERK-1/2 (fold induction over controls) is shown. *P < 0.05 vs. control (Ctrl; n = 3). E and F: Western blot analyses showing that inhibition of MEK1 by PD98059 (PD) abolished Shh-induced ERK-1/2 activation. HDLECs were stimulated with 50 ng/ml Shh in the absence or presence of PD98059 for 3 h. Representative Western blots (E) and quantitative data (F) are shown. *P < 0.05 vs. control (n = 3). G: immunofluorescent staining showing protein expression of p-ERK-1/2 in HDLECs after stimulation with Shh (50 ng/ml) in the absence or presence of PD98059 (10 µM) for 3 h. Scale bar = 30 µm. H–J: Western blot analyses showing the expression of proliferating cell nuclear antigen (PCNA) and cyclin D1 in HDLECs after stimulation with Shh (50 ng/ml) in the absence or presence of PD98059 (10 mM) for 48 h. Representative Western blots (H) and quantitative data (I and J) are shown. *P < 0.05 vs. controls; †P < 0.05 vs. the Shh group (n = 3).
To delineate the downstream signaling of Shh in HDLECs, we next investigated the expression and activation of ERK-1/2. As shown in Fig. 6, C and D, ERK-1/2 was rapidly phosphorylated and activated by Shh in HDLECs as early as 30 min and reached the peak at 3 h after treatment, whereas expression of total ERK-1/2 was unaltered. We next used PD98059, a specific inhibitor of ERK-1/2 upstream kinases MEK1 and MEK2 (37), for incubation with HDLECs. As shown in Fig. 6, E–G, PD98059 effectively inhibited the activation of ERK-1/2 in HDLECs, as confirmed by Western blot analyses and immunofluorescence staining. Moreover, induction of PCNA and cyclin D1 by Shh was abolished when HDLECs were pretreated with PD98059 (10 µM) for 30 min (Fig. 6, H–J). These results suggest that Shh-induced lymphatic endothelial cell proliferation requires ERK-1/2 activation, but it does not depend on canonical hedgehog signaling.
Blockade of Shh signaling inhibits lymphangiogenesis in vivo.
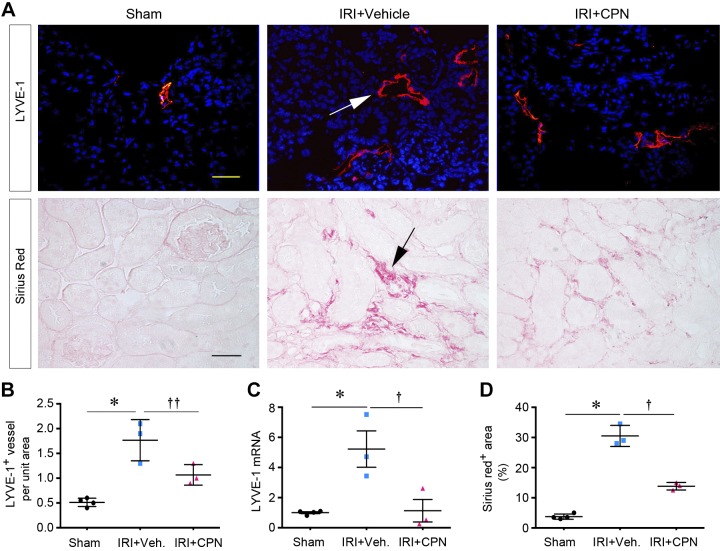
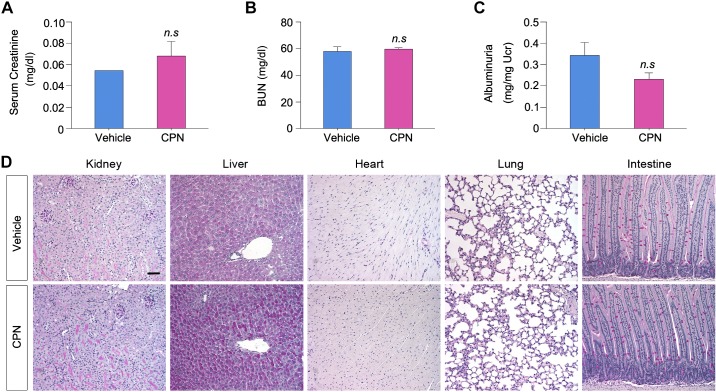
We next investigated whether blockade of Shh signaling can inhibit lymphangiogenesis in vivo. To this end, we studied the influence of Smo inhibitor CPN on lymphangiogenesis in the fibrotic kidney 10 days after IRI, as previously reported (43). CPN was administrated by daily intraperitoneal injections starting 3 days after IRI, a time point when kidney function begins to recover after AKI (35). As shown in Fig. 7, A and C, the numbers and sizes of LYVE-1+ lymphatic vessels were increased at 10 days after IRI compared with the sham group. However, the density of LYVE-1+ lymphatic vessels was reduced in the treatment group injected with CPN. Similarly, renal expression of LYVE-1 mRNA was also substantially induced at 10 days after IRI injury, which was inhibited after CPN treatment (Fig. 7C). Blockade of Shh signaling by CPN was able to ameliorate fibrosis (Fig. 7, A and D), consistent with a previous report (43), suggesting that inhibition of lymphangiogenesis by CPN is associated with a mitigation of renal fibrosis. Of note, blockade of Shh signaling by CPN did not affect kidney morphology and function in normal mice (Fig. 8, A–C) or have any adverse effect on the integrity of several major organs including the liver, heart, lung, and intestine (Fig. 8D).
Fig. 7.
Blockade of sonic hedgehog (Shh) signaling inhibits renal lymphangiogenesis and fibrosis after ischemia-reperfusion injury (IRI). A: inhibition of Shh signaling by cyclopamine (CPN) reduced renal lymphangiogenesis and fibrosis in vivo. Top: immunofluorescent staining showing that CPN inhibited lymphangiogenesis in the fibrotic kidneys 10 days after IRI. Bottom: Sirius red staining showing that CPN ameliorated renal fibrotic lesions 10 days after IRI. B: quantitative determination of lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1)+ vessels in the different groups. *P < 0.05 vs. sham controls; ††P < 0.1 vs. IRI + vehicle (Veh; n = 3–4). C: quantitative RT-PCR analyses showing that CPN inhibited renal expression of LYVE-1 mRNA after IRI injury. *P < 0.05 vs. sham controls (n = 4). D: quantitative determination of renal fibrotic lesions in the different groups. *P < 0.05 vs. sham controls; †P < 0.05 vs. IRI + Veh (n = 3–4).
Fig. 8.
Blockade of sonic hedgehog (Shh) signaling by cyclopamine (CPN) does not affect normal kidney morphology and function in mice. Mice were subjected to daily injection of CPN at 5 mg/kg body wt for 10 days. A–C: CPN did not affect the levels of serum creatinine (A), blood urea nitrogen (BUN) (B), and urinary albumin (C) in mice. No significant (n.s.) difference was found between vehicle and CPN-treated groups. D: representative micrographs showing the structure of major organs including the kidney, liver, heart, lung, and intestine in vehicle- or CPN-treated groups. Scale bar = 50 μm.
DISCUSSION
Kidney fibrosis is known to be associated with the formation and expansion of new lymphatic vessels, which is often accompanied by vascular rarefaction. This observation suggests that capillary endothelial cells of lymphatic origin are quite distinct from those of the vascular system. While the factors regulating vascular endothelial cells are well documented (5, 33), the mediators that control the proliferation of lymphatic endothelial cells are poorly defined. Earlier studies were largely focused on the VEGF-C/VEGF-D and VEGFR-3 axis in regulating the proliferation of the renal lymphatic endothelium (21). The results presented in this study show that Shh, an extracellular signaling protein secreted by the injured tubular epithelium (9, 43), is a novel mitogen that selectively promotes lymphatic endothelial cell proliferation both in vitro and in vivo. Our findings identify Shh as a new lymphangiogenic factor mediating the growth and expansion of lymphatic vessels after kidney injury.
One of the novel and interesting findings of the present study is that Shh selectively promotes the proliferation of lymphatic, but not vascular, endothelial cells (Figs. 3 and 4). This is consistent with the observation that lymphangiogenesis coexists with vascular rarefaction in CKD (Fig. 1). Of note, because CD31 is a nondiscriminatory endothelial marker and is also expressed by the lymphatic endothelium, the vascular rarefaction assessed by CD31 staining in the present study is likely to be underestimated. The lymphatic endothelium in the kidney has unique structural features. Unlike the vascular endothelium with a continuous basement membrane and supported with pericytes and smooth muscle cells (31), there are often gaps between cells in the lymphatic endothelium. In the normal kidney, lymphatic vessels are mainly localized near the interlobular artery/vein and arcuate artery/vein. However, lymphatic vessels can be easily found in the renal cortical region in the diseased kidney via lymphangiogenesis after injury. Such lymphangiogenesis often occurs at the site of renal inflammation and correlates closely with the severity of fibrosis (30). Although not specifically examined in the present study, previous studies have pointed out that newly formed lymphatic vessels sprout from the preexisting local lymphatic network, with no or little contributions from bone marrow-derived endothelial progenitor cells (14). In this context, identification of Shh as a mitogen for lymphatic, but not vascular, endothelial cells is of significance, as it represents a new class of factors that specifically regulate the growth and expansion of lymphatic vessels under pathological conditions.
Shh is the best characterized member among the three hedgehog ligands, which include Indian hedgehog (Ihh) and Desert hedgehog (Dhh) (44). Earlier studies have shown that Shh is induced predominantly in the renal tubular epithelium in a variety of CKD induced by UUO, IRI, ADR, and 5/6 nephrectomy as well as in human kidney biopsies in patients with CKD (4, 9, 29). Using Gli1-LacZ knockin reporter mice, we and others have previously identified interstitial fibroblasts/pericytes as the responding cells of canonical hedgehog signaling in diseased kidneys (9, 10, 29). Indeed, Shh is able to specifically promote interstitial fibroblast proliferation in vitro and markedly augment renal fibrosis in vivo (43). Interestingly, we found that the growth and expansion of new lymphatic vessels are in adjacent with and surrounded by renal tubules with a high level of Shh in injured kidneys (Fig. 2), suggesting that there may be a connection between tubule-derived Shh and lymphangiogenesis after kidney injury. This speculation is validated by the finding that Shh selectively promotes lymphatic, but not vascular, endothelial cell proliferation in vitro (Figs. 3 and 4). Therefore, we have identified lymphatic endothelial cells as a new target of tubule-derived Shh. As such, Shh mediates intercellular communication between injured/stressed renal tubules and the lymphatic endothelium.
It should be pointed out that although Shh is able to promote the proliferation of both interstitial fibroblasts and lymphatic endothelial cells, it apparently uses two dissimilar signal routes. While Shh triggers fibroblast activation and proliferation through the Gli-dependent, canonical pathway, Shh does not stimulate Gli1 expression in lymphatic endothelial cells (Fig. 6), suggesting that it takes the Gli-independent noncanonical route. Notably, Smo inhibitor CPN abolished the mitogenic effect of Shh in HDLECs (Fig. 5), suggesting that Smo activation is required for Shh-mediated lymphangiogenesis. This observation is in harmony with an earlier report (28) showing that Shh activates the GTPases Rac1 and RhoA in a Gli-independent manner through coupling of smoothened to Gi proteins and exerts changes to the actin cytoskeleton. Such cytoskeletal changes promote migration in fibroblasts and tubulogenesis in endothelial cells (27, 28). We found that Shh stimulates ERK-1/2 phosphorylation and activation in lymphatic endothelial cells, and such activation of ERK signaling is likely to be a result of the activation of Smo and required for mediating the mitogenic action of Shh (Fig. 6). Although the molecular details underlying how Shh activates ERK-1/2 in lymphatic endothelial cells remain to be delineated, they clearly involve Smo activation, as CPN abolishes the action of Shh. Therefore, we can conclude that Shh selectively induces the proliferation and expansion of the lymphatic endothelium through a Gli-independent, noncanonical pathway in which activation of Smo and ERK-1/2 is obligatory.
A role for tubule-derived Shh in promoting lymphangiogenesis is also confirmed in CKD in vivo, as blockade of Shh/Smo signaling with the specific small-molecule inhibitor CPN reduces the formation and expansion of lymphatic vessels. Of interest, inhibition of lymphangiogenesis by CPN is associated with a reduction of renal fibrosis (Fig. 7), suggesting a positive correlation of renal lymphangiogenesis with fibrotic lesions in CKD (22, 25, 30, 38). It is worthwhile to stress that lymphatic endothelial cells in vivo respond to Shh stimulation through the noncanonical pathway because the Gli1-positive cells are limited to interstitial fibroblasts/pericytes after kidney injury, as illustrated in Gli1-LacZ knockin reporter mice (9, 10, 29). Shh also induces VEGFR-3 expression in lymphatic endothelial cells (Fig. 3). This raises the possibility that Shh may have a synergistic effect with VEGF-C and VEGF-D on lymphangiogenesis. This issue deserves further investigation.
In summary, we show here that tubule-derived Shh is a novel lymphangiogenic factor that selectively promotes lymphatic, but not vascular, endothelial cell proliferation. This action of Shh is mediated by the Gli-independent, ERK-1/2-dependent, noncanonical pathway. Our results provide unique insights into the mediator and mechanism governing lymphangiogenesis and suggest that blockade of Shh/Smo signaling is a new strategy for curtailing lymphangiogenesis in fibrotic CKD.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-064005 and DK-106049.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.Z. and Y.L. conceived and designed research; H.Z., D.Z., Y.W., H.M., and Y.Y. performed experiments; H.Z., D.Z., and Y.L. analyzed data; H.Z. prepared figures; H.Z. drafted manuscript; H.Z. and Y.L. interpreted results of experiments; Y.L. edited and revised manuscript; Y.L. approved final version of manuscript.
REFERENCES
- 1.Akishima Y, Ito K, Zhang L, Ishikawa Y, Orikasa H, Kiguchi H, Akasaka Y, Komiyama K, Ishii T. Immunohistochemical detection of human small lymphatic vessels under normal and pathological conditions using the LYVE-1 antibody. Virchows Arch 444: 153–157, 2004. doi: 10.1007/s00428-003-0950-8. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1: 219–227, 2002. doi: 10.1016/S1535-6108(02)00051-X. [DOI] [PubMed] [Google Scholar]
- 3.Aukland K, Bogusky RT, Renkin EM. Renal cortical interstitium and fluid absorption by peritubular capillaries. Am J Physiol Renal Physiol 266: F175–F184, 1994. doi: 10.1152/ajprenal.1994.266.2.F175. [DOI] [PubMed] [Google Scholar]
- 4.Bai Y, Lu H, Lin C, Xu Y, Hu D, Liang Y, Hong W, Chen B. Sonic hedgehog-mediated epithelial-mesenchymal transition in renal tubulointerstitial fibrosis. Int J Mol Med 37: 1317–1327, 2016. doi: 10.3892/ijmm.2016.2546. [DOI] [PubMed] [Google Scholar]
- 5.Ballermann BJ, Obeidat M. Tipping the balance from angiogenesis to fibrosis in CKD. Kidney Int Suppl 4: 45–52, 2014. doi: 10.1038/kisup.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bando H, Brokelmann M, Toi M, Alitalo K, Sleeman JP, Sipos B, Gröne HJ, Weich HA. Immunodetection and quantification of vascular endothelial growth factor receptor-3 in human malignant tumor tissues. Int J Cancer 111: 184–191, 2004. doi: 10.1002/ijc.20211. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Fu H, Wu S, Zhu W, Liao J, Hong X, Miao J, Luo C, Wang Y, Hou FF, Zhou L, Liu Y. Tenascin-C protects against acute kidney injury by recruiting Wnt ligands. Kidney Int 95: 62–74, 2019. doi: 10.1016/j.kint.2018.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coso S, Bovay E, Petrova TV. Pressing the right buttons: signaling in lymphangiogenesis. Blood 123: 2614–2624, 2014. doi: 10.1182/blood-2013-12-297317. [DOI] [PubMed] [Google Scholar]
- 9.Ding H, Zhou D, Hao S, Zhou L, He W, Nie J, Hou FF, Liu Y. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol 23: 801–813, 2012. doi: 10.1681/ASN.2011060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabian SL, Penchev RR, St-Jacques B, Rao AN, Sipilä P, West KA, McMahon AP, Humphreys BD. Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol 180: 1441–1453, 2012. doi: 10.1016/j.ajpath.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu H, Tian Y, Zhou L, Zhou D, Tan RJ, Stolz DB, Liu Y. Tenascin-C is a major component of the fibrogenic niche in kidney fibrosis. J Am Soc Nephrol 28: 785–801, 2017. doi: 10.1681/ASN.2016020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y, Tang C, Cai J, Chen G, Zhang D, Dong Z. Rodent models of AKI-CKD transition. Am J Physiol Renal Physiol 315: F1098–F1106, 2018. doi: 10.1152/ajprenal.00199.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P, Salven P, Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res 64: 3737–3740, 2004. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 15.Karpanen T, Alitalo K. Lymphatic vessels as targets of tumor therapy? J Exp Med 194: F37–F42, 2001. doi: 10.1084/jem.194.6.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 113: 5650–5659, 2009. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 17.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, Laakkonen P, Petrova T, Langer B, Raab I. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol 15: 603–612, 2004. doi: 10.1097/01.ASN.0000113316.52371.2E. [DOI] [PubMed] [Google Scholar]
- 18.Kim DJ, Kang JM, Park SH, Kwon HK, Song SJ, Moon H, Kim SM, Seo JW, Lee YH, Kim YG, Moon JY, Lee SY, Son Y, Lee SH. Diabetes aggravates post-ischaemic renal fibrosis through persistent activation of TGF-β1 and Shh signalling. Sci Rep 7: 16782, 2017. doi: 10.1038/s41598-017-16977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim KE, Koh YJ, Jeon BH, Jang C, Han J, Kataru RP, Schwendener RA, Kim JM, Koh GY. Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol 175: 1733–1745, 2009. doi: 10.2353/ajpath.2009.090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinashi H, Falke LL, Nguyen TQ, Bovenschen N, Aten J, Leask A, Ito Y, Goldschmeding R. Connective tissue growth factor regulates fibrosis-associated renal lymphangiogenesis. Kidney Int 92: 850–863, 2017. doi: 10.1016/j.kint.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Kinashi H, Ito Y, Sun T, Katsuno T, Takei Y. Roles of the TGF-β-VEGF-C pathway in fibrosis-related lymphangiogenesis. Int J Mol Sci 19: 2487, 2018. doi: 10.3390/ijms19092487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kneedler SC, Phillips LE, Hudson KR, Beckman KM, Lopez Gelston CA, Rutkowski JM, Parrish AR, Doris PA, Mitchell BM. Renal inflammation and injury are associated with lymphangiogenesis in hypertension. Am J Physiol Renal Physiol 312: F861–F869, 2017. doi: 10.1152/ajprenal.00679.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramann R. Hedgehog Gli signalling in kidney fibrosis. Nephrol Dial Transplant 31: 1989–1995, 2016. doi: 10.1093/ndt/gfw102. [DOI] [PubMed] [Google Scholar]
- 24.Le Clef N, Verhulst A, D’Haese PC, Vervaet BA. Unilateral renal ischemia-reperfusion as a robust model for acute to chronic kidney injury in mice. PLoS One 11: e0152153, 2016. doi: 10.1371/journal.pone.0152153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AS, Lee JE, Jung YJ, Kim DH, Kang KP, Lee S, Park SK, Lee SY, Kang MJ, Moon WS, Kim HJ, Jeong YB, Sung MJ, Kim W. Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int 83: 50–62, 2013. [Erratum in Kidney Int 88: 72–84, 2015.] doi: 10.1038/ki.2012.312. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polizio AH, Chinchilla P, Chen X, Kim S, Manning DR, Riobo NA. Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J Biol Chem 286: 19589–19596, 2011. doi: 10.1074/jbc.M110.197111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polizio AH, Chinchilla P, Chen X, Manning DR, Riobo NA. Sonic Hedgehog activates the GTPases Rac1 and RhoA in a Gli-independent manner through coupling of smoothened to Gi proteins. Sci Signal 4: pt7, 2011. doi: 10.1126/scisignal.2002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauhauser AA, Ren C, Lu D, Li B, Zhu J, McEnery K, Vadnagara K, Zepeda-Orozco D, Zhou XJ, Lin F, Jetten AM, Attanasio M. Hedgehog signaling indirectly affects tubular cell survival after obstructive kidney injury. Am J Physiol Renal Physiol 309: F770–F778, 2015. doi: 10.1152/ajprenal.00232.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakamoto I, Ito Y, Mizuno M, Suzuki Y, Sawai A, Tanaka A, Maruyama S, Takei Y, Yuzawa Y, Matsuo S. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int 75: 828–838, 2009. doi: 10.1038/ki.2008.661. [DOI] [PubMed] [Google Scholar]
- 31.Stolz DB, Sims-Lucas S. Unwrapping the origins and roles of the renal endothelium. Pediatr Nephrol 30: 865–872, 2015. doi: 10.1007/s00467-014-2798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki Y, Ito Y, Mizuno M, Kinashi H, Sawai A, Noda Y, Mizuno T, Shimizu H, Fujita Y, Matsui K, Maruyama S, Imai E, Matsuo S, Takei Y. Transforming growth factor-β induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int 81: 865–879, 2012. doi: 10.1038/ki.2011.464. [DOI] [PubMed] [Google Scholar]
- 33.Tanabe K, Sato Y, Wada J. Endogenous antiangiogenic factors in chronic kidney disease: potential biomarkers of progression. Int J Mol Sci 19: 1859, 2018. doi: 10.3390/ijms19071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HN, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, Coggeshall M, Dandona L, Dicker DJ, Erskine HE, Ferrari AJ, Fitzmaurice C, Foreman K, Forouzanfar MH, Fraser MS, Fullman N, , et al.; GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 8: 1459–1544, 2016. [Erratum in Lancet 389: PE1, 2017.] doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol 303: F1487–F1494, 2012. doi: 10.1152/ajprenal.00352.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams CS, Leek RD, Robson AM, Banerji S, Prevo R, Harris AL, Jackson DG. Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. J Pathol 200: 195–206, 2003. doi: 10.1002/path.1343. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Dai C, Liu Y. Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am J Pathol 163: 621–632, 2003. doi: 10.1016/S0002-9440(10)63689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yazdani S, Poosti F, Kramer AB, Mirković K, Kwakernaak AJ, Hovingh M, Slagman MC, Sjollema KA, de Borst MH, Navis G, van Goor H, van den Born J. Proteinuria triggers renal lymphangiogenesis prior to the development of interstitial fibrosis. PLoS One 7: e50209, 2012. doi: 10.1371/journal.pone.0050209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834, 2010. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 40.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest 124: 878–887, 2014. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou D, Fu H, Xiao L, Mo H, Zhuo H, Tian X, Lin L, Xing J, Liu Y. Fibroblast-specific β-catenin signaling dictates the outcome of AKI. J Am Soc Nephrol 29: 1257–1271, 2018. doi: 10.1681/ASN.2017080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou D, Fu H, Zhang L, Zhang K, Min Y, Xiao L, Lin L, Bastacky SI, Liu Y. Tubule-derived Wnts are required for fibroblast activation and kidney fibrosis. J Am Soc Nephrol 28: 2322–2336, 2017. doi: 10.1681/ASN.2016080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou D, Li Y, Zhou L, Tan RJ, Xiao L, Liang M, Hou FF, Liu Y. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol 25: 2187–2200, 2014. doi: 10.1681/ASN.2013080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou D, Tan RJ, Liu Y. Sonic hedgehog signaling in kidney fibrosis: a master communicator. Sci China Life Sci 59: 920–929, 2016. doi: 10.1007/s11427-016-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou D, Tian Y, Sun L, Zhou L, Xiao L, Tan RJ, Tian J, Fu H, Hou FF, Liu Y. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J Am Soc Nephrol 28: 598–611, 2017. doi: 10.1681/ASN.2016030354. [DOI] [PMC free article] [PubMed] [Google Scholar]