Abstract
Meprin metalloproteases have been implicated in the pathophysiology of diabetic kidney disease (DKD). Single-nucleotide polymorphisms in the meprin-β gene have been associated with DKD in Pima Indians, a Native American ethnic group with an extremely high prevalence of DKD. In African American men with diabetes, urinary meprin excretion positively correlated with the severity of kidney injury. In mice, meprin activity decreased at the onset of diabetic kidney injury. Several studies have identified meprin targets in the kidney. However, it is not known how proteolytic processing of the targets by meprins impacts the metabolite milieu in kidneys. In the present study, global metabolomics analysis identified differentiating metabolites in kidney tissues from wild-type and meprin-β knockout mice with streptozotocin (STZ)-induced type 1 diabetes. Kidney tissues were harvested at 8 wk post-STZ and analyzed by hydrophilic interaction liquid chromatography ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Principal component analysis identified >200 peaks associated with diabetes. Meprin expression-associated metabolites with strong variable importance of projection scores were indoxyl sulfate, N-γ-l-glutamyl-l-aspartic acid, N-methyl-4-pyridone-3-carboxamide, inosine, and cis-5-decenedioic acid. N-methyl-4-pyridone-3-carboxamide has been previously implicated in kidney injury, and its isomers, 4-PY and 2-PY, are markers of peroxisome proliferation and inflammation that correlate with creatinine clearance and glucose tolerance. Meprin deficiency-associated differentiating metabolites with high variable importance of projection scores were cortisol, hydroxymethoxyphenylcarboxylic acid-O-sulfate, and isovaleryalanine. The data suggest that meprin-β activity enhances diabetic kidney injury in part by altering the metabolite balance in kidneys, favoring high levels of uremic toxins such as indoxyl sulfate and N-methyl-pyridone-carboxamide.
Keywords: diabetic kidney injury, meprin β, meprin metalloproteases, metabolites, streptozotocin
INTRODUCTION
Diabetic kidney injury is the leading cause of end-stage renal disease. However, not all patients with diabetes develop kidney injury. Currently, there is no diagnostic tool for predicting patients at risk for diabetic kidney disease (DKD) to facilitate early targeted interventions that could slow progression to end-stage renal disease. Existing clinical tests for diagnosis of DKD such as the urinary albumin-to-creatinine ratio (UACR) are neither sensitive nor accurate predictors. The development of more sensitive and reliable biomarkers for the early diagnosis of DKD requires knowledge of the underlying cellular and molecular mechanisms. Due to the paucity of kidney biopsies, most studies that develop biomarkers of DKD rely on urine and blood samples. However, diabetes affects multiple organs and biomarkers present in biofluids (urine and blood) could be influenced by system-wide changes rather than pathophysiological changes in kidney tissue. Rodent models of DKD are thus valuable in gaining knowledge on kidney-specific changes that could be leveraged in the development of diagnostic tools.
Meprin metalloproteases have been implicated in the pathology of kidney disease including DKD (54, 65) and ischemia-reperfusion (I/R)-induced acute kidney injury (13, 44). Meprins are most abundantly expressed in the brush-border membranes (BBMs) of proximal kidney tubules and small intestines. In fact, meprins are the most abundant proteins in the BBM of proximal kidney tubules. Meprins are also expressed in podocytes under certain pathological conditions, e.g., glomerulonephritis (61) and DKD (59). Other locations with documented meprin expression include leukocytes (monocytes and macrophages) (71), which play a role in inflammation, an underlying cause of diabetic kidney injury. Meprins are comprised of two subunits, α and β, which are encoded by two distinct genes. They result in two protein isoforms, meprin A (α-α or α-β) and meprin B (β-β), with distinct and overlapping substrates. For common substrates, the meprin isoforms have unique cleavage sites. In humans, single-nucleotide polymorphisms in the meprin-β gene have been associated with DKD in Pima Indians, a Native American ethnic group with an extremely high incidence of type 2 diabetes and DKD (26). Urinary excretion of meprins and plasma levels of inflammatory markers mediated by the TNF-α/NF-κB signaling axis also correlated with the severity of kidney injury in diabetic African American men (16). A decrease in expression of meprins was also demonstrated at the onset of diabetic kidney injury in mice (54). Additionally, disruption of both meprin-α and meprin-β genes resulted in higher mortality rates and more severe kidney injury in mice with streptozotocin (STZ)-induced type 1 diabetes (22). Furthermore, glomerular expression of meprin A has been demonstrated in the kidneys of mice with STZ-induced type 1 diabetes but not in nondiabetic controls (59). A role for meprin-β in tubulointerstitial fibrosis has also been suggested in other fibrosis-associated renal injury, with meprin-β being downregulated in collagen type IVA3 knockout mice, which develop Alport’s syndrome and renal pathology (67). In mice, expression of meprin-β exacerbates acute kidney injury induced by I/R (13). Taken together, existing data suggest that meprins play a role in the progression of DKD.
Identified meprin targets in the kidney include extracellular matrix (ECM) proteins (e.g., collagen type IV, nidogen-1, laminin, and fibronectin) (37, 45, 48, 50, 77), cytoskeletal proteins (e.g., villin and actin) (62), tight junction proteins (e.g., occludin and E-cadherin) (6, 28, 39), proinflammatory cytokines (e.g., IL-1β, IL-6, and IL-18) (4, 34, 46), chemokines [e.g., monocyte chemoattractant protein (MCP)-1], AGC kinase family proteins (e.g., the catalytic subunit of PKA and PKC) (12, 19, 59), and proteins that interact with the hypoxia response factors [e.g., osteosarcoma amplified (OS)-9] (23). The diversity of kidney meprin substrates suggests that meprins could modulate kidney injury via a variety of mechanisms under different pathological conditions. The objective of the present study was to use a global metabolomics approach in analysis of kidney tissues from diabetic wild-type (WT) and meprin-β knockout (βKO) mice to gain insights into the meprin-β-mediated metabolic changes that impact the pathology of diabetic kidney injury.
MATERIALS AND METHODS
Experimental animals.
Male WT mice on a C57BL/6 background were purchased from Charles River Laboratories at the age of 6 wk. WT mice express normal levels of both meprin A (α-α and α-β) and meprin B (β-β). Meprin βKO mice were initially obtained from the laboratory of Dr. Judith Bond (Pennsylvania State University College of Medicine, Hershey, PA) and bred at the Laboratory Animal Resource Unit (LARU) of North Carolina A&T State University. Meprin βKO mice are deficient in meprin B (β-β) and the heterodimeric isoform of meprin A (α-β) but express the homomeric isoform of meprin A (α-α). Mice were housed at the LARU in group cages of up to 5 mice/cage. Mice were provided with rat chow and water ad libitum and maintained under a 12:12-h light-dark cycle. All protocols used were reviewed and approved by the North Carolina A&T Institutional Animal Care and Use Committee.
Induction of type 1 diabetes.
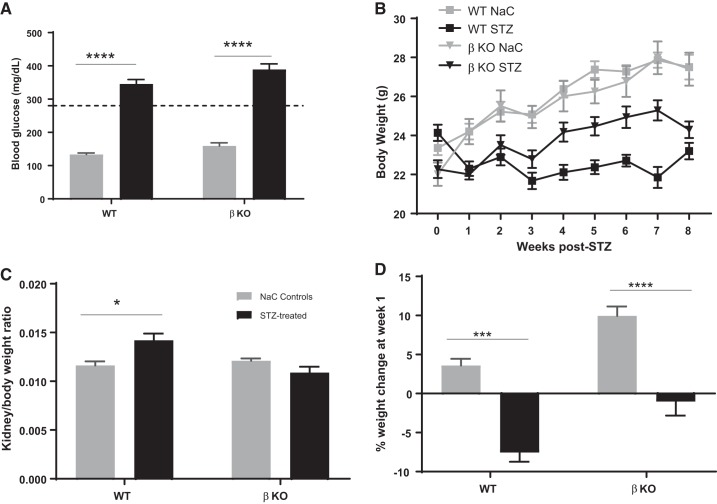
Low-dose STZ was used to induce type 1 diabetes as previously described by Tesch and Allen (73). To this end, 8-wk-old male mice (n = 8 for each genotype) were injected with STZ (50 mg/kg body wt) in freshly prepared 10 mM sodium citrate buffer (pH 4.5) for 5 consecutive days. Mice were deprived of food for 6 h prior to the STZ injection. Control mice (n = 6 for each genotype) were injected with sodium citrate buffer. A standard glucose meter and glucose sticks (Relion) were used to determine daytime 6-h fasting glucose levels at 10 days post-STZ injection (Fig. 1A). Mice with fasting glucose ≥ 280 mg/dl (dashed line on Fig. 1A) were considered diabetic. Body weights were monitored on a weekly basis (Fig. 1B). At 8 wk post-STZ, mice were euthanized and kidneys were harvested. The kidneys were individually weighed, and renal hypertrophy was determined by the kidney weight-to-body weight ratios (Fig. 1C). Although the kidney weight-to-body weight ratio can be indicative of other pathologies such as hyperplasia, renal hypertrophy is characteristic of advanced diabetic nephropathy and is often used in combination with other parameters. We therefore used it in combination with biochemical assessments for kidney injury. The time point for tissue harvest was selected based on a previous study (14) that showed significant increases in biomarkers of kidney injury in this mouse model at 8 wk post-STZ injection. Furthermore, this corresponds to the onset/early stages of kidney injury (before clinical disease) and is well suited for identifying biomarkers for the early diagnosis of diabetic kidney injury. While there is no specific time point for the “early onset of kidney injury,” we obtained blood and urine samples at 4 wk post-STZ injection and performed bioassays for various markers of kidney injury. We were therefore confident that kidney injury had occurred as early as 4 wk based on the data that we obtained (Fig. 2). We did not want to wait until all the characteristic pathological changes had occurred because our goal was to determine changes in the early stages of diabetic kidney injury. A 2-mm midsection of each kidney was fixed in Carnoy’s fixative (60% ethanol, 30% chloroform, and 10% acetic acid) overnight at 4°C and then transferred to 70% ethanol. Sections were paraffin embedded at the Wake Forest University Pathology Laboratory, and 4-mm sections were cut onto PLUS slides for subsequent immunohistochemical analysis. The kidney tissue for metabolomics analysis was snap frozen in liquid nitrogen and stored at −80°C.
Fig. 1.
Assessment of diabetic status, body weights, and renal hypertrophy. A: blood glucose levels were assayed at 10 days after streptozotocin (STZ) injection. B: STZ-injected mice with blood glucose levels ≤ 280 mg/dl (dashed line) were eliminated from the study. Mice were weighed weekly. C: kidneys were harvested at 8 wk post-STZ and individually weighed, and body weight-to-kidney weight ratios were determined. D: weight change at week 1. WT, wild type; βKO, meprin-β knockout; NaC, sodium citrate. *P = 0.0277; ***P = 0.001; ****P < 0.0001.
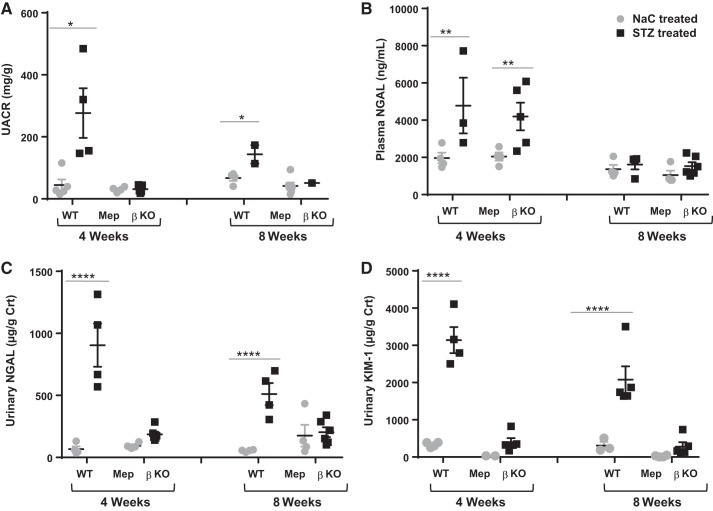
Fig. 2.
Biomarkers of kidney injury. Urine and blood samples were collected at 4 and 8 wk after streptozotocin (STZ) injection, and ELISA was used to determine levels of urinary albumin, creatinine, neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecule-1 (KIM-1) as well as plasma NGAL. A: urinary albumin-to-creatinine ratios (UACRs) were determined by normalizing urinary albumin to creatinine levels. B–D: urinary NGAL (B and C) and KIM-1 (D) levels were also normalized to levels of creatinine (Crt). WT, wild type; KO, knockout. *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.0001.
Biochemical assessment for kidney injury.
To evaluate kidney function, blood and urine samples were collected at 4 and 8 wk post-STZ injection. Blood samples were obtained by tail nicking into heparin tubes and centrifuged to obtain plasma, which was then stored at −80°C until used. Spot urine samples were obtained by bladder massage onto sterile petri dishes and stored at −80°C until used. ELISAs were used to determine the levels of albumin and creatinine, which were then used to compute UACRs. Additionally, we assayed for two biomarkers of kidney injury: neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1). Creatinine assays used a calorimetric assay (Diazyme Laboratories, Poway CA), whereas commercially available ELISA kits (R&D Systems, Minneapolis, MN) were used for NGAL and KIM-1. All assays were performed according to the manufacturers’ instructions. The standard curves for NGAL and KIM-1 were plotted using a four-parameter logistic curve fit (4-PL, GraphPad Prism 7.0).
Immunohistological staining and microscopic analysis.
Immunohistochimical analysis used a modified version of the Vectastain Elite ABC Universal Kit Protocol (Vector Laboratories, Burlingame CA). In summary, slide sections were deparaffinized through xylene, 100% ethanol, 95% ethanol, and water. Antigen unmasking was achieved by boiling in 10 mM sodium citrate buffer (pH 6.0) for 10 min. Endogenous peroxidase activity was quenched using 80% methanol with 6% H2O2 at room temperature for 20 min. Nonspecific binding sites were blocked by incubation in 5% normal horse serum in PBS at room temperature for 30 min in a humidity chamber. Primary antibodies were diluted in PBS buffer with 5% normal serum. Sections were incubated in primary antibodies overnight at 4°C. Rabbit polyclonal anti-KIM-1 antibodies and rabbit polyclonal anti-TNF receptor (TNFR)1 antibodies were obtained from Abcam (Cambridge, UK) and diluted at 1:100. Slides were then rinsed three times in PBS for 10 min each and incubated in universal biotinylated secondary antibodies diluted 1:50 for 30 min at room temperature. Elite ABC reagents were used for staining, and hematoxylin was used as counterstaining for nuclei. Coverslips were mounted and allowed to dry at room temperature overnight. Tissue sections were evaluated by light microscopy using a Keyence BZ-X700 microscope system and imaged using BZ-X Analyzer software (Itasca, IL). The intensity of staining was quantified using ImageJ (National Institutes of Health), and data were analyzed using two-way ANOVA (GraphPad Prism software).
Metabolomics analysis of kidney tissue lysates.
Kidney tissues were homogenized by bead beating in 2 µl/mg 50:50 acetonitrile-water. Tissue homogenates were protein precipitated, and supernatants were lyophilized overnight and reconstituted in 95:5 acetonitrile-water. All samples were prepared and analyzed in a randomized order. Supernatants were analyzed by hydrophilic interaction liquid chromatography ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS) methods (79, 80). Progenesis QI software (Nonlinear Dynamics) was used to align, pick peaks, deconvolute, and annotate compounds. Inconsistent lock mass spray prevented the data alignment of three injections, leading to differing numbers of animals in each group between positive and negative modes (Table 1). The criteria for selecting compounds for further analysis was as follows: retention time > 1 min, peak width between 0.1 and 2 min, abundance > 200 in at least 4 of 5 pooled quality control samples, and coefficient of variation < 0.4 among quality control samples. Multivariate analysis variable importance in projection (VIP) scores of >2 and univariate statistics (Wilcoxon rank sum P < 0.05) were used to prioritize compounds for identification. The fold change (FC) was calculated relative to nondiabetic controls and pooled internal loading controls. All raw and processed analytical data and associated metadata have been uploaded to the publicly accessible National Institutes of Health Common Fund Metabolomics Data Repository (https://www.metabolomicsworkbench.org/) under Metabolomics Workbench Project ID PR0004395. The data are directly accessible at the following website: https://www.metabolomicsworkbench.org/data/DRCCMetadata.php?Mode=Project&ProjectID=PR000395.
Table 1.
Summary of multivariate models and fit statistics
| Model | Number of Compounds | Total Number of Samples | Number of Components | R2X | R2Y | Q2 | Number of Samples in Group 2 |
Number of Samples in the Reference Group | Reference Group |
|---|---|---|---|---|---|---|---|---|---|
| Positive | |||||||||
| 3A: PCA All Samples | 1556 | 31 | 5 | 0.707 | |||||
| 3B: WT STZ vs. WT NaC | 1556 | 12 | 1 + 1 | 0.516 | 0.981 | 0.915 | 8 | 4 | NaC |
| 3C: βKO STZ vs. KO NaC | 1556 | 14 | 1 + 1 | 0.518 | 0.957 | 0.852 | 8 | 6 | NaC |
| 3D: βKO NaC vs. WT NaC | 1556 | 10 | 1 + 0 | 0.157 | 0.826 | −0.045 | 6 | 4 | WT |
| 3E: βKO STZ vs. WT STZ | 1556 | 16 | 1 + 5 | 0.682 | 1 | 0.818 | 8 | 8 | WT |
| Negative | |||||||||
| 41A: PCA All Samples | 921 | 30 | 5 | 0.734 | |||||
| 4B: WT STZ vs. WT NaC | 921 | 13 | 1 + 0 | 0.393 | 0.95 | 0.915 | 8 | 5 | NaC |
| 4C: βKO STZ vs. KO NaC | 921 | 12 | 1 + 0 | 0.3 | 0.879 | 0.576 | 8 | 4 | NaC |
| 4D: βKO NaC vs. WT NaC | 921 | 9 | 1 + 0 | 0.171 | 0.845 | −0.096 | 4 | 5 | WT |
| 4E: βKO STZ vs. WT STZ | 921 | 16 | 1 + 4 | 0.712 | 0.994 | 0.719 | 8 | 8 | WT |
PCA, principal component analysis; STZ, streptozotocin; βKO, meprin-β knockout; WT, wild type; NaC, sodium citrate.
Statistical analysis.
Two-way ANOVA with Tukey’s pair-wise comparisons (GraphPad Prism 7.0 software) was used for analysis of data relating to blood glucose, body weights, renal hypertrophy, and biomarkers of kidney injury. Principle component analysis (PCA) was performed using SIMCA 14.1 (Umetrics, Umeå, Sweden) to evaluate data structure and supervised multivariate analysis. Orthogonal partial least squares-discriminant analysis (OPLS-DA) was used to determine important group differentiating metabolites. Univariate statistics was completed in SAS 9.4 (SAS Institute, Cary, NC). Hypotheses were tested with exact Wilcoxon rank sum or Wilcoxon rank sum tests.
RESULTS
Meprin expression is associated with higher levels of kidney injury biomarkers.
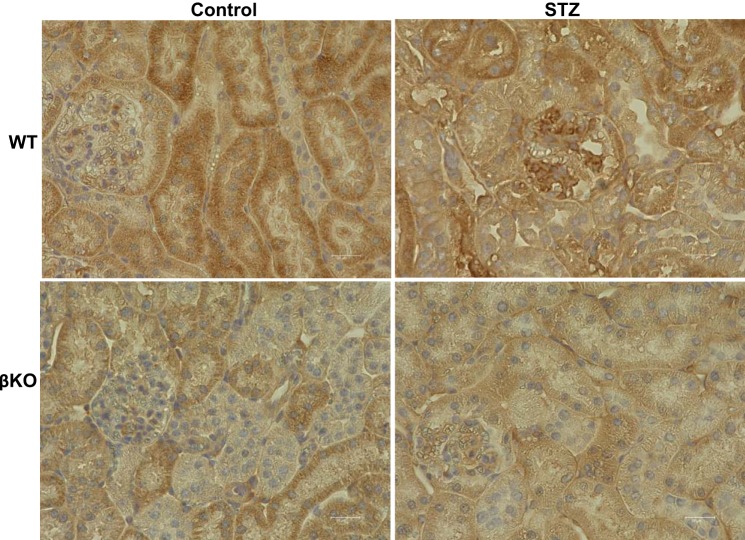
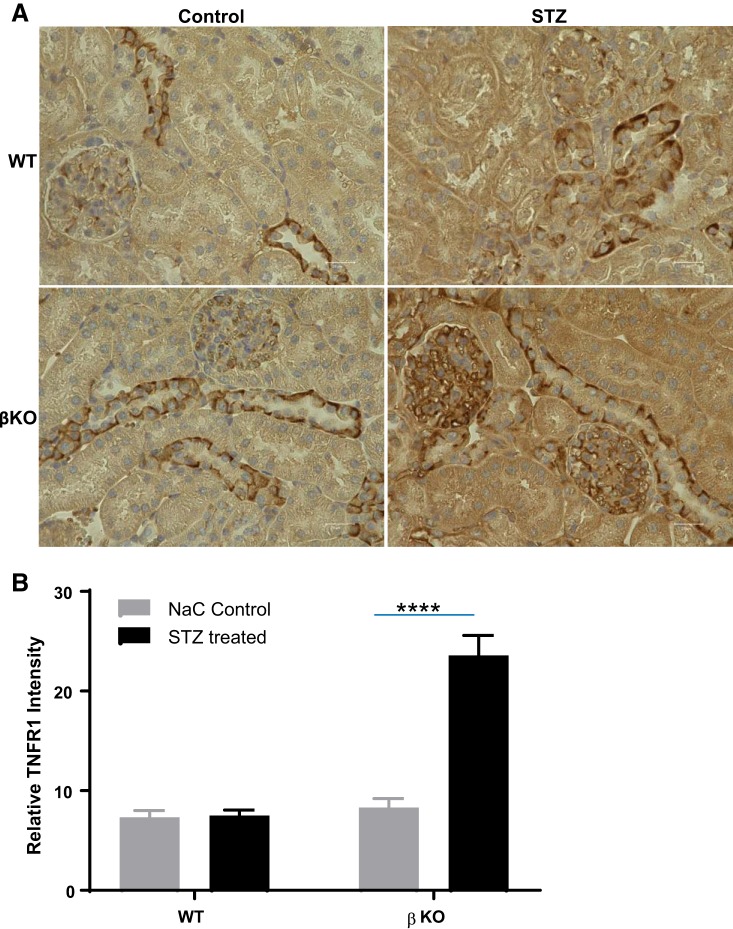
Assays for fasting blood glucose levels confirmed diabetes in STZ-injected mice (Fig. 1A). Time of day (nighttime vs. daytime) has been shown to impact blood glucose levels (70). Blood glucose levels in control mice in the present study reflect the range for daytime fasting. Furthermore, the assays used a glucometer, which has been shown to give higher readings when compared a laboratory assays (74). Interestingly, STZ-injected WT mice demonstrated a significantly greater weight loss than their βKO counterparts relative to sodium citrate vehicle-treated nondiabetic controls for each genotype (Fig. 1B). Weight loss was evident at 1 wk post-STZ injection (Fig. 1D). Diabetic WT mice never recovered from this weight loss and continued to underperform over the 8-wk period. Renal hypertrophy, as demonstrated by body weight-to-kidney weight ratios, was significant in diabetic WT mice (P < 0.05) mice but not in their βKO counterparts (Fig. 1C). However, this could be due to the significant body weight loss in WT mice rather than renal hypertrophy per se. For biochemical assessment of kidney injury, we determined the UACR and assayed for two markers of kidney injury, NGAL and KIM-1, at 4 and 8 wk post-STZ injection. The UACR was significantly higher in WT mice (P = 0.012) but not in βKO mice relative to their sodium citrate-treated nondiabetic controls. Compared with STZ-treated βKO mice, STZ-treated WT mice had significantly higher levels of UACR (P = 0.006; Fig. 2A). There were significant increases in plasma NGAL for both WT and βKO mice at 4 wk post-STZ (P = 0.018; Fig. 2B). Interestingly, the increases were not observed at 8 wk post-STZ. In contrast, urinary NGAL-to-creatinine ratios were significantly higher in diabetic WT mice at both 4 and 8 wk post-STZ (P = 0.0001) but not in βKO mice at either time point (Fig. 2C). A similar pattern was observed for urinary KIM-1-to-creatinine ratios, which significantly increased in WT mice but not in βKO mice at both time points (P = 0.0001 at 4 wk and P = 0.0003 at 8 wk; Fig. 2D). For all three kidney injury biomarkers, levels were higher at 4 wk than at 8 wk, suggesting that reparative mechanisms could be in play. Immunohistochemical analysis showed an increased staining intensity for KIM-1 in diabetic WT mice but not in their βKO counterparts at 8 wk post-STZ (Fig. 3), confirming the ELISA data. We also observed genotype differences in the expression of TNFR1. Overall, there were higher levels of TNFR1 in sections from diabetic βKO mice compared with nondiabetic controls and their WT counterparts (Fig. 4). Because TNF causes changes in glomerular endothelial permeability (83), we quantified levels of TNFR1 in 10 renal corpuscles from each mouse and found a significant increase in TNFRI in diabetic βKO mice (P = 0.0001; Fig. 4B).
Fig. 3.
Representative immunohistological staining for kidney injury molecule-1 (KIM-1) in kidney tissue sections from diabetic mice and sodium citrate (NaC)-treated controls. There was a modest increase in KIM-1 staining intensity in proximal tubules from wild-type (WT) streptozotocin (STZ)-treated mice. Levels of KIM-1 were also higher in glomeruli of STZ-treated WT mice compared with their NaC-treated nondiabetic counterparts. In contrast, KIM-1 staining intensities were comparable in NaC-treated and STZ-treated meprin-β knockout (βKO) mice.
Fig. 4.
Immunolocalization of TNF receptor (TNFR)1 in kidney tissue from wild-type (WT) and meprin-β knockout (βKO) mice with streptozotocin (STZ)-induced type 1 diabetes. A: immunohistochemical staining with anti-TNFR1 antibodies was used to evaluate expression of TNFR1. B: intensity of TNFR staining in glomeruli was quantified using ImageJ, and the data were analyzed by two-way ANOVA. ****P = 0.0001.
Meprin-β expression is associated with significant changes in metabolite profiles in kidney tissue.
Lysates from 32 kidney tissue samples were analyzed by hydrophilic interaction liquid chromatography UPLC-QTOF-MS metabolomics (Table 1). Three injections, one in positive mode and two in negative mode, were excluded for inconsistent lock mass spray, which prevented the data alignment. Unsupervised PCA of normalized, deconvoluted compound ion intensities for the remaining samples demonstrated that STZ treatment/diabetes was the greatest source of variation in the data (Figs. 5A and 6A). Supervised analysis (OPLS-DA) was completed for each of four pairwise comparisons (Figs. 5 and 6, B–E). With one exception, all models had a predictive Q2 of >0.5, indicating that they pass recommended tolerances. OPLS-DA analysis was unable to fit a good model to distinguish vehicle-treated WT metabolite profiles from their βKO counterparts (Fig. 6D), suggesting comparable baseline metabolites levels. Metabolites differentiating βKO from WT kidney tissue were determined based on changes after STZ-induced diabetes (models B and C) and on differences between tissues from diabetic mice for the two genotypes (model E). Multivariate statistical analysis demonstrated that the metabolic patterns could be discriminated based on diabetic kidney status and on genotype. Metabolites that changed in kidney tissues from both WT and βKO mice were considered to be associated with diabetic kidney injury independent of meprin expression/activity. On the other hand, meprin-β has been implicated in metabolites with changes that were unique to either WT kidneys or βKO kidneys only.
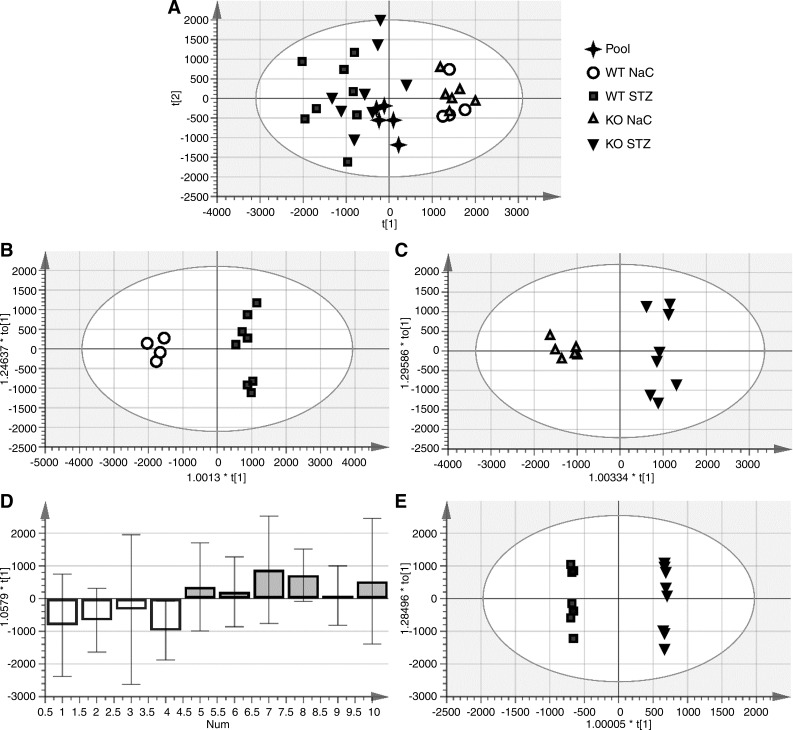
Fig. 5.
Principal component analysis (PCA) of kidney lysates in positive mode. Orthogonal partial least squares-discriminant analysis was used to determine important group differentiating metabolites. A: combined PCA for all samples demonstrating distinct clustering by genotype and treatment [sodium citrate (NaC)-treated control vs. streptozotocin (STZ)-treated diabetic mice]. B: PCA differentiating NaC-treated control and STZ-treated diabetic mice within the wild-type (WT) genotype. C: metabolites that differentiated NaC- versus STZ-treated mice within the meprin-β knockout (βKO) genotype. D: metabolites that differentiated the genotypes (WT vs. βKO) within the NaC-treated control group. E: metabolites that differentiated the genotypes (WT vs. βKO) within the STZ-treated diabetic group. Annotated metabolites are shown in Tables 2 and 3.
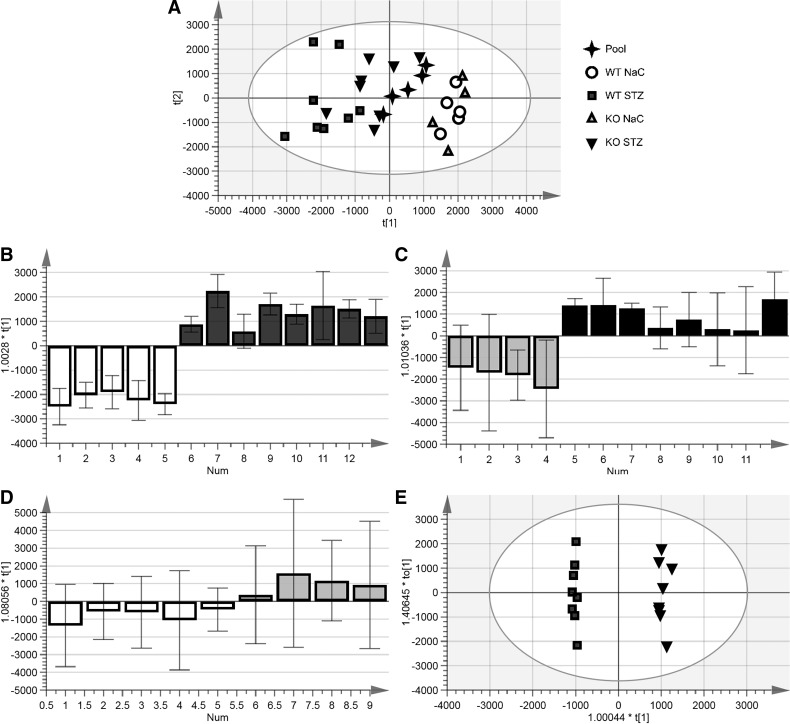
Fig. 6.
Principal component analysis (PCA) of kidney lysates in negative mode. Supervised multivariate analysis was used to determine differentiating metabolites. A: clustering of metabolites for all kidney lysates regardless of genotype or treatment. B: metabolites differentiating sodium citrate (NaC)-treated control and streptozotocin (STZ)-treated diabetic mice within the wild-type (WT) genotype. C: metabolites differentiating NaC-treated control and STZ-treated diabetic mice within the meprin-β knockout (βKO) genotype. D: metabolites that differentiated WT and βKO genotypes within the NaC-treated control group. E: PCA differentiating WT and βKO genotypes within the STZ-treated group. Annotated metabolites are shown in Tables 2 and 3.
Metabolites with diabetes-induced changed profiles in meprin-expressing kidney tissue.
A total of 550 metabolites (22 annotated and 533 unknown metabolites) had significant changes in levels associated with diabetic status in WT but not βKO kidney tissues and could thus be attributed to meprin-β expression/activity. Of the 22 identified metabolites, 13 metabolites had a FC of ≥1.5 (Table 2). Of these, indoxy sulfate had the highest differentiating power (FC = 1.7 and VIP = 5.6) followed by cis-5-decenedioic acid (FC = 1.8 and VIP = 2.1), d-1,5-anhydrofructose (FC = 1.7 and VIP = 1.9), and 3-(3,5-dihydroxyphenyl)-1-propanoic acid sulfate (FC = 1.9 and VIP = 1.5). Although inosine and N-γ-l-glutamyl-l-aspartic acid only had FC = 1.1, it was associated with very high predictive value scores (VIP = 4.8 and VIP = 5.2, respectively). In contrast, dibutyl malate, lysophosphatidylcholine, and isovalerylalanine had high FC values (−2.5, −2.1, and −2.1 respectively) but low VIP scores (0.9, 1.2, and 1.7, respectively), suggesting low predictive power.
Table 2.
Annotated metabolites that differentiate STZ diabetic kidney tissue from NaC-treated control tissue and associate with meprin-β expression or deficiency
| Metabolite | VIP | P Value† | FC* |
|---|---|---|---|
| WT mice only | |||
| Dibutyl malate | 0.9 | 0.002 | −2.5 |
| Lysophosphatidylcholine [16:1(9Z)] | 1.2 | 0.004 | −2.1 |
| 3-Methoxytyrosine | 1 | 0.011 | 2 |
| 3-Methoxy-4-hydroxyphenylethyleneglycol sulfate | 0.9 | 0.003 | 2 |
| 3-(3,5-Dihydroxyphenyl)-l-propanoic acid sulfate | 1.5 | 0.011 | 1.9 |
| Cis-5-Decenedioic acid | 2.1 | 0.008 | 1.8 |
| 5-Hydroxytryptophol | 1 | 0.008 | −1.8 |
| Indoxyl sulfate | 5.6 | 0.002 | 1.7 |
| d-1,5-Anhydrofructose | 1.9 | 0.004 | 1.7 |
| 2,4-Dimethylpimelic acid | 0.8 | 0.006 | −1.6 |
| l-Acetylcarnitine | 1.1 | 0.006 | 1.5 |
| 9′-Carboxy-γ-chromanol | 0.9 | 0.008 | 1.5 |
| Prenyl glucoside | 0.4 | 0.028 | −1.5 |
| 3-Aminosalicylic acid | 0.9 | 0.016 | 1.3 |
| N1-methyl-4-pyridone-3-carboxamide | 0.8 | 0.048 | 1.2 |
| Caffeic acid 3-sulfate | 0.5 | 0.006 | 1.2 |
| Lotaustralin | 1.1 | 0.004 | −1.2 |
| 7-Methoxy-5-prenyloxycoumarin | 0.5 | 0.028 | −1.2 |
| Methyl bisnorbiotinyl ketone | 0.4 | 0.016 | 1.1 |
| Inosine | 4.8 | 0.093 | 1.1 |
| Phenylpropionylglycine | 0.6 | 0.045 | 1.1 |
| N-γ-l-glutamyl-l-aspartic acid | 5.2 | 0.03 | −1.1 |
| 533 Unknowns | |||
| βKO mice only | |||
| Cortisol | 2.3 | 0.028 | 3 |
| Isovalerylalanine | 1.7 | 0.048 | −2.1 |
| Hydroxymethoxyphenylcarboxylic acid-O-sulfate | 3 | 0.028 | 1.8 |
| Cervonoyl ethanolamide | 0.5 | 0.026 | −1.8 |
| Dihydroferulic acid 4-sulfate | 1 | 0.048 | 1.6 |
| Ethyl-7-epi-12-hydroxyjasmonate glucoside | 0.8 | 0.008 | 1.6 |
| 5-Methoxysalicylic acid | 0.7 | 0.016 | 1.4 |
| Wyerol | 0.3 | 0.010 | 1.1 |
| 212 Unknowns |
WT, wild type; βKO, meprin-β knockout; VIP, variable importance of projection; FC, fold change; STZ, streptozotocin; NaC, sodium citrate.
FC is relative to nondiabetic controls; a VIP score of ≥2 is considered a strong predictor of diabetic complications such as diabetic kidney disease.
P value was by an exact Wilcoxon rank sum test.
Diabetes-induced changes in metabolite levels that associate with meprin-β deficiency.
Compared with WT kidneys, the number of metabolites with diabetes-induced changed profiles in βKO kidneys only was much lower (total of 220 metabolines: 8 annotated and 212 unknown metabolites; Table 2). Six of the identified metabolites had FC ≥ 1.5, but only two had VIP ≥ 2, namely, cortisol (FC = 3 and VIP = 2.3) and hydroxymethoxyphenylcarboxylic acid-O-sulfate (FC = 1.8 and VIP = 3.0). Because these metabolites only changed levels in βKO kidneys, the changes can be attributed to meprin-β deficiency.
Metabolites with diabetes-induced changed profiles independent of meprin-β expression or deficiency.
A total of 482 compounds (17 annotated and 465 unknown compounds) were important to separating diabetic kidney tissue from nondiabetic sodium citrate-treated controls for both WT and βKO mice. However, only five compounds had 1.5 ≤ FC and a 2 ≤ VIP in both genotypes (Table 3). The levels of three of these (indole-3-carboxilic acid-O-sulfate, hippuric acid, and nicotine imine) increased, whereas the levels of two (guanosine and glycerophosphocholine) decreased. Although FC for betaine was only 1.4, it was associated with strong VIP scores in both genotypes (9.6 in WT and 8.4 in βKO). In contrast, although indole-3-carboxilic acid-O-sulfate had the highest FC (FC = 3.5 in WT and FC = 3.9 in βKO), it had low VIP scores (VIP = 1.0 in WT and VIP = 0.5 in βKO).
Table 3.
Differentiating metabolites that associate with diabetic status independent of meprin-β expression (changed levels in the kidneys of both WT and βKO mice)
| Metabolite | WT Mice |
βKO Mice |
||||
|---|---|---|---|---|---|---|
| VIP | P value† | FC* | VIP | P value† | FC* | |
| Indole-3-carboxilic acid-O-sulfate | 1 | 0.002 | 3.5 | 0.5 | 0.004 | 3.9 |
| Hippuric acid | 2.9 | 0.006 | 2.3 | 3.8 | 0.109 | 1.8 |
| Isoferulic acid 3-sulfate | 1.3 | 0.019 | 2.3 | 1.8 | 0.028 | 2.1 |
| Guanosine | 3.1 | 0.004 | −2.3 | 3 | 0.01 | −2 |
| Ergothioneine | 1.8 | 0.004 | 2.1 | 2.6 | 0.01 | 1.6 |
| Nicotine imine | 9.8 | 0.016 | 1.9 | 8.9 | 0.01 | 1.6 |
| 3-Methoxy-4-hydroxyphenylethyleneglycol sulfate | 3.2 | 0.011 | 1.9 | 3.8 | 0.073 | 2 |
| Fenoprofen glucuronide | 0.8 | 0.006 | 1.9 | 1.4 | 0.004 | 2.2 |
| Hydroxyphenyllactic acid | 0.8 | 0.011 | 1.8 | 0.9 | 0.028 | 1.5 |
| 4-Hydroxybenzoic acid-4-O-sulfate | 0.9 | 0.003 | 1.8 | 1.1 | 0.008 | 1.6 |
| Glycerophosphocholine | 10.4 | 0.008 | −1.8 | 11 | 0.01 | −1.8 |
| Betaine | 9.6 | 0.004 | 1.6 | 8.4 | 0.01 | 1.4 |
| Tyrosyl-baline | 0.5 | 0.008 | 1.6 | 0.5 | 0.01 | 1.5 |
| 4-Methoxyphenylacetic acid | 0.5 | 0.006 | 1.6 | 0.6 | 0.028 | 1.5 |
| 2,6-Dihydroxybenzoic acid | 0.5 | 0.006 | 1.6 | 0.6 | 0.028 | 1.3 |
| Phosphatidylcholine [20:5(5Z,8Z,11Z,14Z,17Z)/18:3(6Z,9Z,12Z)] | 0.8 | 0.028 | 1.3 | 1.3 | 0.016 | 1.2 |
| Apigenin 7-sulfate | 0.4 | 0.011 | 1.2 | 0.5 | 0.028 | 1.2 |
| 465 Unknowns | ||||||
WT, wild type; βKO, meprin-β knockout; VIP, variable importance of projection; FC, fold change.
FC is relative to nondiabetic controls; VIP score ≥ 2 is considered a strong predictor of diabetic complications such as diabetic kidney disease.
P value was by an exact Wilcoxon rank sum test.
DISCUSSION
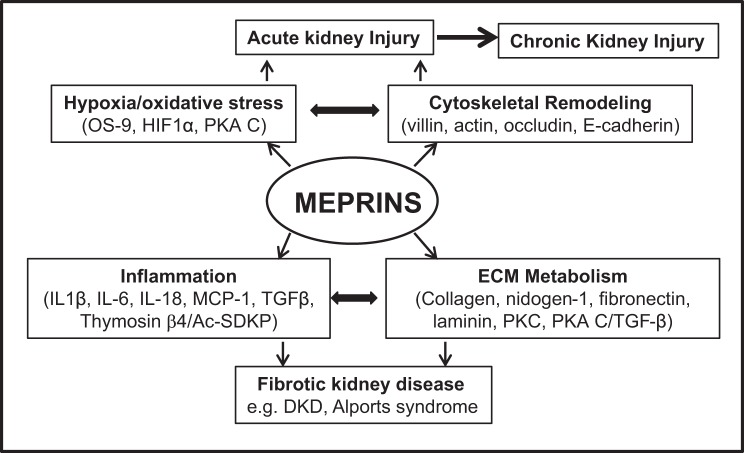
The present study profiled metabolites associated with meprin-β metalloprotease expression in kidney tissue at 8 wk after diabetes induction. This time point reflects the onset/early stages of kind injury before clinical disease and is well suited for evaluating cellular and molecular mechanisms in the progression of kidney injury. Identifying biomarkers in these early stages of kidney injury would be useful in the development of diagnostic tools for early diagnosis of diabetic kidney injury. Furthermore, these times points have been used for assessing various signaling mechanisms and testing drug targets for kidney injury in rodent models of STZ-induced type 1 diabetes (31, 38, 42, 47, 63, 69, 70, 86) and obesity-induced type 2 diabetes (15, 82). Meprin metalloproteases have emerged as susceptibility genes for DKD (14, 54, 65) and have been proposed as potential biomarkers of kidney injury (7, 53). Meprins are the most abundantly expressed proteins in BBMs of proximal kidney tubules and are also expressed in glomeruli in DKD (59) and in glomerulonephritis (61). Furthermore, meprins are expressed in leukocytes (monocytes and macrophages) (71), suggesting a role in inflammation, an underlying cause of diabetic kidney injury. Meprins could thus impact the pathology of DKD at both glomerular and tubulointerstitial locations. Knowledge of the meprin targets in the kidneys has provided insights on potential underlying cellular and molecular mechanisms (Fig. 7). Known meprin-β substrates include ECM proteins (e.g., collagen, nidogen-1, laminin, and fibronectin) (3, 48, 50, 77), proinflammatory cytokines (e.g., IL-1β, IL-6, and IL-18) (34, 36, 46), anti-inflammatory modulators [e.g., thymosin-β4/N-acetyl-seryl-aspartyl-proline (Ac-SDKP)] (51), and signaling molecules (e.g., PKC and the catalytic subunit of PKA) (2, 12, 18, 19, 59). The existing data thus point to a role for meprins in inflammation and ECM metabolism, two mechanisms associated with renal fibrosis in DKD.
Fig. 7.
Summary of potential meprin involvement in kidney injury based on identified meprin substrates present in the kidney. The identified substrates suggest that meprins modulate inflammation and extracellular matrix metabolism, key underlying causes of fibrosis and kidney injury in diabetes. DKD, diabetic kidney disease; MCP-1, monocyte chemoattractant protein 1; OS-9, osteosarcoma amplified-9; TGF-β, transforming growth factor-β; HIF-1α, hypoxia-inducible factor-1α.
Determination of the UACR remains the gold standard for diagnosis of diabetic kidney injury, but it is not sensitive for early prediction of DKD. Recent advances, based on proteomic approaches, have identified promising new biomarkers present in urine and serum, such as NGAL, KIM-1, and cystatin C, that could be used for the early detection of DKD (10, 11, 32, 55, 57, 60). However, these protein markers do not shed sufficient light on the pathophysiological mechanisms of the injury that would facilitate more targeted interventions (8). For instance, NGAL is elevated in DKD but not in obese children with elevated microalbuminuria (30). Similarly, KIM-1 is indicative of tubular cell injury but does not shed much light on the causes of the injury. The value of metabolomics approaches in evaluating kidney injury is supported by recent metabolomics profiling of serum and kidney lysates, which have provided evidence of the pathways that lead to loss of kidney function, inflammation, and recovery (9, 87). For example, in a mouse model of I/R-induced acute kidney injury, global metabolomics profiling of kidney lysates and plasma were shown not only to be good predictors of early kidney injury but also to be of potential diagnostic value (81).
In the present study, mass spectrometry and PCA of kidney tissue lysates identified >200 peaks associated with diabetic kidney injury in WT and βKO mice. Levels of several metabolites significantly changed only in kidney tissues from WT mice and could thus be attributed to meprin-β expression/activity. Those with high VIP scores (VIP ≥ 2) include inosine, indoxyl sulfate, N-γ-l-glutamyl-l-aspartic acid, cis-5-decenedioic acid, and N-methyl-pyridone-carboxamide. Inosine is a metabolite derived from adenosine, and increased levels suggest that meprin-β influences AMP conversion to adenosine and ultimately to inosine. Previous studies have shown that patients on hemodialysis with elevated levels of inosine and hypoxanthine were likely to represent more hypoxic and oxidative stress (20). Another differentiating metabolite, indoxyl sulfate, is a protein-bound tryptophan-derived metabolite that is generated by intestinal microorganisms (microbiota). Indoxyl sulfate is a well-characterized uremic toxin that is associated with reduced renal clearance. Animal studies have demonstrated an association between indoxyl sulfate accumulation and increased fibrosis and oxidative stress (27, 41, 84). High serum levels of indoxyl sulfate have also been shown to correlate with the progression of CKD in human patients (56). The fact that the levels of indoxyl sulfate only increased in kidneys from WT mice and not βKO mice could suggests that meprin-β expressed in the BBM of small intestines could influence the gut microbiota, subsequently resulting in toxicity, which causes injury in the kidneys. N-methyl-2-pyridone-5-carboxamide (2-PY) is one of the end products of nicotinamide-adenine dinucleotide degradation and has previously been implicated in kidney injury. Isomers of N-methyl-pyridone-carboxamide, 4-PY and 2-PY, are markers of peroxisome proliferation and inflammation and correlate with creatinine clearance as well as glucose concentrations in oral glucose tolerance tests (64, 75). Furthermore, 2-PY and 4-PY are considered uremic toxins, and their concentrations are many times elevated in patients with chronic renal failure compared with controls (17, 52, 66, 68, 72). Importantly, the antidiabetic drug vildagliptin lowered the concentrations of both isomers in urine (64). Increased levels of cis-5-decenedioic acid in kidney tissue from WT mice suggest a role for meprins in fatty acid metabolism. Cis-5-decenedioic acid is a monounsaturated dicarboxylic acid derived from the oxidation of oleic and linoleic acids (43) and is associated with medium-chain acyl-CoA dehydrogenase deficiency (76), an inborn error of metabolism. Levels of hydroxymethoxyphenylcarboxylic acid-O-sulfate and cortisol only increased in kidney tissues from meprin-β-deficient mice, suggesting that the increase is associated with meprin deficiency, but their role in kidney injury has not been documented. However, the high cortisol levels could be indicative of increased stress in meprin-β-deficient mice. A subset of metabolites had significant changes associated with diabetes in both WT and βKO mice, indicating that these changes are independent of meprin-β expression or deficiency. These include two metabolites (glycerophosphocholine and betaine) that annotated as osmolytes and hippuric acid. Changes in levels of glycerophosphocholine and betaine have been reported in rats with lithium-induced nephrogenic diabetes insipidus (40) and rats with chronic renal failure treated with N-acetylcysteine (78). Hippuric acid is a product of amino acid metabolism by gut microbiota and has been shown to be part of metabolite panels that change in kidney injury. Urinary levels of hippuric acid have also been shown to significantly increase in acute kidney injury (85).
Previous data from our group using meprin-αβ double-KO mice showed that meprin-αβ-deficient mice had higher mortality rates and more severe kidney injury associated with STZ-induced type 1 diabetes (14). In the present study, we only worked with meprin-β-deficient mice, and the data showed higher levels of kidney injury markers in meprin-β-expressing kidneys. This is similar to the pattern shown in I/R-induced acute kidney injury, where meprin-β expression exacerbated kidney injury (13), but contradicts our data with diabetic meprin-αβ double-KO mice (14). In the present study, all biomarkers of kidney injury (UACR, NGAL, and KIM-1) were significantly higher in WT mice compared with meprin-β deficient mice at 4 wk post-STZ, but the differences were not as significant at 8 wk. This suggests that reparative mechanisms could kick in and serve to restore kidney function at the later time points. However, taken together, the data also suggest potential meprin isoform-specific influences in kidney injury and repair. While meprin-αβ double-KO mice are deficient in all meprin isoforms, βKO mice still express the homomeric isoform of meprin A (α-α), which has anti-inflammatory activities (35, 51). For example, meprin A inactivated MCP-1 (35) and proteolytic processing of thymosin-β4 by meprin A releases the anti-inflammatory peptide Ac-SDKP (51). In contrast, meprin B has both proinflammatory (e.g., activates IL-1β and IL-18) (4, 36) and anti-inflammatory activities (e.g., inactivates IL-6). It is likely that disruption of the meprin-β gene shifts the balance toward proinflammatory activities modulated by meprin A (α-α), which would protect the kidneys from injury. This hypothesis is supported by a previous study (5) that demonstrated that imbalances in meprin A and meprin B influence the severity of inflammatory bowel disease. Another interesting observation was the increased TNFR1 expression in kidney sections from diabetic βKO mice but not their WT counterparts. Levels of TNF-α and TNFRs have been implicated in the progression of kidney injury in both type 1 and type 2 diabetes (1, 21, 24, 58). Elevated levels of TNFR1 and TNFR2 are strong predictors of progression to end-stage renal disease in patients with diabetes (29). Furthermore, TNFR1 associates with markers of inflammation in the pathogenesis of diabetic kidney disease (25). The kidney weight-to-body weight ratios were only significant in WT mice, but this could be due to the greater loss of body weight associated with STZ injection in WT mice compared with their βKO counterparts rather than renal hypertrophy per se. The differences in body weight losses in the first week was a surprising observation for us. Furthermore, the loss in body weight was greatest at 1 wk post-STZ injection, a time when kidney injury is not expected to have occurred. At this point, we do not have the answer for this difference but hypothesize that it is related to dietary processing by gut microbiota. We plan to conduct additional studies to determine whether meprin-β, which is abundantly expressed in BBMs of small intestines, interacts with gut microbiota to influence production of uremic toxins, which then accumulate in diabetic kidneys.
In conclusion, data from the present study show distinct changes in the metabolite profiles of kidney lysates from mice with diabetic kidney injury compared with nondiabetic controls. More importantly, the metabolite profiles for meprin-β-expressing kidneys with diabetic kidney injury are distinct from their βKO counterparts, suggesting that meprin-β alters metabolic pathways in the kidneys of diabetic mice. An interesting observation was the increased levels of metabolites that originate in gut microbiota, indicating that the meprin-β present in BBMs of small intestines contributes to the high levels of uremic toxins in diabetic kidney tissue. These changes could explain in part how meprin-β modulates the pathophysiology of kidney injury. The goal for the present study was not to simply document changes in metabolites that others have established but rather to determine if the expression of or deficiency of meprin-β metalloproteases made a difference in the metabolite profiles and thus gain insights on how meprins modulate kidney injury. To our knowledge, there are no previous data on meprin-associated metabolite changes in the diabetic kidney. This is an area that our group continues to work on, and the present data serve as a basis for future studies.
GRANTS
This work was supported National Institute of General Medical Science (NIGMS) Grants SC-GM-3102049 and SC1-GM-118271 and National Institute on Minority Health and Health Disparities Grant U54-MD-008621 (CFDA 93.307) and Subawards Grant HU-140004 (to E. M. Ongeri), National Institute of Diabetes and Digestive and Kidney Diseases Grant U24-DK-097193 (to S. Sumner), National Center for Advancing Translational Sciences Grant UL1-TR-001111 (to J. Buse and S. Sumner), and NIGMS Grant K01-GM-109320 (to J. Gooding).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S. and E.M.O. conceived and designed research; J.G., L.C., F.A., J.-M.M., M.F., C.W., and E.M.O. performed experiments; J.G., L.C., Z.A., S.M., and E.M.O. analyzed data; J.G., L.C., S.S., and E.M.O. interpreted results of experiments; J.G. and E.M.O. prepared figures; L.C. and E.M.O. drafted manuscript; J.G., L.C., F.A., J.-M.M., S.S., and E.M.O. edited and revised manuscript; J.G., L.C., F.A., J.-M.M., M.F., C.W., Z.A., S.M., S.S., and E.M.O. approved final version of manuscript.
REFERENCES
- 1.Al-Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, Min W, Pober JS, Bradley JR. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J 19: 1637–1645, 2005. doi: 10.1096/fj.05-3841com. [DOI] [PubMed] [Google Scholar]
- 2.Alhanaty E, Patinkin J, Tauber-Finkelstein M, Shaltiel S. Degradative inactivation of cyclic AMP-dependent protein kinase by a membranal proteinase is restricted to the free catalytic subunit in its native conformation. Proc Natl Acad Sci USA 78: 3492–3495, 1981. doi: 10.1073/pnas.78.6.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambort D, Stalder D, Lottaz D, Huguenin M, Oneda B, Heller M, Sterchi EE. A novel 2D-based approach to the discovery of candidate substrates for the metalloendopeptidase meprin. FEBS J 275: 4490–4509, 2008. doi: 10.1111/j.1742-4658.2008.06592.x. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S, Bond JS. Prointerleukin-18 is activated by meprin beta in vitro and in vivo in intestinal inflammation. J Biol Chem 283: 31371–31377, 2008. doi: 10.1074/jbc.M802814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee S, Jin G, Bradley SG, Matters GL, Gailey RD, Crisman JM, Bond JS. Balance of meprin A and B in mice affects the progression of experimental inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 300: G273–G282, 2011. doi: 10.1152/ajpgi.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao J, Yura RE, Matters GL, Bradley SG, Shi P, Tian F, Bond JS. Meprin A impairs epithelial barrier function, enhances monocyte migration, and cleaves the tight junction protein occludin. Am J Physiol Renal Physiol 305: F714–F726, 2013. doi: 10.1152/ajprenal.00179.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker-Pauly C, Broder C, Prox J, Koudelka T, Tholey A. Mapping orphan proteases by proteomics: meprin metalloproteases deciphered as potential therapeutic targets. Proteomics Clin Appl 8: 382–388, 2014. doi: 10.1002/prca.201300079. [DOI] [PubMed] [Google Scholar]
- 8.Bennett MR, Devarajan P. Proteomic analysis of acute kidney injury: biomarkers to mechanisms. Proteomics Clin Appl 5: 67–77, 2011. doi: 10.1002/prca.201000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blydt-Hansen TD, Sharma A, Gibson IW, Mandal R, Wishart DS. Urinary metabolomics for noninvasive detection of borderline and acute T cell-mediated rejection in children after kidney transplantation. Am J Transplant 14: 2339–2349, 2014. doi: 10.1111/ajt.12837. [DOI] [PubMed] [Google Scholar]
- 10.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 4: 337–344, 2009. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Fazio MR, Nicocia G, Buemi M. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res 32: 91–98, 2009. doi: 10.1159/000209379. [DOI] [PubMed] [Google Scholar]
- 12.Boyd S, Newman R, Ongeri E. Protein kinase C alpha is a target for the meprin B metalloproteinase (690.14) (Abstract). FASEB J 28, Suppl 1: 690.14, 2014. [Google Scholar]
- 13.Bylander J, Li Q, Ramesh G, Zhang B, Reeves WB, Bond JS. Targeted disruption of the meprin metalloproteinase beta gene protects against renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 294: F480–F490, 2008. doi: 10.1152/ajprenal.00214.2007. [DOI] [PubMed] [Google Scholar]
- 14.Bylander JE, Ahmed F, Conley SM, Mwiza JM, Ongeri EM. Meprin metalloprotease deficiency associated with higher mortality rates and more severe diabetic kidney injury in mice with STZ-induced type 1 diabetes. J Diabetes Res 2017: 9035038, 2017. doi: 10.1155/2017/9035038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao A, Wang L, Chen X, Guo H, Chu S, Zhang X, Peng W. Ursodeoxycholic acid ameliorated diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress. Biol Pharm Bull 39: 1300–1308, 2016. doi: 10.1248/bpb.b16-00094. [DOI] [PubMed] [Google Scholar]
- 16.Cao L, Sedighi R, Boston A, Premadasa L, Pinder J, Crawford GE, Jegede OE, Harrison SH, Newman RH, Ongeri EM. Undiagnosed kidney injury in uninsured and underinsured diabetic African American men and putative role of meprin metalloproteases in diabetic nephropathy. Int J Nephrol 2018: 6753489, 2018. doi: 10.1155/2018/6753489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrey EA, Smolenski RT, Edbury SM, Laurence A, Marinaki AM, Duley JA, Zhu L, Goldsmith DJ, Simmonds HA. Origin and characteristics of an unusual pyridine nucleotide accumulating in erythrocytes: positive correlation with degree of renal failure. Clin Chim Acta 335: 117–129, 2003. doi: 10.1016/S0009-8981(03)00294-8. [DOI] [PubMed] [Google Scholar]
- 18.Chestukhin A, Litovchick L, Muradov K, Batkin M, Shaltiel S. Unveiling the substrate specificity of meprin beta on the basis of the site in protein kinase A cleaved by the kinase splitting membranal proteinase. J Biol Chem 272: 3153–3160, 1997. doi: 10.1074/jbc.272.6.3153. [DOI] [PubMed] [Google Scholar]
- 19.Chestukhin A, Muradov K, Litovchick L, Shaltiel S. The cleavage of protein kinase A by the kinase-splitting membranal proteinase is reproduced by meprin beta. J Biol Chem 271: 30272–30280, 1996. doi: 10.1074/jbc.271.47.30272. [DOI] [PubMed] [Google Scholar]
- 20.Choi JY, Yoon YJ, Choi HJ, Park SH, Kim CD, Kim IS, Kwon TH, Do JY, Kim SH, Ryu DH, Hwang GS, Kim YL. Dialysis modality-dependent changes in serum metabolites: accumulation of inosine and hypoxanthine in patients on haemodialysis. Nephrol Dial Transplant 26: 1304–1313, 2011. doi: 10.1093/ndt/gfq554. [DOI] [PubMed] [Google Scholar]
- 21.Coca SG, Nadkarni GN, Huang Y, Moledina DG, Rao V, Zhang J, Ferket B, Crowley ST, Fried LF, Parikh CR. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol 28: 2786–2793, 2017. doi: 10.1681/ASN.2016101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conley S, Han J, Hurley S, Ongeri EM. Meprin deficient mice have a more severe form of diabetic nephropathy (Abstract). FASEB J 27, Suppl 1: 702.5, 2013. [Google Scholar]
- 23.Conley S, Martin B, Ongeri EM. Meprins cleave OS-9 present in mouse kidneys subjected to ischemia reperfusion acute kidney injury (Abstract). FASEB J 27, Suppl 1: 705.7, 2013. [Google Scholar]
- 24.DiPetrillo K, Coutermarsh B, Gesek FA. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am J Physiol Renal Physiol 284: F113–F121, 2003. doi: 10.1152/ajprenal.00026.2002. [DOI] [PubMed] [Google Scholar]
- 25.Doody A, Jackson S, Elliott JA, Canavan RJ, Godson C, Slattery D, Twomey PJ, McKenna MJ, le Roux CW, Docherty NG. Validating the association between plasma tumour necrosis factor receptor 1 levels and the presence of renal injury and functional decline in patients with Type 2 diabetes. J Diabetes Complications 32: 95–99, 2018. doi: 10.1016/j.jdiacomp.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Red Eagle AR, Hanson RL, Jiang W, Han X, Matters GL, Imperatore G, Knowler WC, Bond JS. Meprin β metalloprotease gene polymorphisms associated with diabetic nephropathy in the Pima Indians. Hum Genet 118: 12–22, 2005. doi: 10.1007/s00439-005-0019-7. [DOI] [PubMed] [Google Scholar]
- 27.Edamatsu T, Fujieda A, Itoh Y. Phenyl sulfate, indoxyl sulfate and p-cresyl sulfate decrease glutathione level to render cells vulnerable to oxidative stress in renal tubular cells. PLoS One 13: e0193342, 2018. doi: 10.1371/journal.pone.0193342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.George JN, Ongeri EM. Degradation of tight junction proteins in meprin betatransfected kidney cells subjected to hypoxia (Abstract). FASEB J 27, Suppl 1: 954.9, 2013. [Google Scholar]
- 29.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, Cullere X, Johnson AC, Crabtree G, Smiles AM, Mayadas TN, Warram JH, Krolewski AS. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23: 516–524, 2012. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goknar N, Oktem F, Ozgen IT, Torun E, Kuçukkoc M, Demir AD, Cesur Y. Determination of early urinary renal injury markers in obese children. Pediatr Nephrol 30: 139–144, 2015. doi: 10.1007/s00467-014-2829-0. [DOI] [PubMed] [Google Scholar]
- 31.Guo H, Wang Y, Zhang X, Zang Y, Zhang Y, Wang L, Wang H, Wang Y, Cao A, Peng W. Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKα-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci Rep 7: 6852, 2017. doi: 10.1038/s41598-017-07061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haase M, Mertens PR. Biomarkers: more than just markers! Nephrol Dial Transplant 30: 33–38, 2015. doi: 10.1093/ndt/gfu085. [DOI] [PubMed] [Google Scholar]
- 34.Herzog C, Haun RS, Kaushal V, Mayeux PR, Shah SV, Kaushal GP. Meprin A and meprin α generate biologically functional IL-1β from pro-IL-1β. Biochem Biophys Res Commun 379: 904–908, 2009. doi: 10.1016/j.bbrc.2008.12.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herzog C, Haun RS, Shah SV, Kaushal GP. Proteolytic processing and inactivation of CCL2/MCP-1 by meprins. Biochem Biophys Rep 8: 146–150, 2016. doi: 10.1016/j.bbrep.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herzog C, Kaushal GP, Haun RS. Generation of biologically active interleukin-1beta by meprin B. Cytokine 31: 394–403, 2005. doi: 10.1016/j.cyto.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Herzog C, Marisiddaiah R, Haun RS, Kaushal GP. Basement membrane protein nidogen-1 is a target of meprin β in cisplatin nephrotoxicity. Toxicol Lett 236: 110–116, 2015. doi: 10.1016/j.toxlet.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu CC, Yang HT, Ho JJ, Yin MC, Hsu JY. Houttuynia cordata aqueous extract attenuated glycative and oxidative stress in heart and kidney of diabetic mice. Eur J Nutr 55: 845–854, 2016. [Erratum in Eur J Nutr 56: 2007, 2017.] doi: 10.1007/s00394-015-0994-y. [DOI] [PubMed] [Google Scholar]
- 39.Huguenin M, Müller EJ, Trachsel-Rösmann S, Oneda B, Ambort D, Sterchi EE, Lottaz D. The metalloprotease meprinbeta processes E-cadherin and weakens intercellular adhesion. PLoS One 3: e2153, 2008. doi: 10.1371/journal.pone.0002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang GS, Yang JY, Ryu DH, Kwon TH. Metabolic profiling of kidney and urine in rats with lithium-induced nephrogenic diabetes insipidus by 1H-NMR-based metabonomics. Am J Physiol Renal Physiol 298: F461–F470, 2010. doi: 10.1152/ajprenal.00389.2009. [DOI] [PubMed] [Google Scholar]
- 41.Ji C, Luo Y, Zou C, Huang L, Tian R, Lu Z. Effect of astragaloside IV on indoxyl sulfate-induced kidney injury in mice via attenuation of oxidative stress. BMC Pharmacol Toxicol 19: 53, 2018. doi: 10.1186/s40360-018-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang W, Li Z, Zhao W, Chen H, Wu Y, Wang Y, Shen Z, He J, Chen S, Zhang J, Fu G. Breviscapine attenuatted contrast medium-induced nephropathy via PKC/Akt/MAPK signalling in diabetic mice. Am J Transl Res 8: 329–341, 2016. [PMC free article] [PubMed] [Google Scholar]
- 43.Jin SJ, Tserng KY. Metabolic origins of urinary unsaturated dicarboxylic acids. Biochemistry 29: 8540–8547, 1990. doi: 10.1021/bi00489a006. [DOI] [PubMed] [Google Scholar]
- 44.Kaushal GP, Haun RS, Herzog C, Shah SV. Meprin A metalloproteinase and its role in acute kidney injury. Am J Physiol Renal Physiol 304: F1150–F1158, 2013. doi: 10.1152/ajprenal.00014.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaushal GP, Walker PD, Shah SV. An old enzyme with a new function: purification and characterization of a distinct matrix-degrading metalloproteinase in rat kidney cortex and its identification as meprin. J Cell Biol 126: 1319–1327, 1994. doi: 10.1083/jcb.126.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keiffer TR, Bond JS. Meprin metalloproteases inactivate interleukin 6. J Biol Chem 289: 7580–7588, 2014. doi: 10.1074/jbc.M113.546309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SM, Lee SH, Lee A, Kim DJ, Kim YG, Kim SY, Jeong KH, Lee TW, Ihm CG, Lim SJ, Moon JY. Targeting T helper 17 by mycophenolate mofetil attenuates diabetic nephropathy progression. Transl Res 166: 375–383, 2015. doi: 10.1016/j.trsl.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 48.Köhler D, Kruse M, Stöcker W, Sterchi EE. Heterologously overexpressed, affinity-purified human meprin alpha is functionally active and cleaves components of the basement membrane in vitro. FEBS Lett 465: 2–7, 2000. doi: 10.1016/S0014-5793(99)01712-3. [DOI] [PubMed] [Google Scholar]
- 50.Kruse MN, Becker C, Lottaz D, Köhler D, Yiallouros I, Krell HW, Sterchi EE, Stöcker W. Human meprin alpha and beta homo-oligomers: cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem J 378: 383–389, 2004. doi: 10.1042/bj20031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar N, Nakagawa P, Janic B, Romero CA, Worou ME, Monu SR, Peterson EL, Shaw J, Valeriote F, Ongeri EM, Niyitegeka JM, Rhaleb NE, Carretero OA. The anti-inflammatory peptide Ac-SDKP is released from thymosin-β4 by renal meprin-α and prolyl oligopeptidase. Am J Physiol Renal Physiol 310: F1026–F1034, 2016. doi: 10.1152/ajprenal.00562.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lenglet A, Liabeuf S, Bodeau S, Louvet L, Mary A, Boullier A, Lemaire-Hurtel AS, Jonet A, Sonnet P, Kamel S, Massy ZA. N-methyl-2-pyridone-5-carboxamide (2PY)-major metabolite of nicotinamide: an update on an old uremic toxin. Toxins (Basel) 8: 339, 2016. doi: 10.3390/toxins8110339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madoux F, Tredup C, Spicer TP, Scampavia L, Chase PS, Hodder PS, Fields GB, Becker-Pauly C, Minond D. Development of high throughput screening assays and pilot screen for inhibitors of metalloproteases meprin α and β. Biopolymers 102: 396–406, 2014. doi: 10.1002/bip.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathew R, Futterweit S, Valderrama E, Tarectecan AA, Bylander JE, Bond JS, Trachtman H. Meprin-alpha in chronic diabetic nephropathy: interaction with the renin-angiotensin axis. Am J Physiol Renal Physiol 289: F911–F921, 2005. doi: 10.1152/ajprenal.00037.2005. [DOI] [PubMed] [Google Scholar]
- 55.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- 56.Namba S, Okuda Y, Morimoto A, Kojima T, Morita T. [Serum indoxyl sulfate is a useful predictor for progression of chronic kidney disease]. Rinsho Byori 58: 448–453, 2010. [PubMed] [Google Scholar]
- 57.Nauta FL, Boertien WE, Bakker SJ, van Goor H, van Oeveren W, de Jong PE, Bilo H, Gansevoort RT. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care 34: 975–981, 2011. doi: 10.2337/dc10-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navarro-González JF, Jarque A, Muros M, Mora C, García J. Tumor necrosis factor-alpha as a therapeutic target for diabetic nephropathy. Cytokine Growth Factor Rev 20: 165–173, 2009. doi: 10.1016/j.cytogfr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Niyitegeka JM, Bastidas AC, Newman RH, Taylor SS, Ongeri EM. Isoform-specific interactions between meprin metalloproteases and the catalytic subunit of protein kinase A: significance in acute and chronic kidney injury. Am J Physiol Renal Physiol 308: F56–F68, 2015. doi: 10.1152/ajprenal.00167.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noto A, Cibecchini F, Fanos V, Mussap M. NGAL and metabolomics: the single biomarker to reveal the metabolome alterations in kidney injury. BioMed Res Int 2013: 612032, 2013. doi: 10.1155/2013/612032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oneda B, Lods N, Lottaz D, Becker-Pauly C, Stöcker W, Pippin J, Huguenin M, Ambort D, Marti HP, Sterchi EE. Metalloprotease meprin beta in rat kidney: glomerular localization and differential expression in glomerulonephritis. PLoS One 3: e2278, 2008. doi: 10.1371/journal.pone.0002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ongeri EM, Anyanwu O, Reeves WB, Bond JS. Villin and actin in the mouse kidney brush-border membrane bind to and are degraded by meprins, an interaction that contributes to injury in ischemia-reperfusion. Am J Physiol Renal Physiol 301: F871–F882, 2011. doi: 10.1152/ajprenal.00703.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patschan D, Schwarze K, Henze E, Becker JU, Patschan S, Müller GA. eEOC-mediated modulation of endothelial autophagy, senescence, and EnMT in murine diabetic nephropathy. Am J Physiol Renal Physiol 307: F686–F694, 2014. doi: 10.1152/ajprenal.00650.2013. [DOI] [PubMed] [Google Scholar]
- 64.Pelantová H, Bugáňová M, Holubová M, Šedivá B, Zemenová J, Sýkora D, Kaválková P, Haluzík M, Železná B, Maletínská L, Kuneš J, Kuzma M. Urinary metabolomic profiling in mice with diet-induced obesity and type 2 diabetes mellitus after treatment with metformin, vildagliptin and their combination. Mol Cell Endocrinol 431: 88–100, 2016. doi: 10.1016/j.mce.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Red Eagle AR, Hanson RL, Jiang W, Han X, Matters GL, Imperatore G, Knowler WC, Bond JS. Meprin beta metalloprotease gene polymorphisms associated with diabetic nephropathy in the Pima Indians. Hum Genet 118: 12–22, 2005. doi: 10.1007/s00439-005-0019-7. [DOI] [PubMed] [Google Scholar]
- 66.Rutkowski B, Slominska E, Szolkiewicz M, Smolenski RT, Striley C, Rutkowski P, Swierczynski J. N-methyl-2-pyridone-5-carboxamide: a novel uremic toxin? Kidney Int Suppl 63: S19–S21, 2003. doi: 10.1046/j.1523-1755.63.s84.36.x. [DOI] [PubMed] [Google Scholar]
- 67.Sampson NS, Ryan ST, Enke DA, Cosgrove D, Koteliansky V, Gotwals P. Global gene expression analysis reveals a role for the alpha 1 integrin in renal pathogenesis. J Biol Chem 276: 34182–34188, 2001. doi: 10.1074/jbc.M102859200. [DOI] [PubMed] [Google Scholar]
- 68.Slominska EM, Carrey EA, Foks H, Orlewska C, Wieczerzak E, Sowinski P, Yacoub MH, Marinaki AM, Simmonds HA, Smolenski RT. A novel nucleotide found in human erythrocytes, 4-pyridone-3-carboxamide-1-beta-D-ribonucleoside triphosphate. J Biol Chem 281: 32057–32064, 2006. doi: 10.1074/jbc.M607514200. [DOI] [PubMed] [Google Scholar]
- 69.Song G, Han P, Sun H, Shao M, Yu X, Wang W, Wang D, Yi W, Ge N, Li S, Yi T. Astragaloside IV ameliorates early diabetic nephropathy by inhibition of MEK1/2-ERK1/2-RSK2 signaling in streptozotocin-induced diabetic mice. J Int Med Res 46: 2883–2897, 2018. doi: 10.1177/0300060518778711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun C, Li X, Liu L, Conet M, Guan Y, Fan Y, Zhou Y. Effect of fasting time on measuring mouse blood glucose level. Int J Clin Exp 9: 4186–4189, 2016. [Google Scholar]
- 71.Sun Q, Jin HJ, Bond JS. Disruption of the meprin alpha and beta genes in mice alters homeostasis of monocytes and natural killer cells. Exp Hematol 37: 346–356, 2009. doi: 10.1016/j.exphem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Synesiou E, Fairbanks LD, Simmonds HA, Slominska EM, Smolenski RT, Carrey EA. 4-Pyridone-3-carboxamide-1-β-d-ribonucleoside triphosphate (4PyTP), a novel NAD metabolite accumulating in erythrocytes of uremic children: a biomarker for a toxic NAD analogue in other tissues? Toxins (Basel) 3: 520–537, 2011. doi: 10.3390/toxins3060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton) 12: 261–266, 2007. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- 74.Togashi Y, Shirakawa J, Okuyama T, Yamazaki S, Kyohara M, Miyazawa A, Suzuki T, Hamada M, Terauchi Y. Evaluation of the appropriateness of using glucometers for measuring the blood glucose levels in mice. Sci Rep 6: 25465, 2016. doi: 10.1038/srep25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsalik EL, Willig LK, Rice BJ, van Velkinburgh JC, Mohney RP, McDunn JE, Dinwiddie DL, Miller NA, Mayer ES, Glickman SW, Jaehne AK, Glew RH, Sopori ML, Otero RM, Harrod KS, Cairns CB, Fowler VG, Rivers EP, Woods CW, Kingsmore SF, Langley RJ. Renal systems biology of patients with systemic inflammatory response syndrome. Kidney Int 88: 804–814, 2015. doi: 10.1038/ki.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tserng KY, Jin SJ, Kerr DS, Hoppel CL. Abnormal urinary excretion of unsaturated dicarboxylic acids in patients with medium-chain acyl-CoA dehydrogenase deficiency. J Lipid Res 31: 763–771, 1990. [PubMed] [Google Scholar]
- 77.Walker PD, Kaushal GP, Shah SV. Meprin A, the major matrix degrading enzyme in renal tubules, produces a novel nidogen fragment in vitro and in vivo. Kidney Int 53: 1673–1680, 1998. doi: 10.1046/j.1523-1755.1998.00949.x. [DOI] [PubMed] [Google Scholar]
- 78.Wan C, Xue R, Zhan Y, Wu Y, Li X, Pei F. Metabolomic analysis of N-acetylcysteine protection of injury from gadolinium-dtpa contrast agent in rats with chronic renal failure. OMICS 21: 540–549, 2017. doi: 10.1089/omi.2017.0114. [DOI] [PubMed] [Google Scholar]
- 79.Want EJ, Coen M, Masson P, Keun HC, Pearce JT, Reily MD, Robertson DG, Rohde CM, Holmes E, Lindon JC, Plumb RS, Nicholson JK. Ultra performance liquid chromatography-mass spectrometry profiling of bile acid metabolites in biofluids: application to experimental toxicology studies. Anal Chem 82: 5282–5289, 2010. doi: 10.1021/ac1007078. [DOI] [PubMed] [Google Scholar]
- 80.Want EJ, Wilson ID, Gika H, Theodoridis G, Plumb RS, Shockcor J, Holmes E, Nicholson JK. Global metabolic profiling procedures for urine using UPLC-MS. Nat Protoc 5: 1005–1018, 2010. doi: 10.1038/nprot.2010.50. [DOI] [PubMed] [Google Scholar]
- 81.Wei Q, Xiao X, Fogle P, Dong Z. Changes in metabolic profiles during acute kidney injury and recovery following ischemia/reperfusion. PLoS One 9: e106647, 2014. doi: 10.1371/journal.pone.0106647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woods TC, Satou R, Miyata K, Katsurada A, Dugas CM, Klingenberg NC, Fonseca VA, Navar LG. Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am J Nephrol 49: 331–342, 2019. doi: 10.1159/000499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu C, Wu X, Hack BK, Bao L, Cunningham PN. TNF causes changes in glomerular endothelial permeability and morphology through a Rho and myosin light chain kinase-dependent mechanism. Physiol Rep 3: e12636, 2015. doi: 10.14814/phy2.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yisireyili M, Takeshita K, Saito S, Murohara T, Niwa T. Indole-3-propionic acid suppresses indoxyl sulfate-induced expression of fibrotic and inflammatory genes in proximal tubular cells. Nagoya J Med Sci 79: 477–486, 2017. doi: 10.18999/nagjms.79.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y, Gu L, Jiang Y, Bi K, Chen X. Quantitative analysis of biomarkers of liver and kidney injury in serum and urine using ultra-fast liquid chromatography with tandem mass spectrometry coupled with a hydrophilic interaction chromatography column: Application to monitor injury induced by Euphorbiae pekinensis Radix. J Sep Sci 39: 3936–3945, 2016. doi: 10.1002/jssc.201600470. [DOI] [PubMed] [Google Scholar]
- 86.Zhao J, Zhang QL, Shen JH, Wang K, Liu J. Magnesium lithospermate B improves the gut microbiome and bile acid metabolic profiles in a mouse model of diabetic nephropathy. Acta Pharmacol Sin 40: 507–513, 2019. doi: 10.1038/s41401-018-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao YY. Metabolomics in chronic kidney disease. Clin Chim Acta 422: 59–69, 2013. doi: 10.1016/j.cca.2013.03.033. [DOI] [PubMed] [Google Scholar]