Abstract
Laboratory mice are used to identify causes of urinary dysfunction including prostate-related mechanisms of lower urinary tract symptoms. Effective use of mice for this purpose requires a clear understanding of molecular, cellular, anatomic, and endocrine contributions to voiding function. Whether the prostate influences baseline voiding function has not been specifically evaluated, in part because most methods that alter prostate mass also change circulating testosterone concentrations. We performed void spot assay and cystometry to establish a multiparameter “baseline” of voiding function in intact male and female 9-wk-old (adult) C57BL/6J mice. We then compared voiding function in intact male mice to that of castrated male mice, male (and female) mice treated with the steroid 5α-reductase inhibitor finasteride, or male mice harboring alleles (Pbsn4cre/+; R26RDta/+) that significantly reduce prostate lobe mass by depleting prostatic luminal epithelial cells. We evaluated aging-related changes in male urinary voiding. We also treated intact male, castrate male, and female mice with exogenous testosterone to determine the influence of androgen on voiding function. The three methods used to reduce prostate mass (castration, finasteride, and Pbsn4cre/+; R26RDta/+) changed voiding function from baseline but in a nonuniform manner. Castration feminized some aspects of male urinary physiology (making them more like intact female mice) while exogenous testosterone masculinized some aspects of female urinary physiology (making them more like intact male mice). Our results provide evidence that circulating testosterone is responsible in part for baseline sex differences in C57BL/6J mouse voiding function while prostate lobe mass in young, healthy adult mice has a lesser influence.
Keywords: animal model, cystometry, lower urinary tract symptoms, void spot assay
INTRODUCTION
Lower urinary tract symptoms (LUTS) are prevalent in adult men and women, negatively affect quality of life, and associate with depression (9, 37). It was once believed that LUTS in aging men derive almost exclusively from prostatic enlargement and urethral occlusion (36). The rationale supporting this belief was that prostatic volume (31) and LUTS prevalence and severity (14) increase with age (12) and that prostate resection generally alleviates LUTS in aging men (26). Recent studies have suggested a disease process more complex than previously appreciated. Prostatic volume does not strongly correlate with urodynamic patterns or LUTS severity when measured in the same cohort of men (1, 14). Some men with above average prostate volume do not experience clinically significant LUTS, while others with below average prostate volumes experience severe LUTS (10, 38). It is now becoming clear that male LUTS arise from multiple mechanisms in addition to prostatic enlargement (20). There is growing support for the roles of prostatic urethral collagen accumulation (23), inflammation (30), and smooth muscle hypercontracticility (5, 6) in progressive LUTS. There is also a growing need for validated model systems to rigorously test these new mechanisms and new pharmacological interventions.
The use of mice to study urinary voiding dysfunction is extensive and widespread. A search of the term “urology” in PubMed revealed 17,815 publications in 2018: 27 publications were studies in the dog, 575 in the rat, and 1,003 in the mouse. The practice of using male mice for human urinary voiding translational studies has been controversial because not all aspects of mouse and human prostate anatomy are the same. Similarities include anatomic location (base of the bladder) and a narrowing of the urethra in the region where prostatic ducts drain (prostatic urethra). Major differences include prostatic encapsulation and compaction. A portion of the mouse prostate gland lies within a muscular sphincter (rhabdosphinter), but the majority of prostate tissue branches into four bilaterally symmetrical prostate lobes (anterior, dorsal, lateral, and ventral) that are not encapsulated (32, 40). The human prostate gland, in contrast, is spherical and has a fibromuscular capsule (27). Several studies have demonstrated a clear influence of prostate pathologies on mouse urinary voiding behaviors (19, 22, 29, 33). Influence of the mouse prostate on baseline voiding function has not been specifically evaluated, in part because many methods to alter prostate mass also alter circulating testosterone concentrations.
To address these issues, we used multiple experimental groups and contemporary methods to determine the influence of androgens and prostatic mass on baseline voiding function, lower urinary tract anatomy, and histology. Histology will be described in more detail in a future paper (H. Ruetten, K. A. Wegner, H. L. Zhang, P. Wang, J. Sandhu, S. Sandhu, J. Morkrid, B. Mueller, Z. Wang, J. Macoska, R. E. Peterson, D. E. Bjorling, W. A. Ricke, P. C. Marker, and C. M. Vezina, unpublished observations). This report exclusively discusses anatomy and physiology and provides an expansive urinary physiology data set in wild-type C57BL/6J mice that can be mined for hypothesis generation and validation (see Tables 2–10). We first evaluated baseline voiding function in 9-wk-old male and female C57BL/6J control mice. We then used three different approaches to reduce prostatic mass in male mice: surgical castration, treatment with a steroid 5α-reductase inhibitor (finasteride), and a genetic approach to ablate prostate luminal epithelial cells. We also treated intact male, castrated male, and female mice with testosterone to control for the influence of androgen. We identified clear sex differences in baseline urinary function, even though relative bladder mass and volume do not significantly differ between male and female mice. Exogenous testosterone changed several parameters of female mouse urinary function in the direction of intact male mice (masculinization). Castration feminized male mouse urinary function, but neither finasteride treatment nor a genetically induced reduction in prostate mass feminized male mouse urinary function. We conclude that circulating testosterone is responsible, in part, for sex differences in baseline mouse voiding function while prostatic lobe mass plays a lesser role.
Table 2.
Update to male and female baseline urination characteristics
| Intact Male Mice | Intact Female Mice | P Value | |
|---|---|---|---|
| Gross assessment | |||
| n | 13 | 7 | |
| Body mass, g | 24.45 (SD 1.77) | 18.13 (SD 1.06) | <0.0001* |
| Bladder | |||
| Mass, % body mass | 0.15 (SD 0.02) (n = 6) | 0.13 (SD 0.03) | 0.1639 |
| Volume, mm3/g body mass) | 39.86 (SD 26.90) | 22.72 (SD 14.31) | 0.1366 |
| Void spot assay | |||
| n | 9 | 7 | |
| Count | 32.11 (SD 38.01) | 22.86 (SD 3.07) | 0.4890 |
| Total area, cm2 | 11.49 (SD 9.51) | 23.84 (SD 5.64) | 0.0089* |
| Percent area in center | 15.78 (SD 29.91) | 2.48 (SD 3.92) | 0.0524† |
| Percent area in corners | 61.14 (SD 39.78) | 65.12 (SD 25.64) | 0.8220 |
| Spots of a certain size (area) | |||
| 0−0.1 cm2 | 28.11 (SD 33.90) | 16.29 (SD 1.80) | 0.9740 |
| 0.1−0.25 cm2 | 1.00 (SD 1.41) | 1.57 (SD 1.14) | 0.2940 |
| 0.25−0.5 cm2 | 0.67 (SD 0.99) | 0.86 (SD 0.37) | 0.2550 |
| 0.5−1 cm2 | 0.67 (SD 1.11) | 0.43 (SD 0.53) | >0.9999 |
| 1−2 cm2 | 0.33 (SD 0.99) | 0.29 (SD 0.48) | 0.5500 |
| 2−3 cm2 | 0.44 (SD 1.02) | 0.29 (SD 0.77) | 0.8500 |
| 3−4 cm2 | 0.11 (SD 0.33) | 0 (SD 0) | >0.9999 |
| 4+ cm2 | 0.78 (SD 0.66) | 3.14 (SD 0.69) | <0.0010 |
| Cystometry | |||
| n | 6 | 6 | |
| Void duration | 0.64 (SD 0.20) | 0.41 (SD 0.05) | 0.0252* |
| Intervoid interval | 5.10 (SD 2.89) | 3.50 (SD 1.13) | 0.2430 |
| Baseline pressure | 3.61 (SD 0.76) | 2.68 (SD 1.98) | 0.2800 |
| Normalized threshold pressure | 3.35 (SD 0.78) | 9.36 (SD 2.84) | <0.0001* |
| Normalized peak void pressure | 18.37 (SD 5.05) | 25.35 (SD 2.72) | 0.0160* |
| Nonvoiding contractions | 2.26 (SD 1.76) | 2.4 (SD 1.49) | 0.8840 |
| Voided volume, ×10−2 | 7.60 (SD 4.26) | 4.72 (SD 1.25) | 0.1660 |
| Compliance, ×10−2 | 1.92 (SD 1.20) | 0.52 (SD 0.24) | 0.0025* |
| Volume flow rate, ×10−3 | 1.98 (SD 0.73) | 1.96 (SD 0.51) | 0.9390 |
| Mass-based flow rate | 23.70 (SD 10.02) | 16.80 (SD 9.72) | 0.2530 |
| Efficiency, % | 96.40 (SD 9.75) | 104.20 (SD 10.39) | 0.2080 |
Values are averages (SD); n, number of animals.
Statistically significant difference.
Changes that approached significance (trend).
Table 10.
Impact of testosterone on castrated male urinary physiology
| Castrated Male Mice | Castrated Male Mice With Testosterone | P Value | |
|---|---|---|---|
| Gross assessment | |||
| n | 14 | 8 | |
| Body mass, g | 21.51 (SD 1.53) | 23.65 (SD 1.95) | 0.0023* |
| Bladder volume, mm3/g body mass | 18.04 (SD 11.71) | 33.57 (SD 19.49) | 0.0192* |
| Prostate mass, % body mass | 0.03 (SD 0) | 0.21 (SD 0.06) | <0.0001* |
| Anterior mass, % body mass | 0.02 (SD 0) | 0.10 (SD 0.03) | <0.0001* |
| Ventral mass, % body mass | 0.01 (SD 0) | 0.05 (SD 0.03) | <0.0001* |
| Dorsal mass, % body mass | 0.01 (SD 0) | 0.04 (SD 0) | <0.0001* |
| Lateral mass, % body mass | 0.00 (SD 0) | 0.02 (SD 0) | 0.0009* |
| Seminal vesicle mass, % body mass | 0.04 (SD 0) | 0.35 (SD 0.20) | <0.0001* |
| Void spot assay | |||
| n | 11 | 4 | |
| Count | 42.00 (SD 20.23) | 50.30 (SD 29.80) | 0.5462 |
| Total area, cm2 | 20.50 (SD 7.66) | 22.60 (SD 17.24) | 0.7419 |
| Percent area in center | 2.34 (SD 2.21) | 7.35 (SD 11.86) | 0.8645 |
| Percent area in corners | 77.40 (SD 13.13) | 72.80 (SD 30.60) | 0.6846 |
| Spots of a certain size (area) | |||
| 0−0.1 cm | 38.10 (SD 19.37) | 44.00 (SD 26.40) | 0.6410 |
| 0.1−0.25 cm | 1.36 (SD 1.43) | 2.00 (SD 1.64) | 0.5509 |
| 0.25−0.5 cm | 0.64 (SD 0.50) | 1.00 (SD 2.00) | 0.5692 |
| 0.5−1 cm | 0.46 (SD 0.70) | 0 (SD 0) | 0.3956 |
| 1−2 cm | 0.18 (SD 0.40) | 0.25 (SD 0.50) | >0.9999 |
| 2−3 cm | 0 (SD 0) | 0.50 (SD 1.00) | 0.2667 |
| 3−4 cm | 0 (SD 0) | 0 (SD 0) | >0.9999 |
| 4+ cm | 1.27 (SD 0.46) | 2.50 (SD 1.74) | 0.1692 |
| Cystometry | |||
| n | 7−8 | 8 | |
| Void duration | 0.42 (SD 0.08) | 0.51 (SD 0.11) | 0.1049 |
| Intervoid interval | 2.77 (SD 0.34) | 3.00 (SD 1.27) | 0.6444 |
| Baseline pressure | 2.21 (SD 0.74) | 2.92 (SD 1.73) | 0.3111 |
| Normalized threshold pressure | 4.75 (SD 2.06) | 3.32 (SD 1.64) | 0.1437 |
| Normalized peak void pressure | 17.20 (SD 2.12) | 16.00 (SD 4.81) | 0.5400 |
| Nonvoiding contractions | 1.46 (SD 0.71) | 3.58 (SD 1.58) | 0.0039* |
| Voided volume, ×10−2 | 4.05 (SD 0.29) | 4.60 (SD 1.81) | 0.4184 |
| Compliance, ×10−2 | 0.89 (SD 0.53) | 1.34 (SD 0.88) | 0.1840 |
| Volume flow rate, ×10−3 | 1.69 (SD 0.42) | 1.53 (SD 0.51) | 0.5159 |
| Mass-based flow rate | 23.4 (SD 8.39) | 20.60 (SD 12.93) | 0.6126 |
| Efficiency, % | 96.8 (SD 8.75) | 90.74 (SD 16.43) | 0.3993 |
Values are averages (SD); n, number of animals.
Statistically significant difference.
MATERIALS AND METHODS
Mice.
All experiments were conducted under a protocol approved by the University of Wisconsin Animal Care and Use Committee and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were housed in Udel Polysulfone microisolator cages on racks or in Innocage disposable mouse cages on an Innorack; room lighting was maintained on a 12:12-h light-dark cycles, room temperature was maintained at 20.5 ± 5°C, and humidity was 30–70%. Mice were fed no. 8604 Teklad Rodent Diet (Harlan Laboratories, Madison, WI), and feed and water were available ad libitum. Cages contained corn cob bedding. All end point measurements were collected in 9-wk-old mice unless specified otherwise.
All mice used in this study were purchased from Jackson Laboratories (Bar Habor, ME) and included C57BL/6J (stock no. 000664), Tg(Pbsn-cre)4Prb/J (Pbsn4cre; stock no. 026662) bred onto the C57BL/6J background for four to five generations (42), B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (R26R-Tdtomato; Jax stock no. 007914) (24), and Gt(ROSA)26Sortm1(DTA)Jpmb/J (R26R-Dta; Jax stock no. 006331) (13). The genotype of mice with depleted prostatic epithelial cells was Pbsn4cre/+; R26RDta/+, and their respective littermate controls were Pbsn4cre/+; R26RTdTomato/+.
Castration/sham castration.
Castration was performed at 6 wk of age. Mice were anesthetized with isoflurane and given ketoprofen (0.5 mg/kg sc) as an analgesic. A midline incision was made in the scrotum, and the testes were either removed (castrate) or examined (sham controls). The scrotum was closed using a simple interrupted pattern.
Testosterone capsule preparation and implantation.
Capsules were prepared as previously described (17). Silastic tubing (Silastic Laboratory Tubing, 1.57 mm inside diameter × 3.18 mm outside diameter, catalog no. 508-008, Dow Corning) was cut to 16 mm. The wooden stick of cotton tipped applicators (catalog no. 23-400-100, Fisher Scientific) was cut into 5-mm pieces and inserted 3 mm into the tubing to plug the ends. Ten millimeters of the sham capsule were left empty. Ten millimeters of the testosterone capsule was filled with 4-androsten-17β-ol-3-one (testosterone, ≥99% pure, catalog no. A6950-000, Steraloids). Silastic capsules were sealed with silastic medical adhesive, type A (product no. A-100, Dow Corning, purchased from Factor II). Testosterone-filled silastic capsules have been shown to effectively increase testosterone in C57BL/6J mice when implanted as previously described (3).
Mice were anesthetized with isoflurane for silastic capsule implantation and given ketoprofen (0.5 mg/kg sc). An incision was made on the caudal aspect of the back just to the right of midline. Capsules were inserted parallel to the spine, and the incision was closed with wound clips.
Finasteride treatment.
Finasteride (J63454, Alfa Aesar, Ward Hill, MA) was dissolved in 100% ethanol and diluted in corn oil to make a 10% ethanol/90% corn oil dosing solution. The solution was stored at 4°C for the duration of the experiment. Mice were given finasteride (50 µl of a 40 µg/µl solution) daily via oral gavage.
Void spot assay.
We followed the recommended guidelines of reporting void spot assay (VSA) data (11, 16, 39). VSA was performed in the vivarium where mice were housed 1 day before cystometry (CMG) and euthanasia. Whatman grade 540 (no. 057163-W, Fisher Scientific) filter papers (27 × 16 cm) were placed in the bottom of Udel Polysulfone microisolator cages. Mice were placed in the cage (singly housed) with food ad libitum but no water for 4 h starting from 8 to 11 AM GMT. VSA was performed once a week starting at 6 wks of age, allowing for three acclimation sessions before the session at 9 wk of age, which was used for analysis. Filter papers were dried and imaged with an Autochemi AC1 Darkroom ultraviolet imaging cabinet (UVP, Upland, CA) equipped with an Auto Chemi Zoom lens 2UV and an epi-illuminator. Image capture settings were adjusted using UVP VisonWorksLS image-acquisition software. Images were captured using an ethidium bromide filter set (570–640 nm) and 365-nm epi-illumination. Void Whizzard was downloaded from http://imagej.net/Void_Whizzard and run according to the user guide (39). Analyzed parameters included: total spot count, total void area (in cm2), percent area in the center of the paper, percent area in the corners of the paper, and mass distribution of spots (0–0.1, 0.1–0.25, 0.25–0.5, 0.5–1, 1–2, 2–3, 3–4, and 4+ cm).
Anesthetized CMG.
CMG was performed with minimal alterations to previously published protocols (4, 34). Mice were anesthetized with urethane (1.43 g/kg sc). Thirty minutes after mice had been dosed urethane, an incision was made in the ventral abdomen to expose the bladder. Bladder length and diameter were measured for volume calculation. A purse-string suture was placed in the bladder dome. Polyethylene cystostomy tubing (PE-50, outer diameter: 0.58 mm and inner diameter: 0.28 mm) was inserted into the bladder through the center of the suture and purse string secured to hold the tubing in place with 2–3 mm of tubing within the bladder. The abdominal wall and skin were closed separately in a simple interrupted pattern. The exterior tubing was secured to the ventral abdominal skin with two simple interrupted sutures. Mice were placed on a heat pad for 1 h after the procedure.
The exposed tube was connected to a three-way stopcock, and the other two arms of the stopcock were connected to an infusion pump (Harvard Apparatus, Holliston, MA) and pressure transducer (Memscap). Intravesical pressure was recorded continuously using a PowerLab data collection system (ADInstruments, Colorado Springs, CO). Room temperature sterile saline (0.9%) was infused into the bladder at a rate of 0.8 ml/h.
Mice were placed in lateral recumbency above a force transducer (model FT03, Grass Instruments) with a three-dimensional printed urine collection funnel. The force transducer was calibrated with known volumes of saline to create a pressure-volume conversion. The mass of voided urine was recorded continuously using PowerLab.
At least 1 h of voiding activity was recorded. Three to five consecutive voids, occurring after stabilization of micturition cycles, were used for analyses. Multiple parameters were measured, including void duration, intervoid interval, baseline pressure, normalized threshold pressure, normalized peak void pressure, number of nonvoiding contractions, voided volume, compliance, volume flow rate, mass-based flow rate, and efficiency (calculations used for the analysis of cystometric tracings are shown in Fig. 1 and Table 1).
Fig. 1.
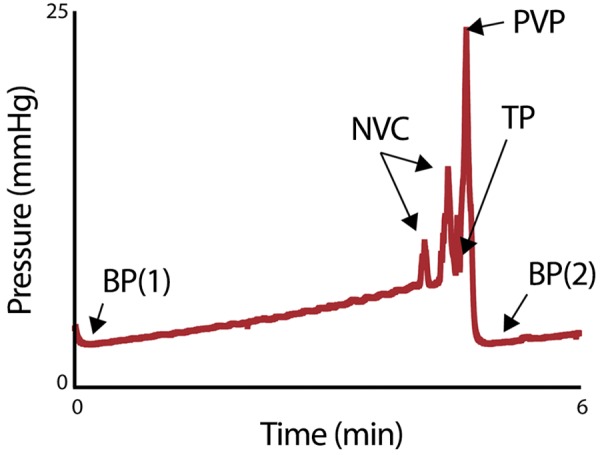
A cystometry trace with annotated features that were used to calculate the cystometry end points shown in Table 1. BP(1) and BP(2) are baseline pressures: the lowest bladder pressure before bladder filling [BP(1)] and immediately after voiding is complete [BP(2)]. NVC indicates a nonvoiding contraction, a bladder pressure spike occurring without urination. TP is the threshold pressure, the pressure when voiding initiates. PVP is the peak void pressure, the maximal bladder pressure achieved during voiding.
Table 1.
Formulas for calculated cystometry parameters
| Cystometry Parameter | Abbreviation | Units | Description |
|---|---|---|---|
| Baseline pressure | BP | mmHg | Lowest pressure of trace immediately after void; also defines starting point of new void (see Fig. 1, BP) |
| Number of nonvoiding contractions | nNVC | No units | Number of spikes in pressure before voiding that did not result in urine excretion (see Fig. 1, NVC) |
| Threshold pressure | TP | mmHg | Pressure at the start of urination (see Fig. 1, TP) |
| Peak void pressure | PVP | mmHg | Greatest pressure achieved during void (see Fig. 1, PVP) |
| Normalized peak void pressure | NPVP | mmHg | PVP − BP |
| Normalized threshold pressure | NTP | mmHg | TP − BP |
| Void duration | VD | min | [time at BP of void(n+1)] − (time of TP of voidn) |
| Voided volume | VV | ml | (mass of urine trace after trace) − (mass of urine trace before void) |
| Intervoid interval | IVI | min | [time at PVP of void(n+1)] − (time at PVP of voidn) |
| Infusion rate | IR | ml/min | Rate at which the pump infuses saline |
| Infusion duration | ID | min | TP − BP |
| Infused volume | IV | ml | IR × ID |
| Efficiency | E | % | VV/IV |
| Compliance | C | ml/mmHg | IV/(TP − BP) |
| Volume flow rate | VFR | ml/min | VV/VD |
| Mass-based flow rate | MBFR | mg/min | Slope of urine mass trace during active voiding |
Statistical analysis.
Statistical analyses were performed with Graph Pad Prism 8.0.2 (Graphpad Software, La Jolla, CA). A Shapiro-Wilk test was used to test for normality, and transformation was applied to normalize data when possible. The F-test was used to test for homogeneity of variance for pairwise comparisons. Welsh’s correction was applied when variances was unequal. When variance was equal, comparisons between two groups were made using Student’s t-test. The Mann-Whitney test was applied when data could not be normalized through transformation. Bartlett’s test was used to test for homogeneity of variance for multiple comparisons. Welsh’s ANOVA was applied when variance was unequal followed by Tamhane’s T2 multiple-comparisons test. When variance was equal, comparisons between groups were made using ordinary one-way ANOVA followed by Sidak’s multiple-comparisons test. If data could not be normalized through transformation, the Kruskal-Wallis test was applied with Dunn’s multiple-comparisons test. P values of <0.05 were considered statistically significant. We have also noted changes that approach significance (P < 0.10) when they are consistent with our hypothesis. These changes are indicated in the tables and referred to in text as “trends” accompanied by the appropriate P value. All numeric data are presented as means (SD).
RESULTS AND DISCUSSION
Male and female mouse baseline urinary voiding physiology characteristics.
We evaluated several voiding parameters in control (defined as sham castrated and sham testosterone-implanted intact) male and control (defined as sham testosterone-implanted intact) female mice to determine the impact of sex on urinary voiding physiology (Fig. 2A and Fig. 3A and Table 2). Body mass was greater in male mice than in female mice, consistent with previous findings in C57BL/6J mice (4). Relative bladder mass and volumes were determined by normalizing to body mass. Male and female relative bladder weight and volume did not significantly differ, consistent with a previous study (7).
Fig. 2.
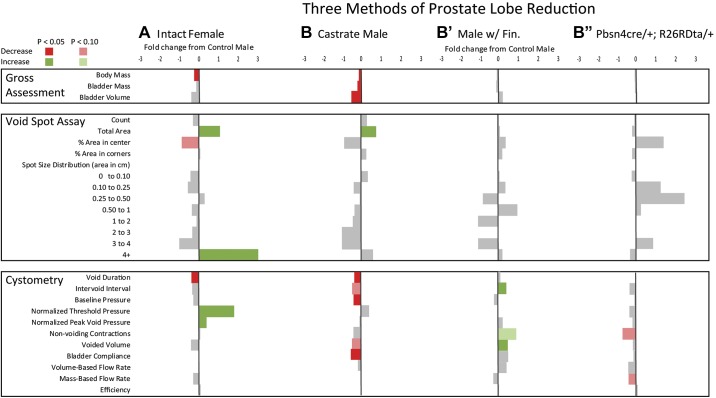
Prostate mass reduction minimally and variably impacts baseline male mouse urinary physiology. Summary statistics for anatomic and physiological differences across experimental groups (B, B′, and B″) evaluating prostate lobe mass reductions compared with male to female baseline differences (A) are shown. n = 6–13 intact male mice (with sham castration and sham testosterone capsule implantation) and 6–7 female mice (with sham testosterone capsule implantation) were compared to determine baseline sex differences. B: castrated male mice (Castrate Male) were compared with sham castrated male mice to determine if castration feminized (directional changes in characteristics consistent with female mice compared with male mice) mouse urinary function. B′: mice treated with the steroid 5α-reductase inhibitor finasteride (Males w/ Fin) were compared with mice treated with finasteride carrier to see if a second method of prostate size reduction was consistent with changes due to castration or feminization of urinary function. B″: male mice genetically engineered to produce diphtheria toxin in luminal epithelial cells, resulting in their depletion (Pbsn4cre/+; R26RDta/+), were compared with male mice genetically engineered to produce red fluorescent protein in their luminal epithelial cells to see if a third method of prostate size reduction was consistent with castration, finasteride treatment, or feminization of urinary function. All results are mean fold differences relative to their respective male control values. Differences that were significantly higher than intact male mice are shown in green and those that were lower than intact male mice are shown in red. End points that did not significantly differ from intact male mice are shown in gray. The numeric fold difference relative to intact male mice is indicated by the bar size according to the legend at the top of the figure. Female urinary physiology was different from male urinary physiology at baseline. Castration caused some changes consistent with feminization of urinary function. Three different methods of prostate mass reduction held no consistent effects on urinary physiology. Statistical analyses were performed with Graph Pad Prism 8.0.2 (Graphpad Software, La Jolla, CA). A Shapiro-Wilk test was used to test for normality, and transformation was applied to normalize data when possible. The F-test was used to test for homogeneity of variance for pairwise comparisons. Welsh’s correction was applied when variances was unequal. When variance was equal, comparisons between two groups were made using Student’s t-test. The Mann-Whitney test was applied when data could not be normalized through transformation.
Fig. 3.
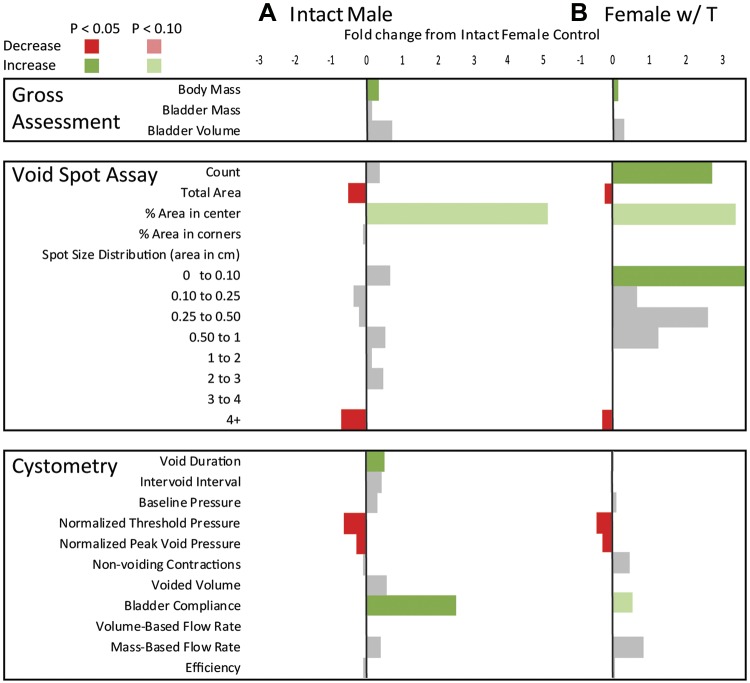
Exogenous testosterone supplementation masculinizes female urinary physiology. Summary statistics for anatomic and physiological differences across experimental groups evaluating if exogenous testosterone supplementation in female mice would masculinize urinary function are shown. A: 6–13 intact male mice (with sham castration and sham testosterone capsule implantation) and 6–7 female mice (with sham testosterone capsule implantation) were compared for baseline differences in urinary function. B: 5–7 female mice were implanted with 1-cm Silastic capsules containing crystalline testosterone (Female w/ T) for 3 wk and compared with 6–7 female mice with sham testosterone capsule implants. All results are mean fold differences relative to intact female control values. Differences that were significantly higher than intact female mice are shown in green and those that were lower than intact female mice are shown in red. End points that did not significantly differ from intact female mice are shown in gray. The numeric fold difference relative to intact female mice is indicated by the bar size according to the legend at the top of the figure. Male urinary physiology was different from female urinary physiology at baseline. The female voiding parameters affected by exogenous testosterone included many of the same parameters that distinguished male from female voiding function. Statistical analyses were performed with Graph Pad Prism 8.0.2 (Graphpad Software, La Jolla, CA). A Shapiro-Wilk test was used to test for normality, and transformation was applied to normalize data when possible. The F-test was used to test for homogeneity of variance for pairwise comparisons. Welsh’s correction was applied when variances was unequal. When variance was equal, comparisons between two groups were made using Student’s t-test. The Mann-Whitney test was applied when data could not be normalized through transformation.
We used VSA as a first approach to evaluate voiding behaviors. The VSA procedure has been refined considerably in recent years. Methodology improvements minimize experimental bias, new software enables rigorous and unbiased assessment (11, 39), and the 12 end-point measurements collected in this study confer a more robust and multidimensional perspective than the five or less measurements collected in prior comparisons of male and female C57BL/6J mice (4, 7). We identified several differences between control male and female mice. Female mice deposited more total urine (measured by area, in cm2) in a 4-h period than male mice. Our findings differed from those of a previous study (4) involving older mice and using a method of VSA analysis that excluded urine spots of <0.66 cm2. It is worth noting that we have recently shown that small void spots are not caused by mice tracking urine from deposited voids, a rationale for their previous exclusion (39).
Previous studies that excluded small and large void spots, or spots in cage corners, did not observe male-female differences in voiding patterns (4, 7). When we evaluated the spatial distribution of voids (center, corners, and in between), we found that female mice trended toward depositing less urine in the center of the cage (P = 0.0524). We also evaluated the categorical distribution of VSA spots (0–0.1, 0.1–0.25, 0.25–0.5, 0.5–1, 1–2, 2–3, 3–4, and 4+ cm2) and found that female mice deposited a greater proportion of large voids (4+ cm2) than male mice.
We next used anesthetized CMG to evaluate voiding function. This method has also evolved in recent years. New practices include 1) a novel method of urethane delivery (subcutaneously) that minimizes spontaneous body movements and their associated influence on intravesicular pressures, 2) computer-generated traces that improve the accuracy of measured trace characteristics, and 3) simultaneous collection of intravesical pressure and voided urine mass allowing for the calculation of additional void characteristics. We now routinely collect 11 CMG end-point measurements compared with the four to five measurements collected in previous studies. We found that peak void pressures were higher in female mice than in male mice, consistent with a previous study (4). Also consistent with previous studies, we did not observe sex differences in intervoid interval, number of nonvoiding contractions, and voided volume (4, 7). Novel findings from this study are that female mice have higher threshold pressures, shorter void durations, and less bladder compliance than male mice.
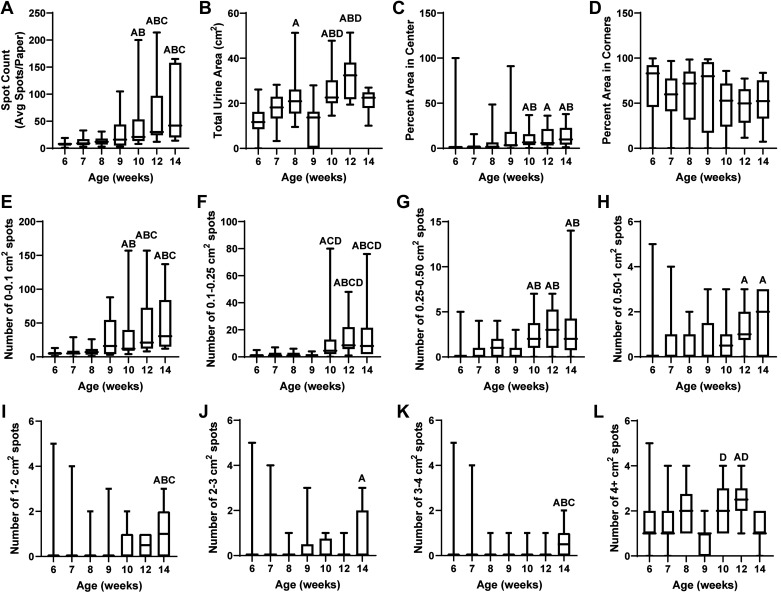
The mouse urinary voiding behaviors reported in this study are specific to 9-wk-old C57BL/6J mice and are not necessarily generalizable to other mouse strains, ages, or health statuses. Sex differences and mouse urinary phenotype have been previously reported to be strain specific (4). Age and disease state in a genetically, surgically, or otherwise altered mice could also impact sex differences in mice. We have previously documented differences in voiding between 3-, 6-, and 9-wk-old male C57BL/6J mice (16). We recently documented aging-related voiding dysfunction between 2- and 24-mo-old male C57BL/6J mice (22). Here, we honed in on urinary function in sexually mature young adult (1.5 to 3.5-mo-old) mice. We performed VSA on 6-, 7-, 8-, 9-, 10-, 12-, and 14-wk old male C57BL/6J mice, and all measured end points changed with age (Fig. 4 and Table 3). These age-related changes in healthy adult male mice could be due to changing hormones, changes in body, bladder, or prostate mass, or changes in behavior. Therefore, age is a critical factor to control for in studies of urinary function.
Fig. 4.
Aging (from 6 to 14 wk old) alters void spot assay (VSA) parameters in male C57BL/6J mice. Thirty mice underwent VSA at 6 and 7 wk, 20 mice underwent VSA at 8 and 10 wk, and 10 mice underwent VSA at 9, 12, and 14 wk of age. The relationship between age and each VSA parameter is shown as box and whisker plots. Age groups were compared, with significant differences as follows: Afrom 6-wk-old mice, Bfrom 7-wk-old mice, Cfrom 8-wk-old mice, and Dfrom 9 wk-old-mice. Six- to nine-week-old mice and ten- to fourteen-week-old mice were statistically similar in VSA characteristics. Spot count (A), total urine area (B), percent area in the center (C), and all spot sizes (E−L) were altered with age. Bartlett’s test was used to test for homogeneity of variance for multiple comparisons. Welsh’s ANOVA was applied when variance was unequal followed by Tamhane’s T2 multiple-comparisons test. When variance was equal, comparisons between groups were made using ordinary one-way ANOVA followed by Sidak’s multiple-comparisons test. If data could not be normalized through transformation, the Kruskal-Wallis test was applied with Dunn’s multiple-comparisons test.
Table 3.
Impact of age on void spot assay characteristics
| 6-wk-old Group | 7-wk-old Group | 8-wk-old Group | 9-wk-old Group | 10-wk-old Group | 12-wk-old Group | 14-wk-old Group | |
|---|---|---|---|---|---|---|---|
| Count | 8.33 (SD 4.82) | 11.97 (SD 8.22) | 13.20 (SD 7.42) | 32.10 (SD 40.16) | 53.45 (SD 61.09)*† | 65.90 (SD 64.61)*†‡ | 75.10 (SD 61.28)*†‡ |
| Total area, cm2 | 12.68 (SD 5.86) | 17.34 (SD 6.68) | 21.89 (SD 10.20)* | 11.50 (SD 10.02) | 25.91 (SD 8.41)*†§ | 31.69 (SD 9.74)*†§ | 21.01 (SD 5.38) |
| Percent area in center | 5.26 (SD 18.18) | 2.41 (SD 3.78) | 6.31 (SD 11.49) | 15.80 (SD 31.53) | 10.74 (SD 11.23)*† | 11.38 (SD 12.49)* | 14.08 (SD 12.90)*† |
| Percent area in corners | 69.67 (SD 32.53) | 59.22 (SD 23.55) | 57.87 (SD 33.32) | 61.10 (SD 42.06) | 48.17 (SD 27.91) | 47.64 (SD 22.33) | 51.56 (SD 25.90) |
| Spots of a certain size (area) | |||||||
| 0−0.1 cm2 | 5.37 (SD 3.56) | 7.57 (SD 6.41) | 8.10 (SD 5.77) | 28.10 (SD 35.73) | 35.45 (SD 44.27)*† | 44.20 (SD 49.21)*†‡ | 50.20 (SD 44.75)*†‡ |
| 0.1−0.25 cm2 | 1.10 (SD 1.26) | 1.80 (SD 1.92) | 1.55 (SD 1.74) | 1.00 (SD 1.49) | 11.95 (SD 19.68)*‡§ | 14.30 (SD 14.14)*†‡§ | 16.20 (SD 22.55)*†‡§ |
| 0.25−0.5 cm2 | 0.23 (SD 0.60) | 0.37 (SD 0.88) | 1.00 (SD 1.16) | 0.67 (SD 1.04) | 2.25 (SD 1.70)*† | 3.00 (SD 2.37)*† | 3.30 (SD 4.08)*† |
| 0.5−1 cm2 | 0.20 (SD 0.38) | 0.47 (SD 0.88) | 0.45 (SD 0.63) | 0.67 (SD 1.17) | 0.75 (SD 0.89) | 1.20 (SD 0.92)* | 1.60 (SD 1.26)* |
| 1−2 cm2 | 0.07 (SD 0.27) | 0.23 (SD 0.88) | 0.15 (SD 0.49) | 0.33 (SD 1.04) | 0.50 (SD 0.76) | 0.50 (SD 0.54) | 1.10 (SD 1.11)*†‡ |
| 2−3 cm2 | 0 (SD 0) | 0.07 (SD 0.27) | 0.05 (SD 0.22) | 0.44 (SD 1.08) | 0.25 (SD 0.45) | 0.10 (SD 0.32) | 0.80 (SD 1.14)* |
| 3−4 cm2 | 0.03 (SD 0.16) | 0.07 (SD 0.27) | 0.05 (SD 0.22) | 0.11 (SD 0.35) | 0.15 (SD 0.36) | 0.10 (SD 0.32) | 0.60 (SD 0.70)*†‡ |
| 4+ cm2 | 1.33 (SD 0.66) | 1.40 (SD 0.77) | 1.85 (SD 0.94) | 0.78 (SD 0.70) | 2.15 (SD 0.98)§ | 2.50 (SD 0.85)*§ | 1.30 (SD 0.66) |
Values are averages (SD); n, number of animals.
Significant difference from the 6-wk-old group;
significant difference from the 7-wk-old group;
significant difference from the 8-wk-old group;
significant difference from the 9-wk-old group.
Prostate mass reduction minimally impacts baseline male mouse urinary physiology.
We hypothesized that the prostate may contribute to sex differences in C57BL/6J mouse voiding behaviors. We used three strategies to reduce prostate mass and evaluate the resulting impact on voiding function: castration, 5α-reductase inhibitor (finasteride) treatment, and genetic prostatic luminal cell ablation. We specifically tested whether our three methods reduced prostate mass as expected, caused a consistent directional change in VSA and/or CMG voiding characteristics, and whether the directional change was consistent with “feminization” of urinary physiology (i.e., same directional change as female mice compared with male mice).
The influence of castration on male voiding function is shown in Fig. 2B and Table 4. Castration, more than any other method to reduce prostate lobe mass, caused the greatest magnitude of prostate lobe mass reduction but also reduced body, bladder, seminal vesicle mass, and bladder volume, as previously reported in Swiss mice (25, 28). Castrated (with sham testosterone implant) and control (sham castrated and sham testosterone-implanted intact) male mice did not overwhelmingly differ in VSA-measured voiding function, but there were some differences consistent with “feminization” of male urinary physiology. Castrated male mice, like female mice, deposited more urine in a 4-h monitoring period compared with control male mice. This is consistent with a previous finding of increased urine mass/time in castrated Swiss mice (28). Castrated mice were similar to control male mice in the percentage of voids deposited in the center of the cage and the number of 4+-cm2 spots. Castrated male and intact female mice had a shorter void duration and lower bladder compliance than control male mice. However, castrated male mice had lower baseline bladder pressure than control male mice, an end point that distinguished them from female mice. Additionally, castrated male mice trended toward a shorter intervoid interval and lower voided volume than control male mice (P = 0.0968 and P = 0.0977, respectively).
Table 4.
Impact of castration on male urinary physiology
| Intact Male Mice | Castrated Male Mice | P Value | |
|---|---|---|---|
| Gross assessment | |||
| n | 13 | 14 | |
| Body mass, g | 24.45 (SD 1.77) | 21.51 (SD 1.53) | <0.0001* |
| Bladder | |||
| Mass, % body mass) | 0.15 (SD 0.02) | 0.12 (SD 0) | 0.0190* |
| Volume, mm3/g body mass | 39.86 (SD 26.90) | 18.04 (SD 11.71) | 0.0041* |
| Prostate mass, % body mass | 0.20 (0.04) | 0.03 (SD 0) | <0.0001* |
| Anterior mass, % body mass | 0.09 (SD 0) | 0.02 (SD 0) | <0.0001* |
| Ventral mass, % body mass | 0.04 (SD 0) | 0.01 (SD 0) | <0.0001* |
| Dorsal mass, % body mass | 0.04 (SD 0) | 0.01 (SD 0) | <0.0001* |
| Lateral mass, % body mass | 0.01 (SD 0) | 0.00 (SD 0) | 0.0003* |
| Seminal vesicle mass, % body mass | 0.62 (SD 0.14) | 0.04 (SD 0) | <0.0001* |
| Void spot assay | |||
| n | 9 | 11 | |
| Count | 32.11 (SD 38.01) | 42.00 (SD 20.23) | 0.1025 |
| Total area, cm2 | 11.49 (SD 9.51) | 20.50 (SD 7.66) | 0.0301* |
| Percent area in center | 15.78 (SD 29.91) | 2.34 (SD 2.21) | 0.1567 |
| Percent area in corners | 61.14 (SD 39.78) | 77.40 (SD 13.13) | 0.2177 |
| Spots of a certain size (area) | |||
| 0−0.1 cm2 | 28.11 (SD 33.90) | 38.10 (SD 19.37) | 0.1472 |
| 0.1−0.25 cm2 | 1.00 (SD 1.41) | 1.36 (SD 1.43) | 0.5651 |
| 0.25−0.5 cm2 | 0.67 (SD 0.99) | 0.64 (SD 0.50) | 0.6534 |
| 0.5−1 cm2 | 0.67 (SD 1.11) | 0.46 (SD 0.70) | 0.9591 |
| 1−2 cm2 | 0.33 (SD 0.99) | 0.18 (SD 0.40) | >0.9999 |
| 2−3 cm2 | 0.44 (SD 1.02) | 0 (SD 0) | 0.1895 |
| 3−4 cm2 | 0.11 (SD 0.33) | 0 (SD 0) | 0.4500 |
| 4+ cm2 | 0.78 (SD 0.66) | 1.27 (SD 0.46) | 0.1328 |
| Cystometry | |||
| n | 6 | 7–8 | |
| Void duration | 0.64 (SD 0.20) | 0.42 (SD 0.08) | 0.0311* |
| Intervoid interval | 5.10 (SD 2.89) | 2.77 (SD 0.34) | 0.0968† |
| Baseline pressure | 3.61 (SD 0.76) | 2.21 (SD 0.74) | 0.0040* |
| Normalized threshold pressure | 3.35 (SD 0.78) | 4.75 (SD 2.06) | 0.1087 |
| Normalized peak void pressure | 18.37 (SD 5.05) | 17.20 (SD 2.12) | 0.6145 |
| Nonvoiding contractions | 2.26 (SD 1.76) | 1.46 (SD 0.71) | 0.3254 |
| Voided volume, ×10−2 | 7.60 (SD 4.26) | 4.05 (SD 0.29) | 0.0977† |
| Compliance, ×10−2 | 1.92 (SD 1.20) | 0.89 (SD 0.53) | 0.0359* |
| Volume flow rate, ×10−3 | 1.98 (SD 0.73) | 1.69 (SD 0.42) | 0.3871 |
| Mass-based flow rate | 23.70 (SD 10.02) | 23.4 (SD 8.39) | 0.9452 |
| Efficiency, % | 96.40 (SD 9.75) | 96.8 (SD 8.75) | 0.9381 |
Values are averages (SD); n, number of animals.
Statistically significant difference.
Changes that approached significance (trend).
We next reduced prostate mass by treating mice for 2 wk with finasteride (100 mg/kg BID via oral gavage) to block the conversion of testosterone to the more potent ligand dihydrotestosterone and reduce androgen concentration in a nonsurgical manner. This dosing paradigm reflects that of a previous study (18) in rats, which reported 24 h of sustained serum finasteride concentrations after a single 100 mg/kg oral dose and a prostate mass reduction 2 wk after treatment. The results are shown in Fig. 2C and Table 5. Male mice treated with finasteride had significantly smaller seminal vesicles and smaller anterior, ventral, and lateral prostates than control male mice (treated with finasteride carrier). Dorsal prostate mass did not differ between groups. VSA-measured voiding function in male mice with finasteride did not differ from control male mice. CMG-measured end points differed between groups [male mice with finasteride had a significantly larger intervoid interval and voided volume and trended (P = 0.0755) toward more nonvoiding contractions than control male mice]. The finasteride-mediated directional changes in voiding function were different than those caused by castration. We treated female mice (which have a very low baseline level of testosterone) with finasteride and performed VSA and CMG to determine the impact of finasteride on female urinary function. Finasteride caused some changes in VSA-measured voiding function in female mice but no changes in CMG-measured function (Table 6).
Table 5.
Impact of finasteride on male urinary physiology
| Male Mice With Oil | Male Mice With Finasteride | P Value | |
|---|---|---|---|
| Gross assessment | |||
| n | 6 | 9 | |
| Body mass, g | 24.67 (SD 0.88) | 23.53 (SD 2.19) | 0.2562 |
| Bladder | |||
| Mass, % body mass | 0.13 (SD 0.02) | 0.12 (SD 0.03) | 0.3701 |
| Volume, mm3/g body mass | 33.71 (SD 22.66) | 41.35 (SD 19.83) | 0.5016 |
| Prostate mass, % body mass | 0.21 (SD 0.02) | 0.14 (SD 0.03) | 0.0009* |
| Anterior mass, % body mass | 0.11 (SD 0.02) | 0.07 (SD 0) | 0.0004* |
| Ventral mass, % body mass | 0.05 (SD 0.02) | 0.04 (SD 0) | 0.0467* |
| Dorsal mass, % body mass | 0.03 (SD 0) | 0.03 (SD 0) | 0.3465 |
| Lateral mass, % body mass | 0.02 (SD 0) | 0.01 (SD 0) | 0.0292* |
| Seminal vesicle mass, % body mass | 0.72 (SD 0.07) | 0.33 (SD 0.09) | <0.0001* |
| Void spot assay | |||
| n | 7 | 9 | |
| Count | 23.20 (SD 13.02) | 24.10 (SD 30.3) | 0.1692 |
| Total area, cm2 | 17.00 (SD 11.45) | 18.40 (SD 13.23) | 0.8209 |
| Percent area in center | 8.82 (SD 19.95) | 12.10 (SD 33) | 0.8845 |
| Percent area in corners | 48.90 (SD 28.84) | 58.50 (SD 39.9) | 0.3610 |
| Spots of a certain size (diameter) | |||
| 0−0.1 cm | 19.90 (SD 11.54) | 21.00 (SD 27.87) | 0.8658 |
| 0.1−0.25 cm | 0.89 (SD 0.93) | 1.22 (SD 1.92) | >0.9999 |
| 0.25−0.5 cm | 0.89 (SD 0.82) | 0.22 (SD 0.45) | 0.1312 |
| 0.5−1 cm | 0.11 (SD 0.29) | 0.22 (SD 0.66) | >0.9999 |
| 1−2 cm | 0.11 (SD 0.29) | 0 (SD 0) | >0.9999 |
| 2−3 cm | 0.11 (SD 0.29) | 0.11 (SD 0.33) | >0.9999 |
| 3−4 cm | 0.11 (SD 0.29) | 0 (SD 0) | >0.9999 |
| 4+ cm | 1.11 (SD 0.82) | 1.33 (SD 0.99) | 0.8013 |
| Cystometry | |||
| n | 9 | 9 | |
| Void duration | 0.63 (SD 0.21) | 0.70 (SD 0.39) | 0.8371 |
| Intervoid interval | 3.35 (SD 1.32) | 4.74 (SD 1.26) | 0.0394 |
| Baseline pressure | 3.96 (SD 0.48) | 3.13 (SD 1.53) | 0.1590 |
| Normalized threshold pressure | 3.64 (SD 2.01) | 3.65 (SD 1.68) | 0.9965 |
| Normalized peak void pressure | 13.00 (SD 2.97) | 15.90 (SD 5.22) | 0.2011 |
| Nonvoiding contractions | 1.21 (SD 0.66) | 2.33 (SD 0.59) | 0.0755† |
| Voided volume, ×10−2 | 4.24 (SD 2.07) | 6.44 (SD 1.41) | 0.0168 |
| Compliance, ×10−2 | 1.24 (SD 0.66) | 1.89 (SD 0.93) | 0.1280 |
| Volume flow rate, ×10−3 | 1.31 (SD 0.81) | 1.87 (SD 0.69) | 0.1377 |
| Mass-based flow rate | 22.80 (SD 10.89) | 17.10 (SD 5.22) | 0.1497 |
| Efficiency, % | 108.00 (SD 61.2) | 110.00 (SD 20.55) | 0.8371 |
Values are averages (SD); n, number of animals.
Statistically significant difference.
Changes that approached significance (trend).
Table 6.
Impact of finasteride on female urinary physiology
| Female Mice With Oil | Female Mice With Finasteride | P Value | |
|---|---|---|---|
| Gross assessment | |||
| n | 7 | 5 | |
| Body mass, g | 18.49 (SD 1.11) | 17.50 (SD 0.69) | 0.0953† |
| Bladder | |||
| Mass, % body mass | 0.12 (SD 0) | 0.12 (SD 0.02) | >0.9999 |
| Volume, mm3/g body mass | 17.82 (SD 10.27) | 17.27 (SD 7.24) | 0.9162 |
| Void spot assay | |||
| n | 7 | 5 | |
| Count | 23.30 (SD 15.16) | 12.20 (SD 7.06) | 0.1337 |
| Total area, cm2 | 22.20 (SD 7.20) | 11.70 (SD 3.38) | 0.0083* |
| Percent area in center | 0.65 (SD 0.66) | 0.49 (SD 0.74) | 0.1698 |
| Percent area in corners | 67.50 (SD 33.34) | 73.70 (SD 18.85) | 0.7023 |
| Spots of a certain size (diameter) | |||
| 0−0.1 cm | 17.90 (SD 13.28) | 8.50 (SD 5.99) | 0.1464 |
| 0.1−0.25 cm | 1.29 (SD 1.88) | 0.50 (SD 0.76) | 0.6166 |
| 0.25−0.5 cm | 0.14 (SD 0.37) | 0.17 (SD 0.38) | >0.9999 |
| 0.5−1 cm | 0.29 (SD 0.48) | 0.33 (SD 0.47) | >0.9999 |
| 1−2 cm | 0.29 (SD 0.48) | 0.50 (SD 0.76) | 0.7797 |
| 2−3 cm | 0.14 (SD 0.37) | 0.83 (SD 0.69) | 0.0862† |
| 3−4 cm | 0.71 (SD 1.88) | 0 (SD 0) | >0.9999 |
| 4+ cm | 2.57 (SD 0.78) | 1.33 (SD 0.47) | 0.0087* |
| Cystometry | |||
| n | 7 | 5 | |
| Void duration | 0.51 (SD 0.13) | 0.47 (SD 0.07) | 0.5419 |
| Intervoid interval | 2.90 (SD 1.48) | 2.49 (SD 0.67) | 0.5803 |
| Baseline pressure | 5.70 (SD 3.31) | 4.88 (SD 1.86) | 0.6330 |
| Normalized threshold pressure | 11.3 (SD 4.95) | 8.22 (SD 1.68) | 0.2212 |
| Normalized peak void pressure | 25.2 (SD 3.49) | 21.70 (SD 3.08) | 0.0965† |
| Nonvoiding contractions | 3.63 (SD 2.96) | 1.64 (SD 1.05) | 0.3422 |
| Voided volume, ×10−2 | 4.10 (SD 1.69) | 3.72 (SD 0.94) | 0.6595 |
| Compliance, ×10−2 | 0.29 (SD 0.10) | 0.37 (SD 0.13) | 0.2640 |
| Volume flow rate, ×10−3 | 1.39 (SD 0.53) | 1.37 (SD 0.49) | 0.9335 |
| Mass-based flow rate | 17.90 (SD 23.86) | 7.99 (SD 2.46) | 0.4346 |
| Efficiency, % | 111.00 (SD 12.04) | 114.00 (SD 14.4) | 0.7246 |
Values are averages (SD); n, number of animals.
Statistically significant difference.
Changes that approached significance (trend).
To address the impact of reducing prostate mass without surgery or hormone treatment, we used a genetic strategy to deplete prostatic luminal epithelial cells (Pbsn4cre/+; R26RDta/+). The results are shown in Fig. 2D and Table 7. Cre-driven epithelial cell death reduced dorsal and lateral prostate mass without significantly changing the mass of other prostate lobes or seminal vesicle. There were no statistical differences in VSA- or CMG-measured voiding function between Pbsn4cre/+; R26RDta/+ mice and their genetic controls (Pbsn4cre/+; R26RTd/+).
Table 7.
Impact of genetic prostatic luminal cell ablation on male urinary physiology
| Pbsn4cre/+;R26RTd/+ Mice | Pbsn4cre/+;R26RDta/+ Mice | P Value | |
|---|---|---|---|
| Gross assessment | |||
| n | 8 | 4 | |
| Body mass, g | 25.58 (SD 1.67) | 24.35 (SD 1.6) | 0.1414 |
| Bladder | |||
| Mass, % body mass) | 0.15 (SD 0.03) | 0.14 (SD 0) | 0.5442 |
| Volume, mm3/g body mass | 35.52 (SD 17.42) | 36.98 (SD 20.22) | 0.8992 |
| Prostate mass, % body mass | 0.22 (SD 0.06) | 0.20 (SD 0.02) | 0.3434 |
| Anterior mass, % body mass | 0.11 (SD 0) | 0.12 (SD 0.02) | 0.4629 |
| Ventral mass, % body mass | 0.05 (SD 0.03) | 0.06 (SD 0) | 0.6707 |
| Dorsal mass, % body mass | 0.05 (SD 0) | 0.03 (SD 0) | 0.0222* |
| Lateral mass, % body mass | 0.01 (SD 0) | 0.00 (SD 0) | 0.0242* |
| Seminal vesicle mass, % body mass | 0.76 (SD 0.11) | 0.72 (SD 0.06) | 0.5431 |
| Void spot assay | |||
| n | 8 | 4 | |
| Count | 78.10 (SD 85.11) | 73.00 (SD 109.4) | 0.3677 |
| Total area, cm2 | 36.60 (SD 10.04) | 30.60 (SD 213.92) | 0.4060 |
| Percent area in center | 5.47 (SD 6.50) | 13.50 (SD 26.6) | 0.2141 |
| Percent area in corners | 69.40 (SD 18.36) | 56.00 (SD 41) | 0.4413 |
| Spots of a certain size (diameter) | |||
| 0−0.1 cm | 67.30 (SD 74.95) | 51.30 (SD 72.2) | 0.3455 |
| 0.1−0.25 cm | 6.00 (SD 11.37) | 14.00 (SD 27.4) | 0.3939 |
| 0.25−0.5 cm | 1.25 (SD 1.38) | 4.50 (SD 8.34) | 0.8990 |
| 0.5−1 cm | 1.00 (SD 1.07) | 1.25 (SD 1.9) | >0.9999 |
| 1−2 cm | 0 (SD 0) | 0 (SD 0) | >0.9999 |
| 2−3 cm | 0 (SD 0) | 0 (SD 0) | >0.9999 |
| 3−4 cm | 0.13 (SD 0.37) | 0.25 (SD 0.5) | >0.9999 |
| 4+ cm | 2.50 (SD 0.76) | 1.75 (SD 0.5) | 0.1293 |
| Cystometry | |||
| n | 8 | 4 | |
| Void duration | 0.44 (SD 0.17) | 0.44 (SD 0.14) | 0.9972 |
| Intervoid interval | 5.32 (SD 2.26) | 3.76 (SD 1.74) | 0.2546 |
| Baseline pressure | 2.44 (SD 0.74) | 2.34 (SD 1.24) | 0.7471 |
| Normalized threshold pressure | 5.68 (SD 4.50) | 3.65 (SD 1.54) | 0.4083 |
| Normalized peak void pressure | 19.70 (SD 9.93) | 16.70 (SD 9.6) | 0.6277 |
| Nonvoiding contractions | 6.60 (SD 5.83) | 2.12 (SD 1.14) | 0.0695† |
| Voided volume, ×10−2 | 6.97 (SD 3.08) | 6.14 (SD 2.48) | 0.6720 |
| Compliance, ×10−2 | 1.81 (SD 0.99) | 1.59 (SD 0.90) | 0.7230 |
| Volume flow rate, ×10−3 | 3.69 (SD 2.88) | 2.21 (SD 0.43) | 0.3574 |
| Mass-based flow rate | 31.70 (SD 7.04) | 19.90 (SD 13.26) | 0.0672† |
| Efficiency, % | 107.00 (SD 23.22) | 114.00 (SD 25.6) | 0.6029 |
Values are averages (SD); n, number of animals.
Statistically significant difference.
Changes that approached significance (trend).
The results of the three different methods of prostate mass reduction show the complex interplay of hormones and anatomic urinary tract variation that result in altered urinary function in male mice. We considered two possible reasons for the surprising observation that the three methods of prostate reduction had inconsistent and minimal effects on urinary function. The first explanation is that off-target effects from methods used to reduce prostate size may have masked the effect of prostate size reduction. The second explanation is that variability in the localization and extent of prostate mass reduction among our three methods lead to inconsistent outcomes with regard to voiding function, and we will explore this possibility in a future paper (H. Ruetten, K. A. Wegner, H. L. Zhang, P. Wang, J. Sandhu, S. Sandhu, J. Morkrid, B. Mueller, Z. Wang, J. Macoska, R. E. Peterson, D. E. Bjorling, W. A. Ricke, P. C. Marker, and C. M. Vezina, unpublished observations). It is worth noting that we exclusively evaluated prostate mass reductions in our study; increases in prostate mass were not examined and could have discrete impacts on urinary function (41).
Exogenous testosterone supplementation masculinizes female urinary physiology.
Because male castration feminized some of male urinary physiology, and because other methods of prostate mass reduction failed to recapitulate the resulting changes in male voiding function, we next tested if circulating testosterone underlies sex differences in mouse urinary function. We supplemented female mice with exogenous testosterone. We assessed supplemented mice for directional changes in VSA/CMG parameters consistent with “masculinization” of urinary physiology (i.e., the parameters changed in the same direction as male mice). Female mice were divided into two groups: control (with sham testosterone capsule implants) or with Silastic testosterone capsule implants (female mice with testosterone). Capsules were implanted at 6 wk of age, and assessment took place at 9 wk of age. The results are shown in Fig. 3B and Table 8.
Table 8.
Impact of testosterone on female urinary physiology
| Intact Female Mice | Female Mice With Testosterone | P Value | |
|---|---|---|---|
| Gross assessment | |||
| n | 7 | 7 | |
| Body mass, g | 18.13 (SD 1.06) | 20.66 (SD 0.85) | 0.0004* |
| Bladder | |||
| Mass, % body mass | 0.13 (SD 0.03) | 0.13 (SD 0.03) | >0.9999 |
| Volume, mm3/g body mass | 22.72 (SD 14.31) | 30.43 (SD 15.45) (n = 6) | 0.3534 |
| Void spot assay | |||
| n | 7 | 7 | |
| Count | 22.86 (SD 3.07) | 91.00 (SD 52.78) | <0.0001* |
| Total area, cm2 | 23.84 (SD 5.64) | 17.82 (SD 4.13) | 0.0415* |
| Percent area in center | 2.48 (SD 3.92) | 11.55 (SD 10.98) | 0.0524† |
| Percent area in corners | 65.12 (SD 25.64) | 66.84 (SD 25.72) | 0.9025 |
| Spots of a certain size (area) | |||
| 0−0.1 cm | 16.29 (SD 1.80) | 81.00 (SD 40.82) | <0.0001* |
| 0.1−0.25 cm | 1.57 (SD 1.14) | 2.71 (SD 3.73) | 0.9930 |
| 0.25−0.5 cm | 0.86 (SD 0.37) | 3.29 (SD 7.41) | 0.4700 |
| 0.5−1 cm | 0.43 (SD 0.53) | 1.00 (SD 1.40) | 0.6941 |
| 1−2 cm | 0.29 (SD 0.48) | 0.29 (SD 0.77) | 0.7220 |
| 2−3 cm | 0.29 (SD 0.77) | 0.29 (SD 0.77) | >0.9999 |
| 3−4 cm | 0 (SD 0) | 0.29 (SD 0.48) | 0.4620 |
| 4+ cm | 3.14 (SD 0.69) | 2.14 (SD 0.69) | 0.0189* |
| Cystometry | |||
| n | 6 | 5 | |
| Void duration | 0.41 (SD 0.05) | 0.42 (SD 0.04) | 0.7880 |
| Intervoid interval | 3.50 (SD 1.13) | 3.39 (SD 1.25) | 0.8860 |
| Baseline pressure | 2.68 (SD 1.98) | 2.99 (SD 1.68) | 0.7860 |
| Normalized threshold pressure | 9.36 (SD 2.84) | 5.13 (SD 2.53) | 0.0207* |
| Normalized peak void pressure | 25.35 (SD 2.72) | 17.76 (SD 1.99) | 0.0006* |
| Nonvoiding contractions | 2.40 (SD 1.49) | 3.68 (SD 0.96) | 0.1700 |
| Voided volume, ×10−2 | 4.72 (SD 1.25) | 4.88 (SD 2.19) | 0.8870 |
| Compliance, ×10−2 | 0.52 (SD 0.24) | 0.83 (SD 0.25) | 0.0714† |
| Volume flow rate, ×10−3 | 1.96 (SD 0.51) | 1.94 (SD 0.76) | 0.9580 |
| Mass-based flow rate | 16.80 (SD 9.72) | 32.50 (SD 27.50) | 0.1620 |
| Efficiency, % | 104.20 (SD 10.39) | 111.80 (SD 24.40) | 0.5050 |
Values are averages (SD); n, number of animals.
Statistically significant difference.
Changes that approached significance (trend).
Female mice with testosterone had a significantly greater body mass but similar bladder mass and volume compared with female control mice. A previous study (28) has reported that testosterone does not change female mouse body weight but increases bladder weight. However, the present study used mice of a different age, strain, environment, and method of testosterone delivery.
Testosterone supplementation caused several changes in female voiding function as measured by VSA. Similar to intact male mice, female mice with testosterone deposited significantly less urine during a 4-h monitoring period, voided fewer spots >4 cm2, and voided a larger percentage in the center of the paper than female control mice. Also similar to male mice, female mice with testosterone had a significantly lower threshold pressure, lower peak void pressure, and higher bladder compliance than control female mice. Female mice with testosterone, unlike male mide, had more void spots, specifically more 0- to 0.1-cm2 spots, than female control mice.
Female voiding parameters affected by exogenous testosterone included many of the same parameters that distinguished male from female voiding function. Thus, we conclude that even though female lower urinary tract anatomy differs from that of male lower urinary tract anatomy, exogenous testosterone “masculinizes” female voiding patterns. The clear relationship between testosterone and sex differences in urinary function was a surprising finding of this study. Androgen receptor expression and activity have been well described in the reproductive tract, but expression and activity in the urinary tract are not well characterized. A previous report (8) has documented androgen receptor expression in occasional stromal cells of the bladder and weak staining in the kidney. Another study (15) found that pelvic ganglia contain androgen sensitive autonomic nerves. This finding raises the possibility that androgens can masculinize autonomic signaling in the female lower urinary tract to drive urinary voiding patterns similar to intact male mice. Further studies to compare androgen receptor expression of the entire male and female lower urinary tract are needed to better elucidate the influence of androgens on autonomic signaling in the bladder, prostate, and urethra.
A VSA feature not accounted for by “masculinization” of urinary function was the increase in spot count, specifically small spots (0–0.1 cm2). We implanted testosterone capsules into 8-wk-old male mice (male mice with testosterone) and assessed voiding 1 wk later (Table 9). Male mice with testosterone trended toward an increase in total void spot count (P = 0.0760) and small (0–0.1 cm2) spot count (P = 0.0696) compared with male control mice (sham castrated and implanted with sham testosterone capsules), making increased voiding frequency a common feature of exogenous testosterone supplementation in female mice and intact male mice (overall P < 0.0001 and P = 0.0760 and small P < 0.0001 and P = 0.0696).
Table 9.
Impact of testosterone on intact male urinary physiology
| Intact Male Mice | Male Mice With Testosterone | P Value | |
|---|---|---|---|
| Gross assessment | |||
| n | 13 | 8 | |
| Body mass, g | 24.45 (SD 1.77) | 24.55 (SD 1.58) | 0.9015 |
| Bladder volume, mm3/g body mass | 39.86 (SD 26.90) | 48.33 (SD 25.20) | 0.3996 |
| Prostate mass, % body mass | 0.20 (SD 0.04) | 0.27 (SD 0.08) | 0.0009* |
| Anterior mass, % body mass | 0.09 (SD 0) | 0.13 (SD 0.03) | <0.0001* |
| Ventral mass, % body mass | 0.04 (SD 0) | 0.05 (SD 0.03) | 0.1341 |
| Dorsal mass, % body mass | 0.04 (SD 0) | 0.05 (SD 0.03) | 0.7933 |
| Lateral mass, % body mass | 0.01 (SD 0) | 0.03 (SD 0.03) | 0.0829† |
| Seminal vesicle mass, % body mass | 0.62 (SD 0.14) | 0.81 (SD 0.31) | 0.4025 |
| Void spot assay | |||
| n | 9 | 8 | |
| Count | 32.10 (SD 38.10) | 83.50 (SD 83.41) | 0.0760† |
| Total area, cm2 | 11.50 (SD 9.51) | 24.49 (SD 9.64) | 0.0325* |
| Percent area in center | 15.80 (SD 29.91) | 4.26 (SD 1.75) | 0.2043 |
| Percent area in corners | 61.10 (SD 39.90) | 67.35 (SD 33.88) | 0.7805 |
| Spots of a certain size (area) | |||
| 0−0.1 cm | 28.10 (SD 33.90) | 76.00 (SD 77.75) | 0.0696† |
| 0.1−0.25 cm | 1.00 (SD 1.41) | 2.50 (SD 3.56) | 0.3021 |
| 0.25−0.5 cm | 0.67 (SD 0.99) | 1.00 (SD 1.16) | 0.4476 |
| 0.5−1 cm | 0.67 (SD 1.11) | 0.50 (SD 0.82) | >0.9999 |
| 1−2 cm | 0.33 (SD 0.99) | 1.00 (SD 2.01) | 0.2028 |
| 2−3 cm | 0.44 (SD 1.02) | 0.50 (SD 1.41) | >0.9999 |
| 3−4 cm | 0.11 (SD 0.33) | 0.50 (SD 1.41) | 0.7692 |
| 4+ cm | 0.78 (SD 0.66) | 1.50 (SD 0.82) | 0.2042 |
| Cystometery | |||
| n | 6−7 | 8 | |
| Void duration | 0.64 (SD 0.21) | 0.63 (SD 0.08) | 0.9266 |
| Intervoid interval | 5.10 (SD 3.12) | 5.67 (SD 1.90) | 0.6714 |
| Baseline pressure | 3.61 (SD 0.82) | 2.91 (SD 1.33) | 0.2477 |
| Normalized threshold pressure | 3.35 (SD 0.87) | 3.84 (SD 1.56) | 0.4781 |
| Normalized peak void pressure | 18.40 (SD 5.45) | 21.26 (SD 5.37) | 0.3205 |
| Nonvoiding contractions | 2.26 (SD 1.90) | 5.09 (SD 1.05) | 0.0029* |
| Voided volume, ×10−2 | 7.60 (SD 4.26) | 7.56 (SD 3.25) | 0.9874 |
| Compliance, ×10−2 | 1.92 (SD 1.20) | 2.23 (SD 0.99) | 0.6342 |
| Volume flow rate, ×10−3 | 1.98 (SD 0.73) | 2.08 (SD 0.91) | 0.8395 |
| Mass-based flow rate | 23.70 (SD 10.02) | 18.26 (SD 7.38) | 0.2621 |
| Efficiency, % | 96.40 (SD 9.75) | 117.30 (SD 54.84) | 0.4630 |
Values are averages (SD); n, number of animals.
Statistically significant difference.
Changes that approached significance (trend).
To determine whether restoration of physiological testosterone induces voiding frequency in castrated male mice, we implanted castrated male mice with testosterone capsules (castrated mice with testosterone) at 8 wk of age and assessed mice at 9 wk of age (Table 10). Spot count did not increase when castrated mice were supplemented with testosterone. Therefore, testosterone increases voiding frequency when increased to supraphysiological concentrations but not when depleted and then returned to physiological concentrations.
A “frequent voider” pattern, as detected by VSA (>100 urine spots deposited on a filter paper in a 4-ho monitoring period), was previously noted in ~10% of 9-wk-old C57BL/6J mice (16). In our study, 14.29% of testosterone-treated female mice and 25% of testosterone-treated male mice were frequent voiders, but no control female mice and just 11% of control male mice were frequent voiders. These results further support the notion that supraphysiological testosterone increases voiding frequency and are consistent with increased voiding frequency in rats and dogs supplemented with testosterone (21).
In conclusion, we used surgical, pharmacological, and genetic approaches to reduce mouse prostate mass and also exposed C57BL/6J mice to exogenous testosterone to determine the influence of the prostate and testosterone on voiding function. We characterized male and intact female urinary phenotypes using contemporary methodologies and highlighted unique sex differences in urinary voiding phenotype.
Urological researchers and practitioners are beginning to appreciate that not all male LUTS arise from urethral occlusion by an enlarged prostate and that additional factors [prostatic urethra collagen accumulation (23), prostatic inflammation (30), and prostate smooth muscle contraction (5, 6)] also drive LUTS. Mice are instrumental in studying these alternative mechanisms. It is possible, for example, to induce prostatic inflammation and drive urinary frequency and pelvic pain in mice modeling LUTS symptomology (2, 19, 35). It is also possible to knockout or overexpress genes and cell types to validate mechanisms arising from clinical studies and to use mice for preclinical safety and efficacy trials of novel therapeutics. Mice fuel a creative cycle of translational research resulting in specific targeted therapies for men. To take full advantage of our models, further baseline studies are needed to determine baseline molecular and cellular contributions to voiding function in our mouse models.
GRANTS
This work was supported by National Institutes of Health grants: U54 DK104310, Summer Program In Undergrduate Urologic Research (U54 DK104310S1), R01ES001332, R01DK099328, F31ES028594, TL1TR002375, and University of Wisconsin-Madison, School of Veterinary Medicine.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.R., K.A.W., and C.M.V. conceived and designed research; H.R., K.A.W., H.L.Z., P.W., B.M., and Z.W. performed experiments; H.R., K.A.W., H.L.Z., J.K.S., and S.K.S. analyzed data; H.R., K.A.W., H.L.Z., and C.M.V. interpreted results of experiments; H.R. prepared figures; H.R. and H.L.Z. drafted manuscript; H.R., K.A.W., B.M., and C.M.V. edited and revised manuscript; H.R., K.A.W., H.L.Z., P.W., J.K.S., B.M., Z.W., J.M., R.E.P., D.E.B., W.A.R., P.C.M., and C.M.V. approved final version of manuscript.
REFERENCES
- 1.Barry MJ, Cockett AT, Holtgrewe HL, McConnell JD, Sihelnik SA, Winfield HN. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol 150: 351–358, 1993. doi: 10.1016/S0022-5347(17)35482-4. [DOI] [PubMed] [Google Scholar]
- 2.Bell-Cohn A, Mazur DJ, Hall C, Schaeffer AJ, Thumbikat P. Uropathogenic Escherichia coli-induced fibrosis, leading to lower urinary tract symptoms, is associated with type 2 cytokine signaling. Am J Physiol Renal Physiol 316: F682–F692, 2019. doi: 10.1152/ajprenal.00222.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benice TS, Raber J. Testosterone and dihydrotestosterone differentially improve cognition in aged female mice. Learn Mem 16: 479–485, 2009. doi: 10.1101/lm.1428209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorling DE, Wang Z, Vezina CM, Ricke WA, Keil KP, Yu W, Guo L, Zeidel ML, Hill WG. Evaluation of voiding assays in mice: impact of genetic strains and sex. Am J Physiol Renal Physiol 308: F1369–F1378, 2015. doi: 10.1152/ajprenal.00072.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caine M, Pfau A, Perlberg S. The use of alpha-adrenergic blockers in benign prostatic obstruction. Br J Urol 48: 255–263, 1976. doi: 10.1111/j.1464-410X.1976.tb10214.x. [DOI] [PubMed] [Google Scholar]
- 6.Campbell MF, Walsh PC. Campbell’s Urology. Philadelphia, PA: Saunders, 1998. [Google Scholar]
- 7.Cornelissen LL, Misajet B, Brooks DP, Hicks A. Influence of genetic background and gender on bladder function in the mouse. Auton Neurosci 140: 53–58, 2008. doi: 10.1016/j.autneu.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Dart DA, Waxman J, Aboagye EO, Bevan CL. Visualising androgen receptor activity in male and female mice. PLoS One 8: e71694, 2013. doi: 10.1371/journal.pone.0071694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girman CJ, Jacobsen SJ, Tsukamoto T, Richard F, Garraway WM, Sagnier PP, Guess HA, Rhodes T, Boyle P, Lieber MM. Health-related quality of life associated with lower urinary tract symptoms in four countries. Urology 51: 428–436, 1998. doi: 10.1016/S0090-4295(97)00717-6. [DOI] [PubMed] [Google Scholar]
- 10.Gnyawali D, Sharma U. Correlation of prostate volume with ‘international prostate symptom score’ and ‘benign prostatic hyperplasia-impact index’ in benign prostatic hyperplasia. J Soc Surgeons Nepal 71: 6−10, 2016. doi: 10.3126/jssn.v17i1.15174. [DOI] [Google Scholar]
- 11.Hill WG, Zeidel ML, Bjorling DE, Vezina CM. Void spot assay: recommendations on the use of a simple micturition assay for mice. Am J Physiol Renal Physiol 315: F1422–F1429, 2018. doi: 10.1152/ajprenal.00350.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacs JT, Coffey DS. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl 2, S2: 33–50, 1989. doi: 10.1002/pros.2990150506. [DOI] [PubMed] [Google Scholar]
- 13.Ivanova A, Signore M, Caro N, Greene ND, Copp AJ, Martinez-Barbera JP. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis 43: 129–135, 2005. doi: 10.1002/gene.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobsen SJ, Girman CJ, Lieber MM. Natural history of benign prostatic hyperplasia. Urology 58, Suppl 1: 5–16, 2001. doi: 10.1016/S0090-4295(01)01298-5. [DOI] [PubMed] [Google Scholar]
- 15.Keast JR, Saunders RJ. Testosterone has potent, selective effects on the morphology of pelvic autonomic neurons which control the bladder, lower bowel and internal reproductive organs of the male rat. Neuroscience 85: 543–556, 1998. doi: 10.1016/S0306-4522(97)00631-3. [DOI] [PubMed] [Google Scholar]
- 16.Keil KP, Abler LL, Altmann HM, Bushman W, Marker PC, Li L, Ricke WA, Bjorling DE, Vezina CM. Influence of animal husbandry practices on void spot assay outcomes in C57BL/6J male mice. Neurourol Urodyn 35: 192–198, 2016. doi: 10.1002/nau.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keil KP, Mehta V, Branam AM, Abler LL, Buresh-Stiemke RA, Joshi PS, Schmitz CT, Marker PC, Vezina CM. Wnt inhibitory factor 1 (Wif1) is regulated by androgens and enhances androgen-dependent prostate development. Endocrinology 153: 6091–6103, 2012. doi: 10.1210/en.2012-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumazaki M, Ando H, Ushijima K, Maekawa T, Motosugi Y, Takada M, Tateishi M, Fujimura A. Influence of dosing time on the efficacy and safety of finasteride in rats. J Pharmacol Exp Ther 338: 718–723, 2011. doi: 10.1124/jpet.111.182865. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Yang G, Bushman W. Prostatic inflammation induces urinary frequency in adult mice. PLoS One 10: e0116827, 2015. doi: 10.1371/journal.pone.0116827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepor H. Pathophysiology of lower urinary tract symptoms in the aging male population. Rev Urol 7, Suppl 7: S3–S11, 2005. [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Tian Y, Guo S, Gu H, Yuan Q, Xie X. Testosterone-induced benign prostatic hyperplasia rat and dog as facile models to assess drugs targeting lower urinary tract symptoms. PLoS One 13: e0191469, 2018. doi: 10.1371/journal.pone.0191469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu TT, Thomas S, Mclean DT, Roldan-Alzate A, Hernando D, Ricke EA, Ricke WA. Prostate enlargement and altered urinary function are part of the aging process. Aging (Albany NY) 11: 2653–2669, 2019. doi: 10.18632/aging.101938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma J, Gharaee-Kermani M, Kunju L, Hollingsworth JM, Adler J, Arruda EM, Macoska JA. Prostatic fibrosis is associated with lower urinary tract symptoms. J Urol 188: 1375–1381, 2012. doi: 10.1016/j.juro.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magari T, Shibata Y, Arai S, Kashiwagi B, Suzuki K, Suzuki K. Time-dependent effects of castration on the bladder function and histological changes in the bladder and blood vessels. Asian J Androl 16: 457–460, 2014. doi: 10.4103/1008-682X.123676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer EK, Kroeze SG, Chopra S, Bottle A, Patel A. Examining the ‘gold standard’: a comparative critical analysis of three consecutive decades of monopolar transurethral resection of the prostate (TURP) outcomes. BJU Int 110: 1595–1601, 2012. doi: 10.1111/j.1464-410X.2012.11119.x. [DOI] [PubMed] [Google Scholar]
- 27.McNeal JE. Normal histology of the prostate. Am J Surg Pathol 12: 619–633, 1988. doi: 10.1097/00000478-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Mucignat-Caretta C, Bondì M, Caretta A. Endocrine status affects bladder size and postvoid residual urinary volume in mice. Horm Behav 46: 11–18, 2004. doi: 10.1016/j.yhbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson TM, Ricke EA, Marker PC, Miano JM, Mayer RD, Timms BG, vom Saal FS, Wood RW, Ricke WA. Testosterone and 17β-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology 153: 5556–5565, 2012. doi: 10.1210/en.2012-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol 54: 1379–1384, 2008. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oesterling JE, Jacobsen SJ, Chute CG, Guess HA, Girman CJ, Panser LA, Lieber MM. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA 270: 860–864, 1993. doi: 10.1001/jama.1993.03510070082041. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira DS, Dzinic S, Bonfil AI, Saliganan AD, Sheng S, Bonfil RD. The mouse prostate: a basic anatomical and histological guideline. Bosn J Basic Med Sci 16: 8–13, 2016. doi: 10.17305/bjbms.2016.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricke WA, Lee CW, Clapper TR, Schneider AJ, Moore RW, Keil KP, Abler LL, Wynder JL, López Alvarado A, Beaubrun I, Vo J, Bauman TM, Ricke EA, Peterson RE, Vezina CM. In utero and lactational TCDD exposure increases susceptibility to lower urinary tract dysfunction in adulthood. Toxicol Sci 150: 429–440, 2016. doi: 10.1093/toxsci/kfw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritter KE, Wang Z, Vezina CM, Bjorling DE, Southard-Smith EM. Serotonin receptor 5-HT3A affects development of bladder innervation and urinary bladder function. Front Neurosci 11: 690, 2017. doi: 10.3389/fnins.2017.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudick CN, Berry RE, Johnson JR, Johnston B, Klumpp DJ, Schaeffer AJ, Thumbikat P. Uropathogenic Escherichia coli induces chronic pelvic pain. Infect Immun 79: 628–635, 2011. doi: 10.1128/IAI.00910-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro E, Lepor H. Pathophysiology of clinical benign prostatic hyperplasia. Urol Clin North Am 22: 285–290, 1995. [PubMed] [Google Scholar]
- 37.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol 20: 327–336, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Turkbey B, Huang R, Vourganti S, Trivedi H, Bernardo M, Yan P, Benjamin C, Pinto PA, Choyke PL. Age-related changes in prostate zonal volumes as measured by high-resolution magnetic resonance imaging (MRI): a cross-sectional study in over 500 patients. BJU Int 110: 1642–1647, 2012. doi: 10.1111/j.1464-410X.2012.11469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wegner KA, Abler LL, Oakes SR, Mehta GS, Ritter KE, Hill WG, Zwaans BM, Lamb LE, Wang Z, Bjorling DE, Ricke WA, Macoska J, Marker PC, Southard-Smith EM, Eliceiri KW, Vezina CM. Void spot assay procedural optimization and software for rapid and objective quantification of rodent voiding function, including overlapping urine spots. Am J Physiol Renal Physiol 315: F1067–F1080, 2018. doi: 10.1152/ajprenal.00245.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wegner KA, Cadena MT, Trevena R, Turco AE, Gottschalk A, Halberg RB, Guo J, McMahon JA, McMahon AP, Vezina CM. An immunohistochemical identification key for cell types in adult mouse prostatic and urethral tissue sections. PLoS One 12: e0188413, 2017. doi: 10.1371/journal.pone.0188413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood RW, Baggs RB, Schwarz EM, Messing EM. Initial observations of reduced uroflow in transgenic adenocarcinoma of murine prostate. Urology 67: 1324–1328, 2006. doi: 10.1016/j.urology.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev 101: 61–69, 2001. doi: 10.1016/S0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]