Abstract
Glucagon-like peptide-1 (GLP-1), an incretin hormone, has diuretic and natriuretic effects. The present study was designed to explore the possible underlying mechanisms for the diuretic and natriuretic effects of GLP-1 via renal nerves in rats. Immunohistochemistry revealed that GLP-1 receptors were avidly expressed in the pelvic wall, the wall being adjacent to afferent renal nerves immunoreactive to calcitonin gene-related peptide, which is the dominant neurotransmitter for renal afferents. GLP-1 (3 μM) infused into the left renal pelvis increased ipsilateral afferent renal nerve activity (110.0 ± 15.6% of basal value). Intravenous infusion of GLP-1 (1 µg·kg−1·min−1) for 30 min increased renal sympathetic nerve activity (RSNA). After the distal end of the renal nerve was cut to eliminate the afferent signal, the increase in efferent renal nerve activity during intravenous infusion of GLP-1 was diminished compared with the increase in total RSNA (17.0 ± 9.0% vs. 68.1 ± 20.0% of the basal value). Diuretic and natriuretic responses to intravenous infusion of GLP-1 were enhanced by total renal denervation (T-RDN) with acute surgical cutting of the renal nerves. Selective afferent renal nerve denervation (A-RDN) was performed by bilateral perivascular application of capsaicin on the renal nerves. Similar to T-RDN, A-RDN enhanced diuretic and natriuretic responses to GLP-1. Urine flow and Na+ excretion responses to GLP-1 were not significantly different between T-RDN and A-RDN groups. These results indicate that the diuretic and natriuretic effects of GLP-1 are partly governed via activation of afferent renal nerves by GLP-1 acting on sensory nerve fibers within the pelvis of the kidney.
Keywords: glucagon-like peptide-1, hemodynamics, renal afferent, sodium and water homeostasis, sympathetic nerve activity
INTRODUCTION
Disorders concerned with altered cardiovascular regulation involving both the heart and kidney are known as cardiorenal syndrome, in which the dysfunction of one organ induces the dysfunction of the other (47, 48, 56). However, the precise underlying mechanisms involved in the interaction between heart and kidney are poorly understood. Recent studies have revealed a crucial role for renal nerves to link the heart and kidney (13, 42, 43, 53, 64, 65). It has been recently demonstrated that in the pressure overload model of heart failure, sympathetic nerve activation led to activation of renal collecting duct epithelial cells to elicit the production of the cytokine colony-stimulating factor that protects cardiomyocytes (13). However, renal denervation has been shown to disrupt this process (13). In addition, other studies have shown that renal denervation affects the expression of β-adrenoceptors in the heart (65) or neprilysin activity in the kidney (43, 53) and improves cardiac function in heart failure (42, 64). These studies suggested that intact renal nerves (either via afferent and/or efferent nerves) possibly facilitate the interaction between the heart and kidney bidirectionally via a potential neural pathway.
Glucagon-like peptide-1 (GLP-1), an incretin hormone, is released from the enteroendocrine cells in response to meals and controls glucose and energy homeostasis, including regulation of insulin secretion from pancreatic islets and feeding behavior (11, 17, 25, 58). GLP-1-related medicines are now widely used for treatment of diabetes and obesity. Recently, cardiovascular outcome studies of GLP-1 receptor (GLP-1R) agonists have shown that GLP-1R agonists reduce cardiovascular events and also prevent progression of chronic kidney disease in patients with type 2 diabetes (34, 36, 37). Studies in humans and animal models of hypertension, diabetes, and obesity have shown that administration of GLP-1/GLP-1R agonists induce diuresis and natriuresis (15, 16, 33, 54, 63). These renal effects of GLP-1/GLP-1R agonists might contribute to maintaining/protecting the cardiorenal system. Several possible mechanisms for diuretic and natriuretic effects of GLP-1/GLP-1R agonists have been postulated. It has been well documented that GLP-1 has direct effects on the kidney; GLP-1 causes renal vasodilatation, increases renal blood flow, increases glomerular filtration rate (GFR), inhibits Na+/H+ exchanger isoform 3 (NHE3)-medicated Na+/H+ exchange in proximal tubule, and decreases Na+, bicarbonate, and water reabsorption (4, 5, 9, 21, 57). In addition, in rodents, GLP-1R agonist directly acts on atrial cardiomyocytes to induce secretion of atrial natriuretic peptide (27), consequently causing diuretic and natriuretic responses. On the other hand, GLP-1/GLP-1R agonists also reportedly act on neural pathways by acting on weak blood-brain barrier (BBB) areas within the brain (18, 24, 51) and vagal afferent fibers (19, 23, 26). These reports prompted us to hypothesize that GLP-1 may also act on afferent and/or efferent renal nerves involved in the interaction between the kidney and heart to regulate diuretic and natriuretic effects and cardiovascular function.
The present study was conducted to assess the contribution of renal nerves to diuretic and natriuretic responses as well as hemodynamic effects of intravenously infused GLP-1. First, we investigated whether GLP-1Rs are expressed in the renal pelvic wall where afferent sensory renal nerves are densely innervated (10, 28). Second, we determined whether GLP-1 activates afferent renal nerve activity and whether GLP-1-induced diuresis and natriuresis and hemodynamic changes are affected by renal denervation. To elucidate the difference in the contribution of afferent versus efferent renal nerves, we performed either total renal denervation (T-RDN) with acute surgical sectioning of the renal nerves or selective afferent renal denervation (A-RDN) with perivascular application of capsaicin on the renal nerves, as previously described (12, 64).
METHODS
Animals.
All procedures used for this study were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and conducted according to National Institutes of Health guiding principles for research involving animals. Male Sprague-Dawley rats weighing 220−250 g were purchased from Sasco Breeding laboratories (Omaha, NE). Animals were housed on a 12:12-h light-dark cycle at ambient 22°C with 30−40% relative humidity. Laboratory chow and tap water were available ad libitum.
Cell transfection.
Human embryonic kidney (HEK)-293 cells seeded with 60% confluence were transfected with rat GLP-1R plasmid (plasmid catalog no. 14944, 2.5 μg/well in a 12-well plate, Addgene, Watertown, MA) or empty pcDNA3 plasmid as its corresponding negative control vector using Opti-MEM and Lipofectamine 3000 (Life Technologies, Carlsbad, CA) as transfection reagents.
Immunohistochemistry for GLP-1R in HEK-293 cells.
To confirm the specificity of mouse monoclonal anti-GLP-1R antibody (sc-390774, Santa Cruz Biotechnology, Santa Cruz, CA), we performed immunocytochemistry on cultured HEK-293 cells transfected with rat GLP-1R plasmid or empty pcDNA3 plasmid as its corresponding negative control vector. Adherent HEK-293 cells were grown on laminin-coated 6-mm Transwell-Clear inserts (Corning, Corning, NY) overnight. Cells were washed twice with PBS after 48 h of plasmid transfection and then fixed in 4% paraformaldehyde for 20 min at room temperature. Next, cells were permeabilized with 0.02% Triton X-100 for 20 min and then blocked in 1% BSA solution for 30 min at room temperature. After blockade was completed, cells were incubated in a 1:100 dilution of GLP-1R antibody solution at 4°C for 3 h followed by a 1:200 dilution of anti-mouse Alexa 488-conjugated secondary antibody (Life Technologies) for 1 h at room temperature in dark. Nuclei were stained with DAPI. Finally, coverslips were mounted onto frosted glass microscope slides using Fluoromount-G mounting medium (Southern Biotechnology). Images were captured by an Olympus fluorescence microscope equipped with a digital camera for obtaining photomicrographs of staining (Qimaging).
Western blot analysis for assessment of GLP-1R protein in HEK293 cell lysates.
To confirm the specificity of GLP-1R antibody in Western blot analysis, samples were prepared from cultured HEK-293 transfected with rat GLP-1R plasmid or empty pcDNA3 plasmid as its corresponding negative control vector. Cells harvested after 48 h of transfection by trypsinization were processed for whole cell protein isolation using RIPA buffer (Boston Bio-product, Ashland, MA). Proteins were quantified by a Pierce BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, MA), and 40 μg of proteins were boiled with 4× denaturing Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) and loaded onto a 15% SDS-PAGE gel. After electrophoresis, gels were transferred onto a PVDF membrane, and transferred PVDF membranes were then blocked with 5% nonfat dried milk in Tris-buffered saline (TBS; Bio-Rad Laboratories) for 1 h at room temperature. Membranes were incubated with primary antibody (anti-GLP-1R, 1:1,000, sc-390774, Santa Cruz Biotechnology, and anti-GAPDH, 1:5,000, MAB374, Millipore, Burlington, MA) in TBS overnight at 4°C. The respective secondary antibodies with horseradish peroxide conjugates were diluted at 1:5,000 or 1:10,000 in TBS and incubated at room temperature for 3 h. A Super Signal West Femto Chemiluminescent kit (ThermoFisher Scientific) was used to develop the probed membranes using a Molecular Imager ChemiDoc XRS imaging system (Bio-Rad Laboratories). Chemiluminescent images were captured using Image Laboratory software (version 6, Bio-Rad Laboratories). Expression of GLP-1R was calculated as the ratio of intensity of GLP-1 relative to the intensity of GAPDH.
Double-labeling immunohistochemistry for calcitonin gene-related peptide and GLP-1R in the kidney.
The kidneys of normal rats were removed and fixed in HistoChoice MB tissue fixative (Amresco, Solon, OH). Paraffin-embedded tissue sections (5 µm) were washed in xylene before rehydration in a series of ethanol washes. For double-labeling immunohistochemistry for calcitonin gene-related peptide (CGRP) and GLP-1R, primary rabbit anti-CGRP antibody (1:500, BML-CA1134, Enzo Life Sciences, Farmingdale, NY) together with anti-GLP-1R antibody (1:100, sc-390774, Santa Cruz Biotechnology) were incubated with kidney sections overnight. After a wash, sections were incubated with secondary Alexa 488 goat anti-rabbit antibody or Alexa 594 goat anti-mouse antibody (1:500, Molecular Probes, Carlsbad, CA) for 2 h. After being washed, sections were mounted on slides. Distribution of immunofluorescence within the kidneys was viewed using an Olympus fluorescence microscope equipped with a digital camera to obtain photomicrographs of staining (Qimaging).
Immunohistochemistry for GLP-1R in the pancreas.
The pancreas of normal rats was removed, frozen on dry ice, and then stored at −80°C. Frozen tissue sections of 7 μm were cut with a freezing microtome and adhered on slides. Sections were fixed with acetone at 4°C for 10 min. After a wash, sections were blocked with 0.03% Triton X-100 and 5% goat serum in PBS at room temperature for 60 min and incubated with primary mouse anti-GLP-1R antibody (1:100, sc-390774, Santa Cruz Biotechnology) overnight at 4°C. After being washed, sections were incubated with secondary Alexa 594 goat anti-mouse antibody (1:500, Molecular Probe) for 2 h in the dark. Nuclei were stained with DAPI. Finally, coverslips were mounted onto frosted glass microscope slides using Fluoromount-G mounting medium (Southern Biotechnology). Images were captured by an Olympus fluorescence microscope equipped with a digital camera for obtaining photomicrographs of staining (Qimaging).
Western blot analysis for the assessment of GLP-1R protein in the kidney.
The kidneys of normal rats were removed, frozen on dry ice, and then stored at −80°C. Proteins extracted from the kidney cortex and pelvis were used for Western blot analysis as described above for GLP-1R (mouse anti-GLP-1R, 1:2,000, sc-390774, Santa Cruz Biotechnology). Expression of GLP-1R was calculated as the ratio of intensity of GLP-1 relative to the intensity of β-actin (mouse anti-β-actin, 1:2,000, sc-47778, Santa Cruz Biotechnology).
Intrapelvic injection of GLP-1 and direct recording of afferent renal nerve activity.
A separate group of normal rats was used for these experiments. Rats were anesthetized by an injection of urethane (0.75 g/kg ip) and α-chloralose (70 mg/kg ip). Body temperature was maintained 36–38°C by a heated stage. After tracheal intubation, animals were allowed to breathe independently. The right femoral artery was cannulated with PE-50 polyethylene tubing and connected to a pressure transducer. Mean arterial pressure (MAP) and heart rate (HR) were simultaneously recorded on a PowerLab data-acquisition system (8SP, ADInstruments, Colorado Springs, CO). The right femoral vein was cannulated with PE-50 tubing for intravenous infusion of saline (0.9% NaCl) at a rate of 50 μl/min throughout the experiment. Intrapelvic injection and direct recording of afferent renal nerve activity were performed as previously described (3, 14, 64). Briefly, the left kidney was exposed through a retroperitoneal flank incision. The renal pelvis was cannulated with a 32-gauge triple-lumen catheter (ReCathCo, Allison Park, PA) via the ureter. Two of three lumens were attached with 1-ml syringes for intrapelvic injection of the drugs, and the rest of the lumens were open for withdrawal of infusate and urine flowing freely out of the catheter. A renal nerve bundle near the renal hilus was isolated and carefully placed on a bipolar platinum electrode. The nerve/electrode preparation was electrically isolated from the surrounding tissue with WACKER SilGel mixture (604 and 601). The electrical signal was amplified with high- and low-frequency cutoffs of 1,000 and 100 Hz, respectively (Grass amplifier). The rectified output (resister capacitor) filtered time constant (0.5 s) was then recorded and integrated using PowerLab (8si, ADInstruments, Sydney, NSW, Australia). First, the basal renal nerve discharge recording was obtained. Then, the central end of the nerve was cut to eliminate efferent nerve signal, leaving only afferent nerve discharge and background noise. Finally, the background noise was determined by cutting the distal end of the nerve. The value of afferent renal nerve activity (ARNA) was calculated by subtracting the background noise from the actual recorded value. The ARNA response to intrapelvic injection of GLP-1 (3 μM in saline, Enzo Life Science) with or without 5 min prior injection of the GLP-1R antagonist exendin 9–39 (300 μM in saline, Bachem, Torrance, CA) was expressed as the percent changes from the baseline ARNA value and also normalized to the peak ARNA response to intrapelvic capsaicin (100 μM in 5% ethanol and 95% saline, Sigma, St. Louis, MO) thus expressed as a percentage of maximum activation of renal afferent nerves (ARNAmax). The intrapelvic injection of the drugs was performed over 30 s.
Total, efferent, and ARNA recording during intravenous infusion of GLP-1.
Rats were anesthetized with urethane (0.75 g/kg ip) and α-chloralose (70 mg/kg ip). Body temperature was maintained at 36–38°C by a heated stage. After tracheal intubation, animals were allowed to breathe independently. The right femoral artery was cannulated with PE-50 tubing for the monitoring of MAP and HR. The right femoral vein was cannulated with PE-50 tubing for intravenous infusion of saline (50 µl/min) throughout the experiment except the period of GLP-1 infusion (1 µg·kg−1·min−1) for 30 min. The renal nerve discharge recording was obtained as described above. The total renal sympathetic nerve activity (RSNA) recording was performed without cutting the renal nerve in normal rats (n = 5). The efferent renal nerve activity (ERNA) recording was obtained after cutting the distal end of the nerve to eliminate the afferent nerve signal in a different group of normal rats (n = 5). The ARNA recording was obtained as mentioned above in another different group of normal rats (n = 6). The peak RSNA, ERNA, or ARNA response to intravenous infusion of GLP-1 was expressed as the percent change from the baseline RSNA, ERNA, or ARNA value.
Acute T-RDN.
Acute T-RDN was performed on the left kidney by surgical sectioning of the left renal nerves followed by perivascular application of 100% ethanol. This technique has been previously used to reduce norepinephrine levels in the kidney by >95% (32, 41, 65).
Chronic A-RDN.
Rats were anesthetized with isoflurane gas starting in an anesthesia chamber from 3% to 4%. During the procedure, isoflurane (2−2.5%, gas vaporizer) was used. Afferent renal denervation was performed as previously described (11, 62) with slight modifications. Briefly, the kidneys were exposed through a midline incision in the abdomen. The renal artery and vein were isolated using a Parafilm barrier. A small piece of filter paper soaked in a capsaicin (Sigma) solution (33 mM in 10% ethanol, 10% Tween 80, and 80% saline) was wrapped around the renal artery and vein for 10 min. After 10 min of capsaicin exposure, the filter paper was removed. The same procedure was repeated on the contralateral side.
Measurement of hemodynamic, diuretic, and natriuretic responses to GLP-1.
Rats were assigned randomly to one of the following three groups: normal, normal + T-RDN, or normal + selective A-RDN, with n = 6–8 rats/group. Rats were anesthetized with Inactin (100 mg/kg ip). Body temperature was maintained at 36–38°C by a heated stage. After tracheal intubation, animals were allowed to breathe independently. The right femoral artery was cannulated with PE-50 tubing for the monitoring of MAP and HR. The right femoral vein was cannulated with PE-50 tubing for 0.9% saline infusion (50 µl/min) to establish steady-state urine production and GLP-1 infusion (1 µg·kg−1·min−1).
The kidneys were exposed through a retroperitoneal flank incision. Subsequently, both ureters were cannulated with PE-10 tubing. Surgery was completed within ~60 min. Urine was collected in preweighed tubes from both left and right kidney via ureteral catheters, and urine volume was measured gravimetrically. After two urine collections (15 min each) over a 30-min stabilization period, rats were administered intravenous infusion of GLP-1 (1 µg·kg−1·min−1) for 30 min. Urine was collected at 10, 20, and 30 min during the infusion of GLP-1 followed by 40-, 50-, and 60-min collections after the termination of GLP-1 infusion. The Na+ concentration in each of the urine samples was analyzed using an ion-selective electrode (Beckman ion analyzer, Brea, CA). Blood was collected in a citrate-containing tube. Urinary and plasma levels of creatinine were determined using commercially available assay kits (ab65340, Abcam, Cambridge, MA) to calculate creatinine clearance (CrCl).
Validation of A-RDN.
To test the efficacy of A-RDN by capsaicin to cause selective ablation of afferent renal nerves, at the end of the renal function experiments, the kidneys were removed, frozen on dry ice, and then stored at −80°C. Protein extracts of the renal pelvis were used for Western blot analysis as described above for CGRP (primary antibody: 1:2,000, rabbit anti-CGRP, BML-CA1134, Enzo Life Sciences) and tyrosine hydroxylase (TH; 1:1,000, mouse anti-TH, sc-25269, Santa Cruz Biotechnology) to quantify the extent of damage to the efferent adrenergic innervation caused by selective A-RDN in the pelvic wall of the kidney.
Statistical analysis.
Data are expressed as means ± SE. Differences between groups and within groups were assessed by a t-test or one- or two-way ANOVA followed by the Bonferroni multiple-comparisons test for post hoc analysis of significance (Prism 7, GraphPad Software). P values of <0.05 were indicative of statistical significance.
RESULTS
Validation for the specificity of the GLP-1R antibody.
HEK-293 cells transfected with GLP-1R were used to validate the specificity of commercially available GLP-1R antibody (sc-390774, Santa Cruz Biotechnology). Immunohistochemistry, using cell transfection, demonstrated that there was robustly positive staining in GLP-1R-transfected cells but not in untransfected cells (Fig. 1A). Western blot analysis using these cell lysates demonstrated that the expression level of GLP-1R was increased by 4.98-fold in GLP-1R-transfected cells compared with untransfected cells (GLP-1R/GAPDH: 0.33 ± 0.06 vs. 0.07 ± 0.01, n = 3, P < 0.05; Fig. 1B). These results validated the specificity and reactivity of GLP-1R antibody for rat GLP-1R.
Fig. 1.
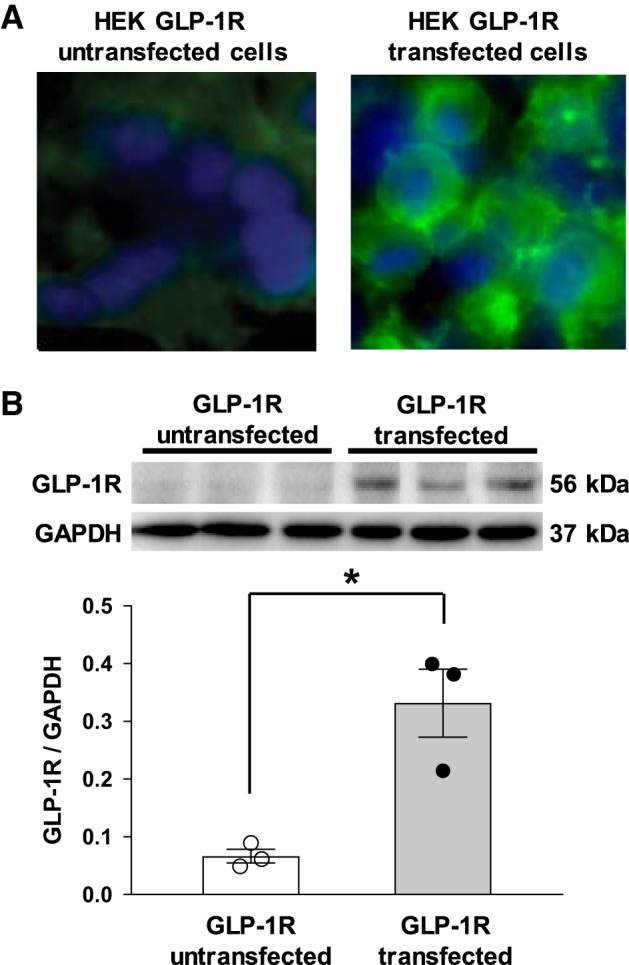
A: immunostaining for glucagon-like peptide-1 receptor (GLP-1R) in human embryonic kidney (HEK)-293 cells transfected with rat GLP-1R plasmid (right) and empty pcDNA3 plasmid as the control (left). GLP-1R is shown in green; DAPI is shown in blue. B: GLP-1R protein expression in HEK-293 cells transfected with rat GLP-1R plasmid and empty pcDNA3 plasmid as the control. n = 3. *P < 0.05.
GLP-1Rs are expressed in the renal pelvic wall.
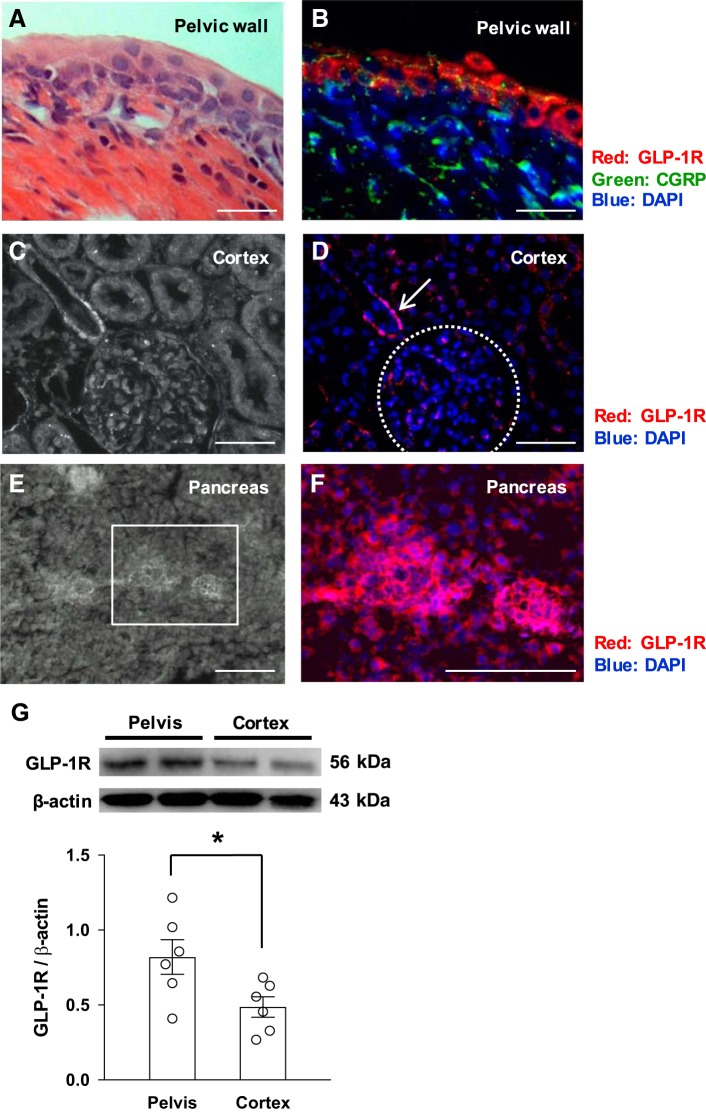
Immunohistochemistry for GLP-1R and CGRP, which is the dominant neurotransmitter for renal afferent nerves (10, 35, 55), revealed that GLP-1Rs were expressed in the pelvic wall being adjacent to afferent renal nerve fibers immunoreactive to CGRP (Fig. 2, A and B). There was relatively sparse expression of GLP-1R in all the other areas of the kidney, including the renal cortex, where there was some minimal staining (Fig. 2, C and D). GLP-1Rs were expressed in the pancreas as a known source tissue for positive control (Fig. 2, E and F). Western blot analysis for GLP-1R revealed that GLP-1R was expressed in both renal pelvis and cortex, with a greater degree of expression in the pelvis compared with cortex (Fig. 2G), consistent with the immunohistochemical data.
Fig. 2.
A and B: hematoxylin and eosin staining of the renal pelvic wall (A) and near-adjacent section (B) showing immunoreaction to glucagon-like peptide-1 receptor (GLP-1R; red) and calcitonin gene-related peptide (CGRP; green). C and D: images of the renal cortex in white light (C) and the same section (D) showing immunoreaction to GLP-1R (red). Arrow shows the immunoreaction to GLP-1R. The dotted circle shows glomeruli. E: immunofluorescent image of pancreas in white light. F: magnified image of the boxed portion of E. GLP-1R is shown in red. Nuclei were stained with DAPI (blue). G: GLP-1R protein expression in the pelvis and cortex of the kidney. Scale bars = 50 μm in A and B and 100 μm in C–F. n = 6. *P < 0.05.
Intrapelvic injection of GLP-1 increases ARNA.
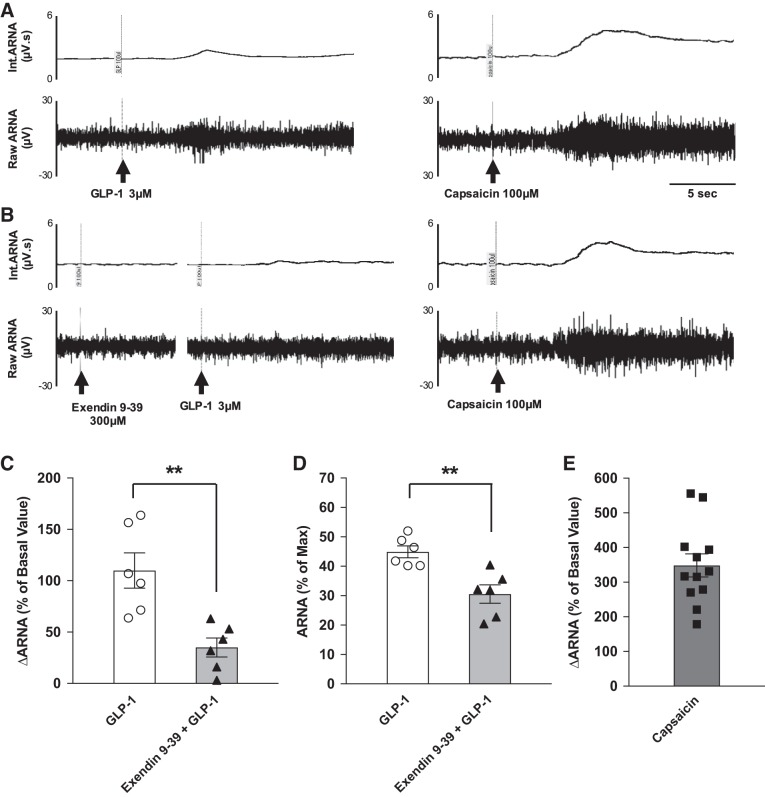
GLP-1 infused directly into the left renal pelvis increased ipsilateral ARNA. ARNA increased by 110.0% of the basal value. This represented 44.9% of ARNAmax as evaluated by injection of capsaicin (transient receptor potential V1 receptor agonist, 100 µM) into the renal pelvis. Prior injection of the GLP-1R antagonist exendin 9–39 (300 µM) attenuated ∆ARNA to 35.1% of the basal value. This represented 30.5% of ARNAmax (Fig. 3).
Fig. 3.
A and B: raw tracings of integrated (Int) and raw afferent renal nerve activity (ARNA) of the responses to intrapelvic injection of glucagon-like peptide-1 (GLP-1; 3 µM) alone (A) and with prior exendin 9–39 (300 µM; B). At the end of the measurement, capsaicin (100 µM) was injected to establish the peak level of ARNA (ARNAmax). C: summary data for ARNA changes from baseline, expressed as a percentage of the basal value, in response to GLP-1 and exendin 9–39 + GLP-1. D: summary data for peak ARNA responses to GLP-1 and exendin 9–39 + GLP-1, expressed as a percentage of the peak ARNA response to intrapelvic injection of capsaicin (ARNAmax). E: summary data for ARNA changes from baseline, expressed as a percentage of the basal value, in response to capsaicin. n = 6. **P < 0.01 vs. GLP-1 injection.
Intravenous infusion of GLP-1 increases total RSNA, ERNA, and ARNA.
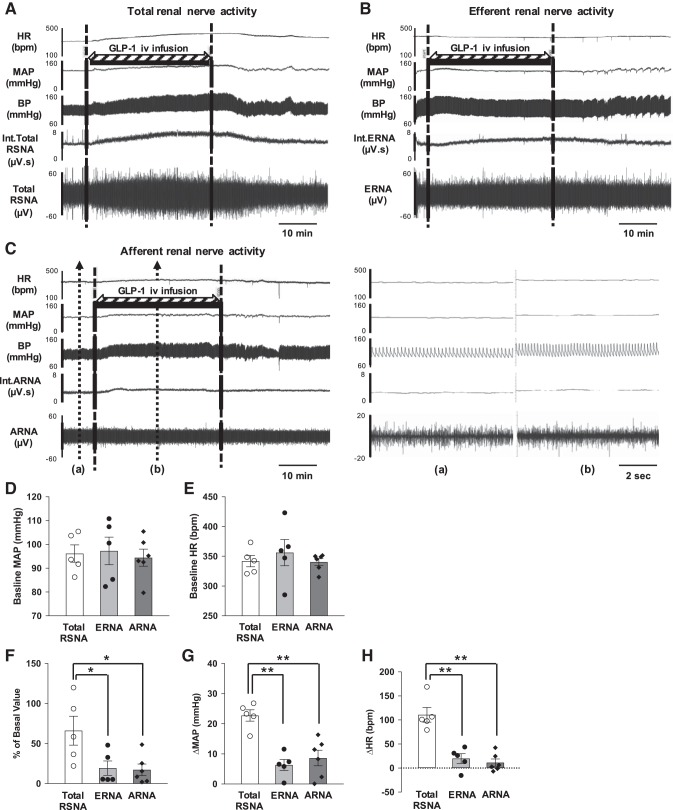
Three different groups of rats were used to measure total RSNA (n = 5), ERNA (n = 5), and ARNA (n = 6) responses to intravenous infusion of GLP-1 (Fig. 4). Baseline MAP and HR were not different among the three groups (Fig. 4, D and E). Intravenous infusion of GLP-1 increased total RSNA (68.1 ± 20.0% of the basal value), ERNA (17.0 ± 9.0% of the basal value), and ARNA (17.3 ± 6.6% of the basal value). Increases in ERNA and ARNA were significantly smaller than that of the RSNA response to GLP-1 (Fig. 4F). Intravenous infusion of GLP-1 increased MAP (22.8 ± 1.7 mmHg) and HR (110 ± 14 beats/min) in rats without cutting the renal nerve to obtain RSNA. After the distal or proximal end of the nerve was cut to obtain ERNA or ARNA, increases in MAP and HR to GLP-1 were significantly attenuated (MAP: 6.3 ± 1.6 mmHg and HR: 20 ± 9 beats/min or MAP 8.6 ± 2.4 mmHg and HR 17 ± 7 beats/min, respectively; Fig. 4, G and H).
Fig. 4.
A: raw tracings of changes in total renal sympathetic activity (RSNA), mean arterial pressure (MAP), and heart rate (HR) to intravenous infusion of glucagon-like peptide-1 (GLP-1) in a normal rat with intact renal nerves. B: raw tracings of changes in efferent renal nerve activity (ERNA), MAP, and HR to intravenous infusion of GLP-1 in a normal rat after the renal nerves were sectioned (proximal end recording). C: raw tracings of changes in afferent renal nerve activity (ARNA), MAP, and HR to intravenous infusion of GLP-1 in a normal rat after the renal nerves were sectioned (distal end recording). Magnified tracings at baseline (a) and during GLP-1 infusion (b) with short timescale recordings are shown. D and E: baseline MAP and HR in the three groups of normal rats (total RSNA, ERNA, and ARNA recording groups). F: summary data for the effect of GLP-1 on changes in total RSNA, ERNA, and ARNA. G and H: summary data for the effect of GLP-1 on changes in MAP and HR in the three groups of normal rats (total RSNA, ERNA, and ARNA recording groups). n = 5–6. *P < 0.05 and **P < 0.01 vs. normal rats with intact renal nerves. bpm, beats/min; BP, blood pressure; Int, integrated.
Acute T-RDN enhances diuretic and natriuretic responses to GLP-1.
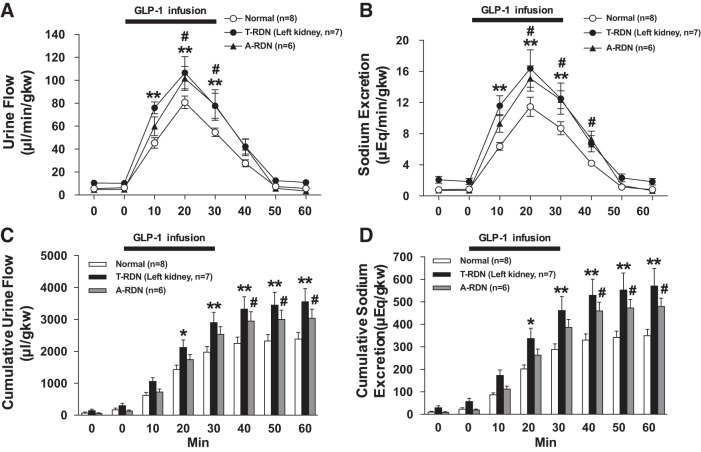
Acute T-RDN was performed by surgical sectioning followed by perivascular application of 100% ethanol on the left renal nerves. Data of urine flow and Na+ excretion in the T-RDN group from left denervated kidneys were compared with data from intact kidneys in a normal group of rats. T-RDN increased baseline levels of cumulative urine flow and Na+ excretion (310.8 ± 52.5 vs. 179.3 ± 39.1 μl/g kidney wt and 59.1 ± 10.5 vs. 25.1 ± 3.9 μeq/g kidney wt, respectively, n = 7–8, P < 0.05), consistent with our previous reports (66, 67). Intravenous infusion of GLP-1 induced diuresis and natriuresis in rats with and without T-RDN. Both diuresis and natriuresis responses to GLP-1 were significantly increased in rats with T-RDN compared with the normal group of rats (urine flow at 20 min: 106.5 ± 12.2 vs. 80.8 ± 5.2 µl·min−1·g kidney wt−1 and Na+ excretion at 20 min: 16.3 ± 2.1 vs. 11.5 ± 1.1 μeq·min−1·g kidney wt−1, n = 7–8, P < 0.01; Fig. 5, A and B). Cumulative urine flow and Na+ excretion also showed significantly greater diuresis and natriuresis in the T-RDN group compared with the normal group (Fig. 5, C and D).
Fig. 5.
A and B: urine flow (A) and Na+ excretion (B) in response to intravenous infusion of glucagon-like peptide-1 (GLP-1) in rats with or without total renal denervation (T-RDN) or afferent renal nerve denervation (A-RDN). C and D: cumulative values of urine flow (C) and Na+ excretion (D) in response to GLP-1 injection in rats with or without T-RDN or A-RDN. n = 6–8. *P < 0.05 and **P < 0.01 between normal and T-RDN groups; #P < 0.05 between normal and A-RDN groups by two-way ANOVA followed by a Bonferroni test. gkw, g kidney wt.
Chronic A-RDN enhances diuretic and natriuretic responses to GLP-1.
A-RDN was performed by bilateral perivascular application of capsaicin on the renal nerves. Ten days after A-RDN, there were enhanced diuretic and natriuretic responses to GLP-1 (urine flow at 20 min: 101.7 ± 9.6 vs. 80.8 ± 5.2 μl·min−1·g kidney wt−1 and Na+ excretion at 20 min: 15.1 ± 1.5 vs. 11.5 ± 1.1 μeq·min−1·g kidney wt−1, n = 6–8, P < 0.05; Fig. 5, A and B). The increase in urine flow rate and Na+ excretion responses to GLP-1 were not significantly different between T-RDN and A-RDN groups. Figure 6 shows the reduction of CGRP in the pelvic wall of capsaicin-treated rats. Consistent with these observations, CGRP protein expression was also decreased by 64% in capsaicin-treated rats. These results indicate that capsaicin treatment was effective in A-RDN. There was no significant difference in TH protein expression in the renal pelvic wall between the two groups (normal and A-RDN) that was indicative of intact efferent noradrenergic renal innervation.
Fig. 6.
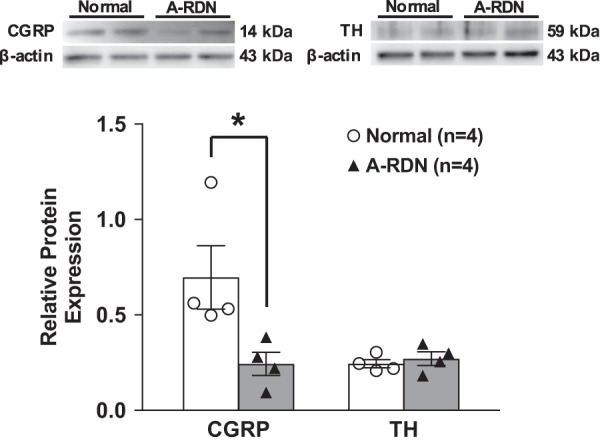
Relative calcitonin gene-related peptide (CGRP) and tyrosine hydroxylase (TH) protein expression in the renal pelvic wall in rats with or without afferent renal nerve denervation (A-RDN). n = 4. *P < 0.05 vs. normal rats.
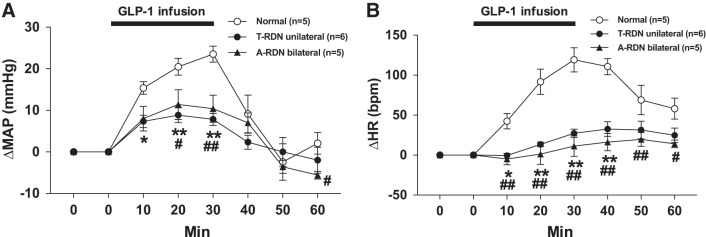
MAP and HR, increased by intravenous infusion of GLP-1, are attenuated with T-RDN and A-RDN.
MAP and HR were significantly increased during intravenous infusion of GLP-1 in normal rats (Fig. 7 and Table 1), consistent with previous reports (8, 59, 61). These changes in MAP and HR to GLP-1 were significantly attenuated by either T-RDN (∆MAP: 23 ± 2 vs. 8 ± 1 mmHg and ∆HR: 119 ± 13 vs. 27 ± 5 beats/min, n = 5–6, P < 0.01) or A-RDN (∆MAP: 23 ± 2 vs. 10 ± 3 mmHg and ∆HR 119 ± 13 vs. 11 ± 11 beats/min, n = 5, P < 0.01). These changes in MAP and HR to GLP-1 were not significantly different between the two groups (T-RDN and A-RDN). Table 2 shows the changes of CrCl during intravenous infusion of GLP-1. T-RDN increased baseline CrCl (1,745 ± 140 vs. 1,217 ± 158 µl·min−1·g kidney wt−1, n = 5, P < 0.05). Intravenous infusion of GLP-1 significantly increased CrCl in both normal and T-RDN rats. The increase in CrCl was significantly greater in rats with T-RDN than normal rats (2,720 ± 205 vs. 2,183 ± 170 µl·min−1·g kidney wt−1, n = 5, P < 0.05).
Fig. 7.
A and B: blood pressure (A) and heart rate (HR; B) changes in response to glucagon-like peptide-1 (GLP-1) injection in rats with or without total renal denervation (T-RDN) or afferent renal nerve denervation (A-RDN). n = 5–6. *P < 0.05 and **P < 0.01 between normal and T-RDN groups; #P < 0.05 and ##P < 0.01 between normal and A-RDN groups by two-way ANOVA followed by a Bonferroni test. bpm, beats/min; MAP, mean arterial pressure.
Table 1.
Hemodynamic changes during intravenous infusion of glucagon-like peptide-1
| Normal | Total Renal Denervation (Unilateral) | Afferent Renal Nerve Denervation (Bilateral) | |
|---|---|---|---|
| n | 5 | 6 | 5 |
| Mean arterial pressure, mmHg | |||
| Baseline | 99 ± 3 | 96 ± 3 | 98 ± 2 |
| 10 min | 115 ± 4† | 104 ± 2* | 107 ± 3* |
| 20 min | 120 ± 4† | 106 ± 2†‡ | 110 ± 4† |
| 30 min | 123 ± 3† | 105 ± 3*§ | 109 ± 3†‡ |
| 40 min | 108 ± 6* | 99 ± 3 | 106 ± 2 |
| 50 min | 97 ± 5 | 97 ± 4 | 95 ± 2 |
| 60 min | 101 ± 3 | 95 ± 3 | 93 ± 2 |
| Heart rate, beats/min | |||
| Baseline | 324 ± 10 | 340 ± 12 | 339 ± 8 |
| 10 min | 367 ± 6† | 339 ± 12 | 330 ± 13 |
| 20 min | 416 ± 11† | 353 ± 12§ | 339 ± 17§ |
| 30 min | 443 ± 10† | 367 ± 11§ | 349 ± 16§ |
| 40 min | 435 ± 10† | 373 ± 13*§ | 354 ± 14§ |
| 50 min | 393 ± 25† | 371 ± 12* | 357 ± 10 |
| 60 min | 382 ± 20† | 364 ± 10 | 351 ± 8 |
Values are means ± SE; n = 5–6 for each group of rats.
P < 0.05 and
P < 0.01 compared with baseline;
P < 0.05 and
P < 0.01 compared with the normal group.
Table 2.
Changes of creatinine clearance during intravenous infusion of glucagon-like peptide-1
Values are means ± SE of creatinine clearance (in μl·min−1·g kidney wt−1); n = 5 for each group of rats.
P < 0.05 compared with baseline;
P < 0.05 compared with the normal group.
DISCUSSION
We have shown that GLP-1Rs are avidly expressed in the renal pelvic wall, being adjacent to afferent renal nerve fibers immunoreactive to CGRP. Intrapelvic injection of GLP-1 increased ARNA that was attenuated by prior injection of the GLP-1R antagonist exendin 9–39. Intravenous infusion of GLP-1 also increased ARNA, suggesting that GLP-1 in the circulation could reach the renal pelvis to activate the afferent renal nerve. Furthermore, total RSNA had an increased response to intravenous infusion of GLP-1, and after the distal end of the nerve was cut to eliminate the afferent signal, the increase in ERNA to GLP-1 was significantly attenuated, suggesting that increased total RSNA appears to be mediated by renal afferent signals. GLP-1-induced diuresis and natriuresis were enhanced by both T-RDN and A-RDN, and these changes to GLP-1 were not significantly different between T-RDN and A-RDN. This study indicates that the diuretic and natriuretic effects of GLP-1 are partly governed via afferent renal nerve activation, potentially by GLP-1 acting on sensory nerve endings within the pelvis of the kidney.
We have demonstrated that GLP-1Rs are avidly expressed in the pelvis compared with the cortex of the kidney by immunohistochemistry and Western blot analysis. The precise localization of GLP-1R has been a subject of debate because of lack of specificity of conventional polyclonal antibodies against GLP-1R (1, 16, 45, 46). In the present study, we used a newly developed monoclonal antibody and confirmed specificity of the antibody by positive staining in GLP-1R-transfected HEK-293 cells while observing no staining in untransfected cells. Double-labeling immunohistochemistry for GLP-1R and CGRP revealed that there is no unequivocal colocalization of GLP-1Rs and afferent renal nerve fibers. These findings imply that GLP-1 signaling might affect the internal environment, including the chemoreceptors in the pelvis, and thus interact with afferent renal nerves. Further elucidation of this signaling remains to be examined.
Intrapelvic injection of GLP-1 increased ARNA, and this response was attenuated by prior injection of the GLP-1R antagonist exendin 9-39, suggesting that GLP-1 acted on afferent renal nerve via canonical GLP-1R in the pelvis. It is known that some cardiovascular actions of GLP-1, including vasodilatation in rodents, are mediated by a GLP-1R-independent mechanism (2). However, the effect of GLP-1 on renal afferent nerve demonstrated in the present study appears to be a GLP-1R-dependent mechanism.
In the present study, intravenous infusion of GLP-1 induced diuresis and natriuresis, with attendant increase in CrCl. These results agree with previous reports that demonstrated that GLP-1 directly acts on afferent arterioles or proximal tubules in the kidney to induce diuresis and natriuresis and increase CrCl and GFR (4, 5, 9, 21, 57). Moreover, we found that intravenous infusion of GLP-1 concomitantly increased RSNA, MAP, and HR. Furthermore, GLP-1-induced diuresis and natriuresis were enhanced by T-RDN. We propose that the increase in RSNA leads to an antidiuretic effect by increased Na+ reabsorption and renal vascular resistance because of increased ERNA (22, 40). Hence, T-RDN inhibited the antidiuretic effect by a GLP-1-induced increase in RSNA, thereby eliciting enhanced GLP-1-induced diuresis and natriuresis. Moreover, GLP-1-induced diuresis and natriuresis were enhanced by A-RDN similar to T-RDN, suggesting that afferent renal nerves are critical for the GLP-1-induced increase in RSNA. Taken together, it is plausible that intravenous infusion of GLP-1 increases ERNA at least in part by increased ARNA. Consistent with this hypothesis, ERNA recording obtained by cutting the distal end of the renal nerve to eliminate the afferent signal demonstrated that the increase in ERNA during intravenous infusion of GLP-1 was diminished compared with the increase in total RSNA. It is notable that even after the distal end of the renal nerve was cut, intravenous infusion of GLP-1 still moderately increased ERNA, MAP, and HR, suggesting that GLP-1 could increase sympathetic outflow possibly via a central action either crossing the BBB and/or vagal afferent pathway. This observation indicates that intravenous infusion of GLP-1 moderately increases ERNA via a central action in the A-RDN group, which has intact efferent fibers, but not in the T-RDN group. Hence, in the A-RDN group, the moderate antidiuretic effect of GLP-1 by central activation of ERNA is still maintained. These findings are consistent with the observation that changes in cumulative urine flow and Na+ excretion to GLP-1 are slightly increased in the T-RDN group compared with the A-RDN group, although these are not statistically different (∆cumulative urine flow at 60 min: 1,174 ± 368 vs. 655 ± 250 μl/g kidney wt, P = 0.26; and ∆cumulative Na+ excretion at 60 min: 220 ± 72 vs. 130 ± 32 μeq/g kidney wt, P = 0.25, n = 6–7). These observations and previous findings (4, 5, 9, 21, 27, 57) collectively indicate that GLP-1 acts on renal artery and tubules in the kidney and/or atrium in the heart to induce diuresis and natriuresis. Subsequently, GLP-1 increases ERNA via afferent renal nerve and the BBB/vagal afferent pathway, thereby inhibiting diuresis and natriuresis as a negative feedback mechanism.
We showed that GLP-1-induced increases in diuresis, natriuresis, and CrCl were enhanced by T-RDN. In contrast, a previous study (38) reported that the diuretic, natriuretic, and GFR responses to GLP-1 were attenuated in the denervated kidney. There are several differences between our study and this previous study in terms of the methodology and baseline conditions that may explain the disparate responses between these studies. First, rats were anesthetized with urethane and α-chloralose or Inactin in our study, whereas they were anesthetized with ketamine and thiobutabarbital in the previous study. Second, basal MAP was 96 ± 3 mmHg and infusion of GLP-1 significantly increased MAP, HR, and RSNA in our study, whereas basal MAP was 130 ± 4 mmHg and infusion of GLP-1 had no significant effect on MAP in the previous study (38). There was a clear increase in MAP in our study. Hence, the different results between our study and the previous study could reflect the different hemodynamic and sympathetic parameters under baseline conditions, which may likely be at a relatively higher levels in terms of basal sympathetic nerve activity in the previous report, masking the excitatory component of the response.
We observed that MAP and HR were increased during intravenous infusion of GLP-1, and these results agree with other previous reports in which acute administration of GLP-1 leads to transient increases in MAP and HR (8, 59, 61). Moreover, we found that MAP and HR increased by intravenous infusion of GLP-1 were attenuated by T-RDN and A-RDN, in which the degrees of these responses to GLP-1 were not significantly different between T-RDN and A-RDN. These findings suggest that afferent renal nerve activation by GLP-1 increases sympathetic outflow to the heart and arterioles in addition to the kidney, possibly via central nervous system reflex pathways. We have previously reported that electrical stimulation of afferent renal nerve activates rostral ventrolateral medulla-projecting paraventricular nucleus neurons specifically in the hypothalamus (60). It is conceivable that this afferent neural pathway from the kidneys could contribute to increasing sympathetic outflow by acute administration of GLP-1 demonstrated in present study.
On the other hand, we demonstrated that T-RDN increased diuresis and natriuresis despite a decrease in MAP because of GLP-1, suggesting that GLP-1-induced diuresis and natriuresis were not mainly mediated by pressure natriuresis per se but appear to be regulated by RSNA.
In the present study, T-RDN was performed by acute surgical cutting of the left renal nerves. This protocol has a possibility that there are compensatory changes in contralateral ERNA, which affects the changes of MAP and HR (7, 28, 49). However, the present study demonstrated that unilateral T-RDN, as well as bilateral A-RDN, attenuated MAP and HR increased by GLP-1, suggesting that unilateral T-RDN is possibly sufficient to interrupt GLP-1-mediated renal afferent input to the central nervous system and reduce sympathetic outflow to affect GLP-1-mediated hemodynamic changes. This finding is supported with a previous report (49) showing that unilateral T-RDN restores global autonomic balance and improves baroreflex function in rabbits with heart failure. To address the potential additive effect of bilateral T-RDN, further investigations comparing the effect of unilateral to bilateral T-RDN on GLP-1-mediated hemodynamic changes are needed.
Regarding the physiological role of GLP-1 in the kidney, there are some reports from the point of view of the “gastrointestinal-renal axis” in the regulation of Na+ balance and blood pressure (4, 9, 31, 50, 62). One of these reports demonstrated that intravenous infusion of the GLP-1R antagonist exendin 9–39 decreased urine flow, Na+ excretion, and GFR via reduction of PKA-mediated inhibition of NHE3 transport function in the proximal tubule of the kidney in normal rats (9), suggesting that endogenous GLP-1 exerts tonic natriuretic action. It is of interest that renal nerve stimulation leads to the activation of NHE3 via ANG II type 1 receptors in normal rats (44). Another study (31) reported that compared with intraperitoneal Na+ administration, oral Na+ loading induced a significant increase in the plasma GLP-1 level and resulted in more prominent natriuresis and a smaller increase in blood Na+ concentration in rats. The present study is the first to demonstrate that GLP-1 acts on its receptor in the pelvis of the kidney and modulates RSNA. These findings suggest the possibility that GLP-1 produced in the gut acts on the renal arteriole via the circulation and then could be filtered by glomerulus and act on tubules and pelvis within the kidney. It should be noted that the dose of 3 µM GLP-1 injected into the renal pelvis in the present study is higher than physiological plasma levels of GLP-1, which are from 1 to 10 pM with fasting to 15−50 pM under postprandial conditions (20, 30, 39). The dose of GLP-1 in the renal pelvis has not been determined and may be concentrated more than anywhere else in the kidney. A previous report (38) has suggested that intravenous infusion of GLP-1 at 1 μg·kg−1·min−1 for 20 min increases circulating levels of GLP-1 from <125 pg/ml to 18.8 ± 1.4 ng/ml. The effect of intravenous infusion of GLP-1 at 1 μg·kg−1·min−1 is comparable to that of intraperitoneal injection of the GLP-1R agonist exendin-4 at 5 μg/kg on renal functions: urine flow, Na+ excretion, renal plasma flow, GFR, and urinary cAMP excretion (5). In patients with diabetes, the maximum plasma concentrations after intraperitoneal injection of exendin-4 at 5 and 10 μg/kg, which are clinical doses, are 121 and 286 pg/ml, respectively (29). Taken together, the GLP-1R agonist has a 70–165 times greater effect on the kidney than GLP-1. After intraperitoneal injection of liraglutide, another GLP-1R agonist, at 15 μg/kg as a clinical dose, the maximum plasma concentration reaches to 10−15 nM (6, 52), which can be estimated to be comparable to the concentration of 2.5 μM GLP-1. Furthermore, we demonstrated that intravenous infusion of GLP-1 at 1 μg·kg−1·min−1 for 30 min increased ARNA. These findings suggest that the doses of GLP-1 used in present study increase ARNA and ERNA and might be relevant to pharmacological doses of GLP-1. This implies as a possibility that the GLP-1R agonist could exhibit a substantial effect similar to 3 μM GLP-1 used in the present study. Hence, the findings demonstrated in the present study are likely relevant to GLP-1 administration in the pharmacological range in addition to potentially physiological levels. The overall physiological role and function of GLP-1 in the kidney remain to be determined, and further investigations are needed.
In conclusion, we have shown that GLP-1R is expressed in the renal pelvic wall and that intrapelvic injection of GLP-1 activates renal afferent nerves. Intravenous infusion of GLP-1 increases RSNA, MAP, and HR, and T-RDN enhances diuretic and natriuretic responses and attenuates pressor and tachycardiac responses to intravenous infusion of GLP-1. Furthermore, selective A-RDN has a comparable effect with T-RDN on GLP-1-induced diuresis and natriuresis and hemodynamic changes. It is concluded that diuretic and natriuretic responses to GLP-1 are regulated in an inhibitory manner by neural circuitry of afferent and efferent renal nerves. These effects of GLP-1 on renal nerves contribute to the regulation of Na+ and water balance and may possibly maintain homeostasis in fluid balance and blood pressure under physiological and pathophysiological conditions.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK-114663 and P01-HL-62222 and an endowed McIntyre Professorship (to K. P. Patel) and Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad (to K. Katsurada).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.K. and K.P.P. conceived and designed research; K.K., S.S.N., N.M.S., H.Z., and X.L. performed experiments; K.K., S.S.N., N.M.S., H.Z., and X.L. analyzed data; K.K., S.S.N., N.M.S., H.Z., X.L., and K.P.P. interpreted results of experiments; K.K., N.M.S., H.Z., and X.L. prepared figures; K.K. and K.P.P. drafted manuscript; K.K. and K.P.P. edited and revised manuscript; K.K., S.S.N., N.M.S., H.Z., X.L., and K.P.P. approved final version of manuscript.
REFERENCES
- 1.Baggio LL, Yusta B, Mulvihill EE, Cao X, Streutker CJ, Butany J, Cappola TP, Margulies KB, Drucker DJ. GLP-1 receptor expression within the human heart. Endocrinology 159: 1570–1584, 2018. doi: 10.1210/en.2018-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117: 2340–2350, 2008. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 3.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68: 1415–1423, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carraro-Lacroix LR, Malnic G, Girardi AC. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol 297: F1647–F1655, 2009. doi: 10.1152/ajprenal.00082.2009. [DOI] [PubMed] [Google Scholar]
- 5.Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol 301: F355–F363, 2011. doi: 10.1152/ajprenal.00729.2010. [DOI] [PubMed] [Google Scholar]
- 6.Damholt B, Golor G, Wierich W, Pedersen P, Ekblom M, Zdravkovic M. An open-label, parallel group study investigating the effects of age and gender on the pharmacokinetics of the once-daily glucagon-like peptide-1 analogue liraglutide. J Clin Pharmacol 46: 635–641, 2006. doi: 10.1177/0091270006288215. [DOI] [PubMed] [Google Scholar]
- 7.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 8.Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab 24: 15–30, 2016. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Farah LX, Valentini V, Pessoa TD, Malnic G, McDonough AA, Girardi AC. The physiological role of glucagon-like peptide-1 in the regulation of renal function. Am J Physiol Renal Physiol 310: F123–F127, 2016. doi: 10.1152/ajprenal.00394.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson M, Bell C. Ultrastructural localization and characterization of sensory nerves in the rat kidney. J Comp Neurol 274: 9–16, 1988. doi: 10.1002/cne.902740103. [DOI] [PubMed] [Google Scholar]
- 11.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101: 515–520, 1998. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol 308: R112–R122, 2015. doi: 10.1152/ajpregu.00427.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiu K, Shibata M, Nakayama Y, Ogata F, Matsumoto S, Noshita K, Iwami S, Nakae S, Komuro I, Nagai R, Manabe I. A heart-brain-kidney network controls adaptation to cardiac stress through tissue macrophage activation. Nat Med 23: 611–622, 2017. doi: 10.1038/nm.4326. [DOI] [PubMed] [Google Scholar]
- 14.Goodwill VS, Terrill C, Hopewood I, Loewy AD, Knuepfer MM. CNS sites activated by renal pelvic epithelial sodium channels (ENaCs) in response to hypertonic saline in awake rats. Auton Neurosci 204: 35–47, 2017. doi: 10.1016/j.autneu.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 89: 3055–3061, 2004. doi: 10.1210/jc.2003-031403. [DOI] [PubMed] [Google Scholar]
- 16.Hirata K, Kume S, Araki S, Sakaguchi M, Chin-Kanasaki M, Isshiki K, Sugimoto T, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Uzu T. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun 380: 44–49, 2009. doi: 10.1016/j.bbrc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 18.Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13: 33, 2012. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki Y, Goswami C, Yada T. Glucagon-like peptide-1 and insulin synergistically activate vagal afferent neurons. Neuropeptides 65: 77–82, 2017. doi: 10.1016/j.npep.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki Y, Sendo M, Dezaki K, Hira T, Sato T, Nakata M, Goswami C, Aoki R, Arai T, Kumari P, Hayakawa M, Masuda C, Okada T, Hara H, Drucker DJ, Yamada Y, Tokuda M, Yada T. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat Commun 9: 113, 2018. doi: 10.1038/s41467-017-02488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen EP, Poulsen SS, Kissow H, Holstein-Rathlou NH, Deacon CF, Jensen BL, Holst JJ, Sorensen CM. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am J Physiol Renal Physiol 308: F867–F877, 2015. doi: 10.1152/ajprenal.00527.2014. [DOI] [PubMed] [Google Scholar]
- 22.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol 1: 731–767, 2011. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 23.Kakei M, Yada T, Nakagawa A, Nakabayashi H. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Auton Neurosci 102: 39–44, 2002. doi: 10.1016/S1566-0702(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 24.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 18: 7–14, 2002. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 25.Katsurada K, Maejima Y, Nakata M, Kodaira M, Suyama S, Iwasaki Y, Kario K, Yada T. Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem Biophys Res Commun 451: 276–281, 2014. doi: 10.1016/j.bbrc.2014.07.116. [DOI] [PubMed] [Google Scholar]
- 26.Katsurada K, Yada T. Neural effects of gut- and brain-derived glucagon-like peptide-1 and its receptor agonist. J Diabetes Investig 7, Suppl 1: 64–69, 2016. doi: 10.1111/jdi.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 19: 567–575, 2013. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 28.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol 308: R79–R95, 2015. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kothare PA, Linnebjerg H, Isaka Y, Uenaka K, Yamamura A, Yeo KP, de la Peña A, Teng CH, Mace K, Fineman M, Shigeta H, Sakata Y, Irie S. Pharmacokinetics, pharmacodynamics, tolerability, and safety of exenatide in Japanese patients with type 2 diabetes mellitus. J Clin Pharmacol 48: 1389–1399, 2008. doi: 10.1177/0091270008323750. [DOI] [PubMed] [Google Scholar]
- 30.Kuhre RE, Gribble FM, Hartmann B, Reimann F, Windeløv JA, Rehfeld JF, Holst JJ. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am J Physiol Gastrointest Liver Physiol 306: G622–G630, 2014. doi: 10.1152/ajpgi.00372.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutina AV, Golosova DV, Marina AS, Shakhmatova EI, Natochin YV. Role of vasopressin in the regulation of renal sodium excretion: interaction with glucagon-like peptide-1. J Neuroendocrinol 28: 1, 2016. doi: 10.1111/jne.12367. [DOI] [PubMed] [Google Scholar]
- 32.Li YF, Mayhan WG, Patel KP. Role of the paraventricular nucleus in renal excretory responses to acute volume expansion: role of nitric oxide. Am J Physiol Heart Circ Physiol 285: H1738–H1746, 2003. doi: 10.1152/ajpheart.00727.2002. [DOI] [PubMed] [Google Scholar]
- 33.Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 38: 132–139, 2015. doi: 10.2337/dc14-1958. [DOI] [PubMed] [Google Scholar]
- 34.Mann JFE, Fonseca V, Mosenzon O, Raz I, Goldman B, Idorn T, von Scholten BJ, Poulter NR. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation 138: 2908–2918, 2018. doi: 10.1161/CIRCULATIONAHA.118.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marfurt CF, Echtenkamp SF. Sensory innervation of the rat kidney and ureter as revealed by the anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) from dorsal root ganglia. J Comp Neurol 311: 389–404, 1991. doi: 10.1002/cne.903110309. [DOI] [PubMed] [Google Scholar]
- 36.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375: 1834–1844, 2016. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 37.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375: 311–322, 2016. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno C, Mistry M, Roman RJ. Renal effects of glucagon-like peptide in rats. Eur J Pharmacol 434: 163–167, 2002. doi: 10.1016/S0014-2999(01)01542-4. [DOI] [PubMed] [Google Scholar]
- 39.Muskiet MHA, Tonneijck L, Smits MM, van Baar MJB, Kramer MHH, Hoorn EJ, Joles JA, van Raalte DH. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 13: 605–628, 2017. doi: 10.1038/nrneph.2017.123. [DOI] [PubMed] [Google Scholar]
- 40.Osborn JW, Foss JD. Renal nerves and long-term control of arterial pressure. Compr Physiol 7: 263–320, 2017. doi: 10.1002/cphy.c150047. [DOI] [PubMed] [Google Scholar]
- 41.Patel KP, Carmines PK. Renal interstitial hydrostatic pressure and sodium excretion during acute volume expansion in diabetic rats. Am J Physiol Regul Integr Comp Physiol 281: R239–R245, 2001. doi: 10.1152/ajpregu.2001.281.1.R239. [DOI] [PubMed] [Google Scholar]
- 42.Patel KP, Xu B, Liu X, Sharma NM, Zheng H. Renal denervation improves exaggerated sympathoexcitation in rats with heart failure: a role for neuronal nitric oxide synthase in the paraventricular nucleus. Hypertension 68: 175–184, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polhemus DJ, Trivedi RK, Gao J, Li Z, Scarborough AL, Goodchild TT, Varner KJ, Xia H, Smart FW, Kapusta DR, Lefer DJ. Renal sympathetic denervation protects the failing heart via inhibition of neprilysin activity in the kidney. J Am Coll Cardiol 70: 2139–2153, 2017. doi: 10.1016/j.jacc.2017.08.056. [DOI] [PubMed] [Google Scholar]
- 44.Pontes RB, Crajoinas RO, Nishi EE, Oliveira-Sales EB, Girardi AC, Campos RR, Bergamaschi CT. Renal nerve stimulation leads to the activation of the Na+/H+ exchanger isoform 3 via angiotensin II type I receptor. Am J Physiol Renal Physiol 308: F848–F856, 2015. doi: 10.1152/ajprenal.00515.2014. [DOI] [PubMed] [Google Scholar]
- 45.Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155: 1280–1290, 2014. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 46.Pyke C, Knudsen LB. The glucagon-like peptide-1 receptor−or not? Endocrinology 154: 4–8, 2013. doi: 10.1210/en.2012-2124. [DOI] [PubMed] [Google Scholar]
- 47.Roubille F, Morena M, Leray-Moragues H, Canaud B, Cristol JP, Klouche K. Pharmacologic therapies for chronic and acute decompensated heart failure: specific insights on cardiorenal syndromes. Blood Purif 37, Suppl 2: 20–33, 2014. doi: 10.1159/000361061. [DOI] [PubMed] [Google Scholar]
- 48.Salman IM. Cardiovascular autonomic dysfunction in chronic kidney disease: a comprehensive review. Curr Hypertens Rep 17: 59, 2015. doi: 10.1007/s11906-015-0571-z. [DOI] [PubMed] [Google Scholar]
- 49.Schiller AM, Haack KK, Pellegrino PR, Curry PL, Zucker IH. Unilateral renal denervation improves autonomic balance in conscious rabbits with chronic heart failure. Am J Physiol Regul Integr Comp Physiol 305: R886–R892, 2013. doi: 10.1152/ajpregu.00269.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul Pept 141: 120–128, 2007. doi: 10.1016/j.regpep.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, Schäffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 124: 4473–4488, 2014. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 81: 161–168, 2008. doi: 10.1016/j.diabres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Sharp TE III, Polhemus DJ, Li Z, Spaletra P, Jenkins JS, Reilly JP, White CJ, Kapusta DR, Lefer DJ, Goodchild TT. Renal denervation prevents heart failure progression via inhibition of the renin-angiotensin system. J Am Coll Cardiol 72: 2609–2621, 2018. doi: 10.1016/j.jacc.2018.08.2186. [DOI] [PubMed] [Google Scholar]
- 54.Skov J, Dejgaard A, Frøkiær J, Holst JJ, Jonassen T, Rittig S, Christiansen JS. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab 98: E664–E671, 2013. doi: 10.1210/jc.2012-3855. [DOI] [PubMed] [Google Scholar]
- 55.Su HC, Wharton J, Polak JM, Mulderry PK, Ghatei MA, Gibson SJ, Terenghi G, Morrison JF, Ballesta J, Bloom SR. Calcitonin gene-related peptide immunoreactivity in afferent neurons supplying the urinary tract: combined retrograde tracing and immunohistochemistry. Neuroscience 18: 727–747, 1986. doi: 10.1016/0306-4522(86)90066-7. [DOI] [PubMed] [Google Scholar]
- 56.Takahama H, Kitakaze M. Pathophysiology of cardiorenal syndrome in patients with heart failure: potential therapeutic targets. Am J Physiol Heart Circ Physiol 313: H715–H721, 2017. doi: 10.1152/ajpheart.00215.2017. [DOI] [PubMed] [Google Scholar]
- 57.Thomson SC, Kashkouli A, Singh P. Glucagon-like peptide-1 receptor stimulation increases GFR and suppresses proximal reabsorption in the rat. Am J Physiol Renal Physiol 304: F137–F144, 2013. doi: 10.1152/ajprenal.00064.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72, 1996. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 59.Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res 114: 1788–1803, 2014. doi: 10.1161/CIRCRESAHA.114.301958. [DOI] [PubMed] [Google Scholar]
- 60.Xu B, Zheng H, Liu X, Patel KP. Activation of afferent renal nerves modulates RVLM-projecting PVN neurons. Am J Physiol Heart Circ Physiol 308: H1103–H1111, 2015. doi: 10.1152/ajpheart.00862.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 110: 43–52, 2002. doi: 10.1172/JCI0215595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang J, Jose PA, Zeng C. Gastrointestinal-renal axis: role in the regulation of blood pressure. J Am Heart Assoc 6: 6, 2017. doi: 10.1161/JAHA.117.005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 21: 1125–1135, 2003. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Zheng H, Katsurada K, Liu X, Knuepfer MM, Patel KP. Specific afferent renal denervation prevents reduction in neuronal nitric oxide synthase within the paraventricular nucleus in rats with chronic heart failure. Hypertension 72: 667–675, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng H, Liu X, Sharma NM, Patel KP. Renal denervation improves cardiac function in rats with chronic heart failure: effects on expression of β-adrenoceptors. Am J Physiol Heart Circ Physiol 311: H337–H346, 2016. doi: 10.1152/ajpheart.00999.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng H, Liu X, Rao US, Patel KP. Increased renal ENaC subunits and sodium retention in rats with chronic heart failure. Am J Physiol Renal Physiol 300: F641–F649, 2011. doi: 10.1152/ajprenal.00254.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng H, Li YF, Zucker IH, Patel KP. Exercise training improves renal excretory responses to acute volume expansion in rats with heart failure. Am J Physiol Renal Physiol 291: F1148–F1156, 2006. doi: 10.1152/ajprenal.00501.2005. [DOI] [PubMed] [Google Scholar]