Abstract
The role of dopamine D1-like receptors (DR) in the regulation of renal Na+ transporters, natriuresis, and blood pressure is well established. However, the involvement of the angiotensin 1–7 (ANG 1−7)-Mas receptor in the regulation of Na+ balance and blood pressure is not clear. The present study aimed to investigate the hypothesis that ANG 1–7 can regulate Na+ homeostasis by modulating the renal dopamine system. Sprague-Dawley rats were infused with saline alone (vehicle) or saline with ANG 1–7, ANG 1–7 antagonist A-779, DR agonist SKF38393, and antagonist SCH23390. Infusion of ANG 1–7 caused significant natriuresis and diuresis compared with saline alone. Both natriuresis and diuresis were blocked by A-779 and SCH23390. SKF38393 caused a significant, SCH23390-sensitive natriuresis and diuresis, and A-779 had no effect on the SKF38393 response. Concomitant infusion of ANG 1–7 and SKF38393 did not show a cumulative effect compared with either agonist alone. Treatment of renal proximal tubules with ANG 1–7 or SKF38393 caused a significant decrease in Na+-K+-ATPase and Na+/H+ exchanger isoform 3 activity. While SCH23390 blocked both ANG 1–7- and SKF38393-induced inhibition, the DR response was not sensitive to A-779. Additionally, ANG 1–7 activated PKG, enhanced tyrosine hydroxylase activity via Ser40 phosphorylation, and increased renal dopamine production. These data suggest that ANG 1–7, via PKG, enhances tyrosine hydroxylase activity, which increases renal dopamine production and activation of DR and subsequent natriuresis. This study provides evidence for a unidirectional functional interaction between two G protein-coupled receptors to regulate renal Na+ transporters and induce natriuresis.
Keywords: Na+-K+-ATPase, Na+/H+ exchanger, natriuresis, renal tubules
INTRODUCTION
It is well established that the renal dopamine system plays a pivotal role in maintaining body fluid and electrolyte balance and long-term blood pressure regulation, especially during the Na+-replete condition (17, 40, 53). Renal dopamine activates D1-like receptors (DR) and inhibits tubular Na+ transporters such as Na+-K+-ATPase and Na+/H+ exchanger isoform 3 (NHE3) and causes natriuresis and diuresis (10, 23, 36, 51). It is believed that the renal dopaminergic system is a local independent system that acts in an autocrine or paracrine manner (2, 3, 12). Renal dopamine production is dependent on tubular uptake of l-3,4-dihydroxyphenylalanine (l-DOPA) and enzymatic activity of aromatic l-amino acid decarboxylase (AADC), which converts l-DOPA to dopamine (2, 3, 12). The proximal tubules exhibit a high concentration of AADC and are considered the primary source of renal dopamine (3, 12, 13, 17). It has been demonstrated that renal tubule-specific deletion of AADC in mice leads to the development of hypertension and salt sensitivity (57). Although it has been postulated that other transporters belonging to solute carrier superfamily, which include basolateral organic cation transporters (OCT1, OCT2, and OCT3) and apical transporters (OCTN1, OCTN2, and OCTN3), also play a role in dopamine transport, their impact on Na+ regulation is not clear (30, 31, 55). It is widely perceived that l-DOPA, freely filtered by the glomerulus, is transported into proximal tubules mainly by Na+-independent l-amino acid transporter (LAT)2, making it a rate-limiting step in dopamine synthesis (3, 11, 27, 45). However, recent studies have shown that an alternative pathway involving tyrosine hydroxylase, an enzyme that converts tyrosine to l-DOPA, a rate-limiting step in neuronal dopamine synthesis, could be contributing to tubular dopamine production (52).
Renal dopamine interacts with angiotensin II (ANG II) receptors, both type 1 and type 2, to regulate Na+ balance and blood pressure (15, 35, 40, 41, 46, 50). Although the interaction with ANG II type 1 receptor is antagonistic in that dopamine suppresses ANG II-mediated antinatriuretic pathway, the interaction with type 2 receptors is synergistic, as DR stimulation induces natriuresis via the ANG type 2 receptor (15, 35, 40, 41, 46, 50). However, little is known about the interaction of renal dopamine with the angiotensin 1–7 (ANG 1−7)-Mas receptor (MasR) system. The role of ANG 1–7 in renal Na+ is controversial, with some studies suggesting antinatriuretic effect, whereas others showing a natriuretic effect (21, 37, 38, 43). It has been shown that ANG 1–7 can activate serine/threonine protein kinases and inhibits renal proximal tubular Na+ transporters; however, the mechanism remains unclear (14, 34). It has been previously reported that ANG 1–7 activates PKG, and PKG has been shown to stimulate tyrosine hydroxylase by phosphorylating the enzyme at Ser40 (22, 26). Activation of tyrosine hydroxylase converts tyrosine to l-DOPA, a rate-limited step in dopamine synthesis. Interestingly, both PKG and tyrosine hydroxylase are highly expressed in epithelial cells (1, 16, 20, 29, 52). Therefore, in the present study, we tested the hypothesis that ANG 1–7-MasR signaling could increase renal dopamine production via tyrosine hydroxylase stimulation, which would activate renal DR and induce natriuresis and diuresis.
MATERIALS AND METHODS
Materials.
ANG 1–7 (Asp-Arg-Val-Tyr-Ile-His-Pro), A-779 (D-Ala7-ANG 1–7, a selective ANG 1–7 antagonist), ouabain (a Na+-K+-ATPase inhibitor), S3226 {3-[2-(3-guanidino-2-methyl-3-oxo-propenyl)-5-methyl-phenyl]-N-isopropylidene-2-methyl-acrylamide dihydrochloride, an NHE3 inhibitor}, KT5823 (a PKG inhibitor), 3-hydroxybenzylhydrazine dihydrochloride (HBH; an AADC inhibitor), 3-iodo-l-tyrosine (a tyrosine hydroxylase inhibitor), KT5720 (a PKA inhibitor), monoclonal anti-tyrosine hydroxylase antibody (catalog no. T2928), anti-phosphotyrosine hydroxylase (pSer40) antibody (catalog no. SAB4503789), secondary antibodies, and other analytic grade chemicals, unless otherwise mentioned, were purchased from Millipore Sigma (St. Louis MO). PKG-1 antibody (catalog no. 3248) was purchased from Cell Signaling (Danvers, MA).
Surgical procedure for ANG 1–7 renal responses.
Eight-week-old male Sprague-Dawley rats (Harlan, Indianapolis, IN) were group housed and had free access to water and normal rodent diet. All of the experimental procedures were approved by the Institutional Animal Care and Use Committee. Surgeries were performed as previously described in detail (39); briefly, rats were anesthetized with an isoflurane-oxygen mixture, and blood pressure was measured by catheterizing the left carotid artery with a solid-state pressure transducer connected to power laboratory (Analog Devices, Colorado Springs, CO). For drug administration, the left jugular vein was catheterized with PE-50 tubing; for urine collection, a midline abdomen incision was made to catheterize the urinary bladder. Throughout the surgery, animals were infused with normal saline (1% body wt·ml−1·h−1), and blood pressure and heart rate were continuously monitored. To determine the effect of ANG 1–7 on Na+ and water excretion, rats were infused with saline alone or saline with ANG 1–7 (1 μg·kg−1·min−1) or SKF38393 (1 μg·kg−1·min−1). A-779 (100 μg/kg), SCH23390 (100 μg/kg), or KT5823 (10 μg/kg) were administered intravenously as a single bolus dose immediately before the initiation of ANG 1–7 or SKF38393 infusion. The procedure consisted of a 40-min stabilization period after the surgery followed by a 45-min collection of urine in the absence of drugs (saline alone) followed by another 45-min period urine collection during drug infusion. Urine and plasma Na+ concentration were measured by atomic absorbance spectroscopy (Perkin-Elmer AA400), and creatinine levels were measured by creatinine analyzer (model 2, Beckman, Brea, CA). Urinary volume was measured by Rainin electronic pipet (Mettler-Toledo Rainin, Oakland CA). Glomerular filtration rate (GFR; in ml/min) was calculated on the basis of the clearance of creatinine, and the fractional excretion of Na+ (FENa; in %) was calculated on the basis of the clearance of Na+ and creatinine. Urinary dopamine was measured by HPLC-MS, as previously described (6).
Na+-K+-ATPase and NHE3 activity.
A separate group of rats was used to prepare renal proximal tubules, as detailed in our previous publications (6–8). Renal proximal tubules were incubated for 10 min at 37°C with ANG 1–7 (0.1 µmol/l), SKF38393 (0.1 µmol/l), A-779 (10.0 µmol/l), SCH23390 (10.0 µmol/l), KT5823 (1.0 µmol/l), HBH (100 µmol/l), and 3-iodo-l-tyrosine (100.0 µmol/l). Na+-K+-ATPase activity was determined by the method of Quigley and Gotterer (47), with slight modifications, as previously reported (6–8). Tubules were lysed by rapid freezing and thawing with liquid nitrogen, and protein was assayed by using a BCA kit (ThermoFisher Scientific, Waltham, MA). The lysed tubular suspension (0.1 mg protein/ml) was used to assay ouabain (4 mmol/l)-sensitive Na+-K+-ATPase activity using end-point phosphate hydrolysis of ATP (4 mmol/l) (6–8). NHE3 activity was measured in proximal tubular brush-border membranes, as previously described (7).
Tyrosine hydroxylase and PKG expression and activity.
Tyrosine hydroxylase expression and phosphorylation were determined by ELISA or Western blot analysis, according to our previously published standardized protocol (5, 9). Briefly, microplates were coated with an antigen (equal amount of cell lysate protein) and incubated with anti-tyrosine hydroxylase or anti-phospho-tyrosine hydroxylase (pSer40) antibody and quantitated by ELISA (5). We also coated microplates with an anti-tyrosine hydroxylase or anti-phosphotyrosine hydroxylase (pSer40) antibody followed by incubation with an equal amount of cell lysate protein followed by ELISA (5). Renal proximal tubular tyrosine hydroxylase activity was measured as previously described by Baillien et al. (4). Briefly, proximal tubules were homogenized in potassium phosphate buffer (50 mmol/l, pH 6.0), and 80 mg (1 mg/ml protein concentration) were added to an assay mixture containing 25 mmol/l l-tyrosine, ferrous ammonium sulfate (10 mmol/l), catalase (3,200 units), ascorbic acid (1 mmol/l), tetrahydrobiopterin, and 0.1 mmol/l HBH dissolved fresh in a small volume of 0.01 mol/l HCl and 50 mmol/l potassium phosphate buffer at pH 6.0 without and with 3-iodo-l-tyrosine (100 µmol/l). The mixture was incubated for 15 min at 37°C, and the reaction was stopped by adding chilled 10% trichloroacetic acid. l-DOPA was measured by HPLC-MS, as previously described by Haavik and Flatmark (28) and detailed in our previous publication (6). PKG expression was determined by ELISA (5), and activity was measured as previously described by Fiscus and Murad (25) and as previously detailed (9). Renal proximal tubular PKA activity was determined, as previously detailed by Corbin and Reimann (18), in the absence and presence of forskolin (10 µmol/l), KT5720 (0.3 µmol/l), and KT5823 (1.0 µmol/l).
Statistical analysis.
Differences between means were evaluated using ANOVA followed by a post hoc Newman-Keuls multiple test. P values of <0.05 were considered statistically significant. For in vivo experiments (drug infusion), 10–12 rats were used in each group; for ex vivo experiments involving proximal tubules, 6–8 rats were used in each group. Experiments involving biochemical analysis were performed in quadruplicate. ELISA was performed in quintuplicate i.e., at least five wells were used for a single sample.
RESULTS
Effect of ANG 1–7 on urine flow, urinary Na+ excretion, fractional excretion of Na+, and urinary dopamine excretion.
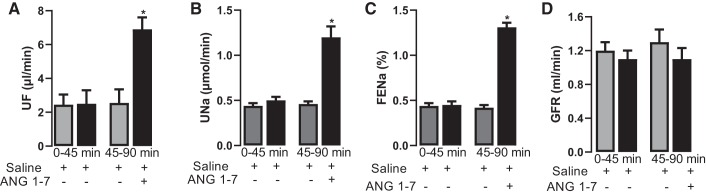
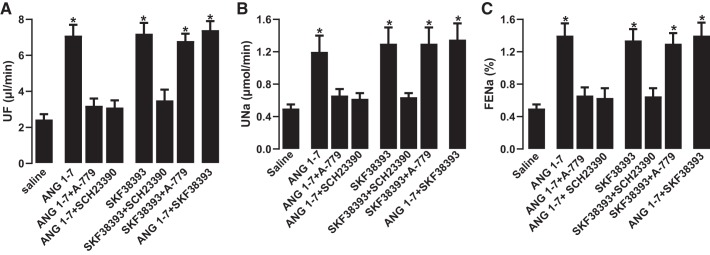
Intravenous administration of ANG 1–7 significantly increased urine flow, urinary Na+ excretion, and FENa compared with saline infusion alone (Fig. 1, A–C). ANG 1–7 had no effect on GFR (Fig. 1D). ANG 1–7-mediated increases in urine volume, urinary Na+ excretion, and FENa were abolished by the ANG 1–7 antagonist A-779 and the DR blocker SCH23390 (Fig. 2, A–C). SKF38393-mediated natriuresis and diuresis were blocked by SCH23390 but were insensitive to A-779 (Fig. 2, A–C). Concomitant administration of ANG 1–7 and SKF38393 did not have a cumulative effect on natriuresis or diuresis compared with ANG 1–7 or SKF38393 alone (Fig. 2, A–C). A-779 per se had no effect, whereas SCH23390 alone or in combination with A-779 reduced urine flow, urinary Na+ excretion, or FENA, but the difference was not statistically significant compared with saline (data not shown). ANG 1–7 and SKF38393 had no effect on blood pressure compared with saline (mean arterial pressure: 107.33 ± 5.36 mmHg with saline, 101.03 ± 6.01 mmHg with ANG 1−7, and 105.65 ± 4.88 mmHg with SKF38393). A-779 and SCH23390 also had no significant effect on blood pressure (data not shown).
Fig. 1.
Effect of angiotensin 1−7 (ANG 1–7) on urine flow (UF), urinary Na+ excretion (UNa), fractional excretion of Na+ (FENa), and glomerular filtration rate (GFR). Rats were infused with saline, and urine was collected for 45 min to establish a baseline. One group was continued on saline, whereas the other group was administered ANG 1–7 in saline for 45 min. UF (A), UNa (B), FENa (C), and GFR (D) were measured as described in materials and methods. n = 10–12 rats. *P < 0.05 vs. saline by repeated-measures ANOVA followed by a post hoc Newman-Keuls test.
Fig. 2.
Effect of angiotensin 1−7 (ANG 1–7) and dopamine D1-like receptor (DR) agonist SKF38393 on urine flow (UF), urinary Na+ excretion (UNa), and fractional excretion of Na+ (FENa). Rats were infused with saline alone or saline with ANG 1–7, SKF38393, ANG 1–7 antagonist A-779, and DR blocker SCH23390. After drug infusion was initiated, urine was collected for 45 min to measure UF (A), UNa (B), and FENa (C). n = 10–12 rats. *P < 0.05 vs. saline by one-way ANOVA followed by post hoc Newman-Keuls test.
ANG 1–7 administration caused a profound increase in urinary dopamine excretion compared with saline (dopamine: 4.63 ± 0.41 pmol/min with saline and 8.91 ± 0.71 pmol/min with ANG 1–7, P < 0.05 vs. saline). The increase in dopamine production was blocked by A-779 and KT5823, but dopamine production was not affected by SCH23390 (ANG 1–7 + A-779: 5.32 ± 0.51 pmol/min, ANG 1–7 + KT5823: 5.03 ± 0.44 pmol/min, and ANG 1–7 + SCH23390: 9.1 ± 0.89 pmol/min, P < 0.05 vs. saline). In the absence of ANG 1–7, SKF38393, SCH23390, A-779, and KT5823 per se had no significant effect on basal dopamine excretion (data not shown).
Effect of ANG 1–7 on renal Na+ transporters.
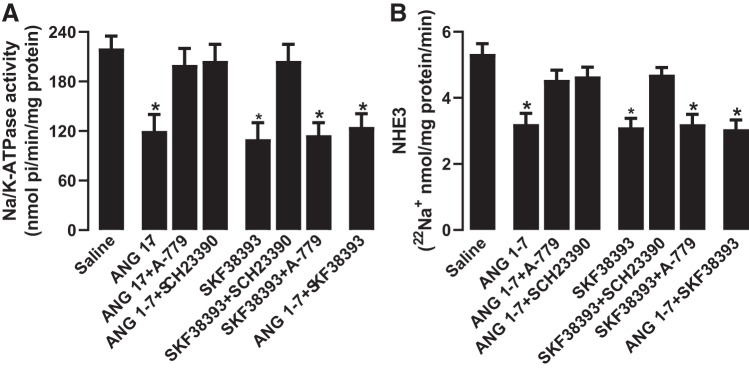
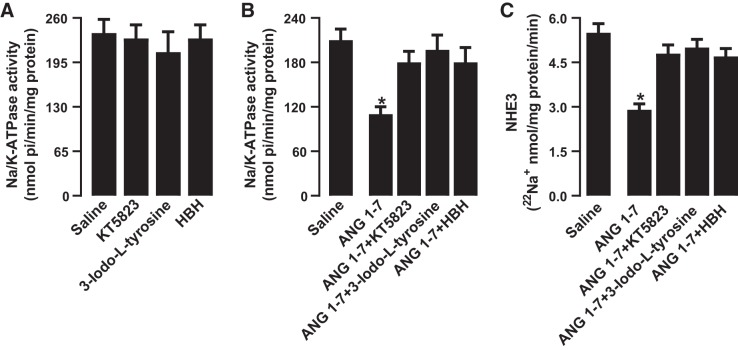
Incubation of renal proximal tubules with ANG 1–7 or SKF38393 inhibited Na+-K+-ATPase and NHE3 activity significantly compared with saline (Fig. 3, A and B). The inhibitory effect of ANG 1–7 on Na+ transporters was sensitive to both A-779 and SCH23390 (Fig. 3, A and B). However, SCH23390 but not A-779 blocked DR-mediated inhibition of Na+ transporters. Concomitant incubation of proximal tubules with ANG 1–7 and SKF38393 did not have a cumulative inhibitory effect on Na+ transporters (Fig. 3, A and B). A-779 and SCH23390 had no effect on basal Na+-K+-ATPase (saline: 225.33 ± 16.36 nmol·min−1·mg protein−1, A-779: 239.29 ± 21.22 nmol·min−1·mg protein−1, and SCH23390: 241.69 ± 19.89 nmol·min−1·mg protein−1) and NHE3 activity (saline: 5.3 ± 0.42 22Na+ nmol·mg protein−1·min−1, A-779: 4.9 ± 0.39 22Na+ nmol·mg protein−1·min−1, and SCH23390: 5.1 ± 0.44 22Na+ nmol·mg protein−1·min−1).
Fig. 3.
Effect of angiotensin 1−7 (ANG 1–7) and dopamine D1-like receptor (DR) agonist SKF38393 on renal proximal tubular Na+ transporters Na+-K+-ATPase and Na+/H+ exchanger isoform 3 (NHE3). Proximal tubules were incubated with ANG 1–7 and SKF38393 in the presence and absence of ANG 1–7 antagonist A-779 and DR blocker SCH23390. Na+-K+-ATPase activity was measured directly in lysed tubules (A), whereas NHE3 activity was measured in brush-border membranes isolated after incubation of tubules with the desired drugs (B). n = 6–8 rats. *P < 0.05 vs. saline by one-way ANOVA followed by a post hoc Newman-Keuls test. Na+-K+-ATPase and NHE3 were assayed in quadruplicate from each rat.
Effect of ANG 1–7 on PKG and tyrosine hydroxylase expression and activity.
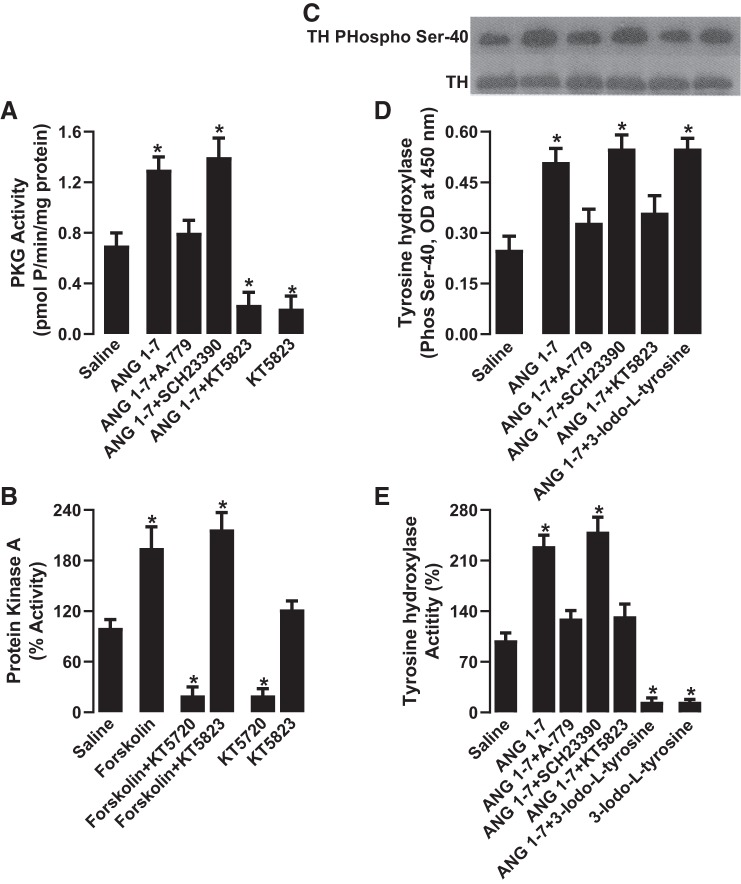
ANG 1–7 had no effect on PKG expression in renal proximal tubules [PKG-1 (α/β), optical density at 450 nm: 0.67 ± 0.11 with saline and 0.74 ± 0.13 with ANG 1–7]. However, ANG 1–7 significantly increased proximal tubular PKG activity, which was blocked by A-779 and the PKG inhibitor KT5823 but was insensitive to SCH23390 (Fig. 4A). KT5823 was able to inhibit PKA activity at a higher concentration. As shown in Fig. 4B, the concentration of KT5823 (1.0 µmol/l) used in the present study had no effect on PKA activity, whereas KT5720, a more specific PKA inhibitor, reduced both basal activity and forskolin-induced activation of PKA. ANG 1–7 did not affect tyrosine hydroxylase expression [optical density at 450 nm: 0.83 ± 0.17 with saline and 0.91 ± 0.13 with ANG 1–7; Fig. 4C, bottom, and Supplemental Fig. S1 (Supplemental Data are available online at https://doi.org/10.6084/m9.figshare.9201893.v2)]. However, ANG 1–7 significantly increased tyrosine hydroxylase Ser40 phosphorylation (Fig. 4, C, top, and D, and Supplemental Fig. S2) activity (Fig. 4E), both of which were blocked by A-779 and KT5823, whereas SCH23390 had no effect (Fig. 4, C–E). The tyrosine hydroxylase inhibitor 3-iodo-l-tyrosine had no effect on ANG 1–7-mediated Ser40 phosphorylation (Fig. 4, C, top, and D) but reduced basal tyrosine hydroxylase activity and abolished ANG 1–7-induced stimulation (Fig. 4E). Basal tyrosine hydroxylase activity was not affected by A-779, SCH23390, or KT5823 (data not shown).
Fig. 4.
Effect of angiotensin 1−7 (ANG 1–7) on PKG and tyrosine hydroxylase (TH) expression and activity. Renal proximal tubules were incubated with ANG 1–7 in the presence and absence of ANG 1–7 antagonist A-779, DR blocker SCH23390, PKG inhibitor KT5823, and TH inhibitor 3-iodo-l-tyrosine. Renal proximal tubular PKG activity (A) and PKA activity (B) are shown. TH expression and phosphorylation were determined by Western blot analysis (C) and ELISA (D). TH activity was determined by HPLC-MS (E). n = 6–8 rats. *P < 0.05 vs. saline by one-way ANOVA followed by a post hoc Newman-Keuls test. PKG and TH activity were assayed in quadruplicate from each rat; expression and Ser40 phosphorylation (ELISA) were performed in quintuplicate from each rat.
Effect of PKG, tyrosine hydroxylase, and dopamine decarboxylase inhibition on ANG 1–7-mediated inhibition of renal Na+ transporters.
Exposure of proximal tubules to PKG, tyrosine hydroxylase, and AADC inhibitors KT5823, 3-iodo-l-tyrosine, and HBH had no effect on basal Na+-K+-ATPase (Fig. 5A) or NHE3 activity (data not shown). However, inhibition of PKG, tyrosine hydroxylase, and AADC abolished ANG 1–7-mediated regulation of both Na+-K+-ATPase and NHE3 (Fig. 5, B and C). Inhibitors of PKG, tyrosine hydroxylase, and AADC failed to abolish SKF38393-induced inhibition of Na+-K+-ATPase or NHE3 (data not shown).
Fig. 5.
Role of dopamine synthesis enzymes on angiotensin 1−7 (ANG 1–7)-induced inhibition of Na+ transporters Na+-K+-ATPase and Na+/H+ exchanger isoform 3 (NHE3). Proximal tubules were incubated with ANG 1–7 and SKF38393 in the presence and absence of PKG inhibitor KT5823, tyrosine hydroxylase inhibitor 3-iodo-l-tyrosine, and aromatic l-amino acid decarboxylase inhibitor 3-hydroxybenzylhydrazine dihydrochloride (HBH). Na+-K+-ATPase activity was measured directly in lysed tubules (A and B), whereas NHE3 activity was measured in brush-border membranes isolated after incubation of tubules with the desired drugs (C). n = 6–8 rats. *P < 0.05 vs. saline by one-way ANOVA followed by a post hoc Newman-Keuls test. Na+-K+-ATPase and NHE3 were assayed in quadruplicate from each rat.
DISCUSSION
The present study demonstrates that ANG 1–7-MasR signaling increases renal dopamine production by stimulating PKG-tyrosine hydroxylase activity. Moreover, the increased dopamine production via DR inhibits renal Na+ transporters Na+-K+-ATPase and NHE3 and induces natriuresis and diuresis in response to ANG 1–7-MasR stimulation. Our results suggest that ANG 1–7-MasR signaling regulates renal Na+ excretion by modulating local dopamine production.
ANG 1–7, a relatively newer member of the renin-angiotensin-aldosterone system, is enzymatically generated directly from ANG I and ANG II by neprilysin and angiotensin-converting enzyme 2, respectively, or indirectly by angiotensin-converting enzyme 2-mediated conversion of ANG I to ANG 1–9, which is converted to ANG 1–7 by neprilysin or angiotensin-converting enzyme (48, 56). Proximal tubules are exposed to circulating ANG 1–7, as well as ANG 1–7, from the glomerular filtrate. In addition, kidneys are exposed to locally generated ANG 1–7, explaining the higher renal versus circulating ANG 1–7 levels. The role of ANG 1–7, unlike ANG II, in kidney electrolyte regulation is not clear. While earlier reports have suggested antinatriuretic and diuretic properties of renal ANG 1–7 in rats, recent reports have shown that the ANG 1–7 deficit could contribute to ANG II-mediated Na+ and water retention and the subsequent increase in blood pressure (32). The exact mechanisms for these discrepancies are not known; however, the variability in animal model, experimental site (ex vivo versus in vivo), and drug administration route could be possible causes for the variable outcome of the ANG 1–7 effect on renal Na+ regulation. In the present study, we found that acute ANG 1–7 administration caused robust natriuresis and diuresis without affecting GFR or blood pressure, suggesting a tubular effect. As expected, intravenous infusion of SKF38393 caused significant natriuresis and diuresis. Interestingly, the effects of ANG 1–7 were abolished by both ANG 1–7 antagonist and DR blocker; however, the effect of DR agonist was insensitive to ANG 1–7 antagonist. These data suggest that ANG 1–7-MasR-mediated Na+ excretion involves DR activation, while the natriuretic response to SKF38393 is independent of ANG 1–7-MasR signaling. These findings are novel, as they identify a unidirectional, as opposed to mutual, interaction between two G protein-coupled receptors to increase renal Na+ excretion.
To identify the mechanisms for the ANG 1–7-MasR and DR interaction in increasing Na+ excretion, we assessed the effect of ANG 1–7-MasR signaling on renal tubular Na+ transporters. The exposure of renal proximal tubules to ANG 1–7 or SKF38393 inhibited Na+-K+-ATPase and NHE3 activity. Interestingly, ANG 1–7-induced inhibition of Na+ transporters was sensitive to both A-779 and SCH23390, whereas the DR effect was independent of ANG 1–7 signaling. These data show that ANG 1–7-MasR inhibits renal tubular transporters via DR activation and is in agreement with aforementioned in vivo finding. To find a link between ANG 1–7-MasR and DR signaling as it relates to renal Na+ regulation, we first measured urinary dopamine excretion. We found that ANG 1–7-treated rats exhibited a significant increase in the urinary dopamine level, which was blocked by A-779 but insensitive to SCH23390, suggesting that increased dopamine production involves ANG 1–7-MasR signaling but is independent of DR. Taken together, our data show that ANG 1–7-MasR stimulation increases renal dopamine production, which activates DR, causing inhibition of Na+-K+-ATPase and NHE3 activity. The role of ANG 1–7 in dopamine production and renal Na+ regulation is conflicting. In contrast to our data, Pawlak et al. (44) reported that AT1R blockade is needed for ANG 1–7-mediated dopamine production in the rat hypothalamus, and Stragier et al. (49) showed that conversion of ANG 1–7 to ANG 3–7 is responsible for dopamine production in the rat striatum. Lara et al. (33, 34) have shown that ANG 1–7 via ANG II type 1 receptors stimulates Na+-ATPase in adult pig renal tubules and inhibits Na+-K+-ATPase in Madin-Darby canine kidney cells; however, the same group failed to observe ANG 1–7-mediated inhibition of Na+-K+-ATPase in pig renal tubules (14). Consistent with our study, DelliPizzi et al. (19) have shown renal natriuretic effects of ANG 1–7 in rats. The exact mechanisms for these discrepancies are not clear; however, the plausible explanation could be differences in the animal model, central versus peripheral effects of ANG 1–7, water, and Na+-replete versus -deplete conditions, and local renin-angiotensin concentration. Nevertheless, we suggest that DR-dependent inhibition of Na+ transporters could, in part, be responsible for ANG 1–7-MasR-mediated natriuresis and diuresis, as these transporters are responsible for >60% of transcellular proximal tubular Na+ reabsorption (24, 54).
In neuronal cells, the rate-limiting step of dopamine synthesis involves tyrosine hydroxylase-mediated conversion of tyrosine to l-DOPA, which is decarboxylated to dopamine by AADC (42). However, it is widely perceived that in the kidney, dopamine is locally synthesized and involves apical uptake of filtered l-DOPA, mainly via LAT1 and LAT2 (1–3, 11–13, 27, 45). The involvement of transporters belonging to the solute carrier superfamily has also been suggested (30, 31, 55). However, the role of tyrosine hydroxylase, which is highly expressed in epithelial cells, has not been fully assessed in renal dopamine synthesis (1, 20, 29, 52). The activity of tyrosine hydroxylase is highly regulated by serine phosphorylation, involving various serine/threonine kinases and phosphatases (22). While an increase in phosphorylation at Ser40 is known to activate the enzyme, the role of Ser19 or Ser31 is not clear (22). Here, we found that ANG 1–7 had no effect on tyrosine hydroxylase expression but increased enzyme Ser40 phosphorylation and activity. ANG 1–7 also increased PKG activity in renal tubules without affecting PKG expression. ANG 1–7-mediated activation of both tyrosine hydroxylase and PKG were sensitive to A-779 but independent of DR signaling. Additionally, ANG 1–7-MasR-mediated Ser40 phosphorylation and activation were blocked by PKG inhibition. These data provide strong evidence that the ANG 1–7-MasR pathway increases dopamine production via activation of tyrosine hydroxylase involving PKG.
To further substantiate the involvement of PKG-tyrosine hydroxylase in ANG 1–7-Mas R-mediated Na+ excretion, we assessed ANG 1–7-induced inhibition of Na+-K+-ATPase and NHE3 in the absence and presence of PKG, tyrosine hydroxylase, and AADC inhibitors. We found that ANG 1–7-MasR-mediated inhibition of renal Na+ transporters was sensitive to PKG, tyrosine hydroxylase, and AADC inhibitors, whereas these inhibitors had no effect on DR signaling. Additionally, infusion of PKG inhibitor abolished the ANG 1–7-mediated increase in urinary dopamine excretion. These data suggest that ANG 1–7-MasR-mediated renal Na+ regulation involves the modulation of local renal dopamine synthesis.
Limitations.
The transport of renal tubular l-DOPA and tyrosine is complex and involves both apical and basolateral Na+-dependent and -independent transporters. A detailed study is warranted to identify the involvement of individual transporters in ANG 1–7-MasR signaling, as it relates to renal dopamine synthesis and Na+ regulation. The investigation of these transporters is beyond the scope of this study.
Conclusions.
Our data show that ANG 1–7-MasR invokes robust natriuresis and diuresis via activation of renal DR. Mechanistically, ANG 1–7-MasR increased PKG activity, which activates tyrosine hydroxylase by Ser40 phosphorylation. Activation of tyrosine hydroxylase leads to increased dopamine production and DR stimulation, which, in turn, inhibit renal Na+ transporters Na+-K+-ATPase and NHE3. These phenomena lead to natriuresis and diuresis in response to ANG 1–7. While ANG 1–7-MasR-mediated renal Na+ regulation is dependent on DR stimulation, SKF38393-DR-induced Na+ excretion does not involve ANG 1–7-MasR signaling. Taken together, this study provides novel insights into the complexity of renal Na+ and dopamine regulation involving ANG 1–7-MasR and DR interaction.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-139808.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A.B. conceived and designed research; A.A.B. and A.D.D. performed experiments; A.A.B. and A.D.D. analyzed data; A.A.B. and A.D.D. interpreted results of experiments; A.A.B. prepared figures; A.A.B. drafted manuscript; A.A.B., A.D.D., and M.L. edited and revised manuscript; A.A.B., A.D.D., and M.L. approved final version of manuscript.
REFERENCES
- 1.Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol 62: 621–647, 2000. doi: 10.1146/annurev.physiol.62.1.621. [DOI] [PubMed] [Google Scholar]
- 2.Armando I, Nowicki S, Aguirre J, Barontini M. A decreased tubular uptake of dopa results in defective renal dopamine production in aged rats. Am J Physiol Renal Physiol 268: F1087–F1092, 1995. doi: 10.1152/ajprenal.1995.268.6.F1087. [DOI] [PubMed] [Google Scholar]
- 3.Armando I, Villar VA, Jose PA. Dopamine and renal function and blood pressure regulation. Compr Physiol 1: 1075–1117, 2011. doi: 10.1002/cphy.c100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baillien M, Foidart A, Balthazart J. Regional distribution and control of tyrosine hydroxylase activity in the quail brain. Brain Res Bull 48: 31–37, 1999. doi: 10.1016/S0361-9230(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 5.Banday AA. Chronic insulin treatment phosphorylates the renal Na-K-ATPase α1-subunit at serine 16/23 and reduces its activity involving PI3-kinase-dependent PKC activation. Am J Physiol Renal Physiol 311: F958–F966, 2016. doi: 10.1152/ajprenal.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banday AA, Lau YS, Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt-sensitive hypertension in Sprague-Dawley rats. Hypertension 51: 367–375, 2008. doi: 10.1161/HYPERTENSIONAHA.107.102111. [DOI] [PubMed] [Google Scholar]
- 7.Banday AA, Lokhandwala MF. Angiotensin II-mediated biphasic regulation of proximal tubular Na+/H+ exchanger 3 is impaired during oxidative stress. Am J Physiol Renal Physiol 301: F364–F370, 2011. doi: 10.1152/ajprenal.00121.2011. [DOI] [PubMed] [Google Scholar]
- 8.Banday AA, Lokhandwala MF. Oxidative stress causes renal angiotensin II type 1 receptor upregulation, Na+/H+ exchanger 3 overstimulation, and hypertension. Hypertension 57: 452–459, 2011. doi: 10.1161/HYPERTENSIONAHA.110.162339. [DOI] [PubMed] [Google Scholar]
- 9.Banday AA, Lokhandwala MF. Oxidative stress impairs cGMP-dependent protein kinase activation and vasodilator-stimulated phosphoprotein serine-phosphorylation. Clin Exp Hypertens 41: 5–13, 2019. doi: 10.1080/10641963.2018.1433197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barati MT, Ketchem CJ, Merchant ML, Kusiak WB, Jose PA, Weinman EJ, LeBlanc AJ, Lederer ED, Khundmiri SJ. Loss of NHERF-1 expression prevents dopamine-mediated Na-K-ATPase regulation in renal proximal tubule cells from rat models of hypertension: aged F344 rats and spontaneously hypertensive rats. Am J Physiol Cell Physiol 313: C197–C206, 2017. doi: 10.1152/ajpcell.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88: 249–286, 2008. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- 12.Carranza A, Karabatas L, Barontini M, Armando I. Decreased tubular uptake of L-3,4-dihydroxyphenylalanine in streptozotocin-induced diabetic rats. Horm Res 55: 282–287, 2001. doi: 10.1159/000050014. [DOI] [PubMed] [Google Scholar]
- 13.Carranza A, Nowicki S, Barontini M, Armando I. l-Dopa uptake and dopamine production in proximal tubular cells are regulated by β2-adrenergic receptors. Am J Physiol Renal Physiol 279: F77–F83, 2000. doi: 10.1152/ajprenal.2000.279.1.F77. [DOI] [PubMed] [Google Scholar]
- 14.Caruso-Neves C, Lara LS, Rangel LB, Grossi AL, Lopes AG. Angiotensin-(1-7) modulates the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule. Biochim Biophys Acta 1467: 189–197, 2000. doi: 10.1016/S0005-2736(00)00219-4. [DOI] [PubMed] [Google Scholar]
- 15.Chen K, Deng K, Wang X, Wang Z, Zheng S, Ren H, He D, Han Y, Asico LD, Jose PA, Zeng C. Activation of D4 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Hypertension 65: 153–160, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Burnett JC. Particulate guanylyl cyclase A/cGMP signaling pathway in the kidney: physiologic and therapeutic indications. Int J Mol Sci 19: E1006, 2018. doi: 10.3390/ijms19041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi MR, Kouyoumdzian NM, Rukavina Mikusic NL, Kravetz MC, Rosón MI, Rodríguez Fermepin M, Fernández BE. Renal dopaminergic system: pathophysiological implications and clinical perspectives. World J Nephrol 4: 196–212, 2015. doi: 10.5527/wjn.v4.i2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbin JD, Reimann EM. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol 38: 287–290, 1974. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- 19.DelliPizzi AM, Hilchey SD, Bell-Quilley CP, DelliPizzi AM, Hilchey SD, Bell-Quilley CP. Natriuretic action of angiotensin (1–7). Br J Pharmacol 111: 1–3, 1994. doi: 10.1111/j.1476-5381.1994.tb14014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Marco GS, Vio CP, Dos Santos OF, Schor N, Casarini DE. Catecholamine production along the nephron. Cell Physiol Biochem 20: 919–924, 2007. doi: 10.1159/000110452. [DOI] [PubMed] [Google Scholar]
- 21.Dibo P, Marañón RO, Chandrashekar K, Mazzuferi F, Silva GB, Juncos LA, Juncos LI. Angiotensin-(1−7) inhibits sodium transport via Mas receptor by increasing nitric oxide production in thick ascending limb. Physiol Rep 7: e14015, 2019. doi: 10.14814/phy2.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem 91: 1025–1043, 2004. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- 23.Felder CC, Albrecht F, Eisner GM, Jose PA. The signal transducer for the dopamine-1 regulated sodium transport in renal cortical brush border membrane vesicles. Am J Hypertens 3: 47S–50S, 1990. doi: 10.1093/ajh/3.6.47S. [DOI] [PubMed] [Google Scholar]
- 24.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Dominguez Rieg JA, Rieg T. Renal tubular NHE3 is required in the maintenance of water and sodium chloride homeostasis. Kidney Int 92: 397–414, 2017. doi: 10.1016/j.kint.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiscus RR, Murad F. cGMP-dependent protein kinase activation in intact tissues. Methods Enzymol 159: 150–159, 1988. doi: 10.1016/0076-6879(88)59016-X. [DOI] [PubMed] [Google Scholar]
- 26.Gironacci MM, Valera MS, Yujnovsky I, Peña C. Angiotensin-(1−7) inhibitory mechanism of norepinephrine release in hypertensive rats. Hypertension 44: 783–787, 2004. doi: 10.1161/01.HYP.0000143850.73831.9d. [DOI] [PubMed] [Google Scholar]
- 27.Gomes P, Soares-da-Silva P. Na+-independent transporters, LAT-2 and b0,+, exchange l-DOPA with neutral and basic amino acids in two clonal renal cell lines. J Membr Biol 186: 63–80, 2002. doi: 10.1007/s00232-001-0136-8. [DOI] [PubMed] [Google Scholar]
- 28.Haavik J, Flatmark T. Rapid and sensitive assay of tyrosine 3-monooxygenase activity by high-performance liquid chromatography using the native fluorescence of DOPA. J Chromatogr A 198: 511–515, 1980. doi: 10.1016/S0021-9673(00)80522-1. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi M, Yamaji Y, Kitajima W, Saruta T. Aromatic l-amino acid decarboxylase activity along the rat nephron. Am J Physiol Renal Physiol 258: F28–F33, 1990. doi: 10.1152/ajprenal.1990.258.1.F28. [DOI] [PubMed] [Google Scholar]
- 30.Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3). J Pharmacol Exp Ther 308: 2–9, 2004. doi: 10.1124/jpet.103.053298. [DOI] [PubMed] [Google Scholar]
- 31.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 24: 1227–1251, 2007. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 32.Kuczeriszka M, Kompanowska-Jezierska E, Sadowski J, Prieto MC, Navar LG. Modulating role of Ang1-7 in control of blood pressure and renal function in AngII-infused hypertensive rats. Am J Hypertens 31: 504–511, 2018. doi: 10.1093/ajh/hpy006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lara LS, Correa JS, Lavelle AB, Lopes AG, Caruso-Neves C. The angiotensin receptor type 1-Gq protein-phosphatidyl inositol phospholipase Cbeta-protein kinase C pathway is involved in activation of proximal tubule Na+-ATPase activity by angiotensin(1−7) in pig kidneys. Exp Physiol 93: 639–647, 2008. doi: 10.1113/expphysiol.2007.040584. [DOI] [PubMed] [Google Scholar]
- 34.Lara LS, De Carvalho T, Leão-Ferreira LR, Lopes AG, Caruso-Neves C. Modulation of the Na+-K+-ATPase activity by angiotensin-(1−7) in MDCK cells. Regul Pept 129: 221–226, 2005. doi: 10.1016/j.regpep.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Wang W, Chen W, Jiang X, Zhang Y, Wang Z, Yang J, Jones JE, Jose PA, Yang Z. Regulation of blood pressure, oxidative stress and AT1R by high-salt diet in mutant human dopamine D5 receptor transgenic mice. Hypertens Res 38: 394–399, 2015. doi: 10.1038/hr.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu X, Ye Z, Zheng S, Ren H, Zeng J, Wang X, Jose PA, Chen K, Zeng C. Long-term exposure of fine particulate matter causes hypertension by impaired renal D1 receptor-mediated sodium excretion via upregulation of G-protein-coupled receptor kinase type 4 expression in Sprague-Dawley rats. J Am Heart Assoc 7: e007185, 2018. doi: 10.1161/JAHA.117.007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magaldi AJ, Cesar KR, de Araújo M, Simões e Silva AC, Santos RA. Angiotensin-(1−7) stimulates water transport in rat inner medullary collecting duct: evidence for involvement of vasopressin V2 receptors. Pflugers Arch 447: 223–230, 2003. doi: 10.1007/s00424-003-1173-1. [DOI] [PubMed] [Google Scholar]
- 38.Mansoori A, Oryan S, Nematbakhsh M. Role of Mas receptor antagonist (A779) on pressure diuresis and natriuresis and renal blood flow in the absence of angiotensin II receptors type 1 and 2 in female and male rats. J Physiol Pharmacol 65: 633–639, 2014. [PubMed] [Google Scholar]
- 39.Marwaha A, Banday AA, Lokhandwala MF. Reduced renal dopamine D1 receptor function in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol 286: F451–F457, 2004. doi: 10.1152/ajprenal.00227.2003. [DOI] [PubMed] [Google Scholar]
- 40.Matsuyama T, Ohashi N, Ishigaki S, Isobe S, Tsuji N, Fujikura T, Tsuji T, Kato A, Miyajima H, Yasuda H. The relationship between the intrarenal dopamine system and intrarenal renin-angiotensin system depending on the renal function. Intern Med 57: 3241–3247, 2018. doi: 10.2169/internalmedicine.0994-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natarajan AR, Eisner GM, Armando I, Browning S, Pezzullo JC, Rhee L, Dajani M, Carey RM, Jose PA. The renin-angiotensin and renal dopaminergic systems interact in normotensive humans. J Am Soc Nephrol 27: 265–279, 2016. doi: 10.1681/ASN.2014100958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuhuber W, Wörl J. Monoamines in the enteric nervous system. Histochem Cell Biol 150: 703–709, 2018. doi: 10.1007/s00418-018-1723-4. [DOI] [PubMed] [Google Scholar]
- 43.O’Neill J, Corbett A, Johns EJ. Dietary sodium intake modulates renal excretory responses to intrarenal angiotensin (1−7) administration in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 304: R260–R266, 2013. doi: 10.1152/ajpregu.00583.2011. [DOI] [PubMed] [Google Scholar]
- 44.Pawlak R, Napiorkowska-Pawlak D, Takada Y, Urano T, Nagai N, Ihara H, Takada A. The differential effect of angiotensin II and angiotensin 1−7 on norepinephrine, epinephrine, and dopamine concentrations in rat hypothalamus: the involvement of angiotensin receptors. Brain Res Bull 54: 689–694, 2001. doi: 10.1016/S0361-9230(01)00489-0. [DOI] [PubMed] [Google Scholar]
- 45.Pinto V, Pinho MJ, Soares-da-Silva P. Renal amino acid transport systems and essential hypertension. FASEB J 27: 2927–2938, 2013. doi: 10.1096/fj.12-224998. [DOI] [PubMed] [Google Scholar]
- 46.Pokkunuri I, Chugh G, Rizvi I, Asghar M. Age-related hypertension and salt sensitivity are associated with unique cortico-medullary distribution of D1R, AT1R, and NADPH-oxidase in FBN rats. Clin Exp Hypertens 37: 1–7, 2015. doi: 10.3109/10641963.2014.977489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quigley JP, Gotterer GS. A comparison of the (Na+-K+)-ATPase activities found in isolated brush border and plasma membrane of the rat intestinal mucosa. Biochim Biophys Acta 255: 107–113, 1972. doi: 10.1016/0005-2736(72)90012-0. [DOI] [PubMed] [Google Scholar]
- 48.Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/angiotensin-(1−7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1−7). Physiol Rev 98: 505–553, 2018. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stragier B, Hristova I, Sarre S, Ebinger G, Michotte Y. In vivo characterization of the angiotensin-(1−7)-induced dopamine and gamma-aminobutyric acid release in the striatum of the rat. Eur J Neurosci 22: 658–664, 2005. doi: 10.1111/j.1460-9568.2005.04188.x. [DOI] [PubMed] [Google Scholar]
- 50.Tang L, Zheng S, Ren H, He D, Zeng C, Wang WE. Activation of angiotensin II type 1 receptors increases D4 dopamine receptor expression in rat renal proximal tubule cells. Hypertens Res 40: 652–657, 2017. doi: 10.1038/hr.2017.13. [DOI] [PubMed] [Google Scholar]
- 51.Taub M, Garimella S, Kim D, Rajkhowa T, Cutuli F. Renal proximal tubule Na,K-ATPase is controlled by CREB-regulated transcriptional coactivators as well as salt-inducible kinase 1. Cell Signal 27: 2568–2578, 2015. doi: 10.1016/j.cellsig.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taveira-da-Silva R, da Silva Sampaio L, Vieyra A, Einicker-Lamas M. l-Tyr-induced phosphorylation of tyrosine hydroxylase at Ser40: an alternative route for dopamine synthesis and modulation of Na+/K+-ATPase in kidney cells. Kidney Blood Press Res 44: 1–11, 2019. doi: 10.1159/000497806. [DOI] [PubMed] [Google Scholar]
- 53.Tiu AC, Bishop MD, Asico LD, Jose PA, Villar VAM. Primary pediatric hypertension: current understanding and emerging concepts. Curr Hypertens Rep 19: 70, 2017. doi: 10.1007/s11906-017-0768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Armando I, Upadhyay K, Pascua A, Jose PA. The regulation of proximal tubular salt transport in hypertension: an update. Curr Opin Nephrol Hypertens 18: 412–420, 2009. doi: 10.1097/MNH.0b013e32832f5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev 84: 987–1049, 2004. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- 56.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1−7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol 300: R804–R817, 2011. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest 121: 2845–2854, 2011. doi: 10.1172/JCI57324. [DOI] [PMC free article] [PubMed] [Google Scholar]