Abstract
The specific pathogenesis of idiopathic nephrotic syndrome (NS) is poorly understood, and the role of immune mediators remains contentious. However, there is good evidence for the role of a circulating factor, and we recently postulated circulating proteases as candidate factors. Immunosuppressive therapy with glucocorticoids (GCs) and T cell inhibitors are widely used in the clinical treatment of NS. Given that T helper (CD4+) cells expressing IL-17A (so-called Th17 cells) have recently been reported to be resistant to GC treatment, and GC resistance remains a major challenge in the management of NS, we hypothesized that Th17 cells produce a circulating factor that is capable of signaling to the podocyte and inducing deleterious phenotypic changes. To test this, we generated human Th17 cells from healthy volunteers and added the supernatants from these T cell cultures to conditionally immortalized human podocytes in vitro. This demonstrated that podocytes treated with Th17 cell culture supernatant, as well as with patient disease plasma, showed significant stimulation of JNK and p38 MAPK pathways and an increase in motility, which was blocked using a JNK inhibitor. We have previously shown that nephrotic plasma elicits a podocyte response via protease-activated receptor-1 (PAR-1). Stimulation of PAR-1 in podocytes elicited the same signaling response as Th17 cell culture supernatant treatment. Equally, protease inhibitors with Th17 cell culture treatment blocked the signaling response. This was not replicated by the reagents added to Th17 cell cultures or by IL-17A. Hence, we conclude that an undefined soluble mediator produced by Th17 cells mimics the deleterious effect of PAR-1 activation in vitro. Given the association between pathogenic subsets of Th17 cells and GC resistance, these observations have potential therapeutic relevance for patients with NS.
Keywords: circulating factor, nephrotic syndrome, protease-activated receptor-1, podocyte, T helper 17
INTRODUCTION
Nephrotic syndrome (NS) is defined by the triad of nephrotic range proteinuria, hypoalbuminemia, and edema. As such, structural and functional defects in the glomerular filtration barrier result in an inability to restrict urinary protein loss (7). Although the specific pathogenesis of idiopathic NS is not fully understood, podocytes are widely regarded to be the target cell, as they contribute to both the final layer of the glomerular filtration barrier and also to the formation of the glomerular basement membrane (1). Further support for their role is evidenced by ultrastructural changes seen histologically in podocytes in NS that render them unable to restrict urinary protein loss (5). This process of ultrastructural change is termed foot process effacement, whereby the foot processes retract and the nature of the slit diaphragm that bridges the gap between neighboring foot processes is changed. This is considered a migratory event and contributes to the overall measure of podocyte motility (19). Focal adhesion kinase (FAK) is a tyrosine kinase that plays a critical role in regulating cell motility, and podocyte-specific deletion of FAK leads to a decrease in cell spreading and motility (17). Notably, mice with this genotype were protected against proteinuria and foot process effacement after podocyte injury (17), and, therefore, podocyte motility in vitro can be used as a surrogate marker for podocyte foot process effacement in vivo (13).
Patients with NS are commonly treated with immunosuppressive glucocorticoids (GCs), which are thought to interfere with production of an immune cell-derived circulating soluble factor(s) but that have also been reported to have direct effects on podocytes (21, 22). Efficacious treatment with GCs is partially determined by the form of NS, in particular whether it is genetic or nongenetic (the former results in GC resistance, whereas the latter can be GC sensitive, can be GC resistant, and, interestingly, can also be initially GC sensitive and become GC resistant). The efficacy of GCs also varies in inflammatory diseases affecting the lungs, gut, and central nervous system, and recent reports have highlighted the role of cytokines expressed by T helper (Th; CD4+) cells in defining GC responsiveness in these conditions, with coexpression of interferon (IFN)-γ and IL-17A in Th17 cells being characteristic of GC resistance. Similarly, it has been suggested that a bias toward Th17 cells correlates with increased proteinuria in minimal change NS (16).
Given the evidence to support the role of a circulating factor produced by cells of the immune system in the development of nongenetic NS, we speculated that CD4+ Th cells are a candidate source of such a soluble mediator. Moreover, this hypothesis provides scope for a Th17-driven mechanism of GC resistance in NS (i.e., a subset of Th17 cells will not be suppressed in GC-resistant patients, and GC treatment itself may provide a selection pressure to enrich these GC-resistant Th17 cells). Therefore, any factors released into the circulation by Th17 cells, and that are capable of damaging the podocyte, will continue to do so despite GC therapy. We have previously suggested that the unknown factor is a protease that signals via protease-activated receptor-1 (PAR-1) as plasma obtained from patients with NS posttransplant during relapse, but not in remission, signals via PAR-1 to increase podocyte motility (8). VASP is a key phosphorylation target in this signaling response and was thought to be responsible for mediating the increase in podocyte motility seen after relapse nephrotic plasma treatment. Hence, in the present study, we sought to interrogate whether soluble mediators from human Th17 cell cultures were capable of inducing the same signaling pathways and podocyte responses in vitro as PAR-1 activation, our goal being to establish whether Th17 cells are a potential source of a circulating factor that can both injure the podocyte and mediate GC resistance in NS.
METHODS
Cell isolation and FACS.
Approximately 80 ml of whole blood was collected from healthy volunteers after informed consent in accordance with a National Health Service Research Ethic Committee-approved protocol at University Hospitals Bristol National Health Service Foundation Trust, United Kingdom (04/Q2002/84). CD4+ T cells were enriched by negative selection using RosetteSep Human CD4+ T cell Enrichment Cocktail (StemCell Technologies) according to the manufacturer’s instructions, washed in wash buffer (PBS supplemented with 1% FCS), and then stained with antibodies for 30 min at 4°C using anti-CD4 (1:50 dilution, clone OKT-4, eBioscience), anti-CD3 (1:100 dilution, clone UCHT1, BD Bioscience), and biotinylated anti-chemokine (C-C motif) receptor (CCR)6 (CD196, 1:50 dilution, clone 11A9, BD Bioscience)/streptavidin-APC (1:200 dilution, BD Bioscience). 7-Aminoactinomycine D (1:400 dilution; Life Technologies, UK) was used to discern living cells. Cells were then sorted by fluorescence-activated cell sorting (FACS) into living CD4+CCR6− and CD4+CCR6+ cells (BD Influx, BD Bioscience).
Cell culture and supernatant generation.
After FACS, cells were resuspended to 2 × 106 cells/ml in complete RPMI-1640 (Invitrogen) supplemented with 10% (vol/vol) FCS, l-glutamine, and penicillin-streptomycin (all PAA Laboratories) and stimulated with plate-bound 5 µg/ml anti-CD3 and 5 µg/ml anti-CD28 antibodies (eBioscience). CD4+CCR6− cells did not receive supplementary cytokines. These cells were expected to express IFN-γ on anti-CD3/anti-CD28 stimulation and were designated Th0. In contrast, CD4+CCR6+ cells received a polarizing cytokine cocktail of 20 ng/ml IL-6, 10 ng/ml IL-23, 10 ng/ml IL-1β (R&D Systems), 100 ng/ml anti-IFN-γ, and 100 ng/ml anti-IL-4 (eBioscience) to generate dual IL-17 and IFN-γ expression for 3 days (designated Th17). Thereafter, cells were transferred to fresh plates and cultured in complete RPMI-1640 containing 50 ng/ml IL-2 only (for Th0) or 50 ng/ml IL-2 plus polarizing cytokine cocktail (for Th17) for 4 days. This 7-day process was repeated once, and cell supernatants were collected at day 14 and frozen.
Flow cytometry.
Intracellular cytokine production from Th17 and Th0 cells was assessed at day 14. Briefly, cells were incubated with 20 ng/ml phorbol 12-myristate 13-acetate (PMA), 1 µM ionomycin, and 2 µM protein transport inhibitor (Monensin, BD Bioscience) for 4 h at 37°C. After stimulation, cells were fixed, permeabilized (Cytofix/perm solution, BD Bioscience), and stained with anti-IL-17 (1:50 dilution, clone eBio63CAP17, eBioscience) and anti-IFN-γ (1:50, clone 4S.B3, eBioscience). All samples were acquired using a BD LSR II (BD Bioscience), and data analysis was run using FlowJo 7.6 software (Treestar).
Podocyte cell culture.
Conditionally immortalized wild-type human podocytes were cultured in whole podocyte medium: RPMI-1640 supplemented with insulin transferrin selenium, penicillin-streptomycin, and 10% (vol/vol) FCS at the permissive temperature of 33°C until confluent (20). Subsequently they were switched to the nonpermissive temperature of 37°C until they began to differentiate, as previously described (18). Podocytes were cultured for 10–14 days at the nonpermissive temperature before being used experimentally.
Treatment and generation of lysate for Western blot analysis.
Culture supernatants were diluted 1:2 in whole podocyte medium (as described above) and applied to the differentiated podocytes. After an incubation period of between 5 and 60 min, cells were lysed and protein was extracted using a Triton X-100-based extraction buffer (50 mM Trizma, 1.23 mM EDTA, 120 mM NaCl, 50 mM NaF, 40 mM sodium β-glycerophosphate, 1.33 mM benzamidine, and 16 mM Triton X-100) supplemented with protease and phosphatase inhibitor cocktails (P-1860, P-0044, and P-5726, Sigma). The culture was scraped, and the lysate was centrifuged to remove any membrane fragments and other contaminants. The cell culture lysate was snap frozen in liquid nitrogen. Lysates were run on 10% acrylamide gels unless otherwise indicated.
Scratch assay.
Podocytes were cultured in six-well plates. Scratch assays were performed once the podocytes had spent 14 days at the nonpermissive temperature of 37°C. In brief, the media were aspirated, and a mechanical wound was inflicted by scratching the monolayer with a pipette tip. Podocytes were then washed in PBS twice to remove any debris and promigratory factors. The diluted Th0/Th17 cell culture supernatant was then added to the wells, and the scratch area was imaged after 0 h (control) and 12 h using a Leica DMIRB microscope and a Zeiss Axiocam ERc 5S camera. The area of the clear zone was measured over time, and podocyte migration was assessed by reduction in the area, indicating more motile cells. The experiment was repeated eight times with different batches of supernatant. Each repeat had at least three but up to six replicates.
Inhibitors.
The p38 MAPK inhibitor SB203580 (Sigma) was used at a dose of 10 µM (2), and the JNK inhibitor SP600125 (Tocris) was used at a concentration of 1 µM (3). The PAR-1 receptor antagonist vorapaxar (ADV-465750877, Sigma) was used at a concentration of 15 μM. The PAR-1 inhibitor FR171113 (SML-0028, Sigma) was used at a concentration of 15 µM. Th17 culture supernatants were pretreated with the inhibitors for 30 min before being added to podocytes.
ELISA.
The IL-23 ELISA (no. 88-7237-22, eBioscience) was performed according to the manufacturer’s protocol.
PAR-1 agonist.
The PAR-1 agonist (PAR-3931-PI, Peptides) is a short peptide that contains the sequence of the tethered ligand. Podocytes were treated with 15 µM for up to 60 min.
Viability assay.
The CellTiter 96 AQueous One Solution Cell Proliferation (MTS) Assay (G-3582, Promega) was performed to assess cell viability. Podocytes were cultured for 14 days at the nonpermissive temperature of 37°C in 96-well plates. They were then treated for 24 h before MTS tetrazolium [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)] was added to the wells. Viable cells metabolize this substrate to produce formazan, which was read at an absorbance of 490 nm using the Dynex Technologies Opsys MR plate reader.
Graphs and statistics.
Graphs show means ± SE unless otherwise indicated and were compiled and analyzed (see figures) using GraphPad Prism (version 5.01). Asterisks in the figures denote the level of significance within a graph: *P < 0.05, **P < 0.01, and ***P < 0.001. When different sets of comparisons are being made within the same graph, pound/hash signs (#) are used in place of asterisks. Interactions were assessed using Bonferroni’s multiple-comparison test unless otherwise stated.
RESULTS
Th17 cell culture supernatant and patient disease plasma stimulates p38 MAPK and JNK signaling pathways.
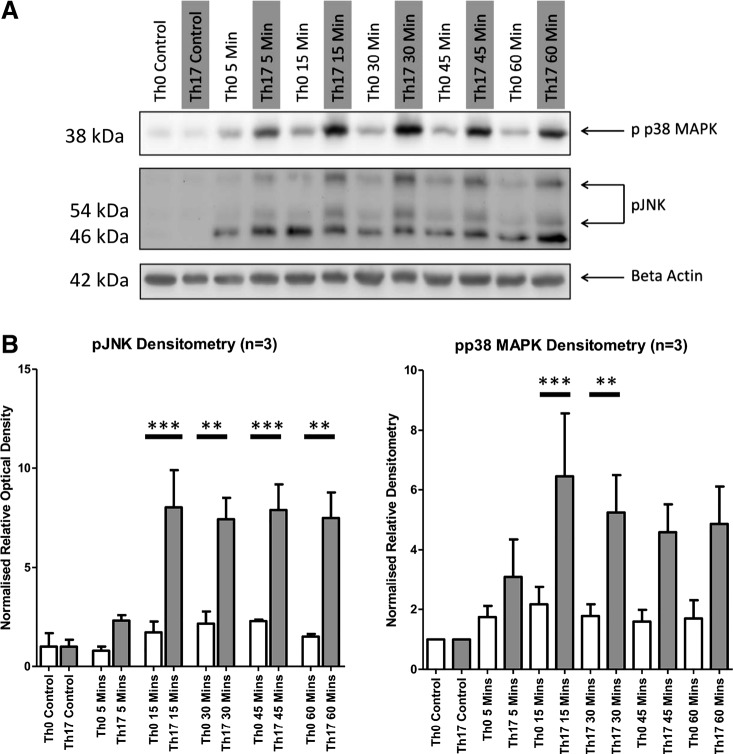
The addition of Th17 cell culture supernatant (from healthy volunteers) to podocytes in vitro significantly stimulated the stress response kinases p38 MAPK and JNK in podocytes at 30 and 15 min, respectively (Fig. 1). Neither Th0 nor Th17 cell culture supernatant treatments had a significant effect on podocyte viability.
Fig. 1.
Podocyte signaling response to T helper (Th)17 cell culture supernatant. The addition of Th17 cell culture supernatant to podocytes elicited a significant response in both phosphorylated (p-)p38 MAPK (A) and pJNK (B) signaling pathways relative to Th0 cell culture supernatant treatment. Statistical significance was measured using one-way ANOVA and a post hoc Bonferroni’s multiple-comparison test.
Paxillin is phosphorylated at the JNK-specific site but not the p38 MAPK-specific site.
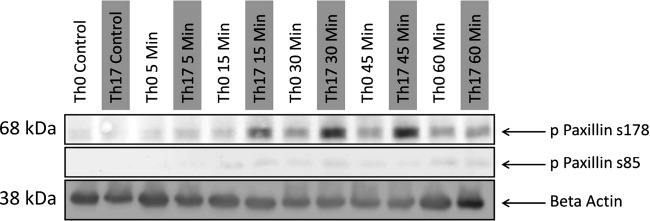
The p38 MAPK and JNK signaling pathways converge downstream; p38 MAPK and JNK have specific target phosphorylation sites on paxillin S85 and S178, respectively. Paxillin is a signaling platform that is localized to focal adhesion sites (4). It plays an important role in organizing the cytoskeleton and adhesion changes that are necessary for cellular migration. We therefore examined whether Th0 and Th17 supernatants could phosphorylate paxillin. This revealed that the S178 phosphorylation site was phosphorylated at 15, 30, and 45 min after the addition of Th17 cell culture supernatant (Fig. 2). As this is coincident with the phosphorylation of JNK (Fig. 1), it is feasible that JNK is phosphorylating paxillin at S178 in podocytes and that this is providing a promigratory signal (9). The S85 site was only minimally phosphorylated in podocytes treated with either Th0 or Th17 supernatant (Fig. 2). It is unclear why paxillin is not phosphorylated by p38 MAPK at S85. Given the clear stimulation of p38 MAPK shown in Fig. 1, it was certainly expected that S85 phosphorylation on paxillin would be detected.
Fig. 2.
JNK phosphorylation induced by T helper (Th)17 cell culture supernatant leads to phosphorylation of paxillin in podocytes. Both p38 MAPK and JNK have specific phosphorylation sites on paxillin: at S85 and S178, respectively. Only the JNK-specific site at S178 is phosphorylated (p). Results are representative of two blots.
Th17 cell culture supernatant significantly increases podocyte motility via JNK signaling.
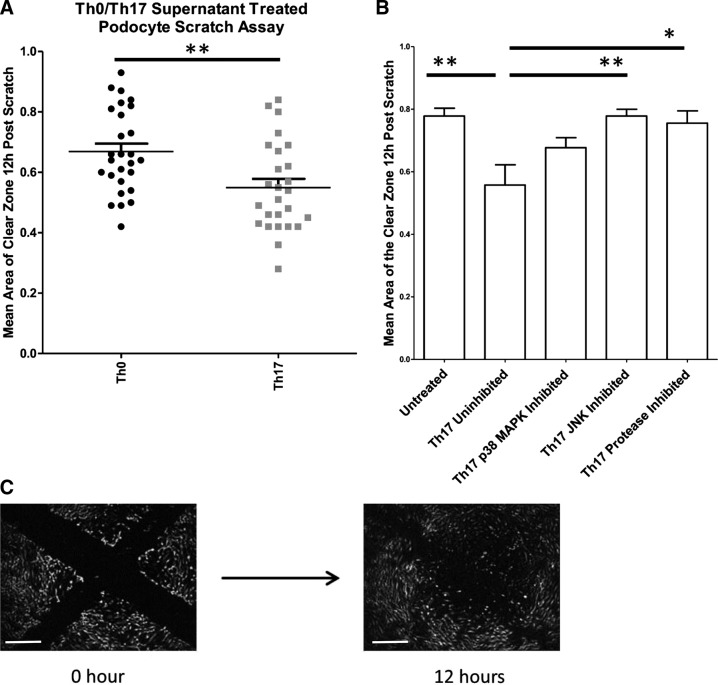
Foot process effacement is an early response of the podocyte to injury and is a key event in the development of proteinuria. Podocyte motility in vitro was assessed using a wound healing assay (Fig. 3A) to determine whether exposure to Th17 cell supernatant rendered podocytes more motile. Collated data from scratch assay experiments using Th0/Th17 supernatants from eight separate donors showed that Th17 supernatant treatment significantly increased the motility of podocytes relative to Th0 treatment (Fig. 3B). To determine the role of JNK and p38 MAPK in this process, podocytes were treated with Th17/Th0 culture supernatants that had been pretreated (30 min) with either JNK inhibitor or p38 MAPK inhibitor. The JNK inhibitor blocked the motility response to Th17 cell culture supernatant (Fig. 3C). This observation suggests that the JNK signaling pathway is a crucial step in mediating the response to the secreted factor and generating a motile phenotype.
Fig. 3.
T helper (Th)17 cell culture supernatant significantly increases podocyte motility relative to Th0 cell culture supernatant. A: podocytes treated with Th17 cell culture supernatant were significantly more motile than those treated with Th0 cell culture supernatant (n = 8, P = 0.0007 by an unpaired t-test). The area of the clear zone is measured in pixels. The area at 12 h was normalized to 0 h. Units are arbitrary. B: to establish the importance of the signaling events detected by Western blot, podocytes were treated with Th17 supernatant and inhibitors against p38 MAPK and JNK. Motility was measured in these podocytes using a wound healing assay. JNK inhibition significantly reduced the Th17-mediated increase in podocyte motility. Statistical significance was measured using one-way ANOVA and a post hoc Bonferroni’s multiple-comparison test. The area of the clear zone is measured in pixels. The area at 12 h was normalized to 0 h. Units are arbitrary. C: a typical set of images that were taken to measure podocyte motility. The area of the clear zone 12 h postscratch is expressed as a decimal of the area at 0 h. Scale bars = 500 μm.
Neither IL-17 nor constituents of the Th17 medium are responsible for the increase in motility.
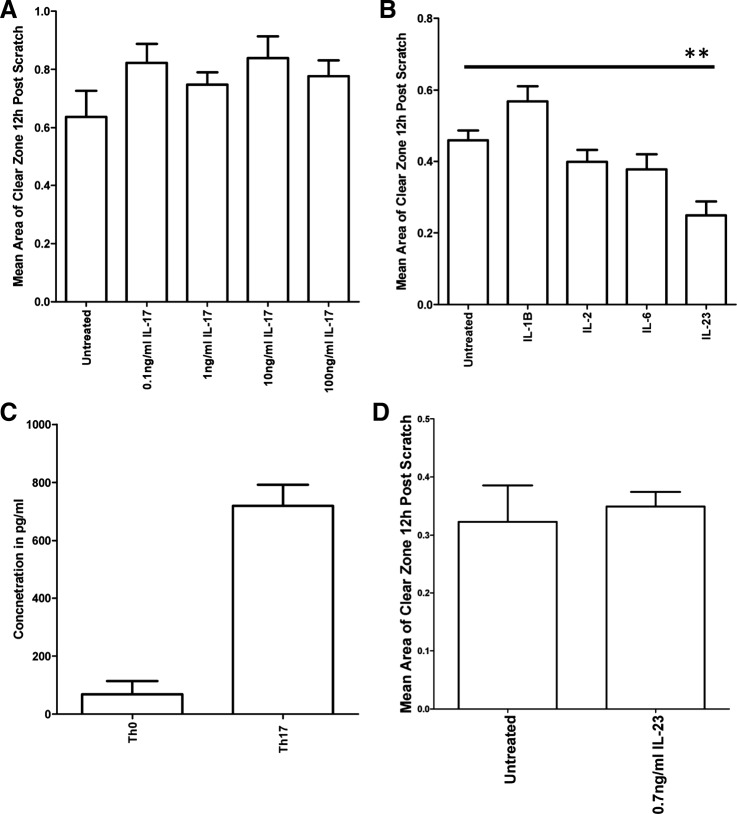
Th17 cells produce a variety of cytokines including IL-17A, making this a candidate effector protein present in the Th17 cell culture supernatant. However, IL-17A alone had no significant effect on podocyte motility at any of the doses tested (0.1−100 ng/ml; Fig. 4A). We also tested the effect on podocyte motility of each single cytokine used to polarize Th17 cells (IL-1B, IL-2, IL-6, and IL-23). IL-23 (used at 10 ng/ml) was the only cytokine to significantly increase podocyte motility (Fig. 4B). To interrogate the IL-23 effect further, we sought to investigate whether this was the factor in the Th17 supernatant causing the increased motility shown in Fig. 4. However, since the supernatants are diluted before addition to podocytes, we first measured the actual concentration of IL-23 in diluted Th17 supernatants (Fig. 4C) and then added this concentration (0.7 ng/ml) to podocytes. However, at this lower dose, IL-23 had no significant effect on podocyte motility (Fig. 4D).
Fig. 4.
IL-23 can significantly increase podocyte motility, although not at the dose present initially in the T helper (Th)17 cell culture supernatant. A: IL-17 had no significant effect on podocyte motility at any of the doses tested (n = 3). B: IL-23 was the only supplementary cytokine that was capable of significantly increasing podocyte motility (six replicates, P = 0.0041 by an unpaired t-test). However, podocytes were treated with a 10 ng/ml dose of IL-23, which is the concentration at which IL-23 is added to the Th17 cell culture medium. By the time the supernatant was applied to the podocytes, the medium was in the presence of Th17 cells for 48 h and through one freeze-thaw cycle. C: therefore, an ELISA was performed to determine the concentration of IL-23 that remained in the Th17 cell culture supernatant. There was 0.7 mg/ml IL-23 in the Th17 cell culture supernatant. D: at this dose, IL-23 had no significant effect on podocyte motility.
PAR-1 agonist treatment elicits the same response as Th17 cell culture supernatant treatment.
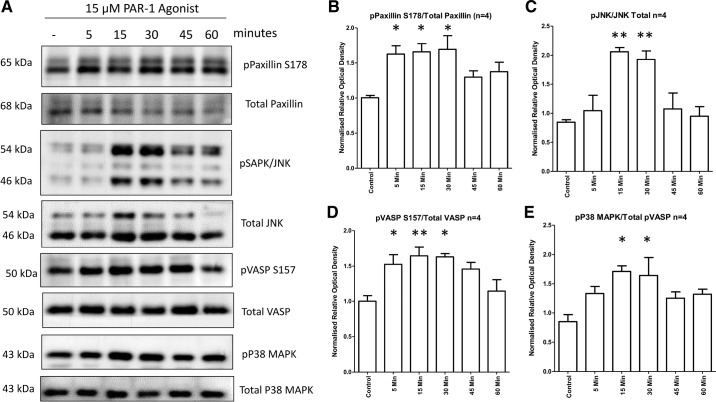
Previous work has suggested that the circulating factor implicated in nongenetic forms of NS signals via PAR-1 (8). Podocytes were treated with PAR-1 agonist to ascertain to what extent signaling and behavioral responses to the Th17 cell culture supernatant could be replicated. The signaling responses of the podocytes to PAR-1 agonist treatment mimicked the response to Th17 cell culture supernatant treatment: phospho-JNK, phospho-p38 MAPK, and phospho-paxillin S178 pathways were all stimulated (Fig. 5, A–C), whereas phospho-paxillin S85 was unaffected (Fig. 5D). This indicates that the JNK signaling pathway and PAR-1 activation mimic the hypermotile phenotype and altered signaling observed in podocytes treated with Th17 cell supernatants.
Fig. 5.
The protease-activated receptor-1 (PAR-1) agonist signaling response mimics the T helper (Th)17 cell culture supernatant response. Conditionally immortalized podocytes were treated with 15 μM PAR-1 agonist for the indicated time course. Control lysate was generated from untreated podocytes. The data shown are representative blots (A) with combined densitometry normalized to the β-actin load control (B−E). B: phosphorylated (p-)JNK signaling. C: p-p38 MAPK signaling. D: p-paxillin S178. E: p-paxillin S85. Signaling responses for each were dynamically like those elicited by Th17 cell culture supernatant treatment. Data represent four independent experiments. Statistical significance was measured using one-way ANOVA and a post hoc Bonferroni’s multiple-comparison test.
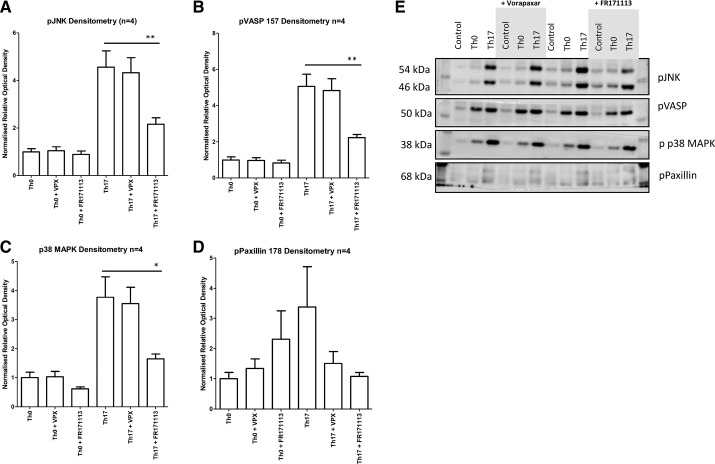
PAR-1 inhibition blocks the podocyte’s signature response to Th17 cell culture supernatant.
To further investigate the role of PAR-1 in response to Th0/Th17 cell culture supernatant, podocytes were treated with supernatants along with the intracellularly acting PAR-1 inhibitor FR171113 and the United States Food and Drug Administration-approved PAR-1 antagonist vorapaxar. The significant increase in phospho-JNK, phospho-p38 MAPK, and phospho-paxillin S178 and VASP was seen in response to the addition of Th17 cell culture supernatant relative to Th0 cell culture supernatant, and FR171113 significantly reduced the phosphorylation of VASP, p38 MAPK, and JNK in response to Th17 cell culture supernatant (Fig. 6). Surprisingly, vorapaxar treatment had no significant effect on the signaling response. Figure 6, A–D, shows the densitometry of the targeted proteins, with Fig. 6E showing representative blots.
Fig. 6.
Protease-activated receptor-1 (PAR-1) inhibition blocks the signature T helper (Th)17 cell culture supernatant response. Th17 cell culture supernatant treatment of podocytes significantly increased phosphorylation of JNK [phospho-JNK (p-JNK); A], VASP S157 [phospho-VASP (p-VASP) 157; B], p38 MAPK [phospho-p38 MAPK (p-p38 MAPK); C], and paxillin S178 [phospho-paxillin (p-paxillin) S178; D]. Conversely, inhibition of PAR-1 by FR171113 significantly reduced each Th17 response (densitometry based on four blots, by one-way ANOVA and a post hoc Bonferroni multiple-comparison test). Representative blots of proteins were studied (E). VPX, vorapaxar.
DISCUSSION
This work suggests that Th17 cells release a hitherto unknown factor that stimulates JNK, p38 MAPK, paxillin, and, importantly, VASP signaling pathways, inducing deleterious effects on podocyte morphology and function akin to that which occurs in NS.
It is envisaged that a subset of pathogenic Th17 cells expand and release a hitherto unknown serine protease that could possibly cleave PAR-1 on the podocyte. This induces a series of pathological signaling events that result in foot process effacement, increased podocyte motility, and proteinuria. Such a situation is consistent with current thinking on steroid-sensitive, steroid-resistant, and steroid-dependent NS.
A role for Th17 cells in NS is becoming increasingly clear. IL-17 has been implicated in causing podocyte damage; indeed, blockade of IL-17, which is predominantly secreted by Th17 cells, improves albuminuria in a model of diabetic nephropathy (15, 23).
The glomerular filtration barrier restricts passage of proteins and macromolecules based on their size and charge. Molecules such as insulin (5 kDa) pass freely through the barrier. Molecules as large as myoglobin (16.9 kDa) pass through relatively uninhibited. Only molecules larger than 60 kDa are restricted to a great extent. Hence, a serine protease with a molecular mass lower than 17 kDa or so would be able to pass through the filtration barrier and stimulate signaling in the podocyte (6).
We have interrogated signaling pathways and podocyte motility in vitro, as a proxy for foot process effacement in vivo, and shown that Th17 cell culture supernatant significantly increased podocyte motility and induced clearly defined signaling pathways in keeping with PAR-1 activation. These data suggest that a soluble mediator generated by Th17 cells affects podocytes in a manner deleterious to barrier function within the glomerulus and is a novel candidate mechanism of NS pathogenesis. However, there is yet to be a consensus on the role of immune mediators in NS. Nonetheless, the active phase of minimal change NS has been linked to an increase in IL-17A-producing Th17 cells, and an increase in Th17 cells and their markers has also been reported during active disease (12, 13). In addition, an increase in the expression of other proinflammatory markers, such as TNF-α and IL-6, during proteinuria has also been reported (14).
Th17 cell culture supernatant significantly stimulated JNK and p38 MAPK signaling pathways in podocytes compared with Th0 supernatant. Although costimulation of these pathways can indicate cell stress, a viability assay showed that neither Th0 nor Th17 cell culture supernatant treatment had a deleterious effect on podocyte viability. The p38 MAPK signaling pathway is known to be stimulated in response to podocyte injury, and Koshikawa et al. (14) showed increased phospho-p38 MAPK in podocyte nuclei in biopsies from patients with minimal change nephropathy, membranous nephropathy, and focal segmental glomerulosclerosis relative to healthy controls. Additionally, they found that marked p38 MAPK phosphorylation preceded proteinuria in experimental models of NS and that the proteinuria could be blocked by the administration of p38 MAPK inhibitor (14). Consistent with this, we observed that administration of Th17 cell culture supernatant to podocytes in vitro significantly increased p38 MAPK signaling 30 min posttreatment relative to Th0 treatment.
The p38 MAPK and JNK pathways converge downstream at paxillin and mediate phosphorylation of paxillin at S85 and S178, respectively. This has been associated with altered cell motility as S85A mutant neuronal cells display neurite outgrowth in response to nerve growth factor administration (10), whereas in S178A mutant epithelial cells and keratocytes cell migration is inhibited (9). This suggests that paxillin plays a key role in the coordination of migratory responses. However, we showed that podocytes treated with Th17 cell culture supernatant only phosphorylated paxillin at S178 and not at S85, suggesting that the JNK signaling response was more important for generating the increased motility.
JNK was significantly phosphorylated by 15 min after Th17 cell culture supernatant treatment, and, by 15 min, JNK had phosphorylated its target site on paxillin (S178). This phosphorylation event has been associated with increased cell migration in epithelial cells and could therefore be driving the increased podocyte motility seen in response to Th17 cell culture supernatant treatment. Indeed, when podocytes were treated with JNK inhibitor, there was a significant reduction in Th17-induced podocyte motility. This supports the notion that the JNK pathway is key to generating the motile phenotype in podocytes.
Furthermore, this study aligns Th17 cells with our previous observation that the circulating factor implicated in relapsing forms of NS signals via PAR-1 (8). In the present study, podocytes treated with PAR-1 agonist mimicked the response to Th17 cell culture supernatant treatment consistent with PAR-1 being involved in the Th17 supernatant-mediated podocyte changes, and we extended our previous results with patient relapse/remission plasma to show the same MAPK/JNK/paxillin signaling. Accordingly, direct inhibition of PAR-1 using FR171113 significantly reduced the signaling response to Th17 cell culture supernatant and patient plasma treatment. Although further study is needed, FR171113 appears to be a specific inhibitor of PAR-1 with no apparent side effects (12).
Vorapaxar had no significant effect on the signaling response of the podocyte to Th17 cell culture supernatant treatment (Fig. 6). This was somewhat surprising given that vorapaxar is a commercially available United States Food and Drug Administration-approved drug used to reduce the risk of stroke or myocardial infarction, where it targets PAR-1 in platelets. Only one dose of vorapaxar was tested, owing to the scarcity of the Th0 and Th17 cell culture supernatants. Ideally, more doses of the antagonist would be tested. Vorapaxar competes with the tethered ligand for the binding of the active site. Therefore, large concentrations of vorapaxar may be necessary to compete with the very high localized concentration of the tethered ligand (24). Vorapaxar is an orthosteric inhibitor that binds to the ligand-binding site (active site) of PAR-1. As such, this inhibitor blocks the binding of the tethered ligand and hence autoactivation of the receptor. FR171113 is an intracellular allosteric inhibitor. FR171113 appears to be a selective inhibitor given its effects on clotting in guinea pigs: FR171113 inhibits thrombin-mediated platelet aggregation and was not seen to have any adverse effects (11).
The ability of FR171113, and inability of vorapaxar, to block PAR-1 signaling could be informative as to the identity and mechanism of the circulating factor. As described, we may not have seen inhibition of signaling since we were not using a sufficient concentration of vorapaxar. Alternatively, the unknown factor(s) in the Th17 cell culture supernatant could be cleaving the extracellular domain of PAR-1 at an alternative site. This would cause what may remain of the tethered ligand to bind the receptor elsewhere than the active site.
FR171113, however, binds allosterically to PAR-1 and blocks the conformational change in the activated form of the receptor. In this way, FR171113 can block the activation of the receptor independent of the way in which the receptor has been activated, whereas vorapaxar can only inhibit classical activation of PAR-1 by the tethered ligand binding the active site.
Importantly, the response of podocytes to Th17 cell supernatant was not mediated by the reagents added to the media of our human Th17 cells cultures or by IL-17A itself. This suggests that another soluble mediator produced by Th17 cells and absent in Th0 cell supernatants is mediating the observed effects on podocyte signaling and motility. Our inhibitor experiments suggest that this factor may be a protease that signals via the JNK pathway to induce a state of podocyte hypermotility.
A bias toward the Th17 phenotype in the regulatory T/Th17 axis has been shown in minimal change NS (16).
Although far from conclusive, the inhibitor experiments presented here suggest a possible role for PAR-1 in the signal transduction pathway between the circulating factor and podocyte damage.
If Th17 cells play a role in the pathogenesis of nongenetic NS, they may also affect response to treatment. Given that GCs remain the standard firstline therapy for NS, and that subsets of Th17 cells have been recently reported across a range of conditions to be candidate mediators of GC resistance, this has potential translational relevance for patients with GC refractory disease. In summary, we have demonstrated, for the first time, that human Th17 cells are able to directly cause podocyte damage via signaling pathways recently described to be activated by plasma from patients with NS (8). We consequently propose that Th17 cells are a candidate mediator of NS pathogenesis with the potential to also influence the efficacy of GCs administered in the treatment of active disease.
GRANTS
L. Schewitz-Bowers, P. Lait, and R. Lee are partially supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology.
DISCLAIMERS
The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute of Health Research, or the Department of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.M., G.I.W., R.W.J.L., and M.A.S. conceived and designed research; C.J.M., M.C., P.J.L., and L.P.S.-B. performed experiments; C.J.M. analyzed data; C.J.M., G.I.W., R.W.J.L., and M.A.S. interpreted results of experiments; C.J.M. prepared figures; C.J.M. drafted manuscript; C.J.M., G.I.W., P.J.L., L.P.S.-B., R.W.J.L., and M.A.S. edited and revised manuscript; C.J.M., G.I.W., M.C., P.J.L., L.P.S.-B., R.W.J.L., and M.A.S. approved final version of manuscript.
REFERENCES
- 1.Abrahamson DR. Role of the podocyte (and glomerular endothelium) in building the GBM. Semin Nephrol 32: 342–349, 2012. doi: 10.1016/j.semnephrol.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkenkamp KU, Tuyt LM, Lummen C, Wierenga AT, Kruijer W, Vellenga E. The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway. Br J Pharmacol 131: 99–107, 2000. doi: 10.1038/sj.bjp.0703534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correia AC, Moonen JR, Brinker MG, Krenning G. FGF2 inhibits endothelial-mesenchymal transition through microRNA-20a-mediated repression of canonical TGF-β signaling. J Cell Sci 129: 569–579, 2016. doi: 10.1242/jcs.176248. [DOI] [PubMed] [Google Scholar]
- 4.Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci 121: 2435–2444, 2008. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deegens JK, Dijkman HB, Borm GF, Steenbergen EJ, van den Berg JG, Weening JJ, Wetzels JF. Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int 74: 1568–1576, 2008. doi: 10.1038/ki.2008.413. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert SJ, Weiner DE. National Kidney Foundation’s primer on kidney diseases (7th ed.). Philadelphia, PA: Elsevier, 2018. [Google Scholar]
- 7.Haraldsson B, Nyström J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 8.Harris JJ, McCarthy HJ, Ni L, Wherlock M, Kang H, Wetzels JF, Welsh GI, Saleem MA. Active proteases in nephrotic plasma lead to a podocin-dependent phosphorylation of VASP in podocytes via protease activated receptor-1. J Pathol 229: 660–671, 2013. doi: 10.1002/path.4149. [DOI] [PubMed] [Google Scholar]
- 9.Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature 424: 219–223, 2003. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- 10.Huang C, Borchers CH, Schaller MD, Jacobson K. Phosphorylation of paxillin by p38MAPK is involved in the neurite extension of PC-12 cells. J Cell Biol 164: 593–602, 2004. doi: 10.1083/jcb.200307081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato Y, Kita Y, Nishio M, Hirasawa Y, Ito K, Yamanaka T, Motoyama Y, Seki J. In vitro antiplatelet profile of FR171113, a novel non-peptide thrombin receptor antagonist. Eur J Pharmacol 384: 197–202, 1999. doi: 10.1016/S0014-2999(99)00658-5. [DOI] [PubMed] [Google Scholar]
- 12.Kato Y, Kita Y, Hirasawa-Taniyama Y, Nishio M, Mihara K, Ito K, Yamanaka T, Seki J, Miyata S, Mutoh S. Inhibition of arterial thrombosis by a protease-activated receptor 1 antagonist, FR171113, in the guinea pig. Eur J Pharmacol 473: 163–169, 2003. doi: 10.1016/S0014-2999(03)01973-3. [DOI] [PubMed] [Google Scholar]
- 13.Kistler AD, Peev V, Forst AL, El Hindi S, Altintas MM, Reiser J. Enzymatic disease of the podocyte. Pediatr Nephrol 25: 1017–1023, 2010. doi: 10.1007/s00467-009-1425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koshikawa M, Mukoyama M, Mori K, Suganami T, Sawai K, Yoshioka T, Nagae T, Yokoi H, Kawachi H, Shimizu F, Sugawara A, Nakao K. Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J Am Soc Nephrol 16: 2690–2701, 2005. doi: 10.1681/ASN.2004121084. [DOI] [PubMed] [Google Scholar]
- 15.Lavoz C, Matus YS, Orejudo M, Carpio JD, Droguett A, Egido J, Mezzano S, Ruiz-Ortega M. Interleukin-17A blockade reduces albuminuria and kidney injury in an accelerated model of diabetic nephropathy. Kidney Int 95: 1418–1432, 2019. doi: 10.1016/j.kint.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 16.Liu LL, Qin Y, Cai JF, Wang HY, Tao JL, Li H, Chen LM, Li MX, Li XM, Li XW. Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol 139: 314–320, 2011. doi: 10.1016/j.clim.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Ma H, Togawa A, Soda K, Zhang J, Lee S, Ma M, Yu Z, Ardito T, Czyzyk J, Diggs L, Joly D, Hatakeyama S, Kawahara E, Holzman L, Guan JL, Ishibe S. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J Am Soc Nephrol 21: 1145–1156, 2010. doi: 10.1681/ASN.2009090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni L, Saleem M, Mathieson PW. Podocyte culture: tricks of the trade. Nephrology (Carlton) 17: 525–531, 2012. doi: 10.1111/j.1440-1797.2012.01619.x. [DOI] [PubMed] [Google Scholar]
- 19.Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem 279: 34827–34832, 2004. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 20.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Schönenberger E, Ehrich JH, Haller H, Schiffer M. The podocyte as a direct target of immunosuppressive agents. Nephrol Dial Transplant 26: 18–24, 2011. doi: 10.1093/ndt/gfq617. [DOI] [PubMed] [Google Scholar]
- 22.Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW. Direct effects of dexamethasone on human podocytes. Kidney Int 70: 1038–1045, 2006. doi: 10.1038/sj.ki.5001655. [DOI] [PubMed] [Google Scholar]
- 23.Yan J, Li Y, Yang H, Zhang L, Yang B, Wang M, Li Q. Interleukin-17A participates in podocyte injury by inducing IL-1β secretion through ROS-NLRP3 inflammasome-caspase-1 pathway. Scand J Immunol 87: e12645, 2018. doi: 10.1111/sji.12645. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Srinivasan Y, Arlow DH, Fung JJ, Palmer D, Zheng Y, Green HF, Pandey A, Dror RO, Shaw DE, Weis WI, Coughlin SR, Kobilka BK. High-resolution crystal structure of human protease-activated receptor 1. Nature 492: 387–392, 2012. doi: 10.1038/nature11701. [DOI] [PMC free article] [PubMed] [Google Scholar]