Abstract
Many neurons receive synchronous input from heterogeneous presynaptic neurons with distinct properties. An instructive example is the crustacean stomatogastric pyloric circuit pacemaker group, consisting of the anterior burster (AB) and pyloric dilator (PD) neurons, which are active synchronously and exert a combined synaptic action on most pyloric follower neurons. Previous studies in lobster have indicated that AB is glutamatergic, whereas PD is cholinergic. However, although the stomatogastric system of the crab Cancer borealis has become a preferred system for exploration of cellular and synaptic basis of circuit dynamics, the pacemaker synaptic output has not been carefully analyzed in this species. We examined the synaptic properties of these neurons using a combination of single-cell mRNA analysis, electrophysiology, and pharmacology. The crab PD neuron expresses high levels of choline acetyltransferase and the vesicular acetylcholine transporter mRNAs, hallmarks of cholinergic neurons. In contrast, the AB neuron expresses neither cholinergic marker but expresses high levels of vesicular glutamate transporter mRNA, consistent with a glutamatergic phenotype. Notably, in the combined synapses to follower neurons, 70–75% of the total current was blocked by putative glutamatergic blockers, but short-term synaptic plasticity remained unchanged, and although the total pacemaker current in two follower neuron types was different, this difference did not contribute to the phasing of the follower neurons. These findings provide a guide for similar explorations of heterogeneous synaptic connections in other systems and a baseline in this system for the exploration of the differential influence of neuromodulators.
NEW & NOTEWORTHY The pacemaker-driven pyloric circuit of the Jonah crab stomatogastric nervous system is a well-studied model system for exploring circuit dynamics and neuromodulation, yet the understanding of the synaptic properties of the two pacemaker neuron types is based on older analyses in other species. We use single-cell PCR and electrophysiology to explore the neurotransmitters used by the pacemaker neurons and their distinct contribution to the combined synaptic potentials.
Keywords: acetylcholine, central pattern generator, glutamate, stomatogastric
INTRODUCTION
Within neural circuits, the activity pattern of any neuron is shaped by input from multiple presynaptic neurons. Inputs from distinct presynaptic neurons may have different signs or strengths and may be subject to different modulatory modifications (Johnson et al. 2011). Even when multiple presynaptic neurons are active synchronously, differences in the strength, dynamics, and plasticity rules of the synapses modify the total influence on the postsynaptic neuron (Abbott et al. 1997; Markram et al. 1998; Puccini et al. 2007). These differences depend on neurotransmitter types, presence of cotransmitters, postsynaptic receptors, and the network activity and modulatory state. Consequently, properties of distinct presynaptic inputs are important factors in defining the neural circuit output and its plasticity in response to different brain states and behavioral needs (Nadim and Bucher 2014).
The pyloric circuit of the crustacean stomatogastric ganglion provides a noteworthy example of flexibility resulting from multiple synaptic inputs (Daur et al. 2016). The pyloric circuit oscillatory activity is driven by a pacemaker group, consisting of one anterior burster (AB) and two pyloric dilator (PD) neurons. These neurons are connected through gap junction-mediated electrical coupling and produce synchronous bursting oscillations. Previous studies of this neural circuit in the spiny lobster Panulirus interruptus have shown that the AB and PD neurons have distinct neurotransmitter types: the AB neuron is glutamatergic, whereas the PD neurons are cholinergic (Eisen and Marder 1982; Marder and Eisen 1984). Consequently, the postsynaptic influences of these two neuron types are distinct in their strength and kinetics (Rabbah and Nadim 2007). However, most postsynaptic targets of the pyloric pacemaker group, including the lateral pyloric (LP) and pyloric constrictor (PY) neurons, receive synaptic input from both AB and PD neurons. As a result, although these postsynaptic neurons experience a composite synaptic input from the pacemaker group, the relative contributions of the AB and PD synapses to this total current are distinct in their strength, kinetics, and response to modulatory inputs (Rabbah and Nadim 2007).
Most recent studies of the pyloric circuit use the crab Cancer borealis as the experimental species (Blitz 2017; Follmann et al. 2017; Haddad and Marder 2018; Haley et al. 2018; Lett et al. 2017; Li et al. 2018; Martinez et al. 2019; Otopalik et al. 2017; White et al. 2017). In C. borealis, however, the neurotransmitters used by the pacemaker AB and PD neurons have not entirely been described, and the relative contribution of the AB and PD synapses to the total pacemaker synaptic output exerted on the follower pyloric neurons remains unquantified. Previous studies have explored the neurotransmitter response of muscles innervated by the PD neuron (Lingle 1980), and an initial detailed study of the pyloric circuit of C. borealis showed that these muscles are sensitive to cholinergic agonists, but not to glutamate, and their evoked excitatory junctional potentials are partially blocked by curare (Hooper et al. 1986). However, there has been no investigation of neurotransmitter identity from a molecular perspective, and the relative synaptic output of the PD neuron in the crab nervous system has not been quantified. In this study, we extend knowledge of neurotransmitter identities of the AB and PD neurons in C. borealis through gene expression analyses, explore the relative contribution of the AB and PD synapses to the total synaptic input in the LP and PY neurons, and examine the short-term dynamics of these synapses. Additionally, we explore whether differences of the pacemaker synapses to the LP and PY neurons influence the relative phasing of these follower neurons.
METHODS
Adult male crabs (C. borealis) were acquired from local distributors and maintained in aquaria filled with chilled artificial seawater until ready for use. Before dissection, crabs were placed on ice, and the dissection was performed using standard protocols as previously described (Blitz and Nusbaum 2012; Tseng and Nadim 2010). The dissected stomatogastric nervous system was secured using 0.1-mm Minutien Pins (Fine Science Tools) in a Dow-Corning-lined 100-mm petri dish filled with Cancer saline (11 mM KCl, 440 mM NaCl, 13 mM CaCl2·2H2O, 26 mM MgCl2·6H2O, 11.2 mM Trizma base, and 5.1 mM maleic acid, adjusted to pH 7.4). The STG was desheathed, exposing the somata of the neurons for intracellular impalement. Preparations were superfused with chilled (11–13°C) physiological Cancer saline.
Extracellular recordings were obtained from identified motor nerves by using stainless steel electrodes placed inside and outside of a petroleum jelly well created to electrically isolate a small section of the nerve and were amplified using a differential alternating current amplifier (model 1700; A-M Systems). Individual pyloric neurons were impaled with sharp glass microelectrodes and identified via their activity patterns, axonal projections in recorded motor nerves, and synaptic interactions with other neurons within the network (Weimann et al. 1991). Intracellular glass microelectrodes were prepared using the Flaming-Brown micropipette puller (P97; Sutter Instruments) and filled with 0.6 M K2SO4 and 20 mM KCl. Current injections were done using microelectrodes with a resistance of 15–22 MΩ; membrane potential measurements were performed using microelectrodes with a resistance of 25–30 MΩ. Intracellular recordings were performed using Axoclamp 2B and 900A amplifiers (Molecular Devices) and digitized using the Digidata acquisition board and pClamp 9 software (Molecular Devices).
Comparison of synapses.
The synapses between pyloric neurons have two components: spike mediated and graded (Graubard et al. 1980). In this study we focus on the graded component, the dominant mode of transmission in pyloric synapses (Manor et al. 1997; Rosenbaum and Marder 2018; Zhao et al. 2011), which we measured in the presence of 10−7 M tetrodotoxin (TTX; Biotium). TTX blocks sodium channels and therefore removes all action potential activity. In the presence of TTX, all pyloric neurons become quiescent at a resting potential of around −60 mV. TTX also blocks all descending modulatory input to the STG.
Our measurements of synaptic potentials include synaptic inputs from both AB and PD neurons. We controlled the membrane potential of the pacemaker ensemble by controlling the PD neuron in two-electrode voltage-clamp mode (Li et al. 2018). We measured the postsynaptic potential (due to both pacemaker neuron types) simultaneously in the LP and PY neurons (Rabbah and Nadim 2007). The PD neuron was voltage-clamped at −60 mV. The voltage-clamped PD neuron was then depolarized with a train of five pulses (duration: 500 ms; interpulse interval: 500 ms) with amplitudes ranging from 15 to 45 mV in 5-mV intervals. The postsynaptic neurons (PY and LP) were each held at −50 mV, away from the synaptic reversal potential of around −80 mV, to record the synaptic current using a single electrode in discontinuous current clamp (DCC). Following measurement of the synapses in control saline (including 10−7 M TTX), to isolate the cholinergic component of the total postsynaptic potential, we repeated our measurements after 30-min superfusion in 10−7 M TTX and 10−5 M picrotoxin (PTX; Sigma-Aldrich). PTX putatively blocks glutamatergic synapses in the STG of both C. borealis and P. interruptus (Bidaut 1980; Cleland and Selverston 1995; Marder and Paupardin-Tritsch 1978; Rinberg et al. 2013; Temporal et al. 2012), and in the latter species has been found to selectively block synaptic inhibition from the AB neuron but not that from the PD neuron (Rabbah and Nadim 2007). After measurements in PTX, the preparations were superfused with control saline for 45 min for measurements in wash conditions.
To quantify the synaptic strength, we fit the difference between the postsynaptic baseline voltage and the peak amplitude of the postsynaptic potential, evaluated at all presynaptic voltages, with a Boltzmann sigmoidal equation:
For each condition, we evaluated the inhibitory postsynaptic potential maximum amplitude (IPSPmax), activation midpoint value (Vmid), and activation slope factor (VC).
Short-term synaptic dynamics were measured in both LP and PY neurons in response to a train of five 500-ms square pulses with 500-ms interpulse intervals (Rabbah and Nadim 2005). The extent of synaptic depression in both LP and PY neurons was quantified as a ratio of the response of the fifth pulse to that of the first pulse.
Measuring the synaptic reversal potential.
To record synaptic reversal potentials, we voltage-clamped the follower LP neuron in normal saline at different holding potentials from −120 to −30 mV, in individual sweeps of 12 s each, and recorded the total current. We were interested in the synaptic input from the pacemakers to this neuron, which is dominant during the PD neuron burst. We therefore monitored the PD neuron bursting activity on the motor nerve pdn.
Holding the LP neuron at hyperpolarized potentials results in the slow activation of hyperpolarizing-activated inward current (Ih). Although Ih is not a large current in this neuron, we blocked its activation with 5 mM CsCl in saline (Peck et al. 2006). Holding the LP neuron at depolarized potentials in normal saline inhibits the pacemakers. This inhibition may be initially strong enough, but the pacemaker activity returns after 2–3 s, so we restricted our measurements to the final 5 s of each sweep.
For each sweep, we low pass filtered the current traces with a cutoff frequency of 500 Hz, divided the traces into segments from the onset of the PD burst to the onset of the subsequent PD burst, normalized time in each segment by the period of that cycle, and resampled the data at 1,000 points per cycle. The traces shown in figures are therefore plotted in phase (time divided by period), with phase 0 (and phase 1) corresponding to the onset of the PD burst (and subsequent burst) on the pyloric dilator nerve (pdn). These cycles were then averaged to produce the current averaged across multiple cycles, for each holding potential.
To separate the synaptic current from the total current, we subtracted the baseline current, defined as the mean value of the current in phases 0.3–0.45, which corresponds to the beginning of the LP neuron burst were it not voltage clamped. A single synaptic current value was measured as the peak of the baseline-subtracted current during the PD neuron burst, as monitored on pdn, and plotted against the holding potential. A linear fit to this current-voltage (I-V) curve identified the synaptic reversal potential in the LP neuron. We did not use the holding potentials of −40 and −30 mV in our analysis, because the activation of voltage-gated currents in the LP neuron produced a clear nonlinearity in the I-V curve. The measurement of the synaptic reversal potential was repeated in the same neuron after bath application of 10−5 M PTX for 30 min.
Dynamic clamp experiments.
For these experiments, the synaptic input from the pacemaker neurons to the LP and PY neurons was blocked by bath application of 10−5 M PTX and injection of a −10-nA direct current into the PD neuron to remove the residual synaptic output. We used dynamic clamp to inject artificial versions of the synaptic input from the pacemaker neurons simultaneously into the LP and PY neurons. This current (Idyn) was modeled in the standard Hodgkin-Huxley formalism as
where was the maximal synaptic conductance and the reversal potential Edyn was set to −80 mV. The synaptic conductance waveform w(t) was a predefined waveform, as previously described (Martinez et al. 2019). For each value of , Idyn was applied repetitively with a cycle period of 1 s for at least 30 s, and only the last five cycles were analyzed. Dynamic clamp was implemented using the NetSuite software (Gotham Scientific) on a 64-bit Windows 7 personal computer using an NI PCI-6070-E board (National Instruments).
Harvesting identified neurons.
The PD, LP, PY, and AB neurons were identified as described above. Because of their small soma size, the AB neurons were filled with Alexa Fluor 488 at a concentration of 4 mg/mL (ThermoFisher Scientific) by electrophoresis for 20 min with a constant −20-nA direct current, to ease identification for harvesting after electrophysiological identification. Cells then were collected as described previously (Schulz et al. 2007). Briefly, after electrophysiological identification of neurons, a petroleum jelly well was built around the STG. The ganglion was then exposed to ~2.5 mg/mL protease (Sigma Aldrich) diluted in Cancer saline to digest the connective tissue. After loosening of the neurons by protease digestion, the protease was thoroughly washed away from the ganglion and the saline in the well was replaced by 70% ethylene glycol diluted in Cancer saline over the course of ~15–20 min. The petri dish was then placed at −20°C for 1 h. A total of five PD, five LP, six PY, and seven AB neurons were hand dissected from the STG (n = 7 ganglia) using fine forceps. Each neuron was placed in 400 μL of lysis buffer and stored at −80°C. Neurons of the same type were harvested from different animals with the following exceptions. In each of two animals, two PY neurons were harvested, and one pair of PD neurons was taken from a single animal.
cDNA synthesis and preamplification.
Total RNA was isolated from each neuron using the Quick-RNA MicroPrep kit (Zymo Research) per the manufacturer’s instructions. Reverse transcription of total RNA was then performed using a mixture of oligo-dT and random hexamer primers (qScript cDNA Supermix; QuantaBio). Half of the cDNA produced from each neuron (10 µL) was then preamplified using PerfeCTa PreAmp Supermix (QuantaBio) according to the manufacturer’s instructions (reaction volume 20 μL) with a 0.5 µM final concentration of each gene-specific primer (Table 1). This protocol utilizes a 14-cycle PCR reaction primed with a pool of target-specific primers to enrich subsequent quantitative PCR (qPCR) reactions when starting sample is limited.
Table 1.
Forward and reverse primer pairs and associated probe sequences
| Gene | Forward Primer | Reverse Primer | Probe | Labeling |
|---|---|---|---|---|
| vGluT | GCGTTCGTGGACCTTCTAC | TCAGCCACCCTGTAATGGAA | ATCACAGCCAACCTACTTCAGCGAG | CAL Fluor Gold 540-BHQ1 |
| ChAT | GGACCGCCTGGCTAAGTAC | TCGCGGAGTCCCATAAGG | AGGCGGCGCTCAAGCTTCAGAC | CAL Fluor Red 610-BHQ2 |
| vAChT | GCGTCAGCTGCTTCTTCCT | CAGCAGTGCCGTGTCTATGAG | TTCGCCAGCAACTACTGGGTGTT | Quasar 670-BHQ2 |
| ACHE | GGGCAACATGGGCATGTAC | GGTCACCACCGAAGAATTCAATG | AGGCGCTGGCCATCAAGTGGATAC | Quasar 705-BHQ2 |
Primer and probe sequences target Cancer borealis acetylcholinesterase (AChE), vesicular acetylcholine transporter (vAChT), choline acetyltransferase (ChAT), and vesicular glutamate transporter (vGluT).
Multiplex primer and probe design.
Genes of interest were identified from the C. borealis nervous system transcriptome (Northcutt et al. 2016). Primer and probe sequences targeting C. borealis acetylcholinesterase (AChE), vesicular acetylcholine transporter (vAChT), choline acetyltransferase (ChAT), and vesicular glutamate transporter (vGluT) were designed using the RealTimeDesign qPCR assay design software (LGC Biosearch Technologies). Primer pairs and probes for the genes of interest were designed to be run in a single qPCR reaction. These assays relied on the following probe fluorophore-quencher pairs to detect PCR products produced during the qPCR reaction: FAM-BHQ1, CAL Fluor Gold 540-BHQ1, CAL Fluor Red 610-BHQ2, Quasar 670-BHQ2, and Quasar 705-BHQ2. Forward and reverse primer pairs and associated probe sequences are reported in Table 1.
Quantitative PCR.
After preamplification, cDNA samples were diluted 7.5 times in nuclease-free water (final volume 150 µL). qPCR reactions (10 µL) were carried out using PerfeCTa Multiplex qPCR ToughMix according to the manufacturer’s instructions (5 times; QuantaBio). These reactions included preamplified cDNA (2.5 μL), forward and reverse primers (2.5 μM), and probes (0.3125 μM each probe). Reactions were run in triplicate on 96-well plates using a CFX96 Touch real-time PCR detection system (Bio-Rad). Cycling conditions for qPCR reactions were as follows: 95°C for 3 min, 40 cycles of 95°C for 15 s, and 58°C for 1 min. Fluorescence measurements were taken at the end of each cycle.
Conversion of quantitation cycle (Cq) value to absolute copy number for each gene was estimated by interpolating Cq values for each gene into a standard curve of known copy number from 106 to 101 copies. We also accounted for the sample amount and the 14-cycle preamplification in our final estimation.
Recording, analysis, and statistics.
Electrophysiology data were acquired using pClamp 9 (Molecular Devices) and sampled at 5 kHz on a personal computer using a Digidata 1332A (Molecular Devices). Statistical and graphical analyses were done using GraphPad Prism 7.0 and Origin 8.5. For statistical analysis, mean ± SEM and P value are reported, unless otherwise indicated.
RESULTS
The electrically coupled AB and PD neurons comprise the pacemaker ensemble of the pyloric network. These neurons oscillate synchronously and together drive the rhythmic activity of the network by simultaneously inhibiting the pyloric follower neurons, including the LP and PY neurons. A cycle of the triphasic pyloric rhythm is generated by the synchronous burst of the pacemaker neurons, followed by a burst in the LP neuron and then a burst in the PY neurons (Fig. 1A). Our goal was to confirm that, in C. borealis, the AB and PD neurons use different neurotransmitters (ACh and glutamate, respectively) and to identify the relative contributions of the AB and PD neurons to the composite synapses in the follower neurons LP and PY.
Fig. 1.
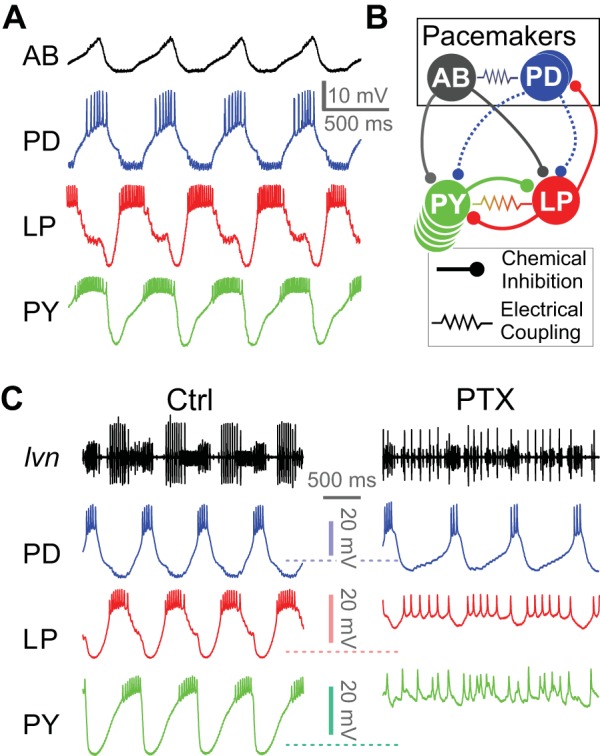
Jonah crab pyloric circuit activity, connectivity and response to pharmacological block of glutamatergic synapses. A: intracellular recordings of the anterior burster (AB), pyloric dilator (PD), lateral pyloric (LP), and pyloric constrictor (PY) neurons during ongoing pyloric activity. B: schematic diagram of the pyloric circuit, consisting of the AB and PD pacemaker ensemble neurons and the follower LP and PY neurons. Putative cholinergic synapses from the PD neuron are shown as dashed curves. C: simultaneous extracellular recording from the lateral ventricular nerve (lvn; containing the axons of PD, LP, and PY neurons) and intracellular recordings of the PD, LP, and PY neurons in control saline (Ctrl) and in the presence of 10−5 M picrotoxin (PTX), which blocks glutamatergic transmission in the stomatogastric ganglion (STG). Rhythmic activity in PD continues in PTX, but the LP-induced inhibitory postsynaptic potentials disappear. Residual rhythmicity remains in the LP and PY neurons.
Single-cell expression of neurotransmitter genes from STG neurons.
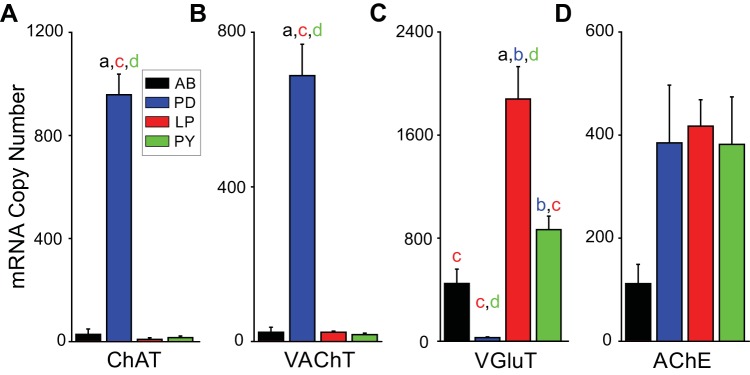
We first determined expression of mRNA levels for genes associated with neurotransmitter phenotype in single neurons: 1) AChE, 2) vGluT, 3) ChAT, and 4) vAChT. In these experiments, individual cells were identified and then assayed for gene expression (see methods). Figure 2 shows the mean level of expression across the four neurons of interest. To determine if the neurons were cholinergic, we measured two markers for ACh expression: ChAT and vAChT. ChAT is responsible for the synthesis of ACh, whereas vAChT is responsible for the transport of ACh from the cytoplasm into synaptic vesicles. Generally, neurons that express these two genes are considered to release ACh (Arvidsson et al. 1997). The PD neuron showed significantly higher expression of the two ACh markers compared with other pyloric neurons [ChAT: P < 0.05, F(3,17) = 3.127, one-way ANOVA (Fig. 2A); vAChT: P < 0.001, F(3,19) = 64.13, one-way ANOVA (Fig. 2B)]. AB, LP, and PY neurons were effectively negative for detection of ChAT and vAChT expression.
Fig. 2.
mRNA copy number for glutamatergic and cholinergic neurotransmission differs among pyloric circuit neurons in the Jonah crab. A: mRNA copy numbers for choline acetyltransferase (ChAT). The anterior burster (AB), lateral pyloric (LP), and pyloric constrictor (PY) neurons show little expression, but the pyloric dilator (PD) neuron has higher ChAT copy numbers (P < 0.05). B: mRNA copy number for vesicular acetylcholine transporter (vAChT). As with ChAT, the PD neuron has the largest copy number for vAChT (P < 0.0001). C: mRNA copy number for glutamate vesicular transporter (vGluT). The LP and PY neurons have large numbers of vGluT mRNA, whereas the PD neuron expresses little vGluT mRNA compared with the other neuron types (P < 0.0001). D: mRNA copy number for acetylcholinesterase (AChE) is not significantly different among the 4 neuron types (P = 0.066). For all measurements: AB (a), n = 7; PD (b), n = 5; LP (c), n = 5; and PY (d), n = 6. Groups labeled a–d indicate a significant difference (P < 0.05) from one another through post hoc analysis. Error bars are SD.
To determine glutamate expression across pyloric neurons we measured vGluT. vGluT is responsible for the transport of glutamate from the cytoplasm into synaptic vesicles and is commonly used a marker of neurons that use glutamate as a neurotransmitter (Jing et al. 2015; Kolodziejczyk et al. 2008). PD neurons showed minimal expression for vGluT, whereas all three other neurons showed significantly greater expression [P < 0.001, F(3,19) = 19.27, one-way ANOVA; Fig. 2C]. This result strongly suggests that in C. borealis, as in P. interruptus, the two pacemaker neurons PD and AB express different neurotransmitters and that each pacemaker neuron may differentially inhibit the follower neurons. Finally, the four neurons did not differ significantly in AChE expression (P = 0.066, F = 2.86, one-way ANOVA: Fig. 2D), which suggests that all four neuron types receive cholinergic synaptic input.
Examining the PD-evoked LP and PY neuron IPSP amplitudes.
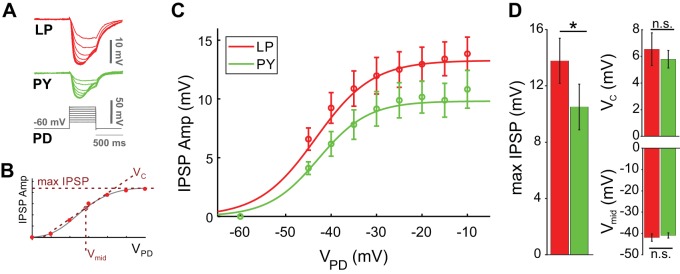
To measure the synaptic strength, we first examined the differences in amplitude of the pacemaker-evoked IPSPs in the LP and PY neurons in response to the PD neuron depolarization. The IPSP amplitude elicited in both LP and PY neurons increased with increased PD depolarization as expected from a graded synapse (Fig. 3, A and B). To measure the synaptic activation curve, in each experiment and for each of the two synapses, we fit the peak synaptic currents at all presynaptic membrane potentials with a Boltzmann sigmoidal curve (Fig. 3B; see methods) to estimate the postsynaptic potential maximum amplitude (max IPSP), the half-activation voltage (Vmid), and the slope factor (VC). The synaptic potential measured in the LP neuron was 36% larger in amplitude than that in the PY neuron (P = 0.033, Student’s t test; n = 12 experiments). However, there was no difference in the Vmid or VC values (Vmid: P = 0.221; VC: P = 0.797, Student’s t test; n = 12 experiments).
Fig. 3.
The pacemaker-induced graded inhibitory postsynaptic potential (IPSP) is different in the follower lateral pyloric (LP) and pyloric constrictor (PY) neurons. A: voltage trace of the pyloric dilator (PD) depolarization and the simultaneously recorded IPSPs in the LP and PY neurons. B: to estimate the IPSP maximum amplitude (max IPSP), the half-activation voltage (Vmid) and the slope factor (VC), in each experiment we fit both the LP and PY peak IPSP amplitudes at all PD presynaptic membrane potentials (VPD) with a Boltzmann sigmoidal curve. C: with increased PD depolarization, both the LP (red) and PY (green) IPSP increased in amplitude as expected in a graded synapse. D: max IPSP for the LP (red) neuron was 36% larger than for the PY (green) neuron (P = 0.033). There was no significant difference (n.s.) in the Vmid (P = 0.221) or VC values (P = 0.797; n = 12 experiments). *P < 0.05.
Does the PD neuron have a functional synapse onto the LP and PY neurons?
The AB and PD neurons make a compound inhibitory synapse to both LP and PY neurons in the spiny lobster P. interruptus that consists of a comparable contribution from each of the two presynaptic neurons (Rabbah and Nadim 2007). Our results showed that in the crab C. borealis, the AB and PD neurons have different mRNA expressions for proteins associated with glutamatergic vs. cholinergic transmission (Fig. 2). We therefore examined the relative contributions of the AB and PD neurons to the total synaptic effect in the LP and PY neuron in C. borealis.
We used PTX (10−5 M) to block the glutamatergic synapses, putatively from the AB neuron, and measured the IPSPs using the presynaptic pulse protocol described above. In both synapses, PTX drastically reduced the amplitude of the IPSP but did not completely block it (Fig. 4A). For IPSPs measured in both the LP and the PY neurons, this effect was consistent at all presynaptic potentials (Fig. 4B). A comparison of the synaptic activation curves in control and PTX showed that PTX significantly decreased the max IPSP amplitude in both synapses [LP: F(2,18) = 18.47, P < 0.0001, n = 12; PY: F(2,27) = 16.51, P < 0.0001, n = 11, one-way RM-ANOVA]. The strength of the pacemaker induced synapse in the LP neuron in PTX was 24% of the control value, whereas in the PY neuron it was 29% of control. The effect of PTX on both synapses was partially washable (Fig. 4). There was no difference in the Vmid or VC values in control and PTX for both the LP and PY neurons (Vmid: LP, P = 0.115; PY, P = 0.068; VC: LP, P = 0.989; PY, P = 0.464; one-way RM-ANOVA).
Fig. 4.
Blocking glutamatergic synapses blocks most but not all of the pacemaker-induced postsynaptic potential in the follower neurons. A: voltage trace of the pyloric dilator (PD) depolarization with a 50-mV pulse and the recorded inhibitory postsynaptic potentials (IPSPs) in the lateral pyloric (LP) and pyloric constrictor (PY) neurons in control, 10−5 M picrotoxin (PTX), and wash conditions. PTX reduced the amplitude of the IPSP in both LP and PY but did not eliminate it. B: synaptic activation curve of the IPSP in the LP and PY neurons in the 3 conditions (Ctrl, PTX, and wash). In both neurons, PTX reduced the IPSP amplitude but did not eliminate it. Activation curves are averages of fits of both the LP and PY peak IPSP amplitudes to individual experiments at all PD presynaptic membrane potentials (VPD) with a Boltzmann sigmoidal curve. Shaded area shows the 95% confidence interval. C: the strength of the pacemaker-induced synapse was reduced significantly in PTX in both the LP (24% of control, P < 0.0001) and PY neurons (29% of control, P < 0.0001). There was no significant difference (n.s.) in the half-activation voltage (Vmid; LP, P = 0.115; PY, P = 0.068) or slope factor (VC) values (LP, P = 0.989; PY, P = 0.464) between Ctrl and PTX for both the LP and PY neurons. *P < 0.05; ***P < 0.0001.
Characterization of synaptic dynamics.
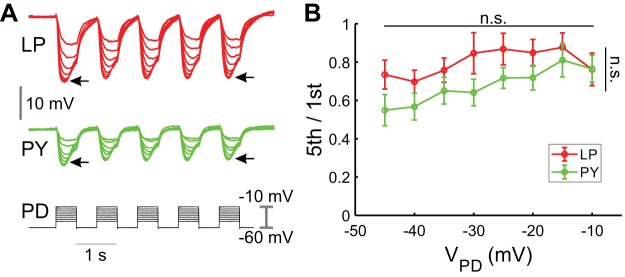
To characterize the extent of short-term dynamics of the pacemaker synaptic input to follower LP and PY neurons, we depolarized the PD neuron to different membrane potentials with consecutive square pulses (five 500-ms pulses with 500-ms interpulse intervals; see methods) and recorded the IPSPs in the LP and PY neurons (Fig. 5A). The second and subsequent IPSPs in both the PY and LP neurons were smaller in amplitude than those elicited by the first pulse, indicating that both synapses had short-term depression. The extent of synaptic depression was quantified as a ratio of the amplitudes of the fifth (steady state) and first IPSPs (5th/1st) for each neuron (Mamiya et al. 2003). Although there was some variation in this paired-pulse ratio, the level of depression was statistically independent of the presynaptic voltage amplitude or the postsynaptic cell type (LP and PY: P = 0.9095, n = 12, two-way ANOVA; Fig. 5B).
Fig. 5.
Pacemaker-induced postsynaptic potentials in the lateral pyloric (LP) and pyloric constrictor (PY) show similar short-term depression. A: the pyloric dilator (PD) neuron was depolarized with a series of 5 consecutive square pulses of different amplitudes, and the inhibitory postsynaptic potentials (IPSPs) in both the PY and LP neurons were recorded simultaneously. The extent of the synaptic depression was quantified by taking the ratio of the amplitude of the fifth to the first IPSP (5th/1st; denoted with arrows). B: across all voltages measured, the level of depression (5th/1st) was independent of the presynaptic voltage (VPD) or the postsynaptic neuron type (P = 0.9095). n.s., No significant difference.
To examine the contribution of the cholinergic synaptic output to synaptic depression, we compared the level of depression in the LP and PY neuron IPSPs in control and PTX saline (Fig. 6A). Because presynaptic voltage amplitude did not influence depression levels, we only show the results for the 40-mV amplitude pulses. Once again, even though there was some variability in the paired-pulse ratio (5th/1st), we found no significant difference between depression levels in control, PTX, or wash in either the LP or PY neuron IPSPs (LP: P = 0.066, n = 12; PY: P = 0.973, n = 11, one-way RM-ANOVA; Fig. 6B). This result indicates that the level of depression is not significantly different for the total synaptic output and the PTX-insensitive component to these follower neurons.
Fig. 6.
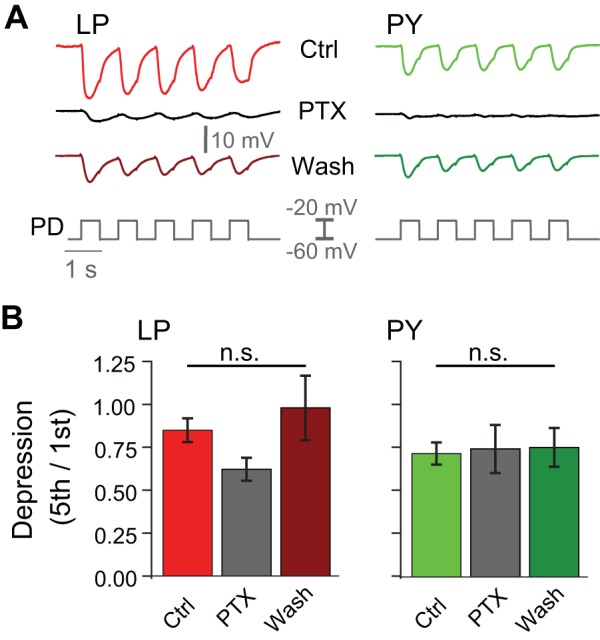
Short-term plasticity of the pacemaker-induced synapses is independent of blocking glutamatergic synapses. A: inhibitory postsynaptic potentials (IPSPs) recorded in the lateral pyloric (LP) and pyloric constrictor (PY) neurons in response to 5 consecutive 40-mV square-pulse depolarizations in the pyloric dilator (PD) neuron in control (Ctrl), 10−5 M picrotoxin (PTX), and wash conditions. B: no significant difference (n.s.) was found between depression levels (5th/1st, ratio of the amplitude of the fifth to the first IPSP) in control, PTX, or wash conditions in either the LP or PY neuron IPSPs (LP, P = 0.066; PY, P = 0.973).
Synaptic reversal potential.
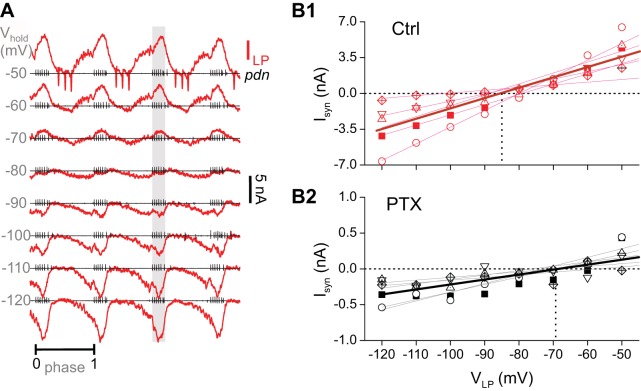
The original report of the distinct neurotransmitter types of the pyloric pacemaker neurons in the spiny lobster reported a distinct synaptic reversal potential for the AB and PD synapses (Marder and Eisen 1984). We therefore examined if the cholinergic and glutamatergic components of the pyloric pacemaker synapse in crab also have distinct reversal potentials. We measured the reversal potential of the pacemaker synapse in the follower LP neuron during ongoing pyloric rhythmic activity, as described previously (Goaillard et al. 2009). The LP neuron was voltage clamped at different holding potentials, and the total current was measured (Fig. 7A). Because the LP neuron is postsynaptic to both the pyloric pacemaker neurons and the PY neuron (Fig. 1), we measured the synaptic current only during the bursting activity of the PD neuron (shaded area in Fig. 7A) and estimated the reversal potential by doing a linear fit to the peak synaptic current in this region (Fig. 7B). In control saline, the mean reversal potential of the total synaptic current from the pacemaker neurons was −87.7 (SD ±9.1) mV (Fig. 7B). In the presence of PTX, the reversal potential of this synapse in the same preparations was significantly more depolarized at −71.4 (SD ±4.0) mV (paired Student’s t test, P = 0.0293, n = 5 experiments).
Fig. 7.
Pacemaker synaptic current has a significantly more positive reversal potential in picrotoxin (PTX). A: example recordings of baseline-subtracted currents (ILP) in the lateral pyloric (LP) neuron, measured in voltage clamp at different holding potentials (Vhold) during ongoing pyloric activity. Simultaneous recordings of the motor nerve pdn show spiking activity of the pyloric dilator (PD) neuron. Traces at each holding potential are normalized to the cycle period, calculated from the onsets of consecutive PD bursts (phases 0 and 1). Each pdn recording is positioned at the 0 current trace of ILP. The pacemaker-elicited synaptic current is calculated as the mean ILP value within the PD burst (shaded region). B: synaptic currents (Isyn) plotted as a function of holding potential (VLP) in control saline (Ctrl; B1) and in 10−5 M PTX (B2). Note the smaller current scale in PTX. Each symbol represents an individual experiment for which currents were measured in both control and PTX (n = 5 experiments). Thin lines indicate linear fits to each experiment. Thick line is the fit to all points. Horizontal dotted line is 0 current. Vertical dotted line is the mean reversal potential.
Are there functional consequences to the differences in the pacemaker synapses to the LP and PY neurons?
The LP and PY neurons always fire in distinct phases of the pyloric rhythm, with the bursting activity of the LP neuron preceding that of PY (see, e.g., Fig. 1). Such a difference in phase is not dictated by an asymmetry of input due to synaptic connectivity (Fig. 1B). However, the strength of an inhibitory synapse to a follower neuron could influence its postinhibitory rebound and therefore its activity phase in the oscillatory network. To examine if the difference in the synaptic strength of the pacemaker input to the LP and PY neurons, as demonstrated in Fig. 3, is a determinant of their different firing phases, we functionally removed the pacemaker synaptic input to these neurons (by bath application of PTX and hyperpolarization of the PD neuron; see methods) and injected an artificial synaptic conductance input to both neurons. A full description of the method is given previously (Martinez et al. 2019).
We found that in response to inhibitory synaptic input to both neurons, with identical maximal conductance, the LP neuron always rebounded to produce a burst before the PY neuron (Fig. 8A). There was no distinction in the burst end of both neurons (not quantified), which was always at the onset of the synaptic inhibition. This was presumably because PTX blocks the synaptic interaction between the PY and LP neurons, which is required for the termination of the LP burst.
Fig. 8.
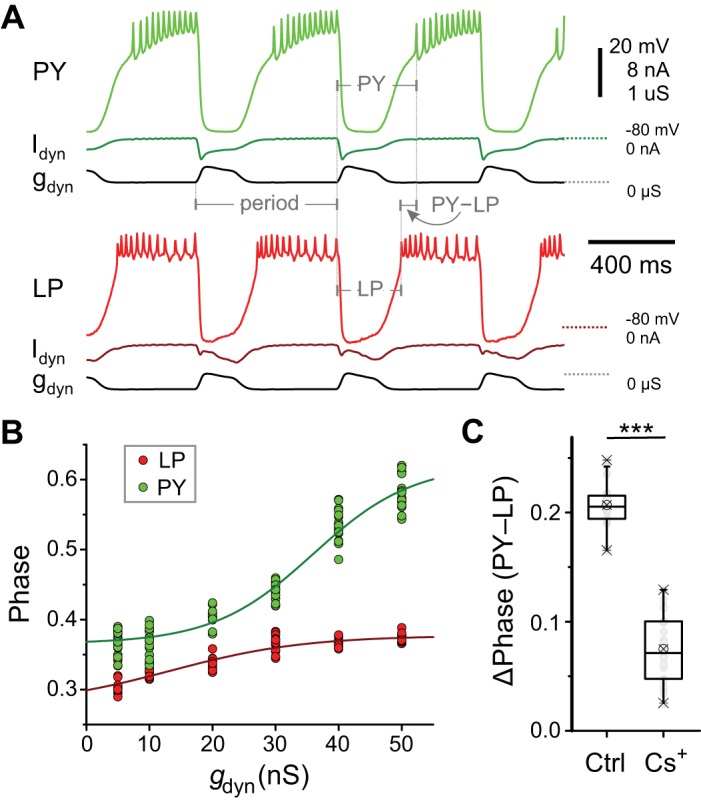
Does the difference between the pacemaker inputs to lateral pyloric (LP) and pyloric constrictor (PY) neurons play a functional role in determining their relative activity phase? A: simultaneous dynamic clamp injection of an artificial synaptic conductance (gdyn) into the synaptically isolated LP and PY neurons. The synaptic conductance consists of a stereotyped waveform injected with a fixed cycle period. The onset burst phase of the LP and PY neurons is calculated as the time from the onset of the gdyn waveform in each cycle, divided by the period. The phase difference between the LP and PY neuron burst onset is the time latency between the two (PY − LP), divided by the period. B: the phase of the LP neuron burst onset is always earlier than that of the PY neuron at all values of . C: the phase difference (Δphase) between the LP and PY neuron burst onsets decreases after the hyperpolarizing-activated inward current (Ih) is blocked (Cs+). ***P < 0.001. Ctrl, control; Idyn, current injected by dynamic clamp.
We quantified the burst onset phase of the two neurons for different maximal conductances of artificial synaptic input (gdyn; Fig. 8B). These results showed that at any given synaptic input strength, the LP burst always led the PY burst [P < 10−6, F(1,9) = 3109, two-way RM-ANOVA, Holm–Sidak post hoc analysis]. Additionally, for gdyn > 20 nS, the LP neuron could never burst later than PY, no matter how much the two synaptic strengths differed. Thus our finding in Fig. 3, that the total pacemaker synaptic input to the LP neuron is stronger than that to PY, was of no consequence to the determination of the activity phases of these two neurons.
Notably, the difference between the activity phases of the LP and PY neurons was significantly smaller after Ih was blocked in both neurons and identical dynamic clamp synaptic inputs were applied (Student’s paired t test, P < 10−6; Fig. 8C). This result supports the primary role of the intrinsic properties of these two neurons in determining their relative activity phases, as was previously suggested in the lobster pyloric circuit (Rabbah and Nadim 2005).
DISCUSSION
Synaptic interactions are primary determinants of neuronal activity and circuit output (Nadim and Bucher 2014). Neurons that receive heterogeneous inputs naturally respond to a compound synaptic influence, which could be shaped by both the activity times of the presynaptic neurons and the properties of their synapses. In oscillatory networks, synaptic input arriving from synchronously active presynaptic neurons provides an instructive example of the direct influence of distinct synapses on the total compound synapse. A clear example of this is seen in pacemaker-driven oscillations involving a heterogenous group of pacemaker neurons (Rabbah and Nadim 2007).
Previous work in the spiny lobster P. interruptus pyloric network demonstrated that the pacemaker group AB and PD neurons use different neurotransmitters to inhibit their circuit targets: the glutamatergic AB neuron produces fast inhibition, whereas the cholinergic PD neuron provides a delayed inhibition (Eisen and Marder 1982). In recent years, the Jonah crab C. borealis has become a primary species for studying properties of the stomatogastric nervous system. This shift has been attributed to numerous technical advantages of this species as a model system, as well as a number of seminal discoveries, including the identification and characterization of several modulatory projection neurons (Blitz and Nusbaum 2011; Nusbaum et al. 2001), the demonstration of ionic current expression variability and correlations (Khorkova and Golowasch 2007; Schulz et al. 2006; Temporal et al. 2012), the characterization of rules of peptide neuromodulation (Gray et al. 2017; Swensen and Marder 2000, 2001) and comodulation (Li et al. 2018), and the development of molecular techniques (Northcutt et al. 2016; Schulz et al. 2007). Surprisingly, several basic circuit properties in the stomatogastric nervous system of this species remain unexplored and are assumed to be similar to those discovered in related species such as the spiny lobster. Among these assumptions is that the pyloric PD neurons are cholinergic and that their contribution to the total synaptic output of the pyloric pacemaker group is as described in the spiny lobster. Given the differences in the circuit connectivity observed between the two systems (Marder and Bucher 2007; Stein 2009) and the central role of the pacemaker synapses in the pyloric circuit, it was important to determine if the findings from the spiny lobster pacemaker synapses apply to the Jonah crab.
Our data confirmed that, as in P. interruptus, in C. borealis the AB neuron is glutamatergic whereas the PD neuron is cholinergic. The differential use of neurotransmitters by the pacemaker neurons allows for the distinct regulation of the contribution of the AB or PD synapses to the follower neurons. For example, the combined pacemaker synapse to the LP and PY neurons has a relative contribution from AB and PD synapses that depends on the pacemaker cycle frequency and duty cycle. Additionally, the pyloric circuit activity is modified by endogenous neuromodulators, several of which modulate the activity and synaptic output of the PD and AB neurons (Harris-Warrick and Johnson 2010; Li et al. 2018). In fact, in some cases, the AB and PD synapses are modulated differentially. For example, dopamine enhances the AB-to-LP synapse but almost abolishes the PD-to-LP synapse (Harris-Warrick et al. 1998). Consequently, the compound pacemaker synapse provides multiple degrees of flexibility for the influence of the pacemaker neurons on their targets, including most follower pyloric neurons.
The neurotransmitter phenotype of STG neurons was first described through enzyme activity assays (Marder 1976) and pharmacology of synaptic physiology (Marder and Eisen 1984). These studies revealed that PD is cholinergic and that AB, LP, and PY are likely glutamatergic. Since this early work, new molecular tools have made it possible to perform gene expression studies on multiple targets (Schulz et al. 2007) and even full transcriptomes in individual neurons (Cadwell et al. 2016). In the present work, we took advantage of single-cell qPCR to measure the expression of mRNA that is commonly used to define neurons into cholinergic and glutamatergic phenotypes (e.g., Li et al. 1995; Roghani et al. 1994; Yamaguchi et al. 2007). We found that the PD neuron expresses high levels of ChAT and vAChT but virtually no vGluT. This supports the original work using enzyme assays and synaptic pharmacology to identify the neurotransmitter phenotype in this neuron. Furthermore, we showed that AB, LP, and PY neurons express relatively high levels of vGluT but almost undetectable quantities of ChAT and vAChT. These results are consistent with previous work that suggested, but did not definitively show, that these neurons are glutamatergic (Eisen and Marder 1982; Marder and Eisen 1984). Given that PD neurons have now been shown to express mRNA consistent with cholinergic phenotype, have enzymatic activity for transporting acetylcholine, and have been shown physiologically to use ACh as a neurotransmitter, our findings strongly suggest that mRNA expression of our candidate genes is, indeed, predictive of neurotransmitter phenotype in neurons of the STG. Therefore, we are confident that in this and future work, we can use these markers to define neurotransmitter phenotype in STG cells in the absence of direct synaptic or functional enzyme data.
To explore the functional strength of the AB and PD synapses to the follower neurons, we took advantage of PTX, a putative blocker of glutamatergic synapses in the stomatogastric ganglion (Bidaut 1980; Cleland and Selverston 1995; Marder and Paupardin-Tritsch 1978; Rinberg et al. 2013; Temporal et al. 2012). We found that in C. borealis there is a functional synapse onto the pyloric follower LP and PY neurons in the presence of PTX. The contribution of the PTX-insensitive component to the total compound synapse was ~24% in the LP neuron and ~29% in the PY neuron. This finding is consistent with the observation that in PTX, the synapse from the pacemakers is often incapable of driving the follower neurons to produce a rhythmic oscillation (e.g., Fig. 1; Rinberg et al. 2013).
The pyloric follower neurons LP and PY rebound from pacemaker inhibition to produce activity bursts at two different phases of each cycle, with the LP burst leading that of PY (see, e.g., Fig. 1). It is natural to assume that this phase difference is partly due to different synaptic input strengths from the pacemaker neurons. Previous studies of the P. interruptus pyloric circuit had shown that the synaptic input from the pacemakers to these two neurons is not different (Rabbah and Nadim 2007). Our results, comparing the corresponding synapses in C. borealis, show that in this species the pacemaker synaptic drive of the LP neuron is in fact stronger than that of PY. This is somewhat unexpected, because a weaker synaptic inhibition should allow for an earlier rebound, which is contrary to what is seen in the LP and PY neuron burst phases. However, using dynamic clamp injection of an artificial inhibitory synaptic conductance into the two neurons, we found that, independent of the strength of synaptic input, the LP neuron always rebounds from inhibition to burst at an earlier phase than the PY neuron (Fig. 7), as had been shown in P. interruptus (Rabbah and Nadim 2005). Synaptic inhibition interacts with numerous intrinsic ionic current dynamics to produce postinhibitory rebound (Martinez et al. 2019). These include, the transient potassium current IA, the Ih current, and low-threshold calcium currents such as the T current. It is plausible, for example, that smaller Ih or larger IA levels in the PY neuron would compensate for the weaker inhibitory drive of this neuron to produce a delayed phase (Zhang et al. 2009). Consistent with this hypothesis, blocking Ih reduced the differences in the activity phases of the LP and PY neurons (Fig. 7C). However, we did not test the effect of blocking IA because of the negative effect of its blockers on these neurons.
It is known that in P. interruptus, both IA and Ih levels in these neurons are modified by the same monoaminergic neuromodulators that also modulate the synaptic output from the AB and PD neurons (Harris-Warrick and Johnson 2010; Parker et al. 2019). It is therefore plausible that in modulated states, the synaptic input from the pacemakers may have different relative strengths and different interactions with the intrinsic voltage-gated currents, thus leading to shifts in the relative phases of the follower LP and PY neurons.
To further examine the differences in the pacemaker synaptic outputs, we measured the reversal potentials of the pacemaker synapses to the LP neuron. A comparison of the synaptic reversal potential in control saline and PTX showed a significant shift from −88 to −71 mV. With the assumption that PTX indeed blocks the glutamatergic component, this indicates that the cholinergic synapse has a much more positive reversal potential than the glutamatergic synapse. Although this result further supports the distinct identities of the two pacemaker output synapses, it contrasts with the classical results from the spiny lobster, where the glutamatergic reversal potential was measured at −70 mV (mean value) but the cholinergic response simply became smaller or disappeared but never reversed, even at −100 mV (Marder and Eisen 1984). However, those experiments were done in current clamp where, considering the modulatory muscarinic increase of excitability in pyloric neurons (Bal et al. 1994; Rosenbaum and Marder 2018; Swensen and Marder 2000), it would have been impossible to completely separate the synaptic effect. A more recent study used voltage clamp to measure the synaptic reversal potential of the pacemaker to LP synapse in C. borealis and did separate the AB and PD synaptic contributions (Goaillard et al. 2009). However, Goaillard et al. did not actually measure the reversal potentials, but rather separated the AB and PD contributions, assuming that the AB synapse reversed at −70 mV and the PD synapse reversed at −90 mV, using the measured AB synapse reversal potential and the extrapolated PD synapse reversal potential from the lobster data of Marder and Eisen (1984). Our result that the cholinergic reversal potential is in fact more positive than the glutamatergic one in the crab implies caution for the separation of mixed synaptic currents without directly measuring the reversal potentials.
In oscillatory circuits, short-term dynamics facilitate changes in synaptic strength as a function of network frequency (Abbott et al. 1997; Rothman et al. 2009). As in previous studies, our results show that the pacemaker synapses to these follower neurons showed depression (Li et al. 2018; Tseng et al. 2014). We also demonstrated that the level of depression is independent of the presynaptic voltage amplitude, the follower neuron type, and the presence of PTX. Thus the PTX-sensitive and -insensitive components have similar short-term dynamics, which may indicate a coordinated adjustment of the synaptic strength as a function of network frequency.
Pacemaker neurons are involved in rhythm generation in a variety of networks (Del Negro et al. 2018; Grace 2016; Jarabo and Martin 2017; Koshiya and Smith 1999; Snider et al. 2018). In most networks, these neurons consist of a heterogeneous population that have multiple cotransmitters or, even in those with the same neurotransmitter identity, have distinct synaptic dynamics. Because these pacemaker neurons can vary in their synaptic release dynamics or be differentially targeted by neuromodulators, their synaptic influence is subject to levels flexibility similar to that seen in the pyloric pacemaker neurons.
Conclusions.
Our work builds on previous studies of the pyloric network in other species of decapod crustaceans that reported on the neurotransmitter content, ion selectivity, and the short-term dynamics of the pacemaker to follower neuron synapses (Eisen and Marder 1982; Harris-Warrick and Johnson 2010; Li et al. 2018; Marder and Eisen 1984; Nadim and Manor 2000; Tseng et al. 2014). The heterogeneity in neurotransmitter type and release properties of the Jonah crab pyloric pacemaker neurons provides this circuit with mechanisms to differentially control the relative contribution of each synapse. A systematic analysis of the differential neuromodulation and analysis of the synaptic dynamics are needed to provide a fuller understanding of the differential roles of the two pacemaker synapses in the biological context. Furthermore, a similar analysis could be applied to understand the synaptic properties of disparate synchronized input in other oscillatory networks to unravel the contribution of synaptic dynamics and its neuromodulation to circuit output.
GRANTS
This work was supported by National Institute of Mental Health Grants MH060605 and MH046742-29.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M., D.S., and F.N. conceived and designed research; D.M., J.M.S., and F.N. performed experiments; D.M., J.M.S., and F.N. analyzed data; D.M., J.M.S., D.S., and F.N. interpreted results of experiments; D.M., J.M.S., and F.N. prepared figures; D.M. and F.N. drafted manuscript; D.M., J.M.S., D.S., and F.N. edited and revised manuscript; D.M., J.M.S., D.S., and F.N. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of D. Martinez: Dept. of Biomedical Sciences and Dalton Cardiovascular Research Center, Univ. of Missouri, 134 Research Park Dr., Columbia, MO 65211.
REFERENCES
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science 275: 220–224, 1997. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol 378: 454–467, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Bal T, Nagy F, Moulins M. Muscarinic modulation of a pattern-generating network: control of neuronal properties. J Neurosci 14: 3019–3035, 1994. doi: 10.1523/JNEUROSCI.14-05-03019.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidaut M. Pharmacological dissection of pyloric network of the lobster stomatogastric ganglion using picrotoxin. J Neurophysiol 44: 1089–1101, 1980. doi: 10.1152/jn.1980.44.6.1089. [DOI] [PubMed] [Google Scholar]
- Blitz DM. Circuit feedback increases activity level of a circuit input through interactions with intrinsic properties. J Neurophysiol 118: 949–963, 2017. doi: 10.1152/jn.00772.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. Neural circuit flexibility in a small sensorimotor system. Curr Opin Neurobiol 21: 544–552, 2011. doi: 10.1016/j.conb.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. Modulation of circuit feedback specifies motor circuit output. J Neurosci 32: 9182–9193, 2012. doi: 10.1523/JNEUROSCI.1461-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, Yilmaz M, Reimer J, Shen S, Bethge M, Tolias KF, Sandberg R, Tolias AS. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat Biotechnol 34: 199–203, 2016. doi: 10.1038/nbt.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Selverston AI. Glutamate-gated inhibitory currents of central pattern generator neurons in the lobster stomatogastric ganglion. J Neurosci 15: 6631–6639, 1995. doi: 10.1523/JNEUROSCI.15-10-06631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daur N, Nadim F, Bucher D. The complexity of small circuits: the stomatogastric nervous system. Curr Opin Neurobiol 41: 1–7, 2016. doi: 10.1016/j.conb.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci 19: 351–367, 2018. doi: 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JS, Marder E. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. III. Synaptic connections of electrically coupled pyloric neurons. J Neurophysiol 48: 1392–1415, 1982. doi: 10.1152/jn.1982.48.6.1392. [DOI] [PubMed] [Google Scholar]
- Follmann R, Goldsmith CJ, Stein W. Spatial distribution of intermingling pools of projection neurons with distinct targets: a 3D analysis of the commissural ganglia in Cancer borealis. J Comp Neurol 525: 1827–1843, 2017. doi: 10.1002/cne.24161. [DOI] [PubMed] [Google Scholar]
- Goaillard JM, Taylor AL, Schulz DJ, Marder E. Functional consequences of animal-to-animal variation in circuit parameters. Nat Neurosci 12: 1424–1430, 2009. doi: 10.1038/nn.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 17: 524–532, 2016. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard K, Raper JA, Hartline DK. Graded synaptic transmission between spiking neurons. Proc Natl Acad Sci USA 77: 3733–3735, 1980. doi: 10.1073/pnas.77.6.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M, Daudelin DH, Golowasch J. Activation mechanism of a neuromodulator-gated pacemaker ionic current. J Neurophysiol 118: 595–609, 2017. doi: 10.1152/jn.00743.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad SA, Marder E. Circuit Robustness to Temperature Perturbation Is Altered by Neuromodulators. Neuron 100: 609–623.e3, 2018. doi: 10.1016/j.neuron.2018.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley JA, Hampton D, Marder E. Two central pattern generators from the crab, Cancer borealis, respond robustly and differentially to extreme extracellular pH. eLife 7: e41877, 2018. doi: 10.7554/eLife.41877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Johnson BR. Checks and balances in neuromodulation. Front Behav Neurosci 4: 47, 2010. doi: 10.3389/fnbeh.2010.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Johnson BR, Peck JH, Kloppenburg P, Ayali A, Skarbinski J. Distributed effects of dopamine modulation in the crustacean pyloric network. Ann N Y Acad Sci 860: 155–167, 1998. doi: 10.1111/j.1749-6632.1998.tb09046.x. [DOI] [PubMed] [Google Scholar]
- Hooper SL, O’Neil MB, Wagner R, Ewer J, Golowasch J, Marder E. The innervation of the pyloric region of the crab, Cancer borealis: homologous muscles in decapod species are differently innervated. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 159: 227–240, 1986. doi: 10.1007/BF00612305. [DOI] [PubMed] [Google Scholar]
- Jarabo P, Martin FA. Neurogenetics of Drosophila circadian clock: expect the unexpected. J Neurogenet 31: 250–265, 2017. doi: 10.1080/01677063.2017.1370466. [DOI] [PubMed] [Google Scholar]
- Jing J, Alexeeva V, Chen SA, Yu K, Due MR, Tan LN, Chen TT, Liu DD, Cropper EC, Vilim FS, Weiss KR. Functional characterization of a vesicular glutamate transporter in an interneuron that makes excitatory and inhibitory synaptic connections in a molluscan neural circuit. J Neurosci 35: 9137–9149, 2015. doi: 10.1523/JNEUROSCI.0180-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Brown JM, Kvarta MD, Lu JY, Schneider LR, Nadim F, Harris-Warrick RM. Differential modulation of synaptic strength and timing regulate synaptic efficacy in a motor network. J Neurophysiol 105: 293–304, 2011. doi: 10.1152/jn.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorkova O, Golowasch J. Neuromodulators, not activity, control coordinated expression of ionic currents. J Neurosci 27: 8709–8718, 2007. doi: 10.1523/JNEUROSCI.1274-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczyk A, Sun X, Meinertzhagen IA, Nässel DR. Glutamate, GABA and acetylcholine signaling components in the lamina of the Drosophila visual system. PLoS One 3: e2110, 2008. doi: 10.1371/journal.pone.0002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature 400: 360–363, 1999. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- Lett KM, Garcia VJ, Temporal S, Bucher D, Schulz DJ. Removal of endogenous neuromodulators in a small motor network enhances responsiveness to neuromodulation. J Neurophysiol 118: 1749–1761, 2017. doi: 10.1152/jn.00383.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Bucher D, Nadim F. Distinct co-modulation rules of synapses and voltage-gated currents coordinate interactions of multiple neuromodulators. J Neurosci 38: 8549–8562, 2018. doi: 10.1523/JNEUROSCI.1117-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Holtzman DM, Kromer LF, Kaplan DR, Chua-Couzens J, Clary DO, Knüsel B, Mobley WC. Regulation of TrkA and ChAT expression in developing rat basal forebrain: evidence that both exogenous and endogenous NGF regulate differentiation of cholinergic neurons. J Neurosci 15: 2888–2905, 1995. doi: 10.1523/JNEUROSCI.15-04-02888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle C. The sensitivity of decapod foregut muscles to acetylcholine and glutamate. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 138: 187–199, 1980. doi: 10.1007/BF00657037. [DOI] [Google Scholar]
- Mamiya A, Manor Y, Nadim F. Short-term dynamics of a mixed chemical and electrical synapse in a rhythmic network. J Neurosci 23: 9557–9564, 2003. doi: 10.1523/JNEUROSCI.23-29-09557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor Y, Nadim F, Abbott LF, Marder E. Temporal dynamics of graded synaptic transmission in the lobster stomatogastric ganglion. J Neurosci 17: 5610–5621, 1997. doi: 10.1523/JNEUROSCI.17-14-05610.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Cholinergic motor neurones in the stomatogastric system of the lobster. J Physiol 257: 63–86, 1976. doi: 10.1113/jphysiol.1976.sp011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69: 291–316, 2007. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Marder E, Eisen JS. Transmitter identification of pyloric neurons: electrically coupled neurons use different transmitters. J Neurophysiol 51: 1345–1361, 1984. doi: 10.1152/jn.1984.51.6.1345. [DOI] [PubMed] [Google Scholar]
- Marder E, Paupardin-Tritsch D. The pharmacological properties of some crustacean neuronal acetylcholine, γ-aminobutyric acid, and l-glutamate responses. J Physiol 280: 213–236, 1978. doi: 10.1113/jphysiol.1978.sp012381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci USA 95: 5323–5328, 1998. doi: 10.1073/pnas.95.9.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Anwar H, Bose A, Bucher DM, Nadim F. Short-term synaptic dynamics control the activity phase of neurons in an oscillatory network. eLife 8: e46911, 2019. doi: 10.7554/eLife.46911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadim F, Bucher D. Neuromodulation of neurons and synapses. Curr Opin Neurobiol 29: 48–56, 2014. doi: 10.1016/j.conb.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadim F, Manor Y. The role of short-term synaptic dynamics in motor control. Curr Opin Neurobiol 10: 683–690, 2000. doi: 10.1016/S0959-4388(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Northcutt AJ, Lett KM, Garcia VB, Diester CM, Lane BJ, Marder E, Schulz DJ. Deep sequencing of transcriptomes from the nervous systems of two decapod crustaceans to characterize genes important for neural circuit function and modulation. BMC Genomics 17: 868, 2016. doi: 10.1186/s12864-016-3215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci 24: 146–154, 2001. doi: 10.1016/S0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- Otopalik AG, Goeritz ML, Sutton AC, Brookings T, Guerini C, Marder E. Sloppy morphological tuning in identified neurons of the crustacean stomatogastric ganglion. eLife 6: e22352, 2017. doi: 10.7554/eLife.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AR, Forster LA, Baro DJ. Modulator-gated, SUMOylation-mediated, activity-dependent regulation of ionic current densities contributes to short-term activity homeostasis. J Neurosci 39: 596–611, 2019. doi: 10.1523/JNEUROSCI.1379-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JH, Gaier E, Stevens E, Repicky S, Harris-Warrick RM. Amine modulation of Ih in a small neural network. J Neurophysiol 96: 2931–2940, 2006. doi: 10.1152/jn.00423.2005. [DOI] [PubMed] [Google Scholar]
- Puccini GD, Sanchez-Vives MV, Compte A. Integrated mechanisms of anticipation and rate-of-change computations in cortical circuits. PLoS Comput Biol 3: e82, 2007. doi: 10.1371/journal.pcbi.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbah P, Nadim F. Synaptic dynamics do not determine proper phase of activity in a central pattern generator. J Neurosci 25: 11269–11278, 2005. doi: 10.1523/JNEUROSCI.3284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbah P, Nadim F. Distinct synaptic dynamics of heterogeneous pacemaker neurons in an oscillatory network. J Neurophysiol 97: 2239–2253, 2007. doi: 10.1152/jn.01161.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg A, Taylor AL, Marder E. The effects of temperature on the stability of a neuronal oscillator. PLoS Comput Biol 9: e1002857, 2013. doi: 10.1371/journal.pcbi.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghani A, Feldman J, Kohan SA, Shirzadi A, Gundersen CB, Brecha N, Edwards RH. Molecular cloning of a putative vesicular transporter for acetylcholine. Proc Natl Acad Sci USA 91: 10620–10624, 1994. doi: 10.1073/pnas.91.22.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum P, Marder E. Graded transmission without action potentials sustains rhythmic activity in some but not all modulators that activate the same current. J Neurosci 38: 8976–8988, 2018. doi: 10.1523/JNEUROSCI.2632-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JS, Cathala L, Steuber V, Silver RA. Synaptic depression enables neuronal gain control. Nature 457: 1015–1018, 2009. doi: 10.1038/nature07604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, Marder E. Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci 9: 356–362, 2006. doi: 10.1038/nn1639. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, Marder EE. Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. Proc Natl Acad Sci USA 104: 13187–13191, 2007. doi: 10.1073/pnas.0705827104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider KH, Sullivan KA, Obrietan K. Circadian regulation of hippocampal-dependent memory: circuits, synapses, and molecular mechanisms. Neural Plast 2018: 7292540, 2018. doi: 10.1155/2018/7292540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein W. Modulation of stomatogastric rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195: 989–1009, 2009. doi: 10.1007/s00359-009-0483-y. [DOI] [PubMed] [Google Scholar]
- Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci 20: 6752–6759, 2000. doi: 10.1523/JNEUROSCI.20-18-06752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Marder E. Modulators with convergent cellular actions elicit distinct circuit outputs. J Neurosci 21: 4050–4058, 2001. doi: 10.1523/JNEUROSCI.21-11-04050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temporal S, Desai M, Khorkova O, Varghese G, Dai A, Schulz DJ, Golowasch J. Neuromodulation independently determines correlated channel expression and conductance levels in motor neurons of the stomatogastric ganglion. J Neurophysiol 107: 718–727, 2012. doi: 10.1152/jn.00622.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng HA, Martinez D, Nadim F. The frequency preference of neurons and synapses in a recurrent oscillatory network. J Neurosci 34: 12933–12945, 2014. doi: 10.1523/JNEUROSCI.2462-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng HA, Nadim F. The membrane potential waveform of bursting pacemaker neurons is a predictor of their preferred frequency and the network cycle frequency. J Neurosci 30: 10809–10819, 2010. doi: 10.1523/JNEUROSCI.1818-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann JM, Meyrand P, Marder E. Neurons that form multiple pattern generators: identification and multiple activity patterns of gastric/pyloric neurons in the crab stomatogastric system. J Neurophysiol 65: 111–122, 1991. doi: 10.1152/jn.1991.65.1.111. [DOI] [PubMed] [Google Scholar]
- White RS, Spencer RM, Nusbaum MP, Blitz DM. State-dependent sensorimotor gating in a rhythmic motor system. J Neurophysiol 118: 2806–2818, 2017. doi: 10.1152/jn.00420.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci 25: 106–118, 2007. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bose A, Nadim F. The influence of the A-current on the dynamics of an oscillator-follower inhibitory network. SIAM J Appl Dyn Syst 8: 1564–1590, 2009. doi: 10.1137/090760994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Sheibanie AF, Oh M, Rabbah P, Nadim F. Peptide neuromodulation of synaptic dynamics in an oscillatory network. J Neurosci 31: 13991–14004, 2011. doi: 10.1523/JNEUROSCI.3624-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]