Abstract
Prosaccades are saccadic eye movements made reflexively in response to the sudden appearance of visual stimuli, whereas antisaccades are saccades that are directed to a location opposite a stimulus. Bibi and Edelman (Bibi R, Edelman JA. J Neurophysiol 102: 3101–3110, 2009) demonstrated that decreases in reaction time resulting from training prosaccades along one spatial axis (horizontal or vertical) could transfer to prosaccades made along the other axis. To help determine whether visual or motor-related processes underlie this facilitation, in the present study we trained participants to make prosaccades and probed their performance (reaction time, error rate) on antisaccade trials and vice versa. Subjects were probed for the effects of training on saccade performance before, during, and after 12 sessions of training. Training on prosaccades improved performance on both pro- and antisaccade tasks. Antisaccade training, with either a classic step task or a gap task, improved performance on gap prosaccades, though by less than it improved antisaccade performance, but had limited effect on an overlap prosaccade task. Across all subjects, training on one task only rarely had an adverse impact on an untrained task. These findings suggest that the predominant effect of saccade training is to facilitate fixation disengagement and motor preparation processes while having little impact on visual input to the saccadic system.
NEW & NOTEWORTHY This is the first systematic examination of whether training of prosaccades and antisaccades is task specific or instead transfers to the other saccade type. It finds that training tends to improve performance of all saccade types tested. These behavioral results provide insight into saccade neurophysiology, suggesting that saccade training enhances processes related to motor excitation and inhibition.
Keywords: antisaccade, gap, motor learning, reaction time, saccade
INTRODUCTION
Saccades are rapid eye movements that shift gaze to focus images of interest in the visual scene onto the fovea, the portion of the retina with the highest density of photoreceptors (Leigh and Zee 2015). Although saccades can be driven by a combination of voluntary and involuntary factors, they are generally made to visual stimuli, both those that are stable and those appearing suddenly in the environment. Visuomotor responsiveness can be assessed with a reactive prosaccade task, in which saccades are made to a suddenly appearing visual stimulus. Saccade reaction time (RT) in response to a suddenly appearing stimulus is often highly variable, particularly for naive subjects (reviewed in Leigh and Zee 2015), but with a small amount of practice is generally considerably less than 200 ms (Bibi and Edelman 2009). Saccade RT variability is thought to result, at least in part, from the amount of time until an activity threshold is reached by saccade-related neurons of the superior colliculus (SC) and frontal eye fields (FEF) (Carpenter and Williams 1995; Leigh and Zee 2015; Schall and Thompson 1999).
A temporal “gap” can be used in a prosaccade task to reduce saccade RT (Saslow 1967). In a gap task there is a small interval in time (100–200 ms) between the disappearance of the fixation point and target appearance. It is thought that this stimulus disappearance decreases saccade RT by, in part, disengaging inhibitory fixation processes before target appearance, so that the target can be reacted to more quickly (Mayfrank et al. 1986).
The minimum RT of saccades in primates is ∼75–80 ms, a limit thought to be governed by the anatomical and synaptic limitations of the visual and oculomotor systems (Edelman and Keller 1996). Saccades with very short latencies (<120 ms) are termed “express” saccades and are thought to be triggered directly by the visual response present in intermediate layer output neurons in the SC (Edelman and Keller 1996). Express saccades occur more frequently with a task using the temporal gap (Fischer and Boch 1983) but do not require it (Amatya et al. 2011).
Although it was first thought that in both human and monkey a saccade had to be prepared spatially to have RTs in the express range (Paré and Munoz 1996), Bibi and Edelman (2009) demonstrated that vector-specific saccade training could generalize to other saccade directions, at least for humans. They trained one set of participants on saccades along the vertical axis, making upward or downward movements, and another set of participants on horizontal saccades (leftward, rightward) in a gap task. For the trained directions, RT decreased with training and express saccade production increased. Performance on the untrained directions was probed before, during, and after training. Results showed that the effects of training transferred to the untrained directions. Moreover, after training, express saccade production was almost as high, and saccade RT was almost as low, for saccades made in blocks of trials with eight possible saccade target locations as for those with two possible locations. These results indicate that motor preparation for saccades can spatially generalize, suggesting that performance is increased by some combination of enhanced disinhibition of the saccadic system during the gap, improved generalization of motor preparation across space, and increased visual responsiveness.
Mechanisms of response inhibition and executive control over the saccadic system can be assessed by observing the generation of antisaccades, which can be performed in the laboratory by instructing subjects to make a saccade in a direction opposite to that of a suddenly appearing stimulus (Hallett 1978; Leigh and Zee 2015; Munoz and Everling 2004). For an antisaccade to be performed, the reflexive prosaccade toward the stimulus must be suppressed and a saccade in the opposite direction, toward blank space, must be performed voluntarily. Pro- and antisaccades depend on partially overlapping sets of mechanisms that may interact and compete. In the antisaccade task, there are two processes that allow a volitional saccade to be performed in the direction opposite the appearance of a stimulus: the suppression of the reflexive prosaccade and the inversion of the stimulus vector to allow the performance of the antisaccade. For these reasons, directional errors in an antisaccade task, where saccades are made inappropriately to the visual stimulus, are commonplace, and successfully made antisaccades tend to have RTs 50–100 ms greater than prosaccades (Leigh and Zee 2015). As with prosaccades, when a gap is used in an antisaccade task, RTs decrease. However, the increased disinhibition to the saccade system can also increase directional error rates (Everling et al. 1998; Fischer and Hartnegg 2000). Impairments in the ability of a subject to perform antisaccades can be indicative of neurological or psychiatric disease (Everling and Fischer 1998; Guitton et al. 1985; Munoz and Everling 2004).
Bibi and Edelman (2009) speculated that the training effects they found for prosaccades were a result of some combination of enhanced saccade disinhibition and enhanced spatially generalized motor preparation. This could be mediated neurophysiologically by enhanced baseline activity in the more caudal SC or the FEF (Dorris et al. 1997; Everling and Munoz 2000), as well by signals in the rostral, fixation-related area of the SC or in the FEF (Dias and Bruce 1994; Dorris and Munoz 1995). For convenience, we refer to both of these properties as “motor-specific properties.” However, it is also possible that factors related to visual processing, in particular those related to feedforward pathways originating in occipital cortex and terminating in saccade-related areas (Leigh and Zee 2015), could have also played a role in improving saccade RT performance. Such potentiation of vision-related signals could perhaps be mediated by an enhanced ability to deploy visual attention (Kristjansson 2011).
The relative role of visual and motoric factors can be determined by training subjects to perform prosaccades and then probing performance on antisaccades (or vice versa). If training improved motoric processes, then it is likely that training on prosaccades would transfer to antisaccades, since motor activation is necessary for both tasks. On the other hand, if training affected visual factors, then training on the prosaccade task should increase errors toward the original target in the antisaccade task and saccade RT should be unaffected, or even increase, as correctly made antisaccades would have longer RTs. Analogous arguments could be made regarding the effect of antisaccade training on prosaccades, in which antisaccade training should slow prosaccade RT. It is also possible that training on a single task could enhance both visual and motor processes, in which some combination of the above effects may occur.
We assessed these possibilities by using a training/probing protocol similar to that of Bibi and Edelman (2009). Subjects were trained on a gap prosaccade or antisaccade task and probed on the other task before, during, and after training. We also examined the effect of training on gap prosaccade and standard antisaccade tasks on performance in an overlap prosaccade task, where the fixation point disappears after target appearance. This was done to test whether training effects of the gap task on prosaccades were limited to facilitating processes occurring during the gap period. If so, then training on a gap prosaccade task would not affect performance in an overlap prosaccade task. Second, we examined whether including a gap antisaccade training protocol would have additional benefits in improving performance in a gap prosaccade task or whether it would instead further strengthen inhibitory processes and increase prosaccade RT. For consistency, we probed performance in gap prosaccade, standard (step) antisaccade, and overlap prosaccade tasks in all three training protocols (gap prosaccade, step antisaccade, gap antisaccade).
MATERIALS AND METHODS
General Methods: Subjects, Eye Movement Recording, and Visual Stimulus Display
A total of three experiments were conducted. Participants had never taken part in an eye movement study. Subjects ranged in age from 19 to 25 yr (sex breakdowns for each experiment are indicated below). Experiments were conducted under a protocol reviewed and approved by The City College of the City University of New York Institutional Review Board, and subjects gave written informed consent before participation. Participants were seated 60 cm from a computer monitor with their head stabilized through the use of chin and cheek rests. They were seated in a fluorescent-lit room and surrounded by a black curtain to help prevent visible reflections on the computer screen.
Eye movement data were recorded by videooculography (EyeLink II; SR Research) at 500 Hz. Before data collection, calibration was done with a nine-point (3 × 3) calibration procedure. Experiments were conducted with software routines for visual display and data collection written in the Experiment Builder experimental programming environment (SR Research) running on a Dell Precision T1600 computer with stimuli displayed on a Compaq P1220 monitor at a frame rate of 100 Hz. Data analysis was performed with custom routines written for MATLAB.
Trial types.
Three trial types were used in all three experiments: Pro Gap, Anti Step, and Pro Overlap. An additional trial type, Anti Gap, was used only for experiment 3.
In the Pro Gap task, the trial began with the presentation of a central green “fixation” square 0.5° in width. Subjects were required to fixate this stimulus within a 2° × 2° electronic window within 1 s of its appearance. The stimulus then remained onscreen for 500–800 ms before disappearing for 150–200 ms (“gap”). The target stimulus appeared (1° wide) at 10° eccentricity randomly to the left or right of the fixation point. Subjects were required to make a saccade to the stimulus within 400 ms of its appearance and land within a 4°-wide electronic window centered at the target location. Three hundred milliseconds after this saccade landed, the target disappeared and the trial ended. Subjects were required to maintain fixation during this 300-ms interval. After an intertrial interval of 700 ms, a new trial began.
The Pro Overlap task was identical to the Pro Gap task except that there was no gap; instead, the fixation point remained onscreen until the initiation of a saccade.
The Anti Step task was identical to the Pro Gap task except that 1) the central fixation point was red to indicate that the subject should look 180° away from the suddenly appearing stimulus, 2) no gap was present (instead, the stimulus appeared when the fixation point disappeared), and 3) a visual stimulus (0.5° × 0.5°) was presented at the desired saccade goal after the saccade was complete to provide spatial feedback to the subject to help increase the accuracy of the non-visually guided antisaccades.
Finally, the Anti Gap task was identical to the Anti Step task except that a gap of 150–200 ms was present between fixation point disappearance and target appearance, as in the Pro Gap task.
Session types.
There were two different session types in all three experiments: Training and Probe sessions (Fig. 1). The Training sessions involved the presentation of only one type of trial. Twelve Training sessions were run in the course of the experiment. Fifteen blocks of 20 trials were run. In the Probe sessions, both trained and untrained tasks were run, to test the transfer of the training in the Training sessions to the various trial types. Probe sessions were run before training, after 6 Training sessions, and after all 12 Training sessions. Probe sessions also consisted of a total of 15 trial blocks each consisting of 20 trials, with 5 blocks each of the Pro Gap, Pro Overlap, and Anti Step trials (trial type was constant within a block).
Fig. 1.
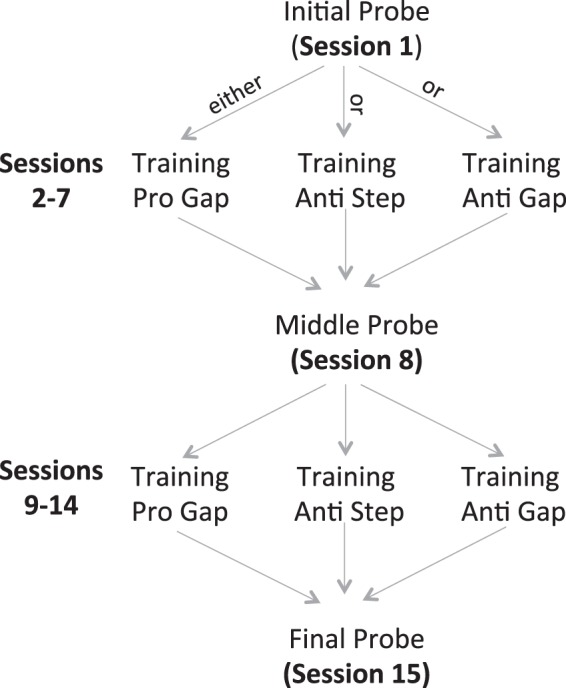
Sequencing of Probe and Training sessions in each of 3 experiments. Subject performance in Pro Gap, Anti Step, and Pro Overlap tasks was probed before, in the middle of, and after training. Participants were trained in 1 of 3 training protocols (experiment 1: Pro Gap, experiment 2: Anti Step, and experiment 3: Anti Gap).
The entire process of training and probing took place within a period of 5 wk. This method of probing and training is similar to our previous work (Bibi and Edelman 2009).
Experiment 1: Influence of Gap Prosaccade Training on Antisaccade and Overlap Prosaccade Performance
Seven subjects (3 men, 4 women) participated. Training sessions consisted of Pro Gap trials. Each of the three Probe sessions consisted of equal numbers of Pro Gap, Pro Overlap, and Anti Step trials (100 trials each). As mentioned above, these trials were run in blocks of a single trial type of 20 trials, with five blocks of each of three tasks. Blocks alternated between the three trial types, and their order was counterbalanced across subjects.
Experiment 2: Effect of (Step) Antisaccade Training on Prosaccade Performance
Six subjects (3 men, 3 women) participated in this experiment. The Probe sessions were the same as in experiment 1. The Training sessions, however, included only Anti Step trials. The experiment was otherwise identical to experiment 1.
Experiment 3: Gap Antisaccade Training
Six subjects (3 men, 3 women) participated in this experiment. Training used Anti Gap trials. The experiment was otherwise identical to experiments 1 and 2.
Data Analysis
Data analysis was performed with routines from MATLAB (MathWorks). All stated statistically significant differences were significant at α = 0.05.
We used two types of statistical analysis approaches. Our general approach was to compare performance before and after training in a particular task, using analysis of variance (ANOVA) with order of probe (before training or after training) as a fixed factor and subject as a random factor. This approach allowed us to use every saccade in the relevant data sets and still analyze aggregate effects. We also examined data trends on an individual subject basis, using nonparametric tests (Wilcoxon rank sum) to ensure that differences were not driven by only one or two subjects. Correlations between antisaccade error rates and RT were observed by using the Pearson product moment to determine whether there was a speed/accuracy trade-off in subject performance. Saccades with RTs <75 ms were deemed anticipatory saccades and were not analyzed further.
Saccade Reaction Time
To compute the start and end of each saccade, a saccade radial velocity trace was obtained by differentiating the horizontal and vertical components of eye position separately with a central difference algorithm implemented in MATLAB. Next, the Pythagorean theorem was used to calculate radial velocity as a function of time. We then used MATLAB routines to estimate the eye position trace just after the time of target appearance to determine the first point at which velocity exceeded 35°/s. Next, the trace was evaluated backward in time until the first point below 15°/s was found. The end of the saccade was determined in an analogous manner, but with time reversed.
As saccade RTs better fit a log-normal distribution than a normal distribution, we computed a central tendency of RT for a particular data set by first log-transforming the data, calculating the mean, and then computing the inverse of the log-transform of the mean. For simplicity, we henceforth use “mean” to refer to the central tendency of a RT data set calculated in such a manner. A log-transform of RT was also calculated when performing statistical tests that rely on an assumption of normality.
Finally, to examine the probability distributions of saccade RT in more detail, we fit the RT distributions with an ex-Gaussian model, which characterizes an RT distribution by mean (μ), dispersion (σ, akin to variance), and a term, τ, reflecting the extent of the right tail of the distribution. Mathematically, the ex-Gaussian function is the convolution of a Gaussian distribution and an exponential distribution (Jóhannesson et al. 2012). This model fit our RT distributions reasonably well (see Figs. 4, 5, 10, and 11). Measures of chi-square goodness of fit showed much better fits with an ex-Gaussian model than with normal or log-normal distributions (personal observations).
Fig. 4.
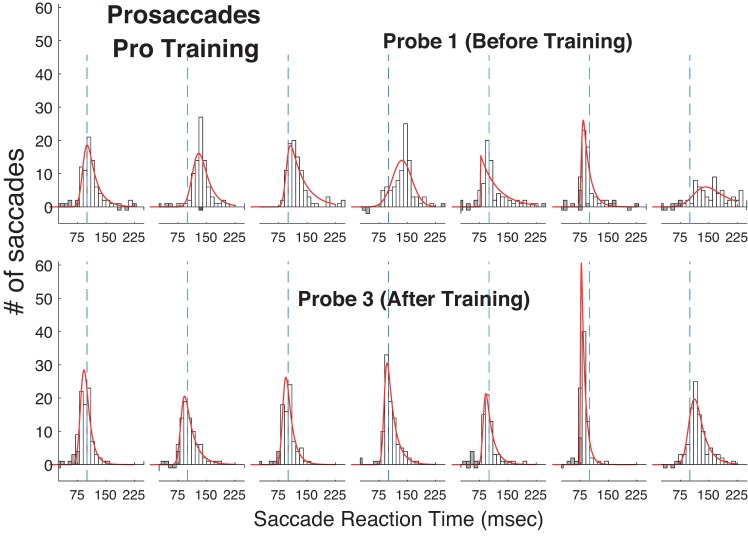
Histograms of reaction time (RT) in the Pro Gap task before (top) and after (bottom) training in the Pro Gap task. Each of the 7 columns shows data for 1 of the 7 subjects (left to right, S1→S7). Number of correctly executed saccades is shown by open bars extending up. Number of saccades made in the wrong direction is shown by bars extending down. Anticipatory saccades (RT < 75 ms) are denoted by shaded bars. Vertical dashed line denotes 100 ms. Red lines indicate ex-Gaussian curve fits.
Fig. 5.
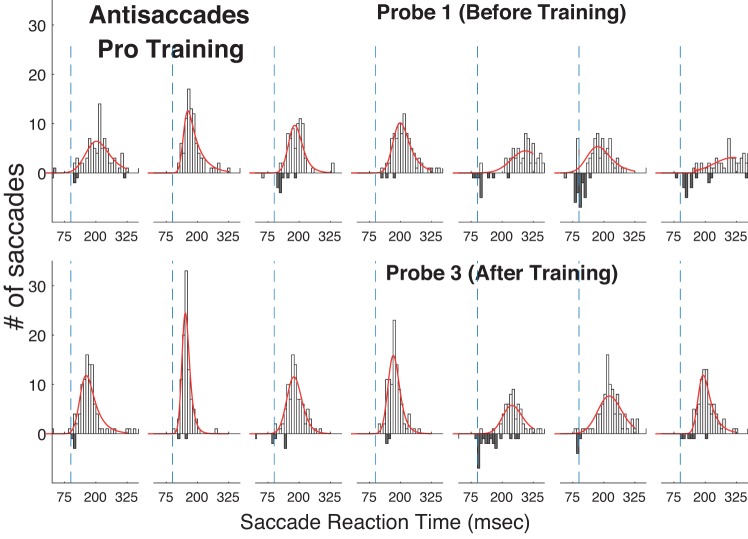
Histograms of reaction time (RT) in the Anti Step task before (top) and after (bottom) training in the Pro Gap task. Number of saccades made in the wrong direction is shown by bars extending down. Conventions as in Fig. 4.
Fig. 10.
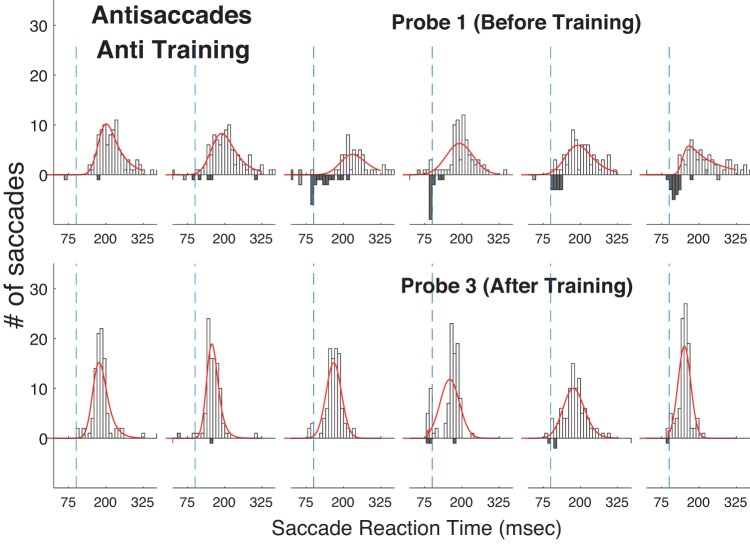
Histograms of reaction time in the Anti Step task before (top) and after (bottom) training in the Anti Step task in experiment 2. Conventions as in Fig. 4.
Fig. 11.
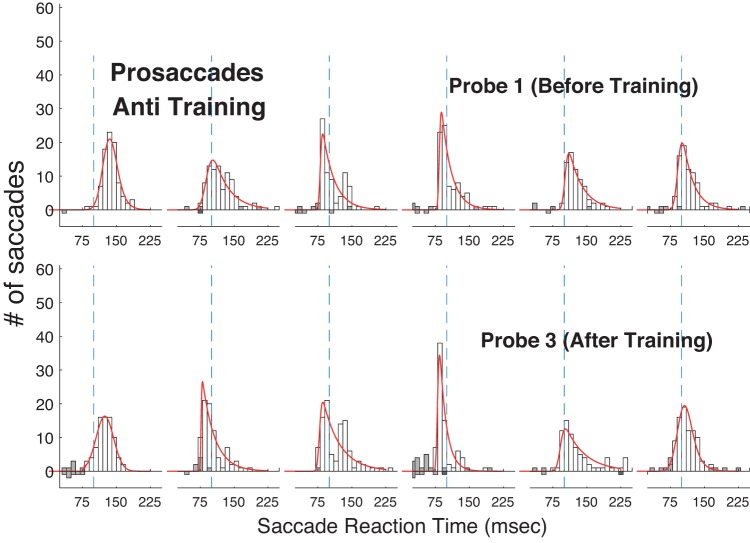
Histograms of reaction time in the Pro Gap task before (top) and after (bottom) training in the Anti Step task in experiment 2. Conventions as in Fig. 4.
Data will be made available upon request (jedelman@ccny.cuny.edu).
RESULTS
Experiment 1: Effect of Gap Prosaccade Training
Reaction time.
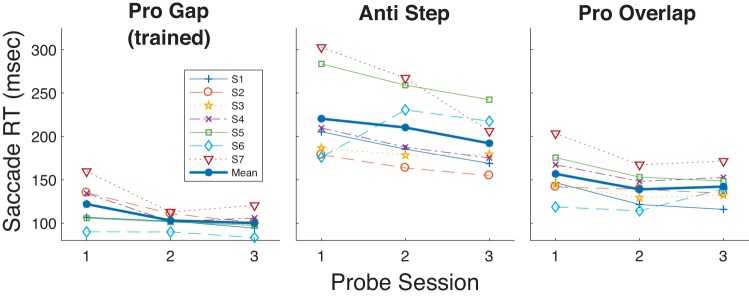
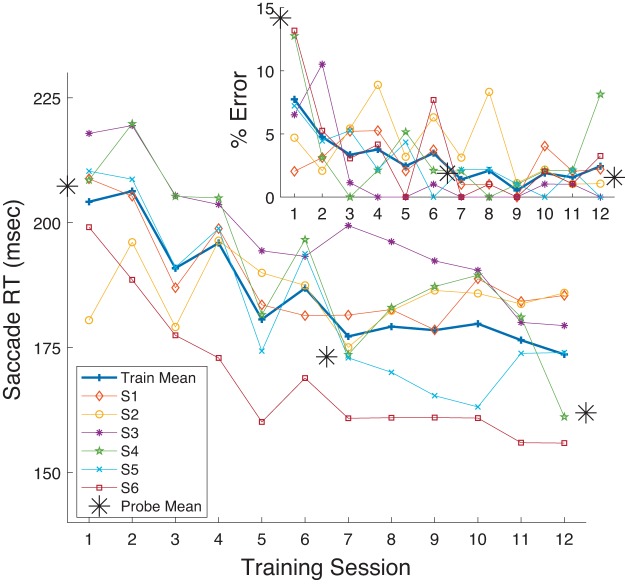
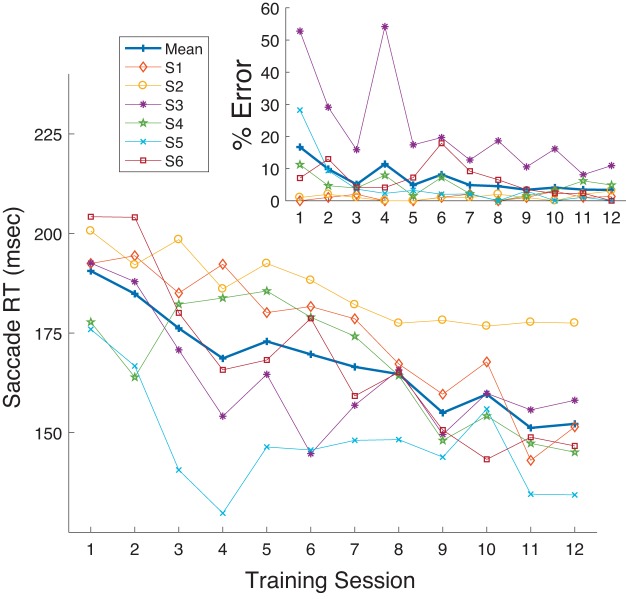
As observed in the Training sessions, over the course of gap prosaccade training all subjects showed an overall decrease in gap RT across the 12 Training sessions (Fig. 2), although for one subject RT was already so low before training that a decrease during training was scarcely evident. As measured in the Probe sessions, training decreased RT in the Pro Gap task (overall mean: Probe 1: 122 ms, Probe 3: 100 ms).
Fig. 2.
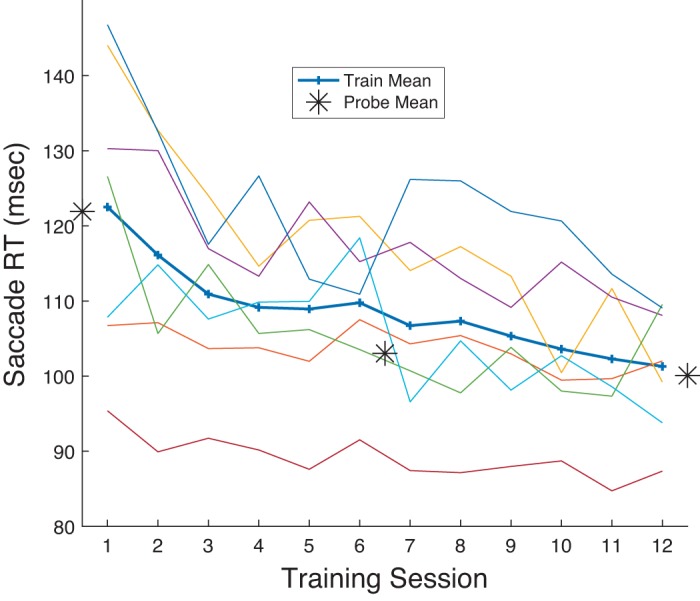
Mean reaction time (RT; see materials and methods) in the Pro Gap task as a function of Training session (sessions 2–7 and 9–14 as seen in Fig. 1) for the 7 subjects in experiment 1. Each thin line shows RT for 1 participant. Thick line shows grand mean across subjects. The 3 asterisks indicate equivalent data for the 3 Probe sessions.
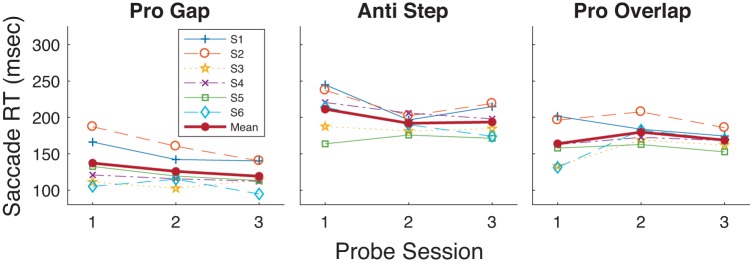
If training enhanced visual processes, then we might expect increased RT in the antisaccade task. However, prosaccade training also decreased RT in the untrained Anti Step task (Probe 1: 220 ms, Probe 3: 192 ms) as well as in the Overlap task (Probe 1: 157 ms, Probe 3: 142 ms; Table 1, Fig. 3). We verified this transfer of training by comparing performance on a subject-by-subject basis before and after training (i.e., Probe 1 vs. Probe 3) in the two untrained tasks with the Wilcoxon rank sum task, a nonparametric test obviating normality in the data. For both the Anti Step task and Pro Overlap task Wilcoxon rank sum tests RT was found to have significantly decreased for five of seven subjects and significantly increased for one of seven subjects. We also verified these general trends across all subjects by computing a mixed-model two-factor ANOVA with Probe number (3 vs. 1) as the fixed factor and subject as the random factor. The main effect of probe number was highly significant for both the Anti Step [df = 1; sum of squares (SS) = 3.01; F = 23.1; P < 10−3] and Pro Overlap (df = 1; SS = 9.99; F = 19.4; P < 10−3) tasks.
Table 1.
Summary of mean reaction time and directional error (for Anti Step task) observed in initial and final Probe sessions for all 3 experiments
| Pro Gap (RT) |
Anti Step (RT) |
Anti Step (% error) |
Pro Overlap (RT) |
|||||
|---|---|---|---|---|---|---|---|---|
| Training Protocol | Probe 1 | Probe 3 | Probe 1 | Probe 3 | Probe 1 | Probe 3 | Probe 1 | Probe 3 |
| Experiment 1: Pro Gap | 122 | 100 | 220 | 192 | 12.0 | 8.1 | 157 | 142 |
| Experiment 2: Anti Step | 115 | 107 | 207 | 162 | 14.2 | 1.6 | 138 | 140 |
| Experiment 3: Anti Gap | 137 | 119 | 211 | 194 | 13.1 | 1.8 | 164 | 169 |
RT, reaction time (in ms).
Fig. 3.
Mean reaction time (RT; see materials and methods) as a function of Probe session (sessions 1, 8, and 15 as seen in Fig. 1) for the 3 probed tasks in experiment 1. Each of the 7 participants’ data and grand mean are shown.
Note also from Fig. 2 that performance on Pro Gap trials in the Training sessions was comparable to that seen at the correspondingly timed Probe trials. As Probe trials required task switching between blocks of trials, namely, shifts from performing prosaccade to antisaccade trials and vice versa, the data provide no evidence that this form of task switching affected saccade performance.
To examine more closely the mechanisms underlying the RT decrease due to training, we plotted the full RT distributions for the Pro Gap task (Fig. 4) and the Anti Step task (Fig. 5) before training (Probe 1) and after training (Probe 3) and fit the RT distributions with an ex-Gaussian model (see materials and methods). Table 2 shows the average across seven subjects of the three parameters for the Pro Gap, Anti Step, and Pro Overlap tasks. Training had similar effects on the model parameters for all three tasks, although the fits suggest that prosaccade training had a larger effect on reducing the right tail of the distribution for the prosaccade tasks than it did for the antisaccade task.
Table 2.
Mean ex-Gaussian parameter fits for RT distributions for the 3 probed tasks before (Probe 1) and after (Probe 3) training for experiment 1
| Pro Gap |
Anti Step |
Pro Overlap |
||||
|---|---|---|---|---|---|---|
| Fit Parameter, ms | Probe 1 | Probe 3 | Probe 1 | Probe 3 | Probe 1 | Probe 3 |
| μ | 100.0 | 86.43 | 199.1 | 170.6 | 123.8 | 119.2 |
| σ | 11.9 | 6.57 | 38 | 23.9 | 24.4 | 18.9 |
| τ | 29.1 | 16.9 | 30.5 | 26.8 | 48.2 | 33.0 |
μ, Mean; σ, dispersion; τ, extent of right tail of distribution.
Effect of gap prosaccade training on antisaccade directional error rate.
If prosaccade training enhanced visual processes, then we might expect directional error rate in the antisaccade task to increase after training, particularly if, as we have seen, prosaccade training decreased antisaccade RT. However, there was no overall RT-accuracy trade-off in the effects of Pro Gap training on Anti Step saccades. Directional errors in the Anti Step task also decreased with Pro Gap training (Table 1, Fig. 6), with error rate dropping from 12.0% in Probe 1 to 8.1% in Probe 3. There was a significant decrease in two of six subjects and a significant increase in one of six subjects, although it should be stressed that for a saccade data set of a given size tests of proportion will have much less statistical power than tests of RT.
Fig. 6.
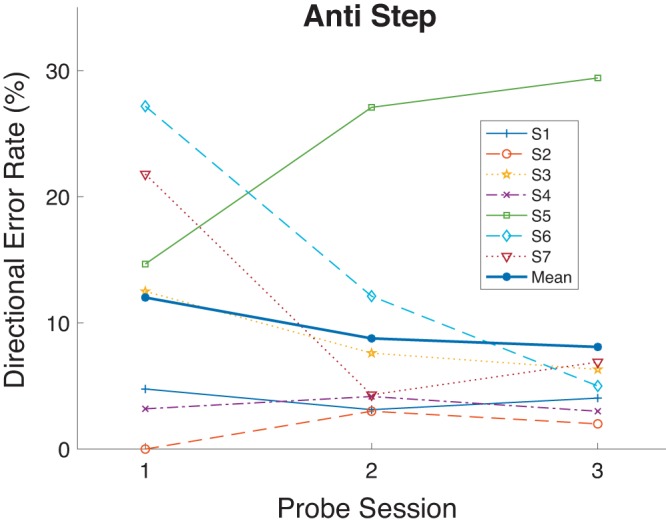
Directional error rate as a function of Probe session (sessions 1, 8, and 15 as seen in Fig. 1) for the Anti Step task in experiment 1. Each participant’s data and grand mean are shown.
We used bootstrap analysis to estimate the confidence interval of this change in proportion of direction errors in the Anti Step task across the all subjects. The distributions of directional error status (i.e., correct/incorrect saccade direction) were resampled with replacement 1,000 times for each subject for Probe 1 and for Probe 3. For each of these 1,000 resamplings we then 1) calculated the proportion of directional errors for Probe 1 and Probe 3 for each subject, 2) used these to calculate grand means of directional error proportions across the seven subjects for Probe 1 and Probe 3, and finally 3) calculated the difference in grand means of directional error proportion between Probe 1 and Probe 3.
This yielded 1,000 estimates of the difference in directional error proportion across the seven subjects. The standard deviation of these 1,000 estimates is approximately equal to the standard error of the difference in directional error proportion of trials between Probes 1 and 3, which can then be used to calculate the 95% confidence interval of this difference. Using this method, we calculated this confidence interval to be entirely positive [0.007, 0.072]. This suggests that there was a modest improvement in saccade direction performance in the antisaccade task with prosaccade training.
Relationship between effects of training on different saccade tasks.
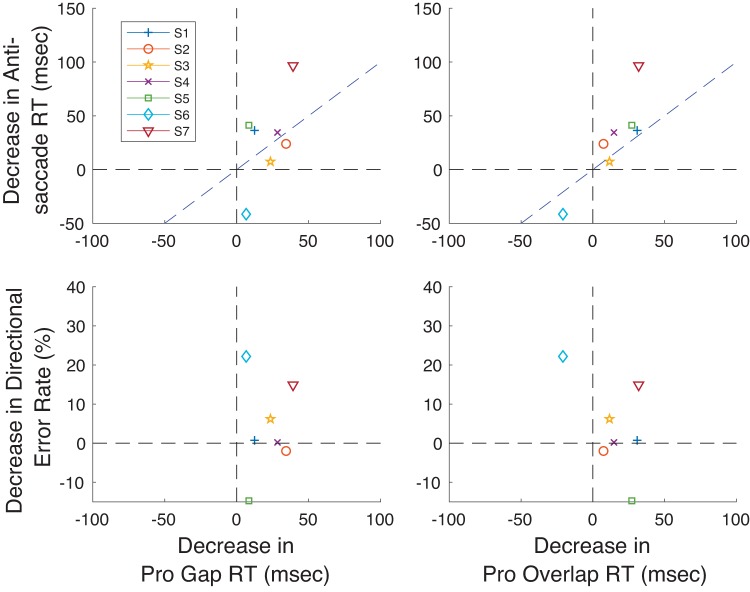
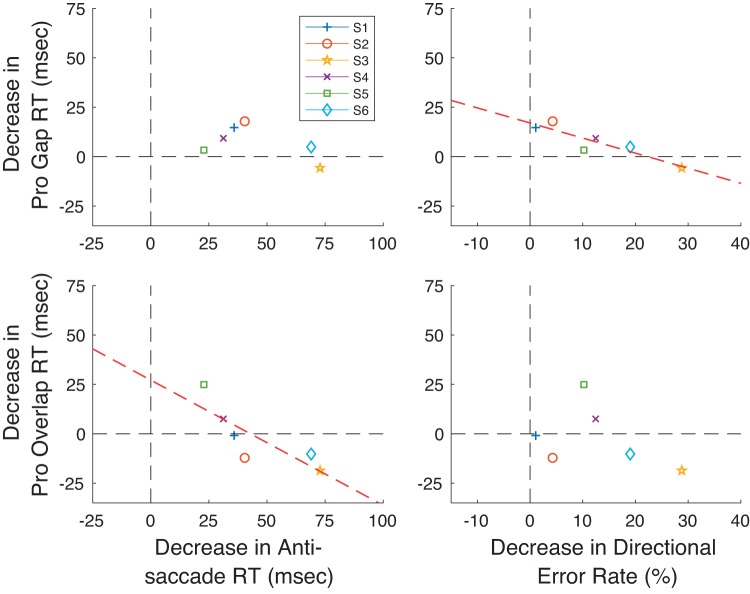
Finally, we analyzed whether there was a trade-off between performance improvement in the Pro Gap task or Pro Overlap task and improvements in the Anti Step task across subjects. We reasoned that subjects who showed particularly strong improvements in the Pro tasks might improve less in the Anti Step task. Figure 7 portrays these results, showing the relationship between change in RT in the Pro tasks and that in the Anti Step task (Fig. 7, top) and the relationship between changes in RT in the Pro tasks and change in directional error rate in the Anti Step task (Fig. 7, bottom). These graphs show that improvements in Pro task performance tend to, if anything, be accompanied by improvements in Anti Step task performance, although one subject (S6) differed from the other subjects in showing a large speed-accuracy trade-off in the Anti Step Task (increase in RT; large decrease in directional error) and an increase in RT in the Pro Overlap task, and another (S5) showed the opposite speed-accuracy trade-off in antisaccade performance (decrease in RT; increase in directional error). Otherwise, the data show that improvements in performance in the Pro Gap task with training tended to transfer to improvements in the Anti Step task (along with improvements in the Pro Overlap task). A subject-by-subject correlation of change in antisaccade RT versus change in the directional error rate of antisaccades showed only an extremely weak relationship between the two measures [r: −0.27, prob: 0.54, slope: −0.96 ms/%, y-intercept (yint): 32.1 ms].
Fig. 7.
Relation between changes in pro- and antisaccade performance from Probe 1 to Probe 3 for the 7 subjects in experiment 1. Data for the Pro Gap and Pro Overlap tasks are shown on left and right, respectively. Data for relationships between prosaccade RT change and antisaccade RT and directional rate change are shown at top and bottom, respectively.
Experiment 2: Effect of Step Antisaccade Training
Reaction time.
Experiment 2 was a partial flip of experiment 1, testing whether training subjects on the Anti Step task would yield improvements on the untrained prosaccade tasks. As expected, we found that antisaccade RT decreased considerably during the Training sessions (Fig. 8). As measured by improved performance in the Anti Step Probe tasks, training led to reductions in its saccade RT in Probe 1 (207 ms) compared with Probe 3 (162 ms; Table 1, Fig. 9). A Wilcoxon rank sum test showed that this was statistically significant for all six subjects. As was the case for Pro Gap saccades in experiment 1, performance on Anti Step trials in the Training sessions was comparable to that seen at the correspondingly timed Probe trials, indicating that task switching did not affect saccade performance.
Fig. 8.
Mean reaction time (RT) in the Anti Step task as a function of Training session (sessions 2–7 and 9–14 as seen in Fig. 1) for all subjects in experiment 2. Each thin line shows RT for 1 participant. Thick line shows grand mean across subjects. Inset: corresponding data for directional error rate. The 3 asterisks indicate equivalent data for the 3 Probe sessions.
Fig. 9.
Mean reaction time (RT) as a function of Probe session (sessions 1, 8, and 15 as seen in Fig. 1) for the 3 probed tasks in experiment 2. Each participant’s data and grand mean are shown.
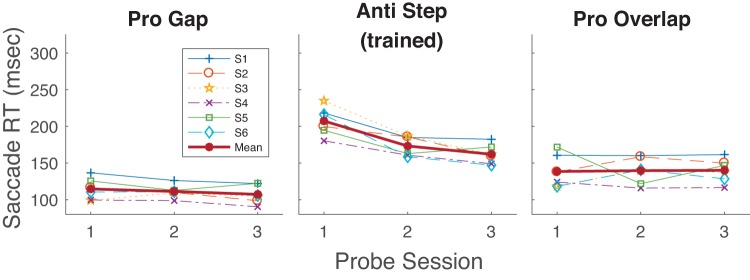
If antisaccade training attenuated visual processes so as to suppress an unwanted prosaccadic reflex, we would expect antisaccade training to result in increased prosaccade RT. However, Anti Step training also decreased the RT of Pro Gap saccades in the Probe tasks, though by a much smaller amount (Probe 1: 115 ms; Probe 3: 107 ms). Analysis of the effect of antisaccade training on the Pro Gap task showed significant decreases in three of six subjects and an overall decrease assessed by the two-factor mixed-model (probe number × subject) ANOVA (df = 1; SS = 12.4; F = 17.4; P < 10−3).
However, there was no such trend for the Pro Overlap task, where RTs showed no decrease with antisaccade training (Probe 1: 138 ms; Probe 3: 140 ms), with two of six subjects showing significant decreases, one of six showing an increase, and no trend revealed in the two-factor ANOVA (df = 1; SS = 0.23; F = 0.33; P = 0.56).
As in experiment 1, we attempted to assess the mechanisms of the improvement through training by fitting the RT histograms for the three tasks for Probe 1 and Probe 3 (Figs. 10 and 11; histograms for the Pro Overlap task are not shown) with curves from an ex-Gaussian distribution (Table 3). We found that training decreased all three parameters of the fit for the Anti Step task, particularly the τ parameter, which reflects the size of the long-RT tail of the distribution. Parameters for the Pro Gap task changed little, whereas the parameters for the Pro Overlap curve fits changed in different directions. Interestingly, the biggest difference in effects of training on the three tasks appeared to be on the τ parameter, where the decrease for the Anti Step task was much greater than that observed in the prosaccade tasks.
Table 3.
Mean ex-Gaussian parameter fits for RT distributions for the 3 probed tasks before (Probe 1) and after (Probe 3) training for experiment 2
| Pro Gap |
Anti Step |
Pro Overlap |
||||
|---|---|---|---|---|---|---|
| Fit Parameter, ms | Probe 1 | Probe 3 | Probe 1 | Probe 3 | Probe 1 | Probe 3 |
| μ | 95.7 | 90.8 | 171.9 | 155.5 | 102.2 | 113.9 |
| σ | 7.0 | 7.98 | 29.1 | 24.1 | 9.54 | 19.3 |
| τ | 24.5 | 23.2 | 45.7 | 11.8 | 49.4 | 38.6 |
μ, Mean; σ, dispersion; τ, extent of right tail of distribution.
Directional error rate.
There was an overall reduction in directional error rate in the trained task after training (Probe 1: 14.2%, Probe 3: 1.6%; Table 1, Fig. 12). The virtual elimination of directional errors with training was not surprising given that training was performed with the same task. This was significant by a proportions test for four of six subjects, with the two other subjects starting with very low directional error rates before training. As was the case for experiment 1, there were few errors regardless of training in the prosaccade tasks.
Fig. 12.
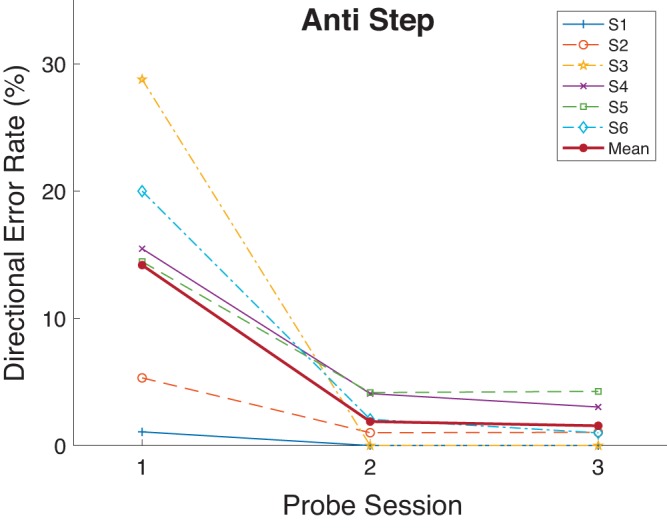
Directional error rate as a function of Probe session (sessions 1, 8, and 15 as seen in Fig. 1) for the Anti Step task in experiment 2. Each participant’s data and grand mean are shown.
Relation between changes in antisaccade and prosaccade performance.
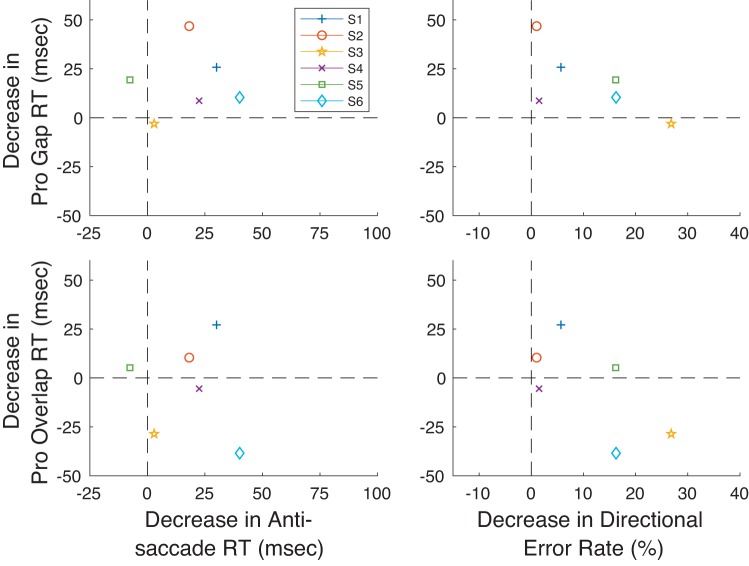
As for experiment 1, we analyzed whether there was a trade-off between performance improvements in the Anti Step task and the change in prosaccade performance across the subjects. We reasoned that subjects who showed particularly strong improvements in the Anti Step task might perform worse or improve less in the prosaccade tasks. Unlike experiment 1, we found some hints that subjects who improve more in the Anti Step task improve less (or get worse) in the prosaccade tasks. Figure 13 portrays these results, showing the relationship between changes in median RT (Fig. 13, left) and the relationship between changes in RT in the Pro tasks and change in directional error rate in the Anti Step task (Fig. 13, right) [note that this is a different convention from that used for the analogous figure for experiment 1 (Fig. 7)]. We found that, even with this small number of subjects, a statistically significant inverse relationship was found between the change in Anti Step RT and that of Pro Overlap RT, albeit with a slope considerably greater than −1 and a positive y-intercept (r: −0.82, prob: 0.044, slope: −0.63, yint: 27.1 ms). We also found a negative correlation between change in directional error rate for Anti Step trials and change in Pro Gap RT (r: −0.91, prob: 0.012, slope: −0.76 ms/%, yint: 17.0 ms). It should be noted that similar effects cannot be ruled out for the other two comparisons (Anti Step RT vs. Pro Gap RT and Anti Step direction error vs. Pro Overlap RT) given the small number of subjects, and indeed we found the slopes and y-intercepts for these two comparisons to be similar to those of the other prosaccade task.
Fig. 13.
Relation between changes in pro- and antisaccade performance from Probe 1 to Probe 3 for the 6 subjects in experiment 2. Data for the Pro Gap and Pro Overlap tasks are shown at top and bottom, respectively (note difference in convention from Fig. 7). Data for relationships between prosaccade RT change and antisaccade RT and directional rate change are shown at left and right, respectively. Data for each subject are shown. Oblique lines are shown in plots where correlations between the 2 measures achieved statistical significance (see text for details).
Finally, we found that improvements in antisaccade RT and directional error rate had a positive correlation across subjects very close to significance (r: 0.77, prob: 0.074, slope: 1.57 ms/%, yint: 25.5 ms).
Experiment 3: Effect of Gap Antisaccade Training
Experiment 3 repeated experiment 2 but used a gap antisaccade task for training instead of a step antisaccade task. A gap antisaccade task is particularly challenging, since the gap disinhibits the saccadic system, facilitating reflexive prosaccades and thus potentially increasing saccade directional errors. We reasoned that the additional saccade inhibition needed to perform this task correctly could result in prosaccades with longer RTs and thus that gap antisaccade training could result in a trade-off between changes in antisaccade and prosaccade performance that we did not observe in experiment 2.
As in experiments 1 and 2, the latencies for the trained saccade (Anti Gap in this case) improved across the training sessions (mean RT: session 1: 191 ms, session 12: 152 ms; Fig. 14). The Probe trials assessed the effect of Anti Gap training on Pro Gap, Anti Step, and Pro Overlap saccades. Note that unlike experiments 1 and 2, no Probe task was identical to the Training task.
Fig. 14.
Mean reaction time (RT) in the Anti Gap task as a function of Training session (sessions 2–7 and 9–14 as seen in Fig. 1) in experiment 3. Each thin line shows RT for 1 participant. Thick line shows grand mean across subjects. Inset: corresponding data for directional error rate.
Training decreased Anti Step RT overall from Probe 1 (211 ms) to Probe 3 (194 ms; Table 1, Fig. 15). Wilcoxon rank sum tests showed that four of six participants had a statistically significant decrease in RT, whereas one of six showed a significant increase. Mixed-model ANOVA analysis showed an overall decrease in RT from Probe 1 to Probe 3 across the six subjects (df = 1; SS = 1.91; F = 19.4; P < 10−3). Note that this difference (18 ms) is much smaller than the effect of Anti Step training on Anti Step performance as seen in experiment 2 (45 ms) and, surprisingly, appears to be smaller even that seen in experiment 1 (33 ms), where the Pro Gap task was trained, although the differing sets of subjects in the three tasks could certainly also contribute to these differences. Anti Gap training also decreased Pro Gap RT overall (Probe 1: 137 ms; Probe 3: 119 ms), with five of six participants showing a significant decrease in RT (Wilcoxon rank sum) and an overall decrease across the six participants [2-factor (probe number × subject) mixed-model ANOVA, main effect of Probe: df = 1; SS = 10.4; F = 42.2; P < 10−3]. Given that Pro Gap RT is much smaller than Anti Step RT before training, it is particularly striking that Anti Gap RT training had a similar overall effect on the two tasks. Finally, training had a minimal or nonexistent effect on Pro Overlap RT (Probe 1: 164 ms; Probe 3: 169 ms), with one of six subjects showing a significant decrease in RT and two of six showing a significant increase. No overall RT change was found across the six subjects [2-factor (probe number × subject) mixed-model ANOVA, main effect of Probe: df = 1; SS = 0.96; F = 0.04; P = 0.84].
Fig. 15.
Mean reaction time (RT) as a function of Probe session (sessions 1, 8, and 15 as seen in Fig. 1) for the 3 probed tasks in experiment 3. Each participant’s data and grand mean are shown.
We confirmed that the mean net RT improvement with training in the Pro Gap task was greater than that found in the Pro Overlap task (18 ms vs. −5 ms) by a pairwise comparison of the RT change across the six subjects (t = 3.14; df = 5; P = 0.026). That significance was striking given the similarity of the two prosaccade tasks and that there were only six subjects.
Ex-Gaussian curve fits for the three probed tasks before and after training showed effects similar to those for experiment 2, with particularly strong effects on σ and τ for the Anti Step task, modest reductions in all parameters for the Pro Gap task, and a reduction in τ but increases in the other parameters for the Pro Overlap task (Table 4).
Table 4.
Mean ex-Gaussian parameter fits for RT distributions for the 3 probed tasks before (Probe 1) and after (Probe 3) training for experiment 3
| Pro Gap |
Anti Step |
Pro Overlap |
||||
|---|---|---|---|---|---|---|
| Fit Parameter, ms | Probe 1 | Probe 3 | Probe 1 | Probe 3 | Probe 1 | Probe 3 |
| μ | 111.3 | 103.5 | 182.4 | 177.7 | 128.4 | 137.1 |
| σ | 13.9 | 12.9 | 29.9 | 21.1 | 17.6 | 24.2 |
| τ | 33.3 | 20.4 | 37.2 | 20.7 | 45.9 | 40.8 |
μ, Mean; σ, dispersion; τ, extent of right tail of distribution.
Directional error rate.
Antisaccade directional error rate decreased in the Anti Step task (Probe 1: 13.1%, Probe 3: 1.8%; Table 1, Fig. 16). Unlike the case of RT, the decrease in directional error rate for the Anti Step in experiment 3 is similar to that found in experiment 2. In the Step Antisaccade task, three of six subjects showed significant RT improvement in a proportion test. One individual (S2) showed few directional errors even before training. As in the previous experiments, there were few prosaccade directional errors made at any stage of the experiment.
Fig. 16.
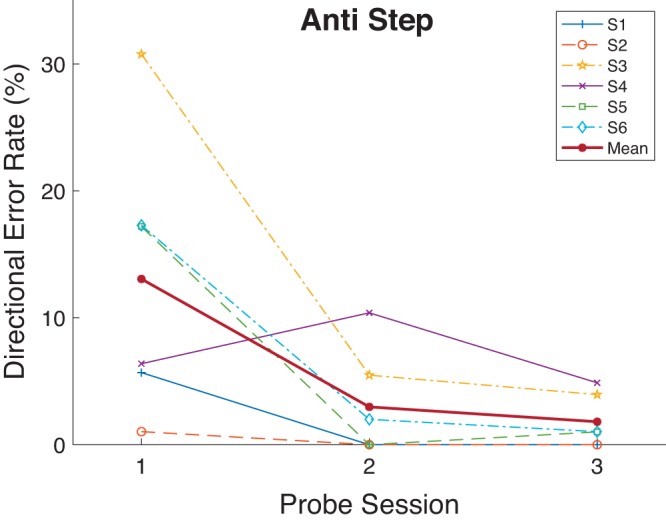
Directional error rate as a function of Probe session (sessions 1, 8, and 15 as seen in Fig. 1) for the Anti Step task in experiment 3. Each participant’s data and grand mean are shown.
Relation between changes in task performance.
The relationships between changes in performance were somewhat different here from those found in experiment 2. There was essentially no relationship between antisaccade RT improvements and changes in prosaccade RT (Fig. 17, left). There were, however, suggestive though statistically insignificant correlations between the decrease in Anti Step directional error and prosaccade RT (Fig. 17, right; Anti Step vs. Pro Gap: r: −0.69, prob: 0.13, slope: −1.16 ms/%, yint: 31.0 ms; Anti Step vs. Pro Overlap: r: −0.65, prob: 0.17, slope: −1.56 ms/%, yint: 12.6 ms). Finally, unlike the case of experiment 2, there was no positive relationship from subject to subject between improvements in Anti Step RT and Anti Step directional error rate (r: −0.41, prob: 0.43, slope: −0.69 ms/%, yint: 25.5 ms).
Fig. 17.
Relation between changes in pro- and antisaccade performance from Probe 1 to Probe 3 in experiment 3. Other conventions as in Fig. 13.
DISCUSSION
These results support the idea that improvements in RT for reflexive saccades are primarily the result of changes in brain centers involved in saccadic programming and disinhibition rather than an increase in speed of visual processing. We found little evidence that prosaccade and antisaccade training have opposing effects on the visuomotor system. In virtually all cases subjects showed significant improvement in performance after training sessions in the trained task, in terms of both RT and, in the case of antisaccades, directional error rates. Moreover, training either improved or had little change on untrained saccade types, although the transfer of training was incomplete.
Effect of Gap Prosaccade Training
Training a gap prosaccadic task over 12 sessions decreased prosaccade RT (Figs. 2–4). This is an agreement with a previous observation showing that training in a gap saccade task led to improvements in performance (Bibi and Edelman 2009). Improvements appeared to occur after the first few sessions of training (Figs. 2 and 3). This improvement generally did not come at the expense of increases in errors of saccade direction (Figs. 5 and 6) or in saccade RT in the antisaccade task (Figs. 3 and 5), where the saccade was made in a direction opposite to that of the suddenly appearing target. Moreover, we found no indication that subjects whose prosaccade performance improved more suffered greater decreases in antisaccade performance (Fig. 7). Training also appeared to transfer to improvements in the prosaccade overlap task (Fig. 3). The change of parameters (μ, σ, τ) of the ex-Gaussian curve fits from Probe 1 to Probe 3 were similar for all probed tasks (Table 2), indicating that the mechanisms governing the reduction of RT were similar.
Effect of Antisaccade Training and Comparison of Training Protocols
Training on the Anti Step task (experiment 2) had a dramatic effect on antisaccade performance, assessed in terms of both RT (Figs. 8–10) and directional error (Figs. 8–10 and 12), with the improvement in the latter particularly dramatic. However, the effect of antisaccade training on performance in the prosaccade tasks was less clear. We found an overall improvement in RT in the Pro Gap task (Figs. 9 and 11), though smaller than that seen in experiment 1. However, the effect on the Pro Overlap task (Fig. 9) was minimal. As assessed by the ex-Gaussian curve fits, the effects on parameters σ and τ by training was quite different for the Pro Gap and Overlap tasks than for the trained Anti Step task (Table 3).
When assessing the relationship between the change in antisaccade performance from subject to subject, two findings emerged (Fig. 13): First, there was a statistically significant inverse relationship between the amount of increase in Pro Overlap RT with training and the decrease in Anti Step RT (Fig. 13, bottom left). This suggests that there is some trade-off in performance changes between step antisaccades and overlap prosaccades riding on top of a generalized improvement in overlap prosaccade performance. It should be noted that this relationship had a slope much less than −1, indicating a weak trade-off in performance, and a very positive y-intercept, indicating that Pro Overlap RTs are increasing much less than antisaccade RTs are decreasing.
Second, we found a statistically significant inverse relationship across subjects between improvement of antisaccade directional error rate and that of gap prosaccade RT (Fig. 13, top right), again riding on top of an overall decrease in gap prosaccade RT. It should be noted that the small number of subjects limits the power of our statistical tests, so it is still possible that these relationships between changes in antisaccade performance and changes in prosaccade performance may generalize across prosaccade types. Indeed, the slopes for both regressions of prosaccade RT change against antisaccade RT change are negative (although slopes are more positive than −1 and the y-intercepts of the relationships are positive).
We reasoned that training with a gap antisaccade task (Fig. 14) could have a stronger inhibitory impact on visual inputs to the saccadic system to prevent reflexive prosaccadic responses than training on the step antisaccade task, thus increasing the negative impact of training on prosaccades. Instead, we found results in experiment 3 that were similar to experiment 2: gap prosaccade RTs decreased after training, and the effect on overlap prosaccade RTs was marginal (Figs. 15 and 17). Comparing the results of experiment 3 with those of experiment 2 suggests that the use of the gap increased the effect of training on the Pro Gap task but decreased the effect on the two nongap tasks (Anti Step and Pro Overlap); this is particularly seen in terms of the mean value and ex-Gaussian curve fit value τ, which measures the size of the rightward tail of the distribution (Table 4). This suggests that training on a gap task could result in a performance improvement on gap tasks, both Pro and Anti, although to confirm this one would have to measure the effects of gap prosaccade training on gap antisaccades.
Moreover, saccade training appeared to have an equal if not greater impact on gap prosaccades than overlap prosaccades regardless of the training protocol. It would be expected that gap training (experiments 1 and 3) would have a greater effect on the Pro Gap task than on the Pro Overlap task, but it is more surprising that the effect of step antisaccade training on gap prosaccades also appeared to be greater than the effect on overlap prosaccades (Fig. 9). This pattern of results is particularly striking given that gap prosaccade RTs before training are much closer to the minimum saccade RT (~80 ms). One possibility is that the primary effect of training is to help disinhibit the saccade system, and enhanced disinhibition would manifest most strongly during a gap task, when the fixation point was removed before target appearance. Testing saccade training using a prosaccade overlap task is necessary to confirm the generality of this effect.
It should be noted that antisaccade directional errors almost completely disappeared for all subjects after antisaccade training (Figs. 12 and 16). Thus the x-axes in the plots in Figs. 13 and 17, right, are highly reflective of the rate of directional error before training. It could thus be argued that subjects who have already mastered the antisaccade task can use the antisaccade training to improve their performance for saccade tasks in general, including prosaccade tasks.
Relation to Previous Work on Antisaccade Training
There has been limited work on the effects of training on antisaccades and the transfer of training between prosaccades and antisaccades. The most comprehensive study is that of Dyckman and McDowell (2005). They probed gap prosaccade and gap antisaccade performance before, during, and after training in a prosaccade task, an antisaccade task, or a fixation task. The numbers of probe and training sessions were smaller than ours (8 vs. 12), and they were run in a more compressed time frame of 2 wk. Training resulted in relatively small changes in RT, and improvements in antisaccade directional rate after antisaccade training were smaller than those found here. In contrast with our results, prosaccade training resulted in an increased rate of antisaccade directional errors. However, the overall RTs in these tasks were much larger than those found here, with antisaccade RTs averaging >300 ms and gap prosaccade RTs averaging >225 ms. This may have been the result of their long fixation and postsaccade periods during both training and probe sessions, which in our experience tends to decrease arousal and thus increase saccade RT. Because of these longer RTs, it is possible that other rate-limiting processes were evident in those studies that may have resulted in different amounts of training transfer. Our much faster RTs, particularly in the gap prosaccade task, suggest that participants are operating near the performance limits of the oculomotor system and that the sensory and motor processes operating are those essential for rapid saccade initiation. It is thus difficult to relate our results to those of Dyckman and McDowell (2005). Other studies have tended to focus on clinical subpopulations suffering from motor deficits. For example, children with dyslexia reduce their antisaccade directional error rate more after antisaccade training than after prosaccade training (Fischer and Hartnegg 2000). Again, performance in these studies appears to be operating in a parameter regime different from ours.
The limited evidence of trade-offs in improvement between prosaccade and antisaccade performance found here was not expected given previous results of studies of prosaccades and antisaccades in various human subpopulations, both clinical and nonclinical. It has been shown previously that Parkinson disease patients who demonstrated an impaired ability to suppress reflexive prosaccades in an antisaccade task also showed reduced RT in prosaccade tasks (Chan et al. 2005). In addition, so-called “express saccade makers,” namely, subjects who made a high percentage of express saccades in a prosaccadic overlap task, also tended to have above-normal error rates on an antisaccade task (Knox et al. 2012). This may suggest that certain human subpopulations have a greater effective visual input into the saccade system. We had speculated that our training on a prosaccade task could result in participant behavior more similar to those of such human subpopulations and that training on an antisaccade could lead to behavior less similar. Instead, we found a very limited trade-off in performance changes in pro- and antisaccades.
Implications for Understanding of Mechanisms Underlying Improvements in Saccade Performance with Training
Prosaccade training could facilitate rapid saccade production through enhancement of visual processes, motor processes, or both. If training in the gap prosaccade task resulted in increases in visual activity or, more specifically, enhancements of visual inputs into the saccadic motor system, perhaps mediated by visual attention, then antisaccade performance in experiment 1 might have worsened, since increases in visual responsiveness might have increased error rates. Likewise, if training on step or gap antisaccades caused decreases in effective visual input to the motor system, then training in experiments 2 and 3 should result in increases in RT in prosaccades. Other than small increases in RT in some subjects in the Overlap task (experiments 2 and 3), these patterns of generalized excitation and inhibition did not occur, suggesting that the changes in visual input into the saccadic system were minor. That said, we did find some trade-offs in performance changes between pro- and antisaccades in the antisaccade training protocols, suggesting that the effects of antisaccade training may in part be mediated by a slight reduction in visual inputs to the saccadic system.
It should be noted that cognitive processes that interface with the visual and motor processes might also play a role in enhanced performance after training. The partial transfer of training of one saccade type to another would then suggest that such cognitive processes would be involved in both prosaccade and antisaccade generation. Given the differences in these tasks, it is difficult to conceive of what these might be. One possibility is that training could have improved processes of task switching, in this context the ability to switch from performing prosaccades to antisaccades and back again across separate blocks of trials. Note that we ran different trial types in the Probe sessions, but in separate blocks of trials, so we would expect task switching-related performance costs to be low. Indeed, examination of Figs. 2 and 8 suggests that task switching costs were nonexistent.
Neuronal Substrates
In monkey, neurons in both the SC and the FEF have been shown to be involved in disengaging fixation (Dias and Bruce 1994; Munoz and Wurtz 1993). Moreover, previous work has shown that in both the SC and FEF greater activity before target appearance is associated with faster RTs during a gap task (Everling and Munoz 2000). The ability to disengage fixation may be improved by both pro- and antisaccade training, and this increased ability, perhaps mediated by changes in responses related to fixation disengagement in SC and FEF, may transfer to the other saccade type.
The motivation for this work was to help uncover the mechanisms underlying reduction in RT with saccade training. Previous work in this laboratory on saccade training demonstrated that express saccade production during a gap prosaccade task did not require vector-specific saccade training or preparation (Bibi and Edelman 2009). This suggested that training did not result in changes at specific loci within a visual or saccadic map. The results in the present study suggest that the predominant contribution to these improvements in RT result from enhanced mechanisms for fixation disengagement and non-spatially specific saccade generation, whereas enhancements of visual input to the saccadic system appear to play a much smaller role. Modulations of visual input may occur more readily during training on an antisaccade task, although here too more motor-specific processes appear to play a predominant role. Given what is known about the neurophysiology of the saccadic system, it is thus likely that the primary substrate of changes with training is in motor and fixation-related areas in brain structures such as the SC and FEF.
GRANTS
This work was supported by National Institute on Minority Health and Health Disparities Grant 5G12 MD-007603.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.M. and J.A.E. conceived and designed research; S.M.M. performed experiments; S.M.M. and J.A.E. analyzed data; S.M.M. and J.A.E. interpreted results of experiments; S.M.M. and J.A.E. prepared figures; S.M.M. and J.A.E. drafted manuscript; S.M.M. and J.A.E. edited and revised manuscript; S.M.M. and J.A.E. approved final version of manuscript.
REFERENCES
- Amatya N, Gong Q, Knox PC. Differing proportions of ‘express saccade makers’ in different human populations. Exp Brain Res 210: 117–129, 2011. doi: 10.1007/s00221-011-2609-z. [DOI] [PubMed] [Google Scholar]
- Bibi R, Edelman JA. The influence of motor training on human express saccade production. J Neurophysiol 102: 3101–3110, 2009. doi: 10.1152/jn.90710.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RH, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature 377: 59–62, 1995. doi: 10.1038/377059a0. [DOI] [PubMed] [Google Scholar]
- Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP. Deficits in saccadic eye-movement control in Parkinson’s disease. Neuropsychologia 43: 784–796, 2005. doi: 10.1016/j.neuropsychologia.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Dias EC, Bruce CJ. Physiological correlate of fixation disengagement in the primate’s frontal eye field. J Neurophysiol 72: 2532–2537, 1994. doi: 10.1152/jn.1994.72.5.2532. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. A neural correlate for the gap effect on saccadic reaction times in monkey. J Neurophysiol 73: 2558–2562, 1995. doi: 10.1152/jn.1995.73.6.2558. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Paré M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17: 8566–8579, 1997. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyckman KA, McDowell JE. Behavioral plasticity of antisaccade performance following daily practice. Exp Brain Res 162: 63–69, 2005. doi: 10.1007/s00221-004-2105-9. [DOI] [PubMed] [Google Scholar]
- Edelman JA, Keller EL. Activity of visuomotor burst neurons in the superior colliculus accompanying express saccades. J Neurophysiol 76: 908–926, 1996. doi: 10.1152/jn.1996.76.2.908. [DOI] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Munoz DP. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. J Neurophysiol 80: 1584–1589, 1998. doi: 10.1152/jn.1998.80.3.1584. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia 36: 885–899, 1998. doi: 10.1016/S0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci 20: 387–400, 2000. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Boch R. Saccadic eye movements after extremely short reaction times in the monkey. Brain Res 260: 21–26, 1983. doi: 10.1016/0006-8993(83)90760-6. [DOI] [PubMed] [Google Scholar]
- Fischer B, Hartnegg K. Effects of visual training on saccade control in dyslexia. Perception 29: 531–542, 2000. doi: 10.1068/p2666c. [DOI] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res 58: 455–472, 1985. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res 18: 1279–1296, 1978. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Jóhannesson ÓI, Ásgeirsson ÁG, Kristjánsson A. Saccade performance in the nasal and temporal hemifields. Exp Brain Res 219: 107–120, 2012. doi: 10.1007/s00221-012-3071-2. [DOI] [PubMed] [Google Scholar]
- Knox PC, Amatya N, Jiang X, Gong Q. Performance deficits in a voluntary saccade task in Chinese “express saccade makers”. PLoS One 7: e47688, 2012. [Erratum in PLoS One 7: annotation/98b3e349-5e61-47a0-a8e5-ebcd6940da21, 2012.] doi: 10.1371/journal.pone.0047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson A. The intriguing interactive relationship between visual attention and saccadic eye movements. In: Oxford Handbook of Eye Movements, edited by Liversedge SP, Gilchrist I, Everling S. Oxford, UK: Oxford Univ. Press, 2011, p. 455–469. [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements. New York: Oxford Univ. Press, 2015. [Google Scholar]
- Mayfrank L, Mobashery M, Kimmig H, Fischer B. The role of fixation and visual attention in the occurrence of express saccades in man. Eur Arch Psychiatry Neurol Sci 235: 269–275, 1986. doi: 10.1007/BF00515913. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5: 218–228, 2004. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 70: 559–575, 1993. doi: 10.1152/jn.1993.70.2.559. [DOI] [PubMed] [Google Scholar]
- Paré M, Munoz DP. Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol 76: 3666–3681, 1996. doi: 10.1152/jn.1996.76.6.3666. [DOI] [PubMed] [Google Scholar]
- Saslow MG. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. J Opt Soc Am 57: 1024–1029, 1967. doi: 10.1364/JOSA.57.001024. [DOI] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci 22: 241–259, 1999. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]