Abstract
Like all skeletal muscles, the diaphragm muscle accomplishes a range of motor behaviors by recruiting different motor unit types in an orderly fashion. Recruitment of phrenic motor neurons (PhMNs) is generally assumed to be based primarily on the intrinsic properties of PhMNs with an equal distribution of descending excitatory inputs to all PhMNs. However, differences in presynaptic excitatory input across PhMNs of varying sizes could also contribute to the orderly recruitment pattern. In the spinal cord of Sprague-Dawley rats, we retrogradely labeled PhMNs using cholera toxin B (CTB) and validated a robust confocal imaging-based technique that utilizes semiautomated processing to identify presynaptic glutamatergic (Glu) terminals within a defined distance around the somal membrane of PhMNs of varying size. Our results revealed an ~10% higher density of Glu terminals at PhMNs in the lower tertile of somal surface area. These smaller PhMNs are likely recruited first to accomplish lower force ventilatory behaviors of the diaphragm as compared with larger PhMNs in the upper tertile that are recruited to accomplish higher force expulsive behaviors. These results suggest that differences in excitatory synaptic input to PhMNs may also contribute to the orderly recruitment of diaphragm motor units.
NEW & NOTEWORTHY The distribution of excitatory glutamatergic synaptic input to phrenic motor neurons differs across motor neurons of varying size. These findings support the size principle of motor unit recruitment that underlies graded force generation in a muscle, which is based on intrinsic electrophysiological properties of motor neurons resulting from differences in somal surface area. A higher density of glutamatergic inputs at smaller, more excitable motor neurons substantiates the earlier and more frequent recruitment of these units.
Keywords: 3D-reconstruction, glutamate, neuromotor control, phrenic motor neurons, presynaptic inputs, spinal cord
INTRODUCTION
Motor neurons are recruited on the basis of their size (i.e., Henneman “size principle”) (Henneman 1957; Stuart and Enoka 1983). In their initial experiments, Henneman and colleagues observed a graded increase of unitary action potential amplitude recorded from the ventral root of decerebrate cats following motor neuron activation by stretching hindlimb muscle spindles. They concluded that smaller motor neurons (as indicated by their lower axonal conduction velocities) have a lower activation threshold and are recruited before larger motor neurons with higher activation thresholds. Smaller motor neurons have lower membrane capacitance and higher membrane resistance compared with larger motor neurons and would allow for a greater change in membrane potential for a given synaptic current. In addition to differences in intrinsic electrophysiological properties of motor neurons, differences in presynaptic terminal density might also contribute to observed differences in motor neuron recruitment independently of size (Binder et al. 1993; Heckman and Binder 1993; Hultborn et al. 2004). However, such an analysis of excitatory presynaptic terminal density across motor neurons based on motor neuron size has not been undertaken.
The diaphragm is innervated by phrenic motor neurons (PhMNs) located from C3–C5 cervical spinal segments. Dick and colleagues (Dick et al. 1987; Jodkowski et al. 1987) showed that PhMN recruitment in the cat was size dependent. Based on single-unit action potential recordings from the C5 ventral root during spontaneous breaths, they observed that early recruited units displayed slower axonal conduction velocities compared with units recruited later. Subsequently, Sieck and colleagues (Sieck 1995; Sieck and Fournier 1989; Sieck et al. 1990) found that the cat diaphragm comprises different motor unit types that are recruited in an orderly fashion to accomplish a range of motor behaviors. In support of the size principle for motor unit recruitment in rat diaphragm, Seven et al. (2013) observed that the amplitude of motor unit action potentials recruited earlier during inspiration was lower than that of those recruited later during inspiration or of motor units recruited during more forceful motor behavior. The transdiaphragmatic pressures generated by the diaphragm during ventilatory (breathing) behaviors require recruitment of only smaller PhMNs comprising fatigue-resistant slow (type S) and fast (type FR) motor units (Fournier and Sieck 1988; Mantilla et al. 2010; Mantilla and Sieck 2011; Seven et al. 2014; Sieck 1988a, 1989, 1994; Sieck et al. 2013; Sieck and Fournier 1989). In contrast, higher force airway clearance behaviors of the diaphragm require recruitment of larger PhMNs comprising more fatigable fast motor units (types FInt and FF). Thus fundamental size-dependent differences are expected to underlie these orderly recruitment patterns which have classically been described using the size principle.
The main excitatory input to PhMNs is glutamatergic (Glu), arising primarily from the dorsolateral medulla (Feldman et al. 1985; McCrimmon et al. 1989). As mentioned, size-dependent recruitment of PhMNs assumes equal presynaptic excitatory input to PhMNs. The goal of this study is to employ a robust technique that utilizes confocal imaging and semiautomated procedures to quantify the density of presynaptic Glu terminals (identified by vesicular Glu transporter, VGLUT expression) at retrogradely labeled PhMNs in rats. Our null hypothesis was that there are no size-dependent differences in Glu presynaptic terminal density at PhMNs.
MATERIALS AND METHODS
Experimental animals.
Adult 3-mo-old female (n = 4) and male (n = 4) Sprague-Dawley rats obtained from Envigo (Indianapolis, IN) were used in this study. All procedures were conducted in accordance with the American Physiological Society's Animal Care Guidelines and were approved by the Institutional Animal Care and Use Committee at the Mayo Clinic. Animals were housed in cages under a 12:12-h light-dark cycle with ad libitum access to food and water. Animals were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) via intramuscular injection for all experimental procedures.
PhMN labeling.
PhMNs were retrogradely labeled by injecting cholera toxin subunit B (CTB) solution (List Biological, Campbell, CA) bilaterally into the diaphragm muscle, as previously described (Mantilla et al. 2009; Prakash et al. 1993, 2000). Briefly, following induction of anesthesia, a midline laparotomy was performed such that the inferior surface of the diaphragm was exposed. With the use of a 10-μL Hamilton syringe, three to four injections of 5 μL of CTB (0.2%) were injected at three to four sites equally spread across the dorsal-to-ventral surface of the muscle to ensure adequate labeling of the entire motor neuron pool. Muscle and skin layers were sutured using 3–0 Vicryl (polyglactin 910). Carprofen (50 mg/ml) was administered ad libitum in the drinking water and started 48 h before surgery. Animals also received Penicillin G (25,000 U/kg) intramuscularly and parenteral dextrose and saline injections subcutaneously following surgery. Animals were maintained on a heating pad until alert and awake. At 72 h following intradiaphragm injection, animals were deeply anesthetized, euthanized by exsanguination, and transcardially perfused with chilled 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4). The spinal cord was resected from C2 to T1, postfixed in 4% paraformaldehyde for 24 h, and transferred to 30% sucrose in 0.1 M PBS (pH 7.4) for 3 days at 4°C. Spinal cords were subsequently embedded in cryomolds (VWR, Radnor, PA), sectioned longitudinally at 70 μm, and stored in 0.1 M PB solution in 24-well plates until processing.
Immunohistochemistry.
Immunohistochemical detection of synaptic input at PhMNs was based on previously reported techniques (Issa et al. 2010; Kinkead et al. 1998; Prakash et al. 2000; Zhan et al. 2000). Briefly, spinal cord sections were blocked for 1 h in 10% normal donkey serum in Tris-buffered saline (TBS) containing 0.3% Triton X-100 (TBS-Tx), followed by incubation overnight (16–24 h) with the appropriate primary antibodies in 5% normal donkey serum TBS-Tx. An anti-CTB (goat polyclonal, catalog no. 703, lot no. 7032A9, 1:5000; List Biologicals) was used to label PhMNs. An anti-vesicular Glu transporter 1 (VGLUT-1) antibody (mouse monoclonal IgG2a, catalog no. 135 311, lot no. 135311/28, 1:400; Synaptic Systems, Goettingen, Germany) and anti-vesicular Glu transporter 2 (VGLUT-2) antibody (mouse monoclonal IgG1, MAB5504, lot no. 2817471, 1:400; Millipore, Billerica, MA) were used to identify Glu synapses at PhMNs due to the predominant VGLUT-1 and VGLUT-2 expression at Glu synapses around motor neurons (Herzog et al. 2004; Issa et al. 2010; Oliveira et al. 2003). After being washed three times in TBS (15 min each), sections were incubated with donkey Alexa Fluor 488 anti-goat and Alexa Fluor 594 anti-mouse secondary antibodies (IgG; Jackson ImmunoResearch Laboratories, West Grove, PA) for 3 h. Tissue sections were then washed three times in TBS before being mounted with ProLong Gold antifade mountant (Thermo Fisher, Waltham, MA) and coverslipped. For all primary/secondary antibody pairs, additional studies not including the primary antibody (blank) or alternately using Alexa Fluor-conjugated secondary antibodies were conducted to confirm the specificity of immunostaining. Positive (rat cerebellum) and negative (rat liver) tissue sections were also stained to confirm staining specificity if VGLUT-1 and VGLUT-2 antibodies. Blank sections were processed in parallel for all immunohistochemical reactions and animals.
Confocal imaging.
Labeled PhMNs in the cervical spinal cord sections were visualized with an Olympus FV2000 laser confocal microscope (Olympus Life Sciences Solutions, Waltham, MA) equipped with argon (488 nm) and green HeNe (543 nm) lasers. Three-dimensional (3D) imaging techniques have been previously reported (Fogarty et al. 2018; Issa et al. 2010; Mantilla et al. 2018; Prakash et al. 1993, 1994, 2000; Sieck et al. 1999; Zhan et al. 2000). All images were acquired at 12-bit resolution in an array of 1024 × 1024 pixels using a ×60 Plan Apo (numerical aperture 1.40, working distance 0.15 mm) oil-immersion objective lens with a step size of 0.5 µm (voxel dimensions: 0.207 × 0.207 × 0.5 µm). Sequential two-channel imaging was performed using a dichroic mirror beam splitter that allows transmission of 555–615 nm (reflects 480–555 and 615–800 nm) and appropriate bandpass emission filters (495–535 nm and 575–630 nm long pass for Alexa 488 and 594, respectively). Since image analysis is highly dependent on image quality, several steps were taken to ensure images were of optimal quality. To reduce cross talk, laser illumination was done sequentially for the imaging of each optical slice. The empirically calculated point spread function for the ×60 lens was used to set the step size, as previously reported (Prakash et al. 1993, 1994; Sieck et al. 1999). Laser intensity and photomultiplier settings were adjusted to maintain black level of the blank sections (no primary antibody) to less than 10% of the dynamic range and to prevent saturation. All PhMNs were imaged in the rostrocaudal direction using FluoView software version 5.0 (Olympus). Registration was verified empirically using multiwavelength microbead calibration techniques similar to previous reports (Prakash et al. 1993, 1994, 2000; Sieck et al. 1999; Zhan et al. 2000).
PhMN size measurements.
A total of 200 PhMNs from 8 animals (25 PhMNs per animal) were analyzed from both ipsilateral and contralateral sides of the PhMN pool. Confocal image stacks were analyzed using NIS Elements AR imaging software (version 5.00; Nikon Instruments, Melville, NY). The procedures for PhMN somal volume measurements have been described previously (Fogarty et al. 2018; Issa et al. 2010; Mantilla et al. 2018; Prakash et al. 1993, 1994, 2000). Surface areas of motor neuron somata were estimated for a prolate spheroid by measuring the major and minor diameters. In a subset of animals, VGLUT density was compared across the soma and primary dendrite compartment in 30 PhMNs (n = 30 PhMNs, 5 PhMNs each from 6 animals). Dendritic diameter was measured from a maximum intensity projection image of each primary dendritic segment (measured at a distance of 15 µm from the somata), which was considered a cylindrical object for surface area and volume measurements (Fogarty et al. 2018; Issa et al. 2010; Mantilla et al. 2018; Prakash et al. 2000; Zhan et al. 2000).
PhMN and dendrite sampling.
Selection of PhMNs for VGLUT analysis was conducted in a systematic and unbiased fashion. Only labeled PhMNs with a distinct mid-nuclear section that were not cut during spinal cord sectioning were sampled for synaptic distribution analyses. Additionally, PhMNs adjoining CTB-labeled dendrites (<6 μm from the motor neuron surface in any direction) were excluded to facilitate identification of VGLUT terminals associated with the index PhMN.
In additional analyses, we systematically identified PhMNs with one or more fully CTB-labeled primary dendrite of 15 μm or greater length. We previously reported that there are four to six primary dendrites per phrenic motor neuron (Fogarty et al. 2018; Issa et al. 2010; Mantilla et al. 2018; Prakash et al. 2000). Only one dendrite per PhMN was selected for analyses of dendritic VGLUT terminal density. In most instances, only one dendrite per PhMN met the labeling and length criteria, and when needed, the dendritic segment that was the most clearly distinguishable from the PhMN soma was selected.
Analysis of VGLUT terminal distribution.
A custom macro was developed on the NIS elements platform to perform semiautomated processing of confocal stacks to create a 2.5-µm shell around CTB-labeled PhMNs and isolate VGLUT terminals within this shell. To perform this task, an edge detection-based threshold algorithm was employed to create a region of interest (ROI) in the green channel (CTB) for each optical slice of the confocal stack. Subsequently, a dilated ROI was created in each optical slice, originating from the outer edge of the motor neuron and radiating outwards in the XY direction, along with a contracted ROI in the inward XY direction. These ROIs were then superimposed onto the red channel and all VGLUT terminals in the shell were isolated. According to electron microscopy (EM) literature (Ellenberger et al. 1990; Goshgarian and Rafols 1984; Tai and Goshgarian 1996), the synaptic cleft measures ~20 nm and Glu synapses range from 0.5 to 2 µm in diameter. To ensure that the entire synapse directly opposing the motor neuron is identified, the dilation shell width was set to 2.5 µm. The inner shell in conjunction with the other shell allowed inclusion of any VGLUT terminals present at the membrane that would have otherwise been cut off due slight discrepancies in thresholding to the motor neuron boundary. To improve the signal-to-noise ratio, images were processed using the deconvolution module in the NIS-Elements AR software package (Nikon). A blind deconvolution algorithm (Point Scan Confocal, 3 iterations) was used in which the most probable combination of point spread function is estimated for the given image and obviates the need to obtain a point spread function for the optical system for each image acquisition session. Following deconvolution, the 3D object measurement plug-in in the NIS-Elements AR software package was utilized to identify VGLUT terminals at the PhMN. Based on previously reported sizes of synapses at PhMNs in studies utilizing EM (Ellenberger et al. 1990; Goshgarian and Rafols 1984; Tai and Goshgarian 1996), the minimum size of a synapse was set to be 1 µm3, volume measurements were floored to exclude all identified VGLUT terminals smaller than 1 µm3, and the maximum size of a synapse was set to be 6 µm3. To avoid situations where objects were not discrete enough to be counted as two distinct objects, any object greater than 6 µm3 was counted as an additional synapse in increments of multiples of 6 µm3. VGLUT terminal density was obtained by normalizing counts to the shell volume of each motor neuron compartment. Total numbers of VGLUT terminals at the soma were estimated using VGLUT terminal density and estimated surface area for that PhMN. Images were converted to 8-bit in NIS Elements for presentation only. No thresholding or postimaging processing was applied for any images. Each channel was pseudocolored in Adobe Photoshop by changing the color gamut (RGB). Only brightness and contrast levels were adjusted linearly if needed to facilitate presentation of multiple colors.
Statistical analysis.
All statistical evaluations were performed with JMP statistical software (version 14.0; SAS Institute, Cary, NC) using a mixed linear regression with repeated measures model with animal as a random effect. For each PhMN, the total number of VGLUT terminals, PhMN somal surface area, and VGLUT terminal density were primary outcome measures. The study statistical design was powered to consider the within-subject variance to detect a 10% difference at P < 0.05. Accordingly, we stereologically sampled 25 phrenic motor neurons per animal, which provided more than sufficient power to reject our null hypothesis that there were no differences in glutamatergic inputs (VGLUT terminals) across PhMNs. This statistical model also provided very robust analyses of clustered data (e.g., VGLUT terminal number and density clustered by somal surface area tertiles or by animal sex). All experimental data are means ± SE, unless otherwise specified. With the mixed linear model, statistical significance was established at the 0.05 level and adjusted for any violation of the assumption of sphericity in repeated measures using the Greenhouse-Geisser correction. When appropriate, post hoc analyses were conducted using Tukey–Kramer honestly significant difference.
RESULTS
Labeling of PhMNs by intramuscular injection.
Robust PhMN labeling was observed using CTB, allowing easy identification of individual PhMNs. Characteristics of the phrenic pool were consistent with previous reports (Fogarty et al. 2018; Lindsay et al. 1991; Mantilla et al. 2018; Prakash et al. 1993, 1994, 2000). PhMNs were typically clustered in groups of three to five motor neurons in the rostrocaudal column in the gray matter of the cervical spinal cord, and soma and primary dendrites were clearly visible. Although intramuscular CTB injections resulted in extensive PhMN labeling, individual PhMNs could be easily discerned (Fig. 1). There were no apparent somatotopic differences in the distribution of small to large PhMNs, with PhMN clusters appearing to be heterogeneous in composition across motor neuron size. In sagittal sections of the spinal cord, we observed a proportion of contralateral dendritic projections from PhMNs crossing the central canal, consistent with previous reports (Boulenguez et al. 2007; Prakash et al. 2000). The dendritic fields of PhMNs overlapped extensively and were bundled in the rostrocaudal axis orientation.
Fig. 1.
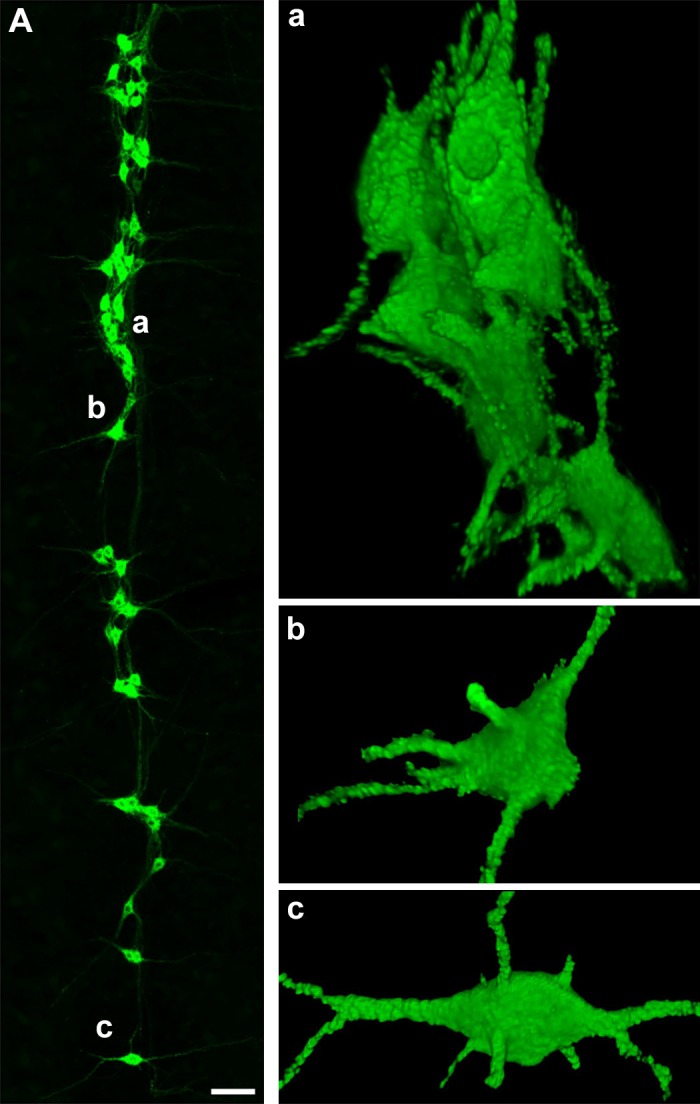
Retrogradely labeled phrenic motor neuron (PhMN) pool. A, left: representative maximum projection image of a 70-µm-thick section of the right cervical (C3–C4) spinal cord showing retrogradely labeled PhMNs in green. Three-dimensional reconstructions of PhMNs marked as a, b, and c are shown at right. Note that the clustering of PhMNs (a) makes it difficult to isolate individual PhMNs for vesicular glutamate transporter analysis compared with the isolated PhMNs selected for analyses (b and c). Scale bar, 100 μm.
In a subset of four animals, an average of 204 ± 21 PhMNs were labeled on one side of the spinal cord using the intramuscular CTB injection approach (total: 804 PhMNs; Fig. 2A). The mean long-axis diameter of PhMNs was 42.8 ± 0.3 µm, and the mean short-axis diameter was 24.8 ± 0.1 µm. The mean somal surface area was 2,942 ± 269 µm2, and the average somal volume was 14,193 ± 197 µm3. To evaluate VGLUT innervation of PhMNs of varying size, a total of 200 PhMNs were sampled from eight rats (n = 25 PhMNs/animal), where the PhMNs had a distinct soma with a visible mid-nuclear section and were devoid of overlapping dendrites. The mean long-axis diameter of sampled PhMNs was 43.4 ± 0.7 µm, and the mean short-axis diameter was 25.1 ± 0.3 µm. The mean somal surface area of sampled PhMNs was 3,019 ± 602 µm2 with no statistically significant difference across animals (F7,197 = 0.55; P = 0.80). The average somal volume was 14,803 ± 444 µm3. Additionally, there was no significant difference between mean somal surface areas of all PhMNs that were labeled with CTB and those that were stereologically sampled for the assessment of VGLUT density (F1,241 = 0.84; P = 0.36; Fig. 2A).
Fig. 2.
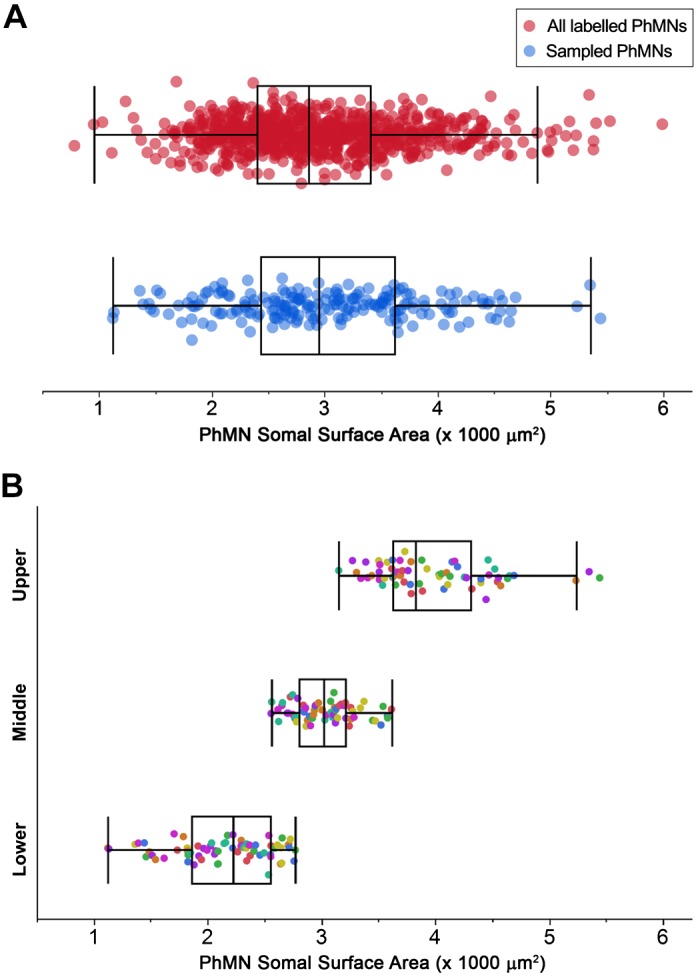
Phrenic motor neuron (PhMN) somal surface area. A: distribution of somal surface areas for all cholera toxin B-labeled PhMNs measured on one side of the spinal cord in a subset of 4 animals (n = 804 PhMNs; red) compared with that of all PhMNs sampled for analysis of (vesicular glutamate transporter VGLUT) density (n = 200 PhMNs from 8 rats; blue). There were no differences in PhMN somal surface area introduced by the sampling for VGLUT analyses (P = 0.36). B: distribution of somal surface areas for the 200 PhMNs used for VGLUT analysis. Presynaptic VGLUT terminals were quantified at individual PhMNs, grouped into lower tertile (n = 72), middle tertile (n = 64), and upper tertile (n = 64) for each animal (indicated by different colors). Boxplots in A and B represent outlier whisker plots of data set in which boundaries are set at the 25th and 75th quartiles, and the median is represented as a line. Note differences across animals in PhMN somal surface area.
PhMNs were further clustered into tertiles of PhMN surface area for each animal to segregate the assessment of presynaptic Glu innervation (Fig. 2B). The tertile grouping reflects the expected proportions of the different types of motor units that would be recruited to accomplish a range of motor behaviors, based on multiple previous studies from our group (Fournier and Sieck 1988; Khurram et al. 2019; Mantilla et al. 2010; Sieck 1991, 1994; Sieck and Fournier 1989). In accordance with our recruitment model, PhMNs in the lower tertile would comprise motor units that are activated during lower force ventilatory behaviors, followed by the middle tertile motor neurons activated to accomplish higher force ventilatory behaviors such as breathing against an occluded airway and deep breaths. The upper tertile of motor neurons likely comprise motor units recruited to accomplish higher force expulsive behaviors. The mean PhMN somal surface areas for the groups were as follows: lower, 2,162 ± 50 µm2 (n = 72); middle, 3,029 ± 34 µm2 (n = 64); and upper, 3,973 ± 63 µm2 (n = 64). Furthermore, PhMNs sampled for VGLUT analysis represented a comparable distribution of the entire labeled the PhMN pool, as assessed by comparing somal surface area distribution of sampled PhMNs with that of all labeled PhMNs on the ipsilateral side of the spinal cord for four animals.
VGLUT terminals at PhMNs.
VGLUT immunoreactivity had a bouton-like appearance and was visible surrounding soma and dendrites of PhMNs in Rexed’s laminae VIII and IX, consistent with previous reports of VGLUT immunoreactivity in the spinal cord (Alvarez et al. 2004; Herzog et al. 2004; Issa et al. 2010) (Fig. 3). VGLUT terminals were isolated within a 2.5-μm shell around identified PhMNs using the workflow described above. These 3D measurements are consistent with the size of synaptic terminals previously identified in the PhMN pool using EM techniques (Ellenberger et al. 1990; Goshgarian and Rafols 1984; Tai and Goshgarian 1996) (Fig. 4, A and B). Along the Z-axis, synapses typically spanned two to three optical slices (0.5 µm per slice) with some synapses spanning up to five consecutive slices. The sphericity of VGLUT terminals was calculated for each terminal (Fig. 4C), and there was a clear reduction in sphericity for VGLUT terminals with a volume >5.9 μm3, consistent with apparent aggregation of neighboring terminals. Of note, this terminal volume cutoff is consistent with morphometric data derived from EM studies (Goshgarian and Rafols 1984; Tai and Goshgarian 1996). Accordingly, terminals with a volume of 6 μm3 or greater were counted as an additional synapse in increments of multiples of 6 mm3 for VGLUT density and number measurements.
Fig. 3.
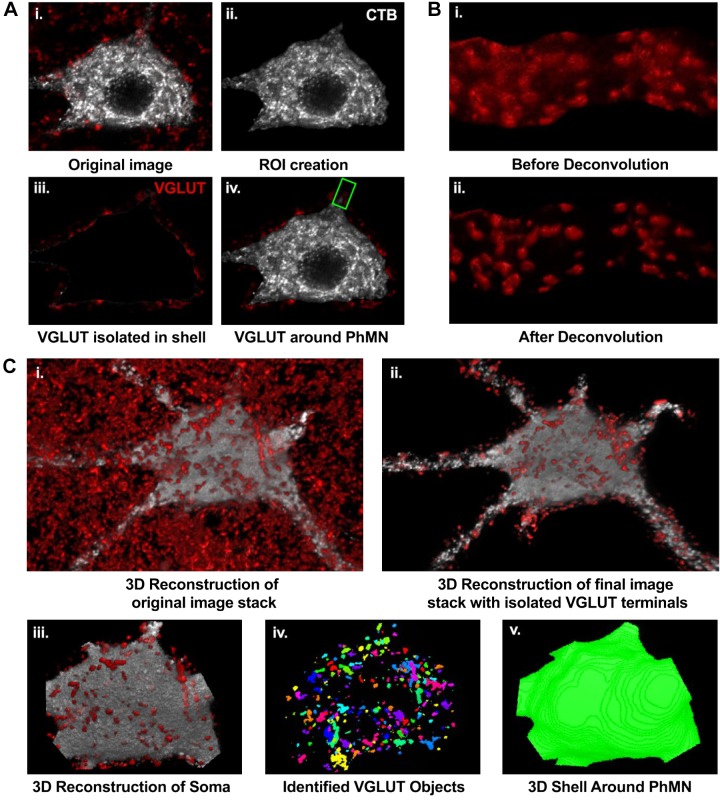
Method for 3-dimensional (3D) analyses of vesicular glutamate transporter (VGLUT) terminals at a single phrenic motor neuron (PhMN). A: representative image of single optical slice (i) with visible VGLUT terminals (red) surrounding a single cholera toxin B (CTB)-labeled PhMN (grayscale). Semiautomated processing for VGLUT terminal isolation included thresholding and creation of a region of interest (ROI) encompassing the CTB-labeled PhMN soma (ii), application of a mask to segregate VGLUT-immunoreactive terminals in the PhMN ROI dilated by 2.5 µm (iii), and single-slice image with isolated VGLUT terminals for a selected PhMN (iv). The green ROI represents the selected dendrite for additional steps exemplified in B. Note the first 2 steps were repeated for each optical slice in the image stack for each sampled PhMN. B: all image stacks containing VGLUT terminals segregated at PhMN soma and selected primary dendrites (see materials and methods for details) were subjected to deconvolution (i) in 3D (blind deconvolution using Point Scan Confocal, 3 iterations). Representative image (ii) shows the improved resolution of VGLUT terminals after deconvolution. C: representative 3D reconstruction for the entire PhMN and surrounding VGLUT terminals (i), with segregated terminals to the surrounding shell (ii). With use of the 3D volume measurement plug-in in NIS elements software, the PhMN soma was selected (iii), individual VGLUT terminals were identified (iv), allowing their dimensions to be quantified, and the volume of the shell surrounding the PhMN was obtained in 3D (v).
Fig. 4.
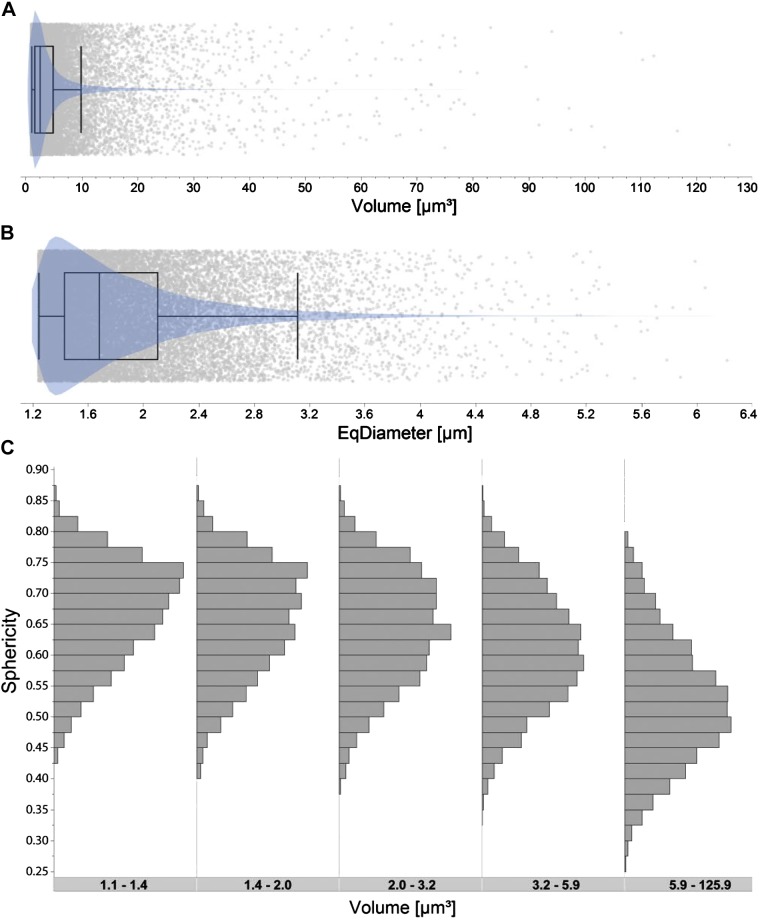
Vesicular glutamate transporter (VGLUT) terminal morphology. A: outlier whisker and violin plots (probability density) of the volume of identified VGLUT terminals (n = 22,905) for all sampled phrenic motor neurons (PhMNs; n = 200 from 8 animals). B: outlier whisker and violin plots of measured equivalent diameter (EqDiameter; diameter of a sphere with the same volume) for each identified VGLUT terminal. Boundaries of outlier whisker plots are set at the 25th and 75th quartiles, and the median is represented as a line. Note highly skewed distribution of VGLUT terminal volume and EqDiameter (A and B). C: distribution of sphericity (ratio of surface area of VGLUT terminal and surface area of a sphere with the same volume) values for identified VGLUT terminals at PhMNs for each quintile of VGLUT object volume (µm3). Note reduced sphericity with increasing VGLUT terminal volume (P < 0.001 for all pairwise comparisons), with the largest reduction in sphericity for the upper quintile indicative of aggregated VGLUT terminals.
In a subset of PhMNs (n = 30 PhMNs from 6 animals), isolated VGLUT terminals at primary dendrites were also quantified. There was a small, yet statistically significant difference in major axis length and terminal volume across soma and primary dendrites (Table 1) with VGLUT terminals at the PhMN soma being larger than at the primary dendrites. Other comparisons of VGLUT terminal morphometrics at the somal versus primary dendrites of PhMNs were not significant.
Table 1.
VGLUT terminal dimensions by PhMN compartment
| Compartment |
||
|---|---|---|
| Soma | Primary Dendrite | |
| Equivalent diameter, µm | 1.832 ± 0.012 | 1.780 ± 0.023 |
| Elongation | 2.503 ± 0.019 | 2.456 ± 0.045 |
| Sphericity | 0.625 ± 0.002 | 0.634 ± 0.005 |
| Major axis length, µm | 3.633 ± 0.039 | 3.419 ± 0.075* |
| Minor axis length, µm | 1.872 ± 0.019 | 1.772 ± 0.034 |
| VGLUT terminal volume, µm3 | 3.108 ± 0.018 | 2.754 ± 0.105* |
Data are means ± SE. Vesicular glutamate transporter (VGLUT) terminal dimensions are summarized from somal and dendritic compartments from 30 PhMNs (n = 2,675 terminals). Equivalent diameter is the diameter of a sphere with the same volume for each identified VGLUT terminal. Elongation is the ratio of maximum to minimum Feret diameter. Sphericity is the ratio of surface area of the VGLUT object to that of a sphere with the same volume.
There was a significant effect of PhMN compartment on major axis length (F1,2615 = 5.47; P = 0.019) and VGLUT terminal volume (F1,2615 = 4.95; P = 0.026), but not for other dimensions (P > 0.074).
VGLUT terminal density decreases with PhMN size.
VGLUT terminal density at the soma was examined across PhMNs (Fig. 5A). One-way ANOVA of VGLUT terminal density by animal showed no difference in density across animals (F1,7 = 2.02; P = 0.054). Density of VGLUT terminals was compared across PhMN somal surface area and by sex using a mixed linear model, with animal as a random effect. There was no effect of sex (F1,5.98 = 0.63; P = 0.46) and no interaction between PhMN somal surface area and sex (F1,188.9 = 0.09; P = 0.77). Sex was thus dropped for subsequent analysis. PhMN somal surface area had a significant effect on VGLUT terminal density (F1,191.5 = 19.16; P < 0.001). The mean VGLUT terminal density at the soma of PhMNs was 0.071 ± 0.001 µm−2.
Fig. 5.
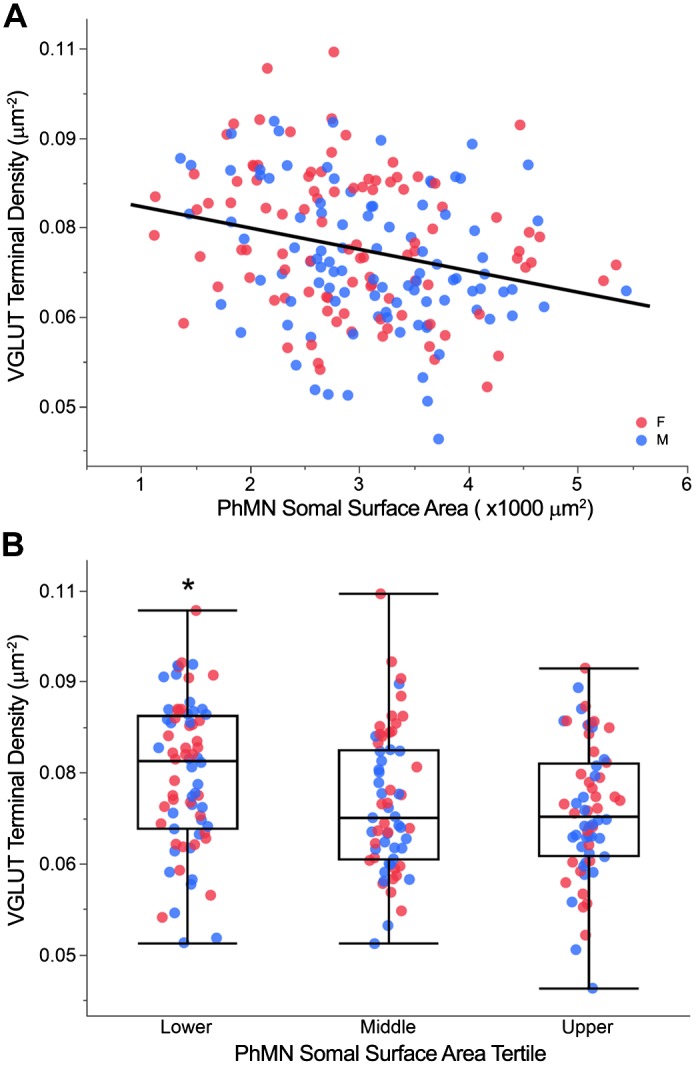
Vesicular glutamate transporter (VGLUT) terminal density across phrenic motor neurons (PhMNs). A: scatterplot of VGLUT terminal density vs. PhMN somal surface area (n = 200 PhMNs from 8 animals). VGLUT terminal density is negatively correlated with PhMN somal surface area (P < 0.001; r2 = 0.12), with no difference between female (red) and male (blue) rats. B: outlier whisker box plot of VGLUT terminal density across individual PhMNs grouped by tertile of somal surface area for each animal. Boundaries of outlier whisker plots are set at the 25th and 75th quartiles, and the median is represented as a line. *P < 0.05, significantly different compared with upper tertile.
VGLUT terminal density was significantly different across PhMN tertiles by size (F2,190 = 4.86; P = 0.009; Fig. 5B). Post hoc analysis revealed statistically significant pairwise differences in the VGLUT terminal density, with the mean VGLUT terminal density at the PhMN soma generally decreased with increasing PhMN tertile: lower tertile, 0.075 ± 0.001 µm−2; middle tertile, 0.071 ± 0.002 µm−2; and upper tertile, 0.069 ± 0.001 µm−2. PhMNs in the lower tertile had an ~10% higher VGLUT terminal density compared with PhMNs in upper tertile.
Total number of VGLUT terminals varies across PhMNs according to size.
Total number of VGLUT terminals was compared across PhMN sizes using a mixed linear model, with animal as a random effect (Fig. 6A). PhMN somal surface area had a significant effect on the number of estimated VGLUT terminals (F1,193.9 = 440.52; P < 0.001). The mean number of VGLUT terminals at the PhMN soma was 213 terminals.
Fig. 6.
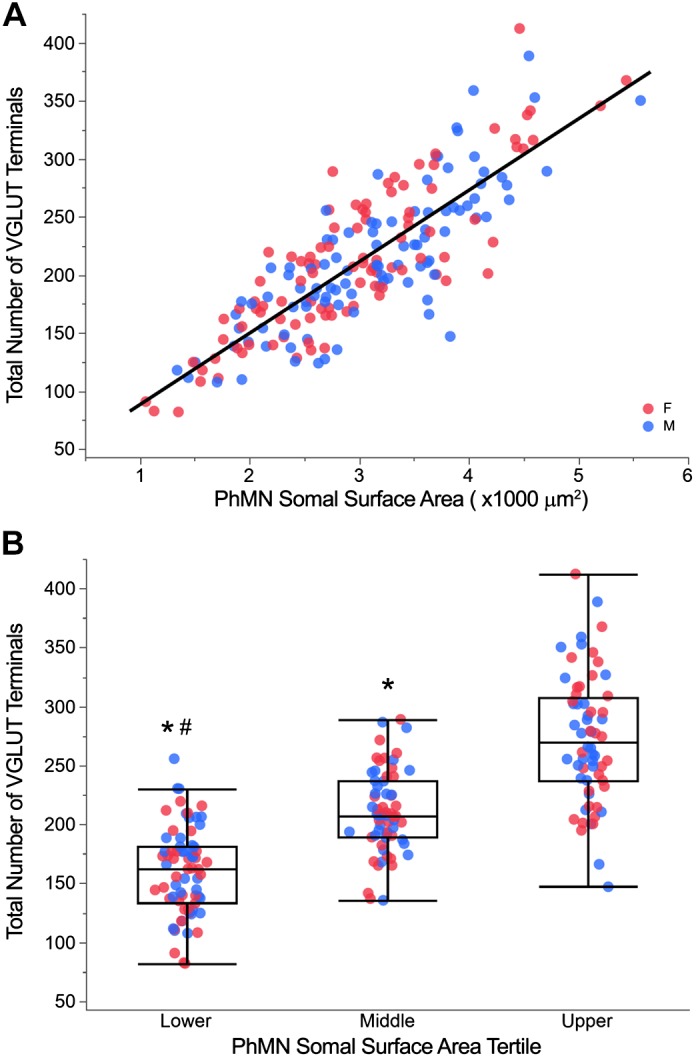
Total number of vesicular glutamate transporter (VGLUT) terminals across phrenic motor neurons (PhMNs). A: scatterplot of total number of VGLUT terminals vs. PhMN somal surface area (n = 200 PhMNs from 8 animals). The total number of VGLUT terminals is positively correlated with PhMN somal surface area (P < 0.001; r2 = 0.69), with no difference between female (red) and male (blue) rats. B: outlier whisker box plot of total number of VGLUT terminals across individual PhMNs grouped by tertile of somal surface area for each animal. Boundaries of outlier whisker plots are set at the 25th and 75th quartiles, and the median is represented as a line. *P < 0.05, significantly different compared with upper tertile (Tukey HSD). #P < 0.05, significantly different compared with middle tertile (Tukey HSD).
When total number of VGLUT terminals was compared across PhMN groups, there was an overall effect of the model (P < 0.001; Fig. 6B). The total number of VGLUT terminals was significantly different across PhMNs grouped in tertiles according to somal surface area (F2,190 = 118.10; P < 0.001). The mean number of VGLUT terminals across the lower, middle, and upper tertiles of PhMN size was 160 ± 6, 213 ± 6, and 273 ± 6 terminals, respectively.
VGLUT synapses at PhMN soma and primary dendrites.
The distribution of VGLUT-labeled synapses was compared across somal and primary dendritic compartments (Fig. 7). There was no effect of PhMN somal surface area (P = 0.97) or neuronal compartment (P = 0.56) on VGLUT terminal density. The mean VGLUT terminal density was 0.058 ± 0.004 and 0.060 ± 0.002 µm−2 at the soma and primary dendrites, respectively. Accordingly, it is estimated that ~185 VGLUT terminals are located at the soma of PhMNs. Based on estimated PhMN dendritic tree surface area representing 87% of total PhMN surface area (Mantilla et al. 2018), ~1,240 VGLUT terminals are located in the dendritic tree.
Fig. 7.
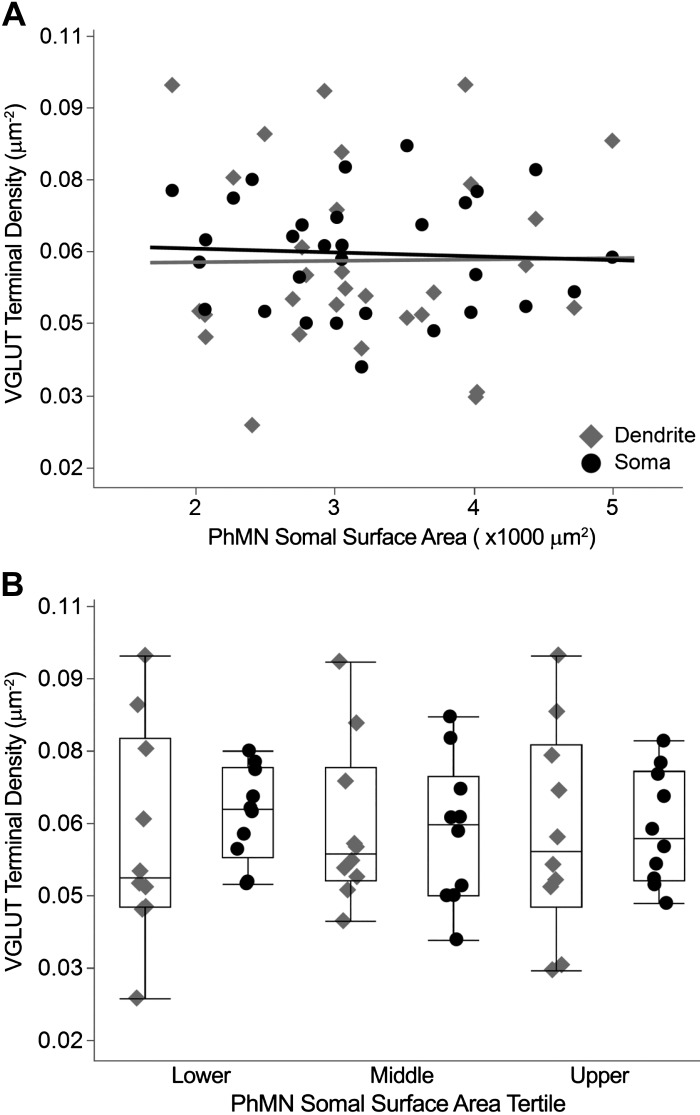
Vesicular glutamate transporter (VGLUT) terminal density in dendritic vs. somal compartments of phrenic motor neurons (PhMNs). A: scatterplots of VGLUT terminal density at PhMNs in dendritic (diamonds) and somal (circles) compartments vs. PhMN somal surface area (n = 30 PhMNs from 8 rats). There was no difference in VGLUT terminal density across compartments. B: outlier whisker box plots of VGLUT terminal density across compartments for PhMNs grouped by tertile of somal surface area per animal, with no differences observed. Boundaries of outlier whisker plots are set at the 25th and 75th quartiles, and the median is represented as a line.
DISCUSSION
The present study found that in rats, smaller PhMNs have a higher density of Glu presynaptic terminals (VGLUT immunoreactive) compared with larger PhMNs. These results suggest that in addition to size-dependent differences in membrane capacitance and resistance, a greater presynaptic input to smaller PhMNs could contribute to their earlier recruitment. This observation is consistent with the long-standing dogma dictating orderly recruitment of motor neurons from small to larger somal size within a motor neuron pool. Accordingly, motor units comprising smaller PhMNs would be more easily recruited to accomplish lower force ventilatory behaviors that are life-sustaining and have a high duty cycle. In contrast, motor units comprising larger motor neurons have lower density of VGLUT terminal innervation and higher activation thresholds, restricting their recruitment to higher force expulsive behaviors such as sneezing and coughing.
PhMN labeling.
Intramuscular diaphragm injections of CTB resulted in extensive labeling of PhMNs spanning C3–C5 segments of the spinal cord with an average of 204 PhMNs per side, which is consistent with previous studies from our laboratory (Alvarez-Argote et al. 2016; Mantilla et al. 2009, 2017, 2018; Martínez-Gálvez et al. 2016; Rana et al. 2017). CTB binds to GM1 gangliosides at nerve terminals that are internalized and retrogradely transported in lipid rafts to motor neurons (Sheikh et al. 1999; Zhang et al. 1995). This uptake mechanism results in reliable nonmembranous labeling of motor neuron soma and proximal dendrites, but inconsistent labeling of more distal dendrites. Thus retrograde CTB labeling is limited with respect to visualization of the entire dendritic tree. However, this is offset by the larger sample of PhMNs obtained. Moreover, in a recent study, we confirmed that there are no differences in CTB uptake at neuromuscular junctions associated with different motor unit types (Gonzalez Porras et al. 2019), so there was no biasing toward any motor neuron type.
Morphometric features of PhMNs were consistent with previous reports from our group and others (Fogarty et al. 2018; Furicchia and Goshgarian 1987; Goshgarian and Rafols 1981; Issa et al. 2010; Mantilla et al. 2018; Prakash et al. 2000). PhMNs displayed “open” nucleus features, including extensive overlap of dendritic fields and a striking rostrocaudal orientation within the nucleus (Mannen 1978). PhMNs also displayed clustering of cell bodies and dendritic bundling. These features might provide the anatomical substrate for activity integration from supraspinal inputs to synchronize PhMN output and facilitate interactions with motor neurons innervating accessory respiratory muscles (Scheibel and Scheibel 1970, 1971).
Calculations of PhMN surface area and volume were based on measurements of long-axis and short-axis diameters and were consistent with our previous studies (Issa et al. 2010; Mantilla et al. 2018). These calculations of PhMN somal surface area assumed a prolate spheroid shape, which we previously found differed by <10% from measurements based on 3D confocal reconstruction of PhMNs (Issa et al. 2010; Mantilla et al. 2018; Prakash et al. 1993).
3D image analysis.
In identifying Glu synapses directly apposing PhMNs, an appropriate ROI was defined on the basis of morphological properties of synaptic boutons derived from EM studies (Ellenberger et al. 1990; Fogarty et al. 2013; Goshgarian and Rafols 1984; Tai and Goshgarian 1996). A dilation shell of 2.5 µm from the projected surface of PhMNs was selected on the basis of EM measurements of the long axis of synapses on PhMNs (0.5–2 µm). This dilation shell accounted for the entire long axis of a synapse as well as the synaptic cleft and plasma membrane.
Uniformity of antibody penetration across the thickness of the sections was confirmed for each tissue section using Z-plane 3D reconstructions of VGLUT immunolabeling. In the case of EM, high-resolution images allow for identification of synaptic sites with greater certainty than confocal microscopy. However, this comes at the cost of a smaller sample of PhMNs and procedures that are more complex and time consuming. Furthermore, associating synaptic terminal density with motor neuron somal surface area would be difficult using EM. Confocal microscopy allows identification of synapses in 3D, facilitating a more comprehensive analysis of synapse volume as well as accurate identification of synapses with the long axis located in the Z plane.
Glu synapses at PhMNs.
VGLUT1 and VGLUT2 are the most abundant Glu transporters in the spinal cord ventral horn (Herzog et al. 2004; Oliveira et al. 2003). Glu terminals are also the most abundant excitatory neurotransmitter at PhMNs, comprising ~50% of all synaptic contacts (Tai and Goshgarian 1996). Glu immunoreactivity using both VGLUT1 and VGLUT2 antibodies was consistent with previous reports in the spinal cord (Alvarez et al. 2004; Herzog et al. 2004; Issa et al. 2010).
In EM studies, where Glu synapses were identified using anti-Glu immunogold labeling techniques (Bae et al. 1999; Shigenaga et al. 2005), Glu boutons at PhMNs were distributed evenly between the primary, intermediate, and distal dendrites. In the present study, intermediate and distal dendrites could not be easily identified using CTB labeling However, compared with EM studies, we confirmed comparable VGLUT terminal densities at PhMN soma and proximal dendrites.
EM based ultrastructural analysis of Glu terminals at PhMNs has been reported, albeit with a very small sample size (Goshgarian and Rafols 1984; Tai and Goshgarian 1996). Glu terminals at PhMNs are predominantly symmetrically shaped with diameters ranging from 1 to 2 µm, with some boutons as large as 4 µm. Consistent with these observations, we found that ~70% of all VGLUT terminals at PhMNs had an equivalent diameter of 1–2 µm, with ~29% of terminals ranging between 2 and 4 µm and <1% of all terminals displaying a diameter >4.0 µm. The total number of Glu terminals at the somal surface of PhMNs observed in our study (213 terminals) was comparable to that (154 terminals) reported by Goshgarian and Rafols (1984).
We examined VGLUT1 and VGLUT2 terminals at PhMNs as an initial evaluation of excitatory inputs to this motor neuron pool. It is important to note that we do assume a relationship between the density of Glu terminals and synaptic input (as defined by an active synapse resulting in an excitatory postsynaptic current) to PhMNs. Our study identified differences in Glu inputs (VGLUT terminals) across PhMNs of varying size, with ~10% higher density in motor neurons in the lower tertile (compared with upper tertile) of motor neuron somal surface area. Differences in input density can be expected to mainly impact the safety margin for synaptic transmission, with a ~10% increase in synaptic current expected to increase the likelihood of motor neuron activation in response to the summed current. Relatively small differences in resting membrane potential are sufficient to exceed threshold for activation. Indeed, a higher density of glutamatergic inputs at smaller, more PhMNs together with their intrinsic size-related electrophysiological properties (e.g., membrane capacitance and input resistance) ensures their earlier and more frequent recruitment during inspiratory efforts.
Source of Glu inputs to PhMNs.
For PhMNs involved in breathing, monosynaptic excitatory inputs derive primarily from the medullary rostral ventral respiratory group (rVRG) (Dobbins and Feldman 1994; Ellenberger and Feldman 1988; Ellenberger et al. 1990; Lipski et al. 1994; Tian and Duffin 1996a, 1996b). Anterograde tracing has shown that descending bulbospinal axons traverse in the lateral and ventromedial white matter of the cervical spinal cord (Feldman et al. 1985; Fuller et al. 2009). There are also interneuron populations in the upper cervical spinal cord that receive medullary inputs (Tian and Duffin 1996a) and send inputs to PhMNs (Dobbins and Feldman 1994; Lane et al. 2008; Lipski et al. 1993).
Studies have also identified the neurophysiological coupling of intercostal and PhMN circuits (Decima et al. 1969; Remmers 1970). Using recombinants of pseudorabies, Lane et al. (2008) confirmed the presence of bilateral propriospinal inputs between intercostal motor neurons innervating the three most caudal intercostal muscles in rats and the PhMNs. The intercostal to phrenic reflex, which is mediated by tracts traveling in the ventrolateral funiculus, could provide modulation of PhMN output based on integration from various central and peripheral sites sending feedback to the intercostal motor neuron pool. These propriospinal inputs appear to be widely distributed to PhMNs since intercostal afferent stimulation leads to a normal recruitment order of PhMNs (Decima et al. 1969; Remmers 1970, 1973).
Most skeletal muscles contain muscle spindles that respond to both static and dynamic changes in muscle length. These spindles send primary 1a afferents with direct synapses on their respective motor neuron pools to modulate motor output. However, it should be noted that there are few if any muscle spindles in the diaphragm (Goshgarian and Roubal 1986; Sieck and Gransee 2012). Hence, there is a dearth of afferent feedback on PhMNs via 1a afferents, as observed by termination of afferent inputs from the phrenic nerve in laminae I–III of the dorsal horn (Goshgarian et al. 1986).
Contribution of synaptic inputs to motor unit recruitment.
According to the size principle, motor unit recruitment is primarily determined by the intrinsic size-related properties of motor neurons (Henneman 1957; Stuart and Enoka 1983). However, the size principle does not exclude differences in the distribution of descending glutamatergic inputs, which may also help shape recruitment order of diaphragm motor units (Lee and Fuller 2011; Sieck 1988a, 1991). Systematic studies of synaptic potential and currents have shown that excitatory inputs to all motor neurons might be nonuniformly distributed (Burke 1981). Within lumbar motor neuron pools, the distribution of 1a afferents varies across motor unit type (Heckman and Binder 1988). However, as mentioned above, the diaphragm muscle has few if any muscle spindles. Other excitatory propriospinal input (e.g., muscle spindle input from intercostal muscles) may affect PhMNs, but the size-related or motor unit type-related distribution of this input has not been examined. Electrophysiological studies of synaptic currents from descending tracts (corticospinal and rubrospinal) show much greater effects on motor neurons innervating fast (type F) motor units compared with slow (type S) motor units (Binder et al. 1998; Powers et al. 1993). The distribution of these supraspinal excitatory inputs on PhMNs has not been systematically explored.
In a study by Hilaire et al. (1983), cross-correlation of the inspiratory-related discharge of brain stem premotor neurons and the discharge of PhMNs suggested a relationship between the timing of premotor activation (e.g., early vs. late inspiration) and the recruitment of PhMNs. Monteau et al. (1985) also observed greater synchrony in the discharge of medullary premotor neurons and excitatory postsynaptic potentials in PhMNs recruited early during inspiration, whereas the correlation was less robust for PhMNs recruited later during inspiration. These findings suggest that there may be a certain degree of selectivity in the projection of inspiratory-related descending premotor inputs on PhMNs. Unfortunately, these previous studies focused on inspiratory-related premotor input to PhMNs and did not consider the pool of PhMNs that are likely not involved in ventilatory behaviors (i.e., larger PhMNs).
Most recently, distinct centers have been identified in the brain stem that modulate activation of PhMNs during sigh behavior and provide additional evidence in support of nonuniform supraspinal control of motor behaviors that could contribute to selective diaphragm motor unit recruitment (Li et al. 2016). Recent studies from our laboratory showed that unilateral cervical spinal cord hemisection (C2SH) that disrupts descending input to PhMNs resulted in selective impairment in lower force ventilatory behaviors, whereas higher force behaviors such as sigh were largely unimpaired (Hernandez-Torres et al. 2017). These findings are consistent with a certain degree of selectivity in descending excitatory inputs across PhMNs.
Conclusion.
There exist a number of sources of Glu input to PhMNs with the predominant inspiratory-related input coming from the medullary rVRG. It is likely that the distribution of these excitatory glutamatergic inputs to PhMNs is largely uniform and that intrinsic properties of PhMNs (somal surface area) are primarily involved in governing the orderly recruitment of diaphragm motor units. However, in this study, we show that Glu terminal density to smaller PhMNs is higher compared with that to larger PhMNs, and this difference in excitatory input may contribute, in part, to the orderly recruitment of diaphragm motor units, especially during ventilatory (inspiratory related) motor behaviors.
GRANTS
This study was funded by National Institute of Health Grants R01 HL96750, R01 HL146114, AG057052, and AG044615.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.R., C.B.M., and G.C.S. conceived and designed research; S.R. performed experiments; S.R. analyzed data; S.R., C.B.M., and G.C.S. interpreted results of experiments; S.R. prepared figures; S.R., C.B.M., and G.C.S. drafted manuscript; S.R., C.B.M., and G.C.S. edited and revised manuscript; S.R., C.B.M., and G.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Heather M. Gransee and Dr. Matthew J. Fogarty for scientific input and relevant discussion, and Dr. Wen-Zhi Zhan and Yun-Hua Fang for technical assistance.
REFERENCES
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol 472: 257–280, 2004. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- Alvarez-Argote S, Gransee HM, Mora JC, Stowe JM, Jorgenson AJ, Sieck GC, Mantilla CB. The impact of midcervical contusion injury on diaphragm muscle function. J Neurotrauma 33: 500–509, 2016. doi: 10.1089/neu.2015.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YC, Nakamura T, Ihn HJ, Choi MH, Yoshida A, Moritani M, Honma S, Shigenaga Y. Distribution pattern of inhibitory and excitatory synapses in the dendritic tree of single masseter α-motoneurons in the cat. J Comp Neurol 414: 454–468, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. How different afferent inputs control motoneuron discharge and the output of the motoneuron pool. Curr Opin Neurobiol 3: 1028–1034, 1993. doi: 10.1016/0959-4388(93)90177-Z. [DOI] [PubMed] [Google Scholar]
- Binder MD, Robinson FR, Powers RK. Distribution of effective synaptic currents in cat triceps surae motoneurons. VI. Contralateral pyramidal tract. J Neurophysiol 80: 241–248, 1998. doi: 10.1152/jn.1998.80.1.241. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Gauthier P, Kastner A. Respiratory neuron subpopulations and pathways potentially involved in the reactivation of phrenic motoneurons after C2 hemisection. Brain Res 1148: 96–104, 2007. doi: 10.1016/j.brainres.2007.02.060. [DOI] [PubMed] [Google Scholar]
- Burke RE. Motor units: anatomy, physiology and functional organization. In: Handbook of Physiology, The Nervous System, Motor Control, edited by Peachey LD. Bethesda, MD: American Physiological Society, 1981, p. 345–422. doi: 10.1002/cphy.cp010210. [DOI] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Intercostal-to-phrenic reflexes in the spinal cat. Acta Physiol Scand 75: 568–579, 1969. doi: 10.1111/j.1748-1716.1969.tb04412.x. [DOI] [PubMed] [Google Scholar]
- Dick TE, Kong FJ, Berger AJ. Recruitment order of diaphragmatic motor units obeys Henneman’s size principle. In: Respiratory Muscles and Their Neuromotor Control, edited by Sieck GC, Gandevia SC, Cameron WE. New York: Liss, 1987, p. 239–247. [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–86, 1994. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol 269: 47–57, 1988. doi: 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, Goshgarian HG. Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. J Comp Neurol 302: 707–714, 1990. doi: 10.1002/cne.903020403. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci 5: 1993–2000, 1985. doi: 10.1523/JNEUROSCI.05-08-01993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Hammond LA, Kanjhan R, Bellingham MC, Noakes PG. A method for the three-dimensional reconstruction of Neurobiotin™-filled neurons and the location of their synaptic inputs. Front Neural Circuits 7: 153, 2013. doi: 10.3389/fncir.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Omar TS, Zhan WZ, Mantilla CB, Sieck GC. Phrenic motor neuron loss in aged rats. J Neurophysiol 119: 1852–1862, 2018. doi: 10.1152/jn.00868.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59: 1055–1066, 1988. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir Physiol Neurobiol 165: 245–253, 2009. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furicchia JV, Goshgarian HG. Dendritic organization of phrenic motoneurons in the adult rat. Exp Neurol 96: 621–634, 1987. doi: 10.1016/0014-4886(87)90224-X. [DOI] [PubMed] [Google Scholar]
- Gonzalez Porras MA, Fogarty MJ, Gransee HM, Sieck GC, Mantilla CB. Frequency-dependent lipid raft uptake at rat diaphragm muscle axon terminals. Muscle Nerve 59: 611–618, 2019. doi: 10.1002/mus.26421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, Moran MF, Prcevski P. Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH, and respiratory rate in the adult rat. Exp Neurol 93: 440–445, 1986. doi: 10.1016/0014-4886(86)90206-2. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of the albino rat: a correlative HRP and Golgi study. J Comp Neurol 201: 441–456, 1981. doi: 10.1002/cne.902010309. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The ultrastructure and synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J Neurocytol 13: 85–109, 1984. doi: 10.1007/BF01148320. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Roubal PJ. Origin and distribution of phrenic primary afferent nerve fibers in the spinal cord of the adult rat. Exp Neurol 92: 624–638, 1986. doi: 10.1016/0014-4886(86)90304-3. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Analysis of effective synaptic currents generated by homonymous Ia afferent fibers in motoneurons of the cat. J Neurophysiol 60: 1946–1966, 1988. doi: 10.1152/jn.1988.60.6.1946. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Computer simulations of motoneuron firing rate modulation. J Neurophysiol 69: 1005–1008, 1993. doi: 10.1152/jn.1993.69.4.1005. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science 126: 1345–1347, 1957. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Hernandez-Torres V, Gransee HM, Mantilla CB, Wang Y, Zhan WZ, Sieck GC. BDNF effects on functional recovery across motor behaviors after cervical spinal cord injury. J Neurophysiol 117: 537–544, 2017. doi: 10.1152/jn.00654.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Landry M, Buhler E, Bouali-Benazzouz R, Legay C, Henderson CE, Nagy F, Dreyfus P, Giros B, El Mestikawy S. Expression of vesicular glutamate transporters, VGLUT1 and VGLUT2, in cholinergic spinal motoneurons. Eur J Neurosci 20: 1752–1760, 2004. doi: 10.1111/j.1460-9568.2004.03628.x. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Gauthier P, Monteau R. Central respiratory drive and recruitment order of phrenic and inspiratory laryngeal motoneurones. Respir Physiol 51: 341–359, 1983. doi: 10.1016/0034-5687(83)90028-2. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res 143: 77–95, 2004. doi: 10.1016/S0079-6123(03)43008-2. [DOI] [PubMed] [Google Scholar]
- Issa AN, Zhan WZ, Sieck GC, Mantilla CB. Neuregulin-1 at synapses on phrenic motoneurons. J Comp Neurol 518: 4213–4225, 2010. doi: 10.1002/cne.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE, Berger AJ. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol 58: 105–124, 1987. doi: 10.1152/jn.1987.58.1.105. [DOI] [PubMed] [Google Scholar]
- Khurram OU, Fogarty MJ, Rana S, Vang P, Sieck GC, Mantilla CB. Diaphragm muscle function following mid-cervical contusion injury in rats. J Appl Physiol (1985) 126: 221–230, 2019. doi: 10.1152/japplphysiol.00481.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci 18: 8436–8443, 1998. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511: 692–709, 2008. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD. Neural control of phrenic motoneuron discharge. Respir Physiol Neurobiol 179: 71–79, 2011. doi: 10.1016/j.resp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Janczewski WA, Yackle K, Kam K, Pagliardini S, Krasnow MA, Feldman JL. The peptidergic control circuit for sighing. Nature 530: 293–297, 2016. doi: 10.1038/nature16964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay AD, Greer JJ, Feldman JL. Phrenic motoneuron morphology in the neonatal rat. J Comp Neurol 308: 169–179, 1991. doi: 10.1002/cne.903080204. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J, Kruszewska B, Zhang X. Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Exp Brain Res 95: 477–487, 1993. doi: 10.1007/BF00227141. [DOI] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res 640: 171–184, 1994. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Mannen H. Three-dimensional reconstruction of individual neurons in higher mammals. Int Rev Cytol Suppl: 329–372, 1978. [PubMed] [Google Scholar]
- Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Impact of glutamatergic and serotonergic neurotransmission on diaphragm muscle activity after cervical spinal hemisection. J Neurophysiol 118: 1732–1738, 2017. doi: 10.1152/jn.00345.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 173: 101–106, 2010. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol 179: 57–63, 2011. doi: 10.1016/j.resp.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Gransee HM, Prakash YS, Sieck GC. Phrenic motoneuron structural plasticity across models of diaphragm muscle paralysis. J Comp Neurol 526: 2973–2983, 2018. doi: 10.1002/cne.24503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods 182: 244–249, 2009. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Gálvez G, Zambrano JM, Diaz Soto JC, Zhan WZ, Gransee HM, Sieck GC, Mantilla CB. TrkB gene therapy by adeno-associated virus enhances recovery after cervical spinal cord injury. Exp Neurol 276: 31–40, 2016. doi: 10.1016/j.expneurol.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Smith JC, Feldman JL. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. J Neurosci 9: 1910–1921, 1989. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteau R, Khatib M, Hilaire G. Central determination of recruitment order: intracellular study of phrenic motoneurons. Neurosci Lett 56: 341–346, 1985. doi: 10.1016/0304-3940(85)90266-6. [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hökfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse 50: 117–129, 2003. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Powers RK, Robinson FR, Konodi MA, Binder MD. Distribution of rubrospinal synaptic input to cat triceps surae motoneurons. J Neurophysiol 70: 1460–1468, 1993. doi: 10.1152/jn.1993.70.4.1460. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol (1985) 89: 563–572, 2000. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Smithson KG, Sieck GC. Measurements of motoneuron somal volumes using laser confocal microscopy: comparisons with shape-based stereological estimations. Neuroimage 1: 95–107, 1993. doi: 10.1006/nimg.1993.1003. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Smithson KG, Sieck GC. Application of the Cavalieri principle in volume estimation using laser confocal microscopy. Neuroimage 1: 325–333, 1994. doi: 10.1006/nimg.1994.1017. [DOI] [PubMed] [Google Scholar]
- Rana S, Sieck GC, Mantilla CB. Diaphragm electromyographic activity following unilateral midcervical contusion injury in rats. J Neurophysiol 117: 545–555, 2017. doi: 10.1152/jn.00727.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE. Inhibition of inspiratory activity by intercostal muscle afferents. Respir Physiol 10: 358–383, 1970. doi: 10.1016/0034-5687(70)90055-1. [DOI] [PubMed] [Google Scholar]
- Remmers JE. Extra-segmental reflexes derived from intercostal afferents: phrenic and laryngeal responses. J Physiol 233: 45–62, 1973. doi: 10.1113/jphysiol.1973.sp010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Developmental relationship between spinal motoneuron dendrite bundles and patterned activity in the hind limb of cats. Exp Neurol 29: 328–335, 1970. doi: 10.1016/0014-4886(70)90062-2. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Developmental relationship between spinal motoneuron dendrite bundles and patterned activity in the forelimb of cats. Exp Neurol 30: 367–373, 1971. doi: 10.1016/S0014-4886(71)80015-8. [DOI] [PubMed] [Google Scholar]
- Seven YB, Mantilla CB, Sieck GC. Recruitment of rat diaphragm motor units across motor behaviors with different levels of diaphragm activation. J Appl Physiol (1985) 117: 1308–1316, 2014. doi: 10.1152/japplphysiol.01395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Non-stationarity and power spectral shifts in EMG activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol 185: 400–409, 2013. doi: 10.1016/j.resp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh KA, Deerinck TJ, Ellisman MH, Griffin JW. The distribution of ganglioside-like moieties in peripheral nerves. Brain 122: 449–460, 1999. doi: 10.1093/brain/122.3.449. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Moritani M, Oh SJ, Park KP, Paik SK, Bae JY, Kim HN, Ma SK, Park CW, Yoshida A, Ottersen OP, Bae YC. The distribution of inhibitory and excitatory synapses on single, reconstructed jaw-opening motoneurons in the cat. Neuroscience 133: 507–518, 2005. doi: 10.1016/j.neuroscience.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Sieck GC. Diaphragm muscle: structural and functional organization. Clin Chest Med 9: 195–210, 1988a. [PubMed] [Google Scholar]
- Sieck GC. Recruitment and frequency coding of diaphragm motor units during ventilatory and non-ventilatory behaviors. In: Respiratory Control, edited by Swanson GD, Grodins FS, Hughson RL. New York: Plenum, 1989, p. 441–450. [Google Scholar]
- Sieck GC. Neural control of the inspiratory pump. Physiology 6: 260–264, 1991. doi: 10.1152/physiologyonline.1991.6.6.260. [DOI] [Google Scholar]
- Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med 15: 641–659, 1994. [PubMed] [Google Scholar]
- Sieck GC. Organization and recruitment of diaphragm motor units. In: The Thorax, edited by Roussos C. New York: Dekker, 1995, p. 783–820. [Google Scholar]
- Sieck GC, Ferreira LF, Reid MB, Mantilla CB. Mechanical properties of respiratory muscles. Compr Physiol 3: 1553–1567, 2013. doi: 10.1002/cphy.c130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol (1985) 66: 2539–2545, 1989. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M, Zhan WZ. Estimated recruitment of diaphragm motor units (Abstract). FASEB J 4: A951, 1990. [Google Scholar]
- Sieck GC, Gransee HM. Respiratory Muscles: Structure, Function and Regulation. Colloquium Series on Integrated Systems Physiology: From Molecule to Function to Disease, edited by Granger DN, Granger J. San Rafael, CA: Morgan & Claypool Life Sciences, 2012,. vol. 34. doi: 10.4199/C00057ED1V01Y2012ISP034. [DOI] [Google Scholar]
- Sieck GC, Mantilla CB, Prakash YS. Volume measurements in confocal microscopy. Methods Enzymol 307: 296–315, 1999. doi: 10.1016/S0076-6879(99)07019-6. [DOI] [PubMed] [Google Scholar]
- Stuart DG, Enoka RM. Motoneurons, motor units, and the size principle. In: The Clinical Neurosciences, edited by Rosenberg RN. New York: Churchill Livingstone, 1983, p. 471–517. [Google Scholar]
- Tai Q, Goshgarian HG. Ultrastructural quantitative analysis of glutamatergic and GABAergic synaptic terminals in the phrenic nucleus after spinal cord injury. J Comp Neurol 372: 343–355, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Tian GF, Duffin J. Connections from upper cervical inspiratory neurons to phrenic and intercostal motoneurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 110: 196–204, 1996a. doi: 10.1007/BF00228551. [DOI] [PubMed] [Google Scholar]
- Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 111: 178–186, 1996b. doi: 10.1007/BF00227296. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Mantilla CB, Zhan P, Bitton A, Prakash YS, de Troyer A, Sieck GC. Regional differences in serotonergic input to canine parasternal intercostal motoneurons. J Appl Physiol (1985) 88: 1581–1589, 2000. doi: 10.1152/jappl.2000.88.5.1581. [DOI] [PubMed] [Google Scholar]
- Zhang RG, Westbrook ML, Westbrook EM, Scott DL, Otwinowski Z, Maulik PR, Reed RA, Shipley GG. The 2.4 A crystal structure of cholera toxin B subunit pentamer: choleragenoid. J Mol Biol 251: 550–562, 1995. doi: 10.1006/jmbi.1995.0455. [DOI] [PubMed] [Google Scholar]