Abstract
Spinal cord stimulation (SCS) is used clinically to limit chronic pain, but fundamental questions remain on the identity of axonal populations recruited. We developed an ex vivo adult mouse spinal cord preparation to assess recruitment following delivery of clinically analogous stimuli determined by downscaling a finite element model of clinical SCS. Analogous electric field distributions were generated with 300-µm × 300-µm electrodes positioned 200 µm above the dorsal column (DC) with stimulation between 50 and 200 µA. We compared axonal recruitment using electrodes of comparable size and stimulus amplitudes when contacting the caudal thoracic DC and at 200 or 600 μm above. Antidromic responses recorded distally from the DC, the adjacent Lissauer tract (LT), and in dorsal roots (DRs) were found to be amplitude and site dependent. Responses in the DC included a unique component not seen in DRs, having the lowest SCS recruitment amplitude and fastest conduction velocity. At 200 μm above, mean cathodic SCS recruitment threshold for axons in DRs and LT were 2.6 and 4.4 times higher, respectively, than DC threshold. SCS recruited primary afferents in all (up to 8) caudal segments sampled. Whereas A and C fibers could be recruited at nearby segments, only A fiber recruitment and synaptically mediated dorsal root reflexes were observed in more distant (lumbar) segments. In sum, clinically analogous SCS led to multisegmental recruitment of several somatosensory-encoding axonal populations. Most striking is the possibility that the lowest threshold recruitment of a nonprimary afferent population in the DC are postsynaptic dorsal column tract cells (PSDCs) projecting to gracile nuclei.
NEW & NOTEWORTHY Spinal cord stimulation (SCS) is used clinically to control pain. To identify axonal populations recruited, finite element modeling identified scaling parameters to deliver clinically analogous SCS in an ex vivo adult mouse spinal cord preparation. Results showed that SCS first recruited an axonal population in the dorsal column at a threshold severalfold lower than primary afferents. These putative postsynaptic dorsal column tract cells may represent a previously unconsidered population responsible for SCS-induced paresthesias necessary for analgesia.
Keywords: dorsal column, Lissauer tract, neuromodulation, pain, postsynaptic dorsal column, SCS
INTRODUCTION
Peripheral somatosensory afferents enter the spinal cord via dorsal roots, and many issue axon collaterals into the most prominent white matter tract, known as the dorsal column (DC). The DC contains axons of large-diameter Aαβ myelinated primary afferents that encode complex sensory information on touch and proprioception (Willis and Coggeshall 2004). Though these myelinated primary afferents are the predominant DC constituent, unmyelinated axons and ascending projections from spinobulbar postsynaptic dorsal column (PSDC) tract neurons also reside in the DC (Enevoldson and Gordon 1989; Giesler et al. 1984; Patterson et al. 1989, 1990; Willis and Coggeshall 2004). PDSC tract neurons are known to receive multiconvergent synaptic inputs from various primary afferents (Willis and Coggeshall 2004). Immediately lateral to the DC is the Lissauer tract (LT), which largely comprises primary afferent axon collaterals (predominantly C fibers) and is implicated in the regulation of spinal receptive field size and gating of pain transmission (Lidierth 2007; Wall et al. 1999).
Epidural spinal cord stimulation (SCS) can depress refractory pain signaling via electrodes positioned at the posterior (dorsal) epidural space above the DC. Approximately 50,000 stimulators are implanted yearly for patients diagnosed with various pain syndromes, with failed back surgery syndrome having among the highest success rates (Cameron 2004; Turner et al. 2004). SCS is thought to recruit DC Aβ fibers and inhibit pain via the gate control theory of pain. This theory postulates that antidromic Aβ fiber excitation inhibits nociceptive transmission through the action of inhibitory interneurons within the dorsal horn (Melzack and Wall 1965; Mendell 2014). It has been assumed that Aβ fiber recruitment from a single dermatome is the core mechanism of SCS-induced pain control even though SCS may also recruit other neuronal populations (e.g., PSDC tract cells) and despite its inability to explain many clinical observations (Jensen and Brownstone 2018; Meyerson and Linderoth 2006).
Most experimental inquiries into mechanisms underpinning the actions of SCS utilize in vivo studies with anesthetized rodents in well-characterized models of neuropathic pain (Guan et al. 2010; Song et al. 2009; Zhang et al. 2015). Several have used amplitudes scaled relative to motor threshold to assess modulation of nociceptive transmission in pain models (Crosby et al. 2015; Sato et al. 2014; Song et al. 2013a, 2013b; Stiller et al. 1996; Yuan et al. 2014). However, basic questions about the identity of axons recruited have not been convincingly addressed. In the present study, we used dimensional electrode scaling and model-based identification of SCS parameters to inform experimental assessments of axon fiber recruitment properties in DC, dorsal roots (DRs), and LT using “clinically analogous” SCS. LT’s proximity to DC, and subsequently the SCS electrode, potentially presents an alternate site of recruitment and modulation.
We examined variability in axonal recruitment to several preclinical SCS parameters including stimulation site, stimulation distance, amplitude, polarity, and pulse duration. Results from these studies answer fundamental questions regarding the recruitment of different fiber populations and segmental circuits.
Parts of this work have been presented previously in abstract form (Idlett et al. 2016, 2017).
MATERIALS AND METHODS
Preclinical electrode scaling.
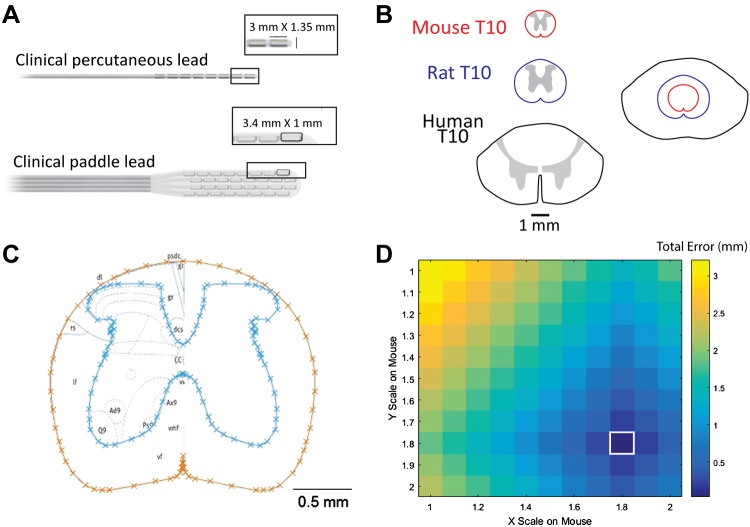
To conduct preclinical experiments in the mouse with clinically analogous SCS, the electrode dimensions were scaled with reference to clinical contact sizes and the mediolateral and dorsoventral dimensions of the adult mouse spinal cord (Fig. 1, A and B). For both rat and mouse, T10 spinal cross section profiles were generated by tracing the gray matter and white matter boundaries over one hemisphere of the T10 spinal segment, according to the cross section from the anatomical atlas (Watson et al. 2009), reflecting the traced profile of the hemisphere over the midline of the segment, and concatenating the original and reflected tracings to form a symmetric cross-section (Fig. 1C). The magnitude of the differences in the lengths of the major and minor axes between an unscaled rat T10 spinal segment and scaled mouse T10 spinal segment was plotted as a function of the mediolateral and dorsoventral scaling factors applied to the mouse spinal segment (Fig. 1D). Major and minor axis lengths for both the rat and mouse spinal cord were determined respectively from the maximum mediolateral and dorsoventral extents of the white matter tracings. The scaling factor generating the minimum error in cross-sectional area between the mouse and rat spinal segment (1.8) was then related to the human-to-rat scaling factor of 1:2.5 previously determined; with the overall scaling between human and mouse spinal cord being 1:4.5. An atlas of the rat and mouse spinal cord (Watson et al. 2009) provides high-resolution images at all spinal levels from rat and mouse as well as a specific scaling factor between human and rat thoracic cord, from which we derived the human-to-rat translation factor and then determined that the rat and mouse thoracic cord are qualitatively anatomically similar. However, no direct scaling data from human to mouse was included in the reference, hence the intermediate step. Based on these scaling factors, verbatim scaled contacts will have lengths of 0.67 mm and widths of 0.30 mm. Because DCs are organized by dermatome in the medial-lateral direction (Feirabend et al. 2002; Niu et al. 2013; Wesselink et al. 1998), and because approaches to improve the spatial selectivity of SCS have focused on constraining the medial-lateral dimension of the activation region (Holsheimer et al. 1998; Sankarasubramanian et al. 2011), subsequent model simulations were conducted using square contacts with side lengths corresponding to the smaller medial-lateral dimension of the scaled-down clinical electrodes (0.3 mm × 0.3 mm). Constraining electrode dimensions seemed advantageous for experimental studies of recruitment because this allowed for greater specificity in target of SCS, aiding in analysis and understanding of experimental outcomes.
Fig. 1.
Preclinical electrode scaling. A: examples of a 1 × 8 clinical cylindrical percutaneous lead and a 4 × 8 clinical paddle lead provided by Boston Scientific. The contact dimensions are 3 mm × 1.35 mm and 3.18 mm × 1.4 mm, respectively. The appropriate size of spinal cord stimulation (SCS) electrodes for subsequent computational modeling and preclinical experimentation was determined by scaling the dimensions of these contacts with respect to the cross-sectional area of the mouse spinal cord. B: comparison of relative sizes of human, rat, and mouse T10 spinal cord. C: example mouse cross section generated from an anatomic atlas of the spinal cord. Cyan and orange lines depict gray and white matter boundaries, respectively. D: rat and mouse error comparisons for major and minor axes: magnitude of the differences in the lengths of the major and minor axes between an unscaled rat T10 spinal cord and scaled mouse T10 spinal cord, plotted as a function of the mediolateral (major) and dorsoventral (minor) scaling factors applied to the mouse spinal cord. Colors denote magnitudes of the total difference in lengths of the major and minor axes measured in mm. This analysis supports a rat-to-mouse scaling factor of 1.8 (white box). When related to the previously determined human-to-rat scaling factor, the human-to-mouse scaling factor is 4.5.
FEM model: mouse spinal cord.
A three-dimensional finite element model (FEM) of both the in vivo mouse spinal cord and the ex vivo mouse spinal cord recording setup was created in COMSOL Multiphysics (version 5.1; COMSOL, Burlington, MA) to compare the electric fields (and thereby currents) generated by the scaled-down electrodes in the experimental preparation with those expected to be generated by the same electrode in an in vivo mouse and by a commercial epidural lead in a human. For the in vivo FEM, the dimensions of the cerebrospinal fluid (CSF) space, dura, epidural fat, and vertebral bony layers were generated by downscaling the x and y dimensions of the corresponding anatomical features from a previously published FEM of the human thoracic spinal cord (Lee et al. 2011) by factors of 4 and 4.5, respectively, derived from the previously described scaling procedure. Dirichlet boundary conditions (V = 0) were used at the lateral and dorsoventral boundaries of the model, and Neumann boundary conditions (n·J = 0) were used at the rostrocaudal boundaries of the model. For the ex vivo FEM, the model was scaled according to the actual experimental setup and consisted of an insulated rectangular chamber measuring 76 mm in length, 45 mm in width, and 8 mm in depth filled with artificial CSF (aCSF) and a 1-mm-diameter ground wire placed 10 mm from the center of the stimulation lead. In the ex vivo model, the faces of the ground wire were set to Dirichlet boundary conditions (V = 0), and the other outer boundaries of the model were set to Neumann boundary conditions (n·J = 0).
An extruded mouse spinal cord 35 mm in length and a model of the experimental stimulation lead were preserved across both the in vivo and ex vivo models. The dimensions of the gray and white matter of the mouse spinal cord were obtained by directly tracing the left half of a slice image of the mouse T10 spinal cord (Watson et al. 2009) and reflecting the tracing over the midline using MATLAB (version R2015a; The MathWorks, Natick, MA) before being imported into COMSOL. Furthermore, the gray matter was displaced 20 µm ventrally from the original tracing to allow the spacing between the dorsal gray matter and white matter boundary to fulfill the minimum mesh element size requirement of the finest mesh setting in COMSOL (~15.2 µm). Stimulating leads were modeled as conductive thin cubic domains with a side length of 0.3 mm surrounded by a rectangular insulating substrate with outer dimensions of 2.4 mm × 0.6 mm × 0.5 mm and suspended from an insulating cylindrical electrode mount 0.4 mm in diameter and centered on the rectangular substrate. The ventral surfaces of each contact on each lead were each set to a uniform current density with Neumann boundary conditions corresponding to the total stimulation amplitude (50, 200 µA) divided by the surface area of each lead (Pelot et al. 2018). Stimulation currents from each of the two contacts were set to the same amplitude at opposite polarities to depict the electric field generated by bipolar stimulation in the spinal cord proximal to the leads. Domain conductivities representing tissues and materials of interest are shown in Table 1, and domain permittivities were set equal to the permittivity of free space, consistent with the quasi-static assumption (Bossetti et al. 2007). Results for each model were then obtained by solving for Laplace’s equation (∇·σ∇V = 0) over an adaptively scaled tetrahedral mesh.
Table 1.
Tissue conductivities and isotropy used in finite element models of the mouse spinal cord
| Tissue | Isotropy and Conductivity, S/m |
|---|---|
| Gray matter | σ = 0.23 |
| White matter | σx = 0.083, σy = 0.083, σz = 0.60 |
| CSF | σ = 1.70 |
| Dura | σ = 0.6 |
| Epidural fat | σ = 0.04 |
| Conductor | σ = 5 × 106 |
| Insulator | σ = 1 × 10−7 |
| Bone | σ = 0.02 |
Values are σ if isotropic and σx, σy, σz if anisotropic; values were based on and updated from Lee et al. (2011).
Constant-current stimulators provide compliance voltages that automatically adjust to the tissue impedance. Boundary conditions of the model were set with this in mind. Because significant clinical variability exists in patient perception thresholds, the theoretical outcomes of the model are representative of electric field strengths expected. The amplitude of current delivered to the mouse model was scaled using the intended experimental geometry for the voltages and electric fields to conform to the clinical ranges without severe discrepancies. One clear limitation of the mouse model is that it represents a scaling from this normalized, exemplary range.
Spinal cord isolation.
All procedures were approved by the Emory University Institutional Animal Care and Use Committee: C57/Bl6 mice (both sexes, n = 35, postnatal day 60 and older) were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) mix by intraperitoneal injection following light anesthetization in an isoflurane chamber. To induce tissue hypothermia, the dorsal skin overlying the vertebral column was removed and mice were placed in an ice bath until there was a noticeable slowing of the respiration rate (2–3 min) before decapitation and isolation of spinal cord in a dish containing ice-cold, oxygenated (95% O2-5% CO2), low-Ca2+ aCSF containing (in mM) 128 NaCl, 1.9 KCl, 13.3 MgSO4, 1.1 CaCl2, 1.2 KH2PO4, 10 glucose, and 26 NaHCO3. The isolated cord was equilibrated to room temperature for 1 h and then pinned dorsal side up in a Sylgard-lined recording chamber while superfused with an oxygenated aCSF containing (in mM) 128 NaCl, 1.9 KCl, 1.3 MgSO4, 2.4 CaCl2, 1.2 KH2PO4, 10 glucose, and 26 NaHCO3 at ~40 ml/min. All experiments were undertaken at room temperature.
Histological test of spinal cord viability.
In four experiments, 8 h following spinal cord isolation and experimentation, the spinal cord was fixed in 2% paraformaldehyde for 2 h, rinsed with phosphate buffer solution (PBS), sectioned with a vibrating blade microtome (Leica VT1000S) in 300-µm-thick slices, stained 30–60 min in 1:250 Neurotrace (N-21482; Molecular Probes) mixed in PBS with 0.3% Triton, and then washed twice in PBS without Triton. Captured images of stained slices allowed assessment of neuronal anatomical integrity (Nikon Eclipse E800 microscope with DXM1200 camera).
Electrophysiology.
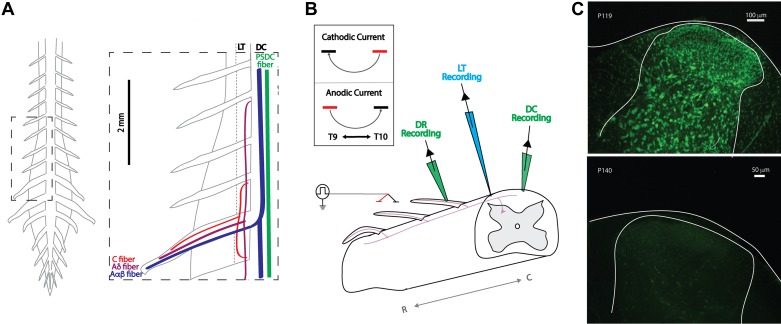
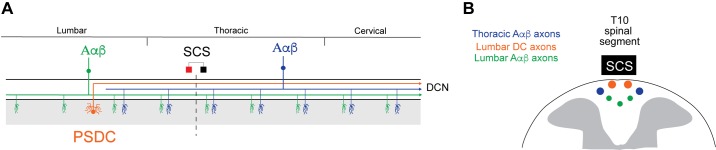
The DC largely comprises myelinated Aαβ primary afferents fibers that transmit proprioceptive and mechanosensitive information but also includes ascending projections from PSDC tract neurons and unmyelinated axons, whereas the neighboring LT mostly consists of C fibers with short-range projections and Aδ nociceptors with long-range projections (Lidierth 2007) (Fig. 2A).
Fig. 2.
Ex vivo adult mouse preparation: experimental setup and viability. A: schematic of gross anatomy of spinal cord from dorsal surface and simplified organization of axon fiber populations subject to recruitment by spinal cord stimulation (SCS; inset). Axonal recruitment with SCS was assessed in dorsal roots (DR), dorsal column (DC), and Lissauer tract (LT). Whereas the DC predominately carries nonnociceptive sensory information, the neighboring LT contains projection from nociception transmitting primary afferents. PSDC, postsynaptic dorsal column. B: schematic of general experimental paradigm. Two electrodes (~1.5 mm apart) are placed on or above the medial DC in a region between T9 and L3. This is used to simulate a bipolar SCS system. Responses to SCS are recorded via glass suction electrodes on the DC and DRs and by a microelectrode on LT. Inset: orientation of bipole (cathode, black; anode, red) and direction of current flow for cathodic and anodic stimulation used for experimental studies. R and C denote rostral and caudal, respectively. C: postexperimentation Nissl staining revealed persistent cellular viability 8 h after cord isolation (top) but dramatic deterioration when superfused for 2 h in the absence of oxygenation (bottom).
Experimental characterization of axonal recruitment was conducted with clinically analogous SCS by using a bipolar electrode constructed of two glass electrodes (300-μm tip diameters, separation 1.5–2 mm) filled with aCSF and positioned (rostrocaudally) medial over the DC, typically in caudal thoracic segments (T9–T11; Fig. 2B). Circular glass electrodes were used for experimental studies due to the repeatability of the construction process relative to manual cutting of 300 μm × 300 μm electrodes. A constant-current stimulator (Hammar et al. 2011) was used to deliver a single monophasic pulse of bipolar SCS while contacting (0 μm) or above the spinal cord DC (200 or 600 μm). Stimulation amplitudes were incrementally increased from 1 to 500 μA with 50-, 200-, and 500-μs pulse durations for assessments of recruitment threshold. The advantage of a constant-current stimulation protocol, based on modeling studies, is that resistivity values normally seen for dura and arachnoid do not significantly affect spinal cord current densities (Coburn and Sin 1985). Thus our experimental arrangement excludes the presence of dura but still is expected to realistically appraise neural system recruitment at defined electrode configurations and distances from the spinal cord at the stimulus intensities applied.
Glass recording suction electrodes were positioned in the lumbar spinal cord on the DC (tip diameter 200 μm), DR (tip diameter 200–250 μm), and LT (tip diameter 1–2 μm, 3–5 MΩ). Targeted recording of LT was accomplished by positioning a glass microelectrode lateral to the DC, in between dorsal root entry zones. Appropriate positioning was confirmed by stimulating the ipsilateral spinal cord one to three spinal segments rostral to the recording site and observing that the conduction velocity of evoked response was slower than that recorded in DR and DC of the same segment. Recruitment threshold was defined as the lowest stimulus amplitude producing a >50% response rate in any recording location. Since threshold was typically lowest for DC axonal recruitment (see results), relative thresholds for recruitment were standardized as the multiple of DC recruitment threshold (TDC) necessary to elicit responses in LT and DR. All recorded data were digitized at 50 kHz (Digidata 1322A 16-bit data acquisition system; Molecular Devices) with pClamp acquisition software (v. 10.7; Molecular Devices). Recorded signals were amplified (5,000 times) and low-pass filtered at 3 kHz using in-house amplifiers. Unless otherwise stated, reported results are for cathodic stimulation 200 μm above the cord with 200-μs pulse duration. Recruitment order trends for cathodic and anodic stimulation were similar, despite differences in polarity. Cathodic stimulation, in some cases, recruited populations at a similar or lower threshold, and SCS experimental studies utilizing monophasic stimulation frequently report observations based on cathodic stimulation (Song et al. 2009; Stiller et al. 1996; Yakhnitsa et al. 1999; Yuan et al. 2014). This is likely due to knowledge that cathodic stimulation recruits neural tissue at lower amplitudes than anodic stimulation (McIntyre and Grill 1999; Ranck 1975; Rattay 1998). For these reasons, we chose to focus our reporting on cathodic stimulation. In all data presented, the number of animals utilized for analysis is represented by the noted n value. For each animal, a representative value was determined by averaging the response from multiple sweeps/trials within that animal (a minimum of 5).
Electrode configurations for estimating conduction velocity.
Central conduction velocity (CV) was calculated on the basis of distance between the site of SCS and the recording electrode. Specifically, central CV of recruited axons was measured between the cathodic electrode used for stimulation and distal recording electrodes placed either near the midline of the dorsal column or more laterally at the dorsal root entry zone. To estimate peripheral CV, we positioned two suction electrodes on individual DRs at least 3 mm apart (L3, L4, or L5) to calculate differences in time of arrival of the antidromic afferent volley (Waddell et al. 1989). CVs of different volley components were calculated post hoc to identify the afferent classifications (i.e., Aαβ, Aδ, C) using published findings of primary afferent CV in dorsal roots at room temperature (Pinto et al. 2008b; Waddell et al. 1989). The fastest conducting components observed likely include antidromic responses of proprioceptive (groups I and II; denoted as Aα) and cutaneous afferents (Aβ). For uniformity with previous publications, we label the fastest conducting myelinated components as Aαβ fibers responses.
Statistical differences in recruitment threshold, with respect to SCS stimulation distance (0, 200, and 600 μm), were analyzed using ordinary one-way ANOVA with Tukey’s correction for multiple comparisons. Differences between the relative recruitment thresholds of DC, DR, and LT were analyzed using the Friedman test with Dunn’s correction for multiple comparisons. Analysis of CV differences was performed using a paired t test. Unless otherwise stated, the results are means ± SD with statistical significance being ascribed at P < 0.05.
RESULTS
Anatomical evidence of viability.
Nissl positive neuronal staining was observed in tissue fixed 8 h after experimentation. As a control, a spinal cord isolation was conducted using the same procedures, except the cord was superfused without oxygen for 2 h, which led to a near complete absence of Nissl stain (Fig. 2C). Demonstrated viability and increased access to the spinal neuraxis without anesthetics supported the use of this ex vivo model for electrophysiological investigations on SCS.
Preclinical finite element analysis model.
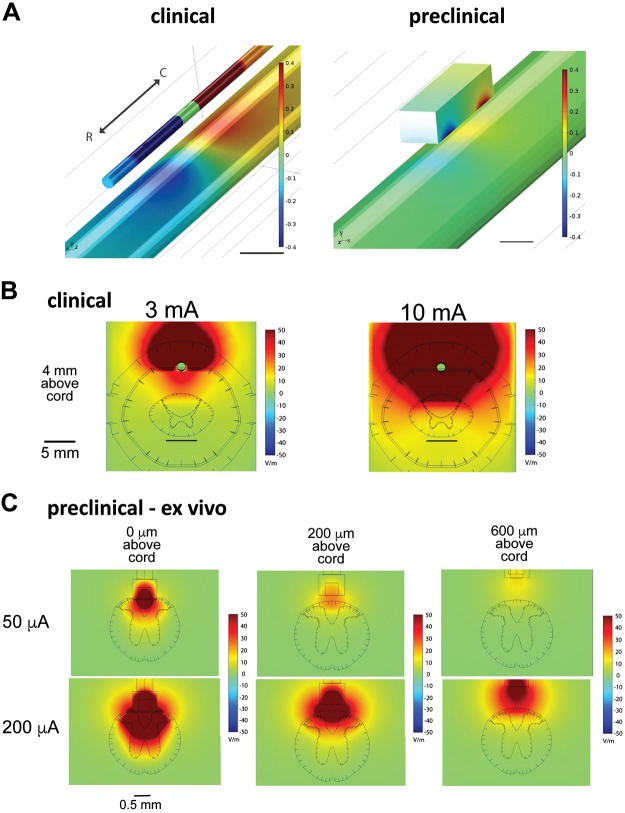
Preclinical stimulation was simulated using a bipolar electrode consisting of two square contacts, dimensions of which are noted in materials and methods, with an edge-to-edge spacing of 1.2 mm and positioned epidurally over the dorsal surface at the midline of the spinal cord, for both the ex vivo and in vivo models described in materials and methods. The clinical standard for SCS electrode placement involving SCS at conventional frequencies associated with paresthesia-based SCS involves implantation of the electrode at the T7–T8 vertebral segments, with the intent of targeting the dorsal column fibers coursing through the T9–T10 spinal cord level. Using a mouse model of the T9–T10 spinal cord is consistent with this therapeutic approach and with prior literature (Canbay et al. 2014). The electric field magnitudes generated during ex vivo preclinical SCS (50 to 200 µA), with a CSF fluid layer of 0, 200, or 600 µm, were compared with those generated in the clinical model with a typical range of SCS amplitudes (3 and 10 mA). Results from analysis were used to guide experimental investigations in the ex vivo adult mouse spinal cord. Qualitatively, preclinical SCS positioned 200 μm (50–200 μA) above the spinal cord in the ex vivo model generated electric field distributions comparable to those generated by clinical SCS (Fig. 3). Preclinical SCS 0 μm above the spinal cord with the same amplitudes (50–200 μA) suggests broader recruitment of populations in the DC and dorsal horn than would be observed in the clinical SCS, indicating that lower stimulation amplitudes than those modeled would be advised for analogous recruitment, whereas stimulation 600 μm above suggests that higher amplitudes of stimulation would be necessary to capture the range of electric field distributions observed in the clinical case. Comparisons between ex vivo and in vivo preclinical cases suggest that although 200 µm is suitable for the ex vivo preclinical SCS, 600 µm in vivo most closely mimics the relative CSF thickness in human and that the bone, fat, and dura contribute to the generation of comparable electric field distributions with stimulation this distance above the cord (Supplemental Fig. S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.8323535).
Fig. 3.
Finite element modeling of electric field magnitudes identifies stimulation amplitudes for clinically analogous spinal cord stimulation (SCS) in the adult mouse. A: rostrocaudal orientation of bipole for clinical (left) and preclinical (right) SCS. The anode (red) is rostral over the spinal cord (T9), and the cathode (blue) is caudal over the spinal cord (T10). Scales are capped at ±0.4 V; scale bars are 5 (left) and 0.5 mm (right). B: clinical SDS. Electric field magnitudes in the human spinal cord during stimulation were generated using a finite element model of clinical bipolar SCS with a clinical 1 × 8 percutaneous lead with amplitudes in the typical range. C: preclinical ex vivo SCS. Electric field magnitudes in the spinal cord during SCS were generated using a finite element model of the ex vivo mouse spinal cord (absence of dura, epidural fat, and bone) in a rectangular bath of cerebrospinal fluid, equivalent to experimental conditions. Properties, mesh settings, and boundary conditions of the finite element model are consistent with those of the clinical model except that all dimensions have been scaled down by a factor of 4.5. Stimulation was simulated using a bipolar electrode consisting of 2 square contacts of side length 0.3 mm with an edge-to-edge spacing of 1.2 mm and positioned epidurally 0, 200, or 600 μm over the dorsal surface of the midline of the spinal cord. The fields generated at 200 μm above the spinal cord are most comparable to those generated in clinical conditions.
SCS recruits afferents that invade multiple segmental dorsal roots and antidromically conduct at velocities consistent with all classes of primary afferents.
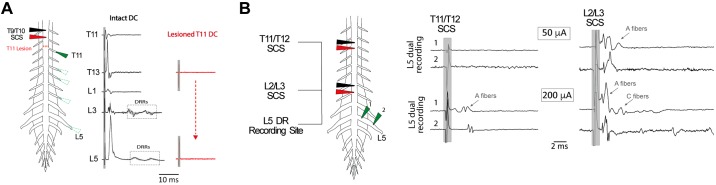
Multisegmental recruitment was directly assessed with bipolar SCS (50 and 200 µA, 200 µs) positioned 0 (n = 5) or 200 µm (n = 3) above the DC. Afferent recruitment was seen in all caudal DRs sampled, the most caudal being eight spinal segments away (Fig. 4). In the example shown, T9/T10 SCS recruitment was abolished following DC lesion above T11, demonstrating that stimulation recruited axons at the stimulation site with subsequent antidromic propagation to DRs. CVs were divided into three categories approximating reported peripheral CVs of Aαβ (>4.2 m/s), Aδ (0.56–4.2 m/s), and C fibers (<0.56 m/s) at room temperature (Pinto et al. 2008b).
Fig. 4.
Spinal cord stimulation (SCS) recruits primary afferents that invade multiple segmental dorsal roots and conduct at velocities consistent with all classes of sensory fibers. A: SCS directly activates afferent axons that project to multiple caudal spinal segments. A single pulse of SCS (200 μA, 200 μs) was applied 200 μm above T9/T10, and antidromic responses in select caudal dorsal roots (DRs; T11–L5) were recorded before (black) and after (red) dorsal column (DC) lesioning above T11 with iridectomy scissors. Lesion of the DC blocked all stimulus-evoked responses in DRs. Each trace is the average response of 10 sweeps. B: distance-dependent recruitment of afferent fiber populations can be identified by differences in peripheral CV. In the experiment shown, bipolar SCS was applied 200 μm above T11/T12 or L2/L3 at amplitudes of 50 and 200 µA (500 μs). T11/T12 SCS led to the recruitment of A fibers in the L5 DR, but only at the higher stimulus amplitude (arrow). In comparison, SCS at a closer segment (L2/L3) recruited A fibers at the lower intensity with higher magnitude stimulation leading to recruitment of slower conducting C fibers (arrows). Each trace is the average of 6 sweeps. The peripheral conduction velocities estimated using this configuration are summarized in Table 2. Shaded box identifies the location of stimulation artifact.
To accurately identify afferent classes on the basis of peripheral CV, we used the two-point recording method in DRs (Fig. 4B; Table 2). We observed a rostrocaudal distance dependence of electrode position on afferent recruitment. By reducing stimulus amplitude (50 μA), we also observed that low-amplitude SCS only recruited A fibers in DRs from nearby spinal segments (0–3 segments away; n = 8) and that higher amplitude SCS (200 μA) was required to recruit A fibers in more distant DRs (n = 7; Fig. 4B; Table 2). Higher amplitude and longer pulse duration SCS also recruits slower conducting Aδ and C fibers in DRs of nearby segments (Fig. 4B; Table 2). Although recruitment of C fibers was not observed in DRs more distal to SCS, we cannot exclude the possibility that multisegmentally projecting unmyelinated C fibers (Mandadi et al. 2013) were undetectable due to dispersion of the volley.
Table 2.
Distance-dependent recruitment of different classes of primary afferents with SCS
| SCS 7–9 Segments Rostral (n = 7) | |||||
|---|---|---|---|---|---|
| Amplitude, μA | 50 | 200 | |||
| Duration, μs | 200 | 500 | 50 | 200 | 500 |
| Aαβ | 9.6 ± 3.7 | 12.9 ± 4.8 | |||
| Aδ | 3.3 ± 0.3 | 3.4 ± 0.9 | |||
| C | |||||
| SCS 0–3 Segments Rostral (n = 8) | |||||
|---|---|---|---|---|---|
| Amplitude, μA | 50 | 200 | |||
| Duration, μs | 200 | 500 | 50 | 200 | 500 |
| Aαβ | 12.7 ± 8.0 | 11.7 ± 4.5 | 12.2 ± 8.3 | 15.3 ± 8.0 | 15.7 ± 7.7 |
| Aδ | 2.3 ± 1.2 | 2.4 ± 0.9 | |||
| C | 0.4 ± 0.03 | ||||
Peripheral conduction velocity (CV) of dorsal root afferents determined using the two-point method on lumbar roots, with SCS positioned 200 μm above the dorsal column. Values are means ± SD for CV of the fastest components in each class of afferents when SCS was applied closer to (0–3 segments rostral; n = 8 animals) or farther from (7–9 segments rostral; n = 7) the DR recording site (for schematic, see Fig. 4B). Afferent classification was determined as described previously (Pinto et al. 2008b). In cases where recruitment was observed in less than half of experiments, no value (null) is listed to best depict the general recruitment result.
SCS evokes synaptically mediated recruitment of primary afferents at thresholds comparable to direct antidromic activation.
Dorsal root potentials (DRPs) and dorsal root reflexes (DRRs), resulting from primary afferent depolarization (PAD), were observed with SCS. These longer latency, synaptically mediated events had variable onset latency and amplitude (observed in 13 of 23 animals). Recordings at the dorsal root entry zone (DREZ) permitted observation of both events, with arrival of suprathreshold DRRs coinciding with the presence of the DRP (Fig. 5, A and Bi). Recordings away from the DREZ led to observations of only DRRs. The jitter and sensitivity of this activity to bicuculline (10 μM; n = 4) confirmed it as synaptically mediated (Fig. 5B).
Fig. 5.
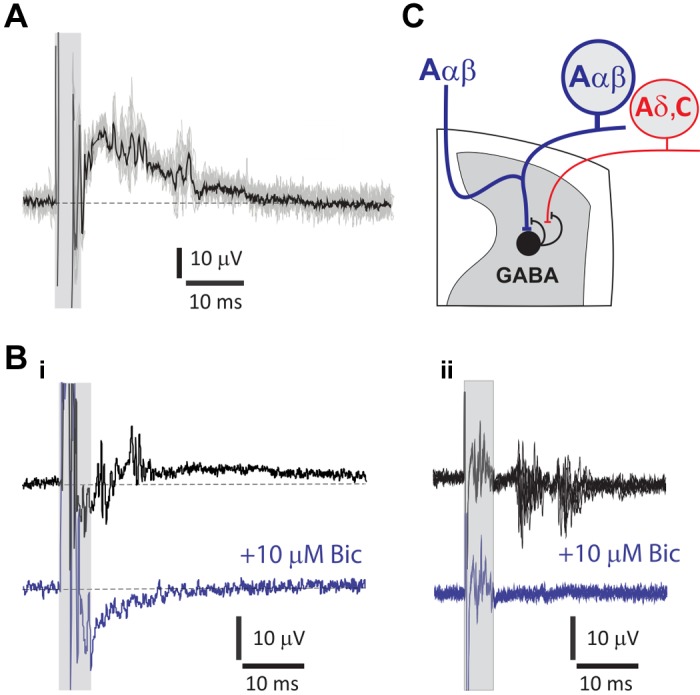
Spinal cord stimulation (SCS) recruited GABAergic presynaptic inhibitory pathways observed as bicuculline-sensitive dorsal root potentials (DRPs) and dorsal root reflexes (DRRs). A: SCS-generated DRP with suprathreshold DRRs recorded at the L5 dorsal root entry zone. By convention, negativity is presented upwards to convey the DRP as primary afferent depolarization. Shaded box indicates the location of stimulus artifact and truncated direct antidromic axonal volley preceding the slow DRP. The average response (black) is presented overlying 10 individual events (gray) to demonstrate variability in timing of individual DRRs. B: SCS evokes DRP and DRRs that are blocked following application of the GABAA receptor antagonist bicuculline (Bic; 10 μM). i: averages of 10 sweeps from the T13 dorsal root entry zone. Both DRP and DRRs are blocked after the addition of Bic (blue). ii: Bic-sensitive DRRs are shown in the absence of an underlying DRP. Shown are 6 events overlaid from an L2 DR recording obtained farther from the root entry zone. In both i and ii, shaded box indicates the location of stimulation artifact and direct primary afferent recruitment. C: schematic depicting possible circuitry responsible for GABAergic actions on afferent axons leading to a form of presynaptic inhibition called primary afferent depolarization (PAD), experimentally observed as DRPs and DRRs. SCS antidromically recruits Aαβ afferent axons responsible for recruiting the spinal circuit leading to PAD. Aαβ afferents synaptically recruit last-order GABAergic interneurons directly or via interposed interneurons (not shown). GABAergic axo-axonic synapses on primary afferents activate Bic-sensitive GABAA receptors that mediate Cl− efflux to produce PAD. Because these experiments cannot identify the afferent axons generating PAD, possible actions on all afferent classes are shown.
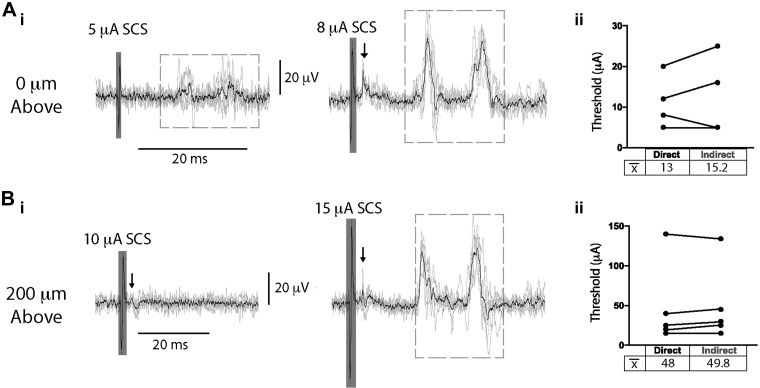
Two distinct populations of DRRs were commonly observed in lumbar DRs, the earliest event being 9.0 ± 1.6 ms after the onset of the direct antidromic response, with the second DRR event occurring 9.2 ± 1.2 ms later. Thresholds for direct antidromic axonal recruitment and synaptically mediated (indirect) DRRs were comparable, with mean threshold for DRR recruitment being numerically only 3.8 ± 2.2 μA higher than the antidromic event (n = 5; P > 0.05, paired t test; Fig. 6). This suggests that the indirect recruitment is activated by excitation of low-threshold primary afferents. The population(s) of axons receiving primary afferent depolarization was not identified, but the presence of two different populations of DRRs suggests that one population may be initiating PAD, whereas two separate populations could be receiving PAD (Fig. 5C), although it is also possible that the second DRR event reflects recurrent activity initiated by the first DRR event or doublet firing within the same recruited axons.
Fig. 6.
Spinal cord stimulation (SCS) evokes direct and indirect primary afferent recruitment at comparable thresholds. SCS was applied contacting (0 μm; A) or 200 µm above (B) T9/T10 during recording of a lumbar dorsal root (DR). Stimulus amplitude was incrementally increased (1–200 µA, 200 µs; i), and the recruitment threshold for direct primary afferent recruitment and synaptically mediated primary afferent activity (indirect) was identified post hoc (ii). Mean threshold (x̄) for indirect recruitment was not significantly higher than that observed for direct recruitment when SCS was applied contacting (0 μm; n = 4 animals, 15.2 ± 10.1 vs. 13.0 ± 6.9 μA, respectively) or 200 µm above (n = 5 animals; 49.8 ± 48.3 vs. 48.0 ± 52.3 μA, respectively; Aii and Bii, paired t test). Arrows indicate direct recruitment, dashed boxes denote indirect recruitment, and shaded boxes depict stimulation artifact. Black lines are the average of 6 traces.
Characterization of dorsal column recruitment with SCS.
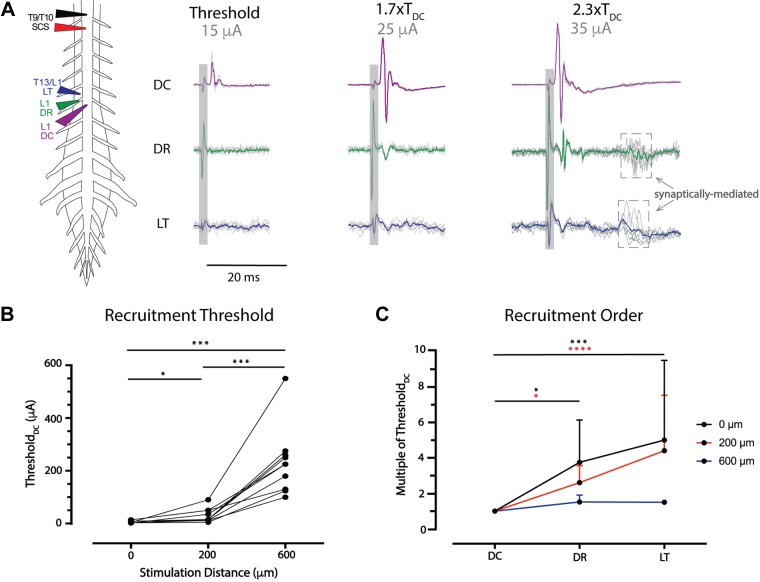
DC recruitment was also characterized in the adult mouse spinal cord as depicted in Fig. 7A, left. Recruitment of axons in DR, DC, and LT was compared when SCS was contacting (0 μm), 200 μm, or 600 µm above the spinal cord (n = 12). Direct recruitment of axons in LT was identified on the basis of a lack of variability in onset latency and amplitude (Fig. 7A, bottom right). Recruitment order and relative thresholds of recruitment was then determined by increasing the magnitude of constant-current SCS (200-μs pulse width; Fig. 7A). Interestingly, when SCS electrodes were contacting or above the DC, axonal recruitment was first seen in the DC regardless of stimulation polarity (n = 12). The mean threshold current for recruitment with SCS contacting the DC was lower for cathodic (6.3 ± 4.3 µA) than anodic (9.1 ± 3.7 µA) stimuli (P < 0.05, n = 12). When stimulation was positioned 200 or 600 µm above the spinal cord, recruitment thresholds were similar at 30.5 ± 26.6 (cathodic) vs. 26.3 ± 13.3 µA (anodic; P = 0.213, n = 12) or 231.9 ± 127.5 (cathodic) vs. 278.5 ± 106.0 µA (anodic; P = 0.125, n = 10), respectively. Compared with thresholds observed with SCS contacting the spinal cord DC, threshold values were ~3–5 times higher at 200 µm above (P < 0.05) and 31–37 times higher at 600 µm above the DC (P < 0.001; Fig. 7B). Thresholds for SCS positioned 600 µm above the DC were 8–11 times that seen with placement 200 µm above (P < 0.001).
Fig. 7.
Spinal cord stimulation (SCS) recruitment thresholds are lower for dorsal column (DC) axons. A: example configuration for investigations of recruitment threshold and order. To identify the recruitment threshold of dorsal axon tracts, bipolar SCS (T9/T10, 1–600 µA, 200 µs) was applied contacting or above the spinal cord, with simultaneous recordings of dorsal root (DR), Lissauer tract (LT), and DC at the same segmental level. In the example, DC (purple) is recruited first at threshold (TDC), with DR (green) recruitment observed at 1.7× TDC and overt LT (blue) recruitment observed at 2.3× TDC. Shaded box identifies the location of stimulation artifact; arrows point to dashed boxes denoting the presence of indirect recruitment/reflexes. The colored trace is an average of 6 sweeps. B: ThresholdDC was determined with stimulation 0, 200, or 600 µm above T9/T10 (n = 12 animals). TDC significantly increased at 200 µm above and 600 µm above the spinal cord. *P < 0.05; ***P < 0.001 (matched one-way ANOVA, Tukey’s multiple comparison). C: DC recruitment was observed at the lowest threshold and significantly lower than threshold for DR and LT with SCS at 0 (n = 12) or 200 µm (n = 12) above the spinal cord. With SCS 600 µm above, recruitment beyond DC was observed in only n = 2 of 10 cases. Values are mean and SD for the test conditions. *P < 0.05; ***P < 0.001; ****P < 0.0001 (Friedman test with Dunn’s multiple comparisons).
Relative thresholds for dorsal root and Lissauer tract recruitment.
We also compared the relative recruitment of antidromic events in DR and LT relative to the threshold seen in dorsal column (TDC) when SCS was contacting or positioned above the DC (n = 12; Fig. 7, A and C). When SCS was contacting the DC, events in DR and LT were recruited at 3.8 ± 2.4 (P < 0.05) and 5.0 ± 4.5 (P < 0.001) times TDC, respectively. At 200 µm above the DC, events in DR and LT were recruited at 2.6 ± 1.0 (P < 0.05) and 4.4 ± 3.1 (P < 0.0001) times TDC, respectively. In comparison, there were no differences in relative recruitment threshold between DR and LT when SCS was touching (P > 0.9) or 200 μm above the DC (P = 0.26). At 600 µm above, only DC recruitment was seen in most experiments (n = 7/10), even at maximal current amplitudes (>200 μA). However, when DR and LT recruitment was seen, both thresholds were similar to TDC, being 1.5 ± 0.4 (n = 5) and 1.5 ± 0.1 (n = 2) times TDC. No axonal recruitment was elicited in 3 of 10 experiments with placement 600 µm above the DC.
Comparison of the lowest threshold axons in dorsal column and dorsal roots.
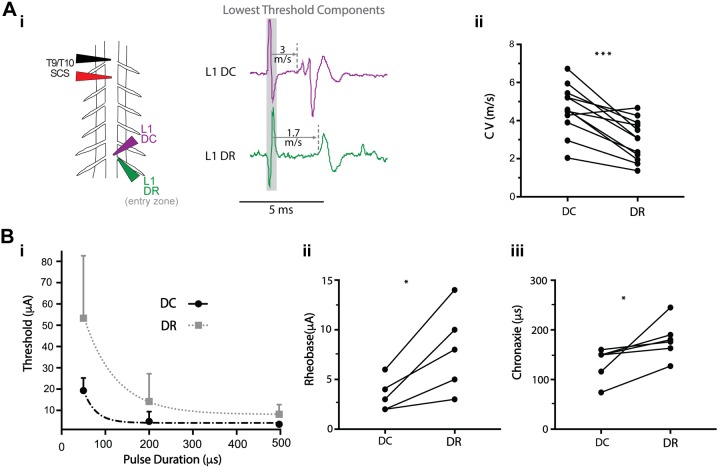
The one-point method was used to compare the central CVs of the first recruited components in DC and DR (at entry zone) at the same segmental level (Fig. 8A). On average, the CV of the first detectable recruited DC component was 43–69% faster that the first observed DR component. This observation was independent of stimulation duration and distance (P < 0.01). Results of this analysis are summarized in Table 3.
Fig. 8.
The lowest threshold recruited fibers for spinal cord stimulation (SCS) in dorsal column (DC) and dorsal roots (DRs) differ in conduction velocity (CV) and relative recruitment properties. A: the CV of the first recruited component in DC is greater than that of the DR (n = 12; i), as confirmed by paired t test (ii; ***P = 0.0001, SCS position 0 µm above). Table 3 presents the results from different stimulation conditions. B: the strength-duration relationship for the first recruited populations in DC and DR differed (n = 5 animals) with rheobase (ii) and chronaxie (iii) being lower in DC than in DR (Wilcoxon matched-pairs signed-rank test,*P < 0.05, SCS positioned 0 µm above). Strength-duration curves for DC and DR (i) are plotted by fitting a one-phase decay exponential model (root mean square error 4.6 and 16.4, respectively) to the mean and SD of the threshold values for 50-, 200-, and 500-μs pulses.
Table 3.
Central CV for SCS-evoked responses in DC and DR
| Mean CV, m/s |
|||||
|---|---|---|---|---|---|
| Stimulation Distance, μm |
Pulse Duration, μs | DC | DR | n | P Value |
| 0 (contacting DC) | 50 | 4.9 ± 1.7 | 2.9 ± 1.3 | 12 | <0.0001 |
| 200 | 4.6 ± 1.3 | 3.0 ± 1.1 | 12 | 0.0001 | |
| 200 | 200 | 4.3 ± 0.9 | 3.0 ± 0.8 | 12 | <0.0001 |
| 600 | 200 | 5.0 ± 1.2 | 3.1 ± 0.9 | 5 | 0.0089 |
Conduction velocity (CV) was measured with simultaneous recordings in the dorsal column (DC) and the dorsal root (DR) entry zone at sites caudal (L1 or L2) to the SCS electrode (T9/T10). Values are means ± SD (n = no. of animals) for CV of the lowest threshold fibers in the DC and DR at room temperature. Statistical analysis was performed using the one-tailed paired t test for samples where both DC and DR recruitment were observed.
The strength-duration relationship of the lowest threshold detectable axons in the DC and DR was assessed with SCS contacting the DC (Fig. 8B). Rheobase and chronaxie were determined by fitting a one-phase decay exponential to the cathodic threshold values in DC and DR with SCS pulse durations of 50, 200, and 500 µs (n = 5). Both rheobase and chronaxie values were significantly lower for DC axons. Mean values for rheobase were 3.5 ± 1.5 µA for DC and 8.0 ± 3.8 µA for DR (P < 0.05). Chronaxie values were 133.3 ± 32.8 and 180.2 ± 38.6 µs for DC and DR, respectively (P < 0.05).
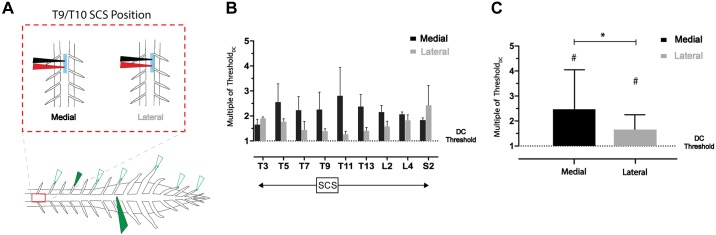
To assess possible differences in recruitment with medial and lateral electrode placement along much of the rostrocaudal axis, we recorded from nine DRs sampled between T3 and S2 (n = 4) while stimulating with our SCS electrode at T9–T10 and simultaneously recording from DC at L3 (Fig. 9A). Across DR recordings, recruitment thresholds (relative to DC threshold) were lower with lateral compared with medial placement (except at T3), with the largest shift occurring in thoracic roots closest to the site of stimulation (Fig. 9B). With all samples grouped across animals (medial/lateral, 36 samples each), there was a significant difference between DR relative recruitment threshold for medial and lateral placement (P < 0.05, Friedman test with Dunn’s multiple comparisons). Nonetheless, DC threshold was always lower than DR recruitment threshold regardless of SCS mediolateral placement (P < 0.0001; Fig. 9C).
Fig. 9.
The low dorsal column (DC) threshold observed is independent of mediolateral position of spinal cord stimulation (SCS). A: schematic of experimental paradigm. Nine DRs across T3–S2 spinal segments were recorded with a simultaneous recording of the L3 DC. Recruitment thresholds of both populations were assessed with medial and lateral SCS placement (T9/T10). B: thresholds of DRs rostral and caudal to the site SCS were greater, relative to the DC threshold (dotted line at y = 1; n = 4 animals). C: grouped samples from n = 4 animals (9 roots sampled each, 36 total samples for both medial and lateral placement) show that although lateral placement leads to a reduction in the relative DR threshold (*P < 0.05), both medial and lateral placement have higher thresholds than DC (dotted line at y = 1) for recruitment (#P < 0.0001, Friedman test with Dunn’s multiple comparisons). Bar charts display means and SD.
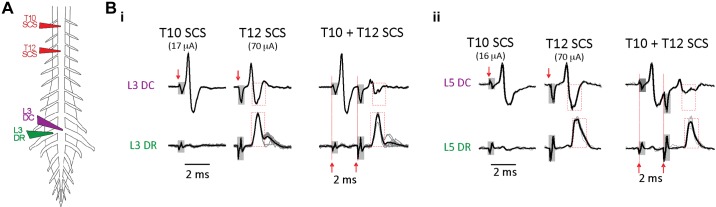
To further demonstrate that the DC contains an axonal population distinct from those in the DR, we undertook dual-site SCS collision experiments to selectively block conduction in the lowest threshold axons not projecting to DRs (n = 4). DC-contacting monopolar SCS electrodes were placed two thoracic segments apart (T10 and T12) for dual-site stimulation (Fig. 10). Caudally conducting axonal volleys were recorded and compared in the DC and DR at the same lumbar segment. Stimuli were adjusted so that T10 SCS only recruited an antidromic volley in the DC while higher intensity T12 SCS recruited volleys in both DC and DR. When T10 SCS preceded T12 SCS by a 2-ms interval, preferential conduction block in the DC volley was seen in all experiments (to 22 ± 8% of control amplitude), leaving the DR volley largely unaffected (volleys 93 ± 7% of control amplitude; P < 0.05). Figure 10B shows examples recorded at L3 and L5 spinal segments. Thus conduction block of the lowest threshold DC axons does not impair antidromic recruitment of dorsal root afferents.
Fig. 10.
Collision testing further supports lowest threshold axons in dorsal column (DC) as being distinct from dorsal root (DR) axons. Conduction block of the lower threshold DC axons at the same segment as recruited DR does not block antidromic recruitment of DR afferents. A: electrode configuration for comparison of recruited axonal volleys recorded in the DC and DR of the same lumbar segment following monopolar spinal cord stimulation (SCS) at T10 and T12 thoracic segments. B: shown are comparison axonal volleys recruited in the DC (top row) or DR (bottom row) of the same lumbar segment when T10 and T12 SCS stimuli were delivered individually or sequentially (T10 then T12) with a 2-ms interstimulus interval. Stimulus onsets are marked by red arrows, and stimulus artifacts are identified as shaded boxes. i: T10 SCS stimulation at lower intensity preferentially evoked an antidromic volley only in the L3 DC (left), whereas T12 SCS at higher intensity evoked recorded volleys in the L3 DR and L3 DC (middle). Amplitudes of the T12 SCS evoked volleys in DC and DR are shown bounded by a red dashed box for comparison with amplitudes observed after collision testing when T10 SCS preceded T12 SCS (right). There was preferential conduction block of axons in their refractory state in the DC volley, whereas the DR volley was largely unaffected (compare red boxed regions). ii: preferential conduction block of the DC volley was also seen at the L5 spinal segment.
Overall, these experiments show that T9/10 thoracic SCS recruited a distinct population of axons in lumbar DC with lower threshold and faster CV than antidromically identified primary afferent responses recorded in homologous segmental lumbar DR axons. Because SCS-recruited DC axons also had lower threshold than antidromically activated primary afferents that originated from DRs spanning the 19 spinal segments sampled (T3–S2), it is highly unlikely these axons are of primary afferent in origin.
DISCUSSION
We developed an isolated ex vivo adult mouse spinal cord preparation as an accessible platform to characterize the axonal populations recruited following delivery of SCS using electrodes scaled to generate clinically comparable electric fields. To our knowledge, these are the first studies employing quantitative methods to scale multiple SCS parameters for experimental studies on recruitment in smaller mammalian models.
With SCS electrodes placed above the caudal thoracic cord (T9/T10), primary experimental observations include the following: 1) Compared with afferent recruitment near the SCS electrode (0–3 segments away), higher stimulus amplitudes were required for antidromic recruitment of Aαβ afferents at more caudal dorsal roots (7–9 segments away). These higher amplitudes also led to antidromic recruitment of slower conducting fibers near the stimulation site (Table 2). 2) In lumbar cord dorsal roots, direct antidromic activation of Aαβ afferents also corresponded with recruitment of bicuculline-sensitive (GABAergic) presynaptically inhibitory actions on terminals of unidentified primary afferents (recorded as DRPs and DRRs). 3) The lowest threshold, fastest conducting axons recruited at lumbar segments were preferentially recruited in the DC and at thresholds significantly lower than seen for afferents in DRs or the LT. These axons retained the lowest recruitment threshold with SCS placed more laterally, compared with primary afferent recruitment that originated from distant DRs (spanning T3–S2 spinal segments). Based on known anatomy and process of exclusion, they are possibly PSDC ascending tract cells. The implications of these observations are elaborated below.
Factors affecting multisegmental recruitment of primary afferents: evidence beyond convention.
The capacity for SCS electrodes to antidromically recruit afferents across many spinal segments demonstrates that SCS is capable of broad axonal activation beyond the segment/dermatome of interest. Whereas, clinically, stimulation parameters are determined by identifying the regime that leads to a region with the greatest paresthesia perception (e.g., paresthesia dermatome focus) overlapping with the region of pain, the complete paresthesia perception can cover a broader dermatomal area (Lee et al. 2011; Moffitt et al. 2009). This suggests that the population of axons responsible for the paresthesia can be recruited simultaneously at multiple spinal segments, although one segment may have the greatest recruitment. We also show that the higher stimulus amplitudes and longer pulse durations required for antidromic recruitment of faster conducting myelinated fibers at more distant segments can also recruit putative pain-encoding unmyelinated C fibers closer to the stimulation site (Fig. 4). This observation predicts that SCS aimed at selective recruitment of myelinated fibers would benefit from use of lower magnitude/short pulse durations placed at spinal segments closer to the targeted site for modulation.
The strength-duration relationship predicts that increases in pulse duration and amplitude enhance axonal recruitment. Clinically, increased SCS duration has resulted in observations of the elicited paresthesia spreading to multiple dermatomes (Yearwood et al. 2010). Longer pulse durations resulted in marked increased recruitment of smaller diameter medial DC axons, which is consistent with sacral shifts in paresthesia focus (Lee et al. 2011). Also, higher stimulation amplitudes have been associated with increased cross-sectional area of DC recruitment (Holsheimer 2002). We observed differences in rheobase and chronaxie for DC compared with DR populations. Such measures are not normally estimated with extracellular stimulation because obtained values strongly depend on distance of axons from stimulation site. Nonetheless, SCS leads to threshold and CV differences between DC and DR populations, and differences quantified on the basis of a strength duration curve may be of instructive importance for subsequent consideration of stimulation strategies. For example, shorter duration, higher intensity pulses could increase separation of DC from DR, whereas longer duration pulses at lower stimulus intensity may more uniformly recruit both populations.
Computational studies also suggest that DR/primary afferent recruitment may be related to discomfort thresholds in patients with SCS (Holsheimer 2002; Howell et al. 2014), and this may be due to co-recruitment of Aδ fibers. Our observation that the increased stimulus strength required to recruit Aαβ fibers from distant caudal segments also recruits smaller diameter (Aδ, C) fibers from nearby segments (Fig. 4B) may underlie unwanted side effects at an off-target dermatome (Dimitrijevic et al. 1980).
Recruitment requiring increased stimulus amplitudes from more distant segments is consistent with earlier observations (BeMent and Ranck 1969; Horch et al. 1976). Because smaller diameter afferent axons have higher thresholds for activation by extracellular stimuli (Hursh 1939; McNeal 1976), these observations have been interpreted as a distance-dependent reduction in diameter of ascending axons (Hildebrand and Skoglund 1971; Hwang et al. 1975). Recruitment differences may also reflect somatotopic differences in axon diameter of functionally distinct Aαβ fiber populations (Niu et al. 2013).
Our experimental results with clinically analogous SCS suggest that a non-primary afferent DC axon population is recruited first (detailed in Are axons of PSDC neurons the lowest threshold axons recruited with SCS?). SCS-evoked responses in DR (Aαβ) and LT (presumably Aδ) are then subsequently recruited at severalfold higher intensity (Fig. 7). Although recruitment beyond DC was not always observed with SCS 600 μm above the cord, when broader recruitment was observed, DR and LT were recruited at comparable relative thresholds. Aβ and Aδ recruitment thresholds were also reported to be similar following DC stimulation in rat (Baba et al. 1994). Although Aδ fibers are not thought to project in the DC (Abraira and Ginty 2013; Bai et al. 2015; however, for monkey and cat, see Horch et al. 1976; Light and Perl 1979), that LT is immediately adjacent and contains Aδ fibers that project many segments (Lidierth 2007) may support their similar recruitment threshold by SCS.
Observed negligible recruitment thresholds differences in DR (Aαβ) and LT (presumably Aδ) is a significant observation since the primary conceptual basis of SCS modulation of pain is via selective recruitment of Aβ fibers that act via inhibitory mechanisms associated with the gate control theory of pain (Mendell 2014). We are unaware of experimental literature that demonstrates SCS selectively recruits Aβ afferent fibers.
Aαβ primary afferent recruitment coincides with recruitment of spinal circuits mediating presynaptic inhibition of primary afferents.
We related clinically analogous SCS to segmental distribution of recruited afferents fiber classes. Although we observed broad rostrocaudal antidromic recruitment of primary afferents originating from many spinal segments, we focused on assessment of axonal recruitment that would correspond to an equivalent expected distribution in humans with failed back surgery syndrome (Moffitt et al. 2009). In mouse this roughly corresponds to L1–L3 segments (McHanwell and Biscoe 1981). We show that T10 SCS at the lower modeled intensity of 50 μA recruited the lowest threshold primary afferents as well as spinal circuits that have been associated with presynaptic depression of primary afferent input (described below). These results are consistent with demonstration of dermatomal overlap and activation of circuitry that could modulate pain signaling. Although one might view this as encouraging, whether such afferent recruitment is causally linked to pathways that generate paresthesia and/or modulate pain is unknown (also see Are orthodromic actions of PSDC neurons responsible for SCS-induced paresthesia?).
PAD is thought to arise predominantly from GABAergic axo-axonic synapses acting on GABAA receptors that cause a Cl− efflux constituting an ionotropic form of afferent presynaptic inhibition (Rudomin and Schmidt 1999; Willis 1999). Figure 5C depicts simplified representation of circuitry generating axo-axonic presynaptic inhibition of primary afferents. PAD was experimentally recorded as a slow depolarizing DRP and/or DRR. DRRs represent suprathreshold spiking arising from an underlying slower depolarizing DRP (Rudomin and Schmidt 1999), and their expression here is likely due to bath temperature (Eccles et al. 1961; Fink et al. 2014).
An important observation was that threshold for recruitment of the lowest threshold (Aαβ) primary afferents was comparable to threshold for recruitment of interneuronal circuits responsible for generating presynaptic inhibition by PAD and producing DRRs (Fig. 5). Whereas SCS recruited Aαβ afferents to activate the circuitry mediating PAD, the identity of the primary afferent axons receiving presynaptic inhibition via PAD cannot be determined from these experiments. They likely include negative feedback onto functionally homologous Aαβ primary afferents (Rudomin and Schmidt 1999) but also could include actions on C fibers, and hence suppress central actions of nociceptors (Witschi et al. 2011).
Are axons of PSDC neurons the lowest threshold axons recruited with SCS?
We observed that the lowest threshold axons recruited at lumbar segments with SCS had differentiable recruitment properties (CV, rheobase, chronaxie) compared with DR afferent axons. That these axons had lower threshold and were distinct from primary afferent axons recorded along the rostrocaudal axis explored (T3–S2) argues against them being collateral branches of primary afferents originating from more rostral or caudal segments (Bai et al. 2015; Niu et al. 2013; Shortland and Wall 1992). Dual-site SCS collision experiments demonstrated the ability to selectively block these DC axons independently of DR afferent axon recruitment, further supporting their axonal identity as distinct from primary afferents.
Postsynaptic dorsal column tract cells (PSDCs) are ascending tract neurons that project via the DC to brain stem gracile nuclei (Willis and Coggeshall 2004) and may be the non-primary afferent axonal population recruited by SCS. Aαβ primary afferent axons enter the DC, project both rostrally and caudally, and regularly issue axon collaterals out to gray matter as they project (Bai et al. 2015; Niu et al. 2013). In contrast, PSDCs are not thought to issues collaterals after entering the DC (see Fig. 11; Giesler et al. 1984). In the absence of branching, PSDCs may retain the higher values of CV reported here and previously (Angaut-Petit 1975; Brown and Fyffe 1981; Giesler and Cliffer 1985; Jankowska et al. 1979).
Fig. 11.
Factors associated with differences in spinal cord stimulation (SCS) recruitment of Aαβ afferents and non-primary afferent fiber populations. A: shown are anatomical considerations in relation to axonal recruitment results. SCS recruited Aαβ axons originating from both rostral and caudal spinal segments. An additional non-primary afferent population was recruited at lower threshold in the lumbar dorsal column (DC). Based on known anatomy, these are likely postsynaptic dorsal column (PSDC) tract cells. PSDC axonal recruitment would be expected to have direct orthodromic actions in brain stem gracile nuclei (DCN) that associate with sensory integrative actions within the lumbar cord. PSDC antidromic axonal recruitment may have segmental synaptic actions on lumbar circuits via local intraspinal axon collaterals. Aαβ afferent axons would have widespread rostrocaudal spinal synaptic actions, including via GABAergic presynaptic inhibition of primary afferent signaling, and also project directly to the DCN. B: cross section of dorsal spinal cord at level of SCS (T10) depicts how differences in axon depth and diameter may explain relative recruitment threshold and conduction velocity (CV) in these axonal populations. Lumbar DC axons (putative PSDCs) have the lowest threshold and fastest CV. Lumbar Aαβ axons have slower CV that may be explained by a smaller axon diameter, whereas their higher threshold may be due to greater depth and/or smaller diameter. Thoracic Aαβ axons had higher threshold values relative to DC axons. This is partly explained by lateral location but also may be due to greater location depth. Thoracic Aαβ CV was not estimated.
Are orthodromic actions of PSDC neurons responsible for SCS-induced paresthesia?
The possibility that PSDCs are the non-primary afferent DC axon population recruited at intensities lower than Aαβ primary afferents (Fig. 7; see below) may indicate their importance in SCS induced modulation of pain that could be independent of peripheral side effects. Assuming activation of Aαβ afferents is responsible for evoking motor reflexes that define motor threshold in clinical SCS approaches (Yang et al. 2011), and given that SCS is effective at amplitude values below motor threshold, recruited axons may include PSDCs. Because PDSCs receive multimodal afferent input (Angaut-Petit 1975; Brown et al. 1983; Giesler and Cliffer 1985; Jankowska et al. 1979; Noble and Riddell 1988) and project to DC gracile nuclei (Giesler et al. 1984), their orthodromic activation by SCS could be directly responsible for initiating paresthesias. Lumbar PSDCs represent ~30% of the cells that project to the gracile nucleus, whereas only ~25% of lumbar enlargement long system primary afferents reach the gracile nucleus (Bai et al. 2015; Giesler et al. 1984). Importantly, because PSDC neurons are largely absent in the thoracic cord (Giesler et al. 1984), PSDC axons recruited at T10 would originate from caudal segments consistent with locations of observed paresthesia reported clinically (Kumar et al. 1991; North et al. 1993). In contrast, the observed broad rostrocaudal recruitment of sensory axons with T10 SCS (Fig. 9) is consistent with anatomical projections of Aαβ fibers in the DC known to originate from many segments above and below the SCS site (Bai et al. 2015; Niu et al. 2013). It would be harder to causally link broad afferent recruitment to the selective generation of paresthesias caudal to the SCS site.
An interesting possibility is that paresthesias and depression of pain perception are both due to PSDC actions. Because PSDC tract neurons only issue local axon collaterals near their cell bodies in the dorsal horn (Brown and Fyffe 1981), antidromic recruitment of PSDCs located in lumbar segments could synaptically interact with other spinal neurons that then modulate lumbar spinal sensory transmission (Noble and Riddell 1988). It is also possible that PSDCs activate supraspinal antinociceptive mechanisms, including that of the serotonergic system (Barchini et al. 2012; Saadé et al. 2015; Song et al. 2011). Overall, preferential PSDC recruitment may represent a critical but currently unexplored neural circuitry underlying conventional SCS-induced paresthesia and analgesia.
Factors associated with experiments conducted in the isolated adult mouse spinal cord.
We developed an ex vivo adult mouse spinal cord preparation that permitted detailed electrophysiological investigations without anesthetic interference. To enhance viability, studies were conducted at room temperature. The concomitant slowing of CV aided separation of Aαβ, Aδ, and C fiber volley components when recorded along relatively short distances but did not provide the actual values of CV that would be seen in vivo. Guidelines for classifying primary afferents in DRs at room temperature were obtained from a study that characterized CV in peripheral nerve and DRs at 22 and 37°C (Pinto et al. 2008a). Nissl staining suggested that dorsal horn neurons are preserved, and electrophysiological recordings demonstrated neuronal participation in synaptically mediated DRRs. However, it is likely that circuit excitability may be depressed via unknown mechanisms, including cord transection-induced spinal shock (Ditunno et al. 2004). Recordings of afferent evoked population synaptic responses as field potentials in the dorsal horn suggested limited recruitment of polysynaptic pathways unless excitability was increased pharmacologically (preliminary observations). Given that distinct descending brain stem systems are known to be involved in SCS pain modulation (Barchini et al. 2012), removal of brain stem represents a further limitation of the isolated spinal cord model system. However, modern genetic approaches enable studies on neuromodulatory actions from brain stem in the isolated spinal cord via optogenetic activation of their descending axons (François et al. 2017).
Traditional preclinical SCS studies are primarily undertaken in the rat in vivo. They vary in their electrode design (ball, paddle, monopolar, bipolar, etc.) and often assign SCS intensity as a percentage of motor threshold (e.g., 70–90%) (Crosby et al. 2015; Song et al. 2013a, 2013b; Stiller et al. 1996; Yuan et al. 2014). Our experimental paradigm leveraged computational modeling to inform detailed recruitment studies to realistically appraise neural system recruitment with clinically analogous stimuli at defined electrode configurations and distances from the spinal cord, features not present in previous preclinical studies of SCS. To achieve this, we strategically developed a model system that allowed us to explore this with optimal access to the DC, DRs, and SCS distance above the cord. Obtained results inform subsequent SCS studies in mouse using scaled electrodes and effective stimulus frequencies to determine whether the delivered currents have modulatory actions on known afferents/pathways including, in mouse, in vivo pain models.
Importantly, although our experimental arrangement excludes the presence of dura, at the constant-current stimulus intensities applied, resistivity values normally seen for dura do not significantly affect spinal cord current densities (Coburn and Sin 1985). Changes from the modeled electrodes (square) to those utilized for experiments (circular) account for an overall surface area difference of 0.02 mm2. Although it is possible that this difference could affect recruitment when SCS is contacting the cord surface, there is little evidence to suggest that a difference of this scale would significantly impact relative recruitment thresholds and recruitment order of axonal populations during stimulation through CSF. The main point of the electrode scaling technique was to quantitatively estimate relevant dimensions and parameters for studies with the mouse spinal cord. Previously, rodent studies frequently employed contacts with dimensions that are larger than what would be recommended for a mouse (Cui et al. 1996; Stiller et al. 1996; Yang et al. 2011), including those comparable to electrodes used clinically (Song et al. 2009, 2013a, 2013b; Yuan et al. 2014).
Summary.
In summary, we paired clinically analogous SCS with an experimentally accessible adult mouse spinal cord preparation to provide detailed studies on the variability in axonal recruitment to several preclinical SCS parameters, including stimulation site, stimulation distance, amplitude, polarity, and pulse duration. Results demonstrate a hierarchical sensitivity of recruitment of various axonal populations and introduce the possibility that the primary actions of SCS may be independent of primary afferent recruitment. Figure 11 summarizes the proposed anatomical relation between relative axonal recruitment.
GRANTS
This material is based on work supported by National Science Foundation Graduate Research Fellowship Grant DGE-1650044, National Institutes of Health Computational Neuroscience Training Grant 5T90DA032466, Boston Scientific Neuromodulation, and the Alfred P. Sloan Foundation.
DISCLOSURES
Funding for this work was provided by Boston Scientific. T. Zhang, N. Brill, W. Gu, and M. Moffitt are employees of Boston Scientific.
AUTHOR CONTRIBUTIONS
S.I., T.Z., N.B., W.G., M.M., and S.H. conceived and designed research; S.I., M.H., J.Q., and S.H. performed experiments; S.I., M.H., and S.H. analyzed data; S.I. and S.H. interpreted results of experiments; S.I., T.Z., N.B., and S.H. prepared figures; S.I. and S.H. drafted manuscript; S.I., T.Z., N.B., W.G., and S.H. edited and revised manuscript; S.I., T.Z., J.Q., N.B., W.G., M.M., and S.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Elzbieta Jankowska for suggestions on including collision experiments and Mike Sawchuk for undertaking histological processing of spinal cord.
REFERENCES
- Abraira VE, Ginty DD. The sensory neurons of touch. Neuron 79: 618–639, 2013. doi: 10.1016/j.neuron.2013.07.051 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angaut-Petit D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat’s fasciculus gracilis. Exp Brain Res 22: 471–493, 1975. doi: 10.1007/BF00237349 . [DOI] [PubMed] [Google Scholar]
- Baba H, Yoshimura M, Nishi S, Shimoji K. Synaptic responses of substantia gelatinosa neurones to dorsal column stimulation in rat spinal cord in vitro. J Physiol 478: 87–99, 1994. doi: 10.1113/jphysiol.1994.sp020232 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Lehnert BP, Liu J, Neubarth NL, Dickendesher TL, Nwe PH, Cassidy C, Woodbury CJ, Ginty DD. Genetic identification of an expansive mechanoreceptor sensitive to skin stroking. Cell 163: 1783–1795, 2015. doi: 10.1016/j.cell.2015.11.060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchini J, Tchachaghian S, Shamaa F, Jabbur SJ, Meyerson BA, Song Z, Linderoth B, Saadé NE. Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience 215: 196–208, 2012. doi: 10.1016/j.neuroscience.2012.04.057 . [DOI] [PubMed] [Google Scholar]
- BeMent SL, Ranck JB Jr. A quantitative study of electrical stimulation of central myelinated fibers. Exp Neurol 24: 147–170, 1969. doi: 10.1016/0014-4886(69)90012-0 . [DOI] [PubMed] [Google Scholar]
- Bossetti CA, Birdno MJ, Grill WM. Analysis of the quasi-static approximation for calculating potentials generated by neural stimulation. J Neural Eng 5: 44–53, 2007. doi: 10.1088/1741-2560/5/1/005 . [DOI] [PubMed] [Google Scholar]
- Brown AG, Brown PB, Fyffe RE, Pubols LM. Receptive field organization and response properties of spinal neurones with axons ascending the dorsal columns in the cat. J Physiol 337: 575–588, 1983. doi: 10.1113/jphysiol.1983.sp014643 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol 321: 31–47, 1981. doi: 10.1113/jphysiol.1981.sp013970 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg 100, Suppl Spine: 254–267, 2004. doi: 10.3171/spi.2004.100.3.0254 . [DOI] [PubMed] [Google Scholar]
- Canbay S, Gürer B, Bozkurt M, Comert A, Izci Y, Başkaya MK. Anatomical relationship and positions of the lumbar and sacral segments of the spinal cord according to the vertebral bodies and the spinal roots. Clin Anat 27: 227–233, 2014. doi: 10.1002/ca.22253 . [DOI] [PubMed] [Google Scholar]
- Coburn B, Sin WK. A theoretical study of epidural electrical stimulation of the spinal cord–Part I: Finite element analysis of stimulus fields. IEEE Trans Biomed Eng 32: 971–977, 1985. doi: 10.1109/TBME.1985.325648 . [DOI] [PubMed] [Google Scholar]
- Crosby ND, Weisshaar CL, Smith JR, Zeeman ME, Goodman-Keiser MD, Winkelstein BA. Burst and tonic spinal cord stimulation differentially activate GABAergic mechanisms to attenuate pain in a rat model of cervical radiculopathy. IEEE Trans Biomed Eng 62: 1604–1613, 2015. doi: 10.1109/TBME.2015.2399374 . [DOI] [PubMed] [Google Scholar]
- Cui JG, Linderoth B, Meyerson BA. Effects of spinal cord stimulation on touch-evoked allodynia involve GABAergic mechanisms. An experimental study in the mononeuropathic rat. Pain 66: 287–295, 1996. doi: 10.1016/0304-3959(96)03069-2 . [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Faganel J, Sharkey PC, Sherwood AM. Study of sensation and muscle twitch responses to spinal cord stimulation. Int Rehabil Med 2: 76–81, 1980. doi: 10.3109/09638288009163961 . [DOI] [PubMed] [Google Scholar]
- Ditunno JF, Little JW, Tessler A, Burns AS. Spinal shock revisited: a four-phase model. Spinal Cord 42: 383–395, 2004. doi: 10.1038/sj.sc.3101603 . [DOI] [PubMed] [Google Scholar]
- Eccles JC, Kozak W, Magni F. Dorsal root reflexes of muscle group I afferent fibres. J Physiol 159: 128–146, 1961. doi: 10.1113/jphysiol.1961.sp006797 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enevoldson TP, Gordon G. Postsynaptic dorsal column neurons in the cat: a study with retrograde transport of horseradish peroxidase. Exp Brain Res 75: 611–620, 1989. doi: 10.1007/BF00249912 . [DOI] [PubMed] [Google Scholar]
- Feirabend HK, Choufoer H, Ploeger S, Holsheimer J, van Gool JD. Morphometry of human superficial dorsal and dorsolateral column fibres: significance to spinal cord stimulation. Brain 125: 1137–1149, 2002. doi: 10.1093/brain/awf111 . [DOI] [PubMed] [Google Scholar]
- Fink AJ, Croce KR, Huang ZJ, Abbott LF, Jessell TM, Azim E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 509: 43–48, 2014. doi: 10.1038/nature13276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- François A, Low SA, Sypek EI, Christensen AJ, Sotoudeh C, Beier KT, Ramakrishnan C, Ritola KD, Sharif-Naeini R, Deisseroth K, Delp SL, Malenka RC, Luo L, Hantman AW, Scherrer G. A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 93: 822–839.e6, 2017. doi: 10.1016/j.neuron.2017.01.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesler GJ Jr, Cliffer KD. Postsynaptic dorsal column pathway of the rat. II. Evidence against an important role in nociception. Brain Res 326: 347–356, 1985. doi: 10.1016/0006-8993(85)90044-7 . [DOI] [PubMed] [Google Scholar]
- Giesler GJ Jr, Nahin RL, Madsen AM. Postsynaptic dorsal column pathway of the rat. I. Anatomical studies. J Neurophysiol 51: 260–275, 1984. doi: 10.1152/jn.1984.51.2.260 . [DOI] [PubMed] [Google Scholar]
- Guan Y, Wacnik PW, Yang F, Carteret AF, Chung CY, Meyer RA, Raja SN. Spinal cord stimulation-induced analgesia: electrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology 113: 1392–1405, 2010. doi: 10.1097/ALN.0b013e3181fcd95c . [DOI] [PubMed] [Google Scholar]
- Hammar I, Krutki P, Drzymala-Celichowska H, Nilsson E, Jankowska E. A trans-spinal loop between neurones in the reticular formation and in the cerebellum. J Physiol 589: 653–665, 2011. doi: 10.1113/jphysiol.2010.201178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C, Skoglund S. Calibre spectra of some fibre tracts in the feline central nervous system during postnatal development. Acta Physiol Scand Suppl 364: 5–41, 1971. doi: 10.1111/j.1365-201X.1971.tb10977.x . [DOI] [PubMed] [Google Scholar]
- Holsheimer J. Which neuronal elements are activated directly by spinal cord stimulation. Neuromodulation 5: 25–31, 2002. doi: 10.1046/j.1525-1403.2002._2005.x . [DOI] [PubMed] [Google Scholar]
- Holsheimer J, Struijk JJ, Wesselink WA. Analysis of spinal cord stimulation and design of epidural electrodes by computer modeling. Neuromodulation 1: 14–18, 1998. doi: 10.1111/j.1525-1403.1998.tb00026.x . [DOI] [PubMed] [Google Scholar]
- Horch KW, Burgess PR, Whitehorn D. Ascending collaterals of cutaneous neurons in the fasciculus gracilis of the cat. Brain Res 117: 1–17, 1976. doi: 10.1016/0006-8993(76)90552-7 . [DOI] [PubMed] [Google Scholar]
- Howell B, Lad SP, Grill WM. Evaluation of intradural stimulation efficiency and selectivity in a computational model of spinal cord stimulation. PLoS One 9: e114938, 2014. [Erratum in PLoS One 10: ee0123485, 2015.] doi: 10.1371/journal.pone.0114938 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh JB. Conduction velocity and diameter of nerve fibers. Am J Physiol 127: 131–139, 1939. doi: 10.1152/ajplegacy.1939.127.1.131. [DOI] [Google Scholar]
- Hwang YC, Hinsman EJ, Roesel OF. Caliber spectra of fibers in the fasciculus gracilis of the cat cervical spinal cord: a quantitative electron microscopic study. J Comp Neurol 162: 195–203, 1975. doi: 10.1002/cne.901620203 . [DOI] [PubMed] [Google Scholar]
- Idlett S, Halder M, Sawchuk M, Quevedo J, Gu W, Moffitt M, Hochman S. Relating dorsal column stimulation to the recruitment and modulation of sensory signaling in the isolated in vitro adult mouse spinal cord. Program No. 145.02 2016 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2016. [Google Scholar]
- Idlett SH, Sawchuk M, Zhang T, Brill N, Gu W, Hochman S. Computational model-driven stimulation parameters for clinically-analogous SCS in mouse: are postsynaptic dorsal column tract cells recruited first? International Neuromodulation Society 13th World Congress Edinburgh, Scotland, May 27–June 1, 2017. [Google Scholar]
- Jankowska E, Rastad J, Zarzecki P. Segmental and supraspinal input to cells of origin of non-primary fibres in the feline dorsal columns. J Physiol 290: 185–200, 1979. doi: 10.1113/jphysiol.1979.sp012767 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Brownstone RM. Mechanisms of spinal cord stimulation for the treatment of pain: still in the dark after 50 years. Eur J Pain 23: 652–659, 2018. doi: 10.1002/ejp.1336 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Nath R, Wyant GM. Treatment of chronic pain by epidural spinal cord stimulation: a 10-year experience. J Neurosurg 75: 402–407, 1991. doi: 10.3171/jns.1991.75.3.0402 . [DOI] [PubMed] [Google Scholar]
- Lee D, Hershey B, Bradley K, Yearwood T. Predicted effects of pulse width programming in spinal cord stimulation: a mathematical modeling study. Med Biol Eng Comput 49: 765–774, 2011. doi: 10.1007/s11517-011-0780-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidierth M. Long-range projections of Aδ primary afferents in the Lissauer tract of the rat. Neurosci Lett 425: 126–130, 2007. doi: 10.1016/j.neulet.2007.08.029 . [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol 186: 133–150, 1979. doi: 10.1002/cne.901860203 . [DOI] [PubMed] [Google Scholar]
- Mandadi S, Hong P, Tran MA, Bráz JM, Colarusso P, Basbaum AI, Whelan PJ. Identification of multisegmental nociceptive afferents that modulate locomotor circuits in the neonatal mouse spinal cord. J Comp Neurol 521: 2870–2887, 2013. doi: 10.1002/cne.23321 . [DOI] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philos Trans R Soc Lond B Biol Sci 293: 477–508, 1981. doi: 10.1098/rstb.1981.0082 . [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM. Excitation of central nervous system neurons by nonuniform electric fields. Biophys J 76: 878–888, 1999. doi: 10.1016/S0006-3495(99)77251-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal DR. Analysis of a model for excitation of myelinated nerve. IEEE Trans Biomed Eng 23: 329–337, 1976. doi: 10.1109/TBME.1976.324593 . [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 150: 971–979, 1965. doi: 10.1126/science.150.3699.971 . [DOI] [PubMed] [Google Scholar]
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain 155: 210–216, 2014. doi: 10.1016/j.pain.2013.12.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson BA, Linderoth B. Mode of action of spinal cord stimulation in neuropathic pain. J Pain Symptom Manage 31: S6–S12, 2006. doi: 10.1016/j.jpainsymman.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Moffitt MA, Lee DC, Bradley K. Spinal cord stimulation: engineering approaches to clinical and physiological challenges. In: Implantable Neural Prostheses 1. Biomedical and Medical Physics, Biomedical Engineering, edited by Greenbaum E, Zhou D. New York: Springer, 2009, p. 155–194. [Google Scholar]
- Niu J, Ding L, Li JJ, Kim H, Liu J, Li H, Moberly A, Badea TC, Duncan ID, Son YJ, Scherer SS, Luo W. Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway. J Neurosci 33: 17691–17709, 2013. doi: 10.1523/JNEUROSCI.3429-13.2013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R, Riddell JS. Cutaneous excitatory and inhibitory input to neurones of the postsynaptic dorsal column system in the cat. J Physiol 396: 497–513, 1988. doi: 10.1113/jphysiol.1988.sp016974 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RB, Kidd DH, Zahurak M, James CS, Long DM. Spinal cord stimulation for chronic, intractable pain: experience over two decades. Neurosurgery 32: 384–394, 1993. doi: 10.1227/00006123-199303000-00008 . [DOI] [PubMed] [Google Scholar]
- Patterson JT, Coggeshall RE, Lee WT, Chung K. Long ascending unmyelinated primary afferent axons in the rat dorsal column: immunohistochemical localizations. Neurosci Lett 108: 6–10, 1990. doi: 10.1016/0304-3940(90)90697-8 . [DOI] [PubMed] [Google Scholar]
- Patterson JT, Head PA, McNeill DL, Chung K, Coggeshall RE. Ascending unmyelinated primary afferent fibers in the dorsal funiculus. J Comp Neurol 290: 384–390, 1989. doi: 10.1002/cne.902900307 . [DOI] [PubMed] [Google Scholar]
- Pelot NA, Thio BJ, Grill WM. Modeling current sources for neural stimulation in COMSOL. Front Comput Neurosci 12: 40, 2018. doi: 10.3389/fncom.2018.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto V, Derkach VA, Safronov BV. Role of TTX-sensitive and TTX-resistant sodium channels in Aδ- and C-fiber conduction and synaptic transmission. J Neurophysiol 99: 617–628, 2008a. doi: 10.1152/jn.00944.2007 . [DOI] [PubMed] [Google Scholar]
- Pinto V, Szûcs P, Derkach VA, Safronov BV. Monosynaptic convergence of C- and Aδ-afferent fibres from different segmental dorsal roots on to single substantia gelatinosa neurones in the rat spinal cord. J Physiol 586: 4165–4177, 2008b. doi: 10.1113/jphysiol.2008.154898 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98: 417–440, 1975. doi: 10.1016/0006-8993(75)90364-9 . [DOI] [PubMed] [Google Scholar]
- Rattay F. Analysis of the electrical excitation of CNS neurons. IEEE Trans Biomed Eng 45: 766–772, 1998. doi: 10.1109/10.678611 . [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–37, 1999. doi: 10.1007/s002210050933 . [DOI] [PubMed] [Google Scholar]
- Saadé NE, Barchini J, Tchachaghian S, Chamaa F, Jabbur SJ, Song Z, Meyerson BA, Linderoth B. The role of the dorsolateral funiculi in the pain relieving effect of spinal cord stimulation: a study in a rat model of neuropathic pain. Exp Brain Res 233: 1041–1052, 2015. doi: 10.1007/s00221-014-4180-x . [DOI] [PubMed] [Google Scholar]
- Sankarasubramanian V, Buitenweg JR, Holsheimer J, Veltink P. Electrode alignment of transverse tripoles using a percutaneous triple-lead approach in spinal cord stimulation. J Neural Eng 8: 016010, 2011. doi: 10.1088/1741-2560/8/1/016010 . [DOI] [PubMed] [Google Scholar]
- Sato KL, Johanek LM, Sanada LS, Sluka KA. Spinal cord stimulation reduces mechanical hyperalgesia and glial cell activation in animals with neuropathic pain. Anesth Analg 118: 464–472, 2014. doi: 10.1213/ANE.0000000000000047 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortland P, Wall PD. Long-range afferents in the rat spinal cord. II. Arborizations that penetrate grey matter. Philos Trans R Soc Lond B Biol Sci 337: 445–455, 1992. doi: 10.1098/rstb.1992.0120 . [DOI] [PubMed] [Google Scholar]
- Song Z, Ansah OB, Meyerson BA, Pertovaara A, Linderoth B. Exploration of supraspinal mechanisms in effects of spinal cord stimulation: role of the locus coeruleus. Neuroscience 253: 426–434, 2013a. doi: 10.1016/j.neuroscience.2013.09.006 . [DOI] [PubMed] [Google Scholar]
- Song Z, Ansah OB, Meyerson BA, Pertovaara A, Linderoth B. The rostroventromedial medulla is engaged in the effects of spinal cord stimulation in a rodent model of neuropathic pain. Neuroscience 247: 134–144, 2013b. doi: 10.1016/j.neuroscience.2013.05.027 . [DOI] [PubMed] [Google Scholar]
- Song Z, Meyerson BA, Linderoth B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain 152: 1666–1673, 2011. doi: 10.1016/j.pain.2011.03.012 . [DOI] [PubMed] [Google Scholar]
- Song Z, Ultenius C, Meyerson BA, Linderoth B. Pain relief by spinal cord stimulation involves serotonergic mechanisms: an experimental study in a rat model of mononeuropathy. Pain 147: 241–248, 2009. doi: 10.1016/j.pain.2009.09.020 . [DOI] [PubMed] [Google Scholar]
- Stiller CO, Cui JG, O’Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of gamma-aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery 39: 367–374, 1996. doi: 10.1097/00006123-199608000-00026 . [DOI] [PubMed] [Google Scholar]
- Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain 108: 137–147, 2004. doi: 10.1016/j.pain.2003.12.016 . [DOI] [PubMed] [Google Scholar]
- Waddell PJ, Lawson SN, McCarthy PW. Conduction velocity changes along the processes of rat primary sensory neurons. Neuroscience 30: 577–584, 1989. doi: 10.1016/0306-4522(89)90152-8 . [DOI] [PubMed] [Google Scholar]
- Wall PD, Lidierth M, Hillman P. Brief and prolonged effects of Lissauer tract stimulation on dorsal horn cells. Pain 83: 579–589, 1999. doi: 10.1016/S0304-3959(99)00170-0 . [DOI] [PubMed] [Google Scholar]
- Watson C, Paxinos G, Kayalioglu G (Editors). The Spinal Cord: a Christopher and Dana Reeve Foundation Text and Atlas. London: Academic, 2009. [Google Scholar]
- Wesselink WA, Holsheimer J, Boom HB. Analysis of current density and related parameters in spinal cord stimulation. IEEE Trans Rehabil Eng 6: 200–207, 1998. doi: 10.1109/86.681186 . [DOI] [PubMed] [Google Scholar]
- Willis WD Jr, Coggeshall RE. Sensory pathways in the dorsal funiculus. In: Sensory Mechanisms of the Spinal Cord. Boston: Springer, 2004, p. 597–664. [Google Scholar]
- Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 124: 395–421, 1999. doi: 10.1007/s002210050637 . [DOI] [PubMed] [Google Scholar]
- Witschi R, Punnakkal P, Paul J, Walczak JS, Cervero F, Fritschy JM, Kuner R, Keist R, Rudolph U, Zeilhofer HU. Presynaptic α2-GABAA receptors in primary afferent depolarization and spinal pain control. J Neurosci 31: 8134–8142, 2011. doi: 10.1523/JNEUROSCI.6328-10.2011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakhnitsa V, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates dorsal horn neuronal hyperexcitability in a rat model of mononeuropathy. Pain 79: 223–233, 1999. doi: 10.1016/S0304-3959(98)00169-9 . [DOI] [PubMed] [Google Scholar]
- Yang F, Carteret AF, Wacnik PW, Chung CY, Xing L, Dong X, Meyer RA, Raja SN, Guan Y. Bipolar spinal cord stimulation attenuates mechanical hypersensitivity at an intensity that activates a small portion of A-fiber afferents in spinal nerve-injured rats. Neuroscience 199: 470–480, 2011. doi: 10.1016/j.neuroscience.2011.09.049 . [DOI] [PubMed] [Google Scholar]
- Yearwood TL, Hershey B, Bradley K, Lee D. Pulse width programming in spinal cord stimulation: a clinical study. Pain Physician 13: 321–335, 2010. . [PubMed] [Google Scholar]
- Yuan B, Liu D, Liu X. Spinal cord stimulation exerts analgesia effects in chronic constriction injury rats via suppression of the TLR4/NF-κB pathway. Neurosci Lett 581: 63–68, 2014. doi: 10.1016/j.neulet.2014.08.023 . [DOI] [PubMed] [Google Scholar]
- Zhang TC, Janik JJ, Peters RV, Chen G, Ji RR, Grill WM. Spinal sensory projection neuron responses to spinal cord stimulation are mediated by circuits beyond gate control. J Neurophysiol 114: 284–300, 2015. doi: 10.1152/jn.00147.2015 . [DOI] [PMC free article] [PubMed] [Google Scholar]