Abstract
Electrophysiological analysis has revealed much about the broad coding and neural ensemble dynamics that characterize gustatory cortical (GC) taste processing in awake rats and about how these dynamics relate to behavior. With regard to mice, however, data concerning cortical taste coding have largely been restricted to imaging, a technique that reveals average levels of neural responsiveness but that (currently) lacks the temporal sensitivity necessary for evaluation of fast response dynamics; furthermore, the few extant studies have thus far failed to provide consensus on basic features of coding. We have recorded the spiking activity of ensembles of GC neurons while presenting representatives of the basic taste modalities (sweet, salty, sour, and bitter) to awake mice. Our first central result is the identification of similarities between rat and mouse taste processing: most mouse GC neurons (~66%) responded distinctly to multiple (3–4) tastes; temporal coding analyses further reveal, for the first time, that single mouse GC neurons sequentially code taste identity and palatability, the latter responses emerging ~0.5 s after the former, with whole GC ensembles transitioning suddenly and coherently from coding taste identity to coding taste palatability. The second finding is that spatial location plays very little role in any aspect of taste responses: neither between- (anterior-posterior) nor within-mouse (dorsal-ventral) mapping revealed anatomic regions with narrow or temporally simple taste responses. These data confirm recent results showing that mouse cortical taste responses are not “gustotopic” but also go beyond these imaging results to show that mice process tastes through time.
NEW & NOTEWORTHY Here, we analyzed taste-related spiking activity in awake mouse gustatory cortical (GC) neural ensembles, revealing deep similarities between mouse cortical taste processing and that repeatedly demonstrated in rat: mouse GC ensembles code multiple aspects of taste in a coarse-coded, time-varying manner that is essentially invariant across the spatial extent of GC. These data demonstrate that, contrary to some reports, cortical network processing is distributed, rather than being separated out into spatial subregion.
Keywords: gustatory cortex, mice, population coding, taste
INTRODUCTION
Mice are the most commonly used subjects in vertebrate neuroscience research. The use of mice affords researchers relatively easy access to the molecular and genetic underpinnings of network activity, learning-related plasticity, and behavior (Kandel et al. 2014; Stevens 1996). As taste is a particularly good sensory system with which to study these three topics (see below and Carleton et al. 2010; Maffei et al. 2012), it is surprising that there has been almost no extensive analysis of central taste electrophysiology in awake mice (although see Kusumoto-Yoshida et al. 2015). There have been several in vivo analyses of mouse gustatory cortical (GC) taste responses based on calcium imaging data, but the results of these analyses, although exciting, have been difficult to reconcile with one another: work from one group suggests extreme spatial separation of responses to different tastes (Chen et al. 2011; Peng et al. 2015), whereas work from other groups suggests broad and nonmapped responses (Fletcher et al. 2017; Lavi et al. 2018; Livneh et al. 2017).
Furthermore, calcium imaging in general lacks the temporal sensitivity necessary for evaluation of fast single-trial response dynamics, a potentially important limitation given the evidence that GC taste responses in awake rats contain precisely such dynamics. More specifically, the rat work has reliably revealed that 1) responses to multiple tastes appear not only in the same region, but also within the same single neurons (Accolla et al. 2007; Fontanini and Katz 2006; Jezzini et al. 2013; Katz et al. 2001; Samuelsen et al. 2013; Samuelsen and Fontanini 2017), 2) these responses progress through a series of firing-rate “epochs,” coding in turn the presence, physical properties, and palatability of a taste across 1–1.5 s (Bahar et al. 2004; Katz et al. 2001; Maier and Katz 2013; Sadacca et al. 2012; Samuelsen et al. 2013), 3) late-epoch palatability-related firing is uniquely affected by experience-driven shifts of taste palatability (Bahar et al. 2004; Fontanini and Katz 2006; Grossman et al. 2008; Moran and Katz 2014), and 4) in single trials, the onset of palatability is a sudden, coherent network phenomenon, the timing of which predicts and likely drives behavior (Jones et al. 2007; Li et al. 2016; Sadacca et al. 2016). A test of whether mouse GC neurons respond in a like manner has not yet been performed and cannot be performed using imaging data.
For the current study, we recorded the responses of small ensembles of GC neurons in awake mice to presentations of stimuli representing four major taste modalities (sweet, salty, sour, and bitter). Our data reveal, first of all, that taste responses in mouse GC recapitulate the dynamics of taste responses in rat GC: taste delivery evoked broad and dynamic responses that coded first taste identity and then taste palatability across 1.0–1.5 s; furthermore, these single-neuron dynamics reflected coherent ensemble transitions: in individual trials, multiple neurons in each ensemble hopped from one firing rate to another coherently, midtrial. We took care to map the spatial extent of GC and were therefore also able to show that these response properties are observed uniformly along the anterior-posterior and dorsal-ventral axes; there were no regions of noticeably sparse responsiveness and no regions in which responses failed to reflect the general dynamics.
These results offer novel insight into mouse cortical taste processing: they support recent imaging studies suggesting that mouse taste responses are broad and nongustotopic (Fletcher et al. 2017; Lavi et al. 2018; Livneh et al. 2017), but they also go beyond this to suggest that mouse cortical taste processing is dynamic and that therefore “breadth” measures fail to fully describe GC taste function in awake mice (i.e., breadth meaningfully changes across the “stages” of the response). These insights can be used, in conjunction with tools available only in the mouse (among mammals), to delve into the undoubtedly rich relationship between molecular and network analyses of sensory function.
MATERIALS AND METHODS
Subjects
The experimental subjects were C57BL/6J (n = 2) and Stk11tm1.1Sjm (n = 4) male and female mice. The mice were purchased from Jackson (Bar Harbor, ME). Stk11f/f mice have conditional floxed allele between exons 3 and 6, which was inactive through the entire set of experiments. These mice were backcrossed after development by the donating investigator for at least four generations to C57BL/6J mice and were bred to C57BL/6J for at least one generation on arrival to Jackson (see also Nakada et al. 2010). After the mice were purchased from Jackson, they were further backcrossed for three to four generations to C57BL/6J before a colony was established. We consider both mice strains to have minimal differences in genetic background. Importantly, no behavioral or neural taste response differences distinguished the strains; they are therefore collapsed into a single group for purposes of our experiments. On arrival, the mice were placed on a 12:12-h light-dark cycle and given ad libitum access to food and water except during experimentation, at which time water access was restricted (see Fluid Delivery Protocol) while food remained available ad libitum (note that animals reliably consume less food when thirsty). Experiment procedures started once mice were 60–80 days old. All procedures were approved by the Brandeis University Institutional Animal Care and Use Committee and in accord National Institutes of Health guidelines.
Surgery
Mice were anesthetized with an intraperitoneal injection of ketamine-xylazine mix (20 mg/mL ketamine, 2.5 mg/mL xylazine, and 0.5 mg/mL acepromazine; total injection volume 5 µL/g) and stabilized in a stereotaxic frame. A midline incision on the scalp exposed the skull, and 2 trephine holes were made dorsal to GC. Multielectrode bundles (16 Formvar-coated, 25-µm-diameter nichrome wires) were gradually lowered into the holes to a spot just above GC: the anterior-posterior (AP) position was ~1.2 mm in front of bregma for anterior GC and ~0.4 mm for posterior GC; the medial-lateral (ML) positioning was ±3 mm for all mice. Bundles were then lowered to a dorsal-ventral (DV) position of −2.25 mm from the pia mater (before recording sessions, bundles were further lowered by 0.25–0.5 mm to reach dorsal GC and 0.75–1.00 mm to reach ventral GC; see Fig. 1 for examples of the identification of the various anatomic locations) and cemented to the skull, along with one intraoral cannula (flexible plastic tubing inserted into the cheek to allow controlled delivery of tastes to the tongue of awake mice; see below).
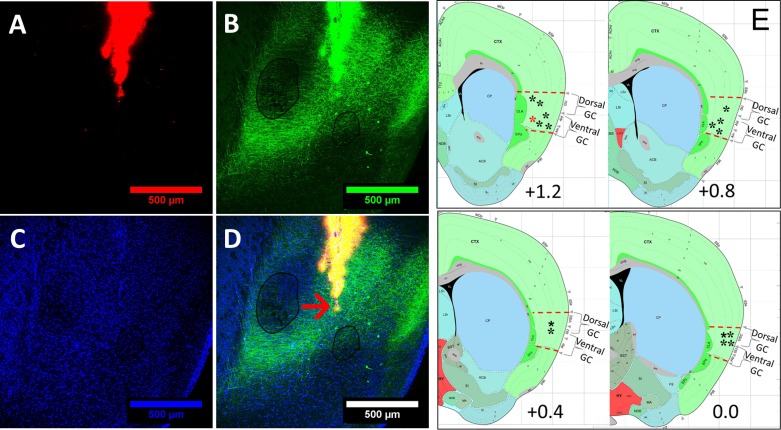
Fig. 1.
Localization of electrode bundles in mouse gustatory cortex (GC). A: to determine the recording site in GC, electrode bundles were coated with Dil (a red fluorescent tracer), which later showed the track traveled by the multielectrode array in GC. B: to assist the separation between dorsal and ventral subregions of GC, basolateral amygdala was infected with AAV2/5::GFP; resultant green fluorescent protein (GFP)-labeled axons were then visible in ventral GC. C: background staining of the same slice with DAPI (a nonspecific nuclear dye). D: in a triple-labeled slice, an arrow indicates the final location of the tips of the electrodes. E: a schematic representation of all electrode bundles (asterisks; red asterisk represents electrode from D) on top of a schematic of a coronal slice containing GC [bregma +1.6 mm to bregma 0.0 mm; every 0.4 mm were collapsed to a single representative image; Allen Institute for Brain Science (2008), mouse brain atlas, P56, coronal; coronal level 42, 46, 50, 54; https://mouse.brain-map.org/experiment/siv?id=100142143&imageId=102162354&imageType=atlas&initImage=atlas&showSubImage=y&contrast=0.5,0.5,0,255,4].
In a subset of mice, basolateral amygdala (AP = −1.4 mm, ML = ±3.4 mm, 200-nL injections at DV = 4.3 and 4.6 mm from the dura) was infected with AAV2/5::Camk2α-GFP to visualize basolateral amygdala axons in the ventral GC. This was done to make the separation of dorsal from ventral subregions of GC easier to visualize.
Mice were given 7 days to recover from the surgery. During the 1st 3 days of this recovery, injections of meloxicam (2 mg/kg ip) and penicillin (0.1 mL), both administered subcutaneously, provided pain and infection management, respectively.
Fluid Delivery Protocol
To accommodate the requirement of electrophysiological recording, experiments were conducted in in a Faraday cage constructed with standard aluminum insect netting with dimensions as 26 × 24 × 33 cm (length × width × height). Fluid was delivered through a nitrogen-pressurized system of polyethylene tubes; flow was controlled by solenoid valves opened by a Raspberry Pi computer installed with customizable Python scripts that control the timing and quantity of each taste delivery (construction details and code available on request; see endnote). During the experiment, mice were allowed to move about the chamber without restraint.
Water deprivation and habituation.
During the 3 days following recovery from surgery, sessions consisted of 60 deliveries of 15-µL aliquots (hereafter, trials) of water across 30 min. This procedure habituated the mice to the experimental environment and to intraoral cannula delivery of fluid and ensured the mice had motivation for liquid consumption while recording. Following the last water-delivery session, electrodes were lowered 250–1,000 µm, and mice were returned to home cage. To ensure rehydration, 2 and 6 h following experimental sessions, mice were given 30-min access to water in the home cages.
On the following day, the recording experiment commenced. The procedure was identical to that described above except that water was replaced with four different taste stimuli, which were sweet (0.2 M sucrose), salty (0.1 M sodium chloride), sour (0.02 M citric acid), and bitter (0.001 M quinine). Noted that we did not use water trials in the current experiment because delivery of a few aliquots of tastes per minute to an awake, salivating rodent renders rinse trials unnecessary. A total of 15 trials of each taste were delivered, in random order (sampling without replacement). These tastes and concentrations were chosen to provide compatibility to our prior rat research and because they provided a broad range of both quality and palatability.
A subset of mice (N = 4) received two identical recording sessions separated by 1 day. In these cases, the electrodes were lowered ~250–500 µm immediately after the first recording session. When data from these two sessions were compared (as a specific part of the study; see below), no substantive between-day differences were noted; the data from the two sessions were therefore otherwise combined.
Acquisition of Electrophysiological Data
Voltage signals from the microelectrodes were collected at 30,000 samples per second. With the use of a 32-channel analog-to-digital converted chip (RHD2132), these signals were digitally amplified and filtered before being saved to the hard drive of an Ubuntu computer connected to an Intan recording system (RHD2000 Evaluation System and amplifier boards; Intan Technologies). We retained all waveforms from these raw voltage signals with at least a 3:1 signal-to-noise ratio and sorted the waveforms into distinct units using a semisupervised spike-sorting strategy: recorded voltage data were filtered between 300 and 3,000 Hz, grouped into potential clusters by a Gaussian mixture model with strict noise tolerance, and refined manually such that conservatism was enhanced by a requirement that the variability in spiking be random (and thus not reflecting biased cluster cutting; for the details of the recording system and Python scripts, see Mukherjee et al. 2017). To further ensure the isolated waveforms represent single neuron records, we also calculated the interspike intervals and removed units for which ≥1% of the intervals disobeyed the biological constraints on the neural refractory period.
Previous use of such techniques in the paradigm used here has been shown to produce data representative of single neurons (Fontanini and Katz 2006; Katz et al. 2001; Mukherjee et al. 2019). A total of 185 single units were isolated for the experiments across 10 sessions. As has been done many times previously, isolated units were categorized as being either putative pyramidal neurons or putative interneurons on the basis of wave shape and average interspike interval (e.g., Barthó et al. 2004; Sirota et al. 2008).
Statistical Analysis of Neural Data
Our analysis roadmap involves two major portions. For the first part, we examined the breadth of taste coding tuning curve of mice GC neurons. To achieve this goal, we started with t tests and ANOVAs, the basic workhorses of the neuroscientist’s statistical toolbox. These techniques have been successfully used in determining taste specificity along the rodent taste pathways from the nucleus of the solitary tract (e.g., Geran and Travers 2006) to forebrain, including limbic system (Lundy and Norgren 2004; Norgren 1970) and GC (Yamamoto et al. 1984). Appreciating the fact that taste stimuli are complex and composed of both quality and palatability (valence) features (e.g., Katz and Sadacca 2011), in the second set of analyses, we follow our own previous work (Fontanini et al. 2009; Li et al. 2013; Sadacca et al. 2012, 2016) to build a more sophisticated characterization of GC coding. Only through these more advanced analyses is the subtlety of the relationship between GC activity and various taste properties revealed.
Taste responsiveness.
A single neuron was deemed “taste responsive” if firing rates changed with taste delivery. Paired t tests were conducted to determine whether the evoked firing rate (2 s postdelivery) significantly differed from its baseline firing rate (2 s before delivery). Note that, in many previous electrophysiological studies of taste firing (in both rat and mouse), this analysis has involved using the multiple seconds of the response to a lengthy (≤20 s) taste delivery as the “sample” from which confidence intervals around a single “average response rate” were calculated, an approach that necessarily limited detectable taste responses to the largest and most long-lasting (Gannon and Contreras 1995; Ninomiya et al. 1992; Tordoff et al. 2015); here, our delivery of multiple trials of each taste allowed a more robust analysis (Fontanini et al. 2009; Katz et al. 2001; Li et al. 2013; Sadacca et al. 2016) and one in which individual time points occurring before behavioral responses can be examined separately (see below). The α-level was set at P < 0.05. This analysis was first performed on firing averaged across taste (providing a general quantification of the neuron’s sensitivity to taste input) and then again for each individual taste response.
Of course, this analysis (like all of those used to study electrophysiology) is imperfect. For one thing, this particular analysis assumes independence of samples, which, strictly speaking, trials are not. Fortunately, our test is robust to this assumption, and any lack of trial-to-trial independence is likely to make responses appear less taste distinctive than they truly are; furthermore, the tests below, which suffer even less from this assumption, provide a converging measure of the validity of our results. Similarly, although this analysis generates a relatively large number of statistical comparisons (another ubiquitous issue for studies of simple coding), the combination of this with the more unified, more conservative analyses described below ensures valid recognition of the nature of mouse GC taste coding.
Taste specificity.
The above analysis provides one estimate of the number of tastes to which a neuron responds, an estimate that has been used as the entirety of statistical validation of taste specificity used in previous studies (e.g., Yamamoto et al. 1984). This approach fails to provide a rigorous description of the taste specificity of the neuron’s firing, however: it is now well-recognized that the reaching of conclusions regarding differences between responses using only separate tests of each response (e.g., concluding that “because the neuron responds to both X and Y, its responses to X and Y are the same” or that “because the neuron responds significantly to X but not to Y, its responses to X and Y are significantly different”) is statistically invalid (Nieuwenhuis et al. 2011).
We instead tested (as we have using rat data: Li et al. 2013; Piette et al. 2012; Sadacca et al. 2012) whether single neuron’s responses were “taste specific” via simple, direct comparison of the firing rates elicited by the four tastes. Specifically, taste-induced firing was divided into four consecutive 500-ms bins, and a repeated-measures ANOVA was conducted with taste and time as within-subject variables; a neuron’s responses were categorized as taste specific if either the main effect of taste or the taste-by-time interaction was significant (P < 0.01): the former significant effect indicates simple taste specificity, i.e., at least one taste induced an amount of spiking that differed significantly from that driven by at least one other taste; the latter indicates “temporal coding” (in quotation marks because the suggestion is not that taste identification comes from analysis of the time course of firing but that cortical taste processing is poorly characterized using only overall firing-rate averages; see discussion), that the time course of the response to one taste was different from the time course of the response to at least one other taste (note that a set of responses could be identical in average rate but differ in time course, in which case the taste main effect would be nonsignificant, whereas the taste-by-time interaction would be significant). Post hoc tests (Tukey honestly significant difference) revealed precisely which responses differed from which.
Note that this analysis, although nonstandard in the field (largely because our approach is unusual in collecting multiple replicates of data with high temporal sensitivity), avoids the above-described pitfalls to which other analyses of response breadth fall prey, allowing uncommonly valid statements of how single-neuron responses to multiple tastes differ from one another. It is beyond the scope of this report to catalog and critique techniques used to assess neural coding in the taste field, but suffice it to say that all have their limitations and that the one described here provides a direct assessment of a subtle form of the question “is the firing of this neuron taste specific?” without introducing specific biases toward answering that question with a “yes.”
Entropy.
We employed the oft-used entropy (H) metric as a direct evaluation of the breadth of single-neuron tuning. H was computed as previously described by Smith and Travers (1979) using the following formula (see also Wilson and Lemon 2013):
where K is a constant (K = 1.66 for a 4-taste battery) and P is the proportional response to each taste (i). Taste-evoked responses used for entropy analyses were calculated by subtracting mean firing rates for the 2 s immediately preceding taste delivery from the 1st 2 s of postdelivery activity. Pi is derived by converting the evoked response for a particular cell into a proportion of the total response to all 4 tastes. A low H value indicates higher selectivity (H = 0 means that a unit responds purely to 1 taste), whereas a high H value represents broader tuning (H = 1 means that a unit responds to all tastes).
Note that this entropy analysis assumes that responses are positive going from a 0 baseline, whereas neural sensory responses may be negative going as measured from a spontaneous firing baseline; that is, responses may be inhibitory. We dealt with this issue simply by taking the absolute value of any inhibitory response for use in the calculation of an H index (Wilson and Lemon 2013). In practice, this solution was a conservative one: inhibitory responses, which are necessarily magnitude-limited by the 0-firing floor effect, will tend to look small in this analysis, despite the fact that they reflect particularly entropic responses (those that vary from substantially below to above 0); thus the use of absolute values will most likely lead to underestimation of a neuron’s breadth of responsiveness.
However, when combined with the above analyses, entropy provides a rich description of GC neuron taste responsiveness. For instance, a neuron that responds to all tastes equally would have high entropy and broad taste responsiveness but would likely be responding not to taste quality per se but to some other aspect of the stimulus. Accordingly, its firing would almost certainly fail to be deemed taste specific according to the taste main effect in a two-way ANOVA.
Finally, it is worth noting that any particular H between 0 and 1 can conceivably be described to represent broad or narrow coding, depending on one’s perspective. To enhance interpretability of H distributions, we therefore compared our results with those of identical analyses performed on simulated data sets (created based on the real data) made up of units with known breadth.
Time course of palatability-related responding.
Most previous studies of taste electrophysiology have made use of anesthetized animals; many of these studies have therefore necessarily ignored (for the most part) evaluation of psychological aspects of coding (e.g., Yokota et al. 1997). When palatability coding has been examined, that examination has typically involved inferences drawn from multivariate data-reduction techniques (multidimensional scaling and cluster analysis) without significance testing (e.g., Chang and Scott 1984; Geran and Travers 2006; Ho et al. 2011).
Once more taking a cue from our work on rats (e.g., Fontanini et al. 2009; Li et al. 2016; Piette et al. 2012; Sadacca et al. 2012, 2016), we performed a simpler, direct analysis using a moving window, an analysis that allowed us to identify time periods in which GC taste responses reflected the hedonic value of the tastes. We first determined the palatability of each taste used in this study for the strain of mice used here: a set of 32 mice of the same strain but separate from those used in the electrophysiological experiment were mildly water restricted for 3 days (2 30-min periods of access to water per day: 1 in the morning and another in the afternoon). Across the following 4 days, mice were exposed to each of the tastes (sucrose, NaCl, citric acid, or quinine; 1/day) for 30 min, and consumption was measured by weight. A subsequent 1-way ANOVA revealed strong interstimulus differences in consumption [F(3,28) = 32.97, P < 0.01]. Although this protocol does not, strictly speaking, rule out postingestive effects on consumption, the preferences determined here precisely matched the palatability ranking determined by lick analysis in brief-access tests performed in mice (Sinclair et al. 2015; Tordoff et al. 2015) and rats (Li et al. 2013): sucrose > NaCl > citric acid > quinine. Given as well the very fact that the relationship between these results and cortical coding proved identical to that previously observed using the brief-access assay to acquire preference data (see results), it is reasonable to conclude that the test validly measured taste preferences in our mice.
We then, for each window of neural firing (window size: 250 ms; step size: 25 ms), performed a Spearman product-moment correlation between the ranked firing rates to each taste and the palatability rankings (Spearman was used, rather than the parametric Pearson, because palatability rankings are ordinal variables). Note that, for this analysis, it matters not to what degree an individual taste was or was not preferred to water; the calculated correlation, like the two-way ANOVA used to measure taste specificity of firing, cleanly measures the palatability relatedness of firing by relating the activity driven by each taste to that driven by the others. However, because this procedure is another that results in a relatively large number of actual tests, we limited the chances of type I errors by requiring that the test achieve significance for three consecutive bins before responses could be deemed palatability related.
Modeling of, and Firing-Rate Change-Point Identification in, Ensemble Firing Data
GC taste processing is dynamic: rat cortical responses have been shown to undergo a pair of sudden and coherent firing-rate transitions in the 1.5 s immediately following taste delivery; the state before the first transition is the same for all tastes, reflecting taste “detection;” the states attained following the first and second transitions are both taste specific, with the last state further reflecting taste palatability (and both predicting and driving taste behavior in single trials; see Li et al. 2016; Sadacca et al. 2016). To detect whether such transitions between states are also facets of mouse cortical taste processing, we performed a simple change-point analysis on ensemble spike data. This analysis is summarized here; for complete details, see Mukherjee et al. (2019).
Trials of ensemble spiking data were categorized in terms of which neuron spiked in each 1-ms bin; an index of 0 corresponded to no spikes from any neuron. If more than one neuron spiked in a time bin, a highly uncommon occurrence, given the relatively low firing rates of GC neurons, we randomly selected one spiking neuron to index that bin (Jones et al. 2007; Sadacca et al. 2016). We then fit a change-point model to the 1st 1.7 s of posttaste delivery ensemble activity, describing ensemble firing-rate transitions between states as categorical distributions with emissions. We initially considered the possibility that our mouse GC data might be best fit by models containing fewer or more than two change points. To compare the quality of the fits of the different models with the ensemble spiking data, we computed the Akaike information criterion (AIC) using the formula AIC = 2 × k − 2 × LL, wherein k represents number of parameters and LL is log likelihood of a particular model, and then examined the properties of the identified change points.
As the results of this analysis confirmed the appropriateness of the 3-state (i.e., 2-change-point) model (see results), we constructed a more extensive model constrained on the basis of the rat cortical findings described above: specifically, we constrained the change from state 1 to state 2 (change point 1, or CP1) to happen within the interval (50–600 ms) and constrained the 2nd change point (CP2) to occur within the interval (CP1 + 0.2 s to 1,500 ms). This is equivalent to placing uniform priors over the intervals that define CP1 and CP2, corresponding to the timing of sudden, coherent firing-rate transitions in GC ensembles (Jones et al. 2007, Sadacca et al. 2016). With these assumptions, the expectation-maximization algorithm, the most widely used approach to perform inference in such models with latent variables (CP1 and CP2), was employed to fit the change-point model (Bishop 2006).
Note that to facilitate a central test of this model, the above-described constraints on the timing of state changes are broad and deliberately not centered on the average timing of the transitions as previously observed in rats: specifically, on the basis of our previous work, we predicted CP1 to occur on average at ~200 ms postdelivery and predicted CP2 to occur on average at ~900 ms postdelivery. If ensemble spiking fails to go through genuinely sudden, coherent firing transitions, any change points identified by the analysis would instead appear (on average) in the middle of the available interval. This result, or any change-point timings, would falsify the prediction that mouse GC ensemble taste responses resemble rat GC ensemble taste data.
Histology
At the end of each experiment, mice were deeply anesthetized and perfused transcardially with 0.9% saline for 1 min followed by 4% paraformaldehyde. Brains were extracted and postfixed in 4% paraformaldehyde for 24–48 h, after which coronal brain slices (60 µm) containing the GC were sectioned on a vibratome. Sections were rinsed five or six times over 90 min (PBS-0.3% Triton X-100), mounted on charged glass slides, and coverslipped with mounting medium including DAPI (VECTASHIELD). The placement of electrodes was determined by localizing Dil (a lipophilic dye coated on electrodes during implantation). To visualize electrode bundle locations, bilateral GC sections were viewed by confocal fluorescence microscopy with a Leica SP5 spectral confocal microscope/resonant scanner with 405 (for DAPI), 546 (for Dil dye), and 488 (for green fluorescent protein) lasers equipped with x-y-z movement stage.
RESULTS
As shown in Table 1, a total of 185 units were isolated across 10 sessions in 6 mice (10–37 neurons per ensemble); 4 mice received 2 sessions each (in which case sessions were recorded from separate DV locations in the GC; see materials and methods). Our recordings spanned much of the GC region (AP bregma +1.6 to 0.0 mm; DV 2.25–3.25 mm from the surface of the brain; see Fig. 1) receiving input from taste thalamus (Chen et al. 2011; Fletcher et al. 2017) or from the amygdala (Mátyás et al. 2014). Prior work has shown these regions to be important for taste behavior and learning in mice (Neseliler et al. 2011).
Table 1.
Number of neurons included in each population ensemble
| Mouse | Recording Session | No. Neurons |
|---|---|---|
| WT2 | 1 | 10 |
| 2 | 22 | |
| WT3 | 1 | 10 |
| 2 | 17 | |
| S7 | 1 | 14 |
| S12 | 1 | 25 |
| 2 | 17 | |
| S13 | 1 | 18 |
| 2 | 15 | |
| S14 | 1 | 37 |
Basic Characterization of Taste-Evoked Responses in the Gustatory Cortex of Mice
Figure 2 presents raster plots and associated peristimulus time histograms (PSTHs) of the taste responses of three simultaneously recorded GC neurons. The first two are regularly spiking (putative) pyramidal neurons, and the third is a fast-spiking (putative) inhibitory interneuron. Note that we did not rigorously identify every recorded neuron as a (putative) pyramidal cell or interneuron using baseline firing rates and action potential widths here; we have done so previously, and this prior work revealed no evidence of major cell-type specificity with regard to any of the analyses performed below (Fontanini and Katz 2006; Jones et al. 2007; Katz et al. 2001).
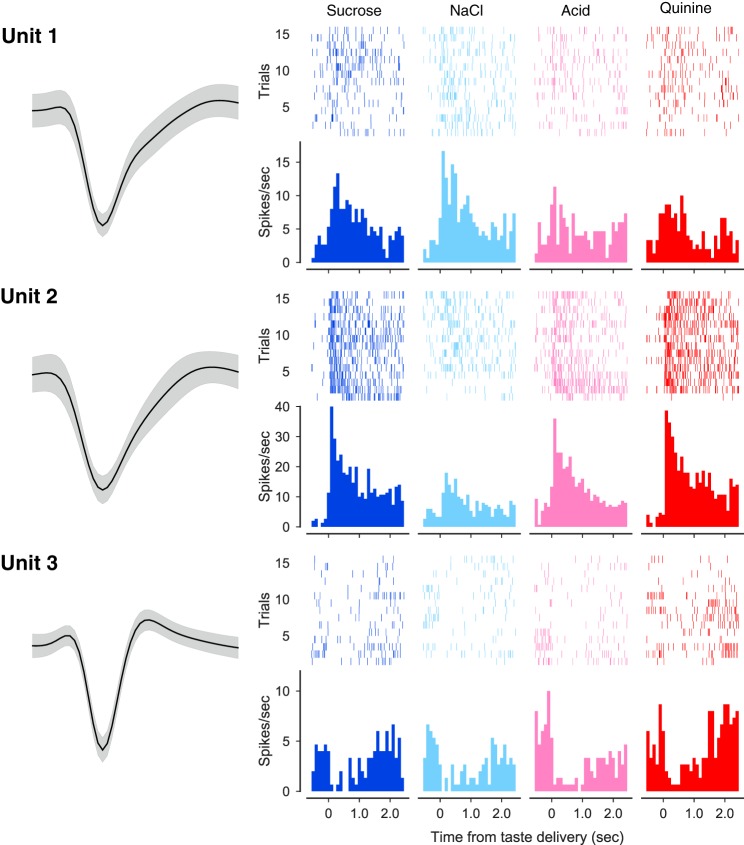
Fig. 2.
Simultaneously recorded gustatory cortex neurons. Left column shows the average action potential waveforms (surrounded by confidence intervals) for 3 neurons recorded simultaneously from the same electrode bundle; each of them had interspike interval violations <1%. Remaining 4 columns show the responses of these neurons to sweet (sucrose), salty (sodium chloride), sour (citric acid), and bitter (quinine) tastes. Top half of each response display is spike time raster plots (each row is a single trial, and each dot is an action potential), below which are associated peristimulus time histograms (firing rate in spikes per second in the y-axes; time poststimulus in the x-axes). Each neuron’s responses were taste specific (see materials and methods), and each individual neuron responded distinctly.
Each of these neurons’ responses were significantly (see materials and methods) taste specific: unit 1 responded to all four tastes but responded more strongly to NaCl and sucrose than to the remaining tastes; unit 2, on the other hand, responded strongly to sucrose, citric acid, and quinine but far less to NaCl; unit 3, meanwhile, was inhibited to differing degrees and for differing lengths of interval by all four tastes.
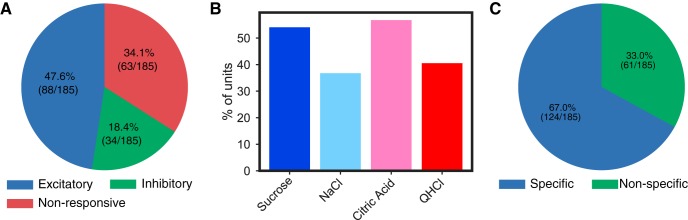
Even the most basic examination of the full sample of responses revealed that a large portion (just under 2/3) of mouse GC neurons respond to taste stimulation (significant paired t tests comparing pre- with poststimulus firing across all trials summed across tastes; Fig. 3A), with excitatory responses (enhanced firing) outnumbering inhibitory (reduced firing) by almost 3 to 1. With the use of repeated-measures ANOVAs to directly compare each neuron’s average and time-varying response to different tastes, we were able to identify an almost identical percentage of neurons as being “taste neurons” (i.e., responding distinctly to ≥1 taste; see materials and methods and Fig. 3C). Although these particular t tests and ANOVAs will not necessarily identify precisely the same neurons, together they suggest that the vast majority of taste responses in mouse GC are truly taste related, as opposed to reflecting somatosensory or general cognitive factors.
Fig. 3.
Mouse gustatory cortical (GC) neurons respond to tastes. A: a pie chart showing the percentage and distribution of GC neurons that produced excitatory or inhibitory responses to taste stimulation. B: similar percentages of mouse GC neurons respond to sweet, salty, sour, and bitter (quinine, QHCl) tastes; ≥35% of the sample responded to each taste. C: 2-way within-neuron ANOVAs comparing responses to the different tastes as a function of time (taste × time, P < 0.01) revealed that the majority of mouse GC neuron taste responses were also taste specific (i.e., different for different tastes).
Despite being taste specific, these responses were broad: significant firing-rate modulations in individual neurons were seldom restricted to only one, or even two, of the four basic tastes delivered. Had they been narrow, then the rate of significant responses to each individual taste (determined using simple t tests, as above, applied to each individual PSTH) would be expected to be around one quarter of this 66% (that is, we would expect ~16% of the neurons to have sucrose responses, another ~16% to have NaCl responses, etc.). A far higher percentage of neurons responded to each taste, however (Fig. 3B), a result consistent with the suggestion that most taste neurons in mouse GC must respond to more than one taste on average.
Because this sort of analysis carries with it a host of (commonly ignored) caveats (see materials and methods), we performed multiple convergent tests of this conclusion. First, we directly analyzed the number of tastes that evoked significant responses in each individual neuron (by pre- vs. poststimulus t tests). According to this analysis, 28.6% of our mouse GC neurons responded significantly to one taste, 26.5% responded to two tastes, 16.8% to three tastes, and 14.1% to all four tastes (Fig. 4A). Although it is inappropriate to reach conclusions concerning taste specificity from this method (which does not directly compare responses to different tastes; see materials and methods), these results confirm that the majority of GC neurons that respond to taste stimulation respond to more than one taste.
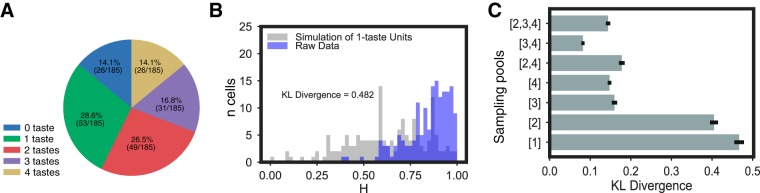
Fig. 4.
Gustatory cortical (GC) neurons code tastes broadly. A: independent paired t tests comparing pre- and posttaste firing rates reveal that the majority of mouse GC neurons respond to >1 taste (even when totally nonresponsive neurons are included in the comparison). B: the distribution of the breadth of taste coding [i.e., entropy (H), x-axis] in GC neurons (purple bars, y-axis = number of neurons) is highly skewed toward broad tuning (a low H indicates narrowly responsive neuron, and a high H indicates a neuron responsive to several tastes). Overlain (gray bars) is the distribution of response entropies for an equivalently sized set of simulated units that respond to only 1 taste. Difference of the 2 distributions was calculated using the Kullback–Leibler (KL) divergence and was highly significant according to a χ2 test conducted on the data. C: KL divergences between the collected data and a range of simulated data sets in which taste responses were simulated from units sampled from the population of neurons responding to particular numbers of tastes (sampling pools).
We went on to calculate response entropy (H) for each neuron, using standard techniques (Samuelsen et al. 2013; Smith and Travers 1979); low H values indicate narrowly responsive neurons, and high H values indicate broadly tuned neurons. The distribution for our neural sample proved to be highly positively skewed, with the vast majority of GC neurons’ H values suggesting broad responsiveness; the distribution was similar when only neurons identified as responding in a taste-specific manner were included (Fig. 4B).
To enhance the interpretability of this last result (see materials and methods), we plotted (Fig. 4B) the distribution of entropies against the H values of a simulated set of neurons modeled on those real neurons identified as responsive to a single taste. This distribution, indicated by χ2 analysis, is significantly different from the distribution of the full set of real data (χ2 = 82.75, P < 0.01), which, in fact, most resembles (according to the Kullback–Leibler divergence) simulated data sets modeled on neurons that respond to three or four tastes (Fig. 4C).
This analysis also reveals that even neurons that in Fig. 4A are listed as responsive to only one taste are not 100% unresponsive to other tastes. Were this not the case, that is, were neural firing totally unaffected by administration of three out of the four tastes, the calculated entropy would equal 0.0; instead, many “one-taste” neurons actually produced subsignificant firing-rate changes to multiple other tastes, a fact that leads even these one-taste neurons to show entropy values >0.5 (see detailed discussion in Smith and Travers 1979). Thus Fig. 4, B and C, is consistent with the suggestion that mouse GC neurons tend toward broad tuning.
Dynamics of Taste-Evoked Responses in Mice GC
In revealing GC taste neurons to be broadly tuned “coarse coders,” the above data are consistent both with previous reports of taste responses in rat GC (Bahar et al. 2004; Fontanini and Katz 2006; Jones et al. 2007; Piette et al. 2012; Samuelsen et al. 2013; Yamamoto et al. 1985) and with calcium imaging data from mouse GC (Fletcher et al. 2017; although see Chen et al. 2011). However, further examination reveals that the above analyses, and the conclusions drawn from them, inadequately describe taste processing in mouse GC in that they ignore meaningful response dynamics that unfold across the 1st 1.5 s of poststimulus time (the period leading up to the making of consumption decisions and production of consumption-related behaviors; see Grill and Norgren 1978; Li et al. 2016; Travers and Norgren 1986), response dynamics that can only be reliably observed using multiple trials of electrophysiological data.
Even simple visual scrutiny of mouse GC taste PSTHs suggests the presence of such interpretable firing-rate dynamics, with response magnitudes changing through time in taste-specific manners. An example is shown in Fig. 5A: a mouse GC neuron that responded strongly to NaCl during the first postdelivery second only and strongly to quinine during the second postdelivery second only. When this pattern of firing is averaged across time, both of these responses “wash out,” and the neuron incorrectly registers as being responsive to citric acid only; this result confirms the need to move beyond analyses that average across time, if the purpose is to comprehensively characterize taste specificity of firing in periods leading up to behavioral responses (see discussion).
Fig. 5.
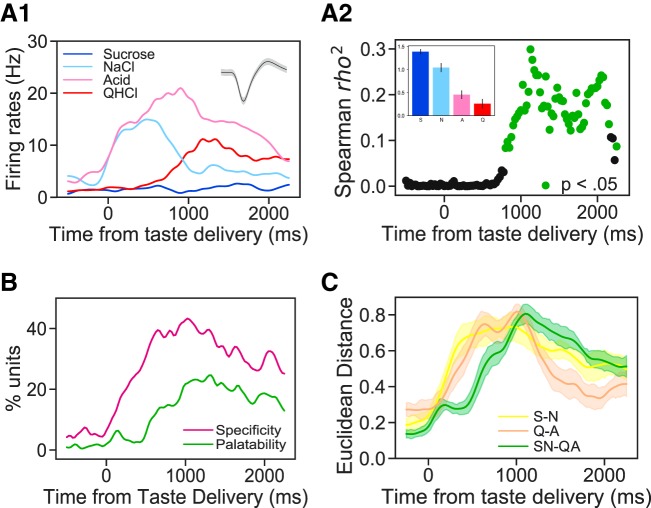
Mouse gustatory cortical (GC) responses evolve through successive epochs of taste quality and taste palatability coding. A1: overlain peristimulus time histograms (y-axis, firing rate; x-axis, time poststimulus) from a representative GC neuron that responded significantly to all tastes but sucrose (S). A2: moving window analysis of the correlation (r2; y-axis) between firing rates and taste palatability (determined by taste intake; see inset) for the neuron shown in A1. Green dots denote significantly non-0 correlations. B: percentages of the entire sample of GC responses that show significant taste specificity (magenta) and palatability relatedness (green). Taste specificity starts to rise soon after taste delivery and peaks at 500 ms; in contrast, palatability-related firing appears only following a delay (~500 ms) and peaks at ~1 s poststimulation. C: response differences (Euclidean separation in multidimensional scaling solutions; see materials and methods for details) between same-palatability taste qualities (S-N and Q-A) emerge soon after stimulus delivery; between-palatability differences, on the other hand (SN-QA), emerge later, confirming that identity-related activity is followed by palatability-related firing. A, citric acid; N, sodium chloride; Q or QHCl, quinine.
Previous work using rats has suggested that an intrinsic part of GC taste response dynamics is the relatively late transition from taste-specific firing into palatability-related firing. To rigorously evaluate the hypothesis that mouse GC taste responses start taste specific and then become palatability-related, we first ascertained behavioral preferences for our battery of four tastes (which differed in both chemical identity and palatability, sucrose and sodium chloride representing palatable tastes and citric acid and quinine representing nonpalatable tastes) in the same strain of mice being used in the electrophysiological experiments, using a single-bottle consumption test (see materials and methods). We then correlated this vector of relative palatabilities (note that we are concerned here, as we were when evaluating taste specificity of firing above, with differences between tastes and not with absolute levels of palatability) with firing rates to the same battery of tastes. For this purpose, firing was calculated for small bins across the 2-s response epoch (using a moving-window analysis as explained in materials and methods and used previously in Fontanini et al. 2009; Li et al. 2016; Piette et al. 2012; Sadacca et al. 2012, 2016).
The result of this analysis brought to bear on the neuron shown in Fig. 5A1 is shown in Fig. 5A2 (with the empirical taste preference data presented as an inset): this single neuron fired in a taste-specific manner almost from the moment of taste delivery, but firing did not become significantly palatability related (green dots) until several hundred milliseconds later, and palatability relatedness only reached an asymptote a full second after taste delivery. In this case, the ordering was “aversive high,” which appeared with approximately the same frequency in our neural sample as “aversive low.” As described above, this pattern reflects the fact that firing rates were modulated at least twice in succession following taste delivery: the first firing-rate modulation resulted in taste specificity, and the second in palatability relatedness.
Figure 5B, which presents the results of this analysis performed on the entire data set, confirms the representativeness of the example: the incidence of taste specificity rises almost immediately following taste stimulus delivery and approaches peak levels as early as 500 ms into the response; palatability relatedness, on the other hand, rises more slowly, beginning to trend upward ~500 ms after stimulus delivery and approaching a peak ~1,000 ms into the response.
We performed one further test of this result, analyzing the distinctiveness of different subsets of taste responses for each neuron at each point in response time; distinctiveness was quantified in terms of normalized Euclidean distances separating two taste responses in a multidimensional scaling solution. Given the above results, we predicted that the distinctiveness of individual pairs of tastes would rise quickly but that the distinctiveness of palatability differences, that is, comparisons of the averaged responses to the palatable tastes (sucrose and NaCl) with the averaged responses to the aversive tastes (quinine and citric acid), would emerge only later. Figure 5C reveals that this is precisely what we observed: the distinctiveness of codes for sucrose and NaCl (yellow line ± SE) rose quickly in postdelivery time, as did the distinctiveness of codes for quinine and citric acid (beige line ± SE); the rise in the distinctiveness of palatabilities (green line ± SE), meanwhile, was delayed. Note as well that following the rise of palatability distinctiveness, the distinctiveness of individual “same-palatability” taste pairs fell precipitously, confirming that general taste specificity becomes refined across time into palatability relatedness.
Finally, we performed a control analysis that examined the distance between palatability-unrelated groups (i.e., sucrose-citric acid and quinine-NaCl) and found that the distinctiveness peaked at a relatively low value and remained stable at that level across time, confirming that “the later rise” of distance between palatability-distinct taste groups is not simply due to the random grouping effect but reflects the nature of temporal dynamic in taste processing.
Thus we conclude that mouse GC codes taste properties sequentially across 1.5 s of poststimulus time, with taste specificity appearing before palatability relatedness. Ancillary analysis confirms that many individual neurons respond distinctly in the two epochs, coding both taste identity early and taste palatability late. In fact, 50% of the neurons that produce significantly taste-specific firing also produce significantly palatability-related firing (as in the example neuron shown in Fig. 5A). This result is novel and, in fact, can currently only be recognized using electrophysiology: the subtle difference in responses that we report, and shifts in those differences across a few hundred milliseconds, are almost impossible to discern with calcium imaging data.
Ensemble Properties of Mouse Cortical Taste Dynamics
Having established the existence of reliable, interpretable single-neuron dynamics in mouse GC, we moved on to asking whether these single-neuron dynamics are, in fact, reflections of coherent ensemble activity (as they are in rats; see Sadacca et al. 2016). To answer this question, we applied multisignal change-point (CP) modeling procedures (Mukherjee et al. 2019) to data sets comprising simultaneously recorded neural ensembles, to test whether transitions between the above-observed firing-rate epochs are 1) coherent between neurons, 2) sudden and jumplike, and 3) trial specific in latency.
We first compared our ability to model the data using two CPs (the predicted model given the above results and previous work on rats) with that achieved using models with fewer or more CPs, with the logic being that if our hypotheses are correct, a two-transition model should fit the data particularly well. Initial assessment of the quality of model fit making use of the Akaike information criterion (AIC) confirmed that mouse GC ensemble taste data are typically well-characterized as going through a set of 3 states separated by 2 CPs (2 sudden transitions of firing that were coherent across simultaneously recorded neural ensembles). Although in a subset of instances a 3-CP model fit equally well, scrutiny of these successful 3-CP fits revealed that, in over half of the trials, multiple CPs were clustered with minimal time separations; they were essentially 2-CP solutions with 1 of the CPs identified twice. Similar results were observed in a subset of data sets in which the fit of a 4-CP model was examined. Clearly, mouse GC taste responses are best described as going through a sequence of 3 firing-rate states separated by sudden, coherent ensemble firing-rate transitions.
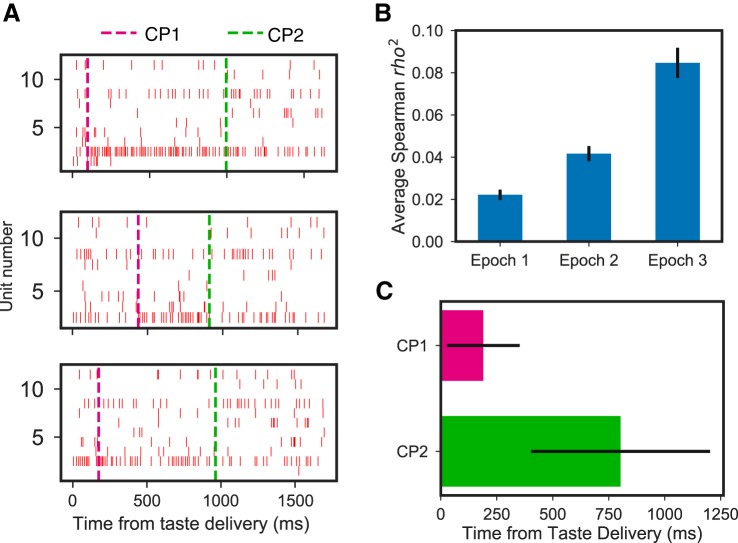
Our analysis of the data as characterized by the 2-CP model demonstrates further features of the taste code in mouse GC. Figure 6A shows an example of 3 quinine trials showing within-trial firing-rate transitions. Clearly, the transitions happen at different times on different trials. To test whether the transitions relate to the earlier-described epochs, we once again correlated palatability with firing but did so separately for the data in each successive state (putting all state 2 firing into 1 analysis, for example). The results of these analyses are displayed in Fig. 6B; a 1-way ANOVA revealed that the palatability correlation varied with state, F(2,368) = 42.80, P < 0.01, and post hoc comparisons (Tukey honestly significant difference test) further revealed that this significance is largely driven by the fact that the correlation with palatability is significantly higher following the 2nd transition than before (P < 0.05). The fact that palatability correlations are modestly higher following the 1st transition than before reflects the simple fact that the very earliest 100–200 ms of GC responses (i.e., the brief period preceding even the 1st transition) are, as they are in rats, totally nonspecific; almost any taste specificity will enhance correlations with palatability beyond those chance correlations observed with nonspecific firing.
Fig. 6.
Epochs of mouse cortical taste responses reflect coherent state sequences in gustatory cortical (GC) ensembles. A: representative single trials of the response of 1 GC ensemble (1 neuron per row; hash marks = individual action potentials) to the taste of quinine, revealing sudden, simultaneous changes in firing rates in several neurons. Magenta and green dashed lines indicate the 1st change point (CP1: from taste detection to identity) and 2nd change point (CP2: from taste identity to palatability), respectively. B: coefficient of determination (r2) between mean firing rates and palatability for each of the 3 states demonstrate, consistent with our hypotheses and work on rat GC, that palatability coding is achieved with state 3 and that taste specificity (which necessarily connotes a basic level of non-0 palatability coding) is achieved with state 2. C: average state-to-state transition times across all trials revealed through change-point analysis.
The average transition times for CP1 and CP2 occurred at ~190 and ~800 ms, respectively (Fig. 6C). Note that these average transition times differed distinctly from the time point that represented the middle of the available intervals (i.e., 325 ms for CP1 and 925 ms for CP2; see materials and methods). This finding proves that the CPs are not simply artifacts of the procedure itself; rather, these latencies are good matches for our prior rat data and for our single-neuron results (Fig. 5C) and confirm our expectation that mouse GC ensembles undergo fairly sudden and coherent shifts in firing while responding to tastes.
These results suggest that even dynamic analysis of single-neuron taste responses in mouse GC, as described above, fail to completely characterize taste processing. Rather than being independent coding elements, single-neuron responses are likely reflections of population coding, an unsurprising fact given the mesoscopic (intracortical connectivity) and macroscopic (between-region feedback) circuitry involved in taste but one that has not been recognized previously.
Spatial Analysis of Mouse Cortical Taste Response Properties
GC is a distributed, heterogeneous region that can be subdivided according to cytoarchitectural and connectivity criteria in the dorsal-ventral and anterior-posterior axes (Allen et al. 1991; Maffei et al. 2012). The imaging literature has failed to provide clarity on the significance of these anatomic subdivisions: one set of authors has suggested that mouse cortical taste coding, unlike that of rat (Accolla et al. 2007), is cleanly gustotopic (Chen et al. 2011; Wang et al. 2018), whereas other research groups suggest otherwise (Fletcher et al. 2017; Lavi et al. 2018).
To explore the potential spatial dependencies of GC taste coding using electrophysiology, we compared taste-evoked firing along the two primary axes of GC. In the dorsal-ventral dimension, we were able to look within-subject, as our electrode bundles could be driven ventrally between tasting sessions. Figure 7 shows a pair of example neurons recorded from dorsal GC and a second pair recorded from ventral GC in the same mouse. It can be seen that each of these responses are quite broad, suggesting basic similarities between dorsal and ventral GC.
Fig. 7.
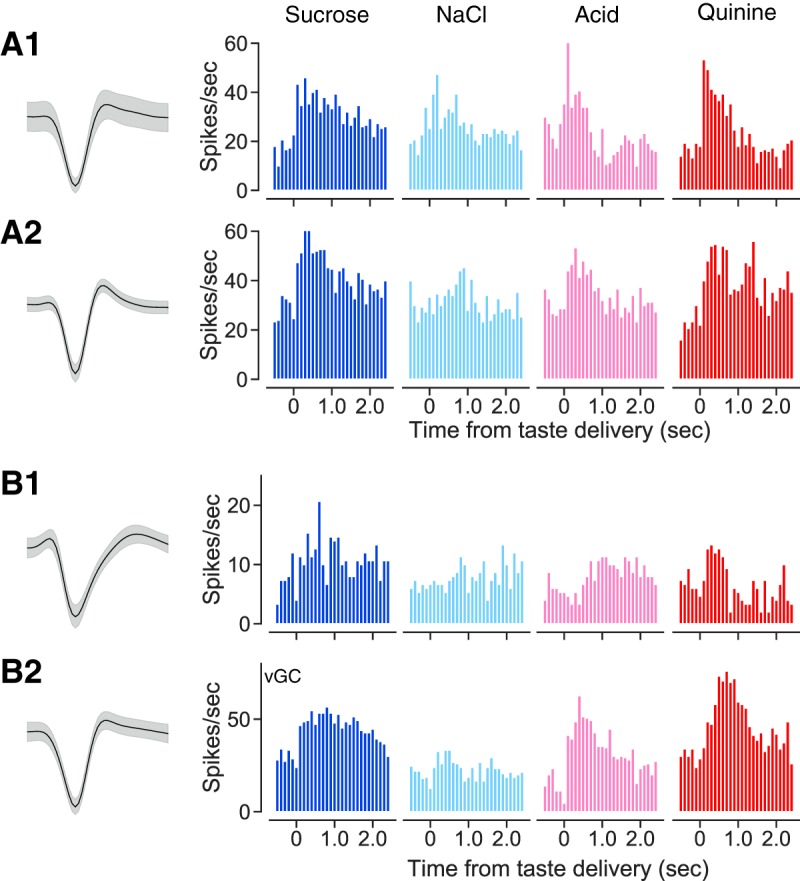
Broadly responsive ensembles of gustatory cortical (GC) neurons can be recorded from dorsal GC (A1 and A2) and ventral GC (vGC; B1 and B2) of the same mouse. A1 and A2: representative neurons (wave shapes and peristimulus time histograms; conventions as for Fig. 2) recorded in dorsal GC. B1 and B2: representative neurons recorded from the same mouse in a 2nd session.
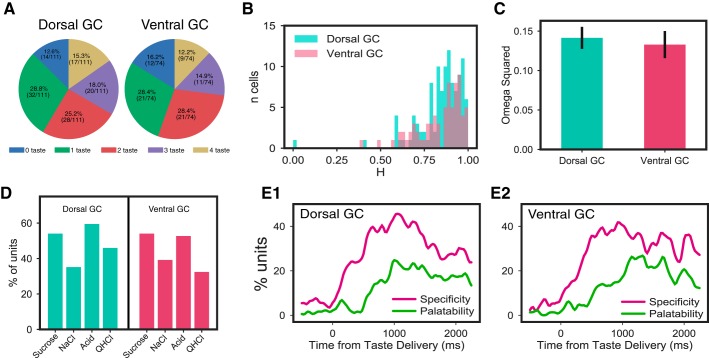
Closer analysis confirmed that both dorsal and ventral GC contained neurons that produced broad, dynamic taste responses. Analysis of the entire sample divided into dorsal and ventral recordings revealed no striking differences in breadth of responsiveness, measured either in terms of number of tastes responded to (Fig. 8A; χ2 = 1.15, P = 0.885) or response entropy (Fig. 8B; χ2 < 1). The magnitudes of taste specificity revealed by ANOVA were similar across dorsal and ventral neurons (Fig. 8C; F < 1), and the pattern of how many neurons responded to which taste did not differ significantly between subregion (Fig. 8D); in both dorsal and ventral GC, single neurons were more likely to respond to both palatable and aversive tastes than either alone (data not shown). Finally, dorsal and ventral neural samples showed similar time courses of response evolution from quality to palatability, measured in terms of the number of neurons showing such responses at each time point (Fig. 8, E1 and E2).
Fig. 8.
Dorsal and ventral gustatory cortical (GC) taste responses in mice have similar properties. A: percentages of dorsal and ventral neurons with significant responses to 0, 1, 2, 3, and 4 tastes. χ2 Tests revealed no significant differences between the distributions. B: response entropies (H) for dorsal and ventral GC neurons were not significantly different, according to χ2 tests comparing the distributions. C: magnitudes of taste specificity [expressed as ω2 (effect size) from repeated-measures ANOVAs; see materials and methods for details] for dorsal and ventral GC neurons. t Test revealed no difference. D: distribution of relative responsiveness to the 4 tastes is virtually identical in dorsal and ventral GC; a χ2 test did not suggest any differences in which subregion responded to which tastes. QHCl, quinine. E1 and E2: in both dorsal and ventral GC, taste specificity starts to rise immediately after taste delivery and peaks at 500 ms, and in both, the emergence and peaking of palatability-related firing was delayed (see Fig. 5).
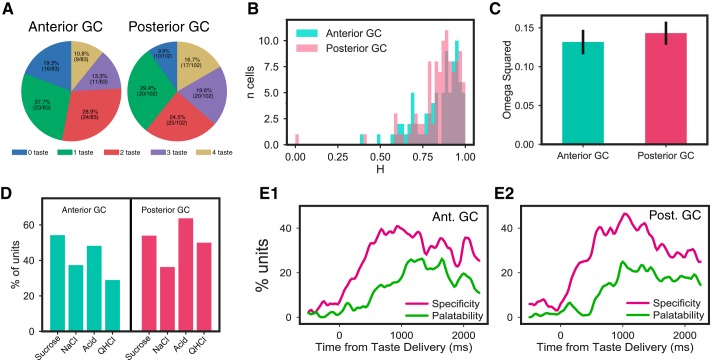
The results of our analysis of the anterior-posterior dimension were analogous. We compared taste responses between bregma +1.6 and bregma 0.0, treating everything forward of 0.8 as anterior. This analysis revealed no significant differences between anterior/posterior breadth of responsiveness, measured either in terms of number of tastes responded to (Fig. 9A; χ2 = 5.51, P = 0.239) or response entropy (Fig. 9B; χ2 < 1); furthermore, neurons from anterior and posterior GC subdivisions had similar magnitudes of taste specificity (Fig. 9C; F < 1), similar patterns of how many neurons responded to each taste (Fig. 9D), and similar time courses of response evolution from quality to palatability (Fig. 9, E1 and E2).
Fig. 9.
Anterior and posterior gustatory cortical (GC) taste responses in mice have similar properties. A: percentages of anterior and posterior neurons with significant responses to 0, 1, 2, 3, and 4 tastes. χ2 Tests revealed no significant differences between the distributions. B: response entropies (H) for anterior and posterior GC neurons were not significantly different, according to χ2 tests comparing the distributions. C: magnitudes of taste specificity [expressed as ω2 (effect size) from repeated-measures ANOVAs; see materials and methods for details] for anterior and posterior GC neurons. t Test revealed no difference. D: distribution of relative responsiveness to the 4 tastes in posterior GC was similar to that in anterior GC; although responsiveness to the 2 more aversive tastes was somewhat higher in posterior GC, a χ2 test did not reveal any significant differences in which subregion responded to which tastes. QHCl, quinine. E1 and E2: in both anterior and posterior GC, taste specificity starts to rise immediately after taste delivery and peaks at 500 ms, and in both, the emergence and peaking of palatability-related firing was delayed (see Fig. 5).
Together, these results suggest that the details of taste coding are relatively insensitive to anatomic location within mouse GC. Neurons across the anterior-posterior and dorsal-ventral axes code taste modalities in a broad and temporally complex manner. Of course, the lack of significant difference between anatomic subregions does not equal rigorously identified “sameness,” and the possibility remains that subtle differences between anatomic subregions (or between species, for that matter) remain to be identified. Nonetheless, these analyses lead us to conclude that whatever differentiates different parts of gustatory insular cortex (see discussion), it is neither the breadth nor dynamics of single-neuron taste responsiveness.
DISCUSSION
Mice have become the most common nonhuman mammalian species studied by neuroscientists, a fact that can no doubt be attributed to the accessibility of mouse genetics, which allows researchers, with (relative) ease, to study the underlying molecular mechanisms of cellular, network, and behavioral phenomena (Kandel et al. 2014; Stevens 1996). As the sense with (arguably) the tightest link to behavior and learning (Carleton et al. 2010; Katz and Sadacca 2011; Maffei et al. 2012), taste is a particularly good system with which to study these topics in a unified manner. Furthermore, this work can take advantage of the extensive progress that has been made toward understanding key principles of taste coding in awake rats (Accolla et al. 2007; Bahar et al. 2004; Fontanini and Katz 2006; Jones et al. 2007; Katz et al. 2001; Li et al. 2016; Moran and Katz 2014; Sadacca et al. 2012; Samuelsen et al. 2012). It is somewhat surprising, therefore, that there has been almost no electrophysiological work done on mouse cortical taste coding and that the imaging studies performed thus far have failed to provide consensus on basic features of gustatory sensory coding (Chen et al. 2011; Fletcher et al. 2017; Lavi et al. 2018).
In fact, although electrophysiological responses to stimuli including tastes have been examined in anesthetized mice (Wilson and Lemon 2013), the current study represents the first thorough investigation of single-neuron spiking responses to a broad battery of taste stimuli by primary gustatory cortical (GC) neurons in awake mice (but see Kusumoto-Yoshida et al. 2015). By delivering a battery of four basic tastes (sweet, salty, sour, and bitter) directly to the tongue of freely moving mice while making recordings from a multielectrode bundle designed to record the activity of small ensembles of single neurons, we are able to confirm the conclusion reached by Fletcher et al. (2017) and Livneh et al. (2017), namely, that mouse GC codes taste in a largely nonsparse manner: approximately two-thirds of recorded GC responses conveyed information about taste identity; by and large, these responses were broadly tuned: over half of the recorded neurons responded to more than one taste, and over half of taste neurons produced firing patterns that were distinctive for three of four tastes.
However, we were able to go further, to reveal aspects of mouse GC taste responses that are largely hidden in calcium imaging data. As detailed in materials and methods, the fine-grained temporal resolution and high signal-to-noise ratio offered by electrophysiology allowed us to show that the time courses of taste responses contain information about the taste (see Fig. 5A for an example): that between-taste differences in time course are not random. Specifically, mouse GC taste responses evolve in a characterizable way across 1–1.5 s. We identify separate epochs of GC responses related to the physiochemical and psychological properties of the tastes in our stimulus battery; this work adds to previous analyses that identified onset and “steady-state” responses in rodent taste responses (phases that are general to all sensory modalities; see Anderson et al. 2000; Katz et al. 2001; Sanchez-Vives and McCormick 2000), showing in more detail the dynamics and content of these broad phases. A large portion (50%) of the recorded neurons coded stimulus identity and stimulus palatability sequentially, rather than being active in only one coding epoch.
These results suggest that the processing of quality and palatability is likely not performed by independent neural ensembles. In fact, population ensemble analysis (Fig. 6) shows that taste processing instantiates a temporal hierarchy in the activity of the coordinate action of distributed ensembles of GC neurons, a very different processing profile from that suggested in recent imaging studies in GC, in which taste identity and palatability were proposed to be confined to specific anatomic locations (Chen et al. 2011; Wang et al. 2018), or what has been revealed in the orbitofrontal cortex, a cortical area considered as the secondary taste cortex and only found to respond to palatability-related sensory stimuli (Rolls et al. 1999; Rolls and Grabenhorst 2008).
Our study adds to a developing foundation for comparative analysis of mice and rat GC taste processing. Although the anatomies of the two species’ taste systems are similar, little work has been done comparing taste coding between the two species. In all of our current results, we observed a striking similarity between mouse cortical taste coding and that extensively described in rat data (Accolla et al. 2007; Bahar et al. 2004; Fontanini and Katz 2006; Jones et al. 2007; Katz et al. 2001; Piette et al. 2012; Sadacca et al. 2012; Samuelsen et al. 2013; Yamamoto et al. 1985). Both species code tastes broadly, and these codes reflect identity and taste palatability in separate, sequential epochs. It may well be that differences will emerge with regard to the impact of taste learning on neural coding (which we will examine in the future). Although it is not our task in this paper to relate these basic findings to those that have come before in rat (for that, see the above references), our results are also broadly consistent with the many previous studies of both mouse and rat taste electrophysiology: few of these previous studies examined the coding content of subepochs of taste responses (mainly for methodological reasons; see materials and methods), focusing instead on using clustering techniques to identify “neuron types;” nonetheless, the findings of these studies are perfectly compatible with the findings here, which provide a different (and behaviorally relevant; see Li et al. 2016; Mukherjee et al. 2019; Sadacca et al. 2016) way of looking at the responses.
It is worth noting that concerns about the generality of our finding might be raised on the basis of the fact that different strains of mice have different taste preferences (e.g., Johnson et al. 2010; Sclafani 2006) and would therefore be expected to respond differently to tastes. Although this is a legitimate concern, we consider the likelihood that strain differences would significantly impact the results presented in our paper to be remarkably low, for at least two reasons. First, given that the response profiles of two entirely different species (rats vs. mice), species with notably different responses to sapid stimuli, are essentially the same, it would be exceedingly surprising to see subtle strain differences having a large impact. Furthermore, the noted strain differences are unlikely to affect the fact that palatability coding follows after quality coding; subtle differences in preferences have been shown to impact only the specifics of which taste responses most resemble which taste responses in the late coding epoch (see Fontanini and Katz 2006; Grossman et al. 2008; Moran and Katz 2014). Accordingly, it would be exceedingly surprising to see subtle strain differences having large impact on the dynamic taste processing.
Note that these data do not address the question of whether the coding of taste is necessarily temporal coding per se (although see Di Lorenzo et al. 2009; Erickson et al. 1994; Hallock and Di Lorenzo 2006; Lemon and Smith 2006): mouse GC taste responses become taste specific at approximately 0.15–0.2 s after taste delivery, a latency that accords well with the absolute minimum behavioral reaction times to tastes (Graham et al. 2014; Halpern and Tapper 1971; Perez et al. 2013; Tapper and Halpern 1968; Weiss and Di Lorenzo 2012); firing in that approximately 0.2- to 1.0-s epoch is best described as “across-fiber patterns” (and not as “labeled lines;” see Erickson 1963, 1982). Rather, our data suggest that the term taste coding far underdescribes the work in which GC is involved, in that GC responses chart the transformation of taste information into an action (i.e., palatability) code. Evidence supporting this conception comes from rat studies showing that the onset of palatability-related firing both predicts (Sadacca et al. 2016) and drives (Li et al. 2016) the onset of taste behavior.
Finally, we extend the above-described analyses into the spatial domain (taking this work beyond that addressed in electrophysiological work on rat GC), probing for possible differences in how dorsal/ventral and anterior/posterior subregions of GC code tastes, because 1) GC subregions form distinct connections with other brain regions along the dorsal/ventral and anterior/posterior axes (Allen et al. 1991; Haley et al. 2016; Maffei et al. 2012) and 2) the literature on imaging of mouse GC fails to support consistent conclusions on the matter (Chen et al. 2011; Fletcher et al. 2017; Lavi et al. 2018; Livneh et al. 2017). Our comparisons revealed remarkably little spatial variance of coding, consistent with Fletcher et al. (2017; see also Accolla et al. 2007 and Lavi et al. 2018): neurons across the heart of GC produced similarly broad, temporally complex taste responses.
This invariance in the face of nonuniform input, most notably, dorsal GC receives mainly thalamic input (Allen et al. 1991; Chen et al. 2011; Fletcher et al. 2017), whereas ventral GC receives mainly amygdala input (Allen et al. 1991; Mátyás et al. 2014; see Fig. 1), is striking given the difference in information carried in those two pathways; thalamus appears to deliver mainly quality-related firing to GC (Liu and Fontanini 2015; Samuelsen et al. 2013), whereas amygdala-cortical axons are more important for palatability coding (Piette et al. 2012). We have no specific explanation for this lack of difference, although it suggests that GC taste responses are to a large extent a function of distributed network processing, rather than of direct sensory or effective input; i.e., whatever information is carried in each pathway, intracortical connectivity ensures that this information is spread across a broader spatial region. This interpretation is supported by the very finding that GC neurons code multiple aspects of tastes in a temporally distributed fashion, which suggests convergence of pathways, and population coding analysis that reveals the temporal codes to be a reliable sequence of coherent network states (see Grossman et al. 2008; Jones et al. 2007; Moran and Katz 2014; Sadacca et al. 2016). It is also notable, however, that amygdalar inactivation eliminates palatability-related firing in only a majority of GC neurons, enhancing them in the remainder (Piette et al. 2012); perhaps a mapping of this effect would reveal that the palatability-related firing that survives amygdalar inactivation will be localized to dorsal, thalamorecipient regions of GC.
Together, our results shed a new light on the structure of taste coding in mice. They show that GC neurons are broadly tuned but go on to show that GC neurons code not only multiple tastes, but also multiple aspects of taste processing (taste identity and palatability), the latter in meaningful response dynamics. We also show that these responses are distributed through a broad spatial extent of GC; cortical network processing is distributed, rather than being separated out into spatial subregions. At the largest “big picture” level, these results suggest that the functional unit of sensory processing in GC is ensembles composed of neurons that are responsive to multiple tastes, in which properties of the processing emerge transiently in specific time epochs.
GRANTS
This research was supported by the National Institute on Deafness and Other Communication Disorders Grant DC-006666.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.L., J.-Y.L., and D.B.K. conceived and designed research; D.L., J.-Y.L., J.W., and N.M. performed experiments; D.L., J.-Y.L., J.W., N.M., and D.B.K. analyzed data; D.L., J.-Y.L., J.W., N.M., and D.B.K. interpreted results of experiments; D.L., J.-Y.L., and N.M. prepared figures; D.L., J.-Y.L., and D.B.K. drafted manuscript; D.L., J.-Y.L., S.B.N., and D.B.K. edited and revised manuscript; D.L., J.-Y.L., J.W., N.M., S.B.N., and D.B.K. approved final version of manuscript.
ENDNOTE
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at the Web site of the authors, which at the time of publication they indicate is: https://github.com/narendramukherjee/blech_clust. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the Web site address, or for any links to or from it.
REFERENCES
- Accolla R, Bathellier B, Petersen CC, Carleton A. Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci 27: 1396–1404, 2007. doi: 10.1523/JNEUROSCI.5188-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol 311: 1–16, 1991. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- Allen Institute for Brain Science Allen Mouse Brain Atlas (Online) 2008. http://mouse.brain-map.org/.
- Anderson J, Lampl I, Reichova I, Carandini M, Ferster D. Stimulus dependence of two-state fluctuations of membrane potential in cat visual cortex. Nat Neurosci 3: 617–621, 2000. doi: 10.1038/75797. [DOI] [PubMed] [Google Scholar]
- Bahar A, Dudai Y, Ahissar E. Neural signature of taste familiarity in the gustatory cortex of the freely behaving rat. J Neurophysiol 92: 3298–3308, 2004. doi: 10.1152/jn.00198.2004. [DOI] [PubMed] [Google Scholar]
- Barthó P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 92: 600–608, 2004. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Bishop CM. Pattern Recognition and Machine Learning. New York: Springer, 2006. [Google Scholar]
- Carleton A, Accolla R, Simon SA. Coding in the mammalian gustatory system. Trends Neurosci 33: 326–334, 2010. doi: 10.1016/j.tins.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang FC, Scott TR. Conditioned taste aversions modify neural responses in the rat nucleus tractus solitarius. J Neurosci 4: 1850–1862, 1984. doi: 10.1523/JNEUROSCI.04-07-01850.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gabitto M, Peng Y, Ryba NJ, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science 333: 1262–1266, 2011. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM, Platt D, Victor JD. Information processing in the parabrachial nucleus of the pons. Ann N Y Acad Sci 1170: 365–371, 2009. doi: 10.1111/j.1749-6632.2009.03903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RP. Sensory neural patterns and gustation. In: Olfaction and Taste: Proceedings of the First International Symposium Held at the Wenner-Gren Center, Stockholm, September 1962, edited by Zotterman Y. Oxford, UK; London; New York; Paris: Pergamon, 1963, vol. 1, p. 205–213. doi: 10.1016/B978-1-4831-9834-7.50021-4. [DOI] [Google Scholar]
- Erickson RP. Studies on the perception of taste: do primaries exist? Physiol Behav 28: 57–62, 1982. doi: 10.1016/0031-9384(82)90102-0. [DOI] [PubMed] [Google Scholar]
- Erickson RP, Di Lorenzo PM, Woodbury MA. Classification of taste responses in brain stem: membership in fuzzy sets. J Neurophysiol 71: 2139–2150, 1994. doi: 10.1152/jn.1994.71.6.2139. [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Ogg MC, Lu L, Ogg RJ, Boughter JD Jr. Overlapping representation of primary tastes in a defined region of the gustatory cortex. J Neurosci 37: 7595–7605, 2017. doi: 10.1523/JNEUROSCI.0649-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini A, Grossman SE, Figueroa JA, Katz DB. Distinct subtypes of basolateral amygdala taste neurons reflect palatability and reward. J Neurosci 29: 2486–2495, 2009. doi: 10.1523/JNEUROSCI.3898-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini A, Katz DB. State-dependent modulation of time-varying gustatory responses. J Neurophysiol 96: 3183–3193, 2006. doi: 10.1152/jn.00804.2006. [DOI] [PubMed] [Google Scholar]
- Gannon KS, Contreras RJ. Sodium intake linked to amiloride-sensitive gustatory transduction in C57BL/6J and 129/J mice. Physiol Behav 57: 231–239, 1995. doi: 10.1016/0031-9384(94)00279-E. [DOI] [PubMed] [Google Scholar]
- Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol 96: 2513–2527, 2006. doi: 10.1152/jn.00607.2006. [DOI] [PubMed] [Google Scholar]
- Graham DM, Sun C, Hill DL. Temporal signatures of taste quality driven by active sensing. J Neurosci 34: 7398–7411, 2014. doi: 10.1523/JNEUROSCI.0213-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143: 263–279, 1978. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Grossman SE, Fontanini A, Wieskopf JS, Katz DB. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J Neurosci 28: 2864–2873, 2008. doi: 10.1523/JNEUROSCI.4063-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley MS, Fontanini A, Maffei A. Laminar- and target-specific amygdalar inputs in rat primary gustatory cortex. J Neurosci 36: 2623–2637, 2016. doi: 10.1523/JNEUROSCI.3224-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock RM, Di Lorenzo PM. Temporal coding in the gustatory system. Neurosci Biobehav Rev 30: 1145–1160, 2006. doi: 10.1016/j.neubiorev.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Halpern BP, Tapper DN. Taste stimuli: quality coding time. Science 171: 1256–1258, 1971. doi: 10.1126/science.171.3977.1256. [DOI] [PubMed] [Google Scholar]
- Ho AS, Hori E, Nguyen PH, Urakawa S, Kondoh T, Torii K, Ono T, Nishijo H. Hippocampal neuronal responses during signaled licking of gustatory stimuli in different contexts. Hippocampus 21: 502–519, 2011. doi: 10.1002/hipo.20766. [DOI] [PubMed] [Google Scholar]
- Jezzini A, Mazzucato L, La Camera G, Fontanini A. Processing of hedonic and chemosensory features of taste in medial prefrontal and insular networks. J Neurosci 33: 18966–18978, 2013. doi: 10.1523/JNEUROSCI.2974-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Sherwood A, Smith DR, Wosiski-Kuhn M, Gallagher M, Holland PC. An analysis of licking microstructure in three strains of mice. Appetite 54: 320–330, 2010. doi: 10.1016/j.appet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LM, Fontanini A, Sadacca BF, Miller P, Katz DB. Natural stimuli evoke dynamic sequences of states in sensory cortical ensembles. Proc Natl Acad Sci USA 104: 18772–18777, 2007. doi: 10.1073/pnas.0705546104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell 157: 163–186, 2014. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Katz DB, Sadacca BF. Taste. In: Neurobiology of Sensation and Reward, edited by Gottfried JA. Boca Raton, FL: CRC/Taylor & Francis, 2011. [PubMed] [Google Scholar]
- Katz DB, Simon SA, Nicolelis MA. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci 21: 4478–4489, 2001. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumoto-Yoshida I, Liu H, Chen BT, Fontanini A, Bonci A. Central role for the insular cortex in mediating conditioned responses to anticipatory cues. Proc Natl Acad Sci USA 112: 1190–1195, 2015. doi: 10.1073/pnas.1416573112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi K, Jacobson GA, Rosenblum K, Lüthi A. Encoding of conditioned taste aversion in cortico-amygdala circuits. Cell Rep 24: 278–283, 2018. doi: 10.1016/j.celrep.2018.06.053. [DOI] [PubMed] [Google Scholar]
- Lemon CH, Smith DV. Influence of response variability on the coding performance of central gustatory neurons. J Neurosci 26: 7433–7443, 2006. doi: 10.1523/JNEUROSCI.0106-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Maier JX, Reid EE, Katz DB. Sensory cortical activity is related to the selection of a rhythmic motor action pattern. J Neurosci 36: 5596–5607, 2016. doi: 10.1523/JNEUROSCI.3949-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Yoshida T, Monk KJ, Katz DB. Lateral hypothalamus contains two types of palatability-related taste responses with distinct dynamics. J Neurosci 33: 9462–9473, 2013. doi: 10.1523/JNEUROSCI.3935-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fontanini A. State dependency of chemosensory coding in the gustatory thalamus (VPMpc) of alert rats. J Neurosci 35: 15479–15491, 2015. doi: 10.1523/JNEUROSCI.0839-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey GJ, Diaz VE, Jikomes N, Resch JM, Lowell BB, Andermann ML. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 546: 611–616, 2017. doi: 10.1038/nature22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy RF Jr, Norgren R. Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol 91: 1143–1157, 2004. doi: 10.1152/jn.00840.2003. [DOI] [PubMed] [Google Scholar]
- Maffei A, Haley M, Fontanini A. Neural processing of gustatory information in insular circuits. Curr Opin Neurobiol 22: 709–716, 2012. doi: 10.1016/j.conb.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JX, Katz DB. Neural dynamics in response to binary taste mixtures. J Neurophysiol 109: 2108–2117, 2013. doi: 10.1152/jn.00917.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mátyás F, Lee J, Shin HS, Acsády L. The fear circuit of the mouse forebrain: connections between the mediodorsal thalamus, frontal cortices and basolateral amygdala. Eur J Neurosci 39: 1810–1823, 2014. doi: 10.1111/ejn.12610. [DOI] [PubMed] [Google Scholar]
- Moran A, Katz DB. Sensory cortical population dynamics uniquely track behavior across learning and extinction. J Neurosci 34: 1248–1257, 2014. doi: 10.1523/JNEUROSCI.3331-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee N, Wachutka J, Katz DB. Impact of precisely-timed inhibition of gustatory cortex on taste behavior depends on single-trial ensemble dynamics. eLife 8: e45968, 2019. doi: 10.7554/eLife.45968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee N, Wachutka J, Katz DB. Python meets systems neuroscience: affordable, scalable and open-source electrophysiology in awake, behaving rodents. In: Proceedings of the 16th Python in Science Conference. Austin, TX: July 10–16, 2017, p. 98–105. [Google Scholar]
- Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 468: 653–658, 2010. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neseliler S, Narayanan D, Fortis-Santiago Y, Katz DB, Birren SJ. Genetically induced cholinergic hyper-innervation enhances taste learning. Front Syst Neurosci 5: 97, 2011. doi: 10.3389/fnsys.2011.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers EJ. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci 14: 1105–1107, 2011. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Nomura T, Katsukawa H. Genetically variable taste sensitivity to d-amino acids in mice. Brain Res 596: 349–352, 1992. doi: 10.1016/0006-8993(92)91571-U. [DOI] [PubMed] [Google Scholar]
- Norgren R. Gustatory responses in the hypothalamus. Brain Res 21: 63–77, 1970. doi: 10.1016/0006-8993(70)90021-1. [DOI] [PubMed] [Google Scholar]
- Peng Y, Gillis-Smith S, Jin H, Tränkner D, Ryba NJ, Zuker CS. Sweet and bitter taste in the brain of awake behaving animals. Nature 527: 512–515, 2015. doi: 10.1038/nature15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez IO, Villavicencio M, Simon SA, Gutierrez R. Speed and accuracy of taste identification and palatability: impact of learning, reward expectancy, and consummatory licking. Am J Physiol Regul Integr Comp Physiol 305: R252–R270, 2013. doi: 10.1152/ajpregu.00492.2012. [DOI] [PubMed] [Google Scholar]
- Piette CE, Baez-Santiago MA, Reid EE, Katz DB, Moran A. Inactivation of basolateral amygdala specifically eliminates palatability-related information in cortical sensory responses. J Neurosci 32: 9981–9991, 2012. doi: 10.1523/JNEUROSCI.0669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Browning AS, Hernadi I, Lenard L. Responses to the sensory properties of fat of neurons in the primate orbitofrontal cortex. J Neurosci 19: 1532–1540, 1999. doi: 10.1523/JNEUROSCI.19-04-01532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol 86: 216–244, 2008. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Sadacca BF, Mukherjee N, Vladusich T, Li JX, Katz DB, Miller P. The behavioral relevance of cortical neural ensemble responses emerges suddenly. J Neurosci 36: 655–669, 2016. doi: 10.1523/JNEUROSCI.2265-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadacca BF, Rothwax JT, Katz DB. Sodium concentration coding gives way to evaluative coding in cortex and amygdala. J Neurosci 32: 9999–10011, 2012. doi: 10.1523/JNEUROSCI.6059-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Fontanini A. Processing of intraoral olfactory and gustatory signals in the gustatory cortex of awake rats. J Neurosci 37: 244–257, 2017. doi: 10.1523/JNEUROSCI.1926-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Gardner MP, Fontanini A. Effects of cue-triggered expectation on cortical processing of taste. Neuron 74: 410–422, 2012. doi: 10.1016/j.neuron.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Gardner MP, Fontanini A. Thalamic contribution to cortical processing of taste and expectation. J Neurosci 33: 1815–1827, 2013. doi: 10.1523/JNEUROSCI.4026-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034, 2000. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiol Behav 87: 734–744, 2006. doi: 10.1016/j.physbeh.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Sinclair MS, Perea-Martinez I, Abouyared M, St John SJ, Chaudhari N. Oxytocin decreases sweet taste sensitivity in mice. Physiol Behav 141: 103–110, 2015. doi: 10.1016/j.physbeh.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60: 683–697, 2008. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Senses 4: 215–229, 1979. doi: 10.1093/chemse/4.3.215. [DOI] [Google Scholar]
- Stevens CF. Spatial learning and memory: the beginning of a dream. Cell 87: 1147–1148, 1996. doi: 10.1016/S0092-8674(00)81808-5. [DOI] [PubMed] [Google Scholar]
- Tapper DN, Halpern BP. Taste stimuli: a behavioral categorization. Science 161: 708–710, 1968. doi: 10.1126/science.161.3842.708. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Aleman TR, Ellis HT, Ohmoto M, Matsumoto I, Shestopalov VI, Mitchell CH, Foskett JK, Poole RL. Normal taste acceptance and preference of PANX1 knockout mice. Chem Senses 40: 453–459, 2015. doi: 10.1093/chemse/bjv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Norgren R. Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behav Neurosci 100: 544–555, 1986. doi: 10.1037/0735-7044.100.4.544. [DOI] [PubMed] [Google Scholar]
- Wang L, Gillis-Smith S, Peng Y, Zhang J, Chen X, Salzman CD, Ryba NJ, Zuker CS. The coding of valence and identity in the mammalian taste system. Nature 558: 127–131, 2018. doi: 10.1038/s41586-018-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MS, Di Lorenzo PM. Not so fast: taste stimulus coding time in the rat revisited. Front Integr Nuerosci 6: 27, 2012. doi: 10.3389/fnint.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM, Lemon CH. Modulation of central gustatory coding by temperature. J Neurophysiol 110: 1117–1129, 2013. doi: 10.1152/jn.00974.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. I. Response characteristics. J Neurophysiol 51: 616–635, 1984. doi: 10.1152/jn.1984.51.4.616. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. II. Information processing of taste quality. J Neurophysiol 53: 1356–1369, 1985. doi: 10.1152/jn.1985.53.6.1356. [DOI] [PubMed] [Google Scholar]
- Yokota T, Eguchi K, Satoh T. Sensitivity of rat cortical neurons in distinguishing taste qualities by individual and correlative activities. Chem Senses 22: 363–373, 1997. doi: 10.1093/chemse/22.4.363. [DOI] [PubMed] [Google Scholar]