Abstract
Decisions about actions typically involve a period of deliberation that ends with the commitment to a choice and the motor processes overtly expressing that choice. Previous studies have shown that neural activity in sensorimotor areas, including the primary motor cortex (M1), correlates with deliberation features during action selection. However, the causal contribution of these areas to the decision process remains unclear. Here, we investigated whether M1 determines choice commitment or whether it simply reflects decision signals coming from upstream structures and instead mainly contributes to the motor processes that follow commitment. To do so, we tested the impact of a disruption of M1 activity, induced by continuous theta burst stimulation (cTBS), on the behavior of human subjects in 1) a simple reaction time (SRT) task allowing us to estimate the duration of the motor processes and 2) a modified version of the tokens task (Cisek P, Puskas GA, El-Murr S. J Neurosci 29: 11560–11571, 2009), which allowed us to estimate subjects’ time of commitment as well as accuracy criterion. The efficiency of cTBS was attested by a reduction in motor evoked potential amplitudes following M1 disruption compared with those following a sham stimulation. Furthermore, M1 cTBS lengthened SRTs, indicating that motor processes were perturbed by the intervention. Importantly, all of the behavioral results in the tokens task were similar following M1 disruption and sham stimulation, suggesting that the contribution of M1 to the deliberation process is potentially negligible. Taken together, these findings favor the view that M1 contribution is downstream of the decision process.
NEW & NOTEWORTHY Decisions between actions are ubiquitous in the animal realm. Deliberation during action choices entails changes in the activity of the sensorimotor areas controlling those actions, but the causal role of these areas is still often debated. With the use of continuous theta burst stimulation, we show that disrupting the primary motor cortex (M1) delays the motor processes that follow instructed commitment but does not alter volitional deliberation, suggesting that M1 contribution may be downstream of the decision process.
Keywords: action selection, motor cortex, movement preparation, TMS, urgency
INTRODUCTION
The physical world provides animals with a variety of action opportunities, constantly requiring them to make decisions, some of which are critical for survival. For instance, the choice of a car driver to turn left or right in front of a sudden obstacle may have dramatic consequences on her/his life and on that of the pedestrians around. The driver will have to quickly deliberate and commit to one action.
Deliberation about actions is thought to entail a competition between distinct neural populations within the motor system (Pezzulo and Cisek 2016; Svoboda and Li 2018). In this view, separate action opportunities increase activity of distinct populations, which compete against each other, possibly through mutual inhibition (Michelet et al. 2010). An action is eventually selected and executed when activity in the related population reaches a critical decision threshold (Laming 1968; Ratcliff 1978; Stone 1960).
In line with this hypothesis, a compendium of studies has shown that the dorsal premotor (PMd), but also the primary motor cortex (M1), display a buildup of choice-selective activity during the decision process. The rate of this buildup depends on the amount of sensory evidence favoring the selection of each action in the environment (Alamia et al. 2019; Derosiere et al. 2018; Donner et al. 2009; Gould et al. 2012; Tosoni et al. 2014; Wyart et al. 2012). According to this view, in the car driver example above, the presence of pedestrians on the right side of the street would increase the activity of the population coding for the movement of rotating the wheel toward the left, and possibly weaken the activity of the population favoring the opposite rightward rotation movement. Ultimately, the driver will commit to turning left and execute the related action, to avoid hitting the group of people.
Importantly, making decisions often requires balancing the desire to take time to deliberate accurately (i.e., to accumulate sensory evidence and make the best choice) with the urge to act (Churchland et al. 2008; Forstmann et al. 2008; Hanks et al. 2011; Thura and Cisek 2014a). During a speeded decision, the urge to act increases as time passes (Cisek et al. 2009; Ditterich 2006; Drugowitsch et al. 2012; Seideman et al. 2018; Thura et al. 2012), but the overall level of urgency also varies depending on the context (Murphy et al. 2016; Thura et al. 2014; Thura and Cisek 2016, 2017). In the situation described earlier, the driver’s level of urgency will be higher if the obstacle suddenly appears close to the car than if it appears far away. As evident in this example, adjustments in urgency alter the balance between decision speed and accuracy, i.e., the so-called speed-accuracy trade-off: choices are more likely to be incorrect when time pressure is elevated, whereas accuracy improves when temporal demands permit long deliberation (e.g., Hanks et al. 2011; Seideman et al. 2018).
At the neural level, several lines of evidence indicate that higher levels of urgency during deliberation about action choices modulate neural activity in PMd and M1 (Murphy et al. 2016; Steinemann et al. 2018; Thura and Cisek 2014a, 2016). In these areas, activity is globally amplified at baseline and then builds up at a faster rate when urgency is high compared with when it is low, reducing the time needed to reach decision threshold but at the cost of accuracy (Thura and Cisek 2016). Recent findings suggest that the basal ganglia (Thura and Cisek 2017; van Maanen et al. 2016) and the locus coeruleus (Hauser et al. 2018; Murphy et al. 2016) may contribute to generate such a modulation of motor cortical activity.
Together, these data indicate that the motor cortical areas combine both the sensory evidence signals guiding the choice with the urgency-related signals determining the best time to commit to that choice, suggesting a crucial role of these areas in the decision-making process. To date, however, a causal test of this role is lacking.
Here, we investigated whether M1 causally contributes to deliberation about action choices or whether it simply reflects decision signals coming from upstream areas, such as PMd, the basal ganglia, or the locus coeruleus. It has previously been shown that disrupting M1 activity by means of continuous theta burst stimulation (cTBS) causes finger responses to slow down (Huang et al. 2005; Lakhani et al. 2014; McAllister et al. 2013). However, it is a matter of debate whether this effect should be interpreted as a slowing down of processes involved in deciding which action to perform (i.e., in the deliberation process) or as a slowing down of the motor processes that follow commitment (i.e., of movement initiation and execution). In fact, a slowing down of the deliberation process has been associated with reduced urgency during volitional decision behavior (Hanks et al. 2011; Seideman et al. 2018; Thura and Cisek 2014a, 2016). If M1 is causally involved in the decision process, then M1 disruption might lengthen deliberation in a manner consistent with reduced urgency compared with a sham cTBS session. Conversely, if M1 is mainly involved in initiating and executing selected actions, then its disruption should have no effect on the deliberation portion of response time and should only slow down the motor processes that follow commitment to an action. These hypotheses were tested by characterizing action choices in a modified version of the tokens task (Cisek et al. 2009), which is specifically designed to estimate subjects’ time of commitment and their accuracy criterion and infer from those variables their urgency functions (Thura and Cisek 2014a).
MATERIALS AND METHODS
Participants
Nineteen healthy, right-handed subjects participated in this study (ten women; 24 ± 3.5 yr old). Participants were financially compensated for their participation and earned additional money depending on their performance in a decision-making task (see Task section below). The protocol was approved by the institutional review board of the Catholic University of Louvain, Brussels, Belgium, and required written, informed consent, in compliance with the principles of the Declaration of Helsinki.
Experimental Design
Experiments were conducted in a quiet and dimly lit room. Subjects were seated at a table in front of a 21-in. cathode-ray tube computer screen. The display was gamma-corrected, and its refresh rate was set at 100 Hz. The computer screen was positioned at a distance of 70 cm from the subject’s eyes and was used to display stimuli during the decision-making task. Left and right forearms were rested on the surface of the table with the palms facing the table. A computer keyboard was positioned upside-down under the dominant (i.e., right) hand with the response keys F9 and F8 under the index and middle fingers, respectively (Fig. 1).
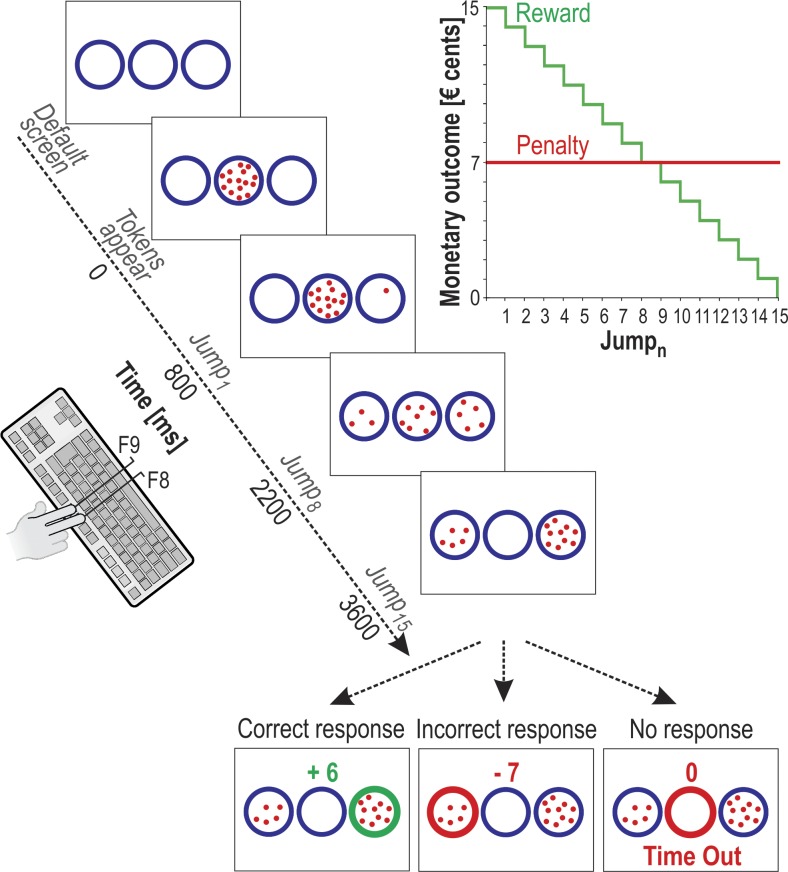
Fig. 1.
Schematic of the tokens task. In each trial, 15 tokens jumped 1 by 1 every 200 ms from the central circle to 1 of the 2 lateral circles (i.e., Jump1 to Jump15). Subjects had to indicate by a right index or right middle finger key press (i.e., F9 and F8 keys, respectively) which lateral circle they thought would receive more tokens (i.e., left or right circle, respectively) at the end of the trial. They could provide their response whenever they wanted between Jump1 and Jump15. For a correct response, the subjects earned, in euro cents, the number of tokens remaining in the central circle at the time of the response. Hence, the reward received for a correct response decreased over time, as depicted on the upper right side of the figure (green trace). Example presented on the lower left side of the figure represents a correct response provided between Jump9 and Jump10 (i.e., the score indicates that 6 tokens remained in the central circle at the moment the right circle was chosen). In contrast, if subjects chose the incorrect lateral circle, they lost €0.07, regardless of their reaction time. As such, the penalty score was fixed, as shown in red on the upper right side of the figure: the lower middle example represents an incorrect choice of the left circle. Thus the reward-to-penalty ratio decreased over time, producing an increasing sense of urgency over the course of a trial. In the absence of response (“Time Out” trial, lower right side example), subjects were neither rewarded nor penalized (score = 0). For representative purposes, the Time Out message is depicted below the circles in this example, although it was presented above them in the experiment.
Task
The task used in the current study is a variant of the tokens task (Cisek et al. 2009) and was implemented by means of LabVIEW 8.2 (National Instruments, Austin, TX). The sequence of events in each trial is depicted in Fig. 1. Between trials, subjects were always presented with a default screen consisting of three circles (4.5 cm diameter), displayed for 2,000 ms on a white background. Fifteen randomly arranged tokens (0.3 cm diameter) then appeared in the central circle. After a delay of 800 ms, the tokens began to jump, one by one every 200 ms from the center to one of the two lateral circles (i.e., Jump1 to Jump15). The subjects’ task was to indicate by a right index or right middle finger key press which lateral circle they thought would ultimately receive the majority of the tokens (i.e., F9 and F8 key presses to choose left and right circles, respectively). They could provide their response as soon as they felt sufficiently confident but between Jump1 and Jump15. Once the response was provided, the tokens kept on jumping every 200 ms until the central circle was empty. At this time, the selected circle was highlighted in either green or red depending on whether the response was correct or incorrect, respectively, and a score was displayed above the central circle to provide the subjects with further feedback of their performance. In correct trials, subjects received a positive score (i.e., a monetary reward) that was equal to the number of tokens remaining in the central circle at the time of the response (in euro cents). Conversely, incorrect responses led to a fixed penalty of 7¢ regardless of the reaction time (RT). Thus the longer the subjects waited to provide a response, the lower was the reward-to-penalty ratio, generating an increasing sense of urgency as time passed within each trial. In the absence of any response before Jump15, the central circle was highlighted in red and a “time out” message appeared on the top of the screen. The subjects were neither rewarded nor penalized in these trials. The feedback cue remained on the screen for 1,000 ms and then disappeared at the same time as the tokens did, denoting the end of the trial. Subjects were told that they would receive a monetary reward at the end of the experiment corresponding to their final score. Each trial lasted 6,600 ms.
Blocks and Sessions
The study included 3 sessions, conducted on separate days at a 24-h interval. Testing always occurred at the same time of the day for a given subject, to avoid variations that could be due to changes in chronobiological states (Derosiere et al. 2015a; Schmidt et al. 2006). Each session comprised 4 blocks of 50 trials, with each block lasting ~5.5 min. Subjects also performed 4 blocks of 5 trials of a simple reaction time (SRT) task, 2 at the beginning and 2 at the end of each session. In the SRT task, subjects were presented with the same display as in the tokens task described above. However, after 50 ms in the central circle, the 15 tokens all jumped together into 1 of the 2 lateral circles at the same time. Subjects were instructed to respond to this “GO signal” by pressing the corresponding key as fast as possible. Importantly, the 15 tokens always jumped into the same lateral circle in all trials of a given SRT block, and subjects were told which lateral circle this would be in advance. This SRT task allowed us to estimate the sum of the delays attributable to the sensory and motor processes in the absence of a choice (see Cisek et al. 2009; Thura et al. 2014).
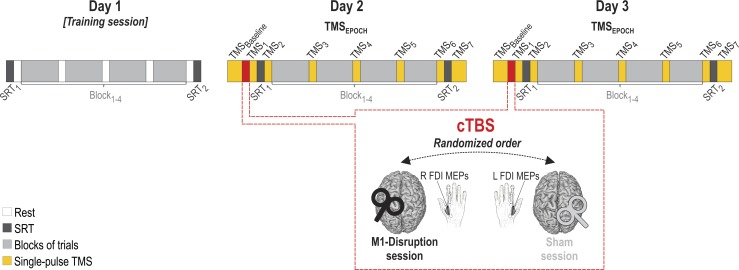
Day 1 served as a training session. Days 2 and 3 corresponded to the actual experimental sessions with the cTBS intervention (Fig. 2). cTBS was applied before subjects engaged in the blocks of trials, either over the left M1 hand area (M1-Disruption session) or over the right primary somatosensory cortex (S1), 2 cm behind the right M1 area (Sham session; Alexandre et al. 2015; Derosière et al. 2014; Torta et al. 2013), in a randomized order. The Sham session allowed us to ensure that the putative behavioral effects observed following M1 cTBS were not due to the tactile and auditory sensations elicited by the transcranial magnetic stimulation (TMS) pulses (Derosiere et al. 2017a, 2017b).
Fig. 2.
Experimental protocol. Subjects came to the laboratory for 3 consecutive days (days 1–3). On each day, they performed the tokens task during 4 blocks of 50 trials (Block1–4; light gray rectangles). They also performed simple reaction time blocks at the beginning and at the end of each session (SRT1 and SRT2, respectively; dark gray rectangles). Day 1 served as a training session and did not involve any continuous theta burst stimulation (cTBS). cTBS train was applied for 40 s at the beginning of days 2 and 3 (red rectangles), either over the left (L) primary motor cortex (M1-Disruption session; black coil) or over the right (R) primary somatosensory cortex (Sham session; gray coil), in a randomized order. Motor evoked potentials (MEPs) were elicited at different time points (TMSEPOCHS) throughout the sessions (TMSBaseline and TMS1–7; yellow rectangles), either in the R first dorsal interosseous (FDI) muscle (M1-Disruption session) or in the L FDI (Sham session), by applying single-pulse TMS over the L or R M1, respectively. Note that in the Sham session, this implied targeting different sites for the cTBS intervention (R S1) and the MEP assessments (R M1; coil position not shown on the figure). M1, primary motor cortex; S1, primary somatosensory cortex; TMS, transcranial magnetic stimulation.
TMS Procedure
TMS was delivered through a 2-by-75-mm figure-of-eight coil connected to a MagPro X100 stimulator (MagVenture, Farum, Denmark). The coil was placed tangentially on the scalp with the handle oriented toward the back of the head and laterally at a 45° angle away from the midline. At the beginning of each session, the M1 hand area was localized by identifying the optimal spot (called the “hotspot”) for eliciting motor evoked potentials (MEPs) in the first dorsal interosseous (FDI) muscle of the right hand (M1-Disruption session) or the left hand (Sham session). To do so, we relied on markers disposed on an electroencephalography (EEG) cap fitted on the participant’s head (Duque et al. 2010, 2014; Vandermeeren et al. 2009). We first applied the stimulation with the center of the coil over the C3 or C4 location of the EEG cap (i.e., corresponding to the right and left M1 areas, respectively). Stimulation intensity was increased until consistent MEP responses were obtained at this location. We then moved the coil by steps of ~0.5 cm around this location in both the rostrocaudal and mediolateral axis. Stimulation was applied with the previously defined intensity at each new location, and MEP amplitudes were visually screened. The hotspot was defined as the location at which the largest and most consistent MEP amplitudes could be obtained. The coil was then held at this location, and the edges of its shape were marked on tapes disposed on the EEG cap. These marks allowed us to localize the hotspot at any required time during the session. Once the hotspot was found, we determined the resting motor threshold (rMT). The rMT was defined as the minimal intensity required to evoke MEPs of 50 µV peak to peak in the targeted muscle on 5 out of 10 consecutive trials at the hotspot (Grandjean et al. 2018; Rossini et al. 1994, 2015; Rothwell et al. 1991; Vassiliadis et al. 2018).
The cTBS procedure consisted of a series of short TMS trains (3 pulses at 50 Hz) repeated every 200 ms for a total duration of 40 s (600 pulses) at an intensity of 80% of rMT (Derosiere et al. 2017a, 2017b; Huang et al. 2005; Solopchuk et al. 2017). Such an intervention has been shown to inhibit the stimulated cortical area, producing a temporary “virtual lesion,” often effective as soon as the train is over (Derosiere et al. 2017a, 2017b; Do et al. 2018; Sasaki et al. 2018) and lasting for between 20 min (Clerget et al. 2013; Oberman et al. 2011; Zénon et al. 2015) and 45 min (Huang et al. 2005).
To monitor the inhibitory effect of cTBS on motor activity, single TMS pulses were applied at the M1 hotspot at 115% of the rMT to elicit MEPs at different time points in the M1-Disruption and Sham sessions (Klein et al. 2014; Labruna et al. 2014; Quoilin et al. 2016, 2018). In the M1-Disruption session, MEPs were recorded in the right FDI following TMS over left M1, to evaluate the impact of left M1 cTBS on left motor excitability. In the Sham session, MEPs were obtained from the left FDI following TMS over right M1, to control for the absence of effect of right S1 cTBS on right M1 excitability.
The time points at which MEPs were elicited in the M1-Disruption and Sham sessions were comparable (Fig. 2). In both sessions, 20 MEPs were elicited at the beginning of the session (i.e., just before cTBS; TMSBaseline). Then, 15 MEPs were elicited just following cTBS (TMS1), after the 2 initial SRT blocks (TMS2), and after each block of trials of the tokens task (TMS3–6). Finally, 20 additional MEPs were evoked following the 2 last SRT blocks (TMS7). These 7 TMSEPOCHS (TMS1–7) fell 1, 3, 11, 19, 27, 35, and 37 min after the cTBS intervention, respectively.
Data Collection
Electromyography (EMG) was used to measure the peak-to-peak amplitude of FDI MEPs elicited by single TMS pulses over the contralateral M1. EMG activity was recorded from surface electrodes placed over the right FDI or the left FDI (M1-Disruption or Sham sessions, respectively). EMG data were collected for 1,000 ms on each trial, starting 300 ms before the TMS pulse. EMG signals were amplified, band-pass filtered online (10–500 Hz), and digitized at 2,000 Hz for offline analysis.
Data Analysis
Motor evoked potential data.
MEP data were collected with Signal 3.0 (Cambridge Electronic Design, Cambridge, United Kingdom) and analyzed with custom Signal scripts. MEP amplitudes were measured for each TMS pulse. Trials with background EMG activity >20 μV on average (root mean square), in the 200-ms window preceding the TMS artifact, were excluded from the analysis. Of the trials, 3.63 ± 5.43% were discarded based on this criterion. The amplitude of MEPs elicited at each time point was averaged to obtain a measure of motor excitability at TMSBaseline and at TMS1–7 in the M1-Disruption and Sham sessions (Fig. 2). For each session, we then expressed MEP amplitudes obtained at TMS1–7 (i.e., after the cTBS intervention) in percentage of the amplitudes measured at TMSBaseline (i.e., before the cTBS intervention).
The disruptive impact of cTBS on cortical activity varies between subjects (Do et al. 2018; Jannati et al. 2017; Rocchi et al. 2018). Here, we aimed at only including individuals in which cTBS effectively disrupted M1. To do so, we discarded subjects presenting percentage MEP amplitudes exceeding 2.5 SD above the mean of the group in the M1-Disruption session at one of the TMSEPOCHS or more. This led to the rejection of three subjects who exhibited average MEP amplitudes of 148.3 ± 12.5, 139.5 ± 10.6, and 188.6 ± 20.9% following the cTBS intervention in the M1-Disruption session (all TMSEPOCHS averaged together), reflecting thus a large increase (rather than the targeted decrease) in motor excitability. The analyses of the MEP and behavioral data were performed on the remaining pool of subjects (n = 16).
Behavioral data.
Behavioral data were collected with LabVIEW 8.2 (National Instruments), stored in a database (Microsoft SQL Server 2005, Redmond, WA), and analyzed with custom MATLAB scripts (MathWorks, Natick, MA). Because day 1 served as a training session (see section Blocks and Sessions), the behavioral analyses focused on the data acquired on days 2 and 3 (i.e., in the M1-Disruption and Sham sessions).
SRT Task
For each subject, we computed the mean SRT for both fingers (right index and right middle fingers), defined as the difference between the time at which subjects pressed the key and the time at which the 15 tokens appeared simultaneously in the lateral circle, obtained at the beginning (SRT1) and at the end (SRT2) of each session. This SRT allowed us to quantify the impact of M1 cTBS on the motor processes that follow commitment to an action in the absence of a choice.
Tokens Task
Classification of the trial types based on the temporal profile of the success probability.
The task allows us to calculate, at each moment in time, the “success probability” pi(t) associated with choosing each lateral circle i. For a total of 15 tokens, if at a particular moment in time the right (R) circle contains NR tokens, the left (L) circle contains NL tokens, and the central (C) circle contains NC tokens, then the probability that the circle on the right will ultimately be the correct one is described as follows:
| (1) |
For some of the analyses, we grouped trials according to the temporal profile of pi(t). That is, although the side of each token jump was completely random in each trial, we could classify some trials as belonging to one of two specific types a posteriori. Trials were categorized as “obvious” when the pi(t) was >0.6 after Jump2 and >0.75 after Jump5; that is, the initial token jumps consistently favored the correct circle. Other trials were categorized as “ambiguous” when the initial jumps were balanced between the lateral circles, keeping the pi(t) close to 0.5 until late in the trial: pi(t) remained between 0.5 and 0.66 up to Jump7 in these trials.
Decision time, percentage of correct choices, and percentage of time out trials.
For each session (M1-Disruption and Sham) and each trial type (obvious and ambiguous; trials that were neither obvious nor ambiguous were not considered here), we analyzed the following behavioral variables: the decision time (DT), the percentage of correct choices (%Correct), and the percentage of time out trials (%TO). To evaluate the DT, we first calculated the RT during the tokens task by computing the difference between the time at which subjects pressed the key and Jump1. We then subtracted from this tokens RT, the mean RT obtained in the SRT task on the same day (SRT1 and SRT2 pooled together), providing us with an estimate of DT, reflecting the duration of the deliberation process for each subject. Note that we used a monetary reward in the tokens but not in the SRT task, which might have led us to slightly underestimate the DT. That is, previous studies have shown that monetary reward can boost motor processes (Reppert et al. 2018; Summerside et al. 2018; Yoon et al. 2018). Hence, the latter might have been faster in the tokens than in the SRT task used to estimate it. Thus we might have subtracted a too large value from the tokens RT, shortening the DT. Still, this putative underestimation of DT applies for both the M1-Disruption and the Sham sessions and has thus no biasing impact on our data.
Sensory evidence at decision time.
Sensory evidence refers to the available information supporting the correct choice. In the tokens task, the sensory evidence is determined by the difference between the number of tokens in each lateral circle; the more the correct circle contains a large number of tokens, compared with the other lateral circle, the higher the evidence. Given that tokens jump one by one in this task, sensory evidence changes after each jump. We can estimate the evidence based on which subjects made their decision by computing after each jump a first-order approximation of the real probability function (Eq. 1), called the sum of log-likelihood ratios (SumLogLR), and then compute this quantity at decision time (Cisek et al. 2009):
| (2) |
In this equation, p(ek|S) is the likelihood of a token event ek (a token jumping into either the selected or unselected lateral circle) during trials in which the selected lateral circle S is correct and p(ek|U) is the likelihood of ek during trials in which the unselected circle U is correct. The SumLogLR is proportional to the difference between the number of tokens that moved toward each lateral circle before the decision. Hence, the lower the amount of sensory evidence in favor of the chosen lateral circle, the lower the SumLogLR.
To characterize the decision policy of the subjects in the Sham and M1-Disruption sessions, we determined the level of sensory evidence at the time of commitment (i.e., at DT). To do so, we binned trials as a function of the total number of tokens that moved before the decision and calculated the average SumLogLR for each bin as performed in previous studies exploiting the tokens task (e.g., Thura and Cisek 2014a, 2017). Seven bins were defined, with the first bin (Bin1) including responses provided between Jump5 and Jump6, the second bin (Bin2) including responses provided between Jump6 and Jump7, and so on until the last bin (Bin7) covering the period between Jump11 and Jump12. SumLogLR at DT preceding Jump5 or following Jump12 were not considered for this analysis because part of the subjects did not respond at these timings. Importantly, the SumLogLR at DT was computed based on every trial where a response was provided [i.e., for correct and incorrect responses, in obvious and ambiguous trials, as well as in other trials with different pi(t)].
Estimation of urgency functions.
According to recent models of decision making, action choices result from the combination of signals that track the available sensory evidence and the level of urgency that grows over time (Cisek et al. 2009; Ditterich 2006; Drugowitsch et al. 2012). For instance, in a minimal implementation of the urgency-gating model (Cisek et al. 2009; Thura et al. 2012), evidence is multiplied by a linearly increasing urgency signal and then compared with a threshold. The result can be expressed as follows:
| (3) |
where yi is the neural activity for choices to target i, Ni is the number of tokens in target i, t is the number of seconds elapsed since the start of the trial, m and b are the slope and y-intercept of the urgency signal, and [ ]+ denotes half-wave rectification (which sets all negative values to 0). When yi for any target crosses the threshold T, that target is chosen.
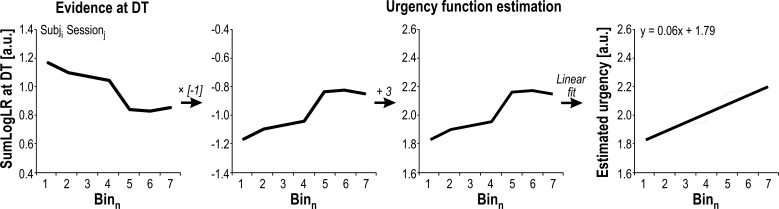
A direct prediction of such urgency-based models is that decisions made with low levels of evidence should be associated with high levels of urgency and vice versa. That is, one core assumption is that a high urgency should push one to commit to a choice even if evidence for that choice is weak. Hence, the SumLogLR at DT values (i.e., reflecting the available sensory evidence at the time of commitment in the tokens task) can be exploited to estimate the level of urgency at DT. Here, we first multiplied the SumLogLR at DT values by −1 (i.e., to “rectify” them; please see Fig. 3), given the theoretical inverse relationship between sensory evidence and urgency at DT. We then added a constant of 3 to the rectified curves to obtain positive urgency values. Finally, we fitted a linear regression over the rectified positive values. We extracted the intercept and the slope of these so-called urgency functions, which we used as estimates of the initial level and the growth rate of the urgency signal, respectively. Second-order polynomial regressions were also performed on the rectified SumLogLR data but did not yield significantly better fits [i.e., group-level Bayesian information criterion values for linear and polynomial fits were 5.75 ± 1.61 and 5.74 ± 1.79 arbitrary units (a.u.), respectively; t14 = 0.02, P = 0.984].
Fig. 3.
Urgency function estimation. We exploited the sum of log-likelihood ratios (SumLogLR) values to estimate the level of urgency at decision time (DT) for each subject (Subj) i and each session j. To do so, we followed 4 steps (shown from left to right). First, SumLogLR values were obtained for different bins of DT. Then, these values were multiplied by −1. Next, a constant of 3 was added to obtain positive values. Finally, a linear regression was fitted over the positive values. Equation of the regression allowed us to extract the intercept and the slope of the obtained urgency function (1.79 and 0.06 in this example, respectively). a.u., Arbitrary units.
Statistical Analysis
All statistical analyses were performed with custom R scripts (R version 3.4.1, car and BayesFactor packages). All data were examined for normality and homogeneity of variance using skewness, kurtosis, and Brown–Forsythe tests. The significance level for all tests was set at P < 0.05, except when Bonferroni corrections were applied. All results are expressed as means ± SE.
Motor evoked potential data.
MEP data (expressed in percentage of MEPs at TMSBaseline) were analyzed using a two-way repeated-measure ANOVA (ANOVARM) with SESSION (M1-Disruption, Sham) and TMSEPOCH (TMS1–7) as within-subject factors. Moreover, the percentage MEP values obtained for each TMSEPOCH were compared against 100% using Bonferroni-corrected single-sample Student’s t tests to identify any significant suppression in the M1-Disruption and in the Sham session.
Behavioral data.
The SRT data were analyzed using a two-way ANOVARM with SESSION (M1-Disruption, Sham) and SRTEPOCH (SRT1, SRT2) as within-subject factors. The DT, the %Correct, and the %TO data were analyzed using two-way ANOVARM with SESSION (M1-Disruption, Sham) and TRIAL (obvious, ambiguous) as within-subject factors. The SumLogLR at DT was analyzed using a two-way ANOVARM with SESSION (M1-Disruption, Sham) and BIN (Bin1–7) as within-subject factors. When appropriate, Tukey honestly significant difference post hoc tests were used to detect paired differences in these ANOVAs. Furthermore, the intercept and the slope of the urgency functions were compared between the two sessions using Student’s t tests.
RESULTS
Motor Evoked Potential Data
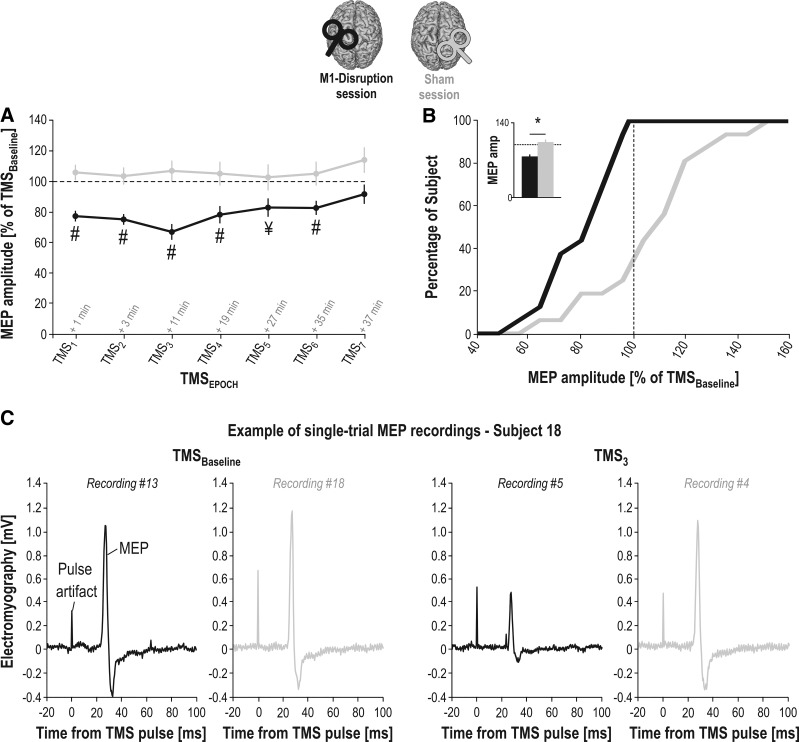
The ANOVARM revealed a main effect of the factor SESSION on the percentage MEP amplitudes (F1,15 = 15.41; P = 0.001; Fig. 4). As such, percentage MEP amplitudes were lower following cTBS in the M1-Disruption session (78.78 ± 3.04%) than in the Sham session (105.53 ± 5.32%; TMS1–7 timings pooled together). The effect size (Cohen’s d) for this factor was 1.5, indicating a large effect of SESSION (Cohen 1988). This effect on percentage MEP amplitudes did not depend on time as the ANOVARM did not reveal any effect of the factor TMSEPOCH (F6,90 = 1.99, P = 0.075) nor interaction with the factor SESSION (F6,90 = 0.72, P = 0.637). Hence, percentage MEP amplitudes remained stable following the cTBS intervention; they were consistently lower in the M1-Disruption than in the Sham session, regardless of the time at which MEPs were considered during the course of the experiment.
Fig. 4.
Motor evoked potential amplitudes (MEP amp). A: mean value of MEPs (in percentage of MEPs at TMSBaseline) elicited after the continuous theta burst stimulation (cTBS) intervention in the 1st dorsal interosseous muscle at each of the TMSEPOCHS (TMS1–7) in the M1-Disruption (black traces) and Sham (gray traces) sessions. Note the significant disruption of MEPs with respect to baseline (i.e., dashed horizontal line) in the M1-Disruption session [#significantly different from 100 at P < 0.0036 (Bonferroni-corrected); ¥significantly different from 100 at P < 0.05 (uncorrected; P = 0.017)]. B: cumulative percentage of subjects. MEPs obtained at TMS1–7 are pooled together. Note that all subjects included in the analysis showed percentage MEP amplitudes <100% in the M1-Disruption (i.e., a disruptive effect), although the same subjects did not show any effect in the Sham session, as also shown in the inset representing the group-level average with the effect of SESSION (*significantly different at P < 0.05). Error bars represent SE. C: example of single-trial MEP recordings. Each trace depicts a raw electromyographic signal in a representative subject (no. 18), starting 20 ms before the TMS pulse and ending 100 ms after it. Artifact caused by the pulse is reflected as a peak occurring at time 0; the MEP occurs ~22 ms later. The 4 recordings display MEPs elicited at TMSBaseline (left) or TMS3 (right) in the M1-Disruption (black traces) or Sham (gray traces) session. In this subject, cTBS had a strong effect; average percentage MEP amplitudes at TMS3 were much smaller in the M1-Disruption session (66 ± 12.8%) compared with the Sham session (112.6 ± 5.7%). M1, primary motor cortex; TMS, transcranial magnetic stimulation.
Additional single-sample Student’s t tests against 100% (run for each TMSEPOCHS; Bonferroni-corrected at P < 0.0035) showed that, as expected, the difference in MEP amplitude between the two sessions reported above was due to a selective suppression of MEPs in the M1-Disruption but not in the Sham session. As such, percentage MEP amplitudes were significantly lower than 100% at almost all timings in the M1-Disruption session, except for TMS5 and TMS7 [i.e., at TMS1–4 and TMS6; all P values = (0.000008 0.002)]. Conversely, amplitudes were never significantly different from 100% (i.e., from TMSBaseline) in the Sham session [all P values = (0.163 0.826)], indicating that right S1 cTBS had no impact on right M1 activity, as previously reported (Derosiere et al. 2017a, 2017b).
Behavioral Data
SRT task.
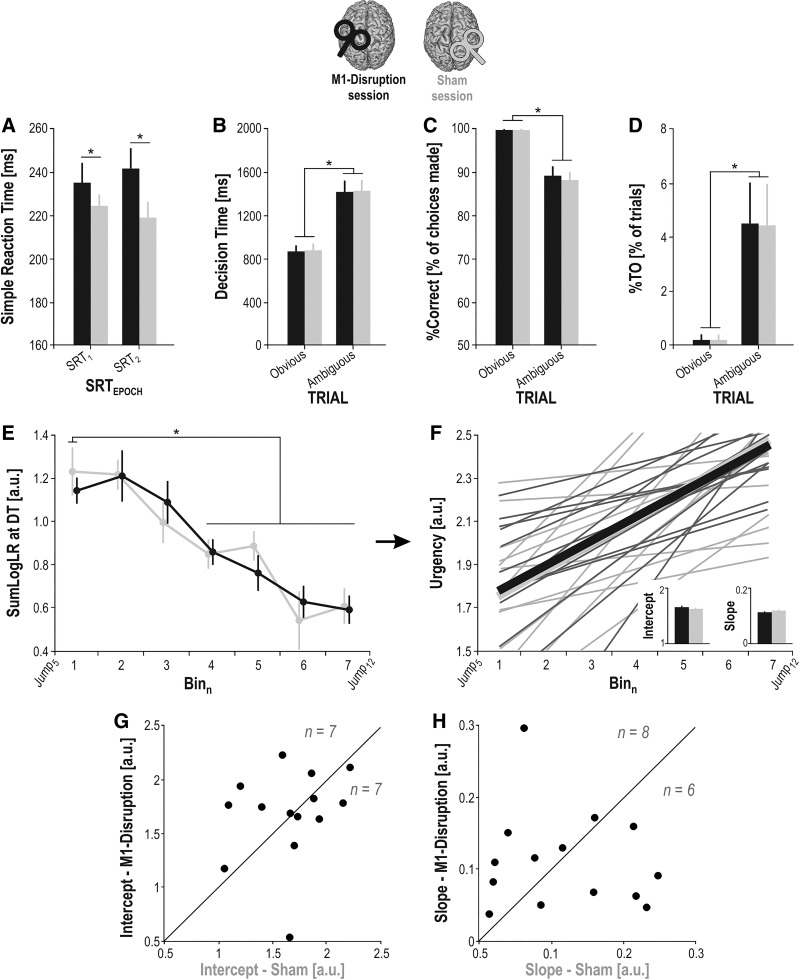
The ANOVARM revealed a main effect of the factor SESSION on the SRT data (F1,15 = 5.34, P = 0.035; Fig. 5A). Indeed, SRTs were significantly prolonged in the M1-Disruption session (237.3 ± 7.4 ms) compared with the Sham session (220.8 ± 5.6 ms; SRT1 and SRT2 pooled together). The effect size (Cohen’s d) for this factor was 0.6, indicating a medium to large effect of SESSION. The impact of M1 disruption on SRTs did not vary over the course of the session. As such, the ANOVARM did not reveal any significant effect of the factor SRTEPOCH (F1,15 = 0.02, P = 0.892) nor of its interaction with the factor SESSION (F1,15 = 1.78, P = 0.202). These findings indicated that M1-Disruption altered the motor processes underlying initiation and/or execution of the cued movements (Huang et al. 2005; Lakhani et al. 2014; McAllister et al. 2013).
Fig. 5.
Behavioral results. A: mean simple reaction time (SRT; obtained in the SRT task) measured at each of the SRTEPOCHS (SRT1–2) in the M1-Disruption (black bars) and Sham (gray bars) sessions. B: mean decision time (DT) measured in each TRIAL (obvious, ambiguous) in the M1-Disruption (black bars) and Sham (gray bars) sessions. C and D: same as B for the percentages of correct choices (%Correct) and of time out trials (%TO), respectively. E: sum of log-likelihood ratios (SumLogLR) at DT measured in each Binn of decision time (i.e., between Jump5 and Jump12; see main text) in the M1-Disruption (black traces) and Sham (gray traces) sessions. F: urgency functions computed based on the rectified SumLogLR at DT for the M1-Disruption (black traces) and Sham (gray traces) sessions. Small bar graphs on the bottom right represent the group-level average intercept and slope of the functions. Light lines illustrate individual estimated urgency functions, and bold lines illustrate the mean urgency functions averaged across population. G: individual intercept values, represented for the M1-Disruption session (y-axis) as a function of the values for the Sham session (x-axis). Points above the diagonal (n = 7/14) represent the subjects showing a higher intercept in the M1-Disruption than following Sham session, whereas points below the diagonal (n = 7/14) represent the subjects showing a lower intercept in the M1-Disruption than following Sham session. H: same as G for the slope values. *Significant difference (P < 0.05). Error bars represent SE. a.u., Arbitrary units; M1, primary motor cortex.
Tokens task.
decision time.
As expected, the ANOVARM revealed a main effect of the factor TRIAL on the DT data (F1,15 = 119.30, P < 0.00001; Fig. 5B). Indeed, DTs were significantly shorter in obvious trials (871.77 ± 57.14 ms) than in ambiguous ones (1,426.57 ± 88.59 ms; M1-Disruption and Sham sessions pooled together). Importantly, the ANOVARM did not reveal any significant effect of the factor SESSION (F1,15 = 0.24, P = 0.631) nor of its interaction with the factor TRIAL (F1,15 = 0.02, P = 0.888). Hence, the time taken by the subjects to deliberate depended on the trial type they encountered (i.e., obvious vs. ambiguous) but was not affected by M1 disruption.
percentage of correct choices.
We found a significant main effect of the factor TRIAL for the %Correct data (F1,15 = 31.727, P = 0.00005; Fig. 5C). Indeed, %Correct was significantly higher in obvious trials (99.80 ± 0.13%) than in ambiguous ones (88.61 ± 1.80%; M1-Disruption and Sham sessions pooled together). However, neither the factor SESSION (F1,15 = 0.05, P = 0.497) nor its interaction with the factor TRIAL were significant (F1,15 = 0.44, P = 0.517). Hence, M1 disruption did not alter the accuracy of the decision process.
percentage of time out trials.
The %TO data revealed a similar pattern as the variables described above. Indeed, the %TO was significantly lower in obvious (0.20 ± 0.13%) than in ambiguous trials (4.43 ± 1.42%; M1-Disruption and Sham sessions pooled together), as confirmed by the ANOVARM (factor TRIAL: F1,15 = 7.47, P = 0.015; Fig. 5D). Moreover, there was no effect of the factor SESSION (F1,15 = 0.01, P = 0.920) or interaction with the factor TRIAL (F1,15 = 0.01, P = 0.922). Hence, the proportion of trials in which subjects refrained from responding was unaffected by M1-Disruption.
sensory evidence at decision time (sumloglr at dt) and urgency.
The amount of sensory evidence based on which subjects made their decision was estimated using the SumLogLR (computed at DT): the higher the SumLogLR, the higher the evidence at DT (Cisek et al. 2009; Thura et al. 2012, 2014). SumLogLR values are presented for each Binn in Fig. 5E (see materials and methods), separately for the M1-Disruption and the Sham sessions. Note that 2 subjects were excluded from this analysis as they responded too early on most trials, resulting in a lack of SumLogLR values after Jump9 in these participants. Hence, SumLogLR analyses were run on 14 subjects.
Overall, fast decisions were made based on more sensory evidence than slow decisions, as confirmed by the ANOVARM showing a main effect of the factor BIN on the SumLogLR at DT [F6,78 = 12.86, P < 0.00001; Fig. 5E; Bayes factor (BF) value >100]. Indeed, Tukey HSD post hoc tests showed that the SumLogLR at DT was significantly higher at Bin1 (1.18 ± 0.06 a.u.) than for any other bin after Bin4 (all SumLogLR at DT ≤ 0.84 ± 0.05 a.u.; M1-Disruption and Sham sessions pooled together). Hence, the amount of sensory evidence based on which subjects made their choices decreased as a function of time. Here again, the ANOVARM did not reveal any significant effect of the factor SESSION (F6,78 = 0.04, P = 0.852) nor of its interaction with the factor BIN (F6,78 = 0.41, P = 0.868). Hence, subjects made their decisions based on a similar amount of sensory evidence in both sessions, suggesting a preservation of the urgency drive during deliberation with M1 disruption.
To further confirm this finding, we obtained a simple approximation of the urgency signal underlying the subjects’ decisions by fitting a linear regression over the rectified version of SumLogLR at DT for each session (M1-Disruption, Sham) and extracted the intercept and the slope of the regression functions (see materials and methods and Fig. 5, F–H). Again, Student’s t tests did not reveal any significant impact of the session on the intercept (t14 = 0.48, P = 0.798) or the slope (t14 = −0.22, P = 0.832) of the urgency functions.
verifying that m1 disruption does not impact deliberation using bayesian analyses.
The ANOVARM and the t tests revealed that M1 disruption did not significantly alter the behavioral data measured in the tokens task, suggesting that M1 is not functionally involved in the deliberation process underlying action choices. To confirm this result, a BF value was computed for each analysis involving the factor SESSION (10 BF values obtained in total), providing us with a ratio of the likelihood probability of the null hypothesis (i.e., H0: the probability that data do not exhibit an effect of SESSION) over the alternative hypothesis (i.e., H1: the probability that data exhibit the effect; Morey and Rouder 2011). A BF value of 1 would reflect an equal probability that H0 and H1 are correct, whereas a BF value >1 would reflect a higher probability that H0 is correct. In accordance with conventional interpretation of BF values (Jeffreys 1998), a BF value ranging between 1 and 3 is interpreted as indicating anecdotal evidence in favor of H0, a value between 3 and 10 as indicating substantial evidence for H0, and a value between 10 and 30 a strong evidence for H0.
Table 1 summarizes the BF values obtained for each factor tested. The average BF value was of 5.66 ± 1.74 (all BF values ranged between 3.46 and 21.28), indicating substantial to strong evidence for an absence of difference in subjects’ behavior between the M1-Disruption and the Sham sessions. Hence, Bayesian analyses further reinforce the conclusion that M1 disruption did not influence the deliberation process underlying decision making but solely altered the motor processes that follow commitment to an action.
Table 1.
Bayes factor values
| Factor Tested | DT | %Correct | %TO | SumLogLR | Urgency Intercept | Urgency Slope |
|---|---|---|---|---|---|---|
| SESSION | 3.87 | 3.77 | 3.91 | 5.23 | 3.46 | 3.48 |
| SESSION × TRIAL | 3.91 | 3.77 | 3.91 | |||
| SESSION × TIME | 21.28 |
First column specifies the factors tested for which a Bayes factor (BF) value was computed. Other columns represent the BF values obtained for each behavioral measure in the tokens task. Overall, BF values ranged between 3.46 and 21.28, indicating substantial to strong evidence for a lack of effect of the SESSION on subjects’ behavior. %Correct, percentage of correct choices; %TO, percentage of time out trials; DT, decision time; SumLogLR, sum of log-likelihood ratios.
DISCUSSION
Previous studies have shown that neural activity in motor cortical areas, including M1, is strongly altered during decisions between actions (Alamia et al. 2019; Derosiere et al. 2018; Donner et al. 2009; Gould et al. 2012; Klein-Flügge and Bestmann 2012; Murphy et al. 2016; Steinemann et al. 2018; Thura and Cisek 2014a, 2016; Tosoni et al. 2014; Wyart et al. 2012). To date, however, the specific contribution of motor cortical areas to the decision process remains debated. Here, we asked whether M1 causally influences deliberation during action choices or whether this area mostly contributes to the motor processes overtly expressing commitment. To do so, we tested the impact of a disruption of M1 activity, induced by continuous theta burst stimulation (cTBS), on the behavior of human subjects in 1) a simple reaction time (SRT) task allowing us to estimate the duration of the motor processes and 2) a modified version of the tokens task (Cisek et al. 2009), which allowed us to estimate subjects’ time of commitment as well as their accuracy criterion.
Subjects were generally faster and more accurate in obvious than in ambiguous trials, suggesting that late decisions relied on weaker sensory evidence compared with early decisions. This is confirmed by the systematic analysis of the sensory evidence available at DT, which indicates that subjects committed to a choice based on less sensory evidence as time elapsed during the course of a trial. This dropping of the accuracy criterion is consistent with previous studies in which similar tasks were used (e.g., Cisek et al. 2009; Gluth et al. 2013; Murphy et al. 2016; Thura et al. 2012, 2014) and supports recent models postulating that urgency grows over time during speeded decisions (Churchland et al. 2008; Ditterich 2006; Drugowitsch et al. 2012; Hanks et al. 2011).
The cTBS intervention reduced MEP amplitudes during the entire M1 disruption session but never following a sham stimulation. Moreover, M1 cTBS lengthened the SRTs (i.e., compared with when sham cTBS was performed), indicating that motor processes that are known to involve M1 were successfully perturbed by the intervention (Huang et al. 2005; Lakhani et al. 2014; McAllister et al. 2013). Notably, in the present study, motor responses were recorded through key presses. Hence, RTs involved two periods, occurring before and after movement initiation (Spieser et al. 2017). As a consequence, it is sensible to assume that the lengthening of SRT observed here might reflect an increase of 1) the time needed for initiating the required motor response, 2) the duration of the execution, or 3) both.
Critically, all of the behavioral data collected in the tokens task were similar in the two cTBS sessions, whether M1 was disrupted or not. Based on this finding, the contribution of M1 to decision making could be negligible. Hence, past reports of decision-related changes in M1 may reflect the influence of signals coming from upstream structures rather than an actual involvement in the deliberation process itself (Thura and Cisek 2017; van Maanen et al. 2016). In a similar vein, our recent work shows that M1 disruption negatively alters value-based choices but only when action values are freshly acquired. Such an effect of M1 disruption does not occur anymore following consolidation. This suggests that M1 contribution to value-based decision making may vanish as subjects become more proficient at using the value information (Derosiere et al. 2015b, 2017a, 2017b). Thus, in well-learned decision-making tasks, the causal involvement of M1 might be restricted to the motor processes that follow commitment to an action.
However, there are alternative explanations for the lack of effect of M1 disruption on decision behavior in the present study. First, the behavioral variables extracted from the tokens task (e.g., DT, decision accuracy, sensory evidence at DT, etc.) may not be sensitive enough to reflect the changes in decision behavior following disruption of motor cortical activity, contrary to the reaction times obtained in the SRT task. In line with this alternative interpretation, the absence of effect of M1 disruption in the tokens task would be due to a lack of sensitivity of the behavioral variables obtained in this specific task. However, the results of another study applying microstimulation in the premotor and motor cortex of nonhuman primates succeeded in altering decision behavior using the same task (Thura and Cisek 2014b). Hence, this suggests that the behavioral variables extracted from the tokens task are sensitive to the disruption of motor cortical activity.
Also, we cannot rule out the possibility that the cTBS intervention led to some fast reorganization of the decision network following M1 disruption. According to this idea, M1 might still be part of the network involved in the deliberation process but some compensatory mechanisms may have occurred in this network following M1 disruption, leading to similar decision behaviors after M1 and sham cTBS (Bestmann et al. 2004; Briend et al. 2017; Derosiere et al. 2017a; Rastogi et al. 2017). One way to tackle this issue in the future would be to exploit online repetitive TMS techniques (Duque et al. 2010, 2013), which allow one to perturb neural activity at a specific moment during the decision process, leaving less time for compensatory mechanisms to occur. As such, previous work has shown that whereas online microstimulation of a decision-related area alters behavior during perceptual decision making (i.e., the lateral intraparietal area; Hanks et al. 2006), (offline) inactivation of the same area does not (Katz et al. 2016).
Now, if the role of M1 is truly negligible, where in the brain are decisions about actions determined? Among many possible areas, PMd emerges as a promising candidate. First, single-cell recordings in behaving monkeys (Thura and Cisek 2014a) have shown that during deliberation, activity of some PMd neurons tuned for a particular action reflects the unfolding sensory evidence favoring that action. This observation also makes it possible that this decision-related activity influences M1 neurons through corticocortical projections (Duque et al. 2012; Martinez-Garcia et al. 2015). Second, the same studies found that PMd activity related to the selected target reaches a peak ~280 ms before movement initiation regardless of decision difficulty, whereas a peak of M1 activity occurs ~140 ms later. Third, neurons in the globus pallidus internus, which are insensitive to sensory information during deliberation, become directionally tuned around the time of the PMd activity peak (Thura and Cisek 2017). Altogether, these results suggest that PMd might be one of the primary sites where decision commitment is determined.
In agreement with this hypothesis, and as mentioned above, a recent study found that microstimulation of PMd neurons alters the deliberation duration, especially if current is applied shortly before commitment time (Thura and Cisek 2014b). Crucially, stimulation has much less influence on decision duration if it is applied long before commitment or between commitment and movement onset. Relevant for the present work, this study also shows similar time-dependent effect of M1 microstimulation on decision durations, but the effect size is much smaller when M1 is stimulated compared with PMd. Finally, other nonprimary motor areas may be causally involved in the deliberation process, including the presupplementary motor area (Tosun et al. 2017). Investigating their precise contribution represents an interesting issue for future investigations.
In conclusion, we show that the offline disruption of M1 activity delays motor processes that follow commitment to an action but does not alter volitional decision behavior. Taken together, these findings suggest that the contribution of M1 might be downstream of the decision process. Future studies should use online disruption protocols to deal with the putative network reorganization that may have occurred following offline M1 disruption in the present study and broaden their investigation to the role of nonprimary motor areas in deliberation, especially the PMd and the presupplementary motor area.
GRANTS
This work was supported by grants from the Fonds Spéciaux de Recherche of the Université Catholique de Louvain, the Belgian National Funds for Scientific Research (FRS-FNRS: MIS F.4512.14), and the Fondation Médicale Reine Elisabeth. G. Derosiere was a postdoctoral fellow supported by the FNRS.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.D., D.T., P.C., and J.D. conceived and designed research; G.D. performed experiments; G.D. analyzed data; G.D., D.T., P.C., and J.D. interpreted results of experiments; G.D. prepared figures; G.D. and J.D. drafted manuscript; G.D., D.T., P.C., and J.D. edited and revised manuscript; G.D., D.T., P.C., and J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
Preprint is available at https://doi.org/10.1101/501205.
REFERENCES
- Alamia A, Zénon A, VanRullen R, Duque J, Derosiere G. Implicit visual cues tune oscillatory motor activity during decision-making. Neuroimage 186: 424–436, 2019. doi: 10.1016/j.neuroimage.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Alexandre F, Derosiere G, Papaiordanidou M, Billot M, Varray A. Cortical motor output decreases after neuromuscular fatigue induced by electrical stimulation of the plantar flexor muscles. Acta Physiol (Oxf) 214: 124–134, 2015. doi: 10.1111/apha.12478. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci 19: 1950–1962, 2004. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Briend F, Leroux E, Delcroix N, Razafimandimby A, Etard O, Dollfus S. Impact of rTMS on functional connectivity within the language network in schizophrenia patients with auditory hallucinations. Schizophr Res 189: 142–145, 2017. doi: 10.1016/j.schres.2017.01.049. [DOI] [PubMed] [Google Scholar]
- Churchland AK, Kiani R, Shadlen MN. Decision-making with multiple alternatives. Nat Neurosci 11: 693–702, 2008. doi: 10.1038/nn.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Puskas GA, El-Murr S. Decisions in changing conditions: the urgency-gating model. J Neurosci 29: 11560–11571, 2009. doi: 10.1523/JNEUROSCI.1844-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerget E, Andres M, Olivier E. Deficit in complex sequence processing after a virtual lesion of left BA45. PLoS One 8: e63722, 2013. doi: 10.1371/journal.pone.0063722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, NJ: Erlbaum, 1988. [Google Scholar]
- Derosière G, Alexandre F, Bourdillon N, Mandrick K, Ward TE, Perrey S. Similar scaling of contralateral and ipsilateral cortical responses during graded unimanual force generation. Neuroimage 85: 471–477, 2014. doi: 10.1016/j.neuroimage.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Derosiere G, Farrugia N, Perrey S, Ward T, Torre K. Expectations induced by natural-like temporal fluctuations are independent of attention decrement: evidence from behavior and early visual evoked potentials. Neuroimage 104: 278–286, 2015a. doi: 10.1016/j.neuroimage.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Derosiere G, Klein PA, Nozaradan S, Zénon A, Mouraux A, Duque J. Visuomotor correlates of conflict expectation in the context of motor decisions. J Neurosci 38: 9486–9504, 2018. doi: 10.1523/JNEUROSCI.0623-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosiere G, Vassiliadis P, Demaret S, Zénon A, Duque J. Learning stage-dependent effect of M1 disruption on value-based motor decisions. Neuroimage 162: 173–185, 2017a. doi: 10.1016/j.neuroimage.2017.08.075. [DOI] [PubMed] [Google Scholar]
- Derosiere G, Zénon A, Alamia A, Duque J. Primary motor cortex contributes to the implementation of implicit value-based rules during motor decisions. Neuroimage 146: 1115–1127, 2017b. doi: 10.1016/j.neuroimage.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Derosiere G, Zenon A, Alamia A, Klein PA, Duque J. Contribution of primary motor cortex to perceptual and value-based decision processes (Abstract). 11th National Congress of the Belgian Society for Neuroscience Mons, Belgium, May 22, 2015b. doi: 10.3389/conf.fnins.2015.89.00056. [DOI] [Google Scholar]
- Ditterich J. Stochastic models of decisions about motion direction: behavior and physiology. Neural Netw 19: 981–1012, 2006. doi: 10.1016/j.neunet.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Do M, Kirkovski M, Davies CB, Bekkali S, Byrne LK, Enticott PG. Intra- and inter-regional priming of ipsilateral human primary motor cortex with continuous theta burst stimulation does not induce consistent neuroplastic effects. Front Hum Neurosci 12: 123, 2018. doi: 10.3389/fnhum.2018.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner TH, Siegel M, Fries P, Engel AK. Buildup of choice-predictive activity in human motor cortex during perceptual decision making. Curr Biol 19: 1581–1585, 2009. doi: 10.1016/j.cub.2009.07.066. [DOI] [PubMed] [Google Scholar]
- Drugowitsch J, Moreno-Bote R, Churchland AK, Shadlen MN, Pouget A. The cost of accumulating evidence in perceptual decision making. J Neurosci 32: 3612–3628, 2012. doi: 10.1523/JNEUROSCI.4010-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Cazares C, Ivry RB. Dissociating the influence of response selection and task anticipation on corticospinal suppression during response preparation. Neuropsychologia 65: 287–296, 2014. doi: 10.1016/j.neuropsychologia.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci 32: 806–816, 2012. doi: 10.1523/JNEUROSCI.4299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci 30: 3793–3802, 2010. doi: 10.1523/JNEUROSCI.5722-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Olivier E, Rushworth M. Top-down inhibitory control exerted by the medial frontal cortex during action selection under conflict. J Cogn Neurosci 25: 1634–1648, 2013. doi: 10.1162/jocn_a_00421. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, Wagenmakers EJ. Striatum and pre-SMA facilitate decision-making under time pressure. Proc Natl Acad Sci USA 105: 17538–17542, 2008. doi: 10.1073/pnas.0805903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluth S, Rieskamp J, Büchel C. Deciding not to decide: computational and neural evidence for hidden behavior in sequential choice. PLoS Comput Biol 9: e1003309, 2013. [Correction in PLoS Comput Biol 13: e1005476, 2017.] doi: 10.1371/journal.pcbi.1003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould IC, Nobre AC, Wyart V, Rushworth MF. Effects of decision variables and intraparietal stimulation on sensorimotor oscillatory activity in the human brain. J Neurosci 32: 13805–13818, 2012. doi: 10.1523/JNEUROSCI.2200-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean J, Derosiere G, Vassiliadis P, Quemener L, Wilde Y, Duque J. Towards assessing corticospinal excitability bilaterally: validation of a double-coil TMS method. J Neurosci Methods 293: 162–168, 2018. doi: 10.1016/j.jneumeth.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Hanks TD, Ditterich J, Shadlen MN. Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. Nat Neurosci 9: 682–689, 2006. doi: 10.1038/nn1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks TD, Mazurek ME, Kiani R, Hopp E, Shadlen MN. Elapsed decision time affects the weighting of prior probability in a perceptual decision task. J Neurosci 31: 6339–6352, 2011. doi: 10.1523/JNEUROSCI.5613-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser TU, Moutoussis M, Purg N, Dayan P, Dolan RJ. Beta-blocker propranolol modulates decision urgency during sequential information gathering. J Neurosci 38: 7170–7178, 2018. doi: 10.1523/JNEUROSCI.0192-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 45: 201–206, 2005. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Jannati A, Block G, Oberman LM, Rotenberg A, Pascual-Leone A. Interindividual variability in response to continuous theta-burst stimulation in healthy adults. Clin Neurophysiol 128: 2268–2278, 2017. doi: 10.1016/j.clinph.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys H. Theory of Probability (3rd ed.). Oxford, UK: Clarendon, 1998. [Google Scholar]
- Katz LN, Yates JL, Pillow JW, Huk AC. Dissociated functional significance of decision-related activity in the primate dorsal stream. Nature 535: 285–288, 2016. doi: 10.1038/nature18617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PA, Petitjean C, Olivier E, Duque J. Top-down suppression of incompatible motor activations during response selection under conflict. Neuroimage 86: 138–149, 2014. doi: 10.1016/j.neuroimage.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Klein-Flügge MC, Bestmann S. Time-dependent changes in human corticospinal excitability reveal value-based competition for action during decision processing. J Neurosci 32: 8373–8382, 2012. doi: 10.1523/JNEUROSCI.0270-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna L, Lebon F, Duque J, Klein PA, Cazares C, Ivry RB. Generic inhibition of the selected movement and constrained inhibition of nonselected movements during response preparation. J Cogn Neurosci 26: 269–278, 2014. doi: 10.1162/jocn_a_00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani B, Bolton DA, Miyasike-Dasilva V, Vette AH, McIlroy WE. Speed of processing in the primary motor cortex: a continuous theta burst stimulation study. Behav Brain Res 261: 177–184, 2014. doi: 10.1016/j.bbr.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Laming DR. Information Theory of Choice Reaction Time. New York: Wiley, 1968. [Google Scholar]
- Martinez-Garcia M, Insabato A, Pannunzi M, Pardo-Vazquez JL, Acuña C, Deco G. The encoding of decision difficulty and movement time in the primate premotor cortex. PLoS Comput Biol 11: e1004502, 2015. doi: 10.1371/journal.pcbi.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister CJ, Rönnqvist KC, Stanford IM, Woodhall GL, Furlong PL, Hall SD. Oscillatory beta activity mediates neuroplastic effects of motor cortex stimulation in humans. J Neurosci 33: 7919–7927, 2013. doi: 10.1523/JNEUROSCI.5624-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet T, Duncan GH, Cisek P. Response competition in the primary motor cortex: corticospinal excitability reflects response replacement during simple decisions. J Neurophysiol 104: 119–127, 2010. doi: 10.1152/jn.00819.2009. [DOI] [PubMed] [Google Scholar]
- Morey RD, Rouder JN. Bayes factor approaches for testing interval null hypotheses. Psychol Methods 16: 406–419, 2011. doi: 10.1037/a0024377. [DOI] [PubMed] [Google Scholar]
- Murphy PR, Boonstra E, Nieuwenhuis S. Global gain modulation generates time-dependent urgency during perceptual choice in humans. Nat Commun 7: 13526, 2016. [Erratum in Nat Commun 8: 14299, 2017.] doi: 10.1038/ncomms13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman L, Edwards D, Eldaief M, Pascual-Leone A. Safety of theta burst transcranial magnetic stimulation: a systematic review of the literature. J Clin Neurophysiol 28: 67–74, 2011. doi: 10.1097/WNP.0b013e318205135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo G, Cisek P. Navigating the affordance landscape: feedback control as a process model of behavior and cognition. Trends Cogn Sci 20: 414–424, 2016. doi: 10.1016/j.tics.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Quoilin C, Lambert J, Jacob B, Klein PA, Duque J. Comparison of motor inhibition in variants of the instructed-delay choice reaction time task. PLoS One 11: e0161964, 2016. doi: 10.1371/journal.pone.0161964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoilin C, Wilhelm E, Maurage P, de Timary P, Duque J. Deficient inhibition in alcohol-dependence: let’s consider the role of the motor system!. Neuropsychopharm 43: 1851–1858, 2018. doi: 10.1038/s41386-018-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi A, Cash R, Dunlop K, Vesia M, Kucyi A, Ghahremani A, Downar J, Chen J, Chen R. Modulation of cognitive cerebello-cerebral functional connectivity by lateral cerebellar continuous theta burst stimulation. Neuroimage 158: 48–57, 2017. doi: 10.1016/j.neuroimage.2017.06.048. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. A theory of memory retrieval. Psychol Rev 85: 59–108, 1978. doi: 10.1037/0033-295X.85.2.59. [DOI] [Google Scholar]
- Reppert TR, Servant M, Heitz RP, Schall JD. Neural mechanisms of speed-accuracy tradeoff of visual search: saccade vigor, the origin of targeting errors, and comparison of the superior colliculus and frontal eye field. J Neurophysiol 120: 372–384, 2018. doi: 10.1152/jn.00887.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi L, Ibáñez J, Benussi A, Hannah R, Rawji V, Casula E, Rothwell J. Variability and predictors of response to continuous theta burst stimulation: a TMS-EEG study. Front Neurosci 12: 400, 2018. doi: 10.3389/fnins.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout AL, Marsden CD, Murray NM, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126: 1071–1107, 2015. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp Exp Physiol 76: 159–200, 1991. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kodama S, Togashi N, Shirota Y, Sugiyama Y, Tokushige SI, Inomata-Terada S, Terao Y, Ugawa Y, Hamada M. The intensity of continuous theta burst stimulation, but not the waveform used to elicit motor evoked potentials, influences its outcome in the human motor cortex. Brain Stimul 11: 400–410, 2018. doi: 10.1016/j.brs.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Münch M, de Quervain DJ, Wirz-Justice A, Cajochen C. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci 26: 8976–8982, 2006. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seideman JA, Stanford TR, Salinas E. Saccade metrics reflect decision-making dynamics during urgent choices. Nat Commun 9: 2907, 2018. doi: 10.1038/s41467-018-05319-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solopchuk O, Alamia A, Dricot L, Duque J, Zénon A. cTBS disruption of the supplementary motor area perturbs cortical sequence representation but not behavioural performance. Neuroimage 163: 34–40, 2017. doi: 10.1016/j.neuroimage.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Spieser L, Servant M, Hasbroucq T, Burle B. Beyond decision! Motor contribution to speed-accuracy trade-off in decision-making. Psychon Bull Rev 24: 950–956, 2017. doi: 10.3758/s13423-016-1172-9. [DOI] [PubMed] [Google Scholar]
- Steinemann NA, O’Connell RG, Kelly SP. Decisions are expedited through multiple neural adjustments spanning the sensorimotor hierarchy. Nat Commun 9: 3627, 2018. doi: 10.1038/s41467-018-06117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M. Models for choice-reaction time. Psychometrika 25: 251–260, 1960. doi: 10.1007/BF02289729. [DOI] [Google Scholar]
- Summerside EM, Shadmehr R, Ahmed AA. Vigor of reaching movements: reward discounts the cost of effort. J Neurophysiol 119: 2347–2357, 2018. doi: 10.1152/jn.00872.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K, Li N. Neural mechanisms of movement planning: motor cortex and beyond. Curr Opin Neurobiol 49: 33–41, 2018. doi: 10.1016/j.conb.2017.10.023. [DOI] [PubMed] [Google Scholar]
- Thura D, Beauregard-Racine J, Fradet CW, Cisek P. Decision making by urgency gating: theory and experimental support. J Neurophysiol 108: 2912–2930, 2012. doi: 10.1152/jn.01071.2011. [DOI] [PubMed] [Google Scholar]
- Thura D, Cisek P. Deliberation and commitment in the premotor and primary motor cortex during dynamic decision making. Neuron 81: 1401–1416, 2014a. doi: 10.1016/j.neuron.2014.01.031. [DOI] [PubMed] [Google Scholar]
- Thura D, Cisek P.. Micro-stimulation of premotor and motor cortex delays the commitment to an action choice. 2014 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2014b, Program No. 651.04. [Google Scholar]
- Thura D, Cisek P. Modulation of premotor and primary motor cortical activity during volitional adjustments of speed-accuracy trade-offs. J Neurosci 36: 938–956, 2016. doi: 10.1523/JNEUROSCI.2230-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thura D, Cisek P. The basal ganglia do not select reach targets but control the urgency of commitment. Neuron 95: 1160–1170.e5, 2017. doi: 10.1016/j.neuron.2017.07.039. [DOI] [PubMed] [Google Scholar]
- Thura D, Cos I, Trung J, Cisek P. Context-dependent urgency influences speed-accuracy trade-offs in decision-making and movement execution. J Neurosci 34: 16442–16454, 2014. doi: 10.1523/JNEUROSCI.0162-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torta DM, Legrain V, Algoet M, Olivier E, Duque J, Mouraux A. Theta burst stimulation applied over primary motor and somatosensory cortices produces analgesia unrelated to the changes in nociceptive event-related potentials. PLoS One 8: e73263, 2013. doi: 10.1371/journal.pone.0073263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosoni A, Corbetta M, Calluso C, Committeri G, Pezzulo G, Romani GL, Galati G. Decision and action planning signals in human posterior parietal cortex during delayed perceptual choices. Eur J Neurosci 39: 1370–1383, 2014. doi: 10.1111/ejn.12511. [DOI] [PubMed] [Google Scholar]
- Tosun T, Berkay D, Sack AT, Çakmak YÖ, Balcı F. Inhibition of pre-supplementary motor area by continuous theta burst stimulation leads to more cautious decision-making and more efficient sensory evidence integration. J Cogn Neurosci 29: 1433–1444, 2017. doi: 10.1162/jocn_a_01134. [DOI] [PubMed] [Google Scholar]
- van Maanen L, Fontanesi L, Hawkins GE, Forstmann BU. Striatal activation reflects urgency in perceptual decision making. Neuroimage 139: 294–303, 2016. doi: 10.1016/j.neuroimage.2016.06.045. [DOI] [PubMed] [Google Scholar]
- Vandermeeren Y, Davare M, Duque J, Olivier E. Reorganization of cortical hand representation in congenital hemiplegia. Eur J Neurosci 29: 845–854, 2009. doi: 10.1111/j.1460-9568.2009.06619.x. [DOI] [PubMed] [Google Scholar]
- Vassiliadis P, Grandjean J, Derosiere G, de Wilde Y, Quemener L, Duque J. Using a double-coil TMS protocol to assess preparatory inhibition bilaterally. Front Neurosci 12: 139, 2018. doi: 10.3389/fnins.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyart V, de Gardelle V, Scholl J, Summerfield C. Rhythmic fluctuations in evidence accumulation during decision making in the human brain. Neuron 76: 847–858, 2012. doi: 10.1016/j.neuron.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Geary RB, Ahmed AA, Shadmehr R. Control of movement vigor and decision making during foraging. Proc Natl Acad Sci USA 115: E10476–E10485, 2018. [Correction in Proc Natl Acad Sci USA 115: E11884, 2018.] doi: 10.1073/pnas.1812979115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zénon A, Klein PA, Alamia A, Boursoit F, Wilhelm E, Duque J. Increased reliance on value-based decision processes following motor cortex disruption. Brain Stimul 8: 957–964, 2015. doi: 10.1016/j.brs.2015.05.007. [DOI] [PubMed] [Google Scholar]