Abstract
In a prior study using laser scanning photostimulation, we found a pronounced cell type-specific mediolateral asymmetry in the local synaptic connectivity in the superficial laminae of the spinal dorsal horn (Kosugi M, Kato G, Lukashov S, Pendse G, Puskar Z, Kozsurek M, Strassman AM. J Physiol 591: 1935–1949, 2013). To obtain information on dorsal horn organization that might complement findings from microelectrode studies, voltage-sensitive dye imaging was used in the present study to examine patterns of activity evoked by focal electrical stimulation, in the presence and absence of synaptic blocking agents, at different positions in transverse, parasagittal, and horizontal slices of the dorsal horn of 2- to 3-wk -old male rats. A pronounced difference in responsiveness was found between medial and lateral dorsal horn, in that medial sites in the superficial dorsal horn showed much larger synaptic responses to focal stimulation than lateral sites. This difference appeared to be a result of a difference in the intrinsic elements of the dorsal horn, rather than a difference in the inputs from the white matter, because the stimulus intensities were subthreshold for evoking synaptic responses from stimulation at sites in the white matter, although it is also possible that the greater responsiveness is due, at least in part, to activation of Aβ primary afferent fibers that pass through the medial dorsal horn. The results raise the possibility of differences between medial and dorsal horn that need to be taken into account in the interpretation of studies of dorsal horn organization.
NEW & NOTEWORTHY We used voltage-sensitive dye imaging to obtain information on spatial aspects of dorsal horn organization that are difficult to examine with single-cell approaches because of the limitations of microelectrode sampling. The most noteworthy finding was a previously unreported, extreme difference between medial and lateral dorsal horn in responsiveness to focal stimulation that appears to result, at least in part, from a greater degree of excitability or local connectivity in medial dorsal horn.
Keywords: nociception, pain, somatosensory, superficial dorsal horn
INTRODUCTION
The spinal dorsal horn is the first central relay site in the somatosensory pathway, containing the central projections of primary afferent sensory neurons that innervate peripheral tissues and the central neurons that receive these projections and in turn project to higher levels (Todd 2010, 2017; Willis and Coggeshall 2004). In addition to primary afferent inputs, dorsal horn neurons also receive descending projections from supraspinal levels, as well as propriospinal and local projections from dorsal horn interneurons (Willis and Coggeshall 2004). The complex circuitry of the dorsal horn has been studied intensively by using a wide array of anatomical and electrophysiological techniques, but is still not well understood, particularly in regard to the organization of local connectivity (Braz et al. 2014; Todd 2010, 2017).
In recent years further advances have been made in the understanding of dorsal horn circuitry by using novel approaches, including genetic methods of transneuronal tracing (Braz et al. 2005; Bráz and Basbaum 2009; Cordero-Erausquin et al. 2009; Peirs et al. 2015), paired microelectrode recording and stimulation (Lu and Perl 2003, 2005; Luz et al. 2010; Santos et al. 2007, 2009), and mapping of local synaptic inputs with laser scanning photostimulation (Kato et al. 2007, 2009, 2013; Kosugi et al. 2013). Microelectrode recording techniques such as paired recordings and laser scanning photostimulation have the ability to identify laminar and cell type-specific patterns of connectivity with single-cell resolution and, in addition, to distinguish excitatory from inhibitory connections, but they are subject to the limitations of microelectrode sampling.
The present study was prompted in part by our prior finding of a mediolateral asymmetry in the intrinsic organization of dorsal horn circuitry, in a study that used laser scanning photostimulation and intracellular labeling to map the origin of local synaptic inputs to morphologically characterized neurons of the superficial dorsal horn (Kosugi et al. 2013). That study found that lamina I neurons receive greater local excitatory synaptic input from interneurons that are medial rather than lateral to their own cell body. In addition, the lamina I neurons displayed a matching asymmetry or medial bias in the distribution of their dendritic fields. The neuronal sample in that study was taken from a roughly central position along the mediolateral axis of the dorsal horn, and potentially it would be of interest to determine whether these asymmetries differed for neuronal samples at different mediolateral positions; however, such investigation of spatial organization would be extremely cumbersome with microelectrode techniques.
In the present study, we have used voltage-sensitive dye imaging (VSDI) in an attempt to obtain information on certain aspects of the spatial organization of the dorsal horn organization that might complement findings obtained from microelectrode studies. Although VSDI lacks the single-cell resolution of microelectrode techniques, it can potentially provide information about larger scale spatial organization that can be difficult to obtain in microelectrode studies. For this purpose, we used VSDI to examine patterns of activity evoked by focal electrical stimulation at different mediolateral and laminar positions within the dorsal horn, as well as patterns in the rostrocaudal distribution of activity evoked by focal stimulation in parasagittal and horizontal slices.
MATERIALS AND METHODS
All experimental procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center. The methods used for obtaining spinal cord slices from rats were similar to those described previously (Bentley and Gent 1994; Yoshimura and Jessell 1989). A portion of the spinal cord was removed from 15- to 20-day-old urethane-anesthetized (1.5 g/kg ip) male Sprague-Dawley rats. The rats were killed by exsanguination (transcardial perfusion with cold Krebs solution) immediately before spinal cord removal. A vibratome (Vibratome 3000 Plus; formerly from Vibratome) was used to cut 300-µm transverse slices or 300- to 400-µm parasagittal or horizontal slices from the lumbar enlargement. In some experiments, transverse slices were made with L5 dorsal roots attached. The slices were stained for 25–50 min at room temperature with 0.1 mg/mL Di-4-ANEPPS (Invitrogen) in a Krebs solution equilibrated with 95% O2 and 5% CO2. The Krebs solution contained (in mM) 117 NaCl, 3.6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose. After staining, slices were transferred to a perfusion chamber for imaging and were perfused continuously with the Krebs solution at a flow rate of ~5 mL/min.
Slices were stimulated electrically by either focal stimulation or dorsal root stimulation. Focal stimuli were delivered at selected sites within the dorsal horn by using micropipettes made from either 1.5-mm-diameter standard glass tubing (WPI; tip 3–10 µm), for monopolar stimulation, or theta glass tubing (Sutter Instruments; tip 12–20 µm), for bipolar stimulation. The micropipette tip was positioned ~40 µm below the surface of the slice. For focal stimulation, the stimulus was a 30-ms train of 10 × 1-ms pulses, 333 Hz. Dorsal root stimulation consisted of a single 1-ms pulse delivered through a suction electrode. Pulse trains were used for focal stimulation rather than single pulses, because in preliminary experiments it was found that VSD responses could be evoked by focal stimulation at much lower stimulus intensities when trains were used (not illustrated). The responses evoked by stimulus trains at these relatively lower intensities were largely 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide (NBQX) sensitive and thus synaptically mediated; the direct (nonsynaptic) activation that necessarily preceded this synaptic activity was apparently more difficult to detect with our imaging methods. Increasing the stimulus intensity to higher levels produced an additional, NBQX-resistant (nonsynaptic) response component in the immediate vicinity of the electrode tip (e.g., see Fig. 1A). Thus the use of stimulus trains rather than single pulses appeared to be advantageous for maximizing the synaptically mediated component of the response relative to the nonsynaptic component, especially when viewing the activity following rather than during the train.
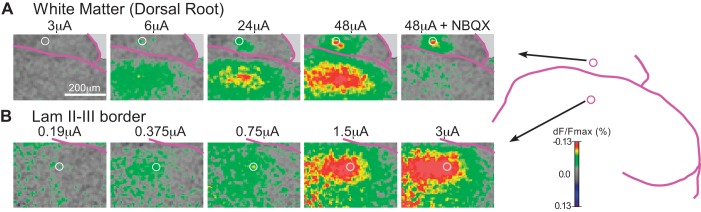
Fig. 1.
Comparison of the voltage-sensitive dye (VSD) response evoked by graded intensities of focal electrical stimulation in the white matter (A; ~50 µm dorsal to the border between the white matter and the dorsal horn) vs. the dorsal horn, in the approximate region of the lamina II/III border (B; ~120 µm ventral to the white matter border), in a transverse slice (right side). In most experiments, stimulus threshold for evoking a response was ~8-fold lower at sites in the dorsal horn (lamina II or II/III border region) than in the overlying white matter. In all figures, focal stimulation consisted of a 30-ms train of ten 1-ms anodal pulses, 333 Hz. Time point illustrated is the end of the stimulus train. Circle in each image marks the stimulating electrode position. Images are transverse slice through right dorsal horn (lateral direction is to the right; ×20 objective). VSD images represent the average of 16 stimulus trials delivered with a 16-s intertrial interval. dF/Fmax, %change in fluorescence; Lam, lamina; NBQX, 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide.
Slices were imaged with an Olympus BX51WI microscope using either a ×20 [1.0 numerical aperture (NA), working distance (WD) 2 mm] or ×10 (0.6 NA, WD 3 mm) water-immersion objective (XLUMPlanFLN 20XW or XLUMPLFL 10XW; Olympus) and, in most experiments, a ×0.63 coupler (DX63; Diagnostic Instruments-Spot Imaging, Sterling Heights, MI). Anatomical images for estimation of laminar borders and selection of dorsal horn sites for focal stimulation were acquired with infrared-differential interference contrast (IR-DIC) optics using a QICam IR FAST 1394 camera (QImaging, Surrey, BC, Canada). VSD images were acquired with the Brainvision MiCAM02-HR imaging and acquisition system (Scimedia, Costa Mesa, CA). Most experiments were done with the acquisition setting of a 3.7-ms sampling rate and a field of 184 × 124 pixels. Excitation, dichroic, and emission filters were used for the VSDI (no. FF01-510/84, FF562-Di03, and BLP01-561R, respectively; Semrock, Rochester, NY). For VSDI, images were acquired from 50 ms before the start of stimulation until 350 ms after the start of stimulation. Illumination was provided by a 100-W halogen lamp (Olympus) and controlled with a shutter (SmartShutter; Sutter Instrument, Novato, CA). Images were averaged over 16 stimulus trials, with an intertrial interval of 16 s. Change in fluorescence was calculated as ΔF/F by the Brainvision software. Each stimulus site was typically stimulated at several intensities (16 trials for each intensity), which were delivered in ascending order.
The following agents were added sequentially to the Krebs solution to investigate components of synaptic transmission: strychnine (glycine antagonist, 300 nM; Sigma-Aldrich), bicuculline (GABAA antagonist, 10 µM; Sigma-Aldrich), d-2-amino-5-phosphonovalerate [AP5; N-methyl-d-aspartate (NMDA) antagonist, 50 µM; Ascent Scientific, Princeton, NJ], and NBQX (non-NMDA antagonist, 10 µM; Ascent Scientific). These agents were applied in the following four steps, with no washout in between: 1) bicuculline, 2) bicuculline + strychnine, 3) bicuculline + strychnine + AP5, and 4) bicuculline + strychnine + AP5 + NBQX. In some experiments, the first step of bicuculline alone was omitted, and instead, strychnine and bicuculline were applied together in a single step. A VSD stimulus sequence (consisting of 16 stimulus trials, with a total duration of ~4 min, as described above) was initiated 10 min after each of the four solution changes.
One slice was used per animal. Throughout, n refers to the number of animals (slices) that contributed to that data set. Each figure that shows VSD images was obtained from a different animal. In each figure that shows more than one VSD image, the images in that figure were obtained from the same slice. The number of stimulus trials in each slice was limited to avoid any decline in of the health and responsiveness of the slice. Thus mediolateral stimulus series (comparing medial and lateral sites) and dorsoventral series were done in separate experiments, because mapping along both axes would require excessive time.
RESULTS
VSD images were recorded while focal electrical stimulation was delivered (30-ms train consisting of 10 × 1-ms pulses, 333 Hz) at sites in the dorsal horn, in transverse (Figs. 1–4), parasagittal (Figs. 6 and 7), and horizontal slices (Fig. 8) of the spinal lumbar enlargement. For comparison, responses were also recorded to stimulation of the dorsal root, in transverse slices (single-pulse stimulation, 1 ms; Fig. 5).
Fig. 4.
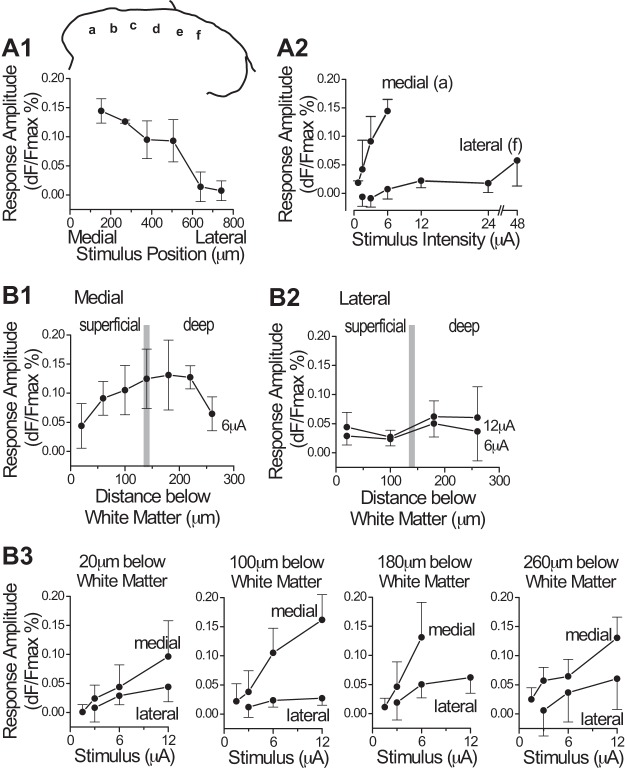
Comparison of response amplitudes at stimulation sites in medial vs. lateral dorsal horn. A1: plot of response amplitude (mean and SD) evoked by stimulation at 6 positions (a–f) across the mediolateral axis of the dorsal horn, in the superficial laminae, for one stimulus intensity (6 µA). Estimated laminar position in this stimulus series was the innermost part of lamina II, ~90 µm below the white matter border. Stimulus positions (a–f) are marked on a transverse outline of the right dorsal horn (inset above graph). For the 6 positions, n = 6, 2, 5, 3, 3, and 4 slices, respectively. The data were compiled from 6 slices (6 animals). Two of the slices were stimulated at all 6 positions, and the other 4 slices were each stimulated at 2–3 positions. A2: plots of response amplitude as a function of stimulus intensity for a medial and a lateral position (positions a and f, n = 6 and 4 slices, respectively). Plots are from the same data plotted in A1. B1–B3: plots from a set of experiments in which stimuli were delivered at different depths below the white matter (i.e., distance from white matter border, as measured along an axis perpendicular to white matter border), at either a medial or a lateral position. Medial sites were similar in mediolateral position to a in A1; lateral sites were at the mediolateral position of f or further lateral; n = 8 and 4 slices (animals) for medial and lateral dorsal horn, respectively (separate slices for medial and lateral sites; separate experiments from A1 and A2). Each slice was stimulated at 3–6 depths below the white matter; n = 4, 6, 8, 6, 5, 3, and 4 slices for the 7 sites in B1, and n = 4, 4, 4, and 2 slices for the 4 sites in B2. In each slice, each site was stimulated at 3–5 stimulus intensities (i.e., not all of the plotted intensities in A2 and B3 were tested at each site). Time point used for these measurements was 30 ms after the end of the 30-ms stimulus train. dF/Fmax, %change in fluorescence.
Fig. 6.
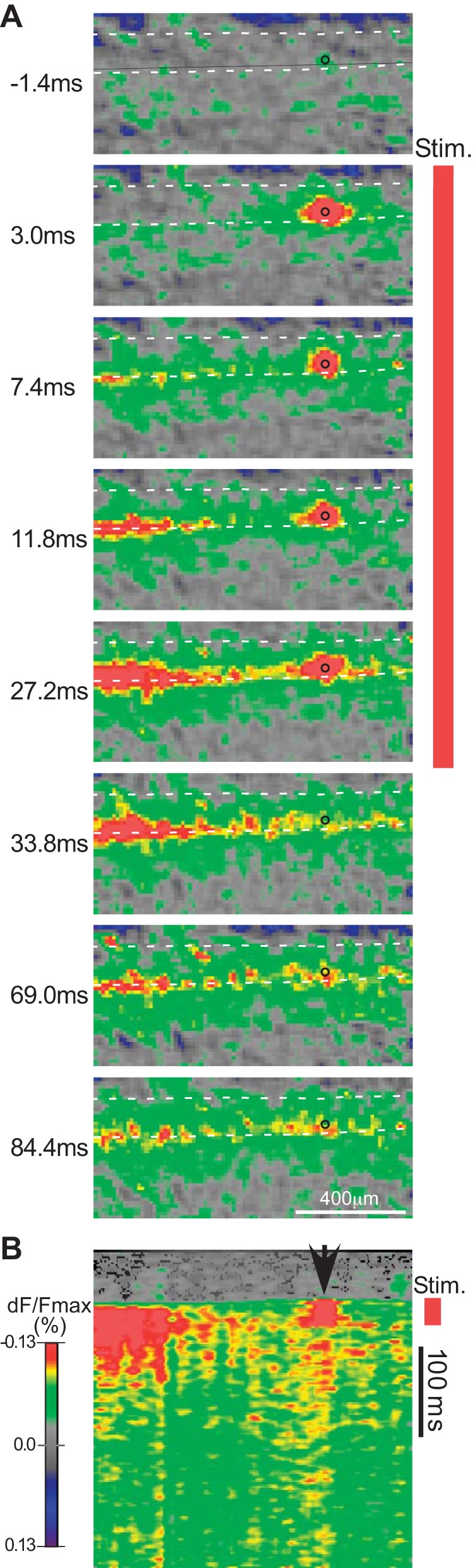
A: time series of voltage-sensitive dye (VSD) images during and after focal electrical stimulation in a parasagittal slice, to illustrate the development of the “patchy” or discontinuous distribution of activity along the rostrocaudal axis. Time points shown at left of each image are relative to the onset of the 30-ms stimulus train (Stim.; indicated by the red vertical bar). Note that the time intervals between the images are not equal throughout the series. Circle on each image marks the stimulus position. Dashed lines mark approximate position of the laminae I–II borders. Dorsal direction is at top. B: plot of time course of activity for the same stimulus trial shown in A. Time is represented on the y-axis (the dorsoventral axis is not represented in this plot). Activity is shown for a single rostrocaudally oriented line drawn approximately parallel to the laminar borders through the zone of peak activity (black line in top image). Arrowhead marks the rostrocaudal position of the stimulus. Distance calibration is same as in A. Stimulus intensity is 36 µA, which is considerably higher than the intensities used for the experiments illustrated in transverse slices (Figs. 1–4). All VSD images, ×10 objective.
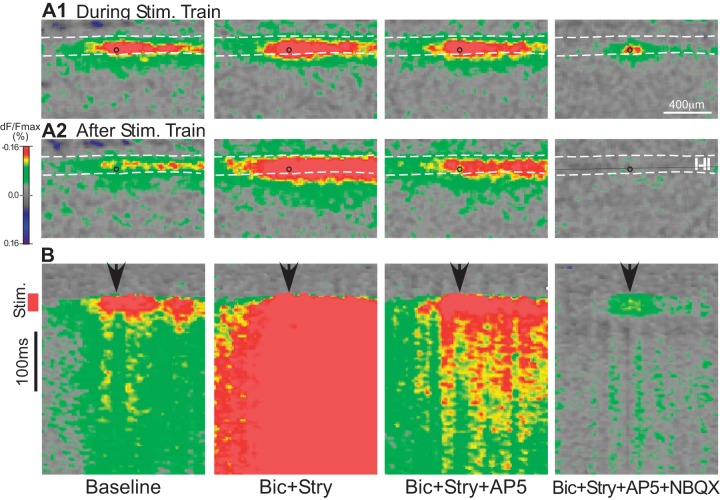
Fig. 7.
Effect of chemical blockade of synaptic transmission on voltage-sensitive dye (VSD) responses evoked by focal electrical stimulation in the superficial dorsal horn, in a parasagittal slice. A: activity during (A1) and 12 ms after (A2) the end of the stimulus train. Circle on each image marks the stimulus position. Dashed lines mark approximate position of the laminae I–II borders. B: plot of time course of activity for the stimulus trial shown in A. Time is represented on the y-axis (the dorsoventral axis is not represented in this plot). Activity is shown for a single rostrocaudally oriented line drawn approximately parallel to the laminar borders through the zone of peak activity (black line in rightmost image of A2 image). Stimulus (Stim.) train is indicated by the red vertical bar. Arrowheads mark the rostrocaudal position of the stimulus. Distance calibration is same as in A. Stimulus intensity is 24 µA. All VSD images, ×10 objective. AP5, d-2-amino-5-phosphonovalerate (50 µM); Bic, bicuculline (10 µM); dF/Fmax, %change in fluorescence; NBQX, 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide (10 µM); Stry, strychnine (300 nM).
Fig. 8.
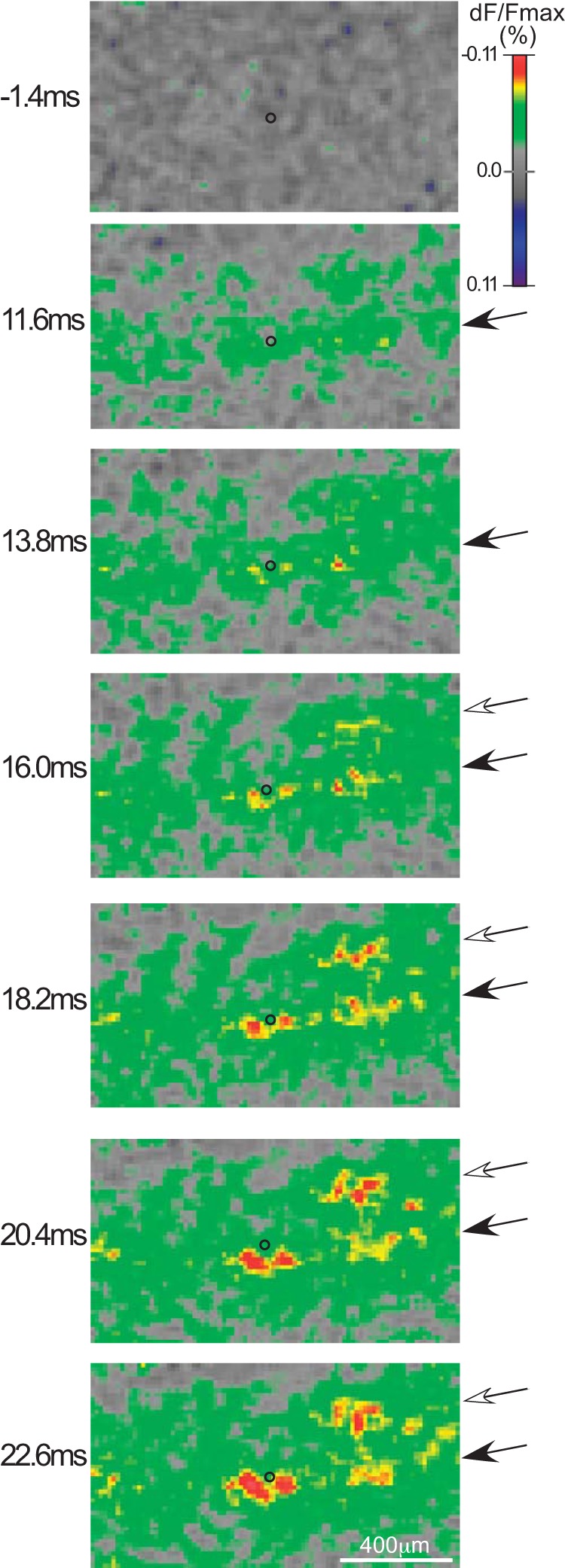
A time series of voltage-sensitive dye (VSD) images during focal electrical stimulation in a horizontal slice through lamina II. Medial direction is at top and rostral direction is to the left. Time points at top left of each VSD image are relative to the onset of the 30-ms stimulus train. Circle on each image marks the stimulus position. Closed arrow indicates a rostrocaudally oriented patchy band of activity that spreads from the stimulus site, and open arrow indicates a more medial zone of activity that develops somewhat later in the stimulus train. Stimulus intensity is 24 µA. All VSD images, ×10 objective. dF/Fmax, %change in fluorescence.
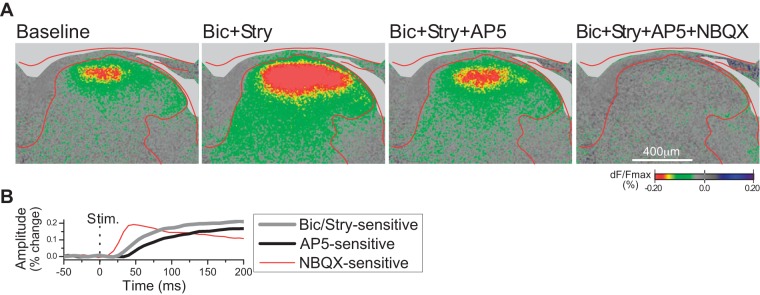
Fig. 5.
A: voltage-sensitive dye (VSD) responses evoked by single-shock stimulation (1 ms, 1.4 mA) of the L5 dorsal root, and effect of chemical blockade of synaptic transmission, in a transverse slice (right side; ×10 objective). (Note that all other figures show responses evoked by focal stimulation in the dorsal horn.) Time point illustrated is 140 ms after the stimulus. B: time course of actions of the synaptic inhibitors bicuculline (Bic; 10 µM), strychnine (Stry; 300 nM), d-2-amino-5-phosphonovalerate (AP5; 50 µM), and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide (NBQX; 10 µM), obtained by sequential subtraction of data shown in A. Curve for Bic/Stry was obtained by subtracting the baseline response (first VSD image) from the response with Bic+Stry (second VSD image). AP5 curve was obtained from the difference of the response before and after addition of AP5 (subtraction of third panel from second panel), and similarly for the NBQX curve (subtraction of fourth panel from third panel). Response amplitude was measured from a circular area of ~70-µm diameter within the zone of peak response, in the superficial dorsal horn. dF/Fmax, %change in fluorescence; Stim., stimulus onset.
Figure 1 shows an experiment in which focal stimulation was delivered at a series of ascending intensities to a site in the dorsal horn (lamina II/III border region, ~120 µm ventral to white matter; Fig. 1B) and a site in the overlying white matter (Fig. 1A). The time point shown in all of the images in Fig. 1 is the end of the 30-ms stimulus train. The dorsal horn site had a much lower response threshold, and the response showed a much greater increase with small increases in intensity, compared with the response evoked from the site in the white matter. Stimulation in the dorsal horn (90–120 µm below the white matter border) produced responses at stimulus intensities that were four- to eightfold lower than those required to produce comparable responses when stimulating in the overlying white matter (20–60 µm dorsal to the dorsal border of the dorsal horn); response threshold was fourfold lower in two slices and eightfold lower in five slices. We take this as evidence that dorsal horn neurons have a lower threshold to focal stimulation than axons in the white matter for evoking a VSD response, and the responses evoked by focal stimulation in the dorsal horn at these relatively low currents (≤6 µA) are primarily from activation of dorsal horn neurons rather than activation of axons.
Figure 1 also shows that the response evoked from the site in the white matter had two components: an NBQX-resistant (nonsynaptic) component in the area surrounding the electrode tip and a separate, broader NBQX-sensitive zone of activation in the subjacent dorsal horn. Figure 2 shows that the VSD response evoked by low-intensity (3 µA) stimulation within the dorsal horn is also largely NBQX sensitive (meaning that the direct nonsynaptic activation that necessarily preceded the synaptic activity was mostly below the threshold for detection at these imaging settings). In that experiment, the synaptic blockers bicuculline, strychnine, AP5, and NBQX were each added to the bath sequentially. Both the response enhancement by blockade of inhibitory transmission (bicuculline, strychnine) and the response suppression by blockade of excitatory transmission (AP5 and NBQX) confirm that the response is largely synaptically mediated. In addition to the NBQX-sensitive activity, a substantial NBQX-resistant response around the electrode tip could be evoked from sites in the dorsal horn as well as the white matter, but for the most part was recruited only at relatively high stimulus intensities (usually >12 µA).
Fig. 2.
Effect of chemical blockade of synaptic transmission on voltage-sensitive dye (VSD) responses evoked by focal electrical stimulation in the superficial dorsal horn, in a transverse slice (right side; ×10 objective). Time point illustrated is 15 ms after the end of the 30-ms stimulus train. Stimulus amplitude was 3 µA; cross in each image marks the stimulating electrode position. AP5, d-2-amino-5-phosphonovalerate (50 µM); Bic, bicuculline (10 µM); dF/Fmax, %change in fluorescence; NBQX, 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide (10 µM); Stry, strychnine (300 nM).
For stimulus sites similar to that in Fig. 2 (~100 µm below the white matter border, which we judged to be at or slightly above the lamina II/III border), the effect of each of the four synaptic blockers, expressed as percent change in response amplitude relative to the immediately preceding response, was bicuculine, 102 (46)%; strychnine, 27 (22)%; AP5, −30 (4)%; and NBQX, −79 (16)% [mean (SD), n = 4 slices]. In separate slices, the effect of the synaptic blockers was tested at sites in the deep dorsal horn, either 240 or 360 µm below the white matter border. The effects were bicuculline, 45 (48)%; strychnine, 36 (49)%; AP5, −31 (16)%; and NBQX, −68 (14)% for sites 240 µm below the white matter (n = 14) and bicuculline, 34 (47)%; strychnine, 65 (48)%; AP5, −34 (10)%; and NBQX, −76 (14)% for sites 360 µm below the white matter (n = 14). Statistical comparison showed no significant differences between the three laminar positions in these effects, but there was a trend toward a greater effect of bicuculline at the most superficial site compared with the two deeper sites (P = 0.052, ANOVA).
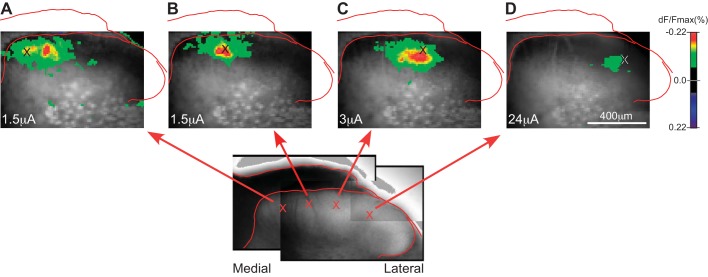
Figures 3 and 4 show the results of experiments in which focal stimulation was delivered to sites at different mediolateral positions across the superficial dorsal horn. Response amplitude was greater with stimulation at medial sites than at lateral sites (Fig. 4A). For the purposes of describing this relationship, the dorsal horn may be described as having a medial portion that is covered by white matter (e.g., stimulation sites in Fig. 3, A–C) and a lateral portion that has almost no overlying white matter (e.g., stimulation site in Fig. 3D). Stimulation in this more lateral portion of the dorsal horn (from the lateral edge of the white matter to the lateral edge of the dorsal horn) evoked extremely small responses (Fig. 4A1), and, unlike at more medial stimulation sites, the response amplitude increased only minimally with large increases in stimulus current (Fig. 4A2). This large difference in responsiveness was present independently of the order of stimulation (lateral sites before or after medial sites). Figure 4B shows the results of a separate set of experiments in which stimuli were delivered at a series of sites at different depths (distances below the white matter), in either medial or lateral dorsal horn (where medial and lateral series were done in separate experiments, to limit the duration that each slice was studied). The difference in responsiveness between medial and lateral sites was present at all depths tested. In experiments with stimulation at lateral sites, at least one medial site was stimulated afterward to confirm the responsiveness of the slice.
Fig. 3.
Mediolateral asymmetry of voltage-sensitive dye (VSD) responses evoked by focal electrical stimulation at different positions across the mediolateral axis of the dorsal horn. Inset: a composite infrared-differential interference contrast image of the slice from which VSD images in A–D were obtained. Cross on each image marks the stimulus position. Stimulus intensity is shown at bottom left in A–D (1.5, 1.5, 3, and 24 µA, respectively); intensity was increased for the more lateral positions because of the higher response threshold at these positions. Distribution of activity evoked from stimulation at the most medial site (A) shows a pronounced lateral asymmetry that reverses direction with stimulation at more lateral sites (C and D). Time point illustrated is immediately after the end of the 30-ms stimulus train. Images show transverse slice through right dorsal horn (×10 objective). dF/Fmax, %change in fluorescence.
For comparison, Fig. 5 shows responses to dorsal root stimulation (stimulation of the entire cut end of the dorsal root with a suction electrode). As with focal stimulation within the dorsal horn, dorsal root stimulation produced larger responses in the medial than the lateral portion of the dorsal horn (Fig. 5A, left, baseline). Inhibitory synaptic blockade (bicuculline plus strychnine) enhanced responses to dorsal root stimulation throughout the mediolateral extent of the dorsal horn, confirming that all portions were capable of generating responses.
In addition to this difference in response amplitude, the responses evoked by focal stimulation at different mediolateral positions also differed in the mediolateral distribution of the activity relative to the stimulation site. The zone of activity evoked by stimulation at far medial sites displayed a lateral asymmetry, in that it extended farther in the lateral than in the medial direction relative to the stimulation site, and the area of maximal response amplitude was displaced laterally from the stimulation site (Fig. 3A). As the stimulus position was moved progressively in the lateral direction, this asymmetry disappeared (Fig. 3B) and then reversed direction to display a medial asymmetry at more centrally located sites near the lateral edge of the white matter (Fig. 3C; also apparent for the site in Fig. 1A, which is a similar position). As a measure of this asymmetry, a calculation was made of the weighted mean (center of the distribution) of the response area along the mediolateral axis. Sites ~200 µm lateral to the medial edge of the dorsal horn (e.g., Fig. 3A) had a response center that was 9 ± 5 µm lateral to the stimulation site, whereas sites ~400 µm lateral to the medial edge of the dorsal horn (similar to Fig. 3C) had a response center that was 4 ± 3 µm medial to the stimulation site (P < 0.0001, unpaired t test; n = 10 and 5 animals, respectively, for the 2 sites).
To examine the distribution of activity along the rostrocaudal axis, experiments were done in parasagittal slices (Figs. 6 and 7). The slices were made from a central position along the mediolateral axis of the dorsal horn. Figure 6 shows an example of a response evoked by focal stimulation in the superficial dorsal horn in a parasagittal slice. A time sequence is shown to illustrate the progressive development of the response during the stimulus train and the initial decay of the response after the stimulus train. Focal stimulation in the superficial dorsal horn in parasagittal slices typically evoked a zone of activity that extended for relatively long distances (>1 mm) along the rostrocaudal axis (Figs. 6 and 7). Evoking this rostrocaudally extensive response zone required a range of relatively high stimulus intensities (24–36 µA), which is a range that is sufficiently high to produce substantial activation of axons, based on the experiments described earlier (cf. Fig. 1A). Such elongated response zones could not be evoked along the dorsoventral axis or the mediolateral axis (in transverse slices), even at high stimulus intensities. The rostrocaudal distribution was also distinctive in that the activity along the other two axes generally showed a single peak zone and a fairly consistent decline with distance from this peak zone, whereas activity along the rostrocaudal axis could extend for some distance without decline and, in addition, could show distinct peaks at some distance from the stimulation site. For example, in the slice in Fig. 6, in addition to the usual high-amplitude zone around the stimulation site, there is a second peak near the left edge of the imaged area. Whereas the first peak (around the stimulus site) was already present at maximum amplitude at the earliest imaged time point after the start of the stimulus train (3 ms) and declined abruptly at the end of the train, the second peak was not recruited until later in the stimulus train and persisted for some period after the end of the train. The example in Fig. 7 further shows that the area of activity that is present around the stimulus site during the train is relatively resistant to NBQX (Fig. 7A1, far right), whereas the other activity, including nearly all activity that persists after the stimulus train, is highly sensitive to NBQX blockade.
An additional distinctive feature of the activity along the rostrocaudal axis is that it tended to exhibit a “patchy” distribution, with multiple discrete zones of higher activity separated by zones of lower activity. This patchy appearance tended to be most evident in the period after the end of the stimulus train, as the response starts to decay (e.g., time point 69 ms in Fig. 6). This patchy quality is illustrated more clearly in the vertical time displays shown in Figs. 6B and 7B. These displays show the time course of activity (represented on the vertical axis) measured along a single rostrocaudal line drawn through the center of the superficial dorsal horn. In these time displays, the individual patches appear as vertical “columns,” illustrating that each individual “patch” of activity maintains a fairly constant rostrocaudal position over time. This constancy of position suggests that the position of the patches is not random, as might be the case if the patchiness were merely the result of the random breaking up of the zone of peak activity during the decay of the response after the end of the stimulus train, but instead might reflect an underlying property of the spatial organization of the dorsal horn. These repeated patches of activity are possibly suggestive of a modular organization in the circuitry of the dorsal horn, such as has been previously proposed (Hantman et al. 2004; Lu and Perl 2005; Lu et al. 2013), if each patch represented a component of a repeating unit that received excitatory input from other positions along the rostrocaudal axis.
The spatial organization of activity evoked by focal stimulation was further examined in horizontal slices. The rostrocaudally elongated zones of activity that were visible in parasagittal slices were not evoked as consistently and were not as clearly visible in horizontal slices, probably because of the difficulty in getting a consistent plane of section through the superficial dorsal horn parallel to the dorsal horn laminae (which are curved mediolaterally and so not perfectly horizontal). Figure 8 shows such an experiment in a horizontal slice, in a time series to show the development of activity during the stimulus train. Only the more medial portion of the dorsal horn is contained in this imaging field (roughly, the portion that is covered by white matter). Initially, there is a rostrocaudally oriented zone of activity that appears at the beginning of the stimulus train, in line with the position of the stimulus (closed arrow). As the stimulus train continues, and this rostrocaudal zone of activity increases and becomes more distinct, a second rostrocaudal zone appears, less distinct but roughly parallel to the first zone, at a more medial position (open arrow).
DISCUSSION
VSDI was used in the present study in an attempt to obtain information about certain aspects of dorsal horn organization that would complement prior findings from single-cell approaches. The present study was prompted in part by our prior finding of a mediolateral asymmetry in the intrinsic circuitry of the dorsal horn, in a study that used a combination of intracellular labeling and mapping of local interneuronal inputs by laser scanning photostimulation (Kosugi et al. 2013). That prior study found that lamina I neurons receive greater local excitatory synaptic input from interneurons that are medial rather than lateral to their own cell body, and exhibit a matching medial asymmetry in their dendritic fields. The present VSDI results also revealed a type of mediolateral asymmetry in dorsal horn organization: medial sites in the superficial dorsal horn showed a much greater responsiveness to focal stimulation than lateral sites such that responses could be evoked at much lower stimulus intensities, and response amplitude showed much greater increases with increasing stimulus intensity. The transition in responsiveness is fairly abrupt and occurs approximately at the lateral edge of the white matter, roughly midway along the mediolateral axis of the dorsal horn.
In the following we discuss two issues concerning this difference in responsiveness in medial vs. lateral dorsal horn: 1) synaptic mediation of the responses and 2) the role of intrinsic vs. extrinsic elements of the dorsal horn.
Synaptic mediation.
As noted above, VSDI responses could be evoked at relatively low stimulus intensities (typically <6 µA) from medial but not lateral sites in the superficial dorsal horn. Experiments with synaptic blockers showed that the activity that could be evoked at these relatively low intensities was largely synaptically mediated. Any synaptic activity must necessarily be preceded by direct (nonsynaptic) activation of neural elements that in turn exert the synaptic effects, but apparently under our recording conditions (gain setting, etc.), using low stimulus intensities and examining activity following rather than during the stimulus train, the proportion of the VSDI activity that was a result of the direct activation was relatively small, and the majority of the imaged activation was from the synaptic rather than the direct component of the response. This relative predominance of the synaptic over the direct component of the response may be partly attributable to the use of stimulus trains rather than single pulses, which may have produced a relative potentiation of the synaptic responses. Thus the neural elements that are directly activated by the electrical stimulus greatly amplify the VSDI response through their synaptic actions. This suggests that a relatively small number of directly activated neurons can produce synaptic activation of a substantially larger number of postsynaptic neurons during stimulation at sites in the medial portion of the superficial dorsal horn.
Activation of dorsal horn neurons.
Our results also suggest that the neural elements that were directly activated by these relatively low stimulus intensities (and whose direct activation produced the imaged synaptic activity) were predominantly dorsal horn neurons, rather than axons that entered the dorsal horn from the white matter, because stimulation in the white matter produced substantial VSDI responses only at higher stimulus intensities. One explanation for this would be if there were a higher threshold to electrical stimulation of axons of passage vs. the cell body or axon initial segment. Such a difference would be consistent with the concept that the axon initial segment is normally the site of impulse initiation (Stuart and Sakmann 1994) as a result of having a higher density of voltage-gated sodium channels and thus a lower threshold (Kole et al. 2008). On the basis of this concept and our findings on the threshold difference between white matter and dorsal horn sites, we interpret our results as evidence that the difference in responsiveness at medial vs. lateral sites is primarily a result of a difference in properties of the intrinsic elements of the superficial dorsal horn, rather than a difference in the properties of extrinsic inputs.
Although the VSDI responses to the low-intensity stimuli were largely synaptically mediated, the greater responsiveness of the medial sites could potentially result from a difference in either one of the two steps involved in the generation of the synaptic activity, i.e., a difference in 1) the amount of direct neuronal activation that is produced by the electrical stimulus, such as would result from greater membrane excitability, or 2) a difference in the amount of synaptic activity evoked by the directly activated neurons, as the result of a greater number or greater strength of local synaptic connections or a greater membrane excitability of the postsynaptic neurons. Another possible cause of greater direct activation would be greater neuronal density, but this seems an extremely unlikely explanation because the neuronal density appears quite uniform across the superficial dorsal horn (Molander et al. 1984; Rexed 1952), and any such difference would have to be very large to cause the extreme mediolateral difference in responsiveness. Our data do not allow us to determine the relative contributions of membrane excitability vs. synaptic connectivity to the difference in responsiveness.
The medial and lateral parts of the lumbar dorsal horn are known to differ in their extrinsic inputs, in that they receive primary afferent projections originating from different parts of the body surface (Swett and Woolf 1985). For the most part, prior studies have not reported differences between the medial and lateral dorsal horn in any aspects of their neuronal properties or intrinsic organization, although such possible differences generally have not been the focus of prior studies. One notable exception is the recent finding that a specific population of interneuron, the excitatory dynorphin neuron, is present only in the medial part of the superficial dorsal horn, at the level of the lumbar enlargement (L4) (Boyle et al. 2017). The authors note that this area corresponds specifically to the region that receives input from glabrous skin of the hind paw and further show that this interneuronal population is absent at spinal levels (L2) that lack glabrous skin input. One further intriguing anatomical mediolateral difference is the presence of an intense band of transient receptor potential vanilloid type 1 (TRPV1) expression in the inner part of the superficial laminae of the medial but not the lateral lumbar dorsal horn (Tominaga et al. 1998). However, TRPV1 is expressed by primary afferents, so this finding is not directly relevant to possible differences in intrinsic properties of dorsal horn neurons. The mediolateral differences found in the present study, as well as those found in the studies of Boyle et al. (2017) and Tominaga et al. (1998), suggest that studies of dorsal horn organization, particularly those that focus on intrinsic circuitry, need to recognize the possibility of substantial differences between medial and lateral dorsal horn.
One additional mediolateral difference in dorsal horn organization concerns extrinsic rather than intrinsic elements: primary afferent axons segregate by size in the dorsal root entry zone before entering the dorsal horn such that large myelinated fibers course medially in the white matter along the dorsal border of the medial dorsal horn and then curve ventrally along the dorsal horn’s medial border, whereas smaller fibers course laterally along the dorsal border of the lateral dorsal horn (Light and Perl 1979). Furthermore, in their course over the medial dorsal horn, a substantial number of the large fibers depart from the main bundle and turn ventrally to pass through the superficial laminae of the medial dorsal horn en route to their projection targets in the deep laminae. Given that large myelinated (Aβ) fibers have much lower thresholds to electrical stimulation than the smaller primary afferent fibers, activation of these fibers could potentially explain the greater responsiveness of medial vs. lateral dorsal horn found in the present study. This would mean that the synaptic (NBQX sensitive) component of the VSD responses represents, at least in part, the Aβ-mediated responses of dorsal horn neurons. One question would be why stimulation just above (20–60 µm) the white matter border fails to activate the Aβ fibers and evoke low-threshold synaptic responses in the dorsal horn, whereas stimulation below the white matter border is much more effective. One explanation would be that the fibers are traveling at an oblique angle relative to the plane of section of the slice such that fibers just above the white matter border either were cut before entering the dorsal horn or entered the dorsal horn at a plane that was too far below the surface of the slice for detection of VSD responses. The present data do not allow us to rule out this possibility, and so activation of Aβ fibers must be considered a possible explanation, at least in part, for the higher responsiveness of medial vs. lateral dorsal horn.
It is difficult to make any direct connection between the present results and our prior finding of a greater amount of local (interneuronal) excitatory input to lamina I neurons from regions that are medial vs. lateral to the neuron’s soma (Kosugi et al. 2013), because that prior study did not sample neurons across a range of mediolateral positions. Nonetheless, it is possible that a medial bias such as was found in the previous study could contribute to the greater responsiveness of medial sites found in the present study, if it reflected a general tendency of neurons across the medial part of the dorsal horn to receive and engage in local excitatory connectivity.
In addition to the extreme medial-lateral difference in responsiveness discussed above, another type of mediolateral asymmetry that was evident in the responses to focal stimulation at sites within the medial dorsal horn was a relatively small but consistent mediolateral displacement of the center of the VSD response relative to the electrode position, which changed direction as the electrode was moved across the dorsal horn such that the response area tended to extend laterally from the electrode position for the most medial stimulation sites and tended to extend medially for more central sites. The neural elements that are directly activated by the stimulus current would be expected to be distributed more or less symmetrically around the electrode, so this asymmetry is more likely to reflect an asymmetry in the distribution of the postsynaptic targets of the directly activated neural elements. One possibility would be that this asymmetry reflects a pattern of opposite trajectories in the axons that enter the dorsal horn from the white matter at different mediolateral positions: axons that enter far medially tend to then course laterally for some distance through the superficial dorsal horn as they make synaptic contacts, and the reverse for axons that enter further laterally (according to this hypothesized explanation). However, as noted above, the relatively low stimulus intensities used in this portion of the study seem to be mostly subthreshold for activating axons from the white matter. If one nonetheless considers the possibility that the observed asymmetric response areas are in fact a result, at least in part, of the trajectories (and resulting synaptic effects) of axons that entered from the white matter, this would mean that there is an extreme difference between medial and lateral dorsal horn in the efficacy of synaptic contacts made by extrinsic axonal inputs. The response pattern evoked by dorsal root stimulation would seem to argue against this possibility, at least for primary afferent inputs. Although the dorsal root response was greater in medial than lateral dorsal horn, the difference was not nearly as extreme as the difference in responsiveness to focal stimulation within the dorsal horn.
An alternative explanation for the asymmetric response area would be a mediolateral asymmetry in the direction of local connectivity such that neurons in the most medial part of the superficial dorsal horn tend to make excitatory synaptic connections preferentially onto neurons that are lateral to their cell body, and this reverses direction for neurons at more central locations. A mediolateral asymmetry in excitatory local connectivity was in fact found in our previous study, but the direction of that asymmetry was in the opposite direction from that proposed above: in a neuronal sample that was distributed in a relatively central mediolateral position (similar location to site in Fig. 3C), there was a strong tendency for lamina I neurons to receive excitatory synaptic input from neurons that are medial to their cell bodies (Kosugi et al. 2013), which would predict a laterally biased asymmetry for evoked postsynaptic VSD responses (opposite to the direction of asymmetry in Fig. 3C). No mediolateral asymmetry was found in the origin of local synaptic inputs for other populations of neurons in the superficial dorsal horn in that study. It should be noted that that study was done in mice, whereas the present study is in rats. It thus appears that the asymmetric response area in the present study cannot be readily explained on the basis of findings on local connectivity obtained from prior studies with single-cell techniques.
A fundamental limitation of the VSDI method is that it lacks cellular resolution, and so the cellular basis for the mediolateral asymmetries, as well as other findings, cannot be determined from these data. Nonetheless, the present data revealed a large difference between medial and lateral dorsal horn in responsiveness to focal stimulation while providing evidence that this difference results from properties of intrinsic elements of the dorsal horn, and it was also useful for mapping the change in responsiveness across the dorsal horn (i.e., identifying the transition point, around the lateral edge of the white matter/dorsal root). Such spatial information was not available from prior single-cell studies and would be cumbersome to obtain in such studies because it would require obtaining separate neuronal samples from multiple mediolateral positions.
The dorsal horn has a somatotopic organization such that, in the lumbar enlargement, medial and lateral dorsal horn receive primary afferent input from distal and proximal hindlimb, respectively (Bon et al. 2002; Swett and Woolf 1985; Takahashi et al. 2003). Sensory discrimination is in general greater in distal vs. proximal/axial body regions, which may be attributable in part to a greater peripheral innervation density (Wang et al. 1997), as well as an overall greater area of representation centrally (Mountcastle 1957; Weinstein 1968). The greater responsiveness we observed in medial vs. lateral dorsal horn would be expected to produce a central amplification of inputs from distal relative to proximal portions of the hindlimb, and thus might also contribute to the enhancement of distal sensory function.
Focal stimulation in the superficial laminae in parasagittal slices evoked rostrocaudally extensive response areas that were dependent on synaptic activity (and thus represent postsynaptic activity evoked by neural elements in the vicinity of the electrode that were directly activated by the electrical stimulus). These zones are evidence of axons that course rostrocaudally and evoke postsynaptic excitation of dorsal horn neurons over a relatively large rostrocaudal expanse (1 mm or greater, comparable to the length of a single segment). We suspect that these axons are extrinsic to the dorsal horn (e.g., primary afferents), rather than originating from dorsal horn neurons, because the rostrocaudally extensive response zones were only evoked at relatively high stimulus currents, in the range required to evoke synaptic responses from stimulus sites in the white matter (which is several times higher than the range necessary for evoking synaptic responses from stimulus sites within the dorsal horn; see earlier discussion of activation of dorsal horn neurons vs. axons from the white matter). Superficial dorsal horn neurons themselves can also give rise to rostrocaudally extensive local axonal projections, but, as we have argued above, we would expect dorsal horn neurons to be activated by lower currents, and so the high currents required to evoke the extensive rostrocaudal response zones would seem to indicate that these responses are not primarily generated by neurons whose cell bodies reside within the superficial dorsal horn. Such an apparent inability of the local interneuronal projections to generate the longer range VSD responses in the present study could be considered consistent with the finding that lamina I local circuit neurons, which have profuse local projections over a similar rostrocaudal range (about one segment), are inhibitory (Szucs et al. 2013). Strong excitatory interneuronal connections also exist within the superficial dorsal horn, but it appears these tend to exert their greatest influences within a range that is more restricted than the extensive zones found in the present study (Kato et al. 2007, 2009; Kosugi et al. 2013; Luz et al. 2010). Ascending tract lamina I projection neurons, which are excitatory (Szucs et al. 2013), do have collateral branches that can project to dorsal horn sites over much longer ranges (several segments), but these projections are much sparser than the shorter range projections of the inhibitory local circuit neurons (Szucs et al. 2010, 2013), and consistent with this, long-range excitatory connections between lamina I neurons are relatively uncommon (Antal et al. 2016). Gutierrez-Mecinas et al. (2018) have also shown that longer, multisegmental propriospinal projections are almost entirely excitatory and, furthermore, found that those projections target most heavily the lateral spinal nucleus rather than the dorsal horn.
The possibility that these extensive postsynaptic response zones are evoked by primary afferents is consistent with anatomical findings that the central projection territories of individual primary afferents, particularly in the superficial laminae, can be extensive rostrocaudally while tending to have a much more restricted mediolateral expanse (Koerber and Mirnics 1995; Light 1992; Sugiura et al. 1986; Wilson et al. 1996). Such a parasagittal orientation, with a greater rostrocaudal than mediolateral expanse, is a general characteristic of the neuropil in superficial laminae, also commonly being exhibited by the dendritic fields, as well as the local axonal projections, of individual dorsal horn neurons (Light 1992; Schneider 1992; Strassman et al. 1994). This parasagittal orientation of the neural elements, with a restricted mediolateral expanse, would contribute to the precise somatotopic mapping of receptive field position across the mediolateral axis of the dorsal horn.
GRANTS
This work was supported by NIH Grant R01 NS057454 (to A. M. Strassman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M., G.K., and A.M.S. conceived and designed research; M.M. performed experiments; M.M. and A.M.S. analyzed data; M.M., G.K., and A.M.S. interpreted results of experiments; M.M. and A.M.S. prepared figures; M.M. and A.M.S. drafted manuscript; M.M., G.K., and A.M.S. edited and revised manuscript; M.M., G.K., and A.M.S. approved final version of manuscript.
REFERENCES
- Antal Z, Luz LL, Safronov BV, Antal M, Szücs P. Neurons in the lateral part of the lumbar spinal cord show distinct novel axon trajectories and are excited by short propriospinal ascending inputs. Brain Struct Funct 221: 2343–2360, 2016. [Erratum in Brain Struct Funct 221: 2399–2400, 2016.] doi: 10.1007/s00429-015-1046-3. [DOI] [PubMed] [Google Scholar]
- Bentley GN, Gent JP. Electrophysiological properties of substantia gelatinosa neurones in a novel adult spinal slice preparation. J Neurosci Methods 53: 157–162, 1994. doi: 10.1016/0165-0270(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Bon K, Wilson SG, Mogil JS, Roberts WJ. Genetic evidence for the correlation of deep dorsal horn Fos protein immunoreactivity with tonic formalin pain behavior. J Pain 3: 181–189, 2002. doi: 10.1054/jpai.2002.123710. [DOI] [PubMed] [Google Scholar]
- Boyle KA, Gutierrez-Mecinas M, Polgár E, Mooney N, O’Connor E, Furuta T, Watanabe M, Todd AJ. A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 363: 120–133, 2017. doi: 10.1016/j.neuroscience.2017.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron 82: 522–536, 2014. doi: 10.1016/j.neuron.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bráz JM, Basbaum AI. Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity. Neuroscience 163: 1220–1232, 2009. doi: 10.1016/j.neuroscience.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron 47: 787–793, 2005. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Cordero-Erausquin M, Allard S, Dolique T, Bachand K, Ribeiro-da-Silva A, De Koninck Y. Dorsal horn neurons presynaptic to lamina I spinoparabrachial neurons revealed by transynaptic labeling. J Comp Neurol 517: 601–615, 2009. doi: 10.1002/cne.22179. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Polgár E, Bell AM, Herau M, Todd AJ. Substance P-expressing excitatory interneurons in the mouse superficial dorsal horn provide a propriospinal input to the lateral spinal nucleus. Brain Struct Funct 223: 2377–2392, 2018. doi: 10.1007/s00429-018-1629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantman AW, van den Pol AN, Perl ER. Morphological and physiological features of a set of spinal substantia gelatinosa neurons defined by green fluorescent protein expression. J Neurosci 24: 836–842, 2004. doi: 10.1523/JNEUROSCI.4221-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G, Kawasaki Y, Ji RR, Strassman AM. Differential wiring of local excitatory and inhibitory synaptic inputs to islet cells in rat spinal lamina II demonstrated by laser scanning photostimulation. J Physiol 580: 815–833, 2007. doi: 10.1113/jphysiol.2007.128314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G, Kawasaki Y, Koga K, Uta D, Kosugi M, Yasaka T, Yoshimura M, Ji RR, Strassman AM. Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn. J Neurosci 29: 5088–5099, 2009. doi: 10.1523/JNEUROSCI.6175-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G, Kosugi M, Mizuno M, Strassman AM. Three-dimensional organization of local excitatory and inhibitory inputs to neurons in laminae III-IV of the spinal dorsal horn. J Physiol 591: 5645–5660, 2013. doi: 10.1113/jphysiol.2013.256016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber HR, Mirnics K. Morphology of functional long-ranging primary afferent projections in the cat spinal cord. J Neurophysiol 74: 2336–2348, 1995. doi: 10.1152/jn.1995.74.6.2336. [DOI] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci 11: 178–186, 2008. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- Kosugi M, Kato G, Lukashov S, Pendse G, Puskar Z, Kozsurek M, Strassman AM. Subpopulation-specific patterns of intrinsic connectivity in mouse superficial dorsal horn as revealed by laser scanning photostimulation. J Physiol 591: 1935–1949, 2013. doi: 10.1113/jphysiol.2012.244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR. The Initial Processing of Pain and Its Descending Control: Spinal and Trigeminal Systems. Basel: Karger, 1992. [Google Scholar]
- Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol 186: 117–131, 1979. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, Xiong L. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 123: 4050–4062, 2013. doi: 10.1172/JCI70026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci 23: 8752–8758, 2003. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II). J Neurosci 25: 3900–3907, 2005. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz LL, Szucs P, Pinho R, Safronov BV. Monosynaptic excitatory inputs to spinal lamina I anterolateral-tract-projecting neurons from neighbouring lamina I neurons. J Physiol 588: 4489–4505, 2010. doi: 10.1113/jphysiol.2010.197012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol 230: 133–141, 1984. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol 20: 408–434, 1957. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Peirs C, Williams SP, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, Seal RP. Dorsal horn circuits for persistent mechanical pain. Neuron 87: 797–812, 2015. doi: 10.1016/j.neuron.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol 96: 415–495, 1952. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Santos SF, Luz LL, Szucs P, Lima D, Derkach VA, Safronov BV. Transmission efficacy and plasticity in glutamatergic synapses formed by excitatory interneurons of the substantia gelatinosa in the rat spinal cord. PLoS One 4: e8047, 2009. doi: 10.1371/journal.pone.0008047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SF, Rebelo S, Derkach VA, Safronov BV. Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J Physiol 581: 241–254, 2007. doi: 10.1113/jphysiol.2006.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SP. Functional properties and axon terminations of interneurons in laminae III-V of the mammalian spinal dorsal horn in vitro. J Neurophysiol 68: 1746–1759, 1992. doi: 10.1152/jn.1992.68.5.1746. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Potrebic S, Maciewicz RJ. Anatomical properties of brainstem trigeminal neurons that respond to electrical stimulation of dural blood vessels. J Comp Neurol 346: 349–365, 1994. doi: 10.1002/cne.903460304. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature 367: 69–72, 1994. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science 234: 358–361, 1986. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol 231: 66–77, 1985. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- Szucs P, Luz LL, Lima D, Safronov BV. Local axon collaterals of lamina I projection neurons in the spinal cord of young rats. J Comp Neurol 518: 2645–2665, 2010. doi: 10.1002/cne.22437. [DOI] [PubMed] [Google Scholar]
- Szucs P, Luz LL, Pinho R, Aguiar P, Antal Z, Tiong SY, Todd AJ, Safronov BV. Axon diversity of lamina I local-circuit neurons in the lumbar spinal cord. J Comp Neurol 521: 2719–2741, 2013. doi: 10.1002/cne.23311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Chiba T, Kurokawa M, Aoki Y. Dermatomes and the central organization of dermatomes and body surface regions in the spinal cord dorsal horn in rats. J Comp Neurol 462: 29–41, 2003. doi: 10.1002/cne.10669. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11: 823–836, 2010. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn. Mol Pain 13: 1744806917693003, 2017. doi: 10.1177/1744806917693003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543, 1998. doi: 10.1016/S0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Wang L, Millecchia R, Brown PB. Correlation of peripheral innervation density and dorsal horn map scale. J Neurophysiol 78: 689–702, 1997. doi: 10.1152/jn.1997.78.2.689. [DOI] [PubMed] [Google Scholar]
- Weinstein S. Intensive and extensive aspects of tactile sensitivity as a function of body part, sex, and laterality. In: The Skin Senses, edited by Kenshalo DR. Springfield, IL: Thomas, 1968, p. 195–222. [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord (3rd ed). New York: Kluwer Academic/Plenum, 2004. [Google Scholar]
- Wilson P, Kitchener PD, Snow PJ. Intraaxonal injection of neurobiotin reveals the long-ranging projections of Aβ-hair follicle afferent fibers to the cat dorsal horn. J Neurophysiol 76: 242–254, 1996. doi: 10.1152/jn.1996.76.1.242. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol 62: 96–108, 1989. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]