Abstract
The corticospinal pathway contributes to the control of grasping in intact humans. After spinal cord injury (SCI), there is an extensive reorganization in the corticospinal pathway; however, its contribution to the control of grasping after the injury remains poorly understood. We addressed this question by using transcranial magnetic stimulation (TMS) over the hand representation of the motor cortex to elicit motor-evoked potentials (MEPs) in an intrinsic finger muscle during precision grip and power grip with the TMS coil oriented to induce currents in the brain in the latero-medial (LM) direction to activate corticospinal axons directly and in the posterior-anterior (PA) and anterior-posterior (AP) directions to activate the axon indirectly through synaptic inputs in humans with and without cervical incomplete SCI. We found prolonged MEP latencies in all coil orientations in both tasks in SCI compared with control subjects. The latencies of MEPs elicited by AP relative to LM stimuli were consistently longer during power compared with precision grip in controls and SCI subjects. In contrast, PA relative to LM MEP latencies were similar between tasks across groups. Central conduction time of AP MEPs was prolonged during power compared with precision grip in controls and SCI participants. Our results support evidence indicating that inputs activated by AP and PA currents are engaged to a different extent during fine and gross grasping in humans with and without SCI.
NEW & NOTEWORTHY The mechanisms contributing to the control of hand function in humans with spinal cord injury (SCI) remain poorly understood. Here, we demonstrate for the first time that the latency of corticospinal responses elicited by transcranial magnetic stimulation anterior-posterior induced currents, relative to latero-medial currents, was prolonged during power compared with precision grip in humans with and without SCI. Gross grasping might represent a stragegy to engage networks activated by anterior-posterior currents after SCI.
Keywords: corticospinal volleys, hand, I waves, motor output, primary motor cortex, transcranial magnetic stimulation
INTRODUCTION
The corticospinal tract contributes to the control of different grasping behaviors (Lemon 2008). For example, in intact humans, studies using transcranial magnetic stimulation (TMS) showed that the size of corticospinal responses elicited in hand muscles changes to a different extent during fine and gross grasping (Datta et al. 1989; Flament et al. 1993; Tazoe and Perez 2017). In addition, the strength of connectivity between cortical areas and the motor cortex differentially changes according to the grasping behavior (Davare et al. 2008). Lesions of the corticospinal tract impair hand motor output (Lang and Schieber 2004). During fine grasping, individuals with spinal cord injury (SCI) show a decreased ability to modulate corticospinal excitability (Bunday et al. 2014) and electromyographic (EMG) activity (Lei and Perez 2018) in hand muscles compared with control subjects. While it is clear that extensive reorganization occurs in the corticospinal pathway following SCI (Oudega and Perez 2012), the extent to which we can engage corticospinal neurons during fine and gross grasping after the injury remains largely unknown.
It is possible to gain insights into transmission in the corticospinal pathway by examing changes in the latency of motor-evoked potentials (MEPs) elicited by TMS using different coil orientations (Di Lazzaro et al. 2012; Di Lazzaro and Rothwell 2014). Studies using epidural (Di Lazzaro et al. 2001) and single motor unit (Day et al. 1989; Hanajima et al. 1998; Sakai et al. 1997) recordings showed that posterior-anterior (PA)-induced TMS currents evoke highly synchronized corticospinal activity whereas anterior-posterior (AP)-induced currents evoke less synchronized activity with peak latencies that are later than those evoked after PA stimulation. Indeed, MEPs elicited with the coil in the AP orientation have longer latency, larger latency dispersion, and higher threshold than MEPs elicited in the PA orientation. To gain insights into changes in corticospinal transmission during gross and fine grasping behaviors in humans with SCI, we examined MEPs elicited by PA and AP currents.
In intact subjects, MEPs elicited by AP currents are consistently longer during power grip compared with precision grip (Federico and Perez 2016) and likely involve contributions from multiple brain areas (Groppa et al. 2012; Volz et al. 2015). Indeed, evidence showed that AP MEP latencies negatively correlate with connectivity between premotor areas and the motor cortex (Volz et al. 2015). Note that these brain regions are more active during power grip compared with precision grip (Ehrsson et al. 2000). Therefore, AP currents might reflect activation of a large range of synaptic inputs from other brain areas active during power grip. In individuals with SCI, recent evidence showed that AP MEP latencies are prolonged compared with LM MEP latencies during index finger abduction, suggesting that it is possible to engage AP inputs during voluntary activity after the injury (Jo et al. 2018). AP MEPs are influenced by subcortical networks (Cirillo and Perez 2015), and it should be noted that individuals with SCI could engage similar subcortical networks to control subjects while performing a power grip compared with a precision grip (Baker and Perez 2017). Thus we hypothesized that changes in MEPs elicited by AP stimulation during gross grasping would be preserved to a similar extent in humans with and without incomplete SCI.
MATERIALS AND METHODS
Subjects
Sixteen subjects with SCI (mean age ± SD = 50.6 ± 14.8 yr, 2 women) and 17 age-matched right-handed control subjects (mean age ± SD = 41.2 ± 15.9 yr, 6 women, P = 0.1) participated in the study. All subjects gave written informed consent before participation, approved by the local ethics committee at the University of Miami (20140997) in accordance with the guidelines established in the Declaration of Helsinki. Individuals with SCI had a chronic (≥1 yr), cervical injury (C2-C8), an intact (score = 2) or impaired (score = 1) but not absent innervation in dermatome C6 using the International Standards for Neurological Classification of Spinal Cord Injury sensory scores, and residual hand motor function (Table 1). Two out of 16 individuals with SCI were categorized by the American Spinal Cord Injury Impairment Scale (AIS) as AIS A (complete injury) due to the lack of sacral sparing (Marino et al. 2003), despite being able to elicit voluntary force with hand muscles. Fourteen individuals were classified as incomplete AIS C and D (Table 1). To test our hypothesis, we examined PA and AP MEP latencies in the first dorsal interosseous (FDI) muscle during precision grip and power grip tasks (Fig. 1A) and compared them with MEP latencies evoked by D-wave activation using LM currents.
Table 1.
Spinal cord injury participants
| SCI Subjects | Age, yr | Sex | Injury Level | AIS | Etiology | Time Since Injury, yr | Maximum FDI MVC, mV |
|---|---|---|---|---|---|---|---|
| 1 | 32 | M | C2–C4 | C | T | 9 | 0.15 |
| 2 | 68 | M | C2–C3 | C | T | 6 | 0.14 |
| 3 | 52 | F | C5–C8 | D | T | 14 | 0.33 |
| 4 | 80 | M | C3–C4 | C | T | 53 | 0.18 |
| 5 | 36 | M | C7–C8 | C | T | 3 | 0.39 |
| 6 | 50 | M | C5–C8 | C | T | 13 | 0.31 |
| 7 | 33 | M | C5–C8 | C | T | 2 | 0.84 |
| 8 | 65 | M | C2–C6 | D | T | 5 | 0.25 |
| 9 | 64 | M | C5–C7 | A | NT | 5 | 0.40 |
| 10 | 49 | M | C4–C6 | D | NT | 3 | 0.29 |
| 11 | 66 | M | C5–C7 | C | T | 11 | 0.44 |
| 12 | 50 | M | C4–C5 | D | T | 4 | 0.20 |
| 13 | 36 | M | C2–C5 | C | T | 4 | 0.16 |
| 14 | 54 | M | C5–C8 | A | T | 18 | 0.42 |
| 15 | 39 | F | C2–C7 | D | NT | 25 | 0.22 |
| 16 | 35 | M | C2–C5 | C | T | 1 | 0.15 |
SCI, spinal cord injury; AIS, American Spinal Injury Association Impairment Scale; FDI, first dorsal interosseous; MVC, maximal voluntary contraction; M, male; F, female; T, traumatic; NT, nontraumatic.
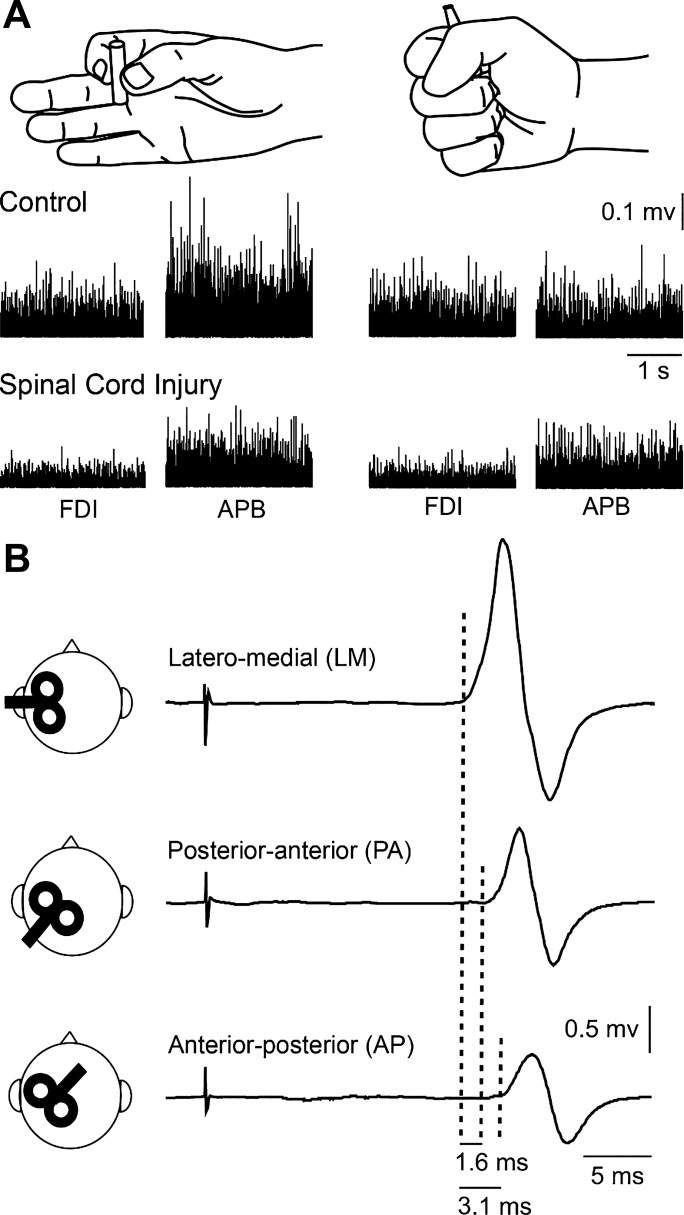
Fig. 1.
Experimental set-up. A: schematic of the hand posture used during testing of precision grip and power grip. Raw EMG traces show activity in the first dorsal interosseous (FDI) muscle in control subject and a participant with spinal cord injury. B: traces show the average of 20 motor-evoked potentials (MEPs) in the FDI tested during precision grip tested with the transcranial magnetic stimulation coil in the latero-medial (LM), posterior-anterior (PA), and anterior-posterior (AP) orientation in a control subject. Note that latencies of MEPs elicited by AP- and PA-directed currents were longer compare with LM. Also note, that latencies of MEPs were longer with AP- compared with PA-directed currents. APB, abductor pollicis brevis.
Experimental procedures
Subjects were seated with both arms flexed at the elbow by 90°. At the beggining of experiments, subjects performed two brief maximal voluntary contractions (MVCs) for 3–5 s with the index finger into abduction and the thumb into abduction, separated by 30 s of rest. MVCs were quantified using background EMG activity in the FDI and abductor pollicis brevis (APB) muscles during index finger and thumb abduction, respectively. During index finger abduction, subjects were instructed to press with the index finger against a custom lever in the abduction direction with the forearm pronated and the wrist restrained by straps. During thumb abduction, subjects were instructed to press with the thumb in the abduction direction with the forearm supinated and the wrist restrained by straps. MVC was measured by calculating the highest mean rectified EMG activity found in the FDI and APB muscles in 1 s during the MVC burst. EMG activity from the FDI muscle was displayed continuously on a computer screen, and verbal feedback was provided to the subjects to ensure that MEPs tested in all coil orientations were acquired at 5% of MVC across conditions. A familiarization trial was completed to ensure that all subjects were able to complete the task using the required level of EMG activity. To compare the trials with similar background EMG activity between tasks, trials in which the background EMG activity (100 ms before the stimulus) was too high or too low (i.e., outside of the means ± 2 SD range) were excluded from the analysis (Bunday et al. 2014); 6.7 ± 3.3% of trials were excluded in controls and 6.9 ± 4.5% in SCI participants.
EMG recordings
EMG was recorded from the FDI and APB muscles through electrodes secured to the skin over the muscle belly (Ag–AgCl, 10-mm diameter). As in previous studies, we tested the right hand in control subjects because no differences have been reported in MEP latencies across hands (Livingston et al. 2010). In individuals with SCI, since both hands are usually affected by the injury (Calabro et al. 2016), we tested the affected hand in which MEPs could be elicited (Jo et al. 2018). The signals were amplified (×200), filtered (30–2,000 Hz), sampled at 10 kHz (CED 1401 with Signal software; Cambridge Electronic Design, Cambridge, UK), and stored on computer for offline analysis.
TMS
TMS was applied using a figure-of-eight coil (loop diameter 70 mm) through a Magstim 2002 magnetic stimulator (Magstim, Whitland, Dyfed, UK) with a monophasic current waveform. Single-pulse TMS was used to elicit MEPs by changing the current flow across the hand area of the primary motor cortex (Di Lazzaro et al. 1998, 2001; Hamada et al. 2013; Sakai et al. 1997; Volz et al. 2015). The following three coil orientations were tested (Fig. 1B): 1) a figure-of-eight coil was held tangentially on the scalp at an angle of 45° to the midline with the handle pointing laterally and posteriorly (PA-induced current in the brain); 2) the position of the figure-of-eight coil handle was reversed around the intersection of coil windings by placing the coil 180° to the PA currents (AP-induced current in the brain); and 3) the handle of the coil was held leftwards 90° from the midsagittal line [LM-induced current in the brain] in all subjects during 5% of MVC (LM = 5.2 ± 0.6% of MVC, PA = 5.1 ± 0.5%, and AP = 5.1 ± 0.5% of MVC; F2,60 = 2.6, P = 0.1). Measurements were performed at the hotspot determined by PA currents since it was shown that the direction of the current does not significantly influence the position of the hotspot (Arai et al. 2005; Sakai et al. 1997). This optimal scalp position determined by PA currents was then marked for reference with a pen on a cap placed on the head and used as a reference to rotate the coil to elicit MEPs in other coil directions (Federico and Perez 2016; Hamada et al. 2013, 2014). The coil was held manually by the experimenter with the head secured to a headrest by straps to limit head movement. The different coil orientations were tested in a randomized order. TMS measurements included active motor threshold (AMT) and MEP onset latency tested at each coil orientation.
MEPs
AMT was determined as the minimum stimulus intensity required to elicit MEP >200 µV peak-to-peak amplitude above the background EMG activity in at least 5 out of 10 consecutive trials in the contracting FDI muscle during index finger abduction (Rothwell et al. 1999). When this is tested during low levels of voluntary contraction, where spinal motoneuron excitability is increased, the effect of temporal summation of descending volleys at the spinal level is minimized (Hamada et al. 2013; Sakai et al. 1997; Wilson et al. 1996). Therefore, MEPs were tested during 5% of MVC using TMS intensities of 150% of the AMT for LM and 110% of the AMT for PA and AP coil orientations (Hamada et al. 2013). A higher stimulus intensity was used for LM to ensure that corticospinal neurons were directly stimulated (D wave) at this coil orientation. The MEP onset latencies tested with transcranial electrical stimulation and LM TMS at 150% AMT were shown to be similar in our previous study (Jo et al. 2018). For two SCI subjects in whom the intensity for LM exceeded the maximum stimulator output, the intensity was set at 100% maximum stimulator output. MEP onset latency was measured for individual trials in each subject and condition. The MEP latency was defined as the time point where rectified EMG signals exceeded 2 SD of the mean background EMG, measured 100 ms before the stimulus artifact. The latency of MEPs elicited by PA- and AP-directed currents was compared with LM to calculate the difference in latencies between PA-LM and AP-LM as a measure of activation of PA- and AP-sensitive inputs, respectively. AP and PA MEP latencies were also compared. We quantified the variability of MEP latencies during each coil orientation in all participants by analyzing MEP latency SD, referred as to latency dispersion. Single TMS pulses were delivered at 0.2 Hz and 20 MEPs were recorded in each coil orientation during precision grip and power grip.
Central and peripheral conduction time
Central conduction time (CCT) and peripheral conduction time (PCT) were calculated from latencies of MEPs, F-wave, and M-max [CCT: MEP latency – (PCT); PCT: (F-wave latency + M-max latency) × 0.5]. F waves were measured during precision grip and power grip using supramaximum stimulus intensity to the ulnar nerve at the wrist (200-μs pulse duration, DS7A; Digitimer) with a monopolar bar electrode with the cathode positioned proximally. Forty stimuli were delivered during each grip task at 1 Hz at an intensity of 150% of the maximal motor response (M-max). During F-wave testing, signals were filtered using a high-pass filter of 100 Hz to identify the earliest F-wave latency for calculating conduction times. The F-wave onset latency was defined as the time at which a signal was ~20 µV above the mean baseline measured 100 ms before the stimulus artifact, and it was estimated in individual trials to identify the response with the shortest latency (Perez and Rothwell 2015).
Data analysis
Normal distribution was tested by the Shapiro-Wilk's test and homogeneity of variances by the Levene’s test of equality and Mauchly’s test of sphericity. When normal distribution could not be assumed, data were log transformed. When sphericity could not be assumed the Greenhouse-Geisser correction statistic was used. Two-way repeated measures ANOVAs were performed to determine the effect of GROUP (controls, SCI) and COIL ORIENTATION (LM, PA, AP) on AMT. Three-way repeated measures ANOVAs were also performed to determine the effect of GROUP, COIL ORIENTATION, and TASK (precision grip, power grip) on MEP latency, MEP latency dispersion, background EMG, CCT, and MEP amplitude. The same test was performed to determine the effect of GROUP, TASK, and COIL ORIENTATION PAIRS (PA-LM and AP-LM) on MEP latency differences. Two-way repeated measures ANOVAs were also performed with factors GROUP and TASK on background EMG activity and PCT. Post hoc comparisons were conducted with multiple t-tests with Bonferroni’s correction. A t-test was used to compare age and MVCs between groups. Group data are presented as means ± SD in the text.
RESULTS
EMG recordings and AMT
Absolute EMG values for MVCs were higher in controls compared with SCI participants for both the FDI (controls = 0.66 ± 0.19 mV, SCI = 0.30 ± 0.18 mV; P < 0.001) and APB (controls = 0.79 ± 0.40 mV, SCI = 0.46 ± 0.29 mV; P < 0.05) muscles.
Repeated measures ANOVA showed no effect of GROUP (F1,29 = 0.03, P = 0.9) or TASK (F1,29 = 0.4, P = 0.5) or in their interaction (F1,29 = 0.1, P = 0.7) on background FDI EMG normalized to MVC. This indicates that similar EMG activity was maintained across groups and tasks (controls: precision grip = 5.1 ± 0.4% MVC; power grip = 5.2 ± 0.4% MVC; SCI: precision grip = 5.1 ± 0.4% MVC; power grip = 5.1 ± 0.5% MVC). Similar results were found for the APB mucle [GROUP (F1,29 = 1.3, P = 0.3), TASK (F1,29 =2.7, P = 0.1), and interaction (F1,29 = 0.1, P = 0.8); controls: precision grip = 6.5 ± 3.3% MVC; power grip = 5.5 ± 3.4% MVC; SCI: precision grip = 7.8 ± 4.9% MVC; power grip = 7.1 ± 3.9% MVC)].
Repeated measures ANOVA showed an effect of GROUP (F1,31 = 20.4, P < 0.001) and COIL ORIENTATION (F1.5,46 =71.9, P < 0.001) but not in their interaction (F1.5,53 = 1.1, P = 0.3) on AMT. Note that AMTs were higher in SCI participants compared with controls in all coil orientations (PA: controls = 44.1 ± 6.7%, SCI = 58.3 ± 10.2%, P < 0.001; AP: controls = 59.9 ± 13.5%, SCI = 76.9 ± 12.1%, P < 0.01; LM: controls = 51.8 ± 9.8%, SCI = 64.4 ± 9.5%, P < 0.01). In addition, AMT was lower for PA- than for LM (controls, P < 0.001; SCI, P < 0.001)- and AP (controls, P < 0.001; SCI, P < 0.001)-directed currents and for LM- than for AP (controls, P < 0.01; SCI, P < 0.001)-directed currents in both groups.
MEP latency in all TMS coil orientations
Figure 2A illustrates MEPs in the FDI muscle in a representative control and SCI subject during precision (black traces) and power (gray traces) grip when testing was conducted with the TMS coil in the LM, PA, and AP orientations. Repeated measures ANOVA showed an effect of GROUP (F1,31 = 18.9, P < 0.001), COIL ORIENTATION (F1.6,62 = 248.4, P < 0.001), TASK (F1,31 = 10.1, P < 0.001) but not in their interaction (F2,62 =0.1, P = 0.3) on MEP latency. Post hoc analysis revealed that MEP latencies were longer in the LM, PA, and AP orientations in SCI compared with control participants (Fig. 2B; Table 2). In both groups, LM MEP latencies were shorter compared with the PA (controls, P < 0.001; SCI, P < 0.001) and AP (controls, P < 0.001; SCI, P < 0.001) and AP MEP latencies were longer than PA (controls, P < 0.001; SCI, P < 0.001), suggesting that after SCI it is possible to engage these different sets of excitatory inputs to corticospinal neurons. Also, note that MEP latencies were longer during power grip compared with precision grip for LM (F1,31 = 7.9, P < 0.01), PA (F1,31 = 10.8, P < 0.01), and AP (F2,62 = 14.9, P < 0.001).
Fig. 2.
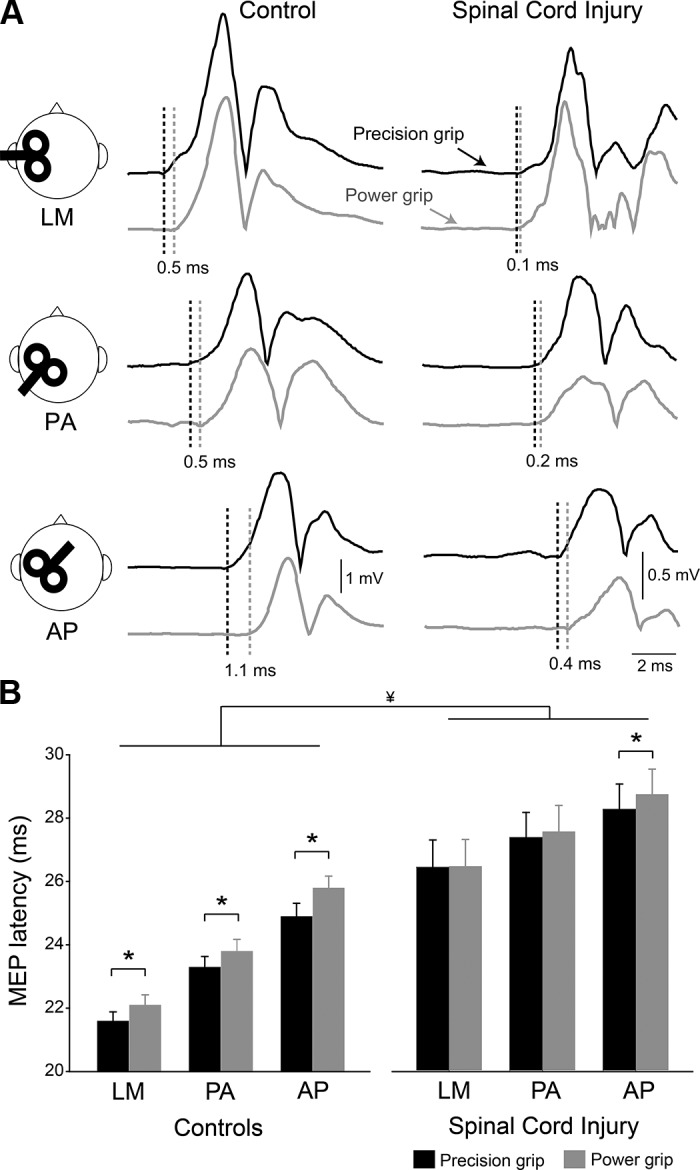
Motor-evoked potential (MEP) latency. A: MEPs elicited in the first dorsal interosseous (FDI) muscle during precision grip and power grip (black and gray traces, respectively) when the TMS coil was oriented in the latero-medial (LM), posterior-anterior (PA), and anterior-posterior (AP) directions in a representative control and SCI subject. Each waveform represents the average of 20 MEPs. B: group data (control, n = 17, SCI, n = 16) shows the latencies of MEP during precision grip (black bar) and power grip (gray bar) with the coil in all directions. Error bars indicate SDs. *P < 0.05, comparison between tasks. ¥P < 0.05, comparison between groups.
Table 2.
MEP latency across TMS coil orientations
| MEP Onset Latency, ms |
|||
|---|---|---|---|
| Group/Grip | LM | PA | AP |
| Controls | |||
| Precision | 21.6 ± 1.2 | 23.3 ± 1.4 | 24.8 ± 1.7 |
| Power | 22.1 ± 1.3* | 23.8 ± 1.5* | 25.8 ± 1.5* |
| SCI | |||
| Precision | 26.2 ± 3.3 | 27.1 ± 3.1 | 28.0 ± 3.4 |
| Power | 26.2 ± 3.2 | 27.3 ± 3.1 | 28.4 ± 3.2* |
Values are means ± SD. MEP, motor-evoked potential; TMS, transcranial magnetic stimulation; LM, latero-medial; PA, posterior-anterior; AP, anterior-posterior; SCI, spinal cord injury,
Significant difference between tasks within each group and coil orientation.
MEP latency differences across coil orientations
Figure 3 shows group and individual data for PA-LM and AP-LM MEP latency differences during precision and power grip in both groups. Note that AP-LM latency difference is larger during power grip than precision grip in both control and SCI participants. Repeated measures ANOVA showed an effect of GROUP (F1,31 = 33.2, P < 0.001), COIL ORIENTATION PAIRS (F1,31 = 107.3, P < 0.001), and TASK (F1,31 = 9.4, P < 0.01) but not in their interaction (F1,31 = 1.9, P = 0.2) on MEP latency differences. Post hoc analysis revealed that both PA-LM and AP-LM MEP latency differences were smaller in SCI compared with control subjects (Table 3). Note that in SCI participants, AP-LM latency difference was further decreased compared with PA-LM (PA-LM = 0.7 ms, AP-LM = 1.5 ms, P < 0.001). Latencies of MEPs elicited by AP relative to LM stimuli were longer during power grip compared with precision grip in controls (P < 0.05) and SCI participants (P < 0.05) while PA relative to LM MEP latencies were similar between tasks in both groups (P > 0.7). Note that during power grip the majority of control (15/17; Fig. 3B) and SCI (12/16; Fig. 3B) participants showed longer AP–LM latency differences compared with precision grip.
Fig. 3.
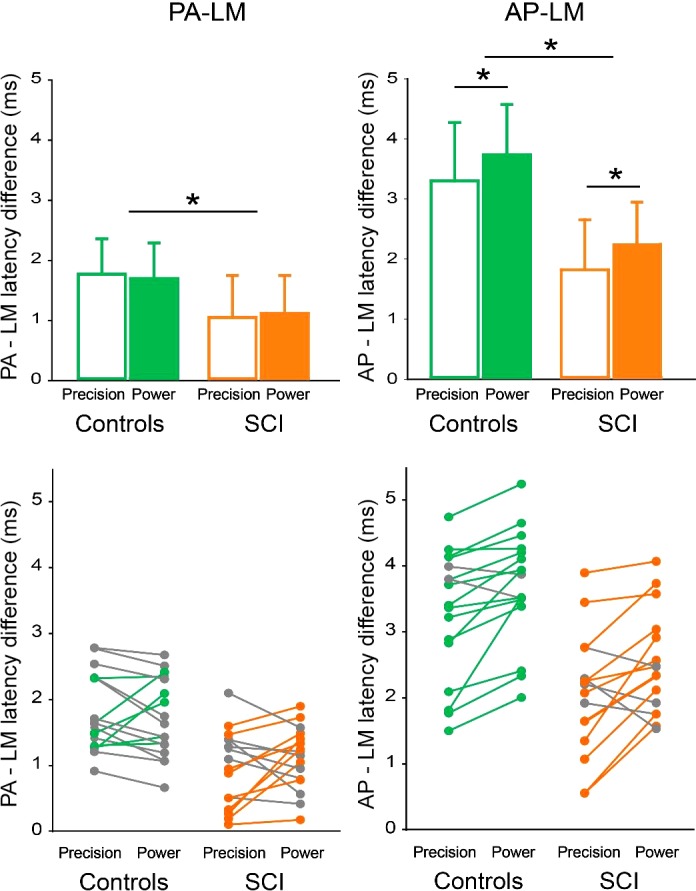
Motor-evoked potential (MEP) latency differences. A: group data [control, n = 17, spinal cord injury (SCI), n = 16] showing posterior-anterior (PA)-latero-medial (LM) and anterior-posterior (AP)-LM MEP latency differences during precision grip and power grip in controls (green bars) and SCI (orange bars). The ordinate shows the MEP latency differences (in milliseconds), and gray circles indicate individual subject within each group. Error bars indicate SD *P < 0.05. B: individual data showing PA-LM and AP-LM MEP latency differences for precision grip and power grip in controls (green) and SCI (orange). Subjects showing changes in opposite direction as those described by green and orange circles are shown in gray.
Table 3.
MEP latency differences across TMS coil orientations
| Group/Grip | Latency Differences, ms |
Latency Differences (EMG-Matched Subgroup), ms |
||
|---|---|---|---|---|
| PA-LM | AP-LM | PA-LM | AP-LM | |
| Controls | ||||
| Precision | 1.7 ± 0.6 | 3.3 ± 1.0 | 1.7 ± 0.5 | 3.2 ± 0.9 |
| Power | 1.7 ± 0.6 | 3.7 ± 0.8* | 1.7 ± 0.5 | 3.8 ± 0.6* |
| SCI | ||||
| Precision | 0.9 ± 0.6 | 1.8 ± 0.8 | 1.1 ± 0.6 | 1.7 ± 0.9 |
| Power | 1.1 ± 0.5 | 2.2 ± 0.7* | 1.3 ± 0.4 | 2.1 ± 0.7* |
Values are means ± SD. MEP, motor-evoked potential; TMS, transcranial magnetic stimulation; EMG, electromyography; PA, posterior-anterior; LM, latero-medial; AP, anterior-posterior; SCI, spinal cord injury,
Significant difference between tasks within each group and coil orientation.
Additionally, in a subgroup of subjects (control, n = 8; SCI, n = 8), we matched the absolute level of EMG activity in the FDI muscle across groups during testing (controls = 0.52 ±0.11 mV, SCI = 0.46 ± 0.21 mV; P = 0.5). Repeated measures ANOVA showed an effect of GROUP (F1,14 = 18.5, P < 0.01), COIL ORIENTATION PAIRS (F1,14 = 43.7, P < 0.001), and TASK (F1,14 = 7.7, P < 0.05) but not in their interaction (F1,14 = 0.1, P = 0.2) on MEP latency differences. Consistent with our previous results, we found that both PA-LM and AP-LM MEP latency differences were smaller in SCI compared with control subjects (Table 3), suggesting that it is less likely that differences in level of background EMG activity across groups contributed to our results.
MEPs latency dispersion
Figure 4A illustrates the dispersion of MEPs latency in the FDI muscle in representative participants during precision grip when testing was conducted with the TMS coil in the LM, PA, and AP orientations. Repeated measures ANOVA showed an effect of GROUP (F1,28 = 25.9, P < 0.001) and COIL ORIENTATION (F2,56 = 37.7, P < 0.001) but not TASK (F1,28 = 0.5, P = 0.5) or in their interaction (F2,56 = 0.4, P = 0.7) on MEP latency dispersion. Post hoc analysis revealed that latency dispersion was larger in SCI than in controls for LM (controls = 0.46 ± 0.09 ms; SCI = 0.95 ± 0.29 ms, P < 0.001), PA (controls = 0.64 ± 0.14 ms; SCI = 1.14 ± 0.36 ms, P < 0.001) and AP (controls = 0.94 ± 0.30 ms; SCI = 1.24 ± 0.32 ms, P < 0.001) MEPs. The latency dispersion was smaller for MEPs tested with LM compared with PA (controls, P < 0.001; SCI, P < 0.01) and AP (controls, P < 0.001; SCI, P < 0.01) stimulation in both groups (Fig. 4B). MEP latency dispersion was larger in the AP compared with the PA orientation in controls (P < 0.01) but not in SCI (P = 0.1) subjects.
Fig. 4.
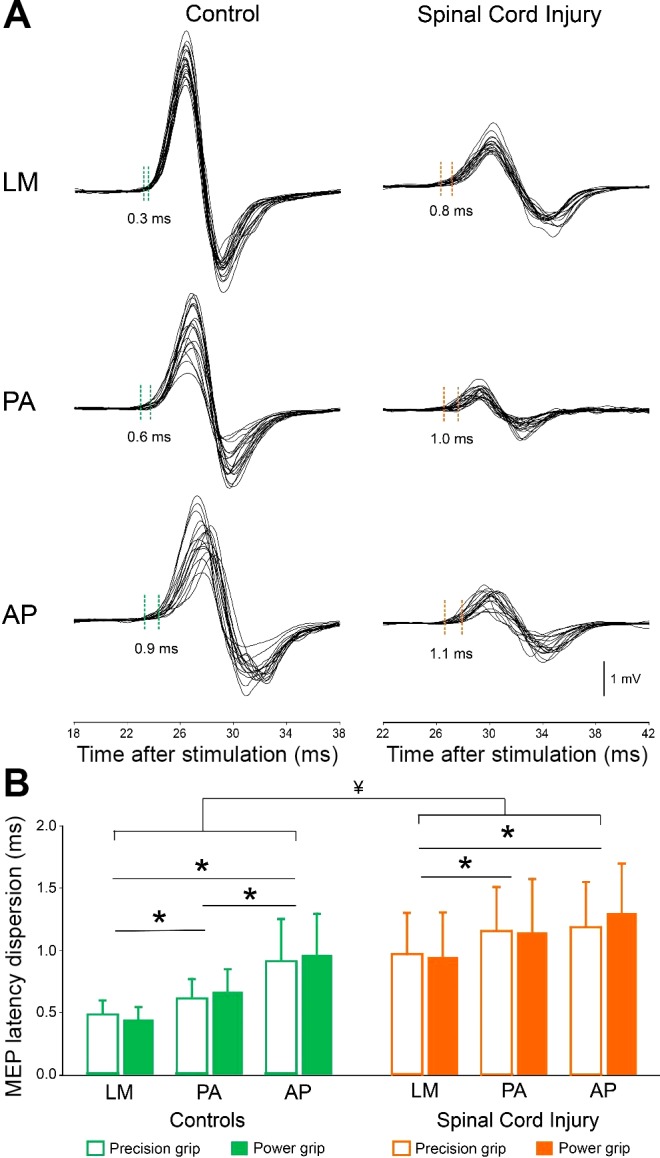
Motor-evoked potential (MEP) latency dispersion. A: waveforms represent 20 MEPs tested with the coil oriented in the latero-medial (LM), posterior-anterior (PA), and anterior-posterior (AP) direction during precision grip in representative control and SCI subject. Dotted lines show the difference between the earliest and latest MEP latency. The abscissa shows the time after stimulation in milliseconds and latency dispersion of MEPs is indicated for each set of 20 MEPs. B: group data show MEP latency dispersion during precision grip and power grip when the coil was oriented in LM, PA, and AP direction. The abscissa shows the coil orientation tested (LM, PA, and AP) in controls (green) and SCI (orange) and the ordinate shows the MEP latency dispersion (in milliseconds). Error bars indicate SD. *P < 0.05, comparison within group. ¥P < 0.001, comparison between groups.
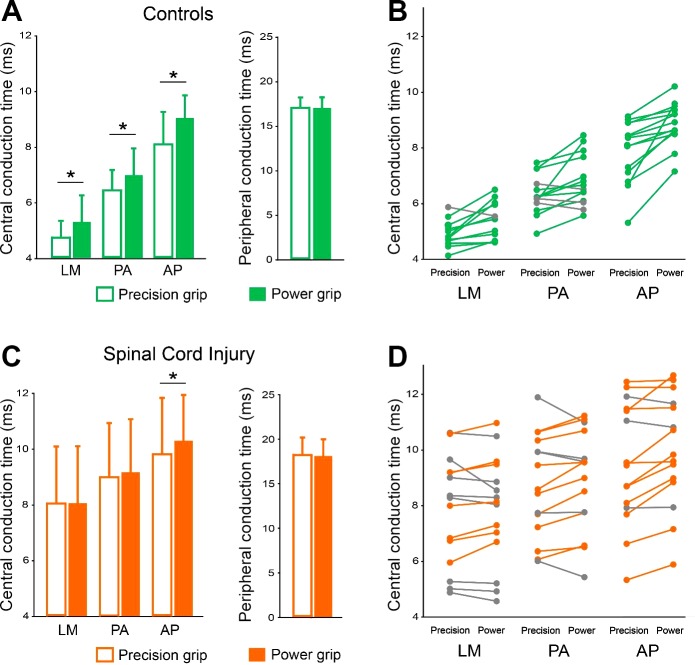
Central and peripheral conduction times
Repeated measures ANOVA showed an effect of GROUP (F1,27 = 16.5, P < 0.001), COIL ORIENTATION (F2,54 =244.3, P < 0.001), and TASK (F1,27 = 24.6, P < 0.001) but not in their interaction (F2,54 = 0.2, P = 0.8) on CCT. Post hoc analysis revealed that CCT was prolonged during power grip compared with precision grip in all coil orientations in controls (LM, P < 0.05, P < 0.05; AP, P < 0.001) but only in AP orientation in SCI participants (LM, P = 0.7; PA, P = 0.2; AP, P < 0.01), whereas we found no effect of GROUP (F1,27 = 3.2, P = 0.1), TASK (F1,27 = 0.4, P = 0.5), or interaction (F1,27 = 0.02, P = 0.9) on PCT (Fig. 5). Note that PCT was similar across tasks in both groups (Table 4).
Fig. 5.
Central (CCT) and peripheral (PCT) conduction time. A–D: group and individual data [control, n = 14; spinal cord injury (SCI), n = 15] shows the CCT and PCT during precision grip and power grip when the TMS coil was oriented in the latero-medial (LM), posterior-anterior (PA), and anterior-posterior (AP) directions in controls (A and B) and in individuals with SCI (C and D). B and D show individual data on PA-LM and AP-LM MEP latency differences for precision grip and power grip in controls (green) and SCI (orange). Subjects showing changes in opposite direction as those described by green and orange circles are shown in gray. Error bars indicate SD. *P < 0.05, comparison between tasks.
Table 4.
Central and peripheral conduction time
| Central Conduction Time, ms |
||||
|---|---|---|---|---|
| Group/Grip | LM | PA | AP | Peripheral Conduction Time, ms |
| Controls | ||||
| Precision | 4.7 ± 0.6 | 6.4 ± 0.7 | 8.1 ± 1.2 | 17.1 ± 1.2 |
| Power | 5.3 ± 1.0* | 7.0 ± 1.0* | 9.1 ± 0.8* | 17.1 ± 1.2 |
| SCI | ||||
| Precision | 8.0 ± 2.0 | 9.0 ± 2.5 | 9.8 ± 1.7 | 18.1 ± 2.0 |
| Power | 8.0 ± 2.0 | 9.2 ± 2.2 | 10.3 ± 2.1* | 18.1 ± 1.9 |
Values are means ± SD. LM, latero-medial; PA, posterior-anterior; AP, anterior-posterior; SCI, spinal cord injury.
Significant difference between tasks within each group and coil orientation.
MEP amplitude
Repeated measures ANOVA showed an effect of GROUP (F1,29 = 26.1, P < 0.001), COIL ORIENTATION (F1.2,35.8 =34.2, P < 0.001), and TASK (F1,29 = 6.2, P < 0.05) but not in their interaction (F2,58 = 0.5, P = 0.6) on MEP amplitude. Post hoc analysis revealed that MEP amplitude was smaller in SCI compared with controls in the LM, PA, and AP orientations (Table 5). In both groups, LM MEP amplitudes were larger compared with the PA (controls, P < 0.01; SCI, P < 0.05) and AP (controls, P < 0.001; SCI, P < 0.05). Additionally, MEP amplitude was smaller during power grip compared with precision grip in controls (P < 0.05) but not in SCI participants (P = 0.3).
Table 5.
MEP amplitude across TMS coil orientations
| Group/Grip | MEP Amplitude, mV |
||
|---|---|---|---|
| Task | LM | PA | AP |
| Controls | |||
| Precision | 4.7 ± 2.6 | 2.1 ± 1.5 | 1.9 ± 1.4 |
| Power | 4.4 ± 2.3 | 1.6 ± 1.3 | 1.3 ± 1.0 |
| SCI | |||
| Precision | 1.3 ± 1.2* | 0.6 ± 0.3* | 0.6 ± 0.4* |
| Power | 1.3 ± 1.1* | 0.5 ± 0.3* | 0.6 ± 0.3* |
Values are means ± SD. MEP, motor-evoked potential; TMS, transcranial magnetic stimulation; LM, latero-medial; PA, posterior-anterior; AP, anterior-posterior; SCI, spinal cord injury.
Significant difference between groups within each coil orientation.
Since changes in MEP amplitude can affect the MEP latency, in a subgroup of subjects (control, n = 7; SCI, n = 7), we matched MEP amplitudes in each coil orientation between groups and tasks and compared MEP latency differences (Table 6). Repeated measures ANOVA showed an effect of GROUP (F1,12 = 12.8, P < 0.01), COIL ORIENTATION PAIRS (F1, 12 = 30.3, P < 0.001), and TASK (F1,12 = 7.9, P < 0.05) but not in their interaction (F1, 12 = 3.1, P = 0.1) on MEP latency differences. We also found that both PA-LM (P < 0.05) and AP-LM (P < 0.01) MEP latency differences were smaller in SCI compared with control subjects. Latencies of MEPs elicited by AP relative to LM stimuli were longer during power grip compared with precision grip (P < 0.01) while PA relative to LM MEP latencies were similar between tasks (P = 0.6). These results are consistent with our previous findings and suggest that it is less likely that differences in MEP amplitude contributed to the main results.
Table 6.
MEP latency differences across TMS coil orientations with amplitude matched
| Latency Differences, ms |
||
|---|---|---|
| Group/Grip | PA-LM | AP-LM |
| Controls | ||
| Precision | 1.57 ± 0.35 | 2.98 ± 0.84 |
| Power | 1.56 ± 0.44 | 3.71 ± 0.68* |
| SCI | ||
| Precision | 0.97 ± 0.42 | 1.83 ± 1.11 |
| Power | 1.06 ± 0.32 | 2.33 ± 1.00* |
Values are means ± SD. MEP, motor-evoked potential; TMS, transcranial magnetic stimulation; PA, posterior-anterior; LM, latero-medial; AP, anterior-posterior; SCI, spinal cord injury.
Significant difference between tasks within each group and coil orientation.
DISCUSSION
We examined the contribution of the corticospinal pathway during precision and power grip by comparing the latency of MEPs elicited by LM-, PA-, and AP-induced currents in the brain in humans with and without chronic incomplete cervical SCI. We found prolonged MEP latencies in all coil orientations in both tasks in SCI compared with control subjects. The latencies of MEPs elicited by AP relative to LM stimuli were consistently longer during power grip compared with precision grip in controls and SCI participants. However, PA relative to LM MEP latencies were similar between tasks across groups. CCT of AP MEPs was prolonged during power compared with precision grip in controls and SCI participants. These findings support the idea that AP- and PA-related inputs are activated to a different extent during power and precision grip in humans with and without SCI.
PA- and AP-induced currents in the brain during grasping in humans with SCI
Our results in control subjects agree with previous findings showing that MEPs elicited by AP currents have a longer latency, larger latency dispersion, and higher threshold than MEPs elicited by PA currents during tonic voluntary activity (Di Lazzaro and Rothwell 2014; Di Lazzaro et al. 2012, 2017) and during precision and power grip (Federico and Perez 2016). We also found that latencies of MEPs elicited by AP relative to the ones elicited by LM stimuli were consistently longer during power grip compared with during precision grip in control subjects, consistent with previous results (Federico and Perez 2016). To gain insights into the contribution of the corticospinal tract to precision and power grip in humans with SCI, here we examined the latencies of MEPs elicited by different induced currents in the brain in this population. We found that latencies of MEPs elicited by AP relative to LM stimuli were prolonged to a similar extent during power grip compared with precision grip in SCI and control subjects. A single TMS pulse applied over the motor cortex evokes temporally synchronized descending waves in the corticospinal tract, which can be recorded from the epidural space (Di Lazzaro et al. 2012; Patton and Amassian 1954). The shortest wave is due to direct stimulation of the corticospinal neuron (D wave) at some distance from the cell body, and later indirect (I) waves (named I1, I2, and I3) possibly arise from transsynaptic activation of corticospinal neurons by intracortical circuits (Di Lazzaro et al. 2012). TMS-induced PA currents across the central sulcus preferentially evoke synchronized corticospinal activity whereas AP currents preferentially evoke less synchronized activity with their peaks partially matching the latency of PA-evoked later activity (Day et al. 1989; Sakai et al. 1997). Thus one possible interpretation of our results is that AP-related inputs can be differentially engaged during precision grip and power grip in both groups. AP MEPs might involve contributions from multiple brain areas including the recruitment of inputs from different cortical areas to the motor cortex (Groppa et al. 2012). For example, AP MEP latencies negatively correlate with connectivity between premotor areas and the motor cortex (Volz et al. 2015), and these brain regions are more active during power grip compared with precision grip (Ehrsson et al. 2000). Thus AP currents might reflect activation of a large range of synaptic inputs from other brain areas active during power grip. It might be also possible that subcortical networks contributed to the MEP latency differences observed in our study. TMS paired-pulse paradigms showed that MEP responses at interstimulus intervals targeting AP-related inputs receive subcortical influences (Cirillo et al. 2016).
One important result to consider is that CCT was prolonged in control subjects during power grip compared with precision grip for LM and PA MEPs and to a larger extent for AP MEPs. The further increase in CCT during power grip for LM responses was intriguing since these MEPs likely reflect direct activation of corticospinal axons. This is supported by the small temporal dispersion of ~0.4 ms found on LM MEPs tested during precision and power grip. Epidural recordings showed that with increments of TMS intensity the latency of evoked D wave remains the same or it can be slightly changed by ~0.5 ms (Burke et al. 1993). The latency of MEPs elicited by electrical stimulation over the motor cortex does not change with increasing stimulus intensities in most subjects (Day et al. 1989). Thus a possibility is that the small increment that we found in CCT of LM MEPs in control subjects during power grip compared with precision grip may have resulted from an involvement of other neural pathways. For example, one might speculate that the reticulospinal pathway favors gross grasping. Reticulospinal outputs are strengthened following a corticospinal lesion and these connections subserve some of the recovery of gross hand motor function (Zaaimi et al. 2018). This is supported by a study showing a pronounced modulation of corticospinal responses by a startle, a stimulus that engages the reticulospinal system, during power grip compared with precision grip in intact humans (Tazoe and Perez 2017). A stronger reticulospinal influence during power grip might be easier to detect after damage to the corticospinal tract as results showed that individuals with SCI can decrease their reaction times in hand muscles while a startle stimulus is presented during power grip but not during precision grip (Baker and Perez 2017). Note that CCT was prolonged during power grip compared with precision grip in our SCI participants on AP MEPs. However, we did not observe prolonged CCT during power grip for LM and PA MEPs in our SCI participants. Note that differences in CCT in LM and AP MEPs in control subjects were small (~0.5 ms), which might be difficult to detect in SCI participants due the increased temporal dispersion of volleys (Cirillo et al. 2016). Several other alternative explanations can contribute to our results and cannot be excluded such as the involvement from a disynaptic pathway through C3–C4 propriospinal neurons (Alstermark et al. 2011) and the contribution of slow and/or indirect corticospinal neurons.
Consistent with our previous results (Jo et al. 2018), we found that overall individuals with SCI showed decreased MEP latency differences in both tasks compared with control subjects and these differences were more pronounced (more affected) for AP compared with PA MEPs. Note that AP MEPs latencies showed less task-dependent changes in SCI compared with control subjects, agreeing with the view that AP MEPs are largely affected after SCI (Jo et al. 2018). Latencies of MEPs elicited by AP relative to LM stimuli were shorter by ~1.5 ms in the majority of our SCI participants compared with controls, which is a similar range to what has been previously reported (Jo et al. 2018). Although at first glance this might seem different from our main results, we propose that this overall decrease might reflect a generalized responsiveness of the corticospinal pathway to receive synaptic inputs from different sources. This agrees with animal data showing remodeling of synaptic structures in the motor cortex after SCI (Kim et al. 2006) and with results in humans with SCI showing an impaired ability to summate excitatory postsynaptic potentials in motoneurons from activation of sensory afferents (Norton et al. 2008).
Functional considerations.
Previous studies suggested that changes in latencies of MEPs elicited by different coil orientations reflect activation of different neuronal inputs to corticospinal neurons (for reviews, see Di Lazzaro et al. 2012, 2017; Di Lazzaro and Rothwell 2014). Therefore, we used these measurements to examine the contribution of the corticospinal tract to the control of grasping in individuals with and without SCI. The corticospinal tract is a major descending pathway contributing to the recovery of function following SCI (Oudega and Perez 2012). Thus understanding how the corticospinal tract is activated following injury has important implications not only for assessment of corticospinal function but also for the development of plasticity-inducing protocols aimed at enhancing recovery of function (Christiansen and Perez 2018).
We found that AP-LM MEP latency differences change to a similar extent in humans with chronic incomplete SCI and controls when they perform power grip but not precision grip. The relationship between these MEP latency changes and functional grasping remains unclear. However, several investigations revealed that MEPs elicited by AP currents can be modulated to a different extent during voluntary motor tasks, motor learning, and plasticity protocols (Di Lazzaro et al. 2011; Federico and Perez 2016; Hamada et al. 2013, 2014; Hannah and Rothwell 2017; Long et al. 2017). AP MEPs likely reflect activity in cortico-cortical pathways transmitting information from multiple cortical (Groppa et al. 2012; Volz et al. 2015) and subcortical (Cirillo and Perez 2015) networks. Thus the MEP latency differences observed in our study might reflect contributions from different networks to power and precision grip. This is consistent with results showing that distinct cortical and subcortical pathways are involved during power and precision grip in intact humans (Tazoe and Perez 2017; Lei and Perez 2018). After SCI, gross grasping behaviors might represent a good strategy to engage multiple neuronal sources involved in hand control compared with more fine dexterous finger movements, consistent with previous results (Baker and Perez 2017). The closer modulation of MEP latency differences between AP and LM responses in SCI compared with control participants during a power grip might reflect contributions to gross grasping from multiple pathways; however, future studies are needed to examine this possibility.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant 1R01NS090622-01 (to M. A. Perez); Paralyzed Veterans of America Grant PVA17_RF_0024 (to H. Jin Jo); and U.S. Department of Veterans Affairs Grants I01RX000815 and I01RX002474 (to M. A. Perez).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.J.J. and M.A.P. conceived and designed research; H.J.J. and M.A.P. performed experiments; H.J.J. and M.A.P. analyzed data; H.J.J. and M.A.P. interpreted results of experiments; H.J.J. and M.A.P. prepared figures; H.J.J. and M.A.P. drafted manuscript; H.J.J. and M.A.P. edited and revised manuscript; H.J.J. and M.A.P. approved final version of manuscript.
REFERENCES
- Alstermark B, Pettersson LG, Nishimura Y, Yoshino-Saito K, Tsuboi F, Takahashi M, Isa T. Motor command for precision grip in the macaque monkey can be mediated by spinal interneurons. J Neurophysiol 106: 122–126, 2011. doi: 10.1152/jn.00089.2011. [DOI] [PubMed] [Google Scholar]
- Arai N, Okabe S, Furubayashi T, Terao Y, Yuasa K, Ugawa Y. Comparison between short train, monophasic and biphasic repetitive transcranial magnetic stimulation (rTMS) of the human motor cortex. Clin Neurophysiol 116: 605–613, 2005. doi: 10.1016/j.clinph.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Baker SN, Perez MA. Reticulospinal contributions to gross hand function after human spinal cord injury. J Neurosci 37: 9778–9784, 2017. doi: 10.1523/JNEUROSCI.3368-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Tazoe T, Rothwell JC, Perez MA. Subcortical control of precision grip after human spinal cord injury. J Neurosci 34: 7341–7350, 2014. doi: 10.1523/JNEUROSCI.0390-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol 470: 383–393, 1993. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L, Perez MA. Targeted-plasticity in the corticospinal tract after human spinal cord injury. Neurotherapeutics 15: 618–627, 2018. doi: 10.1007/s13311-018-0639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J, Calabro FJ, Perez MA. Impaired organization of paired-pulse TMS-induced I-waves after human spinal cord injury. Cereb Cortex 26: 2167–2177, 2016. doi: 10.1093/cercor/bhv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J, Perez MA. Subcortical contribution to late TMS-induced I-waves in intact humans. Front Integr Neurosci 9: 38, 2015. doi: 10.3389/fnint.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta AK, Harrison LM, Stephens JA. Task-dependent changes in the size of response to magnetic brain stimulation in human first dorsal interosseous muscle. J Physiol 418: 13–23, 1989. doi: 10.1113/jphysiol.1989.sp017826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol 586: 2735–2742, 2008. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol 412: 449–473, 1989. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Pilato F, Capone F, Musumeci G, Ranieri F, Ricci V, Bria P, Di Iorio R, de Waure C, Pasqualetti P, Profice P. Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol 105: 2150–2156, 2011. doi: 10.1152/jn.00781.2010. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol 109: 397–401, 1998. doi: 10.1016/S0924-980X(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, Profice P, Tonali P, Rothwell JC. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res 138: 268–273, 2001. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, Pilato F. I-wave origin and modulation. Brain Stimul 5: 512–525, 2012. doi: 10.1016/j.brs.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J Physiol 592: 4115–4128, 2014. doi: 10.1113/jphysiol.2014.274316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Capogna M. Noninvasive stimulation of the human brain: activation of multiple cortical circuits. Neuroscientist, 2017. doi: 10.1177/1073858417717660. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol 83: 528–536, 2000. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Federico P, Perez MA. Distinct corticocortical contributions to human precision and power grip. Cereb Cortex 27: 5070–5082, 2016. [Erratum in Cereb Cortex 28: 689, 2018.] doi: 10.1093/cercor/bhw291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flament D, Goldsmith P, Buckley CJ, Lemon RN. Task dependence of responses in first dorsal interosseous muscle to magnetic brain stimulation in man. J Physiol 464: 361–378, 1993. doi: 10.1113/jphysiol.1993.sp019639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S, Werner-Petroll N, Münchau A, Deuschl G, Ruschworth MF, Siebner HR. A novel dual-site transcranial magnetic stimulation paradigm to probe fast facilitatory inputs from ipsilateral dorsal premotor cortex to primary motor cortex. Neuroimage 62: 500–509, 2012. doi: 10.1016/j.neuroimage.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Hamada M, Galea JM, Di Lazzaro V, Mazzone P, Ziemann U, Rothwell JC. Two distinct interneuron circuits in human motor cortex are linked to different subsets of physiological and behavioral plasticity. J Neurosci 34: 12837–12849, 2014. doi: 10.1523/JNEUROSCI.1960-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex 23: 1593–1605, 2013. doi: 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol 509: 607–618, 1998. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah R, Rothwell JC. Pulse duration as well as current direction determines the specificity of transcranial magnetic stimulation of motor cortex during contraction. Brain Stimul 10: 106–115, 2017. doi: 10.1016/j.brs.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Di Lazzaro V, Perez MA. Effect of coil orientation on motor-evoked potentials in humans with tetraplegia. J Physiol 596: 4909–4921, 2018. doi: 10.1113/JP275798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol 198: 401–415, 2006. doi: 10.1016/j.expneurol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol 91: 1722–1733, 2004. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- Lei Y, Perez MA. Phase-dependent deficits during reach-to-grasp after human spinal cord injury. J Neurophysiol 119: 251–261, 2018. doi: 10.1152/jn.00542.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Livingston SC, Goodkin HP, Ingersoll CD. The influence of gender, hand dominance, and upper extremity length on motor evoked potentials. J Clin Monit Comput 24: 427–436, 2010. doi: 10.1007/s10877-010-9267-8. [DOI] [PubMed] [Google Scholar]
- Long J, Federico P, Perez MA. A novel cortical target to enhance hand motor output in humans with spinal cord injury. Brain 140: 1619–1632, 2017. doi: 10.1093/brain/awx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM; ASIA Neurological Standards Committee 2002 . International standards for neurological classification of spinal cord injury. J Spinal Cord Med 26, Suppl 1: S50–S56, 2003. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain 131: 1478–1491, 2008. doi: 10.1093/brain/awn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol 590: 3647–3663, 2012. doi: 10.1113/jphysiol.2012.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton HD, Amassian VE. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol 17: 345–363, 1954. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- Perez MA, Rothwell JC. Distinct influence of hand posture on cortical activity during human grasping. J Neurosci 35: 4882–4889, 2015. doi: 10.1523/JNEUROSCI.4170-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W; The International Federation of Clinical Neurophysiology . Magnetic stimulation: motor evoked potentials. Electroencephalogr Clin Neurophysiol Suppl 52, Suppl 52: 97–103, 1999. [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res 113: 24–32, 1997. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Tazoe T, Perez MA. Cortical and reticular contributions to human precision and power grip. J Physiol 595: 2715–2730, 2017. doi: 10.1113/JP273679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz LJ, Hamada M, Rothwell JC, Grefkes C. What makes the muscle twich: motor system connectivity and TMS-induced activity. Cereb Cortex 25: 2346–2353, 2015. doi: 10.1093/cercor/bhu032. [DOI] [PubMed] [Google Scholar]
- Wilson SA, Day BL, Thickbroom GW, Mastaglia FL. Spatial differences in the sites of direct and indirect activation of corticospinal neurones by magnetic stimulation. Electroencephalogr Clin Neurophysiol 101, Suppl 101: 255–261, 1996. doi: 10.1016/0924-980X(96)95148-6. [DOI] [PubMed] [Google Scholar]
- Zaaimi B, Dean LR, Baker SN. Different contributions of primary motor cortex, reticular formation, and spinal cord to fractionated muscle activation. J Neurophysiol 119: 235–250, 2018. doi: 10.1152/jn.00672.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]