Abstract
Motor learning is associated with plasticity in both motor and somatosensory cortex. It is known from animal studies that tetanic stimulation to each of these areas individually induces long-term potentiation in its counterpart. In this context it is possible that changes in motor cortex contribute to somatosensory change and that changes in somatosensory cortex are involved in changes in motor areas of the brain. It is also possible that learning-related plasticity occurs in these areas independently. To better understand the relative contribution to human motor learning of motor cortical and somatosensory plasticity, we assessed the time course of changes in primary somatosensory and motor cortex excitability during motor skill learning. Learning was assessed using a force production task in which a target force profile varied from one trial to the next. The excitability of primary somatosensory cortex was measured using somatosensory evoked potentials in response to median nerve stimulation. The excitability of primary motor cortex was measured using motor evoked potentials elicited by single-pulse transcranial magnetic stimulation. These two measures were interleaved with blocks of motor learning trials. We found that the earliest changes in cortical excitability during learning occurred in somatosensory cortical responses, and these changes preceded changes in motor cortical excitability. Changes in somatosensory evoked potentials were correlated with behavioral measures of learning. Changes in motor evoked potentials were not. These findings indicate that plasticity in somatosensory cortex occurs as a part of the earliest stages of motor learning, before changes in motor cortex are observed.
NEW & NOTEWORTHY We tracked somatosensory and motor cortical excitability during motor skill acquisition. Changes in both motor cortical and somatosensory excitability were observed during learning; however, the earliest changes were in somatosensory cortex, not motor cortex. Moreover, the earliest changes in somatosensory cortical excitability predict the extent of subsequent learning; those in motor cortex do not. This is consistent with the idea that plasticity in somatosensory cortex coincides with the earliest stages of human motor learning.
Keywords: EEG, motor cortex, motor skill learning, somatosensory cortex, TMS
INTRODUCTION
There is much evidence that motor skill learning is associated with plasticity in primary motor cortex (Classen et al. 1998; Gribble and Scott 2002; Li et al. 2001; Nudo et al. 1996; Paz and Vaadia 2004; Peters et al. 2017; Rioult-Pedotti et al. 1998). There is also a growing literature suggesting that there is plasticity in the somatosensory system that occurs in conjunction with learning and in some cases predicts motor learning behavior (Ostry and Gribble 2016). During motor skill learning, motor outflow and sensory inflow occur in parallel, and although it is possible that learning-related changes occur independently in motor and somatosensory cortex, it is also possible either that changes in motor cortex play a causal role in sensory change or that changes in somatosensory cortex determine learning-related changes in motor areas of the brain. Evidence consistent with the latter two possibilities is found in studies in which long-term potentiation (LTP) in somatosensory cortex is induced by tetanic stimulation of primary motor cortex and in studies in which LTP in motor cortex results from tetanic stimulation of a number of areas in somatosensory cortex (Iriki et al. 1989; Keller et al. 1990a, 1990b; Sakamoto et al. 1987). To better understand the relative contributions to motor skill acquisition of motor cortical and somatosensory plasticity in humans, we set out to characterize the time course of motor learning-related changes in both somatosensory and motor cortical areas of the brain.
Evidence of learning related plasticity in primary motor cortex is seen in work with nonhuman primates in which learning results in an expanded cortical territory from which movements can be elicited by electrical stimulation and by changes in the directional tuning of neurons (Gribble and Scott 2002; Li et al. 2001; Nudo et al. 1996; Paz and Vaadia 2004). In work with humans, the movements elicited with transcranial magnetic stimulation (TMS) to motor cortex change as a result of training (Classen et al. 1998), and in neuroimaging studies there are learning-related changes in motor areas of the brain (Della-Maggiore and McIntosh 2005; Doyon et al. 2002; Vahdat et al. 2011). Motor learning-related plasticity is likewise found in somatosensory cortex in nonhuman primates, in which the digit representation of fingers engaged in a precise positioning task is expanded (Jenkins et al. 1990). It is also seen in learning-related changes in the excitability of somatosensory cortex (Andrew et al. 2015a, 2015b; Nasir et al. 2013) and in neuroimaging studies in which motor learning results in changes to sensory areas of the brain (Sidarta et al. 2016; Vahdat et al. 2011). In addition, inhibition of somatosensory cortex produces deficits in movements that are specifically related to learning (Mathis et al. 2017). However, on the basis of these studies, it is uncertain how plasticity in motor and somatosensory cortex together relate to learning.
The present study involved a skill learning task in which subjects learned to produce time-varying patterns of force using the index finger. We used electroencephalography (EEG) to measure somatosensory evoked potentials (SEPs) over the course of learning to track changes in the excitability of primary somatosensory cortex. We applied single-pulse TMS to the hand area of primary motor cortex to track changes in corticospinal excitability during learning. If motor skill learning is determined by plasticity in either of these areas, then one ought to see changes in evoked potentials early in learning, and the changes would be expected to predict behavior as well. It was found that the earliest changes during learning occurred in somatosensory cortical responses and that these changes preceded those in motor cortical excitability. Changes to SEPs were correlated with behavioral measures of learning. Changes in motor evoked potentials (MEPs) were not. The findings are consistent with the possibility that somatosensory plasticity coincides with the earliest stages of human motor skill learning.
MATERIALS AND METHODS
Subjects.
Forty subjects participated in the experiment described below (17 men and 23 women, ages 18–28 yr). All subjects were right-handed and reported no history of neurological disorders. The Human Investigation Committee of Yale University approved the experimental protocol. Subjects provided written informed consent.
Experimental procedures.
The experimental goal was to characterize the time course of changes in the excitability of primary motor and primary somatosensory cortex during sensorimotor skill learning. Motor and somatosensory cortical excitability were measured using single-pulse TMS and median nerve stimulation. Although it would be desirable to have measures of both somatosensory and motor cortical excitability in the same subjects, in such a design one is not able to rule out the possibility that somatosensory stimulation affects measures of motor cortical excitability (Hamdy et al. 1998; Iriki et al. 1989; Keller et al. 1990a; Sakamoto et al. 1987) or that motor cortical stimulation affects somatosensory excitability (Keller et al. 1990b). Accordingly, measures of SEPs over the course of learning were obtained from 20 subjects, and measures of MEPs were obtained from 20 different subjects.
Behavioral learning and stimulation were interleaved (Fig. 2). Stimulation was applied after every 30 trials. On each trial, subjects were instructed to press down on a force sensor using their right index finger to reproduce a time varying pattern of forces that was displayed on a monitor (see Force control task). We recorded SEPs using EEG in response to median nerve stimulation at the right wrist (see SEP experiment). MEPs were elicited by single-pulse TMS to the hand area of left primary motor cortex (see MEP experiment). MEPs were recorded from the lumbrical muscle of the right index finger using bipolar electrodes. The first lumbrical muscle flexes the metacarpophalangeal joint and receives motor and sensory innervation from the median nerve. This ensures that median nerve stimulation influences the somatosensory representation of the same muscle that is involved in the motor skill learning task.
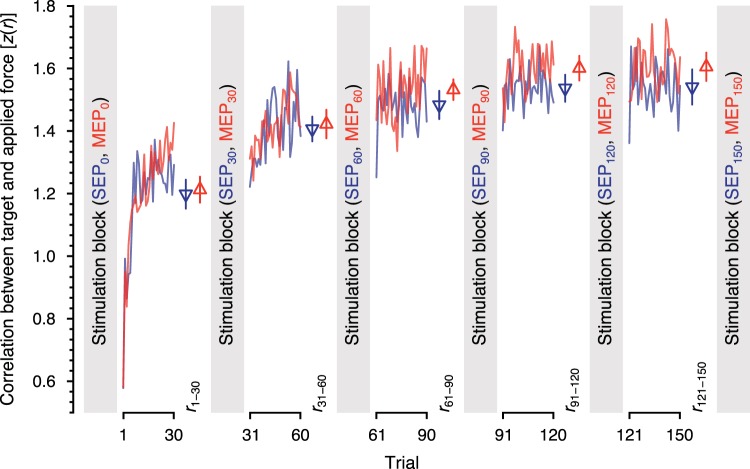
Fig. 2.
Changes in behavioral performance. Data are mean changes to the correlation between the target force profile and the subject applied force for subjects in the somatosensory evoked potential (SEP) experiment (blue) and those in the motor evoked potential (MEP) experiment (red). Triangles and error bars represent mean values and SE in each block of trials. Somatosensory and motor cortical excitability were measured before learning and after every 30 trials (SEP0, MEP0; SEP30, MEP30; SEP60, MEP60; SEP120, MEP120; and SEP150, MEP150). z(r), Fisher r-to-z transform.
Force control task.
Subjects were seated in front of a computer monitor (300 mm high × 375 mm wide) and placed their right index finger on a force sensor (FlexiForce A502 sensor; Tekscan). The force sensor voltage was amplified 10 times and low-pass filtered at 5 Hz (CED1902 amplifier; Cambridge Electronic Design). The processed signal was digitized at 500 Hz with 16-bit resolution (Micro 1401-3 data acquisition unit; Cambridge Electronic Design). The digital signal was presented on the monitor in real time as visual feedback (in red) of the force applied to the sensor (Fig. 1A). The visual representation of applied force moved across the screen at a constant rate. Each trial lasted 3 s.
Fig. 1.
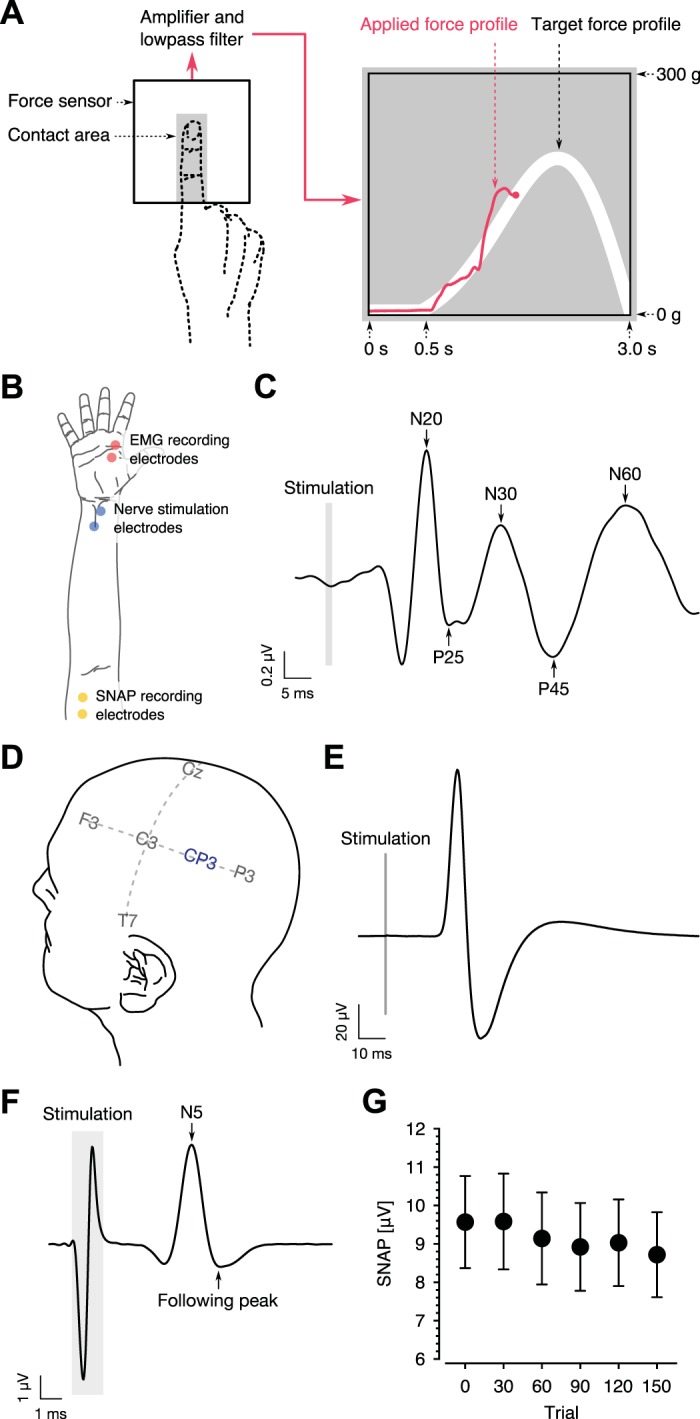
Experimental configuration. A: subjects pressed down on a force sensor with their right index finger (red curve) to match a target force profile (white curve) presented on a monitor. B: adhesive electrodes were placed above the lumbrical muscle of the right index finger (red) in the motor evoked potential (MEP) experiment (EMG, electromyography). Stimulation electrodes were placed on the skin above the median nerve at the right wrist (blue), and adhesive electrodes were placed along the median nerve on the anterior surface of the arm (yellow) in the somatosensory evoked potential (SEP) experiment. C: SEP averaged over all subjects. N20, P25, N30, P45 and N60 potentials were measured to assess somatosensory cortical excitability. D: SEPs were obtained from EEG signals at the CP3 electrode in the SEP experiment. Transcranial magnetic stimulation was delivered to the hand area of left primary motor cortex in the MEP experiment. E: MEP averaged over all subjects. The amplitude of the MEP was measured to assess motor cortical excitability. F and G: sensory nerve action potential (SNAP) averaged over subjects. Peripheral signal conduction was assessed using the amplitude of SNAP.
At the start of each trial, a target force profile was presented on the monitor as a white curve. Subjects pressed the sensor with their index finger to match the target force profile. They were instructed to flex only the metacarpophalangeal joint, which is the joint closest to the palm. On each trial, the target profile consisted of a 500-ms flat line followed by a 2,500-ms unimodal curve (Fig. 1A). The target profile was given as follows:
where t is the elapsed time (in ms) and a and b are control parameters of a Weibull function. By randomly varying a between 240 and 400 and b between 1.5 to 4, and by reversing the function along the time axis, we presented a different target profile on each trial. The parameter a affects the amplitude of the function, whereas the parameter b affects the skew. To keep the total force constant across trials, f(t|a, b) was normalized so that the integral of the function was 2.45 N. To keep initial force constant across trials, the trial began only when subjects maintained a force of <0.098 N for a period of 100 ms. Subjects performed 30 trials in each of five blocks for a total of 150 trials. An intertrial interval was randomized between 750 and 1,250 ms. The rationale for changing target shape on each trial was to create a task comparable to those outside of the laboratory in which the specific movement target varies from trial to trial and information on error for one trial cannot be used directly to update movements for the subsequent trial.
SEP experiment.
Subjects were seated on a chair and placed their right forearm in a supine position on a table. The arm was placed in the same position for each of the six stimulation blocks. Subjects were required to have their eyes closed during simulation to minimize eye-blink artifacts. Adhesive electrodes were placed on the skin above the median nerve at the right wrist (Fig. 1B). Square-wave pulses (0.2 ms) were delivered through these electrodes at a rate of 3 Hz for 3 min (540 in total in each stimulation block) using an isolated square-wave stimulator (Phipps & Bird). The stimulation intensity was just below the minimum intensity that elicited visible muscle contraction. This intensity was determined before the first stimulation block and was kept constant throughout the experiment. EEG was used to record SEPs elicited by median nerve stimulation. The signals were recorded using an active electrode system (ActiveTwo system; BioSemi) and sampled at 4,096 Hz. Thirty-two EEG electrodes were placed over the left hemisphere according to the international 10–20 system. The standard BioSemi reference electrodes were placed laterally on each side of electrode POz. To ensure that changes in the SEP were not the result of systematic peripheral changes such as movement of the electrodes, sensory nerve action potentials (SNAP) were recorded at the same time as SEPs by using active electrodes placed 2 cm apart along the median nerve on the anterior surface of the arm, just above the right elbow (Fig. 1B). The proximal electrode served as the reference, and a ground electrode was placed on the right olecranon.
MEP experiment.
MEPs were also recorded with subjects in a seated position, with the right forearm in a supine position. Single-pulse TMS was delivered to the hand area of left primary motor cortex using a Magstim 200 stimulator (Magstim) and 70-mm figure-eight coil (D-702 coil; Magstim). TMS was delivered 10 times in each MEP recording block. The interstimulus interval ranged from 5 to 12 s. We limited the number of MEP measurements to 10 per block because of cumulative effects of single-pulse TMS on motor cortical excitability (Cuypers et al. 2014; Pellicciari et al. 2016). Surface electromyography (EMG) was recorded using adhesive electrodes placed above the lumbrical muscle belly of the right index finger to capture MEPs elicited by TMS (Fig. 1B). The lumbrical muscle was identified by palpation while subjects produced isometric flexion of the index finger. Placement of the EMG electrodes was verified by the presence of EMG bursts during repetitive finger flexion movements. EMG signals were amplified 13,500 times, bandpass filtered between 16 and 550 Hz, and digitized at 3 kHz, with 12-bit resolution, using the EMG unit of the Brainsight frameless stereotactic system (Rogue Research). The coil position and stimulation intensity were determined before recording and maintained throughout the experiment. The coil placement and resting motor threshold were determined as the minimum intensity that elicited MEPs of >50 μV in the lumbrical muscle in 5 of 10 successive stimulations. The stimulation intensity was maintained at 120% of the resting motor threshold throughout testing. The Brainsight system was used to position the coil.
Behavioral data analysis.
The dependent measure of motor learning in the behavioral task was the Pearson correlation coefficient, computed for a trial between each target force profile and the time-varying force applied by the subject. Since the first 500-ms period was a preparation phase in which the target profile was flat, the correlation was computed using the force profile between 500 and 3,000 ms. Individual values were excluded if they fell outside of ±4 SD from the mean of each 30-trial block. Overall, this resulted in the removal of 1.392% of computed correlations. Motor learning was quantified as the change in average correlation in each block relative to the first block. The correlation values were converted into Fisher z scores for the purpose of statistical tests. The average correlation values were submitted to a mixed-design analysis of variance (ANOVA) with one between-subjects factor (SEP or MEP) and one within-subject factor (blocks 1–30, 31–60, 61–90, 91–120, or 121–150). Bonferroni–Holm-corrected post hoc tests were then applied to assess the number of trials needed for performance to reach an asymptotic level.
SEP analysis.
Each time median nerve stimulation was delivered, we recorded both SEPs and SNAPs. The amplitude of the SEP was computed using the EEG signal at electrode CP3 of the international 10–20 system (Fig. 1D). SNAP amplitudes were computed using the signals recorded at the median nerve just above the right elbow. The continuous EEG signals were passed through a notch filter at 60 Hz and bandpass filtered between 20 and 300 Hz, re-referenced to signals at the left earlobe, and then segmented into epochs between –10 and 70 ms relative to stimulation onset. We subtracted from each epoch the average amplitude in the interval between –10 and 0 ms before stimulation. We rejected epochs in which the maximum resulting voltage exceeded ±30 μV (3.591%). Mean SEPs were computed as the temporal average over the remaining epochs within each 30-trial block. Mean SEP amplitudes were quantified as the peak-to-peak amplitude between the N20 and P25 components (Fig. 1C). These components were chosen because there is consensus that the N20 and P25 components recorded from electrode CP3 reflect activity in primary somatosensory cortex (Allison et al. 1991; Balzamo et al. 2004; Macerollo et al. 2018). The scalp voltage distribution of the N20–P25 component, averaged over all subjects and over all blocks, shows a peak around electrode CP3 (Fig. 3A). We also measured the peak-to-peak amplitudes of the N30–P45 and P45–N60 potentials to examine other possible learning-related changes to the SEP (Fig. 1C). A correlation analysis was conducted to test the relationship between changes in SEPs and measures of behavioral improvement using nonparametric bootstrapping to assess statistical significance.
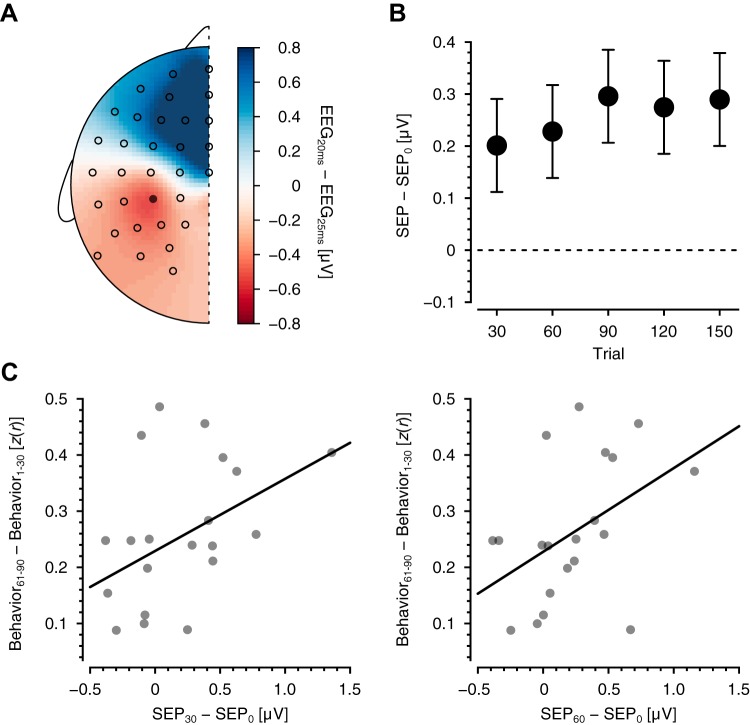
Fig. 3.
Changes in somatosensory cortical excitability. A: scalp voltage distribution associated with the N20–P25 component of the somatosensory evoked potential (SEP). The distribution is the average over all subjects and all stimulation blocks. Circles represent the positions of EEG electrodes, and the closed circle is electrode CP3. B: changes relative to baseline SEP in the N20–P25 potential at CP3 after every 30 behavioral trials. Data are means ± SE. C: relationship between changes in N20–P25 magnitudes at early stages of learning (after trials 30 and 60) and the block at which performance reached asymptote (trials 61–90). z(r), Fisher r-to-z transform.
The preprocessing of SNAP signals was the same as that of SEPs except that a high-pass filter was applied at 0.75 Hz and the peak-to-peak amplitude was quantified between the negative peak at 5 ms after stimulation and the subsequent positive peak (Fig. 1F). One-way repeated-measures ANOVA followed by Bonferroni–Holm-corrected post hoc tests were used to test for changes in SEPs and SNAPs over the course of learning. To evaluate the possibility that measured changes in SEPs were due to changes in peripheral signal conduction, a correlation between changes in SEPs and changes in SNAPs was computed using data from all subjects and all SEP/SNAP blocks.
MEP analysis.
For each MEP block, MEP amplitudes were computed from surface EMG signals at the lumbrical muscle of the right index finger (the first lumbrical muscle; Fig. 1B). MEP amplitudes were quantified as peak-to-peak amplitude of the evoked EMG signal in the interval between 10 and 90 ms after stimulation and were averaged within each block (Fig. 1E). We also tested for changes in MEP onset latency. The onset latency was defined as the interval between the stimulus and the onset of the motor evoked response. One-way repeated-measures ANOVA and Bonferroni–Holm-corrected post hoc tests were used to test for changes in MEPs relative to values obtained in the first block. A correlation analysis was conducted to test the relationship between changes in MEPs and measures of behavioral improvement.
RESULTS
The present experiment was designed to assess the time course of changes to cortical motor and somatosensory excitability during motor skill learning. The state of somatosensory and motor cortex was assessed by measuring SEPs and MEPs at regular intervals between blocks of training trials (Fig. 2). The experimental model of skill learning was a force production task in which subjects pressed down on a force sensor with their right index finger to trace a target force profile that was presented on a monitor (Fig. 1A). The target force profile was different on each trial.
Behavioral improvement in the force production task.
Changes in learning were quantified on a trial-by-trial basis by using the Pearson correlation coefficient computed using a target profile and the applied force. Figure 2 shows the time course of this measure averaged across subjects that were tested in the SEP and MEP conditions. As shown in Fig. 2, low correlations, indicative of large errors, occur early in learning. Performance improves and gradually reaches an asymptotic level somewhere between 60 and 90 trials. ANOVA revealed that the difference in the average correlation values across the five behavioral blocks was statistically reliable (F4,152 = 87.233, P < 10−5) and that the pattern of behavioral improvement did not significantly differ between the subjects receiving median nerve stimulation and subjects receiving TMS (F4,152 = 1.217, P = 0.306). In addition, for these same subjects, no differences in behavior were seen in the first five learning trials (t33.402 = –0.482, P = 0.633). This indicates that any differences in MEP and SEP measures are not attributable to differences initial performance or to differences in amount of learning over the course of the training session. Post hoc tests indicated significant differences between the last block and each of the first and second blocks (F1,152 = 233.503, P < 10−5 for block 1 vs. block 5; F1,152 = 28.700, P < 10−5 for block 2 vs. block 5; P values are corrected) and no reliable difference between the last block and either the third or fourth blocks (F1,152 = 1.363, P = 0.490 for block 3 vs. block 5; F1,152 = 0.421, P = 0.517 for block 4 vs. block 5; P values are corrected). This indicates that subjects successfully learned the force production task, and their performance reached an asymptotic level by the third block (trials 61–90).
Change in SEPs.
SEPs were obtained before learning and again after each 30 behavioral trials (SEP0, SEP30, SEP60, SEP120, and SEP150 in Fig. 2). To assess changes in somatosensory cortical excitability, the mean amplitude of the N20–P25 complex at each measurement point was compared with the baseline mean value. Figure 3B shows the SEP amplitude averaged over subjects in each 30-trial SEP block relative to the first block. An SEP increase relative to baseline was evident at the first measurement point and remained elevated throughout the remainder of the experiment. ANOVA revealed that SEP changed over the course of learning (F5,95 = 3.116, P = 0.0120). Post hoc tests indicated that the SEP increase was reliably different from 0 by trial 30 and beyond (F1,95 = 5.0694, P = 0.0267 for SEP30–SEP0; F1,95 = 6.511, P = 0.0246 for SEP60–SEP0; F1,95 = 10.952, P = 0.00661 for SEP90–SEP0; F1,95 = 9.438, P = 0.00832 for SEP120–SEP0; F1,95 = 10.500, P = 0.00661 for SEP150–SEP0; P values are corrected). Amplitudes of later SEP components, N30–P45 and P45–N60, were not observed to change over the course of the experiment (F5,95 = 2.037, P = 0.0817 for N30–P45; F5,95 = 1.367, P = 0.244 for P45–N60).
We tested for differences in SNAP amplitudes in the same way as was done for SEPs. ANOVA revealed statistically reliable differences in SNAP amplitudes (Fig. 1G; F5,95 = 5.427, P = 0.000196). Post hoc tests indicated that changes in SNAP value relative to baseline were reliably less than 0 after trial 90 and beyond (F1,95 = 0.00527, P = 0.942 for SNAP30–SNAP0; F1,95 = 4.019, P = 0.0957 for SNAP60–SNAP0; F1,95 = 9.241, P = 0.00122 for SNAP90–SNAP0; F1,95 = 6.397, P = 0.0392 for SNAP120–SNAP0; F1,95 = 15.931, P = 0.000647 for SNAP150–SNAP0; P values are corrected). There was no reliable relationship between changes in SEPs and changes in SNAPs (r = 0.132, P = 0.142). The absence of systematic changes in SNAPs until trial 90, in combination with the absence of a relationship between changes in SNAP and SEP measures, argues against the possibility that SEP changes are due to changes in peripheral factors over the course of the experiment.
The relationship between skill learning and SEP change was assessed by computing the correlation between the very first measured changes in SEP (after trials 30 and 60) and measures of asymptotic behavioral improvement (trials 61–90 and beyond; Fig. 3C). There was a significant positive relationship between initial SEP changes (after trials 30 and 60) and asymptotic behavioral values (r = 0.434, P = 0.0384 for SEP30–SEP0; r = 0.445, P = 0.0428 for SEP60–SEP0; P values are corrected). We also tested the correlation of the behavioral improvement from the very first 10 trials to the last 10 trials (trials 1–10 vs. trials 141–150) with SEP change after trial 30 and again after trial 60. In both cases, a statistically reliable correlation was observed (r = 0.364, P = 0.0260 for SEP30; r = 0.364, P = 0.0260 for SEP60). Thus subjects that showed larger changes in primary somatosensory cortex excitability early in learning went on to learn more overall.
Change in motor corticospinal excitability.
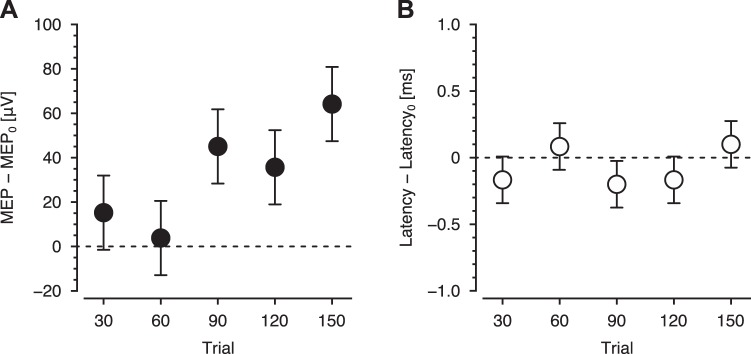
MEPs were obtained before learning and after every 30 trials (Fig. 1A). Changes in the excitability of motor cortex were assessed every 30 trials as the difference in MEP amplitude relative to baseline (MEP0 vs. MEP30, MEP60, MEP120, and MEP150 in Fig. 2). Figure 4A shows mean MEP amplitude averaged over subjects relative to baseline. MEP amplitude was no different from 0 after trials 30 and 60. At trial 90 the amplitude increased above baseline levels, and this increase was maintained afterward. ANOVA revealed that MEP changed over the course of learning (F5,95 = 4.978, P = 0.000430). Post hoc tests indicated that MEP change was significantly different from 0 at trials 90 and 150, and marginally significant at trial 120 (F1,95 = 1.245, P = 0.535 for MEP30–MEP0; F1,95 = 0.121, P = 0.728 for MEP60–MEP0; F1,95 = 9.0398, P = 0.0135 for MEP90–MEP0; F1,95 = 5.386, P = 0.0673 for MEP120–MEP0; F1,95 = 16.115, P = 0.000595 for MEP150–MEP0; P values are corrected). No differences in the latency of MEP onset were observed over the course of the experiment (Fig. 4B; F5,95 = 1.201, P = 0.315). Thus motor corticospinal excitability predominantly increased later in learning.
Fig. 4.
Changes in motor cortical excitability. A: changes in motor evoked potential (MEP) after every 30 behavioral trials. B: changes in latency of MEP onset. Data are means ± SE.
We tested for a correlation between the change in MEP amplitude and behavioral measures of learning. No reliable relationships were detected (uncorrected P > 0.0500) between any combination of MEP changes (e.g., MEP30–MEP0, MEP60–MEP0) and behavioral improvements. Thus, although motor corticospinal excitability increased later in learning, the increased excitability was not related to the measured behavioral improvement.
Proportionate changes in SEPs and MEPs.
The tests for SEP and MEP changes over the course of learning were each repeated using measures of proportionate change relative to baseline rather than as difference scores, which are reported above. In each case, the statistical outcomes were similar (Table 1). Overall, the proportionate change in SEPs was 16% and the proportionate change in MEPs was 27%. SEPs change was identified from the very first measurement and remained elevated throughout, whereas no change in MEP was detected until trial 90. As with difference scores, there was a reliable correlation between proportionate SEP change early in learning (trial 30) and proportionate behavioral improvement (trials 61–90). There was no such relationship between proportionate MEP change and behavior.
Table 1.
Statistical tests on proportionate changes in SEP and MEP
| Measure | Statistic | Corrected P Value |
|---|---|---|
| SEP | ||
| After trial 30 | F1,76 = 8.383 | 0.00494 |
| After trial 60 | F1,76 = 11.225 | 0.00252 |
| After trial 90 | F1,76 = 18.716 | 0.000228 |
| After trial 120 | F1,76 = 14.301 | 0.00123 |
| After trial 150 | F1,76 = 14.128 | 0.00123 |
| Correlation with behavior (after trial 30) | r = 0.364 | 0.0260 |
| MEP | ||
| After trial 30 | F1,76 = 2.791 | 0.184 |
| After trial 60 | F1,76 = 2.914 | 0.184 |
| After trial 90 | F1,76 = 13.300 | 0.00194 |
| After trial 120 | F1,76 = 6.263 | 0.0434 |
| After trial 150 | F1,76 = 18.413 | 0.000259 |
| Correlation with behavior | >0.0500 |
Statistical data are F values for changes in somatosensory evoked potential (SEP) and motor evoked potential (MEP) after every 30 behavioral trials, the Pearson correlation coefficient (r) indicating correlation with behavior, and corrected P values.
DISCUSSION
The present study examined the temporal evolution of primary somatosensory (S1) and primary motor cortex (M1) excitability over the course of human motor learning and found different patterns of MEP and SEP change. An SEP increase was detectable at the first measurement point (trial 30) and showed persistent elevation throughout training. MEPs showed no change early in learning and only showed statistically reliable increases at a later stage (trial 90 and beyond). The changes in SEPs were positively correlated with behavioral improvements in learning, whereas changes in MEPs were not. These findings are supportive of the hypothesis that plasticity in somatosensory cortex is a determining factor at an early stage of motor learning. This also argues against the possibility that somatosensory change is simply a by-product of changes to cortical motor areas, in which case somatosensory change would have been expected to occur after motor cortical change or possibly in parallel.
Evoked potentials in S1, which were assessed using the N20–P25 complex of the SEP, increased early in learning and remained persistently elevated throughout. Subjects who were better learners showed larger SEP increases. These results are in accord with previous findings documenting the involvement of S1 in sensorimotor adaptation and learning. Specifically, SEPs have been found to increase following repetitive typing and tracing tasks (Andrew et al. 2015a, 2015b). In nonhuman primates and rodents, S1 ablation and photoinhibition preserved previously learned motor behavior but disrupted motor learning and adaptation (Mathis et al. 2017; Pavlides et al. 1993).
There is uncertainty regarding the functional role of S1 plasticity in motor learning. The uncertainty arises in part as a result of reciprocal interactions between the somatosensory and motor systems. One possibility is that activity in M1 results in changes to somatosensory cortex. In particular, tetanic stimulation of M1 induces LTP in S1 (Keller et al. 1990b), and M1 activity has also been shown to drive changes in local field potentials in S1 (Zagha et al. 2013). Accordingly, S1 could play a role in learning by receiving signals from M1 or premotor areas. Indeed, previous studies have documented that there were changes to the cortical response in sensory areas and to perception in the context of voluntary movement (Bernier et al. 2009; Blakemore et al. 1999; Palmer et al. 2016; Shergill et al. 2013; Voss et al. 2006). However, it is unlikely that cortical motor activity or movement caused the learning-related SEP change observed in the present study. Our SEP measurement was acquired at rest, and unlike previous studies, the SEP changes observed in the present study were not attenuated but enhanced, and the increases in SEP preceded changes in M1 excitability. These findings thus argue against the possibility that plasticity in S1 occurs as a consequence of movement-related activity in cortical motor areas.
The present results are consistent with the idea that the behavioral improvements which occur early in learning are driven at least in part by changes in somatosensory cortical function. These early sensory changes predict learning and might serve one or more of the following functions. One possibility is that somatosensory cortex is involved in the acquisition and storage of newly learned somatic states (new somatosensory targets) that guide subsequent movements or the improvement of somatic acuity during learning. The alignment of movements following learning with altered estimates of limb position is consistent with this possibility (Ohashi et al. 2019; Ostry et al. 2010), as is the observation that somatosensory perceptual acuity increased in association with learning of a sequence of limb movements and in reinforcement learning (Bernardi et al. 2015; Wong et al. 2011). The idea that somatosensory cortex is involved in coding of position in space is supported by the finding that lesions to somatosensory and posterior parietal cortex result in deficits to position sense (Findlater et al. 2016).
Another possibility is that plasticity in S1 may be related directly to its involvement in the efferent control of movement. Cortical areas 3a, 3b, 1, 2, and 5 and second somatosensory cortex each have outputs to the spinal cord (Murray and Coulter 1981; Rathelot et al. 2017). Corticospinal tract outputs from somatosensory cortex terminate most densely in the intermediate zone of the contralateral spinal cord (Ralston and Ralston 1985) and in this region overlap extensively with projections from motor cortex (Liu and Chambers 1964). Cells in S1 including identified pyramidal tract neurons discharge in advance of the initiation of muscle activity (Fromm and Evarts 1982; Soso and Fetz 1980) and thus may participate in the control of movement through termination on spinal interneurons.
In the present study, M1 excitability was only found to increase late in learning once performance levels reached asymptotic values, suggesting that M1 changes may be associated with the achievement of a high degree of precision in learning. Changes to motor cortical excitability that are restricted to later in learning have been reported previously (Orban de Xivry et al. 2013). Earlier changes in M1 excitability are also observed, but in tasks involving ballistic movement (Bologna et al. 2015) where precision requirements are limited.
Both S1 and M1 cortical excitability increased over the course of learning. Parallel changes related to learning in the somatosensory and motor systems have been observed in various forms, such as in behavioral correlations between somatosensory and motor performance (Mattar et al. 2013; Vahdat et al. 2011) and in coherent electrophysiological activity between S1 and M1 (Arce-McShane et al. 2016). In the present study, these two changes were measured in separate groups that underwent the same learning task. Accordingly, in the present design we cannot assess the extent to which M1 changes might be predicted by changes to S1. However, there is accumulating evidence that inputs to M1 from S1 regulate and may be essential to M1 plasticity. Specifically, in cats, inputs to M1 via intercortical pathways from S1 produce M1 LTP and regulate M1 LTP produced via the thalamocortical pathway (Iriki et al. 1989; Sakamoto et al. 1987). In humans, repetitive somatosensory stimulation applied to the fingers or wrist induces plasticity in hand motor cortex (Lewis and Byblow 2004; Rocchi et al. 2017). In contrast, activity in monkey M1 on its own does not lead to durable changes in M1 circuity as assessed by torque production resulting from intracortical microstimulation (Lucas and Fetz 2013).
Separate groups of subjects were tested for SEP and MEP measurements. We used separate groups to ensure that motor cortical excitability was not affected by median nerve stimulation and that somatosensory cortex excitability was not affected by stimulation to motor cortex. However, the inherent limitation is that a direct comparison cannot be undertaken of MEPs and SEPs over the course of learning. In principle, it would have been desirable to conduct tests of the possible dependence of MEP changes on earlier changes in sensory cortex excitability.
In conclusion, separate assessments of S1 and M1 responses in humans were obtained to characterize the time-varying contribution of these regions to sensorimotor learning. S1 excitability increased early in learning, and these increases covaried with behavioral improvements in performance. M1 motor excitability increased later as performance levels reached asymptotic values and thus may be involved with repetition-based processes in learning. The results are consistent with the idea that plasticity in S1 is an integral component of the early stages of motor skill learning.
GRANTS
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01HD075740 and the Natural Sciences and Engineering Research Council of Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.O., P.L.G., and D.J.O. conceived and designed research; H.O. performed experiments; H.O. analyzed data; H.O., P.L.G., and D.J.O. interpreted results of experiments; H.O., P.L.G., and D.J.O. prepared figures; H.O., P.L.G., and D.J.O. drafted manuscript; H.O., P.L.G., and D.J.O. edited and revised manuscript; H.O., P.L.G., and D.J.O. approved final version of manuscript.
REFERENCES
- Allison T, McCarthy G, Wood CC, Jones SJ. Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain 114: 2465–2503, 1991. doi: 10.1093/brain/114.6.2465. [DOI] [PubMed] [Google Scholar]
- Andrew D, Haavik H, Dancey E, Yielder P, Murphy B. Somatosensory evoked potentials show plastic changes following a novel motor training task with the thumb. Clin Neurophysiol 126: 575–580, 2015a. doi: 10.1016/j.clinph.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Andrew D, Yielder P, Murphy B. Do pursuit movement tasks lead to differential changes in early somatosensory evoked potentials related to motor learning compared with typing tasks? J Neurophysiol 113: 1156–1164, 2015b. doi: 10.1152/jn.00713.2014. [DOI] [PubMed] [Google Scholar]
- Arce-McShane FI, Ross CF, Takahashi K, Sessle BJ, Hatsopoulos NG. Primary motor and sensory cortical areas communicate via spatiotemporally coordinated networks at multiple frequencies. Proc Natl Acad Sci USA 113: 5083–5088, 2016. doi: 10.1073/pnas.1600788113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzamo E, Marquis P, Chauvel P, Régis J. Short-latency components of evoked potentials to median nerve stimulation recorded by intracerebral electrodes in the human pre- and postcentral areas. Clin Neurophysiol 115: 1616–1623, 2004. doi: 10.1016/j.clinph.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Bernardi NF, Darainy M, Ostry DJ. Somatosensory contribution to the initial stages of human motor learning. J Neurosci 35: 14316–14326, 2015. doi: 10.1523/JNEUROSCI.1344-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier PM, Burle B, Vidal F, Hasbroucq T, Blouin J. Direct evidence for cortical suppression of somatosensory afferents during visuomotor adaptation. Cereb Cortex 19: 2106–2113, 2009. doi: 10.1093/cercor/bhn233. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. The cerebellum contributes to somatosensory cortical activity during self-produced tactile stimulation. Neuroimage 10: 448–459, 1999. doi: 10.1006/nimg.1999.0478. [DOI] [PubMed] [Google Scholar]
- Bologna M, Rocchi L, Paparella G, Nardella A, Li Voti P, Conte A, Kojovic M, Rothwell JC, Berardelli A. Reversal of practice-related effects on corticospinal excitability has no immediate effect on behavioral outcome. Brain Stimul 8: 603–612, 2015. doi: 10.1016/j.brs.2015.01.405. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79: 1117–1123, 1998. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Cuypers K, Thijs H, Meesen RL. Optimization of the transcranial magnetic stimulation protocol by defining a reliable estimate for corticospinal excitability. PLoS One 9: e86380, 2014. doi: 10.1371/journal.pone.0086380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, McIntosh AR. Time course of changes in brain activity and functional connectivity associated with long-term adaptation to a rotational transformation. J Neurophysiol 93: 2254–2262, 2005. doi: 10.1152/jn.00984.2004. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 99: 1017–1022, 2002. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlater SE, Desai JA, Semrau JA, Kenzie JM, Rorden C, Herter TM, Scott SH, Dukelow SP. Central perception of position sense involves a distributed neural network - Evidence from lesion-behavior analyses. Cortex 79: 42–56, 2016. doi: 10.1016/j.cortex.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Fromm C, Evarts EV. Pyramidal tract neurons in somatosensory cortex: central and peripheral inputs during voluntary movement. Brain Res 238: 186–191, 1982. doi: 10.1016/0006-8993(82)90781-8. [DOI] [PubMed] [Google Scholar]
- Gribble PL, Scott SH. Overlap of internal models in motor cortex for mechanical loads during reaching. Nature 417: 938–941, 2002. doi: 10.1038/nature00834. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci 1: 64–68, 1998. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- Iriki A, Pavlides C, Keller A, Asanuma H. Long-term potentiation in the motor cortex. Science 245: 1385–1387, 1989. doi: 10.1126/science.2551038. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guíc-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol 63: 82–104, 1990. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- Keller A, Iriki A, Asanuma H. Identification of neurons producing long-term potentiation in the cat motor cortex: intracellular recordings and labeling. J Comp Neurol 300: 47–60, 1990a. doi: 10.1002/cne.903000105. [DOI] [PubMed] [Google Scholar]
- Keller A, Pavlides C, Asanuma H. Long-term potentiation in the cat somatosensory cortex. Neuroreport 1: 49–52, 1990b. doi: 10.1097/00001756-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD. Neurophysiological and behavioural adaptations to a bilateral training intervention in individuals following stroke. Clin Rehabil 18: 48–59, 2004. doi: 10.1191/0269215504cr701oa. [DOI] [PubMed] [Google Scholar]
- Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron 30: 593–607, 2001. doi: 10.1016/S0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Liu CN, Chambers WW. An experimental study of the cortico-spinal system in the monkey (Macaca mulatta). The spinal pathways and preterminal distribution of degenerating fibers following discrete lesions of the pre- and postcentral gyri and bulbar pyramid. J Comp Neurol 123: 257–283, 1964. doi: 10.1002/cne.901230209. [DOI] [PubMed] [Google Scholar]
- Lucas TH, Fetz EE. Myo-cortical crossed feedback reorganizes primate motor cortex output. J Neurosci 33: 5261–5274, 2013. doi: 10.1523/JNEUROSCI.4683-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macerollo A, Brown MJN, Kilner JM, Chen R. Neurophysiological changes measured using somatosensory evoked potentials. Trends Neurosci 41: 294–310, 2018. doi: 10.1016/j.tins.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Mathis MW, Mathis A, Uchida N. Somatosensory cortex plays an essential role in forelimb motor adaptation in mice. Neuron 93: 1493–1503.e6, 2017. doi: 10.1016/j.neuron.2017.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar AA, Darainy M, Ostry DJ. Motor learning and its sensory effects: time course of perceptual change and its presence with gradual introduction of load. J Neurophysiol 109: 782–791, 2013. doi: 10.1152/jn.00734.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Coulter JD. Organization of corticospinal neurons in the monkey. J Comp Neurol 195: 339–365, 1981. doi: 10.1002/cne.901950212. [DOI] [PubMed] [Google Scholar]
- Nasir SM, Darainy M, Ostry DJ. Sensorimotor adaptation changes the neural coding of somatosensory stimuli. J Neurophysiol 109: 2077–2085, 2013. doi: 10.1152/jn.00719.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16: 785–807, 1996. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi H, Valle-Mena R, Gribble PL, Ostry DJ. Movements following force-field adaptation are aligned with altered sense of limb position. Exp Brain Res 237: 1303–1313, 2019. doi: 10.1007/s00221-019-05509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Ahmadi-Pajouh MA, Harran MD, Salimpour Y, Shadmehr R. Changes in corticospinal excitability during reach adaptation in force fields. J Neurophysiol 109: 124–136, 2013. doi: 10.1152/jn.00785.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostry DJ, Darainy M, Mattar AA, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J Neurosci 30: 5384–5393, 2010. doi: 10.1523/JNEUROSCI.4571-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostry DJ, Gribble PL. Sensory plasticity in human motor learning. Trends Neurosci 39: 114–123, 2016. doi: 10.1016/j.tins.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CE, Davare M, Kilner JM. Physiological and perceptual sensory attenuation have different underlying neurophysiological correlates. J Neurosci 36: 10803–10812, 2016. doi: 10.1523/JNEUROSCI.1694-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Miyashita E, Asanuma H. Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. J Neurophysiol 70: 733–741, 1993. doi: 10.1152/jn.1993.70.2.733. [DOI] [PubMed] [Google Scholar]
- Paz R, Vaadia E. Learning-induced improvement in encoding and decoding of specific movement directions by neurons in the primary motor cortex. PLoS Biol 2: e45, 2004. doi: 10.1371/journal.pbio.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicciari MC, Miniussi C, Ferrari C, Koch G, Bortoletto M. Ongoing cumulative effects of single TMS pulses on corticospinal excitability: an intra- and inter-block investigation. Clin Neurophysiol 127: 621–628, 2016. doi: 10.1016/j.clinph.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Peters AJ, Liu H, Komiyama T. Learning in the rodent motor cortex. Annu Rev Neurosci 40: 77–97, 2017. doi: 10.1146/annurev-neuro-072116-031407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston DD, Ralston HJ 3rd. The terminations of corticospinal tract axons in the macaque monkey. J Comp Neurol 242: 325–337, 1985. doi: 10.1002/cne.902420303. [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Dum RP, Strick PL. Posterior parietal cortex contains a command apparatus for hand movements. Proc Natl Acad Sci USA 114: 4255–4260, 2017. doi: 10.1073/pnas.1608132114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci 1: 230–234, 1998. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rocchi L, Erro R, Antelmi E, Berardelli A, Tinazzi M, Liguori R, Bhatia K, Rothwell J. High frequency somatosensory stimulation increases sensori-motor inhibition and leads to perceptual improvement in healthy subjects. Clin Neurophysiol 128: 1015–1025, 2017. doi: 10.1016/j.clinph.2017.03.046. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Porter LL, Asanuma H. Long-lasting potentiation of synaptic potentials in the motor cortex produced by stimulation of the sensory cortex in the cat: a basis of motor learning. Brain Res 413: 360–364, 1987. doi: 10.1016/0006-8993(87)91029-8. [DOI] [PubMed] [Google Scholar]
- Shergill SS, White TP, Joyce DW, Bays PM, Wolpert DM, Frith CD. Modulation of somatosensory processing by action. Neuroimage 70: 356–362, 2013. [Erratum in Neuroimage 197: 827, 2019.] doi: 10.1016/j.neuroimage.2012.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidarta A, Vahdat S, Bernardi NF, Ostry DJ. Somatic and reinforcement-based plasticity in the initial stages of human motor learning. J Neurosci 36: 11682–11692, 2016. doi: 10.1523/JNEUROSCI.1767-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soso MJ, Fetz EE. Responses of identified cells in postcentral cortex of awake monkeys during comparable active and passive joint movements. J Neurophysiol 43: 1090–1110, 1980. doi: 10.1152/jn.1980.43.4.1090. [DOI] [PubMed] [Google Scholar]
- Vahdat S, Darainy M, Milner TE, Ostry DJ. Functionally specific changes in resting-state sensorimotor networks after motor learning. J Neurosci 31: 16907–16915, 2011. doi: 10.1523/JNEUROSCI.2737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M, Ingram JN, Haggard P, Wolpert DM. Sensorimotor attenuation by central motor command signals in the absence of movement. Nat Neurosci 9: 26–27, 2006. doi: 10.1038/nn1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JD, Wilson ET, Gribble PL. Spatially selective enhancement of proprioceptive acuity following motor learning. J Neurophysiol 105: 2512–2521, 2011. doi: 10.1152/jn.00949.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E, Casale AE, Sachdev RN, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron 79: 567–578, 2013. doi: 10.1016/j.neuron.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]