Abstract
Potato is the most widely cultivated non-cereal crop in the world, and like any other crop, it is susceptible to yield losses because of various factors, including pathogen attacks. Among the various diseases of potato, late blight caused by the oomycete Phytophthora infestans is considered as the most devastating disease worldwide. In this study, transgenic potato plants overexpressing the D-galacturonic acid reductase (GalUR) gene with an enhanced level of cellular L-ascorbate (L-AsA) were challenged with Phytophthora infestans to determine the level of stress tolerance induced in those plants. With the onset of pathogen infection, necrotic lesions progressively expanded and became necrotic in the control plants. The transgenic potato lines with enhanced ascorbic acid showed reduced necrotic lesions. Hydrogen peroxide (H2O2) and malondialdehyde (MDA) levels were relatively lower in transgenic plants compared to the untransformed control (UT) plants. The mRNA expressions of pathogenesis-related (PR) genes, such as pathogenesis related 1 (PR1) and phenylalanine ammonia-lyase (PAL) were slightly higher in GalUR overexpressing transgenic lines as compared to the untransformed control plants. Pathogen infection also altered the mRNA expression of genes associated with gibberellic acid (GA) and abscisic acid (ABA) biosynthesis. Furthermore, the increase in various antioxidant enzymes was also observed in the gene expression analysis with the transgenic plants. The complete loss of the pathogen growth and disease occurrence was not observed in our study; however, the findings indicated that an increase in the level of cellular L-ascorbate in the transgenic potato leads to enhanced cellular antioxidants, PR genes and plant defense hormones, such as GA and ABA resulting in the reduction of the disease symptoms caused by the Phytophthora infestans.
Keywords: ascorbic acid, Phytophthora infestans, reactive oxygen species, Solanum tuberosum, transgenic plants
1. Introduction
Potato (Solanum tuberosum L.) stands as the world’s fourth largest grown non-cereal crop [1]. Plant development and production of potatos are significantly affected by both abiotic and biotic stresses [2]. In response to pathogen infection, plants trigger defense responses via the production of various reactive oxygen species (ROS), namely hydrogen peroxide (H2O2), OH−, and O2− molecules. These ROS act as signals for the induction of a hypersensitive response (HR) mediated cell death and activation of defense associated genes to inhibit pathogen growth and their further spreading [3]. Although these active oxygen species act as a signal in favor of pathogen resistance and other normal physiological processes, their exceedance causes potential damage to biological supermolecules for instance lipids, proteins, and nucleic acids [4]. When the ROS activity exceeds beyond their normal levels, antioxidants, for example, ascorbate, glutathione, α-tocopherol, ß-carotene, and antioxidative enzymes, such as superoxide dismutase (SOD), catalases (CAT), and peroxidases (POX) contribute to the detoxification of ROS activity [5,6]. Among the antioxidants, vitamin C (L-ascorbic acid, AsA) is a significant multifunctional antioxidant compound and an important substrate for the detoxification of reactive oxygen species (ROS) involved in stress tolerance [7,8]. It also acts as a signaling molecule in several physiological processes such as cell division, growth regulation, and senescence [9,10]. It is a cofactor for the enzymes violaxanthin de-epoxidase (catalyst for zeaxanthin production), 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase (one of the catalysts in ethylene biosynthesis), and 2-oxoacid-dependent dioxygenases which is involved in abscisic acid (ABA) and gibberellic acid (GA) biosynthesis [11,12,13]. Numerous reports indicate the positive role of AsA and other antioxidants in governing protection to plants against diseases [14].
An exogenous application of AsA, dehydroascorbate, and H2O2 efficiently alleviated the damages caused by the Phytoplasma in the infected potato tubers and enhanced the quality, as well as the tuber yield [15,16]. The previous study illustrated that AsA reduces the severity of Alternaria brassicicola infection in Arabidopsis [17]. Moreover, they reported that vtc1 and vtc2 mutant Arabidopsis plants were highly sensitive to this pathogen due to a lower AsA level, which in turn causes increased cell damage. Accumulation of ascorbic acid provides a defense response to turnip mosaic virus (TMV) in resistant Brassica rapa [18]. The eggplant mottled dwarf virus affects ascorbic acid quality, plant development, and fruit ripening stages in Solanum lycopersicon [19]. Mohammed et al. noticed an inverse correlation between the rate of P. infestans infection and the antioxidant level in tomato (Lycopersicon esculentum Mill.) [20]. Previous studies demonstrated the better performance of D-galacturonic acid reductase (GalUR) transgenic plants under various abiotic stresses [21]. The aforementioned previous findings showed the positive effects of AsA in disease resistance against several plant pathogens. Hence, to determine their performance under biotic stress conditions, we subjected the control and transgenic potato plants to the P. infestans infection, which is the most common pathogen in potato. We observed a reduction in disease severity in the GalUR transgenic potato plants against the potato late blight disease. The molecular analysis of genes related to the pathogenesis resistance (PR) and hormone biosynthesis revealed their altered gene expression in response to the P. infestans attack. Furthermore, transgenic potato plants with enhanced hydrogen peroxide scavenging activity minimizes the foliar damage caused by the P. infestans infection.
2. Materials and Methods
2.1. Plant Material
Transgenic GalUR potato (Solanum tuberosum L. cv. Taedong Valley) tubers over accumulating AsA (Ascorbate) were kindly provided [21,22]. The tubers were sprouted and grown to maturity in the glasshouse conditions. The single-node cuttings from the transgenic potato were cultivated in culture tubes (25 × 150 mm) on Murashige and Skoog (MS) basal medium [23] with 3% (w/v) sucrose. The subcultured shoots were kept in a plant growth chamber (22 ± 2 °C, light/dark cycle of 16 h/8 h). Two months-old in vitro grown GalUR transgenic and untransformed (UT) control plants (15 sets with three replicates) were transplanted into the soil and further maintained in the glasshouse, and were used for all experiments.
2.2. Phytophthora infestans Disease Resistance Studies
P. infestans strain (KACC 40718) acquired from the Korean Agricultural Culture Collection (KACC), Rural Development Administration (RDA), South Korea was routinely subcultured in the rye-agar medium with sucrose (2%) and incubated at 18 ± 1 °C in the dark. The zoospores from 14 day-old cultures were isolated by flooding the culture plate with cold sterile distilled water followed by incubation at 4 °C for 2 h. The solution containing zoospores was removed from the Petri dish and kept on ice until used for potato leaf inoculation. Two months-old in vitro-grown GalUR transgenic and UT control potato plants (15 sets of GalUR transgenic and UT control plants with three replicates) were used for the challenging study. The fully developed leaves from the top third to fifth were detached and inoculated with 10 µL spots (1 × 105 zoospores mL−1 concentration) of freshly isolated zoospores suspension on to the abaxial surface. Then the inoculated leaves were kept in water-saturated filter paper in the sterile Petri dishes. Parallelly, the well-developed leaves third from the top of the intact plants were inoculated with 10 µL of zoospore suspension. The inoculated plants were incubated at 18 ± 1 °C with a 16 h/8 h photoperiod with 95–98% relative humidity (RH) for one day and subsequently maintained at 70% RH. The development of disease and necrosis was examined carefully every day after pathogen inoculation.
2.3. Histological Studies of Infected Leaves
Leaf discs from the infected leaves were stained with lactophenol–trypan blue staining, as described previously [24]. Stained specimens were examined under light microscopy for P. infestans infection.
2.4. Evaluation of Disease Severity
The scoring values for the disease severity in the P. infestans infected leaves of GalUR and UT control potato plants were given based on the previous study [25]. The infected plants were monitored after challenging with the pathogen. The disease severity index (DSI) scoring values (0–5) for the estimation of disease severity were assigned as follows: 0—no visible symptoms or lesions, 1—scarce visible lesions, 2—small lesions with 1% discoloration of leaves, 3—discoloration of leaves up to 10% with increased lesion size, 4—increased leaf discoloration (25%), 5—increased leaf discoloration (>25%), and severe damage. Each treatment had six replicates, and the experiment was repeated twice. The data are presented as means ± standard deviation of n = 6. The data were analyzed using the Kruskall–Wallis non-parametric test at p = 0.002.
2.5. Gene Expression Studies in Infected Samples
Total RNA was extracted from the P. infestans challenged leaf samples of UT control, as well as GalUR transgenic plants using Tri-reagent (Sigma Aldrich) followed by treatment with DNase I. First-strand cDNA synthesis, was carried out using 2 µg of total RNA, oligo dT, and Super Script-II Reverse Transcriptase (Invitrogen). Quantitative real-time polymerase chain reaction (qRT-PCR) analysis was performed using the SYBR PCR kit (Bio-Rad, Hercules) in the CFX 96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules). The PCR reaction conditions are as follows; 95 °C for 10 s, then 38 cycles of 95 °C for 5 s, annealing temperature for 15 s, and 72 °C for 30 s. The primers used for the analysis of the transcripts variation are listed in Table 1. The gene expression variation for PR1 (pathogenesis-related protein 1) and antimicrobial producing PAL (phenylalanine ammonia-lyase), antioxidative genes such as APX (ascorbate peroxidase), GR (glutathione reductase), DHAR (dehydroascorbate reductase), CAT (catalase), SOD (superoxide dismutase), NCED1 (9-cis-epoxycarotenoid dioxygenase 1), and NCED2 (both the NCED1 and NCED2 involved in the catalysis of ABA precursor zeaxanthin biosynthesis), GA20OX1 (involved in the catalysis of GA biosynthesis) were analyzed. The relative gene expressions obtained by the comparative cycles of threshold (Ct) values of GalUR transgenic samples were normalized with the Ct values of the control (WT) plant samples, and the relative gene expression was calculated by using the 2−∆∆Ct method [26]. The experiment was carried out separately in triplicates and was expressed as the mean ± standard deviation (SD). One-way ANOVA analysis followed by Duncan’s test was used to determine significant differences at p ≤ 0.05 significance level.
Table 1.
List of primers used in the study.
| NCBI Accession Number | Primer Name | Sequence (5′–3′) |
|---|---|---|
| X55749 | Actin | F: CTGGTGGTGCAACAACCTTA |
| R: GAATGGAAGCAGCTGGAATC | ||
| AB041343 | APx | F: ACCAATTGGCTGGTGTTGTT |
| R: TCACAAACACGTCCCTCAAA | ||
| AY442179 | CAT | F: TGCCCTTCTATTGTGGTTCC |
| R: GATGAGCACACTTTGGAGGA | ||
| AF354748 | SOD | F: GTTTGTGGCACCATCCTCTT |
| R: GTGGTCCTGTTGACATGCAG | ||
| X76533 | GR | F: GGATCCTCATACGGTGGATG |
| R: TTAGGCTTCGTTGGCAAATC | ||
| DQ512964 | DHAR | F: AGGTGAACCCAGAAGGGAAA |
| R: TATTTTCGAGCCCACAGAGG | ||
| AJ250136 | PR1 | F: GCATCCCGAGCACAAAATTA |
| R: GAAATCACCACTTCCCTTGG | ||
| X63103 | PAL1 | F: TTGCACAAGTTGCATCCATT |
| R: CACCAGCTCTTGCACTTTCA | ||
| AJ291453 | GA20OX1 | F: CAAGATTGTGTTGGCGGACT |
| R: ACTGCTCTGTGCAGGCAACT | ||
| AY662342 | StNCED1 | F: GGAAATCAACAAGAAAAGCCA |
| R: ATATTTGTTGTCACCATAAATGAA | ||
| AY662343 | StNCED2 | F: GGGACTTTCATTAGCTCAAAGGACTTGC |
| R: GCGATGTAAATTTGAATTACTATTATTCGCTCA |
2.6. Estimation of H2O2 and MDA
The quantification of H2O2 content in UT control and GalUR transgenic plant leaf tissues challenged with P. infestans was carried out using a method, as described previously [27]. The MDA content in the UT control and transgenic leaf tissues were determined, as described earlier [28]. The experiments were carried out separately in triplicates and were expressed as the mean ± standard deviation (SD). One-way ANOVA analysis followed by the Duncan’s test was used to determine significant differences at p ≤ 0.05 significance level.
2.7. Estimation of the Plant Hormones GA and ABA
The plant hormones, GA and the ABA were estimated using the protocol as published by Tang et al. [29]. All the experiments were conducted with waters alliance E-2695 Chromatographic station and direct ultraviolet–visible (UV) absorbance detection unit. The HPLC system consisted of C18 reverse-phase column (150 mm × 4.6 mm, 5 μm) with a methanol gradient in 0.6% acetic acid and UV detector set at 254 nm. Standard ABA and GA3 were purchased from Sigma (USA). Three plant sets from three groups were used for the phytohormone analysis. The leaf tissues (100 mg fresh mass) of UT control, GalUR transgenic, and GalUR transgenic plants challenged with P. infestans were ground in liquid nitrogen, homogenized, and then extracted overnight with 30 mL of 80% cold aqueous methanol (<0 °C) in darkness at 4 °C following the standard protocol. The stock solutions of the hormones were prepared at 1.0 mg/mL by dissolving accurately weighed amounts of each compound in methanol (MeOH) and stored at 4 °C. The experiments were carried out separately in triplicates and were expressed as the mean ± standard deviation (SD). One-way ANOVA analysis followed by Duncan’s test was used to determine significant differences at p ≤ 0.05 significance level.
2.8. Statistical Analysis
The experiments include real-time gene expression, HPLC, and biochemical estimations (H2O2 and MDA) which were done separately in triplicates and are expressed as the mean ± standard deviation (SD). One-way ANOVA analysis followed by Duncan’s test was used to determine significant differences at p ≤ 0.05 significance level. Disease severity index data were analyzed using the Kruskal–Wallis non-parametric test at p = 0.002. The statistical analyses were performed separately using the SPSS package program version 25 (SPSS, Chicago, IL, USA).
3. Results
3.1. GalUR Transgenics with Reduced Necrotic Damage to Fungal Pathogen Infection
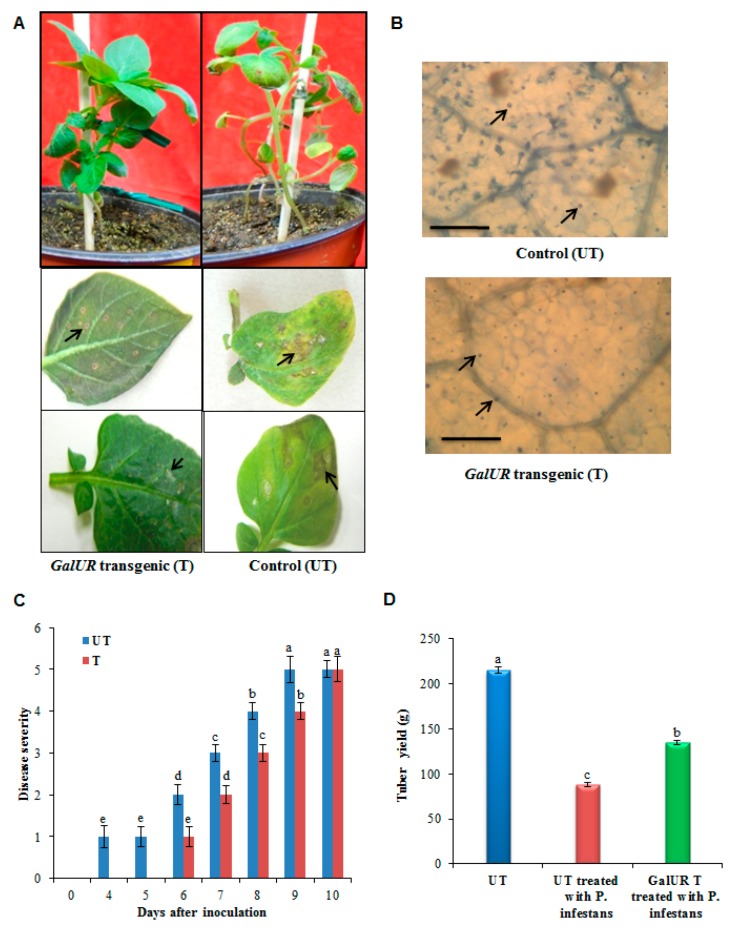
In the present investigation, both transgenic and the UT control plants showed small but visible lesions after four to five days of pathogen inoculation, indicating the successful pathogen infection (Figure 1A). Microscopic visualization revealed the pathogen-induced cell death in the infected leaf tissues. The occurrence of pathogen-induced cell death was significantly higher in the UT control potato leaves compared to the GalUR transgenic potato leaves (Figure 1B). The disease symptom also appeared quicker in UT control plants as compared to the transgenic plants, probably due to the high antioxidant activity observed in the GalUR plants. The lesion size progressively expanded until it became necrotic in the UT control plants, whereas, the infected leaves of thetransgenic plants exhibited reduced lesions and necrosis at the initial stages of pathogenesis (Figure 1A). This indicates that the increased antioxidant potential of GalUR expressing transgenic potato plants to the symptoms caused by P. infestans. Furthermore, the disease severity assessment showed that disease incidence started earlier in the control plants (UT) compared to GalUR transgenic plants. The progressive reduction of necrotic lesions was observed at earlier stages in the GalUR transgenic plants (Figure 1C). Both the UT control and transgenic plants could not diminish the occurrence of the disease; however, a delayed disease progression was observed in the case of the transgenic potato with enhanced L-AsA accumulation (Figure 1C). The tubers were also harvested from both the plants; however, the UT control potato suffered a huge loss of tubers than the transgenic potato (P ≤ 0.05). The untransformed control potato (cultivars Taedong Valley) yielded 215 grams of tubers under the control condition, while the diseased plant yielded 88.2 grams of tubers of smaller size. In contrast, the transgenic potato also suffered yield penalty, which yielded 135 grams tubers which were normal in size (Figure 1D).
Figure 1.
Pathogen infection in control and GalUR potato challenged with P. infestans. (A) GalUR transgenic potato plants showing restricted lesions and necrosis development (arrows) compared to control plants, (B) Microscopic appearances of P. infestans infected potato leaves stained with lactophenol-Trypan blue. The numbers of dead cells were seen in control plants while few dead cells were present in the GalUR potato transgenics after P. infestans infection. Bars = 200 µm, (C) Disease severity in potato plants. Disease range of damage caused by P. infestans after inoculation of leaves of potato plants. The disease severity index (DSI) scoring values (0–5) for the estimation of disease severity were assigned as follows: 0—no visible symptoms or lesions, 1 —scarce visible lesions, 2—small lesions with 1% discoloration of leaves, 3—discoloration of leaves up to 10% with increased lesion size, 4—increased leaf discoloration (25%), 5—increased leaf discoloration (>25%), and severe damage. Each bar represents a means ± standard deviation of n = 6. Different letters indicate a significant difference in disease severity according to a Kruskal–Wallis non-parametric test at p = 0.002, (D) Tubers yield in the untransformed (UT) control and GalUR transgenic potato challenged with P. infestans and UT control plants and the data are presented as means ± SD of three replicates. Different letters indicate a significant difference at p ≤ 0.05.
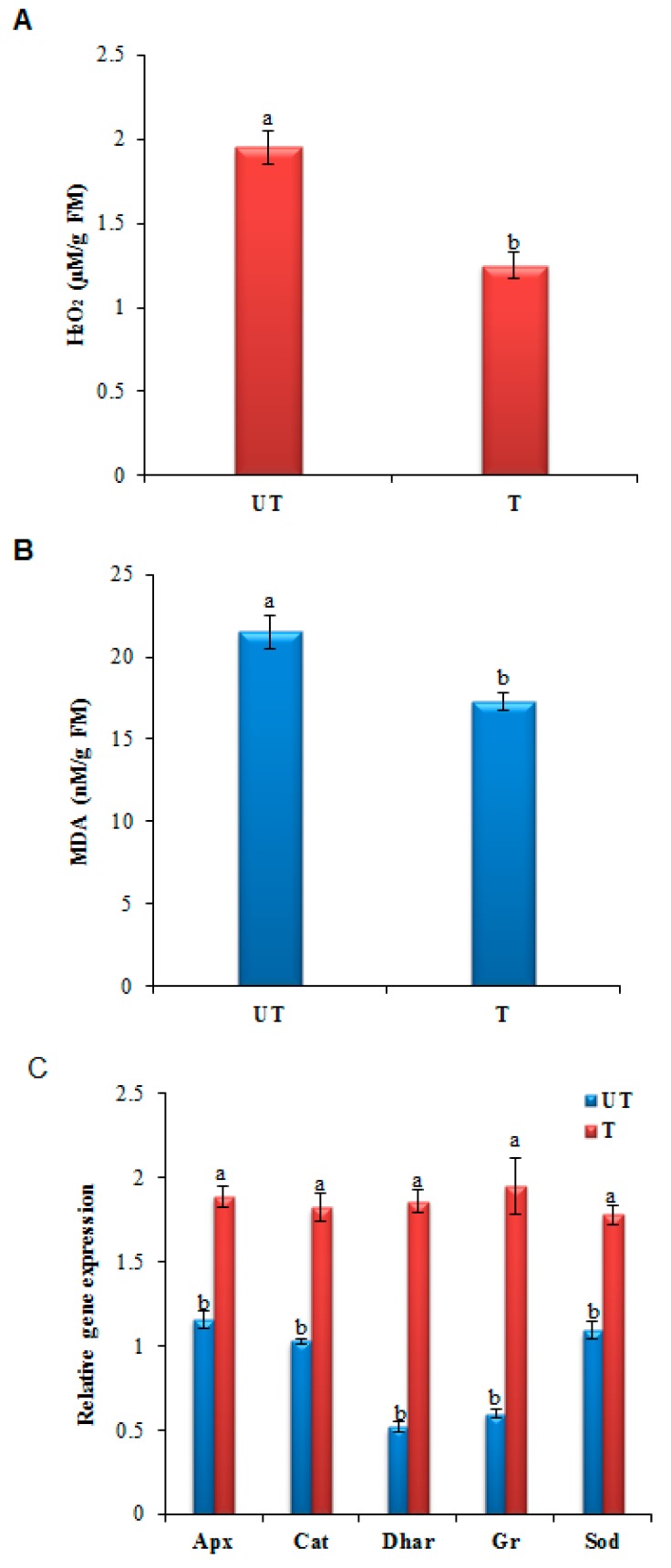
3.2. Reduced Levels of H2O2 and MDA and Enhanced Antioxidant Gene Expression in GalUR-Transgenic Potato Plants
H2O2 is one of the major ROS molecules produced in plants in response to pathogen attack and its concentration was probably below the threshold in the GalUR transgenic potato lines (Figure 2A) due to its removal via the enhanced activity of the cellular antioxidants enzymes and AsA level, which probably protected the plant cells from further cellular damage. The level of MDA recognized as an indicator of lipid peroxidation. The MDA level was lower in GalUR transgenic plants than control plants (Figure 2B). The lower H2O2 and MDA content in transgenic plants compared to the UT control plants (p ≤ 0.05) indicated the enhanced ROS-scavenging activity via the maintenance of higher cellular AsA and the antioxidant enzymes, as evident in the gene expression analysis of the antioxidative genes such as APX (ascorbate peroxidase), GR (glutathione reductase), DHAR (dehydroascorbate reductase), CAT, and SOD (Figure 2C). In addition, the ROS scavenging activity is also associated with the reduced necrotic lesions observed in GalUR transgenics.
Figure 2.
Histograms showing estimated (A) H2O2, (B) MDA content in P. infestans infected GalUR transgenic (T) and untransformed control (UT) potato leaves, (C) genes encoding reactive oxygen species (ROS)-scavenging enzymes, ascorbate peroxidase (APx), catalase (CAT), dehydroascorbate reductase (DHAR), glutathione reductase (GR), and superoxide dismutase (SOD) in P. infestans infected GalUR transgenic (T) and untransformed control (UT) potato plants. Data are presented as means ± SD of three replicates. Different letters indicate a significant difference at p ≤ 0.05.
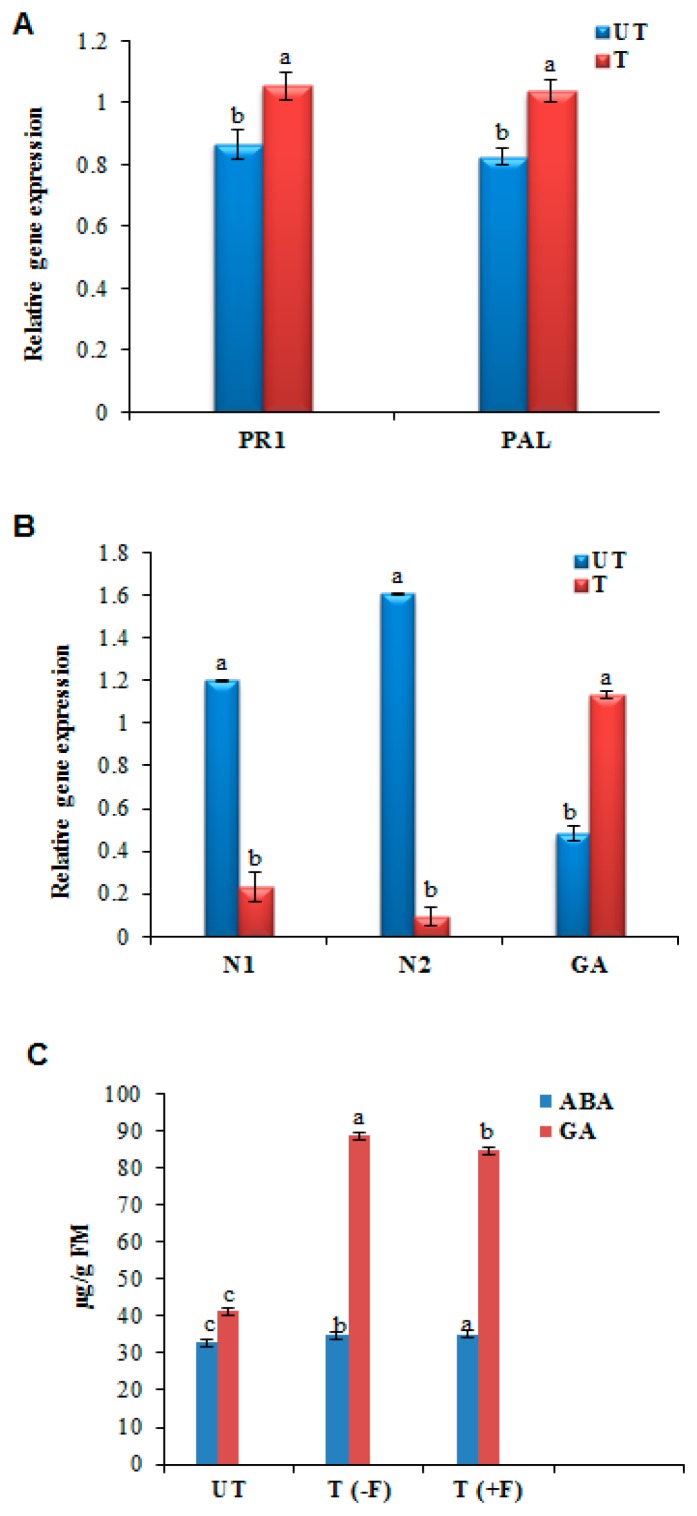
3.3. Altered Expression of PR, Phytohomone Genes, and Estimation of Phytohormones Using HPLC
Gene expression profiles of PR1 and antimicrobial producing PAL (phenylalanine ammonia-lyase) gene was analyzed and the results indicated that the transcripts of PR1 and PAL gene were slightly higher in the GalUR transgenics compared to UT control plants (p ≤ 0.05) (Figure 3A). Since it is known that AsA acts as a cofactor for several phytohormone biosyntheses and they play a certain role in plant immunity, we performed qRT-PCR analysis of genes that are intricate in the regulation of ABA and GA biosyntheses to monitor their transcript levels upon pathogen induction. GA20OX1 expression was higher in the GalUR transgenic and lower in the control plants, whereas the reversible results were observed in the NCED1, NCED2 expression (p ≤ 0.05) (Figure 3B). The chromatographic estimation also showed the presence of the ABA and the GA hormones in the UT control and the GalUR transgenic potato. However, the GA content was more in the case of the GalUR transgenic plants than the UT control, which was 2.3 times more in the GalUR transgenic plants (p ≤ 0.05) (Figure 3C). This may be due to the enhanced level of the AsA in the GalUR transgenic plant, which is a negative regulator of the ABA and a positive regulator of the GA, and hence promotes GA biosynthesis. The gene expression data also showed the same result.
Figure 3.
Quantitative Real-Time gene expression analysis of (A) Pathogenesis related (PR) genes, PR-1 and PAL (phenylalanine ammonia-lyase), (B) Abscisic acid (ABA) biosynthesis pathway genes, N1 (9-cis-epoxycarotenoid dioxygenase 1), N2 (9-cis-epoxycarotenoid dioxygenase 2), and GA biosynthesis pathway gene (Gibberellic acid 20 oxidase), and (C) Estimation of phytohormones such as ABA and GA hormone in the in control (UT) and GalUR transgenic (T) transgenic potato. T (-F) denotes the transgenic plant without P. infestans, and T (+F) denotes the plant infected with the fungal strain. Data are presented as means ± SD of three replicates. Different letters indicate a significant difference at p ≤ 0.05.
4. Discussion
In the current investigation, we planned to study the putative role of AsA in disease tolerance against P. infestans using transgenic potato plants overexpressing GalUR gene. These transgenic potato plants overproduce 1.6–2-fold AsA and exhibit tolerance to various abiotic stresses due to the enhanced ROS-scavenging activity [30]. AsA plays a vital role in detoxification of H2O2 and MDA, which is one of the major ROS molecules produced under pathogen attack in plants to induce hypersensitive response (HR). HR acts as a positive signal to transmit this locally induced resistance at the systemic level. However, the higher H2O2 and MDA concentration should be detoxified by antioxidative systems before it acts as a potent toxic to plants. Here, we report the GalUR transgenic plants with reduced H2O2 and MDA content due to the efficient detoxification by L-ascorbate.
The efficient ROS-scavenging activity of GalUR transgenics was evident from the upregulated transcripts of various antioxidative enzymes such as SOD, GR, DHAR, APX, and CAT compared to control plants (p ≤ 0.05) indicating the importance of antioxidative enzymes and their promising role in the detoxification of ROS (Figure 3C). Sarowar et al. revealed that the tobacco plants overexpressing Capsicum annuum ascorbate peroxidase-like-1 gene (CAPOA1) showed enhanced ROS-scavenging activity due to its two-fold higher peroxidase transcript level and provided increased resistance to Phytophthora nicotianae [31]. Similarly, Zhu et al. suggested that the induction of antioxidant genes, such as DHAR and GR genes were involved in the alleviation of the RNA virus induced disease symptoms [32]. Consistent with our study, we observed the high transcript abundance of peroxidase (APX), DHAR, and GR genes in GalUR transgenics, which directly corresponds to the reduction in disease symptoms. These GalUR transgenics showed reduced necrotic spots compared to the control leaf samples due to the enhanced active oxygen scavenging system. Microscopic visualization analysis showed that pathogen-induced cell death was higher in control plants than GalUR transgenic leaves. Moreover, the lesion formation and its proliferation were delayed in the GalUR transgenic plants. These results clearly indicate the potential scavenging activity of AsA on pathogen-induced excessive ROS molecules. The present observation also overlaps with the previous study, which stated that the exogenous application of AsA reduces the severity of blight diseases and the oomycetes spore count [33]. Another recent study also demonstrated that the AsA treatment at a higher concentration in the initial stage protects plants through effective alleviation of disease symptoms, as well as the inhibition of viral replication [34]. However, in the current investigation, GalUR transgenic plants showed reduced disease severity at the initial stages but did not control the disease.
It has been suggested that PR genes such as PR1, PR5, and PAL accumulate after pathogen attack and play a positive role in conferring resistance against P. infestans [35]. However, in the present study, a slightly higher expression of PR1 and PAL gene transcripts was observed in the non-transgenic plants than the GalUR transgenics perhaps due to the higher H2O2 content in the control potato plants (P≤0.05). AsA acts as a cofactor for the enzymes involved in ABA and GA biosynthesis pathway, it can influence these hormone levels and their signaling pathways. It was reported that the high AsA level downregulates ABA biosynthesis and promotes GA biosynthesis [36]. In accordance with these results, upregulation of GA20Ox1 transcripts and downregulation of NCED1 and NCED2 transcripts were found in the GalUR potato transgenics (P≤0.05). The GA and ABA act as a positive signal for the biotic stress resistance, which was proved in various crops [37,38]. Mohr and Cahill suggested that the pretreatment of the ABA significantly decreased the production of salicylic acid and the lignification’s process in Arabidopsis infected with the Pseudomonas syringae pv. tomato [39]. However, emerging evidence suggests that GA signaling components play major roles in plant disease resistance and susceptibility. Recently, it has been found that Arabidopsis DELLA (aspartic acid–glutamic acid–leucine–leucine–alanine) proteins, which act as negative regulators of GA signaling, control plant immune responses by modulating salicylic acid (SA) and jasmonic acid (JA) dependent defense responses [40]. Moreover, it has been shown that DELLA proteins promote the expression of genes encoding ROS detoxification enzymes, thereby regulating the levels of ROS after biotic or abiotic stress [41]. In consistence with this, the transgenic potato showed lesser necrotic lesions due to the ROS removal via maintenance of the higher L-ascorbate, higher antioxidant enzymes, and enhanced GA level, which not only removed the H2O2 or other ROS, but the disease resistance and susceptibility against the fungus due to GA signaling pathways. It has also been demonstrated that gid1 mutant of rice, defective in GA receptors, accumulates higher GA levels, and shows enhanced resistance to the blast fungus Magnaporthe grisea compared to wild type plants [42]. Consistent with these reports, we observed the higher GA and lower ABA levels in the GalUR transgenic plants might also be the responsible factor for the delayed disease progression in GalUR transgenic plants. The strong ROS detoxification mechanism found in GalUR transgenics reduces the cell death and necrotic symptoms, which in turn reduce the severity of the disease.
5. Conclusions
GalUR transgenic potato plants overproducing AsA with potent antioxidant capacity showed decreased necrotic symptoms and reduced disease lesions in the initial stages induced by P. infestans challenge. These plants exhibited enhanced active oxygen scavenging activity via the increased accumulation of antioxidative enzymes that lead to the efficient detoxification of H2O2, which could otherwise cause severe necrotic death of disease-infected leaves. These plants showed altered hormone biosynthesis, such as the upregulation of the GA and downregulation of ABA. Further, the defense-related PR1 and PAL gene expression was slightly lower in GalUR transgenic plants as compared to control plants. Our findings suggest that the decreased necrotic cell death associated with the reduction in foliar tissue damage was induced through the efficient antioxidant system of the GalUR transgenic potato plants, although it does not essentially control the pathogen growth and disease. Further characterization of these plants is warranted to understand the molecular basis of biotic stress tolerance in plants that accumulate higher levels of AsA.
Author Contributions
Methodology, Validation and Formal Analysis—I.-M.C.; Investigation, Methodology, Formal Analysis—B.V.; Methodology, Validation, and Formal Analysis—C.P.U.; Formal analysis— G.P.; Formal Analysis—G.R.; Methodology, Formal Analysis, Validation, Writing—Original Draft Preparation—M.T.
Funding
This paper was supported by the KU Research Professor Program of Konkuk University, Seoul, South Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zhang H., Xu F., Wu Y., Hu H.-H., Dai X.-F. Progress of potato staple food research and industry development in China. J. Integr. Agric. 2017;16:2924–2932. doi: 10.1016/S2095-3119(17)61736-2. [DOI] [Google Scholar]
- 2.Dahal K., Li X.-Q., Tai H., Creelman A., Bizimungu B. Improving potato stress tolerance and tuber yield under a climate change scenario—A current overview. Front. Plant Sci. 2019;10:563. doi: 10.3389/fpls.2019.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zurbriggen M.D., Carrillo N., Hajirezaei M.R. ROS signaling in the hypersensitive response: When, where and what for? Plant Signal. Behav. 2010;5:393–396. doi: 10.4161/psb.5.4.10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Breusegem F., Dat J.F. Reactive oxygen species in plant cell death1. Plant Physiol. 2006;141:384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asada K. THE water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Boil. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. 3rd ed. Oxford University Press; London, UK: 1999. [Google Scholar]
- 7.Lim B., Smirnoff N., Cobbett C.S., Golz J.F. Ascorbate-deficient vtc2 mutants in Arabidopsis do not exhibit decreased growth. Front. Plant Sci. 2016;7:1025. doi: 10.3389/fpls.2016.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akram N.A., Shafiq F., Ashraf M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017;8:613. doi: 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokunaga T., Miyahara K., Tabata K., Esaka M. Generation and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA for L-galactono-1, 4-lactone dehydrogenase. Planta. 2005;220:854–863. doi: 10.1007/s00425-004-1406-3. [DOI] [PubMed] [Google Scholar]
- 10.Locato V., Cimini S., De Gara L. Strategies to increase vitamin C in plants: From plant defense perspective to food biofortification. Front. Plant Sci. 2013;4:152. doi: 10.3389/fpls.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskling M., Arvidsson P.-O., Åkerlund H.-E. The xanthophyll cycle, its regulation and components. Physiol. Plant. 1997;100:806–816. doi: 10.1111/j.1399-3054.1997.tb00007.x. [DOI] [Google Scholar]
- 12.Davey M.W., Van Montagu M., Inzé D., Sanmartin M., Kanellis A., Smirnoff N., Benzie I.J.J., Strain J.J., Favell D., Fletcher J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000;80:825–860. doi: 10.1002/(SICI)1097-0010(20000515)80:7<825::AID-JSFA598>3.0.CO;2-6. [DOI] [Google Scholar]
- 13.Carr A.C., Maggini S. Vitamin C and Immune Function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallie D.R. L-ascorbic Acid: A multifunctional molecule supporting plant growth and development. Scientifica. 2013;2013:795964. doi: 10.1155/2013/795964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero-Romero M.T., López-Delgado H.A. Ameliorative effects of hydrogen peroxide, ascorbate and dehydroascorbate in Solanum tuberosum infected by Phytoplasma. Am. J. Potato Res. 2009;86:218–226. doi: 10.1007/s12230-009-9075-1. [DOI] [Google Scholar]
- 16.Smirnoff N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free. Radic. Biol. Med. 2018;122:116–129. doi: 10.1016/j.freeradbiomed.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botanga C.J., Bethke G., Chen Z., Gallie D.R., Fiehn O., Glazebrook J. Metabolite profiling of Arabidopsis inoculated with Alternaria brassicicola reveals that ascorbate reduces disease severity. Mol. Plant-Microbe Interact. 2012;25:1628–1638. doi: 10.1094/MPMI-07-12-0179-R. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara A., Togawa S., Hikawa T., Matsuura H., Masuta C., Inukai T. Ascorbic acid accumulates as a defense response to Turnip mosaic virus in resistant Brassica rapa cultivars. J. Exp. Bot. 2016;67:4391–4402. doi: 10.1093/jxb/erw223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarrillo A., Minutolo M., Alioto D., Errico A. Ascorbic acid regulation in leaves and fruits of tomato ecotypes infected by Eggplant mottled dwarf virus. Sci. Hortic. 2017;225:512–524. doi: 10.1016/j.scienta.2017.07.043. [DOI] [Google Scholar]
- 20.Mohammed A.E., Smit I., Pawelzik E., Keutgen A.J., Horneburg B. Organically grown tomato (Lycopersicon esculentum Mill.): Bioactive compounds in the fruit and infection with Phytophthora infestans. J. Sci. Food Agric. 2012;92:1424–1431. doi: 10.1002/jsfa.4720. [DOI] [PubMed] [Google Scholar]
- 21.Upadhyaya C.P., Akula N., Kim H.S., Jeon J.H., Ho O.M., Chun S.C., Kim D.H., Park S.W. Biochemical analysis of enhanced tolerance in transgenic potato plants overexpressing d-galacturonic acid reductase gene in response to various abiotic stresses. Mol. Breed. 2011;28:105–115. [Google Scholar]
- 22.Upadhyaya C.P., Young K.E., Akula N., soon Kim H., Heung J.J., Oh O.M., Aswath C.R., Chun S.C., Kim D.H., Park S.W. Over-expression of strawberry d-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci. 2009;177:659–667. [Google Scholar]
- 23.Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 24.Roetschi A., Si-Ammour A., Mauch-Mani B., Belbahri L., Mauch F. Characterization of an Arabidopsis-Phytophthora Pathosystem: Resistance requires a functional PAD2 gene and is independent of salicylic acid, ethylene and jasmonic acid signalling. Plant J. 2001;28:293–305. doi: 10.1046/j.1365-313X.2001.01148.x. [DOI] [PubMed] [Google Scholar]
- 25.Alejo-Iturvide F., Marquez-Lucio M.A., Morales-Ramirez I., Vazquez-Garciduenas M.S., Olalde-Portugal V. Mycorrhizal protection of chili plants challenged by Phytophthora capsici. Eur. J. Plant Pathol. 2008;120:13–20. doi: 10.1007/s10658-007-9188-7. [DOI] [Google Scholar]
- 26.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Chung I.-M., Venkidasamy B., Thiruvengadam M. Nickel oxide nanoparticles cause substantial physiological, phytochemical, and molecular-level changes in Chinese cabbage seedlings. Plant Physiol. Biochem. 2019;139:92–101. doi: 10.1016/j.plaphy.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Chung I.M., Rekha K., Rajakumar G., Thiruvengadam M. Elicitation of silver nanoparticles enhanced the secondary metabolites and pharmacological activities in cell suspension cultures of bitter gourd. 3 Biotech. 2018;8:412. doi: 10.1007/s13205-018-1439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Y., Wang L., Ma C., Liu J., Liu B., Li H. The Use of HPLC in determination of endogenous hormones in anthers of bitter melon. J. Life Sci. 2011;5:139–142. [Google Scholar]
- 30.Gururani M.A., Upadhyaya C.P., Baskar V., Venkatesh J., Nookaraju A., Park S.W. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J. Plant Growth Regul. 2012;32:245–258. doi: 10.1007/s00344-012-9292-6. [DOI] [Google Scholar]
- 31.Sarowar S., Kim E.N., Kim Y.J., Ok S.H., Kim K.D., Hwang B.K., Shin J.S. Overexpression of a pepper ascorbate peroxidase-like 1 gene in tobacco plants enhances tolerance to oxidative stress and pathogens. Plant Sci. 2005;169:55–63. doi: 10.1016/j.plantsci.2005.02.025. [DOI] [Google Scholar]
- 32.Zhu F., Yuan S., Wang S.-D., Xi D.-H., Lin H.-H. The higher expression levels of dehydroascorbate reductase and glutathione reductase in salicylic acid-deficient plants may contribute to their alleviated symptom infected with RNA viruses. Plant Signal. Behav. 2011;6:1402–1404. doi: 10.4161/psb.6.9.16538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haggag W.M., El-Khair H.A. Application of some natural compounds for management of potato late and early blights. J. Food Agric. Environ. 2007;5:157–163. [Google Scholar]
- 34.Wang S.-D., Zhu F., Yuan S., Yang H., Xu F., Shang J., Xu M.-Y., Jia S.-D., Zhang Z.-W., Wang J.-H., et al. The roles of ascorbic acid and glutathione in symptom alleviation to SA-deficient plants infected with RNA viruses. Planta. 2011;234:171–181. doi: 10.1007/s00425-011-1391-2. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Hadrami A.E.I., Adam L.R., Daayf F. Differential activation and suppression of potato defence responses by Phytophthora infestans isolates representing US-1 and US-8 genotypes. Plant Pathol. 2008;57:1026–1037. doi: 10.1111/j.1365-3059.2008.01866.x. [DOI] [Google Scholar]
- 36.Barth C., De Tullio M., Conklin P.L. The role of ascorbic acid in the control of flowering time and the onset of senescence. J. Exp. Bot. 2006;57:1657–1665. doi: 10.1093/jxb/erj198. [DOI] [PubMed] [Google Scholar]
- 37.Agrawal G.K., Rakwal R., Jwa N.S., Agrawal V.P. Signaling molecules and blast pathogen attack activates rice OsPR1a and OsPR1b genes: A model illustrating components participating during defense/stress response. Plant Physiol. Biochem. 2001;39:1095–1103. doi: 10.1016/S0981-9428(01)01333-X. [DOI] [Google Scholar]
- 38.Yu H.J., Mun J.H., Kwon Y.M., Lee J.S., Kim S.G. Two cDNAs encoding pathogenesis-related proteins of Lithospermum erythrorhizon display different expression patterns in suspension cultures. J. Plant Physiol. 1999;155:364–370. doi: 10.1016/S0176-1617(99)80118-7. [DOI] [Google Scholar]
- 39.Mohr P.G., Cahill D.M. Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes, in Arabidopsis infected with Pseudomonas syringae pv. tomato. Funct. Integr. Genom. 2007;7:181–191. doi: 10.1007/s10142-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 40.Navarro L. A Plant miRNA Contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;312:436–439. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- 41.Achard P., Renou J.-P., Berthomé R., Harberd N.P., Genschik P. Plant DELLAs Restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Boil. 2008;18:656–660. doi: 10.1016/j.cub.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka N., Matsuoka M., Kitano H., Asano T., Kaku H., Komatsu S. gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein (PBZ1) in response to cold stress and pathogen attack. Plant Cell Environ. 2006;29:619–631. doi: 10.1111/j.1365-3040.2005.01441.x. [DOI] [PubMed] [Google Scholar]