Abstract
Hepatocellular carcinoma is the most common primary liver cancer and the fourth leading cause of cancer death worldwide. A total of 70–80% of patients are diagnosed at an advanced stage with a dismal prognosis. Sorafenib had been the standard care for almost a decade until 2018 when the Food and Drug Administration approved an alternative first-line agent namely lenvatinib. Cabozantinib, regorafenib, and ramucirumab also displayed promising results in second line settings. FOLFOX4, however, results in an alternative first-line treatment for the Chinese clinical oncology guidelines. Moreover, nivolumab and pembrolizumab, two therapeutics against the Programmed death (PD)-ligand 1 (PD-L1)/PD1 axis have been recently approved for subsequent-line therapy. However, similar to other solid tumors, the response rate of single agent targeting PD-L1/PD1 axis is low. Therefore, a lot of combinatory approaches are under investigation, including the combination of different immune checkpoint inhibitors (ICIs), the addition of ICIs after resection or during loco-regional therapy, ICIs in addition to kinase inhibitors, anti-angiogenic therapeutics, and others. This review focuses on the use of ICIs for the hepatocellular carcinoma with a careful assessment of new ICIs-based combinatory approaches.
Keywords: hepatocellular carcinoma, immune checkpoint inhibitors, HCC, pembrolizumab, nivolumab, immune microenvironment, targeted therapies
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the fourth leading cause of cancer death worldwide as stated in reports as of 2018. Chronic hepatitis C virus (HCV) or chronic hepatitis B virus (HBV) infections, alcohol abuse, and non-alcoholic steatohepatitis are the main risk factors [1,2]. An association between type 2 diabetes mellitus and HCC has also been reported [3]. Treatment for the early stage includes hepatectomy, liver transplant, hepatic transarterial chemoembolization (TACE), and radiofrequency ablation (RFA).
Nevertheless, 70–80% of patients cannot benefit from such opportunities because they are diagnosed at an advanced stage and can receive only palliative care. Sorafenib has been the standard choice for a decade in advanced HCC, even if other Tyrosine Kinase Inhibitors (TKIs) as well [4] are characterized by possible primary resistance or acquired resistance [5]. Recently, lenvatinib showed similar results in terms of survival in a non-inferiority randomized trial study considering the same subset of patients [6]. Meanwhile, cabozantinib, regorafenib, and an anti-Vascular Endothelial Growth Factor Receptor (VEGFR) 2 molecules namely ramucirumab exhibited promising results in the second-line setting [7,8,9]. However, the response rate of TKIs in HCC remains low and new treatment approaches are needed. As known, cancers cells are able to evade immunosurveillance, promoting tumor growth and progression through the activation of different immune checkpoint pathways. Monoclonal antibodies targeting immune checkpoints have determined an enormous breakthrough in cancer therapeutics, promoting the immune-mediated elimination of tumor cells. Among these, Programmed death (PD)-ligand 1 (PD-L1)/PD1 and Cytotoxic T-Lymphocyte Antigen (CTLA)-4 inhibitors are actually used in several malignancies, whereas molecules able to disrupt other co-inhibitory signalling pathways are under investigation, such as T cell immunoglobulin and immune-receptor tyrosine-based inhibitory motif domain (TIGIT), Lymphocyte activation gene-3 (LAG3), and T cell immunoglobulin containing the mucin domain 3 (TIM-3). In the era of immunotherapy, immune checkpoint inhibitors (ICIs) have also been tested for HCC patients [10], in particular, nivolumab and pembrolizumab result in approved for second-line therapy. However, similar to other gastrointestinal malignancies, [11] the HCC response rate of ICIs as a monotherapy is low, therefore new combinatory approaches comprising TKIs, the addition of different ICIs, anti-angiogenic therapeutics, locoregional therapy, other kinase inhibitors, chemotherapy, and other drugs are currently under intensive investigations.
2. From Liver Immune System to HCC Immune Disorders
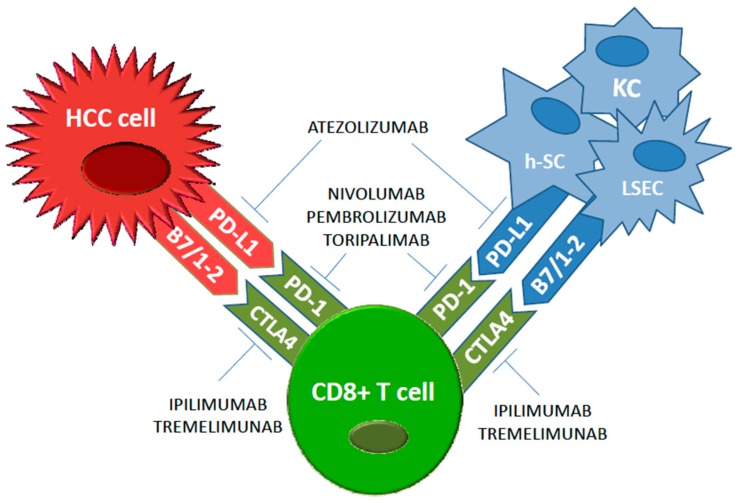
The liver is an organ with a specific blood supply that influences its immune microenvironment. In particular, 75% of the blood vascularises the liver through the portal vein that drains into smaller diameter structures called sinusoids. Therefore, a great number of antigens are in contact through the liver sinusoidal endothelial cells (LSECs) with the liver immune microenvironment, consisting in hepatic stellate cells (h-SCs) [12], Kupffer cells [13], fibroblasts [14], dendritic cells (DCs) [15], and lymphocytes [16]. The tumour microenvironment actively participates in drug-resistance acquisition in solid tumours [17,18,19]. Both lymphocytes and DCs present multiple subtypes with different pro-tumorigenic functions [20,21] including both the response to pathogenic non-self antigens and the tolerance to self-antigens. As far as immune-tolerance is concerned, the hepatic microenvironment shows a high expression of the hepatocyte growth factor and colony-stimulating factor 1, which promote a tolerigenic phenotype that is required to overcome autoimmune mechanisms due to antigenic hyperstimulation coming from the bowel [22]. In addition, HCV and HBV infections lead to frequent chronic inflammatory liver insult resulting in a deregulation of T cell activities with an increase of the expression of ICIs [23]. At the same time, HCC patients exhibit a more immunosuppressed microenvironment of the liver [24] with an increased number of regulatory T cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSC) which correlate with tumor progression and poor prognosis [25,26]. Given the tight correlation between the endothelial and epithelial function in immune response modulation against different cancer types [27,28,29], extensive investigation in HCC patients pinpoint LSECs as immune tolerance inducer of CD8-positive T cells to tumor-associated antigens, inducing T-regs, and also increase PD-L1 expression which correlate with recurrence after surgery and poor prognosis in advanced HCC (Figure 1) [30].
Figure 1.
Main immune checkpoints and relative immune checkpoint inhibitors in hepatocellular carcinoma (HCC).
3. Checkpoint Inhibitors
Efficacy and safety of ICIs have been evaluated in several trials in HCC (Table 1). In September 2017, based on the results of a phase I/II nonrandomized multi-institution study (CheckMate 040), the Food and Drug Administration (FDA) approved a full human immunoglobulin G4 monoclonal antibody direct to PD1, namely Nivolumab for HCC patients who progressed on or after sorafenib. The trial included 48 patients in a dose-escalation phase and 214 patients in a dose-expansion phase. Among the dose-escalation phase, nivolumab resulted in a disease control rate of 55% and an objective response rate of 10%, with a median OS (OS) of the entire cohort of 7.6 months that increase to 9.8 months in sorafenib-naïve patients [31]. CheckMate 459, a multi-centre, randomized, phase III trial assessed nivolumab compared with sorafenib as a first-line treatment in 1009 HCC patients, results are currently in process (NCT02576509) [32], nonetheless, a press release reported that the study did not achieve statistical significance for its primary endpoint of OS. More recently, the FDA granted accelerated approval for pembrolizumab, another antibody targeting PD-1, for patients with HCC previously treated with sorafenib. In the nonrandomized, open-label, phase II KEYNOTE-224 trial including 104 patients who progressed on or were intolerant to sorafenib, pembrolizumab demonstrated an objective response of 17% with one complete response and 17 partial response (PR), while 46 patients experienced a stable disease (SD). The median progression-free survival (PFS) was 4.9 months and the median OS was 12.9 months [33]. However, similar to the phase III CheckMate 459, the phase 3 Keynote-240 trial comparing pembrolizumab to a placebo in second-line HCC did not meet its coprimary endpoints of OS and PFS [34]. The monoclonal antibody anti-Cytotoxic T-Lymphocyte Antigen (CTLA) 4, tremelimumab was assessed in a phase II multi-center clinical trial including 20 patients with advanced HCC from hepatitis C viral aetiology. The infusion of tremelimumab at the dose of 15 mg/Kg IV every 90 days resulted in 18% of PR and a 60% of SDwith a PFS of 6.48 months (95% CI 3.95–9.14). Interestingly, a significant drop in the viral load was observed [35]. In addition, at the recent ESMO 2019, data concerning the use of ICIs in first-line treatment was examined. The CheckMate 459, a Phase 3 Study comparing Nivolumab (NIVO) vs. Sorafenib has shown amedian OS of 16.4 months for nivolumab and 14.7 months for SORAFENIB, with higher ORR and lower toxicities [36].
Table 1.
Clinical trials of immune checkpoint inhibitors in HCC.
| Treatments | Mediators | Design | Setting | Primary Outcomes (Finished/Ongoing) | Reference |
|---|---|---|---|---|---|
| NIVOLUMAB | Anti-PD1 | Dose-escalation Dose-expansion phase trial | Second line in sorafenib pretreated patients | DCR: 55%; ORR: 10%, mOS 9.8 months (finished) | 31 |
| NIVOLUMAB vs. SORAFENIB | Anti-PD1 | Phase III trial | First line treatment | mOS (ongoing, preliminary negative results) | 32 |
| PEMBROLIZUMAB | Anti-PD1 | Phase II trial | Second line in sorafenib pretreated patients | ORR: 17%; mPFS: 4.9 months; mOS: 12.9 months (finished) | 33 |
| PEMBROLIZUMAB vs. PLACEBO | Anti-PD1 | Phase III trial | Second line | Improved OS (HR: 0.78; p = 0.0238) and PFS (HR: 0.78; p = 0.0209)—does not meet significance per the prespecified statistical plan (ongoing, preliminary negative results) | 34 |
| TORIPALIMAB | Anti-PD1 | Phase II trial | Adiuvant setting | Recurrence-freesurvival (ongoing) | 41 |
| TREMELIMUMAB | Anti-CTLA4 | Phase II trial | Pretreated advanced HCC from hepatitis C viral etiology | 18% of PR and a 60% of SD (finished) | 35 |
| NIVOLUMAB plus IPILIMUMAB | Anti-CLA4 plus Anti-PD1 | Phase I-II trial | Neoadjuvant treatment | Delay to surgery Incidence of treatment-emergent adverse events (ongoing) | 38 |
| NIVOLUMAB plus IPILIMUMAB | Anti-CLA4 plus Anti-PD1 | Phase II trial | Neoadjuvant treatment | The percentage of subjects with tumor shrinkage after drug treatment study (ongoing) | 39 |
| ATEZOLIZUMAB plus BEVACIZUMAB | Anti-PD1 plus antiangiogenic drug | Phase II trial | First line treatment | 61% PR with a relatively positive tolerability (finished) | 45 |
| ATEZOLIZUMAB plus BEVACIZUMAB vs. SORAFENIB | Anti-PD1 plus antiangiogenic drug | Phase III trial | Metastatic and/or unresectable HCC (first line) | OS/PFS (ongoing) | 46 |
| DURVALUMAB (D) plus BEVACIZUMAB (B) vs. D vs. placebo | Anti-PDL1 plus antiangiogenic drug | Phase III trial | Adjuvant setting | Recurrence-free survival (ongoing) | 47 |
| LENVATINIB plus PEMBROLIZUMAB | Anti-PD1 plus TKI | Phase 1b trial | unresectable HCC (first line) | 46% of PR and 46% of SD (finished) | 48 |
| LENVATINIB (L) plus PEMBROLIZUMAB vs. L | Anti-PD1 plus TKI | Phase III trial | Advanced HCC (first line) | PFS/OS (ongoing) | 49 |
| NIVOLUMAB (N) vs. IPILIMUMAB (I) + N vs. SORAFENIB N vs. CABOZANTINIB (C) + N vs. SORAFENIB (CP−A) vs. N+C+I | Anti-PD1 and Anti-CTLA4 plus TKI | Phase I-II trial | CP-A HCC CP-B HCC |
Safety and Tolerability (ongoing) | 50 |
| TACE, radiofrequency ablation, or cryoablation plus TREMELIMUMAB | Anti-CTLA4 plus interventional radiological procedures | Phase 1b trial | Locally advanced HCC | 23.5% of PR (finished) | 56 |
| PEMBROLIZUMAB plus TACE | Anti-PD1 plus interventional radiological procedures | Phase I-II trial | Locally advanced HCC | Safety and Tolerability (ongoing) | 52 |
| NIVOLUMAB plus TACE | Anti-PD1 plus interventional radiological procedures | Early phase I trial | Locally advanced HCC | Safety and Tolerability (ongoing) | 53 |
| PEMBROLIZUMAB plus yttrium90 radioembolization | Anti-PD1 plus interventional radiological procedures | Early phase I trial | Locally advanced HCC | PFS (ongoing) | 54 |
| NIVOLUMAB plus yttrium90 radioembolization | Anti-PD1 plus interventional radiological procedures | Early phase I trial | Locally advanced HCC | Recurrence rate (ongoing) | 55 |
| SHR-1210 + ApatinibSHR-1210 + FOLFOX4 or GEMOX regimen | Anti-PD1 plus chemotherapy or TKI | Phase II trial | Advanced Primary Liver Cancer | Safety and Tolerability (ongoing) | 57 |
Abbreviation: CP: Child-Pugh; CTLA4: Cytotoxic T-Lymphocyte Antigen-4; DCR: disease control rate; HCC: hepatocellular carcinoma; mOS: median Overall survival; mPFS: median Progression Free Survival; ORR: overall response rate; PD-1: Programmed death 1; PD-L1: Programmed death-ligand 1; PR: partial response; SD: stable disease; TACE: transarterial chemoembolization; TKI: tyrosine Kinase Inhibitor.
Abbreviation: CP: Child-Pugh; CTLA4: Cytotoxic T-Lymphocyte Antigen-4; DCR: disease control rate; HCC: hepatocellular carcinoma; mOS: median Overall survival; mPFS: median Progression Free Survival; ORR: overall response rate; PD-1: Programmed death 1; PD-L1: Programmed death-ligand 1; PR: partial response; SD: stable disease; TACE: transarterial chemoembolization; TKI: tyrosine Kinase Inhibitor.
Unfortunately, the response rate of single agent ICI remains low, differently from the circulating CD8+ T-cells that increased after ICIs treatment, none activity enhancement have been observed for intrahephaticCD8+ T-cells. The combination of anti-CTLA4 and antibody targeting the PD1/PD-L1 axis are also under investigation, based on preclinical studies demonstrating that the 2 pathways are not overlapping, indeed it seems that the combination has a synergistic effect able to reverse the refractoriness of intrahepatic CD8+ T-cells [37]. The combination of the anti-CLA4 antibody ipilimumab and nivolumab is currently assessed in patients undergoing hepatic resection as a neoadjuvant treatment (NCT03682276, NCT03510871) [38,39]. Recently, monthly tremelimumab 75 mg in combination with the anti-PD-L1 durvalumab 1500 mg for 4 doses followed by monthly durvalumab 1500 mg monotherapy until progression has been assessed in patients with advanced HCC who had received at least one prior therapy. Disease control rate is 60% with a median PFS of 7.8 months (95% CI 2.6 to 10.6 months) and median OS of 15.9 (95% CI 7.1 to 16.3 months).
Combined immune checkpoint inhibition (ICI) with tremelimumab and durvalumab in patients with advanced hepatocellular carcinoma (HCC) or biliary tract carcinomas (BTC) [40].
Instead, in the adjuvant setting, for patients who have undergone a remedial resection, toripalimab a anti-PD-1 antibody has being assessed in the JUPTER-04 trial with the primary end-point consisting in recurrence-free survival [41].
4. Combined Approaches with Checkpoint Inhibitors
Despite the fact that the impact of ICIs on the treatment of malignancies is unprecedented, unlike melanoma and non-small cell lung cancer, the response rate in HCC remains low. In regards to this, as well as for other malignancies, researchers are assessing combined approaches to increase the efficacy of ICIs [42]. The combination of other therapeutics with ICIS is able to modify the immune microenvironment of the tumor, up-regulating T cells with effector functions, and decreasing the immunosuppressive cells expression, and so changing a cold tumor into a hot one The combination of ICIs with anti-Vascular Endothelial Growth Factor (VEGF) therapy is a major approach under investigation for HCC patients using the immunomodulatory effects of an anti-VEGF drug as a means of decreasing CD4+ regulatory T-Lymphocytes and MDSCs as well as the activation and differentiation of DCs [43,44]. The combination of atezolizumab, a fully humanized, engineered monoclonal antibody of IgG1 isotype anti-PD-L1 and bevacizumab, a monoclonal antibody anti-VEGF, resulted in 61% partial response among 21 HCC patients with a relatively positive tolerability characterized by 35% of the subjects experiencing grade III/IV adverse events [45]. The combination of atezolizumab plus bevacizumab is also being assessed in a phase III trial, open-label, multi-center, randomized study, with sorafenib in the control arm, in pts with locally advanced or metastatic and/or unresectable HCC (NCT03434379) [46]. Another randomized phase III EMRALD-2 (NCT03847428) [47] is assessing the role of another ICIs targeting PD-L1, namely durvalumab, in addition to bevacizumab in the adjuvant setting.
Ater sorafenib, other TKIs have been approved for HCC patients [6,9]. Interestingly, evidence suggests that these small molecule inhibitors could improve tumor immunogenicity through the increase of antigen expression and the activation of cytotoxic activity of CD8+T-cells. Several trials are exploring the combination of ICIs with TKIs approved for HCC patients, such as regorafenib, cabozantinib, and levatinib. Preliminary results of a phase 1b trial of lenvatinib with pembrolizumab demonstrated that among the 13 patients treated, 6 (46%) achieved a PR and 6 (46%) a SD [48]. A phase 3 assessing this combination for the first-line treatment of patients with advanced HCC is ongoing [49]. Two cohorts were added to the phase I-IINCT01658878clinical trial with the aim to assess the tolerability and the effectiveness of nivolumab in combination with sorafenib and with cabozantinib [50].
Another combined approach under investigation is the addition of ICIsto locoregional therapies, in particular, the post-transarterial chemoembolization (TACE), which is associated with antigen release and the exposure of damage-associated molecular patterns. A phase I trial concerning HCC patients treated with TACE, radiofrequency ablation, or cryoablation in combination with tremelimumb, showed 23.5% (4 of 17 patients) of PR [51]. A tumor biopsy at 6 weeks showed an increase of CD8+ T-cells infiltration in patients who showed a clinical benefit. Others trials combing ICIs with locoregional therapy are ongoing: pembrolizumab with TACE (phase I/II, NCT03397654) [52], nivolumab with TACE (phase I, NCT03143270) [53], pembrolizumab with yttrium-90 radioembolization (phase II, NCT03099564) [54], and nivolumab with yttrium-90 radioembolization (phase II, NCT03812562) [55], among others.
Even if HCC is known as a malignancy which is highly refractory to chemotherapy, based on the results of the EACH study, FOLFOX4 has been recently recommended as a clinical practice guideline by the China FDA 8.6% of partial response and 38.6% of stable disease have been reported with a median OS of 5.7 months [56]. Interestingly, similar to other chemotherapeutic agents, oxaliplatin can induce an antitumor immune response, activating DCs, promoting the antitumor CD4+T cells phenotype, and by the down-regulation of MDSC and regulatory T cells. Regarding this factor, a monoclonal antibody directed againstPD-1 namely SHR-1210 combined with FOLFOX4 is under investigation in Chinese patients with advanced HCC (NCT03092895) [57].
Finally, in addition to the co-inhibitory receptors CTLA-4 and PD-1 other co-inhibitory molecules have been described, including TIGIT, LAG3, and TIM-3 [27]. The latter has been shown to be involved in HCC progression, with high infiltration of TIM-3 positive cells correlating with poor prognosis. The combination of anti-PD-L1/anti-PD1 therapy with therapeutics targeting TIM-3 (NCT03099109) [58] and LAG-3 (NCT03005782and NCT01968109) [59,60] is under investigation.
5. Conclusions and Future Directions
As discussed in this manuscript, ICIs are under intensive investigation for HCC patients, as well as other malignancies, ICIs arealready approved for lines subsequent to the first, such as nivolumab and pembrolizumab. However, in the same way as in other solid tumors, the response rate is low, therefore several strategies to improve the efficacy of ICIs are under investigation. Several prospective clinical trials are assessing the efficacy and safety of PD1 blockade in combination with CTLA-4 blockade, since this combination should enhance the results of single agent anti-PD1. This combination should enhance OS and long term survival rates of both localized and metastatic HCC. In any case, it will take several years before obtaining not only results from these data, but also discovering predictive factors able to select patient candidates to ICIs therapy. The combinations of different ICIs with anti-angiogenic therapeuticsor of kinase inhibitors, are now being tested in HCC as well, with the aim to improve the anticancer effectiveness and prolong cancer survival. Interestingly, a unique and promising combinatory approach that is being assessed in HCC patients is the addition of ICIs to locoregional therapy.
In the next years, several preclinical and clinical studies on HCC biology and treatment will address tailored therapies and combinations for these malignancies. In this scenario, immune oncology will have a special role in HCC. Even if, currently, the immunotherapy remains a non-curative treatment for HCC patients, the better tolerability of ICIs may display a crucial role in the improvement of the quality of life.
Acknowledgments
This research project was supported in part by Apulian Regional Project “Medicina di Precisione”.
Author Contributions
Conceptualization, V.L., O.B., A.A.; methodology, V.L., O.B., A.A.; Writing—Original Draft preparation, V.L., O.B., A.G., A.L., S.D., R.M., A.G.S., A.A.; supervision, V.L., O.B., A.A.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen C., Wang G. Mechanisms of hepatocellular carcinoma and challenges and opportunities for molecular targeted therapy. World J. Hepatol. 2015;7:1964–1970. doi: 10.4254/wjh.v7.i15.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadei Gardini A., Marisi G., Scarpi E., Scartozzi M., Faloppi L., Silvestris N., Masi G., Vivaldi C., Brunetti O., Tamberi S., et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin. Pharmacother. 2015;16:2719–2725. doi: 10.1517/14656566.2015.1102887. [DOI] [PubMed] [Google Scholar]
- 4.Quatrale A.E., Porcelli L., Silvestris N., Colucci G., Angelo A., Azzariti A. EGFR tyrosine kinases inhibitors in cancer treatment: In vitro and in vivo evidence. Front. Biosci. 2011;16:1962–1972. doi: 10.2741/3833. [DOI] [PubMed] [Google Scholar]
- 5.Brunetti O., Gnoni A., Licchetta A., Longo V., Calabrese A., Argentiero A., Delcuratolo S., Solimando A.G., CasadeiGardini A., Silvestris N. Predictive and Prognostic Factors in HCC Patients Treated with Sorafenib. Preprints. 2019 doi: 10.20944/preprints201909.0071.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.-W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in firstline treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 noninferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Alfa G.K., Meyer T., Cheng A.L., El-Khoueiry A.B., Rimassa L., Ryoo B.Y., Cicin I., Merle P., Chen Y., Park J.W., et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu A.X., Park J.O., Ryoo B.Y., Yen C.J., Poon R., Pastorelli D., Blanc J.F., Chung H.C., Baron A.D., Pfiffer T.E., et al. REACH Trial Investigators. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., Pracht M., Yokosuka O., Rosmorduc O., Breder V., et al. RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 10.Longo V., Gnoni A., Casadei Gardini A., Pisconti S., Licchetta A., Scartozzi M., Memeo R., Palmieri V.O., Aprile G., Santini D., et al. Immunotherapeutic approaches for hepatocellular carcinoma. Oncotarget. 2017;8:33897–33910. doi: 10.18632/oncotarget.15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basile D., Garattini S.K., Bonotto M., Ongaro E., Casagrande M., Cattaneo M., Fanotto V., De Carlo E., Loupakis F., Urbano F., et al. Immunotherapy for colorectal cancer: Where are we heading? Expert Opin. Biol. Ther. 2017;17:709–721. doi: 10.1080/14712598.2017.1315405. [DOI] [PubMed] [Google Scholar]
- 12.Mogler C., König C., Wieland M., Runge A., Besemfelder E., Komljenovic D., Longerich T., Schirmacher P., Augustin H.G. Hepatic stellate cells limit hepatocellular carcinoma progression through the orphan receptor endosialin. EMBO Mol. Med. 2017;9:741–749. doi: 10.15252/emmm.201607222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu K., He X., Lei X.Z., Zhao L.S., Tang H., Liu L., Lei B.J. Pathomorphological study on location and distribution of Kupffer cells in hepatocellular carcinoma. World J. Gastroenterol. 2003;9:1946. doi: 10.3748/wjg.v9.i9.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo N., Araki K., Kuwano H., Shirabe K. Cancer-associated fibroblasts in hepatocellular carcinoma. World J. Gastroenterol. 2016;22:6841. doi: 10.3748/wjg.v22.i30.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau A.H., Thomson A.W. Dendritic cells and immune regulation in the liver. Gut. 2003;52:307–314. doi: 10.1136/gut.52.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirabe K., Motomura T., Muto J., Toshima T., Matono R., Mano Y., Takeishi K., Ijichi H., Harada N., Uchiyama H., et al. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: Pathology and clinical management. Int. J. Clin. Oncol. 2010;15:552–558. doi: 10.1007/s10147-010-0131-0. [DOI] [PubMed] [Google Scholar]
- 17.Son B., Lee S., Youn H., Kim E., Kim W., Youn B. The role of tumor microenvironment in therapeutic resistance. Oncotarget. 2017;8:3933. doi: 10.18632/oncotarget.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang W.B., Yao M., Cheng N. Priming cancer cells for drug resistance: Role of the fibroblast niche. Front. Biol. 2014;9:114–126. doi: 10.1007/s11515-014-1300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porcelli L., Iacobazzi R.M., Di Fonte R., Serratì S., Intini A., Solimando A.G., Brunetti O., Calabrese A., Leonetti F., Azzariti A., et al. CAFs and TGF-β Signaling Activation by Mast Cells Contribute to Resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer. Cancers. 2019;11:330. doi: 10.3390/cancers11030330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang N., Figini M., Shangguan J., Wang B., Sun C., Pan L., Ma Q., Zhang Z. Dendritic cells based immunotherapy. Am. J. Cancer Res. 2017;7:2091. [PMC free article] [PubMed] [Google Scholar]
- 21.Argentiero A., De Summa S., Di Fonte R., Iacobazzi R.M., Porcelli L., Da Vià M., Brunetti O., Azzariti A., Silvestris N., Solimando A.G. Gene Expression Comparison between the Lymph Node-Positive and-Negative Reveals a Peculiar Immune Microenvironment Signature and a Theranostic Role for WNT Targeting in Pancreatic Ductal Adenocarcinoma: A Pilot Study. Cancers. 2019;11:942. doi: 10.3390/cancers11070942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertino G., Demma G., Ardiri A., Proiti M., Mangia A., Gruttadauria S., Toro A., Di Carlo I., Malaguarnera G., Bertino N., et al. The immune system in hepatocellular carcinoma and potential new immunotherapeutic strategies. BioMed Res. Int. 2015;2015:731469. doi: 10.1155/2015/731469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Miamen A.G., Dong H., Roberts L.R. Immunotherapeutic approaches to hepatocellular carcinoma treatment. Liver Cancer. 2012;1:226–237. doi: 10.1159/000343837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardini A.C., Scarpi E., Faloppi L., Scartozzi M., Silvestris N., Santini D., De Stefano G., Marisi G., Negri F.V., Foschi F.G., et al. Immune inflammation indicators and implication for immune modulation strategies in advanced hepatocellular carcinoma patients receiving sorafenib. Oncotarget. 2016;7:67142–67149. doi: 10.18632/oncotarget.11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong P., Ma L., Liu L., Zhao G., Zhang S., Dong L., Xue R., Chen S. CD86/CD206, Diametrically Polarized Tumor-Associated Macrophages, Predict Hepatocellular Carcinoma Patient Prognosis. Int. J. Mol. Sci. 2016;17:320. doi: 10.3390/ijms17030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arihara F., Mizukoshi E., Kitahara M., Takata Y., Arai K., Yamashita T., Nakamoto Y., Kaneko S. Increase in CD14+HLA-DR-/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol. Immunother. 2013;62:1421–1430. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mai J., Virtue A., Shen J., Wang H., Yang X.F. An evolving new paradigm: Endothelial cells–conditional innate immune cells. J. Hematol. Oncol. 2013;6:61. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann K., Rudolph C., Neumann C., Janke M., Amsen D., Scheffold A. Liver sinusoidal endothelial cells induce immunosuppressive IL-10-producing Th1 cells via the Notch pathway. Eur. J. Immunol. 2015;45:2008–2016. doi: 10.1002/eji.201445346. [DOI] [PubMed] [Google Scholar]
- 29.Leone P., Di Lernia G., Solimando A.G., Cicco S., Saltarella I., Lamanuzzi A., Ria R., Frassanito M.A., Ponzoni M., Ditonno P., et al. Bone marrow endothelial cells sustain a tumor-specific CD8+ T cell subset with suppressive function in myeloma patients. OncoImmunology. 2019;8:e1486949. doi: 10.1080/2162402X.2018.1486949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Q., Wang X.Y., Qiu S.J., Yamato I., Sho M., Nakajima Y., Zhou J., Li B.Z., Shi Y.H., Xiao Y.S., et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin. Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 31.El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.Y., Choo S.P., Trojan J., Welling T.H., 3rd, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. NCT02576509: An Investigational Immuno-Therapy Study of Nivolumab Compared to Sorafenib as a First Treatment in Patients with Advanced Hepatocellular Carcinoma. [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02576509.
- 33.Zhu A.X., Finn R.S., Edeline J., Cattan S., Ogasawara S., Palmer D., Verslype C., Zagonel V., Fartoux L., Vogel A., et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 34.Finn R.S., Ryoo B.Y., Merle P., Kudo M., Bouattour M., Lim H.Y., Breder V.V., Edeline J., Chao Y., Ogasawara S., et al. Results of KEYNOTE-240: Phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC) J. Clin. Oncol. 2019;37:4004. doi: 10.1200/JCO.2019.37.15_suppl.4004. [DOI] [Google Scholar]
- 35.Sangro B., Gomez-Martin C., De la Mata M., Iñarrairaegui M., Garralda E., Barrera P., Riezu-Boj J.I., Larrea E., Alfaro C., Sarobe P., et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Fizazi K., Maillard A., Penel N., Baciarello G., Allouache D., Daugaard G., van de Wouw A., Soler G., Vauleon E., Chaigneau L., et al. A phase 3 trial of empiric chemotherapy with cisplatin and gemcitabine or systemic treatment tailored by molecular gene expression analysis. Ann. Oncol. 2019;30(Suppl. 5):v851–v934. doi: 10.1093/annonc/mdz394. [DOI] [Google Scholar]
- 37.Nakamoto N., Cho H., Shaked A., Olthoff K., Valiga M.E., Kaminski M., Gostick E., Price D.A., Freeman G.J., Wherry E.J., et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoSPathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. NCT03682276: Safety and Bioactivity of Ipilimumab and Nivolumab Combination Prior to Liver Resection in Hepatocellular Carcinoma (PRIME-HCC) [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02576509.
- 39. NCT03510871: Nivolumab Plus Ipilimumab as Neoadjuvant Therapy for Hepatocellular Carcinoma (HCC) [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03510871.
- 40.Floudas C.S., Xie C., Brar G., Morelli M.P., Fioravanti S., Walker M., Mabry-Hrones D., Wood B.J., Levy E.B., Krishnasamy V.P., et al. Combined immune checkpoint inhibition (ICI) with tremelimumab and durvalumab in patients with advanced hepatocellular carcinoma (HCC) or biliary tract carcinomas (BTC) J. Clin. Oncol. 2019;37:336. doi: 10.1200/JCO.2019.37.4_suppl.336. [DOI] [Google Scholar]
- 41. NCT03859128: Oripalimab or Placebo as Adjuvant Therapy in Hepatocellular Carcinoma after Curative Hepatic Resection (JUPITER 04) [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03510871.
- 42.Longo V., Brunetti O., Azzariti A., Galetta D., Nardulli P., Leonetti F., Silvestris N. Strategies to Improve Cancer Immune Checkpoint Inhibitors Efficacy, Other Than Abscopal Effect: A Systematic Review. Cancers. 2019;11:539. doi: 10.3390/cancers11040539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J., Yan J., Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitale G., Dicitore A., Gentilini D., Cavagnini F. Immunomodulatory effects of VEGF: Clinical implications of VEGF-targeted therapy in human cancer. Cancer Biol. Ther. 2010;9:694–698. doi: 10.4161/cbt.9.9.11691. [DOI] [PubMed] [Google Scholar]
- 45.Stein S., Pishvaian M.J., Lee M.S., Lee K.H., Hernandez S., Kwan A., Liu B., Grossman W., Iizuka K., Ryoo B.Y. Safety and clinical activity of 1L atezolizumab + bevacizumab in a phase Ib study in hepatocellular carcinoma (HCC) J. Clin. Oncol. 2018;36:4074. doi: 10.1200/JCO.2018.36.15_suppl.4074. [DOI] [Google Scholar]
- 46. NCT03434379: A Study of Atezolizumab in Combination with Bevacizumab Compared with Sorafenib in Patients with Untreated Locally Advanced or Metastatic Hepatocellular Carcinoma [IMbrave150] (IMbrave150) [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03434379.
- 47. NCT03847428: Assess Efficacy and Safety of Durvalumab Alone or Combined with Bevacizumab in High Risk of Recurrence HCC Patients after Curative Treatment (EMERALD-2) [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03847428.
- 48.Ikeda M., Sung M.W., Kudo M., Kobayashi M., Baron A.D., Finn R.S., Kaneko S., Zhu A.X., Kubota T., Kraljevic S., et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC) J. Clin. Oncol. 2018;36:4076. doi: 10.1200/JCO.2018.36.15_suppl.4076. [DOI] [Google Scholar]
- 49.Llovet J.M., Kudo M., Cheng A.L., Finn R.S., Galle P.R., Kaneko S., Meyer T., Qin S., Dutcus C.E., Chen E., et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): Phase 3 LEAP-002 study. J. Clin. Oncol. 2019;37 doi: 10.1200/JCO.2019.37.15_suppl.TPS4152. [DOI] [Google Scholar]
- 50. NCT01658878: An Immuno-Therapy Study to Evaluate the Effectiveness, Safety and Tolerability of Nivolumab or Nivolumab in Combination with Other Agents in Patients with Advanced Liver Cancer (CheckMate040) [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01658878.
- 51.Duffy A.G., Ulahannan S.V., Makorova-Rusher O., Rahma O., Wedemeyer H., Pratt D., Davis J.L., Hughes M.S., Heller T.E., Gindi M., et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. NCT03397654: Study of Pembrolizumab Following TACE in Primary Liver Carcinoma (PETAL) [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03397654.
- 53. NCT03143270: A Study to Test the Safety and Feasibility of Nivolumab with Drug Eluting Bead Transarterial Chemoembolization in Patients with Liver Cancer. [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03143270.
- 54.NCT0333446: Pembrolizumab Plus Y90 Radioembolization in HCC Subjects. [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT0333446.
- 55. NCT03812562: Nivolumab and Yttrium-90 in Treating Patients with Liver Cancer Undergoing Surgical Resection. [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03812562.
- 56.Qin S., Bai Y., Lim H.Y., Thongprasert S., Chao Y., Fan J., Yang T.S., Bhudhisawasdi V., Kang W.K., Zhou Y., et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J. Clin. Oncol. 2013;31:3501–3508. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 57. NCT03092895: A Study of SHR-1210 in Combination with a Patinib or Chemotherapy in Subjects with Advanced PLC or BTC. [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03812562.
- 58. NCT03099109: A Study of LY3321367 Alone or with LY3300054 in Participants with Advanced Relapsed/Refractory Solid Tumors. [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03099109.
- 59. NCT03005782: Study of REGN3767 (Anti-LAG-3) with or without REGN2810 (Anti-PD1) in Advanced Cancers. [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03005782.
- 60. NCT01968109: An Investigational Immuno-therapy Study to Assess the Safety, Tolerability and Effectiveness of Anti-LAG-3 with and without Anti-PD-1 in the Treatment of Solid Tumors. [(accessed on 12 September 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01968109.