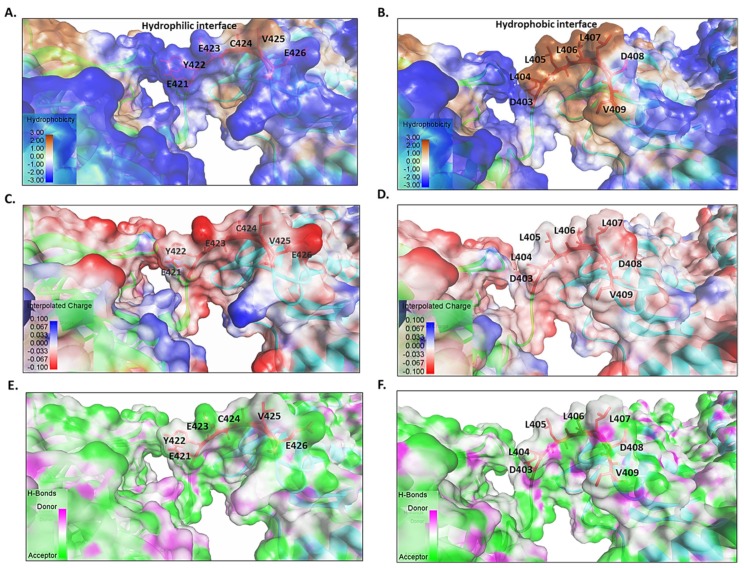
Figure 3.
Comparative surface features of linker motifs of PfHsp70-1 (canonical Hsp70) and PfHsp70-z (Hsp110). PfHsp70-z, an Hsp110, possesses a more hydrophilic linker surface interface (A) as opposed to the more hydrophobic linker surface of canonical Hsp70, represented by PfHsp70-1 (B). The glutamate residues on the surface of PfHsp70-z account for a negatively charged linker surface (C) as opposed to that of PfHsp70-1 which is mostly neutral (D). The linker of PfHsp70-z (Hsp110) (E) possesses more hydrogen binding sites as compared to the linker for the canonical Hsp70 (PfHsp70-1) (F).