Abstract
Potassium is an essential macronutrient that has been partly overshadowed in root science by nitrogen and phosphorus. The current boom in potassium-related studies coincides with an emerging awareness of its importance in plant growth, metabolic functions, stress tolerance, and efficient agriculture. In this review, we summarized recent progress in understanding the role of K+ in root growth, development of root system architecture, cellular functions, and specific plant responses to K+ shortage. K+ transport is crucial for its physiological role. A wide range of K+ transport proteins has developed during evolution and acquired specific functions in plants. There is evidence linking K+ transport with cell expansion, membrane trafficking, auxin homeostasis, cell signaling, and phloem transport. This places K+ among important general regulatory factors of root growth. K+ is a rather mobile element in soil, so the absence of systemic and localized root growth response has been accepted. However, recent research confirms both systemic and localized growth response in Arabidopsis thaliana and highlights K+ uptake as a crucial mechanism for plant stress response. K+-related regulatory mechanisms, K+ transporters, K+ acquisition efficiency, and phenotyping for selection of K+ efficient plants/cultivars are highlighted in this review.
Keywords: potassium, root growth, root system architecture, deficiency, KT/HAK/KUP transporters
1. Introduction
Potassium is a macronutrient that may constitute up to 10% of plant dry weight [1]. It is a major inorganic cation in the plant cytoplasm, essential for activity of various enzymes, including those participating in primary metabolism [2]. It contributes significantly to turgor regulation, which is important for many plant processes, such as stomatal function [3,4], cell volume growth [5,6,7], existence of cytoplasm-plasma membrane-cell wall continuum [8], and plant movements [9]. To fulfil the diverse developmental and physiological functions of K+ in plants, broad spectrum of K+ transporters and channels evolved to orchestrate K+ transport [10,11,12,13].
Root system growth and development relies on K+ at various levels. Protein synthesis and enzyme activity of root cells need adequate cytoplasmic K+ levels to maintain the cytoplasmic pH [14] and the anionic charge of proteins [15]. Cell expansion in the elongation zone requires turgor pressure, which builds up via osmotically active substances, including K+ [16,17]. In the root maturation zone, root hairs grow apically via the action of K+ fluxes [18,19,20]. K+ affects R:S ratio (root to shoot biomass partitioning) via phloem transport [21,22]. Moreover, adaptive changes of root system architecture (RSA) and root hair coverage evolved in plants to enhance K+ uptake in potassium limiting conditions [10,23].
Soil K+ bioavailability is often low (especially in acidic soils) and limited mostly to the topsoil as most of soil potassium is incorporated in minerals [24,25]. K+ limitation is thus a common problem affecting agricultural production [25,26]. Plants engage high-affinity K+ transporters, modulate K+ channel transport properties [27,28,29,30], and change root system architecture (RSA) to cope with K+ deficiency [31]. Some growth responses to low K+ provide functional adjustments of the root system to enhance K+ acquisition efficiency. Sensing local K+ availability in rhizosphere triggers local root growth [32], but preferential branching to K+ rich patches seems to be mild compared to N or P local response [33,34]. K+ limitation negatively impacts root elongation and the number of first order lateral roots [35,36,37], but the response varies among species, cultivars, ecotypes, and even root types [35,38]. Suppressed cell volume growth or limited phloem delivery of assimilates to belowground organs may participate in root growth inhibition [21,22]. K+ scarcity also increases plant susceptibility to biotic and abiotic stresses [26,39].
In this review, we focused on K+ involvement in root growth and root system architecture establishment at various levels, from cell growth up to root system response to stress factors. The root system responses to low K+ stress are highlighted.
2. Potassium and Root Cell Expansion
The crucial role of osmotically active K+ cation in turgor-driven cell expansion is well known (see e.g., [5,17,40]). Two voltage-dependent, inward-rectifying Shaker K+ channels, KAT1 and KAT2 (K+ CHANNEL IN ARABIDOPSIS THALIANA1, 2), and K+ transporter KT2/KUP2/SHY3 (K+ TRANSPORT2/K+ UPTAKE2/SHORT HYPOCOTYL3) were shown to participate in auxin-induced cell expansion in the hypocotyls and leaves of Arabidopsis thaliana [6,7,41]. Kup2/6/8 triple mutants display significantly larger leaf epidermal cells, which indicates the involvement of these KT/HAK/KUP (K+ TRANSPORT/HIGH-AFFINITY K+/K+ UPTAKE) transporters in K+ efflux and modulation of volume growth [6]. KT2/KUP2/SHY3 expression is not limited to the shoot. It is expressed in the root tip [7] and might, therefore, be involved in root cell expansion the same way as in the shoot.
KAT1 functions mostly in the shoot, including in the guard cells [42]; expression of KAT1 in roots is weak [41]. However, similar Shaker K+ channel KC1 (K+ RECTIFYING CHANNEL1) is active in roots. KC1 is predominantly expressed in root hairs and the endodermis, and modulates root hair K+ uptake [43]. KC1 is a silent regulatory subunit of the heterotetrameric channel AKT1 (ARABIDOPSIS K+ TRANSPORTER1), an inward-rectifying K+ channel of the Shaker family. AKT1 plays a dominant role in root K+ uptake over a broad range of K+ external concentrations [44,45,46]. The assembly of the AKT1/KC1 heterotetramer is triggered by low external K+ availability and modifies AKT1 transport properties so that it prevents K+ leakage under these circumstances [29,47]. AKT1 is involved in root hair elongation, but the mechanism is not clear yet [19].
Moreover, a direct link exists between secretion and K+ channel activity. SNARE (SOLUBLE NSF ATTACHMENT PROTEIN RECEPTOR; NSF-N-ETHYLMALEIMIDE-SENSITIVE FACTOR) protein SYP121 (SYNTAXIN OF PLANT121), a component of exocytosis regulation machinery controlling vesicle fusion with the target membrane [48], regulates trafficking of K+ channel KAT1 and its distribution within plasma membrane subdomains [49]. Moreover, SYP121 directly interacts with the voltage-sensing domain of KAT1 and KC1 channels via FxRF motif and shifts their voltage-driven gating [50,51,52,53]. The SYP121 binding affects the stability of the KAT1 channel in open or closed states and promotes its activity [54]. Binding of SYP121 to KC1 enhances secretion in feedback, highlighting the interplay between exocytosis and K+ transport [52,53]. In contrast, binding of the R-SNARE protein VAMP721 (VESICLE-ASSOCIATED MEMBRANE PROTEIN721) to KC1 or KAT1 suppresses their activities [55].
SNARE-dependent control of K+ uptake thus presents not only a regulatory pathway of K+ acquisition in plants subjected to K+ deficiency [23], but also a mechanism to coordinate membrane expansion and cell wall material exocytosis with K+ uptake and generation of turgor pressure to drive cell growth [53,56]. SYP121 also coordinates the trafficking of some plasma membrane localized aquaporins (PIP2-7) and thus fine-tunes the water permeability of the membrane [57].
3. Potassium and Root Hair Growth
Root hairs are short-lived tubular emergences of rhizodermal cells that serve many important roles in root-soil contact, nutrient uptake, and microbial interactions [58]. Long root hairs are advantageous for K+ acquisition under K+ shortage, as was shown by comparing different crop species [59] and even rice (Oryza sativa L.) genotypes differing in average length of root hairs [60].
Root hair establishment starts with the determination of trichoblast cell fate orchestrated by the action of transcription and other regulatory factors. It continues with the formation of a bulge in the cell wall and specialized tip growth of the emerging hair. Many aspects of this process, including trichoblast genetic determinants, cell wall loosening, deposition of new cell wall material, Ca2+ signaling, cytoskeleton action, pH gradients, and other processes, have been studied and reviewed thoroughly [58,61,62]. The bulge formation and tip growth require turgor pressure generation, and, here, K+ comes to the scene as the main osmotically active inorganic ion of the plant cell [62]. Apoplast acidification was shown to activate K+ channels [63] and an acidic environment is typical for bulge outgrowth domains [64].
Among others, the AKT1 channel is present in root hairs and participates in K+ uptake [44,45,46]. Surprisingly, akt1 plants exhibit a K+-dependent root hair phenotype that is not fully consistent with the proposed role of the AKT1/KC1 channel in triggering root hair growth via K+ uptake. Akt1 mutants possess longer root hairs under zero external K+ levels but shorter root hairs under very high K+ levels. At 100 mM external K+ root hairs of akt1 mutants completely fail to even elongate. The authors hypothesized that AKT1 negatively regulates root hair elongation in the absence of K+, but is required to sustain root hair tip growth under K+ levels above 50 mM [19].
K+ transporter mutant trh1 (tiny root hair1) also shows a strong root hair phenotype. Trichoblast cell fate determination pathways are not affected in trh1 plants, but root hairs fail to elongate and remain very short [18,65,66]. TRH1/KUP4 is a member of the KT/HAK/KUP family of K+ transporters [30,67,68]. Based on a complementation study in Saccharomyces cerevisiae, TRH1 can mediate high-affinity K+ uptake [18], but the trh1 root hair phenotype is insensitive to K+ supply [18,19]. It can be rescued by external auxin application [69] or phosphorus deficiency, but not iron deficiency [70]. This observation, together with agravitropic root growth, mis-localization of the PIN1 (PIN-FORMED1) auxin efflux carrier, and auxin over-accumulation in the root tip of trh1 plants, highlights TRH1 function in auxin homeostasis maintenance within root apex as necessary to sustain the growth of root hairs [18,65]. The importance of auxin signaling in root hair growth regulation is evident [71] and supported by root hairs defective phenotypes of other auxin mutants, e.g., tir1 (TRANSPORT INHIBITOR RESPONSE1), an auxin receptor mutant [72]. However, the effect of TRH1 might be also indirect via modification of K+ homeostasis in meristematic cells, as discussed below.
TRH1 may act as an auxin efflux carrier involved in shoot to root auxin translocation [69]. In roots, it localizes at plasmalemma in a polarized manner. It co-localizes with PIN1 on the basal side of cells in stele in the elongation zone. In the meristematic zone, the preferential localization is in the rhizodermis and cortex. TRH1 might therefore contribute to both acropetal and basipetal auxin transport within the root apex [65] and coordinate environmental cues, auxin signaling, and root hair development [66]. TRH1 seems to function as a homodimer: its assembly is driven by strong interactions between the C-terminal cytoplasmic domains of each of the TRH1 subunits [66].
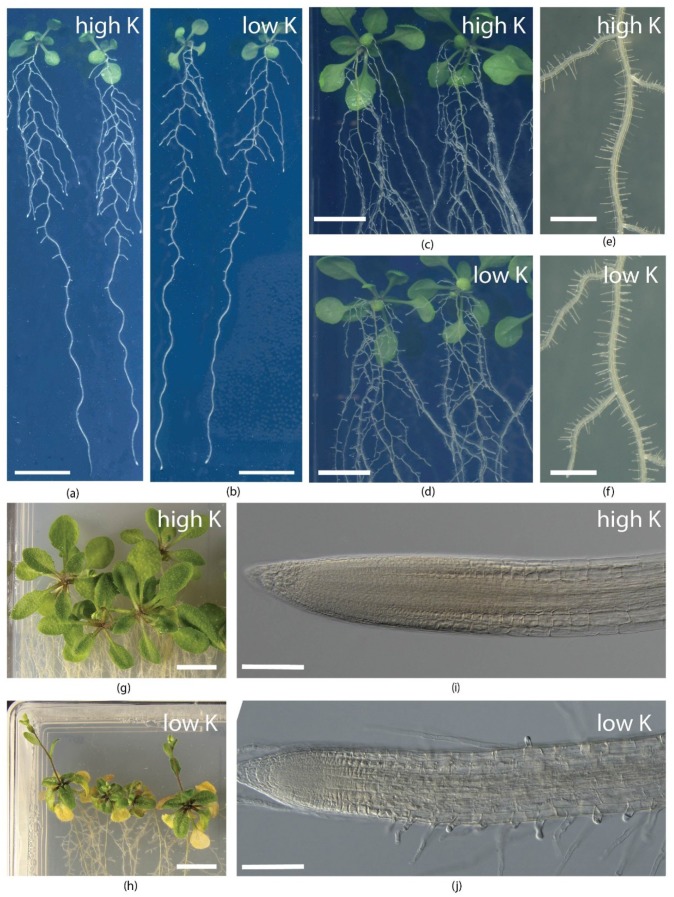
It must be mentioned that root hair growth responds to K+ soil status [73], see Figure 1. K+ deficiency stimulates root hair growth [59] in an ethylene-dependent manner [36,74]. This response relies significantly, but not completely, on EIN2 (ETHYLENE INSENSITIVE2), a positive regulator of the ethylene pathway [36,75,76]. K+ deficient plants increase ethylene levels [77] and ROS (reactive oxygen species) signaling is involved downstream of ethylene [74,78].
Figure 1.
Symptoms of K+ deprivation in Arabidopsis thaliana: Root systems of 10-day-old in vitro plants on (a) high-K and (b) low-K media show preferential inhibition of the first-order lateral root growth in low-K. Root system branching of 16-day-old in vitro plants on (c) high-K and (d) low-K media with enhanced branching to higher orders in low-K. Root hairs of in vitro plants on (e) high-K and (f) low-K media. Shoots of 16-day-old in vitro plants on (g) high-K and (h) low-K media with symptoms of K+ deficiency on leaves in low-K. Lateral root apex of (i) high-K and (j) low-K plants. High-K medium: 0.2x strength MS (Murashige and Skoog) with 4 mM K+; low-K medium: 0.2x strength MS with 15 μM K+. Media were supplemented with 1% agar and 1% sucrose. Scale bars: 1 cm (a–d, g–h); 2 mm (e–f); 100 μm (i–j).
4. Root System Growth and Architecture under Potassium Deprivation
A common plant response to nutrient deficiency is preferential biomass allocation into the root system (increase in root:shoot ratio) to forage for nutrients from a larger volume of soil [2]. The opposite is often true for low K+ stress [79,80]. Although there are also studies showing only a minor effect [59,81], root growth retardation is a common response to K+ limiting [79,80]. One possible explanation is the fact that carbohydrates are retained in the shoots of K+ deprived plants [21,22,82].
Mechanisms to successfully cope with K+ deficiency are diverse among species and genotypes. Among the most important are enhanced K+ uptake, ability to keep a certain level of root meristem proliferation under low K+, chemical modifications of the rhizosphere, and effective K+ translocation within the plant body [83]. K+-efficient genotypes might combine these strategies [83,84]. For example, some tomato varieties resistant to K+ deficiency enhanced uptake capacity per root area, while others enhanced root system growth in comparison to sensitive varieties [85,86]. In soybean, almost no change in RSA was reported, but K+ uptake and translocation was considerably higher in K+ efficient cultivars [87,88]. Most plants combined both strategies. More intensive root system proliferation was found in K+-efficient genotypes in various plant species, including important crops, such as rice [89], maize [81], tobacco [90], and Medicago truncatula [91]. Same species are also capable of facilitating K+ uptake under K+ limiting conditions by activation of high-affinity K+ transporters [92,93,94,95,96]. From these data, it becomes clear that enhanced root system proliferation must be supported by more effective K+ uptake to some extent.
Change of RSA is not a simple increase of root biomass, but rather a stimulation of branching to higher orders (Figure 1). In a resistant rice genotype, a higher abundance of fine roots within the root system was observed under K+ deprivation [80,89]. In Arabidopsis thaliana and tobacco, length and number of first order lateral roots decreases, while number of second order laterals increases in K+ depleted plants [31,90], see Figure 1. The elongation of the main root seems to be inhibited or unaffected, at least in studies with Arabidopsis thaliana (Col-0) as the model plant [35,36,37,77]. The inhibition is well documented, particularly in seedlings [32], while later developmental stages are less sensitive. Thanks to extensive analysis of Arabidopsis thaliana ecotypes, large variability in RSA under K+ stress has been revealed. The ecotypes can be, with some simplification, classified into two clusters. Ecotypes from cluster I compromise lateral root growth in favor of maintaining growth of the main root (e.g., ecotype Col-0). Ecotypes from cluster II do the opposite, which results in lateral roots overgrowing the tip of the main root [35]. Preferential growth of laterals, branching to higher orders, and long dense root hairs in low-K plants resemble low-P responses and seem reasonable for efficient K+ capture from soil, as both Pi and K+ are predominantly bioavailable in the topsoil [25,97].
Moreover, localized proliferation of roots to nutrient-rich patches of heterogenous soil is a strategy that enhances nutrient acquisition efficiency. This proliferation is well documented for nitrogen, in both NH4+ and NO3- forms [98,99,100]. For K+, which is rather mobile within the soil [24], such a localized response was considered to be absent. This conclusion was in accordance with a study on barley, which did not distinctly respond to localized K+ supply, although localized N and P supply triggered massive lateral root growth into the enriched area [33]. Similar results were obtained with soybean in split-root culture [34]. On the other hand, lateral roots of Arabidopsis thaliana showed arrested growth on low-K+ media when the root system was split between low- and high-K+ media [32]. Therefore, localized growth response to K+ exists, at least in some species, but appears weak.
Local proliferation of lateral roots into nutrient-rich patches depends on the root’s ability to perceive availability of a particular ion. High-affinity transporters AMT1.3 (AMMONIUM TRANSPORTER1.3) and NRT1.1 (NITRATE TRANSPORTER1.1, also known as CHL1 and NPF6.3) perform this sensoric role for NH4+ and NO3−, respectively [98,100]. Analogously, AKT1 and HAK5 (HIGH-AFFINITY K+ 5) arise as candidates for K+ sensors as these two transport proteins share the majority of K+ uptake under K+ deficient conditions [27,28,46]. A study by Li and colleagues assigned the sensoric role to AKT1 [32]. In the Arabidopsis thaliana akt1 mutant, the localized response to K+ in media is abolished [32], as well as the proliferation of second order lateral roots in low K+ conditions [31].
Besides the aforementioned RSA responses, root anatomy might also be affected by K+ status of the plant. K+-deprived roots may develop more extensive cortical aerenchyma, as was described in maize [101]. This trend seems an adaptive strategy of building “cheap” root biomass to explore more soil. Enhanced cortical senescence also occurs under N and P deficiency and improves growth under nutrient deficiency [97,101,102]. Root endodermal suberization also changes in response to nutrient availability. In Arabidopsis thaliana, K+ shortage suppresses the suberization and thus affects root transport properties [103,104].
5. How Does K+ Deprivation Regulate Root System Growth and Architecture?
Regulatory mechanisms underlying root growth responses to low K+ stress are gradually being unraveled. The inhibition of root growth correlates with limited K+ acquisition from environmental and internal K+ status. Plants with disrupted high-affinity K+ uptake, e.g., athak5 Arabidopsis thaliana or oshak1 rice, have lower tissue K+ levels and shorter roots compared to wild-type genotypes in low K+ conditions [27,105,106]. Reduced root growth of K+-deprived plants was further linked to decreased auxin concentrations in plant tissue [107] and disruption of auxin maxima in the root tip, as observed in Arabidopsis thaliana and tobacco [32,108]. In Arabidopsis thaliana, degradation of the AtPIN1 transporter was detected [32] and a drop in NtPIN1 transcription was reported in tobacco [108]. Auxin signaling thus seems to be affected, directly or indirectly, by K+ level in the cells of root apical meristem (RAM). Activity of RAM gradually diminishes under low K+ stress [35], see Figure 1. Stimulation of RAM activity (e.g., by overexpression of positive regulators [109]) enhances root growth of K+ deprived plants. In rice, overexpression of WUSCHEL-related homeobox gene OsWOX11 stimulated root growth and K+ uptake due to larger root surface area, and increased low K+ stress tolerance [110].
Auxin response machinery is affected by low K+ stress as well [107]. Among others, the MYB77 transcription factor is downregulated under K+ deprivation [77]. MYB77 is expressed in primary and lateral roots and MYB77 protein interacts with ARFs (AUXIN RESPONSE FACTORs) to enhance expression of auxin-responsive genes [107]. Lower activity of this auxin response modulator appears to be linked with a decreased number of lateral roots upon K+ deprivation. Myb77 knock-out plants have lower lateral root numbers compared to the wild-type in low K+ conditions [107].
Ethylene is another growth regulator involved in low K+ stress responses [36]. Ethylene triggers upregulation of high-affinity K+ transporter HAK5 in low K+ environments [36]. Ethylene insensitive mutants, ein1-1 and etr1-1 (ethylene insensitive 1-1; ethylene response1-1), do not show diminished root growth under K+ deficiency [36], which is inconsistent with the direct role of K+ status on meristematic cells mentioned above. Regulation of HAK5 also relies on ROS [37,77]. K+-deprived roots have higher H2O2 levels [77] and altered expression of key peroxidase RCI3 (RARE COLD INDUCIBLE GENE3), which regulates transcription of HAK5. RCI3 is upregulated under low K+ stress, but rci3 knock-out mutants or RCI3-ox overexpressing plants have similar root growth when compared to wild-type plants under K+ deficiency [37]. On the other hand, reactive nitrogen species NO inhibited lateral root growth in low K+ tobacco [90]. In Arabidopsis thaliana, NO is involved in iron toxicity-induced disruption of K+ homeostasis (reduced K+ retention) within the root tip, which results in root growth inhibition [111].
From the presented data we can conclude that a K+ shortage modulates auxin transport and RAM maintenance pathways, at least partially through ethylene and NO signaling, and thus decreases the activity of the meristem. However, the complexity of the regulatory network and its hierarchy still await further clarification. Interaction and crosslinking between nutrient response pathways is emerging, but current knowledge is still incomplete. In Arabidopsis thaliana, the loss of nitrate transporter NRT1.5/NPF7.3 led to a lower number of lateral roots in low K+ plants [112]. NRT1.5 participates in NO3− xylem loading [113] and modulates K+ homeostasis under low K+ via regulation of K+ root-to-shoot translocation and coordination of NO3−/K+ absorption [112,114,115]. The exact mechanism linking NRT1.5 with lateral root primordia is unknown. K+ shortage in nrt1.5 roots is one possible explanation [112], but the role of NRT1.5 is not limited to low K+ stress only. NRT1.5/NPF7.3 expression increases under low Pi, and the nrt1.5 mutant exhibits fewer lateral roots also in low Pi conditions as well [116].
6. Phloem Transport
Growth of heterotrophic roots relies on the delivery of carbohydrates via the phloem. K+ seems to take part in carbohydrate phloem loading and is transported in the phloem in large quantities. After identification of K+ retranslocation within the plant body via the phloem [117], K+ fluxes were quantified for several plant species and growth conditions. The amount of recirculated K+ is usually more than 40% of the root acquired K+, carried by the ascending xylem transpiration stream [118,119]; it may rise up to 70% in saline conditions [120,121].
Concentration of K+ in the phloem serves as a feedback signal for shoot demand of K+ [122]. Phloem-supplied K+ supports root growth to some extent and its utilization might even exceed K+ supply from soil under certain circumstances [118]. Phloem-transported K+ could also feed individual roots growing in K+ depleted soil patches. However, the physiological relevance of this process is not fully understood. For example, barley plants supplied with radiolabeled K+ in split-root culture showed only a minor allocation of the tracer to K-depleted roots compared to shoot [123].
It is generally accepted that K+ starvation decreases the rate of photosynthesis and carbohydrate export from source leaves via the phloem, as shown in different plant species [21,22,82,124,125]. Among others, K+ enrichment enhanced the rate of phloem exudation in Ricinus communis [126,127], increased export of 14CO2-derived compounds from leaves in sugar beet [128], and increased the velocity of C transfer in the trunk of Eucalyptus grandis [129]. Moreover, Arabidopsis thaliana plants defective in heavy metal-binding protein NAKR1 (SODIUM POTASSIUM ROOT DEFECTIVE1) over-accumulated K+ in the shoots, which results in impaired phloem loading, a smaller root system, and late flowering [130]. These data indicate a crosslink between K+ recycling and transport of assimilates, which is important for root growth. NaKR1 is expressed in phloem companion cells all over the plant and its action is essential for proper K+ distribution [130]. NaKR1 also regulates the expression of FT (FLOWERING LOCUS T) via miR156-SPL3 (SQUAMOSA PROMOTER BINDING PROTEIN-LIKE3) module in a partially K+-dependent manner, which indicates a link between K+ status, sucrose export, and regulation of flowering. Accordingly, Arabidopsis thaliana loss-of-function mutants in AKT2/3, a phloem-specific K+ channel, exhibit decreased sucrose phloem loading, reduced K+ dependence of phloem membrane potential, and delayed flowering [131,132]. It is proposed that the transmembrane gradient of K+ between apoplast and sieve element/companion cell complex acts as an additional energy source (decentralized energy storage) for sucrose transport, which increases in importance under ATP-limited conditions [133]. Post-translational modulation of AKT2/3 channel gating properties modulates K+ permeability, which allows the utilization of K+ gradient to overcome local energy limitation [133,134]. This described mechanism is in agreement with AKT2/3 being expressed predominantly in the phloem of photosynthetically-active parts of the shoot; its expression gradually increases after the onset of a light period, and is higher in shaded compared to illuminated leaves [132].
7. Root System Resistance to Stress
K+ supply improves plant resistance to some stress factors. In this chapter we present examples of stresses that limit root growth and are alleviated by K+ supply. Among others, sufficient K+ availability increases plant salt tolerance. High salinity, mostly caused by NaCl, triggers Na+ accumulation and K+ loss in plants tissues [135]. Plants tend to keep Na+/K+ ratio low in autotrophic parts by Na+ xylem loading restriction in roots [136], Na+ retrieval from xylem sap [137,138], and Na+ retranslocation from shoot back to roots [139]. The Na+/K+ ratio in plants is considered a good measure of the saline stress experienced by the plant [140].
Root growth is inhibited by salinity in most plant species [141], but halophytes may exhibit an opposite response [142]. Reduced root elongation or a decreased number of lateral roots are common responses, but mild salinity can increase the number of lateral roots as part of a stress-induced morphogenic response (SIMR) [143]. Preferential allocation of Na+ into the root system contributes to salinity-induced root growth inhibition, but seems favorable for long-term survival. Arabidopsis thaliana hkt1 (high-affinity K+ transporter1) mutants have lower Na+ content in the roots and increased shoot Na+ concentration. HKT1 mediates high-affinity K+/Na+ cotransport and low-affinity Na+ uptake [144]. It retrieves Na+ from xylem sap and contributes to shoot-to-root Na+ retranslocation [139]. Roots of hkt1 plants grow faster under short-term Na+ exposure, but leaves are hypersensitive to salt stress over the course of long-term experiments and root performance decreases over time [137].
Management of the Na+/K+ ratio in roots is crucial to sustaining root growth and enhancing salt tolerance [140]. The sos2 mutant, one of the salt overly sensitive mutants of Arabidopsis thaliana, is very sensitive to low K+ conditions and exhibits strong root growth inhibition in saline conditions [145]. SOS2 is not directly involved in K+ transport but encodes a serine/threonine protein kinase, which is upregulated under salt stress [146]. SOS2 modulates the activity of H+/Na+ antiporter SOS1 [147,148] and activates H+/Ca2+ antiporter CAX1 (CALCIUM EXCHANGER1) [149].
K+ supply also alleviates adverse effects of other competing ions (e.g., Cs+, NH4+). Excess NH4+ leads to cessation of root growth, especially when K+ or NO3− are scarce [150,151,152]. Activity of the root apical meristem as well as cell volume growth are negatively affected [153,154], comprising a general phenomenon called ammonium toxicity syndrome. This syndrome is caused by multiple mechanisms, such as energy demanding NH3 cycling on the plasmatic membrane [150], carbon depletion [155], pH changes [156], and induced starvation of Ca2+, Mg2+, and K+ [157]. K+ starvation is an important factor in the syndrome and even a small enhancement in K+ accessibility alleviates NH4+ toxicity [158,159,160]. K+ uptake is disrupted under high external NH4+ levels due to K+/NH4+ competition for K+ channels [159,161].
Cs+ is another element competing for K+ uptake mechanisms. High-affinity K+ transporters mediate most of the Cs+ uptake, e.g., AtHAK5 in Arabidopsis thaliana [105] or OsHAK1 in rice [162]. Cs+ is not naturally occurring in soils, but has become present in the environment as a pollutant since the testing of nuclear bombs and the meltdowns of nuclear power plants [163,164]. When concentrated enough, Cs+ becomes toxic and inhibits root growth [105]. Similarly to Na+, Cs+ toxicity depends on the Cs+/K+ ratio [165], and it is alleviated by K+ supply [105,166]. K+ outcompetes Cs+ in uptake [167] and high-K plants suppress expression of high-affinity transporters [27]. Plant accumulation of Cs+ and the risk of its entrance to the food chain is reduced at elevated K+ levels [166].
It is generally supposed that an appropriate supply of K+ would have a positive influence on biotic stress resistance, but experimental data are highly dependent on host/pest pairs and evaluation methods, with effects ranging from positive to negative (reviewed e.g., [26,82,168]). Enhanced resistance of K+ replete plants may reside in the greater energy reserves of plants, increased mechanical resistance of plant bodies, and lower concentrations of simple saccharides and amino acids within tissues [82]. On the other hand, plant defense and K+ deficiency overlap in jasmonic acid (JA) and ethylene signaling. Enhancement of plant defenses by K+ starvation is therefore possible [82,169]. Arabidopsis thaliana accumulates jasmonic acid and glucosinolates under K+ starvation [170] and might be more resistant to pests. Enhanced resistance to biotic stress was indeed confirmed for Pi-starved Arabidopsis thaliana, which also accumulates jasmonic acid [171]. Methyl jasmonate (bioactive metabolite of JA) strongly inhibits root growth [172], which is in agreement with the general root growth response in most plants under K+ or Pi deficiency [79]. These findings suggest that priming by nutrient stress may lead to enhanced resistance to biotic stress in Arabidopsis thaliana, although overall root growth might be suppressed.
Acknowledgments
The authors thank Lena Hunt for correction of English.
Author Contributions
M.S. and E.T. contributed equally to the manuscript; A.S. comprised to finalization of the text.
Funding
The work was supported by Czech Ministry of Education, Youth and Sports—Project LO1417.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Leigh R.A., Wyn Jones R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984;97:1–13. doi: 10.1111/j.1469-8137.1984.tb04103.x. [DOI] [Google Scholar]
- 2.Marschner H. Mineral Nutrition of Higher Plants. Academic Press Ltd.; London, UK: 1995. pp. 1–889. [Google Scholar]
- 3.Cotelle V., Leonhardt N. 14-3-3 proteins in guard cell signaling. Front. Plant Sci. 2016;6:1210. doi: 10.3389/fpls.2015.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blatt M.R. Plant physiology: redefining the enigma of metabolism in stomatal movement. Curr. Biol. 2016;26:R107–R109. doi: 10.1016/j.cub.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K., Kinoshita T. The regulation of plant cell expansion: Auxin-induced turgor-driven cell elongation. In: Rose R.J., editor. Molecular Cell Biology of the Growth and Differentiation of Plant Cells. CRC Press; Boca Raton, London, UK: 2016. pp. 156–173. [Google Scholar]
- 6.Osakabe Y., Arinaga N., Umezawa T., Katsura S., Nagamachi K., Tanaka H., Ohiraki H., Yamada K., Seo S.U., Abo M. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell. 2013;25:609–624. doi: 10.1105/tpc.112.105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elumalai R.P., Nagpal P., Reed J.W. A mutation in the Arabidopsis KT2/KUP2 potassium transporter gene affects shoot cell expansion. Plant Cell. 2002;14:119–131. doi: 10.1105/tpc.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z., Persson S., Sánchez-Rodríguez C. At the border: The plasma membrane–cell wall continuum. J. Exp. Bot. 2015;66:1553–1563. doi: 10.1093/jxb/erv019. [DOI] [PubMed] [Google Scholar]
- 9.Nieves-Cordones M., Andrianteranagna M., Cuellar T., Cherel I., Gibrat R., Boeglin M., Moreau B., Paris N., Verdeil J.L., Zimmermann S., et al. Characterization of the grapevine Shaker K+ channel VvK3.1 supports its function in massive potassium fluxes necessary for berry potassium loading and pulvinus-actuated leaf movements. New Phytol. 2019;222:286–300. doi: 10.1111/nph.15604. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Wu W.-H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013;64:451–476. doi: 10.1146/annurev-arplant-050312-120153. [DOI] [PubMed] [Google Scholar]
- 11.Szczerba M.W., Britto D.T., Kronzucker H.J. K+ transport in plants: physiology and molecular biology. J. Plant Physiol. 2009;166:447–466. doi: 10.1016/j.jplph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Wu W.H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017;39:123–128. doi: 10.1016/j.pbi.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Gierth M., Maser P. Potassium transporters in plants - Involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 2007;581:2348–2356. doi: 10.1016/j.febslet.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Walker D.J., Black C.R., Miller A.J. The role of cytosolic potassium and pH in the growth of barley roots. Plant Physiol. 1998;118:957–964. doi: 10.1104/pp.118.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maathuis F.J., Sanders D. Mechanisms of potassium absorption by higher plant roots. Physiol. Plant. 1996;96:158–168. doi: 10.1111/j.1399-3054.1996.tb00197.x. [DOI] [Google Scholar]
- 16.Pritchard J. The control of cell expansion in roots. New Phytol. 1994;127:3–26. doi: 10.1111/j.1469-8137.1994.tb04255.x. [DOI] [PubMed] [Google Scholar]
- 17.Dolan L., Davies J. Cell expansion in roots. Curr. Opin. Plant Biol. 2004;7:33–39. doi: 10.1016/j.pbi.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Rigas S., Debrosses G., Haralampidis K., Vicente-Agullo F., Feldmann K.A., Grabov A., Dolan L., Hatzopoulos P. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell. 2001;13:139–151. doi: 10.1105/tpc.13.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desbrosses G., Josefsson C., Rigas S., Hatzopoulos P., Dolan L. AKT1 and TRH1 are required during root hair elongation in Arabidopsis. J. Exp. Bot. 2003;54:781–788. doi: 10.1093/jxb/erg066. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S., Zhang M.L., Ma T.L., Wang Y. Phosphorylation of ARF2 relieves its repression of transcription of the K+ transporter gene HAK5 in response to low potassium stress. Plant Cell. 2016;28:3005–3019. doi: 10.1105/tpc.16.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cakmak I., Hengeler C., Marschner H. Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J. Exp. Bot. 1994;45:1251–1257. doi: 10.1093/jxb/45.9.1251. [DOI] [Google Scholar]
- 22.Cakmak I., Hengeler C., Marschner H. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J. Exp. Bot. 1994;45:1245–1250. doi: 10.1093/jxb/45.9.1245. [DOI] [Google Scholar]
- 23.Wang Y., Wu W.H. Plant sensing and signaling in response to K+-deficiency. Mol. Plant. 2010;3:280–287. doi: 10.1093/mp/ssq006. [DOI] [PubMed] [Google Scholar]
- 24.Brady N.C., Weil R.R. The Nature and Properties of Soils. 13th ed. Pearson Education Inc. Prentice Hall; Upper Saddle River, NJ, USA: 2002. pp. 1–960. [Google Scholar]
- 25.Römheld V., Kirkby E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil. 2010;335:155–180. doi: 10.1007/s11104-010-0520-1. [DOI] [Google Scholar]
- 26.Wang M., Zheng Q., Shen Q., Guo S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013;14:7370–7390. doi: 10.3390/ijms14047370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gierth M., Maser P., Schroeder J.I. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 2005;137:1105–1114. doi: 10.1104/pp.104.057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyo Y.J., Gierth M., Schroeder J.I., Cho M.H. High-affinity K+ transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol. 2010;153:863–875. doi: 10.1104/pp.110.154369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma T., Dreyer I., Riedelsberger J. The role of K+ channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Front. Plant Sci. 2013;4:224. doi: 10.3389/fpls.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santa-Maria G.E., Oliferuk S., Moriconi J.I. KT-HAK-KUP transporters in major terrestrial photosynthetic organisms: A twenty years tale. J. Plant Physiol. 2018;226:77–90. doi: 10.1016/j.jplph.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Kellermeier F., Armengaud P., Seditas T.J., Danku J., Salt D.E., Amtmann A. Analysis of the root system architecture of Arabidopsis provides a quantitative readout of crosstalk between nutritional signals. Plant Cell. 2014;26:1480–1496. doi: 10.1105/tpc.113.122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., Wu W.H., Wang Y. Potassium channel AKT1 is involved in the auxin-mediated root growth inhibition in Arabidopsis response to low K+ stress. J. Int. Plant Biol. 2017;59:895–909. doi: 10.1111/jipb.12575. [DOI] [PubMed] [Google Scholar]
- 33.Drew M. Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol. 1975;75:479–490. doi: 10.1111/j.1469-8137.1975.tb01409.x. [DOI] [Google Scholar]
- 34.Fernández F.G., Brouder S.M., Volenec J.J., Beyrouty C.A., Hoyum R. Soybean shoot and root response to localized water and potassium in a split-pot study. Plant Soil. 2011;344:197–212. doi: 10.1007/s11104-011-0740-z. [DOI] [Google Scholar]
- 35.Kellermeier F., Chardon F., Amtmann A. Natural variation of Arabidopsis root architecture reveals complementing adaptive strategies to potassium starvation. Plant Physiol. 2013;161:1421–1432. doi: 10.1104/pp.112.211144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung J.Y., Shin R., Schachtman D.P. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell. 2009;21:607–621. doi: 10.1105/tpc.108.063099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim M.J., Ciani S., Schachtman D.P. A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Mol. Plant. 2010;3:420–427. doi: 10.1093/mp/ssp121. [DOI] [PubMed] [Google Scholar]
- 38.Hackett C. A study of the root system of barley: I. Effects of nutrition on two varieties. New Phytol. 1968;67:287–299. doi: 10.1111/j.1469-8137.1968.tb06384.x. [DOI] [Google Scholar]
- 39.Cakmak I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005;168:521–530. doi: 10.1002/jpln.200420485. [DOI] [Google Scholar]
- 40.Christian M., Steffens B., Schenck D., Burmester S., Böttger M., Lüthen H. How does auxin enhance cell elongation? Roles of auxin-binding proteins and potassium channels in growth control. Plant Biol. 2006;8:346–352. doi: 10.1055/s-2006-923965. [DOI] [PubMed] [Google Scholar]
- 41.Philippar K., Ivashikina N., Ache P., Christian M., Lüthen H., Palme K., Hedrich R. Auxin activates KAT1 and KAT2, two K+-channel genes expressed in seedlings of Arabidopsis thaliana. Plant J. 2004;37:815–827. doi: 10.1111/j.1365-313X.2003.02006.x. [DOI] [PubMed] [Google Scholar]
- 42.Pilot G., Lacombe B., Gaymard F., Chérel I., Boucherez J., Thibaud J.B., Sentenac H. Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J. Biol. Chem. 2001;276:3215–3221. doi: 10.1074/jbc.M007303200. [DOI] [PubMed] [Google Scholar]
- 43.Reintanz B., Szyroki A., Ivashikina N., Ache P., Godde M., Becker D., Palme K., Hedrich R. AtKC1, a silent Arabidopsis potassium channel α-subunit modulates root hair K+ influx. Proc. Natl. Acad. Sci. USA. 2002;99:4079–4084. doi: 10.1073/pnas.052677799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagarde D., Basset M., Lepetit M., Conejero G., Gaymard F., Astruc S., Grignon C. Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J. 1996;9:195–203. doi: 10.1046/j.1365-313X.1996.09020195.x. [DOI] [PubMed] [Google Scholar]
- 45.Sentenac H., Bonneaud N., Minet M., Lacroute F., Salmon J.-M., Gaymard F., Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- 46.Hirsch R.E., Lewis B.D., Spalding E.P., Sussman M.R. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- 47.Geiger D., Becker D., Vosloh D., Gambale F., Palme K., Rehers M., Anschuetz U., Dreyer I., Kudla J., Hedrich R. Heteromeric AtKC1· AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J. Biol. Chem. 2009;284:21288–21295. doi: 10.1074/jbc.M109.017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rehman R.U., Stigliano E., Lycett G.W., Sticher L., Sbano F., Faraco M., Dalessandro G., Di Sansebastiano G.P. Tomato Rab11a characterization evidenced a difference between SYP121-dependent and SYP122-dependent exocytosis. Plant Cell Physiol. 2008;49:751–766. doi: 10.1093/pcp/pcn051. [DOI] [PubMed] [Google Scholar]
- 49.Sutter J.U., Campanoni P., Tyrrell M., Blatt M.R. Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. Plant Cell. 2006;18:935–954. doi: 10.1105/tpc.105.038950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honsbein A., Sokolovski S., Grefen C., Campanoni P., Pratelli R., Paneque M., Chen Z., Johansson I., Blatt M.R. A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell. 2009;21:2859–2877. doi: 10.1105/tpc.109.066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grefen C., Chen Z., Honsbein A., Donald N., Hills A., Blatt M.R. A novel motif essential for SNARE interaction with the K+ channel KC1 and channel gating in Arabidopsis. Plant Cell. 2010;22:3076–3092. doi: 10.1105/tpc.110.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grefen C., Karnik R., Larson E., Lefoulon C., Wang Y., Waghmare S., Zhang B., Hills A., Blatt M.R. A vesicle-trafficking protein commandeers Kv channel voltage sensors for voltage-dependent secretion. Nat. Plants. 2015;1:15108. doi: 10.1038/nplants.2015.108. [DOI] [PubMed] [Google Scholar]
- 53.Huisman R., Bisseling T. Growth and development: Close relations of secretion and K+ Nat. Plants. 2015;1:15113. doi: 10.1038/nplants.2015.113. [DOI] [PubMed] [Google Scholar]
- 54.Lefoulon C., Waghmare S., Karnik R., Blatt M.R. Gating control and K+ uptake by the KAT1 K+ channel leaveraged through membrane anchoring of the trafficking protein SYP121. Plant Cell Environ. 2018;41:2668–2677. doi: 10.1111/pce.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang B., Karnik R., Wang Y., Wallmeroth N., Blatt M.R., Grefen C. The Arabidopsis R-SNARE VAMP721 interacts with KAT1 and KC1 K+ channels to moderate K+ current at the plasma membrane. Plant Cell. 2015;27:1697–1717. doi: 10.1105/tpc.15.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karnik R., Waghmare S., Zhang B., Larson E., Lefoulon C., Gonzalez W., Blatt M.R. Commandeering channel voltage sensors for secretion, cell turgor, and volume control. Trends Plant Sci. 2017;22:81–95. doi: 10.1016/j.tplants.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hachez C., Laloux T., Reinhardt H., Cavez D., Degand H., Grefen C., De Rycke R., Inzé D., Blatt M.R., Russinova E. Arabidopsis SNAREs SYP61 and SYP121 coordinate the trafficking of plasma membrane aquaporin PIP2; 7 to modulate the cell membrane water permeability. Plant Cell. 2014;26:3132–3147. doi: 10.1105/tpc.114.127159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Datta S., Kim C.M., Pernas M., Pires N.D., Proust H., Tam T., Vijayakumar P., Dolan L. Root hairs: development, growth and evolution at the plant-soil interface. Plant Soil. 2011;346:1–14. doi: 10.1007/s11104-011-0845-4. [DOI] [Google Scholar]
- 59.Høgh-Jensen H., Pedersen M.B. Morphological plasticity by crop plants and their potassium use efficiency. J. Plant Nutr. 2003;26:969–984. doi: 10.1081/PLN-120020069. [DOI] [Google Scholar]
- 60.Klinsawang S., Sumranwanich T., Wannaro A., Saengwilai P. Effects of root hair length on potassium acquisition in rice (Oryza sativa L.) Appl. Ecol. Environ. Res. 2018;16:1609–1620. doi: 10.15666/aeer/1602_16091620. [DOI] [Google Scholar]
- 61.Balcerowicz D., Schoenaers S., Vissenberg K. Cell fate determination and the switch from diffuse growth to planar polarity in Arabidopsis root epidermal cells. Front. Plant Sci. 2015;6:1163–1176. doi: 10.3389/fpls.2015.01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schoenaers S., Balcerowicz D., Vissenberg K. Molecular mechanisms regulating root hair tip growth: A comparison with pollen tubes. In: Obermeyer G., Feijó J., editors. Pollen Tip Growth: From Biophysical Aspects to Systems Biology. Springer International Publishing; Cham, Switzerland: 2017. pp. 167–243. [Google Scholar]
- 63.Hoth S., Dreyer I., Dietrich P., Becker D., Müller-Röber B., Hedrich R. Molecular basis of plant-specific acid activation of K+ uptake channels. Proc. Natl. Acad. Sci. USA. 1997;94:4806–4810. doi: 10.1073/pnas.94.9.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bibikova T.N., Jacob T., Dahse I., Gilroy S. Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development. 1998;125:2925–2934. doi: 10.1242/dev.125.15.2925. [DOI] [PubMed] [Google Scholar]
- 65.Rigas S., Ditengou F.A., Ljung K., Daras G., Tietz O., Palme K., Hatzopoulos P. Root gravitropism and root hair development constitute coupled developmental responses regulated by auxin homeostasis in the Arabidopsis root apex. New Phytol. 2013;197:1130–1141. doi: 10.1111/nph.12092. [DOI] [PubMed] [Google Scholar]
- 66.Daras G., Rigas S., Tsitsekian D., Iacovides T.A., Hatzopoulos P. Potassium transporter TRH1 subunits assemble regulating root-hair elongation autonomously from the cell fate determination pathway. Plant Sci. 2015;231:131–137. doi: 10.1016/j.plantsci.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 67.Nieves-Cordones M., Rodenas R., Chavanieu A., Rivero R.M., Martinez V., Gaillard I., Rubio F. Uneven HAK/KUP/KT protein diversity among angiosperms: species distribution and perspectives. Front. Plant Sci. 2016;7:127. doi: 10.3389/fpls.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grabov A. Plant KT/KUP/HAK potassium transporters: single family - multiple functions. Ann. Bot. 2007;99:1035–1041. doi: 10.1093/aob/mcm066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vicente-Agullo F., Rigas S., Desbrosses G., Dolan L., Hatzopoulos P., Grabov A. Potassium carrier TRH1 is required for auxin transport in Arabidopsis roots. Plant J. 2004;40:523–535. doi: 10.1111/j.1365-313X.2004.02230.x. [DOI] [PubMed] [Google Scholar]
- 70.Müller M., Schmidt W. Environmentally induced plasticity of root hair development in Arabidopsis. Plant Physiol. 2004;134:409–419. doi: 10.1104/pp.103.029066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee R.D., Cho H.T. Auxin, the organizer of the hormonal/environmental signals for root hair growth. Front. Plant Sci. 2013;4:448. doi: 10.3389/fpls.2013.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dharmasiri N., Dharmasiri S., Weijers D., Lechner E., Yamada M., Hobbie L., Ehrismann J.S., Jurgens G., Estelle M. Plant development is regulated by a family of auxin receptor F box proteins. Dev. Cell. 2005;9:109–119. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 73.Hodge A., Berta G., Doussan C., Merchan F., Crespi M. Plant root growth, architecture and function. Plant Soil. 2009;321:153–187. doi: 10.1007/s11104-009-9929-9. [DOI] [Google Scholar]
- 74.Schachtman D.P. The role of ethylene in plant responses to K+ deficiency. Front. Plant Sci. 2015;6:1153. doi: 10.3389/fpls.2015.01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alonso J.M., Hirayama T., Roman G., Nourizadeh S., Ecker J.R. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- 76.Guzman P., Ecker J.R. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shin R., Schachtman D.P. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA. 2004;101:8827–8832. doi: 10.1073/pnas.0401707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shin R., Berg R.H., Schachtman D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005;46:1350–1357. doi: 10.1093/pcp/pci145. [DOI] [PubMed] [Google Scholar]
- 79.Hermans C., Hammond J.P., White P.J., Verbruggen N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006;11:610–617. doi: 10.1016/j.tplants.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 80.Jordan-Meille L., Martineau E., Bornot Y., Lavres J., Abreu-Junior C., Domec J.-C. How does water-stressed corn respond to potassium nutrition? A shoot-root scale approach study under controlled conditions. Agriculture. 2018;8:180. doi: 10.3390/agriculture8110180. [DOI] [Google Scholar]
- 81.Zhao X.-h., Qiu H., Wen J., Wang X., Du Q., Wang J., Wang Q. Response of root morphology, physiology and endogenous hormones in maize (Zea mays L.) to potassium deficiency. J. Integr. Agr. 2016;15:785–794. doi: 10.1016/S2095-3119(15)61246-1. [DOI] [Google Scholar]
- 82.Amtmann A., Troufflard S., Armengaud P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 2008;133:682–691. doi: 10.1111/j.1399-3054.2008.01075.x. [DOI] [PubMed] [Google Scholar]
- 83.Rengel Z., Damon P.M. Crops and genotypes differ in efficiency of potassium uptake and use. Physiol. Plant. 2008;133:624–636. doi: 10.1111/j.1399-3054.2008.01079.x. [DOI] [PubMed] [Google Scholar]
- 84.Zorb C., Senbayram M., Peiter E. Potassium in agriculture - Status and perspectives. J. Plant Physiol. 2014;171:656–669. doi: 10.1016/j.jplph.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 85.Chen J., Gabelman W.H. Morphological and physiological characteristics of tomato roots associated with potassium-acquisition efficiency. Sci. Hort. 2000;83:213–225. doi: 10.1016/S0304-4238(99)00079-5. [DOI] [Google Scholar]
- 86.Chen J., Gabelman W. Isolation of tomato strains varying in potassium acquisition using a sand-zeolite culture system. Plant Soil. 1995;176:65–70. doi: 10.1007/BF00017676. [DOI] [Google Scholar]
- 87.Fernández F.G., Brouder S.M., Volenec J.J., Beyrouty C.A., Hoyum R. Root and shoot growth, seed composition, and yield components of no-till rainfed soybean under variable potassium. Plant Soil. 2009;322:125–138. doi: 10.1007/s11104-009-9900-9. [DOI] [Google Scholar]
- 88.Liu C., Tu B., Wang X., Jin J., Li Y., Zhang Q., Liu X., Ma B. Potassium translocation combined with specific root uptake is responsible for the high potassium efficiency in vegetable soybean. Crop Pasture Sci. 2019;70:516–525. doi: 10.1071/CP19042. [DOI] [Google Scholar]
- 89.Jia Y.B., Yang X.E., Feng Y., Jilani G. Differential response of root morphology to potassium deficient stress among rice genotypes varying in potassium efficiency. J. Zhejiang Uni. Sci. B. 2008;9:427–434. doi: 10.1631/jzus.B0710636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song W., Xue R., Song Y., Bi Y., Liang Z., Meng L., Dong C., Wang C., Liu G., Dong J., et al. Differential response of first-order lateral root elongation to low potassium involves nitric oxide in two tobacco cultivars. J. Plant Growth Regul. 2018;37:114–127. doi: 10.1007/s00344-017-9711-9. [DOI] [Google Scholar]
- 91.Garcia K., Ané J.M. Polymorphic responses of Medicago truncatula accessions to potassium deprivation. Plant Signal. Behav. 2017;12:e1307494. doi: 10.1080/15592324.2017.1307494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y., Wang Y., Li B., Xiong C., Eneji A.E., Zhang M., Li F., Tian X., Li Z. The cotton high-affinity K+ transporter, GhHAK5a, is essential for shoot regulation of K+ uptake in root under potassium deficiency. Plant Cell Physiol. 2019;60:888–899. doi: 10.1093/pcp/pcz003. [DOI] [PubMed] [Google Scholar]
- 93.Zhang H., Xiao W., Yu W., Yao L., Li L., Wei J., Li R. Foxtail millet SiHAK1 excites extreme high-affinity K+ uptake to maintain K+ homeostasis under low K+ or salt stress. Plant Cell Rep. 2018;37:1533–1546. doi: 10.1007/s00299-018-2325-2. [DOI] [PubMed] [Google Scholar]
- 94.Nieves-Cordones M., Martínez-Cordero M.A., Martínez V., Rubio F. An NH4+-sensitive component dominates high-affinity K+ uptake in tomato plants. Plant Sci. 2007;172:273–280. doi: 10.1016/j.plantsci.2006.09.003. [DOI] [Google Scholar]
- 95.Wang T.-B., Gassmann W., Rubio F., Schroeder J.I., Glass A.D. Rapid up-regulation of HKT1, a high-affinity potassium transporter gene, in roots of barley and wheat following withdrawal of potassium. Plant Physiol. 1998;118:651–659. doi: 10.1104/pp.118.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feng H., Tang Q., Cai J., Xu B., Xu G., Yu L. Rice OsHAK16 functions in potassium uptake and translocation in shoot, maintaining potassium homeostasis and salt tolerance. Planta. 2019;250:549–561. doi: 10.1007/s00425-019-03194-3. [DOI] [PubMed] [Google Scholar]
- 97.Lynch J.P. Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytol. 2019;223:548–564. doi: 10.1111/nph.15738. [DOI] [PubMed] [Google Scholar]
- 98.Lima J.E., Kojima S., Takahashi H., von Wirén N. Ammonium triggers lateral root branching in Arabidopsis in an AMMONIUM TRANSPORTER1; 3-dependent manner. Plant Cell. 2010;22:3621–3633. doi: 10.1105/tpc.110.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krouk G., Crawford N.M., Coruzzi G.M., Tsay Y.-F. Nitrate signaling: adaptation to fluctuating environments. Curr. Opin. Plant Biol. 2010;13:265–272. doi: 10.1016/j.pbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 100.Krouk G., Lacombe B., Bielach A., Perrine-Walker F., Malinska K., Mounier E., Hoyerova K., Tillard P., Leon S., Ljung K. Nitrate-regulated auxin transport by NRT1. 1 defines a mechanism for nutrient sensing in plants. Dev. Cell. 2010;18:927–937. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 101.Postma J.A., Lynch J.P. Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiol. 2011;156:1190–1201. doi: 10.1104/pp.111.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schneider H.M., Postma J.A., Wojciechowski T., Kuppe C., Lynch J.P. Root cortical senescence improves growth under suboptimal availability of N, P, and K. Plant Physiol. 2017;174:2333–2347. doi: 10.1104/pp.17.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barberon M. The endodermis as a checkpoint for nutrients. New Phytol. 2017;213:1604–1610. doi: 10.1111/nph.14140. [DOI] [PubMed] [Google Scholar]
- 104.Barberon M., Vermeer J.E.M., De Bellis D., Wang P., Naseer S., Andersen T.G., Humbel B.M., Nawrath C., Takano J., Salt D.E. Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell. 2016;164:447–459. doi: 10.1016/j.cell.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 105.Qi Z., Hampton C.R., Shin R., Barkla B.J., White P.J., Schachtman D.P. The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a caesium uptake pathway in Arabidopsis. J. Exp. Bot. 2008;59:595–607. doi: 10.1093/jxb/erm330. [DOI] [PubMed] [Google Scholar]
- 106.Chen G., Hu Q., Luo L., Yang T., Zhang S., Hu Y., Yu L., Xu G. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 2015;38:2747–2765. doi: 10.1111/pce.12585. [DOI] [PubMed] [Google Scholar]
- 107.Shin R., Burch A.Y., Huppert K.A., Tiwari S.B., Murphy A.S., Guilfoyle T.J., Schachtman D.P. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell. 2007;19:2440–2453. doi: 10.1105/tpc.107.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song W., Liu S., Meng L., Xue R., Wang C., Liu G., Dong C., Wang S., Dong J., Zhang Y. Potassium deficiency inhibits lateral root development in tobacco seedlings by changing auxin distribution. Plant Soil. 2015:163–173. doi: 10.1007/s11104-015-2579-1. [DOI] [Google Scholar]
- 109.Van der Graaff E., Laux T., Rensing S.A. The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009;10:248. doi: 10.1186/gb-2009-10-12-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen G., Feng H., Hu Q., Qu H., Chen A., Yu L., Xu G. Improving rice tolerance to potassium deficiency by enhancing OsHAK16p:WOX11-controlled root development. Plant Biotechnol. J. 2015;13:833–848. doi: 10.1111/pbi.12320. [DOI] [PubMed] [Google Scholar]
- 111.Zhang L., Li G., Wang M., Di D., Sun L., Kronzucker H.J., Shi W. Excess iron stress reduces root tip zone growth through nitric oxide-mediated repression of potassium homeostasis in Arabidopsis. New Phytol. 2018;219:259–274. doi: 10.1111/nph.15157. [DOI] [PubMed] [Google Scholar]
- 112.Zheng Y., Drechsler N., Rausch C., Kunze R. The Arabidopsis nitrate transporter NPF7. 3/NRT1. 5 is involved in lateral root development under potassium deprivation. Plant Signal. and Behav. 2016;11:2832–2847. doi: 10.1080/15592324.2016.1176819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin S.H., Kuo H.F., Canivenc G., Lin C.S., Lepetit M., Hsu P.K., Tillard P., Lin H.L., Wang Y.Y., Tsai C.B., et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell. 2008;20:2514–2528. doi: 10.1105/tpc.108.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Drechsler N., Zheng Y., Bohner A., Nobmann B., von Wiren N., Kunze R., Rausch C. Nitrate-dependent control of shoot K homeostasis by the nitrate transporter1/peptide transporter family member NPF7.3/NRT1.5 and the stelar K+ outward rectifier SKOR in Arabidopsis. Plant Physiol. 2015;169:2832–2847. doi: 10.1104/pp.15.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meng S., Peng J.S., He Y.N., Zhang G.B., Yi H.Y., Fu Y.L., Gong J.M. Arabidopsis NRT1.5 mediates the suppression of nitrate starvation-induced leaf senescence by modulating foliar potassium level. Mol. Plant. 2016;9:461–470. doi: 10.1016/j.molp.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 116.Cui Y.N., Li X.T., Yuan J.Z., Wang F.Z., Wang S.M., Ma Q. Nitrate transporter NPF7.3/NRT1.5 plays an essential role in regulating phosphate deficiency responses in Arabidopsis. Biochem. Biophys. Res. Commun. 2019;508:314–319. doi: 10.1016/j.bbrc.2018.11.118. [DOI] [PubMed] [Google Scholar]
- 117.Greenway H., Pitman M. Potassium retranslocation in seedlings of Hordeum vulgare. Austr. J. Biol. Sci. 1965;18:235–247. doi: 10.1071/BI9650235. [DOI] [Google Scholar]
- 118.Jeschke W.D., Atkins C.A., Pate J.S. Ion circulation via phloem and xylem between root and shoot of nodulated white lupin. J. Plant Physiol. 1985;117:319–330. doi: 10.1016/S0176-1617(85)80068-7. [DOI] [PubMed] [Google Scholar]
- 119.Jeschke W.D., Wolf O., Hartung W. Effect of NaCI salinity on flows and partitioning of C, N, and mineral ions in whole plants of white lupin, Lupinus albus L. J. Exp. Bot. 1992;43:777–788. doi: 10.1093/jxb/43.6.777. [DOI] [Google Scholar]
- 120.Jeschke W.D., Pate J.S. Cation and chloride partitioning through xylem and phloem within the whole plant of Ricinus communis L. under conditions of salt stress. J. Exp. Bot. 1991;42:1105–1116. doi: 10.1093/jxb/42.9.1105. [DOI] [Google Scholar]
- 121.Wolf O., Munns R., Tonnet M.L., Jeschke W.D. The role of the stem in the partitioning of Na+ and K+ in salt-treated barley. J. Exp. Bot. 1991;42:697–704. doi: 10.1093/jxb/42.6.697. [DOI] [Google Scholar]
- 122.Johansson I., Wulfetange K., Porée F., Michard E., Gajdanowicz P., Lacombe B., Sentenac H., Thibaud J.-B., Mueller-Roeber B., Blatt M.R., et al. External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. Plant J. 2006;46:269–281. doi: 10.1111/j.1365-313X.2006.02690.x. [DOI] [PubMed] [Google Scholar]
- 123.Drew M.C., Saker L.R. Uptake and long-distance transport of phosphate, potassium and chloride in relation to internal ion concentrations in barley: Evidence of non-allosteric regulation. Planta. 1984;160:500–507. doi: 10.1007/BF00411137. [DOI] [PubMed] [Google Scholar]
- 124.Martineau E., Domec J.-C., Bosc A., Dannoura M., Gibon Y., Bénard C., Jordan-Meille L. The role of potassium on maize leaf carbon exportation under drought condition. Acta Physiol. Plant. 2017;39:219–232. doi: 10.1007/s11738-017-2515-5. [DOI] [Google Scholar]
- 125.Berg W.K., Lissbrant S., Cunningham S.M., Brouder S.M., Volenec J.J. Phosphorus and potassium effects on taproot C and N reserve pools and long-term persistence of alfalfa (Medicago sativa L.) Plant Sci. 2018;272:301–308. doi: 10.1016/j.plantsci.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 126.Mengel K., Haeder H.-E. Effect of potassium supply on the rate of phloem sap exudation and the composition of phloem sap of Ricinus communis. Plant Physiol. 1977;59:282–284. doi: 10.1104/pp.59.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mengel K. Effect of potassium on the assimilate conduction to storage tissue. Ber. Deut. Bot. Ges. 1980;93:353–362. [Google Scholar]
- 128.Doman D.C., Geiger D.R. Effect of exogenously supplied foliar potassium on phloem loading in Beta vulgaris L. Plant Physiol. 1979;64:528–533. doi: 10.1104/pp.64.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Epron D., Cabral O.M., Laclau J.P., Dannoura M., Packer A.P., Plain C., Battie-Laclau P., Moreira M.Z., Trivelin P.C., Bouillet J.P., et al. In situ 13CO2 pulse labelling of field-grown eucalypt trees revealed the effects of potassium nutrition and throughfall exclusion on phloem transport of photosynthetic carbon. Tree Physiol. 2016;36:6–21. doi: 10.1093/treephys/tpv090. [DOI] [PubMed] [Google Scholar]
- 130.Tian H., Baxter I.R., Lahner B., Reinders A., Salt D.E., Ward J.M. Arabidopsis NPCC6/NaKR1 is a phloem mobile metal binding protein necessary for phloem function and root meristem maintenance. Plant Cell. 2010;22:3963–3979. doi: 10.1105/tpc.110.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Deeken R., Geiger D., Fromm J., Koroleva O., Ache P., Langenfeld-Heyser R., Sauer N., May S.T., Hedrich R. Loss of the AKT2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta. 2002;216:334–344. doi: 10.1007/s00425-002-0895-1. [DOI] [PubMed] [Google Scholar]
- 132.Deeken R., Sanders C., Ache P., Hedrich R. Developmental and light-dependent regulation of a phloem-localised K+ channel of Arabidopsis thaliana. Plant J. 2000;23:285–290. doi: 10.1046/j.1365-313x.2000.00791.x. [DOI] [PubMed] [Google Scholar]
- 133.Dreyer I., Gomez-Porras J.L., Riedelsberger J. The potassium battery: a mobile energy source for transport processes in plant vascular tissues. New Phytol. 2017;216:1049–1053. doi: 10.1111/nph.14667. [DOI] [PubMed] [Google Scholar]
- 134.Gajdanowicz P., Michard E., Sandmann M., Rocha M., Corrêa L.G.G., Ramírez-Aguilar S.J., Gomez-Porras J.L., González W., Thibaud J.-B., Van Dongen J.T. Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc. Natl. Acad. Sci. USA. 2011;108:864–869. doi: 10.1073/pnas.1009777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lynch J., Läuchli A. Potassium transport in salt-stressed barley roots. Planta. 1984;161:295–301. doi: 10.1007/BF00398718. [DOI] [PubMed] [Google Scholar]
- 136.Shi H., Quintero F.J., Pardo J.M., Zhu J.-K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mäser P., Eckelman B., Vaidyanathan R., Horie T., Fairbairn D.J., Kubo M., Yamagami M., Yamaguchi K., Nishimura M., Uozumi N. Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett. 2002;531:157–161. doi: 10.1016/S0014-5793(02)03488-9. [DOI] [PubMed] [Google Scholar]
- 138.Sunarpi H.T., Motoda J., Kubo M., Yang H., Yoda K., Horie R., Chan W.Y., Leung H.Y., Hattori K., Konomi M. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005;44:928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- 139.Berthomieu P., Conéjéro G., Nublat A., Brackenbury W.J., Lambert C., Savio C., Uozumi N., Oiki S., Yamada K., Cellier F. Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J. 2003;22:2004–2014. doi: 10.1093/emboj/cdg207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shabala S., Pottosin I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014;151:257–279. doi: 10.1111/ppl.12165. [DOI] [PubMed] [Google Scholar]
- 141.Gersani M., Graham E.A., Nobel P.S. Growth responses of individual roots of Opuntia ficus-indica to salinity. Plant Cell Environ. 1993;16:827–834. doi: 10.1111/j.1365-3040.1993.tb00504.x. [DOI] [Google Scholar]
- 142.Rubinigg M., Wenisch J., Elzenga J.T.M., Stulen I. NaCl salinity affects lateral root development in Plantago maritima. Funct. Plant Biol. 2004;31:775–780. doi: 10.1071/FP03222. [DOI] [PubMed] [Google Scholar]
- 143.Zolla G., Heimer Y.M., Barak S. Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J. Exp. Bot. 2010;61:211–224. doi: 10.1093/jxb/erp290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rubio F., Gassmann W., Schroeder J.I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 145.Zhu J.-K., Liu J., Xiong L. Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role of potassium nutrition. Plant Cell. 1998;10:1181–1191. doi: 10.1105/tpc.10.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu J., Ishitani M., Halfter U., Kim C.-S., Zhu J.-K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA. 2000;97:3730–3734. doi: 10.1073/pnas.97.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Qiu Q.-S., Guo Y., Dietrich M.A., Schumaker K.S., Zhu J.-K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA. 2002;99:8436–8441. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Halfter U., Ishitani M., Zhu J.-K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA. 2000;97:3735–3740. doi: 10.1073/pnas.97.7.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cheng N.H., Pittman J.K., Zhu J.K., Hirschi K.D. The protein kinase SOS2 activates the Arabidopsis H+/Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. J. Biol. Chem. 2004;279:2922–2926. doi: 10.1074/jbc.M309084200. [DOI] [PubMed] [Google Scholar]
- 150.Coskun D., Britto D.T., Li M., Oh S., Kronzucker H.J. Capacity and plasticity of potassium channels and high-affinity transporters in roots of barley and Arabidopsis. Plant Physiol. 2013;162:496–511. doi: 10.1104/pp.113.215913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Britto D.T., Kronzucker H.J. NH4+ toxicity in higher plants: a critical review. J. Plant Physiol. 2002;159:567–584. doi: 10.1078/0176-1617-0774. [DOI] [Google Scholar]
- 152.Britto D.T., Kronzucker H.J. Futile cycling at the plasma membrane: a hallmark of low-affinity nutrient transport. Trends Plant Sci. 2006;11:529–534. doi: 10.1016/j.tplants.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 153.Liu Y., Lai N., Gao K., Chen F., Yuan L., Mi G. Ammonium inhibits primary root growth by reducing the length of meristem and elongation zone and decreasing elemental expansion rate in the root apex in Arabidopsis thaliana. PLoS ONE. 2013;8:6031–6042. doi: 10.1371/journal.pone.0061031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li Q., LI B.H., Kronzucker H.J., SHI W.M. Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity. Plant Cell Environ. 2010;33:1529–1542. doi: 10.1111/j.1365-3040.2010.02162.x. [DOI] [PubMed] [Google Scholar]
- 155.Roosta H., Schjoerring J.K. Root carbon enrichment alleviates ammonium toxicity in cucumber plants. J. Plant Nutr. 2008;31:941–958. doi: 10.1080/01904160802043270. [DOI] [Google Scholar]
- 156.Zheng X., He K., Kleist T., Chen F., Luan S. Anion channel SLAH3 functions in nitrate-dependent alleviation of ammonium toxicity in Arabidopsis. Plant Cell Environ. 2015;38:474–486. doi: 10.1111/pce.12389. [DOI] [PubMed] [Google Scholar]
- 157.Roosta H.R., Schjoerring J.K. Effects of ammonium toxicity on nitrogen metabolism and elemental profile of cucumber plants. J. Plant Nutr. 2007;30:1933–1951. doi: 10.1080/01904160701629211. [DOI] [Google Scholar]
- 158.Szczerba M.W., Britto D.T., Balkos K.D., Kronzucker H.J. Alleviation of rapid, futile ammonium cycling at the plasma membrane by potassium reveals K+-sensitive and -insensitive components of NH4+ transport. J. Exp. Bot. 2008;59:303–313. doi: 10.1093/jxb/erm309. [DOI] [PubMed] [Google Scholar]
- 159.Szczerba M.W., Britto D.T., Ali S.A., Balkos K.D., Kronzucker H.J. NH4+-stimulated and-inhibited components of K+ transport in rice (Oryza sativa L.) J. Exp. Bot. 2008;59:3415–3423. doi: 10.1093/jxb/ern190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Balkos K.D., Britto D.T., Kronzucker H.J. Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72) Plant Cell Environ. 2010;33:23–34. doi: 10.1111/j.1365-3040.2009.02046.x. [DOI] [PubMed] [Google Scholar]
- 161.Hoopen F.t., Cuin T.A., Pedas P., Hegelund J.N., Shabala S., Schjoerring J.K., Jahn T.P. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: molecular mechanisms and physiological consequences. J. Exp. Bot. 2010;61:2303–2315. doi: 10.1093/jxb/erq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nieves-Cordones M., Mohamed S., Tanoi K., Kobayashi N.I., Takagi K., Vernet A., Guiderdoni E., Perin C., Sentenac H., Very A.A. Production of low-Cs+ rice plants by inactivation of the K+ transporter OsHAK1 with the CRISPR-Cas system. Plant J. 2017;92:43–56. doi: 10.1111/tpj.13632. [DOI] [PubMed] [Google Scholar]
- 163.Yasunari T.J., Stohl A., Hayano R.S., Burkhart J.F., Eckhardt S., Yasunari T. Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc. Natl. Acad. Sci. USA. 2011;108:19530–19534. doi: 10.1073/pnas.1112058108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vakulovsky S., Nikitin A., Chumichev V., Katrich I.Y., Voitsekhovich O., Medinets V., Pisarev V., Bovkum L., Khersonsky E. Cesium-137 and strontium-90 contamination of water bodies in the areas affected by releases from the Chernobyl nuclear power plant accident: an overview. J. Environ. Radioact. 1994;23:103–122. doi: 10.1016/0265-931X(94)90055-8. [DOI] [Google Scholar]
- 165.Hampton C.R., Bowen H.C., Broadley M.R., Hammond J.P., Mead A., Payne K.A., Pritchard J., White P.J. Cesium toxicity in Arabidopsis. Plant Physiol. 2004;136:3824–3837. doi: 10.1104/pp.104.046672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhu Y.G., Smolders E. Plant uptake of radiocaesium: A review of mechanisms, regulation and application. J. Exp. Bot. 2000;51:1635–1645. doi: 10.1093/jexbot/51.351.1635. [DOI] [PubMed] [Google Scholar]
- 167.Kobayashi R., Kobayashi N.I., Tanoi K., Masumori M., Tange T. Potassium supply reduces cesium uptake in Konara oak not by an alteration of uptake mechanism, but by the uptake competition between the ions. J. Environ. Radioact. 2019;208:6032–6040. doi: 10.1016/j.jenvrad.2019.106032. [DOI] [PubMed] [Google Scholar]
- 168.Perrenoud S. Potassium and Plant Health. International Potash Institute; Bern, Switzerland: 1977. pp. 1–5. [Google Scholar]
- 169.Armengaud P., Breitling R., Amtmann A. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 2004;136:2556–2576. doi: 10.1104/pp.104.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Troufflard S., Mullen W., Larson T.R., Graham I.A., Crozier A., Amtmann A., Armengaud P. Potassium deficiency induces the biosynthesis of oxylipins and glucosinolates in Arabidopsis thaliana. BMC Plant Biol. 2010;10:172–185. doi: 10.1186/1471-2229-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Khan G.A., Vogiatzaki E., Glauser G., Poirier Y. Phosphate deficiency induces the jasmonate pathway and enhances resistance to insect herbivory. Plant Physiol. 2016;171:632–644. doi: 10.1104/pp.16.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Staswick P.E., Su W., Howell S.H. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc. Natl. Acad. Sci. USA. 1992;89:6837–6840. doi: 10.1073/pnas.89.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]