Abstract
Recent advances in synthetic biology and an emerging algal biotechnology market have spurred a prolific increase in the availability of molecular tools for cyanobacterial research. Nevertheless, work to date has focused primarily on only a small subset of model species, which arguably limits fundamental discovery and applied research towards wider commercialisation. Here, we review the requirements for uptake of new strains, including several recently characterised fast-growing species and promising non-model species. Furthermore, we discuss the potential applications of new techniques available for transformation, genetic engineering and regulation, including an up-to-date appraisal of current Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR associated protein (CRISPR/Cas) and CRISPR interference (CRISPRi) research in cyanobacteria. We also provide an overview of several exciting molecular tools that could be ported to cyanobacteria for more advanced metabolic engineering approaches (e.g., genetic circuit design). Lastly, we introduce a forthcoming mutant library for the model species Synechocystis sp. PCC 6803 that promises to provide a further powerful resource for the cyanobacterial research community.
Keywords: CRISPR/Cas, CRISPRi, genetic circuits, genome engineering, inteins, genome-scale models, mutant library, optogenetics, serine integrase, sigma factors, synthetic biology
1. Introduction
Cyanobacteria are a diverse phylum of photosynthetic prokaryotes that are found in a wide variety of marine and freshwater habitats [1,2,3,4]. Oxygenic photosynthesis evolved approximately 2.5 billion years ago in the predecessors to modern-day cyanobacteria [5]. Their early success led to a significant increase in free oxygen (O2) in the Earth’s atmosphere and the subsequent evolution of most aerobic organisms [6,7,8]. Today, cyanobacteria account for 20%–30% of global carbon dioxide (CO2) fixation [9].
The ability of cyanobacteria to convert captured carbon into a wide variety of complex organic molecules makes them promising platforms for the sustainable production of biofuels and high-value chemicals [10,11,12]. Compared to plants, cyanobacteria offer several advantages for biotechnological applications, including (1) higher photosynthetic efficiencies [13,14], (2) capacity to grow in hostile living environments (e.g., in extremes of temperature, salinity and pH) [1,3,4], (3) the ability to be cultured on non-arable land with minimal nutrients [15], and (4) the relatively rapid and inexpensive generation of mutants (predominantly in model species) [16]. Moreover, chloroplasts descend from an internalised cyanobacterium [17], thus certain physiological and biochemical features are conserved in eukaryotic photosynthetic organisms, making cyanobacteria excellent chassis for production of plant-derived natural products, such as terpenes [18,19]. Currently, several companies are investigating the use of cyanobacteria for producing biochemicals, including biofuels (Algenol), inks (Living Ink Technologies) and pigments for food and cosmetics (DIC Corp., Lumen Bioscience, ScotBio). Nevertheless, several hurdles still need to be overcome for more widespread industrial adoption.
One key issue has been the relatively slow growth rates of cyanobacterial species, including those developed as models such as Synechocystis sp. PCC 6803 (PCC 6803) and Synechococcus elongatus PCC 7942 (PCC 7942). For example, PCC 6803 has a doubling-time of ca. 7 h under standard growth conditions [20], compared to 20 min for Escherichia coli and 2 h for Saccharomyces cerevisiae [21,22]. Recently, several genetically tractable cyanobacterial species have been characterised with faster growth rates that are comparable with S. cerevisiae [20,23,24]. In this review, we will discuss these strains and other non-model species that demonstrate the potential for cyanobacteria to close the gap between industrially viable heterotrophic and phototrophic bio-platforms or are useful organisms for investigating unique aspects of cyanobacterial biology and how they adapt to different environments.
A further significant challenge limitation has been the limited availability of molecular tools to engineer cyanobacteria. However, the past few years have seen a rapid proliferation of characterised tools and parts for cyanobacteria, including CRISPR/Cas-based systems [25,26,27,28]. This has driven the widespread adoption of the synthetic biology paradigm for the design of biological tools based on the bottom-up approach of recombining standardised parts or modules (e.g., promoters, ribosome binding sites (RBS), coding sequences and terminators) [29,30,31,32,33,34,35]. Many of those tools were initially developed in E. coli or S. cerevisiae and have been adapted and modified for use in cyanobacteria [36,37]. A significant drawback is that they may behave differently in cyanobacteria and between different cyanobacterial species. Thus, the functionality of all new tools ported for cyanobacterial applications must first be validated [35,38]. Here, we will outline several recent developments in genome engineering using CRISPR/Cas and recombinase approaches, as well as the opportunities and limitation of recent tools developed in E. coli that show promise for cyanobacterial research and biotechnology applications.
To date, 290 draft genomes and 85 full genomes are available online in the CyanoBase database (http://genome.microbedb.jp/cyanobase [39]). The growing availability of cyanobacterial genome sequencing data has helped to foster the development of genome-scale models (GSMs) for a variety of species, ranging from model species (e.g., PCC 6803) to the industrial relevant strain Arthrospira (Spirulina) platensis NIES-39 [40,41,42,43]. As with E. coli [44], GSMs have allowed cyanobacterial researchers to adopt a systems biology approach to propose and predict the outcomes of engineering strategies. Here, we will also highlight examples where GSMs have successfully guided efforts to modify metabolism to improve production in cyanobacteria and how GSMs could drive an increase in available cyanobacterial omics data.
Finally, we will introduce a forthcoming barcoded mutant library in PCC 6803 called CyanoSource. CyanoSource will be a powerful tool to develop a deeper understanding of metabolism in PCC 6803, guide work in other species (including new fast-growing strains) and will set a benchmark for high-throughput research in the cyanobacterial field.
2. “Non-Model” Species: Requirements for Uptake and Genetic Manipulation
Cyanobacterial research has focused primarily on model organisms that are straightforward to culture under laboratory conditions, amenable to genetic modification and can be frozen for long-term storage [16,45,46,47]. The uptake of more recalcitrant, non-model species for laboratory research can present significant challenges. Table 1 outlines some basic features that are desirable for culturing and engineering. Below, we have highlighted several new or known “non-model” cyanobacteria, including their potential benefits, challenges for widespread uptake and industrial usage, and where applicable, progress towards efficient genetic manipulation (Table 2).
Table 1.
Desirable features required for culturing and genetically engineering cyanobacterial strains.
|
Table 2.
Summary of genetic manipulations carried out in model and non-model cyanobacterial species discussed in Section 2.
| Species | Strain | Desirable Features from Table 1 | Transformation Method | Reported Selection Markers | Agar Medium | References |
|---|---|---|---|---|---|---|
| Synechococcus sp. | UTEX 2973 | 1), 2), 3), 4), 5), 6) | Conjugation CRISPR/Cas pilN mutants are naturally transformable |
Apramycin Chloramphenicol Kanamycin Spectinomycin Streptomycin |
BG-11 | [20,33,35,58] |
| PCC 11801 | 1), 2), 3), 5), 6) | Naturally transformable | Spectinomycin | BG-11 | [23] | |
| PCC 11901 | 1), 2), 3), 5), 6) | Naturally transformable | Acrylic acid Spectinomycin |
AD7 | [24] | |
| Nostoc punctiforme | ATCC 29133 | 1), 2), 3), 4), 5), 6) | Conjugation | Chloramphenicol Neomycin sacB markers Streptomycin |
Allen and Arnon | [62,63,90] |
| Cyanothece sp. | PCC 7822 | 1), 2), 3), 5) | Electroporation | Spectinomycin | BG-11 | [66] |
| ATCC 51142 | 1), 2), 3), 4), 6) | Conjugation | Kanamycin | BG-11 ASP2 |
[67] | |
| Arthrospira platensis | C1 | 1), 2), 3), 4), 5) | Electroporation | Spectinomycin | Zarrouk | [53] |
| Leptolyngbya sp. | BL0902 | 1), 2), 3), 4), 5), 6) | Conjugation | Chloramphenicol Erythromycin Neomycin Spectinomycin Streptomycin |
BG-11 | [72,73] |
| Fremyella diplosiphon | SF33 | 1), 2), 3), 4), 6) | Conjugation | Kanamycin Neomycin sacB markers |
BG-11/HEPES | [74,75,91] |
| Marine Synechococcus sp. | WH7803 WH8102 WH8103 |
1), 2), 3), 6) | Conjugation Electroporation |
Kanamycin | SN | [76,77,92] |
| Marine Prochlorococcus sp. | 1), 3) | - | Spectinomycin | Pro99 | [76,93] | |
| Thermosynechococcus elongatus | BP-1 | 1), 2), 3), 4), 5), 6) | Naturally transformable | Chloramphenicol Kanamycin |
BG-11 | [81] |
| PKUAC-SCTE542 | 1), 2), 3), 6) | Naturally transformable | Spectinomycn | BG-11 | [82] | |
| Chlorogloeopsis fritschii | PCC 6912 | 1), 2), 3), 4), 5) | Biolistic Conjugation |
Kanamycin Neomycin |
Allen and Arnon | [83,90] |
| Fischerella muscicola | PCC 7414 | 1), 2), 3), 4), 5) | Biolistic Conjugation |
Kanamycin Neomycin |
Allen and Arnon | [83,90] |
| Chroococcidiopsis thermalis | 1), 2), 3), 4), 5) | Conjugation | Neomycin | BG-11 | [84] | |
| Gloeobacter violaceus | PCC 7421 | 1), 2), 3), 4), 5) | Conjugation | Streptomycin | BG-11 | [89] |
2.1. The Emergence of Fast-Growing and Stress-Tolerant Synechococcus Strains
Cyanobacterial strains that can achieve growth rates comparable with heterotrophic microbes could be of significant value to basic research and the biotechnology industry. Recently, three new Synechococcus strains have been reported with high growth rates: Synechococcus elongatus UTEX 2973 (UTEX 2973), Synechococcus elongatus PCC 11801 (PCC 11801) and Synechococcus sp. PCC 11901 (PCC 11901). UTEX 2973 was first described in 2015 as a fast-growing, stress-tolerant strain that was re-isolated from a previously characterised fast-growing strain that had lost the ability for fast growth [20,55]. Under high light (>500 µmol photons m−2 s−1) and high temperatures (38–42 °C), UTEX 2973 can achieve doubling times similar to that of S. cerevisiae (ca. 2 h) during the early growth phase [20,24,56]. Under those conditions, UTEX 2973 can produce biomass twice as fast as its close relative PCC 7942, despite only a small number of nucleotide differences between the two genomes [57]. However, growth was not examined for longer periods of time (i.e., >24 h). At lower temperatures (30 °C), growth of UTEX 2973 is slower than PCC 7942 or PCC 6803 at 300 or 500 µmol photons m−2 s−1 [35], and slower than 6803 when cultured for longer periods (up to 10 days) under high light (750 µmol photons m−2 s−1) [24]. UTEX 2973 is not naturally competent, but is amenable to conjugation and genome editing by CRISPR/Cas [20,58]. Furthermore, naturally transformable mutants of UTEX 2973 have been described [33], while three point mutations in PCC 7942 can reportedly lead to growth rates similar to that of UTEX 2973 [57]. The latter finding could have a significant impact on cyanobacterial research as PCC 7942 is a naturally transformable and widely used model species.
PCC 11801 was isolated from India and first described in 2018 [23]. Similarly to UTEX 2973, PCC 11801 is tolerant to high light and temperatures and exhibits fast growth rates (i.e., a doubling time of 2.3 h). Furthermore, PCC 11801 is tolerant of high levels of NaCl (i.e., it can grow at sea salt concentrations (ca. 0.7 M), whereas PCC 7942 cannot) and it is naturally transformable. The genome sequence of PCC 11801 is highly similar to PCC 7942 and UTEX 2973 (ca. 83%). Thus, existing plasmid vectors used for engineering PCC 7942 are widely compatible in PCC 11801.
One of the best-performing strains reported to date is PCC 11901, which was isolated in Singapore from an estuarine environment enriched with nitrogen and phosphorous compounds [24]. PCC 11901 is tolerant to high light levels, achieves fast growth rates comparable to UTEX 2973, and is tolerant to a wide range of salinities, similar to PCC 11801. Although UTEX 2973 grows faster than PCC 11901 during the early growth phase, PCC 11901 reportedly outperforms UTEX 2973 when grown for longer time periods (i.e., >24 h). PCC 11901 accumulated 2–3 times more biomass when grown alongside PCC 6803, PCC 7942, Synechococcus sp. PCC 7002 (PCC 7002) and UTEX 2973, achieving an OD730 = 101 and a biomass of 18.3 g dry weight per litre. PCC 11901 is naturally transformable with efficiencies similar to that of PCC 7002, as demonstrated by the generation of markerless mutants using methods previously described in PCC 7002 [59].
2.2. Nostoc punctiforme ATCC 29133
Nostoc punctiforme ATCC 29133 (ATCC 29133) is a nitrogen (N2)-fixing, heterocyst-forming cyanobacterium which forms a symbiotic relationship within the coralloid roots of plants. ATCC 29133 is a useful organism for investigating symbiotic relationships between plants and N2-fixing cyanobacteria [60]. It has also been key to understanding the biosynthetic pathway of scytonemin, a natural sunscreen against UV damage produced by many cyanobacterial species [61]. Transformation of ATCC 29133 was first described in 1994 [62] and has since been further developed to generate scytonemin-deficient mutants [63]. Mutants were obtained via conjugation, either by random transposon insertion into the open reading frame of the scytonemin biosynthesis operon or by allelic exchange. ATCC 29133 appears amenable to selection using chloramphenicol, neomycin, streptomycin and sacB markers.
2.3. Cyanothece sp.
Cyanothece species are a group of unicellular cyanobacteria that can perform photosynthesis and fix N2 within the same cell via temporal separation of the two processes. N2 fixation occurs during the dark period when O2 levels are low. They are natural contributors to N2 fixation in rice paddies, and, therefore, could play an important role in reducing agricultural fertiliser use [64,65]. A genetic transformation protocol for producing targeted gene knockouts in Cyanothece sp. PCC 7822 was developed in 2010 [66], where a single-stranded DNA fragment encoding a spectinomycin resistance cassette was electroporated into cells, leading to integration of the cassette at random points in the genome via non-homologous recombination. Despite testing Cyanothece sp. ATCC 51142, PCC 7424, PCC 7425, PCC 8801 and PCC 8802, mutants could only be generated in PCC 7822 via this technique. More recently, Liberton et al. [67] reported a method for generating targeted mutations in Cyanothece sp. ATCC 51142 using triparental mating. A plasmid encoding two methylases was required in order to make the cargo plasmid more resistant to digestion. Using this system, a kanamycin resistance cassette was inserted into a targeted chromosomal site.
2.4. Arthrospira sp.
Arthrospira species are the source of high-value nutraceuticals (e.g., Spirulina) and natural blue pigments in food (e.g., the phycobiliprotein, C-phycocyanin) [68,69]. They tolerate high levels of alkalinity and can be cultured in a variety of closed or open (e.g., race way pond) environments. Nevertheless, Arthrospira sp. are highly resistant to genetic modification due to an abundance of native restriction–modification systems that can rapidly degrade heterologous DNA [70]. The most efficient transformation system reported to date used a Tn5 transposase expression cassette to generate random integration events in the genome of Arthrospira platensis C1 and selection via a spectinomycin resistance cassette [53]. DNA degradation was minimised by encapsulation in liposomes and mixing with a type 1 restriction inhibitor prior to electroporation. Transformed cells resistant to spectinomycin were reportedly stable for several months. It is tempting to speculate that delivery of a CRISPR/Cas system with this approach could enable targeted genome editing in Arthrospira sp.
2.5. Leptolyngbya sp.
Leptolyngbya species are widely distributed in terrestrial and freshwater environments [71], and are, therefore, of great ecological interest. Leptolyngbya sp. BL0902 is also used for the production of biomass and bioproducts, as it can grow at a range of industrially viable temperatures, tolerate high salt concentrations, pH extremes and variable light conditions. Growth rates in the laboratory and in outdoor ponds are similar to those of Arthrospira species [72]. Conjugal transformation of Leptolyngbya sp. BL0902 (BL0902) with broad host range vectors based on RSF1010 was successfully carried out in 2012 [72,73], although two antibiotics were required to limit the appearance of spontaneous resistant mutants. Conjugation was used for generation of a transposon library and introduction of an expression plasmid.
2.6. Fremyella diplosiphon
Fremyella diplosiphon is a filamentous, heterocyst-forming, freshwater species that can adjust its photosynthetic receptors and antenna to differences in light intensity and quality. Detailed methods for genetic manipulation are available that allow for the generation of unmarked mutants [74]. First, the plasmid of interest is methylated to protect it from digestion in F. diplosiphon. The plasmid is then introduced via triparental mating and transconjugates selected on plates containing neomycin [75]. Unmarked mutants can then be generated using sacB.
2.7. Marine Synechococcus sp. and Prochlorococcus sp.
Marine Synechococcus and Prochlorococcus genera are responsible for approximately a quarter of ocean primary productivity [2], and are, therefore, of great academic interest. Genetic manipulation of Prochlorococcus sp. (including the introduction of a heterologous plasmid) has not been reported. However, Prochlorococcus strains can be cultured on semi-solid agar plates in the presence of specific ‘helper’ heterotrophic bacteria [76], fulfilling the initial requirement for genetic manipulation. Gene deletion has been reported in the marine Synechococcus species WH7803, WH8102 and WH8103 [77,78]. Plasmids were introduced into cells via biparental mating or electroporation. Transformants were plated on semi-solid (0.3% w/v) agar plates and kanamycin was used as the selectable marker. Via this method, self-replicating and suicide plasmids were introduced, which facilitated targeted mutations.
2.8. Thermosynechococcus elongatus
Thermosynechococcus elongatus BP-1 is a thermophilic cyanobacterium with optimal growth at 55 °C, making it ideal for biotechnology applications that require high temperatures [79]. T. elongatus BP-1 proteins are also ideal for purification and crystallographic studies, due to their increased stability at high temperatures [80]. A natural transformation method for chromosomal integration has been developed for T. elongatus BP-1, with either kanamycin or chloramphenicol used for selection of transformants [81]. More recently, T. elongatus PKUAC-SCTE542 has been highlighted as a naturally transformable strain with high growth rates that is sensitive to spectinomycin [82].
2.9. Chlorogloeopsis fritschii and Fischerella muscicola
Chlorogloeopsis fristchii sp. PCC 6912 and Fischerella muscicola PCC 7414 are two of the most complex species of cyanobacteria, in that they are filamentous, heterocyst-forming strains able to undergo multiplanar cell division and thereby create multiseriate filaments [83]. Introduction of expression plasmids via conjugation and biolistic DNA transfer methods has been reported in both species. Conjugation was made possible by the partial removal of the exopolysaccharide sheath by introducing a salt washing step.
2.10. Chroococcidiopsis thermalis
Chroococcidiopsis thermalis is found in environments with extremes of temperature (both hot and cold) [84]. Furthermore, these extremophile cyanobacteria can survive long periods of desiccation and high levels of solar radiation that few other organisms can tolerate. C. thermalis incorporates chlorophyll f in its photosystems, allowing absorption of far-red light not available to other photosynthetic organisms [85]. Chroococcidiopsis species have been suggested as possible candidates for terraforming other planets [86]. Expression plasmids have been introduced into several strains of C. thermalis via conjugation [84].
2.11. Gloeobacter violaceus PCC 7421
Gloeobacter violaceus PCC 7421 (PCC 7421) is a primordial cyanobacterium that lacks thylakoid membranes [87]. PCC 7421 localises proteins involved in photosynthesis and respiration to specific regions of the cytoplasmic membrane [88]. Despite its very slow growth, a method of transforming PCC 7421 with an expression vector via conjugation has been developed [89].
3. Current and Future Strategies for Genome Engineering in Cyanobacteria
3.1. CRISPR/Cas Genome Editing in Cyanobacteria
The RNA-guided CRISPR/Cas family of enzymes has been the driving force for a revolutionary step change in precision genome editing capacity in almost every field of biology, including photosynthetic biology [94,95,96]. Briefly, all CRISPR/Cas genome editing systems exploit the Class II family of CRISPR-associated endonuclease (Cas) enzymes (comprising types II, V and VI Cas) [97,98]. The type II-A Cas of Streptococcus pyogenes (SpCas9) was first demonstrated as a site-specific RNA-guided DNA cleavage tool by Jinek et al. [99]. Since then, a vast array of CRISPR/Cas technologies have been produced and continue to be developed at a rapid pace [100,101,102,103,104,105]. Genome editing studies using CRISPR/Cas have now been reported in several cyanobacterial species, including PCC 6803, PCC 7942, UTEX 2973 and the filamentous strain Nostoc (Anabaena) PCC 7120, from four separate labs (for recent reviews, see [25,27,106]).
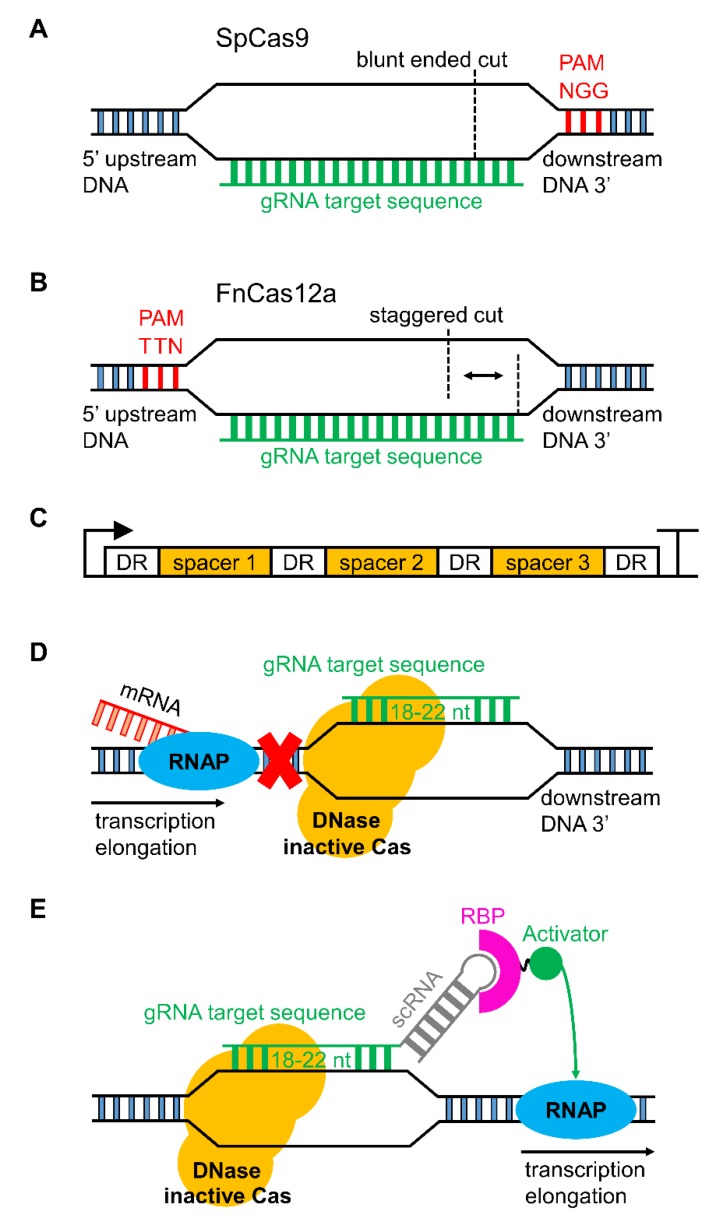
We will not cover the specific mechanisms of all the available CRISPR/Cas tools here (for reviews, see [94,96]). We will focus on Cas9, which confers a blunt-ended double-stranded break (DSB) in DNA (Figure 1A), and type V-A Cas (Cas12a, or previously Cpf1), which produces a staggered DSB (Figure 1B) [107]. Use of CRISPR/Cas systems for gene editing relies on a synthetic single-guide RNA (sgRNA or gRNA), which, for Cas9, is a fusion of a crRNA (CRISPR-RNA) and a tracrRNA (trans-activating crRNA). For CRISPR/Cas9 systems, gRNAs are commonly expressed from a DNA template, with each gRNA transcribed from a single expression cassette. The crRNA component is customised for targeting a specific genomic locus and the tracrRNA acts as a scaffold for recruitment of Cas9. In contrast, Cas12a enzymes possess an intrinsic RNase activity that facilitates autoprocessing of gRNAs that can be expressed from ‘spacer arrays’ (Figure 1C) and a tracrRNA fusion is not required. Spacer arrays are comprised of spacers that code for gRNAs, which are each separated by a direct repeat (DR). The DR facilitates recognition and cleavage of the precursor RNA by Cas12a to form mature gRNAs. To date, all Cas isoforms that target DNA require a 2–6 nucleotide sequence called a protospacer-adjacent motif (PAM) site for Cas to bind DNA and generate a DSB. Depending on the type of Cas used, PAM sites are situated immediately upstream or downstream of the gRNA target locus. As PAM sites are sequence-specific, the choice of Cas enzyme used can impact on the gRNA loci available.
Figure 1.
Basic overview of CRISPR/Cas-based genome editing and CRISPR interference in cyanobacteria. (A) The type II-A CRISPR/Cas system comprising SpCas9 that is targeted by a guide RNA (gRNA, green) upstream of an NGG protospacer adjacent motif (PAM) (red). SpCas9 generates a blunt-ended double-stranded break (DSB) in DNA (black dashed line). (B) The type II-V CRISPR/Cas system comprising FnCas12a that is targeted by a gRNA downstream of a TTN PAM. Cas12a confers a staggered DSB in DNA that results in a five-nucleotide overhang. (C) Illustration of a single expression cassette containing three spacers (i.e., a spacer array, orange) that are flanked by direct repeat regions (DRs, white). Cas12a recognises and cleaves spacers in response to a DR to generate a mature gRNA from each spacer. (D) Overview of transcriptional inhibition by CRISPRi. DNase inactive Cas enzymes (e.g., dCas9 or ddCas12a) are targeted to a DNA locus by a gRNA (typical length indicated as 18–22 nucleotides), which blocks RNA polymerase (RNAP, blue) to prevent mRNA (red) synthesis. (E) Example of transcriptional activation by CRISPRa. DNase inactive Cas enzymes (e.g., dCas9 or ddCas12a) are targeted by a gRNA fused to a scaffold RNA (scRNA, grey). The scRNA recruits an RNA binding protein (RBP, pink) fused to a transcriptional activator (green) (e.g., [108]), which subsequently leads to activation of RNAP and transcription elongation.
In cyanobacteria (as in other prokaryotes that lack an endogenous non-homologous end joining (NHEJ) pathway) [109,110]), CRISPR/Cas has been used as an enhancement tool to improve the frequency of targeted mutation by HR [58,111,112,113]. In brief, expression of Cas and a gRNA mediates cleavage at a specific DNA target site. The co-expressed editing template contains homology flanks (ca. 1 kb in length) and subsequent repair modifies the locus and mutates the PAM site to avoid repeated cleavage. The potential advantages of CRISPR/Cas over established HR strategies in model cyanobacteria such as PCC 6803 [16] are that 1) a markerless mutation is induced at the DNA target site in a single event; 2) multiple sites could be modified simultaneously, provided the appropriate gRNAs and editing templates are co-expressed; and 3) CRISPR/Cas systems could be more efficient for engineering species that are not naturally transformable (e.g., by conjugation or electroporation). Potential drawbacks include toxicity of the Cas enzyme and the time required to cure new mutants of the CRISPR/Cas vector following editing. One approach to accelerate the latter issue is the inclusion of a negative selection marker (e.g., sacB) on the CRISPR/Cas vector [113]. Thus far, SpCas9 is the only Cas9 reported to have been expressed in cyanobacterial strains, including PCC 6803, PCC 7942 and UTEX 2973 (Table 3) [58,111,112]. Expression of SpCas9 has been linked to toxicity and failure to recover colonies following transformation or conjugation in all three species, even at low expression levels. It remains unclear why SpCas9 appears toxic in cyanobacteria, or whether other Cas9 enzymes may be more compatible.
Table 3.
Cyanobacterial species where CRISPR/Cas has been used for gene editing.
| Species and Strain | Cas Type | Expression System | Reference |
|---|---|---|---|
| Synechococcus elongatus PCC 7942 | SpCas9 | episomal | [111] |
| Synechocystis sp. PCC 6803 | FnCas12a | episomal | [114] |
| Synechococcus elongatus UTEX 2973 | |||
| Nostoc sp. PCC 7120 | |||
| Synechococcus elongatus UTEX 2973 | SpCas9 | episomal | [58] |
| Synechocystis sp. PCC 6803 | SpCas9 | chromosomal | [112] |
| Synechococcus elongatus PCC 7942 | FnCas12a | episomal | [57] |
| Synechococcus elongatus UTEX 2973 | |||
| Nostoc sp. PCC 7120 | FnCas12a | episomal | [113] |
In contrast to Cas9, expression of Cas12a in the cyanobacterial strains examined so far does not appear to result in toxicity [57,113,114]. Gene editing using Cas12a from Francisella novicida (FnCas12a) has been demonstrated in PCC 6803, PCC 7942, UTEX 2973 and Nostoc PCC 7120. An additional advantage of Cas12a is that the intrinsic RNase activity allows for the design of a single expression cassette containing multiple gRNAs to target several DNA loci simultaneously (Figure 1C) [115,116,117]. A recent multiplexing study with Cas12a designed an array of 25 gRNAs on a single plasmid that simultaneously edited multiple genomic target sites in mammalian cells [105].
Previously, reports of gene editing with FnCas12a in cyanobacteria have been limited to a single lab [57,114]. However, Niu et al. [113] have recently also demonstrated that Nostoc PCC 7120 can be edited by FnCas12a. A high efficacy was demonstrated for FnCas12a-mediated gene editing of single target sites (83%) by attempting to generate knockout mutants in 26 different genes using 52 gRNAs (two per gene) [113]. In addition, a markerless double-knockout mutant was generated for two genes required for heterocyst formation (hetR (alr2339) and hetN (alr5358)) in a single step following co-conjugation with two CRISPR/FnCas12a editing vectors carrying different antibiotic selection markers. Lastly, a conditional knockout was generated for the essential gene polA (alr1254; DNA polymerase I) by replacing the RBS of the polA promoter with a theophylline-induced riboswitch [118,119]. Successfully transconjugated lines were only viable when grown with theophylline. Transconjugates were then cured of the self-replicating CRISPR/FnCas12a vector by withdrawing antibiotics and using sacB counter selection on sucrose plates.
Thus far, FnCas12a is the only reported Cas12a used in cyanobacteria genome editing studies. One general constraint of CRISPR/Cas editing is the specificity of the PAM site required for DNA cleavage. Of potential interest to cyanobacterial researchers is the growing availability of different Cas12a isoforms (e.g., AsCas12a and LbCas12a from Acidaminococcus sp. and Lachnospiraceae bacterium, respectively) and those engineered to recognise alternative PAM sequences (Table 4) [96,120]. Increased flexibility in PAM recognition will allow for more choice when targeting loci for genome editing [120,121]. New Cas enzymes continue to be identified but remain to be evaluated in cyanobacteria, such as CasX, which also produces a staggered DSB but is smaller than Cas12a [122]. If CasX is less toxic than Cas9, it may provide a useful new set of tools for genome-editing in cyanobacterial species.
Table 4.
The available PAM sequences for cyanobacterial species highlighted in this study for different Cas variants. Only species with full genomes available are shown. The Cas variants indicated are Streptococcus pyogenes Cas9 (SpCas9), Francisella novicida Cas12a (FnCas12a), Acidaminococcus sp. Cas12a (AcCas12a, and variants AsCas12a-RR and ASCas12a-RVR), Lachnospiraceae bacterium Cas12a (LbCas12a) and Deltaproteobacteria bacterium CasX (CasX). The average number of PAM sites (in brackets) per kb of genome are shown (N = A, T, C, G; V = A, C, G; Y = C, T). Genome data was sourced from The European Nucleotide Archive (https://www.ebi.ac.uk/ena) and The National Centre for Biotechnology Information (https://www.ncbi.nlm.nih.gov/genome).
| Cyanobacteria | Genome Size (bp) | SpCas9 (NGG) | FnCas12a (TTN) | AsCas12a and LbCas12a (TTTV) | AsCas12a-RR (TYCV) | ASCas12a-RVR (TATV) | CasX (TTCN) |
|---|---|---|---|---|---|---|---|
| Arthrospira platensis C1 | 6,089,210 | 134 | 168 | 24 | 46 | 21 | 33 |
| Arthospira plantensis NIES 39 | 6,788,435 | 114 | 171 | 37 | 46 | 22 | 33 |
| Chroococcidiopsis thermalis PCC 7203 | 6,315,792 | 89 | 175 | 38 | 41 | 17 | 34 |
| Cyanothece sp. ATCC 51142 | 4,934,271 | 118 | 221 | 35 | 40 | 23 | 39 |
| Cyanothece sp. PCC 7822 | 6,091,620 | 114 | 210 | 33 | 39 | 21 | 36 |
| Gleobacter violaceus PCC 7421 | 4,659,019 | 170 | 89 | 17 | 42 | 6 | 26 |
| Nostoc punctiforme strain ATCC 29133 | 8,234,322 | 114 | 194 | 43 | 40 | 20 | 35 |
| Nostoc sp. PCC 7120 | 6,413,771 | 119 | 191 | 27 | 39 | 39 | 34 |
| Synechococcus elongatus PCC 6301 | 2,696,255 | 142 | 113 | 10 | 41 | 7 | 28 |
| Synechococcus elongatus PCC 7942 | 2,695,903 | 141 | 113 | 10 | 41 | 6 | 28 |
| Synechococcus elongatus PCC 11801 | 2,691,022 | 139 | 115 | 10 | 41 | 7 | 29 |
| Synechococcus sp. PCC 7002 | 3,008,047 | 153 | 163 | 22 | 48 | 11 | 33 |
| Synechococcus sp. PCC 11901 | 3,081,514 | 152 | 163 | 22 | 47 | 11 | 33 |
| Synechococcus sp. UTEX 2973 | 2,690,418 | 142 | 113 | 10 | 41 | 6 | 28 |
| Synechococcus sp. WH 8102 | 2,434,428 | 173 | 86 | 7 | 49 | 4 | 28 |
| Synechocystis sp. PCC 6803 | 3,569,561 | 161 | 174 | 23 | 51 | 12 | 32 |
| Thermosynechococcus elongatus BP-1 | 2,593,857 | 176 | 126 | 13 | 42 | 10 | 27 |
3.2. Serine Integrases for Generating Multiple Knock-ins
The most commonly used methods for genome engineering in cyanobacteria still rely on HR and the use of selective markers. For example, to generate a gene knockout or a knock-in mutant, heterologous DNA must be integrated with a selective marker (e.g., an antibiotic resistance cassette). Thus, the ability to generate mutants in a given species with multiple insertional mutations is limited by the availability and efficacy of selective markers. To overcome this limitation, several methods have been developed for generating markerless mutants, which allows mutant strains to undergo further genetic modifications. One of the most widely used markerless techniques in PCC 6803 uses a two-step HR approach with the negative selection marker sacB, which produces levansucrase, an enzyme conferring sensitivity to sucrose [16]. Nevertheless, generating a fully segregated markerless mutant for a single locus takes ca. 4 weeks to several months, depending on the target locus, while sequential engineering for multi-mutant strains can be very time consuming. Tsujimoto et al. [123] demonstrated an insertion of 20.8 kb in PCC 6803, but this was achieved by a laborious five-step HR process using ca. 4 kb at a time. Therefore, new methods need to be developed to allow large DNA insertions and/or multiple loci engineering in a more efficient timeframe.
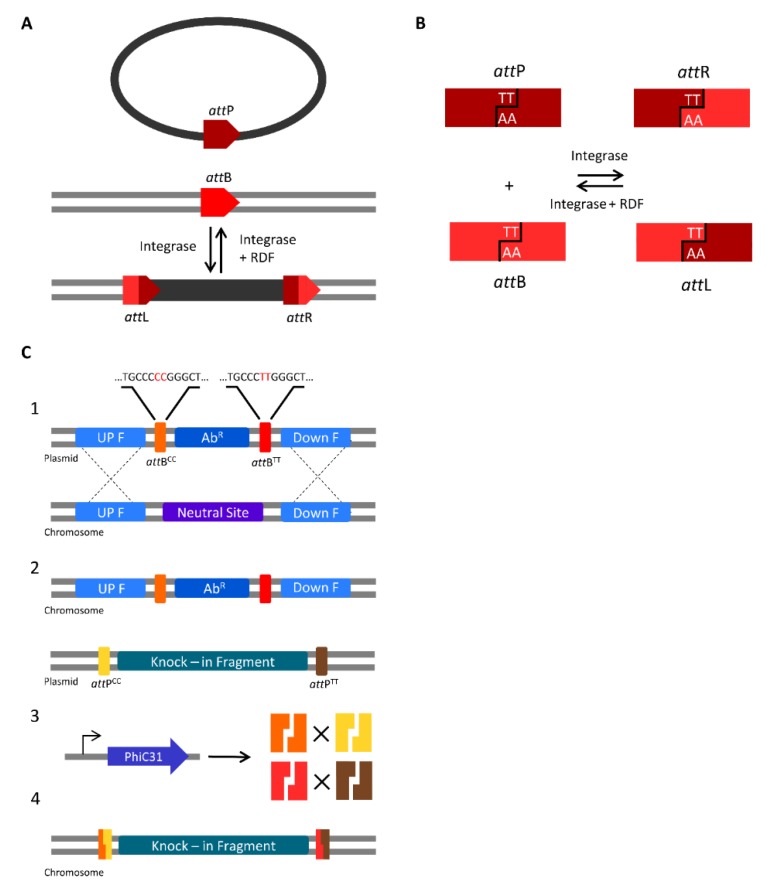
Serine integrases are a subfamily of the site-specific recombinases that catalyse DNA rearrangement through small DNA sequences (<50 bp) called attachments (att) (commonly used for GateWay cloning) [124]. Serine integrases catalyse recombination between the att sites from linear or circular DNA that can result in excision, integration or inversion of DNA sequences, depending on the position and orientation of the att sites. Temperate bacteriophages encode serine integrases to catalyse integration of their DNA into bacterial genomes through recombination of attP (phage) and attB (bacteria) attachment sites, generating attL (left) and attR (right) sites (Figure 2A). Serine integrases bind to the attP and attB sites to make a staggered cut in the central region, generating halves with a two base-pair 3′ overhang. Then, rotation takes place to swap the attP and attB half-sites and finally, the two bp complementary overhangs religate to generate attR and attL sites that cannot recombine again unless a recombination directionality factor (RDF) is present (Figure 2B). Six pairs of nonpalindromic central overlap sequences (TT, CT, GT, CA, CC, and TC) can be used to create orthogonal sites and allows multiple att sites to be used simultaneously (e.g., attPTT will only recombine with attBTT in a specific orientation) [125]. The control of directionality and orthogonality have made serine integrases attractive tools for genome engineering and genetic logic gate design [126,127]. In contrast to CRISPR/Cas-based approaches, site-specific recombination using serine integrases does not rely on endogenous DNA repair pathways, such as NHEJ or HR. Although CRISPR/Cas knock-in approaches are able to generate small insertions in a single step, the size of the insertion remains limited by the efficiency of HR. In addition, unlike Cas9, no toxicity has been reported with the usage of serine integrases in several organisms [124,126,128].
Figure 2.
Mechanism of action of serine integrases and knock-in strategies. (A) DNA rearrangement with serine integrases: attB and attP sites recombine to make attL and attR sites. In the presence of the cognate recombination directionality factor (RDF), the reaction is reversed [124]. (B) The serine integrases bind to the attP and attB sites, generate a staggered 2 bp cut to produce two halves at each site, and then rotate to swap the two halves from each site. If the halves are complementary, relegation occurs to generate attL and attR sites. (C) A serine integrase-mediated knock-in strategy for cyanobacteria. Integration of a landing pad (the landing pad shown carries orthogonal attBCC and attBTT sites) occurs by homologous recombination (HR) at a neutral site in the cyanobacterial chromosome (C1). A self-replicating vector carrying the donor DNA fragment flanked by attPCC and attPTT sites and a PhiC31 integrase expression cassette is introduced into the cyanobacterial strain (C2). Expression of PhiC31 integrase results in the recombination of the orthogonal attB and attP pairs (C3), replacing the “landing pad” with the donor DNA to generate an unmarked knock-in mutant (C4).
Serine integrases have been used for genome engineering in a variety of organisms, including mice [129], Drosophila melanogaster [130], S. cerevisiae, E. coli [126] and Clostridium ljungdahlii [128]. A recent strategy outlined a portable method to simplify the introduction of multiple genomic insertions using the orthogonal att sites of the PhiC31 serine integrase [126]. Firstly, one or more selective markers flanked by two orthogonal attB sites were integrated into the genome of E. coli as “landing pads”. Although Snoeck et al. [126] used HR to introduce the landing pads, other techniques such as CRISPR/Cas have been used for att site integration [128]. Secondly, a donor vector carrying an expression cassette for PhiC31 integrase and the DNA fragment(s) to be inserted flanked by attP orthogonal sites was introduced to generate insertions specific for each corresponding landing pad by attB × attP recombination (Figure 2C).
Using a single landing pad, a 10.3 kb DNA fragment was inserted with 100% efficiency. Simultaneous recombination with three landing pads generated a triple-knock-in mutant (all fragments were ca. 2.5 kb in size), with an efficiency of 75%.
In cyanobacteria, integration of one or more landing pads at a given locus could proceed either by a two-step HR approach if the species is naturally transformable [16], or by CRISPR/Cas-mediated HR [114]. CRISPR/Cas could also be used to insert landing pads at multiple loci in a single step [113], Broad host range vectors able to self-replicate in cyanobacteria (e.g., pPMQAK1) could be used to construct the donor vector [35]. To generate a markerless knock-in mutant following serine intergrase-mediated recombination, the donor vector could be cured from the strain by inclusion of the negative selection marker sacB on the vector backbone. Thus, serine integrases could emerge as a useful tool for the generation of multi-knock-in markerless mutants for large DNA fragments in cyanobacteria. Generating a library of strains with att “landing pads” in combination with high-throughput assembly methods, such as Golden Gate MoClo for the assembly of the donor vectors [35], could significantly speed up the design-build-test cycle.
4. Known and Novel Tools for Regulating Gene Expression in Cyanobacteria
4.1. Gene Regulation with CRISPRi and Synthetic Small Regulatory RNAs
The emergence of CRISPR interference (CRISPRi) and synthetic small regulatory RNA (sRNA) tools in cyanobacterial research has allowed for fine modulation of gene expression at both the transcriptional and translational levels. For translational repression, sRNA tools employ an antisense RNA to bind a specific mRNA transcript target and generate an RNA duplex [131,132]. The RNA duplex suppresses translation and subsequently targets the mRNA transcript for degradation. sRNA-based approaches have been demonstrated in PCC 6803 and PCC 7002, including a paired termini antisense RNA approach and an Hfq-mediated system using sRNAs fused to a MicC scaffold [132,133,134]. Both approaches demonstrated up to 90% reduction in protein expression.
For transcriptional repression, CRISPRi approaches make use of DNase-inactive variants of Cas, called “dead” Cas9 (dCas9) or DNase “dead” Cas12a (ddCas12a; also known as ddCpf1) (Figure 1D) [100,117]. To date, SpdCas9 is the only reported DNase-inactive Cas used in cyanobacterial research, but it has been used successfully to repress gene expression in PCC 6803, PCC 7002, PCC 7942 and Nostoc PCC 7120 [35,133,135,136,137,138]. Unlike SpCas9, SpdCas9 does not appear to be toxic. However, in our lab, we have observed a reduction in growth rates in PCC 6803 when SpdCas9 was expressed at high levels, suggesting that low/medium strength promoters should be used when designing SpdCas9 expression cassettes. Decreased growth rates and changes in cell morphology and division have been observed in E. coli when expressing SpdCas9 at high levels [139].
Multiplexing of gRNAs to target several genes simultaneously with SpdCas9 has been demonstrated in PCC 6803 [137,140]. Kaczmarzyk et al. [137] demonstrated simultaneous repression of six native genes. However, a potential limitation to multiplexing using dCas9 is the need for individual expression cassettes for each gRNA. Kaczmarzyk et al. [137] reported issues with vector recombination in PCC 6803 due to repeated use of common promoters and terminators for each gRNA cassette. ddCas12a may provide an improvement over SpdCas9 in cyanobacteria for multiplexing gRNAs and repression of multiple loci, as demonstrated in E. coli [117]. Cas12a requires a DR length of 19 base pair (bp) to generate mature gRNAs from spacer arrays [141], which is significantly less than that of most promoters and terminators; this may help to reduce plasmid vector size requirements and mitigate recombination issues when multiplexing is required. However, cloning spacer arrays can be challenging due to the multiple repeated sequences, and is not always achievable even using commercial DNA synthesis companies.
CRISPRi using SpdCas9 has been reported to reduce protein expression of heterologous reporter genes (e.g., YFP) between 40% and 99%, depending on the gRNA(s) used [35,140]. Native gene repression has been shown to vary, with a maximum reduction of 94% achieved for glgC in PCC 7942 [136,140]. Yao et al. [136,140] targeted a range of native genes with CRISPRi in PCC 6803 and observed reductions of <90% for slr0942, sll0990, and slr1192, but more modest knock down of 50% for slr0091. Thus, validation of gRNA(s) is important to ensure effective transcriptional repression, which can be time consuming. To achieve more robust and consistent down regulation, it may be advantageous to combine CRISPRi and sRNA to simultaneously modulate transcription and translation. Furthermore, CRISPR/Cas variants have been characterised that target RNA, and thus also can modulate expression at the translational level. For example, Cas13 cleaves single-stranded RNA [142], and in its deactivated form (dCas13), can bind mRNA and suppress translation [143]. Cas13 may provide additional tools for RNA manipulation and additional strategies for gene and multigene repression studies.
Finally, dCas9 has also been used to drive gene expression by so called CRISPR activation (CRISPRa). CRISPRa relies on gRNA(s) modified to include an extended hairpin sequence (termed a scaffold RNA, scRNA) that function, for example, to recruit an RNA binding protein (RBP) fused to a transcriptional activator [108]. Thus, when the dCas9-scRNA complex binds to a target locus, the scRNA recruits the appropriate machinery to drive transcription (Figure 1E). As the dCas9 is unmodified, CRISPRi and CRISPRa could be achieved concurrently with the expression of multiple gRNAs and scRNAs for simultaneous gene repression and activation [108,144]. In E. coli, effective gene activation by CRISPRa does require appropriately positioned PAM sites situated at specific locations upstream of the transcription start site. Recent work shows that shifting the gRNA target site by as little as two nucleotides can lead to a significant loss in activation [145]. Nevertheless, these approaches could improve on current strategies in cyanobacteria to both express heterologous pathways and repress native gene expression for the redirection of metabolic flux towards desired products [137].
4.2. Sigma Factors and RNA Polymerase as Regulatory Tools for Gene Transcription
The highlighted serine integrase recombination and CRISPR-based approaches are examples of promising tools for genome engineering and gene regulation in cyanobacteria. However, applying these approaches often requires careful regulation of the composite parts by inducible and, ideally, orthogonal gene expression systems to generate predictable outputs [146,147,148].
Sigma factors are critical components required for transcription initiation in bacteria that interact with the core RNA polymerase (RNAP) complex to facilitate binding to specific DNA promoter regions [149]. Thus, different sigma factors are involved in driving the transcription of different subsets of genes, and are themselves expressed by different environmental or stress inputs [150,151]. All cyanobacterial sigma factors belong to the sigma 70 family [152], although several others exist in bacteria [153].
Recent reports have provided good evidence that sigma factors may be compatible with RNAP complexes from different bacterial species, paving the way for a potential novel orthogonal expression system in cyanobacteria. Nostoc PCC 7120 contains twelve sigma factors that regulate gene expression according to environmental conditions [154]. In a recent study, Wells et al. [155] tested the sigma factors from Nostoc PCC 7120 in E. coli and observed that several cyanobacterial promoters were able to drive transcription in E. coli only when sigma factors from Nostoc PCC 7120 were co-expressed. Similarly, sigma factors from Bacillus subtilis can be co-expressed in E. coli to construct an orthogonal expression system [156]. Thus, testing non-sigma 70 factors in cyanobacteria may help to identify novel tools for orthogonal transcriptional regulation [157]. For example, E. coli have a sigma 54 factor [158,159], which if functional in cyanobacteria, could allow for transcription from sigma 54-dependant promoters as a novel orthogonal trans-acting expression system [152]. Liu et al. [160] have recently demonstrated in E. coli that sigma 54-dependent promoters can be combined with CRISPRa and have a higher dynamic range compared to sigma 70-dependent promoters.
Similarly, heterologous RNAP systems could be employed. For example, the T7 RNAP is a single subunit RNA polymerase of viral origin that is commonly used as an expression tool in E. coli due to its orthogonality to bacterial transcription machinery [161,162,163]. Use of T7 RNAP has also recently been demonstrated in PCC 6803 and PCC 7942 [164,165]. As in E. coli, native sigma factors in cyanobacteria do not interact with the T7 promoter sequence (PT7), so genes driven by PT7 are transcribed only if the cognate T7 RNAP is expressed. Directed evolution approaches have produced new variants of T7 RNAP with altered promoter recognition characteristics [161,166]. PT7 has also undergone substantial analysis, with Komura et al. [167] testing transcriptional activity of 7847 PT7 variants. T7 RNAP has also been adapted to act as a photoactivatable genetic switch in E. coli with dark-off/light-on properties [168]. The latter system could be of use in cyanobacterial biotechnology; for example, light- or dark-dependent control of protein production (e.g., for light-sensitive bioproducts) with a variety of different promoter strengths.
4.3. The Potential of Optogenetic Systems
Several small-molecule inducible/repressible systems have been characterised in E. coli [169,170,171]. However, only a small number have been characterised in cyanobacteria, and thus far, only in model species [31,37,164,172,173,174]. The commonly used lac operon induction system, which uses isopropyl β–d-1 thiogalactopyranoside (IPTG), has been shown to be leaky and have low induction levels, possibly due to the limited capacity of IPTG to diffuse into cyanobacterial cells [119,175,176]. Metal ion inducible promoters have been tested in several cyanobacterial strains [177,178,179], but these are sometimes not practical as many metals ions are present in standard growth media (e.g., BG11 [180]) and toxicity can be an issue. More recently, an arabinose inducible and a rhamnose inducible promoter were characterised in PCC 6803, which showed tight regulation, linear response and sustained expression [31,37]. However, the relatively low availability of inducible/repressible systems in cyanobacterial species compared to model heterotrophs still limits the progress and development of more advanced synthetic circuits for dynamic control of cellular behaviour [108,181].
In the last decade, optogenetics (i.e., light-controlled regulation) has emerged as a promising tool for tuning synthetic circuits in mammalian and bacterial cell systems [182,183,184,185,186]. Compared with chemical induction systems, optogenetic systems allow for more targeted, rapid and precise control of genetic elements with increased spatial and temporal control, while being minimally invasive and reversible [187,188,189,190]. Optogenetic systems can be classified broadly into two-component systems (TCSs) or one-component systems (OCSs).
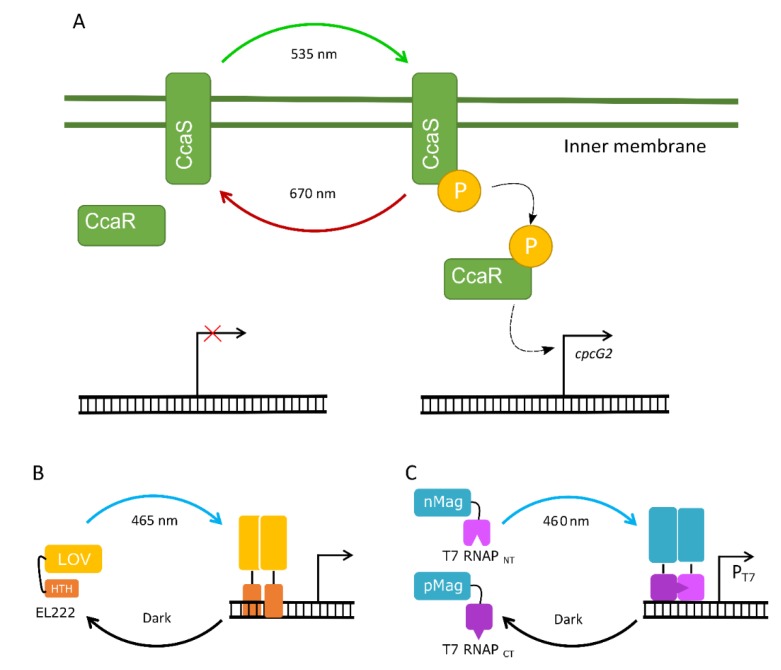
An optogenetic TCS requires two elements: a light-sensing module and a light-responsive module that is activated by the light-sensing module. For example, the green light-inducible CcaS/CcaR TCS in PCC 6803 relies on the membrane-bound histidine kinase CcaS (i.e., the light-sensing module), which is phosphorylated in green light (Figure 3A) [191]. Phosphorylated CcaS subsequently phosphorylates the cytosolic response regulator protein CcaR (i.e., the light-responsive module) that, in turn, activates the expression of the phycobilisome linker gene cpcG2. TCSs have been identified in plants [192], bacteria [191] and fungi that specifically sense UV [193], blue [194,195], green [196,197,198], red [199] or near-infrared light [200]. In contrast, optogenetic OCSs act as both the sensing and responsive modules and are found in the cytosol. So far, only the blue light-activated OCSs that belong to the LOV (Light–Oxygen–Voltage) family of proteins have been characterised in heterotrophic bacteria [187,201], S. cerevisiae [186,202] and Arabidopsis thaliana [203] (Figure 3B,C). Both systems have been used in the control of genetic circuits at multiple levels, such as transcription and protein activity.
Figure 3.
Examples of optogenetic two-component and one-component systems. (A) The two-component green light-inducible CcaS/CcaR system native to Synechocystis sp. PCC 6803. In the presence of green light (535 nm), the histidine kinase CcaS is phosphorylated and, in turn, phosphorylates the response activator CcaR, which results in expression of cpcG2 [191,196]. (B) The EL222 transcription factor from Erythrobacter litoralis HTCC2594 is a one-component system. Blue light induces a conformational change between the LOV and the Helix-Turn-Helix (HTH) DNA binding domain, allowing dimerisation and DNA binding [187]. (C) Split T7 RNA polymerase (RNAP) fused to the Vivid (VVD) photoreceptor from Neurospora crassa is a one-component system. In blue light, the two subunits of VVD (nMag and pMag) interact to assemble the split T7 RNAP (T7 RNAPNT and T7 RNAPCT) as a functional RNA polymerase [204].
Apart from the green light-inducible CcaS/CcaR system, only one other optogenetic TCS has been characterised in PCC 6803, the near-UV activated UirS/UirR system that regulates phototaxis [205]. The latter has not yet been exploited in cyanobacterial research, but the CcaS/CcaR system has been used in PCC 6803 to regulate GFP expression [196], and drive expression of two T4 phage-derived lysis genes to generate a green light-induced cell membrane lytic system [197]. The CcaS/CcaR system has also been used to modulate GFP in a marine species where the CcaS/CcaR system is absent (Synechococcus sp. NKBG 15041c), and demonstrated tight repression under red light and a 20-fold induction of GFP under green light [206]. Thus, cyanobacterial TCSs show promise as tools for transcriptional control in species where those TCSs are not present.
There are also several optogenetic systems characterised in E. coli that could be used in cyanobacterial research. For example, the near-infrared (760 nm) TCS BphP1/PpsR2 from Rhodopseudomonas palustris showed rapid response dynamics and a 2.5-fold dynamic range [200]. Recently, an OCS based on the Vivid (VVD) photoreceptor from the filamentous fungus Neurospora crassa was used to generate a blue-light-inducible T7 RNAP system (Figure 3B) [204]. In the dark, only low levels of gene expression were observed, while high levels of expression were achieved in blue light (460 nm), with an inducible range of >300-fold. Optogenetic systems responsive to different light wavelengths can also be combined to achieve multichromatic gene control [199]. For example, a red–green–blue (RGB) system was constructed in E. coli for production of three different pigments to generate colour biophotographs [188]. The RGB system used a fragmented T7 RNAP that could bind to specific promotors depending on the light input, and demonstrated little crosstalk, high dynamic range and fast responses when induced.
Currently, the main challenge for porting optogenetic systems into cyanobacteria is ensuring compatibility with native light-responsive components. Cyanobacteria naturally produce many of the cofactors required for light-sensing and light-responsive modules [207], which could provide an advantage when porting heterologous optogenetic systems. However, growing cyanobacteria in specific wavelengths (e.g., blue, green, orange, red) will affect photosynthetic efficiencies and growth [208]. Therefore, optimising optogenetic systems might require testing several sources of light to limit any impacts on photosynthesis and achieve the desired outputs.
4.4. Using Inteins to Progress Genetic Circuit Research in Cyanobacteria
Genetic circuits occur in nature and form the basis by which living cells respond and adapt to the surrounding environment [146]. A key goal in synthetic biology is the generation of synthetic genetic circuits that operate as Boolean logic functions to give digital-like control over gene expression in response to environmental stimuli [146,209,210]. Basic Boolean logic functions include ‘AND’ and ‘NOT’ gates: AND gates will give an output signal if all inputs are ‘ON’, while NOT gates will give an output signal only if all inputs are ‘OFF’ [147,211,212]. To date, relatively few synthetic genetic circuits have been constructed in cyanobacteria [213]. These include an oxygen-responsive AND gate in PCC 6803 [214] and four NOT gate variants in PCC 6803, PCC 7942, Nostoc PCC 7120, Synechocystis sp. WHSyn, and Leptolyngbya sp. BL0902 [73]. Currently, two key constraints for making more complex synthetic genetic circuits in cyanobacteria are the relatively small number of characterised inducible expression systems available, and the limited means to integrate multiple input signals (i.e., from different inducible systems) into a single output [215]. Thus, new tools (such as those highlighted in this review) will be required.
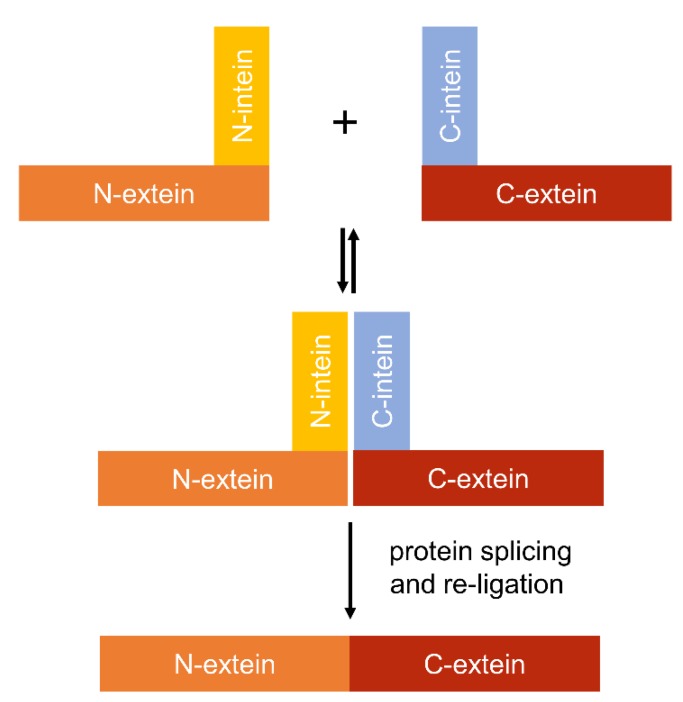
Inteins are naturally occurring polypeptides (100–150 amino acids) within a larger precursor protein that can excise themselves spontaneously from flanking protein regions (exteins (external proteins)) [216]. Inteins have been identified in several cyanobacteria [217], and a variety of other organisms, including other bacterial species, archaea, viruses and eukaryotes [218,219]. Intein excision involves the cleavage of two peptide bonds, resulting in the formation of a new peptide bond, which ligates the flanking exteins together, forming a newly rearranged protein. This auto-catalytic posttranslational modification is referred to as protein splicing and it exists in two forms, cis- and trans-splicing [220]. In the former, the intein coding sequence is embedded in frame with the gene, such that the precursor protein is produced from a single mRNA transcript and translated as two exteins flanking the intein. Upon cis-splicing, the intein is excised from the precursor protein and the flanking exteins are ligated seamlessly to form a new protein. In contrast, trans-splicing events are facilitated by two precursor proteins translated from separate mRNAs, where each encodes a “split intein” fragment (i.e., an N-intein half and a C-intein half) fused to an extein (Figure 4). When the precursor proteins are brought into close proximity, the split inteins re-assemble non-covalently [221]. Trans-splicing then occurs, resulting in excision of the split inteins and ligation of the exteins via a covalent bond to generate an intact protein [221,222].
Figure 4.
Overview of protein trans-splicing with split inteins. Each precursor protein is composed of a split intein (an N-intein half or a C-intein half) fused to an extein (an N-extein half and a C-extein half). When the split inteins are brought into close proximity, they undergo an autocatalytic trans-splicing reaction. During this process, each split intein fragment is cleaved and the extein halves are spliced together to generate an intact protein via a covalent bond.
Split inteins have been used in E. coli to construct a functional two input transcriptional AND gate system using a split T7 RNAP approach [222]. T7 RNAP was separated into two domains, each fused to an N- or a C-intein half, and expressed from two different inducible promoters. Schaerli et al. [222] showed that expression of both T7 RNAP domain-intein fusions was required to reconstitute a functional T7 RNAP and drive transcription of GFP from PT7. Using a similar trans-splicing approach, split inteins have also been used to facilitate re-assembly of split variants of the more complex multidomain transcriptional regulator TetR [223]. Like T7 RNAP, TetR repressible systems have been used in PCC 6803 and PCC 7942 [164]. Thus, these split intein-based gate systems may be straightforward to port into cyanobacteria, provided that the inteins used are orthogonal (i.e., not of cyanobacterial origin) [224]. Split intein strategies could also be used for other transcription factors successfully trailed in cyanobacterial species (e.g., LacI, AraC and RhaS [37,176,214,225]) to construct larger synthetic genetic circuits.
5. Genome-Scale Models
Manipulating cyanobacteria for biotechnology applications is dependent not just on developing better genetic tools but also improving our understanding of cellular metabolism. Genome-scale models (GSMs) are large-scale simulation tools that comprise a global description of the metabolic reactions and pathways of an organism using stoichiometric coefficients and mass balances of the participating metabolites [226]. GSMs are receiving increasing interest due to their predictive power in metabolome and flux changes, thereby making them a valuable tool in metabolic engineering approaches and optimisation for enhanced production of target metabolites [227,228].
Currently, 290 draft genomes and 85 full genomes are available for cyanobacterial species [39]. However, GSMs have only been developed for a small number of these, primarily models such as PCC 6803 [42], PCC 7942 [41] and more recently, UTEX 2973 [43] (Table 5). GSMs from model species can be used as a scaffold to draft GSMs for other cyanobacterial species, as many pathways are conserved [229]. Generating robust and accurate GSMs is an iterative and bottom-up process dependent on the expansion and updating of draft models with available experimental data. Thus, GSM models are continually being improved due to the growing availability of sequencing and omics information.
Table 5.
Genome-scale models currently available for different cyanobacterial strains.
| Cyanobacteria | GSM Name | Reference |
|---|---|---|
| Synechocystis sp. PCC 6803 | iSynCJ816 | [230] |
| imSyn716 | [42] | |
| Synechococcus sp. PCC 7942 | iSyf715 | [231] |
| iJB785 | [41] | |
| iJB792 | [232] | |
| Synechococcus sp. UTEX 2973 | iSyu683 | [233] |
| imSyu593 | [43] | |
| Synechococcus sp. PCC 7002 | iSpy708 | [234] |
| iSpy821 | [235] | |
| Arthrospira platensis NIES-39 | n/a | [40] |
| Nostoc sp. PCC 7120 | n/a | [236] |
Recent cyanobacterial GSMs have attempted to include algorithms to model components of the photosynthetic electron transport chain [41,230,232,235]. However, representing the mechanisms of light capture and electron flow in stoichiometric coefficients is challenging as fluxes cannot be determined experimentally by standard methods (e.g., 13C metabolic flux analysis) [41,237,238]. The model iJB785 for PCC 7942 incorporates the impact of light availability on metabolic flux [41]. When used to estimate whether genes were essential or non-essential for survival, the model achieved an accuracy of 78% based on previous experimental data [239]. Recently, the PCC 7942 model was updated (i.e., iJB792) to include whole-cell light absorbance and the rate of photosynthetic O2 evolution to predict metabolic reaction fluxes [232]. The updated model demonstrated a 98% correlation between simulated and experimental metabolic fluxes under low- (60 μmol photons m−2·s−1) and high-light (600 μmol photons m−2·s−1) conditions. Similarly, the model iSynCJ816 for PCC 6803 can account for changes in energy absorption for different light qualities [230], and achieved a 77% accuracy when compared with experimental results from online databases and literature searches. In comparison, the latest GSM for E. coli (iML1515) can predict gene essentiality in minimal media with 16 different carbon sources with an average accuracy of 94% [44]. The relatively higher accuracy of iML1515 relies on the integration of biochemical, physiological, localisation, genetic, transcriptomic, proteomic and fluxomic data. Currently, protein localisation and transcriptomic data are not included in GSMs for cyanobacteria.
Cyanobacterial GSMs have been used to detect key metabolic differences between species [240] and to identify bottlenecks for the biosynthesis of relevant metabolites [241]. These include a composite GSM for the closely related strains, PCC 7942 and UTEX 2973, based on the model inSyn617 for PCC 6803. The resulting model (iSyu683) identified pathways where resources were allocated differently between PCC 7942 and UTEX 2973, the most prominent difference being carbon uptake rates [233]. An improved model for UTEX 2973 (imSyu593) was recently developed using transient 13C-labeling. The flux elucidation revealed nearly complete conversion (>96%) of the assimilated carbon into biomass compared with only 86% conversion in PCC 6803. Comparison of the UTEX 2973 flux map with that of PCC 6803 revealed differences in the synthesis of key Calvin cycle metabolites, fructose-6-phosphate and sedoheptulose-7-phosphate, and production of amino acids, glycine and serine, from the photorespiratory salvage pathway [43].
GSMs can be used in cyanobacteria to improve bioproduction. Recently, the model iBJ792 for PCC 7942 was used to determine an optimal solution in silico to maximise the production of 2,3-butanediol [232]. GSMs can be also be combined with other computational tools for production optimisation. For example, OptFlux and OptForce are software tools that identify all possible engineering interventions by determining what genes to knock out, or which reaction fluxes in a model need to increase, decrease or fall to zero, to overproduce specific metabolites [242,243]. OptFlux has been combined with GSMs in PCC 6803 to improve the production of n-butanol [138]. OptFlux confirmed that genes targeted for manipulation based on previous experimental data were essential for growth. Subsequent repression of the gene targets arrested growth and redirected carbon flux towards the production of n-butanol, resulting in a 5-fold yield increase compared to the non-repressed strain. Similarly, OptForce was used to increase the production of limonene [244]. Overexpressing three predicted gene targets (two involved in the pentose phosphate pathway and geranyl diphosphate synthase) resulted in a 2.3-fold improvement in limonene production in vivo.
Further improvements in the accuracy of GSMs in cyanobacteria are constrained by the lack of available omics data. For example, a large percentage of the predicted proteins in most cyanobacterial genomes are still annotated as unknown or hypothetical [245]. In addition, transcriptomic data and transcriptional regulatory mechanisms are not integrated in current cyanobacterial GSM models, unlike those for other species such as E. coli (iML1515) [44,246]. Lastly, incorporation of more accurate models for photosynthesis, light-harvesting and electron transport is required [232]. The current expansion of high-throughput omics technologies and automated cloning facilities (e.g., genome foundries) can be used to generate large amounts of experimental data under different growth conditions, which promises to help overcome current limitations for cyanobacterial GSMs.
6. Development of CyanoSource: A Barcoded Mutant Library for Synechocystis sp. PCC 6803
Mutant generation is a key tool in bacterial research and for altering species for biotechnology applications. Individual research laboratories currently generate mutants of interest via a variety of different experimental methods, using a range of plasmid systems in sub-strains that can vary significantly at both the phenotypic and genotypic level (e.g., in PCC 6803 [247,248,249]). This raises the issue of whether studies are directly comparable, a key concern given the growing emphasis on reproducibility within the scientific community. The construction of mutant libraries is a consistent and efficient method for the study of protein properties, regulation and function. Mutant libraries have been generated for model photosynthetic eukaryotes, such as Chlamydomonas reinhardtii [250] and Arabidopsis thaliana [251], and model microbial species including E. coli [252] and S. cerevisiae [253]. The existence of mutant libraries accelerates the pace of research, avoids unnecessary replication between research groups and helps to improve experimental designs. Targeted mutant libraries also provide knowledge on the essential gene set required for survival, further avoiding wasteful laboratory replication [239]. In addition, many research groups, particularly in developing countries, lack the expertise and resources to generate cyanobacterial mutants.
Cyanobacteria remain underdeveloped for fundamental research and viable biotechnological exploitation. Nevertheless, cyanobacterial research is a rapidly growing field with approximately 13,000 new papers published in the last 5 years, making it the third most studied group of autotrophs behind plants and algae. In PCC 6803, only ca. 1050 coding sequences (~30%) have assigned functions, compared to ~80% in E. coli and S. cerevisiae [226]. Of these, only a small proportion have been characterised in a cyanobacterium [39]. The majority have been assigned functions based on studies of homologues in other bacteria, even though the function and importance of characterised genes may differ significantly between phototrophic and heterotrophic bacteria. Transposon libraries have been reported for two model cyanobacterial species [239,254], but these suffer from common issues associated with transposon mutagenesis, including random large insertions, pleiotropic effects, incomplete saturation of the genome and difficulty in recovering individual mutants of interest. A publicly available genome-wide collection of gene knockout mutants would generate a much-needed step change in resource availability and significantly accelerate research in functional genomics and cellular processes in cyanobacteria. It would also supplement existing algal and plant resources, allowing researchers to further examine genes conserved across the photosynthetic lineage.
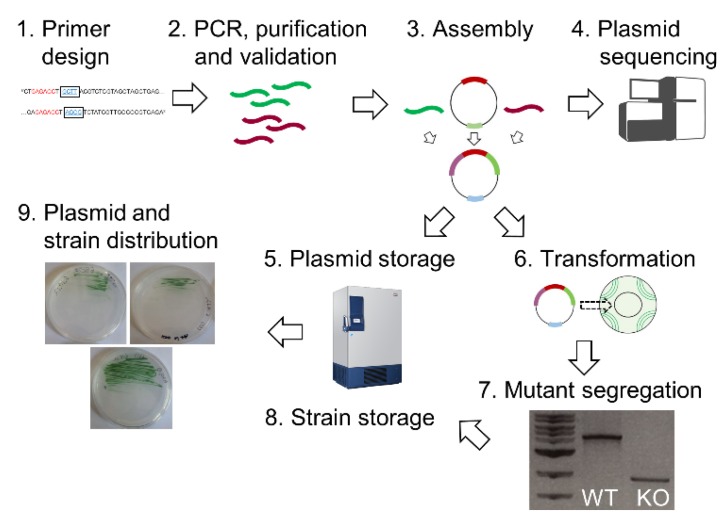
Gene characterisation studies remain challenging and time-consuming but recent developments in automation [255] can streamline and shorten the process of one of the major experimental hurdles: generation of targeted mutants in which the gene of interest has been deleted. DNA assembly and bacterial transformation are complex manual tasks that are time consuming and can suffer from high error rates. Investment by the UK Research and Innovation councils in automated DNA Foundry technologies has provided an opportunity to overcome these challenges [255]. In collaboration with the Earlham DNA Foundry (EDF) and the Edinburgh Genome Foundry (EGF) we will carry out the generation of the plasmids and mutants for this library by using a recently established MoClo system for cyanobacteria [35]. This resource, termed CyanoSource, will target 3456 genes in PCC 6803 (Figure 5). Conditional mutants (i.e., specialised mutants that require an external stimulus to repress a gene) will be constructed for essential genes that cannot be removed. Here, where appropriate, we will use a copper-sensitive promoter that switches off the gene when copper is present [256,257,258]. For the cyanobacterial community, a genome-wide library of mutants and genetic parts generated via automation will guarantee a standard of quality control not otherwise achievable.
Figure 5.
Workflow for the automated production of the CyanoSource resource at two UK DNA Foundry facilities. Primers (1) will be used to generate amplicons carrying BsaI sites (red) and appropriate four-nucleotide overhangs (blue, boxed) for MoClo assembly. PCR products will then be purified and validated (2) and used to assemble suicide vectors (3) for natural transformation of Synechocystis sp. PCC 6803. Vectors will be sequenced by MiSeq (4) and glycerol stocks made for long-term storage (5). PCC 6803 mutants will be generated (6), followed by several rounds of re-streaking to produce segregated mutants that will be confirmed by colony PCR (7), and stored as glycerol stocks (8). For distribution, plasmid stocks are planned to be supplied by Addgene and mutants supplied by the University of East Anglia CyanoSource Hub as streaked plates from glycerol stocks (9).
In addition to automation, this library will be barcoded to allow for the tracking of individual strains within a pooled mutant library. This powerful approach has been described in S. cerevisiae [253] and C. reinhardtii [250], where barcoded libraries can be subjected to different conditions and the relative fitness of individual mutants in a population can be determined via deep sequencing. Construction of this library will begin in November 2019. All plasmids and mutants will be made available to UK and international researchers via a public database, which will be updated throughout the project.
7. Conclusions
Given the ongoing advances in complex synthetic biology tools to finely modulate metabolism in microbes, the future looks bright for progressing both fundamental and applied cyanobacterial research. The topics outlined in this review highlight just some of the current exciting methods that could be used to generate a step change for cyanobacteria researchers by improving transformation efficiencies, gene regulation and capacity for metabolic engineering. Development of the techniques and resources outlined in this review should significantly improve our knowledge of this environmentally important phylum, especially for poorly characterised species. Moreover, their application towards the development of cyanobacteria as a renewable biotechnology platform could have immense implications, not just commercially but also in replacing polluting fossil fuels usage for chemical production and reducing carbon emissions.
Acknowledgments
G.A.R.G., A.A.S.O., and L.A.M. acknowledge funding support from the BBSRC EASTBIO CASE PhD programme [BB/M010996/1], the Consejo Nacional de Ciencia y Tecnología (CONACYT) PhD programme, and the BBSRC Norwich Research Park Doctoral Training Partnership programme [BB/S507404/1], respectively. A.J.M and D.J.L.-S. acknowledge funding from the UK Biotechnology and Biological Sciences Research Council (BBSRC) grants [BB/S020128/1] and [BB/S020365/1], respectively. B.W. acknowledges funding support by the UK BBSRC grant [BB/N007212/1], and UK Research and Innovation grant [MR/S018875/1].
Author Contributions
Conceptualization, G.A.R.G., A.A.S.O., A.J.M.; Writing–Original Draft Preparation, G.A.R.G., A.A.S.O., L.A.M., D.J.L.-S., A.J.M.; Writing–Review & Editing, all authors; Supervision, D.J.L.-S., B.W., A.J.M.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Seckbach J. Algae and Cyanobacteria in Extreme Environments. Springer; Dordrecht, The Netherlands: 2007. Cellular Origin, Life in Extreme Habitats and Astrobiology. [Google Scholar]
- 2.Flombaum P., Gallegos J.L., Gordillo R.A., Rincón J., Zabala L.L., Jiao N., Karl D.M., Li W.K.W., Lomas M.W., Veneziano D., et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA. 2013;110:9824–9829. doi: 10.1073/pnas.1307701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen D., Miller S.R. Photosynthetic temperature adaptation during niche diversification of the thermophilic cyanobacterium Synechococcus A/B clade. ISME J. 2017;11:1053–1057. doi: 10.1038/ismej.2016.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puente-Sánchez F., Arce-Rodríguez A., Oggerin M., García-Villadangos M., Moreno-Paz M., Blanco Y., Rodríguez N., Bird L., Lincoln S.A., Tornos F., et al. Viable cyanobacteria in the deep continental subsurface. Proc. Natl. Acad. Sci. USA. 2018;115:10702–10707. doi: 10.1073/pnas.1808176115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schirrmeister B.E., Gugger M., Donoghue P.C.J. Cyanobacteria and the great oxidation event: Evidence from genes and fossils. Palaeontology. 2015;58:769–785. doi: 10.1111/pala.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knoll A.H. Paleobiological perspectives on early microbial evolution. Cold Spring Harb. Perspect. Biol. 2015;7:1–17. doi: 10.1101/cshperspect.a018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nutman A.P., Bennett V.C., Friend C.R.L., Van Kranendonk M.J., Chivas A.R. Rapid emergence of life shown by discovery of 3700-million-year-old microbial structures. Nature. 2016;537:535–538. doi: 10.1038/nature19355. [DOI] [PubMed] [Google Scholar]
- 8.Dick G.J., Grim S.L., Klatt J.M. Controls on O2 production in cyanobacterial mats and implications for Earth’s oxygenation. Annu. Rev. Earth Planet. Sci. 2018;46:123–147. doi: 10.1146/annurev-earth-082517-010035. [DOI] [Google Scholar]
- 9.Pisciotta J.M., Zou Y., Baskakov I.V. Light-dependent electrogenic activity of cyanobacteria. PLoS ONE. 2010;5:e10821. doi: 10.1371/journal.pone.0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lea-Smith D.J., Howe C.J. The use of cyanobacteria for biofuel production. In: Love J., Bryant J.A., editors. Biofuels and Bioenergy. John Wiley & Sons, Ltd.; Chichester, UK: 2017. pp. 143–155. [Google Scholar]
- 11.Singh R., Parihar P., Singh M., Bajguz A., Kumar J., Singh S., Singh V.P., Prasad S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017;8:1–37. doi: 10.3389/fmicb.2017.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll A.L., Case A.E., Zhang A., Atsumi S. Metabolic engineering tools in model cyanobacteria. Metab. Eng. 2018;50:47–56. doi: 10.1016/j.ymben.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Dismukes G.C., Carrieri D., Bennette N., Ananyev G.M., Posewitz M.C. Aquatic phototrophs: Efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 2008;19:235–240. doi: 10.1016/j.copbio.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Long B.M., Hee W.Y., Sharwood R.E., Rae B.D., Kaines S., Lim Y.L., Nguyen N.D., Massey B., Bala S., von Caemmerer S., et al. Carboxysome encapsulation of the CO2-fixing enzyme Rubisco in tobacco chloroplasts. Nat. Commun. 2018;9:3570. doi: 10.1038/s41467-018-06044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau N.S., Matsui M., Abdullah A.A.A. Cyanobacteria: Photoautotrophic microbial factories for the sustainable synthesis of industrial products. BioMed Res. Int. 2015;2015:754934. doi: 10.1155/2015/754934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lea-Smith D.J., Vasudevan R., Howe C.J. Generation of marked and markerless mutants in model cyanobacterial species. J. Vis. Exp. 2016;2016:e54001. doi: 10.3791/54001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keeling P.J. Diversity and evolutionary history of plastids and their hosts. Am. J. Bot. 2004;91:1481–1493. doi: 10.3732/ajb.91.10.1481. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen A.Z., Mellor S.B., Vavitsas K., Wlodarczyk A.J., Gnanasekaran T., Perestrello Ramos H de Jesus M., King B.C., Bakowski K., Jensen P.E. Extending the biosynthetic repertoires of cyanobacteria and chloroplasts. Plant J. 2016;87:87–102. doi: 10.1111/tpj.13173. [DOI] [PubMed] [Google Scholar]
- 19.Lin P.C., Pakrasi H.B. Engineering cyanobacteria for production of terpenoids. Planta. 2019;249:145–154. doi: 10.1007/s00425-018-3047-y. [DOI] [PubMed] [Google Scholar]
- 20.Yu J., Liberton M., Cliften P.F., Head R.D., Jacobs J.M., Smith R.D., Koppenaal D.W., Brand J.J., Pakrasi H.B. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci. Rep. 2015;5:8132. doi: 10.1038/srep08132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sezonov G., Joseleau-Petit D., D’Ari R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007;189:8746–8749. doi: 10.1128/JB.01368-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snoep J.L., Mrwebi M., Schuurmans J.M., Rohwer J.M., Teixeira de Mattos M.J. Control of specific growth rate in Saccharomyces cerevisiae. Microbiology. 2009;155:1699–1707. doi: 10.1099/mic.0.023119-0. [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal D., Sengupta A., Sohoni S., Sengupta S., Phadnavis A.G., Pakrasi H.B., Wangikar P.P. Genome features and biochemical characteristics of a robust, fast growing and naturally transformable cyanobacterium Synechococcus elongatus PCC 11801 isolated from India. Sci. Rep. 2018;8:16632. doi: 10.1038/s41598-018-34872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Włodarczyk A., Selão T.T., Norling B., Nixon P.J. Unprecedented biomass and fatty acid production by the newly discovered cyanobacterium Synechococcus sp. PCC 11901. bioRxiv. 2019:684944. doi: 10.1101/684944. [DOI] [Google Scholar]
- 25.Behler J., Vijay D., Hess W.R., Akhtar M.K. CRISPR-based technologies for metabolic engineering in cyanobacteria. Trends Biotechnol. 2018;36:996–1010. doi: 10.1016/j.tibtech.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Sun T., Li S., Song X., Diao J., Chen L., Zhang W. Toolboxes for cyanobacteria: Recent advances and future direction. Biotechnol. Adv. 2018;36:1293–1307. doi: 10.1016/j.biotechadv.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Santos-Merino M., Singh A.K., Ducat D.C. New applications of synthetic biology tools for cyanobacterial metabolic engineering. Front. Bioeng. Biotechnol. 2019;7:1–24. doi: 10.3389/fbioe.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijay D., Akhtar M.K., Hess W.R. Genetic and metabolic advances in the engineering of cyanobacteria. Curr. Opin. Biotechnol. 2019;59:150–156. doi: 10.1016/j.copbio.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Markley A.L., Begemann M.B., Clarke R.E., Gordon G.C., Pfleger B.F. Synthetic biology toolbox for controlling gene expression in the cyanobacterium Synechococcus sp. strain PCC 7002. ACS Synth. Biol. 2015;4:595–603. doi: 10.1021/sb500260k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen J., Keasling J.D. Engineering cellular metabolism. Cell. 2016;164:1185–1197. doi: 10.1016/j.cell.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Immethun C.M., DeLorenzo D.M., Focht C.M., Gupta D., Johnson C.B., Moon T.S. Physical, chemical, and metabolic state sensors expand the synthetic biology toolbox for Synechocystis sp. PCC 6803. Biotechnol. Bioeng. 2017;114:1561–1569. doi: 10.1002/bit.26275. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira E.A., Pacheco C.C., Pinto F., Pereira J., Lamosa P., Oliveira P., Kirov B., Jaramillo A., Tamagnini P. Expanding the toolbox for Synechocystis sp. PCC 6803: Validation of replicative vectors and characterization of a novel set of promoters. Synth. Biol. 2018;3:1–15. doi: 10.1093/synbio/ysy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S., Sun T., Xu C., Chen L., Zhang W. Development and optimization of genetic toolboxes for a fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Metab. Eng. 2018;48:163–174. doi: 10.1016/j.ymben.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Roulet J., Taton A., Golden J.W., Arabolaza A., Burkart M.D., Gramajo H. Development of a cyanobacterial heterologous polyketide production platform. Metab. Eng. 2018;49:94–104. doi: 10.1016/j.ymben.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasudevan R., Gale G.A.R., Schiavon A.A., Puzorjov A., Malin J., Gillespie M.D., Vavitsas K., Zulkower V., Wang B., Howe C.J., et al. CyanoGate: A modular cloning suite for engineering cyanobacteria based on the plant MoClo syntax. Plant Physiol. 2019;180:39–55. doi: 10.1104/pp.18.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B., Eckert C., Maness P.C., Yu J. A Genetic toolbox for modulating the expression of heterologous genes in the cyanobacterium Synechocystis sp. PCC 6803. ACS Synth. Biol. 2018;7:276–286. doi: 10.1021/acssynbio.7b00297. [DOI] [PubMed] [Google Scholar]
- 37.Kelly C.L., Taylor G.M., Hitchcock A., Torres-Méndez A., Heap J.T. A rhamnose-inducible system for precise and temporal control of gene expression in cyanobacteria. ACS Synth. Biol. 2018;7:1056–1066. doi: 10.1021/acssynbio.7b00435. [DOI] [PubMed] [Google Scholar]
- 38.Heidorn T., Camsund D., Huang H.H., Lindberg P., Oliveira P., Stensjö K., Lindblad P. Synthetic biology in cyanobacteria. In: Voigt C., editor. Methods in Enzymology. Volume 497. Elsevier; Boston, MA, USA: 2011. pp. 539–579. [DOI] [PubMed] [Google Scholar]
- 39.Fujisawa T., Narikawa R., Maeda S.I., Watanabe S., Kanesaki Y., Kobayashi K., Nomata J., Hanaoka M., Watanabe M., Ehira S., et al. CyanoBase: A large-scale update on its 20th anniversary. Nucleic Acids Res. 2017;45:551–554. doi: 10.1093/nar/gkw1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshikawa K., Aikawa S., Kojima Y., Toya Y., Furusawa C., Kondo A., Shimizu H. Construction of a genome-scale metabolic model of Arthrospira platensis NIES-39 and metabolic design for cyanobacterial bioproduction. PLoS ONE. 2015;10:e0144430. doi: 10.1371/journal.pone.0144430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broddrick J.T., Rubin B.E., Welkie D.G., Du N., Mih N., Diamond S., Lee J.J., Golden S.S., Palsson B.O. Unique attributes of cyanobacterial metabolism revealed by improved genome-scale metabolic modeling and essential gene analysis. Proc. Natl. Acad. Sci. USA. 2016;113:E8344–E8353. doi: 10.1073/pnas.1613446113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gopalakrishnan S., Pakrasi H.B., Maranas C.D. Elucidation of photoautotrophic carbon flux topology in Synechocystis PCC 6803 using genome-scale carbon mapping models. Metab. Eng. 2018;47:190–199. doi: 10.1016/j.ymben.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Hendry J.I., Gopalakrishnan S., Ungerer J., Pakrasi H.B., Tang Y.J., Maranas C.D. Genome-scale fluxome of Synechococcus elongatus UTEX 2973 using transient 13 C-labeling data. Plant Physiol. 2019;179:761–769. doi: 10.1104/pp.18.01357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monk J.M., Lloyd C.J., Brunk E., Mih N., Sastry A., King Z., Takeuchi R., Nomura W., Zhang Z., Mori H., et al. iML1515, a knowledgebase that computes Escherichia coli traits. Nat. Biotechnol. 2017;35:904–908. doi: 10.1038/nbt.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shestakov S.V., Khyen N.T. Evidence for genetic transformation in blue-green alga Anacystis nidulans. MGG Mol. Gen. Genet. 1970;107:372–375. doi: 10.1007/BF00441199. [DOI] [PubMed] [Google Scholar]
- 46.Stevens S.E., Porter R.D. Transformation in Agmenellum quadruplicatum. Proc. Natl. Acad. Sci. USA. 1980;77:6052–6056. doi: 10.1073/pnas.77.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elhai J. Strong and regulated promoters in the cyanobacterium Anabaena PCC 7120. FEMS Microbiol. Lett. 1993;114:179–184. doi: 10.1111/j.1574-6968.1993.tb06570.x. [DOI] [PubMed] [Google Scholar]
- 48.Wendt K.E., Pakrasi H.B. Genomics approaches to deciphering natural transformation in cyanobacteria. Front. Microbiol. 2019;10:1259. doi: 10.3389/fmicb.2019.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taton A., Unglaub F., Wright N.E., Zeng W.Y., Paz-Yepes J., Brahamsha B., Palenik B., Peterson T.C., Haerizadeh F., Golden S.S., et al. Broad-host-range vector system for synthetic biology and biotechnology in cyanobacteria. Nucleic Acids Res. 2014;42:e136. doi: 10.1093/nar/gku673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elhai J., Wolk C.P. Conjugal transfer of DNA to cyanobacteria. In: Packer L., Glazer A.N., editors. Methods in Enzymology. Volume 167. Academic Press; Cambridge, MA, USA: 1988. pp. 747–754. [DOI] [PubMed] [Google Scholar]
- 51.Masukawa H., Inoue K., Sakurai H., Wolk C.P., Hausinger R.P. Site-directed mutagenesis of the Anabaena sp. strain PCC 7120 nitrogenase active site to increase photobiological hydrogen production. Appl. Environ. Microbiol. 2010;76:6741–6750. doi: 10.1128/AEM.01056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandakovic D., Trigo C., Andrade D., Riquelme B., Gómez-Lillo G., Soto-Liebe K., Díez B., Vásquez M. CyDiv, a conserved and novel filamentous cyanobacterial cell division protein involved in septum localization. Front. Microbiol. 2016;7:94. doi: 10.3389/fmicb.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeamton W., Dulsawat S., Tanticharoen M., Vonshak A., Cheevadhanarak S. Overcoming intrinsic restriction enzyme barriers enhances transformation efficiency in Arthrospira platensis C1. Plant Cell Physiol. 2017;58:822–830. doi: 10.1093/pcp/pcx016. [DOI] [PubMed] [Google Scholar]
- 54.Ried J.L., Collmer A. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene. 1987;57:239–246. doi: 10.1016/0378-1119(87)90127-2. [DOI] [PubMed] [Google Scholar]
- 55.Kratz W.A., Myers J. Nutrition and growth of several blue-green algae. Am. J. Bot. 1955;42:282–287. doi: 10.1002/j.1537-2197.1955.tb11120.x. [DOI] [Google Scholar]
- 56.Ungerer J., Lin P.C., Chen H.Y., Pakrasi H.B. Adjustments to photosystem stoichiometry and electron transfer proteins are key to the remarkably fast growth of the cyanobacterium Synechococcus elongatus UTEX 2973. MBio. 2018;9:e02327-17. doi: 10.1128/mBio.02327-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ungerer J., Wendt K.E., Hendry J.I., Maranas C.D., Pakrasi H.B. Comparative genomics reveals the molecular determinants of rapid growth of the cyanobacterium Synechococcus elongatus UTEX 2973. Proc. Natl. Acad. Sci. USA. 2018;115:E11761–E11770. doi: 10.1073/pnas.1814912115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wendt K.E., Ungerer J., Cobb R.E., Zhao H., Pakrasi H.B. CRISPR/Cas9 mediated targeted mutagenesis of the fast growing cyanobacterium Synechococcus elongatus UTEX 2973. Microb. Cell Fact. 2016;15:115. doi: 10.1186/s12934-016-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Begemann M.B., Zess E.K., Walters E.M., Schmitt E.F., Markley A.L., Pfleger B.F. An organic acid based counter selection system for cyanobacteria. PLoS ONE. 2013;8:e76594. doi: 10.1371/journal.pone.0076594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meeks J.C. Symbiosis between nitrogen-fixing cyanobacteria and plants. Bioscience. 1998;48:266–276. doi: 10.2307/1313353. [DOI] [Google Scholar]
- 61.Gao Q., Garcia-Pichel F. Microbial ultraviolet sunscreens. Nat. Rev. Microbiol. 2011;9:791–802. doi: 10.1038/nrmicro2649. [DOI] [PubMed] [Google Scholar]
- 62.Cohen M.F., Wallis J.G., Campbell E.L., Meeks J.C. Transposon mutagenesis of Nostoc sp. strain ATCC 29133, a filamentous cyanobacterium with multiple cellular differentiation alternatives. Microbiology. 1994;140:3233–3240. doi: 10.1099/13500872-140-12-3233. [DOI] [PubMed] [Google Scholar]
- 63.Soule T., Stout V., Swingley W.D., Meeks J.C., Garcia-Pichel F. Molecular genetics and genomic analysis of scytonemin biosynthesis in Nostoc punctiforme ATCC 29133. J. Bacteriol. 2007;189:4465–4472. doi: 10.1128/JB.01816-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reddy K.J., Haskell J.B., Sherman D.M., Sherman L.A. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J. Bacteriol. 1993;175:1284–1292. doi: 10.1128/jb.175.5.1284-1292.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bandyopadhyay A., Elvitigala T., Welsh E., Stöckel J., Liberton M., Min H., Sherman L.A., Pakrasi H.B. Novel metabolic attributes of the genus Cyanothece, comprising a group of unicellular nitrogen-fixing cyanobacteria. MBio. 2011;2:e00214-11. doi: 10.1128/mBio.00214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Min H., Sherman L.A. Genetic transformation and mutagenesis via single-stranded DNA in the unicellular, diazotrophic cyanobacteria of the genus Cyanothece. Appl. Environ. Microbiol. 2010;76:7641–7645. doi: 10.1128/AEM.01456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liberton M., Bandyopadhyay A., Pakrasi H.B. Enhanced nitrogen fixation in a glgX -deficient strain of Cyanothece sp. strain ATCC 51142, a unicellular nitrogen-fixing cyanobacterium. Appl. Environ. Microbiol. 2019;85:e02887-18. doi: 10.1128/AEM.02887-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohmori M., Ehira S. Spirulina: An example of cyanobacteria as nutraceuticals. In: Sharma N.K., Rai A.K., Stal L.J., editors. Cyanobacteria. John Wiley & Sons, Ltd.; Chichester, UK: 2013. pp. 103–118. [Google Scholar]
- 69.Adir N., Bar-Zvi S., Harris D. The amazing phycobilisome. Biochim. Biophys. Acta Bioenerg. 2019 doi: 10.1016/j.bbabio.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 70.Fujisawa T., Narikawa R., Okamoto S., Ehira S., Yoshimura H., Suzuki I., Masuda T., Mochimaru M., Takaichi S., Awai K., et al. Genomic structure of an economically important cyanobacterium, Arthrospira (Spirulina) platensis NIES-39. DNA Res. 2010;17:85–103. doi: 10.1093/dnares/dsq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimura Y., Hirose Y., Misawa N., Osana Y., Katoh H., Yamaguchi H., Kawachi M. Comparison of the terrestrial cyanobacterium Leptolyngbya sp. NIES-2104 and the freshwater Leptolyngbya boryana PCC 6306 genomes. DNA Res. 2015;22:403–412. doi: 10.1093/dnares/dsv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taton A., Lis E., Adin D.M., Dong G., Cookson S., Kay S.A., Golden S.S., Golden J.W. Gene Transfer in Leptolyngbya sp. strain BL0902, a cyanobacterium suitable for production of biomass and bioproducts. PLoS ONE. 2012;7:e30901. doi: 10.1371/journal.pone.0030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taton A., Ma A.T., Ota M., Golden S.S., Golden J.W. NOT gate genetic circuits to control gene expression in cyanobacteria. ACS Synth. Biol. 2017;6:2175–2182. doi: 10.1021/acssynbio.7b00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Busch A.W.U., Montgomery B.L. The Tryptophan-Rich Sensory Protein (TSPO) is involved in stress-related and light-dependent processes in the cyanobacterium Fremyella diplosiphon. Front. Microbiol. 2015;6:1393. doi: 10.3389/fmicb.2015.01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pattanaik B., Montgomery B.L. FdTonB is involved in the photoregulation of cellular morphology during complementary chromatic adaptation in Fremyella diplosiphon. Microbiology. 2010;156:731–741. doi: 10.1099/mic.0.035410-0. [DOI] [PubMed] [Google Scholar]
- 76.Morris J.J., Kirkegaard R., Szul M.J., Johnson Z.I., Zinser E.R. Facilitation of robust growth of Prochlorococcus colonies and dilute liquid cultures by “helper” heterotrophic bacteria. Appl. Environ. Microbiol. 2008;74:4530–4534. doi: 10.1128/AEM.02479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brahamsha B. A genetic manipulation system for oceanic cyanobacteria of the genus Synechococcus. Appl. Environ. Microbiol. 1996;62:1747–1751. doi: 10.1128/aem.62.5.1747-1751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ostrowski M., Mazard S., Tetu S.G., Phillippy K., Johnson A., Palenik B., Paulsen I.T., Scanlan D.J. PtrA is required for coordinate regulation of gene expression during phosphate stress in a marine Synechococcus. ISME J. 2010;4:908–921. doi: 10.1038/ismej.2010.24. [DOI] [PubMed] [Google Scholar]
- 79.Yamaoka T., Satoh K., Katoh S. Photosynthetic activities of a thermophilic blue-green alga. Plant Cell Physiol. 1978;19:943–954. doi: 10.1093/oxfordjournals.pcp.a075684. [DOI] [Google Scholar]
- 80.Liang Y., Kaczmarek M.B., Kasprzak A.K., Tang J., Shah M.M.R., Jin P., Klepacz-Smółka A., Cheng J.J., Ledakowicz S., Daroch M. Thermosynechococcaceae as a source of thermostable C-phycocyanins: Properties and molecular insights. Algal Res. 2018;35:223–235. doi: 10.1016/j.algal.2018.08.037. [DOI] [Google Scholar]
- 81.Onai K., Tabata S., Ishiura M., Kaneko T., Morishita M. Natural transformation of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1: A simple and efficient method for gene transfer. Mol. Genet. Genom. 2004;271:50–59. doi: 10.1007/s00438-003-0953-9. [DOI] [PubMed] [Google Scholar]
- 82.Liang Y., Tang J., Luo Y., Kaczmarek M.B., Li X., Daroch M. Thermosynechococcus as a thermophilic photosynthetic microbial cell factory for CO2 utilisation. Bioresour. Technol. 2019;278:255–265. doi: 10.1016/j.biortech.2019.01.089. [DOI] [PubMed] [Google Scholar]
- 83.Stucken K., Ilhan J., Roettger M., Dagan T., Martin W.F. Transformation and Conjugal Transfer of Foreign Genes into the Filamentous Multicellular Cyanobacteria (Subsection V) Fischerella and Chlorogloeopsis. Curr. Microbiol. 2012;65:552–560. doi: 10.1007/s00284-012-0193-5. [DOI] [PubMed] [Google Scholar]
- 84.Billi D., Friedmann E.I., Helm R.F., Potts M. Gene transfer to the desiccation-tolerant Cyanobacterium Chroococcidiopsis. J. Bacteriol. 2001;183:2298–2305. doi: 10.1128/JB.183.7.2298-2305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nürnberg D.J., Morton J., Santabarbara S., Telfer A., Joliot P., Antonaru L.A., Ruban A.V., Cardona T., Krausz E., Boussac A., et al. Photochemistry beyond the red limit in chlorophyll f–containing photosystems. Science. 2018;360:1210–1213. doi: 10.1126/science.aar8313. [DOI] [PubMed] [Google Scholar]
- 86.Verseux C., Baqué M., Lehto K., De Vera J.P.P., Rothschild L.J., Billi D. Sustainable life support on Mars—The potential roles of cyanobacteria. Int. J. Astrobiol. 2016;15:65–92. doi: 10.1017/S147355041500021X. [DOI] [Google Scholar]
- 87.Rippka R., Waterbury J., Cohen-Bazire G. A cyanobacterium which lacks thylakoids. Arch. Microbiol. 1974;100:419–436. doi: 10.1007/BF00446333. [DOI] [Google Scholar]
- 88.Rexroth S., Mullineaux C.W., Ellinger D., Sendtko E., Rögner M., Koenig F. The plasma membrane of the cyanobacterium Gloeobacter violaceus contains segregated bioenergetic domains. Plant Cell. 2011;23:2379–2390. doi: 10.1105/tpc.111.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Araki M., Shimada Y., Mimuro M., Tsuchiya T. Establishment of the reporter system for a thylakoid-lacking cyanobacterium, Gloeobacter violaceus PCC 7421. FEBS Open Bio. 2013;3:11–15. doi: 10.1016/j.fob.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allen M.B., Arnon D.I. Studies on nitrogen-fixing blue-algae. I. Growth and nitrogen fixation by Anabaena cylindrica Lemm. Plant Physiol. 1955;27:366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bordowitz J.R., Montgomery B.L. Photoregulation of cellular morphology during complementary chromatic adaptation requires sensor-kinase-class protein RcaE in Fremyella diplosiphon. J. Bacteriol. 2008;190:4069–4074. doi: 10.1128/JB.00018-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waterbury J.B., Willey J.M. Isolation and growth of marine planktonic cyanobacteria. Methods Enzymol. 1988;167:100–105. [Google Scholar]
- 93.Moore L.R., Coe A., Zinser E.R., Saito M.A., Sullivan M.B., Lindell D., Frois-Moniz K., Waterbury J., Chisholm S.W. Culturing the marine cyanobacterium Prochlorococcus. Limnol. Oceanogr. Methods. 2007;5:353–362. doi: 10.4319/lom.2007.5.353. [DOI] [Google Scholar]
- 94.Naduthodi M.I.S., Barbosa M.J., van der Oost J. Progress of CRISPR-Cas Based Genome Editing in Photosynthetic Microbes. Biotechnol. J. 2018;13:1700591. doi: 10.1002/biot.201700591. [DOI] [PubMed] [Google Scholar]
- 95.Khumsupan P., Donovan S., McCormick A.J. CRISPR/Cas in Arabidopsis: Overcoming challenges to accelerate improvements in crop photosynthetic efficiencies. Physiol. Plant. 2019;166:428–437. doi: 10.1111/ppl.12937. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y.T., Jiang J.Y., Shi T.Q., Sun X.M., Zhao Q.Y., Huang H., Ren L.J. Application of the CRISPR/Cas system for genome editing in microalgae. Appl. Microbiol. Biotechnol. 2019;103:3239–3248. doi: 10.1007/s00253-019-09726-x. [DOI] [PubMed] [Google Scholar]
- 97.Makarova K.S., Wolf Y.I., Alkhnbashi O.S., Costa F., Shah S.A., Saunders S.J., Barrangou R., Brouns S.J.J., Charpentier E., Haft D.H., et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 2015;13:722–736. doi: 10.1038/nrmicro3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koonin E.V., Makarova K.S. Origins and evolution of CRISPR-Cas systems. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20180087. doi: 10.1098/rstb.2018.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qi L.S., Larson M.H., Gilbert L.A., Doudna J.A., Weissman J.S., Arkin A.P., Lim W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murovec J., Pirc Ž., Yang B. New variants of CRISPR RNA-guided genome editing enzymes. Plant Biotechnol. J. 2017;15:917–926. doi: 10.1111/pbi.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abudayyeh O.O., Gootenberg J.S., Essletzbichler P., Han S., Joung J., Belanto J.J., Verdine V., Cox D.B.T., Kellner M.J., Regev A., et al. RNA targeting with CRISPR-Cas13. Nature. 2017;550:280–284. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eid A., Alshareef S., Mahfouz M.M. CRISPR base editors: Genome editing without double-stranded breaks. Biochem. J. 2018;475:1955–1964. doi: 10.1042/BCJ20170793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abudayyeh O.O., Gootenberg J.S., Kellner M.J., Zhang F. Nucleic acid detection of plant genes using CRISPR-Cas13. CRISPR J. 2019;2:165–171. doi: 10.1089/crispr.2019.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Campa C.C., Weisbach N.R., Santinha A.J., Incarnato D., Platt R.J. Multiplexed genome engineering by Cas12a and CRISPR arrays encoded on single transcripts. Nat. Methods. 2019;6:887–893. doi: 10.1038/s41592-019-0508-6. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y., Malzahn A.A., Sretenovic S., Qi Y. The emerging and uncultivated potential of CRISPR technology in plant science. Nat. Plants. 2019;5:778–794. doi: 10.1038/s41477-019-0461-5. [DOI] [PubMed] [Google Scholar]
- 107.Swarts D.C., van der Oost J., Jinek M. Structural basis for guide RNA processing and seed-dependent DNA targeting by CRISPR-Cas12a. Mol. Cell. 2017;66:221–233.e4. doi: 10.1016/j.molcel.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dong C., Fontana J., Patel A., Carothers J.M., Zalatan J.G. Synthetic CRISPR-Cas gene activators for transcriptional reprogramming in bacteria. Nat. Commun. 2018;9:2489. doi: 10.1038/s41467-018-04901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang W., Bikard D., Cox D., Zhang F., Marraffini L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 2013;31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vento J.M., Crook N., Beisel C.L. Barriers to genome editing with CRISPR in bacteria. J. Ind. Microbiol. Biotechnol. 2019:1–15. doi: 10.1007/s10295-019-02195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li H., Shen C.R., Huang C.H., Sung L.Y., Wu M.Y., Hu Y.C. CRISPR-Cas9 for the genome engineering of cyanobacteria and succinate production. Metab. Eng. 2016;38:293–302. doi: 10.1016/j.ymben.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 112.Xiao Y., Wang S., Rommelfanger S., Balassy A., Barba-Ostria C., Gu P., Galazka J.M., Zhang F. Developing a Cas9-based tool to engineer native plasmids in Synechocystis sp. PCC 6803. Biotechnol. Bioeng. 2018;115:2305–2314. doi: 10.1002/bit.26747. [DOI] [PubMed] [Google Scholar]
- 113.Niu T.C., Lin G.M., Xie L.R., Wang Z.Q., Xing W.Y., Zhang J.Y., Zhang C.C. Expanding the potential of CRISPR-Cpf1-based genome editing technology in the cyanobacterium Anabaena PCC 7120. ACS Synth. Biol. 2019;8:170–180. doi: 10.1021/acssynbio.8b00437. [DOI] [PubMed] [Google Scholar]
- 114.Ungerer J., Pakrasi H.B. Cpf1 Is A versatile tool for CRISPR genome editing ccross diverse species of cyanobacteria. Sci. Rep. 2016;6:39681. doi: 10.1038/srep39681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zetsche B., Gootenberg J.S., Abudayyeh O.O., Slaymaker I.M., Makarova K.S., Essletzbichler P., Volz S.E., Joung J., van der Oost J., Regev A., et al. Cpf1 Is a Single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fonfara I., Richter H., BratoviÄ M., Le Rhun A., Charpentier E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature. 2016;532:517–521. doi: 10.1038/nature17945. [DOI] [PubMed] [Google Scholar]
- 117.Zhang X., Wang J., Cheng Q., Zheng X., Zhao G., Wang J. Multiplex gene regulation by CRISPR-ddCpf1. Cell Discov. 2017;3:17018. doi: 10.1038/celldisc.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakahira Y., Ogawa A., Asano H., Oyama T., Tozawa Y. Theophylline-dependent riboswitch as a novel genetic tool for strict regulation of protein expression in cyanobacterium Synechococcus elongatus PCC 7942. Plant Cell Physiol. 2013;54:1724–1735. doi: 10.1093/pcp/pct115. [DOI] [PubMed] [Google Scholar]
- 119.Ma A.T., Schmidt C.M., Golden J.W. Regulation of gene expression in diverse cyanobacterial species by using theophylline-responsive riboswitches. Appl. Environ. Microbiol. 2014;80:6704–6713. doi: 10.1128/AEM.01697-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao L., Cox D.B.T., Yan W.X., Manteiga J.C., Schneider M.W., Yamano T., Nishimasu H., Nureki O., Crosetto N., Zhang F. Engineered Cpf1 variants with altered PAM specificities. Nat. Biotechnol. 2017;35:789–792. doi: 10.1038/nbt.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kleinstiver B.P., Prew M.S., Tsai S.Q., Topkar V.V., Nguyen N.T., Zheng Z., Gonzales A.P.W., Li Z., Peterson R.T., Yeh J.R.J., et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015;523:481–485. doi: 10.1038/nature14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu J.J., Orlova N., Oakes B.L., Ma E., Spinner H.B., Baney K.L.M., Chuck J., Tan D., Knott G.J., Harrington L.B., et al. CasX enzymes comprise a distinct family of RNA-guided genome editors. Nature. 2019;566:218–223. doi: 10.1038/s41586-019-0908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsujimoto R., Kotani H., Yokomizo K., Yamakawa H., Nonaka A., Fujita Y. Functional expression of an oxygen-labile nitrogenase in an oxygenic photosynthetic organism. Sci. Rep. 2018;8:7380. doi: 10.1038/s41598-018-25396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Merrick C.A., Zhao J., Rosser S.J. Serine integrases: Advancing synthetic biology. ACS Synth. Biol. 2018;7:299–310. doi: 10.1021/acssynbio.7b00308. [DOI] [PubMed] [Google Scholar]
- 125.Colloms S.D., Merrick C.A., Olorunniji F.J., Stark W.M., Smith M.C.M., Osbourn A., Keasling J.D., Rosser S.J. Rapid metabolic pathway assembly and modification using serine integrase site-specific recombination. Nucleic Acids Res. 2014;42:e23. doi: 10.1093/nar/gkt1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Snoeck N., De Mol M.L., Van Herpe D., Goormans A., Maryns I., Coussement P., Peters G., Beauprez J., De Maeseneire S.L., Soetaert W. Serine integrase recombinational engineering (SIRE): A versatile toolbox for genome editing. Biotechnol. Bioeng. 2019;116:364–374. doi: 10.1002/bit.26854. [DOI] [PubMed] [Google Scholar]
- 127.Guiziou S., Mayonove P., Bonnet J. Hierarchical composition of reliable recombinase logic devices. Nat. Commun. 2019;10:456. doi: 10.1038/s41467-019-08391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang H., Chai C., Yang S., Jiang W., Gu Y. Phage serine integrase-mediated genome engineering for efficient expression of chemical biosynthetic pathway in gas-fermenting Clostridium ljungdahlii. Metab. Eng. 2019;52:293–302. doi: 10.1016/j.ymben.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 129.Andreas S., Schwenk F., Küter-Luks B., Faust N., Kühn R. Enhanced efficiency through nuclear localization signal fusion on phage phiC31-integrase: Activity comparison with Cre and FLPe recombinase in mammalian cells. Nucleic Acids Res. 2002;30:2299–2306. doi: 10.1093/nar/30.11.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Groth A.C., Fish M., Nusse R., Calos M.P. Construction of transgenic Drosophila by using the site-specific integrase from phage φC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Na D., Yoo S.M., Chung H., Park H., Park J.H., Lee S.Y. Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nat. Biotechnol. 2013;31:170–174. doi: 10.1038/nbt.2461. [DOI] [PubMed] [Google Scholar]
- 132.Sun T., Li S., Song X., Pei G., Diao J., Cui J., Shi M., Chen L., Zhang W. Re-direction of carbon flux to key precursor malonyl-CoA via artificial small RNAs in photosynthetic Synechocystis sp. PCC 6803. Biotechnol. Biofuels. 2018;11:26. doi: 10.1186/s13068-018-1032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Higo A., Isu A., Fukaya Y., Ehira S., Hisabori T. Application of CRISPR interference for metabolic engineering of the heterocyst-forming multicellular cyanobacterium Anabaena sp. PCC 7120. Plant Cell Physiol. 2018;59:119–127. doi: 10.1093/pcp/pcx166. [DOI] [PubMed] [Google Scholar]
- 134.Zess E.K., Begemann M.B., Pfleger B.F. Construction of new synthetic biology tools for the control of gene expression in the cyanobacterium Synechococcus sp. strain PCC 7002. Biotechnol. Bioeng. 2016;113:424–432. doi: 10.1002/bit.25713. [DOI] [PubMed] [Google Scholar]
- 135.Gordon G.C., Korosh T.C., Cameron J.C., Markley A.L., Begemann M.B., Pfleger B.F. CRISPR interference as a titratable, trans-acting regulatory tool for metabolic engineering in the cyanobacterium Synechococcus sp. strain PCC 7002. Metab. Eng. 2016;38:170–179. doi: 10.1016/j.ymben.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang C.H., Shen C.R., Li H., Sung L.Y., Wu M.Y., Hu Y.C. CRISPR interference (CRISPRi) for gene regulation and succinate production in cyanobacterium S. elongatus PCC 7942. Microb. Cell Fact. 2016;15:196. doi: 10.1186/s12934-016-0595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kaczmarzyk D., Cengic I., Yao L., Hudson E.P. Diversion of the long-chain acyl-ACP pool in Synechocystis to fatty alcohols through CRISPRi repression of the essential phosphate acyltransferase PlsX. Metab. Eng. 2018;45:59–66. doi: 10.1016/j.ymben.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 138.Shabestary K., Anfelt J., Ljungqvist E., Jahn M., Yao L., Hudson E.P. Targeted repression of essential genes to arrest growth and increase carbon partitioning and biofuel titers in cyanobacteria. ACS Synth. Biol. 2018;7:1669–1675. doi: 10.1021/acssynbio.8b00056. [DOI] [PubMed] [Google Scholar]
- 139.Cho S., Choe D., Lee E., Kim S.C., Palsson B., Cho B.K. High-Level dCas9 Expression induces abnormal cell morphology in Escherichia coli. ACS Synth. Biol. 2018;7:1085–1094. doi: 10.1021/acssynbio.7b00462. [DOI] [PubMed] [Google Scholar]
- 140.Yao L., Cengic I., Anfelt J., Hudson E.P. Multiple Gene Repression in Cyanobacteria Using CRISPRi. ACS Synth. Biol. 2016;5:207–212. doi: 10.1021/acssynbio.5b00264. [DOI] [PubMed] [Google Scholar]
- 141.Miao C., Zhao H., Qian L., Lou C. Systematically investigating the key features of the DNase deactivated Cpf1 for tunable transcription regulation in prokaryotic cells. Synth. Syst. Biotechnol. 2019;4:1–9. doi: 10.1016/j.synbio.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Abudayyeh O.O., Gootenberg J.S., Konermann S., Joung J., Slaymaker I.M., Cox D.B.T., Shmakov S., Makarova K.S., Semenova E., Minakhin L., et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353:aaf5573. doi: 10.1126/science.aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cox D.B.T., Gootenberg J.S., Abudayyeh O.O., Franklin B., Kellner M.J., Joung J., Zhang F. RNA editing with CRISPR-Cas13. Science. 2017;358:1019–1027. doi: 10.1126/science.aaq0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martella A., Firth M.A., Taylor B.J.M., Goeppert A.U., Cuomo E.M., Roth R.G., Dickson A.J., Fisher D.I. Systematic evaluation of CRISPRa and CRISPRi modalities enables development of a multiplexed, orthogonal gene activation and repression system. ACS Synth. Biol. 2019;8:1998–2006. doi: 10.1021/acssynbio.8b00527. [DOI] [PubMed] [Google Scholar]
- 145.Fontana J., Dong C., Kiattisewee C., Chavali V.P., Tickman B.I., Carothers J.M., Zalatan J.G. Effective CRISPRa-mediated control of gene expression in bacteria must overcome strict target site requirements. bioRxiv. 2019:770891. doi: 10.1101/770891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bradley R.W., Buck M., Wang B. Tools and principles for microbial gene circuit engineering. J. Mol. Biol. 2016;428:862–888. doi: 10.1016/j.jmb.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 147.Nielsen A.A.K., Der B.S., Shin J., Vaidyanathan P., Paralanov V., Strychalski E.A., Ross D., Densmore D., Voigt C.A. Genetic circuit design automation. Science. 2016;352:aac7341. doi: 10.1126/science.aac7341. [DOI] [PubMed] [Google Scholar]
- 148.Lalwani M.A., Zhao E.M., Avalos J.L. Current and future modalities of dynamic control in metabolic engineering. Curr. Opin. Biotechnol. 2018;52:56–65. doi: 10.1016/j.copbio.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 149.Buck M., Cannon W. Specific binding of the transcription factor sigma-54 to promoter DNA. Nature. 1992;358:19–21. doi: 10.1038/358422a0. [DOI] [PubMed] [Google Scholar]
- 150.Feklístov A., Sharon B.D., Darst S.A., Gross C.A. Bacterial sigma factors: A historical, structural, and genomic perspective. Annu. Rev. Microbiol. 2014;68:357–376. doi: 10.1146/annurev-micro-092412-155737. [DOI] [PubMed] [Google Scholar]
- 151.Davis M.C., Kesthely C.A., Franklin E.A., MacLellan S.R. The essential activities of the bacterial sigma factor. Can. J. Microbiol. 2017;63:89–99. doi: 10.1139/cjm-2016-0576. [DOI] [PubMed] [Google Scholar]
- 152.Stensjö K., Vavitsas K., Tyystjärvi T. Harnessing transcription for bioproduction in cyanobacteria. Physiol. Plant. 2018;162:148–155. doi: 10.1111/ppl.12606. [DOI] [PubMed] [Google Scholar]
- 153.Helmann J.D. Where to begin? Sigma factors and the selectivity of transcription initiation in bacteria. Mol. Microbiol. 2019;112:335–347. doi: 10.1111/mmi.14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Imamura S., Asayama M. Sigma factors for cyanobacterial transcription. Gene Regul. Syst. Biol. 2009;3:65–87. doi: 10.4137/GRSB.S2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wells K.N., Videau P., Nelson D., Eiting J.E., Philmus B. The influence of sigma factors and ribosomal recognition elements on heterologous expression of cyanobacterial gene clusters in Escherichia coli. FEMS Microbiol. Lett. 2018;365:fny164. doi: 10.1093/femsle/fny164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bervoets I., Van Brempt M., Van Nerom K., Van Hove B., Maertens J., De Mey M., Charlier D. A sigma factor toolbox for orthogonal gene expression in Escherichia coli. Nucleic Acids Res. 2018;46:2133–2144. doi: 10.1093/nar/gky010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tripathi L., Zhang Y., Lin Z. Bacterial sigma factors as targets for engineered or synthetic transcriptional control. Front. Bioeng. Biotechnol. 2014;2:33. doi: 10.3389/fbioe.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Iyer L.M., Koonin E.V., Aravind L. Evolution of bacterial RNA polymerase: Implications for large-scale bacterial phylogeny, domain accretion, and horizontal gene transfer. Gene. 2004;335:73–88. doi: 10.1016/j.gene.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 159.Zhao K., Liu M., Burgess R.R. Promoter and regulon analysis of nitrogen assimilation factor, σ54, reveal alternative strategy for E. coli MG1655 flagellar biosynthesis. Nucleic Acids Res. 2010;38:1273–1283. doi: 10.1093/nar/gkp1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu Y., Wan X., Wang B. Engineered CRISPRa enables programmable eukaryote-like gene activation in bacteria. Nat. Commun. 2019;10:3693. doi: 10.1038/s41467-019-11479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meyer A.J., Ellefson J.W., Ellington A.D. Directed evolution of a panel of orthogonal T7 RNA polymerase variants for in vivo or in vitro synthetic circuitry. ACS Synth. Biol. 2015;4:1070–1076. doi: 10.1021/sb500299c. [DOI] [PubMed] [Google Scholar]
- 162.Hussey B.J., McMillen D.R. Programmable T7-based synthetic transcription factors. Nucleic Acids Res. 2018;46:9842–9854. doi: 10.1093/nar/gky785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Temme K., Hill R., Segall-Shapiro T.H., Moser F., Voigt C.A. Modular control of multiple pathways using engineered orthogonal T7 polymerases. Nucleic Acids Res. 2012;40:8773–8781. doi: 10.1093/nar/gks597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim W.J., Lee S.M., Um Y., Sim S.J., Woo H.M. Development of SyneBrick vectors as a synthetic biology platform for gene expression in Synechococcus elongatus PCC 7942. Front. Plant Sci. 2017;8:293. doi: 10.3389/fpls.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jin H., Lindblad P., Bhaya D. Building an inducible T7 RNA polymerase/T7 promoter circuit in Synechocystis sp. PCC6803. ACS Synth. Biol. 2019;8:655–660. doi: 10.1021/acssynbio.8b00515. [DOI] [PubMed] [Google Scholar]
- 166.Pu J., Zinkus-Boltz J., Dickinson B.C. Evolution of a split RNA polymerase as a versatile biosensor platform. Nat. Chem. Biol. 2017;13:432–438. doi: 10.1038/nchembio.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Komura R., Aoki W., Motone K., Satomura A., Ueda M. High-throughput evaluation of T7 promoter variants using biased randomization and DNA barcoding. PLoS ONE. 2018;13:e0196905. doi: 10.1371/journal.pone.0196905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Han T., Chen Q., Liu H. Engineered photoactivatable genetic switches based on the bacterium phage T7 RNA Polymerase. ACS Synth. Biol. 2017;6:357–366. doi: 10.1021/acssynbio.6b00248. [DOI] [PubMed] [Google Scholar]
- 169.Meyer A.J., Segall-Shapiro T.H., Glassey E., Zhang J., Voigt C.A. Escherichia coli “Marionette” strains with 12 highly optimized small-molecule sensors. Nat. Chem. Biol. 2019;15:196–204. doi: 10.1038/s41589-018-0168-3. [DOI] [PubMed] [Google Scholar]
- 170.Miyazaki K. Molecular engineering of the salicylate-inducible transcription factor Sal7AR for orthogonal and high gene expression in Escherichia coli. PLoS ONE. 2018;13:e0194090. doi: 10.1371/journal.pone.0194090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kelly C.L., Liu Z., Yoshihara A., Jenkinson S.F., Wormald M.R., Otero J., Estévez A., Kato A., Marqvorsen M.H.S., Fleet G.W.J., et al. Synthetic chemical inducers and genetic decoupling enable orthogonal control of the rhaBAD Promoter. ACS Synth. Biol. 2016;5:1136–1145. doi: 10.1021/acssynbio.6b00030. [DOI] [PubMed] [Google Scholar]
- 172.Huang H.H., Lindblad P. Wide-dynamic-range promoters engineered for cyanobacteria. J. Biol. Eng. 2013;7:10. doi: 10.1186/1754-1611-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Albers S.C., Gallegos V.A., Peebles C.A.M. Engineering of genetic control tools in Synechocystis sp. PCC 6803 using rational design techniques. J. Biotechnol. 2015;216:36–46. doi: 10.1016/j.jbiotec.2015.09.042. [DOI] [PubMed] [Google Scholar]
- 174.Cao Y.Q., Li Q., Xia P.F., Wei L.J., Guo N., Li J.W., Wang S.G. AraBAD based toolkit for gene expression and metabolic robustness improvement in Synechococcus elongatus. Sci. Rep. 2017;7:18059. doi: 10.1038/s41598-017-17035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huang H.H., Camsund D., Lindblad P., Heidorn T. Design and characterization of molecular tools for a Synthetic Biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res. 2010;38:2577–2593. doi: 10.1093/nar/gkq164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Camsund D., Heidorn T., Lindblad P. Design and analysis of LacI-repressed promoters and DNA-looping in a cyanobacterium. J. Biol. Eng. 2014;8:4. doi: 10.1186/1754-1611-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Blasi B., Peca L., Vass I., Kós P.B. Characterization of stress responses of heavy metal and metalloid inducible promoters in Synechocystis PCC6803. J. Microbiol. Biotechnol. 2012;22:166–169. doi: 10.4014/jmb.1106.06050. [DOI] [PubMed] [Google Scholar]
- 178.Pérez A.A., Gajewski J.P., Ferlez B.H., Ludwig M., Baker C.S., Golbeck J.H., Bryant D.A. Zn2+ -inducible expression platform for Synechococcus sp. strain PCC 7002 based on the smtA promoter/operator and smtB repressor. Appl. Environ. Microbiol. 2017;83:1–14. doi: 10.1128/AEM.02491-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Englund E., Liang F., Lindberg P. Evaluation of promoters and ribosome binding sites for biotechnological applications in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2016;6:36640. doi: 10.1038/srep36640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rippka R., Deruelles J., Waterbury J.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979;111:1–61. doi: 10.1099/00221287-111-1-1. [DOI] [Google Scholar]
- 181.Gao C., Hou J., Xu P., Guo L., Chen X., Hu G., Ye C., Edwards H., Chen J., Chen W., et al. Programmable biomolecular switches for rewiring flux in Escherichia coli. Nat. Commun. 2019;10:3751. doi: 10.1038/s41467-019-11793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Polstein L.R., Gersbach C.A. A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nat. Chem. Biol. 2015;11:198–200. doi: 10.1038/nchembio.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee D., Hyun J.H., Jung K., Hannan P., Kwon H.B. A calcium- and light-gated switch to induce gene expression in activated neurons. Nat. Biotechnol. 2017;35:858–863. doi: 10.1038/nbt.3902. [DOI] [PubMed] [Google Scholar]
- 184.Mansouri M., Strittmatter T., Fussenegger M. Light-controlled mammalian cells and their therapeutic applications in synthetic biology. Adv. Sci. 2019;6:1800952. doi: 10.1002/advs.201800952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tandar S.T., Senoo S., Toya Y., Shimizu H. Optogenetic switch for controlling the central metabolic flux of Escherichia coli. Metab. Eng. 2019;55:68–75. doi: 10.1016/j.ymben.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 186.Zhao E.M., Suek N., Wilson M.Z., Dine E., Pannucci N.L., Gitai Z., Avalos J.L., Toettcher J.E. Light-based control of metabolic flux through assembly of synthetic organelles. Nat. Chem. Biol. 2019;15:589–597. doi: 10.1038/s41589-019-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jayaraman P., Devarajan K., Chua T.K., Zhang H., Gunawan E., Poh C.L. Blue light-mediated transcriptional activation and repression of gene expression in bacteria. Nucleic Acids Res. 2016;44:6994–7005. doi: 10.1093/nar/gkw548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fernandez-Rodriguez J., Moser F., Song M., Voigt C.A. Engineering RGB color vision into Escherichia coli. Nat. Chem. Biol. 2017;13:706–708. doi: 10.1038/nchembio.2390. [DOI] [PubMed] [Google Scholar]
- 189.Rost B.R., Schneider-Warme F., Schmitz D., Hegemann P. Optogenetic tools for subcellular applications in neuroscience. Neuron. 2017;96:572–603. doi: 10.1016/j.neuron.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 190.Yu Q., Wang Y., Zhao S., Ren Y. Photocontrolled reversible self-assembly of dodecamer nitrilase. Bioresour. Bioprocess. 2017;4:36. doi: 10.1186/s40643-017-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hirose Y., Shimada T., Narikawa R., Katayama M., Ikeuchi M. Cyanobacteriochrome CcaS is the green light receptor that induces the expression of phycobilisome linker protein. Proc. Natl. Acad. Sci. USA. 2008;105:9528–9533. doi: 10.1073/pnas.0801826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tilbrook K., Arongaus A.B., Binkert M., Heijde M., Yin R., Ulm R. The UVR8 UV-B Photoreceptor: Perception, signaling and response. Arab. Book. 2013;11:e0164. doi: 10.1199/tab.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ramakrishnan P., Tabor J.J. Repurposing synechocystis PCC6803 UirS-UirR as a UV-violet/green photoreversible transcriptional regulatory tool in E. coli. ACS Synth. Biol. 2016;5:733–740. doi: 10.1021/acssynbio.6b00068. [DOI] [PubMed] [Google Scholar]
- 194.Möglich A., Ayers R.A., Moffat K. Design and signaling mechanism of light-regulated histidine kinases. J. Mol. Biol. 2009;385:1433–1444. doi: 10.1016/j.jmb.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jin X., Riedel-Kruse I.H. Biofilm Lithography enables high-resolution cell patterning via optogenetic adhesin expression. Proc. Natl. Acad. Sci. USA. 2018;115:3698–3703. doi: 10.1073/pnas.1720676115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Abe K., Miyake K., Nakamura M., Kojima K., Ferri S., Ikebukuro K., Sode K. Engineering of a green-light inducible gene expression system in Synechocystis sp. PCC6803. Microb. Biotechnol. 2014;7:177–183. doi: 10.1111/1751-7915.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Miyake K., Abe K., Ferri S., Nakajima M., Nakamura M., Yoshida W., Kojima K., Ikebukuro K., Sode K. A green-light inducible lytic system for cyanobacterial cells. Biotechnol. Biofuels. 2014;7:56. doi: 10.1186/1754-6834-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ong N.T., Tabor J.J. A miniaturized Escherichia coli green light sensor with high dynamic range. ChemBioChem. 2018;19:1255–1258. doi: 10.1002/cbic.201800007. [DOI] [PubMed] [Google Scholar]
- 199.Tabor J.J., Levskaya A., Voigt C.A. Multichromatic control of gene expression in Escherichia coli. J. Mol. Biol. 2011;405:315–324. doi: 10.1016/j.jmb.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ong N.T., Olson E.J., Tabor J.J. Engineering an E. coli near-infrared light sensor. ACS Synth. Biol. 2018;7:240–248. doi: 10.1021/acssynbio.7b00289. [DOI] [PubMed] [Google Scholar]
- 201.Takakado A., Nakasone Y., Terazima M. Sequential DNA binding and dimerization processes of the photosensory protein EL222. Biochemistry. 2018;57:1603–1610. doi: 10.1021/acs.biochem.7b01206. [DOI] [PubMed] [Google Scholar]
- 202.Xu X., Du Z., Liu R., Li T., Zhao Y., Chen X., Yang Y. A single-component optogenetic system allows stringent switch of gene expression in yeast cells. ACS Synth. Biol. 2018;7:2045–2053. doi: 10.1021/acssynbio.8b00180. [DOI] [PubMed] [Google Scholar]
- 203.Papanatsiou M., Petersen J., Henderson L., Wang Y., Christie J.M., Blatt M.R. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science. 2019;363:1456–1459. doi: 10.1126/science.aaw0046. [DOI] [PubMed] [Google Scholar]
- 204.Baumschlager A., Aoki S.K., Khammash M. Dynamic blue light-inducible T7 RNA Polymerases (Opto-T7RNAPs) for precise spatiotemporal gene expression control. ACS Synth. Biol. 2017;6:2157–2167. doi: 10.1021/acssynbio.7b00169. [DOI] [PubMed] [Google Scholar]
- 205.Song J.Y., Cho H.S., Cho J.I., Jeon J.S., Lagarias J.C., Park Y.I. Near-UV cyanobacteriochrome signaling system elicits negative phototaxis in the cyanobacterium Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA. 2011;108:10780–10785. doi: 10.1073/pnas.1104242108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Badary A., Abe K., Ferri S., Kojima K., Sode K. The Development and characterization of an exogenous green-light-regulated gene expression system in marine cyanobacteria. Mar. Biotechnol. 2015;17:245–251. doi: 10.1007/s10126-015-9616-1. [DOI] [PubMed] [Google Scholar]
- 207.Wiltbank L.B., Kehoe D.M. Diverse light responses of cyanobacteria mediated by phytochrome superfamily photoreceptors. Nat. Rev. Microbiol. 2019;17:37–50. doi: 10.1038/s41579-018-0110-4. [DOI] [PubMed] [Google Scholar]
- 208.Luimstra V.M., Schuurmans J.M., Verschoor A.M., Hellingwerf K.J., Huisman J., Matthijs H.C.P. Blue light reduces photosynthetic efficiency of cyanobacteria through an imbalance between photosystems I and II. Photosynth. Res. 2018;138:177–189. doi: 10.1007/s11120-018-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wan X., Volpetti F., Petrova E., French C., Maerkl S.J., Wang B. Cascaded amplifying circuits enable ultrasensitive cellular sensors for toxic metals. Nat. Chem. Biol. 2019;15:540–548. doi: 10.1038/s41589-019-0244-3. [DOI] [PubMed] [Google Scholar]
- 210.Wang B., Buck M. Rapid engineering of versatile molecular logic gates using heterologous genetic transcriptional modules. Chem. Commun. 2014;50:11642–11644. doi: 10.1039/C4CC05264A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Brophy J.A.N., Voigt C.A. Principles of genetic circuit design. Nat. Methods. 2014;11:508–520. doi: 10.1038/nmeth.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xiang Y., Dalchau N., Wang B. Scaling up genetic circuit design for cellular computing: Advances and prospects. Nat. Comput. 2018;17:833–853. doi: 10.1007/s11047-018-9715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xia P., Ling H., Foo J.L., Chang M.W. Synthetic biology toolkits for metabolic engineering of cyanobacteria. Biotechnol. J. 2019;14:1800496. doi: 10.1002/biot.201800496. [DOI] [PubMed] [Google Scholar]
- 214.Immethun C.M., Ng K.M., Delorenzo D.M., Waldron-Feinstein B., Lee Y.C., Moon T.S. Oxygen-responsive genetic circuits constructed in Synechocystis sp. PCC 6803. Biotechnol. Bioeng. 2016;113:433–442. doi: 10.1002/bit.25722. [DOI] [PubMed] [Google Scholar]
- 215.Wang B., Barahona M., Buck M. A modular cell-based biosensor using engineered genetic logic circuits to detect and integrate multiple environmental signals. Biosens. Bioelectron. 2013;40:368–376. doi: 10.1016/j.bios.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Noren C.J., Wang J., Perler F.B. Dissecting the chemistry of protein splicing and its applications. Angew. Chem. Int. Ed. 2000;39:450–466. doi: 10.1002/(SICI)1521-3773(20000204)39:3<450::AID-ANIE450>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 217.Caspi J., Amitai G., Belenkiy O., Pietrokovski S. Distribution of split DnaE inteins in cyanobacteria. Mol. Microbiol. 2003;50:1569–1577. doi: 10.1046/j.1365-2958.2003.03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Perler F.B. InBase: The intein database. Nucleic Acids Res. 2002;30:383–384. doi: 10.1093/nar/30.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Novikova O., Topilina N., Belfort M. Enigmatic distribution, evolution, and function of inteins. J. Biol. Chem. 2014;289:14490–14497. doi: 10.1074/jbc.R114.548255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Saleh L., Perler F.B. Protein splicing in cis and in trans. Chem. Rec. 2006;6:183–193. doi: 10.1002/tcr.20082. [DOI] [PubMed] [Google Scholar]
- 221.Lockless S.W., Muir T.W. Traceless protein splicing utilizing evolved split inteins. Proc. Natl. Acad. Sci. USA. 2009;106:10999–11004. doi: 10.1073/pnas.0902964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schaerli Y., Gili M., Isalan M. A split intein T7 RNA polymerase for transcriptional AND-logic. Nucleic Acids Res. 2014;42:12322–12328. doi: 10.1093/nar/gku884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zeng Y., Jones A.M., Thomas E.E., Nassif B., Silberg J.J., Segatori L. A split transcriptional repressor that links protein solubility to an orthogonal genetic circuit. ACS Synth. Biol. 2018;7:2126–2138. doi: 10.1021/acssynbio.8b00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Carvajal-Vallejos P., Pallissé R., Mootz H.D., Schmidt S.R. Unprecedented rates and efficiencies revealed for new natural split inteins from metagenomic sources. J. Biol. Chem. 2012;287:28686–28696. doi: 10.1074/jbc.M112.372680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Huang H.H., Seeger C., Helena Danielson U., Lindblad P. Analysis of the leakage of gene repression by an artificial TetR-regulated promoter in cyanobacteria. BMC Res. Notes. 2015;8:459. doi: 10.1186/s13104-015-1425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Goodall E.C.A., Robinson A., Johnston I.G., Jabbari S., Turner K.A., Cunningham A.F., Lund P.A., Cole J.A., Henderson I.R. The Essential Genome of Escherichia coli K-12. MBio. 2018;9:e02096-17. doi: 10.1128/mBio.02096-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lee S.Y., Kim H.U. Systems strategies for developing industrial microbial strains. Nat. Biotechnol. 2015;33:1061–1072. doi: 10.1038/nbt.3365. [DOI] [PubMed] [Google Scholar]
- 228.Gu C., Kim G.B., Kim W.J., Kim H.U., Lee S.Y. Current status and applications of genome-scale metabolic models. Genome Biol. 2019;20:1–18. doi: 10.1186/s13059-019-1730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Baroukh C., Muñoz-Tamayo R., Bernard O., Steyer J.P. Mathematical modeling of unicellular microalgae and cyanobacteria metabolism for biofuel production. Curr. Opin. Biotechnol. 2015;33:198–205. doi: 10.1016/j.copbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 230.Joshi C.J., Peebles C.A.M., Prasad A. Modeling and analysis of flux distribution and bioproduct formation in Synechocystis sp. PCC 6803 using a new genome-scale metabolic reconstruction. Algal Res. 2017;27:295–310. doi: 10.1016/j.algal.2017.09.013. [DOI] [Google Scholar]
- 231.Triana J., Montagud A., Siurana M., Fuente D., Urchueguía A., Gamermann D., Torres J., Tena J., de Córdoba P., Urchueguía J. Generation and evaluation of a genome-scale metabolic network model of Synechococcus elongatus PCC7942. Metabolites. 2014;4:680–698. doi: 10.3390/metabo4030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Broddrick J.T., Welkie D.G., Jallet D., Golden S.S., Peers G., Palsson B.O. Predicting the metabolic capabilities of Synechococcus elongatus PCC 7942 adapted to different light regimes. Metab. Eng. 2019;52:42–56. doi: 10.1016/j.ymben.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mueller T.J., Ungerer J.L., Pakrasi H.B., Maranas C.D. Identifying the metabolic differences of a fast-growth phenotype in Synechococcus UTEX 2973. Sci. Rep. 2017;7:41569. doi: 10.1038/srep41569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Vu T.T., Hill E.A., Kucek L.A., Konopka A.E., Beliaev A.S., Reed J.L. Computational evaluation of Synechococcus sp. PCC 7002 metabolism for chemical production. Biotechnol. J. 2013;8:619–630. doi: 10.1002/biot.201200315. [DOI] [PubMed] [Google Scholar]
- 235.Qian X., Kim M.K., Kumaraswamy G.K., Agarwal A., Lun D.S., Dismukes G.C. Flux balance analysis of photoautotrophic metabolism: Uncovering new biological details of subsystems involved in cyanobacterial photosynthesis. Biochim. Biophys. Acta Bioenerg. 2017;1858:276–287. doi: 10.1016/j.bbabio.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 236.Malatinszky D., Steuer R., Jones P.R. A comprehensively curated genome-scale two-cell model for the heterocystous cyanobacterium Anabaena sp. PCC 7120. Plant Physiol. 2017;173:509–523. doi: 10.1104/pp.16.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nogales J., Gudmundsson S., Knight E.M., Palsson B.O., Thiele I. Detailing the optimality of photosynthesis in cyanobacteria through systems biology analysis. Proc. Natl. Acad. Sci. USA. 2012;109:2678–2683. doi: 10.1073/pnas.1117907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Knoop H., Gründel M., Zilliges Y., Lehmann R., Hoffmann S., Lockau W., Steuer R. Flux balance analysis of cyanobacterial metabolism: The metabolic network of Synechocystis sp. PCC 6803. PLoS Comput. Biol. 2013;9:e1003081. doi: 10.1371/journal.pcbi.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rubin B.E., Wetmore K.M., Price M.N., Diamond S., Shultzaberger R.K., Lowe L.C., Curtin G., Arkin A.P., Deutschbauer A., Golden S.S. The essential gene set of a photosynthetic organism. Proc. Natl. Acad. Sci. USA. 2015;112:E6634–E6643. doi: 10.1073/pnas.1519220112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Abernathy M.H., Yu J., Ma F., Liberton M., Ungerer J., Hollinshead W.D., Gopalakrishnan S., He L., Maranas C.D., Pakrasi H.B., et al. Deciphering cyanobacterial phenotypes for fast photoautotrophic growth via isotopically nonstationary metabolic flux analysis. Biotechnol. Biofuels. 2017;10:273. doi: 10.1186/s13068-017-0958-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shabestary K., Hudson E.P. Computational metabolic engineering strategies for growth-coupled biofuel production by Synechocystis. Metab. Eng. Commun. 2016;3:216–226. doi: 10.1016/j.meteno.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ranganathan S., Suthers P.F., Maranas C.D. OptForce: An optimization procedure for identifying All genetic manipulations leading to targeted overproductions. PLoS Comput. Biol. 2010;6:e1000744. doi: 10.1371/journal.pcbi.1000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rocha I., Maia P., Evangelista P., Vilaça P., Soares S., Pinto J.P., Nielsen J., Patil K.R., Ferreira E.C., Rocha M. OptFlux: An open-source software platform for in silico metabolic engineering. BMC Syst. Biol. 2010;4:45. doi: 10.1186/1752-0509-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lin P.C., Saha R., Zhang F., Pakrasi H.B. Metabolic engineering of the pentose phosphate pathway for enhanced limonene production in the cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2017;7:17503. doi: 10.1038/s41598-017-17831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lv Q., Ma W., Liu H., Li J., Wang H., Lu F., Zhao C., Shi T. Genome-wide protein-protein interactions and protein function exploration in cyanobacteria. Sci. Rep. 2015;5:15519. doi: 10.1038/srep15519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Vivek-Ananth R.P., Samal A. Advances in the integration of transcriptional regulatory information into genome-scale metabolic models. Biosystems. 2016;147:1–10. doi: 10.1016/j.biosystems.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 247.Zavřel T., Očenášová P., Červený J. Phenotypic characterization of Synechocystis sp. PCC 6803 substrains reveals differences in sensitivity to abiotic stress. PLoS ONE. 2017;12:e0189130. doi: 10.1371/journal.pone.0189130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Soppa J., Ludt K., Zerulla K. The ploidy level of Synechocystis sp. PCC 6803 is highly variable and is influenced by growth phase and by chemical and physical external parameters. Microbiology. 2016;162:730–739. doi: 10.1099/mic.0.000264. [DOI] [PubMed] [Google Scholar]
- 249.Morris J.N., Eaton-Rye J.J., Summerfield T.C. Phenotypic variation in wild-type substrains of the model cyanobacterium Synechocystis sp. PCC 6803. N. Z. J. Bot. 2017;55:25–35. doi: 10.1080/0028825X.2016.1231124. [DOI] [Google Scholar]
- 250.Li X., Patena W., Fauser F., Jinkerson R.E., Saroussi S., Meyer M.T., Ivanova N., Robertson J.M., Yue R., Zhang R., et al. A genome-wide algal mutant library and functional screen identifies genes required for eukaryotic photosynthesis. Nat. Genet. 2019;51:627–635. doi: 10.1038/s41588-019-0370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Alonso J.M., Stepanova A.N., Leisse T.J., Kim C.J., Chen H., Shinn P., Stevenson D.K., Zimmerman J., Barajas P., Cheuk R., et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 252.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K.A., Tomita M., Wanner B.L., Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006;2 doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Giaever G., Chu A.M., Ni L., Connelly C., Riles L., Véronneau S., Dow S., Lucau-Danila A., Anderson K., André B., et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 254.Watabe K., Mimuro M., Tsuchiya T. Development of a high-frequency in vivo transposon mutagenesis system for Synechocystis sp. PCC 6803 and Synechococcus elongatus PCC 7942. Plant Cell Physiol. 2014;55:2017–2026. doi: 10.1093/pcp/pcu128. [DOI] [PubMed] [Google Scholar]
- 255.Chambers S., Kitney R., Freemont P. The Foundry: The DNA synthesis and construction Foundry at Imperial College. Biochem. Soc. Trans. 2016;44:687–688. doi: 10.1042/BST20160007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kuchmina E., Wallner T., Kryazhov S., Zinchenko V.V., Wilde A. An expression system for regulated protein production in Synechocystis sp. PCC 6803 and its application for construction of a conditional knockout of the ferrochelatase enzyme. J. Biotechnol. 2012;162:75–80. doi: 10.1016/j.jbiotec.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 257.Krynická V., Tichý M., Krafl J., Yu J., Kaňa R., Boehm M., Nixon P.J., Komenda J. Two essential FtsH proteases control the level of the Fur repressor during iron deficiency in the cyanobacterium Synechocystis sp. PCC 6803. Mol. Microbiol. 2014;94:609–624. doi: 10.1111/mmi.12782. [DOI] [PubMed] [Google Scholar]
- 258.Schuergers N., Nürnberg D.J., Wallner T., Mullineaux C.W., Wilde A. PilB localization correlates with the direction of twitching motility in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology. 2015;161:960–966. doi: 10.1099/mic.0.000064. [DOI] [PubMed] [Google Scholar]