Abstract
Introduction
Renal artery embolization is performed before radical nephrectomy (RN) for renal mass in order to induce preoperative infarction and to facilitate surgical intervention through decrease of intraoperative bleeding. Moreover, in metastatic renal cancer it seems to stimulate tumour-specific antibodies, even if no established benefits in clinical response or survival have been reported. The role of preoperative renal artery embolization (PRAE) in management of renal masses has been often debated and its real benefits are still unclear. Nevertheless, in huge and complex renal masses, which are often characterized by a high and anarchic blood supply and rapid local invasion, radical nephrectomy can be challenging even for skilled surgeons. The aim of this prospective randomized study was to evaluate the effectiveness and safety of PRAE in complex masses by comparing perioperative outcomes of RN with and without PRAE.
Materials and methods
From December 2015 to May 2018 we enrolled prospectively 64 patients who underwent RN for localized (T2a-b) or locally advanced (T3 and T4) or advanced (N+, M+) renal cancers. Patients were divided in two groups. The first group included 30 patients who underwent PRAE; in the second group we enrolled 34 patients who did not undergo RN without PRAE. Perioperative outcomes in terms of operative time, blood loss, transfusion rate and length of hospitalization were evaluated. Statistical analysis was performed using GraphPad Prism 6.0 software.
Results
Median blood loss was 250 ml (50-500) and 400 ml (50-1000) in the first and second group, respectively, with a statistically significant difference (p=0.0066). Median surgical time was 200 min (90-390) and 240 min (130-390) in PRAE and No-PRAE group (p=0.06), respectively. No major complications occurred after embolization. Overall complication rate in Group 1 and 2 was 46.7% (14/30) and 50% (17/34), respectively (p=0.34). No major complications occurred in both groups. The mean follow up was 21,5 months.
Conclusions
Our results prove PRAE to be a safe procedure with low complications rate. To our experience, PRAE seems to be a useful tool in surgical management of a large mass and advanced disease.
Keywords: Radical nephrectomy, Embolization, PRAE, Renal masses, Huge mass
1. Introduction
Renal cell carcinoma (RCC) represents 3% of all cancers with the highest incidence in Western countries [1]. In 2012, there were approximately 84,400 new cases of RCC and 34,700 kidney cancer-related deaths in the European Union [2]. Radical nephrectomy (RN) is the gold standard treatment for tumours larger than 7 cm as well as for locally advanced and metastatic diseases [3]. In 1973, renal artery embolization was introduced, to clinical practice by Almgard et al. to induce necrosis in renal neoplasms. [4] The first indications for renal embolization without nephrectomy were limited to treatment of severe symptomatic hematuria and other palliative strategies for metastatic renal cancer [5, 6]; afterwards, it was performed before RN for renal masses in order to induce preoperative infarction and, consequently, to facilitate surgical intervention through decrease of intraoperative bleeding. Moreover, embolization with delayed RN has been carried out in metastatic RCC with the aim to stimulate tumour-specific antibodies, even if no established benefits in clinical response or survival have been reported [7]. Afterwards, the indications for renal embolization have been extended to different conditions such as persistent bleeding, treatment of hemorrhagic angiomyolipomas (AML), arteriovenous fistulae and vascular malformations, before endograft placement for abdominal aortic aneurysm repair, pseudo-aneurysm, medical renal disease as malignant hypertension and severe nephrotic syndrome [5, 6]. However, the role of preoperative renal artery embolization (PRAE) in management of renal masses has been often debated and its real benefit is still unclear. Nevertheless, in huge and complex renal masses, which are often characterized by high and anarchic blood supply and rapid local invasion, RN can be challenging even for skilled surgeons. To our knowledge, there are no prospective and randomized good quality studies reported in the literature. The aim of this prospective randomized study was to evaluate the effectiveness and safety of PRAE in complex masses comparing perioperative outcomes of RN with and without PRAE.
2. Materials and Methods
In a high-volume tertiary institution, a prospective randomized study was carried out to evaluate perioperative data and perioperative complications. The study was approved by the Ethics Committee of the University of Perugia. All subjects signed an informed consent. From December 2015 to May 2018 we prospectively enrolled a total of 64 patients undergoing RN for renal cell cancer (RCC). Patients were randomly assigned to two groups. The first (PRAE group) included patients who underwent PRAE; the second group (No-PRAE group) included patients who did not undergo PRAE. Simple randomization by computer-generated random numbers was performed. Before undergoing RN, abdominal computerized tomography (CT), or magnetic resonance imaging (MRI) in case of kidney failure, were performed in each case. Renal nephrometry score [8] was used to quantify the tumour’s relevant anatomical features as they related to the complexity of the mass, aiding the treatment decision-making. T2 tumours with nephrometry scores of 10-12 were considered high complexity [9] and were included in the study. Other inclusion criteria were locally advanced (T3 and T4) or advanced (N+, M+) renal cancers. Exclusion criteria were T1 masses and bilateral or multi-focal tumours. Heavy BMI or comorbidities were not considered as exclusion criteria. Karnofsky performance status scale [10] and Clavien-Dindo Classification [11] were used to quantify functional status and to evaluate complications, respectively. Laparoscopic surgery was preferred except if minimally invasive approach was technically unsuitable; in these cases a thoracic-phrenic-laparotomy surgery was chosen. The surgical technique did not differ between embolized and non-embolized patients. Lymph node dissection was performed according to the imaging and surgical findings. The endpoint of this study was to evaluate the effect of PRAE on perioperative outcomes in terms of operative time, blood loss, transfusion rate, complications and length of hospitalization. Within the first 2 years after surgery, standard follow-up included physical examination and ultrasound every 3 months as well as CT of the abdomen and thorax every 6 months. After the second year, physical and ultrasound examination was performed every 6 months and CT of the abdomen and thorax once a year. Data were analyzed with GraphPad Prism 6.0. Patient characteristics and perioperative outcomes were analyzed using appropriate comparative tests (T Student, Mann Whitney and Fisher’s exact tests) to verify statistical differences between variables under analysis; the significance threshold was set at 0.05.
2.1. Embolization Technique
Renal artery embolization via inguinal percutaneous access through the femoral artery was performed by the interventional radiologist. PRAE was generally performed the day before surgery under local analgesia. After confirmation of the tumour vascularization with a contrast study, the selective renal artery embolization was performed. Different techniques may be used [12]. In our series Haemostatic Absorbable Gelatin Sponge (Spongostan, Ethicon™, Somerville, NJ, USA), Polyvinyl Alcohol (PVA) Embolization particles (Contour, Boston Scientific™, Marlborough, MA, USA), and metallic spirals were preferred (Figure 1). The embolization of accessory renal artery branches was performed only when it was safe and technically feasible. In case of post infarction syndrome (PIS) our treatment consisted of analgesic therapy with paracetamol 1g every 8 hours and, in case of unremitting pain, by a subcutaneous injection of 5 mg of morphine. Antiemetic and antipyretic drugs were used as needed.
Figure 1.
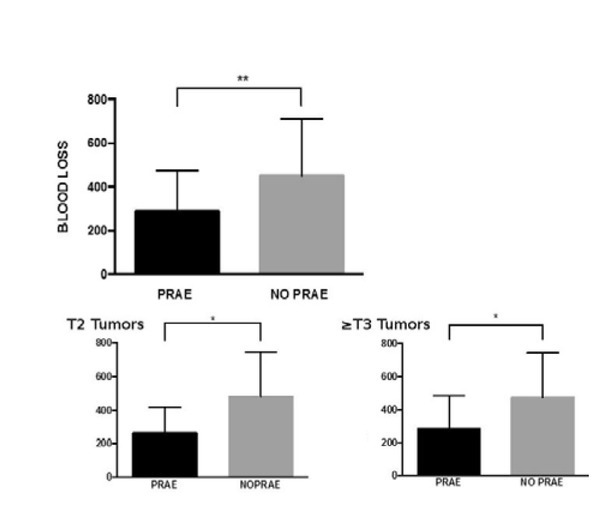
Blood loss report
3. Results
PRAE and No-PRAE group included 30 and 34 patients, respectively. Demographic data were comparable between two groups. Median BMI was 29 kg/m2 (range 20-39) and 28 kg/m2 (range 22-36), respectively for group 1 and 2, median Karnofsky Performance status scale was 90 for both groups. For the PRAE group, median clinical tumour size was 11 cm (ranged from 8 to 17 cm), whereas for the No-PRAE group it was 8.8 cm (ranged from 8 to 16 cm) (p=0.0001). RN was performed after PRAE with a median delay of 21 hrs (ranged from 14 to 30 hrs). PIS occurred in 87% of cases and all of the cases needed pharmacological treatment. No major complications occurred after embolization; there were no cases of coil migration, adjacent organ injury or PRAE-related death. In PRAE group, 12/30 (40%) patients underwent laparoscopic surgery, of which 8 with retroperitoneal approach and 4 with transperitoneal one; the other patients (60%) underwent open trans-peritoneal RN. Lymphadenectomy was carried out in 18/30 (60%) patients. In No-PRAE group, laparoscopic transperitoneal approach was used in 18/34 patients (53%), open surgery in 16/34 (47%); lymphadenectomy was performed in 56% of cases (19/34). Clinical and pathological patients characteristics as well as perioperative
data are showed in Table 1. For the PRAE group, median blood loss was 250 ml (50-500 ml) while for No-PRAE group was 400 ml (50-1000 ml) (Figure 2). Blood loss was significantly higher in No-PRAE group than in PRAE group (p=0.0066). However, no difference was found for transfusion rate (p>0,05). Median surgical time was 205 min (ranged from 90 to 390 min) for the PRAE group versus 240 min (ranged from 130 to 390 min) for the No-PRAE group with no statistically significant difference (p=0.06). Blood loss and surgical time were then compared between the two groups, stratifying according to clinical staging (T2 vs≥T3 lesions). PRAE group showed significantly lower blood loss than No-PRAE group both in T2 and ≥T3 clinical stage (p=0.03) [Figure 1]. On the contrary, surgical time did not differ between T2 (214 min vs 228 min; p=0.54) and ≥T3 lesions (185 min vs 243 min; p=0.08). Median length of hospital stay was 8 days for both groups. This result did not statistically differ (p=0.37) neither by stratifying T2 (p=0.36) from ≥T3 (p=0.25). Overall complication rate was
Table 1.
Demographic, clinical and pathological data
| Characteristic | Total Cohort | % | PRAE group pt. | % | No-PRAE group pt. | % |
|---|---|---|---|---|---|---|
| Patients n | 64 | 100% | 30 | 47% | 34 | 53% |
| Sex Male | 29 | 45.3% | 11 | 37% | 18 | 53% |
| Female | 35 | 54.7% | 19 | 63% | 16 | 47% |
| Age Median | 67.5 | 65.7 | 66.2 | |||
| Range | 19-81 | 19-81 | 38-80 | |||
| Site Right | 30 | 46.8% | 14 | 46.6% | 16 | 47% |
| Left | 34 | 53.2% | 16 | 53.4% | 18 | 53% |
| Median Clinical Size (range) | 10 cm (8-17) | 11 cm (8-17) | 8 cm (8-16) | |||
| PNS | ||||||
| Anemia | 19 | 29.6% | 9 | 30% | 10 | 34% |
| Fever | 20 | 31.2% | 8 | 66% | 12 | 35% |
| Asthenia | 13 | 20.3% | 7 | 23% | 6 | 18% |
| Weight Loss | 9 | 14% | 4 | 13% | 5 | 14% |
| Hypertension | 3 | 4.7% | 3 | 10% | 0 | 0% |
| Ascites/Aedema | 2 | 3.1% | 1 | 3% | 1 | 3% |
| cT Staging | ||||||
| cT2b | 23 | 36% | 11 | 37% | 11 | 33% |
| cT3a | 27 | 42% | 16 | 53% | 12 | 35% |
| cT3b | 9 | 14% | 0 | 0% | 9 | 26% |
| cT4 | 5 | 7% | 3 | 10% | 2 | 6% |
| cN Staging | ||||||
| cN0 | 29 | 44% | 12 | 40% | 15 | 45% |
| cN1 | 27 | 42% | 14 | 47% | 14 | 41% |
| cN2 | 9 | 14% | 4 | 13% | 5 | 14% |
| cM Staging | ||||||
| cM0 | 41 | 64% | 20 | 67% | 21 | 62% |
| cM1 | 23 | 36% | 10 | 33% | 13 | 38% |
| pT Staging | ||||||
| pT0 | 6 | 9% | 2 | 7% | 4 | 12% |
| pT2 | 17 | 26% | 11 | 37% | 4 | 35% |
| pT3 | 40 | 63% | 16 | 53% | 26 | 59% |
| pT4 | 1 | 2% | 1 | 3% | 0 | 0% |
| Grading | ||||||
| G0 | 6 | 9% | 2 | 7% | 4 | 12% |
| G1 | 4 | 6% | 2 | 7% | 1 | 3% |
| G2 | 18 | 28% | 6 | 20% | 10 | 29% |
| G3 | 30 | 47% | 16 | 53% | 14 | 41% |
| G4 | 9 | 14% | 4 | 13% | 5 | 15% |
| pN Staging | ||||||
| pNx | 16 | 25% | 9 | 30% | 7 | 20% |
| pN0 | 37 | 58% | 14 | 47% | 23 | 68% |
| pN1 | 11 | 17% | 7 | 23% | 4 | 12% |
| Margins | ||||||
| R0 | 59 | 92% | 28 | 93% | 31 | 91% |
| R1 | 5 | 2 | 7% | 3 | 9% | |
| 8% | ||||||
| PRAE material | ||||||
| Gelatine Sponge | 26 | 41% | 26 | 87% | n.a | n.a |
| Polyvinyl Alchool | 12 | 19% | 12 | 40% | n.a | n.a |
| Metallic Spirals | 12 | 19% | 12 | 40% | n.a | n.a |
| PIS | 26/30 | 87% | 26 | 87% | n.a | n.a |
| Median Time P-t-S (range) | 21 h | 21 h (14-30) | n.a | |||
| (14-30) | ||||||
| Histotype | ||||||
| RCC | 50 | 78.2% | 22 | 74% | 28 | 82% |
| Oncocytoma | 3 | 4.7% | 1 | 3% | 2 | 6% |
| Cromophobe | 4 | 6.3% | 3 | 10% | 1 | 3% |
| Papillary | 1 | 1.5% | 1 | 3% | 0 | 0% |
| Solitary Fibrous Tumor | 1 | 1.5% | 1 | 3% | 0 | 0% |
| KS | 4 | 6.3% | 2 | 7% | 2 | 6% |
| TCC | 1 | 1.5% | 0 | 0% | 1 | 3% |
| Median Blood loss (range) | 325 cc (50-1000) | 250 cc (50-500) | 400 cc (50-1000) | |||
| Median Surgical Time (range) | 220 min (90-390) | 205 min (90-390) | 240 min (130-390) | |||
| Transfusion Rate | 23/64 | 36% | 9 | 30% | 14 | 41% |
| Intraoperative Transfusion Rate | 13/64 | 20% | 4 | 13% | 9 | 26% |
| Hospital Transfusion Rate | 17/64 | 26% | 7 | 23% | 10 | 29% |
| Median Hospital Stay (range) | 8 d (5-20) | 8 d (6-20) | 8 d (5-15) | |||
| Int. Care Unit stay | 12 | 19% | 8 | 27% | 4 | 12% |
Abbreviation: PNS= para neoplastic syndrome; PIS=post infarction Syndrome; P-t-S= PRAE to Surgery; RCC= renal cell carcinoma; SFT= Solitary Fibrous Tumor; KS= Kidney’s Sarcoma; TCC= transitional cell carcinoma.
Figure 2.
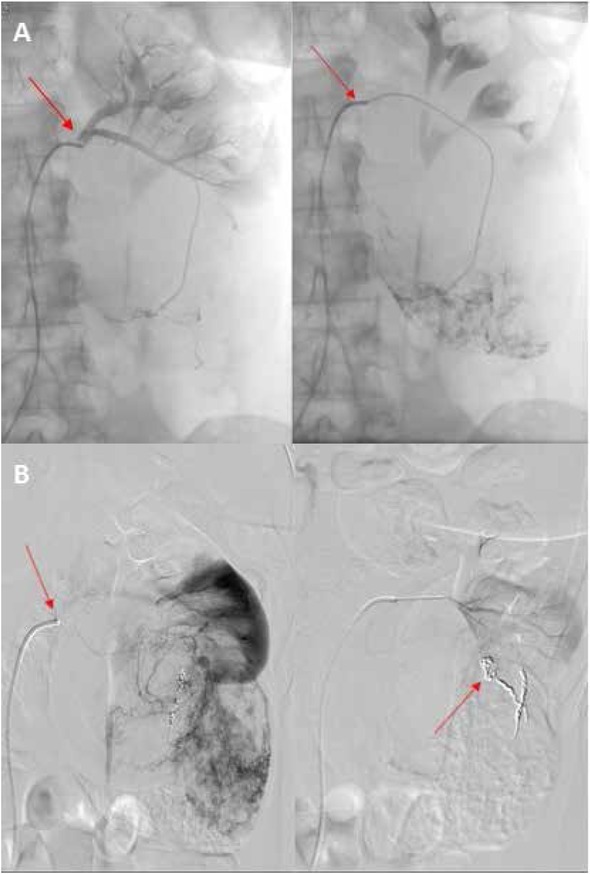
Selective digital subtraction angiography in patient with renal cell carcinoma of left kidney. (A). Postembolization angiography showing complete occlusion of arterial vessels of the left renal mass with Embolization particles (B)
46.7% (14/30) and 50% (17/34) in group 1 and 2, respectively, without any statistically significant difference (p=0.34). No major complication occurred in both groups. The mean follow up was 21,5 months. Finally, the cancer free survival between the two groups had similar results, which were not reported due to the small cohort and the too short follow up.
4. Discussion
PRAE has been introduced to reduce the risk of oncological spread and intraoperative bleeding, in order to facilitate surgery, thus decreasing perioperative morbidity. Nevertheless, in recent literature the real usefulness of PRAE is still debated. Some authors agree that the advantages of PRAE are to decrease intraoperative bleeding with a lower transfusion rate and to reduce operative time [13, 14, 15, 16].
However, most of the studies concerning blood loss from nephrectomy after PRAE are small and non-randomized [18, 19, 20]. We deem that PRAE facilitates RN by reducing the tumour blood supply and therefore, the operative blood loss and blood transfusion requirements; thereby, the operative time may be decreased too, especially in huge and complex renal mass or in tumours with wide blood supply. Other important benefits of PRAE include the potential role of an early ligation of the renal vein before the renal artery has been fully controlled, according to the indications given by Robson [21]. He suggests the renal artery ligation before the vein in order to reduce the oncological spread due to tumour manipulation [22]. This surgical strategy may be really advantageous in case of huge renal mass with anarchic vascular growth or whenever there is a perihilar disease or in a presence of hilar adenopathy, alleviating some of the technical surgical difficulties. Our prospective, randomized study demonstrated that PRAE is a safe and well tolerated procedure with a low, even if not negligible, complications rate.
We found a significant decrease of median blood loss in patients that underwent PRAE (250 ml vs 400 ml, p=0.0066). This finding highlighted one of the most relevant advantages of PRAE, in particular in case of RN for huge and complex mass. Likewise, in our series the surgical time in PRAE group has been reduced (median surgical time was 205 minutes versus 240 minutes) even if the difference is not statistically significant especially for the T2 lesions. Also for T3 tumour a significant difference has not been proved, but the results are quite close reflecting a general aid in the technical difficulties of surgery for the larger masses. In 2014, Zargar et al. reported their results from performing PRAE in locally advanced kidney cancer; moreover, they reviewed the recent literature comparing several studies with different results (Table 2). The authors concluded that PRAE could reduce overall post-operative complications rate (42%), intraoperative blood loss (750cc) and blood transfusion rate [23]. However their findings about blood loss and transfusion rate were higher than our data; this difference could be due to inclusion in their series of inferior vena cava thrombus tumours that seemed to worsen median duration of surgery and blood loss. Likewise, different experiences in recent literature
Table 2.
Review of recent literature data
| Our Series | Zargar et al. | Subramanian | et al. | Schwartz | |||
|---|---|---|---|---|---|---|---|
| PRAE | No PRAE | PRAE | IVC PRAE | PRAE | No PRAE | PRAE | |
| Number of cases | 30 | 34 | 42 | 15 | 135 | 90 | 66 |
| Median surgical Time (min) | 205 | 205 | 192.5 | 258.5 | 390 | 313 | n.a |
| Median Transfusion (U) | 0 | 0 | 2 | 2 | 8 | 4 | 3.9 |
| Median blood loss (cc) | 250 | 400 | 750 | 1550 | 2000 | 1500 | 725 |
| Post-op Complications % | 53.3% | 44% | 45.2% | 53% | 58.43% | 26.29% | n.a |
| Median Hospital Stay (days) | 8 | 8 | 9 | 9 | 19 | 10 | n.a |
| Mortality % | 0 | 0 | 0 | 0 | 17.13% | 3.3% | n.a |
did not clarify if PRAE may improve surgical performance in large masses RN with or without IVC thrombus. We do not usually perform embolization of IVC tumours because venous hypertension reduces significantly the arterial renal flow, erasing potential advantages of PRAE.
Many authors reported minimal morbidity in PRAE due to rare but undeniable detriments as hematoma of the access site, infarction of unpredictable sites as opposite side kidney, bowel, medullar ischemia and vascular lesions, contrast nephrotoxicity, besides other serious complications as coil migrations which are rarely described [16, 17, 22, 23, 24, 25, 26, 27, 28]. Moreover, the risk of failure has to be considered. In our 30 patients who underwent PRAE, complication rate is comparable with patients who did not undergo preoperative embolization. Anyway, complications did not modify the surgical strategy and there were no major complications according to the Clavien-Dindo Classification [6]. The delay to surgery after PRAE is reported from a few hrs to 48 hrs, until several days; RN has been reported being performed also 78 days after PRAE [5]. In our series median time to surgery is about 21 hrs, with a range from 14 to 30 hrs. Timing of 24-48 hrs is recommended because of the risk of emphysematous pyelonephritis that may occur if nephrectomy is performed more than 4 days after renal artery embolization. The renal infarction may cause a post-infarction syndrome (PIS) in almost 90% of patients [24]. Symptoms as flank pain, fever, nausea and vomiting with or without paralytic ileus are the classic presentations of PIS. In our series, PIS occurred in 87% of cases (26/30) and was treated by paracetamol 1g every 8 hours and, in more severe cases, by 5 mg morphine injection on demand. Septic complications of post-infarction syndrome can reach as high as 10% and they depend strictly on the timing of surgery after PRAE [25]. It should be pointed out that the lack of post-infarction syndrome might be indicative of an incomplete embolization due to a collateral vascular circle or a failure of the hemostatic material placement. However, in our study 4 patients did not show PIS: 2 cases showed an efficient embolization and only 2 cases had a series of collateral vessels that did not consent a complete ischemia. Lin et al. reported renal embolization simultaneous to nephrectomy in order to avoid all the complications arising from the waiting time between the two procedures [26]. Although performing both procedures concomitantly in the same surgical act appears to retain the advantages of the PRAE, we preferred to wait about 24 hrs in order to warrant an optimal stationary arrangement of the embolization.
Finally, PRAE also may induce immune response against the tumour. Nakano et al. reported a direct role of the embolization in the modulation of the immune lymphocyte proliferative response and Bakke et al. confirmed an implementation of the natural killer cell activities after embolization [27, 28]. In a retrospective study Zielinski et al. [29] compared 118 patients who underwent PRAE before nephrectomy with a case-matched control group including 116 patients who underwent nephrectomy alone. They found a significant survival benefit in the PRAE group. Nevertheless, this survival benefit applied only to patients with pT2 and pT3 disease and to patients with pT3N+ at the time of surgery. However, these observations are not univocally confirmed in recent literature and the direct role on the overall survival has to be confirmed by prospective and larger cohort studies with a longer follow-up. Even if affected by too short follow up, our data seem to outline a better trend for the PRAE group, but without reaching a statistically significant value. The main limitation of this study was the small sample size and short follow up.
5. Conclusion
The prospective randomized study showed PRAE to be a safe procedure with relatively low complications rate. To our experience, PRAE seemed to be a useful tool in surgical management of huge mass and advanced disease. However further prospective studies with larger sample size and longer follow up are necessary to confirm our results.
Acknowledgements
None
List of Abbreviations
- PRAE
Preoperative Renal Artery Embolization
- RN
Radical Nephrectomy
- RCC
Renal Cell Carcinoma
- CT
Computed Tomography
- MRI
Magnetic Resonance
- AML
Angiomiolypomas
- PIS
Post Infarction Syndrome
- IVC
Intra Vena Cava
- PNS
Para-NeoplasticSyndromes
- P-t-S
PRAE-to-Surgery
- SFT
Solitary Fibrous Tumour
- KS
Kidney Sarcoma
- TCC
Transitional Cell Carcinoma
Footnotes
Competing Interests
The authors declare no conflict of interest.
Authors’ contributions
EM, GC and MA conceived the study and participated in its coordination. MDZ, RC and AB participated in design of the study. MGE, GP, PU and JARdV collected the data. MGE, GP, PU and AB performed statistical analysis. GC, AP and JARdV drafted the manuscript. EM, RC, MA, AP, MDZ and GC critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was not funded
References
- [1].Ferlay J, Soerjomataram I, Ervik M. GLOBOCAN 2010 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer. 2013. et al.
- [2].Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–403. doi: 10.1016/j.ejca.2012.12.027. et al. [DOI] [PubMed] [Google Scholar]
- [3].Boni A, Cochetti G, Ascani S, Del Zingaro M, Quadrini F, Paladini A, Cocca D, Mearini E. Robotic treatment of oligometastatic kidney tumor with synchronous pancreatic metastasis: case report and review of the literature. BMC Surg. 2018;18(1):40. doi: 10.1186/s12893-018-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Almgard LE, Fernstrom I, Haverling M, Ljungqvist A. - Treatment of renal adenocarcinoma by embolic occlusion of the renal circulation. Br J Urol. 1973;45:474–479. doi: 10.1111/j.1464-410x.1973.tb06806.x. [DOI] [PubMed] [Google Scholar]
- [5].Schwartz MJ1, Smith EB, Trost DW, Vaughan ED Jr. – Renal artery embolization: clinical indications and experience from over 100 cases. BJU Int. 2007;99(4):881–886. doi: 10.1111/j.1464-410X.2006.06653.x. [DOI] [PubMed] [Google Scholar]
- [6].Choe J, Shin JH, Yoon HK, Ko GY, Gwon DI, Ko HK, Kim JH, Sung KB. Safety and efficacy of transarterial nephrectomy as an alternative to surgical nephrectomy. Korean J Radiol. 2014;15(4):472–480. doi: 10.3348/kjr.2014.15.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kalman D, Varenhorst E. The role of arterial embolization in renal cell carcinoma. Scand J Urol Nephrol. 1999;33:162–170. doi: 10.1080/003655999750015934. [DOI] [PubMed] [Google Scholar]
- [8].Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- [9].Cantera D, Kutikova A. Manleya B et a. Utility of the R.E.N.A.L.-Nephrometry Scoring System in Objectifying Treatment Decision-Making of the Enhancing Renal Mass. Urology. 2011;78(5):1089–1094. doi: 10.1016/j.urology.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mor V, Laliberte L, Morris JN, Wiemann M. - The Karnofsky performance status scale, an examination of its reliability and validity in a research setting. Cancer. 1984;53(9):2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- [11].Dindo D. MD; Demartines N. Clavien P. - Classification of Surgical Complication. A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. MD and. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Muller A, Rouvière O. - Renal artery embolization-indications, technical approaches and outcomes. Nat Rev Nephrol. 2014 doi: 10.1038/nrneph.231. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [13].Kalman D, Varenhorst E. The role of arterial embolization in renal cell carcinoma. Scand J Urol Nephrol. 1999;33(3):162–170. doi: 10.1080/003655999750015934. [DOI] [PubMed] [Google Scholar]
- [14].Bakal CW, Cynamon J, Lakritz PS, Sprayregen S. Value of preoperative renal artery embolization in reducing blood transfusion requirements during nephrectomy for renal cell carcinoma. J Vasc Interv Radiol. 1993;4:727–731. doi: 10.1016/s1051-0443(93)71958-2. [DOI] [PubMed] [Google Scholar]
- [15].Bergreen PW, Woodside J, Paster SB. - Therapeutic renal infarction. J Urol. 1977;118:372–374. doi: 10.1016/s0022-5347(17)58024-6. [DOI] [PubMed] [Google Scholar]
- [16].Lammer J, Justich E, Schreyer H, Pettek R. - Complications of renal tumor embolization. Cardiovasc. Intervent. Radiol. 1985;8:31–35. doi: 10.1007/BF02552637. [DOI] [PubMed] [Google Scholar]
- [17].Schwartz MJ, Smith EB, Trost DW, Vaughan ED Jr. - Renal artery embolization: clinical indications and experience from over 100 cases. BJU Int. 2007;99(4):881–6. doi: 10.1111/j.1464-410X.2006.06653.x. Epub 2006 Dec 13. [DOI] [PubMed] [Google Scholar]
- [18].Provenza G, Sparagna A, Cunsolo GV, Tierno SM, Centanini F, Bellotti C, Mezzetti G. Renal artery embolization in a gross kidney neoplasm. Case report. 2013;34(9-10):263–266. G Chir. [PMC free article] [PubMed] [Google Scholar]
- [19].Sauk S, Zuckerman DA. - Renal artery embolization. Semin Intervent Radiol. 2011;28(4):396–406. doi: 10.1055/s-0031-1296082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Muller A1, Rouvière O2. Renal artery embolization-indications, technical approaches and outcomes. Nat Rev Nephrol. 2015;11(5):288–301. doi: 10.1038/nrneph.2014.231. [DOI] [PubMed] [Google Scholar]
- [21].Pansadoro A, Cochetti G, D’amico F, Barillaro F, Del Zingaro M, Mearini E. - Retroperitoneal laparoscopic renal tumour enucleation with local hypotension on demand. World J Urol. 2015;33(3):427–432. doi: 10.1007/s00345-014-1325-2. [DOI] [PubMed] [Google Scholar]
- [22].Robson CJ, Churchill BM, Anderson W. - The results of radical nephrectomy for renal cell carcinoma. J Urol. 1969;101(3):297–301. doi: 10.1016/s0022-5347(17)62331-0. [DOI] [PubMed] [Google Scholar]
- [23].Zargar H, Addison B, McCall J, Bartlett A, Buckley B, Rice M. - Renal artery embolization prior to nephrectomy for locally advanced renal cell carcinoma. ANZ J Surg. 2014;84(7-8):564–567. doi: 10.1111/ans.12545. [DOI] [PubMed] [Google Scholar]
- [24].Ginat DT, Saad WE, Turba UC. - Transcatheter renal artery embolization: clinical applications and techniques. Tech Vasc. Interv. Radiol. 2009;12(4):224–239. doi: 10.1053/j.tvir.2009.09.007. [DOI] [PubMed] [Google Scholar]
- [25].Weckermann D, Schlotmann R, Tietze W, Hackel T. - Gas formation after renal artery embolisation: genesis and clinical relevance. Urol.Int. 1992;49:211–214. doi: 10.1159/000282428. [DOI] [PubMed] [Google Scholar]
- [26].Lin PH, Terramani TT, Bush RL, Keane TE, Moore RG, Lumsden AB. - Concomitant intraoperative renal artery embolization and resection of complex renal carcinoma. J Vasc.Surg. 2003;38:446–450. doi: 10.1016/s0741-5214(03)00429-4. [DOI] [PubMed] [Google Scholar]
- [27].Nakano H, Nihira H, Toge T. - Treatment of renal cancer patients by transcatheter embolization and its effects on lymphocyte proliferative responses. J Urol. 1983;130(1):24–27. doi: 10.1016/s0022-5347(17)50935-0. [DOI] [PubMed] [Google Scholar]
- [28].Bakke A, Göthlin JH, Haukaas SA, Kalland T. - Augmentation of natural killer cell activity after arterial embolization of renal c arcinomas. Cancer Res. 1982;42(9):3880–3883. [PubMed] [Google Scholar]
- [29].Zielinski H, Szmigielski S, Petrovich Z. Comparison of preope rative embolization followed by radical nephrectomy with radical nephrectomy alone for renal cell carcinoma. Am J Clin Oncol. 2000;23(1):6–12. doi: 10.1097/00000421-200002000-00002. Feb; [DOI] [PubMed] [Google Scholar]


