SUMMARY
Paediatric swallowing disorders can have several causes, from prematurity and congenital anomalies to gastro-oesophageal reflux and infective or inflammatory pathologies of the upper digestive tract. In neonates, the swallowing process is reflexive and involuntary. Later in infancy, the oral phase comes under voluntary control, while the pharyngeal phase and oesophageal phases remain involuntary. Swallowing difficulties can severely compromise pulmonary health and nutritional intake of paediatric patients. Videofluoroscopic Swallow Study (VFSS) is a radiographic procedure that provides a dynamic view of the swallowing process and is frequently considered to be definitive evaluation for objective assessment of dysphagia in paediatric patients. This review focuses on the different possible aetiologies of paediatric swallowing disorders and related videofluoroscopic swallowing study procedures and appearances.
KEY WORDS: Swallowing disorders, Videofluoroscopic swallowing study, Paediatric, Dysphagia
RIASSUNTO
I disturbi della deglutizione in età pediatrica possono essere dovuti a diverse cause, dalla condizione di prematurità e patologie malformative sino al reflusso gastro-esofageo o a patologie infettive od infiammatorie del primo tratto gastroenterico. Nei neonati il processo della deglutizione è involontario e basato su meccanismi riflessi. In seguito, nell’ infanzia, la fase orale diventa volontaria, mentre le fasi faringea ed esofagea rimangono involontarie. I disordini della deglutizione possono severamente compromettere le capacità respiratorie e l’apporto nutrizionale dei pazienti pediatrici. La videofluorografia è una procedura radiologica che fornisce una valutazione dinamica della deglutizione ed è generalmente considerata come la metodica strumentale definitiva per valutare in modo obiettivo la disfagia nel paziente pediatrico. Questa review mira a descrivere le differenti eziologie della disfagia in età infantile, oltre che a focalizzarsi su i rilievi videofluorografici in queste condizioni patologiche.
PAROLE CHIAVE: Disturbi della deglutizione, Videofluorografia, Pediatria, Disfagia
Introduction
Children are estimated to swallow 600-1,000 times a day 1. Feeding and swallowing are developmental phenomena involving highly complex interactions that begin in embryologic and foetal periods and continue throughout infancy and early childhood 2,3.
Swallowing enables saliva and bolus to be propelled from the mouth through the pharynx into the oesophagus 4. When referring to swallowing, both sensory inputs (as taste, somaesthetic sensitivity, oral stereognosis, vibrotactile detection, propioception, nociception, chemical and thermal sensitivity) and motor outputs (as mastication, respiration and swallowing) are implicated 5.
“Paediatric dysphagia” is not related to a specific diagnosis but refers to any disturbance of the normal swallow sequence in infants and children, as difficulties in transporting a bolus from the oral cavity to the back of the tongue or moving food into the oesophagus, compromising safety and adequacy of nutritional intake 6-10.
Pre- and post-natal development of swallowing mechanisms
Through understanding of the development of feeding and swallowing skills, it is possible to shed light on how and why infants may demonstrate signs of oropharyngeal dysphagia.
During embryologic life, between the 4th and the 7th weeks of gestation, many processes relevant to swallowing development take place.
After the incorporation of the endoderm of the yolk sac into the embryo to form the primordial gut and rupture of pharyngeal membrane to form primitive choanae, separation of oesophagus and trachea from the primitive foregut is essential to avoid liquid aspiration during their passage through oesophagus 11.
Thereafter, the foetal period (from the 9th week of gestation to birth) is characterised by continuous differentiation of tissues and organs 11 and by a dramatic development of swallowing, sucking and oral sensorimotor function; this latter depends from brainstem and cerebral system development and is the fundamental system for correct functioning of the former 5,12.
Sensory cranial nerve input to the brain stem swallowing centre depends on the V, VII, IX and X cranial nerves while primary motor cranial nerve output is provided primarily by the V, VII, IX, X and XII nerves and by the cervical C1-C3 nerves 5. Correct development of cranial nerves is mandatory for adequate swallowing. Myelinisation of the roots of some cranial nerves is seen during the 20th-24th weeks of gestation, and during the 35th-38th weeks the nervous system matures sufficiently to carry out integrative functions as nipple feeding 13.
Moreover, other cerebral regions are implicated in sensory and motor system development such as the nucleus tractus solitarius, nucleus ambiguous, dorsal motor nucleus, hypoglossal nucleus and cerebral cortex 14.
Foetal swallowing is important to regulate amniotic fluid volume and composition, as well as maturation of the foetal gastrointestinal tract and renal foetal system 5,15.
Oral motor skills also develop within a system that changes during post-natal life both in structural growth and neurological control: the successful use of the suckle reflex masters suckling and its coordination with breathing, the child’s motor function (mostly involving his/her tongue) masters the stabilisation of the jaw 16,17.
The swallowing anatomic components of infants are different from adult ones. In the infant, the oral cavity is smaller and teeth have not erupted. We can also typically find a smoother tongue and harder palate. The larynx and hyoid bone are higher in the neck to the oral cavity, while in adults the larynx goes down to a lower area in the neck. The epiglottis is almost attached to the soft palate so that the larynx is open to the nasopharynx 18.
The proper integration of the respiratory and feeding functions is mandatory because during feeding the time left for safe air exchange is reduced, minute ventilation is decreased, exhalation is prolonged and inhalation shortened. Thus, proper maturation and practice of the above functions during the first years of life enhances oral motor patterns, and this latter influences feeding performance 16.
Swallowing requires both voluntary and involuntary actions and can be summed up into four phases (oral, triggering of swallowing reflex, pharyngeal and oesophageal) that involve structures and muscles of the nose, mouth, throat, chest, abdomen and digestive tract 19. The oral phase consists of both preparatory and transit phases. During the preparatory phase, food and/or liquid are prepared in the oral cavity by suckling or mastications in order to form a bolus that, in the transit phase, is moved posteriorly through the oral cavity. During the pharyngeal phase, bolus is transported through the pharynx, and then through the cervical and thoracic oesophagus into the stomach during the oesophageal phase 11,20.
In neonates, the swallowing process is reflexive and involuntary and each of the abovementioned phases may mature at different times and/or rates. Later in infancy, the oral phase is voluntary and triggering of the swallow reflex is generally an involuntary activity, but it can be commanded voluntarily, while the pharyngeal and oesophageal phases remain involuntary 6,11.
A child affected by chronic dysphagia will likely show delayed progression of normal feeding skills, recurrent respiratory disease and, consequently, growth deficiency. Aspiration is one of the abnormalities that may be encountered as an anomaly in the development during post-natal life and consists of passage of ingested material, refluxed contents, or oral secretions through the vocal folds into the lower respiratory tract. Recurrent or chronic aspiration is a serious risk factor in the paediatric population, resulting in infection, chronic lung disease and even death.
The physiological avoidance of aspiration depends not only on anatomical separation of respiratory and digestive tracts in embryologic life, but also on central neural processing. Fluids contacting the laryngeal mucosa evoke laryngeal chemoreflexes 21 resulting in many possible responses such as rapid swallowing, apnoea, laryngeal constriction, hypertension and bradycardia, or cough; as the infant matures the formers reflexes (rapid swallowing and apnoea) become less probable, while cough and laryngeal constriction become more prominent 22. However, sex-related differences have been demonstrated between early oral, tongue, pharyngeal and laryngeal motor activities: oral and upper airway skills emerge earlier in females and the latter (pharyngeal and laryngeal movements) are less rhythmic and complete in males throughout the second semester 23.
Paediatric swallowing disorders: aetiology
An altered swallow sequence may compromise safety, efficiency, or adequacy of nutritional intake. Because swallowing and breathing share a common space in the pharynx, swallowing difficulties can have a bad effect on pulmonary health in addition to impairing nutritional intake 24. Swallowing disorders occur in approximately 1% of children in the general population.
Swallowing disorders in the paediatric population are often different compared to those responsible for adult dysphagia. Many aetiologies should be kept in mind during differential diagnoses 11,25-31 (Table I).
Table I.
Different aetiologies of dysphagia.
| Causes of oropharyngeal dysphagia | |
| Neurological diseases (34.9%) | Motor neuron disease; myopathy; birth asphyxia; cerebral palsy; microcephaly; periventricular leukomalacia |
| Infective/flogistic pathologies | Neurosyphilis; herpetic meningoencephalitis; congenital cytomegalovirus infection; dermatomyositis; epiglottitis |
| Structural disorders (congenital or acquired) | Restricted lingular frenulum; cleft lip/palate; choanal atresia or stenosis (e.g. Charge syndrome); goitre; caustic injuries |
| Causes of esophageal dysphagia | |
| Motility disorders | Achalasia; scleroderma; diffuse oesophageal spasm |
| Intrinsic structural disorders | Diverticula; stenosis; oesophageal plications |
| Extrinsic structural disorders | Vertebral anomalies; foreign body: mediastinal lesions |
| Oesophagitis | Herpes-simplex virus; Candida; gastro-oesophageal reflux disease; Crohn’s disease; eosinophilic oesophagitis; caustic agents |
|
Causes related to prematurity (10-49%) | |
| Low gestational age at birth; low birth weight; comorbidities associated with prematurity | |
| Cardio-respiratory diseases | |
| Broncho-pulmonary dysplasia; laryngo-/tracheo-/bronchomalacia; cyanotic and acyanotic heart defects | |
| Iatrogenic complications | |
| Tracheostomy; feeding tube; respiratory support | |
Clinical assessment
Before exposing the paediatric patient to radiation during videofluoroscopic swallowing study (VFSS), accurate clinical assessment should be made by taking clinical history, evaluating sensorimotor function of the anatomical structure for swallowing and directly observing the child during a meal 32.
A clinical evaluation of feeding should involve a speech language pathologist (SLP) with experience in feeding disorders during an individual session or during a clinical group session by a feeding team 20.
In order to assess different potential causes of peadiatric dysphagia, the clinician has to focus on physiological-medical disorders, behavioural disturbances and developmental issues 20.
Medical disorders may be chronic, temporary, or progressive and affect many systems related to swallowing including the respiratory, nervous and/or metabolic systems, digestive tract and craniofacial structures. Behavioural disorders must be considered as a possible contributing cause of dysphagia: the patient may adopt aggressive or unfit behaviour, refuse to be fed or have little motivation to engage in feeding-based activities. The paediatric patient may also develop inadequate skills for swallowing because of privation of correct practice for acquisition of mature skills or as a consequence of a medical or behavioural disorder. Schedule for Oral-Motor Assessment (SOMA) and the Dysphagia Disorder Survey are two of the more common assessment tools that the clinician can use to examine swallowing function in the paediatric population 33,34. Nevertheless, it must be said that often clinicians do not use formal assessment tools when evaluating feeding skills in children with suspected dysphagia. Several studies also highlight the inaccuracy of clinical evaluation alone in predicting airway involvement, given that silent aspiration is not uncommon in the paediatric population. When altered swallowing function is suspected in the paediatric patient, instrumental assessment should be requested to confirm the presence of dysphagia and detect aspiration risk 11.
Videofluoroscopic swallowing study
VFSS is considered to be the best instrumental evaluation for objective swallowing assessment, and not just in paediatric patients 20,35-37.
It allows concurrent visualisation of the oral, pharyngeal and oesophageal stages of swallowing 9 and is essential to confirm airway protection adequacy and exclude swallowing dysfunction after clinical evaluation of feeding 38.
Therefore, VFSS provides crucial diagnostic information 39 and leads to a reduction in chest infections risk by detecting clinically “silent” tracheal aspiration (aspiration before, during, or after swallowing in the absence of cough or visible signs of choking) 40,41, especially in neurologically-based feeding disorders.
Thus, the indications for VFSS 20,38 in the paediatric patient comprise:
• observation of oral preparatory, oral transit, pharyngeal and/or oesophageal phases of swallowing;
• patient hostility towards endoscopic examination;
• suspected or diagnosed anatomical anomalies of nasal cavities, oropharyngeal tract or upper oesophageal structures that are a hindrance to endoscopic evaluation;
• suspected swallowing disorder as a contributory cause of a persistent feeding refusal or a respiratory disorder;
• planning treatment to improve swallowing efficiency and reduce the risk of aspiration.
Contraindications for VFFS include:
• patients who have never fed orally;
• impossibility to adopt correct posture during the exam because of medical instability, agitation or lethargy;
• allergies to barium/iodine contrast;
• patient who cannot be transferred to the radiology department 20.
Another commonly used instrumental evaluation of swallowing for paediatric patients is Fiberoptic Endoscopic Evaluation of Swallowing (FEES) 20,37,42, which a sensory testing of laryngeal adductor response (LAR) can be added to (Fiberoptic Endoscopic Evaluation of Swallowing and Sensory test or FEESST).
During FEES an endoscope allows observation of dynamical changes of the larynx and pharynx during the pharyngeal phase of swallowing and passage of bolus.
FEES can be performed at the bedside and repeated in a brief period and in different clinical conditions, so that it should be considered a very valuable instrumental method in follow-up 42.
FEES, on the other hand, allows assessment of the pharyngeal phase only and make indirect considerations about the oesophageal and oral phases; it is only acceptable for either very young children or for older cooperating children and is not very helpful to assess repeated swallowing.
Therefore, the question about whether VFFS can be considered as the gold standard to assess swallowing disorders is still open. Studies have shown that both VFSS and FEES have comparable sensitivity, specificity and predictive abilities 43,44, and a valuable approach may include both examinations as complementary, when available.
Practical and radiological technique
VFSS is a fast radiographic procedure. During the exam, barium contrast agents (administered at various consistencies – from solid to liquid – according to the situation) or, if necessary, hydrosoluble no-ionic iodated agents are transported in the oro-pharyngeal cavity and oesophagus, and the sequential phases of this passage are captured in real time using fluoroscopy 45.
An optimal approach to the patient can be achieved thanks to multidisciplinary management of the procedure by radiologist, radiographer and deglutologist 45-47.
Families have to be prepared for what to expect from the procedure, and advised that for best execution of the exam and cooperation of the children, it is advisable to bring appetising foods to be mixed with the contrast agent, familiar utensils and a seating system that children usually use during meals 20,37.
Moreover, in the radiology department there should be a child-friendly environment, such as a fluoroscopy room with visual distracters (toys, boxes of rewards) and a familiar caregiver 20,37.
At present there is not a unique protocol for VFSS in infants, since the procedure is strongly influenced by individual medical conditions, feeding modality, preferred food consistencies, age and size of the paediatric patient 20,37,38.
Regarding the question of lack of a unique VFSS protocol, in 2013 the International Dysphagia Diet Standardisation Initiative (IDDSI) 48 was founded to develop globally standardised terminology to refer to thickened liquids and texture modified foods used for patients of all ages affected by dysphagia.
During a consensus meeting, a first group of descriptors of texture and flow behaviour were developed to propose a framework to > 3,100 people in 57 countries around the world, obtaining positive feedback. The final IDDSI framework consists of levels from 0 to 7 including both liquids and foods on a continuum and every level is identified by a number, colour codes and a text label. Level 1 (slightly thick liquids) has particular utility for paediatric patients, even if it cannot be always available in all healthcare.
However, during VFSS barium sulphate powder is usually mixed with different textures of liquid, semi-solid and solid food (as cookies or crackers) and administered to the patient. As some authors suggest 49, one-half cup of thin barium can be mixed either with 1½ teaspoons of thickener to obtain a nectar consistency or with 1½ tablespoons thickener to create a honey-like texture.
Density of barium sulfate suspension is often expressed in a weight/weight (w/w) ratio, which indicates the number of grams of active ingredient per 100 g of product; otherwise, it is expressed in a weight/volume (w/v) ratio which expresses the number of grams of active ingredient per 100 mL of product 49. Varibar thin liquid (40% w/v, after reconstitution; E-Z-EM Inc., Westbury, NY) and E-Z-HD (98% w/w; E-Z-EM Inc., Westbury, NY) are barium sulphate suspensions commonly used for VFSS.
Even though nectar and honey-like consistencies can be created using thickener, several barium sulfate suspensions are commercially available such as Varibar® Thin Liquid, Varibar® Nectar, Varibar® Honey, Varibar® Pudding 49. For infants (0-1 year), some authors 38 state that the examination should start with liquids, as this texture often results to be the prevailing one in an infants’ diet, and disparate type of nipples with different flows can be used 1,50.
In patients older than 1 year, it is possible to previously evaluate their food and drink preferences 1. However, the use of the patient’s favourite food mixed with barium may facilitate cooperation to accept other types of food, resulting in a wider range of information. Finally, after having started the study, the radiologist and the SLP may change the volume and viscosity of the barium texture on the basis of patient’s symptoms and signs detected 37.
The patient should not chew gum or eat for several hours prior to VFSS 51 and if the child cannot autonomously feed because of a gastrostomy or nasogastric tube (NG tube), it is recommended 37 to take small tastes of the foods for 1-2 weeks prior to the VFSS. When a NG tube is in place, its removal is not necessary in most cases, as swallowing evaluation can be performed anyway 52 and having the tube repassed is a traumatic manoeuvre for the paediatric patient 37.
Cleft lip and/or palate patients require adequate feeding methods during the VFSS. A special need feeder is a one-way valve bottle designed for infants who have sucking difficulties: it is activated by compression movements alone, so the cleft lip and/or palate patient can overcome the obstacle of sucking dysfunction during feeding 1.
During VFSS, the presence of a family member who feeds the paediatric patient should be recommended, especially in infants, using child preferred utensils, like the baby’s own feeding bottle, thus contributing to make the patient seat in a friendly feeding position to achieve the optimal conditions for VFSS 20.
During the procedure, patient positioning depends on his/her size, age and medical conditions 38. Babies, infants and children up to 3 years should be seated in usual position in their own wheelchair or a preformed seat with secure straps mounted on the X-ray equipment. When the child size exceeds preformed seat dimensions, as the fluoroscopy table is vertically positioned, the patient can sit on a step set on the lower side of the table 37,51.
The child has to be primarily positioned in the lateral view to assess oro-pharyngeal cavity, larynx and cervical oesophageal region. The radiologist activates the fluoroscope for few seconds prior to the administration of barium contrast-impregnated food or liquid and keeps it on as long as the bolus reaches the cervical region of oesophagus 45. During the oral phase, the radiologist must assess bolus containment before the swallow (Fig. 1), the rhythmicity of jaw movements and coordination of tongue movements.
Fig. 1.
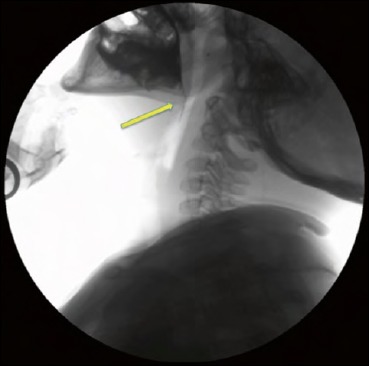
Videofluorography lateral view in a 10-year-old patient. During the oral phase, a leakage of barium in the oesophagus (arrow) indicates inadequacy of bolus containment.
The lips, nasal cavity, cervical spine column and pharyngo-oesophageal segments are, respectively, in the anterior, superior, posterior and inferior limits of the field of view 53,54.
The Antero-Posterior (A-P) view is not always routinely obtained by clinicians, since the diagnostic contribution made by an A-P view essentially concerns assessment of structural and functional symmetry and detection of unilateral abnormalities of the pharyngeal wall, as is the case with pharyngeal paresis or paralysis 45.
Milliampere (mA) and kilovolt (kV) settings are typically dependent on the patient’s age, height and weight.
For a 6-month-old to 5-year-old child, the usual mA and kV settings are 58-60 kVp with 1-1.1 mA, while for a 10-year-old patient these are 62 kVp and 1.5 mA 37. As several authors state, using a pulse rate of 30 pulses/sec is essential to detect rapid aspiration and to recognise any bolus flow event related to the oropharyngeal phase of swallowing 45,55,56 (Fig. 2).
Fig. 2.

Videofluorography lateral view in an 8-year-old patient with Down syndrome. During the pharyngeal phase subepiglottic penetration (arrow) and aspiration are demonstrated with persistence of contrast media in the trachea (arrowhead), in the absence of coughing.
Interpreting results
During VFSS, assessment of swallowing consists in observing the orally preparatory, oral transit, pharyngeal and oesophageal phases 20,37. Bolus formed during orally preparatory phase is held inside the oral cavity and does not move into the open larynx thanks to the base of the tongue and soft palate which close the oral cavity posteriorly 20,57.
During the oral transit phase, an anterior-to-posterior elevation of tongue push the bolus posteriorly toward the pharynx, so that pharyngeal reflex is triggered. Larynx closes by contraction of the aryepiglottic folds.
The pharyngeal phase takes place in less than a second and begins when bolus passes through the anterior faucial arch and reaches the posterior pharyngeal wall; bolus is then pushed toward the cricopharyngeal sphincter by contraction of pharyngeal constrictor muscles. Spill of bolus into the nasopharynx is prevented by elevation of the soft palate and larynx closes true and false vocal cords and aryepiglottic folds to block the way to trachea.
As the oesophageal phase begins, the cricopharyngeal muscle relaxes and bolus moves through cervical and thoracic oesophagus and into the stomach thanks to oesophageal peristalsis 57.
Deterioration in swallowing function can be demonstrated by several abnormalities such as delay in the initiation of the swallowing reflex, residue of contrast-impregnated food and liquid, epiglottal undercoating, penetration and aspiration 37.
The presence of aspiration is characterised by the entry of ingested material below the level of the true vocal folds into the trachea 58 and if aspiration occurs, the material can enter the airway before, during and/or after the pharyngeal swallow 53. When the bolus blocks the patency of the airways a chocking event occurs, exposing infants to a life-threatening condition.
Penetration is present when bolus material enters the laryngeal vestibule down to the level of the true vocal folds, but it does not cross the vocal folds 11 (Fig. 3).
Fig. 3.

Videofluorography lateral view in a 6-month-old patient with perinatal stroke shows transient sub-epiglottic penetration (arrow), in the absence of coughing.
The Penetration-Aspiration Scale 59 is a widely employed interval scale for a reliable quantification of penetration and aspiration events observed during VFSS.
The final 8-point version of the scale is multidimensional since several types of behaviours are evaluated.
Contrast-material not entering the airway is scored 1, while penetration can be scored from 2 to 5. Score 2 is given if contrast material remains above the vocal folds but no residue is visible, while score 3 is given if visible residue remains. If contrast material contacts the vocal folds but is ejected from the airway penetration this is scored 4, while if there is no ejection of material and residue is visible, penetration is scored 5.
Aspiration is a more severe event than penetration: it can be scored from 6 to 8 according to whether aspirated material is partially or totally expelled from the airway (score 6), subglottic residue is visible despite the patient’s effort (score 7) or aspiration occurs without the patient’s attempt to expel contrast material (score 8).
Another abnormality seen on VFSS is epiglottic undercoating which occurs when material penetrates underneath the epiglottis above the laryngeal vestibule 37.
Deteriorated swallowing can be also displayed by a swallow reflex delayed more than 1 sec 37.
On VFSS some patients show a normal swallowing process in the first few swallows but, as feeding progresses, abnormalities appear. On the other hand, certain patients may have greater difficulty during first few swallows and, as they become more organised, improve their function with additional swallows. Thus, during the procedure, multiple swallows have always to be examined 60. If during basic examination no symptoms appear, provocative manoeuvres can be used to evoke swallowing abnormalities, always with caution, such as body position change, always keeping in mind the patient’s individual history.
Protective and therapeutic manoeuvres, such as modifications regarding neck or body position, are available to prevent aspiration and limit considerable risks deriving from a sudden inability to breathe 37.
VFSS must be rapidly aborted when severe aspiration occurs, oxygen level saturation drops, or if the child does not respond to protective or therapeutic manoeuvres 37,51.
The procedure should end after having achieved all the goals of the study, trying to minimize the radiation exposure with the maximum level of clinical and radiological results 45,61.
Radioprotection issues
Videofluoroscopic analysis of swallowing is considered to be the best instrumental evaluation to objectively assess swallowing function after clinical feeding, confirming airway protection adequacy during the event 20. However, there are limitations to the procedure such as cost, time constraints and, mostly, radiation exposure.
Although the radiation dose from VFSS is relatively low, between 0.2 and 0.85 mSv 62-66 (for a chest x-ray acquired in P-A the patient receives a radiation dose of 0.02 mSv) 67 any radiation from medical tests must be minimised to comply with the “As Low As Reasonably Achievable” principle 68. This is particularly true in the paediatric population. Long-term effects of radiation are increasingly acknowledged, particularly in infants, since adverse effects of radiation exposure are known to be age-dependent: children are more sensitive to radiation-induced cancer than adults and the radiogenic risk of developing a radiation-related cancer is 2-3 times higher for a young child compared with an adult exposed to an identical radiation dose 69-71. Therefore, optimisation of the procedure is important to reduce the dose using registration or fluoroscopy with low exposure data, if possible, due to intrinsic high contrast differences between barium and soft tissue. Also, specific age, weight protocols and diagnostic reference levels should be set within each department for the different ages of patients 72-76. In order to maintain a low dose, the radiologist should make the timing of the fluoroscopy coincide with the oral and pharyngeal phases of swallowing. In VFSS, fluoroscopy time has been shown to be highly correlated with kerma area product (KAP) values and is known as a practical tool for monitoring patient radiation dose 77. Guidelines have been adopted to limit radiation exposure times, but multiple variables may influence the duration of the exam. In particular, factors influencing radiation exposure time in VFSS include medical diagnosis category, swallowing impairment severity, the clinician’s experience and use of a standardised protocol.
Conclusions
Feeding and swallowing disorders present in different manners and the underlying aetiology may be difficult to determine. An evaluation of clinical history and physical examination may screen some abnormalities, but often do not provide help in identifying the underlying cause of feeding and swallowing disorders. VFSS is considered to be the best instrumental evaluation for complete assessment from the oral to pharyngeal and oesophageal phases. In addition, the procedure strongly contributes to reducing the risk of chest infections by detecting clinically “silent” tracheal aspiration.
However, behavioural, structural and physiological disorders often coexist, complicating diagnosis and management.
For this reason, a multidisciplinary approach to diagnosis and management is helpful.
Figures and tables
Footnotes
Conflict of interest statement
None declared.
References
- 1.Gower RE. Swallowing evaluations with the pediatric population: a comparison to standard adult protocols. Research papers 2014. Paper 512. 2014. http://opensiuc.lib.siu.edu/gs_rp/512.
- 2.Stevenson RD, Allaire JH. The development of normal feeding and swallowing. Pediatr Clin North Am 1991;38:1439-53. https://doi.org/10.1016/s0031-3955(16)38229-3 10.1016/s0031-3955(16)38229-3 [DOI] [PubMed] [Google Scholar]
- 3.Kahane JC. Postnatal development and aging of the human larynx. Semin Speech Lang 1983; 4:189-203. [Google Scholar]
- 4.Zhu M, Yu B, Yang W, et al. Evaluation of normal swallowing functions by using dynamic high-density surface electromyography maps. Biomed Eng Online 2017;16:133 https://doi.org/10.1186/s12938-017-0424-x 10.1186/s12938-017-0424-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller AJ. The neuroscientific principles of swallowing and dysphagia. San Diego: Singular Publishing Group; 1999. pp. 100-1. [Google Scholar]
- 6.Chantal L. Development of suck and swallow mechanisms in infants. Ann Nutr Metab 2015;66:7-14. https://doi.org/10.1159/000381361 10.1159/000381361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arvedson JC. Feeding with craniofacial anomalies. Arvedson JC, Brodsky LB, editors. Pediatric swallowing and feeding: assessment and management. Second edition Albany, NY: Singular Publishing Group; 2002. pp. 527-61. [Google Scholar]
- 8.Groher ME, Crary MA. Dysphagia: clinical management in adults and children. Second edition Maryland Heights, MO: Mosby/Elsevier; 2010. [Google Scholar]
- 9.Miller CK, Willging JP. Advances in the evaluation and management of pediatric dysphagia. Curr Opin Otolaryngol Head Neck Surg 2003;11:442-6. [DOI] [PubMed] [Google Scholar]
- 10.Dodrill P, Gosa MM. Pediatric dysphagia: physiology, assessment, and management. Ann Nutr Metab 2015;66(Suppl 5):24-31. https://doi.org/10.1159/000381372 10.1159/000381372 [DOI] [PubMed] [Google Scholar]
- 11.Moore KL, Persaud TVN, Torchia MG. The developing human: clinically oriented embryology. Tenth edition Philadelphia: Elsevier; 2003. [Google Scholar]
- 12.Kinney HC, Brody BA, Kloman AS, et al. Sequence of CNS myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol 1988;47:217-34. https://doi.org/10.1097/00005072-198805000-00003 10.1097/00005072-198805000-00003 [DOI] [PubMed] [Google Scholar]
- 13.Miller JL, Sonies BC, Macedonia C. Emergence of oropharyngeal, laryngeal, and swallowing activity in the developing fetal upper aerodigestive tract: an ultrasound evaluation. Early Hum Dev 2003;71:61-87. https://doi.org/10.1016/s0378-3782(02)00110-x 10.1016/s0378-3782(02)00110-x [DOI] [PubMed] [Google Scholar]
- 14.Broussaud DL, Altschuler SM. Central integration of swallow and airway-protective reflexes. Am J Med 2000;108(Suppl 4a):62S-7S. https://doi.org/10.1016/s0002-9343(99)00340-x 10.1016/s0002-9343(99)00340-x [DOI] [PubMed] [Google Scholar]
- 15.Ross MG, Nijland MJM. Development of ingestive behaviour. Am J Physiol 1998;274:879-93. https://doi.org/10.1152/ajpregu.1998.274.4.R879 10.1152/ajpregu.1998.274.4.R879 [DOI] [PubMed] [Google Scholar]
- 16.Kelly BN, Huckabee ML, Jones RD, et al. The first year of human life: coordinating respiration and nutritive swallowing. Dysphagia 2007;22:37-43. https://doi.org/10.1007/s00455-006-9038-3 10.1007/s00455-006-9038-3 [DOI] [PubMed] [Google Scholar]
- 17.Morris SE, Klein MD. Pre-feeding skills: a comprehensive resource for mealtime development. Second edition Tucson, AZ: Therapy Skill Builders; 2000. [Google Scholar]
- 18.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing - normal and abnormal. Phys Med Rehabil Clin N Am 2008;19:691-707. https://doi.org/10.1016/j.pmr.2008.06.001 10.1016/j.pmr.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheppard JJ. Using motor learning approacheS for treating swallowing and feeding disorders: a review. Lang Speech Hear Serv Sch 2008;39:227-36. https://doi.org/10.1044/0161-1461(2008/022) 10.1044/0161-1461(2008/022) [DOI] [PubMed] [Google Scholar]
- 20.American Speech-Language-Hearing Association. Pediatric Dysphagia. www.asha.org/Practice-Portal/Clinical-Topics/Pediatric-Dysphagia.
- 21.Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Med 2001;111(Suppl 8a):69S-77S. https://doi.org/10.1016/s0002-9343(01)00860-9 10.1016/s0002-9343(01)00860-9 [DOI] [PubMed] [Google Scholar]
- 22.Thach BT. Maturation of cough and other reflexes that protect the fetal and neonatal airway. Pulm Pharmacol Ther 2007;20:365-70. https://doi.org/10.1016/j.pupt.2006.11.011 10.1016/j.pupt.2006.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JL, Macedonia C, Sonies BC. Sex differences in prenatal oral-motor function and development. Dev Med Child Neurol 2006;48:465-70. [DOI] [PubMed] [Google Scholar]
- 24.Tutor JD, Srinivasan S, Gosa MM, et al. Pulmonary function in infants with swallowing dysfunction. PLoS One 2015;10:e0123125 https://doi.org/10.1371/journal.pone.0123125 10.1371/journal.pone.0123125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharyya N. The prevalence of pediatric voice and swallowing problems in the United States. Laryngoscope 2015;125:746-50. https://doi.org/10.1002/lary.24931 10.1002/lary.24931 [DOI] [PubMed] [Google Scholar]
- 26.Lefton-Greif MA, Arvedson JC. Pediatric feeding and swallowing disorders: state of health, population trends, and application of the international classification of functioning, disability, and health. Semin Speech Lang 2007;28:161-5. https://doi.org/10.1055/s-2007-984722 10.1055/s-2007-984722 [DOI] [PubMed] [Google Scholar]
- 27.Scerrino G, Tudisca C, Bonventre S, et al. Swallowing disorders after thyroidectomy: what we know and where we are. A systematic review. Int J Surg 2017;41(Suppl 1):S94-S102. https://doi.org/10.1016/j.ijsu.2017.03.078 10.1016/j.ijsu.2017.03.078 [DOI] [PubMed] [Google Scholar]
- 28.Scerrino G, Inviati A, Di Giovanni S, et al. Esophageal motility changes after thyroidectomy; possible associations with postoperative voice and swallowing disorders: preliminary results. Otolaryngol Head Neck Surg 2013;148:926-32. https://doi.org/10.1177/0194599813482299 10.1177/0194599813482299 [DOI] [PubMed] [Google Scholar]
- 29.Mezoff EA. Focus on diagnosis. Pediatr Rev 2012;33:518-20. https://doi.org/10.1542/pir.33-11-518 10.1542/pir.33-11-518 [DOI] [PubMed] [Google Scholar]
- 30.Manikam R, Perman JA. Pediatric feeding disorders. J Clin Gastroenterol 2000;30:34-46. https://doi.org/10.1097/00004836-200001000-00007 10.1097/00004836-200001000-00007 [DOI] [PubMed] [Google Scholar]
- 31.Buchholz DW. Dysphagia associated with neurological disorders. Acta Otorhinolaryngol Belg 1994;48:143-55. [PubMed] [Google Scholar]
- 32.Rugiu MG. Role of videofluoroscopy in evaluation of neurologic dysphagia. Acta Otorhinolaryngol Ital 2007;27:306-16. [PMC free article] [PubMed] [Google Scholar]
- 33.Ko MJ, Kang MJ, Ko KJ, et al. Clinical usefulness of schedule for oral-motor assessment (SOMA) in children with dysphagia. Ann Rehabil Med 2011;35:477-84. https://doi.org/10.5535/arm.2011.35.4.477 10.5535/arm.2011.35.4.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheppard JJ, Hochman R, Baer C. The dysphagia disorder survey: validation of an assessment for swallowing and feeding function in developmental disability. Res Dev Disabil 2014;35:929-42. https://doi.org/10.1016/j.ridd.2014.02.017 10.1016/j.ridd.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 35.Russo S, Lo Re G, Galia M, et al. Videofluorography swallow study of patients with systemic sclerosis. Radiol Med 2009;114:948-59. https://doi.org/10.1007/s11547-009-0416-4 10.1007/s11547-009-0416-4 [DOI] [PubMed] [Google Scholar]
- 36.Lo Re G, Galia M, La Grutta L, et al. Digital cineradiographic study of swallowing in patients with amyotrophic lateral sclerosis. Radiol Med 2007;112:1173-87. https://doi.org/10.1007/s11547-007-0214-9 10.1007/s11547-007-0214-9 [DOI] [PubMed] [Google Scholar]
- 37.Hiorns MP, Ryan MM. Current practice in paediatric videofluoroscopy. Pediatr Radiol 2006;36:911-9. https://doi.org/10.1007/s00247-006-0124-3 10.1007/s00247-006-0124-3 [DOI] [PubMed] [Google Scholar]
- 38.Arvedson JC, Lefton-Greif MA. Pediatric videofluoroscopic swallow studies: a professional manual with caregiver guidelines. San Antonio: Communication Skill Builders/Psychological Corporation; 1998. [Google Scholar]
- 39.Reilly S, Douglas J, Oates J. Evidence based practice in speech pathology. London: Whurr Publishers; 2004. [Google Scholar]
- 40.Logemann JA. Evaluation and treatment of swallowing disorders. Second edition San Diego: College-Hill Press; 1983. [Google Scholar]
- 41.Smith Hammond CA, Goldstein LB. Cough and aspiration of food and liquids due to oral-pharyngeal dysphagia: ACCP evidence-based clinical practice guidelines. Chest 2006;129 (Suppl 1):154S-68S. https://doi.org/10.1378/chest.129.1_suppl.154S 10.1378/chest.129.1_suppl.154S [DOI] [PubMed] [Google Scholar]
- 42.Farneti D, Genovese E. Endoscopic criteria in assessing severity of swallowing disorders. In: Speyer R, Bogaardt H, editors. Seminars in Dysphagia. IntechOpen, September 2nd 2015. https://doi.org/10.5772/60836 10.5772/60836 Available from: https://www.intechopen.com/books/seminars-in-dysphagia/endoscopic-criteria-in-assessing-severity-of-swallowing-disorders. [DOI] [Google Scholar]
- 43.Gomes GF, Rao N, Brady S, et al. Gold-Standard? Analysis of the videofluoroscopic and fiberoptic endoscopic swallow examinations. J Applied Res 2003;3:89-96. [Google Scholar]
- 44.Wu CH, Hsiao TY, Chen JC, et al. Evaluation of swallowing safety with fiberoptic endoscope: comparison with videofluoroscopic technique. Laryngoscope 1997;107:396-401. https://doi.org/10.1097/00005537-199703000-00023 10.1097/00005537-199703000-00023 [DOI] [PubMed] [Google Scholar]
- 45.Martin-Harris B, Jones B. The videofluorographic swallowing study. Phys Med Rehabil Clin N Am 2008;19:769-85. https://doi.org/10.1016/j.pmr.2008.06.004 10.1016/j.pmr.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knechtges PM, Carlos RC. The evolving role of radiologists within the health care system. J Am Coll Radiol 2007;4:626-35. https://doi.org/10.1016/j.jacr.2007.05.014 10.1016/j.jacr.2007.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo Re G, De Luca R, Muscarneri F, et al. Relationship between anxiety level and radiological investigation. Comparison among different diagnostic imaging exams in a prospective single-center study. Radiol Med 2016;121:763-8. https://doi.org/10.1007/s11547-016-0664-z 10.1007/s11547-016-0664-z [DOI] [PubMed] [Google Scholar]
- 48.Cichero JAY, Lam P, Steele CM, et al. Development of International terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: the IDDSI framework. Dysphagia 2017;32:293-314. https://doi.org/10.1007/s00455-016-9758-y 10.1007/s00455-016-9758-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Callahan MJ, Talmadge JM, MacDougall RD, et al. Selecting appropriate gastroenteric contrast media for diagnostic fluoroscopic imaging in infants and children: a practical approach. Pediatr Radiol 2017;47:372-81. https://doi.org/10.1007/s00247-016-3709-5 10.1007/s00247-016-3709-5 [DOI] [PubMed] [Google Scholar]
- 50.Lefton-Greif MA, McGrattan KE, Carson KA, et al. First steps towards development of an instrument for the reproducible quantification of oropharyngeal swallow physiology in bottle-fed children. Dysphagia 2018;33:76-82. https://doi.org/10.1007/s00455-017-9834-y 10.1007/s00455-017-9834-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The American College of Radiology. ACR practice parameter for the performance of the modified barium swallow. 2014. www.acr.org/~/media/7d306289d61341dd9146466186a77dbe.pdf.
- 52.Leder SB, Suiter DM. Effect of nasogastric tubes on incidence of aspiration. Arch Phys Med Rehabil 2008;89:648-51. https://doi.org/10.1016/j.apmr.2007.09.038 10.1016/j.apmr.2007.09.038 [DOI] [PubMed] [Google Scholar]
- 53.Martin-Harris B, Logemann JA, McMahon S, et al. Clinical utility of the modified barium swallow. Dysphagia 2000;15:136-41. https://doi.org/10.1007/s004550010015 10.1007/s004550010015 [DOI] [PubMed] [Google Scholar]
- 54.Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. AJR Am J Roentgenol 1990;154:953-63. https://doi.org/10.2214/ajr.154.5.2108569 10.2214/ajr.154.5.2108569 [DOI] [PubMed] [Google Scholar]
- 55.Mercado-Deane MG, Burton EM, Harlow SA, et al. Swallowing dysfunction in infants less than 1 year of age. Pediatr Radiol 2001;31:423-28. https://doi.org/10.1007/s002470100456 10.1007/s002470100456 [DOI] [PubMed] [Google Scholar]
- 56.Jones B, Donner MW. Interpreting the study. Jones B, Donner MW, editors.. Normal and abnormal swallowing: imaging in diagnosis and therapy. New York, NY: Springer Verlag; 1991. pp. 51-75. [Google Scholar]
- 57.Logemann JA. Manual for the videofluorographic study of swallowing. Second edition Austin, TX: Pro-Ed Inc; 1993. [Google Scholar]
- 58.Robbins J, Coyle J, Rosenbek J, et al. Differentiation of normal and abnormal airway protection during swallowing using the penetration-aspiration scale. Dysphagia 1999;14:228-32. https://doi.org/10.1007/PL00009610 10.1007/PL00009610 [DOI] [PubMed] [Google Scholar]
- 59.Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia 1996;11:93-8. [DOI] [PubMed] [Google Scholar]
- 60.Newman LA, Keckley C, Petersen MC, et al. Swallowing function and medical diagnoses in infants suspected of dysphagia. Pediatrics 2001;108:E106 https://doi.org/10.1542/peds.108.6.e106 10.1542/peds.108.6.e106 [DOI] [PubMed] [Google Scholar]
- 61.O’Donoghue S, Bagnall A. Videofluoroscopic evaluation in the assessment of swallowing disorders in paediatric and adult populations. Folia Phoniatr Logop 1999;51:158-71. https://doi.org/10.1159/000021494 10.1159/000021494 [DOI] [PubMed] [Google Scholar]
- 62.Moro L, Cazzani C. Dynamic swallowing study and radiation dose to patients. Radiol Med 2006;111:123-9. [DOI] [PubMed] [Google Scholar]
- 63.Wright RE, Boyd CS, Workman A. Radiation doses to patients during pharyngeal videofluoroscopy. Dysphagia 1998;13:113-5. https://doi.org/10.1007/PL00009554 10.1007/PL00009554 [DOI] [PubMed] [Google Scholar]
- 64.Weir KA, McMahon SM, Long G, et al. Radiation doses to children during modified barium swallow studies. Pediatr Radiol 2007;37:283-90. https://doi.org/10.1007/s00247-006-0397-6 10.1007/s00247-006-0397-6 [DOI] [PubMed] [Google Scholar]
- 65.Zammit-Maempel I, Chapple CL, Leslie P. Radiation dose in videofluoroscopic swallow studies. Dysphagia 2007;22:13-5. https://doi.org/10.1007/s00455-006-9031-x 10.1007/s00455-006-9031-x [DOI] [PubMed] [Google Scholar]
- 66.Chau KH, Kung CM. Patient dose during videofluoroscopy swallowing studies in a Hong Kong public hospital. Dysphagia 2009;24:387-90. https://doi.org/10.1007/s00455-009-9214-3 10.1007/s00455-009-9214-3 [DOI] [PubMed] [Google Scholar]
- 67.Huda W, Atherton JV. Energy imparted in computed tomography. Med Phys 1995;22:1263-9. https://doi.org/10.1118/1.597564 10.1118/1.597564 [DOI] [PubMed] [Google Scholar]
- 68.United States Nuclear Regulatory Commission, Nuclear Regulatory Commission Regulations. Title 10 code of federal regulations, part 20 standards for protection against radiation. Section 20.1003 Definitions. www.nrc.gov/reading-rm/doc-collections/cfr/part020/part020-1003.html.
- 69.Khong PL, Ringertz H, Donoghue V. Radiological protection in paediatric diagnostic and interventional radiology. Ann ICRP 2013;42:1-63. https://doi.org/10.1016/j.icrp.2012.10.001 10.1016/j.icrp.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 70.Alzen G, Benz-Bohm G. Radiation protection in pediatric radiology. Dtsch Arztebl Int 2011;108:407-14. https://doi.org/10.3238/arztebl.2011.0407 10.3238/arztebl.2011.0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salerno S, Marrale M, Geraci C, et al. Cumulative doses analysis in young trauma patients: a single-centre experience. Radiol Med 2016;121:144-52. https://doi.org/10.1007/s11547-015-0584-3 10.1007/s11547-015-0584-3 [DOI] [PubMed] [Google Scholar]
- 72.European Diagnostic Reference Levels for Paediatric Imaging (PiDRL). European Guidelines on DRLs for Paediatric Imaging. 2016. www.eurosafeimaging.org/wp/wp-content/uploads/2014/02/European-Guidelines-on-DRLs-for-Paediatric-Imaging_Revised_18-July-2016_clean.pdf.
- 73.Colagrande S, Origgi D, Zatelli G, et al. CT exposure in adult and paediatric patients: a review of the mechanisms of damage, relative dose and consequent possible risks. Radiol Med 2014;119:803-10. https://doi.org/10.1007/s11547-014-0393-0 10.1007/s11547-014-0393-0 [DOI] [PubMed] [Google Scholar]
- 74.Salerno S, Marchese P, Magistrelli A, et al. Radiation risks knowledge in resident and fellow in paediatrics: a questionnaire survey. Ital J Pediatr 2015;41:21 https://doi.org/10.1186/s13052-015-0130-x 10.1186/s13052-015-0130-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Granata C, Origgi D, Palorini F, et al. Radiation dose from multidetector CT studies in children: results from the first Italian nationwide survey. Pediatr Radiol 2015;45:695-705. https://doi.org/10.1007/s00247-014-3201-z 10.1007/s00247-014-3201-z [DOI] [PubMed] [Google Scholar]
- 76.Palorini F, Origgi D, Granata C, et al. Adult exposures from MDCT including multiphase studies: first Italian nationwide survey. Eur Radiol 2014;24:469-83. https://doi.org/10.1007/s00330-013-3031-7 10.1007/s00330-013-3031-7 [DOI] [PubMed] [Google Scholar]
- 77.Miller DL, Balter S, Cole PE, et al. Radiation doses in interventional radiology procedures. The RAD-IR study. Part I: overall measures of dose. J Vasc Interv Radiol 2003;14:711-27. https://doi.org/10.1097/01.rvi.0000079980.80153.4b 10.1097/01.rvi.0000079980.80153.4b [DOI] [PubMed] [Google Scholar]


