Abstract
The TIF (transoral incisionless fundoplication) 2.0 procedure is indicated for patients with a hiatal hernia less than 2 cm. Many patients with gastroesophageal reflux disease (GERD) require hiatal hernia repair. This study examined the safety and efficacy when repairing defects in 2 anatomical structures (hiatus and lower esophageal sphincter) in a concomitant set of procedures in patients with hiatal hernias between 2 and 5 cm. Methods. Prospective data were collected from 99 patients who underwent hiatal hernia repair followed immediately by the TIF procedure (HH + -TIF). GERD-HRQL (Health-Related Quality of Life), RSI (Reflux Symptom Index), and GERSS (Gastroesophageal Reflux Symptom Score) questionnaires were administered before the procedure and mailed at 6 and 12 months. Results. Ninety-nine patients were enrolled, and all were symptomatic on PPI medications with hiatal hernias between 2 and 5 cm. Overall baseline GERD-HRQL scores indicated daily bothersome symptoms. At 12-month follow-up, median GERD-HRQL scores improved by 17 points, indicating that subjects had no bothersome symptoms. The median GERSS scores decreased from 25.0 at baseline to 1.0 and 90% of subjects reported having effective symptom control (score <18) at 12 months. Seventy-seven percent of subjects reported effective control of laryngopharyngeal reflux (LPR) symptoms at 12 months with an RSI score of 13 or less. At 12 months, 74% of subjects reported that they were not using proton pump inhibitors. All measures were statistically improved at P < .05. There were no adverse effects reported. Conclusion. HH + TIF provides significant symptom control for heartburn and regurgitation with no long-term dysphagia or gas bloat normally associated with traditional antireflux procedures. Most patients reported durable symptom control and satisfaction with health condition at 12 months.
Keywords: esophageal surgery, flexible endoscopy, gastric surgery, interventional endoscopy, evidence based medicine/surgery, hiatal hernia repair, TIF, transoral incisionless fundoplication
Introduction
Gastroesophageal reflux disease (GERD) is a chronic condition affecting almost 60 million Americans.1 It is one of the most frequent disorders presenting in primary care and community practice settings in the United States and substantially affects patients’ quality of life and productivity.2 Traditional treatment for severe GERD has been medical, with surgery reserved for patients with incomplete symptom control or intolerance to medical therapy. However, medical therapy with proton pump inhibitors (PPIs), has come under scrutiny because of an associated higher incidence of pneumonia,3 osteoporosis and hip fractures,4 Clostridium difficile colitis,5 chronic kidney disease,6 cardiac events if used in conjunction with Plavix,7 and other severe conditions.8-11 PPI therapy also does not stop positional regurgitation; and up to 40% of patients continue to have regurgitation symptoms, despite reduction in the acid content of the refluxed stomach juice.12-15
Hiatal hernia is frequently associated with GERD. The most common is type I sliding hiatal hernia. It occurs when the muscular crura of the diaphragm are weakened and widened, and a portion of the stomach protrudes into the thorax. Previous studies examining the effect of crural repair alone versus fundoplication alone reported each contributes about 50% to the reflux barrier.16,17
GERD unresponsive to medical therapies can be treated by laparoscopic fundoplication, magnetic sphincter augmentation or endoluminal techniques, including transoral incisionless fundoplication (TIF 2.0 procedure) using the EsophyX device (EndoGastric Solutions, Inc, Redmond, WA, USA). The TIF 2.0 procedure was initially contraindicated in patients with a hiatal hernia larger than 2 cm due to concerns over anatomic feasibility and concerns regarding efficacy. In 2017, the Food and Drug Administration approved use of the EsophyX device in patients with hiatal hernias larger than 2 cm in conjunction with laparoscopic repair of the hernia. The HH + TIF–associated repairs allow more GERD patients to benefit from the lower rates of post fundoplication dysphagia and gas bloating associated with the lap Nissen.18
The purpose of our study was to confirm the safety and efficacy of a laparoendoscopic treatment approach for reflux patients with hiatal hernias between 2 and 5 cm. In our prospective study, we evaluated multiple preoperative factors and their relationship to clinical outcomes of HH + TIF in GERD patients with an inadequate response to PPIs over a 12-month time period.
Methods
Prospective data were collected, following institutional review board approval, from patients who underwent HH + TIF at 2 community hospital settings in Indiana and Wisconsin. Baseline demographics, GERD symptoms, endoscopy findings, and operative details were recorded for all patients involved in the study.
Inclusion and Exclusion Criteria
Patients aged 18 to 75 years with moderate to severe typical or atypical GERD symptoms for >1 year were potential subjects for the study. Subjects had to have a hiatal hernia between 2 and 5 cm on endoscopy and ongoing daily PPI use for more than 6 months with either complete or partial symptom control.
Preoperative Evaluation
All subjects underwent a complete preoperative history and physical examination, as well as esophagogastroduodenoscopy or transnasal endoscopy to identify esophagitis and measure hiatal hernia. Specifically, both axial and transverse dimensions of the hiatal defect were recorded along with a visual Hill Grade assessment. The use of GERD medications, such as PPIs, histamine receptor antagonists and antacids, were recorded for each subject as “daily” (dose taken 4-7 days per week), “occasional” (dose taken 1-3 days per week), or “none.” Esophageal manometry and 24-hour pH data were not routinely collected for this cohort.
Three validated questionnaires were administered before the procedure and mailed at 6 and 12 months post-procedure.
The GERD Health-Related Quality of Life Questionnaire (GERD-HRQL) is composed of 10 questions rating symptoms of heartburn and 6 questions rating the symptoms of regurgitation (Table 1). A total score is computed for the heartburn symptoms questions based on a scale of 0 to 5 where 0 = no symptoms and 5 = incapacitation to do daily activities. A reduction of the score by 50 % or greater is considered to indicate a successful intervention.19
Table 1.
Validated Symptom-Assessment Questionnaire GERD-HRQL.
| Symptom-Related Questions | Scoringa | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| GERD-HRQL (Velanovich, 2007)19 | ||||||
| Heartburn score | ||||||
| 1. How bad is the heartburn? 2. Heartburn when lying down? 3. Heartburn when standing up? 4. Heartburn after meals? 5. Does heartburn change your diet? 6. Does heartburn wake you from sleep? 7. Do you have difficulty swallowing? 8. Do you have pain with swallowing? 9. Do you have gassy or bloating feeling? 10. If you take reflux medication, does this affect your daily life? |
||||||
| Regurgitation score | ||||||
| 1. How bad is the regurgitation? 2. Regurgitation when lying down? 3. Regurgitation when standing up? 4. Regurgitation after meals? 5. Does regurgitation change your diet? 6. Does regurgitation wake you from sleep? |
||||||
Abbreviation: GERD-HRQL, Gastroesophageal Reflux Disease–Health-Related Quality if Life Questionnaire.
Scoring definitions: 0 = no symptoms; 1 = noticeable, but not bothersome; 2 = noticeable. Bothersome, but not every day; 3 = bothersome daily; 4 = bothersome and affects daily activities; 5 = incapacitation to do daily activities.
The Gastroesophageal Reflux Symptom Score (GERSS) questionnaire assesses both severity (score of 0 to 3 with 3 = severe) and frequency (0 to 4 with 4 = daily) of heartburn, regurgitation, abdominal distension, dysphagia, and cough (Table 2). To determine total symptom scores, severity and frequency are multiplied for each symptom and are then summed (ie, 1 to 60). A total GERSS score less than 18 is considered good reflux control.20
Table 2.
Validated Symptom Assessment Questionnaire GERSS.a
| Question | Scoring | ||||
|---|---|---|---|---|---|
| 1. Heartburn score | |||||
| How much has heartburn bothered you on a daily basis? | 0 | 1 | 2 | 3 | |
| How often have you experienced heartburn? | 0 | 1 | 2 | 3 | 4 |
| 2. Regurgitation score | |||||
| How much has regurgitation bothered you on a daily basis? | 0 | 1 | 2 | 3 | |
| How often have you experienced regurgitation? | 0 | 1 | 2 | 3 | 4 |
| 3. Abdominal distension score | |||||
| How much has abdominal distention bothered you on a daily basis? | 0 | 1 | 2 | 3 | |
| How often have you experienced abdominal distension? | 0 | 1 | 2 | 3 | 4 |
| 4. Dysphagia score | |||||
| How much has dysphagia bothered you on a daily basis? | 0 | 1 | 2 | 3 | |
| How often have you experienced dysphagia? | 0 | 1 | 2 | 3 | 4 |
| 5. Coughing score | |||||
| How much has coughing bothered you on a daily basis? | 0 | 1 | 2 | 3 | |
| How often have you experienced coughing? | 0 | 1 | 2 | 3 | 4 |
GERSS, Gastroesophageal Reflux Symptom Score.
Scoring definitions for intensity: 0 = not at all; 1 = intermittently; 2 = moderately; 3 = severely. Scoring definitions for frequency: 0 = never; 1 = once monthly; 2 = once weekly; 3 = 2 to 4 days per week; 4 = daily.
In addition, we used the laryngopharyngeal reflux (LPR) questionnaire Reflux Symptom Index (RSI) on our group and wish to report these results as well. The 9-question RSI assesses laryngopharyngeal reflux using 9 questions scored from 0 to 5. A total RSI score of 13 or less is considered normal (Table 3).21
Table 3.
Validated Symptom Assessment Questionnaire RSI.a
| Symptom Related Questions | Scoring | |||||
|---|---|---|---|---|---|---|
| Reflux Symptom Index (Belafsky et al, 2002)21 | 0 | 1 | 2 | 3 | 4 | 5 |
| 1. Hoarseness or problem with your voice? 2. Clearing your throat? 3. Excess throat mucus or postnasal drip? 4. Difficulty swallowing food, liquids, or pills? 5. Coughing after you ate or after lying down? 6. Breathing difficulties or choking episodes? 7. Troublesome or annoying cough? 8. Sensations of something in your throat or a lump in your throat? 9. Heartburn, chest, pain, indigestion, or stomach acid coming up? |
||||||
| Satisfaction | ||||||
| How satisfied are you with your health condition? | S | N | D | |||
Abbreviation: RSI, Reflux Symptom Index.
Scoring definitions: 0 = no symptoms; 1 = noticeable, but not bothersome; 2 = noticeable. Bothersome, but not every day; 3 = bothersome daily; 4 = bothersome and affects daily activities; 5 = incapacitation to do daily activities. Satisfaction: S = satisfied; N = neutral; D = dissatisfied.
Surgical Technique
All patients were under general anesthesia. Intraoperative esophagogastroduodenoscopy (EGD) examination was performed to look for contraindications and again measure and grade the hiatal hernia. Laparoscopic repair was performed with four 5-mm ports, with an optional fifth 5-mm port to facilitate retraction and exposure. A routine complete hiatal dissection was performed. The gastroesophageal junction was circumferentially mobilized, using care to protect and preserve the anterior and posterior vagus nerves as well as the hepatic branch of the vagus, until the gastroesophageal junction was 3 to 4 cm below the hiatus without tension. The hiatal defect was then repaired posteriorly using suture at the discretion of the surgeon. An anterior suture was placed if needed to achieve a snug reapproximation of the crura around the esophagus. The insufflation was released, and ports removed. Incisions were closed and dressings placed.22 Patient was then placed in a partial lateral decubitus position for the remaining endoscopic fundoplasty.
The valve barrier is reconstructed using TIF 2.0 technique with the EsophyX technology as described by Bell and Cadière.23
Briefly, once the EsophyX device was properly positioned within the stomach under endoscopic control, a valve was reconstructed by creating a series of esophagogastric, full-thickness plications circumferentially around the distal esophagus. The number of fasteners used per patient ranged from 13 to 14 at site 1 and 19 to 20 at site 2. Following successful TIF 2.0 valve creation, the device was withdrawn and a final endoscopy was performed to assess the newly created valve (length, circumference, Hill Grade) as well as to assess integrity of the hiatal repair and for any potential persistence of hiatal hernia caused by device introduction through the newly repaired hiatus. Additionally, measurements were obtained to assess the difference in axial length from the incisors to the gastroesophageal junction in comparison with the preoperative measurements.
Each procedure was appropriately documented, and pictures were taken for review and evaluation. Any complications related to the procedure were recorded.
Subjects were released the next day after examination and instructed to consume a traditional postoperative diet of clear liquids for 2 weeks. The usage of PPIs was continued for 14 days following the procedure to allow healing of the gastric mucosa around the fasteners. Following discharge, subjects were asked to adhere to a modified diet of graduating food texture for 4 to 6 weeks in accordance with the hospital’s post-fundoplication standard of care.
Follow-up Evaluation
Follow-up assessments were conducted by mail at 6- and 12-months post-procedure. At each follow-up, subjects were asked to complete the same three questionnaires assessing their current symptoms.
Definition of Endpoints
Scores of ≤2 to each question were indicative of effectively treated symptoms. Symptoms were considered as clinically significantly improved if the total GERD-HRQL, GERSS, and RSI scores are reduced by ≥50% at 6 months (primary) and 12 months (secondary) post-procedure.
Statistical Analysis
All analyses were performed using SAS version 9.4. Results by time and clinical site were summarized based on the underlying distribution for each variable. Data recorded using a scale considered continuous, such as age, were summarized using the mean, standard deviation, median, range and upper and lower 95% confidence intervals. Data recorded using a multinomial or binomial scale, such as the proportion of subjects who reported elimination of symptoms based on the GERD-HRQL Questionnaire, were summarized using counts, percentages, and 95% exact binomial confidence intervals. No data were imputed for patients who withdrew prematurely. The interpretation of the results was predicated on an examination of the confidence intervals.
Results
Subject Disposition
Ninety-nine patients were enrolled, 50 from site 1, 49 from site 2 (Figure 1). The questionnaire response rate was 73% at 6 months, 67% at 12 months, and 48% for both.
Figure 1.
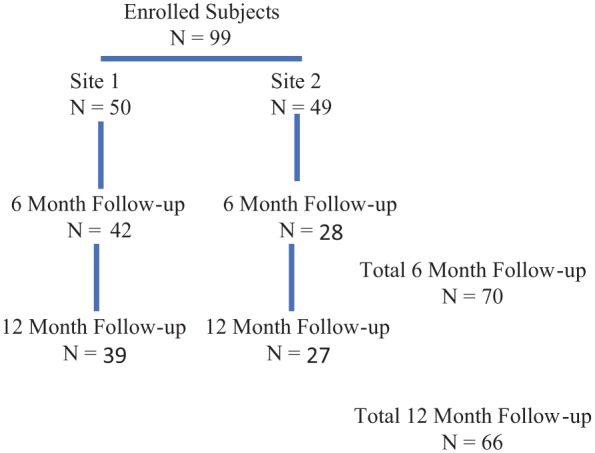
Subject disposition.
Baseline Characteristics
The average age of subjects was approximately 53 years and 55% of subjects were female (Table 4). Overall, mean body mass index was 30 kg/m2. Most subjects were symptomatic on PPI medications with hiatal hernias between 2 and 5 cm. Demographic and baseline characteristics were well balanced between the 2 sites.
Table 4.
Demographic and Baseline Characteristics.
| Characteristic | Overall (N = 99) | Site 1 (n = 50) | Site 2 (n = 49) |
|---|---|---|---|
| Age (years) | |||
| n | 99 | 50 | 49 |
| Mean (SD) | 53.2 (13.07) | 52.5 (12.86) | 53.8 (13.37) |
| Median | 55.0 | 53.5 | 56.0 |
| Sex, n (%) | |||
| Male | 45 (45.0) | 21 (42.0) | 24 (49.0) |
| Female | 54 (55.0) | 29 (58.0) | 25 (51.0) |
| BMI (kg/m2) | |||
| n | 48 | 49 | |
| Mean (SD) | 29.2 (5.00) | 30.8 (4.95) | |
| Median | 29.7 | 31.4 | |
| Duration of GERD symptoms | |||
| 6 months, n (%) | 99 (100.0) | ||
| Hiatal hernia measurements | |||
| Range of axial and/or transverse dimensions (cm) | 2-5 | 2-5 | |
| Type of GERD symptoms, % | |||
| PPI usage, n (%) | |||
| Daily | 63 (63.6) | 38 (76.0) | 25 (51.0) |
| None | 4 (4.0) | 0 | 4 (8.2) |
| Occasional | 2 (2.0) | 2 (4.0) | 0 |
| Twice daily | 29 (29.3) | 10 (20.0) | 19 (38.8) |
| Weekly | 1 (1.0) | 0 | 1 (2.0) |
| Satisfied with health condition, n (%) | |||
| Satisfied | 8 (8.0) | 5 (10.0) | 3 (6.1) |
| Neutral | 38 (38.4) | 18 (36.0) | 20 (41.0) |
| Dissatisfied | 53 (53.5) | 27 (54.0) | 26 (53.1) |
Abbreviations: BMI, body mass index; GERD, gastroesophageal reflux disease; PPI, proton pump inhibitor.
Baseline Work-up
All patients had a baseline EGD. Diagnostic tests were performed to determine eligibility for HH+TIF intervention only. All patients had objectively proven GERD with preoperative testing with pH study or esophagram at site 1. Patients at site 2 were included based on endoscopic findings as well as pH testing. Study design did not include follow-up EGD or repeat diagnostic procedures.
Site 1 (n = 50 Patients)
At site 1, 5 EGDs were normal, 25 patients had Los Angeles grade A esophagitis, 15 had grade B, 5 had grade C. Of the group, 14 had manometry preoperatively, 32 had barium swallows, and 39 had Bravo pH studies.
Site 2 (n = 49 Patients)
At Site 2, 5 EGDs were normal, 26 patients had Los Angeles grade A esophagitis, 13 had grade B, 4 had grade C, and 2 had Barrett’s; 4 patients underwent pH testing.
Symptom-Related Outcomes
GERD-HRQL Scores
Overall GERD-HRQL scores at baseline indicated presence of daily bothersome symptoms. At 6 and 12 months, median GERD-HRQL scores improved by 19 and 17 points, respectively, which indicated that subjects were reporting no symptoms or noticeable, but not bothersome symptoms.
GERD-HRQL has a specific question measuring gas bloat. At baseline, median score was 3.11 and at 6- and 12-month follow-ups, it was reduced to 1.42 and 1.30, respectively (Table 5). Traditional fundoplication patients frequently report an increase in gas bloat.18
Table 5.
GERD-HRQL Questionnaire.
| Survey Period | |||
|---|---|---|---|
| Baseline (N = 99) | 6 Months (n =70) | 12 Months (n = 66) | |
| Change from baseline post-TIF procedure, P < .05 | |||
| Mean (SD) | 25.1 (0.07) | 4.6 (6.33) | 4.6 (5.81) |
| Median (95% CI) | 26.0 (22.9, 27.3) | 2.0 (3.11, 6.12) | 2.0 (3.21, 6.07) |
| Mean (SD) change from baseline | −18.9 (11.39) | −18.3 (11.07) | |
| Median (95% CI) change from baseline | −19.0 (–21.61, –16.13) | −17.0 (–20.99, –15.50) | |
| Median GERD-HRQL scores by individual question | |||
| How bad is the heartburn? | 2.95 | 0.45 | 0.52 |
| Heartburn when lying down? | 2.84 | 0.35 | 0.42 |
| Heartburn when standing up? | 2.57 | 0.32 | 0.33 |
| Heartburn after meals? | 2.87 | 0.32 | 0.45 |
| Does heartburn change your diet? | 2.86 | 0.39 | 0.44 |
| Does heartburn wake you from sleep? | 2.52 | 0.25 | 0.30 |
| Do you have difficulty swallowing? | 1.74 | 0.46 | 0.38 |
| Do you have pain with swallowing? | 1.28 | 0.38 | 0.23 |
| Do you have gassy or bloating feeling? | 3.11 | 1.42 | 1.39 |
| If you take reflux medication, does this affect your daily life? | 2.39 | 0.41 | 0.24 |
| How bad is the regurgitation? | 2.79 | 0.49 | 0.41 |
| Regurgitation when lying down? | 2.48 | 0.41 | 0.41 |
| Regurgitation when standing up? | 2.17 | 0.32 | 0.30 |
| Regurgitation after meals? | 2.39 | 0.35 | 0.27 |
| Does regurgitation change your diet? | 2.27 | 0.30 | 0.36 |
| Did regurgitation wake you from sleep? | 2.23 | 0.25 | 0.26 |
Abbreviations: GERD-HRQL, Gastroesophageal Reflux Disease–Health-Related Quality of Life Questionnaire; TIF, transoral incisionless fundoplication; SD, standard deviation; CI, confidence interval.
GERSS Scores
The median (95% CI) GERSS scores decreased from 25.0 (22.74, 28.84) at baseline to 2.0 (4.20, 9.68) and 1.0 (3.28, 7.32) at months 6 and 12, respectively. At 6 and 12 months, 86% to 90% of subjects reported having effective control of their symptoms (score <18) (Table 6).
Table 6.
GERSS Questionnaire.
| Survey Period | |||
|---|---|---|---|
| Baseline (N = 99) | 6 Months (n = 70) | 12 Months (n = 66) | |
| Change from baseline post-TIF procedure, P < .05 | |||
| Mean (SD) | 25.8 (15.30) | 6.9 (11.39) | 5.3 (8.22) |
| Median (95% CI) | 25.0 (22.74, 28.84) | 2.0 (4.20, 9.68) | 1.0 (3.28, 7.32) |
| Mean (SD) change from baseline | −15.3 (16.49) | −17.9 (16.00) | |
| Median (95% CI) change from baseline | −14.0 (–19.26, –11.27) | −17.0 (–21.83, –13.90) | |
| Median GERSS scores: severity questions | |||
| Abdominal distention | 1.71 | 0.77 | 0.68 |
| Coughing | 1.65 | 0.75 | 0.62 |
| Dysphagia | 1.33 | 0.48 | 0.48 |
| Heartburn | 1.94 | 0.31 | 0.27 |
| Regurgitation | 1.58 | 0.31 | 0.30 |
| Median GERSS scores: frequency questions | |||
| Abdominal distention | 2.42 | 1.21 | 0.85 |
| Coughing | 2.63 | 1.17 | 0.88 |
| Dysphagia | 1.82 | 0.62 | 0.59 |
| Heartburn | 2.85 | 0.48 | 0.42 |
| Regurgitation | 2.38 | 0.46 | 0.33 |
| Proportion of Subjects Who Reported Effective Control of Symptoms Based on the GERSS Questionnaire | 6 Months (n = 69) | 12 Months (N = 66) | |
| Number (%) of subjects with effective control of symptoms (score <18) | 59 (85.5%)a | 60 (90.9)b | |
| 95% CI | 7.17%, 25.04% | 3.41%, 18.74% | |
Abbreviations: GERSS, Gastroesophageal Reflux Symptom Score; TIF, transoral incisionless fundoplication; SD, standard deviation; CI, confidence interval.
Not reported for 31 subjects.
Not reported for 34 subjects.
Reflux Symptom Index
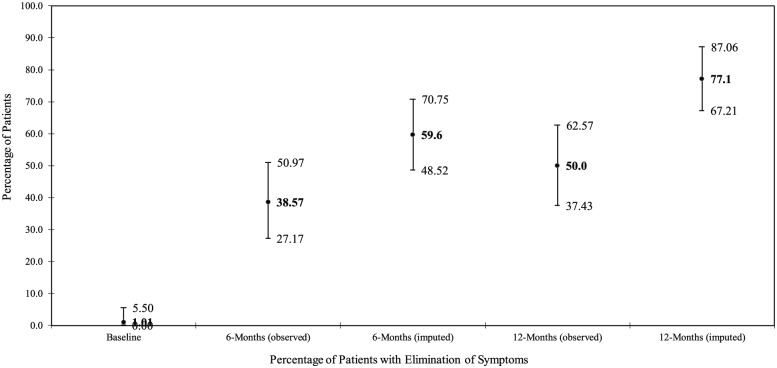
RSI was collected as a measure of atypical GERD or LPR symptoms. Overall, 60% and 77% of subjects reported effective control of LPR symptoms at 6 and 12 months, respectively (ie, RSI score of 13 or less) (Figure 2). Median RSI scores decreased from 26 points at baseline to 15 and 16 points at months 6 and 12, respectively, indicating a return to a normal status of reflux (Table 7).
Figure 2.
Reflux Symptom Index (RSI)—Proportion of subjects who returned to normal based on effective control of laryngopharyngeal reflux (LPR) symptoms (score <13) (proportion with 2-sided 95% exact binominal confidence intervals).
Table 7.
RSI Questionnaire.
| Survey Period | |||
|---|---|---|---|
| Baseline (N = 99) | 6 Months (n = 70) | 12 Months (n = 66) | |
| Change from baseline Post-TIF procedure, P < .05 | |||
| Mean (SD) | 28.5 (12.00) | 11.1 (10.92) | 8.9 (10.93) |
| Median (95% CI) | 29.0 (26.09, 30.88) | 7.0 (8.47, 13.68) | 5.5 (6.27, 11.64) |
| Mean (SD) change from baseline | −16.3 (12.89) | −18.0 (13.21) | |
| Median (95% CI) change from baseline | −15.0 (–19.36, –13.16) | −16.0 (–21.27, –14.73) | |
| Median RSI scores by individual question, P < .05 | |||
| Hoarseness or a problem with your voice | 2.12 | 0.94 | 0.55 |
| Clearing your throat | 3.18 | 1.51 | 1.20 |
| Excess throat mucus or postnasal drip | 3.00 | 1.68 | 1.23 |
| Difficulty swallowing foods, liquids, or pills | 1.68 | 0.65 | 0.62 |
| Coughing after you ate or after lying down | 2.54 | 0.90 | 0.70 |
| Breathing difficulties or choking episodes | 1.91 | 0.45 | 0.48 |
| Troublesome or annoying cough | 2.59 | 1.00 | 0.67 |
| Sensation or something sticking or a lump in your throat | 2.62 | 0.94 | 0.79 |
| Hoarseness or a problem with your voice | 2.12 | 0.94 | 0.55 |
| Clearing your throat | 3.18 | 1.51 | 1.20 |
Satisfaction With Current Health Condition
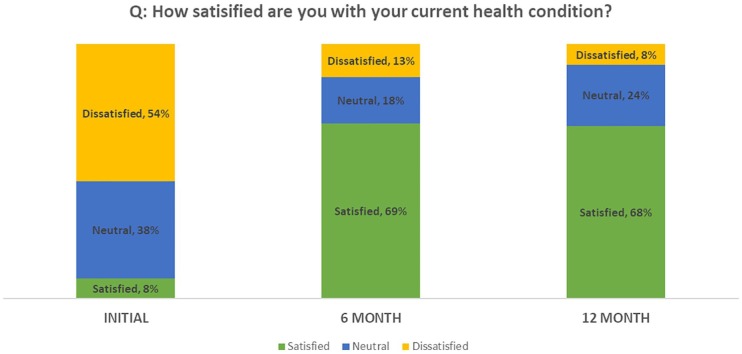
A clinically relevant shift in health condition satisfaction was observed at both post-baseline timepoints. At baseline, only 8% of subjects were satisfied with the current state of their health, whereas approximately 70% of subjects were satisfied with the health condition at months 6 and 12 (Table 8; Figure 3).
Table 8.
Satisfaction with Current Health Condition.
| P < .05 Category | Baseline (N = 99), n (%) | 6 Months (n = 70), n (%) | 12 Months (n = 66), n (%) |
|---|---|---|---|
| Dissatisfied | 53 (53.5) | 9 (13.2) | 5 (7.6) |
| Neutral | 38 (38.4) | 11 (16.2) | 16 (24.2) |
| Satisfied | 8 (8.0) | 48 (70.6) | 45 (68.2) |
Figure 3.
Satisfaction with current health condition.
Heartburn
The primary driver for GERD patients seeking treatment is heartburn. Here are the median scores for heartburn from the three validated questionnaires. Note significant score changes on individual questions. (Table 9).
Table 9.
Heartburn Questions Median Scores.
| Heartburn | Baseline | 6 Months | 12 Months |
|---|---|---|---|
| GERD-HRQL HB Q1 | 2.95 | 0.45 | 0.52 |
| GERD-HRQL HB Q2 | 2.84 | 0.35 | 0.42 |
| GERD-HRQL HB Q3 | 2.57 | 0.32 | 0.33 |
| GERD-HRQL HB Q4 | 2.87 | 0.32 | 0.45 |
| GERD-HRQL HB Q5 | 2.86 | 0.39 | 0.44 |
| GERD-HRQL HB Q6 | 2.52 | 0.25 | 0.30 |
| GERSS HB Q1a | 1.94 | 0.31 | 0.27 |
| GERSS HB Q1b | 2.85 | 0.48 | 0.42 |
| RSI Q9 | 3.42 | 0.72 | 0.47 |
Abbreviations: GERD-HRQL, Gastroesophageal Reflux Disease–Health-Related Quality of Life Questionnaire; RSI, Reflux Symptom Index; HB, heartburn; Q, question.
Regurgitation
Regurgitation is a typical, but difficult, symptom to treat. Table 10 is a collection of median scores on regurgitation and demonstrate improvement when using HH + TIF intervention.
Table 10.
Regurgitation Question Median Scores.
| Regurgitation | Baseline | 6 Months | 12 Months |
|---|---|---|---|
| GERD-HRQL Re Q1 | 2.79 | 0.49 | 0.41 |
| GERD-HRQL Re Q2 | 2.48 | 0.41 | 0.41 |
| GERD-HRQL Re Q3 | 2.17 | 0.32 | 0.30 |
| GERD-HRQL Re Q4 | 2.39 | 0.35 | 0.27 |
| GERD-HRQL Re Q5 | 2.27 | 0.30 | 0.36 |
| GERD-HRQL Re Q6 | 2.23 | 0.25 | 0.26 |
| GERSS Re Q2a | 1.58 | 0.31 | 0.30 |
| GERSS Re Q2b | 2.38 | 0.46 | 0.33 |
Abbreviations: GERD-HRQL, Gastroesophageal Reflux Disease–Health-Related Quality of Life Questionnaire; GERSS, Gastroesophageal Reflux Symptom Score; Re, regurgitation; Q, question.
Dysphagia
Dysphagia is a common symptom in patients with GERD and is queried in the validated instruments slightly differently. The HH + TIF procedure appears to positively affect scores without introducing new-onset symptom (Table 11). This is contrary to findings from traditional fundoplication.18
Table 11.
Dysphagia Questions Median Scores.
| Dysphagia | Baseline | 6 months | 12 months |
|---|---|---|---|
| GERD-HRQL Q7 | 1.74 | 0.46 | 0.38 |
| GERSS Q4a | 1.33 | 0.48 | 0.48 |
| GERSS Q4b | 1.82 | 0.62 | 0.59 |
| RSI Q4 | 1.68 | 0.65 | 0.62 |
Abbreviations: GERD-HRQL, Gastroesophageal Reflux Disease–Health-Related Quality of Life Questionnaire; RSI, Reflux Symptom Index; Q, question.
PPI Usage
Over 60% of subjects reported using PPIs daily at baseline (Table 12). At month 6, only 11% of subjects were using PPIs daily and 70% reported never using PPIs. At month 12, 74% of subjects reported that they were not using PPIs.
Table 12.
Proton Pump Inhibitor (PPI) Usage.
| P < .05 Usagea | Baseline (N = 99), n (%) | Month 6 (n = 70), n (%) | Month 12 (n = 27), n (%) |
|---|---|---|---|
| Daily | 63 (63.6) | 8 (11.4) | NA |
| Never | 4 (4.0) | 49 (70.0) | NA |
| Occasional | 2 (2.0) | 8 (11.4) | NA |
| Twice daily | 29 (29.3) | 3 (4.3) | NA |
| Twice weekly | 1 (1.0) | 2 (2.9) | NA |
| No | NA | 20 (74.1) | |
| Yes | NA | 7 (25.9) |
Abbreviation: NA, not applicable.
Daily = any dose taken 4 to 7 days per week; Occasional = any dose taken 1 to 3 days per week; Never = no PPI medication used.
Adverse Events
There were no intraoperative complications laparoscopic or esophageal. There were no adverse events reported by our subjects. Additionally, subjects did not report any increase in typical post-fundoplication side effects such as gas bloat, inability to belch or inability to vomit following the combined procedure.
Discussion
The reduction and repair of a hiatal hernia repair improves reflux symptoms and reduces the recurrence or progression to larger hernias over time. Patients with large hernias irrespective of reflux symptoms are typically treated with an antireflux procedure at the time of hernia repair to minimize hernia recurrence and to alleviate the development of de novo reflux.17,24,25
Unfortunately, total and even partial fundoplications are associated with a high incidence of troublesome side effects (bloating, flatulence, dysphagia, early satiety) that while mostly transient, have discouraged many patients from accepting surgical treatment of their GERD.22
In the TEMPO randomized controlled trial by Trad et al,26 only GERD patients with hiatal hernias less than 2 cm were enrolled. Trad and colleagues reported significant improvement of regurgitation, atypical symptoms, and heartburn, as evaluated by 2 of the 3 validated, disease-specific questionnaires used in our study (RSI, GERD-HRQL). The results were maintained without significant deterioration over 5-year follow-up. These outcomes were achieved without unwanted SAE and post-fundoplication side effects.26 Testoni et al27 subsequently showed that presence of a hiatal hernia >2 cm was a significant predictor of TIF failure over 6-year follow-up. This would suggest that more uniform response rates might be achieved when applying HH + TIF intervention. The only patient selection criteria that was different between TEMPO patients and our cohort was the presence of a significant hiatal hernia that was repaired just prior to the TIF 2.0 procedure. In our early follow-up, results were similar, and one could hope the durability will be similar.
In our practice, more than half of patients presenting with reflux complaints have a hiatal hernia larger than 2 cm and would not be candidates for a purely endoluminal approach with EsophyX technology. These patients in many instances do not wish to have a traditional laparoscopic fundoplication due to concerns of potential side effects. This cohort did not report gas bloat, inability to belch, or inability to vomit after the combined procedure, which aligns with systematic review data.28 Performing an anatomical repair of the hiatal defect alone avoids the more extensive traditional laparoscopic dissection that would require a larger retroesophageal window creation, a takedown of the short gastrics for complete fundic mobilization that adds increased risk of bleeding and injury to the spleen, and repositioning the bulk of the fundus in the retroesophageal space. This focused, less extensive dissection allows patients to become candidates for an endoluminal approach to valve reconstruction, rather than the supra-physiologic fundoplication. The endoluminal approach allows for a more anatomically correct valve repair which addresses the goals of increasing intraabdominal length, reestablishing the angle of His, and reestablishing a Hill grade 1 valve. Additionally, with this technique, the fasteners delivered to create the fundoplasty are placed while the stomach is inflated, in comparison with traditional fundoplication with the stomach decompressed. The anchoring fasteners placed in this manner are likely less affected by stomach inflation with air ingestion or meals and thus potentially explains the significantly lower incidence of gas bloat following this antireflux procedure. Similarly, since the new valve is more anatomically correct than traditional fundoplication, this would potentially explain the improved ability to belch or vomit following this endoluminal reconstruction. Despite potential concerns over introducing the EsophyX device immediately following the laparoscopic hiatal hernia repair, which mirrors the concerns over bougie usage in traditional fundoplications, there were no esophageal injuries in our study. This is likely attributed to direct visualized introduction via endoscope (vs. blind passage of a bougie) and is consistent with historic safety profile of EsophyX device use.28
The results of this study affirm this concept and define the safety and efficacy of this approach. One of the potential impacts on efficacy and durability of TIF valve reconstruction alone was presence of a larger hiatal hernia. In many occasions, preoperative assessment may underestimate the extent of hiatal disruption. By addressing both aspects of the antireflux barrier, there is potentially a positive impact on efficacy of the TIF procedure due to elimination of any hiatal hernia. Similar results were reported by Ihde et al29 in 2011 in a case series report where 18 of 42 TIF patients had a HH repair in the same anesthesia session. Heartburn was significantly improved or eliminated in 63% of patients and 76% were off daily PPI at 6 months. The hiatal hernia repair patients had the same outcomes as patients with no hiatal hernia.29
Our study provides justification that this approach can be applied in a community setting. Certainly, a weakness of the study is the short-term follow-up of 12 months. Other studies including the TEMPO trial demonstrated longer-term efficacy and durability of TIF alone but determining similar durability and efficacy of this combined approach would need longer-term follow-up and study. Although the majority of patients included in the trial had objective evidence of GERD preoperatively, some patients included based on endoscopic evidence of esophagitis may not have had true abnormal esophageal exposure of gastric content and thus may not have provided appropriately comparable results. Another weakness of our study would be the lack of objective outcomes data such as pH testing and lack of a comparator group (HH + TIF vs fundoplication or TIF 2.0 without hiatal repair). We feel that there are enough data in the literature from randomized controlled trials known as TEMPO,26 RESPECT,30 and European sham31 by Håkanson et al. These studies established strong clinical evidence for TIF 2.0 without hiatal repair that a comparator group was unnecessary. Performing TIF alone in patients with larger hiatal hernias is highly likely to fail if repair is not made in the same anesthesia session29 and would be clinically inappropriate. Ideally, direct comparison with a matched group of fundoplication patients would provide the best comparison; however, in our study the patients had self-selected themselves as not wanting a traditional fundoplication. Finally, our study did not report any objective postprocedure testing. In the setting of a private practice community, any follow up studies would have been the financial responsibility of the patient (or their insurance carrier), therefore objective postoperative studies such as pH testing, esophagram, or endoscopy were not performed. For this study, we instead relied on a very comprehensive symptom evaluation process using 3 validated questionnaires. Objective longer-term postprocedure testing with either pH testing, endoscopy, or esophagram would be the goal of future investigation.
Conclusion
The objective of the present study was to assess the safety and efficacy of a HH + TIF procedure among a cohort of GERD patients with hiatal hernias. Based on our findings, the procedures can be safely performed one after the other, either with a single surgeon doing both components, or performed by a team of surgeon and gastroenterologist. Our study confirmed the efficacy of this approach with regard to quality-of life measure, symptom control, and reduction in PPI use. The subject-reported symptoms of GERD were significantly reduced across all instruments used to assess effectiveness, and the results were durable out to 12 months postprocedure. Quality of life was greatly improved as indicated by the large percentage of subjects who reported improved satisfaction with their current health condition at both 6 and 12 months. By combining laparoscopic hiatal hernia repair with a TIF 2.0 valve reconstruction, a greater population of patients could be considered candidates for surgical treatment of their refractory reflux disease without being exposed to the potential effects of traditional laparoscopic antireflux procedures.
Acknowledgments
The authors wish to thank Mary Jo Anhalt.
Footnotes
Author Contributions: Study concept and design: Peter Janu and Peter Mavrelis
Acquisition of data: Peter Janu, Peter Mavrelis, Ahmad Bassel Shughoury, Kumar Venkat, Daniel Hurwich, Tom Galouzis, James Siatras, Dennis Streeter, and Kathleen Korman
Analysis and interpretation: George Mavrelis, Peter Janu and Peter Mavrelis
Study supervision: Peter Mavrelis
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Drs Peter Janu and Peter Mavrelis receive honorarium from Endogastric Solutions Inc for speaking engagements and teaching.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Peter Janu  https://orcid.org/0000-0001-7356-7399
https://orcid.org/0000-0001-7356-7399
References
- 1. Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1392-1413. [DOI] [PubMed] [Google Scholar]
- 2. Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149:1731-1741.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arj A, Razavi Zade M, Yavari M, Akbari H, Zamani B, Asemi Z. Proton pump inhibitors use and change in bone mineral density. Int J Rheum Dis. 2016;19:864-868. [DOI] [PubMed] [Google Scholar]
- 5. Dos Santos-Schaller O, Boisset S, Seigneurin A, et al. Recurrence and death after Clostridium difficile infection: gender-dependent influence of proton pump inhibitor therapy. Springerplus 2016;5:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lazarus B, Chen Y, Wilson FP, Epaulard O. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937-944. [DOI] [PubMed] [Google Scholar]
- 8. Hsu WH, Wu IC, Kuo CH, et al. Influence of proton pump inhibitor use in gastrointestinal polyps. Kaohsiung J Med Sci. 2010;26:76-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacobs C, Coss Adame E, Attaluri A, Valestin J, Rao SS. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment Pharmacol Ther. 2013;37:1103-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416. [DOI] [PubMed] [Google Scholar]
- 11. Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One. 2015;10:e0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carlsson R, Dent J, Watts R, et al. Gastro-oesophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. International GORD Study Group. Eur J Gastroenterol Hepatol. 1998;10:119-124. [PubMed] [Google Scholar]
- 13. Castell DO, Murray JA, Tutuian R, Orlando RC, Arnold R. Review article: the pathophysiology of gastro-oesophageal reflux disease—oesophageal manifestations. Aliment Pharmacol Ther. 2004;20(suppl 9):14-25. [DOI] [PubMed] [Google Scholar]
- 14. Fass R. Proton-pump inhibitor therapy in patients with gastro-oesophageal reflux disease: putative mechanisms of failure. Drugs. 2007;67:1521-1530. [DOI] [PubMed] [Google Scholar]
- 15. Hunt RH, Yuan Y, Yaghoobi M. GERD: new strategies and new failures. J Clin Gastroenterol. 2007;41(suppl 2):S72-S80. [Google Scholar]
- 16. Woodward ER, Thomas HF, McAlhany JC. Comparison of crural repair and Nissen fundoplication in the treatment of esophageal hiatus hernia with peptic esophagitis. Ann Surg. 1971;173:782-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Müller-Stich BP, Achtstätter V, Diener MK, et al. Repair of paraesophageal hiatal hernias—is a fundoplication needed? A randomized controlled pilot trial. J Am Coll Surg. 2015;221:602-610. [DOI] [PubMed] [Google Scholar]
- 18. Galmiche JP, Hatlebakk J, Attwood S, et al. ; LOTUS Trial Collaborators. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA. 2011;305:1969-1977. doi: 10.1001/jama.2011.626 [DOI] [PubMed] [Google Scholar]
- 19. Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20:130-134. [DOI] [PubMed] [Google Scholar]
- 20. Allen CJ, Parameswaran K, Belda J, Anvari M. Reproducibility, validity and responsiveness of a disease-specific symptom questionnaire for gastroesophageal reflux disease. Dis Esophagus. 2000;13: 265-270. [DOI] [PubMed] [Google Scholar]
- 21. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16:274-277. [DOI] [PubMed] [Google Scholar]
- 22. Swanstrom L, Wayne R. Spectrum of gastrointestinal symptoms after laparoscopic fundoplication. Am J Surg. 1994;167:538-541. [DOI] [PubMed] [Google Scholar]
- 23. Bell RC, Cadière GB. Transoral esophago-gastric fundoplication: technical and safety considerations. Surg Endosc. 2011;25:2387-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furnee EJ, Draaisma WA, Gooszen HG, Hazebroek EJ, Smout AJ, Broeders IA. Tailored or routine addition of an antireflux fundoplication in laparoscopic large hiatal hernia repair: a comparative cohort study. World J Surg. 2011;35:78-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williamson WA, Ellis FH, Jr, Streitz JM, Shahian DM. Paraesophaegeal hiatal hernia: is an antireflux procedure necessary? Ann Thorac Surg. 1993;56:447-451. [DOI] [PubMed] [Google Scholar]
- 26. Trad KS, Barnes WE, Prevou ER, et al. The TEMPO trial at 5 years: transoral fundoplication (TIF 2.0) is safe, durable, and cost-effective. Surg Innov. 2018;25:149-157. doi: 10.1177/1553350618755214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Testoni PA, Testoni S, Mazzoleni G, Vailati C, Passaretti S. Long-term efficacy of transoral incisionless fundoplication with Esophyx (TIF 2.0) and factors affecting outcomes in GERD patients followed for up to 6 years: a prospective single-center study. Surg Endosc. 2015;29:2770-2780. [DOI] [PubMed] [Google Scholar]
- 28. Trad KS. Transoral incisionless fundoplication: current status. Curr Opin Gastroenterol. 2016;32:338-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ihde GM, Besancon K, Deljkich E. Short-term and symptomatic outcomes of transoral incisionless fundoplication with or without hiatal hernia repair in patients with chronic gastroesophageal reflux disease. Am J Surg. 2011;202:740-746. [DOI] [PubMed] [Google Scholar]
- 30. Hunter JG, Kahrilas PJ, Bell RCW, et al. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology. 2015;148:324-333. [DOI] [PubMed] [Google Scholar]
- 31. Håkansson B, Montgomery M, Cadiere GB, et al. Randomised clinical trial: transoral incisionless fundoplication vs. sham intervention to control chronic GERD. Aliment Pharmacol Ther. 2015;42:1261-1270. [DOI] [PubMed] [Google Scholar]