Abstract
Background and Objectives: To perform a systematic review and meta-analysis with the aim of determining the relationship between H. pylori infection and psoriasis. Methods: Pubmed, Embase, China National Knowledge Infrastructure (CNKI), and Web of Science were searched for articles published up to July, 2019. Review Manager 5.3 and Stata 12.0 were used for statistical analyses. Results: The initial database search resulted in 204 articles. Through exclusion and screening, 11 studies involving a total of 1741 participants were finally included in this meta-analysis. The odds ratio (OR) of H. pylori infection rate in the psoriasis group was significantly higher than that in the control group (OR = 1.19, 95% CI 1.15–2.52, P = 0.008). Subgroup analysis showed that no significant difference was detected between the Asia group and the Europe group. As for the methods of H. pylori detection, a statistically significant increase of H. pylori infection in the IgG ELISA test group was detected, compared with the urea breath test group. In addition, analysis based on the severity of psoriasis showed a statistically significant increase of H. pylori infection in moderate and severe psoriasis patients (OR = 2.27; 95% CI: 1.42–3.63, I2 = 27%), but not in the mild psoriasis patients (OR = 1.10; 95% CI: 0.79–1.54, I2 = 0%). Conclusion: H. pylori infection is associated with psoriasis, and psoriasis patients with H. pylori infection have higher Psoriasis Area and Severity Index (PASI) scores. The findings are of considerable significance for the clinical practices.
Keywords: Helicobacter pylori, infection, psoriasis
1. Introduction
Helicobacter pylori is a microaerophilic gram-negative bacterium that naturally colonizes human gastric mucosa. H. pylori infection is a global health problem. In some developing countries, the rate of H. pylori infection can reach 80–90% [1,2]. It is well known that H. pylori infection is closely related to various gastrointestinal diseases, such as duodenal ulcer and gastric cancer [3]. In recent years, the relationship between H. pylori infection and extragastrointestinal diseases has attracted the attention of many researchers. It has been reported that H. pylori infection is associated with esophageal diseases, dementia, autoimmune skin disease, and so on [4,5,6]. In addition, studies suggested a relationship between H. pylori infection and certain skin diseases, which might lead to novel therapeutic strategies for these diseases [7,8,9].
Psoriasis is a common chronic cutaneous disease which seriously affects the life quality of patients. The main clinical manifestations are erythema, papules, and scales [10]. Lesions usually occur in the extensor aspects of elbows and knees but can appear anywhere on the body. The pathogenesis of psoriasis is still unclear. Researchers believe that it is related to genetics, infection, immune abnormalities, and endocrine factors [11]. In terms of treatment, there is currently no cure for psoriasis, however, appropriate symptomatic treatment can control the symptoms [12]. In recent years, researchers have found that H. pylori infection is associated with the pathogenesis of psoriasis. Although several studies have shown that treatments for H. pylori infection could alleviate the clinical symptoms of psoriasis, some studies have produced controversial results [13,14,15].
Thus, this meta-analysis was conducted to estimate the relationship between H. pylori infection and psoriasis, aiming to provide more reliable evidences for clinical psoriasis treatment decision.
2. Materials and Methods
2.1. Search Strategy
We systematically searched Pubmed, Web of Science, Embase, and China National Knowledge Infrastructure (CNKI) for all the relevant studies investigating the association between H. pylori infection and psoriasis published up to July 2019. There were no restrictions on language or publication type. The following search terms were used: (psoriases or psoriasis) and (H. pylori or Helicobacter pylori). The complete search strategy was included in Table S1. Reference lists of identified articles were also manually searched for other relevant articles.
2.2. Selection Criteria
Two independent reviewers reviewed the results of the preliminary searches. Studies were included if they (1) were cohort studies, cross-sectional studies, or case–control studies that assessed the relationship between psoriasis and H. pylori infection; (2) compared at least two groups, including (a) patients with any type of psoriasis diagnosed by clinical manifestations or pathology and (b) participants without psoriasis recruited from hospitals or communities; (3) the full text was available; (4) reported the data of the H. pylori infection rates in the participants with or without psoriasis; (5) diagnosed H. pylori infection by histology, IgG ELISA test, urea breath test, or stool antigen test.
Studies were excluded if they (1) were conference summaries, case reports, reviews, letters, or comments; (2) included the study participants with a specific disease (e.g., cardiovascular diseases, kidney diseases, etc.) or patients who had taken antibiotics, proton pump inhibitors, antacids, or glucocorticoids the preceding 2 weeks; (3) had incomplete or missing information.
In case of duplicate reports, or studies clearly reported results from the same study population. We chose the latest or most complete one.
2.3. Data Extraction and Quality Assessment
Two reviewers (Yu and Ni) used the standardized data abstraction sheets to extract the data independently. Disagreements were resolved by consulting a third reviewer (Zh). The following information from each article was extracted: the first author’s name, published year, location, study design, baseline features of the involved participants, the Psoriasis Area and Severity Index of the patients, and outcomes of H. pylori infection test. The authors were contacted if we could not obtain the required information in original articles.
The Newcastle–Ottawa Scale (NOS) [16] was used to estimate the quality of each included study. The scale scores ranged from 0 to 9. We considered the higher the score, the better the quality of the study. The NOS score of 0–3 was considered poor, while 4–6 was fair and 7–10 was good quality. Two independent reviewers completed the entire quality assessment. Consensus on quality assessment was reached via discussion.
2.4. Statistical Analysis
This meta-analysis was conducted by Review Manager 5.3 and Stata 12.0. We analyzed the outcomes that included incidence of H. pylori infection between psoriasis and control groups and expressed them as an odds ratio (OR) with a 95% confidence interval (CI). P < 0.05 was considered as statistically significant. Heterogeneity among studies was evaluated via chi-square tests and the inconsistency statistic. The levels of heterogeneity assessed by I2 were as follows: 0–25% meant homogeneity; 25–50% meant low heterogeneity; 50–75% meant moderate heterogeneity, and >75% meant high heterogeneity [17]. The random-effects model was used when I2 > 50%, otherwise, the fixed-effects model was adopted. Subgroup analyses were conducted by locations and the methods of H. pylori detection. In addition, potential publication bias was assessed by Begg’s and Egger’s tests. P values < 0.05 were considered statistically significant.
3. Results
3.1. Literature Search and Study Characteristics
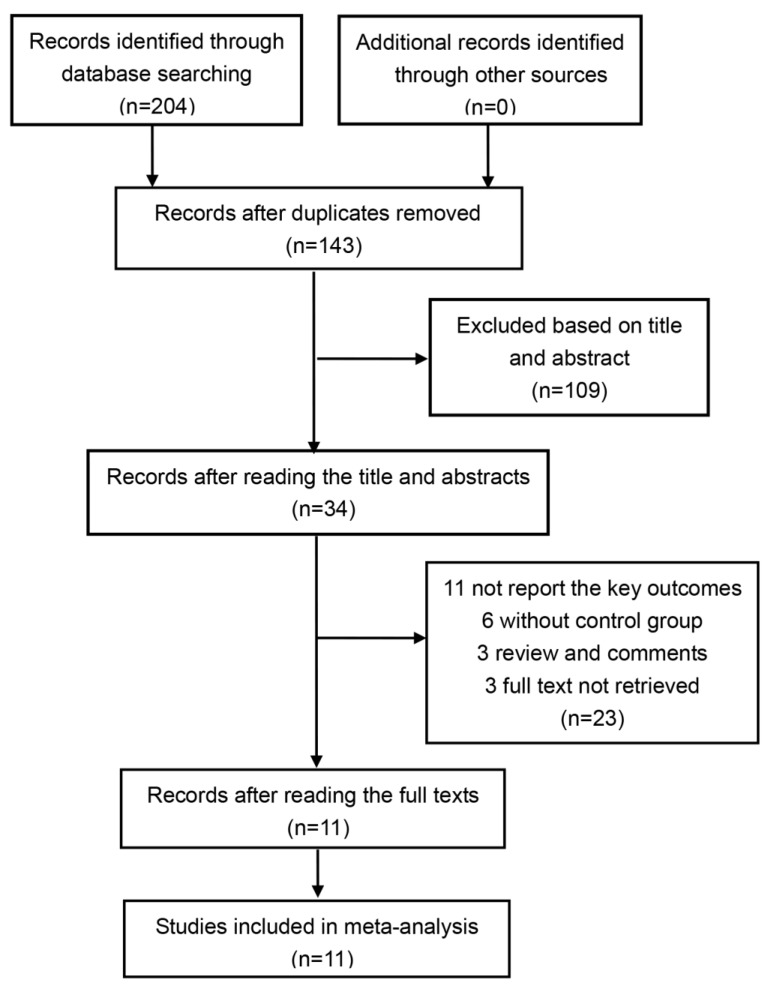
The initial database search generated 204 articles, 61 of which were excluded because of duplicates. After reading the title and abstract, 109 records were excluded via the initial screening. We then screened the whole 34 articles and finally included 11 studies for analysis (1741 participants) [18,19,20,21,22,23,24,25,26,27,28]. Figure 1 details the selection and exclusion process.
Figure 1.
Flowchart of the study selection.
Among the 11 studies included in the meta-analysis, three were cohort study [19,21,27], four were cross-sectional study [20,23,24,25] and four were case–control study [18,22,26,28]. The publication year of the involved studies varied from 2000 [18] to 2017 [27]. The number of the participants in each study ranged from 40 [20] to 450 [21]. Among all the studies, five were from Asia [21,24,25,26,28], four from Europe [18,19,22,23], one from South America [27], and one from Africa [20]. As for the detection method of H. pylori, five studies used the IgG ELISA test [20,23,24,26,27] and five studies used the urea breath test [18,19,22,25,28], while Onsun N et al. [21] used the stool antigen test. All of the included studies were graded as moderate and good quality. Table 1 details the characteristics of each included study.
Table 1.
Characteristics of studies included in the meta-analysis.
| Study (Year) | Location | Study Design | Total Cases (Patients/Control) | Women (%) | Mean Age | Mean PASI | Outcomes | NOS Quality Assessment Score |
|---|---|---|---|---|---|---|---|---|
| Dauden E 2000 [18] | Spain (Europe) | Case-control | 165 (84/81) | NA | NA | NA | Positive urea breath test | 5 |
| Campanati A 2015 [19] | Italy (Europe) | Cohort study | 360 (210/150) | 48.1 | 49.75 | 14.56 ± 4.35 | Positive urea breath test | 9 |
| Ghada F 2010 [20] | Egypt (Africa) | Cross-sectional study | 40 (20/20) | 20 | 26–55 | PASI < 15 = 3 PASI 15-25 = 7 PASI > 25 = 10 |
Positive H.pylori IgG ELISA test | 9 |
| Onsun N 2012 [21] | Turkey (Asia) | Cohort study | 450 (300/150) | 49 | 41.65 | 3.94 ± 4.99 | Positive stool antigen test | 7 |
| Fabrizi G 2001 [22] | Italy (Europe) | Case-control | 49 (20/29) | 44.9 | 5~19 | NA | Positive urea breath test | 6 |
| Zhelezova G 2015 [23] | Bulgaria (Europe) | Cross-sectional study | 49 (25/24) | 32 | 52.2 | NA | Positive H.pylori IgG ELISA test | 7 |
| Qayoom S 2003 [24] | India (Asia) | Cross-sectional study | 100 (50/50) | 44 | 5–60 | NA | Positive H.pylori IgG ELISA test | 8 |
| Türkmen D 2011 [25] | Turkey (Asia) | Cross-sectional study | 113 (56/57) | 42.9 | 38.4 | 5.89 | Positive urea breath test | 8 |
| Azizzadeh M 2014 [26] | Iran (Asia) | Case-control | 122 (61/61) | 54 | 33.3 | 6.6 ± 3.1 | Positive H.pylori IgG ELISA test | 7 |
| Mesquita P 2017 [27] | Brazil (South America) | Cohort study | 147 (126/21) | 57.9 | 50.48 | PASI < 5 = 21 PASI 5-10 = 40 PASI > 10 = 65 |
Positive H.pylori IgG ELISA test | 7 |
| Xunsheng X 2013 [28] | China (Asia) | Case-control | 146 (86/60) | 41 | 16.5–70.5 | 17.42 ± 3.43 | Positive urea breath test | 7 |
NA not available; NOS Newcastle-Ottawa Scale; IgG ELISA IgG enzyme linked immunosorbent.
3.2. Psoriasis and H. pylori Infection Rates
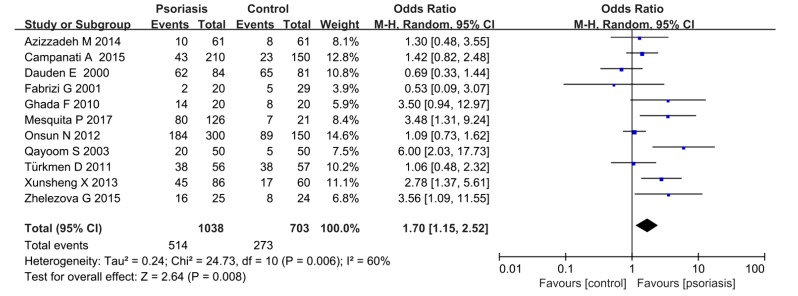
Among the involved cases, 1038 patients were in the psoriasis group and 703 in the control group. The Mantel–Haensze random-effects model was used because of the moderate heterogeneity (I2 = 60%). The H. pylori infection rates in the psoriasis group were 49.5%, while they were 38.8% in the control group. The pooled OR was 1.70 (95% CI 1.15–2.52, P = 0.008) (Figure 2).
Figure 2.
H. pylori infection in patients with or without psoriasis.
3.3. Subgroup Analysis
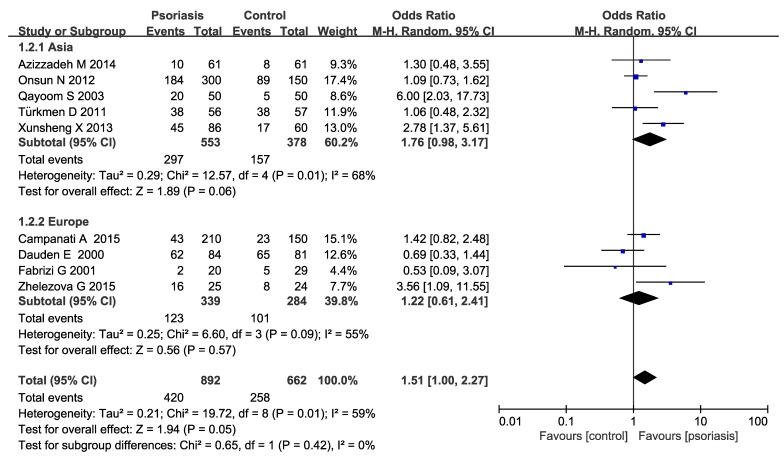
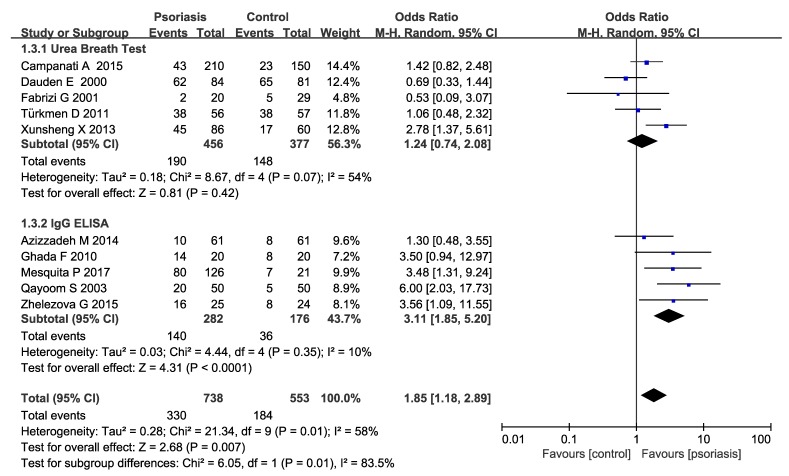
Subgroup analysis were conducted in our meta-analysis based on the following aspects: locations (Asia or Europe) (Figure 3), the methods of H. pylori detection (Figure 4) and the severity of psoriasis according to PASI (mean PASI ≤ 10 mild; mean PASI ≥ 10 moderate and severe; Figure 5). The subgroup analyses of locations showed that there were no significant increases in both the Asia and Europe groups (Asia pooled OR = 1.76, 95% CI 0.98–3.17, P = 0.06; Europe OR = 1.22 95% CI 0.61–2.41, P = 0.57), suggesting that region is not an influencing factor. Ghada et al. and Mesquita et al’s studies were not included in the regional subgroup analysis, since the relevant data were only from one literature. As for the methods of H. pylori detection, the pooled OR was 1.24 (95% CI 0.74–2.08) in urea breath test subgroup, while 3.11 (95% CI 1.85–5.20) in the IgG ELISA test group, indicating a statistically significant increase of H. pylori infection in IgG ELISA test group. The severity of psoriasis showed a statistically significant increase of H. pylori infection in moderate and severe psoriasis patients (OR=2.27; 95% CI: 1.42–3.63, I2 = 27%) but not in the mild psoriasis patients (OR=1.10; 95% CI: 0.79–1.54, I2 = 0%).
Figure 3.
Sub-analysis according to region.
Figure 4.
Sub-analysis according to the H. pylori detection methods.
Figure 5.
Sub-analysis according to the severity of psoriasis.
3.4. Sensitivity Analyses and Publication Bias
By removing one study at a time, we found that none of the studies changed the pooled risk of the H. pylori infection rates substantially. In addition, we found no significant publication bias as assessed by the Begg’s test (P = 0.350) and the Egger’s test (P = 0.154).
4. Discussion
In this meta-analysis, the results showed the incidence of H. pylori in patients with psoriasis was 10.7% higher than that of the control group (pooled RR = 1.70, P < 0.01), which indicated that psoriasis might be associated with H. pylori infection. In the regional subgroup analysis, there were no significant increases in both Asia and Europe groups. With respect to the methods of H. pylori detection, there was a statistically significant increase of H. pylori infection in the IgG ELISA test group compared with the urea breath test group. As for the severity of psoriasis, the infection rate of H. pylori was higher in patients with moderate and severe psoriasis compared with the patients with mild psoriasis, indicating that H. pylori infection and the severity of psoriasis required more attention in clinical treatment.
The heterogeneity was moderate in this study (I2 = 60%), and regression analysis showed that different detection methods were not the source of heterogeneity. However, due to the small number of eligible literatures included in our study, it was not yet certain that different detection methods were not the sources of heterogeneity. In this meta-analysis, urea breath test, stool antigen test, and IgG ELISA test were used to detect H. pylori infection. It has been reported that the urea breath test was currently the best method for detecting H. pylori, with high accuracy and easy operation, but there might be false positive or false negative results. The stool antigen test has good sensitivity and specificity, which can detect the existing infection of H. pylori. The accuracy of this method is considered to be equal to that of the UBT, but currently there is a lack of reagents for the detection of monoclonal antibody with high accuracy. The IgG ELISA test can reflect H. pylori infection over a period of time, meanwhile, it is not affected by recent medication or local gastric lesions. At the same time, although IgG ELISA is not useful for treating patients in clinical practice, it is useful in epidemiological studies. The cause in this case is not relevant to the actual presence of the bacterium, but to the presence during the life [29]. However, H. pylori antibody positive cannot determine the current infection, and antibody negative cannot rule out the initial infection either [30,31,32,33]. Each of the three methods has its own advantages and disadvantages, and different methods might have a certain impact on the results of the study. It is expected that there can be uniform and accurate detection methods to provide more evidence in the future. On the other hand, subgroup analysis suggested that the severity of psoriasis might be a source of heterogeneity. I2 was significantly reduced in the mild group and the moderate and severe group (I2 = 0%, I2 = 27%, respectively). However, due to the limited literatures that conform to this study, more studies are needed to provide evidence of higher quality.
Our results indicate increased H. pylori infection in patients with psoriasis. The potential pathogenesis is as follows: (i) Chronic inflammation. Chronic inflammation caused by H. pylori might result in the release of certain cytokines and activation of immune cells, which might induce other immune-related pathologies [34]. (ii) Heat–shock protein leading to increased production of IL-6. Heat–shock protein (HSP) in H. pylori mediates macrophage-induced release of IL–6, which might lead to infiltration of cytokines from the gastric mucosa [35]. Previous studies have shown that psoriasis might also be mediated by IL–6 [36], which provided a possible relationship between these two diseases. (iii) Cytotoxin-associated gene A (CagA). CagA can cause host cell secretion of IL–8, and IL–8 plays an important role in the formation of psoriasis. IL–8 has chemotaxis to neutrophils and T cells. Meanwhile, it can promote generation of new blood vessels and proliferation of horniness cells [37]. Therefore, H. pylori infection might play a role in the pathogenesis of psoriasis vulgaris by stimulating the immune response of the organism and inducing the production of a large amount of IL–8. In addition, the pathogenesis of psoriasis might be similar to the pathogenesis of H. pylori infection and other skin disorders, for example, the release of vascular mediators like histamines might be the triggering factor for the development of rosacea [7].
The present study proved a significant association between psoriasis and H. pylori infection, in accordance with a previous study by Yong et al [38]. Yong et al did not include children in their study. In contrast to the abovementioned meta-analysis, we conducted subgroup analyses according to the region, H. pylori detection methods, and the severity of psoriasis to explore the corresponding relationship in depth. However, our meta-analysis also has some limitations. First, Scopus was not included in the literature retrieval, and the study finally included only 11 literatures, which might influence the credibility of the outcomes. Next, the variety of population characteristics may lead to clinical heterogeneity. Although subgroup analysis was conducted for breaking through this limitation, its influence may still be incompletely controlled. In addition, not all the studies included the severity of psoriasis, and our analysis did not consider the effect of age and gender factors. The credibility of the evidence can be improved via well-designed meta-analysis in the future, when more high-quality clinical trials are available.
5. Conclusions
Current evidence indicates that H. pylori infection is associated with psoriasis, and psoriasis patients with H. pylori infection may have a higher Psoriasis Area and Severity Index (PASI) score. Future studies should focalize not only on H. pylori infection but also on the role of gut microbiota (plus Helicobacter) in the pathogenesis of psoriasis.
Supplementary Materials
The following are available online at https://www.mdpi.com/1010-660X/55/10/645/s1. Table S1: Systematic literature review search terms and strategy.
Author Contributions
Data curation: M.Y., P.N.; Formal analysis: M.Y.; Funding acquisition: R.Z.; Methodology: S.C.; Project administration: R.Z.; Software: M.Y., P.N.; Supervision: R.Z., G.D.; Writing-original draft: M.Y.; Writing-review & editing: R.Z.
Funding
This study was funded by National Natural Science Foundation of China (81773495).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Burucoa C., Axon A. Epidemiology of Helicobacter pylori infection. Helicobacter. 2017;22:1. doi: 10.1111/hel.12403. [DOI] [PubMed] [Google Scholar]
- 2.Pellicano R., Ribaldone D.G., Fagoonee S., Astegiano M., Saracco G.M., Mégraud F. A 2016 panorama of Helicobacter pylori infection: Key messages for clinicians. Panminerva Med. 2016;58:304–317. [PubMed] [Google Scholar]
- 3.Wang F., Meng W., Wang B., Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P., Megraud F., O’Morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., Bazzoli F., Gasbarrini A., Atherton J., Graham D.Y., et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2016;6:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 5.Leone N., Pellicano R., Brunello F., Cutufia M.A., Berrutti M., Fagoonee S., Rizzetto M., Ponzetto A. Helicobacter pylori seroprevalence in patients with cirrhosis of the liver and hepatocellular carcinoma. Cancer Detect. Prev. 2003;27:494–497. doi: 10.1016/j.cdp.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 6.de Korwin J.D., Ianiro G., Gibiino G., Gasbarrini A. Helicobacter pylori infection and extragastric diseases in 2017. Helicobacter. 2017;22:1. doi: 10.1111/hel.12411. [DOI] [PubMed] [Google Scholar]
- 7.Kutlubay Z., Zara T., Engin B., Serdaroğlu S., Tüzün Y., Yilmaz E., Eren B. Helicobacter pylori infection and skin disorders. Hong Kong Med. J. 2014;20:317–324. doi: 10.12809/hkmj134174. [DOI] [PubMed] [Google Scholar]
- 8.Magen E., Delgado J.S. Helicobacter pylori and skin autoimmune diseases. World J. Gastroenterol. 2014;20:1510–1516. doi: 10.3748/wjg.v20.i6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorgensen A.R., Egeberg A., Gideonsson R., Weinstock L.B., Thyssen E.P., Thyssen J.P. Rosacea is associated with Helicobacter pylori: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2017;31:2010–2015. doi: 10.1111/jdv.14352. [DOI] [PubMed] [Google Scholar]
- 10.Bochenska K., Smolinska E., Moskot M., Jakóbkiewicz-Banecka J., Gabig-Cimińska M. Models in the Research Process of Psoriasis. Int. J. Mol. Sci. 2017;18:2514. doi: 10.3390/ijms18122514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahil S.K., Capon F., Barker J.N. Update on psoriasis immunopathogenesis and targeted immunotherapy. Semin. Immunopathol. 2016;38:11–27. doi: 10.1007/s00281-015-0539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alwan W., Nestle F.O. Pathogenesis and treatment of psoriasis: Exploiting pathophysiological pathways for precision medicine. Clin. Exp. Rheumatol. 2015;33:S2–S6. [PubMed] [Google Scholar]
- 13.Dauden E., Cabrera M.M., Onate M.J., Pajares J.M., García-Díez A. CagA seropositivity in Helicobacter pylori positive patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 2004;18:116–117. doi: 10.1111/j.1468-3083.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- 14.Ali M., Whitehead M. Clearance of chronic psoriasis after eradication therapy for Helicobacter pylori infection. J. Eur. Acad. Dermatol. Venereol. 2008;22:753–754. doi: 10.1111/j.1468-3083.2007.02452.x. [DOI] [PubMed] [Google Scholar]
- 15.Tuzun Y., Keskin S., Kote E. The role of Helicobacter pylori infection in skin diseases: Facts and controversies. Clin. Dermatol. 2010;28:478–482. doi: 10.1016/j.clindermatol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Wells G.S.B., O’Connell D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Ottawa Health Research Institute; Ottawa, ON, Canada: 2019. [Google Scholar]
- 17.Higgins J.P.T., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dauden E., Vazquez-Carrasco M.A., Penas P.F., Pajares J.M., García-Díez A. Association of Helicobacter pylori infection with psoriasis and lichen planus: prevalence and effect of eradication therapy. Arch. Dermatol. 2000;136:1275–1276. doi: 10.1001/archderm.136.10.1275. [DOI] [PubMed] [Google Scholar]
- 19.Campanati A., Ganzetti G., Martina E., Giannoni M., Gesuita R., Bendia E., Giuliodori K., Sandroni L., Offidani A. Helicobacter pylori infection in psoriasis: Results of a clinical study and review of the literature. Int. J. Dermatol. 2015;54:109–114. doi: 10.1111/ijd.12798. [DOI] [PubMed] [Google Scholar]
- 20.Ghada F., Mohamed S., Sameh M., Sanad H. Helicobacter Pylori Infection: A Possible Predisposing Factor in Chronic Plaque-Type Psoriasis. J. Egypt. Women Dermatol. Soc. 2010;7:39–43. [Google Scholar]
- 21.Onsun N., Arda U.H., Su O., Beycan I., Biyik Ozkaya D., Senocak M. Impact of Helicobacter pylori infection on severity of psoriasis and response to treatment. Eur. J. Dermatol. 2012;22:117–120. doi: 10.1684/ejd.2011.1579. [DOI] [PubMed] [Google Scholar]
- 22.Fabrizi G., Carbone A., Lippi M.E., Anti M., Gasbarrini G. Lack of evidence of relationship between Helicobacter pylori infection and psoriasis in childhood. Arch. Dermatol. 2001;137:1529. [PubMed] [Google Scholar]
- 23.Zhelezova G., Yocheva L., Tserovska L., Mateev G., Vassileva S. Prevalence of Helicobacter pylori seropositivity in patients with psoriasis. Probl. Infect. Parasit. Dis. 2015;43:13–17. [Google Scholar]
- 24.Qayoom S., Ahamd Q.M. Psoriasis and Helicobacter pylori. Indian J. Dermatol. Venereol. Leprol. 2003;69:133–134. [PubMed] [Google Scholar]
- 25.Türkmen D., Özcan H., Kekilli E. Relation between psoriasis and Helicobacter pylori. Turk Dermatol. Derg. 2011;5:39–42. doi: 10.5152/tdd.2011.09. [DOI] [Google Scholar]
- 26.Azizzadeh M., Nejad Z.V., Ghorbani R., Pahlevan D. Relationship between Helicobacter pylori infection and psoriasis. Ann. Saudi Med. 2014;34:241–244. doi: 10.5144/0256-4947.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesquita P.M.D., Jorge M.T., Mantese S.A., Berbert A.L., Rodrigues J.J. Relationship of Helicobacter pylori seroprevalence with the occurrence and severity of psoriasis. An. Bras. Dermatol. 2017;92:52–57. doi: 10.1590/abd1806-4841.20174880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xunsheng X., Xiaohong S. Correlation between Helicobacter pylori infection and psoriasis vulgaris. Chin. J. Hosp. Inf. Dis. 2013;5:1100–1102. [Google Scholar]
- 29.Pellicano R., Fagoonee S., Rizzetto M., Ponzetto A. Helicobacter pylori and coronary heart disease: Which directions for future studies? Crit. Rev. Microbiol. 2003;29:351–359. doi: 10.1080/713608015. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y.K., Kuo F.C., Liu C.J., Wu M.C., Shih H.Y., Wang S.S., Wu J.Y., Kuo C.H. Diagnosis of Helicobacter pylori infection: Current options and developments. World J. Gastroenterol. 2015;21:11221–11235. doi: 10.3748/wjg.v21.i40.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes A.I., Vale F.F., Oleastro M. Helicobacter pylori infection-recent developments in diagnosis. World J. Gastroenterol. 2014;20:9299–9313. doi: 10.3748/wjg.v20.i28.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel S.K., Pratap C.B., Jain A.K., Gulati A.K., Nath G. Diagnosis of Helicobacter pylori: What should be the gold standard? World J. Gastroenterol. 2014;20:12847–12859. doi: 10.3748/wjg.v20.i36.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Best L.M., Takwoingi Y., Siddique S., Selladurai A., Gandhi A., Low B., Yaghoobi M., Gurusamy K.S. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst. Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mejias-Luque R., Gerhard M. Immune Evasion Strategies and Persistence of Helicobacter pylori. Curr. Top. Microbiol. Immunol. 2017;400:53–71. doi: 10.1007/978-3-319-50520-6_3. [DOI] [PubMed] [Google Scholar]
- 35.Radic M. Role of Helicobacter pylori infection in autoimmune systemic rheumatic diseases. World J. Gastroenterol. 2014;20:12839–12846. doi: 10.3748/wjg.v20.i36.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J., Zhao Y., Hu J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1 beta and IL-6 production in mice. PLoS ONE. 2013;8:e67078. doi: 10.1371/journal.pone.0067078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cinar L., Kartal D., Gur G. The effect of Helicobacter pylori eradication on psoriasis course. OA Text. 2017;4:1–4. [Google Scholar]
- 38.Yong W.C., Upala S., Sanguankeo A. Association between Psoriasis and Helicobacter pylori Infection: A Systematic Review and Meta-analysis. Indian J. Dermatol. 2018;63:193–200. doi: 10.4103/ijd.IJD_531_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.