Abstract
Neural stem cells (NSCs) play an important role in neural tissue engineering because of their capacity of self-renewal and differentiation to multiple cell lineages. The in vitro conventional neurosphere culture protocol has some limitations such as limited nutrition and oxygen penetration and distribution causing the heterogeneity of cells inside, inaccessibility of internal cells, and inhomogeneous cellular morphology and properties. As a result, cultivation as a monolayer is a better way to study NSCs and obtain a homogeneous cell population. The cadherins are a classical family of homophilic cell adhesion molecules mediating cell-cell adhesion. Here, we used a recombinant human E-cadherin mouse IgG Fc chimera protein that self-assembles on a hydrophobic polystyrene surface via hydrophobic interaction to obtain an E-cadherin-coated culture plate (ECP). The rat fetal NSCs were cultured on the ECP and routine tissue culture plate (TCP) from passage 2 to passage 5. NSCs on TCP formed uniform floating neurospheres and grew up over time, while cells on the ECP adhered on the bottom of the plate and exhibited individual cells with scattering morphology, forming intercellular connections between cells. The cell proliferation and differentiation behaviors that were evaluated by Cell Counting Kit-8 assay (CCK-8), immunofluorescence staining, and real-time quantitative polymerase chain reaction showed NSCs could maintain the capacity for self-renewal and ability to differentiate into neurons, oligodendrocytes, and astrocytes after the long-term in vitro cell culture and passaging. Therefore, our study indicated that hE-cad-Fc could provide a homogeneous environment for individual cells in monolayer conditions to maintain the capacity of self-renewal and differentiation by mimicking the cell–cell interaction.
1. Introduction
Neural stem cells (NSCs) have the capacity for self-renewal and can generate multiple cell lineages including neurons, astrocytes, and oligodendrocytes, accounting for the growth of the developing brain and the regenerative potential of the adult brain in vivo.1−3 The in vitro isolation, expansion, and differentiation of NSCs play an important role in understanding the biological properties of stem cells and provide promising solutions for stem-cell-mediated regenerative medicine, such as treatments for neurodegenerative diseases and spinal cord injury.4,5
Cultivation as free-floating aggregates, known as neurospheres, is a classical approach for expanding NSCs, which is considered to be a more natural environment for the cells because of the three-dimensional niche-like structure.6 Many protocols for culturing NSCs in vitro adopted the method of neurospheres.7−9 However, this method also has some significant limitations. First, the tightly packed neurosphere interiors limit nutrition and oxygen penetration and distribution, which will affect the accuracy of some studies such as proliferation assays and studies of the effects of growth factors and other reagents on cell survival, growth, and differentiation.10 Second, the cells inside the neurospheres cannot be visualized, making it hard to determine their morphology and compare the cell-specific properties.11 In addition, the culture method brings some technical difficulties such as loading uniform numbers of spheres with uniform size into individual wells for high-throughput screening and performing uniform differentiation.12
To solve these problems, culturing NSCs as a monolayer has been investigated to precisely control the in vitro milieu of each cell and obtain a rather homogeneous undifferentiated cell population, which is suitable for studying the properties of cells at the individual cell level. The most common way to perform the monolayer culture is by coating the culture substrates with peptides derived from the extracellular matrix (ECM) such as laminin, fibronectin, and Matrigel, and other substances such as poly-l-ornithine and poly-l-lysine.13−15 D’Aiuto et al. used monolayer cultures of human-iPSC-derived NSCs on Matrigel-coated plates to obtain large-scale generation of neurons, which was ideal for high-throughput screening.12 Xiao et al. also used plates coated with Matrigel for the expansion of NSCs.14 Ray and Gage cultured NSCs from mice and rats on PORN-laminin-coated plastic plates to investigate and compare the cell-specific properties, where the monolayer culture contributed to accurately evaluate cell shape, morphology, and other characteristics.11 Blaschke et al. grew primary rat NSCs as monolayers on polydimechylsiloxane-based gels coated with l-poly-ornithine to study the effects of elastic substrates on NSC functions.16 Similarly, Vay et al. applied l-poly-ornithine and bovine fibronectin to precoat culture dishes to obtain homogeneous and undifferentiated NSCs for further characterization.17 Compared with our study, these attempts to culture NSCs in monolayer conditions relied on signals mediated by cell-substrate contacts with the local ECM. Though these signals could expand NSC cultures and promote neuronal differentiation, they are different from the cell–cell contacts that the cell received in the niche in vivo and in neurospheres in vitro, which are generally provided by cell adhesion molecules between cells such as the cadherin family.
Epithelial cadherin (E-cadherin), which is a member of the cadherin superfamily, a group of cell surface glycoproteins that mediate calcium-dependent cellular adhesion and communications, has been identified as a key factor in cell–cell adhesion by means of β-catenin to participate in signal transduction pathways.18,19 Studies have revealed that cadherin-based substrates such as E-cadherin and N-cadherin could maintain stem cell features.20,21 Embryonic stem (ES) cells were cultured on plates coated with a fusion protein of human E-cadherin and IgG Fc domain (hE-cad-Fc), showing that the cells retained the pluripotency and differentiation features of ES cells.22 The hE-cad-Fc protein was also proved to promote the survival ability and the paracrine function of the human umbilical cord blood-derived stem cell and enhance the adhesion and proliferation of human mesenchymal stem cells (hMSCs).23,24 E-cadherin has been certified to be expressed by NSCs and regulate NSC self-renewal both in vivo and in vitro.25,26 However, few studies were about E-cadherin in the monolayer culture of NSCs. Therefore, our study first investigated the long-term effect of the hE-cad-Fc fusion protein on the self-renewal and differentiation capacities of NSCs via a monolayer culture method, which provided a promising way for a homogeneous cell population harvest and precise cell study.
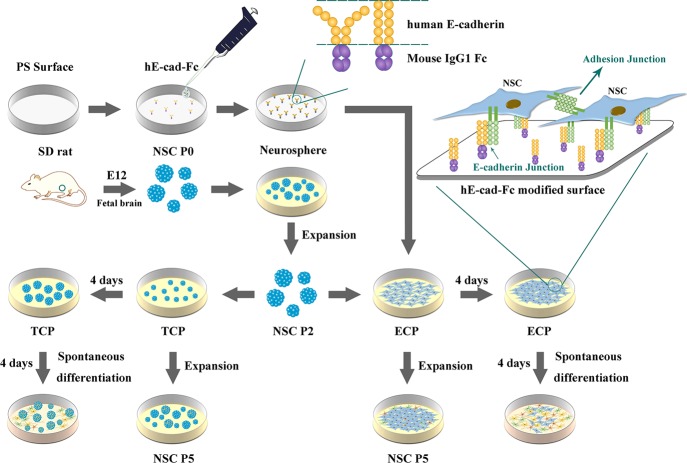
In this study, we applied the human E-cadherin mouse IgG Fc chimera protein (hE-cad-Fc) on the polystyrene (PS) surface to obtain the E-cadherin-coated culture plate (ECP) for the NSC monolayer attachment by mimicking the cell–cell interaction. The properties of the ECP were identified through an optical contact angle meter and by atomic force microscopy (AFM). Primary rat fetal NSCs isolated from the fetal brain cortex of Sprague–Dawley (SD) rats were first expanded on the tissue culture plate (TCP) as neurospheres and then cultured on both the TCP and ECP to compare the cell growth and spontaneous differentiation. Both cell cultures were maintained through five passages and characterized by real-time fluorescence quantitative polymerase chain reaction (RT-PCR). To identify the maintenance of self-renewal ability on the ECP, Cell Counting Kit-8 (CCK-8) was used to detect the cell growth and proliferation of each generation of NSCs cultured on the ECP. Additionally, NSCs of passage 5 (P5) obtained from the ECP were cultured both on the TCP and ECP again and observed by immunofluorescence to identify the stemness for a long-term culture.
2. Materials and Methods
All experiments involving animals were performed in accordance with the Guide for the Care and Use of Laboratory Animals from the Chinese Ministry of Public Health and U.S. National Institutes of Health.
2.1. Preparation of ECPs
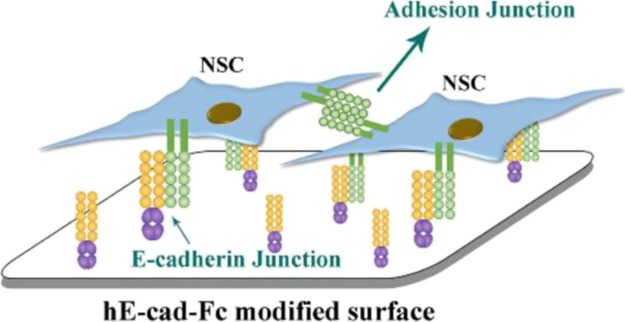
The recombinant human E-cadherin mouse IgG Fc chimera protein (hE-cad-Fc) solution with a concentration of 439.5 μg/mL was kindly provided by SOMAR Corporation (Japan), with a purity of over 82% by SDS-PAGE and was then diluted with sterile phosphate-buffered saline (PBS) with 900 μM of calcium to obtain concentrations of 1.25, 2.5, 5, 10, 15, 20, 25, 30, and 40 μg/mL. ECPs were prepared as described previously.23 Briefly, the diluted hE-cad-Fc solution was added to nontreated PS plates (Iwaki, Japan). The volume of solution was 3 mL for a 3 cm-diameter culture plate and 100 μL for one well of a 48-well plate. The plates were shaken by hands until the solutions covered the bottom of the plates, and then the plates were incubated for 2 h at room temperature. Prior to use, the hE-cad-Fc solution was aspirated and discarded, and the plates were rinsed with PBS with 900 μM of calcium (Figure 1).
Figure 1.
Schematic representation of the study design and the behavior of NSCs on the hE-cad-Fc-modified surface.
After air-drying for 24 h at room temperature, the water contact angle of the modified surface was analyzed by an optical contact angle meter. The topography of the modified surface was characterized by AFM (Bruker Dimension ICON, Billerica, MA). The scan area was 4 μm × 4 μm, and the scan rate was 1.00 Hz.
2.2. NSC Isolation and Culture
NSCs were isolated from Sprague–Dawley rats at gestational day E12 according to a previous protocol.27,28 Briefly, embryonic rat cerebral cortices were separated, dissected, cut into small pieces, and then transferred into cold PBS and digested with 2.5% EDTA/trypsin at 37 °C for 5 min. The dissociated cells were collected by centrifugation and resuspended in a serum-free medium containing neurobasal medium, 2% B27 supplement, 1% glutamine, 20 ng/mL epidermal growth factor (EGF), 20 ng/mL basic fibroblast growth factor (bFGF), and 1% penicillin–streptomycin (Gibco, USA). The cerebral cortical NSCs, taken as passage 0 (P0), were purified and cultured in a 6-well culture plate (Corning, USA) and maintained at 37 °C in a humidified atmosphere containing 5% CO2. When the cells began forming neurospheres, the original culture medium was replaced with a complete culture medium containing DMEM-F12, 2% B27 supplement, 1% N2 supplement, 20 ng/mL EGF, 20 ng/mL bFGF, and 1% PS. After 5 days, the proliferating cells formed huge neurospheres and then the adherent cells on the bottom were discarded. When the neurospheres were large with the center in dark under optical microscopy indicating the cells in the center were not growing well, or some neurospheres began to adhere on the bottom of culture plate, they needed to be passaged immediately. Normally, the NSCs were passaged every 2–3 days. The neurospheres were collected, dissociated to individual cells by mechanical digestion, and cultured in a new T75 culture flask in the fresh complete culture medium. The individual cells grew into new neurospheres in 5 days, and the procedure of the subculture was repeated again to obtain NSCs in passage 2 (P2) for further use. Immunofluorescence staining was used to identify the stemness of the NSCs.
To identify the optimal concentration of hE-cad-Fc for the adhesion and growth of NSCs, the cells were cultured on the ECP with different concentrations and observed with a light microscope (Leica, Germany) everyday for three days. Moreover, CCK-8 assay was performed to observe the proliferation of NSCs on the ECP at day 1, 2, and 3. The optimal concentration was used for further experiments.
To examine the differentiation capacity of NSCs grown on the ECP compared with the TCP, NSCs (P2) were cultured in the TCP and ECP, respectively, at a density of 2 × 104 cells/cm2 in the complete culture medium described above and incubated for 4 days at 37 °C. Then, the spontaneous differentiation of NSCs was induced by applying the culture medium without the EGF and bFGF (differentiation culture medium) for another 4 days. The cells were observed with a light microscope everyday and the culture medium was changed every 3 days. At day 8, the cells were collected for immunofluorescence staining and the RT-PCR.
For the long-term identification of stemness maintenance, NSCs (P2) were then cultured in the TCP and ECP for three passages to obtain NSCs of passage 5 [NSCs (P5)], and the cells were collected for the RT-PCR. The passaging of NSCs was carried out every 5 days. In addition, NSCs on the ECP of each generation from P2 to P5 were used to perform CCK-8 assay to identify the proliferation capacity with the increase of the subculture. NSCs (P5) on ECP were again cultured on TCP and ECP, and immunofluorescence staining was applied.
2.3. Cell Proliferation Assay
For CCK-8 assay, the NSCs of each passage obtained on the ECP were seeded into 48-well E-cad-coated plates at a density of 3 × 104 cells per well (n = 4). At day 1, 3, 5, and 7, the cultured cells were incubated with 300 μL of 10% CCK-8 working solution in the complete culture medium for 2 h at 37 °C in the dark. Then, 100 μL of the supernatant was extracted to a new 96-well plate, and the optical density (OD) value at 450 nm was measured by an EnSpire Multimode Plate Reader (PerkinElmer, USA).
2.4. Immunofluorescence Staining
All samples were fixed with 4% formaldehyde for 20 min and then washed three times in PBS. Then, the fixed cells were permeabilized with 0.1% Triton-X in PBS for 5 min and later washed with PBS three times, and the cells were then blocked with 10% goat serum (Gibco, USA) in PBS for 30 min at room temperature. After removal of the blocking reagent, the cells were incubated with primary antibody dilution, including rabbit anti-Nestin (1:250, ab92391, Abcam), mouse anti-GFAP (1:300, 3670S, CST), rabbit anti-β-tubulin III (1:1000, ab18207, Abcam), rabbit anti-MAP2 (1:1000, ab32454, Abcam), mouse anti-Oligodencyte Marker O4 (1:50, O7139, Sigma), and mouse anti-NF (1:800, Abcam) at 4 °C overnight. The secondary antibodies, goat anti-rabbit IgG H&L (Alexa Fluor 594, 1:200, ab150084, Abcam) and goat anti-mouse IgG H&L (Alexa Fluor 488, 1:200, ab150117, Abcam), were used to react with the cells for 1 h at room temperature. Then, the cells were washed with PBS, and the nucleus was stained with DAPI for 5 min. The samples were visualized by indirect fluorescence under the fluorescent microscope (Leica, Germany), confocal fluorescence microscopy (Zeiss LSM 780, Germany), or fluorescence microscopy Olympus IX81 (Olympus, Japan).
2.5. Real-Time Fluorescence Quantitative PCR
Total mRNA was obtained by the miRcute miRNA Isolation Kit (DP501, Tiangen, Beijing, China) and then reverse-transcribed to cDNA using the FastQuant RT Kit (KR-106, Tiangen, Beijing, China). RT-PCR was performed on the CFX96 Real-Time System (Bio-Rad Laboratories, Inc. China) with iTaq Universal SYBR Green Supermix (172-5122, Bio-Rad Laboratories, Inc. China). The primer pairs used for amplification are listed in Table S1. Three replicate samples were analyzed for each gene (n = 3). Each sample was measured three times, and the average was calculated to serve as the expression level of the gene in the sample. Relative gene expression levels were determined by the 2–ΔΔCt method, and the target genes were normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene as an internal control.
2.6. Statistical Analysis
All numerical data were presented as mean ± standard deviation (SD). SPSS 23.0 software (SPSS Inc., Chicago, IL, USA) was used for all statistical procedures. Statistical comparisons were performed with the t-test for independent samples or one-way analysis of variance for multigroup comparisons, followed by Tukey’s HSD post hoc test (equal variances) or Dunnett’s T3 post hoc test (unequal variances). The values were considered to be significantly different, when the p-value was less than 0.05.
3. Results
3.1. Preparation and Characterization of the E-Cadherin-Coated Substrate
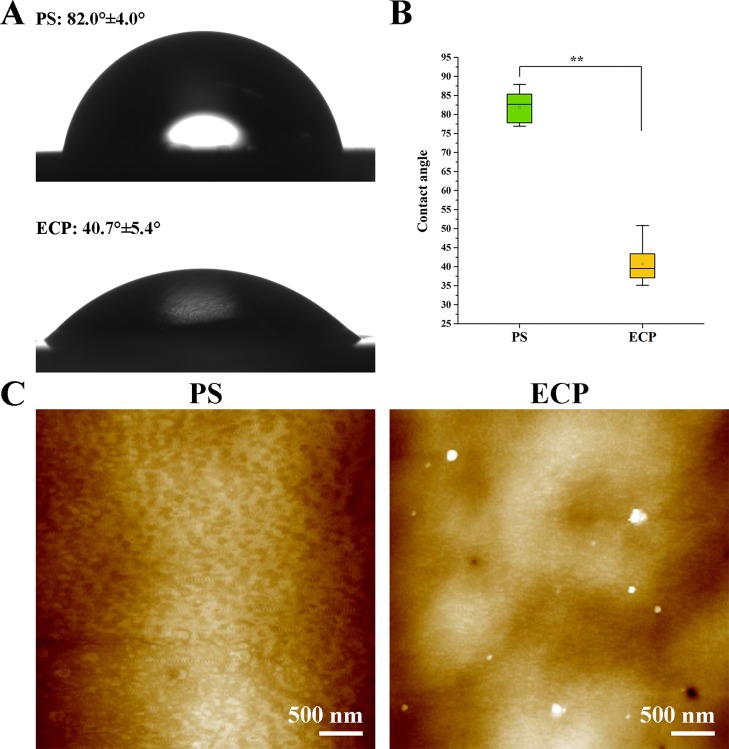
The water contact angle of ECP (40.7° ± 5.4°) was significantly smaller than that of the PS surface (82.0° ± 4.0°), as shown in Figure 2A,B. The topography of the ECP investigated by AFM (Figure 2C) showed uniformly distributed nanostructures with the height of around 10 nm on average compared with the PS surface, which was smooth. These results indicated that hE-cad-Fc was effectively immobilized on the PS surface and could enhance the surface wettability.
Figure 2.
Characterization of the hE-cad-Fc-modified surface. (A) Water contact angles and (B) statistical comparison of PS and ECP. (C) Topography of PS and ECP. The data were reported as mean ± SD, n = 5. *p < 0.05, **p < 0.01.
3.2. Adhesion and Proliferation of NSCs in Response to Different Concentrations of hE-Cad-Fc
The purity of isolated NSCs plays an important role in this study because the cells taken from the cortexes of the fetal SD rats may contain neurons, oligodendrocytes, or astrocytes which could affect the results of differentiation. Expression of the NSC marker Nestin, differentiating neuron markers MAP2 and β-tubulin III, and the astrocyte marker GFAP was examined and analyzed with immunofluorescence staining, as shown in Figure S1. All images were acquired with the same conditions. Although most of the cells stain positive for Nestin, very few of the cells were positive for MAP2, β-tubulin III, and GFAP, which indicated that the isolated NSCs had high purity and could be used for further experiments.
As shown in Figure S2, most NSCs aggregated and formed neurospheres at day 3 when cultured on the ECP with concentrations of 0, 1.25, 2.5, and 5 μg/mL hE-cad-Fc. However, when the concentrations were higher than 10 μg/mL, almost all the NSCs were dispersed, and in monolayer conditions at day 1, there were no significant changes in adhesion with the concentrations increase. CCK-8 results indicated that NSCs grew the fastest and had the highest OD value at day 3 on the ECP with 15 μg/mL of hE-cad-Fc (Figure S3). Therefore, a concentration of 15 μg/mL was better for NSC adhesion and proliferation and used for further experiments in this study.
3.3. Adhesion, Proliferation, and Differentiation of NSCs (P2)
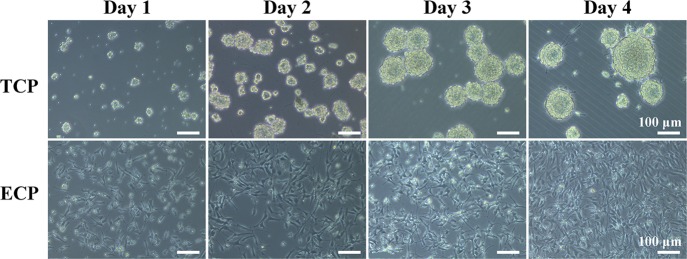
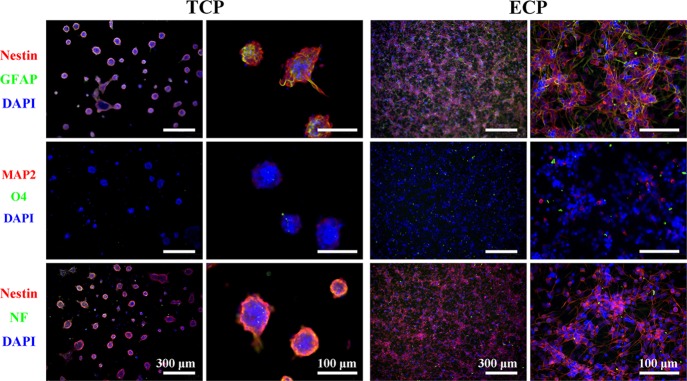
NCSs (P2) were cultured on the TCP and ECP for 8 days. In the first 4 days, they were provided with the complete culture medium with growth factors, and the cells in both kinds of plates showed a good capacity for self-renewal indicated by everyday observation with a light microscope, as shown in Figure 3. In the TCP, the cells grew to neurospheres and the size and shape of neurospheres were very uniform at day 3. At day 4, some neurospheres grew so big that the cells started stretching out from the spheres, which was usually a sign for the subculture, and then the differentiation culture medium was applied. In the ECP, NSCs adhered on the bottom of the plate, exhibited individual cells with scattering morphology, and intercellular connections were formed between the cell-like network through their protrusions, which was different from the cells on the TCP. According to the pictures of NSCs by light microscopy in Figure 3, the diameters of neurospheres on the TCP and the density of NSCs on the ECP were increasing in 4 days obviously, indicating that cells on both the TCP and ECP exhibited fast proliferation.
Figure 3.
Cell adhesion and morphology of NSCs (P2) on the TCP and ECP at day 1, 2, 3, and 4.
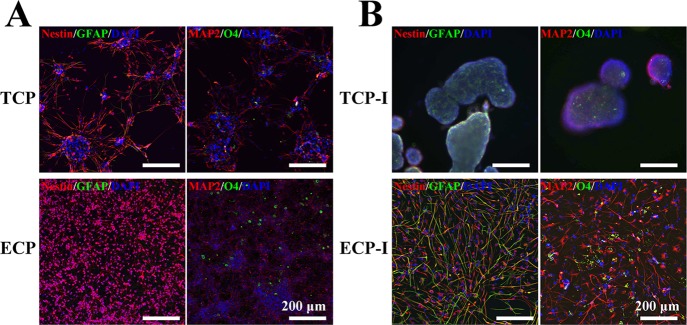
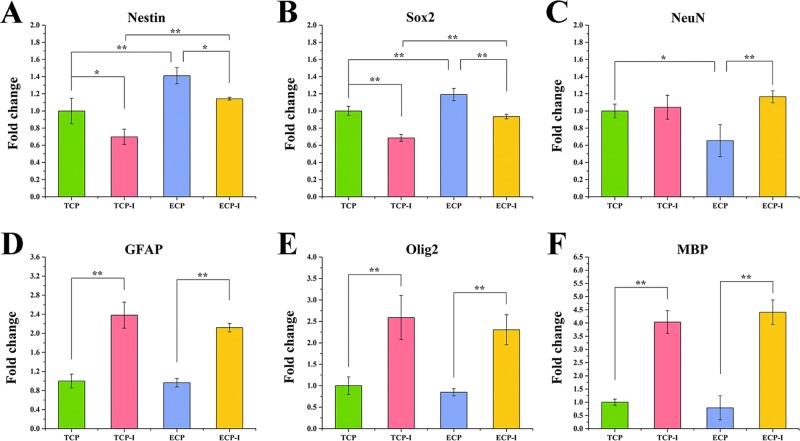
Under EGF and bFGF withdrawal, NSCs on both the TCP and ECP can differentiate into neurons, oligodendrocytes, and astrocytes. After spontaneous differentiation for 4 days, NSCs on the TCP and ECP were stained for the undifferentiated NSC marker Nestin, neuronal marker MAP2, astrocyte marker GFAP, and oligodendrocyte marker O4, as shown in Figure 4A,B, where “TCP” and “ECP” indicated cells supplied with the complete culture medium for all 8 days, while the “TCP-I” and “ECP-I” indicated cells induced by the differentiation culture medium. On the TCP, large numbers of cells migrated out from the neurospheres and grew axons with the increasing culture time, which showed a similar morphology as cells on the ECP. Before differentiation, NSCs showed a higher expression of Nestin and almost no expression of GFAP, MAP2, and O4 on the TCP and ECP, indicating the good stemness maintenance for up to 8 days. After differentiation, cells of the TCP-I and ECP-I exhibited a higher positive staining of MAP2, GFAP, and O4 and lower of Nestin compared with undifferentiated cells, which demonstrated that cells had differentiated into neurons, oligodendrocytes, or astrocytes. This result indicated that NSCs cultured on the ECP could maintain the pluripotency to differentiate into all three neural subtypes. In addition, NSCs of the TCP-I differentiated still in the form of neurospheres, which made it hard to identify the changes of individual cells inside and the percentage of a certain cell type. However, the immunofluorescence staining in Figure 4B showed the NSC (in red), astrocyte (in green), neuron (in red), and oligodendrocyte (in green) and exhibited individual distribution without clear aggregation and inhomogeneity, indicating that different kinds of cells on the ECP could get a relatively uniform distribution after differentiation.
Figure 4.
Immunofluorescence staining of undifferentiated (A) and differentiated (B) NSCs (P2) after the 8-day culture with Nestin (red, NSC marker), MAP2 (red, neuronal marker), GFAP (green, astrocyte marker), and O4 (green, oligodendrocyte marker). The nuclei were stained with DAPI (blue).
Real-time RT-PCR was conducted to measure the relevant gene expression levels in NSCs on the TCP, TCP-I, ECP, and ECP-I after spontaneous differentiation at day 8, where TCP was regarded as a control (Figure 5). The gene expression of Nestin and Sox2 (for NSCs) in the TCP-I and ECP-I exhibited significant downregulation compared with TCP and ECP, while GFAP (for astrocytes), Olig2 (for oligodendrocytes), and MBP (for mature oligodendrocytes) exhibited significant upregulation compared with the TCP and ECP. The expression of NeuN (for neurons) on the TCP was not significantly different from that on TCP-I, but the expression on ECP-I was significantly greater than that on the ECP. These results were consistent with the immunofluorescence staining results. It is worth noting that the expressions of Nestin and Sox2 of NSCs on the ECP were significantly higher than those on the TCP, and the expression of NeuN was lower, indicating that the ECP might have a better performance on maintaining the stemness of NSCs when cultured for up to 8 days.
Figure 5.
Gene expression of neural specific markers in NSCs (P2) cultured on the ECP and TCP, including Nestin (A) and Sox2 (B) for NSCs, NeuN (C) for neurons, GFAP (D) for astrocytes, and Olig2 (E) and MBP (F) for oligodendrocytes. The expression was normalized to GAPDH. *p < 0.05, **p < 0.01.
3.4. Proliferation Capacity of NSCs after Passaging
CCK-8 results of NSCs on the ECP are shown in Figure 6. From passage 2 to passage 5, NSCs exhibited the capacity of proliferation, while the efficiency was different among generations, referred to as NSCs (P2), NSCs (P3), NSCs (P4), and NSCs (P5). The quantities of cells cultured on the ECP initially were controlled to be equal, and the OD values were measured at day 1, 3, 5, and 7. At day 1, there was no significant difference among the four groups. However, the OD value of NSCs (P5) was significantly lower than those of NSCs (P2) and NSCs (P4) at day 3, 5, and 7. Although the OD value of NSCs (P3) was significantly lower than that of NSCs (P5) at day 3, NSCs (P3) had a faster proliferation than NSCs (P5), which was identified by a significantly higher OD value at day 7. NSCs (P2) had the highest OD values at day 3, 5, and 7, which were significantly different from other groups. The OD values of NSCs (P3) and NSCs (P4) were not significantly different at day 5 and 7. The results indicated that NSCs on the ECP could maintain a good capacity of proliferation, however, decreasing with subcultures.
Figure 6.
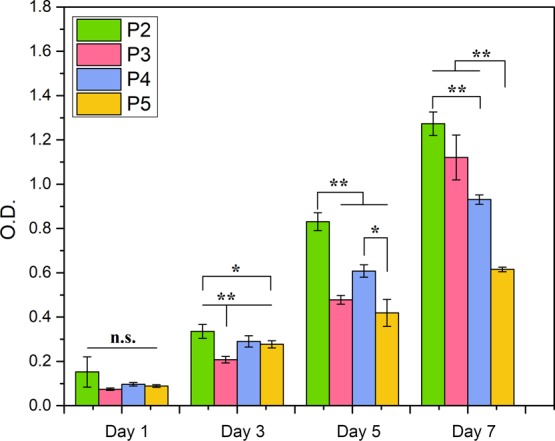
Proliferation capacity of NSCs of different generations on the ECP.
3.5. Characteristics of NSCs (P5)
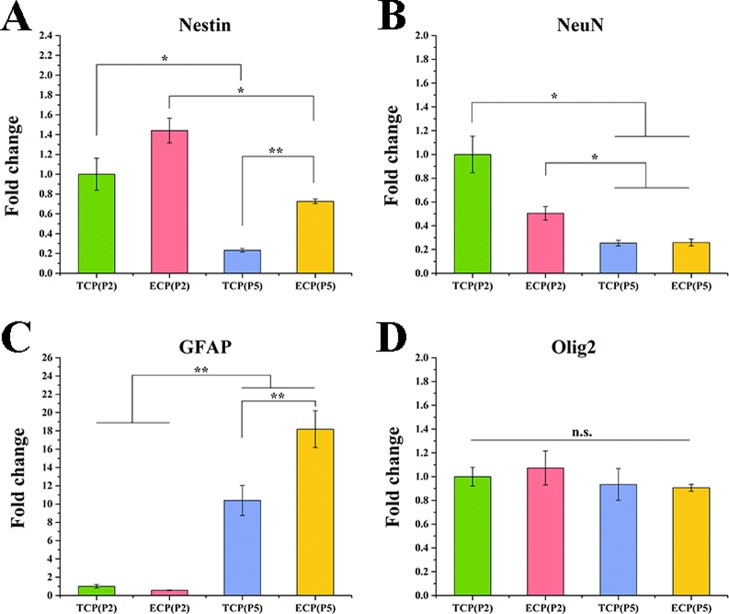
NSCs (P5) obtained from the TCP and ECP were analyzed by the RT-PCR, as shown in Figure 7. The TCP (P2) and ECP (P2) groups represented NSCs (P2) on the TCP and ECP, respectively. TCP (P5) and ECP (P5) groups represented NSCs (P5) on the TCP and ECP, respectively. The expressions of Nestin and NeuN in the TCP (P5) and ECP (P5) were significantly reduced, while the expressions of GFAP were significantly improved, compared with those of TCP (P5) and ECP (P5), respectively. There were no significant differences of the Olig2 expression among the four groups, indicating an increasing inclination toward astrocytes rather than neurons and oligodendrocytes of NSCs during generations. Moreover, the expression of Nestin in ECP (P5) was significantly higher than that in TCP (P5), indicating better stemness maintenance of NSCs on the ECP than the TCP after the same generations of passaging.
Figure 7.
Gene expressions of neural specific markers in NSCs (P5) cultured on the ECP and TCP, including Nestin (A) for NSCs, NeuN (B) for neurons, GFAP (C) for astrocytes, and Olig2 (D) for oligodendrocytes. The expression was normalized to GAPDH. *p < 0.05, **p < 0.01.
To better identify the properties of NSCs (P5) on the ECP, they were again cultured on both the TCP and ECP to observe if NSCs maintained a good capacity of self-renewal according to immunofluorescence staining (Figure 8). It was obvious that NSCs (P5) could still grow to form neurospheres. Most of the cells were still positive for the Nestin marker no matter whether on the TCP or ECP. However, cells positive for GFAP were more than those in Figure 4A, which were consistent with the results of RT-PCR.
Figure 8.
Phenotype marker expressions of NSCs (P5) on the ECP and TCP, including Nestin for NSCs, MAP2 and NF for neurons, GFAP for astrocytes, and O4 for oligodendrocytes. The nuclei were stained with DAPI (blue).
4. Discussion
Recently, optimizing protocols for stable proliferation and long-term stemness maintenance is one of the central goals of stem cell research.29 NSCs on the ordinary tissue culture plastic dish TCP incline to form neurospheres in serum-free media supplemented with the EGF and bFGF. The sizes of the neurospheres are hard to control, which are usually not uniform, especially for the long-term culture. Moreover, the heterogeneity is ineluctable because the cells inside the spheres can be exposed to suboptimal conditions, which are different from the cells outside that can contact with the culture medium directly.30 Therefore, each cell of the neurosphere is likely to locate in different cellular microenvironments, which makes it difficult to get a large population of undifferentiated stem cells, as the interaction with differentiating cells can expose NSCs to paracrine factors that promote differentiation.29 However, the monolayer culture of NSCs has its own unique advantages in some respects which can provide a solution to the above problems. On the one hand, the construction of massive homogeneous cells without affecting the self-renewal and differentiation capacities can be achieved because of the uniform exposure to growth factors in the culture medium. On the other hand, each cell can be directly monitored under monolayer conditions, which is important to study the behavior of stem cells.31
In the present study, we used a recombinant human E-cadherin mouse IgG Fc chimera protein (hE-cad-Fc) that self-assembles on a hydrophobic PS surface via the hydrophobic interaction to prepare an artificial two-dimensional substrate for the NSC monolayer culture by mimicking the cell–cell interaction in vivo and inside neurospheres. The protein was applied onto the PS surface at a suitable concentration and evaluated in terms of the effects on the stemness maintenance and differentiation capacity of NSCs for a long-term culture compared with the routine neurosphere culture on the TCP. According to the results of the light microscope, NSCs exhibited a good adherent morphology in the monolayer condition on the E-cadherin-based substrate, while the cells formed different sizes of neurospheres on the TCP. The proliferation of NSCs was in the form of higher cell density on the ECP while larger neurospheres on the TCP. At first, the neurospheres were relatively uniform in size, but they preferred to stick together and some of the neurospheres grew larger than others. On day 4, large neurospheres began to adhere onto the substrate with the central color darkening, and cells grew from the inside, which was more obvious when cultured continuously. However, the cells on the ECP maintained the monolayer condition with a neat arrangement even for a long term. These results were an indication that the cells in neurospheres were in a heterogeneous microenvironment while those in the monolayer condition were in a relatively homogeneous microenvironment, which could be one explanation for the stemness maintenance on the ECP. The RT-PCR results showed that the NSCs could maintain self-renewal capacity on the E-cadherin-based substrate as well as on regular culture plates and could differentiate to neurons, oligodendrocytes, or astrocytes spontaneously. Compared with the TCP, the higher expressions of Nestin and Sox2 and the lower expression of NeuN on the ECP after 8 days indicated better stemness maintenance for a long-term culture at a certain generation of NSCs. The expression of NeuN in differentiated cells on the ECP was significantly higher than that of undifferentiated cells, but the difference between differentiated and undifferentiated cells on the TCP was not significant, which indicated that the ECP may promote some degree of neuronal differentiation.
To evaluate the long-term effect of the ECP on stemness maintenance, NSCs were cultured for five passages because the NSCs used in various studies were commonly under ten passages, in which the cells could maintain relatively high stemness.32,33 In addition, it was found in our experiments that the morphology uniformity and proliferation rate of the NSCs went down with the increase of passaging especially after five passages (data not shown). The aim of our study was to obtain a homogeneous population of NSCs with the capacities of self-renewal and differentiation for further studies and applications of NSCs. Thus, the cells should be strictly in good condition; otherwise, the results would be affected and would not accurately reflect the role of our material. Therefore, the NSCs under five passages had better biological properties and were enough for observing the long-term stemness for the E-cadherin-based substrate. Compared with NSCs at passage 2, cells at passage 5 on both the TCP and ECP exhibited a decreased expression of Nestin and NeuN, but cells on the ECP showed a significantly higher expression of Nestin than those on the TCP. Moreover, NSCs cultured on the ECP from passage 2 to passage 5 could maintain a good proliferation capacity, and even the cells at passage 5 could form Nestin-positive neurospheres and monolayers, indicating the stemness maintenance for the long-term continuous passage culture. From the above two aspects, the hE-cad-Fc protein could contribute to maintain the self-renewal and differentiation capacities of NSCs in the monolayer condition for a long term, compared to the neurosphere culture. However, the expression of GFAP at passage 5 was higher than that at passage 2 on both substrates, possibly caused by the accumulation of astrocytes during passaging because no inducible factor was used for the differentiation of NSCs.
The choice of 15 μg/mL of the hE-cad-Fc protein was based on both the previous study and our result in Figure S2, in which the cells exhibited good adhesion and proliferation. The immobilization of the hE-cad-Fc protein increased with the concentration rising from 0.1 to 10 μg/mL and became constant from 10 to 30 μg/mL, which indicated that hE-cad-Fc achieved saturation at this concentration. The protein was also proved to have long-term binding stability on the PS surface for at least 5 days.23 The promotion of cell adhesion with the concentration of hE-cad-Fc increase was observed through the light microscope, during which the attached phenotype of NSCs exhibited gradual alterations from the neurosphere to the single cell, as shown in Figure S2. As we only changed the concentration of hE-cad-Fc with other conditions unchanged, we think that the concentration of hE-cad-Fc should be a key factor for NSC adhesion. The general range of the hE-cadherin-Fc concentration was also determined based on the results of immobilization and cell adhesion under different concentrations in the study by Xu.24 However, the adhesion mechanism of NSCs on the E-cadherin-based substrate was not shown and will be investigated in the future. According to their studies, the adhesion of the hMSCs on hE-cadherin-Fc was markedly inhibited compared with the PS surface when an anti-human-E-cadherin antibody was used as a blocker, indicating that the adhesion of hMSCs on the modified surface was mediated by the E-cadherin domain. In addition, the E-cadherin expression in human bone marrow mesenchymal stem cells (hMSCs) on hE-cadherin-Fc was distinctly higher than that on the TCP, indicating that the hE-cadherin-Fc matrix could not only enhance cell adhesion but simultaneously facilitate the expression of E-cadherin. We think it is probably the same with NSCs. The adhesion of cells was achieved via the combination of endogenous E-cadherin and exogenous E-cadherin. The cells could not adhere to the PS surface unless there were E-cadherin domains on the PS surface even with high endogenous E-cadherin. Because the protein immobilization efficiency increased with concentration, more NSCs would adhere on the modified surface with a higher concentration. As a result, the adhesion behavior is influenced by different concentrations of hE-cad-Fc on the substrate. The in-depth mechanism including how the exogenous E-cadherin affects the expression of endogenous E-cadherin of NSCs will be investigated in our subsequent work.
The attempts to culture NSCs in monolayer conditions were also carried out by other studies. Sun et al. characterized the proliferative, differentiative, and passaging capacities of neural stem and progenitor cells (NSPCs) on adherent substrates coated with poly-d-lysine and fibronectin in detail.32 Results showed that adherent NSPCs grew significantly faster than neurospheres in the P1 and P3 passages, but the self-renewal capacity in the adherent culture reduced after the P5 passage, and the growth of cells within neurospheres was slower than that in the adherent culture. Laminin has been used for the monolayer culture of NSCs for many years, which was identified to play important roles in mouse/human NSC propagation and neuronal differentiation, allowing for niche-independent symmetric self-renewal of NSCs.34,35 Sun et al. found that the laminin substrate with EGF and FGF2 could promote the continuous proliferation of human NSCs during one-year expansion, and switching the laminin substrate to gelatin or uncoated substrates could cause cell detachment and neurosphere formation.36 Different from E-cadherin we used in this study, laminins and fibronectins are both proteins of the ECM, related to cell adhesion, growth, and migration.37,38 Poly-d-lysine and poly-ornithine are also chemically synthesized ECM used to facilitate cell adhesion via mimicking cell–matrix interactions.39 The survival, proliferation, self-renewal, and differentiation of NSCs involve a number of processes that require not only cell–matrix interactions but also cell–cell interactions.40 E-cadherin plays an important role in mediating calcium-dependent, homophilic cell–cell adhesion in all epithelial tissues including embryonic stem cells (ESCs).41 In addition, Karpowicz et al. confirmed the presence of E-cadherin in neurospheres via immunocytochemistry and found that the disruption or loss of E-cadherin attachment reduced NSC self-renewal in vivo and in vitro.25 Therefore, the use of the E-cadherin-based substrate could commendably mimic the cell–cell interaction among NSCs in niche and in neurospheres. On the one hand, the advantages of the monolayer culture can be retained, which contributes to expose all cells in the homogeneous microenvironment containing nutrition and growth factors, avoiding the gathering of differentiation paracrine factors within neurospheres. On the other hand, E-cadherin plays a role of maintaining and promoting the self-renewal capacity via its biological characteristics. Our results showed that NSCs on the ECP maintained good self-renewal capacity from passage 2 to passage 5, indicating that E-cadherin could perform well as conventional coating components. In our study, the differentiation of NSCs on the ECP (monolayer culture) and TCP (neurosphere culture) was not obviously different according to the immunofluorescence results, indicating that the culture on the ECP did not affect the spontaneous differentiation of NSCs. However, more quantitative tests are needed to evaluate the differentiation properties under two kinds of culture conditions. Moreover, NSCs of passage 5 either on the TCP or ECP exhibited lower Nestin and NeuN expressions and higher GFAP expression, indicating the inclination of the differentiation to astrocytes, under which circumstance we suggest using earlier passages of NSCs in some strict studies.
The mechanism is being investigated. On the E-cadherin-coated substrate, NSCs exhibited scattering morphologies, which was in correspondence with the ESC and human bone marrow mesenchymal stem cells cultured under the same condition. E-cadherin has long extracellular and cytoplasmic domains, which can establish interactions between cells and at the same time associate with intracellular adaptor proteins such as β-catenin and p120-catenin directly and α-catenin indirectly. Then, the intracellular adaptor proteins link cell–cell adhesion to the actin-myosin network and other intracellular signaling pathways.41 A previous study reported that activity of RhoA and Rac1 contributed to the filopodia extension of mouse ESCs and F9 embryonal carcinoma cells on the E-cadherin surface.42,43 According to the known effects of E-cadherin, we can assume that the homogeneous interaction between cells and coated E-cadherin changes the cytoskeleton via catenin and promotes the activity of RhoA and Rac1, leading to cell scattering, which needs further studies.
Besides the simple culture of cells on plates, ECM proteins and cadherins can also be used to modify complex scaffolds and allow stem cells to grow better for tissue engineering.40 N-cadherin is also a member of the cell adhesion molecule expressed on the surface of NSCs which is involved in cell migration and possesses the ability to promote the growth and differentiation of stem cells. Fusion chimeras of N-cadherin-Fc were used to coat polymer thin films and electrospun scaffolds alone and in combination with L1-Fc. H9 NSCs were cultured on the scaffolds and the neuronal differentiation, neurite outgrowth, and survival during oxidative stress were promoted.44 Self-assembled peptide hydrogels were prepared with N-cadherin mimetic peptide conjugation, which could enhance the chondrogenesis and osteogenesis of hMSCs.45,46 Furthermore, a poly(3,4-ethylenedioxythiophene)/chitosan/gelatin scaffold was designed and modified with laminin to grow NSCs inside. Results showed that the scaffold could promote NSC adhesion and proliferation and enhance differentiation into neurons and astrocytes.33 Similarly, E-cadherin can be applied to the modified natural or synthetic materials to promote the self-renewal and differentiation properties of NSCs and other stem cells in the scaffold, which is of great significance for cell therapy and tissue engineering. Multicellular aggregates constructed with human E-cadherin fusion protein-coated PLGA microparticles could enhance the adhesion and hepatic-specific differentiation of hMSCs, providing a promising method for the liver disease therapy and other endoderm-derived organ construction.47
5. Conclusions
We successfully established the E-cadherin-based substrate through the hydrophobic interaction with hydrophobic PS surfaces for the long-time monolayer culture of NSCs. The hE-cad-Fc protein facilitated the generation of homogeneous population of NSCs growing in the same adherent environment. The cells on the ECP could maintain a good self-renewal capacity after the 8-day culture and passaging, and the cells maintained the ability to differentiate into neurons, oligodendrocytes, and astrocytes. Moreover, the recombinant human E-cadherin mouse IgG Fc chimera protein is a promising choice for the monolayer culture of NSCs and has a potential for maintaining the self-renewal of stem cells in engineered nerve tissue grafts via mimicking the cell–cell interaction.
Acknowledgments
We acknowledge the financial support from the National Key R&D Program of China (grant 2018YFB0704304), the National Natural Science Foundation of China (no. 31771056), and the Tsinghua University Initiative Scientific Research Program (no. 20161080091). We thank Kumiko Matsunaga from SOMAR Corporation SAT Dept. for kindly providing us with the recombinant human E-cadherin mouse Fc chimera protein. We also thank Yue Sun and Jingjing Wang at Center of Biomedical Analysis, Tsinghua University for the help with confocal microscopy.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02053.
Phenotype marker expressions of isolated NSCs for the purity examination, adhesion of NSCs on different concentrations of hE-cad-Fc, proliferation of NSCs on different concentrations of hE-cad-Fc, and sequence of primers for RT-PCR (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Temple S. The development of neural stem cells. Nature 2001, 414, 112–117. 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Gage F. H. Mammalian neural stem cells. Science 2000, 287, 1433. 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Huang L.; Zhang L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog. Neurobiol. 2019, 173, 1–17. 10.1016/j.pneurobio.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. U.; Lee H. J.; Kim Y. B. Neural stem cell-based treatment for neurodegenerative diseases. Neuropathology 2013, 33, 491–504. 10.1111/neup.12020. [DOI] [PubMed] [Google Scholar]
- Rosenzweig E. S.; Brock J. H.; Lu P.; Kumamaru H.; Salegio E. A.; Kadoya K.; Weber J. L.; Liang J. J.; Moseanko R.; Hawbecker S.; Huie J. R.; Havton L. A.; Nout-Lomas Y. S.; Ferguson A. R.; Beattie M. S.; Bresnahan J. C.; Tuszynski M. H. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. 10.1038/nm.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleyrolle L. P.; Reynolds B. A. Isolation, expansion, and differentiation of adult Mammalian neural stem and progenitor cells using the neurosphere assay. Methods Mol. Biol. 2009, 549, 91–101. 10.1007/978-1-60327-931-4_7. [DOI] [PubMed] [Google Scholar]
- Pauly M. G.; Krajka V.; Stengel F.; Seibler P.; Klein C.; Capetian P. Adherent vs. Free-Floating Neural Induction by Dual SMAD Inhibition for Neurosphere Cultures Derived from Human Induced Pluripotent Stem Cells. Front. Cell. Dev. Biol. 2018, 6, 3. 10.3389/fcell.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H.; Hara M. Clusters of neural stem/progenitor cells cultured on a soft poly(vinyl alcohol) hydrogel crosslinked by gamma irradiation. J. Biosci. Bioeng. 2016, 121, 584–590. 10.1016/j.jbiosc.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Shin J.; Choi E. J.; Cho J. H.; Cho A.-N.; Jin Y.; Yang K.; Song C.; Cho S.-W. Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules 2017, 18, 3060–3072. 10.1021/acs.biomac.7b00568. [DOI] [PubMed] [Google Scholar]
- Svendsen C. N.; Skepper J.; Rosser A. E.; ter Borg M. G.; Tyres P.; Ryken T. Restricted growth potential of rat neural precursors as compared to mouse. Dev. Brain Res. 1997, 99, 253. 10.1016/s0165-3806(97)00002-3. [DOI] [PubMed] [Google Scholar]
- Ray J.; Gage F. H. Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol. Cell. Neurosci. 2006, 31, 560–573. 10.1016/j.mcn.2005.11.010. [DOI] [PubMed] [Google Scholar]
- D’Aiuto L.; Zhi Y.; Kumar Das D.; Wilcox M. R.; Johnson J. W.; McClain L.; MacDonald M. L.; Di Maio R.; Schurdak M. E.; Piazza P.; Viggiano L.; Sweet R.; Kinchington P. R.; Bhattacharjee A. G.; Yolken R.; Nimgaonka V. L.; Nimgaonkar V. L. Large-scale generation of human iPSC-derived neural stem cells/early neural progenitor cells and their neuronal differentiation. Organogenesis 2014, 10, 365–377. 10.1080/15476278.2015.1011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C. I.; Yoon H.; Drucker K. L.; Langley M. R.; Kleppe L.; Scarisbrick I. A. The Thrombin Receptor Restricts Subventricular Zone Neural Stem Cell Expansion and Differentiation. Sci. Rep. 2018, 8, 9360. 10.1038/s41598-018-27613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X.; Putatunda R.; Zhang Y.; Soni P. V.; Li F.; Zhang T.; Xin M.; Luo J. J.; Bethea J. R.; Cheng Y.; Hu W. Lymphotoxin beta receptor-mediated NFkappaB signaling promotes glial lineage differentiation and inhibits neuronal lineage differentiation in mouse brain neural stem/progenitor cells. J. Neuroinflammation 2018, 15, 49. 10.1186/s12974-018-1074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L.; Cao L.; Shen Z.; Shao L.; Gao S.; Zhang C.; Lu J.; Li W. Materials for Neural Differentiation, Trans-Differentiation, and Modeling of Neurological Disease. Adv. Mater. 2018, 30, e1705684 10.1002/adma.201705684. [DOI] [PubMed] [Google Scholar]
- Blaschke S.; Vay S. U.; Pallast N.; Rabenstein M.; Abraham J. A.; Linnartz C.; Hoffmann M.; Hersch N.; Merkel R.; Hoffmann B.; Fink G. R.; Rueger M. A. Substrate elasticity induces quiescence and promotes neurogenesis of primary neural stem cells-A biophysical in vitro model of the physiological cerebral milieu. J. Tissue Eng. Regener. Med. 2019, 13, 960–972. 10.1002/term.2838. [DOI] [PubMed] [Google Scholar]
- Pellegrino G.; Trubert C.; Terrien J.; Pifferi F.; Leroy D.; Loyens A.; Migaud M.; Baroncini M.; Maurage C.-A.; Fontaine C.; Prévot V.; Sharif A. A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and gray mouse lemur (Microcebus murinus). J. Comp. Neurol. 2018, 526, 1419–1443. 10.1002/cne.24376. [DOI] [PubMed] [Google Scholar]
- Angst B. D.; Marcozzi C.; Magee A. I. The cadherin superfamily: diversity in form and function. J. Cell Sci. 2001, 114, 629–641. 10.1023/A:1005628808688. [DOI] [PubMed] [Google Scholar]
- van Roy F.; Berx G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 2008, 65, 3756–3788. 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X.-S.; Murakami Y.; Tamai T.; Nagaoka M.; Cho C.-S.; Ito Y.; Akaike T. A fusion protein N-cadherin-Fc as an artificial extracellular matrix surface for maintenance of stem cell features. Biomaterials 2010, 31, 5287–5296. 10.1016/j.biomaterials.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Haque A.; Hexig B.; Meng Q.; Hossain S.; Nagaoka M.; Akaike T. The effect of recombinant E-cadherin substratum on the differentiation of endoderm-derived hepatocyte-like cells from embryonic stem cells. Biomaterials 2011, 32, 2032–2042. 10.1016/j.biomaterials.2010.11.045. [DOI] [PubMed] [Google Scholar]
- Nagaoka M.; Koshimizu U.; Yuasa S.; Hattori F.; Chen H.; Tanaka T.; Okabe M.; Fukuda K.; Akaike T. E-cadherin-coated plates maintain pluripotent ES cells without colony formation. PLoS One 2006, 1, e15 10.1371/journal.pone.0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Mao H.; Qian M.; Hu F.; Cao L.; Xu K.; Shuai Q.; Gao C.; Lang R.; Akaike T.; Yang J. Surface modification with E-cadherin fusion protein for mesenchymal stem cell culture. J. Mater. Chem. B 2016, 4, 4267–4277. 10.1039/c6tb00765a. [DOI] [PubMed] [Google Scholar]
- Xu J.; Zhu C.; Zhang Y.; Jiang N.; Li S.; Su Z.; Akaike T.; Yang J. hE-cadherin-Fc fusion protein coated surface enhances the adhesion and proliferation of human mesenchymal stem cells. Colloids Surf., B 2013, 109, 97–102. 10.1016/j.colsurfb.2013.03.042. [DOI] [PubMed] [Google Scholar]
- Karpowicz P.; Willaime-Morawek S.; Balenci L.; DeVeale B.; Inoue T.; van der Kooy D. E-Cadherin regulates neural stem cell self-renewal. J. Neurosci. 2009, 29, 3885–3896. 10.1523/jneurosci.0037-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.; Wu Z.; Luo L. J.; Huang X.; Qian W. Q.; Wang H.; Li S. H.; Liu J. E-cadherin maintains the activity of neural stem cells and inhibits the migration. Int. J. Clin. Exp. Pathol. 2015, 8, 14247. [PMC free article] [PubMed] [Google Scholar]
- Ren Y.-J.; Zhang H.; Huang H.; Wang X.-M.; Zhou Z.-Y.; Cui F.-Z.; An Y.-H. In vitro behavior of neural stem cells in response to different chemical functional groups. Biomaterials 2009, 30, 1036–1044. 10.1016/j.biomaterials.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Hinsch K.; Zupanc G. K. H. Isolation, cultivation, and differentiation of neural stem cells from adult fish brain. J. Neurosci. Methods 2006, 158, 75–88. 10.1016/j.jneumeth.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Conti L.; Cattaneo E. Neural stem cell systems: physiological players or in vitro entities?. Nat. Rev. Neurosci. 2010, 11, 176–187. 10.1038/nrn2761. [DOI] [PubMed] [Google Scholar]
- Bez A.; Corsini E.; Curti D.; Biggiogera M.; Colombo A.; Nicosia R. F.; Pagano S. F.; Parati E. A. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res. 2003, 993, 18–29. 10.1016/j.brainres.2003.08.061. [DOI] [PubMed] [Google Scholar]
- Bayati V.; Gazor R.; Nejatbakhsh R.; Negad Dehbashi F. Enrichment of skin-derived neural precursor cells from dermal cell populations by altering culture conditions. Stem Cell Invest. 2016, 3, 83. 10.21037/sci.2016.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T.; Wang X.-J.; Xie S.-S.; Zhang D.-L.; Wang X.-P.; Li B.-Q.; Ma W.; Xin H. A comparison of proliferative capacity and passaging potential between neural stem and progenitor cells in adherent and neurosphere cultures. Int. J. Dev. Neurosci. 2011, 29, 723–731. 10.1016/j.ijdevneu.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Wang S.; Guan S.; Li W.; Ge D.; Xu J.; Sun C.; Liu T.; Ma X. 3D culture of neural stem cells within conductive PEDOT layer-assembled chitosan/gelatin scaffolds for neural tissue engineering. Mater. Sci. Eng., C 2018, 93, 890–901. 10.1016/j.msec.2018.08.054. [DOI] [PubMed] [Google Scholar]
- Conti L.; Pollard S. M.; Gorba T.; Reitano E.; Toselli M.; Biella G.; Sun Y.; Sanzone S.; Ying Q.-L.; Cattaneo E.; Smith A. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005, 3, e283 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard S. M.; Conti L.; Sun Y.; Goffredo D.; Smith A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb. Cortex 2006, 16, i112–i120. 10.1093/cercor/bhj167. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Pollard S.; Conti L.; Toselli M.; Biella G.; Parkin G.; Willatt L.; Falk A.; Cattaneo E.; Smith A. Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Mol. Cell. Neurosci. 2008, 38, 245–258. 10.1016/j.mcn.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Li Y.-C.; Tsai L.-K.; Wang J.-H.; Young T.-H. A neural stem/precursor cell monolayer for neural tissue engineering. Biomaterials 2014, 35, 1192–1204. 10.1016/j.biomaterials.2013.10.066. [DOI] [PubMed] [Google Scholar]
- de la Rosa J.; Sáenz Antoñanzas A.; Shahi M. H.; Meléndez B.; Rey J. A.; Castresana J. S. Laminin-adherent versus suspension-non-adherent cell culture conditions for the isolation of cancer stem cells in the DAOY medulloblastoma cell line. Tumor Biol. 2016, 37, 12359–12370. 10.1007/s13277-016-5119-6. [DOI] [PubMed] [Google Scholar]
- Tian L.; Prabhakaran M. P.; Hu J.; Chen M.; Besenbacher F.; Ramakrishna S. Synergistic effect of topography, surface chemistry and conductivity of the electrospun nanofibrous scaffold on cellular response of PC12 cells. Colloids Surf., B 2016, 145, 420–429. 10.1016/j.colsurfb.2016.05.032. [DOI] [PubMed] [Google Scholar]
- Chooi W. H.; Chew S. Y. Modulation of cell-cell interactions for neural tissue engineering: Potential therapeutic applications of cell adhesion molecules in nerve regeneration. Biomaterials 2019, 197, 327–344. 10.1016/j.biomaterials.2019.01.030. [DOI] [PubMed] [Google Scholar]
- Li L.; Bennett S. A. L.; Wang L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adhes. Migr. 2012, 6, 59–73. 10.4161/cam.19583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka M.; Ise H.; Harada I.; Koshimizu U.; Maruyama A.; Akaike T. Embryonic undifferentiated cells show scattering activity on a surface coated with immobilized E-cadherin. J. Cell. Biochem. 2008, 103, 296–310. 10.1002/jcb.21406. [DOI] [PubMed] [Google Scholar]
- Mattias L.; Haque A.; Adnan N.; Akaike T. The effects of artificial E-cadherin matrix-induced embryonic stem cell scattering on paxillin and RhoA activation via alpha-catenin. Biomaterials 2014, 35, 1797–1806. 10.1016/j.biomaterials.2013.11.042. [DOI] [PubMed] [Google Scholar]
- Cherry J. F.; Bennett N. K.; Schachner M.; Moghe P. V. Engineered N-cadherin and L1 biomimetic substrates concertedly promote neuronal differentiation, neurite extension and neuroprotection of human neural stem cells. Acta Biomater. 2014, 10, 4113–4126. 10.1016/j.actbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Zhu M.; Lin S.; Sun Y.; Feng Q.; Li G.; Bian L. Hydrogels functionalized with N-cadherin mimetic peptide enhance osteogenesis of hMSCs by emulating the osteogenic niche. Biomaterials 2016, 77, 44–52. 10.1016/j.biomaterials.2015.10.072. [DOI] [PubMed] [Google Scholar]
- Li R.; Xu J.; Wong D. S. H.; Li J.; Zhao P.; Bian L. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/beta-catenin signaling. Biomaterials 2017, 145, 33–43. 10.1016/j.biomaterials.2017.08.031. [DOI] [PubMed] [Google Scholar]
- Cao L.; Zhang Y.; Qian M.; Wang X.; Shuai Q.; Gao C.; Lang R.; Yang J. Construction of multicellular aggregate by E-cadherin coated microparticles enhancing the hepatic specific differentiation of mesenchymal stem cells. Acta Biomater 2019, 95, 382. 10.1016/j.actbio.2019.01.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.